Abstract
A novel µ-opioid receptor antagonist, methocinnamox (MCAM), attenuates some abuse-related and toxic effects of opioids. This study further characterized the pharmacology of MCAM in separate groups of rats using procedures to examine antinociception, gastrointestinal motility, and withdrawal in morphine-dependent rats. Antinociceptive effects of opioid receptor agonists were measured before and after MCAM (1–10 mg/kg) using warm water tail withdrawal and sensitivity to mechanical stimulation in inflamed paws (complete Freund’s adjuvant). Before MCAM, morphine, fentanyl, and the κ-opioid receptor agonist spiradoline dose dependently increased tail-withdrawal latency from 50°C water; MCAM attenuated the antinociceptive effects of morphine and fentanyl, but not spiradoline. Morphine increased sensitivity to mechanical stimulation and decreased gastrointestinal motility, and MCAM blocked both effects. These antagonist effects of 10 mg/kg MCAM were persistent, lasting for 2 weeks or longer. Withdrawal emerged after discontinuation of morphine treatment or administration of 10 mg/kg MCAM or 17.8 mg/kg naloxone; other than the day of antagonist administration when withdrawal signs were greater in rats that received antagonist compared with rats that received vehicle, there was no difference among groups in directly observable withdrawal signs or decreased body weight. These results confirm that MCAM is a selective µ-opioid receptor antagonist with an exceptionally long duration of action, likely due to pseudoirreversible binding. Despite its sustained antagonist effects, the duration of withdrawal precipitated by MCAM is not different from that precipitated by naloxone, suggesting that the long duration of antagonism provided by MCAM could be particularly effective for treating opioid abuse and overdose.
SIGNIFICANCE STATEMENT
The opioid receptor antagonist MCAM attenuates some abuse-related and toxic effects of opioids. This study demonstrates that MCAM selectively antagonizes multiple effects mediated by µ-opioid receptor agonists for 2 weeks or longer, and like naloxone, MCAM precipitates withdrawal in morphine-dependent rats. Despite this persistent antagonism, withdrawal signs precipitated by MCAM are not significantly different from signs precipitated by naloxone or occurring after discontinuation of morphine, suggesting that using MCAM for opioid abuse or overdose would not produce sustained withdrawal.
Introduction
Currently, three pharmacological options are available for treating opioid use disorder (OUD). Two medications, methadone and buprenorphine, are µ-opioid receptor agonists that mimic some effects of abused opioids. The third medication is the opioid receptor antagonist naltrexone, which blocks the effects of abused opioids. While effective in some patients, they each have limitations. For example, methadone and buprenorphine are diverted and abused, and both have adverse effects, including respiratory depression, which can be exacerbated by alcohol and benzodiazepines (Kintz, 2001; Pirnay et al., 2004; Pelissier-Alicot et al., 2010; Jones et al., 2012, 2014; Jones and McAninch, 2015; Kriikku et al., 2018). Although naltrexone is not abused and is safer than methadone and buprenorphine, induction of treatment must be done carefully to minimize the emergence of withdrawal. Poor compliance and a relatively short duration of action limit the usefulness of naltrexone for treating OUD, although recently developed extended release formulations might improve outcomes. However, because of its competitive, reversible binding to µ-opioid receptors, the antagonist effects of naltrexone can be surmounted by large dose of agonists, such that reinforcing and respiratory-depressant effects of opioids can be achieved by taking more drug, thereby limiting protection by naltrexone. Thus, despite the effectiveness of these medications, the risk of relapse and overdose remains high, indicating that current options are not adequately addressing the opioid crisis (Volkow et al., 2019).
Naloxone is the only medication available for opioid overdose. Like naltrexone, it is a competitive, reversible antagonist at opioid receptors, it precipitates withdrawal in opioid-dependent individuals, and its antagonism can be surmounted by taking more drug. Moreover, its duration of action (≤1 hour) is shorter than many abused opioids, such that opioid-induced toxic effects can reemerge after rescue with naloxone. A medication that reverses and provides long-lasting protection from opioid overdose could be a significant improvement over naloxone.
Methocinnamox (MCAM) is an opioid receptor antagonist that might retain the positive features of naltrexone and naloxone (e.g., safety and no abuse liability) while reducing vulnerabilities associated with surmountability. While each antagonist has affinity for all three types of opioid receptors (µ, κ, and δ) and most of the interactions between antagonist and receptors are reversible, MCAM differs from naltrexone and naloxone in that its interactions with µ-opioid receptors are functionally irreversible (i.e., pseudoirreversible) (Broadbear et al., 2000), suggesting that MCAM would selectively and insurmountably block µ-opioid receptors. In contrast, the surmountable antagonism produced by naltrexone and naloxone is not selective. MCAM does not appear to have efficacy at any opioid receptor and, when given alone, does not produce antinociceptive effects or alter respiration, although it attenuates the reinforcing, antinociceptive, and respiratory-depressant effects of µ-opioid receptor agonists in mice, rats, and nonhuman primates (Broadbear et al., 2000; Peckham et al., 2005; Gerak et al., 2019; Maguire et al., 2019). In the presence of MCAM, large doses of morphine can produce antinociceptive effects, although these effects might be mediated by other receptors (e.g., κ-opioid receptors) (Takemori and Portoghese, 1987; Peckham et al., 2005) rather than reflect surmountability at µ-opioid receptors. Moreover, these antagonist effects are persistent, lasting for a week or more in monkeys (Gerak et al., 2019; Maguire et al., 2019). In nonhuman primates, doses of MCAM larger than those that attenuate the abuse-related and toxic effects of opioids do not attenuate self-administration of nonopioids (e.g., cocaine), decrease responding for food, or alter heart rate, blood pressure, body temperature, or activity (Maguire et al., 2019), suggesting that MCAM is safe and not likely to produce unexpected adverse effects.
While MCAM could safely provide long-lasting protection against the abuse-related and toxic effects of opioids, little is known about the ability of MCAM to block other effects mediated by µ-opioid receptors. Moreover, the impact of sustained, insurmountable blockade of µ-opioid receptors is also unknown. One possible concern regarding the therapeutic use of MCAM for OUD would be how to provide pain relief because MCAM would block the analgesic effects of µ-opioid receptor agonists. In addition, if used to rescue an opioid-dependent individual from overdose, MCAM would precipitate withdrawal, and precipitation of withdrawal by a pseudoirreversible antagonist such as MCAM has not been studied extensively. This study addressed these potential concerns and examined the generality of sustained antagonism by MCAM to another class of µ-opioid receptor agonists currently predominating the opioid crisis (fentanyl) by using two procedures to measure antinociception and other procedures to examine gastrointestinal motility and changes in body temperature as well as precipitation of withdrawal in morphine-dependent rats.
Materials and Methods
Subjects
Male Sprague-Dawley rats (Envigo Inc., Chicago, IL) were maintained under a 14-hour light/10-hour dark cycle; experiments were conducted during the light cycle. Rats were housed individually in colony rooms that were maintained at a constant temperature and humidity. While in their home cage, they had continuous access to water and, with the exception of one study, unlimited access to food (Envigo Teklad, Madison, WI). The eight rats in which gastrointestinal transit was measured did not have unlimited access to food; instead, they were maintained at 375 ± 5 g body weight with daily food rations of 15 g, except on days preceding tests when the ration was decreased to 5 g. All animals used in these studies were maintained in accordance with the Institutional Animal Care and Use Committee at The University of Texas Health Science Center at San Antonio, and the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the US National Institutes of Health (National Research Council, 2011).
Apparatus
Antinociceptive effects were determined using two assays. For the warm water tail-withdrawal procedure, three water baths (EW-14576-00; Cole-Parmer, Vernon Hills, IL) were maintained at constant temperature (40, 50, or 55°C) throughout the experiment, and latency for rats to remove their tails from water maintained at each temperature was measured using a stopwatch. For the paw inflammation procedure, rats were placed on a mesh stand (Model 410; IITC Inc. Life Science) in plastic enclosures (Model 435; IITC Inc. Life Science) for testing, and an electronic Von Frey probe (Model 2396; IITC Inc. Life Science) with a rigid tip (Model 2391; IITC Inc. Life Science) was used to measure paw-withdrawal threshold in grams of force. Paw thickness was measured with a digital caliper (Model 54-101-175; Fowler), and body temperature was measured with a rectal thermometer (PhysiTemp Instruments, Clifton, NJ) before and after sessions.
Procedures
Warm Water Tail Withdrawal.
Antinociceptive effects were assessed by measuring the latency for rats to remove their tails from water maintained at 40, 50, or 55°C. Experiments began with determination of baseline latencies for each of the three temperatures by gently restraining the rats and placing the lower portions of their tails into water baths. The remainder of the session was divided into 30-minute cycles, each of which began with an injection and ended with redetermination of tail-withdrawal latencies from each of the three temperatures. Latencies were measured starting 28 minutes after each injection with the order of presentation of the different temperatures varying nonsystematically across cycles and rats. The first cycle began immediately after obtaining baseline latencies with an injection of vehicle. During some sessions, only vehicle or sham injections were administered for up to six cycles. For other sessions, dose-effect curves were determined. An ineffective dose of drug was given at the beginning of the second cycle with the cumulative dose increasing in one-half log unit increments every cycle.
Three separate groups of eight rats participated in these studies. In the first group, the magnitude and duration of antagonism by MCAM was examined by determining morphine dose-effect curves in the absence of MCAM and at various times after administration of MCAM (1–10 mg/kg). Doses of MCAM were studied in ascending order and separated by 21 days. For each of these morphine dose-effect curves, dosing continued until tail-withdrawal latency from water maintained at 50°C was at least 13 seconds, which insured that the latency reached 80% of the maximum possible effect. On days when MCAM was administered, rats received no other injections; tail-withdrawal latencies were measured 28 and 58 minutes after MCAM. Morphine dose-effect curves were redetermined 1 day later (i.e., 24 hours after MCAM) and every 4 days thereafter until the potency of morphine in the presence of MCAM was not different from its potency in the absence of MCAM (see Data Analyses for details). When rats received 10 mg/kg MCAM, the morphine dose-effect curve remained shifted 21 days later; after determination of that dose-effect curve, the interval between tests with morphine increased to 8 days through day 53 and then to 16 days for one final test 69 days after MCAM administration. After day 21, control tail-withdrawal latencies were measured 4 days before each morphine test with rats receiving only vehicle injections during these intervening sessions.
In a second group of eight rats, 10 mg/kg MCAM was administered before assessing the antinociceptive effects of fentanyl. First, a dose range of fentanyl was identified in the absence of MCAM such that the smallest dose was ineffective and the largest dose increased tail-withdrawal latency from water maintained at 50°C to at least 13 seconds. Thereafter, the dose range for each fentanyl test remained the same regardless of latencies obtained; sessions comprised four cycles, with rats receiving saline at the beginning of the first cycle and cumulative doses of 0.01, 0.032, and 0.1 mg/kg fentanyl at the beginning of the second, third, and fourth cycles, respectively, and ending after the fourth cycle. This fixed dose range was used to measure the duration of antagonism while limiting the amount of agonist administered to avoid other effects that might develop after repeated administration of large doses (e.g., tolerance). On days when MCAM was administered, rats received no other injections and tail-withdrawal latencies were not measured. Fentanyl dose-effect curves were obtained 1 and 5 days later and every 8 days thereafter until the tail-withdrawal latency obtained with a cumulative dose of 0.1 mg/kg fentanyl in the presence of MCAM was not different from the same dose of fentanyl obtained in the absence of MCAM. After day 5, control tail-withdrawal latencies were obtained 4 days before each fentanyl test with rats receiving only vehicle injections during these intervening sessions. For reasons unrelated to the study, one rat died between fentanyl dose-effect curves obtained 29 and 37 days after MCAM.
In a third group of eight rats, the antinociceptive effects of spiradoline and later of morphine were determined at various times after 10 mg/kg MCAM. First, dose-effect curves for spiradoline and morphine were obtained in the absence of MCAM up to the dose that increased tail-withdrawal latency from water maintained at 50°C to at least 13 seconds. On the day that MCAM was administered, rats received no other injections and tail-withdrawal latencies were measured 28 and 58 minutes after MCAM. One day later (i.e., 24 hours after MCAM), spiradoline dose-effect curves were obtained during a four-cycle session beginning with saline and then using the dose range of spiradoline that was identified before MCAM administration (1, 3.2, and 10 mg/kg). Five days after MCAM administration and 4 days after redetermination of the spiradoline dose-effect curve, the antinociceptive effects of morphine were assessed in a similar four-cycle session; morphine was studied in this manner every 8 days until the tail-withdrawal latency obtained with a cumulative dose of 17.8 mg/kg morphine in the presence of MCAM was not different from latency obtained with the same dose of morphine obtained in the absence of MCAM. After day 5, control tail-withdrawal latencies were obtained 4 days before each morphine test with rats receiving only vehicle injections during these intervening sessions.
Paw Inflammation.
Sixteen rats that weighed 250–270 g at the start of the experiment were randomly assigned to two groups with eight rats in each group. Baseline values for body temperature, paw thickness, and paw-withdrawal threshold were determined in all rats before receiving hindpaw injections. Rats were briefly anesthetized with 2%–4% isoflurane, and the plantar surface of the footpad was cleaned with betadine and 70% ethanol. Using a 27-G, 1/2-inch needle, 0.1 ml of complete Freund’s adjuvant (CFA) emulsion or saline was injected subcutaneously to the footpad. The paw side (left or right) associated with the CFA injection was counterbalanced across rats, and saline was injected into the other paw. One day after hindpaw injections, paw thickness was redetermined and then one group of rats received 10 mg/kg MCAM and the other group received vehicle. Paw-withdrawal threshold was tested on days 1, 3, 15, 25, and 30 after MCAM or vehicle. In each of six cycles, an injection was given and 15 minutes later the force that resulted in paw withdrawal was determined three times for each paw.
One day after MCAM or vehicle, four rats from each group were tested with cumulative doses of morphine (1.78–17.8 mg/kg) and the other four rats from each group were tested with cumulative doses of meloxicam (0.56–5.6 mg/kg) to test the selectivity of MCAM. Three days after MCAM or vehicle, rats that had initially received morphine were tested with meloxicam, and rats that had initially received meloxicam were tested with morphine. Fifteen and 30 days after MCAM or vehicle all rats were tested with morphine, and 25 days after MCAM or vehicle all rats were tested with saline given in all cycles.
Gastrointestinal Transit.
On test days, standard rodent chow (Teklad; 75 g; Enivgo) was soaked in warm tap water (120 ml) until homogenized (approximately 90 minutes). Tests were conducted in eight rats in the home cage with bedding removed immediately prior to the session and began with 2-hour access to the wet chow. After 2 hours, any remaining chow was removed from the cage and weighed. The weight of the remaining chow was subtracted from the initial weight to estimate consumption. Rats then received either saline or 10 mg/kg morphine, and fecal boli were collected, counted, and weighed hourly for the next 6 hours. The effects of saline and morphine on fecal output were determined twice with tests separated by at least 1 week. Thereafter, MCAM (10 mg/kg) was administered 5 minutes before morphine (10 mg/kg). Beginning 5 days after MCAM administration, morphine was tested every 4 days (i.e., days 5, 9, 13, 17, 21, 25, and 29) until results of three consecutive tests were not significantly different from results obtained before MCAM administration.
Dependence/Withdrawal.
Three groups of five rats received morphine twice daily at 7:00 AM and 5:00 PM. The 19-day treatment period began with a dose of 3.2 mg/kg per injection, and the dose increased every 3 days in one-fourth log unit increments up to 56 mg/kg per injection twice daily. On the third day of treatment with the largest dose of morphine, rats received an injection of vehicle 100 minutes after the morning injection of morphine, withdrawal signs were monitored, and then rats received 56 mg/kg morphine at 5:00 PM. On the next day, rats received 56 mg/kg morphine at 7:00 AM; a second injection was administered 100 minutes later with rats randomly assigned to receive vehicle, 10 mg/kg MCAM, or 17.8 mg/kg naloxone. Beginning at 5:00 PM on that day and continuing for the next 5 days, rats received saline instead of morphine at 7:00 AM and 5:00 PM. Withdrawal signs were scored 1, 2, 3, and 5 days after the last dose of morphine.
Two individuals, blind to the injection (i.e., vehicle, naloxone, or MCAM) given on the last day of morphine treatment, monitored directly observable withdrawal signs. During observation periods, rats were in their home cage, which was moved into a different room before observations began; food, but not water, was available during sessions. Three discrete observation periods were conducted for each rat beginning 30, 60, and 90 minutes after the injection of vehicle or antagonist, including measurement of body weight and scoring of vocalization during handling. Thereafter, the remaining 13 signs (ptosis, teeth chattering, tongue protrusion, salivation, lacrimation, chromodacryorrhea, jumping, abdominal writhing, wet dog shake, rearing, paw biting, paw tremor, and diarrhea) were scored as present or absent during four 15-second intervals that were separated by 15 seconds. As long as a sign was observed during at least one interval, that sign was recorded as present for the observation period. Thus, the maximum score for each observation period was 14.
Drugs
Morphine sulfate and fentanyl hydrochloride (Drug Supply Program, National Institute on Drug Abuse, Rockville, MD) as well as spiradoline mesylate (Upjohn, Kalamazoo, MI) were dissolved in normal saline and administered intraperitoneally in a volume of 1 ml/kg body weight except when the largest dose of MCAM (10 mg/kg) was given before determination of morphine dose-effect curves up to doses that produced a maximum possible effect. Because very large doses were needed, morphine was dissolved in 10% β-cyclodextrin vehicle such that a larger concentration could be obtained, thereby limiting injection volumes at the largest doses to a maximum of 1.2 ml. Meloxicam sodium salt hydrate was purchased from Sigma-Aldrich Corp. (St. Louis, MO), dissolved in a vehicle containing equal volumes of polyethylene glycol 400 and saline, and administered intraperitoneally. MCAM hydrochloride, which was synthesized by two of the authors (A.D., S.M.H.) according to a previously established procedure (Broadbear et al., 2000), and naloxone hydrochloride (Drug Supply Program, National Institute on Drug Abuse) were dissolved in a vehicle of 10% w/v β-cyclodextrin in saline and administered subcutaneously. Doses are expressed in the form listed previously in milligrams per kilogram of body weight. CFA containing 1 mg of heat-killed and dried Mycobacterium tuberculosis (strain H37Ra, 25177; ATCC) per milliliter was purchased from Sigma Aldrich (product number F5881), and then diluted with saline (1:1 ratio) to a concentration of 0.5 mg/ml M. tuberculosis emulsion.
Data Analyses
Graphs were constructed and analyses were conducted with GraphPad Prism version 7.03 for Windows (GraphPad Software, La Jolla, CA). Significance was set at P < 0.05.
Warm Water Tail Withdrawal.
Tail-withdrawal latencies were converted to a percentage of the maximum possible effect (15 seconds) according to the following expression: [(test latency − control latency)/(15 seconds − control latency)] × 100%, and then averaged across seven or eight rats; mean latencies, expressed as a percentage of the maximum possible effect (mean ± 1 S.E.M.), were plotted as a function of dose. Whenever possible, ED50 values were determined by first fitting straight lines to dose-effect curves for individual rats using the largest dose for which tail-withdrawal latency remained below 25%, the smallest dose for which tail-withdrawal latency exceeded 75%, and all doses in between. The ED50 values were then estimated from these straight lines using linear regression when three or more data points were available or by interpolation when only two points were available and plotted as a function of time since MCAM administration. Potency ratios were calculated for each rat by dividing the ED50 values for morphine or spiradoline obtained after MCAM administration by the ED50 values obtained in the absence of MCAM. A significant change in the potency of morphine or spiradoline was detected when the 95% confidence intervals of the potency ratios averaged among rats did not include 1. For tests in which ED50 values could not be obtained because a limited dose range was studied and tail-withdrawal latencies did not exceed 50% of the maximum possible latency (i.e., 15 seconds) from 50°C water, statistical significance was determined by comparing the percentage of the maximum possible latency obtained following administration of the largest cumulative dose of morphine or fentanyl using one-factor (time since MCAM administration), Geisser-Greenhouse–corrected repeated-measures ANOVA followed by Dunnett’s multiple comparisons test.
Paw Inflammation.
Paw thickness was similar before and after sessions and across the two groups (i.e., rats that received MCAM or vehicle 1 day after hindpaw injections). Consequently, measurements taken before sessions were averaged across groups (95% confidence intervals). The paw that received an injection of CFA was considered significantly inflamed if the thickness of that paw was outside of the 95% confidence interval of the thickness of the vehicle-injected paw. Similarly, paw-withdrawal threshold was obtained at the beginning of each session 15 minutes after a saline injection and was considered significantly lower in the CFA paw when the mean withdrawal threshold was outside of the 95% confidence interval of the value obtained in the vehicle-injected paw. The effects of morphine on antinociception were not significantly different on day 3 compared with day 1 in either MCAM- or vehicle-treated rats (data not shown); therefore, the data were collapsed across these two tests, which allowed for a two-factor repeated-measures ANOVA to be conducted with treatment (MCAM or vehicle) and time (days since MCAM or vehicle) as factors. Change in body temperature was determined by subtracting the body temperature before the session from the body temperature at the end of the session. The effects of morphine on body temperature were not significantly different on day 3 compared with day 1 in either MCAM- or vehicle-treated rats; therefore, the data were collapsed across these two tests, which allowed for a two-factor repeated-measures ANOVA to be conducted with treatment (MCAM or vehicle) and time (days since MCAM or vehicle) as factors.
Gastrointestinal Transit.
Fecal output (fecal boli/6 hours) and the estimated amount of wet chow consumed (g/2 hours) were plotted as a function of time since MCAM administration. Statistical significance was determined using a one-factor (time since MCAM administration) repeated-measures ANOVA followed by Bonferroni’s post hoc test.
Dependence/Withdrawal.
Directly observable signs were combined, which gave a composite score for total withdrawal signs for each rat on each day. This score was determined by counting the number of signs recorded as present by at least one observer during each observation period and then adding across observation periods to obtain a withdrawal score for that day; the maximum possible score for 1 day was 42 [(3 observation periods) × (14 signs)]. Scores were determined for individual rats and then averaged (mean ± 1 S.E.M.) among rats within a treatment condition. Body weight was measured at the beginning of each observation period; the value obtained before the third observation period (i.e., 90 minutes after the injection of vehicle or antagonist on the last day of morphine treatment) was plotted as a function of time. Statistical significance was determined by comparing the number of withdrawal signs observed across all intervals in all three observation periods using two-factor repeated-measures ANOVA; one factor was the injection given before the first observation period on the last day of morphine treatment (MCAM, naloxone, or vehicle) and the other factor was days since that injection. Dunnett’s multiple comparisons test was used for post hoc analyses.
Results
Warm Water Tail Withdrawal.
Tail-withdrawal latencies (mean ± 1 S.E.M.) obtained in the absence of drug were 15, 2.63 ± 0.18, and 1 seconds from water maintained at 40, 50, and 55°C, respectively. When given alone, morphine, fentanyl, and spiradoline dose dependently increased latencies to >90% of control latencies at cumulative doses of 17.8, 0.1, and 10 mg/kg, respectively (Figs. 1, 3, and 4, filled circles). When given alone, MCAM (1–10 mg/kg) did not increase tail-withdrawal latencies 28 or 58 minutes after administration (data not shown).
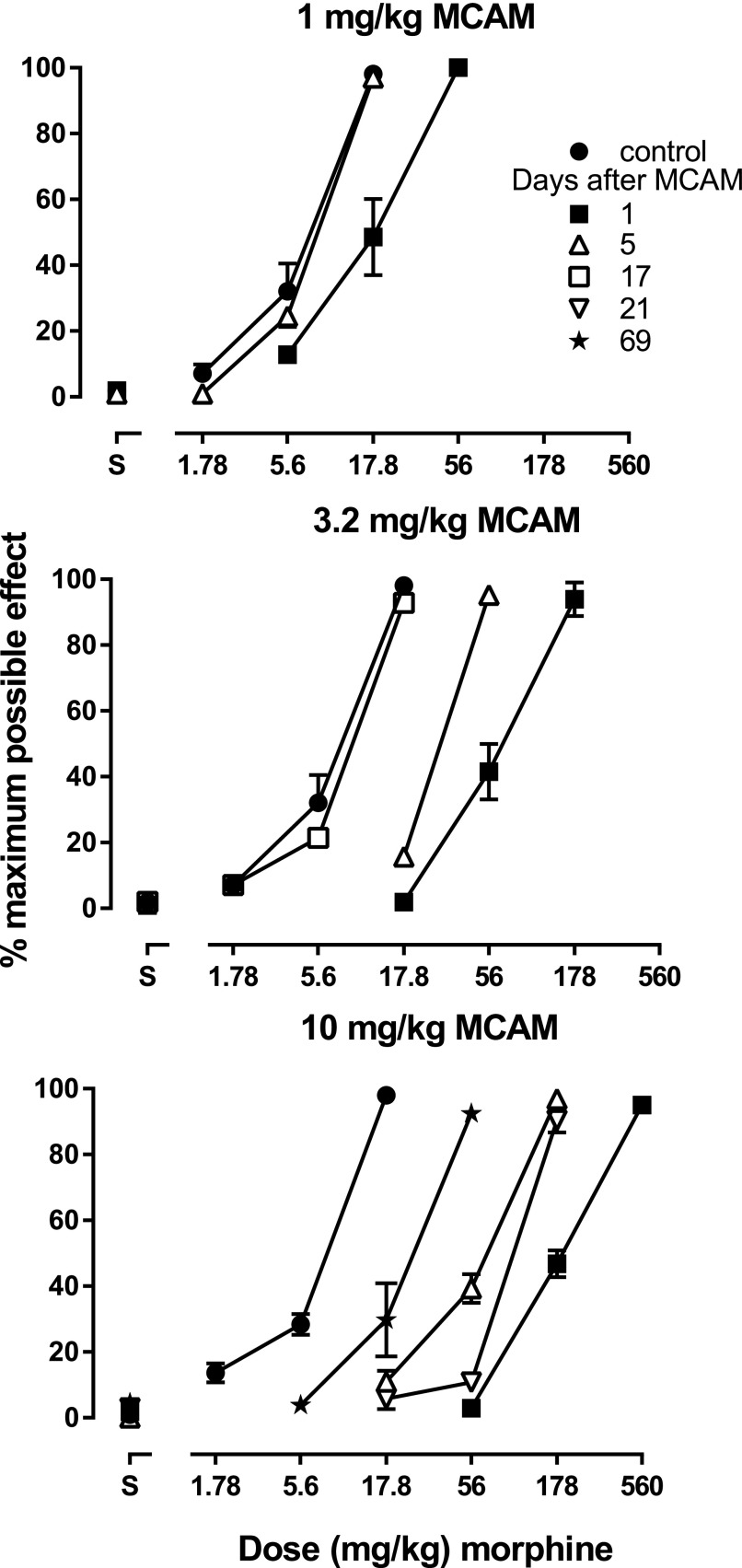
Fig. 1.
Antinociceptive effects of morphine in the absence of MCAM (filled circles) and at various times after MCAM administration. Each panel shows the antagonist effects of a different dose of MCAM. Ordinates: latency to remove tails from 50°C water, expressed as a percentage of control (mean ± 1 S.E.M.) and averaged across eight rats. Abscissae: vehicle (S) or morphine dose in milligrams per kilogram of body weight.
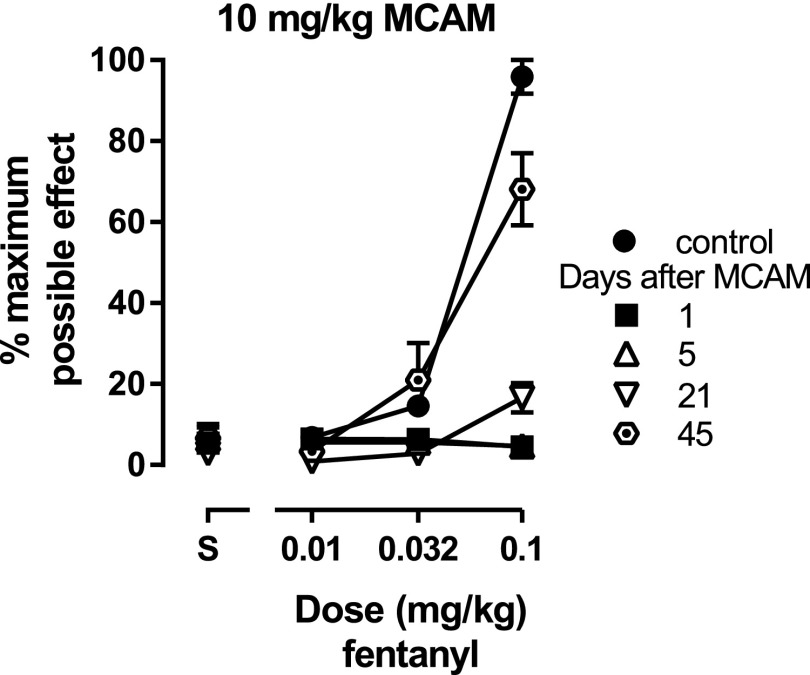
Fig. 3.
Antinociceptive effects of fentanyl in the absence of MCAM (filled circles) and at various times after 10 mg/kg MCAM administration. The dose range for fentanyl tests remained the same, regardless of latencies obtained. Ordinates: latency to remove tails from 50°C water, expressed as a percentage of control (mean ± 1 S.E.M.) and averaged across seven rats for the dose-effect curve determined 45 days after MCAM and across eight rats for all other dose-effect curves. Abscissae: vehicle (S) or fentanyl dose in milligrams per kilogram of body weight.
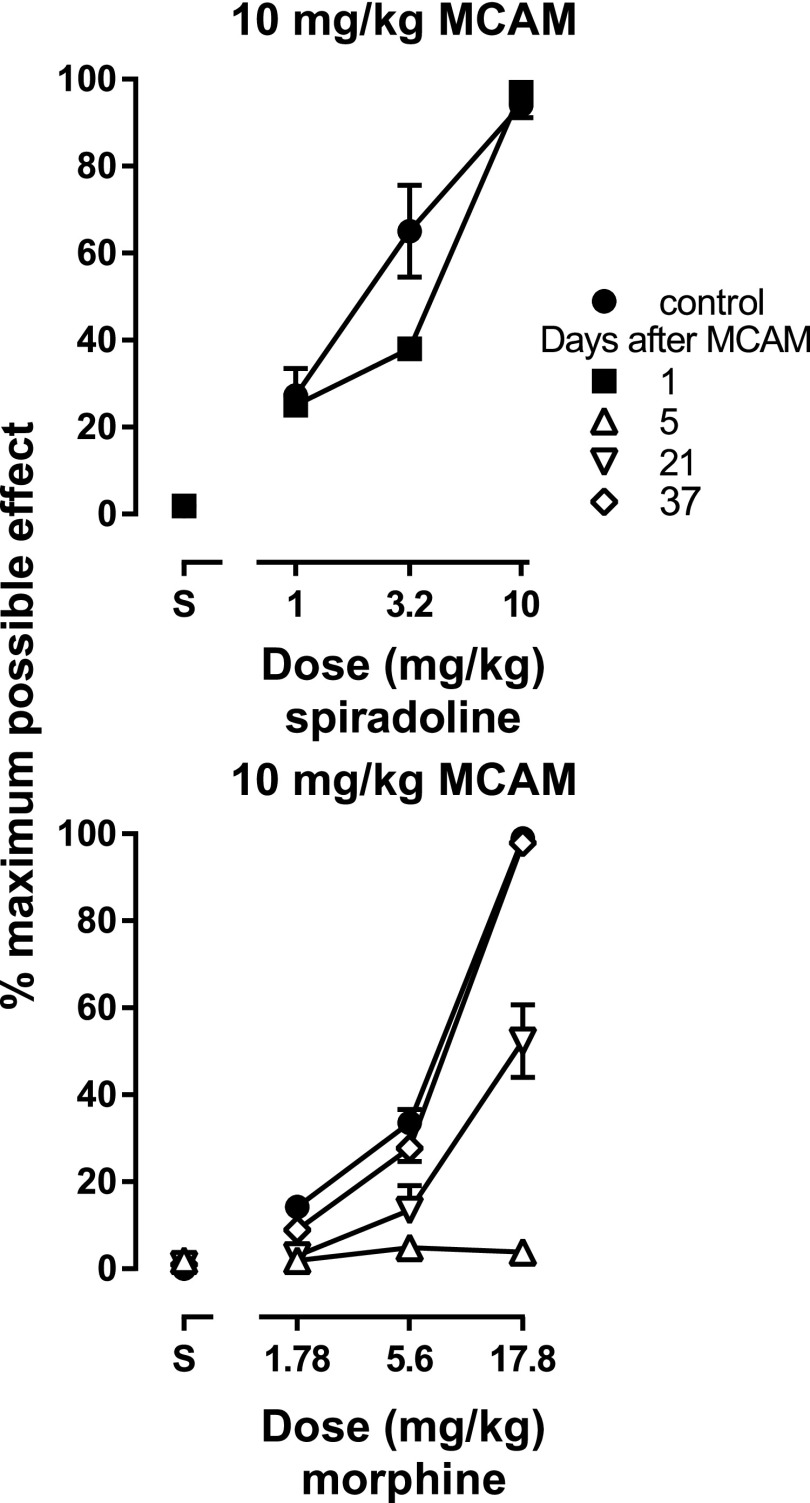
Fig. 4.
Antinociceptive effects of spiradoline (top panel) and morphine (bottom panel) in the absence of MCAM (filled circles) and at various times after 10 mg/kg MCAM administration. The dose range for each agonist test remained the same, regardless of latencies obtained. Ordinates: latency to remove tails from 50°C water, expressed as a percentage of control (mean ± 1 S.E.M.) and averaged across eight rats. Abscissae: vehicle (S) or agonist dose in milligrams per kilogram of body weight.
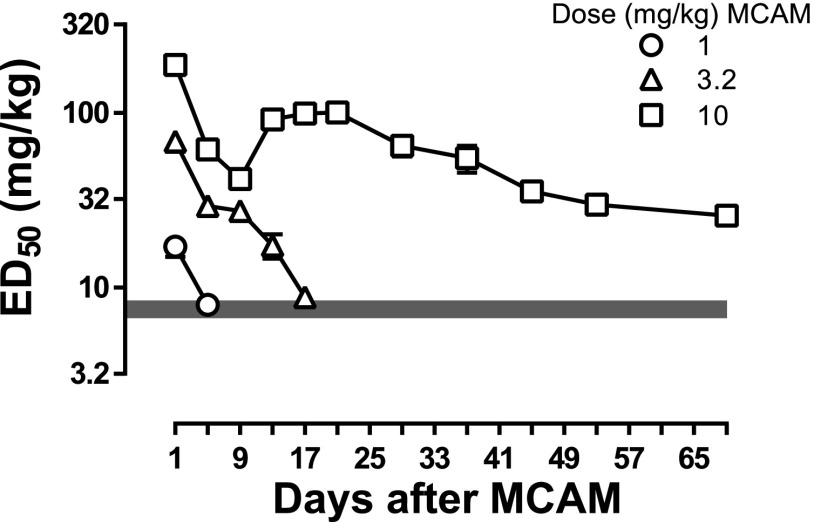
In one group of rats, morphine dose-effect curves were determined at various times after administration of MCAM (1–10 mg/kg). When given 1 day earlier, MCAM dose dependently attenuated the effects of morphine on tail-withdrawal latency from water maintained at 50°C, with each larger dose of MCAM shifting the morphine dose-effect curve further rightward (Fig. 1, all panels, compare filled squares with filled circles). For example, the dose of morphine needed to increase tail-withdrawal latency to >90% was 56 mg/kg after 1 mg/kg MCAM (Fig. 1, top panel, filled squares) and 560 mg/kg after 10 mg/kg MCAM (Fig. 1, bottom panel, filled squares). The magnitude of antagonism diminished over time since MCAM administration. The morphine dose-effect curve recovered and was not different from the control curve within 5 and 17 days of administration of 1 mg/kg MCAM (Fig. 1, top panel, triangles) and 3.2 mg/kg MCAM (Fig. 1, middle panel, open squares), respectively; in contrast, the morphine dose-effect curve remained shifted to the right of the control curve 69 days after administration of 10 mg/kg MCAM (Fig. 1, bottom panel, filled stars). The ED50 values for morphine also demonstrated that the magnitude and duration of antagonism increased with MCAM dose and decreased over time since MCAM administration (Fig. 2). Under these conditions, the potency of morphine returned to control after 1 and 3.2 mg/kg MCAM and remained significantly changed even 69 days after 10 mg/kg MCAM (Table 1). For each of these 21 morphine dose-effect curves, dosing stopped when tail-withdrawal latency from water maintained at 50°C was at least 13 seconds, and at the largest dose of morphine tested under each of these conditions average tail-withdrawal latency from water maintained at 55° ranged from 5.4 ± 1.8% to 30.5 ± 5.3% of the maximum possible effect.
Fig. 2.
ED50 values obtained from morphine dose-effect curves at various times after MCAM administration (1–10 mg/kg; same data as in Fig. 1 and Table 1). The gray bar represents the 95% confidence interval of the mean ED50 from the control morphine dose-effect curves (filled circles, Fig 1). Ordinates: estimates of ED50 values obtained by linear regression of morphine dose-effect curves in milligrams per kilogram of body weight. Abscissa: days since MCAM administration.
TABLE 1.
ED50 values (mean ± 1 S.E.M.) for morphine alone and after MCAM in a warm water tail-withdrawal procedure measuring the latency to remove tails from water maintained at 50°C
Dose ratios (95% confidence intervals) are ED50 values for morphine after MCAM divided by ED50 values for morphine before MCAM.
| Dosing Condition | ED50 | Dose Ratio |
|---|---|---|
| mg/kg | ||
| Morphine alone | 7.52 ± 0.80 | |
| Morphine after 1 mg/kg MCAM | ||
| 1 day | 17.14 ± 2.20 | 2.38 (1.65, 3.08)a |
| 5 days | 7.95 ± 0.53 | 1.14 (0.85, 1.41) |
| Morphine alone | 7.52 ± 0.80 | |
| Morphine after 3.2 mg/kg MCAM | ||
| 1 day | 68.61 ± 8.95 | 10.21 (5.77, 14.29)a |
| 5 days | 29.27 ± 0.55 | 4.47 (2.90, 5.79)a |
| 9 days | 27.38 ± 1.58 | 4.15 (2.67, 5.39)a |
| 13 days | 17.38 ± 2.73 | 2.66 (1.28, 3.85)a |
| 17 days | 8.80 ± 0.14 | 1.33 (0.90, 1.69) |
| Morphine alone | 7.30 ± 0.56 | |
| Morphine after 10 mg/kg MCAM | ||
| 1 day | 187.68 ± 7.56 | 26.76 (21.44, 31.81)a |
| 5 days | 61.68 ± 6.01 | 8.97 (6.01, 11.65)a |
| 9 days | 41.75 ± 3.90 | 6.11 (3.94, 8.10)a |
| 13 days | 91.67 ± 10.56 | 12.65 (9.54, 15.59)a |
| 17 days | 98.95 ± 2.71 | 14.13 (11.36, 16.78)a |
| 21 days | 100.23 ± 3.90 | 14.23 (11.60, 16.77)a |
| 29 days | 64.75 ± 8.59 | 9.29 (5.71, 12.39)a |
| 37 days | 55.06 ± 9.72 | 7.58 (4.63, 10.15)a |
| 45 days | 35.40 ± 3.06 | 4.95 (4.05, 5.81)a |
| 53 days | 29.77 ± 0.59 | 4.25 (3.44, 5.02)a |
| 69 days | 25.73 ± 3.20 | 3.83 (2.02, 5.53)a |
Potency of morphine was significantly changed as evidenced by 95% confidence intervals of the potency ratios that did not include 1.
In a second group of rats the ability of 10 mg/kg MCAM to attenuate the antinociceptive effects of another µ-opioid receptor agonist, fentanyl, was assessed. Because sensitivity to morphine did not recover fully in MCAM-treated rats that were tested with large doses of morphine (as previously described), a limited dose range was used to assess MCAM antagonism of fentanyl (i.e., to avoid the development of tolerance or other effects of large doses of fentanyl that could influence the recovery of sensitivity to fentanyl antinociception). MCAM antagonized the effects of a fixed dose range of fentanyl, shifting the dose-effect curve downward (Fig. 3). Tail-withdrawal latencies at 0.1 mg/kg fentanyl were still significantly decreased 37 days after MCAM and were not different from control 45 days after MCAM [F(1.93,11.6) = 40.7, P < 0.0001] (Table 2).
TABLE 2.
Tail-withdrawal latency from water maintained at 50°C, expressed as a percentage of the maximum possible effect (mean ± 1 S.E.M.), obtained after administration of the largest cumulative dose of fentanyl (0.1 mg/kg; n = 7) or morphine (17.8 mg/kg; n = 8) alone and at various times after administration of 10 mg/kg MCAM
| Dosing Condition | Maximum Possible Effect | |
|---|---|---|
| After 0.1 mg/kg Fentanyl | After 17.8 mg/kg Morphine | |
| % | % | |
| Agonist alone | 95.2 ± 4.8 | 99.0 ± 1.0 |
| Agonist after 10 mg/kg MCAM | ||
| 1 day | 5.2 ± 2.1* | N.D.a |
| 5 days | 0* | 3.8 ± 1.5* |
| 13 days | 10.3 ± 4.0* | 32.7 ± 6.8* |
| 21 days | 13.9 ± 2.9* | 52.3 ± 8.4* |
| 29 days | 33.5 ± 5.0* | 37.6 ± 11.7* |
| 37 days | 65.4 ± 7.6* | 97.9 ± 1.4 |
| 45 days | 68.1 ± 8.9 | N.D.a |
N.D., not determined.
Morphine dose-effect curves were not determined under these conditions.
P < 0.05 compared with the effects of the agonist in the absence of MCAM.
Finally, in a third group of rats, MCAM did not shift the spiradoline dose-effect curve (Fig. 4, top panel); the ED50 values (mean ± 1 S.E.M.) for spiradoline obtained before and 1 day after MCAM administration were 2.51 ± 0.53 and 2.87 ± 0.13 mg/kg, respectively, yielding a potency ratio (95% confidence interval) of 1.56 (0.78, 2.24). In contrast, MCAM antagonized the antinociceptive effects of morphine, as evidenced by the downward shift in the morphine dose-effect curve in these rats 5 days after MCAM (i.e., 4 days after the spiradoline test) (Fig. 4, bottom panel). Tail-withdrawal latencies obtained after administration of 17.8 mg/kg morphine were significantly decreased for 29 days after MCAM and were no longer different from control 37 days after MCAM [F(2.23,15.6) = 41.8, P < 0.0001] (Table 2).
Paw Inflammation.
Before hindpaw injections, there was no difference in thickness between the paw that later received an injection of saline and the paw that later received an injection of CFA. CFA, but not saline, significantly increased paw thickness for up to 31 days (Table 3). Paw-withdrawal threshold in cycle 1 (i.e., 15 minutes after intraperitoneal saline) was used to determine changes in paw sensitivity to mechanical stimulation across days. Before hindpaw injections, there was no difference in withdrawal threshold between the paw that later received an injection of saline and the paw that later received an injection of CFA (Table 3); CFA but not saline increased sensitivity to mechanical stimulation for up to 31 days after hindpaw injections, as indicated by a significantly lower withdrawal threshold for the CFA-injected paw compared with the saline-injected paw (Table 3).
TABLE 3.
Paw thickness determined before sessions and paw-withdrawal threshold determined during the first cycle of the session with saline administered at the beginning of that cycle
Each value represents the mean (95% confidence interval) in eight rats.
| Days Since Hindpaw Injection | Paw Thickness | Withdrawal Threshold | ||
|---|---|---|---|---|
| Saline Paw | CFA Paw | Saline Paw | CFA Paw | |
| mm | mm | g | g | |
| Before | 3.26 (3.02, 3.49) | 3.17 (2.95, 3.39) | 50.73 (40.95, 60.50) | 50.34 (40.48, 60.19) |
| 1 | 3.34 (3.15, 3.53) | 7.55 (7.26, 7.84)a | N.D.b | N.D.b |
| 2 | 3.24 (3.11, 3.36) | 7.61 (7.28, 7.94)a | 52.47 (45.34, 59.60) | 17.59 (13.19, 21.98)a |
| 31 | 3.22 (3.13, 3.30) | 5.91 (5.62, 6.20)a | 37.37 (31.66, 43.09) | 18.54 (13.83, 23.25)a |
Paw thickness or withdrawal threshold was significantly changed when the value of the inflamed (CFA) paw was outside of the 95% confidence interval of the noninflamed (saline) paw.
Withdrawal threshold was not determined 1 day after hindpaw injection.
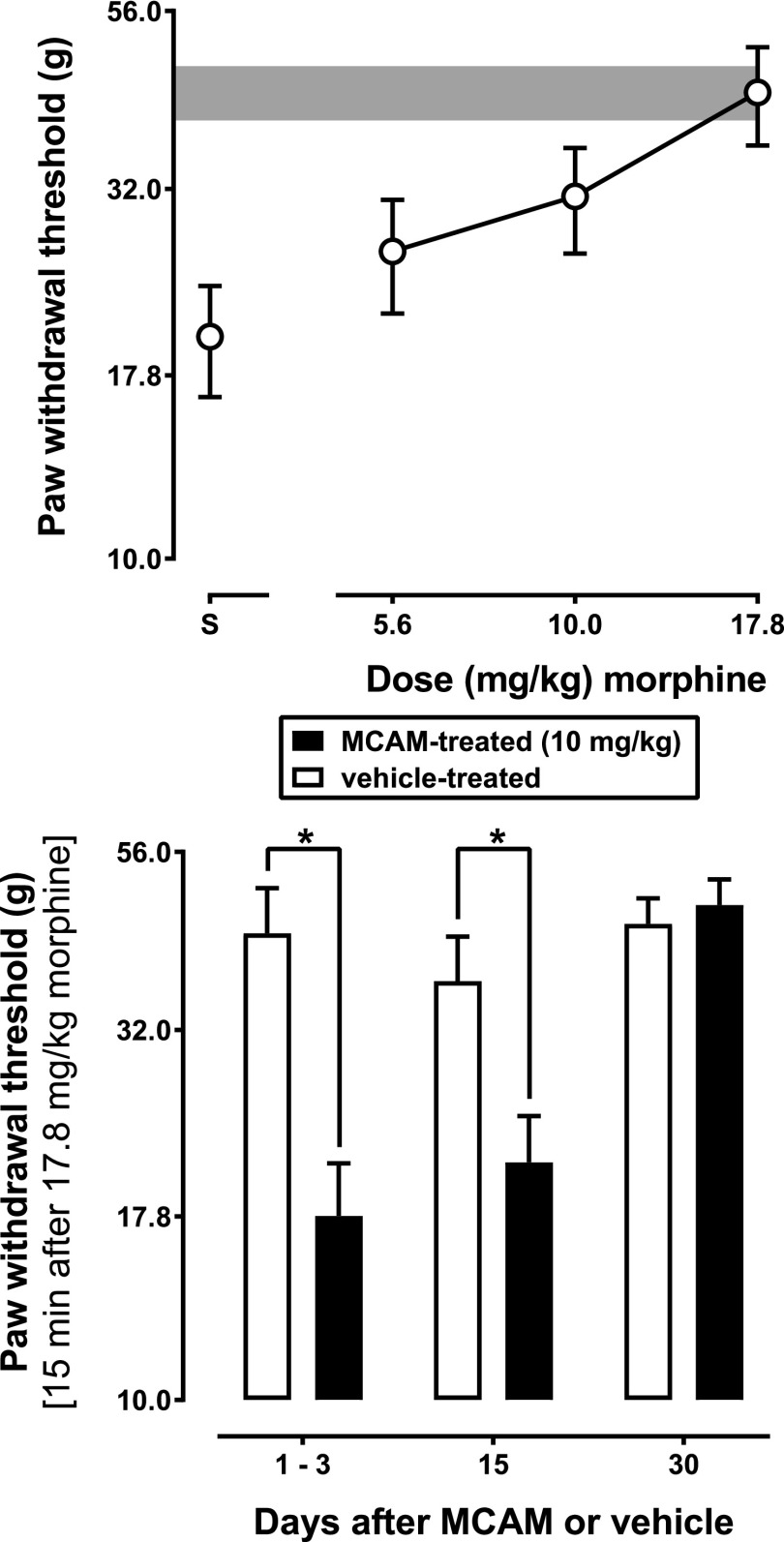
Morphine, but not saline or meloxicam, dose dependently increased paw-withdrawal threshold in vehicle-treated rats (data not shown). There was a significant main effect of treatment [F(1,7) = 6.7, P = 0.036], a significant main effect of time [F(2,14) = 16, P < 0.001], and a significant treatment × time interaction [F(2,14) = 6.6, P = 0.009]. MCAM attenuated the effects of morphine on paw-withdrawal threshold for at least 15 days (Fig. 5), as evidenced by a significant difference between groups on days 1 and 3 [t(14) = 4.6, P = 0.001] as well as on day 15 [t(14) = 2.9, P = 0.035], but not on day 30 [t(14) = 0.49, P > 0.999].
Fig. 5.
Effects of morphine on paw-withdrawal threshold in rats with an inflamed paw. The top panel shows a morphine dose-effect curve obtained 1–3 days after administration of vehicle in rats with an inflamed paw. In the bottom panel, the effects of a cumulative dose of 17.8 mg/kg morphine on paw-withdrawal threshold are shown in rats with an inflamed paw that received either vehicle or MCAM. Ordinate: paw-withdrawal threshold (g) averaged across eight rats; error bars represent mean ± 1 S.E.M. The gray bar represents the 95% confidence interval for the saline paw in the first cycle of sessions in which a morphine dose-effect curve was determined; * indicates that the effects of 17.8 mg/kg morphine were significantly different (P < 0.05) in rats that received 10 mg/kg MCAM compared with rats that received vehicle. Abscissae: days since administration of MCAM or vehicle.
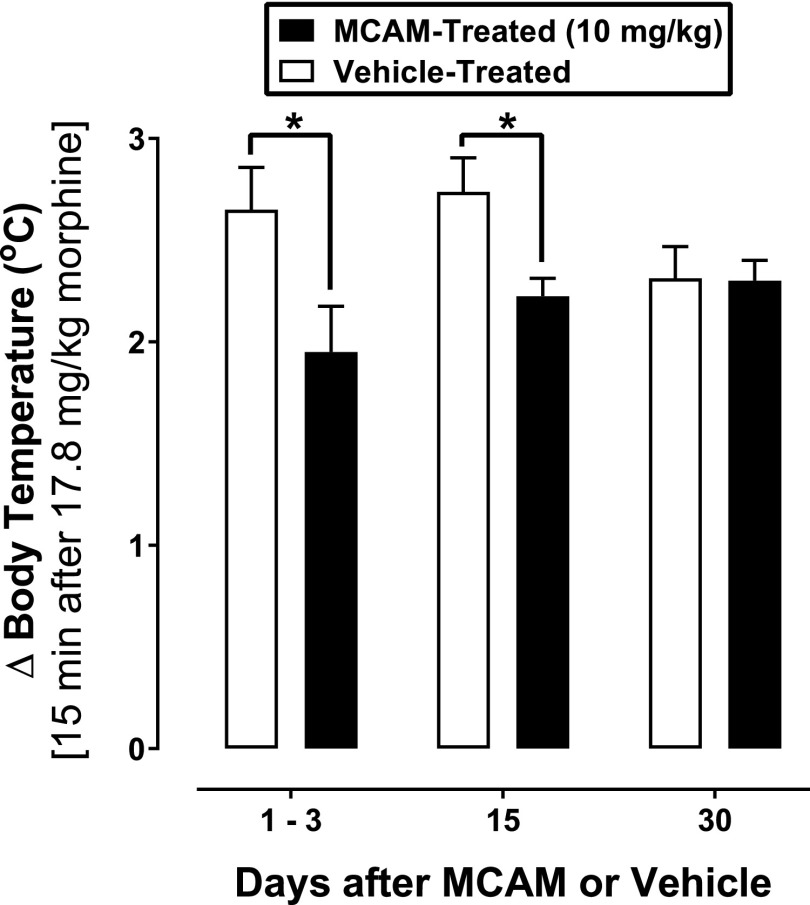
Morphine, but not saline or meloxicam, increased the body temperature in MCAM- and vehicle-treated rats (data not shown). There was a significant main effect of treatment [F(1,7) = 7.7, P = 0.027] but not time [F(2,14) = 1.7, P < 0.317], and a significant treatment × time interaction [F(2,14) = 4.8, P = 0.026]. MCAM blocked the hyperthermic effects of morphine for at least 15 days (Fig. 6), since there was a significant difference between groups on days 1 and 3 [t(14) = 4.3, P = 0.002] and day 15 [t(14) = 3.1, P = 0.021], but not day 30 [t(14) = 0.08, P > 0.999].
Fig. 6.
Changes in body temperature produced by 17.8 mg/kg morphine in rats with an inflamed paw that received either vehicle or MCAM. Ordinate: change in body temperature (°C) averaged across eight rats; error bars represent mean ± 1 S.E.M.; * indicates that the effects of 17.8 mg/kg morphine were significantly different (P < 0.05) in rats that received 10 mg/kg MCAM compared with rats that received vehicle. Abscissae: days since administration of MCAM or vehicle.
Gastrointestinal Transit.
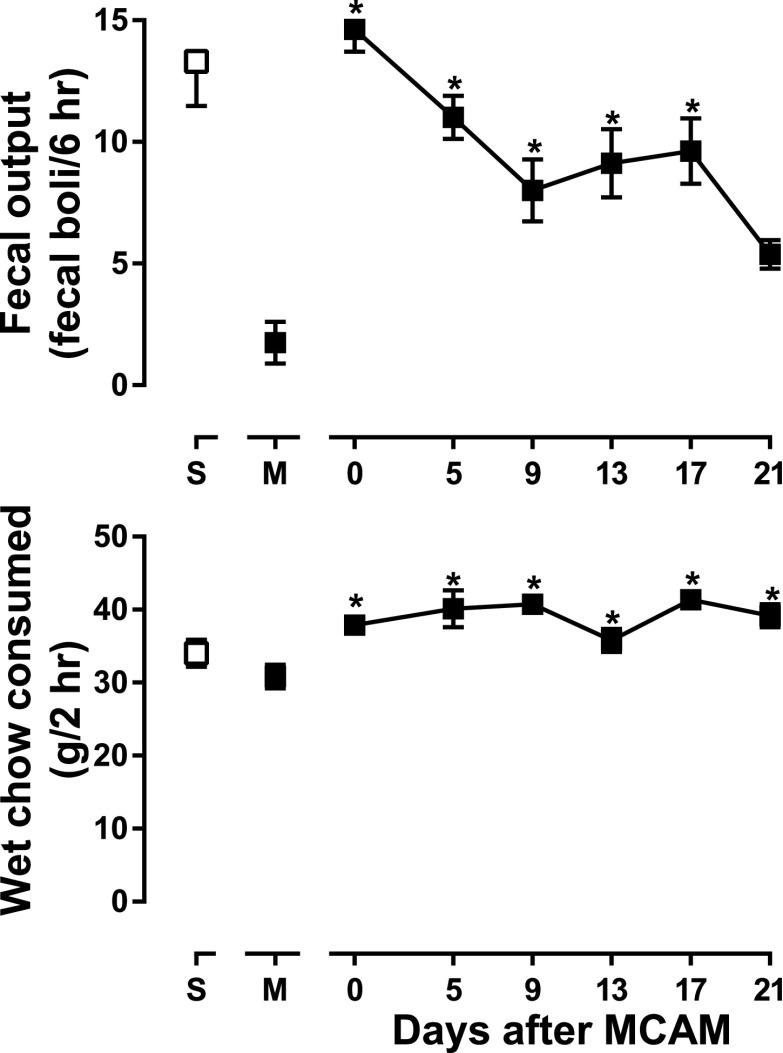
Morphine significantly decreased fecal output compared with saline [t(63) = 7.5, P < 0.001] (Fig. 7, top panel, compare points above M and S). On the day of MCAM administration, morphine did not significantly decrease fecal output [t(63) = 0.9, P > 0.99] (Fig. 7, top panel, point above 0). The effects of morphine on days 0, 5, 9, 13, and 17 after MCAM administration were significantly different from the effects of morphine before MCAM administration [t(63) = 8.4, P < 0.001 for day 0; t(63) = 6.0, P < 0.001 for day 5; t(63) = 4.1, P = 0.001 for day 9; t(63) = 4.8, P < 0.001 for day 13; and t(63) = 5.1, P < 0.001 for day 17], whereas the effects of morphine on day 21 after MCAM administration were not significantly different from the effects of morphine before MCAM administration [t(63) = 2.4, P = 0.19] (Fig. 7, top panel).
Fig. 7.
Fecal output and amount of wet chow consumed in eight rats that received 10 mg/kg MCAM and 10 mg/kg morphine. Ordinates: fecal output (fecal boli/6 hours) and wet chow consumed (g/12 hours); error bars represent mean ± 1 S.E.M.; *P < 0.05 compared with the effects of 10 mg/kg morphine obtained in the absence of MCAM. Abscissae: days since administration of MCAM or vehicle.
Estimated consumption of wet chow remained relatively stable across tests (Fig. 7, bottom panel). On days when morphine alone was tested, consumption was not different compared with saline [t(63) = 2.0, P = 0.50]; however, consumption was slightly but significantly elevated on all tests after MCAM administration compared with the effects of morphine alone [t(63) = 4.3, P < 0.001 for day 0; t(63) = 5.7, P < 0.001 for day 5; t(63) = 6.1, P < 0.001 for day 9; t(63) = 3.0, P = 0.031 for day 13; t(63) = 6.5, P < 0.001 for day 17; and t(63) = 5.2, P < 0.001 for day 21].
Dependence/Withdrawal.
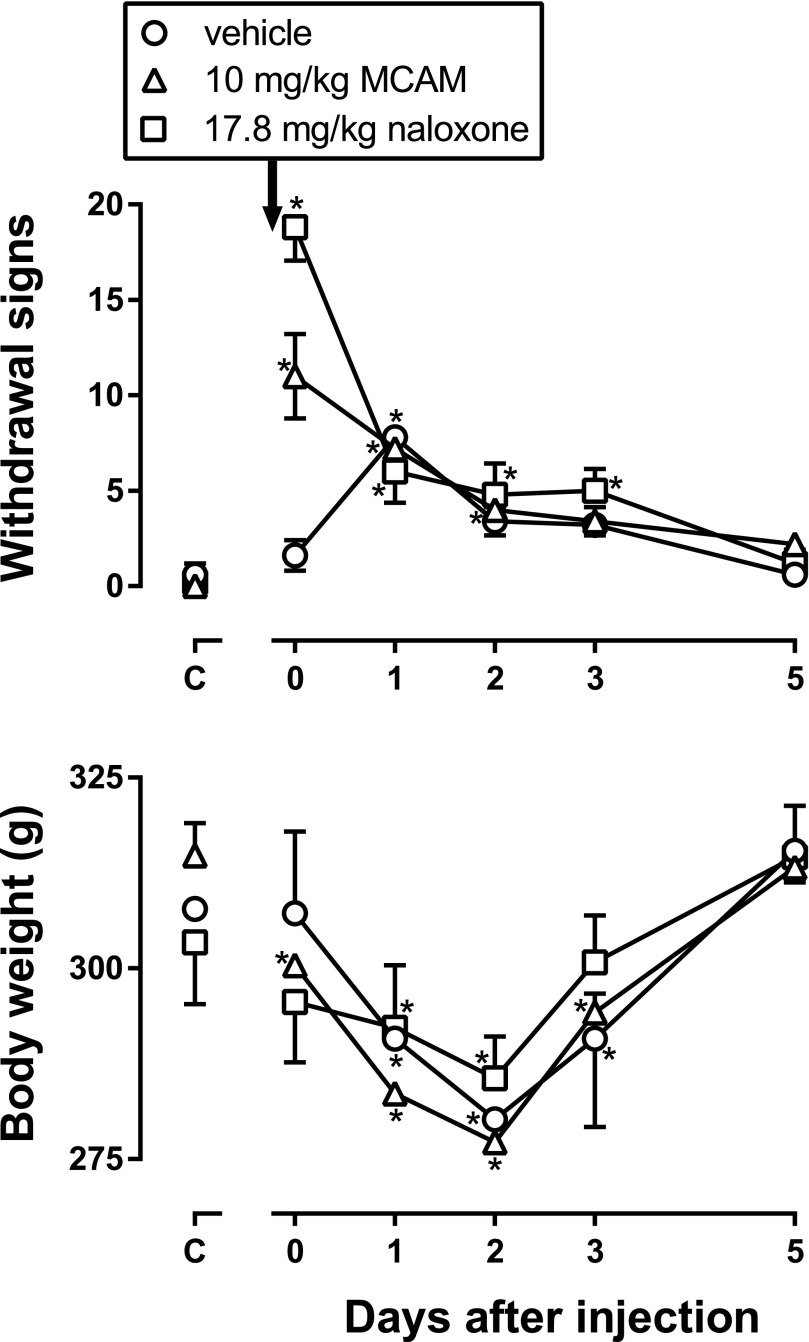
During twice-daily treatment with 56 mg/kg morphine, withdrawal signs did not occur (Fig. 8, top panel, points above C) and body weight was not significantly different among groups (Fig. 8, bottom panel, points above C). MCAM (10 mg/kg) and naloxone (17.8 mg/kg) increased the number of withdrawal signs observed on the day they were administered, compared with the number of withdrawal signs observed before antagonist administration or the number of signs observed in the group that received vehicle instead of an antagonist before the first observation period (Fig. 8, top panel, points above 0). There was a significant main effect of the injection given before the observation periods [i.e., antagonist or vehicle; F(2,8) = 14.63, P = 0.0021], a significant main effect of days since antagonist or vehicle administration [F(5,20) = 29.77, P < 0.0001], and a significant interaction [F(10,40) = 13.43, P < 0.0001]. Post hoc tests revealed that the group receiving MCAM and the group receiving naloxone showed significantly more withdrawal signs on the day antagonists were administered (Fig. 8, day 0) compared with the group that received vehicle. No morphine was administered to any rats after day 0; for all three groups, the number of withdrawal signs was significantly greater 1 day after the last dose of morphine compared with the number observed during morphine treatment (Fig. 8, top panel, for each group compare points above 1 to points above C); however, there was no difference in withdrawal signs among the three groups on any day after discontinuation of morphine treatment (Fig. 8, top panel). Body weight was also significantly decreased by administration of antagonist or discontinuation of morphine treatment (Fig. 8, bottom panel). There was a significant main effect of days since antagonist or vehicle administration [F(5,20) = 99.69, P < 0.0001] and a significant interaction [F(10,40) = 4.60, P = 0.0002], but no main effect of the injection given on the last day of morphine treatment. The largest decrease in body weight occurred 2 days after the last dose of morphine, and this decrease was not different among groups (Fig. 8).
Fig. 8.
Number of withdrawal signs and body weight in five rats that received vehicle, naloxone, or MCAM on the last day of morphine treatment. Ordinates: number of signs or body weight (g); error bars represent mean ± 1 S.E.M. *P < 0.05 compared with effects obtained on the last day of morphine treatment. Abscissae: days since administration of MCAM or vehicle.
Discussion
MCAM is an opioid receptor antagonist that might be useful for treating OUD and overdose because it retains the positive aspects of naltrexone and naloxone (e.g., safety and no abuse liability) (Maguire et al., 2019) and its long duration of antagonist action would block the effects of abused opioids for an extended period, particularly compared with naloxone for which extended release formulations are not available. Although MCAM would be expected to provide long-term protection against the abuse-related and toxic effects of opioids, sustained blockade of µ-opioid receptors could introduce other challenges. For example, MCAM will block the analgesic effects of µ-opioid receptor agonists, rendering them ineffective, and using MCAM to reverse opioid overdose would precipitate withdrawal in opioid-dependent patients. This study examined antagonism of opioid agonists by MCAM using several different procedures in rats.
MCAM attenuated the acute effects of µ-opioid receptor agonists in a dose- and time-related manner. Sustained antagonism by MCAM has been reported (Broadbear et al., 2000; Peckham et al., 2005; Gerak et al., 2019; Maguire et al., 2019); however, the current study extends previous findings in several ways. First, although MCAM was shown to attenuate the antinociceptive effects of morphine in rodents (Broadbear et al., 2000; Peckham et al., 2005), antagonism was monitored for only 2 days. In the current study, MCAM blocked the effects of morphine and fentanyl for several weeks. Two different procedures were used to assess the ability of MCAM to attenuate the antinociceptive effects of µ-opioid receptor agonists (warm water tail withdrawal and CFA-induced hypersensitivity), with 10 mg/kg MCAM antagonizing these effects of morphine for more than 2 weeks. Morphine and other µ-opioid receptor agonists decrease gastrointestinal motility and alter body temperature, producing hypothermia or hyperthermia depending on the conditions. In this study, morphine significantly decreased fecal output and increased body temperature, on average by more than 2.5°C. MCAM produced long-lasting antagonism of the effects of morphine on gastrointestinal motility and body temperature. Thus, MCAM produces sustained antagonism of multiple effects of µ-opioid receptor agonists.
Using the warm water tail-withdrawal procedure, the long duration of action of MCAM was observed. In one study, doses of morphine producing a predetermined level of effect were given frequently to rats that received MCAM; under these conditions, there was progressive and complete recovery of sensitivity to morphine in rats treated with 1 or 3.2 mg/kg MCAM. Although sensitivity to morphine recovered partially in rats treated with 10 mg/kg MCAM, the morphine dose-effect curve did not return fully to control 69 days after MCAM administration. Because MCAM is thought to bind pseudoirreversibly to µ-opioid receptors, its duration of action might be determined by the availability of newly synthesized receptors (Zernig et al., 1994, 1996). One possible explanation for sensitivity to morphine not recovering fully to control after treatment with 10 mg/kg MCAM is that the very large doses of morphine tested relatively frequently (i.e., up to 560 mg/kg every 4 days) desensitized newly synthesized µ-opioid receptors as they became expressed. Although the reason sensitivity to morphine did not recover fully is not evident from this experiment, given this outcome, the duration of action of a single dose of 10 mg/kg MCAM was likely overestimated in this study. Nevertheless, in patients receiving MCAM for opioid abuse or overdose, use or abuse of opioid receptor agonists would not be expected to decrease, and might substantially increase, the time needed for agonist effects to return to control after MCAM administration, which would effectively lengthen the antagonist activity of a large dose of MCAM.
Because sensitivity to morphine did not recover fully in rats treated with 10 mg/kg MCAM and tested with large doses of morphine, the agonist dose range was limited for subsequent experiments, with the largest doses studied being 0.1 mg/kg for fentanyl (Fig. 3) and 17.8 mg/kg for morphine (Fig. 4). Moreover, to reduce further possible changes in sensitivity resulting from repeated testing, agonists were tested less frequently (every 8 days). Under these conditions, MCAM antagonized fentanyl and morphine for at least 30 days, suggesting that a single large dose of MCAM could provide long-term protection against the effects of µ-opioid receptor agonists, regardless of the use of opioids in the presence of MCAM. Recently, there has been an increase in overdose deaths caused by fentanyl and its analogs (Colon-Berezin et al., 2019; Spencer et al., 2019; Zoorob, 2019), resulting in concerns (particularly in the lay press) that currently available opioid receptor antagonists (naltrexone and naloxone) are less effective in attenuating the effects of fentanyl and its analogs compared with their ability to attenuate the effects of other opioids (e.g., heroin). Antagonism across different classes of µ-opioid receptor agonists (morphine- and fentanyl-like opioids) indicates that MCAM would be expected to be equally effective in attenuating the effects of all µ-opioid receptor agonists. Reported differences between fentanyl and other µ-opioid receptor agonists are likely due to the amount taken (e.g., inadvertently) as well as the potency of the opioid agonists, and not due to qualitative differences in effectiveness between morphine- and fentanyl-like opioids or their susceptibility to antagonism by MCAM, naltrexone, or naloxone.
MCAM binds pseudoirreversibly to µ-opioid receptors (Broadbear et al., 2000), and several predictions can be made based on this type of interaction between drugs and receptors. First, because MCAM does not readily dissociate from µ-opioid receptors, its antagonist effects would be expected to be persistent, and in the current study the effects of the largest dose of MCAM lasted at least several weeks. In addition, antagonism by MCAM would be expected to be insurmountable; however, large doses of morphine produced maximal effects the day after administration of a large dose of MCAM. One possible explanation is that the antinociceptive effects of very large doses of morphine are mediated by receptors other than µ-opioid receptors (e.g., κ-opioid receptors) (Takemori and Portoghese, 1987; Stoller et al., 2007). This possibility is consistent with the effects of spiradoline (i.e., unchanged) in the presence of MCAM. Although MCAM also binds to κ- and δ-opioid receptors, its interactions with these receptors are reversible (Broadbear et al., 2000) and MCAM would not still be binding to these receptors the day after administration. That the antinociceptive effects of spiradoline are not changed the day after MCAM suggests that κ-opioid receptors are unchanged and could be mediating the effects of morphine in MCAM-treated rats (Toll et al., 1998).
In morphine-dependent subjects, administration of a µ-opioid receptor antagonist precipitates characteristic signs of withdrawal. In the current study, both MCAM and naloxone precipitated withdrawal in morphine-treated rats. However, the number of withdrawal signs and decreased body weight on subsequent days were not different in rats that received MCAM, naloxone, or vehicle, suggesting that withdrawal precipitated by MCAM is not qualitatively different from either withdrawal precipitated by naloxone or withdrawal after discontinuation of morphine treatment, consistent with results obtained with the irreversible µ-opioid receptor antagonist β-funaltrexamine in morphine-dependent nonhuman primates (Gmerek and Woods, 1985).
Male rats were used in this study. Female rats are less sensitive than male rats to the antinociceptive effects of morphine, and this potency difference is not due to variations in the number of µ-opioid receptors, binding affinity of morphine for these receptors, or ability of morphine to stimulate G proteins (Peckham et al., 2005). Moreover, when given 24 hours before testing, 0.32 mg/kg MCAM decreased the number of µ-opioid receptors by 50%, producing a 3-fold shift to the right in the morphine dose-effect curve in female and male rats; while larger doses of MCAM produced greater rightward shifts in the morphine dose-effect curve in both sexes, they decreased the maximum effect produced by morphine in female, but not in male, rats (Peckham et al., 2005). Similar effects of MCAM were obtained in male rats in the current study. Given that a 30-fold smaller dose of MCAM reduced the number of µ-opioid receptors by 50%, few µ-opioid receptors would be available to interact with morphine 1 day after administration of 10 mg/kg MCAM, and males are more sensitive than females to antinociceptive effects of drugs acting at κ-opioid receptors (Craft and Bernal, 2001).
Because MCAM attenuates the effects of morphine and fentanyl, but not those of spiradoline, persistent antagonism by MCAM is selective for µ-opioid receptor agonists. While these behavioral effects are predicted for a drug with the pharmacological properties of MCAM in vitro, these results also indicate that acute pain could be treated through mechanisms other than µ-opioid receptors in patients taking MCAM for opioid abuse or overdose. These results extend those of previous studies on MCAM (Broadbear et al., 2000; Peckham et al., 2005; Gerak et al., 2019; Maguire et al., 2019) to other measures of µ-opioid receptor agonism and to subjects physically dependent on morphine. Taken together, studies in mice, rats, and nonhuman primates provide compelling support for the potential use of MCAM for treating OUD as well as opioid overdose.
Acknowledgments
The authors thank J. Juarez and A. Nelson for excellent technical assistance.
Abbreviations
- CFA
complete Freund’s adjuvant
- MCAM
methocinnamox
- OUD
opioid use disorder
Authorship Contributions
Participated in research design: Gerak, Minervini, Wooden, France.
Conducted experiments: Gerak, Minervini, Latham, Ghodrati, Lillis, Wooden.
Contributed new reagents or analytic tools: Husbands, Disney.
Performed data analysis: Gerak, Minervini.
Wrote or contributed to the writing of the manuscript: Gerak, Minervini, Wooden, Husbands, Disney, France.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01DA048417 (to C.P.F.) and R01DA07315 (to S.M.H.)] and by the Welch Foundation [Grant AQ-0039 (to C.P.F.)].
All funding sources had no involvement beyond financial support of this study. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or the National Institute on Drug Abuse.
The authors have no conflict of interest.
References
- Broadbear JH, Sumpter TL, Burke TF, Husbands SM, Lewis JW, Woods JH, Traynor JR. (2000) Methocinnamox is a potent, long-lasting, and selective antagonist of morphine-mediated antinociception in the mouse: comparison with clocinnamox, β-funaltrexamine, and β-chlornaltrexamine. J Pharmacol Exp Ther 294:933–940. [PubMed] [Google Scholar]
- Colon-Berezin C, Nolan ML, Blachman-Forshay J, Paone D. (2019) Overdose deaths involving fentanyl and fentanyl analogs—New York City, 2000–2017. MMWR Morb Mortal Wkly Rep 68:37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Bernal SA. (2001) Sex differences in opioid antinociception: κ and ‘mixed action’ agonists. Drug Alcohol Depend 63:215–228. [DOI] [PubMed] [Google Scholar]
- Gerak LR, Maguire DR, Woods JH, Husbands SM, Disney A, France CP. (2019) Reversal and prevention of the respiratory-depressant effects of heroin by the novel μ-opioid receptor antagonist methocinnamox in rhesus monkeys. J Pharmacol Exp Ther 368:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmerek DE, Woods JH. (1985) Effects of β-funaltrexamine in normal and morphine-dependent rhesus monkeys: observational studies. J Pharmacol Exp Ther 235:296–301. [PubMed] [Google Scholar]
- Jones CM, McAninch JK. (2015) Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med 49:493–501. [DOI] [PubMed] [Google Scholar]
- Jones JD, Mogali S, Comer SD. (2012) Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug Alcohol Depend 125:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Paulozzi LJ, Mack KA, Centers for Disease Control and Prevention (CDC) (2014) Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse–related emergency department visits and drug-related deaths—United States, 2010. MMWR Morb Mortal Wkly Rep 63:881–885. [PMC free article] [PubMed] [Google Scholar]
- Kintz P. (2001) Deaths involving buprenorphine: a compendium of French cases. Forensic Sci Int 121:65–69. [DOI] [PubMed] [Google Scholar]
- Kriikku P, Häkkinen M, Ojanperä I. (2018) High buprenorphine-related mortality is persistent in Finland. Forensic Sci Int 291:76–82. [DOI] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, Woods JH, Husbands SM, Disney A, France CP. (2019) Long-lasting effects of methocinnamox on opioid self-administration in rhesus monkeys. J Pharmacol Exp Ther 368:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham EM, Barkley LM, Divin MF, Cicero TJ, Traynor JR. (2005) Comparison of the antinociceptive effect of acute morphine in female and male Sprague-Dawley rats using the long-lasting mu-antagonist methocinnamox. Brain Res 1058:137–147. [DOI] [PubMed] [Google Scholar]
- Pelissier-Alicot A-L, Sastre C, Baillif-Couniou V, Gaulier J-M, Kintz P, Kuhlmann E, Perich P, Bartoli C, Piercecchi-Marti M-D, Leonetti G. (2010) Buprenorphine-related deaths: unusual forensic situations. Int J Legal Med 124:647–651. [DOI] [PubMed] [Google Scholar]
- Pirnay S, Borron SW, Giudicelli CP, Tourneau J, Baud FJ, Ricordel I. (2004) A critical review of the causes of death among post-mortem toxicological investigations: analysis of 34 buprenorphine-associated and 35 methadone-associated deaths. Addiction 99:978–988. [DOI] [PubMed] [Google Scholar]
- Spencer MR, Warner M, Bastian BA, Trinidad JP, Hedegaard H. (2019) Drug overdose deaths involving fentanyl, 2011–2016. Natl Vital Stat Rep 68:1–19. [PubMed] [Google Scholar]
- Stoller DC, Sim-Selley LJ, Smith FL. (2007) Role of kappa and delta opioid receptors in mediating morphine-induced antinociception in morphine-tolerant infant rats. Brain Res 1142:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori AE, Portoghese PS. (1987) Evidence for the interaction of morphine with kappa and delta opioid receptors to induce analgesia in beta-funaltrexamine-treated mice. J Pharmacol Exp Ther 243:91–94. [PubMed] [Google Scholar]
- Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O’Brien A, White A, et al. (1998) Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr 178:440–466. [PubMed] [Google Scholar]
- Volkow ND, Jones EB, Einstein EB, Wargo EM. (2019) Prevention and treatment of opioid misuse and addiction: a review. JAMA Psychiatry 76:208–216. [DOI] [PubMed] [Google Scholar]
- Zernig G, Burke T, Lewis JW, Woods JH. (1996) Mechanism of clocinnamox blockade of opioid receptors: evidence from in vitro and ex vivo binding and behavioral assays. J Pharmacol Exp Ther 279:23–31. [PubMed] [Google Scholar]
- Zernig G, Butelman ER, Lewis JW, Walker EA, Woods JH. (1994) In vivo determination of mu opioid receptor turnover in rhesus monkeys after irreversible blockade with clocinnamox. J Pharmacol Exp Ther 269:57–65. [PubMed] [Google Scholar]
- Zoorob M. (2019) Fentanyl shock: the changing geography of overdose in the United States. Int J Drug Policy 70:40–46. [DOI] [PubMed] [Google Scholar]