Abstract
Conservation of marine species requires the ability to predict the effects of climate-related stressors in an uncertain future. Experiments and observations in modern settings provide crucial information, but lack temporal scale and cannot anticipate emergent effects during ongoing global change. By contrast, the deep-time fossil record contains the long-term perspective at multiple global change events that can be used, at a broad scale, to test hypothesized effects of climate-related stressors. For example, geologically rapid carbon cycle disruption has often caused crises in reef ecosystems, and selective extinctions support the hypothesis that greater activity levels promote survival. Geographical patterns of extinction and extirpation were more variable than predicted from modern physiology, with tropical and temperate extinction peaks observed at different ancient events. Like any data source, the deep-time record has limitations but also provides opportunities that complement the limitations of modern and historical data. In particular, the deep-time record is the best source of information on actual outcomes of climate-related stressors in natural settings and over evolutionary timescales. Closer integration of modern and deep-time evidence can expand the types of hypotheses testable with the fossil record, yielding better predictions of extinction risk as climate-related stressors continue to intensify in future oceans.
This article is part of a discussion meeting issue ‘The past is a foreign country: how much can the fossil record actually inform conservation?’
Keywords: climate change, mass extinctions, ocean acidification, palaeontology, conservation palaeobiology
1. Introduction
Terrestrial and marine ecosystems are in the midst of an extinction crisis [1]. Human activities have caused and continue to cause population declines through overharvesting, habitat degradation and fragmentation, and introduction of invasive species. Anthropogenic carbon dioxide emissions also trigger wide-ranging environmental disruption (often called ‘global change’) that increases extinction risk for species on land and in the ocean. In marine ecosystems, these climate-related stressors of warming, ocean deoxygenation and ocean acidification will continue to intensify in the coming decades, also elevating the extinction risk for vulnerable organisms.
To protect species from extinction, it is crucial to identify organisms that are currently at risk or may be at risk in the future. This is an extremely challenging goal, given multiple and potentially synergistic stressors and the need to extrapolate into an uncertain future. Unsurprisingly, given the urgency of climate change and ocean acidification as two of the factors that might increase extinction risk in the oceans, a tremendous amount of research seeks to constrain the effects of climate-related stressors on marine organisms. This research brings together a range of methods: experimental manipulations in the laboratory and with mesocosms (e.g. [2,3]), observations of ‘natural laboratories' such as low-pH vent sites (e.g. [4,5]), and analysis of large databases (e.g. [6,7]). Although climate-related stressors are not the only causes of increased extinction risk, they could be significant contributors which must, therefore, be considered when assessing conservation needs.
Experimental and observational studies of modern species provide valuable insights into the effects of climate-related stressors on marine organisms, but also have limitations. The scale of the problem is especially challenging: marine global change involves multiple stressors acting on hundreds of thousands of species, throughout the entire ocean and over decades to centuries of evolutionary time (figure 1). Experimental manipulations, and even natural laboratories, can only provide information at small spatial and temporal scales, and in many cases in controlled but artificial conditions [3,8]. Outcomes may depend on the rate of change [9,10], making extrapolations to longer timescales more difficult. Analyses of large databases can assess many species at global spatial scales, but studies using data only from the modern and historical record will rarely be able to evaluate changes over evolutionary timescales.
Figure 1.
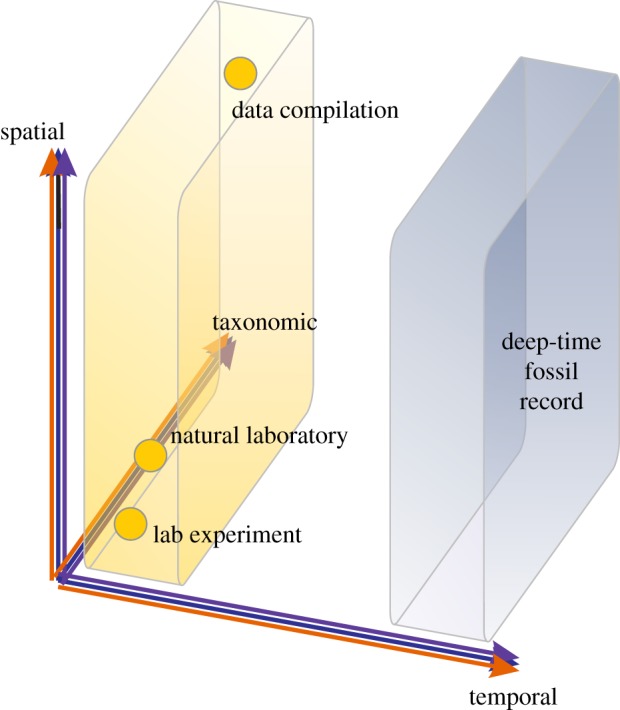
The phenomenon of global change involves multiple climate-related stressors (depicted schematically by multiple arrows for each axis) that operate over a range of spatial, temporal and taxonomic scales. Investigations that use the modern and historical record (yellow box) are mostly conducted across spatial and taxonomic scales but are fundamentally limited in temporal scale. The deep-time fossil record (blue box) reveals actual outcomes over evolutionary timescales, mostly at broad spatial and taxonomic scales. Together, these methods provide complementary information on the biotic response to climate-related stressors.
The deep-time fossil record (pre-Quaternary, or older than about 2.5 Myr) can fill that gap by providing unparalleled scale for evaluating biological impacts of climate-related stressors. Focusing on the marine record, there have been multiple ‘natural experiments’ of geologically rapid global change, with different rates and magnitudes, over the past 300 Myr [11] (figure 2). Some were catastrophic mass extinctions while others had only minimal taxonomic losses. They capture a spectrum of climate-related stressors, from ocean anoxic events to hyperthermals with abrupt ocean warming and acidification. That spectrum of events can test the importance of traits under different climate scenarios and can reveal general principles that apply across a range of perturbations. Although the nature of the deep-time fossil record also limits some inferences, and events in deep time are not exact analogues to modern ecosystems or environmental change, Earth history is the only way to assess the biological impacts of climate-related stressors across myriad taxa, in natural ecosystems, and over evolutionary timescales.
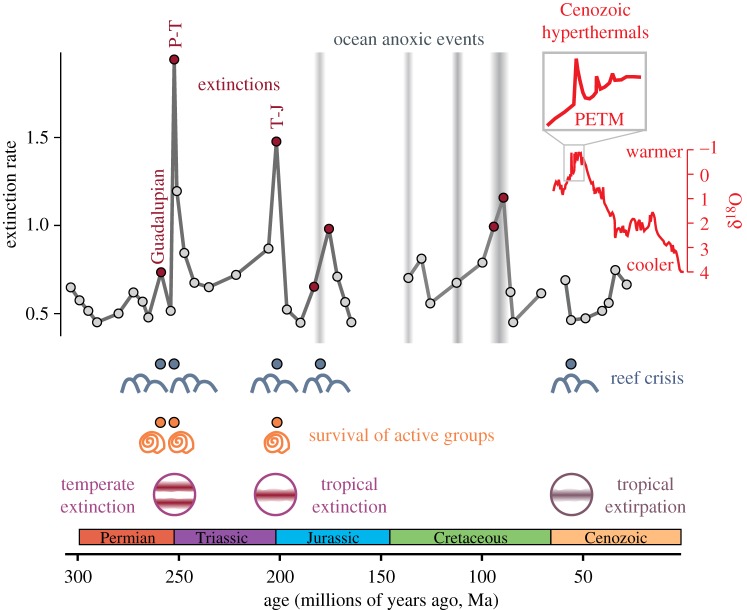
Figure 2.
Global change events in Earth's deep-time history, caused by carbon cycle disruption, ocean warming and deoxygenation, and in some cases acidification. Geologically rapid perturbations of the carbon cycle caused extinction events (red circles), including the Guadalupian, Permian–Triassic (P-T) and Triassic–Jurassic (T-J). Extinction rates are for marine invertebrates, from the Paleobiology Database. Carbon release also led to ocean anoxic events when the duration was more prolonged, especially in the Jurassic and Cretaceous periods, and Cenozoic hyperthermals such as the Palaeocene-Eocene Thermal Maximum (PETM) when the rate of carbon release was more rapid. Climate-related stressors caused reef crises when rates of global change were more rapid, while climate-driven extinctions in the Permian and Triassic resulted in selective survival of more active groups. The T-J extinction and PETM led to selective extinction or extirpation in the tropics, but taxonomic losses were greater at temperate latitudes during P-T extinction.
Evidence from the deep-time record has been incorporated into conservation biology in limited areas, most notably in reef conservation [12,13], but there is unrealized potential for wider use. Quaternary fossil records can provide baseline conditions for natural ecosystems, but both the Quaternary and deep-time records have uses beyond setting pre-anthropogenic baselines [14,15]. Palaeontological studies have included exploratory analyses of extinction selectivity that investigated a variety of traits: feeding, motility and other life-habit attributes; geographical range, palaeolatitudinal distribution and habitat preference; and physiological traits such as buffering of calcification [16,17]. Other studies have quantified selective taxonomic losses that potentially reflect physiological differences, but with the primary goal of evaluating potential environmental ‘kill mechanisms’ during extinctions [18]. These investigations contribute significantly to reconstructions of environmental and biotic change during mass extinctions, but I suggest that the deep-time fossil record is best placed to contribute to conservation biology through more targeted testing of hypotheses generated from experiments or observations in modern settings.
Not all hypotheses can be tested with the deep-time fossil record, however. To take advantage of the fossil record, testable hypotheses must make predictions that can be evaluated at broader temporal and spatial resolution and with extinct organisms that might be only distantly related to extant species. Functional trait-based hypotheses have been promising [19], especially for traits that can be inferred from shell morphology and for traits that can be generalized at higher taxonomic levels (using taxonomy as a proxy for suites of traits that are not or not-easily measurable in fossils). Hypotheses that make predictions, for example, about geographical patterns of risk or about expectations for range shifts, may also be well-suited for testing at deep-time global change events.
2. Case studies: testing functional trait predictions
In today's oceans, reef-building corals are thought to be among the organisms most vulnerable to warming and acidification [20]. This assessment stems in part from the observation that corals are already suffering during bleaching events when ocean heat waves cause widespread mortality [21]. The vulnerability of reef-building corals likely results from their sensitive functional, especially physiological, traits, such as the delicate symbiosis with photosynthetic algae and the rapid calcification of large skeletons. However, although concern over the future of corals and coral reef-building is warranted, there is continuing uncertainty because some corals may have potential for evolutionary adaptation, may respond to future multistressor global change in ways that cannot be predicted from current responses, or may be rescued by more resilient species or populations [12].
The deep-time fossil record has demonstrated that reef ecosystems, and the corals or hypercalcified sponges that built them, are especially vulnerable to global environmental change. Although only one event, the Permian–Triassic extinction 250 Ma, caused total extinction of corals, reef-building organisms typically suffered disproportionate losses during hyperthermal extinctions [22] (figure 2). Even when taxonomic losses were minimal, such as during the Palaeocene-Eocene Thermal Maximum (PETM), the rapid environmental change resulted in decreased reef volume [22]. Although assessing rates over short timescales is challenging in deep time [23], reef crises appear to have been more severe at hyperthermals with more rapid carbon cycle perturbations (Permian–Triassic and Triassic–Jurassic extinctions, PETM) than at those with more protracted warming (Cretaceous ocean anoxic events) [22]. It appears that evolutionary potential, rescue by more resilient populations, and other mechanisms were not sufficient to prevent major reef declines and sometimes widespread extinction during deep-time hyperthermals. These outcomes lend support to some of the more pessimistic projections for future reefs and create more urgency for minimizing the rate of ocean warming and acidification and limiting non-climate stressors to reef ecosystems.
In the younger parts of the deep-time record, a greater proportion of fossil taxa belong to still-living genera or families, which enables more nuanced functional inferences even though most or all fossil species are now extinct. Among corals, this can allow feeding mode, the presence of symbiosis, and even reproductive strategy to be inferred in 50 million-year-old species from their living relatives, testing the importance of those functional traits during time periods that included the PETM and other hyperthermal events [24]. During those time periods, fossil coral species with a broader range of feeding options and mixed reliance on photosymbiosis tended to be more likely to survive, a pattern of selectivity that differed from background extinctions in other time periods [24]. Studies of ancient hyperthermals can complement the focused investigations of extant corals, providing a valuable perspective on actual outcomes during environmental stress over long timescales.
Functional traits are likely important predictors of survival not just among corals, but among all marine organisms. For example, more active organisms might have physiological traits and mechanisms that enable them to cope with elevated CO2 levels [25], and may also be less vulnerable to ocean warming [9]. The underlying physiological traits that cause these different responses to CO2 or temperature stress may be impossible to measure in fossils, but Peck et al. [9] developed a motility- and feeding-based activity quotient that can be generalized even to extinct species. Using this quotient, there was no clear relationship between activity levels and extinction risk over a 150 Myr interval from the Permian to Jurassic, except at three extinctions (Guadalupian, Permian–Triassic and Triassic–Jurassic) when higher levels of activity promoted survival among benthic marine invertebrates [26] (figure 2). However, the relationship between activity and extinction was less clear when including marine vertebrates, perhaps because of vulnerability at different life stages. Sharks preferentially survived the Permian–Triassic and Triassic–Jurassic mass extinctions relative to invertebrates, but bony fishes and invertebrates had similarly elevated extinction rates at the Triassic–Jurassic [27]. These findings suggest that the relevance of activity level, in a broad sense, operates both over the short timescales of experimental work [9,25] and over evolutionary timescales in ancient hyperthermal extinctions.
Although the deep-time record enables testing of hypothesized links between functional traits and extinction risk during global change, there are limitations to the conclusions, just as there are also limitations in controlled laboratory experiments, observations from modern ecosystems, or any type of study. One limitation is that functional traits are often inferred from taxonomic relationships, and at broad taxonomic levels, rather than being directly measurable. For example, activity levels were categorized mostly at the level of taxonomic order or even class, assigning the same value to all epifaunal brachiopods [26] even though there undoubtedly were physiological differences among brachiopod species. Analyses of the deep-time record are also typically performed with data binned into time intervals that are several million years long. This binning is not a major issue when extinction rates are high, because the signal from the event will outweigh background selectivity, but it can be potentially difficult to attribute selectivity to environmental stresses when extinction rates are similar to background levels. As in all studies, the limitations must be considered when applying interpretations in a different context and translating deep-time findings to the modern ocean, but the scale of the record provides complementary strengths that can benefit conservation studies.
3. Case studies: testing geographic predictions
In contrast with functional traits, which must be inferred from morphology or extrapolated from living relatives, the palaeogeographic location of fossil occurrences can be observed directly. This enables testing of hypotheses that use geographical distribution to predict vulnerability to climate-related stressors. For example, are organisms that inhabit the tropics at greater risk of extinction from climate-related stressors [28]? In modern oceans, many tropical organisms have only a small buffer between maximum environmental temperatures and their physiological thermal limits [10,28]. Perhaps physiological plasticity is instead important for survival during global change [29]. If that is the case, could organisms inhabiting more variable habitats, at temperate latitudes for example, be less vulnerable?
These hypotheses are challenging to test solely with modern data, but events in deep time provide natural rates and patterns of global change that can be used to investigate the responses of organisms over evolutionary timescales. At the Triassic–Jurassic extinction, marine invertebrates with a tropical preference were more likely to go extinct [30], providing support for the hypothesis of greater risk in the tropics (figure 2). However, extinction rates were lower in the tropics than at temperate latitudes during the Permian–Triassic extinction, suggesting more complex spatial patterns from multiple stressors, such as the combination of elevated temperature and reduced oxygen [31]. Overall, the relationship in deep time between climate-driven stress and the geographical pattern of extinction is complicated and variable [32].
The combination of palaeogeographic data with general circulation models or Earth-system models can provide powerful tools for testing hypothesized effects of climate-related stressors. Taxonomic losses during the Permian–Triassic extinction may have been more severe at latitudes where the combined effects of warming and deoxygenation were most severe, consistent with the importance of metabolic oxygen supply and demand during global change events [31]. During the PETM, calcareous nannoplankton disappeared from the tropics and became restricted to higher latitudes with lower carbonate saturation state, suggesting that temperature rather than ocean acidification was a key control on their distribution [33].
However, several factors complicate the interpretation of deep-time geographical distribution data. For one, global change events in deep time are represented by fossiliferous rocks preserved and exposed at some geographical locations but missing from others. Ancient terrestrial environments typically have sparser geographical records while rocks from ancient marine environments tend to be geographically more widespread because marine sedimentary basins tend to be deeper, longer-lived and less susceptible to later erosional destruction [34]. As a result, reconstructions of geographical distribution must be done with care, especially when attempting to infer absences, and even more so when inferring the absence of organisms that would have been rare.
The interpretation of geographical extinction patterns can also be confounded by non-random distribution and/or sampling of taxonomic groups that had different extinction rates. For example, corals tended to be especially vulnerable to climate-related stressors and also had predominantly tropical distributions in the past, as today. On the other hand, fossil ostracods had comparatively low extinction rates during the Permian–Triassic crisis, and are most often collected by dissolving limestone rocks that predominantly form in the tropics; as a result, the vast majority of Permian–Triassic ostracods are also known from the ancient tropics. Although geographical patterns of extinction may not be as clear after disentangling taxonomic selectivity, consistent with complex impacts of climate-related stressors, the deep-time record has tremendous potential.
4. Applicability of the deep-time record
The case studies demonstrate situations where the deep-time record can reveal the biological consequences of climate-related stressors, but how can that be applied to answer conservation questions? Can the deep-time fossil record help guide applied species or ecosystem management decisions? Or can it help inform restoration strategies and best practices for conservation interventions? In these areas, the deep-time record is likely of little relevance. The deep-time record also cannot document human impacts on ecosystems or assess the societal consequences of biodiversity loss. However, I argue that the deep-time fossil record can nevertheless provide important guidance for conservation biology. There are several hundred thousand marine species [35], but only a tiny fraction of those species have had their conservation status formally assessed. Similarly, although experiments have documented the risks to selected marine species from warming or acidification, it will be impractical to perform multistressor experiments at the scale needed to evaluate all species. As a result, the vulnerability of the vast majority of marine species remains only loosely constrained, especially the vulnerability to climate-related stressors.
As demonstrated by the case studies, the deep-time fossil record is best positioned to refine assessments of vulnerability to climate-related stressors at broad scales. But predictions of extinction risk are not a simple binary (either known or unknown); instead, our assessments occupy a spectrum of varying degrees of confidence from complete ignorance to complete certainty. Experimental or observational studies in modern settings have the potential to substantially increase the certainty of extinction risk predictions, but only for a few species. Conversely, the enormous scope of the deep-time record can increase the certainty of extinction risk predictions for a huge number of species, likely providing more incremental knowledge gains than possible from controlled experiments. Nevertheless, even incremental improvements in certainty are valuable, especially when helping to refine the extinction risk of unstudied or understudied groups.
Applied conservation decisions require that scientific knowledge be translated into actionable tasks, which often can be challenging to synthesize from basic research. However, this challenge applies not only to evidence from the deep-time fossil record but also to many experimental or observational studies on modern organisms. Nonetheless, these basic research studies provide crucial guidance for understanding the fundamental mechanisms that govern vulnerability to climate-related stressors. Evidence from the deep-time record, or from experiments or observations, will not always translate to specific actions, but decision-making is ultimately strengthened by incorporating diverse evidence from sources with different strengths.
Many marine species lack data on their conservation status, but forecasting future risk is even more difficult given the complexity of environmental and biological systems. Are there emergent behaviours, which cannot be extrapolated from experiments or historical observations, over evolutionary timescales or as conditions change beyond thresholds? Experimental or observational studies can propose hypotheses but are not, on their own, conclusive predictions of the future. The deep-time record, because it contains the actual responses to climate-related stressors over evolutionary timescales, is the best and perhaps the only way to approach these questions. Did a taxonomic group that is hypothesized to be vulnerable actually suffer greater extinction in the past? Was a functional trait that promoted survival in an experiment actually a significant predictor of survival during real global change events? Patterns of taxonomic, functional or geographical selectivity that occurred consistently at multiple deep-time global change events are likely to represent important and general processes governing vulnerability to climate-related stressors. These patterns can also yield insights that would be unavailable from modern evidence alone. For example, the deep-time fossil record strongly implies that such responses are not likely to be adequate to save reef ecosystems over the short term, given continued environmental change at current rates, although reefs will recover over geological timescales. Time is the most significant limitation of studies of the extant fauna, but the long-term perspective of the deep-time record, its greatest strength, fills that gap and provides complementary evidence to investigate the response to climate-related stressors.
5. Conclusion: toward a closer integration of modern and deep-time evidence
Deep-time natural experiments are best placed to test hypotheses over evolutionary timescales. For example, do findings from experiments or observations actually apply over realistic scales of global change? Ancient global change events can be used to test the importance of functional traits, for example supporting predictions that more active organisms are generally less vulnerable to climate-related stressors. The deep-time record is also well-placed to test geographical controls, for example indicating that the relationship between geographical distribution and extinction risk is more complicated than predicted from experiments on modern taxa. Deep-time studies provide the big-picture view, documenting outcomes across multiple taxonomic or functional groups and potentially across multiple events. This perspective can help reveal the general principles underlying vulnerability of marine organisms to climate-related stressors.
But what can be done to expand the utility of the deep-time record and increase the applicability of its findings? Progress toward this objective will be maximized if both biologists and palaeobiologists think about approaches that bridge the temporal and spatial scales between experimental studies and deep-time data.
One goal might be to increase the number of hypotheses that are testable with deep-time data. This will require greater willingness on the part of biologists to make predictions that can be generalized to the broader taxonomic, functional or geographical scales available in deep time. Experimental and observational studies typically ask highly focused questions, and there may be a reluctance to generalize their outcomes because responses can be species-specific, contingent on the combination of stressors, or otherwise nuanced. While those concerns are valid, it is still valuable to synthesize the results of individual studies to generate more broadly applicable and testable predictions. Average differences in the predicted vulnerability of different taxonomic, functional or geographical groups, even if there is variability among responses within each group, can be tested thanks to the vast scope of the deep-time record.
A parallel goal might be to increase the ability of the deep-time fossil record to test hypotheses. This will require palaeobiologists to think creatively about methods for testing hypotheses, especially ways to bridge the gap in scales between high-resolution but short-timescale modern data and long-timescale but coarse-resolution fossil data. For example, continued integration of multiple types of information—palaeobiological, modelling, isotopic and others—will expand the types of questions that can be answered in deep time. Other techniques, such as sclerochronology (the sampling of shell growth increments for geochemical and biological information), can provide a high-resolution archive for testing hypotheses at timescales comparable to some studies of modern organisms. These and other methods can help translate deep-time data to modern settings and questions.
Experiments, observations, historical data, the Quaternary fossil record and deep-time events all provide pieces of information to inform conservation questions. Each method has different strengths and limitations—the deep-time record is not unique in that respect—and some types of data are better suited to particular types of questions. Deep time allows us to ask and answer the question ‘what actually happened when organisms were faced with climate-related stressors?’ The combination of this unique and powerful ability with the precision and nuance of modern studies can open exciting possibilities for conservation biology. A core principle of geology is uniformitarianism (the present is the key to understanding the past), but in a time of rapid environmental disruptions that are unprecedented in the historical record, Earth's deep-time past may actually be the key to understanding our future [36].
Acknowledgements
I thank the contributors to the Paleobiology Database. This is Paleobiology Database publication no. 348.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Barnosky AD, et al. 2011. Has the Earth's sixth mass extinction already arrived? Nature 471, 51–57. ( 10.1038/nature09678) [DOI] [PubMed] [Google Scholar]
- 2.Collard M, Dery A, Dehairs F, Dubois P. 2014. Euechinoidea and Cidaroidea respond differently to ocean acidification. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 174, 45–55. ( 10.1016/j.cbpa.2014.04.011) [DOI] [PubMed] [Google Scholar]
- 3.Queirós AM, et al. 2015. Scaling up experimental ocean acidification and warming research: from individuals to the ecosystem. Glob. Change Biol. 21, 130–143. ( 10.1111/gcb.12675) [DOI] [PubMed] [Google Scholar]
- 4.Crook ED, Cohen AL, Rebolledo-Vieyra M, Hernandez L, Paytan A. 2013. Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. Proc. Natl Acad. Sci. USA 110, 11 044–11 049. ( 10.1073/pnas.1301589110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroeker KJ, Micheli F, Gambi MC, Martz TR. 2011. Divergent ecosystem responses within a benthic marine community to ocean acidification. Proc. Natl Acad. Sci. USA 108, 14 515–14 520. ( 10.1073/pnas.1107789108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sunday JM, Bates AE, Dulvy NK. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830. ( 10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stuart-Smith RD, Edgar GJ, Bates AE. 2017. Thermal limits to the geographic distributions of shallow-water marine species. Nat. Ecol. Evol. 1, 1846–1852. ( 10.1038/s41559-017-0353-x) [DOI] [PubMed] [Google Scholar]
- 8.De Boeck HJ, et al. 2015. Global change experiments: challenges and opportunities. BioScience 65, 922–931. ( 10.1093/biosci/biv099) [DOI] [Google Scholar]
- 9.Peck LS, Clark MS, Morley SA, Massey A, Rossetti H. 2009. Animal temperature limits and ecological relevance: effects of size, activity and rates of change. Funct. Ecol. 23, 248–256. ( 10.1111/j.1365-2435.2008.01537.x) [DOI] [Google Scholar]
- 10.Nguyen KDT, Morley SA, Lai C-H, Clark MS, Tan KS, Bates AE, Peck LS. 2011. Upper temperature limits of tropical marine ectotherms: global warming implications. PLoS ONE 6, e29340 ( 10.1371/journal.pone.0029340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clapham ME, Renne PR. 2019. Flood basalts and mass extinctions. Ann. Rev. Earth Planet. Sci. 47, 275–303. ( 10.1146/annurev-earth-053018-060136) [DOI] [Google Scholar]
- 12.Pandolfi JM. 2015. Incorporating uncertainty in predicting the future response of coral reefs to climate change. Ann. Rev. Ecol. Evol. Syst. 46, 281–303. ( 10.1146/annurev-ecolsys-120213-091811) [DOI] [Google Scholar]
- 13.Pandolfi JM, Kiessling W. 2014. Gaining insights from past reefs to inform understanding of coral reef response to global climate change. Curr. Opin. Environ. Sustain. 7, 52–58. ( 10.1016/j.cosust.2013.11.020) [DOI] [Google Scholar]
- 14.Willis KJ, Birks HJB. 2006. What is natural? The need for a long-term perspective in biodiversity conservation. Science 314, 1261–1265. ( 10.1126/science.1122667) [DOI] [PubMed] [Google Scholar]
- 15.Dietl GP, Flessa KW. 2011. Conservation paleobiology: putting the dead to work. Trends Ecol. Evol. 26, 30–37. ( 10.1016/j.tree.2010.09.010) [DOI] [PubMed] [Google Scholar]
- 16.Clapham ME, Payne JL. 2011. Acidification, anoxia, and extinction: a multiple logistic regression analysis of extinction selectivity during the Middle and Late Permian. Geology 39, 1059–1062. ( 10.1130/G32230.1) [DOI] [Google Scholar]
- 17.Dunhill AM, Foster WJ, Azaele S, Sciberras J, Twitchett RJ. 2018. Modelling determinants of extinction across two Mesozoic hyperthermal events. Proc. R. Soc. B 285, 20180404 ( 10.1098/rspb.2018.0404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knoll AH, Bambach RK, Payne JL, Pruss S, Fischer WW. 2007. Paleophysiology and end-Permian mass extinction. Earth Planet. Sci. Lett. 256, 295–313. ( 10.1016/j.epsl.2007.02.018) [DOI] [Google Scholar]
- 19.Polly PD, et al. 2011. History matters: ecometrics and integrative climate change biology. Proc. R. Soc. B 278, 1131–1140. ( 10.1098/rspb.2010.2233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL. 2011. Projecting coral reef futures under global warming and ocean acidification. Science 333, 418–422. ( 10.1126/science.1204794) [DOI] [PubMed] [Google Scholar]
- 21.Baker AC, Glynn PW, Riegl B. 2008. Climate change and coral reef bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuarine Coast. Shelf Sci. 80, 435–471. ( 10.1016/j.ecss.2008.09.003) [DOI] [Google Scholar]
- 22.Kiessling W, Simpson C. 2011. On the potential for ocean acidification to be a general cause of ancient reef crises. Glob. Change Biol. 17, 56–67. ( 10.1111/j.1365-2486.2010.02204.x) [DOI] [Google Scholar]
- 23.Kemp DB, Eichenseer K, Kiessling W. 2015. Maximum rates of climate change are systematically underestimated in the geological record. Nat. Commun. 6, 9890 ( 10.1038/ncomms9890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss AM, Martindale RC. 2019. Paleobiological traits that determined scleractinian coral survival and proliferation during the late Paleocene and early Eocene hyperthermals. Paleoceanogr. Paleoclim. 34, 252–274. ( 10.1029/2018PA003398) [DOI] [Google Scholar]
- 25.Melzner F, Gutowska MA, Langenbuch M, Dupont S, Lucassen M, Thorndyke MC, Bleich M. 2009. Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6, 2313–2331. ( 10.5194/bg-6-2313-2009) [DOI] [Google Scholar]
- 26.Clapham ME. 2017. Organism activity levels predict marine invertebrate survival during ancient global change extinctions. Glob. Change Biol. 23, 1477–1485. ( 10.1111/gcb.13484) [DOI] [PubMed] [Google Scholar]
- 27.Vázquez P, Clapham ME. 2017. Extinction selectivity among marine fishes during multistressor global change in the end-Permian and end-Triassic crises. Geology 45, 395–398. ( 10.1130/G38531.1) [DOI] [Google Scholar]
- 28.Vinagre C, Leal I, Mendonça V, Madeira D, Narciso L, Diniz MS, Flores AAV. 2016. Vulnerability to climate warming and acclimation capacity of tropical and temperate coastal organisms. Ecol. Indic. 62, 317–327. ( 10.1016/j.ecolind.2015.11.010) [DOI] [Google Scholar]
- 29.Hofmann GE, Todgham AE. 2010. Living in the now: physiological mechanisms to tolerate a rapidly changing environment. Annu. Rev. Physiol. 72, 127–145. ( 10.1146/annurev-physiol-021909-135900) [DOI] [PubMed] [Google Scholar]
- 30.Kiessling W, Aberhan M, Brenneis B, Wagner PJ. 2007. Extinction trajectories of benthic organisms across the Triassic–Jurassic boundary. Palaeogeogr. Palaeoclimatol. Palaeoecol. 244, 201–222. ( 10.1016/j.palaeo.2006.06.029) [DOI] [Google Scholar]
- 31.Penn JL, Deutsch C, Payne JL, Sperling EA. 2018. Temperature-dependent hypoxia explains biogeography and severity of end-Permian marine mass extinction. Science 362, eaat1327 ( 10.1126/science.aat1327) [DOI] [PubMed] [Google Scholar]
- 32.Reddin CJ, Kocsis ÁT, Kiessling W. 2019. Climate change and the latitudinal selectivity of ancient marine extinctions. Paleobiology 41, 633–639. ( 10.1017/pab.2018.34) [DOI] [Google Scholar]
- 33.Gibbs SJ, Bown PR, Ridgwell A, Young JR, Poulton AJ, O'Dea SA. 2016. Ocean warming, not acidification, controlled coccolithophore response during past greenhouse climate change. Geology 44, 59–62. ( 10.1130/G37273.1) [DOI] [Google Scholar]
- 34.Holland SM. 2016. The non-uniformity of fossil preservation. Phil. Trans. R. Soc. B 371, 20150130 ( 10.1098/rstb.2015.0130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costello MJ, Chaudhary C. 2017. Marine biodiversity, biogeography, deep-sea gradients, and conservation. Curr. Biol. 27, R511–R527. ( 10.1016/j.cub.2017.04.060) [DOI] [PubMed] [Google Scholar]
- 36.Doe BR. 1983. The past is the key to the future. Geochim. Cosmochim. Acta 47, 1341–1354. ( 10.1016/0016-7037(83)90293-4) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.