Abstract
Thermal acclimation of plant respiration is highly relevant to climate projections; when included in models, it reduces the future rate of atmospheric CO2 rise. Although all living plant tissues respire, few studies have examined differences in acclimation among tissues, and leaf responses have received greater attention than stems and roots. Here, we examine the short-term temperature acclimation of leaf, stem and root respiration within individuals of eight disparate species acclimated to five temperatures, ranging from 15 to 35 °C. To assess acclimation, we measured instantaneous tissue temperature response curves (14–50 °C) on each individual following a 7-day acclimation period. In leaves and photosynthetic stems, the acclimation temperature had little effect on the instantaneous tissue temperature response of respiration, indicating little to no thermal acclimation in these tissues. However, respiration did acclimate in non-photosynthetic tissues; respiratory rates measured at the acclimation temperature were similar across the different acclimation temperatures. Respiratory demand of photosynthetic tissue increased with acclimation temperature as a result of increased photosynthetic demands, resulting in rates measured at the acclimation temperature that increased with increasing acclimation temperature. In non-photosynthetic tissue, the homeostatic response of respiration suggests that acclimation temperature had little influence on respiratory demand. Our results indicate that respiratory temperature acclimation differs by tissue type and that this difference is the consequence of the coupling between photosynthesis and respiration in photosynthetic, but not non-photosynthetic tissue. These insights provide an avenue for improving the representation of respiratory temperature acclimation in large-scale models.
Keywords: Carbon cycling, climate change, Rd, respiratory demand, terrestrial biosphere models, warming
Our study found that temperature acclimation of respiration differs by tissue type (i.e. leaves, stems and roots). Tissue that does not photosynthesize was found to have more homeostatic responses to temperature than photosynthetic tissue. This was found due to the strong linkage between photosynthetic biochemistry and respiration fluxes. These results suggest that plant respiratory responses to changing temperatures, such as future warming, will be tissue type-specific. The link to photosynthesis found in our study provides an avenue for improving the representation of these responses in carbon cycle models.
Introduction
Respiratory carbon release from the land surface is one of the largest fluxes of carbon dioxide (CO2) between the atmosphere and the Earth’s surface. Respiration by plants makes up about half of this flux (Ciais et al. 2013). As a result, terrestrial biosphere models are highly sensitive to the representation of plant respiratory processes, including respiratory thermal acclimation (Atkin et al. 2008; Booth et al. 2012; Slot et al. 2014; Lombardozzi et al. 2015; Smith et al. 2016; Huntingford et al. 2017), a process that may become increasingly important as climates warm globally (Ciais et al. 2013). While thermal acclimation of plant respiration has been observed in a variety of studies, it is still not often included in terrestrial biosphere model simulations (Smith and Dukes 2013), likely due to a poor mechanistic understanding of the response (Atkin et al. 2005).
Thermal acclimation of respiration is defined as a change in the instantaneous response of respiration to temperature as a result of a longer-term change in temperature (Atkin and Tjoelker 2003; Atkin et al. 2005; Smith and Dukes 2013). This commonly results in some combination of a decrease in the slope of the relationship between respiration and temperature and/or a reduction in respiratory rates measured at a common temperature (Atkin and Tjoelker 2003). This effect can dampen respiratory responses to temperature. As such, respiratory rates measured at a tissue temperature identical to the temperature to which the tissue is acclimated are relatively homeostatic across acclimation temperatures (Loveys et al. 2003; Slot and Kitajima 2015). These acclimation responses tend to be stronger in tissues developed at the new temperature or acclimated to the new temperature for a longer period of time (Atkin and Tjoelker 2003)
Acclimation responses likely depend, in part, on changes in maintenance demand that result from changes in temperature (Lambers et al. 1983; Amthor 1984). For instance, increases in temperature may result in increased maintenance demand to support functioning of non-respiratory enzymes, reducing the degree of respiratory acclimation observed in response to short-term changes in temperature. In support of this, studies (e.g. Loveys et al. 2003) have found that tissues developed at a new temperature show stronger respiratory acclimation than ones that developed before a change in acclimation temperature (Atkin et al. 2005) and respiratory acclimation in leaves tends to increase with time following the transfer to a new temperature regime (Slot and Kitajima 2015). This suggests that higher maintenance requirements in tissues developed before a temperature change may limit the degree of respiratory acclimation, particularly in leaves. Still, it is unclear how these mechanisms may play out in other plant tissues, such as stems and roots.
Respiratory thermal acclimation may differ by tissue type; however, this effect has received little attention in the literature (Atkin and Tjoelker 2003; Smith and Dukes 2013) and terrestrial biosphere models typically simulate stem and root respiration simply as a function of leaf respiration (Atkin et al. 2017). Leaves (e.g. Slot and Kitajima 2015), stems (e.g. Maseyk et al. 2008) and roots (e.g. Jarvi and Burton 2013) have each been observed to acclimate to changes in temperature in previous studies. Nonetheless, comparisons of acclimation among tissue types are rare.
For leaves and photosynthetic stems, acclimation is likely tied to temperature effects on photosynthetic biochemistry (Gifford 2003; Smith and Dukes 2017). Indeed, previous controlled environment (Smith and Dukes 2017) and space-for-time substitution (Atkin et al. 2015) studies have found that the ratio of leaf dark respiration to photosynthetic capacity is similar under different acclimation temperatures. Notably, Smith and Dukes (2017) showed non-homeostatic leaf respiratory responses to five acclimation temperatures in 11 species, an effect that coincided with increases in photosynthetic capacity.
In non-photosynthetic tissues, such as woody stems and roots, one would not expect respiration to be closely linked to photosynthetic processes. Instead, demand for respiratory products in these tissues is more likely to be related to growth and transport (Tjoelker et al. 1999; Covey-Crump et al. 2002; Atkin et al. 2007). If these processes are not influenced by changes in acclimation temperature, one might expect a greater degree of observed acclimation in non-photosynthetic tissues (e.g. as seen by Loveys et al. 2003). Additionally, spectral differences among tissues as well as insultation of roots by soil may result in non-uniform changes in tissue temperatures resulting from a change in air temperature. As acclimation is a response to tissue, not air, temperature, these differences could result in differential acclimation responses among tissues.
In one of the only studies that has examined respiratory temperature acclimation across multiple tissues, Loveys et al. (2003) found greater acclimation of roots than leaves in previously developed, but not newly developed leaves. The authors attributed this effect to the rapid growth and turnover of the roots, such that some portion of the measured roots had developed under the new temperatures (Loveys et al. 2003). However, this may have also been due to an increased demand for respiratory products in leaves due to higher rates of photosynthetic processes, but no change in demand in roots. Stem tissue was not compared to root and leaf tissue. In general, the dearth of studies examining acclimation differences among plant tissues limits our ability to understand and predict how respiratory fluxes will be influenced by temperature.
Here, we examine respiratory thermal acclimation of leaves, stems and roots in response to a short-term (i.e. 7-day) change in temperature in eight species (Betula alleghaniensis, Cucumis sativa, Glycine max, Pinus nigra, Pinus pinaster, Pinus pinea, Pinus sylvestris and Zea mays). We assessed short-term thermal responses to mimic the types of changes that plants may experience over intra-annual timescales and because photosynthesis is known to acclimate over short time periods (Veres and Williams 1984; Battaglia et al. 1996; Atkin et al. 2000; Turnbull et al. 2002; Gunderson et al. 2010). We used a variety of plant species in order to make our results more generalizable across taxa, but did not have reason to expect species to differ in their responses. Individual plants were acclimated to one of five temperatures from 15 to 35 °C and the instantaneous response of respiration to temperature was measured for each tissue from 14 to 50 °C. We hypothesized that acclimation to warmer temperatures would reduce the instantaneous tissue temperature sensitivity of respiration for each tissue. We expected this reduced sensitivity to result in more homeostatic respiratory rates at the acclimation temperatures than would be expected from the instantaneous responses alone. We expected this acclimation to be greater in non-photosynthetic than in photosynthetic tissues because these tissues are not influenced by changes in photosynthetic processes that result from changes in acclimation temperature.
Methods
Growth conditions
We used species that varied in growth form, including trees (B. alleghaniensis, P. nigra, P. pinaster, P. pinea, P. sylvestris) and crops that included herbaceous species (C. sativa, G. max) and a grass (Z. mays) (Table 1). The individuals were grown from seed in a 50 %/50 % mixture of field soil (sandy loam; pH: 6.9) and potting soil (Sungro Metro Mix 510; Sungro Horticulture, Agawam, MA, USA) in 1.9-L pots. Plants were not pot-bound by the end of the experiment. Individuals were germinated and grown for an initial period in controlled environment glass houses at ~25 °C. Relative humidity inside the glass house was 58 % on average over the course of this growing period. The glasshouse was sprayed with reflective paint to reduce the risk of overheating. This also acted to reduce photosynthetically active radiation (PAR). As such, PAR was supplemented using 400-Watt overhead lights, with daily PAR reaching a maximum of ~1500 µmol m−2 s−1. Overhead lights were set to a constant 16/8-h light/dark schedule. Individuals were watered when soil became dry. Individuals were provided fertilizer (Miracle Gro 24-8-16 N-P-K; Scotts Company LLC, Marysville, OH, USA) following initial germination and about every 60 days thereafter to avoid nutrient limitation.
Table 1.
Number of individuals sampled per species per acclimation temperature for dark respiration and, in parentheses, dark respiration and photosynthesis. Ta = acclimation temperature. *Stem respiration was not measured for Z. mays.
| T a = 15 °C | T a = 20 °C | T a = 25 °C | T a = 30 °C | T a = 35 °C | Average | |
|---|---|---|---|---|---|---|
| Betula alleghaniensis | 1 (1) | 3 (3) | 0 (0) | 3 (3) | 1 (1) | 1.6 (1.6) |
| Cucumis sativa | 3 (3) | 2 (2) | 1 (1) | 1 (1) | 2 (2) | 1.8 (1.8) |
| Glycine max | 5 (2) | 2 (0) | 1 (1) | 6 (6) | 1 (1) | 3 (2) |
| Pinus nigra | 2 (2) | 3 (3) | 3 (2) | 2 (2) | 2 (2) | 2.4 (2.2) |
| Pinus pinaster | 1 (1) | 1 (1) | 3 (1) | 2 (2) | 4 (4) | 2.2 (1.8) |
| Pinus pinea | 1 (1) | 1 (1) | 2 (2) | 1 (1) | 0 (0) | 1 (1) |
| Pinus sylvestris | 1 (1) | 1 (1) | 2 (2) | 3 (3) | 3 (3) | 2 (2) |
| Zea mays* | 3 (3) | 2 (2) | 2 (1) | 4 (0) | 3 (0) | 2.8 (1.2) |
| Average | 2.1 (1.8) | 1.9 (1.6) | 1.8 (1.3) | 2.8 (2.3) | 2 (1.6) | 2.1 (1.7) |
Acclimation treatments
The time between germination and transfer to growth chambers differed by species. For trees, this period was ~6 months. As such, all trees were juveniles and ranged from ~30 to 50 cm in height. For annual species, this time period was ~1–2 months. In all cases, species were transferred before the production of reproductive tissues.
After germination in the glass houses and the initial growth period, individuals were transferred to environmentally controlled growth chambers (Conviron E15; Controlled Environments Inc., North Branch, MN, USA) for a 7-day acclimation period. Chambers were set to either 15, 20, 25, 30 or 35 °C (acclimation temperature or Ta; see Table 1 for the number of individuals of each species acclimated to each temperature). Relative humidity was set to 50 %, lights were set to a 16/8-h light/dark schedule with lights increasing in intensity (25 % every 15 min) during the first hour and decreasing in intensity (25 % every 15 min) during the last hour of the 16-h light period. Photosynthetically active radiation was ~1470 µmol m−2 s−1 during peak hours inside the chamber. Plants in the chamber were provided water when soil became dry. Each individual was acclimated to only one of the five acclimation temperatures.
Gas exchange measurements
Following the 7-day acclimation treatment, instantaneous temperature response curves were developed for leaf (Rd,leaf), stem (Rd,stem) and root (Rd,root) dark respiration (Rd). To build these curves, Rd measurements were taken at tissue temperatures of 14, 23, 32, 41 and ~50 °C using two LiCor 6400 portable photosynthesis systems running simultaneously (LiCor Biosciences, Lincoln, NE, USA) with a standard 3 cm × 2 cm chamber and attached light source turned off. The cuvette was sealed with putty to ensure there were no leaks. The lack of leaks was confirmed by leak tests (i.e. blowing on the chamber) during each measurement. All measurements proceeded inside the chamber following a 1-h dark adaptation period prior to the first measurement and during which the entire plant was placed in a dark growth chamber. The chamber was kept dark throughout the course of the measurements. Both the cuvette and growth chamber temperatures were adjusted to alter tissue temperatures. Measurements were made successively at progressively warmer temperatures from 14 to ~50 °C. Full temperature responses of leaves, then stems and then roots were recorded. For root measurements, roots were carefully removed from soil to reduce breakage and measured while attached to the plant. Tissue temperatures were measured using the internal thermocouple on the LiCor 6400, ensuring that tissues were in contact with the thermocouple during measurement. The warmest tissue temperature was set to the maximum temperature attainable by the machine and thus varied by individual (mean ± SE: leaf = 44.49 ± 0.013 °C, stem = 45.36 ± 0.012 °C, root = 45.14 ± 0.011 °C). For each temperature setting, we took 30 measurements over 30 s after matching the infrared gas analyzers. The average of these was used for analysis. One individual per portable photosynthesis system was measured each day for a total of two individuals measured per day. Note that Rd,stem was not measured for Z. mays due to the thickness of the stems. Rd,stem was measured for all other species.
Prior to, but on the same day as, all respiratory measurements, net photosynthesis (Anet) by intercellular CO2 (Ci) curves was taken on each individual. To build these curves, A/Ci measurements were taken at leaf temperatures of 14, 23, 32, 41 and ~50 °C using the LiCor 6400 portable photosynthesis instruments (LiCor Biosciences, Lincoln, NE, USA). Both cuvette and growth chamber temperatures were adjusted to alter leaf temperatures. Responses were measured first at the temperature at which the plant was grown. This measurement was made to ensure stomata were open and responding to changes in CO2 and was discarded prior to analysis. Measurements were then made successively at progressively warmer temperatures from 14 to ~50 °C. Leaf temperatures were measured using the internal thermocouple on the LiCor 6400. The warmest leaf temperature was set to the maximum temperature attainable by the machine and thus varied by individual (mean ± SE: 44.31 ± 0.10 °C). Light inside the chamber was set to a saturating rate of 1200 µmol m−2 s−1. Humidity inside the leaf chamber was maintained at ~60 %, but was occasionally lower at high temperatures. In those cases, water was added to the flow path by adding water (<5 mL) to the soda lime to achieve the highest level of humidity possible. A/Ci curves were generated using leaf chamber CO2 values of (in order): 400, 300, 200, 100, 50, 400, 400, 600, 800, 1000, 1200, 1500 and 2000 µmol mol−1 CO2 for C3 species and 400, 300, 200, 100, 50, 0, 400, 400, 600 and 800 µmol mol−1 CO2 for C4 species. Photosynthetic parameters, including the maximum rate of Rubisco carboxylation (Vcmax), the maximum rate of electron transport for Ribulose-1,5-bisphosphate regeneration (Jmax) and, for C4 species, the maximum rate of phosphoenol pyruvate carboxylation (Vpmax), were fit for each A/Ci curve using the ‘plantecophys’ package in R (Duursma 2015), following Smith and Dukes (2017). For all gas exchange measurements, we ensured that leaf fluxes were at steady state before beginning measurements.
Following gas exchange analyses, tissue inside the chamber was removed and fresh tissue projected area was assessed using a scanner and ImageJ (Schneider et al. 2012); if necessary, gas exchange rates were adjusted accordingly. Tissues were then dried to a constant mass at 65 °C. Fluxes were converted to a dry mass basis (i.e. µmol CO2 g−1 s−1).
Temperature response curve fitting
The temperature responses of leaf, stem and root Rd for each individual plant were fit using a third-order polynomial described by O’Sullivan et al. (2013), as in Smith and Dukes (2017):
| (1) |
where RT (µmol g−1 s−1) is the process rate at the leaf temperature Tl, a corresponds to the exponential rate of RT at 0 °C (µmol g−1 s−1), b is a parameter describing the change in rates with temperature at temperatures near 0 °C and c is a parameter describing the change in this increase with increasing temperature. The average root mean squared error (RMSE) for the Rd temperature response curves of leaves, stems and roots was 0.09, 0.11 and 0.16 µmol g−1 s−1, respectively.
As a proxy for demand for the workings of photosynthetic machinery, the temperature response of Vcmax was also fit using equation 1 for each plant. The average RMSE was 0.13 µmol m2 s−1. Following fitting, Vcmax data were converted to a per-gram trate (i.e. µmol g−1 s−1) to aid in comparison to Rd data.
Data analysis
To test whether the instantaneous response of Rd differed as a result of changes in Ta, we used mixed-model analyses of covariance with the ‘lmer’ function in the ‘lme4’ package (Bates et al. 2015) in R version 3.5.0 (R Development Core Team 2019). The temperature response parameters a, b and c for Rd were used as dependent variables. The species (categorical variable), 7-day temperature to which the individual was acclimated (Ta; i.e. 15, 20, 25, 30 or 35 °C; continuous variable), tissue type (leaf, photosynthetic stem, non-photosynthetic or root; categorical variable) and the interaction between tissue type and Ta were included as predictor variables in each model. Individual was included as a random factor in the models. To explicitly examine the influence of photosynthetic processes on Rd of each tissue we calculated rates of Rd of each tissue and Vcmax at tissue temperatures equal to Ta (Rd,acc and Vcmax,acc). We then calculated the ratio of each tissue’s Rd,acc to Vcmax,acc (Rd,acc/Vcmax,acc) and fit a similar mixed-effects model as above. Following model fitting, significance testing was performed by calculating the Wald χ 2 for each model parameter using the ‘Anova’ function in the ‘car’ package (Fox and Weisberg 2011). For all models, we visually examined residual plots following model fitting to ensure that necessary assumptions for model comparisons were met (Zuur et al. 2009).
Post hoc analyses were done using the ‘emmeans’ package (Lenth 2018). Specifically, post hoc least squared mean slope and intercept values describing the relationship between the response variables and Ta were calculated using the fitted model for each tissue type. This allowed for these calculations to account for all independent variables fit in the model. We examined whether slopes from these models were different from 0 for each tissue type using a t-statistic test with degrees of freedom calculated using Kenward-Roger approximation. This was done using the ‘test.emmGrid’ function in the ‘emmeans’ package (Lenth 2018). We used planned contrasts to compare slopes of photosynthetic versus non-photosynthetic tissues to the acclimation temperatures following our original hypothesis. This was done using t-ratio tests using the ‘contrast’ function in the ‘emmeans’ package (Lenth 2018).
Using the least squared mean slope and intercept values for the relationship between the response variables and Ta, we were able to calculate fitted Rd values at different tissue temperatures (Tt). Specifically, we calculated Rd at Ta = Tt. To assess the degree of acclimation for each, we used the ‘Homeostasis Method’ (Loveys et al. 2003). This involved calculating the ratio of Rd at Ta = Tt to Rd,25 at Ta = 25 °C for plants acclimated to 15, 20, 30 and 35 °C, which we refer to AcclimHomeo. For ease of comparison, Rd,25 at Ta = 25 °C was used in the denominator when calculating AcclimHomeo for plants acclimated to 15 and 20 °C and Rd,25 at Ta = 25 °C was used in the numerator when calculating AcclimHomeo for plants acclimated to 30 and 35 °C. As such, in all cases, AcclimHomeo values of 1 indicate homeostatic Rd rates, with values further from 1 indicating progressively less homeostasis. We used plants acclimated to 25 °C as the reference because this was the temperature at which the plants were germinated and grown prior to being placed in the growth chamber.
Due to poor germination, death and equipment malfunctioning, the number of individuals per species per acclimation temperature differed. On average 2.1 individuals per species per acclimation temperature were measured. This sample size was the result of a choice to maximize the number of species used to assess the generality of our hypotheses, which were not species-specific, but rather tissue-specific. To match this hypothesis, we included acclimation temperature as a continuous variable in our models and did not include any interaction terms with species. Thus, the low number of species per acclimation temperature was not an issue for our models. Table 1 shows the number of individuals per species per acclimation temperature used for the analysis. The mixed-model analyses of variance used here are robust for handling unbalanced designs (Zuur et al. 2009).
All data used for the analyses described here can be found at https://github.com/SmithEcophysLab/tissue_respiration (doi: 10.5281/zenodo3445384).
Results
The basal rate of dark respiration (a)
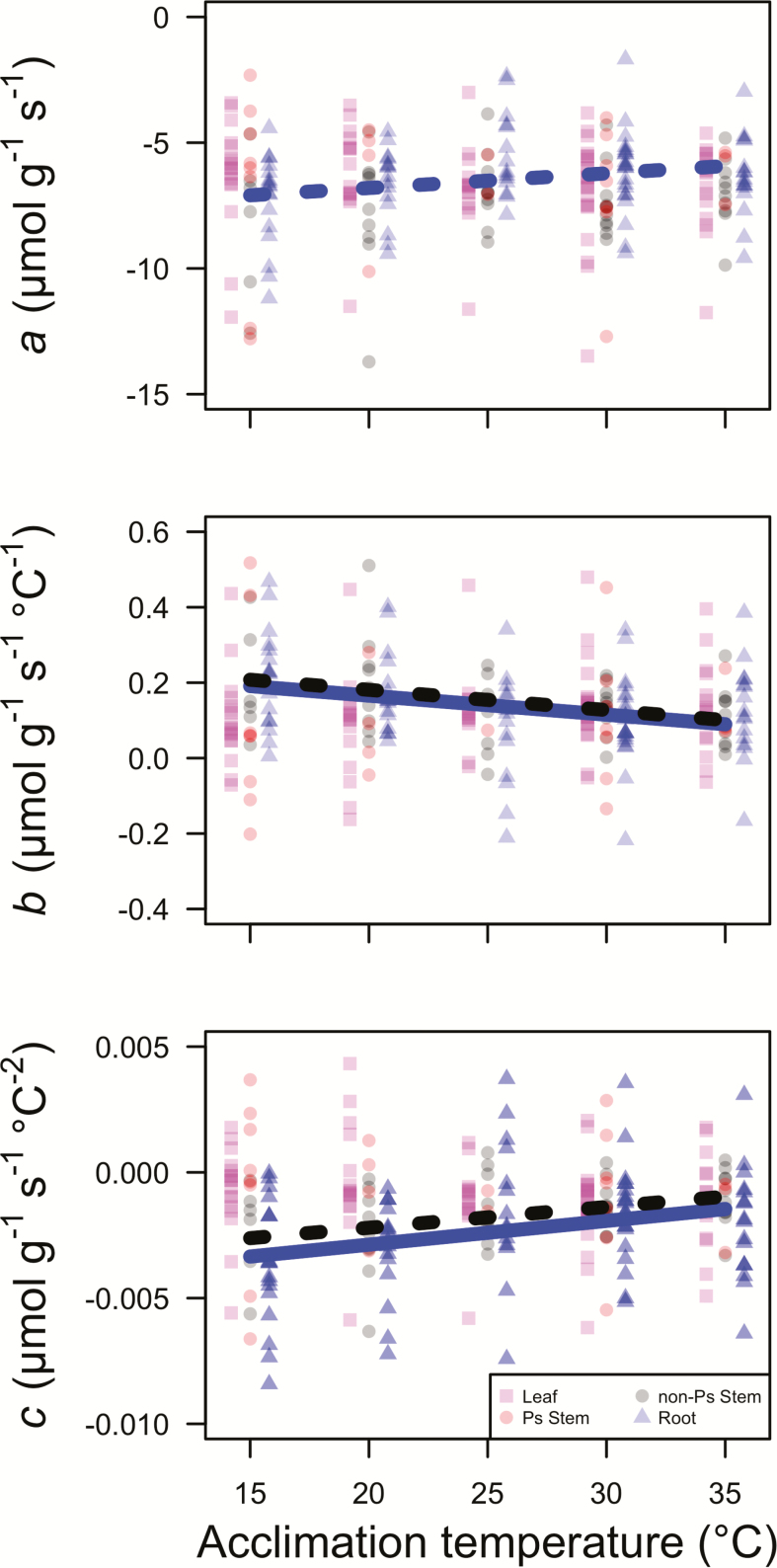
The parameter, a, that describes the rate of Rd at a tissue temperature of 0 °C was not detectably influenced by tissue type, the temperature at which the plants were acclimated (acclimation temperature; Ta), or the interaction between the two factors (P > 0.05 in all cases; Table 2). Post hoc analyses of slopes did indicate a marginally significant increase in a rates with Ta for roots (P = 0.054; Table 3; Fig. 1), but no effect for other tissue types (P > 0.10 in all cases; Table 3; Fig. 1). A planned contrast found no difference between slopes of the a–Ta relationship between photosynthetic and non-photosynthetic tissue (t213 = 1.79, P > 0.05). The a value did differ by species (P < 0.05; Table 2). Post hoc comparisons indicated that the only statistically different (P < 0.05) species combination was between the species with the lowest a rates (P. pinaster; estimated marginal mean a across tissue types = −7.57 µmol g−1 s−1) and the species with the highest a rates (Z. mays; estimated marginal mean a across tissue types = −5.90 µmol g−1 s−1). All other species had statistically similar a rates.
Table 2.
Results from mixed-model analysis of variance testing thermal acclimation of instantaneous temperature response parameters across tissue types*. *P values less than 0.05 are indicated in bold. Ta = acclimation temperature, a corresponds to the exponential rate of RT at 0 °C (µmol g−1 s−1), b is a parameter describing the change in rates with temperature at temperatures near 0 °C and c is a parameter describing the change in this increase with increasing temperature, Rd,acc/Vcmax,acc is the ratio of dark respiration to the maximum rate of Rubisco carboxylation at tissue temperatures equal to the acclimation temperature.
| a | b | c | R d,acc/Vcmax,acc | ||||||
|---|---|---|---|---|---|---|---|---|---|
| df | χ 2 | P | χ 2 | P | χ 2 | P | χ 2 | P | |
| Species | 7 | 17.65 | 0.014 | 9.97 | 0.190 | 10.93 | 0.142 | 40.02 | <0.001 |
| T a | 1 | 1.27 | 0.259 | 2.01 | 0.156 | 2.32 | 0.128 | 14.95 | <0.001 |
| Tissue | 3 | 3.37 | 0.338 | 3.71 | 0.295 | 26.57 | <0.001 | 30.18 | <0.001 |
| T a × Tissue | 3 | 5.38 | 0.146 | 9.68 | 0.021 | 12.08 | 0.007 | 13.03 | 0.004 |
Table 3.
Slopes of the response of the instantaneous temperature response parameters to Ta*. *Slope indicates the least squared mean slope of the relationship between the parameter (i.e. a, b or c) and Ta. The SE is the standard error of the least squared mean slope. Degrees of freedom (df) were estimated using Kenward-Roger approximation. The t-ratio test examined whether the slopes were significantly different from 0. Values with P values less than 0.05 and 0.1 are indicated in bold and italics, respectively.
| a | b | c | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue | Photosynthetic | Slope | SE | df | t-ratio | P | Slope | SE | df | t-ratio | P | Slope | SE | df | t-ratio | P |
| Leaf | Yes | −0.027 | 0.030 | 219.7 | −0.91 | 0.361 | 0.002 | 0.002 | 221.9 | 1.17 | 0.244 | −0.000041 | 0.000032 | 221.9 | −1.29 | 0.200 |
| Stem | Yes | 0.002 | 0.053 | 222.9 | 0.04 | 0.968 | 0.001 | 0.004 | 222.6 | 0.42 | 0.675 | −0.000033 | 0.000056 | 222.6 | −0.59 | 0.555 |
| Stem | No | 0.060 | 0.043 | 222.7 | 1.38 | 0.168 | −0.005 | 0.003 | 222.7 | −1.87 | 0.062 | 0.000083 | 0.000046 | 222.7 | 1.82 | 0.071 |
| Root | No | 0.058 | 0.030 | 219.7 | 1.94 | 0.054 | −0.005 | 0.002 | 221.9 | −2.56 | 0.011 | 0.000094 | 0.000032 | 221.9 | 2.99 | 0.003 |
Figure 1.
The effect of acclimation temperature (Ta) on parameter values describing the instantaneous temperature response of dark respiration (Rd). Leaf, photosynthetic (Ps) stem, non-Ps stem and root parameters are indicated by pink squares, red circles, grey circles and blue triangles, respectively. Leaf and root points are jittered along the x-axis by −0.8 and 0.8 °C, respectively, to improve visibility. Significant (P < 0.05) and marginally significant (P < 0.10) slopes are shown with solid and dashed lines, respectively, with colours corresponding to tissue type (i.e. black = non-Ps stem, blue = root). Slope values are least squared means from the mixed-model analyses of variance.
The thermal response of the instantaneous rate of dark respiration near 0 °C (b)
There was an interaction between tissue type and Ta for the parameter, b, that describes the instantaneous response of Rd to temperature near a tissue temperature of 0 °C (P < 0.05; Table 2). Post hoc analyses of slopes indicated that root b decreased with Ta (P < 0.05; Table 3; Fig. 1), and that non-photosynthetic stem b similarly decreased with Ta, but this response was only marginally significant (P = 0.062; Table 3; Fig. 1). The post hoc slope analysis indicated that leaf and photosynthetic stem b were not significantly influenced by Ta (P > 0.10; Table 3; Fig. 1). These results suggested that non-photosynthetic tissue b was more responsive to Ta than photosynthetic tissue b, an effect confirmed by a planned contrast showing that slopes of the relationship between b and Ta differed between photosynthetic and non-photosynthetic tissue (t216 = −2.67, P < 0.01). The b parameter did not differ by species (P > 0.10; Table 2).
The change in the instantaneous thermal response of dark respiration with increasing temperature (c)
There was a significant interaction between tissue type and Ta on the parameter, c, that describes the change in the instantaneous thermal response of Rd with increasing tissue temperature (P < 0.01; Table 2). Post hoc analyses of slopes indicated that root c increased with Ta (P < 0.01; Table 3; Fig. 1) and that non-photosynthetic stem c similarly increased with Ta, but this effect was only marginally significant (P = 0.071; Table 3; Fig. 1). Leaf and photosynthetic stem c were not influenced by Ta (P > 0.10; Table 3; Fig. 1). These results suggest that non-photosynthetic tissue c was more responsive to Ta than photosynthetic tissue c, an effect confirmed by a planned contrast showing that slopes of the relationship between c and Ta differed between photosynthetic and non-photosynthetic tissue (t216 = 2.96; P < 0.01). The c parameter did not differ by species (P > 0.10; Table 2).
Modelled thermal acclimation of dark respiration
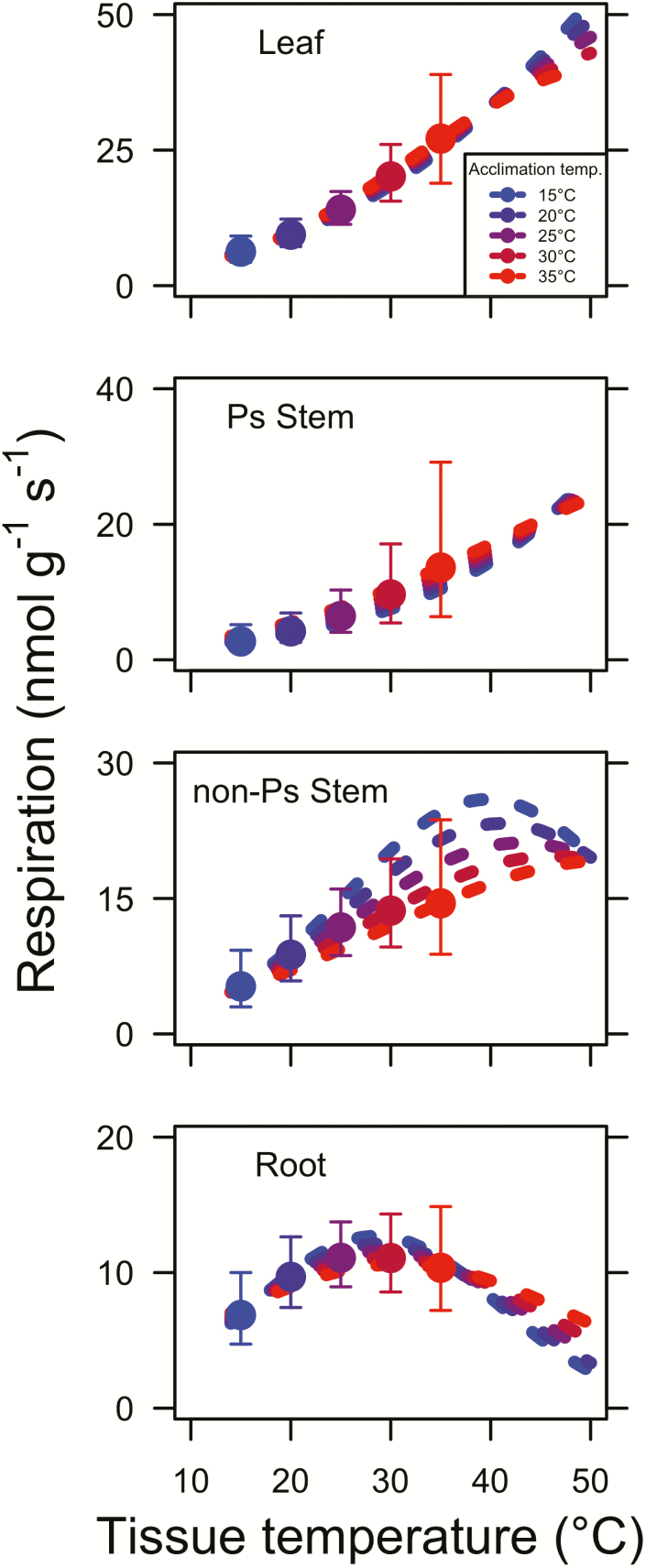
We assessed thermal acclimation of dark respiration for each tissue type by modelling the instantaneous response for each Ta assessed in the study (i.e. 15, 20, 25, 30 and 35 °C). We did this using the least squared mean slope and intercept values for the relationship between parameters a, b and c and Ta from the mixed-model analysis of variance (Table 3) to calculate parameter values at Ta values of 15, 20, 25, 30 and 35 °C. We also calculated modelled respiration rates at Ta equal to the tissue temperature. These calculations showed that instantaneous responses to tissue temperature were strongest in photosynthetic tissues, leaves in particular, and dampened in non-photosynthetic tissue, roots in particular (Fig. 2).
Figure 2.
The instantaneous temperature response of leaf, photosynthetic (Ps) stem, non-photosynthetic stem and root respiration. Instantaneous curves were created using the parameters obtained in Table 3. Points indicate least squared mean (±SE) values for respiration rates measured at leaf temperatures Ta equal to the tissue temperature. Throughout, blue, blue-purple, purple, red-purple and red points and lines indicate values for plants acclimated to 15, 20, 25, 30 and 35 °C, respectively (see inset legend in top panel).
Homeostasis of dark respiration under varying temperatures
This difference in thermal acclimation between photosynthetic and non-photosynthetic tissue was also apparent in AcclimHomeo values (Table 4). Values closer to 1 indicate a greater degree of homeostasis in respiration rates. Leaves and photosynthetic stems had average AcclimHomeo values of 0.58 and 0.55, respectively, while root and non-photosynthetic stems had values of 0.89 and 0.71, respectively (Table 4). This effect was driven by greater homeostasis at warmer temperatures in non-photosynthetic tissue. In fact, across all tissue types, AcclimHomeo tended to be furthest from 1 in plants acclimated to 15 °C (average = 0.48; Table 4). This suggests that low acclimation temperatures tended to reduce Rd regardless of tissue type.
Table 4.
Calculated homeostasis (AcclimHomeo) values for each tissue at each acclimation temperature (Ta)*. *All values are in relation to Rd values at Ta = 25 °C. Values closer to 1 indicate a greater degree of homeostatic acclimation. Ta = acclimation temperature; Ps = photosynthetic.
| T a | Leaf | Ps stem | Non-Ps stem | Root | Average |
|---|---|---|---|---|---|
| 15 °C | 0.45 | 0.42 | 0.45 | 0.62 | 0.48 |
| 20 °C | 0.67 | 0.65 | 0.74 | 0.87 | 0.73 |
| 30 °C | 0.70 | 0.67 | 0.86 | 1.00 | 0.81 |
| 35 °C | 0.52 | 0.47 | 0.82 | 1.07 | 0.72 |
| Average | 0.58 | 0.55 | 0.72 | 0.89 | 0.68 |
The ratio of dark respiration to photosynthetic capacity
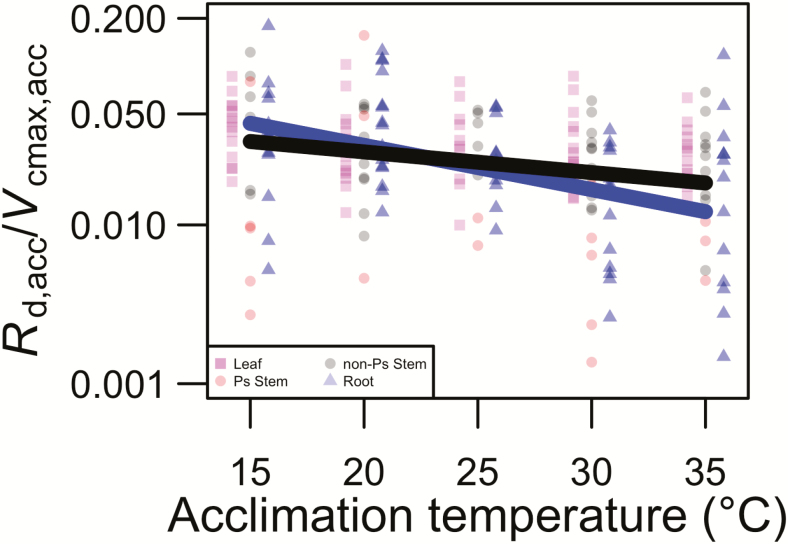
The ratio of dark respiration to the maximum rate of Rubisco carboxylation at the tissue temperature equal to Ta (Rd,acc/Vcmax,acc) depended on Ta in some tissue types, but not others (tissue type × Ta; P < 0.01; Table 2). Post hoc analyses of slopes indicated that root and non-photosynthetic stem Rd,acc/Vcmax,acc decreased with Ta (P < 0.05 in both cases; Fig. 3), but that neither leaf nor photosynthetic stem Rd,acc/Vcmax,acc was influenced by Ta (P > 0.05 in both cases; Fig. 3). These results suggest that non-photosynthetic tissue Rd,acc/Vcmax,acc was more responsive to Ta than photosynthetic tissue Rd,acc/Vcmax,acc. This conclusion was supported by a planned contrast showing that slopes of the relationship between Rd,acc/Vcmax,acc and Ta differed between photosynthetic and non-photosynthetic tissue (t154 = −2.00; P < 0.05). The Rd,acc/Vcmax,acc varied by species (P < 0.01; Table 2). Post hoc comparisons found that the only species that differed significantly in Rd,acc/Vcmax,acc (P < 0.05) was that with the highest ratio (P. pinea; estimated marginal mean Rd,acc/Vcmax,acc across tissue types = 0.041) and that with lowest ratio (Z. mays; estimated marginal mean Rd,acc/Vcmax,acc across tissue types = 0.010).
Figure 3.
The effect of acclimation temperature (Ta) on the ratio of dark respiration to the maximum rate of Rubisco carboxylation at tissue temperature equal to the acclimation temperature (Rd,acc/Vcmax,acc) for each tissue type leaf, photosynthetic (Ps) stem, non-Ps stem and root values are indicated by pink squares, red circles, grey circles and blue triangles, respectively. Leaf and root points are jittered along the x-axis by −0.8 and 0.8 °C, respectively, to improve visibility. Significant (P < 0.05) slopes are shown with solid lines, with colours corresponding to tissue type (i.e. black = non-Ps stem, blue = root). Slope values are least squared means from the mixed-model analyses of variance. The line equations are y = e−0.063x − 2.20 and y = e−0.030x – 2.94 for black (i.e. non-photosynthetic stem) and blue (i.e. root) lines, respectively. Data are plotted on a log scale.
Discussion
Here, we asked: As is commonly assumed by large-scale models (e.g. Oleson et al. 2013), is thermal acclimation of dark respiration (Rd) similar across tissue types (leaves, stems and roots)? And, if not, then why do tissue types differ? Using eight species across four diverse plant functional types, we found that thermal acclimation in response to a short acclimation period (7 days) was apparent in non-photosynthetic tissues, but was not observed in photosynthetic tissues. This pattern is consistent with the results found by Loveys et al. (2003), who found AcclimHomeo ratios of leaves and roots that were nearly equivalent to the results found here. Our stem results provide further insight into the mechanisms driving this response and suggest that photosynthetic tissues have reduced short-term thermal down-regulation of dark respiration, thus decreasing AcclimHomeo values. Photosynthetic data taken on the same individuals showed that increases in Ta resulted in an increase in maximum rates of Rubisco carboxylation (Vcmax) and electron transport (Jmax) (Smith and Dukes 2017). This effect likely increased respiratory demand for photosynthetic processes, which may have limited any respiratory down-regulation.
Past work has shown that dark respiration acclimation of leaves is primarily driven by acclimation of Vcmax (Wang et al. 2018). Indeed, Vcmax has been used as a proxy to model leaf dark respiration for decades (Farquhar et al. 1980; Collatz et al. 1991). Our results support the idea that dark respiration of photosynthetic tissue is highly correlated to Vcmax, as evidenced by the fact that Rd,acc/Vcmax,acc ratios were similar across all acclimation temperature treatments in photosynthetic tissues. As such, Vcmax may be a suitable proxy for simulating dark respiration thermal acclimation of photosynthetic tissue in large-scale models.
However, our results suggest that dark respiration is less sensitive to changes in acclimation temperature in non-photosynthetic tissue than in photosynthetic tissue. This was particularly true for acclimation temperatures between 20 and 35 °C. At these temperatures, rates of dark respiration were relatively homeostatic (AcclimHomeo ratios ranging from 0.74 to 1.07). This may have been because growth and maintenance demands remained similar across these temperatures in these tissues, coupled with reduced respiratory temperature limitation at these temperatures. Indeed, at 15 °C AcclimHomeo values tended to drop in all tissues, indicating that this low acclimation temperature may have induced some degree of temperature limitation to dark respiration.
The strong acclimation responses of non-photosynthetic tissue are consistent with previous stem and root respiratory thermal acclimation studies (e.g. Maseyk et al. 2008; Jarvi and Burton 2013). Our results do not fully clarify the drivers of the observed response. Our results do, however, suggest that non-photosynthetic tissue respiration cannot be modelled using photosynthetic tissue responses, as is commonly done (e.g. Oleson et al. 2013), as this approach may overestimate non-photosynthetic tissue respiration at high temperatures. Further work is necessary to understand the mechanisms driving temperature responses of non-photosynthetic dark respiration in order to reliably simulate this process in models.
Our results may have been related to the length of our acclimation time period. We chose a 7-day acclimation period to simulate short-term (i.e. intra-annual) variation in temperature that these plants might experience in the field. Slot and Kitajima (2015) used a meta-analysis to examine the Rd thermal acclimation of leaves, and found that acclimation increased with increasing duration of the experimental treatment. Thus, the leaves (and potentially other tissues) in our experiment may not have had time to fully adjust to the changed conditions. Additionally, the lack of leaf dark respiration acclimation observed in the photosynthetic tissue in our study contrasts with strong acclimation responses seen in longer-term studies of leaves (Heskel et al. 2016; Reich et al. 2016). However, this does not explain the acclimation seen by non-photosynthetic tissue in our study and, indeed, those studies did not report data on photosynthetic capacity (e.g. Vcmax), which may have helped to explain the respiration acclimation observed. Further studies that examine the timescale of respiratory temperature acclimation across multiple tissue types would support a more refined representation of acclimation in large-scale models.
While the b and c parameters defining the shape of the temperature response curve did not differ by species, the a parameter defining the basal rate did show species specificity. This indicates that the species in this study did not show variation in the shape of the acclimation response, but did vary in the magnitude of their respiratory rates. These results were not surprising given previous reports of wide variation in basal respiration rates among species (e.g. Reich et al. 2007; Atkin et al. 2015; Heskel et al. 2016; Smith and Dukes 2018). The goal of our study was not to determine differences across species, but rather to use multiple species to broadly examine tissue-specific acclimation responses, which was reflected in the design of our statistical models in that species by Ta interactions were not included. Nonetheless, the low number of species used here was a limitation of our study and future work should build upon these results and examine whether tissue-specific temperature acclimation varies by species or plant type.
Taken as a whole, our results support the idea that respiration processes in models need to be timescale- and tissue-dependent. While we provide the data necessary to parameterize such statistical models (https://github.com/SmithEcophysLab/tissue_respiration), we suggest that these data instead be used to develop and test more mechanistic models of plant Rd. Our results, coupled with those from previous studies mentioned above, suggest some core principles acting to drive respiration responses and acclimation to temperature. First, respiratory processes, under many conditions, are likely driven by demand for respiratory products. Respiration in plants acts to support processes such as enzyme turnover, carbohydrate export and growth (Amthor 1984) and many models, at least at the leaf scale, are already designed based on this principle and are capable of acting at timescales longer than instantaneous (i.e. they include acclimation) (Atkin et al. 2017). This mechanism could be extended to non-photosynthetic tissues, which, as we show in this study, are likely to acclimate differently than photosynthetic tissues.
Sources of Funding
N.G.S. acknowledges support from Texas Tech University. This work was supported by a NASA Earth and Space Science Fellowship (NNX13AN65H) and a Purdue Climate Change Research Center Graduate Fellowship to N.G.S. J.S.D.’s contributions were supported in part by Hatch project 1000026 of the United States Department of Agriculture’s National Institute of Food and Agriculture. G.L. acknowledges funding from the National Natural Science Foundation of China (31270564).
Contributions by the Authors
N.G.S., G.L. and J.S.D. designed the study. N.G.S. and G.L. carried out the research and performed the analyses. N.G.S., G.L. and J.S.D. wrote the manuscript.
Conflict of Interest
None declared.
Acknowledgements
We thank D. Kniola, D. Lubelski and R. Rafalski for their laboratory and greenhouse assistance.
Data Accessibility
All data and analysis scripts can be accessed at https://github.com/SmithEcophysLab/tissue_respiration (doi: 10.5281/zenodo3445384).
Literature Cited
- Amthor JS. 1984. The role of maintenance respiration in plant growth. Plant, Cell & Environment 7:561–569. [Google Scholar]
- Atkin OK, Atkinson LJ, Fisher RA, Campbell CD, Zaragoza-Castells J, Pitchford JW, Woodward F, Hurry V.. 2008. Using temperature‐dependent changes in leaf scaling relationships to quantitatively account for thermal acclimation of respiration in a coupled global climate–vegetation model. Global Change Biology 14:2709–2726. [Google Scholar]
- Atkin OK, Bahar NHA, Bloomfield KJ, Griffin KL, Heskel MA, Huntingford C, de la Torre AM, Turnbull MH.. 2017. Leaf respiration in terrestrial biosphere models. In: Tcherkez G, Ghashghaie J, eds. Plant respiration: metabolic fluxes and carbon balance Cham, Switzerland: Springer International Publishing, 107–142. [Google Scholar]
- Atkin OK, Bloomfield KJ, Reich PB, Tjoelker MG, Asner GP, Bonal D, Bönisch G, Bradford MG, Cernusak LA, Cosio EG, Creek D, Crous KY, Domingues TF, Dukes JS, Egerton JJG, Evans JR, Farquhar GD, Fyllas NM, Gauthier PPG, Gloor E, Gimeno TE, Griffin KL, Guerrieri R, Heskel MA, Huntingford C, Ishida FY, Kattge J, Lambers H, Liddell MJ, Lloyd J, Lusk CH, Martin RE, Maksimov AP, Maximov TC, Malhi Y, Medlyn BE, Meir P, Mercado LM, Mirotchnick N, Ng D, Niinemets Ü, O’Sullivan OS, Phillips OL, Poorter L, Poot P, Prentice IC, Salinas N, Rowland LM, Ryan MG, Sitch S, Slot M, Smith NG, Turnbull MH, VanderWel MC, Valladares F, Veneklaas EJ, Weerasinghe LK, Wirth C, Wright IJ, Wythers KR, Xiang J, Xiang S, Zaragoza-Castells J. 2015. Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytologist 206:614–636. [DOI] [PubMed] [Google Scholar]
- Atkin OK, Bruhn D, Hurry VM, Tjoelker MG. 2005. Evans Review No. 2: the hot and the cold: unravelling the variable response of plant respiration to temperature. Functional Plant Biology 32:87–105. [DOI] [PubMed] [Google Scholar]
- Atkin OK, Millar AH, Gardeström P, Day DA. 2000. Photosynthesis, carbohydrate metabolism and respiration in leaves of higher plants. In: Leegood RC, Sharkey TD, von Caemmerer S, eds. Photosynthesis. Dordrecht: Springer, 153–175. [Google Scholar]
- Atkin OK, Scheurwater I, Pons TL. 2007. Respiration as a percentage of daily photosynthesis in whole plants is homeostatic at moderate, but not high, growth temperatures. The New Phytologist 174:367–380. [DOI] [PubMed] [Google Scholar]
- Atkin OK, Tjoelker MG. 2003. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science 8:343–351. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67:1–48. [Google Scholar]
- Battaglia M, Beadle C, Loughhead S. 1996. Photosynthetic temperature responses of Eucalyptus globulus and Eucalyptus nitens. Tree Physiology 16:81–89. [DOI] [PubMed] [Google Scholar]
- Booth BBB, Chris DJ, Mat C, Ian JT, Peter MC, Stephen S, Chris H, Richard AB, Glen RH, Jon L.. 2012. High sensitivity of future global warming to land carbon cycle processes. Environmental Research Letters 7:24002. [Google Scholar]
- Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, Jones C, Le Quéré C, Myneni RB, Piao S, Thornton P.. 2013. Climate change 2013: the physical science basis. Contribution of working group I to the Fifth assessment report of the Intergovernmental Panel on Climate Change (V. Barros, P.M. Midgley, T.F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, eds.). Cambridge, UK and New York, NY: Cambridge University Press. [Google Scholar]
- Collatz GJ, Ball JT, Grivet C, Berry JA. 1991. Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration: a model that includes a laminar boundary layer. Agricultural and Forest Meteorology 54:107–136. [Google Scholar]
- Covey-Crump EM, Attwood RG, Atkin OK. 2002. Regulation of root respiration in two species of Plantago that differ in relative growth rate: the effect of short- and long-term changes in temperature. Plant, Cell & Environment 25:1501–1513. [Google Scholar]
- Duursma RA. 2015. Plantecophys–an R package for analysing and modelling leaf gas exchange data. PLoS One 10:e0143346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S. 2011. An {R} companion to applied regression, 2nd edn. Thousand Oaks, CA: Sage Publications, Inc. [Google Scholar]
- Gifford RM. 2003. Plant respiration in productivity models: conceptualisation, representation and issues for global terrestrial carbon-cycle research. Functional Plant Biology 30:171–186. [DOI] [PubMed] [Google Scholar]
- Gunderson CA, O’Hara KH, Campion CM, Walker A V, Edwards NT. 2010. Thermal plasticity of photosynthesis: the role of acclimation in forest responses to a warming climate. Global Change Biology 16:2272–2286. [Google Scholar]
- Heskel MA, O’Sullivan OS, Reich PB Tjoelker MG, Weerasinghe LK Penillard A Egerton JJG, Creek D, Bloomfield KJ, Xiang J, Sinca F, Stangl ZR, Martinez-de la Torre A, Griffin KL, Huntingford C, Hurry V, Meir P, Turnbull MH, Atkin OK.. 2016. Convergence in the temperature response of leaf respiration across biomes and plant functional types. Proceedings of the National Academy of Sciences of the United States of America 113:3832–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntingford C, Atkin OK, Martinez-de la Torre A, Mercado LM, Heskel MA, Harper AB, Bloomfield KJ O’Sullivan OS Reich PB Wythers KR Butler EE Chen M Griffin KL Meir P Tjoelker MG Turnbull MH Sitch S Wiltshire A Malhi Y.. 2017. Implications of improved representations of plant respiration in a changing climate. Nature Communications 8:1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvi MP, Burton AJ. 2013. Acclimation and soil moisture constrain sugar maple root respiration in experimentally warmed soil. Tree Physiology 33:949–959. [DOI] [PubMed] [Google Scholar]
- Lambers H, Szaniawski RK, de Visser R. 1983. Respiration for growth, maintenance and ion uptake. An evaluation of concepts, methods, values and their significance. Physiologia Plantarum 58:556–563. [Google Scholar]
- Lenth R. 2018. emmeans: estimated marginal means, aka least-squares means. [Google Scholar]
- Lombardozzi DL, Bonan GB, Smith NG, Dukes JS, Fisher RA. 2015. Temperature acclimation of photosynthesis and respiration: a key uncertainty in the carbon cycle‐climate feedback. Geophysical Research Letters 42:8624–8631. [Google Scholar]
- Loveys BR, Atkinson LJ, Sherlock DJ, Roberts RL, Fitter AH, Atkin OK. 2003. Thermal acclimation of leaf and root respiration: an investigation comparing inherently fast‐ and slow‐growing plant species. Global Change Biology 9:895–910. [Google Scholar]
- Maseyk K, Grünzweig JM, Rotenberg E, Yakir D. 2008. Respiration acclimation contributes to high carbon‐use efficiency in a seasonally dry pine forest. Global Change Biology 14:1553–1567. [Google Scholar]
- Oleson KW, Lawrence DM, Bonan GB, Drewniak B, Huang M, Koven CD, Levis S, Li F, Riley WJ, Subin ZM, Swenson SC, Thornton PE.. 2013. Technical description of version 4.5 of the Community Land Model (CLM). Boulder, CO: National Center for Atmospheric Research. [Google Scholar]
- O’Sullivan OS, Weerasinghe KWLK, Evans JR, Egerton JJG, Tjoelker M, Atkin OK. 2013. High-resolution temperature responses of leaf respiration in snow gum (Eucalyptus pauciflora) reveal high-temperature limits to respiratory function. Plant, Cell & Environment 36:1268–1284. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- Reich PB, Sendall KM, Stefanski A, Wei X, Rich RL, Montgomery RA. 2016. Boreal and temperate trees show strong acclimation of respiration to warming. Nature 531:633–636. [DOI] [PubMed] [Google Scholar]
- Reich PB, Wright IJ, Lusk CH. 2007. Predicting leaf physiology from simple plant and climate attributes: a global GLOPNET analysis. Ecological Applications 17:1982–1988. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH image to ImageJ: 25 years of image analysis. Nature Methods 9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot M, Kitajima K. 2015. General patterns of acclimation of leaf respiration to elevated temperatures across biomes and plant types. Oecologia 177:885–900. [DOI] [PubMed] [Google Scholar]
- Slot M, Rey-Sánchez C, Gerber S, Lichstein JW, Winter K, Kitajima K. 2014. Thermal acclimation of leaf respiration of tropical trees and lianas: response to experimental canopy warming, and consequences for tropical forest carbon balance. Global Change Biology 20:2915–2926. [DOI] [PubMed] [Google Scholar]
- Smith NG, Dukes JS. 2013. Plant respiration and photosynthesis in global-scale models: incorporating acclimation to temperature and CO2. Global Change Biology 19:45–63. [DOI] [PubMed] [Google Scholar]
- Smith NG, Dukes JS. 2017. Short-term acclimation to warmer temperatures accelerates leaf carbon exchange processes across plant types. Global Change Biology 23:4840–4853. [DOI] [PubMed] [Google Scholar]
- Smith NG, Dukes JS. 2018. Drivers of leaf carbon exchange capacity across biomes at the continental scale. Ecology 99:1610–1620. [DOI] [PubMed] [Google Scholar]
- Smith NG, Malyshev SL, Shevliakova E, Kattge J, Dukes JS. 2016. Foliar temperature acclimation reduces simulated carbon sensitivity to climate. Nature Climate Change 6:407–411. [Google Scholar]
- Tjoelker MG, Oleksyn J, Reich PB. 1999. Acclimation of respiration to temperature and CO2 in seedlings of boreal tree species in relation to plant size and relative growth rate. Global Change Biology 49:679–691. [Google Scholar]
- Turnbull MH, Murthy R, Griffin KL. 2002. The relative impacts of daytime and night-time warming on photosynthetic capacity in Populus deltoides. Plant, Cell & Environment 25:1729–1737. [Google Scholar]
- Veres JS, Williams GJ. 1984. Time course of photosynthetic temperature-acclimation in Carex eleocharis Bailey. Plant Cell and Environment 7:545–547. [Google Scholar]
- Wang H, Atkin O, Keenan T, Smith N, Wright I, Bloomfield K.. 2018. Thermal acclimation of leaf respiration consistent with optimal plant function. bioRxiv; doi:10.1101/434084. [DOI] [PubMed] [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. In: Gail M, Krickeberg K, Samet JM, Tsiatis A, Wong W, eds. New York, NY: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and analysis scripts can be accessed at https://github.com/SmithEcophysLab/tissue_respiration (doi: 10.5281/zenodo3445384).