Abstract
Opioid misuse and abuse is a major international public health issue. Opioid use disorder (OUD) is largely maintained by a desire to suppress aversive opioid withdrawal symptoms. Opioid withdrawal in patients seeking abstinence from illicit or prescribed opioids is often managed by provision of a μ-opioid agonist/partial agonist in combination with concomitant medications. Concomitant medications are administered based on their ability to treat specific symptoms rather than a mechanistic understanding of the opioid withdrawal syndrome; however, their use has not been statistically associated with improved treatment outcomes. Understanding the central and/or peripheral mechanisms that underlie individual withdrawal symptom expression in humans will help promote medication development for opioid withdrawal management. To support focused examination of mechanistically supported concomitant medications, this review summarizes evidence from preclinical (N = 68) and human (N = 30) studies that administered drugs acting on the dopamine, serotonin, cannabinoid, orexin/hypocretin, and glutamate systems and reported outcomes related to opioid withdrawal. These studies provide evidence that each of these systems contribute to opioid withdrawal severity. The Food and Drug Administration has approved medications acting on these respective systems for other indications and research in this area could support the repurposing of these medications to enhance opioid withdrawal treatment. These data support a focused examination of mechanistically informed concomitant medications to help reduce opioid withdrawal severity and enhance the continuum of care available for persons with OUD.
Introduction
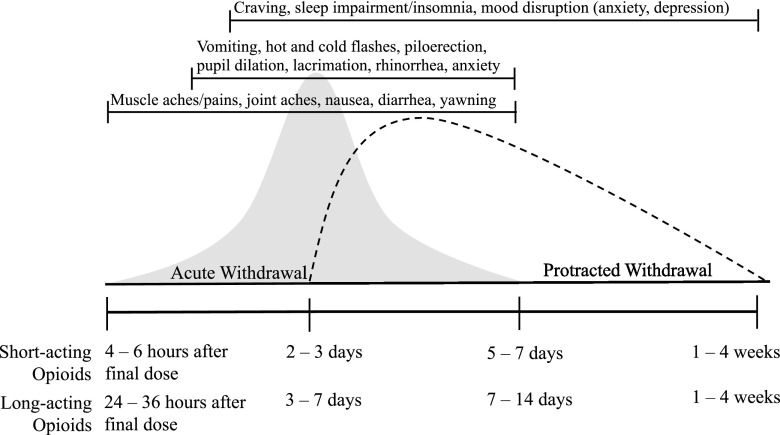
The increased prevalence of opioid use disorder (OUD) and opioid-related morbidity and mortality is a national and international public health crisis. Continuous opioid exposure results in physiologic dependence and a prominent and aversive opioid withdrawal syndrome upon opioid discontinuation. As shown in Fig. 1, opioid withdrawal manifests as an acute syndrome that begins within hours of the final dose and can last for up to 14 days, and is followed by a protracted syndrome that can persist for several additional weeks (Martin and Jasinski, 1969; Jasinski, 1981). Opioid withdrawal symptoms vary in severity and expression across individuals and continued opioid use and opioid relapse in persons with OUD are driven, in part, by a goal of avoiding opioid withdrawal symptoms and associated cravings (Hutcheson et al., 2001; Negus and Banks, 2018). As a result, OUD pharmacotherapies frequently aim to suppress symptoms of acute opioid withdrawal, an approach first reported in 1949 (Powers, 1949) and validated in 1965 (Dole and Nyswander, 1965).
Fig. 1.
Schematic of the human opioid withdrawal syndrome. The prototypical human opioid withdrawal curve is shown here for both the acute (gray) and protracted (dashed line) phases. Although the specific duration and magnitude of withdrawal varies across individuals, the acute withdrawal syndrome generally begins 4–6 (short acting) and 24–36 (long acting) hours after opioid discontinuation, peaks within 2 to 3 (short acting) and 3–7 (long acting) days, and ends within 5–7 (short acting) and 7–14 (long acting) days, as indicated by the vertical lines. Although specific symptoms emerge at different times for different individuals, an expected symptom progression is indicated in the brackets across the top of the figure; the ends of the brackets signify when the clusters of specific symptoms generally emerge and dissipate. Protracted withdrawal is hypothesized to extend for an additional 1–4 weeks following resolution of the acute withdrawal syndrome. Note: additional symptoms may exist that are not included here.
The current standard-of-care treatment of opioid withdrawal management is to administer methadone or buprenorphine, which act as full or partial agonists on the μ-opioid receptor, respectively (Gowing et al., 2016). Both medications suppress symptoms of withdrawal when administered chronically; however, symptoms reemerge when doses are tapered down, which occurs when patients are withdrawn from opioids they have been using illicitly or as prescribed to them for pain management (Farrell, 1994). Several concomitant medications are commonly administered to manage emergent symptoms of acute withdrawal (Hillhouse et al., 2010; Dunn et al., 2011; Schuckit, 2016). These include nonsteroidal anti-inflammatory drugs for muscle aches and pains, promethazine for nausea/vomiting, loperamide for diarrhea, and klonipin or trazodone for sleep disturbance. The use of these medications is based on their perceived effectiveness for individual symptoms rather than a mechanistic understanding of the opioid withdrawal syndrome or empirical evidence of their efficacy in reducing opioid withdrawal severity. The only retrospective evaluation conducted on this topic reported no significant association between these medications and opioid withdrawal outcomes (Hillhouse et al., 2010).
The exceptions to this are two adrenergic agonists. The first is clonidine, which is prescribed off-label (Gold et al., 1978), and the second is lofexidine (Lucemyra), which was approved by the Food and Drug Administration (FDA) for opioid withdrawal in 2018 (Gorodetzky et al., 2017). Administration of clonidine and lofexidine is premised upon a mechanistic understanding that the adrenergic system modulates some opioid withdrawal symptoms through locus coeruleus–mediated noradrenergic hyperactivity (Gold et al., 1979; van Dongen, 1981). The majority of research examining adrenergic agonist withdrawal suppression has focused on clonidine, and there is strong evidence that clonidine reliably suppresses some symptoms of opioid withdrawal while concurrently increasing the severity of other symptoms (Gianutsos et al., 1976; Schulz and Herz, 1977; Bednarczyk and Vetulani, 1978). The net result is that clonidine produces mild-to-moderate opioid withdrawal suppression (Gowing et al., 2016), the degree to which varies across individuals (Dunn et al., 2018b).
Despite the fact that OUD treatment is largely organized around the provision of pharmacotherapy to manage opioid withdrawal, concomitant medications are not selected based on a mechanistic understanding of the central and/or peripheral systems underlying individual opioid withdrawal symptom expression in humans. This review summarizes preclinical animal and human evidence that the dopamine (DA), serotonin (5-HT), cannabinoid, orexin/hypocretin, and glutamate neurotransmitter systems directly contribute to the expression and/or severity of specific opioid withdrawal symptoms. These systems were selected for review because of the body of literature supporting their pivotal involvement in the opioid withdrawal syndrome and the fact that several FDA-approved or investigational medications that act on these systems exist and could be evaluated and ultimately repurposed for an opioid withdrawal indication. The expectation is not that these medications would be used in lieu of opioid agonists but that they would be administered in combination with opioid agonist therapy to replace the aforementioned symptomatic medications and improve opioid withdrawal management. The norepinephrine system is not discussed here because lofexidine has gained recent FDA approval and the GABA system was omitted because the majority of FDA-approved medications acting on that system have prominent abuse liability profiles that limit their adoption in OUD treatment settings. The overarching goal of this review is to provide a resource to support the prospective examination of candidate concomitant medications for opioid withdrawal symptom management.
Materials and Methods
This review reports the results of studies that described opioid withdrawal symptom incidence and/or severity following exposure to agents acting on the aforementioned systems. As shown in Table 1, there is generally good concordance for a number of opioid withdrawal symptom categories between animal and human models of opioid withdrawal, although the expression of symptoms within each category is often species specific. Withdrawal in rodents generally includes increased jumping, self-directed behaviors (increased grooming and penile licking), hyperactivity (locomotion, digging, and rears), vocalization, ptosis, wet dog shakes, teeth chattering, secretions (lacrimation, rhinorrhea, and salivation), respiration, and gastrointestinal motility (increased defecation and loose stools/diarrhea). Human withdrawal expression is comprised of several symptoms (as indicated in Fig. 1; Table 1) that vary across individuals with regard to incidence and severity; no demographic or drug use characteristics have been identified that reliably predict the severity of human opioid withdrawal symptoms. An exception to this is gender, for which there is substantial preclinical evidence to support causal associations between gonadal hormones and opioid effects (Huhn et al., 2018), and some limited human empirical evidence that gender may moderate withdrawal severity (Dunn et al., 2018b).
TABLE 1.
Withdrawal systems and symptoms conserved across species
Only symptoms for which there is correspondence across species are presented; additional symptoms exist that are species specific but are not included here.
| Withdrawal System and Symptoms | Human | Rat | Mouse |
|---|---|---|---|
| Anhedonia | |||
| Brain stimulation threshold | X | X | |
| Conditioned aversion | X | X | |
| Depression | X | ||
| Anxiety/escape behaviors (rodents) | |||
| Anxiety | X | ||
| Burrowing | X | X | |
| Digging | X | X | |
| Jumping | X | X | |
| Rearing | X | X | |
| Changes in body temperature | |||
| Hot and/or cold flashes | X | ||
| Hypothermia | X | X | |
| Piloerection (gooseflesh) | X | X | X |
| Teeth chattering | X | X | |
| Gastrointestional | |||
| Defecation | X | X | |
| Diarrhea, Loose stool | X | X | X |
| Nausea | X | ||
| Vomiting | X | ||
| Hyperactivity, self-directed behaviors | |||
| Chewing | X | X | |
| Grooming | X | X | |
| Licking | X | X | |
| Locomotion | X | X | X |
| Penile licking | X | X | |
| Restlessness | X | ||
| Motivation to use | |||
| Craving | X | ||
| Progressive ratio breakpoint | X | X | X |
| Reinstatement | X | X | |
| Pain, hyperalgesia | |||
| Joint aches and pains | X | ||
| Muscle aches and pains | X | ||
| Reactive to touch | X | X | |
| Ultrasonic vocalizations | X | X | |
| Writhing | X | X | |
| Ptosis | X | X | |
| Secretions | |||
| Lacrimation | X | X | X |
| Rhinorrhea | X | X | X |
| Salivation | X | X | |
| Urination | X | X | |
| Tremors/shaking | |||
| Facial tremor | X | X | |
| Forepaw tremors | X | X | |
| Hand tremors | X | ||
| Head shakes | X | X | |
| Limb shakes | X | X | |
| Wet dog shakes | X | X |
Reviewed papers were identified through PubMed searches inclusive of all years using the following search terms: opioid, opiate, withdrawal AND dopamine, serotonin, 5-HT, cannabinoid, cannabis, THC, orexin, hyopcretin, and glutamate (where THC denotes tetrahydrocannabinol). Eligible studies included outcome data about the opioid withdrawal syndrome and were conducted in rodents, nonhuman primates, or humans with opioid physical dependence and administered an agent that acted on one or more of the systems of interest. A total of 68 preclinical and 30 human studies were identified and reviewed. Preclinical studies examined withdrawal in animals with opioid physical dependence either by administering an opioid antagonist (in a precipitated withdrawal model) or through abrupt opioid discontinuation (in a spontaneous withdrawal model). Since only a limited number of human empirical studies (n = 16) were available for this review, case reports (n = 7), retrospective chart reviews (n = 3), a prospective cohort study (n = 1), an online survey or quality-assurance surveys (n = 2), and a secondary outcome analysis (n = 1) were included to provide breadth of coverage. All of the results discussed subsequently refer to opioid withdrawal symptoms that were observed during the acute (vs. protracted) opioid withdrawal. Specific study details are provided in accompanying tables, which have been organized into preclinical tables for dopamine (Table 2), serotonin (Table 3), cannabinoids (Table 4), orexin (Table 5), glutamate (Table 6), and medications acting on multiple systems (Table 7), and human tables categorized as empirical (Table 8) and nonempirical (Table 9) reports.
TABLE 2.
Preclinical examinations of the dopamine system on the opioid withdrawal syndrome
Only results pertaining to effects of non-opioid drugs on opioid withdrawal symptoms are presented.
| Reference | Drugs Evaluated for Opioid Withdrawal Outcomes (Dose and Route) | Species | Sample Size | Withdrawal Method | Dependence Method | Withdrawal Signs Assessed | Summary of Withdrawal-Specific Results |
|---|---|---|---|---|---|---|---|
| Ary and Lomax (1976) | Apomorphine (20 µg); Dopamine (10, 50, 100, and 200 µg), Pimozide (0.5 and 1 µg, i.p.). Administered to rostral hypothalamus and lateral ventricle unless indicated. | Rat | ≥5 | Precipitated, naloxone (1 mg/kg; i.p.) on day 3 | Morphine (75 mg) subcutaneous implant | Body temperature | Rostral hypothalamus injections: apomorphine (D2 agonist; 20 µg) increased the hypothermia produced by naloxone, pimozide (D2 antagonist; 0.5 and 1 µg) and dopamine had no effect. Lateral ventricle injections: no effects on naloxone-induced hypothermia. |
| Cox et al. (1976) | Apomorphine (1.25 mg/kg) and pimozide (0.5 mg/kg) administered intraperitoneally; pimozide (0.5 µg) administered intracerebroventricularly. | Rat | NR | Precipitated, naloxone (1 mg/kg; i.p.) on day 3 | Morphine (75 mg) subcutaneous implant | Body temperature, chewing, diarrhea, head shakes, facial tremor, grooming, licking, sneezing, teeth chatter, wet dog shakes, and writhing. Total withdrawal score | Apomorphine (D2 agonist) (1.25 mg/kg, i.p.) decreased chewing, teeth chattering, wet dog shakes, and writhing. Pimozide (D2 antagonist) (0.5 mg/kg, i.p.) reduced hypothermia and increased chewing, head shakes, and writhing. Intracerebroventricularly: pimozide (0.5 µg, i.p.) significantly blocked hypothermia. |
| el-Kadi and Sharif (1998) | Apomorphine (0.5, 1, 2.5, 5, 10, 20, and 30 mg/kg); L-DOPA (100 and 500 mg/kg); haloperidol (0.2 and 1.0 mg/kg); pimozide (2 and 5 mg/kg); domperidone (5 and 10 mg/kg); flupenthixol (0.05 and 0.1 mg/kg); sulpride (5 mg/kg). Administered intraperitoneally. | Mouse | 8–10 | Precipitated, naloxone (1 mg/kg, i.p.) on day 7 | Escalating morphine doses up to 160 mg/kg, s.c., by day 6 | Body temperature, body weight, burrows, jumping, and wet dog shakes | L-DOPA (dopamine precursor) (100 and 500 mg/kg) reduced jumping, burrows, body weight loss, hypothermia (100 and 500 mg/kg), and wet dog shakes (500 mg/kg). Apomorphine (D2 agonist) increased burrows and jumping at low doses (2.5 and 5 mg/kg) and decreased them at high doses (10, 20, and 30 mg/kg). Apomorphine also decreased body weight loss (2.5, 5, and 10 mg/kg) and increased wet dog shakes (2.5, 5, 10, and 20 mg/kg) and hypothermia (all doses). Haloperidol (D2 antagonist) reduced jumps (0.2 and 1 mg/kg), wet dog shakes (0.2 and 1 mg/kg), burrows (0.2 and 1 mg/kg), and body weight loss (1 mg/kg). Pimozide (D2 antagonist) decreased jumps (2 and 5 mg/kg), and wet dog shakes, burrows, and body weight loss (5 mg/kg). Domperidone (D2 antagonist) decreased jumping and hypothermia (10 mg/kg) but increased body weight loss (10 mg/kg). Flupenthixol (D2 antagonist) decreased wet dog shakes and burrows (0.05 and 0.1 mg/kg), and jumping, body weight loss, and hypothermia (0.1 mg/kg). Sulpride (D2 agonist) (5 mg/kg) increased all symptoms. |
| Herz et al. (1974) | d-Amphetamine (0.5, 1, 2, and 5 mg/kg); cocaine (5, 10, and 25 mg/kg); L-DOPA (100 mg/kg); apomorphine (1, 2.5, and 5 mg/kg); desipramine (2.5, 5, and 10 mg/kg). Administered intraperitoneally. | Rat | 8–16 | Precipitated, levallorphan (1 mg/kg, i.p.) on day 10 | Morphine subcutaneous implant (dose NR), six pellets implanted over 10 days | Eye twitching, diarrhea, flying, jumping, lacrimation, ptosis, rhinorrhea, salivation, teeth chattering, wet dog shakes, and writhing | d-Amphetamine increased jumping (0.5 and 1, 2 mg/kg) and teeth chattering (1 mg/kg), and decreased wet dog shakes (0.5 and 1 mg/kg). All signs decreased at 2 and 5 mg/kg. Cocaine increased jumping (10 mg/kg) and decreased wet dog shakes (10 mg/kg). All signs decreased at 25 mg/kg. L-DOPA (dopamine precursor) decreased ptosis and diarrhea (100 mg/kg) but increased eye twitching (100 mg/kg). Apomorphine (D2 agonist) decreased ptosis and increased eye twitching at 1 mg/kg, increased ptosis and decreased diarrhea at 2.5 mg/kg, and decreased ptosis and diarrhea but increased eye twitching at 5 mg/kg. Desipramine decreased ptosis and diarrhea but increased eye twitching at all doses, and increased jumping at 5, 10, and 20 mg/kg. |
NR, not reported.
TABLE 3.
Preclinical examinations of the serotonin system on the opioid withdrawal syndrome
Only results pertaining to effects of non-opioid drugs on opioid withdrawal symptoms are presented.
| Reference | Drugs Evaluated for Opioid Withdrawal Outcomes (Dose and Route) | Species | Sample Size | Withdrawal Method | Dependence Method | Withdrawal Signs Assessed | Summary of Withdrawal-Specific Results |
|---|---|---|---|---|---|---|---|
| Cervo et al. (1981) | d-fenfluramine (5 mg/kg), cyproheptadine HLC (10 mg/kg), clonidine (0.1 mg/kg), phentolamine methane sulphonate (5 mg/kg), piperoxane HCL (5, 10 mg/kg), phenoxybenzamine HCL (20 mg/kg), prazosin HCL (10 mg/kg), propranolol (10 mg/kg), haloperidol (1 mg/kg), piribedil monometane sulphonate (60 mg/kg). Administered intraperitoneally. | Rat | ≥18 | Precipitated, naloxone (1 mg/kg, i.p.) on day 11 | Escalating morphine (route NR) doses up to 160 mg/kg by day 7 | Diarrhea, flat posture, jumping, ptosis, salivation, teeth chattering, vocalization on touch, and wet dog shakes | d-fenfluramine (SSRI) reduced jumping only. Clonidine (α2 agonist) reduced diarrhea and ptosis. Phenoxybenzamine (adrenergic antagonist) reduced diarrhea. The adrenergic antagonists piperoxane, phentolamine methane sulphonate, prazosin (α1), propranolol (β1), haloperidol, and peribedil monometane sulphonate (α2) had no effects. |
| el-Kadi and Sharif (1995) | Cyproheptadine (0.5, 1, 5, and 10 mg/kg), methysergide (0.1, 1, 3, and 6 mg/kg). Administered intraperitoneally. | Mouse | 8–10 | Precipitated, naloxone (1 mg/kg, i.p.) on day 7 | Escalating morphine doses up to 160 mg/kg, s.c., by day 6 | Body temperature, body weight, burrowing, jumping, and wet dog shakes | Cyproheptadine (5-HT2A,2C antagonist) increased jumping (0.5 and 1 mg/kg) and hypothermia (all doses) and decreased jumping (5 and 10 mg/kg), wet dog shakes (all doses), burrows (10 mg/kg), and body weight loss (5 and 10 mg/kg). Methysergide (5-HT2 antagonist and 5-HT1A agonist) decreased burrowing, jumping, and wet dog shakes (all doses), and body weight loss (1, 3, and 6 mg/kg) but increased hypothermia (1, 3, and 6 mg/kg). |
| Higgins et al. (1991) | Ondansetron (0.01, 0.1, and 1 mg/kg), MDL 7222 (1 and 3 mg/kg). Administered subcutaneously. | Rat | 6 | Precipitated, naloxone (0.5 mg/kg, s.c.) on days 3 and 4 | Morphine (75 mg) subcutaneous implant | Body temperature, paw shakes, mouth movements, salivation, startle, rhinorrhea, penile grooming, teeth chattering, weight loss, and wet dog shakes | Ondansetron (5-HT3 antagonist) attenuated body weight loss (0.1 and 1 mg/kg). MDL 7222 (5-HT3 antagonist) also attenuated body weight loss (3 mg/kg). |
| Pang et al. (2016) | Glemanserin (0.5 mg/kg). Administered intraperitoneally. | Mouse | 10 | Precipitated, naloxone (5 mg/kg, i.p.) on day 7 | Escalating morphine doses up to 100 mg/kg, s.c., by day 6 | Body grooming, burrowing, digging body, extended posture, face grooming, head shakes, jumping, paw licking, penile licking, rearing, scratching, and wet dog shakes | Glemanserin (5-HT2A antagonist) decreased jumping (0.5 mg/kg). |
| Pinelli et al. (1997) | Ondansetron (0, 1, 2, and 4 mg/kg). Administered intraperitoneally. | Rat | 8 | Precipitated, naloxone (30 mg/kg, i.p.) on day 4 | Escalating morphine doses up to 100 mg/kg, i.p., by day 4 | Body temperature, defecation, jumping, salivation, urine excretion, and wet dog shakes | Ondansetron (5-HT3 antagonist) reduced defecation (1, 2, and 4 mg/kg), body temperature (4 mg/kg), and jumping (2 and 4 mg/kg), but increased wet dog shakes (1, 2, and 4 mg/kg). |
| Romandini et al. (1984) | (+)-Fenfluramine (5 mg/kg); m-chlorophenylpiperazine (CPP; 2.5 mg/kg), Clonidine (0.5 mg/kg). Administered intraperitoneally. | Rat | ≥16 | Precipitated, naloxone (1 mg/kg, i.p.). Experiment 1 tested on Day 3; Experiment 2 on Day 5 | Experiment 1: Morphine (75 mg) subcutaneous implant; Experiment 2: Escalating morphine doses up to 40 mg/kg, i.p., by day 5 | Abnormal posture, diarrhea, jumping, ptosis, salivation, teeth chattering, vocalization on touch, wet dog shakes | (−)-fenfluramine (SSRI) and and m-CPP (5-HT agonist) decreased jumping. Clonidine (α2 agonist) reduced wet dog shakes and increased jumping. |
| Samanin et al. (1980) | Quipazine (5 mg/kg), m-CPP (2.5 mg/kg); clonidine (0.5 mg/kg), haloperidol (0.5 mg/kg), propranolol hydrochloride (10 mg/kg). Administered intraperitoneally. | Rat | NR | Precipitated, naloxone (1 mg/kg, i.p.) on day 11 | Escalating morphine doses up to 160 mg/kg, s.c., by day 7 | Diarrhea, dyspnea, flat posture, jumping, ptosis, teeth chattering, salivation, vocalization on touch, and wet dog shakes | m-CPP (5-HT2C agonist) decreased diarrhea and jumping. Quizapine (5-HT2A/3 agonist) decreased jumping. Clonidine (α2 agonist) decreased diarrhea and ptosis. Propranolol and haloperidol had no effect. |
| Shahidi and Hashemi-Firouzi (2014) | AS19 (3, 5, and 10 mg/kg); SB269970 (1, 3, and 10 mg/kg). Administered intraperitoneally. | Mouse | 8 | Precipitated, naloxone (3 mg/kg, s.c.) on day 5 | Escalating morphine doses up to 45 mg/kg, s.c., by day 5 | Body grooming, body weight, face grooming, head shakes, jumping, limb shakes, sniffing, standing, teeth chattering, and writhing | AS19 (5-HT7 agonist) decreased weight loss (all doses); jumping, head shaking, standing, and writhing (5 and 10 mg/kg); and teeth chattering and limb shaking (10 mg/kg). SB269970 (5-HT7A antagonist) increased teeth chattering (10 mg/kg) and limb shaking (1 mg/kg). |
| Wu et al. (2015) | Lorcaserin (0.5 mg/kg), clonidine (0.05 mg/kg). Administered intraperitoneally. | Mouse | 10 | Precipitated, naloxone (5 mg/kg, i.p.) on day 5 | Escalating diacetyl morphine doses up to 50 mg/kg, s.c., by day 5 | Body grooming, burrowing, digging body, extended posture, head shakes, jumping, paw licking, penile grooming, rearing, and wet dog shakes | Lorcaserin (5-HT2C agonist) decreased jumping and paw licking. Clonidine (α2 agonist) decreased jumping and paw licking. |
| Zhang et al. (2016) | Lorcaserin (0.5, 0.75, and 1.0 mg/kg), SB242084 (1.0 mg/kg), Clonidine (0.2 mg/kg). Administered intraperitoneally. | Mouse | 9 to 10 | Precipitated, naloxone (5 mg/kg, i.p.) on day 7 | Escalating morphine doses up to 100 mg/kg, s.c., by day 6 | Body grooming, burrowing, defecation, digging body, extended posture, face grooming, head shakes, jumping, paw licking, penile licking, piloerection, ptosis, rearing, scratching, urination, vocalization on touch, and wet dog shakes | Lorcaserin (5-HT2C agonist) decreased jumping, burrowing, body grooming, rearing, wet dog shakes, paw licking, penile licking, and scratching (all doses). Clonidine (α2 agonist) decreased jumping, burrowing, body grooming, rearing, wet dog shakes, head shakes, paw licking, penile licking, and scratching (0.2 mg/kg). SB242084 (5-HT2C antagonist; 0.2 mg/kg) pretreatment blocked lorcaserin (0.5 mg/kg) suppression of jumping. |
m-CPP, meta-chlorophenylpiperazine; SSRI, selective serotonin reuptake inhibitor; NR, not reported.
TABLE 4.
Preclinical examinations of the cannabis system on the opioid withdrawal syndrome
Only results pertaining to effects of non-opioid drugs on opioid withdrawal symptoms are presented.
| Reference | Drugs Evaluated for Opioid Withdrawal Outcomes (Doses and Route) | Species | Sample Size | Withdrawal Method | Dependence Method | Withdrawal Signs Assessed | Summary of Withdrawal-Specific Results |
|---|---|---|---|---|---|---|---|
| Bhargava (1976) | THC (2.5, 5, and 10 mg/kg). Administered intraperitoneally. | Mouse | NR | Precipitated, naloxone (30 mg/kg, s.c.) on day 3 | Morphine (75 mg) subcutaneous implant for 3 days | Defecation, jumping, and rearing | THC (all doses) decreased defecation, jumping, and rearing. |
| Cichewicz and Welch (2003) | THC (0, 20, and 50 mg/kg). Administered orally. | Mouse | 6 | Precipitated, naloxone (1 mg/kg, s.c.) 12 hours after final morphine dose | Escalating morphine (oral) doses up to 300 mg/kg by day 7 | Diarrhea and jumping | THC (all does reduced jumping. |
| Del Arco et al. (2002) | AM404 (2 and 10 mg/kg). Administered intraperitoneally. | Mouse | 8–10 | Precipitated, naloxone (1 mg/kg, route NR) on day 6; spontaneous withdrawal on day 6, 7, 8, or 9 | Escalating morphine doses up to 100 mg/kg, i.p., by day 5 | Abdominal constrictions, body weight, jumping, locomotor activity, piloerection, swallowing movements, and wet dog shakes | AM404 (anandamide transport inhibitor) had no effect on naloxone-precipitated withdrawal. AM404 decreased spontaneous withdrawal signs of jumping (all doses) and locomotor activity (10 mg/kg). |
| Gamage et al. (2015) | THC (1, 3, and 10 mg/kg), JZL184 (4 and 40 mg/kg), PF-3845 (1, 3, and 10 mg/kg), and SA-57 (1.25, 5, and 12.5 mg/kg). JZL184, PF-3845, and SA-57 administered intraperitoneally; THC administered subcutaneously. | Mouse | 16–28 | Precipitated, naloxone (0.3, 1 mg/kg, s.c.) on day 2 | Morphine (75 mg) subcutaneous implant for 2 days | Diarrhea, head shakes, jumping, and paw tremors | THC (3 and 10 mg/kg), JZL184 (MAGL inhibitor; 40 mg/kg), SA-57 (dual FAAH/MAGL inhibitor; 5 and 12.5 mg/kg) decreased jumping. PF-3845 (FAAH inhibitor) had no effect. |
| Hine et al. (1975a) | THC (1, 2, 5, and 10 mg/kg). Administered intraperitoneally. | Rat | 7 | Precipitated, naloxone (4 mg/kg, i.p.) on day 3 | Morphine (75 mg) subcutaneous implant for 3 days | Abnormal posture, chewing, defection, diarrhea, ear blanching, ptosis, teeth chattering, vocalization, wet dog shakes, and total withdrawal score | THC (5 and 10 mg/kg) reduced total withdrawal scores, defection, diarrhea, and wet dog shakes. |
| Hine et al. (1975b) | Groups 1 and 2: THC (1 mg/kg). Group 3: THC (10 mg/kg). Administered intraperitoneally. | Rat | 8–11 | Precipitated, naloxone (4 mg/kg, i.p.) on day 13 (group 1) or day 26 (groups 2 and 3) | Group 1: daily methadone injections (10 mg/kg, s.c.) for 13 days. Groups 2 and 3: daily methadone injections (up to 30 mg/kg, s.c.) for 26 days | Abnormal posture, body temperature, chewing, defecation, ear blanching, escapes, ptosis teeth chattering turning, vocalization on touch, wet dog shakes, and total withdrawal score | THC (1 mg/kg) decreased defecation, diarrhea, earn blanching, vocalization on touch, and total withdrawal score in animals in group 1 who received 13 days of methadone exposure. THC (10 mg/kg) decreased defecation, diarrhea, escapes, turns, wet dog shakes, and total withdrawal score in group 3 who received 26 days of methadone exposure. |
| Hine et al. (1975c) | THC (2 mg/kg), cannabidiol (10 mg/kg), THC (2 mg/kg) + cannabidiol (10 mg/kg). Administered intraperitoneally. | Rat | 8–9 | Precipitated, naloxone (4 mg/kg, i.p.) on day 3 | Morphine (75 mg) subcutaneous implant for 3 days | Abnormal posture, audible grinding, chewing, defecation, escapes, diarrhea, ear blanching, ptosis, teeth chattering, vocalization on touch, wet dog shakes, and writhing | THC (2 mg/kg) decreased total withdrawal scores, defecation, and diarrhea. Cannabidiol alone had no effect. THC (2 mg/kg) + cannabidol (10 mg/kg) decreased total withdrawal scores, defecation, ear blanching, escapes, and wet dog shakes. |
| Li et al. (2019) | AM1710 (5 mg/kg). Administered intraperitoneally. | Mouse | 8 | Precipitated, naloxone (5 mg/kg, i.p.) after final morphine dose | Daily morphine injections (10 mg/kg, i.p.) for 12 days | Body temperature, body weight, and jumping | AM1710 (CB2 agonist; 5 mg/kg) decreased jumping. |
| Lichtman et al. (2001) | THC (0, 0.1, 0.3, 1, 3, and 10 mg/kg). Administered subcutaneously. | Mouse | 6 | Precipitated, naloxone (1 mg/kg, i.p.) on day 5 | Morphine (75 mg) subcutaneous implant for 5 days | Diarrhea, head shake, jumping, paw tremor, ptosis, scratching, and writhing | THC dose dependently reduced head shakes and paw tremors (specific doses that produced effects NR). |
| Mas-Nieto et al. (2001) | Rimonbant (10 mg/kg), rimonobant (10 mg/kg) + morphine (dose escalated to 100 mg/kg by day 5). Administered intraperitoneally. | Mouse | 7–10 | Precipitated, naloxone (1 mg/kg, s.c.) on day 6 | Escalating morphine up to 100 mg/kg, i.p., by day 5 | Body tremor, jumping, paw tremor, ptosis, sniffing, and wet dog shakes | Rimonobant (CB1) inverse agonist/antagonist; 10 mg/kg) coadministered with morphine decreased jumping and wet dog shakes. SR141716A had no effect when administered alone. |
| Ramesh et al. (2013) | THC (dose NR), rimonabant (dose NR), JZL184 (4, 16, and 40 mg/kg), PF-3845 (1, 3, and 10 mg/kg), SA-57 (2.5, 5, and 12.5 mg/kg). Rimonabant, JZL184, PF-3845, and SA-57 administered intraperitoneally; THC administered subcutaneously. | Mouse | 10 to 11 | Spontaneous, morphine pellets extracted on day 3 | Morphine (75 mg) subcutaneous implant for 3 days | Body weight, diarrhea, head, shakes, and paw tremors | JZL184 (MAGL inhibitor) decreased body weight, diarrhea, and paw tremors at all doses, and head shakes and jumping at 16 and 40 mg/kg. Rimonabant reversed all effects of JZL184 (40 mg/kg). PF-3845 (FAAH inhibitor) decreased head shakes, jumping, and paw tremors (10 mg/kg). THC blocked all withdrawal signs. JZL184 (4 mg/kg) + PF-3845 (10 mg/kg) decreased all withdrawal signs. SA-57 (dual FAAH/MAGL inhibitor) decreased diarrhea, jumping, and paw flutters (2.5 and 5 mg/kg), and head shakes (5 mg/kg). |
| Rubino et al. (2000) | Rimonobant (10 mg/kg per day). Administered intraperitoneally. | Rat | ≥6 | Precipitated, naloxone (10 mg/kg, i.p.) on day 5 (AM and PM sessions) | Morphine (75 mg) subcutaneous implant for 5 days | Digging, grooming, head shakes, jumping, penile licking, rearing, salivation, teeth chattering, wet dog shakes, writhing, and total withdrawal score | Rimonobant (CB1 inverse agonist/antagonist; 10 mg/kg per day) decreased diarrhea, digging, teeth chattering, penile licking, and total withdrawal score. SR141716A increased salivation. |
| Shahidi and Hasenein (2011) | URB597 (0.03, 0.1, 0.3, 0.5, and 1 mg/kg). Administered intraperitoneally. | Rat | 9 | Precipitated, naloxone (3 mg/kg, s.c.) on day 8 | Escalating morphine doses up to 66 mg/kg, s.c., by day 7 | Body weight, face grooming, jumping paw tremor, penile licking, rearing, sniffing standing, teeth chattering, and wet dog shakes | URB597 (FAAH inhibitor) decreased body weight loss, face grooming, jumping, penis licking, sniffing, and teeth chattering (all doses tested). URB597 also decreased standing and wet dog shakes (0.1, 0.3, 0.5, and 1 mg/kg), paw tremors (0.5 mg/kg), and rearing (1 mg/kg). |
| Vela et al. (1995) | THC (10 mg/kg; experiment 1 only), anandamide (0.1, 1, and 5 mg/kg). Administered intravenously. | Mice | 8–15 | Experiment 1: precipitated, naloxone (1 mg/kg, i.p.) on day 3; experiment 2: precipitated, naloxone (5 mg/kg, s.c.) on day 5 | Experiment 1: morphine (75 mg) subcutaneous implant for days; experiment 2: escalating, morphine (route NR) doses up to 45 mg/kg by day 5 | Body weight and jumping | Experiment 1: anandamide decreased jumping (5 mg/kg) and body weight loss (1 mg/kg). THC (10 mg/kg) decreased jumping and body weight. Experiment 2: anandamide decreased body weight loss (1 and 5 mg/kg); THC, not tested. |
AM404, N-(4-hydroxyphenyl) arachidonylethanolamide; CB2, cannabinoid type 2 receptor; FAAH, fatty acid amide hydrolase; MAGL, monoacylglycerol lipase; NR, not reported.
TABLE 5.
Preclinical examination of the orexin/hypocretin system on the opioid withdrawal syndrome
Only results pertaining to effects of non-opioid drugs on opioid withdrawal symptoms are presented.
| Reference | Drugs Evaluated for Opioid Withdrawal Outcomes (Dose and Route)a | Species | Sample Size | Withdrawal Method | Dependence Method | Withdrawal Signs Assessed | Summary of Withdrawal-Specific Results |
|---|---|---|---|---|---|---|---|
| Ahmadi-Soleimani et al. (2014) | SB-334867 (100 µM/0.2 µl). Direct administration to LPGi nucleus. | Rat | 8–13 | Precipitated, naloxone (2 mg/kg, i.p.). Injection occurred following morphine escalation, specific time NR | Escalating mrophine (drinking water) increasing in concentrations up to 0.4 mg/ml and maintained for 15 days | Chewing, defecation, digging, genital licking, grooming, rearing, sniffing, teeth chattering, and wet dog shakes | SB-334867 decreased chewing, genital licking, grooming, rearing, and sniffing. |
| Azizi et al. (2010) | SB-334867 (100 µM/0.2 µl). Direct administration to locus coereleus. | Rat | 9 | Precipitated, naloxone (1 mg/kg, i.p.) 2 hours after morphine administration on day 10 | Morphine (10 mg/kg) delivered every 12 hours for 10 days | Chewing, diarrhea, head tremor, jumping, rearing, scratching, sniffing, teeth chattering, ptosis, and wet dog shakes | SB-334867 decreased chewing, diarrhea, head tremor, scratching, teeth chattering, and wet dog shakes. |
| Davoudi et al. (2016) | SB-334867 (3 mM/0.2 µl). Bicuculline (15 µM/0.2 µl). Direct administration to locus coereleus. | Rat | 7 | Precipitated, naloxone (3 mg/kg, s.c.) on day 8 | Escalating morphine doses up to 66 mg/kg, s.c., by day 7 | Defecation, head tremor, genital licking, rearing, scratching, sniffing, teeth chatttering, wet dog shake, and writhing | SB-334867 decreased defecation, head tremor, gential licking, rearing, scratching, sniffing, wet dog shakes, and writhing. Bicuculline (GABA antagonist) had no effect by itself but when coadministered with SB-334867 reversed effects on head tremor, rearing, scratching, teeth chattering, wet dog shakes, and writhing. |
| Erami et al. (2012) | SB-334867 (1 mM/5 µl). Administered intracerebroventricularly. | Rat | 8 | Precipitated, nalozone (2 mg/kg, s.c.) on day 10 | Morphine (10 mg/kg, s.c.) delivered every 12 hours for 10 days | Chewing, climbing, defecation, diarrhea, head tremor, jumping, rearing, scratching, sniffing, teeth chattering, and wet dog shakes | SB-334867 decreased chewing, climbing, diarrhea, jumping, rearing, rhinorrhea, and teeth chattering. |
| Ghaemi-Jandabi et al. (2014) | Orexin A (100 µM/200 nl); SB-334867 (100 µM/200 nl). Direct administration to locus coereleus. | Rat | 6 to 7 | Precipiated, OXA (0.2 µl) injection to locus coereleus on day 10 | Morphine (10 mg/kg, s.c.) delivered every 12 hours for 9 days | Chewing, head tremor, paw tremor, rearing, scratching, sniffing, teeth chattering, and wet dog shakes | OXA increased chewing, head tremor, rearing, scratching, and wet dog shakes. SB-33847 decreased chewing, head tremor, paw tremor, scratching, sniffing, rearing, and teeth chattering. |
| Hooshmand et al. (2017) | SB-334867 (3 mM/200 nl) + glutamate (100 nM/200 nl). Direct administration to locus coereleus. | Rat | NR | Precipitated, glutamate (100 nM/200 nl) injection to locus coereleus on day 7 | Escalating morphine up to 66 mg/kg, s.c., by day 7 | Chewing, defecation, head tremor, paw tremor, rearing, scratching, sniffing, teeth chattering, wet dog shakes, and writhing | Glutamate increased chewing, paw tremor rearing, scratching, sniffing, wet dog shakes, and writhing. SB-334867 pretreatment did not attenuate glutamate-precipitated withdrawal during the day (rest phase) but decreased chewing, head tremor, paw tremor, rearing, sniffing, scratching, teeth chattering, wet dog shakes, and writhing during the night (active phase). |
| Hooshmandi et al. (2017) | SB-334867 (0.5 µg/0.5 µl). Direct administration to dorsal hippocampus. | Rat | 12 | Precipitated, naloxone (1.5 mg/kg, i.p.) on day 10 | Morphine (10 mg/kg, s.c.) delivered every 12 hours for 9 days. | Activity, chewing, diarrhea, head tremor, freezing, penile licking, ptosis, rearing, rubbing, scratching, sniffing, teeth chattering, wet dog shakes, and writhing | SB-334867 decreased chewing, diarrhea, freezing, penile licking, ptosis, scratching, teeth chattering, wet dog shakes, and writhing. |
| Laorden et al. (2012) | SB-334867 (20 mg/kg). Administered intraperitoneally. | Rat | 4–8 | Precipitated, naloxone (1 mg/kg, s.c.) on day 6 | Morphine (150 mg) subcutaneous implant for 6 days | Diarrhea, jumping, mastication, paw tremor, piloerection, ptosis, sniffing, teeth chattering, tremor, wet dog shakes, and writhing | SB-334867 decreased body tremor, diarrhea, mastication, piloerection, ptosis, sniffing, wet dog shakes, and writhing. Global withdrawal score also decreased. |
| Sharf et al. (2008) | SB-334867 (0, 20 mg/kg). Direct administration to nucleus accumbens. | Mouse | 9 | Precipitated, naloxone (1 mg/kg, s.c.) 2 hours after morphine administration | Escalating morphine doses up to 100 mg/kg, i.p., for 2.5 days | Backward walking, gnawing, head swoops, jumping, paw tremors, ptosis, tremors, and wet dog shakes | SB-334867 decreased backward walking, ptosis, tremors, wet dog shakes, and global withdrawal score (sum of withdrawal symptoms). |
LPGi, lateral paragigantocellularis; NR, not reported.
All drugs were administered to specific brain regions, except where noted.
TABLE 6.
Preclinical examinations of the glutamate system on the opioid withdrawal syndrome
Only results pertaining to effects of non-opioid drugs on opioid withdrawal symptoms are presented.
| Reference | Drugs Evaluated for Opioid Withdrawal Outcomes (Doses and Route) | Species | Sample Size | Withdrawal Method | Dependence Method | Withdrawal Signs Assessed | Summary of Withdrawal-Specified Results |
|---|---|---|---|---|---|---|---|
| Fundytus and Coderre (1994) | MK-801, GYKI 52466, (S)-4C-PG, and L-AP3 (same dose schedule: 1.6, 8, and 40 nmol). Administered intracerebroventricularly. | Rat | 4–10 | Precipitated, naloxone (1 mg/kg, s.c.), on day 7 | Morphine (36.65 µmol/day) administrated subcutaneously via pump | Jumping, teeth chattering, wet dog shakes, and writing | MK-801 (NMDA antagonist). (S)-4C-PG (metabotropic receptor antagonist), and L-AP3 (metabotropic receptor antagonist) dose dependently reduced teeth chattering and writing, as well as time spent in withdrawal. GYKI 52466 (AMPA/kainate receptor antagonist had no effect. |
| Fundytus and Coderre (1997) | (1S,3R)-ACPD, DHPG, and L-AP4 (same dose scheduled: 0.12, 0.6, and 3 nmol), DCG-IV (4.8 or 24 pmol). Administrated intracerebroventricularly. | Rat | 11–18 | Precipitated, naloxone (1 mg/kg, s.c.), on day 7 | Morphine (36.65 μmol/day) administrated subcutaneously via pump | Diarrhea, eye twitch, salivation, teeth chattering, and writing | (1S,3R)-ACPD (mGlu nonspecific) decreased eye twitch severity and time spent in withdrawal. DCG-IV (mG1u2/3 antagonist) decreased diarrhea, eye twitch, salivation, and time spent in withdrawal. L-AP4 (mGlu4 antagonist) increased eye twitch. DHPG (mGlu1/5 antagonist) had no significant effect. |
| Fundytus et al. (1997) | MCPG, MCCG, and MAP4 (same dose schedule: 1.6, 8, and 40 nmol/day). Administrated intracerebroventricularly. | Rat | 17–20 | Precipitated, naloxone (1 mg/kg, s.c.), on day 7 | Morphine (75 mg) subcutaneous implant for 3 days | Chewing, diarrhea, lacrimation, penile grooming, ptosis, salivation, stretching, teeth chattering, vocalization on touch, wet dogs shakes, and total withdrawal score | MCPG (mGlu nonselective antagonist) decreased jumps, vocalization, wet dog shakes, and time spent in withdrawal. MCCG (mGlu2/3 antagonist) decreased jumping, wet dog shakes, and time spent in withdrawal. MAP4 (mGlu4,6,7,8 antagonist) decreased time spent in withdrawal. |
| Kosten et al. (1995) | Felbamate (100 and 300 mg/kg), D-cycloserine (3 and 10 mg/kg), ± HA-966 (3, 10 mg/kg), Administered intraperitoneally. | Rat | 6 | Precipitated, naloxone (10 mg/kg, s.c.) on day 4 | Morphine (75 mg) subcutaneous implant for 3 days | Chewing, diarrhea, lacrimation, penile grooming, ptosis, salivation, stretching, teeth chattering, vocalization on touch, wet dog shakes, and total withdrawal score | Felbamate (glycine antagonist) decreased frequency of chewing, penile grooming, teeth chatterin, and severity of salivation in a linear manner across doses. D-cycloserine (glycine agonist) decreased withdrawal severity at both doses but not in a linear way. ± HA-966 (partial glycine agonist) had no effect. |
| Kotlinkska and Bochenski (2007) | MTEP (23 and 10 mg/kg), EMQMCM (5 and 20 mg/kg) Administered intraperitoneally. | Mouse | 8–10 | Precipitated, naloxone (4 mp kg, i.p.) on day 3 | Morphine sensitization procedure for 13 days (10 mg/kg, i.p., every 3 days) followed by morphine (37.5 mg) subcutaneous implant for 3 days | Jumping | MTEP (mGlu5 antagonist) 10 mg decreased jumping. EMQMCM (mGlu1 antagonist) had no effect. |
| Leal et al. (2003) | Ibogaine (40 and 80 mg/kg), MK-801 (0.15, and 0.3 mg/kg), Ibogaine (40 mg/kg) + MK-801 (0.15 mg/kg). Administered intraperitoneally. | Mouse | 10–13 | Precipitated, nalaxone (5 mg/kg, i.p.) on day 4 | Escalating morphine doses up to 225 mg/kg, i.p., by day 3 | Jumping | All doses of ibogaine and MK-801 (NMDA antagonist) decreased jumping. Ibogaine (40 mg/kg) and MK-801 (0.15 mg/kg) coadministration decreased jumping at the level observed for the high dose of each drug independently. |
| Medrano et al. (2015) | Ceftriaxone (100 and 200 mg/kg), Topiramate (20 and 40 mg/kg). Administered intraperitoneally. | Rat | 8–10 | Precipitated, naltrexone (10 mg/kg, i.p.) on day 4 | Morphine (200 mg/kg) subcutaneous implant for 3 days | Abnormal posture, body weight, diarrhea, exploring, ear blanching, eye twitching, jumping, lacrimation, mastication, penile erection, ejaculation, piloerection, ptosis, rhinorrhea, teeth chattering, vocalization on touch, and wet dog shakes | Ceftriaxone (glutamate transporter inhibitor) (200 mg/kg) decreased jumping, mastication, teeth chattering, and total withdrawal. Topiramate (glutamate release inhibitor) (40 mg/kg) reduced exploring, jumping, mastication, wet dog shakes, and total withdrawal score. |
| Pałucha-Poniewiera et al. (2009) | ACPT-1 (2.5, 10, 30 mg/kg). Administered intraperitoneally. | Mouse | 8–10 | Precipitated, naloxone (4 mg/kg, i.p.) on day 4 | Morphine (30 mg/kg, i.p.) for 3 days, with an additional dose on day 4 | Body tremor, body weight, jumping, paw shake, and wet dog shakes | ACPT-1 (mGlu2/3 agonist) decreased body tremor (all doses), body weight (10 and 30 mg), jumping (10 mg), paw shakes (10 and 30 mg), and wet dog shakes (all doses). |
| Rasmussen et al. (1996) | LY293558 (0.1, 10, and 30 mg/kg). Administered intraperitoneally. | Rat | 10–12 | Precipitated, naltrexone (10 mg/kg, s.c.) on day 4 | Morphine (150 mg) subcutaneous implant for 2 days | Body weight, chewing, diarrhea, digging, erections, irritability, jumping, lacrimation, ptosis, salivation, stereotyped head movements, teeth chattering, tremor, wet dog shakes, writhing, and total withdrawal score | LY293558 (AMPA antagonist) dose dependently decreased total withdrawal score; specific dose-dependent decreases in chewing, diarrhea, lacrimation, ptosis, salivation, stretching head movements, wet dog shakes, and writhing. |
| Rasmussen et al. (2005) | MPEP (1, 3, and 10 mg/kg), MTEP (1, 3, and 10 mg/kg). Administered intraperitoneally. | Rat | 6–16 | Precipitated, naltrexone (10 mg/kg, s.c.) on day 3 | Morphine (75 mg) subcutaneous implant for 2 days | Body weight, chewing, diarrhea, digging, erection, irritability, jumping, lacrimation, ptosis, salivation, teeth chattering, wet dog shakes, writhing, and total withdrawal score | MPEP (mGlu5 antagonist) reduced total withdrawal score at all doses tested and decreased body weight, chewing, digging, erection (1 mg/kg only), jumping, and teeth chattering (3 mg/kg only). MTEP (mGlu5 antagonist) 3 and 10 mg/kg reduced total withdrawal score and body weight, chewing, digging, erection (1 and 3 mg/kg only), and writhing. |
| Sekiya et al. (2004) | DL-TBOA (1, 3, 10 nmol). Administered intracerebroventricularly. | Rat | 6–7 | Precipitated, naloxone (0.1 mg/kg, i.p.) on day 5 | Morphine (150 mg) subcutaneous impart for 5 days | Backward walking, body weight, ejaculation, head shaking, jumping, paw shaking, stretching, teeth chattering, wet dog shakes | DL-TBOA (glutmate uptake inhibitor) dose dependently increased ejaculation, rhinorrhea, salivation, teeth chattering, and wet dog shakes. |
| Tanganelli et al. (1991) | MK-801 (0.1, 0.3, and 1 mg/kg), glutamatic acid dyethylester (100–500 mg/kg), pyroglutamic acid (500–1000 mg/kg). Administered intraperitoneally. | Mouse | 15–20 | Precipitated, naloxone (3 mg/kg, s.c.) on day 2 | Morphine (75 mg) subcutaneous implant for 2 days | Hyperactivity and jumping | MK-801 (NMDA antagonist) 0.3 and 1 mg/kg decreased jumping. Glutamatic acid dyethylester had no effect. Pyroglutamic acid (non-NMDA antagonist) 1000 mg/kg decreased jumping. |
| Tokuyama et al. (1996) | MK-801 (0.1 mg/kg). Administered intraperitoneally. | Rat | 10–14 | Precipitated, naloxone (48 nmol/5 µl, LC) or glutamate (1 and 10 nmol/5 µl, LC) 2 hours after last opioid infusion | Morphine (26 nmol/µl per hour) or butorphanol (26 nmol/µl per hour) intracerebroventricular osmotic minipump infusions for 3 days | Body weight, escape behavior, locomotion, penis licking, ptosis, rearing, salivation, scratching, teeth chattering, and wet dog shakes | Glutamate increased all withdrawal signs at both doses. MK-801 (NMDA antagonist) decreased all signs of withdrawal that were precipitated by either glutamate or naloxone. |
| Tokuyama et al. (1998) | H-7 (1 and 10 nmol/µl per hour) intracerebroventricular osmotic minipump infusions. | Rat | 7 | Precipitated, naloxone (24 and 48 nmol/5 µl, i.c.v.) or glutamate (1 and 10 nmol/5 µl, LC) 2 hours after opioid infusion | Morphine (26 nmol/µl per hour) or butorphanol (26 nmol/µl per hour) intracerebroventricular osmotic minipump infusion for 3 days | Body weight, escape behavior, locomotion, penile licking, ptosis, rearing, salivation, stretching, teeth, chattering, and wet dog shakes | Glutamate increased all signs except weight loss and salivation. H-7 (cAMP inhibitor) dose dependently decreased escape behavior, locomotion, penis licking, ptosis, rearing, salivation, streching, and teeth chattering. Outcomes are dependent on dependence and withdrawal method used. |
| Vandergriff and Rasmussen (1999) | LY354740 (3, 10, and 30 mg/kg), LY317207 (30 mg/kg). Administered subcutaneously. | Rat | 8 | Precipitated, naltrexone (10 mg/kg, s.c.) on day 3 | Morphine (300 g) subcutaneous implant for 2 days | Body weight, chewing, diarrhea, digging, erections, irritability, jumping, lacrimation, ptosis, salivation, stereotyped head movements, teeth chattering, wet dog shakes, writhing, and total withdrawal score | LY354740 (mG1u2/3 agonist) dose dependently decreased total withdrawal score, chewing, diarrhea, digging, and salivation. Wet dog shakes and ptosis significantly decreased at 30 mg/kg only. LY317207 (inactive LY354740 enantiomer) had no effect. |
| Watanabe et al. (2002) | CNQX (10 and 30 nmol), MK-801 (10 and 30 nmol), D-CPPene (0.001, 0.1, and 0.1 nmol) infusion to central nucleus of the amygdala | Rat | 5–9 | Precipitated, naloxone (0.3 mg/kg, i.p.) on day 4 | Morphine (75 mg) subcutaneous implant for 2 days | Backward walking, body weight, diarrhea, head shakes, jumping, lacrimation, paw shakes, ptosis, rearing, rhinorrhea, salivation, stretching, teeth chattering, and wet dog shakes | CNQX (AMPA/kainate antagonist) decreased backward walk, body weight loss, diarrhea, head shakes, paw shakes, ptosis, rhinorrhea, teeth chattering, and wet dog shakes. MK-801 (NMDA antagonist) and D-CPPene (NMDA antagonist) had no effect. |
CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; LC, locus coeruleus; NR, not reported.
TABLE 7.
Preclinical examinations of medications with multiple mechanisms of action on the opioid withdrawal syndrome
Only results pertaining to effects of non-opioid drugs on opioid withdrawal symptoms are presented.
| Reference | Drugs Evaluated for Opioid Withdrawal Outcomes (Dose and Route) | Species | Sample Size | Withdrawal Method | Dependence Method | Withdrawal Signs Assessed | Summary of Withdrawal-Specific Results |
|---|---|---|---|---|---|---|---|
| Aceto and Bowman (1993) | Buspirone (0.2, 0.4, and 0.8 mg/kg) | Rhesus Monkey | ≥3 | Precipitated, naloxone (0.05 mg/kg, s.c.) 2 to 3 hours after last morphine administration | Morphine (3 mg/kg, s.c.) at minimum for ≥3 months | Ataxia, body sag, fighting, jaw sag, lying down, ptosis, retching, restlessness, rigid abdominal muscles, slowing, vocalization, and wet dog shakes | Buspirone dose dependently reduced rigid abdominal muscles, fighting, lying down, retching, restlessness, and vocalization and increased wet dog shakes. |
| Berthold et al. (1989) | Clonidine (0.01, 0.03, 0.1, and 0.3 mg/kg), prazosin (0.1, 1, and 4 mg/kg), spiroperidol (0.05 and 0.5 mg/kg), 8-OH-DPAT (0.5, 1, 2, 4, 8 mg/kg); RU 24969 fumerate (0.125, 0.25, 0.5., 1, 2, and 4 mg/kg); Idazoxan; (−)-pindolol (1, 2, and 4 mglkg); (+)-SDZ 21-009 (1 mg/kg); (−)-SDZ 21-009 (1 mg/kg); buspironc (1, 2, 5, and 10 mg/kg); ipsapirone (1, 3, and 10 mg/kg); yohimbine (0.5 and 2 mg/kg); flesinoxan (0.5, 1, and 3 mg/kg); Rauwolscine (0.5 and 2 mg/kg), WY 26392 (0.5, 2 mg/kg), haloperidol (0.1, 0.5, and 1.0 mg/kg); pCPA (150 mg/kg). Administered subcutaneously. | Mouse | 10 | Precipitated, naloxone (1 mg/kg, i.p.) on day 5 | Morphine (75 mg) subcutaneous implant for 5 days | Jumping | 8-OH-DPAT (1 mg/kg), RU 24969 (0.25–4 mg/kg), buspirone (2 mg/kg) (5-HT1 agonists), ipsapirone (10 mg/kg), and flesinoxan (all doses) (5-HT1 agonists) decreased jumping. Idazoxan (all doses), prazosin (1 and 4 mg/kg), rauwolscine (2 mg/kg), WY 26392 (all doses), and yohimbine (all doses) (α-adrenoreceptor antagonists) also decreased jumping. Spiroperidol (5-HT1 antagonist) pretreatment had no effect alone but increased jumping from 8-OH-DPAT and buspirone; no effect of spiroperidol pretreatment on RU24969 and idazoxan. Haloperidol pretreatment enhanced 8-OH-DAPAT–related jumping. Pretreatment (−)-pindolol and (−)-SDZ 21-009 (β-adrenoreceptor/5-HT1A and 5-HT1B antagonists) decreased ability of RU 24969 to suppress jumping. (+)-SDZ21-009 did not impact RU 24969. 8-OH-DAPAT unaffected by (−)-pindolol. pCPA, clonidine (α2 agonist), and (−)-pindolol had no effects. |
| Cappendijk et al. (1994) | β-Carboline (20 mg/kg), Ibogaine (40 mg/kg). Administered intraperitoneally. | Rat | 10 | Precipitated, naloxone (4 mg/kg, i.p.) on day 3 | Morphine (75 mg) subcutaneous implant for 3 days | Chewing, diarrhea, grooming, jumping, penile licking, ptosis, rearing, rhinorrhea, teeth- chattering, wet dog shakes, vocalization on touch, and total withdrawal score | β-Carboline (20 mg/kg) decreased the total withdrawal score, chewing, diarrhea, grooming penile licking, rearing, and teeth chattering. Ibogaine decreased the total withdrawal score, chewing, diarrhea, penile licking, and teeth chattering. |
| Dzoljic et al. (1988) | Ibogaine (4, 8, and 16 μg). Administered intracerebroventricularly. | Rat | 10 | Precipitated, naloxone (5 mg/kg, i.p.) on day 3 | Morphine (85 mg) subcutaneous implant for 3 days | Chewing, diarrhea, digging, ejaculation, grooming, head holding, head shakes, jumping, paw tremor, penile licking, ptosis, rearing, rhinorrhea, salivation, scratching, stretching, teeth chattering, urination, vocalization on touch, wet dog shakes, and writhing | Ibogaine significantly reduced jumping and salivation (8 and 16 μg), chewing, digging, head hiding, penile licking, rearing, teeth chattering, and writhing (all doses), relative to cerebrospinal fluid control condition. |
| Francés et al. (1992) | Ibogaine (30 mg/kg). Administered intraperitoneally. | Mouse | 5–32 | Precipitated, naloxone (5 mg/kg, i.p.) on day 1, 2, 3, 4, or 5 (varied based on group) | Escalating morphine doses up to 100 mg/kg, i.p., for up to 5 days (varied based on group) | Body shakes, body weight, dropping, diarrhea, and jumping | Ibogaine (30 mg/kg) increased jumping and did not decrease any withdrawal signs. |
| Glick et al. (1992) | Ibogaine (20, 40, and 80 mg/kg). Administered intraperitoneally. | Rat | 7–17 | Precipitated, naltrexone (1 mg/kg, i.p.) on day 6 | Morphine (50 mg) subcutaneous implant for 5 days | Burying, diarrhea, grooming, paw shaking, teeth chattering, and wet dog shakes | Ibogaine decreased diarrhea, teeth chattering, and wet dog shakes (40, 80 mg/kg), and grooming (all doses). |
| Hine et al. (1975a) | Cannabidiol (10 mg, kg). Administered intraperitoneally | Rat | 7 | Precipitated, naloxone (4 mg/kg, i.p.) on day 3 | Morphine (75 mg) subcutaneous implant for 3 days | Abnormal posture, chewing, defecation, diarrhea, ear blanching, ptosis, teeth chattering, vocalization, wet dog shakes, and total withdrawal score | Cannabidiol (10 mg/kg) had no effect. |
| Hine et al. (1975c) | THC (2 mg/kg), cannabidiol (10 mg/kg), THC (2 mg/kg) + Cannabidiol (10 mg/kg). Administered intraperitoneally. | Rat | 8 to 9 | Precipitated, naloxone (4 mg/kg, i.p.) on day 3 | Morphine (75 mg) subcutaneous implant for 3 days | Abnormal posture, audible grinding, chewing, defecation, escapes, diarrhea, ear blanching, ptosis, teeth chattering, vocalization on touch, wet dog shakes, and writhing | THC (2 mg/kg) decreased total withdrawal scores and defecation, and diarrhea. Cannabidiol alone had no effect. THC (2 mg/kg) + Cannabidiol (10 mg/kg) decreased total withdrawal scores, defecation, ear blanching, escapes, and wet dog shakes. |
| Kang et al. (2008) | Mirtazapine (3, 10, and 30 mg/kg). Administered intraperitoneally. | Rat | 40 | Precipitated, naloxone (1 mg/kg, i.p.) on day 10 | Morphine 10 mg, s.c., twice a day for 10 days | Body weight, chewing, digging, escape attendance, grooming, rearing, scratching, teeth chattering, and wet dog shakes | Mirtazapine reduced chewing (10 and 30 mg/kg), and escape attendance, grooming, and teeth chattering (all doses). Mirtazepine (10 and 30 mg/kg) reduced total withdrawal score. |
| Koyuncuoğlu et al. (1990) | Ketamine (0.5 and 1 mg/kg, i.v.), dextromethorphan (1 and 2 mg/kg, i.p.). | Rat | 10–16 | Precipitated, naloxone (2 mg/kg, i.p.) on day 3 | Morphine (225 mg) subcutaneous implant + F16 for 3 days | Defecation, diarrhea, flying jumping, ptosis, teeth chattering, wet dog shakes, and writhing | Ketamine (1 mg/kg) decreased defecation, jumping, and teeth chattering, but increased wet dog shakes. Dextromethorphan (1 mg/kg) decreased flying and teeth chattering, and (2 mg/kg) decreased all signs except writhing. |
| Leal et al. (2003) | Ibogaine (40 and 80 mg/kg), MK-801 (0.15 and 0.3 mg/kg), ibogaine (40 mg/kg) + MK-801 (0.15 mg/kg). Administered intraperitoneally. | Mouse | 10–13 | Precipitated, naloxone (5 mg/kg, i.p.) on day 4 | Escalating morphine doses up to 225 mg/kg, i.p., by day 3 | Jumping | All doses of ibogaine and MK-801 (NMDA antagonist) decreased jumping. Ibogaine (40 mg/kg) and MK-801 (0.15 mg/kg) coadministration decreased jumping at the level observed for the high dose of each drug independently. |
| Lu et al. (2001) | Venlafaxine (10 and 20 mg/kg). Administered intraperitoneally. | Rat | 12 | Precipitated, naloxone (2 mg/kg, i.p.) on day 5 | Escalating morphine doses up to 40 mg/kg, s.c., by day 5 | Body weight, diarrhea, exploring, irritability, jumping, lacrimation, piloerection, ptosis, teeth chattering, wet dog shakes, and writhing | Venlafaxine (10 and 20 mg/kg) decreased diarrhea, exploring, jumping, piloerection, and shakes. Venlafaxine (20 mg/kg per kilogram) also decreased irritability, lacrimation, wet dog shakes, and writhing. |
| Mash et al. (2016) | Noribogaine (10, 30, 56, and 100 mg/kg). Intragastric administration | Mouse | 5–11 | Precipitated, naloxone (3 mg/kg, i.p.) on day 4 | Escalating morphine doses up to 75 mg/kg, s.c., for 4 days | Body tremors, diarrhea, jumping, and paw tremors | Noribogaine decreased body tremors (30 and 100 mg/kg), paw tremors (100 mg/kg), and jumping (30, 56, and 100 mg/kg). |
| Panchal et al. (2005) | 18-MC (5, 10, and 25 μg/1 μl). Infused into intramedial habenula, locus coeruleus, or interpeduncular nucleus. | Rat | 5–10 | Precipitated, naltrexone (1 mg/kg, i.p.) on day 8 (immediately following intracerebral drug infusion) | Escalating morphine doses up to 80 mg/kg, s.c., 7 days | Burying, diarrhea, grooming, rearing, teeth chattering, and wet dog shakes | Intramedial habenula 18-MC decreased body weight loss (10 μg) and burying (10 μg). Teeth chattering decreased at 5 μg and increased at 10 μg. Intralocus coeruleus 18-MC dose decreased diarrhea (10 μg), burying (all doses), teeth chattering (5 and 20 μg), and wet dog shakes (10 μg). Intrainterpeduncular nucleus 18-MC decreased burying (5 μg) and rearing (10 μg) and increased diarrhea (5 μg) and teeth chattering (20 μg). |
| Parker and Siegel (2001) | Ibogaine (40 mg/kg). Administered intraperitoneally. | Rat | 28 total (individual group size NR) | Precipitated, naloxone (1 mg/kg, s.c.) on day 3 | Morphine (20 mg/ml) subcutaneous implant for 2 days | Mouth movements, penile licking, rearing, teeth chattering, and wet dog shakes | Ibogaine (40 mg/kg) decreased mouth movements, penile licking, and teeth chattering. |
| Rho and Glick (1998) | 18-MC (10, 20, or 40 mg/kg). Administered intraperitoneally. | Rat | 6–8 | Precipitated, naltrexone (1 mg/kg, i.p.) on day 8 | Escalating morphine up to 140 mg/kg, s.c., for 7 days | Body weight, burying, diarrhea, flinching, grooming, teeth chattering, and wet dog shakes | 18-MC decreased body weight loss (40 mg/kg), burying (20 and 40 mg/kg), diarrhea (40 mg/kg), teeth chattering (20 and 40 mg/kg), and wet dog shakes (20 mg/kg). |
| Schreiber et al. (2003) | Mianserin (25 mg/kg), trazodone (50 mg/kg), mianserin (25 mg/kg) + trazodone (50 mg/kg). Administered subcutaneously. | Mouse | >10 | Precipitated, naloxone (1 mg/kg, s.c.) | High morphine group: escalating morphine up to 160 mg/kg, s.c., by day 8; low morphine: escalating morphine up to 40 mg/kg, s.c., by day 8 | Grooming, jumping, and rearing | Mianserin (25 mg/kg), trazodone (50 mg/kg), and mainserin (25 mg/kg) + trazodone (50 mg/kg) combination reduced jumping, rearing, and grooming in both high and low morphine groups. |
| Sharpe and Jaffe (1990) | Ibogaine (5, 10, 20, and 40 mg/kg). Administered subcutaneously. | Rat | 6 | Precipitated, naloxone (0.5 mg/kg, s.c.) on day 3 | Morphine (75 mg) subcutaneous implant for 3 days | Activity, grooming, lacrimation, mouth movement, paw shakes, penile licking, rhinorrhea, salivation, stretching, teeth chattering, wet dog shakes, and total withdrawal score | Ibogaine decreased grooming (10 mg/kg) and increased teeth chattering (5 mg/kg). |
| Streel et al. (2005) | Ketamine (2.5 mg/kg), Midazolam (0.25 mg/kg). Administered intramuscularly | Rat | 10 | Precipitated, naloxone (1 mg/kg, s.c.) on day 3, administered three times (total daily dose 3 mg/kg, s.c.) | Escalating morphine up to 150 mg/kg, s.c., for 3 days | Abnormal posture, cheek tremors, defecation, escape attempts, head lift, jumping, mastication, salivation, sniffing, teeth chattering, urination, vocalization on touch, wet dog shakes, and total withdrawal score | Ketamine (2.5 mg/kg) decreased defecation, urination, and total withdrawal scores. Midazolam (0.25 mg/kg) decreased urination and total withdrawal scores. |
pCPA, para-chlorophenylalanine; 18-MC, 18-methoxyroconaridine; NR, not reported.
TABLE 8.
Summary of human empirical studies on the opioid withdrawal syndrome
Note that only outcomes directly related to opioid withdrawal severity are discussed. Studies may have reported additional outcomes not presented here. All medications administered via oral route unless noted. Participants in all studies had OUD unless noted. Results from all participants are reported unless noted.
| Reference | System | Medication + Dose | Design | Comparator | Sample Size | Male | Withdrawal Type | Additional Ancillary Medications Given | Primary Withdrawal-Specific Outcome | Result |
|---|---|---|---|---|---|---|---|---|---|---|
| % | ||||||||||
| Akerele et al. (2008) | Glutamate | Dextromethorphan (30 mg/day) + quinidine (30 mg/day) | RCT; randomized, double-blind, placebo controlled | Placebo | 31 (22 dextromethorphan + quinidine, nine placebo) | 68 | Spontaneous, withdrawn from morphine (100 mg/day, s.c.) over 4 days | Acetaminophen, antacids (medication and doses NR) | MHOWS, VAS | Dextromethorphan (30 mg/day) + quindine (30 mg/day) did not differ significantly from placebo on any withdrawal measure (MHOWS). |
| Amiri et al. (2014) | Multiple mechanisms | Amantadine (200 mg/day) + clonidine (0.4–1.2 mg/day) | RCT; randomized, double-blind, controlled | Clonidine (0.4–1.2 mg/day) | 69 [only completer sample (n = 60) reported: 30 amanatadine + clonidine, 30 clonidine] | 100 | Spontaneous, withdrawal from illicit opioids over 3 days | Clonazepam (1 mg), acetaminophen (500 mg) | COWS | Data from 60 completers. Amantadine (200 mg/day) + clodine (0.4–1.2 mg/day) reduced withdrawal severity significantly more than clondine (0.4–1.2 mg/day). |
| Bisaga et al. (2001) | Glutamate | Memantine (60 mg/day) | Human laboratory study; Modified multiple baseline, between-subjects comparison | Placebo | Eight (four memantine, four placebo) | 75 | Precipitated (naloxone, 0.4 mg, i.m.) after 4–7 days of stabilization on morphine (30 mg, four times a day) | NR | CINA, OOWS, SOWS | Memantine (60 mg/day) significantly reduced CINA and OOWS ratings of withdrawal relative to placebo when administered 6 and 54 (but not 126) hours after naloxone. Memantine also significantly reduced withdrawal AUC relative to baseline. Memantine did not significantly improve SOWS ratings relative to placebo. |
| Bisaga et al. (2015) | Cannabis | Dronabinol (30 mg/day) | RCT; randomized, double-blind, placebo controlled | Placebo | 60 (40 dronabinol, 20 placebo) | 85 | Spontaneous, withdrawn from buprenophine (8 mg/day) over 2 days transitioned to naltrexone over 4 days | Clonidine (0.8 mg/day), clonazepam (up to SOWS 3.5 mg/day), zolpidem (10 mg/day), and other medicatons (unspecified) | SOWS | Dronabinol (30 mg/day) significantly reduced SOWS ratings relative to placebo during abrupt withdrawal period (days 2–4). |
| Buydens-Branchey et al. (2005) | Multiple mechanisms | Buspirone (30 mg/day; 45 mg/day) | RCT; randomized, double-blind, placebo controlled, four group design | Placebo | 31 (eight placebo, eight methadone taper, eight buspirone 30 mg, seven buspirone 45 mg) | 100 | Spontaneous, placebo and buspirone group abruptly discontinued from methadone (30 mg/day); methadone group tapered off methadone (30 mg/day) over 7 days | NR | OOWS, SOWS | Buspirone (30 mg/day; 45 mg/day) dose dependently reduced OOWS and SOWS ratings to placebo. Buspirone 45 mg/day produced the lowest AUC value overall and conferred the most withdrawal suppression. |
| Chu et al. (2017) | Serotonin | Ondansetron (8 mg, i.v.) | Human laboratory study; double-blind, within-subject, randomized, crossover comparison | Placebo | 33 (non-OUD persons with chronic back pain) | 61 | Precipitated (naloxone, 0.4 mg/70 kg, i.v., or 0.8 mg/70 kg, i.v., if no response to 0.4 mg/70 kg dose) | Docusate sodium (100 mg), metoclopramide (10 mg) | OOWS, SOWS | Ondansetron (8 mg, i.v.) did not significantly reduced naloxone-precipitated withdrawal severity relative to placebo on any measure. |
| Pérez de los Cobos et al. (2001) | Multiple mechanisms | Amantadine (flexible dosing of 200–300 mg/day) | RCT; two successive randomized, double-blind, placebo controlled trails | Trial 1: placebo, trial 2: methadone taper | Trial 1:40 (19 amantadine, 21 placebo); trial 2: 40 (21 amantadine, 19 placebo). Participants in both trials had OUD and cocaine-use disorder. | 81 | Spontaneous, withdrawn from methadone (maximum 50 mg/day) over 12 days | Diazepam (dose NR) | Craving VAS, OOWS, SOWS | Amantadine (200–300 mg/day) was not significantly different from placebo on any withdrawal measure in either trial. |
| Jain et al. (2011) | Glutamate | Memantine (20 mg/day) | Human laboratory study; randomized, double-blind, placebo controlled | Buprenorphine (2 mg, SL) | 62 [only completer sample (n = 45) reported; 25 memantine, 20 buprenorphine] | 100 | Precipitated (naloxone, 0.4 mg, i.v.) following 5-day stabilization on dextropoxyphene (650 mg day) | Zolpidem (10 mg) | OOWS, SOWS | Data from 45 completers. Buprenorphine pretreatment prior to naloxone challenge significantly decreased SOWS ratings. Neither medication significantly decreased withdrawal as rated by the OOWS. |
| Jovaiša et al. (2006) | Multiple mechanisms | Ketamine (0.5 mg/kg bolus following by 0.5 mg/kg per hour infusion) | RCT; randomized, double-blind, placebo controlled | Placebo | 58 [only completer sample (n = 50) reported: 22 ketamine, 28 placebo] | 86 | Rapid anesthetic-assisted detoxification with series of naloxone (1.6 mg, i.v.), naloxone (0.8 mg/h, i.v., infusion), and then naltrexone (100 mg via oro-gastric tube) under isofurane anesthesia | Clonidine (.002 mg), carbamaapine (200 mg), clonazepam (2 mg) | OOWS | Data from 50 completers. Ketamine infusion significantly reduced OOWS ratings to placebo during the first and second, but not third hour postanesthesia. |
| Lin et al. (2008) | Multiple mechanisms | Venlafaxine (300 mg/day) | RCT; randomized, double-blind, placebo controlled | Placebo | 34 (15 venlafaxine, 19 placebo) | 88 | Spontaneous, withdrawn from illicit opioid use over 7 days using lorazepam (1 mg/day), fexofenadine hydrochloride (240 mg/day), flurazepam (30 mg/day), trazodone (100 mg/day) | Chlorpromazine (dose NR); clonidine (0.075 mg), ibuprofen (400 mg), metoclopramide hydrochloride (10 mg), loperamide (2 mg) | OOWS, VAS for withdrawal, time spent sleeping, and ancillary medication utilization | Data from 20 completers reported. Participants who received venlafaxine (300 mg/day) had significantly reduced OOWS and VAS ratings and more sleep relative to placebo. Venlafaxine participants also requested fewer ancillary medications. |
| Lin et al. (2014) | Multiple mechanisms | Dextromethorphan (240 mg/day) | RCT; randomized, double-blind, placebo controlled | Placebo | 80 [only completer sample (n = 65) reported; 33 dextromethorphan, 32 placebo] | 97 | Spontaneous, withdrawn from illicit opioid use over 7 days using clonidine (0.075–0.3 mg/day), lorezepam (1 mg/day), fexofenadine hydrochloride (240 mg/day), nitrazepam (30 mg/day), trazodone (100 mg/day) | Ibuprofen (400 mg/day), lorezepam (1 mg/day), metoclopramide hydrochloride (10 mg/day), loperamide (2 mg/day), trazodone, flurazepam, or chlorpromazine (doses NR); none provided on session days | OOWS, time spent sleeping, and ancillary medication utilization | Data from 65 completers (33 dextromethorphan, 32 placebo) reported. Dextromethorphan (240 mg/day) significantly reduced OOWS ratings relative to placebo on Days 3–6. No group differences observed on time spent sleeping or ancillary medication utilization. |
| Lofwall et al. (2016) | Cannabis | Dronabinol (5, 10, 20, and 40 mg/day) | Human laboratory study; randomized, double-blind, within-subject, placebo controlled | Placebo, oxycodone (30, 60 mg/day) | 18 [only completer sample (n = 12) reported] | 50 | Spontaneous, discontinued from oxycodone (120 mg/day) for each day-long session | None provided on session days | Opioid agonist/antagonist scale, OOWS, short opiate withdrawal scale | Data from 12 completers. Oxycodone decreased all withdrawal ratings. Dronabinol (20, 30 mg/day) significantly reduced withdrawal ratings on the antagonist, OOWS, and short opitate withdrawal scales relative to placebo. |
| Malek et al. (2013) | Glutamate | Dextromethorphan (300 mg/day) + clonidine (0.4–1.2 mg/day) | RCT; randomized, double-blind, controlled | Clonidine (0.4–1.2 mg/day) | 60 (30 dextromethorphan + clonidine, 30 clonodine) | 100 | Spontaneous, withdrawal from illicit opioid use using clonidine (0.4–1.2 mg/day), clonazepam (3 mg/day), acetaminophen (2000 mg/day) over 3 days | NR | COWS | Dexotromethorphan (300 mg/day) + clonidine significantly reduced ratings at 24, 48, and 72 hours relative to clonidine alone. |
| Pozzi et al. (2000) | Multiple mechanisms | Trazodone (600 mg/day) | RCT; randomized, single-blind design | Clonidine (0.45–0.9 mg/day) | 45 (30 trazodone, 15 clonidine) | 80 | Spontaneous, withdrawn from methadone (20 mg/day) over 3 days transitioned to naltrexone over 4 days | Ranitidine (450 mg/day), flurazepam (30 mg), methoclopramide (doses NR), ketorolac or diclofenac (doses NR) | Study-specific observer (12 items) and self-report (13 items) withdrawal rating scales | Trazodone (up to 600 mg/day) significantly reduced self-reported withdrawal relative to clonidine during the first two taper days, no group differences in observer ratings of withdrawal. |
| Rose et al. (2003) | Multiple mechanisms | Buspirone (30 mg/day) | RCT; randomized, double-blind, placebo controlled | Placebo | 20 (group sample size NR) | 100 | Spontaneous, abruptly discontinued from methadone (mean dose 95 mg/day) over 6 days | NR | OOWS, SOWS | Buspirone (30 mg/day) significantly reduced OOWS ratings on days 5–7 and 9, and SOWS ratings on day 8, relative to placebo. |
| Srisurapanont and Jarusuraisin (1998) | Multiple mechanisms | Amitriptyline (up to 100 mg/day) | RCT; randomized, double-blind, parallel design | Lorazepam (up to 4 mg/day) | 27 (13 amitriptyline, 14 lorazepam) | 100 | Spontaneous, abruptly discontinued from methadone (M does 95 mg/day) over 6 days | Analgesis, nonsterois anti-inflammatory drugs (specific medications and doses NR) | Short opiate withdrawal scale and sleep evaluation questionnaire | Amitriptyline (up to 100 mg/day) did not differ significantly from lorazepam (up to 4 mg/day) on any withdrawal outcome. Amitriptyline produced lower ratings on the ease of awakening from sleep; subscale of the sleep evaluation questionnaire relative to lorazepam. |
AUC, area under the curve; CINA, clinical inventory narcotic activity; COWS, clinical opiate withdrawal scale; MHOWS, modified Himmelsbach opioid withdrawal scale; NR, not reported; OOWS, objective opioid withdrawal scale; RCT, randomized controlled trial; SL, sublingual; SOWS, subjective opiate withdrawal scale; VAS, visual analog scale.
TABLE 9.
Summary of non-experimental human studies on the opioid withdrawal syndrome
Note that only outcomes directly related to opioid withdrawal severity are discussed. Studies may have reported additional outcomes not presented here.
| Reference | System | Medication | Study Type | Sample Size | Male | Result |
|---|---|---|---|---|---|---|
| % | ||||||
| Alper et al. (1999) | Multiple mechanisms | Ibogaine | Retrospective chart review | 33 | 67 | Ibogaine (mean = 19.3 ± 6.9 mg/kg) reduced withdrawal severity within 24 hours among 75% of individuals. |
| Birch (1889) | Cannabis | Cannabis | Case report | 1 | 100 | Cannabis reduced opioid withdrawal severity. |
| Brown and Alper (2018) | Multiple mechanisms | Ibogaine | Prospective cohort study | 30 | 83 | Ibogaine (mean = 1540 ± 920) reduced SOWS ratings from mean = 31 to mean = 14 within 3 days. Fifty percent of individuals reported opioid abstinence at 1-month follow-up. |
| Davis et al. (2017)a | Multiple mechanisms | Ibogaine | Survey (online) | 88 | 73 | Individuals stated ibogaine reduced or eliminated withdrawal symptoms (80% of respondents), led to sustained opioid abstinence (30%), and produced sustained reductions in opioid craving (25%). |
| Epstein and Preston (2015) | Cannabis | Cannabis | Secondary outcome from RCT | 116 | 53 | Participants completing a methadone-assisted taper (10-weeks) who did (n = 46) or did not (n = 70) provide a urine sample testing positive for cannabis during the treatment did not vary in their ratings of opioid withdrawal severity during the parent trial. |
| Gossop et al. (1991) | Cannabis | Cannabis | Retrospective chart review | 50 | 70 | Cannabis increased (24% of respondents) or decreased (12%) opioid withdrawal severity. |
| Lalanne et al. (2016)b | Multiple mechanisms | Ketamine | Case report | 1 | 0 | Patient reported ketamine (1 mg/kg) successfully assisted taper off opioid medications. |
| Malcolm et al. (2018) | Multiple mechanisms | Ibogaine | Retrospective chart review | 50 | 61 | Ibogaine (dose NR) eliminated ratings on COWS (78% of individuals), SOWS (68%), and opioid craving (79%) 2 days after abrupt opioid discontinuation. |
| Pinkofsky et al. (2005) | Multiple mechanisms | Quetiapine | Survey (quality assurance) | 107 | 45 | Quetiapine reduced cravings (74% of individuals), anxiety (49%), somatic pain (22%), and insomnia (21%), and improved appetite (13%). Only 4% felt quetiapine had no effect. |
| Quinlan (2012)a | Multiple mechanisms | Ketamine | Case report | 11 | NR | Patients who received ketamine-assisted opioid detoxification reported ketamine was well-tolerated. Patients reported feeling better after 2 months (73% of respondents) and remained abstinent from opioids at 6 months (27%). |
| Sheppard (1994) | Multiple mechanisms | Ibogaine | Case report | 7 | 71 | Ibogaine (700–1800 mg) reduced opioid withdrawal severity at 24–38 hours. |
| Strickler et al. (2018)b | Multiple mechanisms | Ketamine | Case report | 1 | 100 | Ketamine (10 mg/h infusion) + clonidine patch used to successfully taper patient off opioids over 7-day period. |
| Wakim (2012)b | Serotonin | Ondansetron | Case report | 1 | 0 | Ondansetron (16 mg/day) used to successfully taper patients off opioids over 10-day period. |
| Zullino et al. (2002) | Glutamate | Topiramate | Case report | 3 | 67 | Topiramate (up to 500 mg/day) used to successfully taper patients off opioids over 9–14 day period. |
COWS, clinical opiate withdrawal scale; NR, not reported; RCT, randomized controlled trial; SOWS, subjective opiate withdrawal scale.
Patient population had chronic opioid use (population unspecified).
Patient population comprised of persons with acute or chronic pain and opioid use disorder.
Results
Dopamine System
Dopamine neurotransmission occurs via two families of G protein–coupled receptor families, the D1-like receptors that include D1 and D5 receptor subtypes, and the D2-like receptors that include D2, D3, and D4 receptor subtypes. DA is frequently implicated in substance use disorders (for a review, see Volkow et al., 2017) and there is clear evidence of clinically meaningful DA and opioid interaction in humans. For instance, many methadone patients begin using cocaine during methadone maintenance (Leri et al., 2003) and methadone has been shown empirically to increase the reinforcing effects of cocaine (Preston et al., 1996). The following section reviews only preclinical research because no human studies met the inclusion criteria.
Preclinical Withdrawal.
Preclinical behavioral evidence clearly implicates DA in the expression of opioid withdrawal, and drugs acting on this system often exert positive effects at one dose and negative effects at another dose. Drugs that increase DA, including the DA transport reuptake inhibitors amphetamine and cocaine and the DA precursor L-DOPA, dose dependently increase several symptoms while simultaneously decreasing others (Herz et al., 1974; el-Kadi and Sharif, 1998). Numerous studies have reported that low doses of the D2 agonist apomorphine exacerbates withdrawal, evidenced by increased jumping, wet dog shakes, burrows, and hyperthermia, whereas higher doses decrease jumping, burrowing, and other responses (Herz et al., 1974; Ary and Lomax, 1976; Cox et al., 1976; el-Kadi and Sharif, 1998). The D2 receptor antagonists domperidone, fluenthixol, and pimozide also reduce several opioid withdrawal symptoms, whereas the D2 receptor agonist sulpride increased the severity of all symptoms measured (Cox et al., 1976; el-Kadi and Sharif, 1998).
Serotonin System
Extracellular levels of serotonin are moderated by the membrane protein 5-HT transporter, and with the exception of the ionotropic 5-HT3 receptor, 5-HT exerts effects through seven families of G protein–coupled receptors (5-HT1–7) with ≥14 subtypes (for a review, see Berger et al., 2009). A large number of commercially available medications act on the 5-HT system and could be evaluated for OUD withdrawal management. For instance, tricyclic medications that inhibit 5-HT reuptake are recommended and widely used for pain management (Finnerup et al., 2015; Obata, 2017). Several 5-HT medications are also formally approved for mood disturbance and anxiety, two prominent opioid withdrawal symptoms that are present during both the acute and protracted withdrawal phases (Handelsman et al., 1987; Latowsky, 1996; Wesson and Ling, 2003). Intracerebroventricular injections of 5-HT to nondependent rats have also been observed to directly and dose dependently produce wet dog shakes (Drust et al., 1979), which is a prominent sign of opioid withdrawal in rodents.
Preclinical Withdrawal.
Similar to DA, the 5-HT system can both increase and decrease the severity of withdrawal symptoms depending on the dose of drug administered; for instance, administration of the antidepressant fenfluramine, which promotes 5-HT release and inhibits 5-HT reuptake, decreased jumping (Cervo et al., 1981; Romandini et al., 1984) but not wet dog shakes or other symptoms (Romandini et al., 1984). The 5-HT2 receptor agonists meta-chlorophenylpiperazine and lorcaserin also decreased jumping (Samanin et al., 1980; Cervo et al., 1981; Shahidi and Hashemi-Firouzi, 2014; Wu et al., 2015) as well as diarrhea (Samanin et al., 1980) and paw licking (Shahidi and Hashemi-Firouzi, 2014), although another study found that lorcaserin only reduced wet dog shakes, paw licking, and penile grooming but not jumping or other withdrawal signs (Zhang et al., 2016). The 5-HT2A/3 receptor agonist quipazine selectively decreased jumping but did not decrease other signs of opioid withdrawal (Samanin et al., 1980) and the 5-HT7 receptor agonist AS19 has been observed to block jumping, grooming, shaking, teeth chattering, and writhing (Shahidi and Hashemi-Firouzi, 2014).
Similar to agonists of 5-HT receptors, antagonists also block some withdrawal behaviors while exacerbating others. For example, pretreatment with the 5-HT2 antagonist cyproheptadine and the mixed 5HT2 antagonist/5HT1 agonist methysergide reduced jumping, wet dog shakes, and burrows but increased weight loss and hypothermia (el-Kadi and Sharif, 1995). The 5-HT3 antagonist ondansetron reduced jumping, defecation, and wet dog shakes in one study (Pinelli et al., 1997) but had no effect on wet dog shakes, paw shakes, or salivation in another study (Higgins et al., 1991). The 5-HT2A antagonist glemanserin has also been found to selectively reduce jumping and self-directed behaviors (Pang et al., 2016); however, the 5-HT7 receptor–specific antagonist SB269970 increased jumping, grooming, teeth chattering, and shaking (Shahidi and Hashemi-Firouzi, 2014).
Human Withdrawal.
Two studies have reported on whether the 5-HT3 antagonist ondansetron modifies opioid withdrawal severity in humans. The first was a case report that suggested ondansetron might reduce human opioid withdrawal syndrome severity (Wakim, 2012); however, the second was a placebo-controlled within-subject empirical evaluation that found no benefit of ondansetron relative to placebo following naloxone-precipitated withdrawal (Chu et al., 2017).
Cannabinoid System
Arachindonoylethanolamide (anandamide) and 2-arachidonoylglycerol are two primary (but not sole) endogenous cannabinoid ligands that bind to and activate cannabinoid type 1 (CB1) and type 2 receptors. CB1 receptors are often colocalized with μ-opioid receptors (for a review, see Bloomfield et al., 2019), and cannabinoid agonists, antagonists, and endocannabinoid catabolic enzyme inhibitors have all been examined for opioid withdrawal suppression.
Preclinical Withdrawal.
Several studies have reported that Δ9-THC, the primary psychoactive cannabinoid in the cannabis plant and a partial agonist at CB1 receptors, reduced withdrawal-related jumping and other escape behaviors, wet dog shakes, gastrointestinal symptoms, and shaking in rodents (Hine et al., 1975a,b,c; Bhargava, 1976; Lichtman et al., 2001; Cichewicz and Welch, 2003), although additional studies reported no beneficial effect of THC on jumping (Lichtman et al., 2001) or diarrhea (Lichtman et al., 2001; Cichewicz and Welch, 2003). Direct administration of the endogenous cannabinoid anandamide was found to decrease jumping and weight loss (Vela et al., 1995); however, increasing anandamide through inhibition of its catabolic enzymes using the fatty acid amide hydrolase inhibitor URB597, monoacylglycerol lipase inhibitor JZL184, or dual fatty acid amide hydrolase/monoacylglycerol lipase inhibitor SA-57 increased jumping again (Shahidi and Hasanein, 2011; Gamage et al., 2015). In addition, URB597 also decreased signs of wet dog shakes, teeth chattering, tremors, and self-directed behaviors (Shahidi and Hasanein, 2011). However, this effect may be compound specific since the fatty acid amide hydrolase inhibitor PF-3845 had no effect on jumping when administered alone (Gamage et al., 2015), although reductions in jumping, tremors, shakes, and diarrhea were observed when a high dose of PF-3845 was combined with low doses of JZL184 and SA-57 (Ramesh et al., 2013). Further complicating this issue is the fact that the anandamide-transport inhibitor N-(4-hydroxyphenyl) arachidonylethanolamide, which increases synaptic anandamide levels, blocked signs of spontaneous but not naloxone-precipitated withdrawal (Del Arco et al., 2002), suggesting these two syndromes may have different underlying mechanisms. The only study to examine a cannabinoid type 2 receptor–specific agonist (AM1710) on opioid withdrawal found it also reduced jumping (Li et al., 2019).
Finally, chronic exposure to the CB1 inverse agonist/antagonist SR141716A (Rimonobant) selectively reduced jumping (Rubino et al., 2000; Mas-Nieto et al., 2001), wet dog shakes (Mas-Nieto et al., 2001), teeth chattering, self-directed behaviors, and diarrhea (Rubino et al., 2000), while not changing other measured behaviors.
Human Withdrawal.
The human clinical evidence for the therapeutic effectiveness of cannabinoids during opioid withdrawal is limited and focused largely on the cannabis plant or THC. The earliest evidence of a potential therapeutic application of cannabis for opioid withdrawal is a case report from 1889, wherein a patient being withdrawn from the opioid laudanum showed immediate improvement in symptoms following cannabis administration, evidenced by being able to “take a turn around the verandah with the aid of a stick” (Birch, 1889). More recent retrospective reports have provided mixed support for cannabis treatment of opioid withdrawal. One secondary analysis of patients undergoing a methadone taper found no evidence that urine samples, which indicated recent cannabis use, were associated with better outcomes, concluding that cannabis use did not meaningfully improve withdrawal management (Epstein and Preston, 2015). A second retrospective chart review reported that 33% and 66% of patients using cannabis during opioid withdrawal found it to increase and decrease withdrawal severity, respectively (Gossop et al., 1991). Human empirical studies have generally examined whether the synthetic THC product dronabinol improves opioid withdrawal management. One randomized study in patients undergoing opioid-assisted withdrawal reported lower withdrawal ratings in participants receiving dronabinol versus placebo (Bisaga et al., 2015), and a second randomized human laboratory study in participants undergoing abrupt opioid discontinuation found that dronabinol modestly reduced withdrawal relative to placebo (Lofwall et al., 2016). However, dronabinol administration during opioid withdrawal has been observed to produce clinically significant levels of tachycardia (Jicha et al., 2015) and intoxication (Lofwall et al., 2016); suggesting its therapeutic window for this indication may be narrow.
Orexin/Hypocretin System
The orexin (OX)/hypocretin system (hereinafter referred to as orexin) is composed of two endogenous peptides (OXA and OXB) and two receptors (OX1R and OX2R) (for a review, see Wang et al., 2018). Orexin is the most recently discovered of the systems reviewed herein (de Lecea et al., 1998; Sakurai et al., 1998), and there is a correspondingly limited number of pharmacological probes available to assess its role in opioid withdrawal. However, there is substantial rationale for evaluating this system. Orexin signaling generally promotes wakefulness, and the FDA has recently approved the dual OX1R/OX2R antagonist suvorexant (Belsomra) based on evidence that it promotes sleep and improves sleep architecture (Herring et al., 2016). Acute opioid withdrawal significantly disrupts sleep (Oyefeso et al., 1997), and pronounced protracted abnormal rapid eye movement sleep patterns are evident during both preclinical (Khazan and Colasanti, 1972; Colasanti et al., 1975) and human withdrawal (Mehtry et al., 2014). Since less sleep impairment is significantly associated with better withdrawal outcomes (Warden et al., 2012; Dunn et al., 2015), the orexin system is a logical target to address withdrawal-precipitated insomnia. Despite this evidence base, no preclinical or human studies have assessed the role of orexin in opioid-related insomnia; rather, the following studies all provide evidence that the orexin system confers benefits on other non-sleep–related withdrawal symptoms. There are no human studies examining the orexin system during withdrawal; therefore, herein only preclinical studies are reviewed.
Preclinical Withdrawal.
Direct administration of OXA (which preferentially binds to OX1R) increases self-directed behaviors, wet dog shakes, teeth chattering, and tremors, which can be blocked by pretreatment with the OX1R antagonist SB-334867 (Ghaemi-Jandabi et al., 2014). SB-334867 also reduced chewing, diarrhea, jumping, ptosis, shaking, wet dog shakes, and other signs when administered by itself (Azizi et al., 2010; Erami et al., 2012; Ahmadi-Soleimani et al., 2014; Hooshmandi et al., 2017) or prior to naloxone-precipitated withdrawal (Laorden et al., 2012), although in the latter case it did increase teeth chattering (Laorden et al., 2012). This, combined with evidence that direct administration of SB-334867 to the nucleus accumbens reduced self-directed behaviors, wet dog shakes, tremors, ptosis, gnawing, and global withdrawal scores (Sharf et al., 2008), strongly implicates the orexin system in the expression of some withdrawal symptoms. However, when SB-334867 is administered to the locus coeruleus following GABAA antagonist pretreatment, its beneficial effects on withdrawal are attenuated, suggesting that orexin modulation of opioid withdrawal may be due to OX1R and GABA interactions in the locus coeruleus (Davoudi et al., 2016). The complexity of orexin’s role in opioid withdrawal is further illustrated by a study that found OX1R-dependent withdrawal reduction was limited to the active dark cycle phase, suggesting orexin-specific effects on withdrawal may be dependent on circadian rhythms and relegated to wakefulness and possibly drug seeking (Hooshmand et al., 2017).
Glutamate System
The glutamate system is comprised of ionotropic glutamate [(iGlu), AMPA and NMDA] and metabotropic glutamate [(mGlu), mGlu1–5] receptors that are ubiquitously distributed throughout the brain and are being actively investigated for their role in a wide range of substance use disorders (for a review, see Niciu et al., 2012). A convergence of evidence suggests that iGlu antagonists, mGlu2/3 agonists, and mGlu5 antagonists reduce the severity of some opioid withdrawal symptoms.
Preclinical Withdrawal.
Glutamate appears to increase, and glutamate antagonism appears to decrease, opioid withdrawal severity. For instance, increasing glutamate levels by directly injecting glutamate into the locus coeruleus (Tokuyama et al., 1998) or through pretreatment with the glutamate transporter inhibitor DL-TBOA, increases stretching, wet dog shakes, and teeth chattering (Sekiya et al., 2004). Decreasing glutamate levels through administration of the β-lactam antibiotic ceftriaxone (which upregulates the glutamate transporter) decreased jumping and teeth chattering in rats (Medrano et al., 2015), and increasing glutamate by coadministering dihydrokainic acid to block ceftriaxone increased withdrawal symptoms again (Medrano et al., 2015). Opioid withdrawal was also completely suppressed in rats that received an infusion of the protein kinase inhibitor H-7 to reduce glutamate in the locus coeruleus (Tokuyama et al., 1996).
Additional evidence supports targeting the iGlu NMDA and AMPA receptors for opioid withdrawal. Pretreatment with the NMDA receptor antagonist dextromethorphan reduced chewing and paw shakes relative to a control condition and independent of the coadministration of the CYP2D6-inhibitor quinidine, which is generally required for humans to achieve a therapeutic effect of dextromethorphan (Bisaga et al., 2008). The NMDA antagonist MK-801 reduced jumping and the overall duration of withdrawal (Tanganelli et al., 1991; Fundytus and Coderre, 1994; Tokuyama et al., 1996; Leal et al., 2003), although it increased wet dog shakes (Fundytus and Coderre, 1994). Pretreatment with felbamate and D-cycloserine (an antagonist and agonist, respectively) at the glycine site of the NMDA receptor decreased withdrawal, although only felbamate did so in a linear, dose-dependent manner (Kosten et al., 1995). Both AMPA-receptor antagonists topiramate and LY293558 reduced jumping, wet dog shakes, gastrointestinal signs, mastication, ptosis, lacrimation, chewing, and writhing (Rasmussen et al., 1996; Medrano et al., 2015), and microinjection of an AMPA/kainite-glutamate-receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione into the central nucleus of the amygdala significantly reduced teeth chattering, diarrhea, and rhinorrhea (Watanabe et al., 2002). However, intracerebroventricular administration of the AMPA/kainite antagonist GYKI did not improve withdrawal (Fundytus and Coderre, 1994).
Medications targeting iGlu receptors generally have poor receptor specificity and potential for abuse or other negative side effect profiles that may limit their adoption in clinical settings (Herman et al., 1995). Research evaluating mGlu medications as alternatives to iGlu suggest they are also promising targets. For instance, the nonselective mGlu agonist (1S,3R)-ACPD and the selective mGlu2/3 agonist DCG-IV both reduced signs of diarrhea and salivation, as well as the overall duration of opioid withdrawal (Fundytus and Coderre, 1997). The mGlu2/3 receptor agonist LY354740 also dose dependently decreased wet dog shakes, hyperactivity, ptosis, gastrointestinal signs, writhes, salivation, and chews (Vandergriff and Rasmussen, 1999), and pretreatment with the mGlu2/3 receptor agonist ACPT-1 decreased jumping, wet dog shakes, and tremors (Pałucha-Poniewiera et al., 2009). The mGlu antagonists MCPG (nonselective), MCCG (mGlu2/3), MAP4 (mGlu 4, 6, 7, and 8), and MPEP or MTEP (mGlu5 antagonists) reduced several withdrawal signs including jumping, lacrimation, ptosis, and wet dog shakes, as well as time spent in withdrawal (Fundytus et al., 1997; Rasmussen et al., 2005; Kotlinska and Bochenski, 2007). Evidence of mGlu1 receptor antagonists are mixed; while the mGlu1 antagonist (S)-4C-PG reduced teeth chattering and writhing (Fundytus and Coderre, 1994), the mGlu1 antagonist EMQMCM did not significantly modify overall opioid withdrawal scores (Kotlinska and Bochenski, 2007).
Human Withdrawal.
Several studies have examined glutamatergic agents in humans. One series of three case studies reported that withdrawal improved in patients who were transitioned from clonidine to topiramate (Zullino et al., 2002). A second case report found that patients treated with topiramate required fewer concomitant medications during detoxification (suggesting they had lower severity opioid withdrawal) relative to patients treated with other medications (Zullino et al., 2004). Empirical studies support these reports. For instance, the NMDA-antagonist memantine and the partial μ-agonist buprenorphine suppressed opioid withdrawal at comparable levels in a randomized trial, although buprenorphine was significantly more effective when only subjective ratings were evaluated (Jain et al., 2011). Memantine also reduced naloxone-precipitated withdrawal severity in patients with OUD (Bisaga et al., 2001). Dextromethorphan has also been evaluated in randomized studies. The first study observed no difference in withdrawal suppression between dextromethorphan and placebo, although the authors thought a benefit of dextromethorphan might have started to emerge during the protracted period (Lin et al., 2014). The second study found that dextromethorphan + clonidine resulted in a more mild withdrawal syndrome than clonidine alone (Malek et al., 2013), and the third study found no difference between dextromethorphan + quinidine and placebo (Akerele et al., 2008).
Medications with Multiple Mechanisms of Action
Several preclinical and the majority of human studies examining medications for opioid withdrawal management have evaluated medications that act through multiple transmitter systems, which precludes determination as to what system might be mediating the observed effects on withdrawal. However, given that the majority of medications approved for use in humans act on several systems, it is important that these studies be reviewed.
Preclinical Withdrawal.
Most medications reviewed herein have prominent 5-HT effects and show positive signals for opioid withdrawal management. First, buspirone, which has strong affinity for the 5-HT1A receptor and moderate antagonist affinity at the D3 and D4 receptors, attenuated opioid withdrawal symptoms in rhesus monkeys (Aceto and Bowman, 1993) and rats (Berthold et al., 1989). Second, venlafaxine (an antidepressant that inhibits 5-HT and 5-HT noradrenergic reuptake) decreased jumping, hyperactivity, writhing, ptosis, lacrimation, and gastrointestinal symptoms in rats (Lu et al., 2001). Third, mirtazapine (a 5-HT2 and 5HT3 receptor family antagonist that also blocks adrenergic autoreceptors) reduced wet dog shakes, rearing, and grooming in rats (Kang et al., 2008). Fourth, the tetracyclic mianserin, which antagonizes 5-HT and adrenergic receptors, and trazodone (a 5-HT antagonist and reuptake inhibitor) both reduced jumping, hyperactivity, and grooming in mice, although combining the two medications together did not increase either drug’s effect (Schreiber et al., 2003). However, the cannabinoid cannabidiol (an inverse agonist at the CB1 and cannabinoid type 2 receptors), which also acts as a 5HT1A agonist (an allosteric modulator of μ- and δ-opioid receptors) and a positive modulator of the transient receptor vanilloid-1 (Kathmann et al., 2006), did not reduce withdrawal when administered alone but did reduce wet dog shakes and gastrointestional distress when coadministered with THC (Hine et al., 1975a,c). Finally, neither spiroperidol nor haloperidol, two antipsychotic medications with dual action as 5-HT (subtype unknown) and D2 receptor antagonists, had an effect on withdrawal-related jumping (Berthold et al., 1989).
Several additional preclinical studies have examined whether medications with primary NMDA receptor activity reduce withdrawal severity. The most frequently researched compound is ibogaine, an NMDA antagonist that binds to κ-opioid and σ2 receptors and inhibits nicotine receptors as well as 5-HT and DA transport (Mach et al., 1995; Alper, 2001; Bulling et al., 2012). Numerous studies have suggested that ibogaine, its primary metabolite noribogaine, and a related congener 18-methoxyroconaridine, decrease some opioid withdrawal symptoms in preclinical models (Dzoljic et al., 1988; Sharpe and Jaffe, 1990; Glick et al., 1992; Cappendijk et al., 1994; Rho and Glick, 1998; Parker and Siegel, 2001; Leal et al., 2003; Panchal et al., 2005; Mash et al., 2016), although at least one study found that ibogaine increased withdrawal severity in mice (Francés et al., 1992). Ketamine, an NMDA receptor antagonist with cholinergic and opioid activity, has also been observed to reduce withdrawal (Streel et al., 2005), although it may not be more effective than dextromethorphan (Koyuncuoğlu et al., 1990).
Human Withdrawal.
The vast majority of human studies reviewed herein examined medications that act on the DA, 5-HT, and glutamate systems. A retrospective chart review reported the antipsychotic medication quetiapine, which antagonizes D2 and 5-HT2 receptors, benefited 96.3% of the patients undergoing opioid withdrawal, specifically improving opioid craving (73.8%), somatic pain (22.5%), and insomnia (20.5%) (Pinkofsky et al., 2005). Buspirone has also been shown to improve opioid withdrawal symptoms in both an open-label trial of men undergoing withdrawal from methadone (Rose et al., 2003) and in a randomized, controlled comparison of buspirone to placebo in men undergoing withdrawal from methadone (Buydens-Branchey et al., 2005). Amantadine, an NMDA antagonist that increases DA release and inhibits DA reuptake, has been shown in a randomized comparison to suppress more symptoms of withdrawal when coadministered with clonidine, relative to clonidine alone (Amiri et al., 2014). In contrast, a second study that examined amantadine for cocaine use in methadone-maintained patients observed no difference in opioid withdrawal outcomes, although the outcomes may have been impacted by ongoing cocaine (a DA reuptake inhibitor) use in that study (Pérez de los Cobos et al., 2001). An additional study that compared the tricyclic antidepressant amitriptyline (which inhibits norepinephrine and 5-HT reuptake) to the benzodiazepine lorazepam for insomnia management during opioid withdrawal reported no differences between the two medications, although the lack of placebo condition in this study makes it difficult to determine whether either medication improved insomnia (Srisurapanont and Jarusuraisin, 1998). A randomized comparison of trazodone + naltrexone to clonidine + naltrexone for rapid withdrawal from methadone reported trazodone was as effective as clonidine in reducing overall withdrawal symptom severity and outperformed clonidine with regard to insomnia, thirst, and shivers (Pozzi et al., 2000). Finally, a randomized evaluation reported significantly lower withdrawal symptoms among participants who received venlafaxine versus placebo (Lin et al., 2008).
Several studies support targeting the glutamate system for human withdrawal management. For instance, retrospective chart reviews, surveys, and case reports all provide associative evidence that ibogaine reduces the severity of opioid withdrawal in humans (Sheppard, 1994; Alper et al., 1999; Davis et al., 2017; Brown and Alper, 2018; Malcolm et al., 2018), although no empirical studies have been published to formally support these reports. However, the widespread clinical adoption of ibogaine for OUD seems unlikely (Hoelen et al., 2009; Paling et al., 2012; Asua, 2013; Jalal et al., 2013) because it has a very narrow therapeutic window and is dangerous for persons with preexisting cardiovascular problems (Alper et al., 2012) or who plan to combine it with opioids (Mazoyer et al., 2013). Ibogaine also produced significant head and body tremors in many of the studies reviewed herein. Efforts to develop the potentially safer metabolite noribogaine for opioid withdrawal are in the beginning stages and show initial promise (Glue et al., 2015a,b; Mash et al., 2016). Another alternative to ibogaine may be ketamine, which has been supported for opioid withdrawal management in case reports of patients with chronic pain (Quinlan, 2012; Strickler et al., 2018), and a patient with OUD (Lalanne et al., 2016), as well as a randomized study that found it lowered opioid withdrawal significantly more than placebo among patients undergoing rapid naltrexone-assisted detoxification under anesthesia (Jovaiša et al., 2006). The recent FDA approval of the ketamine derivative esketamine for severe depression provides a new potential pathway through which this drug class can be examined for human opioid withdrawal management.
Discussion
The OUD trajectory generally transitions from using opioids for euphoric or pain-relieving qualities to using opioids to avoid withdrawal or craving, and avoidance of withdrawal is believed to motivate continued opioid use despite the potential to incur negative health, financial, judicial, social, and personal consequences. Therefore, opioid withdrawal symptom management is a primary goal of most OUD treatments. This review summarized preclinical and clinical evidence supporting the involvement of the DA, 5-HT, cannabinoid, orexin, and glutamate systems in the opioid withdrawal syndrome. All of the reviewed systems appeared to contribute to some but not all symptoms of withdrawal, suggesting they likely modulate but do not independently drive the opioid withdrawal syndrome. The data reviewed herein suggest it is unlikely that medications acting on these systems will be better at suppressing withdrawal than an opioid agonist. Rather, these data suggest that medications targeting these systems should be evaluated to determine whether their coadministration with opioid agonists can improve opioid withdrawal management. Empirically evaluating medications that work on these systems can help transition the OUD treatment field from using symptomatic concomitant medications (e.g., nonsteroidal anti-inflammatory drugs) to those supported by a mechanistic understanding of opioid withdrawal.
The fact that methadone, buprenorphine, and naltrexone are FDA approved for OUD treatment does not detract from the value of investigating additional pharmacotherapies for opioid withdrawal management—new medications to reduce opioid withdrawal are still needed. Supervised opioid withdrawal is the most frequently used form of OUD treatment (Jones et al., 2015); however, many patients leave treatment prematurely because their withdrawal is not adequately managed and those who complete it experience protracted withdrawal and ongoing cravings (Northrup et al., 2015). This leaves patients at significant risk for relapse and fatal overdose (Degenhardt et al., 2011). Further complicating this issue is the fact that no standardized guidelines for opioid withdrawal exist. Opioid-assisted detoxifications generally coadminister opioids with other concomitant medications to manage emergent withdrawal symptoms, and detoxifications that do not use opioids rely solely on these concomitant medications. Evidence suggests that patients vary considerably in their manifestation of withdrawal (Northrup et al., 2015; Dunn et al., 2018b) and may, therefore, benefit from having a range of mechanistically informed concomitant treatments available. Improved withdrawal management is also critical for improving patient access to extended-release naltrexone, a relapse prevention treatment that blocks exogenous opioid administration and is as effective a treatment as buprenorphine once patients are able to successfully taper off their opioid medications (Tanum et al., 2017; Lee et al., 2018). By increasing patient eligibility for naltrexone, efforts to mechanistically identify concomitant medications during withdrawal could help expand OUD treatment access.
Empirically supported concomitant medications could also be used to help patients who are receiving opioids for chronic pain management, either by augmenting their treatments and potentially reducing their opioid reliance, or by assisting them in transitioning off of long-term opioid therapy in favor of other pain management strategies. For instance, several epidemiologic studies have suggested that medicinal cannabis may produce an opioid-sparing effect (Bachhuber et al., 2014; Bradford and Bradford, 2016; Campbell et al., 2018). This is of major interest in the context of the current opioid crisis, since evidence that chronic opioid exposure may not be an appropriate treatment of pain (Busse et al., 2013) has led to new guidelines recommending patients be transitioned off long-term opioid treatments (Dowell et al., 2016). Notably, interest in the potential opioid-sparing effects of cannabis has prompted several states to legislatively define OUD as an approved indication for medicinal cannabis, despite a lack of empirical support for this approach (Humphreys and Saitz, 2019) and data suggesting cannabis exposure increases the risk of developing OUD (Olfson et al., 2018). Since there are also no standardized guidelines for tapering patients off of their clinically indicated pain medications, a mechanistic understanding of opioid withdrawal symptoms can help support the development of effective pain medication tapering protocols or studies on the opioid-sparing effects of cannabis and other targeted medications.
As shown in Table 10, all of the neurotransmitter systems evaluated here have corresponding medications that are FDA approved for other indications or are being actively investigated in humans, suggesting they could be repurposed for the indication of opioid withdrawal management. Advancing a medication from discovery to market can cost a billion dollars and take more than a decade (Vocci and Ling, 2005; Adams and Brantner, 2006), which is too slow a process to meaningfully impact the current opioid crisis. Examining approved medications for opioid withdrawal will also allow existing data to be leveraged to inform safety, doses, and participant eligibility. Although repurposing a medication may expedite treatment access, the need to examine medications in a randomized design still slows the development process. To circumvent this issue, the use of a human laboratory session to model clinical withdrawal experience could be investigated as a medication development pathway. For instance, a recent study in a human OUD clinical population showed that response to a precipitated naloxone challenge was significantly associated with withdrawal response in a subsequent double-blind randomized and controlled pharmacotherapy examination (Dunn et al., 2018b). A human laboratory model was also used in some of the empirical human studies reviewed herein (Bisaga et al., 2001; Jain et al., 2011; Chu et al., 2017) and has been regularly used to screen medications for alcohol and tobacco use disorders (Perkins et al., 2008, 2013; McKee, 2009; McKee et al., 2012). Together, this evidence suggests that a laboratory model could be a promising method for screening candidate opioid withdrawal medications prior to clinical trial investigations.
TABLE 10.
Summary of outcomes from preclinical and human empirical studies and prospective targets for evaluation
| Neurotransmitter System | Prospective Medication Targets for Evaluation | ||
|---|---|---|---|
| Improved ≥1 Withdrawal Symptoma | Did Not Improve Any Withdrawal Symptoms | Approved for Use in Humans and Acts on Transmitter Systems of Interestb | |
| Dopamine | Buspirone | Sulpride | Acetophenazine |
| d-Amphetamine | Aripiprazolec | ||
| Desipramine | Asenapine | ||
| Domperidone | Clozapinec | ||
| Flupenthixol | Domperidone | ||
| Haloperidol | Droperidol | ||
| L-DOPA | Fluphenazine | ||
| Mianserin | Fluspirilene | ||
| Pimozide | Iloperidone | ||
| Quetiapine | Loxapine | ||
| Lurasidonec | |||
| Mesoridazine | |||
| Methotrimeprazine | |||
| Metoclopramide | |||
| Molindone | |||
| Olanzapinec | |||
| Paliperidone | |||
| Perphenazine | |||
| Pimozide | |||
| Pipotiazine | |||
| Proclorperazine | |||
| Risperidone | |||
| Sulpiride | |||
| Ziprasidonec | |||
| Serotonin | Buspirone | Amitriptyline | Aripriprazolec |
| Cyproheptadine | Brexpiprazole | ||
| Fenfluramine | Chlorpromazine | ||
| Glemanserin | Clozapinec | ||
| Mianserin | Cyclobenzaprine | ||
| Mirtazapine | Cyproheptadine | ||
| Lorcaserin | Desipramine | ||
| Ondansetron | Lisuride | ||
| Quetiapine | Lorcaserin | ||
| Trazodone | Lurasidonec | ||
| Venlafaxine | Mirtazapine | ||
| Olanzapinec | |||
| Palonosetron | |||
| Promethazine | |||
| Risperidone | |||
| Vortioxetine | |||
| Ziprasidonec | |||
| Cannabinoid | Dronabinol | Cannabidiol | Cannabidiol |
| Nabilone | |||
| Orexin | NR | NR | Suvorexant |
| Glutamate | Amantadine | NR | Acamprosate |
| D-Cycloserine | Phenobarbital | ||
| Felbamate | Esketamine | ||
| Ketamine | Guaifenesin | ||
| Memantine | Pentobarbital | ||
| Noribogaine | Perampanel | ||
| Topiramate | Rufinadmide | ||
| Secobarbital | |||
NR, none reported.
Medications may be listed more than once if they act on multiple systems.
The medications included here are approved for use in humans and could be evaluated for possible opioid withdrawal symptom management. Note that each medication should be assessed for its potential side effect profile and contraindications, and that some medications may have abuse liability or other features that might limit their adoption in clinical settings.
Medication acts on more than one target that may alleviate withdrawal.
This review is complicated by the fact that many of the drugs evaluated preclinically are not yet approved for human consumption; although preclinical and human symptoms cluster into similar categories (as shown in Table 1), they may not directly generalize across species. The studies were also extremely heterogeneous in nature, and despite efforts to systematically review all available literature the diverse manner in which these studies were published and indexed may have resulted in some publications being accidentally omitted. Thus, this paper should be conceptualized as a narrative summary to stimulate future investigations. Finally, despite evidence that many opioid effects may be mediated by gonadal hormones (Huhn et al., 2018) the majority of preclinical and human studies reviewed herein evaluated predominately (and often exclusively) male samples. More research is needed to determine whether medications are equally effective for both sexes, particularly in light of data suggesting hormones impact craving and relapse vulnerability for other drugs of abuse (e.g., Carpenter et al., 2006; Franklin et al., 2008).
In summary, given the scale and impact of the opioid crisis and the vast number of people throughout the world who are prescribed opioids chronically for pain management, there is a pressing need to identify mechanistically informed medications that reduce the severity of opioid withdrawal symptoms. This review provides evidence that several non-opioid systems (DA, 5-HT, cannabinoid, orexin, and glutamate) contribute to opioid withdrawal symptom severity and identifies several potential FDA-approved medications (Table 10) that could be evaluated as concomitant medications for opioid withdrawal management. The repurposing of an existing medication is a less-expensive method for drug development than a discovery-to-market approach, and the familiarity and availability of existing medications may help them to be more readily adopted in clinical settings. Developing a solid mechanistically informed treatment regimen for persons with opioid physical dependence is essential to the advancement of patient care and to improving treatment outcomes for persons experiencing opioid withdrawal.
Abbreviations
- CB1
cannabinoid type 1 receptor
- DA
dopamine
- FDA
Food and Drug Administration
- 5-HT
serotonin
- iGlu
ionotropic glutamate
- mGlu
metabotropic glutamate
- OUD
opioid use disorder
- OX
orexin
- THC
tetrahydrocannabinol
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Dunn, Huhn, Bergeria, Gipson, Weerts.
Footnotes
Salary support for this review was provided by the National Institutes of Health National Institute on Drug Abuse [Grants R01DA042751, R01DA035246, R01DA040644, and R34DA042926 (to K.E.D.); UG3DA048734 (to A.S.H. and K.E.D.); and R21DA044479, R03DA045881, and R00DA036569 (to C.D.G.)], and the Arizona Alzheimer’s Consortium (to C.D.G.).
References
- Aceto MD, Bowman ER. (1993) Suppression of opiate withdrawal and cocaine hyperarousal syndromes by buspirone. Possible pharmacotherapeutic applications. Arzneimittelforschung 43:942–945. [PubMed] [Google Scholar]
- Adams CP, Brantner VV. (2006) Estimating the cost of new drug development: is it really 802 million dollars? Health Aff (Millwood) 25:420–428. [DOI] [PubMed] [Google Scholar]
- Ahmadi-Soleimani SM, Ghaemi-Jandabi M, Azizi H, Semnanian S. (2014) Orexin type 1 receptor antagonism in lateral paragigantocellularis nucleus attenuates naloxone precipitated morphine withdrawal symptoms in rats. Neurosci Lett 558:62–66. [DOI] [PubMed] [Google Scholar]
- Akerele E, Bisaga A, Sullivan MA, Garawi F, Comer SD, Thomas AA, Nunes EV, Kleber HD. (2008) Dextromethorphan and quinidine combination for heroin detoxification. Am J Addict 17:176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper KR. (2001) Ibogaine: a review. Alkaloids Chem Biol 56:1–38. [DOI] [PubMed] [Google Scholar]
- Alper KR, Lotsof HS, Frenken GM, Luciano DJ, Bastiaans J. (1999) Treatment of acute opioid withdrawal with ibogaine. Am J Addict 8:234–242. [DOI] [PubMed] [Google Scholar]
- Alper KR, Stajić M, Gill JR. (2012) Fatalities temporally associated with the ingestion of ibogaine. J Forensic Sci 57:398–412. [DOI] [PubMed] [Google Scholar]
- Amiri S, Malek A, Tofighnia F, Habibi Asl B, Seidy A. (2014) Amantadine as augmentation in managing opioid withdrawal with clonidine: a randomized controlled trial. Iran J Psychiatry 9:142–146. [PMC free article] [PubMed] [Google Scholar]
- Ary M, Lomax P. (1976) Dopaminergic sites involved in morphine withdrawal hypothermia. Proc West Pharmacol Soc 19:290–294. [PubMed] [Google Scholar]
- Asua I. (2013) Growing menace of ibogaine toxicity. Br J Anaesth 111:1029–1030. [DOI] [PubMed] [Google Scholar]
- Azizi H, Mirnajafi-Zadeh J, Rohampour K, Semnanian S. (2010) Antagonism of orexin type 1 receptors in the locus coeruleus attenuates signs of naloxone-precipitated morphine withdrawal in rats. Neurosci Lett 482:255–259. [DOI] [PubMed] [Google Scholar]
- Bachhuber MA, Saloner B, Cunningham CO, Barry CL. (2014) Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern Med 174:1668–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarczyk B, Vetulani J. (1978) Antagonism of clonidine to shaking behavior in morphine abstinence syndrome and to head twitches produced by serotonergic agents in the rat. Pol J Pharmacol Pharm 30:307–322. [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL. (2009) The expanded biology of serotonin. Annu Rev Med 60:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold H, Fozard JR, Engel G. (1989) 5-HT1 receptor agonists attenuate the naloxone-induced jumping behaviour in morphine-dependent mice. Eur J Pharmacol 162:19–27. [DOI] [PubMed] [Google Scholar]
- Bhargava HN. (1976) Inhibition of naloxone-induced withdrawal in morphine dependent mice by 1-trans-Δ9-tetrahydrocannabinol. Eur J Pharmacol 36:259–262. [DOI] [PubMed] [Google Scholar]
- Birch E. (1889) The use of Indian hemp in the treatment of chronic chloral and chronic opium poisoning. Lancet 133:625. [Google Scholar]
- Bisaga A, Comer SD, Ward AS, Popik P, Kleber HD, Fischman MW. (2001) The NMDA antagonist memantine attenuates the expression of opioid physical dependence in humans. Psychopharmacology (Berl) 157:1–10. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Kos T, Wójcikowski J, Daniel WA, Popik P. (2008) Brain levels of dextromethorphan and the intensity of opioid withdrawal in mice. Drug Alcohol Depend 95:147–151. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Sullivan MA, Glass A, Mishlen K, Pavlicova M, Haney M, Raby WN, Levin FR, Carpenter KM, Mariani JJ, et al. (2015) The effects of dronabinol during detoxification and the initiation of treatment with extended release naltrexone. Drug Alcohol Depend 154:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield MAP, Hindocha C, Green SF, Wall MB, Lees R, Petrilli K, Costello H, Ogunbiyi MO, Bossong MG, Freeman TP. (2019) The neuropsychopharmacology of cannabis: a review of human imaging studies. Pharmacol Ther 195:132–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford AC, Bradford WD. (2016) Medical marijuana laws reduce prescription medication use in medicare part D. Health Aff (Millwood) 35:1230–1236. [DOI] [PubMed] [Google Scholar]
- Brown TK, Alper K. (2018) Treatment of opioid use disorder with ibogaine: detoxification and drug use outcomes. Am J Drug Alcohol Abuse 44:24–36. [DOI] [PubMed] [Google Scholar]
- Bulling S, Schicker K, Zhang YW, Steinkellner T, Stockner T, Gruber CW, Boehm S, Freissmuth M, Rudnick G, Sitte HH, et al. (2012) The mechanistic basis for noncompetitive ibogaine inhibition of serotonin and dopamine transporters. J Biol Chem 287:18524–18534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse JW, Schandelmaier S, Kamaleldin M, Hsu S, Riva JJ, Vandvik PO, Tsoi L, Lam T, Ebrahim S, Johnston B, et al. (2013) Opioids for chronic non-cancer pain: a protocol for a systematic review of randomized controlled trials. Syst Rev 2:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, Reel-Brander C. (2005) Efficacy of buspirone in the treatment of opioid withdrawal. J Clin Psychopharmacol 25:230–236. [DOI] [PubMed] [Google Scholar]
- Campbell G, Hall W, Nielsen S. (2018) What does the ecological and epidemiological evidence indicate about the potential for cannabinoids to reduce opioid use and harms? A comprehensive review. Int Rev Psychiatry 30:91–106. [DOI] [PubMed] [Google Scholar]
- Cappendijk SL, Fekkes D, Dzoljic MR. (1994) The inhibitory effect of norharman on morphine withdrawal syndrome in rats: comparison with ibogaine. Behav Brain Res 65:117–119. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT. (2006) Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine Tob Res 8:627–638. [DOI] [PubMed] [Google Scholar]
- Cervo L, Rochat C, Romandini S, Samanin R. (1981) Evidence of a preferential role of brain serotonin in the mechanisms leading to naloxone-precipitated compulsive jumping in morphine-dependent rats. Psychopharmacology (Berl) 74:271–274. [DOI] [PubMed] [Google Scholar]
- Chu LF, Sun J, Clemenson A, Erlendson MJ, Rico T, Cornell E, Obasi H, Sayyid ZN, Encisco EM, Yu J, et al. (2017) Ondansetron does not reduce withdrawal in patients with physical dependence on chronic opioid therapy. J Addict Med 11:342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichewicz DL, Welch SP. (2003) Modulation of oral morphine antinociceptive tolerance and naloxone-precipitated withdrawal signs by oral Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther 305:812–817. [DOI] [PubMed] [Google Scholar]
- Colasanti B, Kirchman A, Khazan N. (1975) Changes in the electroencephalogram and REM sleep time during morphine abstinence in pellet-implanted rats. Res Commun Chem Pathol Pharmacol 12:163–172. [PubMed] [Google Scholar]
- Cox B, Ary M, Lomax P. (1976) Dopaminergic involvement in withdrawal hypothermia and thermoregulatory behavior in morphine dependent rats. Pharmacol Biochem Behav 4:259–262. [DOI] [PubMed] [Google Scholar]
- Davis AK, Barsuglia JP, Windham-Herman AM, Lynch M, Polanco M. (2017) Subjective effectiveness of ibogaine treatment for problematic opioid consumption: short- and long-term outcomes and current psychological functioning. J Psychedelic Stud 1:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoudi M, Azizi H, Mirnajafi-Zadeh J, Semnanian S. (2016) The blockade of GABAA receptors attenuates the inhibitory effect of orexin type 1 receptors antagonist on morphine withdrawal syndrome in rats. Neurosci Lett 617:201–206. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, McLaren J. (2011) Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction 106:32–51. [DOI] [PubMed] [Google Scholar]
- Del Arco I, Navarro M, Bilbao A, Ferrer B, Piomelli D, Rodríguez De Fonseca F. (2002) Attenuation of spontaneous opiate withdrawal in mice by the anandamide transport inhibitor AM404. Eur J Pharmacol 454:103–104. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, II, et al. (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95:322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole VP, Nyswander M. (1965) A medical treatment for diacetylmorphine (heroin) addiction. A clinical trial with methadone hydrochloride. JAMA 193:646–650. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R. (2016) CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 315:1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drust EG, Sloviter RS, Connor JD. (1979) Effect of morphine on ‘wet-dog’ shakes caused by cerebroventricular injection of serotonin. Pharmacology 18:299–305. [DOI] [PubMed] [Google Scholar]
- Dunn KE, Finan PH, Andrew Tompkins D, Strain EC. (2018a) Frequency and correlates of sleep disturbance in methadone and buprenorphine-maintained patients. Addict Behav 76:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Saulsgiver KA, Miller ME, Nuzzo PA, Sigmon SC. (2015) Characterizing opioid withdrawal during double-blind buprenorphine detoxification. Drug Alcohol Depend 151:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Strain EC, Heil SH, Higgins ST. (2011) The association between outpatient buprenorphine detoxification duration and clinical treatment outcomes: a review. Drug Alcohol Depend 119:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Weerts EM, Huhn AS, Schroeder JR, Tompkins DA, Bigelow GE, Strain EC. (2018b) Preliminary evidence of different and clinically meaningful opioid withdrawal phenotypes. Addict Biol DOI: 10.1111/adb.12680 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzoljic ED, Kaplan CD, Dzoljic MR. (1988) Effect of ibogaine on naloxone-precipitated withdrawal syndrome in chronic morphine-dependent rats. Arch Int Pharmacodyn Ther 294:64–70. [PubMed] [Google Scholar]
- el-Kadi AO, Sharif SI. (1995) The role of 5-HT in the expression of morphine withdrawal in mice. Life Sci 57:511–516. [DOI] [PubMed] [Google Scholar]
- el-Kadi AO, Sharif SI. (1998) The role of dopamine in the expression of morphine withdrawal. Gen Pharmacol 30:499–505. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL. (2015) No evidence for reduction of opioid-withdrawal symptoms by cannabis smoking during a methadone dose taper. Am J Addict 24:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erami E, Azhdari-Zarmehri H, Rahmani A, Ghasemi-Dashkhasan E, Semnanian S, Haghparast A. (2012) Blockade of orexin receptor 1 attenuates the development of morphine tolerance and physical dependence in rats. Pharmacol Biochem Behav 103:212–219. [DOI] [PubMed] [Google Scholar]
- Farrell M. (1994) Opiate withdrawal. Addiction 89:1471–1475. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, et al. (2015) Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 14:162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francés B, Gout R, Cros J, Zajac JM. (1992) Effects of ibogaine on naloxone-precipitated withdrawal in morphine-dependent mice. Fundam Clin Pharmacol 6:327–332. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Ehrman R, Lynch KG, Harper D, Sciortino N, O’Brien CP, Childress AR. (2008) Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: a retrospective analysis. J Womens Health (Larchmt) 17:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fundytus ME, Coderre TJ. (1994) Effect of activity at metabotropic, as well as ionotropic (NMDA), glutamate receptors on morphine dependence. Br J Pharmacol 113:1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fundytus ME, Coderre TJ. (1997) Attenuation of precipitated morphine withdrawal symptoms by acute i.c.v. administration of a group II mGluR agonist. Br J Pharmacol 121:511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fundytus ME, Ritchie J, Coderre TJ. (1997) Attenuation of morphine withdrawal symptoms by subtype-selective metabotropic glutamate receptor antagonists. Br J Pharmacol 120:1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamage TF, Ignatowska-Jankowska BM, Muldoon PP, Cravatt BF, Damaj MI, Lichtman AH. (2015) Differential effects of endocannabinoid catabolic inhibitors on morphine withdrawal in mice. Drug Alcohol Depend 146:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemi-Jandabi M, Azizi H, Semnanian S. (2014) Blockade of orexin type 1 receptors inhibits the development of morphine tolerance in lateral paragigantocellularis nucleus: an electrophysiological approach. Brain Res 1578:14–22. [DOI] [PubMed] [Google Scholar]
- Gianutsos G, Hynes MD, Lal H. (1976) Enhancement of morphine-withdrawal and apomorphine-induced aggression by clonidine. Psychopharmacol Commun 2:165–171. [PubMed] [Google Scholar]
- Glick SD, Rossman K, Rao NC, Maisonneuve IM, Carlson JN. (1992) Effects of ibogaine on acute signs of morphine withdrawal in rats: independence from tremor. Neuropharmacology 31:497–500. [DOI] [PubMed] [Google Scholar]
- Glue P, Lockhart M, Lam F, Hung N, Hung CT, Friedhoff L. (2015a) Ascending-dose study of noribogaine in healthy volunteers: pharmacokinetics, pharmacodynamics, safety, and tolerability. J Clin Pharmacol 55:189–194. [DOI] [PubMed] [Google Scholar]
- Glue P, Lockhart M, Lam F, Hung N, Hung CT, Tunnicliffe D, Cape G, Devane J, Weis H, Howes J, et al. (2015b) Evaluation of noribogaine safety, pharmacokinetics, and opioid withdrawal effects in methadone-dependent patients. [Google Scholar]
- Gold MS, Redmond DE, Jr, Kleber HD. (1978) Clonidine blocks acute opiate-withdrawal symptoms. Lancet 2:599–602. [DOI] [PubMed] [Google Scholar]
- Gold MS, Redmond DE, Jr, Kleber HD. (1979) Noradrenergic hyperactivity in opiate withdrawal supported by clonidine reversal of opiate withdrawal. Am J Psychiatry 136:100–102. [DOI] [PubMed] [Google Scholar]
- Gorodetzky CW, Walsh SL, Martin PR, Saxon AJ, Gullo KL, Biswas K. (2017) A phase III, randomized, multi-center, double blind, placebo controlled study of safety and efficacy of lofexidine for relief of symptoms in individuals undergoing inpatient opioid withdrawal. Drug Alcohol Depend 176:79–88. [DOI] [PubMed] [Google Scholar]
- Gossop M, Battersby M, Strang J. (1991) Self-detoxification by opiate addicts. A preliminary investigation. Br J Psychiatry 159:208–212. [DOI] [PubMed] [Google Scholar]
- Gowing L, Farrell M, Ali R, White JM. (2016) Alpha2 adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev:CD002024 DOI: 10.1002/14651858.CD002024. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. (1987) Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse 13:293–308. [DOI] [PubMed] [Google Scholar]
- Herman BH, Vocci F, Bridge P. (1995) The effects of NMDA receptor antagonists and nitric oxide synthase inhibitors on opioid tolerance and withdrawal. Medication development issues for opiate addiction. Neuropsychopharmacology 13:269–293. [DOI] [PubMed] [Google Scholar]
- Herring WJ, Connor KM, Snyder E, Snavely DB, Zhang Y, Hutzelmann J, Matzura-Wolfe D, Benca RM, Krystal AD, Walsh JK, et al. (2016) Suvorexant in patients with insomnia: pooled analyses of three-month data from phase-3 randomized controlled clinical trials. J Clin Sleep Med 12:1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz A, Bläsig J, Papeschi R. (1974) Role of catecholaminergic mechanisms in the expression of the morphine abstinence syndrome in rats. Psychopharmacology (Berl) 39:121–143. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Nguyen P, Joharchi N, Sellers EM. (1991) Effects of 5-HT3 receptor antagonists on behavioural measures of naloxone-precipitated opioid withdrawal. Psychopharmacology (Berl) 105:322–328. [DOI] [PubMed] [Google Scholar]
- Hillhouse M, Domier CP, Chim D, Ling W. (2010) Provision of ancillary medications during buprenorphine detoxification does not improve treatment outcomes. J Addict Dis 29:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hine B, Friedman E, Torrelio M, Gershon S. (1975a) Morphine-dependent rats: blockade of precipitated abstinence by tetrahydrocannabinol. Science 187:443–445. [DOI] [PubMed] [Google Scholar]
- Hine B, Torrelio M, Gershon S. (1975b) Attenuation of precipitated abstinence in methadone-dependent rats by delta 9-THC. Psychopharmacol Commun 1:275–283. [PubMed] [Google Scholar]
- Hine B, Torrelio M, Gershon S. (1975c) Differential effect of cannabinol and cannabidiol on THC-induced responses during abstinence in morphine-dependent rats. Res Commun Chem Pathol Pharmacol 12:185–188. [PubMed] [Google Scholar]
- Hoelen DW, Spiering W, Valk GD. (2009) Long-QT syndrome induced by the antiaddiction drug ibogaine. N Engl J Med 360:308–309. [DOI] [PubMed] [Google Scholar]
- Hooshmand B, Azizi H, Javan M, Semnanian S. (2017) Intra-LC microinjection of orexin type-1 receptor antagonist SB-334867 attenuates the expression of glutamate-induced opiate withdrawal like signs during the active phase in rats. Neurosci Lett 636:276–281. [DOI] [PubMed] [Google Scholar]
- Hooshmandi M, Hosseinmardi N, Janahmadi M, Khakpai F, Rohampour K, Doostmohammadi J. (2017) Antagonism of orexin type-1 receptors (OX1Rs) attenuates naloxone-precipitated morphine withdrawal syndrome in rat dorsal hippocampus. Pharmacol Biochem Behav 158:39–48. [DOI] [PubMed] [Google Scholar]
- Huhn AS, Berry MS, Dunn KE. (2018) Systematic review of sex-based differences in opioid-based effects. Int Rev Psychiatry 30:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys K, Saitz R. (2019) Should physicians recommend replacing opioids with cannabis? JAMA 321:639–640. [DOI] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A. (2001) The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci 4:943–947. [DOI] [PubMed] [Google Scholar]
- Jain K, Jain R, Dhawan A. (2011) A double-blind, double-dummy, randomized controlled study of memantine versus buprenorphine in naloxone-precipitated acute withdrawal in heroin addicts. J Opioid Manag 7:11–20. [DOI] [PubMed] [Google Scholar]
- Jalal S, Daher E, Hilu R. (2013) A case of death due to ibogaine use for heroin addiction: case report. Am J Addict 22:302 DOI: 10.1111/j.1521-0391.2012.00330.x. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. (1981) Opiate withdrawal syndrome: acute and protracted aspects. Ann N Y Acad Sci 362:183–186. [DOI] [PubMed] [Google Scholar]
- Jicha CJ, Lofwall MR, Nuzzo PA, Babalonis S, Elayi SC, Walsh SL. (2015) Safety of oral dronabinol during opioid withdrawal in humans. Drug Alcohol Depend 157:179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, McCance-Katz E. (2015) National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health 105:e55–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovaiša T, Laurinėnas G, Vosylius S, Šipylaitė J, Badaras R, Ivaškevičius J. (2006) Effects of ketamine on precipitated opiate withdrawal. Medicina (Kaunas) 42:625–634. [PubMed] [Google Scholar]
- Kang L, Wang D, Li B, Hu M, Zhang P, Li J. (2008) Mirtazapine, a noradrenergic and specific serotonergic antidepressant, attenuates morphine dependence and withdrawal in Sprague-Dawley rats. Am J Drug Alcohol Abuse 34:541–552. [DOI] [PubMed] [Google Scholar]
- Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E. (2006) Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn Schmiedebergs Arch Pharmacol 372:354–361. [DOI] [PubMed] [Google Scholar]
- Khazan N, Colasanti B. (1972) Protracted rebound in rapid movement sleep time and electroencephalogram voltage output in morphine-dependent rats upon withdrawal. J Pharmacol Exp Ther 183:23–30. [PubMed] [Google Scholar]
- Kosten TA, DeCaprio JL, Rosen MI. (1995) The severity of naloxone-precipitated opiate withdrawal is attenuated by felbamate, a possible glycine antagonist. Neuropsychopharmacology 13:323–333. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Bochenski M. (2007) Comparison of the effects of mGluR1 and mGluR5 antagonists on the expression of behavioral sensitization to the locomotor effect of morphine and the morphine withdrawal jumping in mice. Eur J Pharmacol 558:113–118. [DOI] [PubMed] [Google Scholar]
- Koyuncuoğlu H, Güngör M, Sağduyu H, Aricioğlu F. (1990) Suppression by ketamine and dextromethorphan of precipitated abstinence syndrome in rats. Pharmacol Biochem Behav 35:829–832. [DOI] [PubMed] [Google Scholar]
- Lalanne L, Nicot C, Lang JP, Bertschy G, Salvat E. (2016) Experience of the use of Ketamine to manage opioid withdrawal in an addicted woman: a case report. BMC Psychiatry 16:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laorden ML, Ferenczi S, Pintér-Kübler B, González-Martín LL, Lasheras MC, Kovács KJ, Milanés MV, Núñez C. (2012) Hypothalamic orexin-A neurons are involved in the response of the brain stress system to morphine withdrawal. PLoS One 7:e36871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latowsky M. (1996) Improving detoxification outcomes from methadone maintenance treatment: the interrelationship of affective states and protracted withdrawal. J Psychoactive Drugs 28:251–257. [DOI] [PubMed] [Google Scholar]
- Leal MB, Michelin K, Souza DO, Elisabetsky E. (2003) Ibogaine attenuation of morphine withdrawal in mice: role of glutamate N-methyl-D-aspartate receptors. Prog Neuropsychopharmacol Biol Psychiatry 27:781–785. [DOI] [PubMed] [Google Scholar]
- Lee JD, Nunes EV, Jr, Novo P, Bachrach K, Bailey GL, Bhatt S, Farkas S, Fishman M, Gauthier P, Hodgkins CC, et al. (2018) Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet 391:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Bruneau J, Stewart J. (2003) Understanding polydrug use: review of heroin and cocaine co-use. Addiction 98:7–22. [DOI] [PubMed] [Google Scholar]
- Li AL, Lin X, Dhopeshwarkar AS, Thomaz AC, Carey LM, Liu Y, Nikas SP, Makriyannis A, Mackie K, Hohmann AG. (2019) Cannabinoid CB2 agonist AM1710 differentially suppresses distinct pathological pain states and attenuates morphine tolerance and withdrawal. Mol Pharmacol 95:155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Sheikh SM, Loh HH, Martin BR. (2001) Opioid and cannabinoid modulation of precipitated withdrawal in Δ9-tetrahydrocannabinol and morphine-dependent mice. J Pharmacol Exp Ther 298:1007–1014. [PubMed] [Google Scholar]
- Lin SK, Chen CH, Pan CH. (2008) Venlafaxine for acute heroin detoxification: a double-blind, randomized, control trial. J Clin Psychopharmacol 28:189–194. [DOI] [PubMed] [Google Scholar]
- Lin SK, Pan CH, Chen CH. (2014) A double-blind, placebo-controlled trial of dextromethorphan combined with clonidine in the treatment of heroin withdrawal. J Clin Psychopharmacol 34:508–512. [DOI] [PubMed] [Google Scholar]
- Lofwall MR, Babalonis S, Nuzzo PA, Elayi SC, Walsh SL. (2016) Opioid withdrawal suppression efficacy of oral dronabinol in opioid dependent humans. Drug Alcohol Depend 164:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Su WJ, Yue W, Ge X, Su F, Pei G, Ma L. (2001) Attenuation of morphine dependence and withdrawal in rats by venlafaxine, a serotonin and noradrenaline reuptake inhibitor. Life Sci 69:37–46. [DOI] [PubMed] [Google Scholar]
- Mach RH, Smith CR, Childers SR. (1995) Ibogaine possesses a selective affinity for σ2 receptors. Life Sci 57:PL57–PL62. [DOI] [PubMed] [Google Scholar]
- Malcolm BJ, Polanco M, Barsuglia JP. (2018) Changes in withdrawal and craving scores in participants undergoing opioid detoxification utilizing ibogaine. J Psychoactive Drugs 50:256–265. [DOI] [PubMed] [Google Scholar]
- Malek A, Amiri S, Habibi Asl B. (2013) The therapeutic effect of adding dextromethorphan to clonidine for reducing symptoms of opioid withdrawal: a randomized clinical trial. ISRN Psychiatry 2013:546030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Jasinski DR. (1969) Physiological parameters of morphine dependence in man—tolerance, early abstinence, protracted abstinence. J Psychiatr Res 7:9–17. [DOI] [PubMed] [Google Scholar]
- Mash DC, Ameer B, Prou D, Howes JF, Maillet EL. (2016) Oral noribogaine shows high brain uptake and anti-withdrawal effects not associated with place preference in rodents. J Psychopharmacol 30:688–697. [DOI] [PubMed] [Google Scholar]
- Mas-Nieto M, Pommier B, Tzavara ET, Caneparo A, Da Nascimento S, Le Fur G, Roques BP, Noble F. (2001) Reduction of opioid dependence by the CB1 antagonist SR141716A in mice: evaluation of the interest in pharmacotherapy of opioid addiction. Br J Pharmacol 132:1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer C, Carlier J, Boucher A, Péoc’h M, Lemeur C, Gaillard Y. (2013) Fatal case of a 27-year-old male after taking iboga in withdrawal treatment: GC-MS/MS determination of ibogaine and ibogamine in iboga roots and postmortem biological material. J Forensic Sci 58:1666–1672. [DOI] [PubMed] [Google Scholar]
- Medrano MC, Mendiguren A, Pineda J. (2015) Effect of ceftriaxone and topiramate treatments on naltrexone-precipitated morphine withdrawal and glutamate receptor desensitization in the rat locus coeruleus. Psychopharmacology (Berl) 232:2795–2809. [DOI] [PubMed] [Google Scholar]
- Mehtry V, Nizamie SH, Parvez N, Pradhan N. (2014) Sleep profile in opioid dependence: a polysomnographic case-control study. J Clin Neurophysiol 31:517–522. [DOI] [PubMed] [Google Scholar]
- McKee SA. (2009) Developing human laboratory models of smoking lapse behavior for medication screening. Addict Biol 14:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S. (2012) Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine Tob Res 14:1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Banks ML. (2018) Modulation of drug choice by extended drug access and withdrawal in rhesus monkeys: implications for negative reinforcement as a driver of addiction and target for medications development. Pharmacol Biochem Behav 164:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Kelmendi B, Sanacora G. (2012) Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Behav 100:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrup TF, Stotts AL, Green C, Potter JS, Marino EN, Walker R, Weiss RD, Trivedi M. (2015) Opioid withdrawal, craving, and use during and after outpatient buprenorphine stabilization and taper: a discrete survival and growth mixture model. Addict Behav 41:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata H. (2017) Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci 18:2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Wall MM, Liu SM, Blanco C. (2018) Cannabis use and risk of prescription opioid use disorder in the United States. Am J Psychiatry 175:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyefeso A, Sedgwick P, Ghodse H. (1997) Subjective sleep-wake parameters in treatment-seeking opiate addicts. Drug Alcohol Depend 48:9–16. [DOI] [PubMed] [Google Scholar]
- Paling FP, Andrews LM, Valk GD, Blom HJ. (2012) Life-threatening complications of ibogaine: three case reports. Neth J Med 70:422–424. [PubMed] [Google Scholar]
- Pałucha-Poniewiera A, Novák K, Pilc A. (2009) Group III mGlu receptor agonist, ACPT-I, attenuates morphine-withdrawal symptoms after peripheral administration in mice. Prog Neuropsychopharmacol Biol Psychiatry 33:1454–1457. [DOI] [PubMed] [Google Scholar]
- Panchal V, Taraschenko OD, Maisonneuve IM, Glick SD. (2005) Attenuation of morphine withdrawal signs by intracerebral administration of 18-methoxycoronaridine. Eur J Pharmacol 525:98–104. [DOI] [PubMed] [Google Scholar]
- Pang G, Wu X, Tao X, Mao R, Liu X, Zhang YM, Li G, Stackman RW, Jr, Dong L, Zhang G. (2016) Blockade of serotonin 5-HT2A receptors suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in morphine-treated mice. Front Pharmacol 7:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Siegel S. (2001) Modulation of the effects of rewarding drugs by ibogaine. Alkaloids Chem Biol 56:211–225. [DOI] [PubMed] [Google Scholar]
- Pérez de los Cobos J, Duro P, Trujols J, Tejero A, Batlle F, Ribalta E, Casas M. (2001) Methadone tapering plus amantadine to detoxify heroin-dependent inpatients with or without an active cocaine use disorder: two randomised controlled trials. Drug Alcohol Depend 63:187–195. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Karelitz JL, Jao NC, Chengappa KN, Sparks GM. (2013) Sensitivity and specificity of a procedure for early human screening of novel smoking cessation medications. Addiction 108:1962–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Stitzer M, Fonte CA, Briski JL, Scott JA, Chengappa KN. (2008) Development of procedures for early screening of smoking cessation medications in humans. Clin Pharmacol Ther 84:216–221. [DOI] [PubMed] [Google Scholar]
- Pinelli A, Trivulzio S, Tomasoni L. (1997) Effects of ondansetron administration on opioid withdrawal syndrome observed in rats. Eur J Pharmacol 340:111–119. [DOI] [PubMed] [Google Scholar]
- Pinkofsky HB, Hahn AM, Campbell FA, Rueda J, Daley DC, Douaihy AB. (2005) Reduction of opioid-withdrawal symptoms with quetiapine. J Clin Psychiatry 66:1285–1288. [DOI] [PubMed] [Google Scholar]
- Powers BR. (1949) Use of methadone to combat withdrawal symptoms of dilaudid addiction; case report. J Tn State Med Assoc 42:83. [PubMed] [Google Scholar]
- Pozzi G, Conte G, De Risio S. (2000) Combined use of trazodone-naltrexone versus clonidine-naltrexone in rapid withdrawal from methadone treatment. A comparative inpatient study. Drug Alcohol Depend 59:287–294. [DOI] [PubMed] [Google Scholar]
- Preston KL, Sullivan JT, Strain EC, Bigelow GE. (1996) Enhancement of cocaine’s abuse liability in methadone maintenance patients. Psychopharmacology (Berl) 123:15–25. [DOI] [PubMed] [Google Scholar]
- Quinlan J. (2012) The use of a subanesthetic infusion of intravenous ketamine to allow withdrawal of medically prescribed opioids in people with chronic pain, opioid tolerance and hyperalgesia: outcome at 6 months. Pain Med 13:1524–1525. [DOI] [PubMed] [Google Scholar]
- Ramesh D, Gamage TF, Vanuytsel T, Owens RA, Abdullah RA, Niphakis MJ, Shea-Donohue T, Cravatt BF, Lichtman AH. (2013) Dual inhibition of endocannabinoid catabolic enzymes produces enhanced antiwithdrawal effects in morphine-dependent mice. Neuropsychopharmacology 38:1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K, Kendrick WT, Kogan JH, Aghajanian GK. (1996) A selective AMPA antagonist, LY293558, suppresses morphine withdrawal-induced activation of locus coeruleus neurons and behavioral signs of morphine withdrawal. Neuropsychopharmacology 15:497–505. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Martin H, Berger JE, Seager MA. (2005) The mGlu5 receptor antagonists MPEP and MTEP attenuate behavioral signs of morphine withdrawal and morphine-withdrawal-induced activation of locus coeruleus neurons in rats. Neuropharmacology 48:173–180. [DOI] [PubMed] [Google Scholar]
- Rose JS, Branchey M, Wallach L, Buydens-Branchey L. (2003) Effects of buspirone in withdrawal from opiates. Am J Addict 12:253–259. [PubMed] [Google Scholar]
- Rho B, Glick SD. (1998) Effects of 18-methoxycoronaridine on acute signs of morphine withdrawal in rats. Neuroreport 9:1283–1285. [DOI] [PubMed] [Google Scholar]
- Romandini S, Cervo L, Samanin R. (1984) Evidence that drugs increasing 5-hydroxytryptamine transmission block jumping but not wet dog shakes in morphine-abstinent rats: a comparison with clonidine. J Pharm Pharmacol 36:68–70. [DOI] [PubMed] [Google Scholar]
- Rubino T, Massi P, Viganò D, Fuzio D, Parolaro D. (2000) Long-term treatment with SR141716A, the CB1 receptor antagonist, influences morphine withdrawal syndrome. Life Sci 66:2213–2219. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, et al. (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585. [DOI] [PubMed] [Google Scholar]
- Samanin R, Cervo L, Rochat C, Poggesi E, Mennini T. (1980) Reduction in the number of serotonin receptors in the brainstem of morphine dependent rats: relation to blockade of naloxone precipitated jumping by serotonin agonists. Life Sci 27:1141–1146. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Backler MM, Herman I, Shamir D, Rigai T, Bar-Hamburger R, Pick CG. (2003) Mianserin and trazodone significantly attenuate the intensity of opioid withdrawal symptoms in mice. Addict Biol 8:107–114. [PubMed] [Google Scholar]
- Schuckit MA. (2016) Treatment of opioid-use disorders. N Engl J Med 375:357–368. [DOI] [PubMed] [Google Scholar]
- Schulz R, Herz A. (1977) Naloxone-precipitated withdrawal reveals sensitization to neurotransmitters in morphine tolerant/dependent rats. Naunyn Schmiedebergs Arch Pharmacol 299:95–99. [DOI] [PubMed] [Google Scholar]
- Sekiya Y, Nakagawa T, Ozawa T, Minami M, Satoh M. (2004) Facilitation of morphine withdrawal symptoms and morphine-induced conditioned place preference by a glutamate transporter inhibitor DL-threo-β-benzyloxyaspartate in rats. Eur J Pharmacol 485:201–210. [DOI] [PubMed] [Google Scholar]
- Shahidi S, Hasanein P. (2011) Behavioral effects of fatty acid amide hydrolase inhibition on morphine withdrawal symptoms. Brain Res Bull 86:118–122. [DOI] [PubMed] [Google Scholar]
- Shahidi S, Hashemi-Firouzi N. (2014) The effects of a 5-HT7 receptor agonist and antagonist on morphine withdrawal syndrome in mice. Neurosci Lett 578:27–32. [DOI] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Dileone RJ. (2008) Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol Psychiatry 64:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe LG, Jaffe JH. (1990) Ibogaine fails to reduce naloxone-precipitated withdrawal in the morphine-dependent rat. Neuroreport 1:17–19. [DOI] [PubMed] [Google Scholar]
- Sheppard SG. (1994) A preliminary investigation of ibogaine: case reports and recommendations for further study. J Subst Abuse Treat 11:379–385. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M, Jarusuraisin N. (1998) Amitriptyline vs. lorazepam in the treatment of opiate-withdrawal insomnia: a randomized double-blind study. Acta Psychiatr Scand 97:233–235. [DOI] [PubMed] [Google Scholar]
- Streel E, Dan B, Antoniali V, Clement B, Campanella S, Hanak C, Vanderlinden P, Pelc I, Verbanck P. (2005) Effects of anaesthetic agents in interference of naloxone-induced opiate-withdrawal are dose-dependent in opiate-dependent rats. Life Sci 77:650–655. [DOI] [PubMed] [Google Scholar]
- Strickler EM, Schwenk ES, Cohen MJ, Viscusi ER. (2018) Use of ketamine in a multimodal analgesia setting for rapid opioid tapering in a profoundly opioid-tolerant patient: a case report. A A Pract 10:179–181. [DOI] [PubMed] [Google Scholar]
- Tanganelli S, Antonelli T, Morari M, Bianchi C, Beani L. (1991) Glutamate antagonists prevent morphine withdrawal in mice and guinea pigs. Neurosci Lett 122:270–272. [DOI] [PubMed] [Google Scholar]
- Tanum L, Solli KK, Latif ZE, Benth JŠ, Opheim A, Sharma-Haase K, Krajci P, Kunøe N. (2017) Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry 74:1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama S, Wakabayashi H, Ho IK. (1996) Direct evidence for a role of glutamate in the expression of the opioid withdrawal syndrome. Eur J Pharmacol 295:123–129. [DOI] [PubMed] [Google Scholar]
- Tokuyama S, Zhu H, Wakabayashi H, Feng YZ, Ho IK. (1998) The role of glutamate in the locus coeruleus during opioid withdrawal and effects of H-7, a protein kinase inhibitor, on the action of glutamate in rats. J Biomed Sci 5:45–53. [DOI] [PubMed] [Google Scholar]
- Vandergriff J, Rasmussen K. (1999) The selective mGlu2/3 receptor agonist LY354740 attenuates morphine-withdrawal-induced activation of locus coeruleus neurons and behavioral signs of morphine withdrawal. Neuropharmacology 38:217–222. [DOI] [PubMed] [Google Scholar]
- van Dongen PA. (1981) The central noradrenergic transmission and the locus coeruleus: a review of the data, and their implications for neurotransmission and neuromodulation. Prog Neurobiol 16:117–143. [DOI] [PubMed] [Google Scholar]
- Vela G, Ruiz-Gayo M, Fuentes JA. (1995) Anandamide decreases naloxone-precipitated withdrawal signs in mice chronically treated with morphine. Neuropharmacology 34:665–668. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA, Baler R. (2017) The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci 18:741–752. [DOI] [PubMed] [Google Scholar]
- Vocci F, Ling W. (2005) Medications development: successes and challenges. Pharmacol Ther 108:94–108. [DOI] [PubMed] [Google Scholar]
- Wakim JH. (2012) Alleviating symptoms of withdrawal from an opioid. Pain Ther 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wang Q, Ji B, Pan Y, Xu C, Cheng B, Bai B, Chen J. (2018) The orexin/receptor system: molecular mechanism and therapeutic potential for neurological diseases. Front Mol Neurosci 11:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden D, Subramaniam GA, Carmody T, Woody GE, Minhajuddin A, Poole SA, Potter J, Fishman M, Bogenschutz M, Patkar A, et al. (2012) Predictors of attrition with buprenorphine/naloxone treatment in opioid dependent youth. Addict Behav 37:1046–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. (2002) Involvement of glutamate receptors within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Jpn J Pharmacol 88:399–406. [DOI] [PubMed] [Google Scholar]
- Wesson DR, Ling W. (2003) The clinical opiate withdrawal scale (COWS). J Psychoactive Drugs 35:253–259. [DOI] [PubMed] [Google Scholar]
- Wu X, Pang G, Zhang YM, Li G, Xu S, Dong L, Stackman RW, Jr, Zhang G. (2015) Activation of serotonin 5-HT2C receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in heroin-treated mice. Neurosci Lett 607:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Wu X, Zhang YM, Liu H, Jiang Q, Pang G, Tao X, Dong L, Stackman RW., Jr (2016) Activation of serotonin 5-HT2C receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in morphine-dependent mice. Neuropharmacology 101:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zullino DF, Cottier AC, Besson J. (2002) Topiramate in opiate withdrawal. Prog Neuropsychopharmacol Biol Psychiatry 26:1221–1223. [DOI] [PubMed] [Google Scholar]
- Zullino DF, Khazaal Y, Hättenschwiler J, Borgeat F, Besson J. (2004) Anticonvulsant drugs in the treatment of substance withdrawal. Drugs Today (Barc) 40:603–619. [DOI] [PubMed] [Google Scholar]