Summary:
Innate lymphoid cells (ILCs) promote tissue homeostasis and immune defense but also contribute to inflammatory diseases. ILCs exhibit phenotypic and functional plasticity in response to environmental stimuli, yet the transcriptional regulatory networks that control ILC function are largely unknown. Here we integrate gene expression and chromatin accessibility data to infer regulatory interactions between transcription factors (TFs) and genes within intestinal type 1, 2, and 3 ILC subsets. We predicted the “core” TFs driving ILC identities, organized TFs into cooperative modules controlling distinct gene programs, and validated roles for c-MAF and BCL6 as regulators affecting type 1 and type 3 ILC lineages. The ILC network revealed alternative-lineage-gene repression, a mechanism that may contribute to reported plasticity between ILC subsets. By connecting TFs to genes, the TRNs suggest means to selectively regulate ILC effector functions, while our network approach is broadly applicable to identifying regulators in other in vivo cell populations.
Keywords: Gene regulation, ATAC-seq, lineage commitment, Inferelator, lymphocyte development
eTOC blurb:
Innate lymphoid cells contribute to tissue homeostasis and defense against pathogens, processes that require coordinated transcription of thousands of genes. Pokrovskii and colleagues construct and experimentally validate a genome-scale transcriptional regulatory network describing how intestinal ILC gene expression programs and their plasticity are controlled by transcription factors.
Introduction:
Innate lymphoid cells (ILCs) regulate critical aspects of tissue homeostasis, inflammation, and repair (Cherrier et al., 2012; Eberl et al., 2015; van de Pavert and Vivier, 2015; Sonnenberg and Artis, 2015; Spits and Cupedo, 2012; Walker et al., 2013). As the innate counterparts of adaptive T lymphocytes, ILCs lack somatically rearranged antigen receptors and respond rapidly upon encountering specific metabolites or cytokines (Tait Wojno and Artis, 2016; Vivier et al., 2018). ILCs are enriched at mucosal and barrier surfaces, poised to coordinate tissue immunity and homeostasis (Tait Wojno and Artis, 2012).
Although recent studies highlight ILC heterogeneity (Björklund et al., 2016; Gury-BenAri et al., 2016), ILCs can be broadly classified into three principal groups based on signature effector cytokines, functional characteristics, and dependence on lineage-determining transcription factor (TF) expression (Eberl et al., 2015; Spits et al., 2013; Walker et al., 2013). Type 1 ILCs include ILC1 and natural killer (NK) cells that produce interferon gamma (IFNγ) and require the TF T-bet (Tbx21). NK cells are unique in their potent cytolytic function and dependence on eomesodermin (Eomes) (Gordon et al., 2012; Pikovskaya et al., 2016). Type 2 ILCs (ILC2s) require GATA3 and secrete the cytokines interleukin-4 (IL-4), IL-5 and IL-13. ILC3 comprise a heterogeneous group of related cells defined by their dependence on RORγt and production of IL-22 and IL-17 cytokines. During development, the T-helper-like ILCs (ILC1, ILC2, and a subset of ILC3) arise from a common progenitor that diverges from NK cell and lymphoid tissue inducer (LTi) cell progenitors (Constantinides et al., 2014; Klose et al., 2014). Unlike NK cells, these ILCs are dependent on receptor for the alpha chain of IL-7 (IL7Rα). While some of the TFs driving early lineage commitment of ILCs have been discovered (Constantinides et al., 2014, 2015; Fang and Zhu, 2017; Lim et al., 2017; Serafini et al., 2015; Yu et al., 2013), the transcriptional mechanisms that maintain ILC identity and function are less well studied.
An emerging property of ILCs is their apparent functional and phenotypic flexibility in response to changing tissue environments (Lim et al., 2016, 2017; Melo-Gonzalez and Hepworth, 2017; Ohne et al., 2016). For example, ILC1 and ILC3 exist on a continuum marked at its extremes by the exclusive expression of lineage-specifying factors T-bet or RORγt, while intermediate populations express both TFs (Bernink et al., 2015; Klose et al., 2013; Verrier et al., 2016; Vonarbourg et al., 2010). Their interconversion can result in the accumulation of pro-inflammatory IFNγ-producing “ex-ILC3” that drive intestinal inflammation (Bernink et al., 2013; Vonarbourg et al., 2010). This property mirrors the relationship between Th1 and Th17 cells, with conversion of RORγt+ IL-17A-expressing cells into T-bet+ IFNγ-producing Th1-like “pathogenic Th17 cells” in the presence of IL-23 (Hirota et al., 2011). Plasticity between ILC1 and ILC2 as well as ILC1 and NK cells has also been reported (Lim et al., 2017). These findings highlight the need to better understand the molecular mechanisms governing ILC functional plasticity.
Recent studies described epigenetic and transcriptional signatures for ILC subsets and suggested subset-specific TF regulators based on TF-motif analysis of differentially accessible chromatin regions (Gury-BenAri et al., 2016; Koues et al., 2016; Robinette et al., 2015; Shih et al., 2016). However, the TF regulators of individual genes in each ILC subset remain largely unknown. These regulatory relationships are the building blocks of larger gene programs driving ILC function and require broad, unbiased and systematic elucidation.
Transcriptional regulatory networks (TRNs) describe the regulatory interactions between TFs and their target genes. TRN construction is especially challenging in relatively rare ex vivo cell types like ILCs, because many transcriptional regulators are unknown, and it is technically difficult to obtain direct TF occupancy data for those that are. In this context, integration of chromatin accessibility with TF motif databases provides a means to identify putative regulatory regions and the candidate TFs binding to those regions (Pique-Regi et al., 2011). We recently developed a version of the Inferelator algorithm that integrates TF motif analysis of chromatin accessibility and gene expression data to infer TRNs (Miraldi et al., 2019). Importantly, we showed that this method, applied to accessibility and gene expression data alone, can recapitulate a “gold standard” network (Ciofani et al., 2012; Yosef et al., 2013) that was built through the more laborious approach of TF chromatin immunoprecipitation and TF perturbation RNA-seq in related Th17 cells (Miraldi et al., 2019).
In this study, we applied the Inferelator to construct TRNs for five intestinal ILC subsets (CCR6+ ILC3, CCR6− ILC3, ILC1, NK cells, and ILC2) by leveraging published ILC genomics studies and our own intestinal ILC datasets. This unbiased approach recovered the few known TF regulators of subset-specific genes and predicted roles for nearly a hundred additional TFs in ILC regulation. Clustering of TFs, based on shared regulatory interactions, revealed core modules: groups of TFs that cooperatively regulated distinct functional pathways. The combined intestinal ILC network not only identified activators of genes within each subset but also repressors of genes corresponding to alternative ILC lineages. We experimentally validated TFs predicted to repress alternative-lineage genes. These proof-of-concept validation experiments highlight how TRNs can be used to elucidate control points of cell identity and plasticity in mature intestinal ILCs.
Results
Innate lymphoid cell chromatin accessibility landscapes suggest activating and repressive regulatory elements
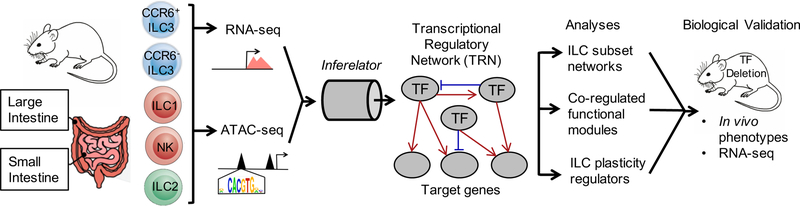
To construct intestinal ILC transcriptional regulatory networks (TRNs), we globally identified putative regulatory elements in small (SI) and large intestine (LI) ILCs by the assay for transposase-accessible chromatin using sequencing (ATAC-seq) (Buenrostro et al., 2013). In parallel, we assessed gene expression by RNA-seq (Figure 1). Five ILC subsets were sort-purified from C57BL/6N mice using surface markers that discriminated lineage-restricted TF expression (Figure S1). Canonical NK cells were negative for IL7rα and other lineage markers but expressed NK1.1, while ILCs stained positive for IL7rα and expressed either KLRG1 and GATA3 (ILC2) or KLRB1B (ILC1-to-LC3 continuum). (Klrb1d encodes Klrb1b in B6 mice.) Expression of NK1.1 and CCR6 among KLRB1B+ cells further discriminated T-bet positive ILC1 from two ILC3 subpopulations that either expressed RORγt alone (CCR6+, which includes LTi cells) or co-expressed RORγt and T-bet (CCR6−).
Figure 1. Study design for generation and biological validation of ILC TRN.
See text and also Figure S1.
From the RNA-seq and ATAC-seq, we identified differentially expressed genes and accessible chromatin regions (peaks) for each ILC subset. Correlation-based clustering yielded expected groupings of ILCs (e.g., ILC1 and NK cells differed more from ILC2 and ILC3 than from each other, Figure S2). Differentially expressed genes included expected lineage-specific TF, cytokine and receptor genes: Rorc, Il23r, Il22, Il1r1, Il1r2 in ILC3; Gata3, Hes1, Pparg, Areg, Il1rl1 (ST2), Bmp2, Bmp7 in ILC2; Tbx21 in ILC1; and Eomes and Gzma in NK. In addition to these canonical ILC-subset markers, hundreds of TFs and immunological signatures distinguished the subsets (Figure S3).
To explore major trends in the ATAC-seq data, we performed principal component analysis (PCA) on all (~65k) accessible peaks identified across the five ILC subsets (Figure 2A, left panel). The largest accessibility pattern distinguished ILC1s and NKs from ILC3, while the second-largest separated ILC2 from ILC1 and ILC3. Approximately 104 peaks were unique to each broad ILC class (ILC1+NK, ILC2, ILC3) (pastel-colored circles in the loadings plot of Figure 2A, totaled in Figure 2B).
Figure 2. Characterization of genomic regulatory elements in ILCs.
(A) SI ILC samples are plotted according to accessibility patterns (Scores). Contributions of individual ATAC-seq peaks to the PC axes are displayed in the Loadings plot; peaks are annotated based on lineage-specific accessibility (pastel circles: pink (ILC1), green (ILC2) and blue (ILC3)) and proximity to lineage-specific ILC3 genes (dark blue dots). (B) Subset-specific ATAC-seq peaks were categorized as distal (pastel color scheme as in (A)) or proximal (+/−10kb gene body, red (ILC1), dark green (ILC2), dark blue (ILC3)) to subset-specific genes. (C) Representative ATAC-seq tracks for each ILC subset (n=2–4 per subset) highlight putative activating and repressive peaks. (D) The overlap between putative activating or repressive elements and activating histone marks (H3K27ac and H3K4me2) (Gury-BenAri et al., 2016) for six scenarios (element active in ILC1, ILC2, ILC3, ILC1 and ILC2, ILC2 and ILC3 or ILC1 and ILC3). Overlap was greater for putative activating versus repressive peaks (odds-ratio 1.62, P<10−3 for Fisher Exact Test, n=3695 (activating) and 872 (repressive) peaks. (E) The table displays subset-specific TFs whose motifs were enriched in subset-specific peaks proximal to subset-specific genes (FDR=10%). See also Figures S2, S3.
We explored the correlation between the accessibility of putative regulatory elements and expression of nearby genes. Subset-specific peaks were assigned to subset-specific genes based on proximity. In the loadings plot (Figure 2A), the majority of these ILC3-gene proximal peaks (dark blue dots) have increased accessibility in ILC3 (pastel blue circles). This trend was exemplified by the ILC3-specific peak lying upstream of the Il17re locus, an ILC3-specific gene (Figure 2C, upper panel). Given the correlation between gene expression and peak accessibility, the accessible region might promote Il17re expression. A motif search in the ILC3-associated peak yielded a Retinoic Acid-Related Orphan Receptor Response Element (RORE), to which the ILC3-expressed TFs RORγt or RORα might bind and potentially promote Il17re expression.
A small fraction of peaks near ILC3 genes had increased accessibility in type 1 ILCs and ILC2 (Figure 2A,B). For example, two peaks, accessible in ILC1 and NK cells, were near Il23r, a gene solely expressed in ILC3 (Figure 2C, lower panel). This pattern might arise from mechanisms of repression, by which type 1 ILC TFs might bind elements that suppress expression of alternative-lineage genes. Based on TF gene expression (Figure S3) and motif analysis, three potential ILC1 and NK TFs (IRF8, TBX21 and EOMES) (Adams et al., 2018; Gordon et al., 2012; Mace et al., 2017) are candidate negative regulators of Il23r in type 1 ILCs. Similar to ILC3, a smaller number of subset-specific peaks negatively correlated with the expression of proximal alternative-lineage genes in type 1 and 2 ILCs (Figure 2B). We examined the overlap between putative activating and repressive elements with iChIP for activating histone marks measured in ILC1, ILC2 and ILC3 (Gury-BenAri et al., 2016). Our putative activating elements had significantly greater overlap with the activating histone marks than the putative repressive elements (Figure 2D).
To infer TFs binding the predicted activating or repressive elements in each ILC subset, we performed TF motif analysis. In Figure 2E, we limit motif enrichment analysis to peaks and genes unique to the three broad subsets: type 1 ILC, ILC2, and ILC3. Similar to motif enrichment analyses by other groups (Gury-BenAri et al., 2016; Koues et al., 2016; Shih et al., 2016), we recovered key known lineage-specifying TFs RORγt, T-bet and GATA3, in addition to other candidate regulators (Figure 2E). By including motif analysis of putative repressive elements, we additionally identified candidate TFs mediating repression of alternative-lineage genes across multiple loci. For example, in uniquely type 1 ILC accessible regions, the T-bet motif was enriched proximal not only to ILC1 genes (suggesting activation) but also ILC2 and ILC3 genes (suggesting repression).
Thus, simple motif analysis, integrating differential accessibility with gene expression, can be used to derive a noisy initial set of TF-gene regulatory interactions (Blatti et al., 2015). However, such a regulatory network would include many sources of error: 1) A TF motif occurrence within a peak indicates the potential for, rather than evidence of, TF binding and regulatory function; 2) TF motifs are often shared by members of a TF subfamily, leading to ambiguity; 3) long-range regulatory interactions are difficult to assign to genes in the absence of 3D-chromatin conformation data; 4) interaction sign prediction (as described above) is imperfect; 5) motif analysis will not recover all TF binding events, as knowledge of TF motifs is incomplete and TFs can bind chromatin indirectly (e.g., as complexes). Thus, we refer to this initial, noisy and incomplete set of TF-gene interactions as a “prior ∆ATAC” network, serving as input to a more robust method for TRN inference (Miraldi et al., 2019).
ILC subsets have shared and unique core regulatory networks
We constructed an ILC TRN using our most recent Inferelator method (Miraldi et al., 2019) (Figure 1). Like the original (Bonneau et al., 2006), this method models gene expression as a function of TF activities, leveraging (partial) correlations between gene expression and TF activity profiles across many sample conditions to learn TF-target gene interactions. As increasing the number of gene expression samples boosts network inference, we incorporated public ILC genomics datasets (Gury-BenAri et al., 2016; Shih et al., 2016) in our analysis (Figure S4A). As a result, our gene expression matrix tripled in size to 62 ILC samples total: 40 from SI, 8 from LI, and the remainder from bone marrow, lung, liver and spleen). Importantly, the Inferelator enables incorporation of prior information (e.g., ∆ATAC prior), and this markedly improved inference in another mammalian context (Miraldi et al., 2019).
The ILC TRN contained 63,832 TF-gene interactions for 445 TFs and 6,418 genes, across subsets (Methods, Figure S4). 218 TFs were not in the ∆ATAC prior but their regulatory interactions had strong support from gene expression modeling alone; thus, our ILC TRN was not limited to TFs with known motifs (29%). Positive interactions outnumbered repressive interactions by 1.6:1. This positive-edge bias was consistent with the distribution of lineage-specific ATAC-seq peaks, which were proximal to genes specific to that lineage more frequently than genes of another lineage (Figures 2B, S4B,C).
To identify the key transcriptional regulators associated with each cell type, we derived “core” TRNs for each of the five ILC subsets. We identified “core” TFs that promoted subset-specific gene expression via gene activation or repression (Miraldi et al., 2019). Our analysis revealed 125 subset-promoting TFs (Figure 3A). The “core” TRN analysis correctly recovered known subset-specific regulators: RORγt in ILC3 (Sawa et al., 2010), GATA3 in ILC2 (Hoyler et al., 2012), T-bet in the type 1 ILC networks (Powell et al., 2012; Sciumé et al., 2012; Townsend et al., 2004) and Eomes uniquely in the NK network (Gordon et al., 2012; Pikovskaya et al., 2016). The ILC networks also accurately predicted several other TFs with known ILC-lineage roles. These include TCF-1 (Tcf7) in CCR6+ ILC3 (Mielke et al., 2013); LEF1 (Held et al., 2003) and BLIMP-1 (Prdm1) (Kallies et al., 2011; Smith et al., 2010) in NK cells; KLF2 in NK cells and ILC1 (Rabacal et al., 2016); and PPARγ in ILC2 (Figure 3A, red text). Our analysis shared predictions with another study (Shih et al., 2016); TFs predicted to regulate ILC2, CD4+ ILC3 and NCR+ ILC3 (ATF3, BACH1, NR1D2, NFKB1) are “core” to ILC2, CCR6+ ILC and CCR6−ILC3 in our analysis as well (Figure 3A, green text). Only a small subset of the 125 TF predictions were previously characterized in ILCs, highlighting the utility of our network approach to expand TF predictions beyond what is possible by standard motif enrichment analysis.
Figure 3. Core TRNs for the ILC subsets.
(A) Core TFs are clustered according to enriched positive regulation of up-regulated genes (red) or negative regulation of down-regulated genes (blue) in a given ILC subset, as both types of regulation would contribute to subset-specific gene expression. The significance of enrichment is displayed in the left panel. The right panel displays the number of positive (red) and negative (blue) regulatory interactions per TF; interactions supported by the ∆ATAC prior are shaded pink (+) and light blue (−). TFs previously associated with an ILC subset are in red text. TFs in green were predicted core regulators of intestinal ILC2, CD4+ ILC3 and NCR+ ILC3 (Shih et al., 2016). Subset-specific and shared core TRNs are displayed for (B) ILC3, (C) ILC1, and (D) ILC2. Core TRNs are limited to select TFs in (A), cytokines, chemokines and receptors. Node color represents z-scored gene expression in the given cell type (red for up-regulation, blue for down-regulation); TF node size is proportional to degree (number of target genes). Edge color denotes positive (red) and negative (blue) regulation. Solid edges are supported by the ∆ATAC prior and gene expression modeling, while dotted edges are supported by gene expression modeling alone. See also Figure S4.
To examine what genes were regulated by core TFs, we visualized the subset-specific TRNs (Figure 3B–3D). Several of the cytokine interactions have been previously verified. For example, RORγt in ILC3 was predicted to regulate its canonical targets, including Il23r, Il17a and Il1r1. Recovery of known TFs and regulatory interactions provided confidence in network quality and the potential to aid in discovery of additional relevant interactions.
TF modules co-regulate distinct functional pathways
Many TFs function in a combinatorial manner to co-regulate distinct gene programs for specific cellular functions (Pope and Medzhitov, 2018). To connect TFs in the TRN to ILC functions, we clustered TFs into “co-regulating modules” based on overlap in their target genes (Miraldi et al., 2019). This analysis identified several groups of TFs with significant overlap in their positive target genes and helped organize the network into easier-to-interpret modules (Figure 4A). To help identify functional roles for each module, we performed gene-set enrichment analysis on the module’s co-regulated target genes (Figures 4A, S5). A cluster composed of TGIF1, NR2F6, IRF7, VDR, MAF, and BCL6 was predicted to positively regulate chemotaxis and cytokine signaling, suggesting that it may be relevant to ILC identity and trafficking. These factors, with the exception of TGIF1, were predicted to be both activators and repressors of ILC3 genes. These TFs were most highly expressed in SI CCR6− ILC3 and are thus most likely to coordinate cytokine and chemokine programs in these cells (Figure 4B). The targets of known circadian regulators NR1D1, RORA, and NR1D2 (Sato et al., 2004; Zhang et al., 2014) were most highly expressed in ILC3, and form another distinct cluster (Figure 4A). A cluster of genes predicted to regulate adipogenesis was most highly expressed in ILC2 (in both SI and LI), and two of the TFs (GATA3 and STAT1) were themselves in the adipogenesis gene set (Figure 4A,C). ILC3 have been proposed to process and present antigen to regulate T cells in response to microbiota (Hepworth et al., 2013, 2015), but the transcriptional regulation of this process is not fully understood. This analysis predicted that a set of TFs (ZFP385A, SPI1, POU2AF1, and GATA2) regulate genes involved in “Antigen presentation”, “Asthma”, “Rheumatoid Arthritis”, and “Intestinal IgA production network”. Indeed, several HLA genes are regulated by this TF module (Figure 4D). Thus, our co-regulator analysis highlights coordinated regulation of key pathways in ILCs, recovering some known TF-pathway associations and many predictions for TF regulation of ILC function.
Figure 4. TF-TF modules co-regulate expression of gene pathways.
(A) The normalized target gene overlap between the top 15 “TF-TF modules” (TFs with significant overlap in positively regulated target genes, see Methods) are annotated with enriched gene pathways in the main panel. In the left panel, TFs are annotated according to positive (red) or negative (blue) regulation of ILC-subset signature genes. The lower panel displays TF gene expression. TF-TF module TRNs are displayed as in Figure 2 for (B) “chemotaxis, cytokine signaling”, (C) “adipogenesis”, and (D) “antigen presentation” modules; nodes are colored according to gene expression in SI CCR6−ILC3, SI ILC2, and LI CCR6− ILC3, respectively. Target genes were included in the TRN visualization if positively regulated by at least two module TFs. See also Figure S5.
The ILC TRN reveals TF repressors of alternate lineage genes
The phenomenon of ILC functional and phenotypic plasticity is poorly understood. We hypothesized that regulatory circuits directing the conversion of one ILC subset into another might function via de-repression of alternative ILC-subset genes. We thus sought to identify TFs in the TRN predicted to act as repressors of such genes. Our analysis identified 41 TF repressors of alternative ILC-subset genes (Figure 5A), greatly expanding candidates beyond the initial ∆ATAC motif analysis (Figure 2E); several candidates did not have motifs in our database and thus were unrecoverable from ATAC-seq analysis alone. We further examined how these negative regulators were predicted to directly regulate one another. We generated a TF-TF lineage-regulating network for some of the most significant alternative-lineage gene repressors identified (Figure 5B). In the repressive TRN, ILC3-specific RORC antagonized ILC1 and NK regulators Tbx21 and Bach2, while ILC1 and NK regulators, including T-bet and BCL6, repressed Rorc. T-bet additionally antagonized the hallmark ILC2 TF Gata3. Reciprocally, ILC2 TFs KLF5, POU2F2 and IRF4 repressed Tbx21 and Rorc. This analysis implicates a number of ILC TFs as potential ILC plasticity regulators. We chose BCL6 and c-MAF as candidates for further validation due to their predicted reciprocal regulation of genes in the ILC1-to-ILC3 continuum as well as for their predicted roles in controlling a module of cytokine and cytokine-receptor genes (Figure 4A,B).
Figure 5. Predicted TF repressors of alternative ILC subset genes.
(A) TFs predicted to be repressors of subset-specific gene expression are clustered according to the significance of negative edge enrichment in genes upregulated per subset (FDR=10−5; blue heatmap, middle panel). The left panel displays TF gene expression in the SI, while the right panel indicates number of target genes per TF, as in Figure 3A. (B) A TRN constructed from a selection of lineage-repressors, RORC and GATA3. TF nodes are colored according to the ILC subset(s) in which the TF is expected to be most active.
c-MAF regulates the balance between intestinal ILC1 and ILC3
c-MAF has critical functions in effector T cells, contributing to Il10 expression in multiple T cell subsets, the differentiation of induced Treg cells (Xu et al., 2018), and repression of Il22 (Rutz et al., 2011). Its function in ILCs has not been explored. Our ILC TRN predicted that c-MAF repressed CCR6+ ILC3 lineage genes while promoting gene signatures of CCR6− ILC3 and ILC1, where c-MAF is most highly expressed (Figure 5A). To test whether c-MAF regulates these cell types, we examined ILCs in the SI lamina propria of mice lacking c-MAF in all lymphocyte populations (Maffl/fl;Il7rcre) (Wende et al., 2012) (Figure 6A). We observed a reduction in the number of RORγt+ T-bet− ILC3 which was accompanied by an increase in the frequencies and total numbers of the other ILC subsets, particularly ILC1, in Maffl/fl;Il7rcre animals compared to Maffl/fl (or Il7rcre) controls (Figure 6A,C, S6A). There was a concomitant increase in IFN-γ and decrease in IL-22 when intestinal ILCs were pushed toward effector cytokine secretion with PMA-Ionomycin stimulation (Figure 6B). Thus, as predicted by the ILC TRN, c-MAF regulates the balance between ILC1 and ILC3, but the net effect of Maf ablation in promoting the type 1 ILC program indicates that c-MAF may positively regulate genes that restrain this fate in T-bet-expressing ILCs. Maffl/fl;Il7rcre ILCs increased expression of T-bet, suggesting that c-MAF may regulate the balance of ILC1 and CCR6+ ILC3 via direct or indirect suppression of Tbx21 (Figure 6D).
Figure 6. Functional roles for c-MAF and BCL6 in intestinal ILCs.
(A) Indicated ILC populations from the SILP of Maffl/fl;Il7rcre or control Maffl/fl mice. Lineage negative is (CD3−TCRβ−TCRγδ−CD11b−CD11c−CD14−CD19−). (B) PMA-Ionomycin stimulated small intestine lamina propria cells, gated on lineage negative population. (C) Absolute numbers of ILC subsets from one representative of three experiments. Statistics calculated by Welch’s t test. Total animals for data generation: ctrl n=14, Maffl/fl;Il7rcre n=13. (D) Histogram depicting T-bet expression in RORγt+T-bet+ ILC3 gate. MFI is mean fluorescence intensity +/− standard deviation. (ctrl n=5, Maffl/fl;Il7rcre n=4 two-tailed t-test P = 10−4) (E, F) ILC populations from the SILP of Bcl6fl/fl;Ncr1cre or control Bcl6fl/fl mice, lineage negative as in (A). (G) Absolute numbers of SILP ILC populations. Each line corresponds to an independent experiment (n=6) containing between 2-4 mice of each genotype (Bcl6fl/fl;Ncr1cre n=15, Ctrls n=14). Each dot represents mean value of an experiment. Statistics calculated by ratio paired t test. See also Figure S6.
BCL6 promotes abundance of intestinal type 1 ILCs
The ILC TRN predicted that BCL6 was a core regulator of NK and CCR6− ILC3 lineages (Figure 3A). BCL6 is critical for controlling germinal center B cell and follicular helper T (Tfh) cell differentiation (Hatzi et al., 2013, 2015a), with a known role for alterative-lineage repression of Th17, Th2 and Th1 programs in Tfh cells (Hatzi et al., 2015b). However, a role for BCL6 in ILC function has not been previously characterized. Conditional inactivation of Bcl6 in NK, ILC1 and NKp46-expressing ILC3 was achieved by crossing Bcl6fl/fl (Hollister et al., 2013) to Ncr1cre (Narni-Mancinelli et al., 2011) mice. Comparison of SI lamina propria ILC populations between Bcl6fl/fl;Ncr1cre (Bcl6 deletion) and control Bcl6fl/fl mice (or Ncr1cre mice) revealed decreased NK and ILC1 cell frequencies and numbers, while there was no change in ILC3 (Figure 6E, F, G). Bcl6 expression was highest in NK, decreasing progressively from ILC1 to CCR6− ILC3 to CCR6+ ILC3 (Figure S3A). Thus, the two ILC populations with highest Bcl6 expression were decreased. Yet the ILC TRN also predicted that BCL6 both positively and negatively regulated ILC3 signature genes (Figures 3A, 5A). Thus we sought to investigate BCL6-dependent gene changes to test the accuracy of target gene prediction by the Inferelator and to shed light on how BCL6 regulates ILC homeostasis and function.
Validation of BCL6 as a modulator of NK cell and ILC3 gene programs
To investigate the role of BCL6 in ILCs, we compared transcriptomes of NK, ILC1, and CCR6− ILC3 from Bcl6-deleted and control mice. Bcl6 deletion led to gene expression changes in each subset, with the greatest number in NK cells (Figure 7A). Although less highly expressed in CCR6− ILC3, deletion of Bcl6 perturbed nearly as many genes (450 versus 474 in NK). Perturbation of Bcl6 in ILC1 led to fewer gene expression changes (103), despite higher Bcl6 expression than in ILC3s (Figure S3A),
Figure 7. Validation of TRN predictions for BCL6.
(A) Up- and down-regulated genes comparing Bcl6 deletion (Bcl6fl/fl;Ncr1cre-) to control (Bcl6fl/fl) per SI ILC subset (FDR=10%). (B) Clustering of 902 differentially expressed genes (Bcl6 deletion versus control, FDR=10% in at least one subset). Genes are mean-normalized per ILC subset to highlight Bcl6-dependent trends. (C) Significance of overlap between TRN-predicted BCL6 targets and the Bcl6-deletion-dependent genes (Fisher Exact Test, left panels) and percent sign agreement for overlapping regulatory predictions (right panel). (D) Positively and negatively-regulated BCL6 targets were (1) inferred from the Bcl6 deletion differential expression analysis in each cell type as well as the union across cell types (FDR=10%) and (2) tested for enrichment in genes upregulated per subset; TRN predictions for BCL6 are provided for reference. (E) BCL6 is a negative regulator of ILC3 lineage genes in both NK cells and CCR6− ILC3, and, for CCR6− ILC3, these genes were enriched for several cytokine pathways. Additionally, BCL6 promotes expression of some CCR6− ILC3 genes in NK cells, while, in CCR6− ILC3, BCL6 promotes NK gene expression. See also Figure S7.
Visualizing the Bcl6-deletion data revealed several trends (Figure 7B). Across subsets, BCL6 has a greater number of putative repressed targets than positive targets, consistent with its known roles as a transcriptional repressor (Chang et al., 1996; Crotty et al., 2010; Hatzi et al., 2015b) (Figure 7B). Some gene targets were conserved across NK, ILC1, and ILC3, while others were unique to NK cells. Most of the putative repressed targets (70%) were conserved among ILCs and included canonical ILC3 genes (e.g., Rora, Stat3, Il17a, Il17f), while 67% of positive targets were only differentially expressed in NK cells (Figure 7B). Although fewer genes were differentially expressed in ILC1 (Figure 7A), Bcl6-dependent gene patterns in ILC1 were qualitatively similar to CCR6− ILC3, suggesting conserved regulatory mechanisms (Figure 7B). BCL6 appeared to promote several genes with known roles in NK cell differentiation and functions (Klf2, Fasl, Clec2d, CD48, CD160, Ptpn6, Klrk1, Klrb1b, Klrb1c, Klrb1c, Klrb1f). These results suggest that BCL6 is mainly a repressor in ILC1 and ILC3, while, in NK cells, BCL6 is both a repressor and activator (Figure 7B, S7C,D).
We next tested whether TRN-predicted BCL6 targets were supported by the Bcl6 deletion data. For each ILC subset, TRN-predicted targets were two-fold more likely to be differentially expressed in Bcl6-deleted cells (P<10−4, Fisher Exact Test, Figure 7C), and the enrichment grew to four-fold upon combining differential genes across the ILC subsets (“Union”) (P<10 −8). These results were robust over a range of FDR cutoffs and TRN model sizes (Figure S7A). Importantly, TRNs built from gene expression data alone (without ATAC-seq) poorly predicted Bcl6-dependent genes (Figure S7B), highlighting the importance of integrating ATAC-seq data to TRN inference. Finally, there was reasonable agreement in the predicted sign of regulatory edge between the ILC TRN and Bcl6 deletion analysis (Figure 7C).
Both up-regulated and down-regulated gene sets in Bcl6 deleted NK cells were enriched for ILC3-signature genes, supporting the ILC TRN prediction that BCL6 is both a positive and negative regulator of ILC3 genes in NK cells (Figure 7D). In CCR6− ILC3, BCL6 also putatively repressed a subset of CCR6− ILC3 signature genes and promoted expression of ILC1 and NK-signature genes. Some promotion of ILC1 and NK-signature genes was observed in NK cells and ILC1 as well (Figure 7B, Figure S7E). BCL6-repressed CCR6−ILC3 genes across NK cells and CCR6− ILC3 significantly overlapped, while the BCL6-activated CCR6−ILC3 genes in NK cells did not overlap with any of the BCL6-repressed genes (Figure 7E). Among TFs suppressed by BCL6, RORA (highest expression in ILC2 and ILC3) was also predicted to repress ILC1 and NK signature genes (Figure 5), highlighting a potential mechanism for increased ILC1 and NK gene expression in Bcl6-deficient ILCs.
BCL6 (and c-MAF) belong to a cluster of TFs predicted to co-regulate cytokine signaling (Figure 4B). GSEA of Bcl6-dependent genes corroborates this prediction in CCR6−ILC3. Indeed, BCL6 targets in CCR6− ILC3 were enriched for IL12-, IL23- and IL-2-signaling pathways, cytokine production, and endogenous TLR signaling (FDR<1%) (Figure 7E, S7F). Taken together, these results validate several BCL6 predictions from the ILC TRN. BCL6 is a modulator of the ILC3 program and activator of NK genes, with distinct roles in ILC3 and NK cells. These data suggest potential mechanisms whereby BCL6 maintains SI ILC1 and NK cells through repression of alternative lineage (ILC3) genes and promotion of select genes required for maintenance of type 1 ILCs.
Discussion
Innate lymphoid cells are important mediators of mucosal immunity, especially in early immune responses. However, they also contribute to diverse pathologies, from autoimmune disease to metabolic syndrome. Previous ILC genomics studies identified genes, gene pathways and putative regulomes of the ILC lineages (Gury-BenAri et al., 2016; Koues et al., 2016; Robinette et al., 2015; Shih et al., 2016). In this study, we integrated our own substantial ILC genomics dataset with previous work (Gury-BenAri et al., 2016; Koues et al., 2016) to construct a genome-scale transcriptional regulatory network (TRN) for gut-resident ILCs. This effort is a proof-of-concept for in vivo application of our TRN inference method (Miraldi et al., 2019). The ILC TRN de novo predicted known regulators of ILC lineages as well as many candidate TFs, two of which, BCL6 and c-MAF, were confirmed to be functionally relevant in the contexts predicted. Moreover, the networks identify specific gene targets of each TF, further facilitating biological hypothesis development and testing. The ILC TRN and co-regulated gene modules are intended to serve as a resource. Networks and gene expression data can be interactively searched, visualized and explored via https://github.com/flatironinstitute/ILCnetworks.
In addition to identifying “core” TFs and TRNs, we uncovered 41 TFs implicated in repression of ILC-lineage-specific gene expression. Both sets of TFs represent potential lineage control points. Not only could they promote maintenance of subset-specific gene expression patterns, but their perturbation could potentially be used to alter ILC lineage identity and functionality. Several autoimmune and inflammatory diseases are associated with altered abundance and activity of ILC subsets (Eberl et al., 2015). For example, maternal antibiotic exposure is associated with a decrease in lung ILC3 and increased susceptibility to infection in the offspring (Gray et al., 2017). Our ILC TRN could be used to design disease interventions that limit the function or abundances of specific ILC subsets through targeting of lineage-promoting or repressing TFs. Constructing TRNs for human ILCs from health and disease states could prove especially valuable. We emphasize that our approach is entirely general and readily applicable to other cell populations in physiological settings, including T helper cells, macrophages, and others.
Gene expression profiling of Bcl6-deletion ILCs supported specific TF-gene regulatory hypotheses from the TRN. The Bcl6-deletion data also provide a potential explanation for the reduction of SI type 1 ILC in Bcl6-deletion animals. BCL6 might contribute to ILC3-to-ILC1 plasticity by repressing ILC3-promoting signaling pathways (e.g., IL-23 signaling). BCL6 also promotes NK and ILC1 genes required for survival or homing. These two mechanisms may be connected. Our TRN analyses suggest that BCL6 represses ILC3 TFs in type 1 ILC, some of which (e.g., RORA) are predicted to repress type 1 signature genes and could alter type 1 ILC accumulation in the intestine.
Notably, BCL6-repression of the ILC3 program mirrored BCL6 repression of the Th17 program in Tfh cells. In both ILCs and Tfh, Rora, Stat3, Il17a, Il17f are BCL6 targets while Rorc is not (Hatzi et al., 2015b). Thus, like other TFs (Walker et al., 2013), BCL6 has some shared regulatory roles between ILC and T helper subsets. Constructing TRNs for intestinal T helper cells would be an opportunity for a comparative analysis of T helper cells and ILCs. In NK cells, the purpose of BCL6 repression of ILC3 genes is less clear, as plasticity between NK and ILC3 (or NK and ILC1) has not been reported. It is possible that NK cells need to express ILC3 signature genes in some conditions.
A number of ILC TRN predictions are consistent with current knowledge of ILC lineage regulation, and a limited number of predictions have been experimentally tested in this study. However, the vast majority of predictions remain to be tested. We anticipate that the ILC TRNs will be useful to unraveling the molecular mechanisms driving complex phenotypes (e.g., due to TF deletion or other experimental perturbations) in ILCs. We hope that these maps of transcriptional regulation increase the pace of discovery in ILC biology, eventually guiding the design of genetic and chemical perturbations to alter subset-specific ILC behaviors in human health and disease.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to the Lead Contact, Richard Bonneau (bonneau@nyu.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
Wild type and Maf deletion experiments.
Maffl/fl and Il7racre mice were kindly provided by C. Birchmeier and H. R. Rodewald (Schlenner et al., 2010; Wende et al., 2012). Maf conditional deletion mice were generated by crossing Maffl/fl to Il7racre animals. C57BL/6 mice were purchased from Taconic labs and along with Maf deletion mice were bred and maintained in the animal facility of the Skirball Institute (New York University School of Medicine) in SPF conditions. All animal procedures were performed in accordance with protocols approved by the Institutional Animal Care and Usage Committee of New York University School of Medicine.
Bcl6 deletion experiments.
Mice were housed under pathogen-free conditions, and experiments were performed using ethical guidelines approved by the Institutional Animal Use and Care Committees of Cincinnati Children’s Hospital Medical Center. Ncr1icre mice were previously described (Narni-Mancinelli et al., 2011) and were kindly provided by Eric Viviér (University of Marseille). Bcl6fl/fl mice were purchased from Jackson laboratories (Bar Harbor, ME). Both strains of mice were on C57BL/6 background. Ncr1icre/icre mice and Bcl6fl/fl mice were bred to obtain Bcl6fl/fl Ncr1wt/icre (Bcl6 deletion) or Bcl6fl/fl Ncr1wt/wt (WT). After breeding, all mice were kept with their littermates (males and females were separated). Bcl6 deletion was verified by examining NCR1 expression and by PCR. Male mice at 16–20 weeks of age were utilized in experiments. In each experiment, an equal number of mice was used.
Isolation of SILP and LILP lymphocytes and TF analysis.
Intestinal tissues were sequentially treated with PBS containing 1 mM DTT at room temperature for 10 min, and 5 mM EDTA at 37°C for 20 min to remove epithelial c ells, and then minced and dissociated in RPMI containing collagenase (1 mg/ml collagenase II; Roche), DNase I (100 µg/ml; Sigma), dispase (0.05 U/ml; Worthington) and 10% FBS with constant stirring at 37°C for 45 min (SI) or 60 min (LI). Le ukocytes were collected at the interface of a 40%/80% Percoll gradient (GE Healthcare). For transcription factor analysis, lamina propria mononuclear cells were first stained for surface markers before fixation and permeabilization, and then subjected to intracellular TF staining according to the manufacturer’s protocol (eBioscience Intracellular Fixation & Permeabilization buffer set from eBioscience).
Sorting of intestinal ILCs
NK, ILC1, ILC2, and ILC3 (CCR6+ and CCR6−) were sorted from the small or large intestine lamina propria preparation using the surface marker panel described in Figure S1. A portion of sorted cells was stained intracellularly with ILC lineage transcription factors to confirm that the correct populations were isolated. Lin−Klrg1hi cells were uniformly Gata3hi and thus mature ILC2 (Hoyler et al., 2012). We have found KLRB1B to be a good marker of ILC1 and ILC3, which can then be further stratified based on expression of NK1.1hi (ILC1) and NK1.1lo-NK1.1neg (ILC3) (Figure S1). ILC1 are T-bethi while ILC3 are RORγt+ and can be either T-bet− (CCR6+) or T-bet+ (CCR6−) (Figure S1). Because of a limitation of 4 sorting streams, ILC1, ILC2, and ILC3 (CCR6+ and CCR6−) were sorted from the same mice while NK cells were sorted from a different
Assessing cytokine production in ILCs
To detect IL-22 and IFNγ from ILCs, isolated LP cells were incubated with or without 50 ng/ml phorbol myristate acetate (PMA) (Sigma), 500 ng/ml Ionomycin (Sigma) in the presence of GolgiSTOP (BD Biosciences) in complete media at 37°C for four hours. Cells were then stained as described below.
Cell staining for flow cytometry
For analysis of ILCs, live LP cells were stained with anti-NK1.1, anti-IL7ra, anti-Klrg1, anti-KLRB1B (clone 2D12, subclone from 2D9) as previously described (Aust et al., 2009; Carlyle et al., 2006), and anti-CCR6. Anti-CD11b, anti-CD11c, anti-CD14, anti-CD19, anti-B220, anti-TCRβ, anti-TCRγδ, and anti-CD3 were used as dump gate. For cytokine and transcription factor staining, cells were stained for surface markers, followed by fixation and permeabilization before cytoplasmic and nuclear factor staining according to the manufacturer’s protocol (FOXP3 staining buffer set from eBioscience) with individually or in combination anti-IL-22, anti-IFNg, anti-RORgt, anti-T-bet, and anti-Gata3. 4′,6-diamidino-2-phenylindole (DAPI) or Live/dead fixable blue (ThermoFisher) was used to exclude dead cells. Flow cytometric analysis was performed on an LSRII (BD Biosciences) or an Aria (BD Biosciences). All data were re-analyzed using FlowJo (Tree Star). Commercially available antibodies used in flow cytometry experiments above are listed in Table S1.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| BV421 Rat Anti-Mouse CD196 (CCR6) (Clone 140706) | BD Biosciences | Cat# 564736 |
| PerCP-Cy5.5 Hamster Anti-Mouse CD3e (Clone 145–2C11 | BD Biosciences | Cat# 551163 |
| Brilliant Violet 605 Rat Anti-Mouse CD3 (Clone 17A2) | BioLegend | Cat# 100237 |
| PerCP-Cyanine5.5 Rant anti-mouse CD11b (Clone M1/70) | eBioscience | Cat# 45–0112-82 |
| PerCP-Cyanine5.5 Hamster anti-mouse CD11c (Clone N418) | eBioscience | Cat# 45–0114-82 |
| PerCP/Cy5.5 Rat anti-mouse CD14 (Clone Sa14–2) | BioLegend | Cat# 123313 |
| BV605 Rat Anti-Mouse CD14 (Clone rmC5–3) | BD Biosciences | Cat# 740357 |
| PerCP-Cyanine5.5 Rat Anti-Mouse CD19 (Clone eBio1D3 (1D3)) | eBioscience | Cat# 45–0193-82 |
| Brilliant Violet 605™ Rat Anti-Mouse CD19 (Clone 6D5) | BioLegend | Cat# 115539 |
| Alexa Fluor 488 Rat Anti-Mouse Gata-3 (Clone TWAJ) | eBioscience | Cat# 53–9966-42 |
| Alexa Fluor 488 Rat Anti-Mouse IFN gamma (Clone XMG1.2) | eBioscience | Cat# 53–7311-82 |
| APC Rat Anti-Mouse IL-22 (Clone IL22JOP) | eBioscience | Cat# 17–7222-82 |
| PE Rat Anti-Mouse CD127 (IL-7Rα) (Clone A7R34) | eBioscience | Cat# 12–1271-82 |
| PE/Cy7 Rat Anti-Mouse CD127 (IL-7Rα) (Clone A7R34) | BioLegend | Cat# 135013 |
| anti-Klrb1b (clone 2D12, subclone from 2D9) | (Aust et al., 2009) | N/A |
| PE Hamster Anti-Mouse KLRG1 (Clone 2F1) | eBioscience | Cat# 12–5893-82 |
| PerCP-eFluor 710 Hamster Anti-Mouse KLRG1 (Clone 2F1) | eBioscience | Cat# 46–5893-82 |
| FITC Hamster Anti-Mouse/human KLRG1 (Clone 2F1) | BioLegend | Cat# 138409 |
| APC-eFluor 780 Anti-Mouse NK1.1 (Clone PK136) | eBioscience | Cat# 47–5941-82 |
| BV421 Anti-Mouse NK1.1 (Clone PK136) | BD Biosciences | Cat# 562921 |
| FITC Anti-Mouse NK1.1 (Clone PK136) | eBioscience | Cat# 11–5941-82 |
| APC Anti-Mouse NK1.1 (Clone PK136) | eBioscience | Cat# 17–5941-82 |
| Brilliant Violet 711 anti-mouse NK-1.1 (Clone PK136) | BioLegend | Cat# 108745 |
| APC Rat Anti-Mouse ROR gamma (t) (Clone B2D) | eBioscience | Cat# 17–6981-82 |
| PerCP-Cyanine5.5 Anti-Mouse/human T-bet (Clone 4B10) | eBioscience | Cat# 45–5825-82 |
| PE Anti-Mouse/human T-bet (Clone 4B10) | eBioscience | Cat# 12–5825-82 |
| PerCP-Cyanine5.5 Hamster Anti-Mouse TCR beta (Clone H57–597) | eBioscience | Cat# 45–5961-82 |
| PerCP-eFluor 710 Hamster Anti-Mouse TCR γ/δ (Clone eBioGL3 (GL-3, GL3)) | eBioscience | Cat# 46–5711-82 |
| Brilliant Violet 605 Hamster Anti-Mouse TCR γ/δ (Clone GL3) | BioLegend | Cat# 118129 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| phorbol myristate acetate (PMA) | Sigma | Cat# P8139; PubChem Substance ID 24278653 |
| Ionomycin calcium salt | Sigma | Cat# I3909; PubChem Substance ID 329815372 |
| BD GolgiStop™ Protein Transport Inhibitor (Containing Monensin) | BD Biosciences | Cat# 554724 |
| Percoll density gradient media | GE Life Sciences | Cat# 17089101 |
| Collagenase D | Roche | Cat# 11088874103 |
| DNAse I | Sigma | Cat# DN25–1G |
| Dispase | Worthington | Cat# LS02104 |
| Critical Commercial Assays | ||
| NEBNext® Library Quant Kit for Illumina | New England Biolabs | Cat# E7630A |
| Ovation RNA-Seq System V2 | Nugen | Cat# 7102–08 |
| Ovation® Ultralow System V2 | Nugen | Cat# 0344NB |
| NEBNext Ultra II Directional RNA Library Prep kit | New England Biolabs | Cat# E7760S |
| Deposited Data | ||
| Raw and analyzed RNA-seq | This paper | GEO: GSE116093 |
| Raw and analyzed RNA-seq | Shih et al., 2016 | GEO: GSE77695 |
| Raw and analyzed RNA-seq | Gury-BenAri et al., 2016 | GEO: GSE85154 |
| Raw and analyzed ATAC-seq | This paper | GEO: GSE116093 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6 | Taconic labs | C57BL/6NTac |
| Mouse: Il7raCre | H. R. Rodewald (Wende et al., 2012) | N/A |
| Mouse: Maffl/fl | C. Birchmeier (Schlenner et al., 2010) | N/A |
| Mouse: B6.129S(FVB)-Bcl6tm1.1Dent/J | Jackson Laboratory | Strain # 023727 |
| Mouse: Ncr1iCre | E. Vivier (Narni-Mancinelli et al., 2011) | N/A |
| Software and Algorithms | ||
| Bowtie2 | Langmead and Salzberg, 2012 | http://bowtie-bio.sourceforge.net/bowtie2/index.shtml |
| SAMtools | Li et al., 2009 | http://www.htslib.org/ |
| Picard | http://broadinstitute.github.io/picard | |
| PeakDEck | McCarthy and O’Callaghan, 2014 | https://www.ccmp.ox.ac.uk/peakdeck |
| HTSeq-count | Anders et al., 2015 | https://github.com/simon-anders/htseq |
| DESeq2 | Love et al., 2014 | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| BEDTools | Quinlan et al., 2010 | https://bedtools.readthedocs.io/en/latest/ |
| STAR aligner | Dobin et al., 2013 | https://github.com/alexdobin/STAR |
| Inferelator-LASSO-StARS | Miraldi et al., 2019 | https://github.com/emiraldi/infTRN_lassoStARS.git |
| jp_gene_viz network visualization software | Karwacz et al., 2017 | https://github.com/simonsfoundation/jp_gene_viz |
| COMBAT | Johnson et al, 2007 | https://rdrr.io/github/Bioconductor-mirror/sva/man/ComBat.html |
| Other | ||
| Resource website for interactive TRN and gene expression visualization | This paper | https://github.com/flatironinstitute/ILCnetworks |
| Active enhancers identified in intestinal ILC1, ILC2 and ILC3 (from Table S2) | Gury-BenAri et al., 2016 | https://ars.els-cdn.com/content/image/1-s2.0-S009286741630993X-mmc2.xlsx |
METHOD DETAILS
ATAC-seq
Sorted ILC populations were prepared according to (Buenrostro et al., 2013). Paired-end 50bp sequences were generated from samples on an Illumina HiSeq2500. Sequences were mapped to the murine genome (mm10) with Bowtie2 (2.2.3) (Langmead and Salzberg, 2012), filtered based on mapping score (MAPQ > 30, Samtools (0.1.19)), and duplicates removed (Picard). For each sample individually, we ran Peakdeck (McCarthy and O’Callaghan, 2014) (parameters –bin 75, -STEP 25, -back 10000, -npBack100000) and filtered peaks with Praw<10−4. To enable quantitative comparison of accessibility across samples, we generated a reference set of accessible regions, taking the union (BEDTools (Quinlan and Hall, 2010)) of peaks detected in individual samples. The reference set of ATAC-seq peaks contained 65,281 potential regulatory loci, ranging from 100 base pairs (bp) to 3750 bp (median length 375 bp). Reads per reference peak were counted with HTSeq-count (Anders et al., 2015). This pipeline resulted in ~14 million uniquely mapped reads per sample, ~42% of which mapped to the identified peaks. We used DESeq2 (Love et al., 2014) to normalize and identify differentially accessible peaks across conditions. Downstream analysis and data visualization were performed in MATLAB R2014B. For PCA analysis (Figure 2), ATAC-seq data were robustly normalized (DESeq2); counts were mean-centered and variance-normalized by the square root of standard deviation. ATAC-seq data were deposited in the GEO database under accession GSE116091.
RNA-seq
For the initial RNA-seq measurements in wildtype ILCs, cells were sorted (FACSAria II, BD) directly into Trizol and snap-frozen, thawed for chloroform extraction and purified using the RNAeasy minElute kit (Qiagen). Samples were evaluated for RNA quality by sampling and analyzing one µl of library by Bioanalyzer (Agilent, Santa Clara, CA), using DNA high sensitivity chip. An accurate quantification of library concentration, was performed by NEBNext Library Quant Kit (New England BioLabs). Libraries were prepared using Nugen Ovation RNAseq System V2 and Nugen Ovation Ultralow Library System kits and sequenced on an Illumina HiSeq2500. Sequences were mapped to the murine genome (mm10) with STAR (Dobin et al., 2013). HTSeq-count (Anders et al., 2015) was used to count reads per gene. DESeq2 (Love et al., 2014) was used to normalize and identify differentially expressed transcripts among ILC lineages. Additional ILC RNA-seq data were downloaded from GEO: GSE85154, GSE77695, and processed similarly. We controlled for batch effects across the three datasets using COMBAT (Johnson et al., 2007). In COMBAT, we modeled batch and “broad” cell type (type 1 ILC (ILC1 and NK), ILC2, and ILC3), and clustering of the resulting data matrix revealed trends that were consistent with prior knowledge not provided to COMBAT (e.g., CD4+ILC3 clustered with CCR6+ILC3, NCR+ILC3 clustered with CCR6-ILC3, and samples clustered based on tissue of origin). The resulting gene expression matrix contained 62 samples (Supplemental Data S1).
For RNA-seq of Bcl6 deletion and control animals, ILC populations were sorted as described above. Briefly, cells were sorted into 100% FBS, and immediately spun at 600 g for 6 min. After removal of supernatant, the cell pellet was lysed with Lysis/Binding Buffer from mirVana miRNA Isolation Kit (Thermo Fisher, Grand Island, NY). Samples were evaluated for RNA quality as described above. The library for RNA-seq was prepared by using NEBNext Ultra II Directional RNA Library Prep kit (New England BioLabs, Ipswich, MA). To study differential gene expression, individually indexed and compatible libraries were proportionally pooled (~25 million reads per sample in general) for clustering in cBot system (Illumina, San Diego, CA). Libraries at the final concentration of 15 pM were clustered onto a single read (SR) flow cell v3 using Illumina TruSeq SR Cluster kit v3, and sequenced to 51 bp using TruSeq SBS kit v3 on Illumina HiSeq system. Sequences were mapped and gene expression analysis performed as described above.
RNA-seq data were deposited in the GEO database under accession GSE116092. Both gene expression matrices (62 wildtype samples and the 18 Bcl6 deletion samples) are available as interactive heatmaps from our network visualization site (https://github.com/flatironinstitute/ILCnetworks).
Core ILC subset gene sets.
ILC subset gene sets were used to develop core networks (Figure 3) and for detection of lineage regulators (Figure 5). They were constructed from our gene expression dataset (20 samples). For a given lineage, ILC subset genes were included in the set if they were more highly expressed (log2(FC)>1, FDR=10%) in that subset relative to at least one other subset and not decreased (log2(FC)<−1, FDR=10%) relative to any other subset in the SI. This analysis yielded overlapping subset-defining gene sets ranging from ~1500-~2500 genes per subset. We also define sets of subset-suppressed genes, which, for a given subset, had to be less expressed in that subset relative to at least one other subset and not increased relative to any other subset in the SI. These core ILC gene signatures of up- and down-regulated genes are contained in Supplemental Data S2.
QUANTIFICATION AND STATISTICAL ANALYSIS
Transcriptional regulatory network inference.
Motif Analysis and Generation of ∆ATAC Prior Matrix.
Peaks were associated with putative transcription-factor (TF) binding events and target genes to generate a “prior” network, , of TF-gene interactions. We collected mouse motifs for 859 TFs from CisBP (Weirauch et al., 2014) and ENCODE (Kheradpour and Kellis, 2014), as described in (Miraldi et al., 2019). We scanned peaks for individual motif occurrences with FIMO (parameters --thresh .00001, --max-stored-scores 500000, and a first-order background model) and included motif occurrences with Praw<10−5 in our analysis. To construct the ∆ATAC prior, core ILC subset ATAC-seq peak sets were defined based on differential accessibility, analogously, with the same parameters, to the core ILC subset gene sets above. For each subset and core TF, we began with all ATAC-seq peaks containing at least one motif occurrence (as the background set), we then tested whether core subset ATAC-seq peaks were enriched nearby core subset genes. Note that to perform the enrichment, genes were mapped to motif-containing peaks; gene sets were converted to “peak sets” and included the union of motif-containing peaks falling within +/−10kb of a gene body in the gene set. We used the hypergeometric CDF to estimate enrichment and applied a nominal significance cutoff of Praw<10−4. TFs significantly enriched in ∆ATAC proximal to core gene sets were included as regulators of the genes in the set that contained a ∆ATAC-proximal TF-motif-containing peak. To detect repressive interactions, we repeated the procedure above looking for enrichment of core ATAC-seq peaks nearby subset-suppressed genes. This procedure was also repeated for gene sets derived from CCR6− ILC3, CCR6+ ILC3 and ILC2 samples from the LI. Finally, we also derived LI- and SI-dependent TF-gene interactions, similarly testing for enrichment between ∆ATAC and ∆gene sets based on LI versus SI accessibility / expression in the three cell types above. The resulting “∆ATAC” prior matrix contained 42,850 TF-gene interactions for 4226 genes and 129 TFs (Supplemental Data S3).
Inference framework.
We built gene expression models according to (Miraldi et al., 2019), using a modified LASSO-StARS framework to solve for TF-gene interaction terms {bik}:
| [Equation 1] |
where, is the-expression-matrix for genes in the prior, is the matrix of inferred TF-gene interaction coefficients, contains-TF-activities, is a matrix-of nonnegative penalties, and ∘ represents a Hadamard (entry-wise matrix) product (Gustafsson et al., 2015; Studham et al., 2014). Representing the LASSO penalty as a Hadamard product involving a matrix of penalty terms, as opposed to a single penalty term, enabled us to incorporate prior information into the model-building procedure. Specifically, a smaller penalty is used if there is evidence for the TF-gene interaction in the prior matrix. This procedure encourages selection of interactions supported by the prior (e.g., containing ATAC-seq evidence), if there is also support in the gene expression data.
For this study, our target gene expression matrix contained the 6418 “core” ILC genes (defined above) for the 62 samples. We considered 445 potential TF regulators (intersection of “core” ILC genes with our curated list of mouse TFs (Miraldi et al., 2019)). Entries of the Λ matrices were limited to two values: the nonnegative value λ, for TF-gene interactions without evidence in the prior, and .25 × λ, for TF-gene interactions with support in the prior. Note that 25 × λ weights interactions in the prior twice as strongly as in our previous Th17 TRN (Miraldi et al., 2019), because, in this context, we have fewer gene expression samples (62 versus 254) and a presumed higher confidence prior (due to ∆ATAC method above).
As described previously, we used the stability-selection method StARS (Liu et al., 2010) with 50 subsamples of size . 63 × |samples| and an instability cutoff = .05 to solve for the TF-gene interactions B. We then ranked TF-gene interactions based on stability as described in (Miraldi et al., 2019) and used out-of-sample prediction (described below) to determine how many interactions to include in the final TRN. To estimate the TF activity matrix, we used two methods: (1) TF gene expression and (2) prior knowledge of TF target gene expression (Arrieta-Ortiz et al., 2015). For (2), we used the following relationship to solve for TF activities: X = PA [Equation 2], where is the (∆ATAC) prior. We generated-separate TRNs using each of the TF activity methods and rank combined TRNs using the maximum (Miraldi et al., 2019). Based on gene-expression prediction (described below, Figure S4D,E), our ILC TRN size was set to an average of 10 TFs per gene (total 63,832 TF-gene interactions). At this model size, 25,113 TRNs interactions (39%) had ∆ATAC prior support, and the TRN contained 59% of the 42,850 prior interactions (Supplemental Data S4). Thus, many ∆ATAC are filtered out (41% of the prior) because they are not supported by the gene expression data and new interactions (61% of the final TRN) are learned from gene expression patterns alone.
Gene Expression Prediction.
TRN model quality was computationally assessed by out-of-sample gene expression prediction. To ensure robustness of results, four separate out-of-sample gene expression prediction tasks were designed, where, for each independently, ILC TRN models were built in the absence of (1) all ILC1 (5 samples) from (Gury-BenAri et al., 2016), (2) all ILC2 (5 samples) from (Gury-BenAri et al., 2016), (3) all ILC3 (6 samples) from (Gury-BenAri et al., 2016), and (4) all eight large intestine (LI) samples. For each out-of-sample prediction challenge, models were built using prior-based TFA (TFA=P+A) and TF mRNA (see “Inference Framework”). Because the LI gene expression data had been used to construct the ∆ATAC prior, TF-gene interactions based on gene sets derived from the LI samples were omitted and the reduced prior was used to train the model.
Training TFA matrices were mean-centered and variance-normalized according to the training-set means and standard deviations . Target gene expression vectors were mean-centered according to the training-set mean . Predictive performance was quantified with as a function of mean model size (TFs / gene) (Figure S4D,E). In brief, for each model-size cutoff, we regressed the vector of normalized training gene expression data onto the reduced set of normalized training TFA estimates to arrive at a set of multivariate linear coefficients . is defined as:
| [Equation 3a] |
where
| [Equation 3b] |
and
| [Equation 3c] |
Any indicates that the Inferelator model has predictive benefit over the null model (simply using mean gene expression from the observed training data). For all leave-out gene expression prediction tasks, media increased dramatically from model-size two to five TFs / gene and began to plateau at 10 TFs / gene, suggesting 10 TFs / gene as model-size cutoff (Figure S4D,E). We note that ILC TRN analyses (Figures 3–5, 7) were robust to variation in this cutoff (e.g., Figure S7A).
TF-TF Module Analysis
To identify co-regulation of gene pathways by TFs, TF-TF modules were constructed (Miraldi et al., 2019). In brief, for positive and negative edges separately, we calculated a background-normalized overlap score zij (N between TF i and TF j as:
| [Equation 4] |
where zi|j (N is the z-score of the overlap between TF i and TF j, using the mean and standard deviation associated with the overlaps of TF j to calculate the z-score. We filtered the normalized overlap matrix so that it contained only TFs with at least one significant overlap (FDR = 10%). We then converted the similarity matrix of normalized overlaps to a distance matrix for hierarchical clustering using Ward distance. To arrive at a final number of clusters, we calculated the mean silhouette score for solutions over a range of total clusters and selected the solution that maximized silhouette score. For positive interactions, this analysis lead to 57 clusters, and 12 clusters for negative interactions. We estimated the significance of individual clusters based on cluster size and overlap between TFs in the cluster (Miraldi et al., 2019). We only report positive TFTF clusters, because, as observed previously (Miraldi et al., 2019), positive TF-TF clusters were orders of magnitude more significant than TF-TF clusters based on repressive interactions.
Supplementary Material
Supplemental Data S1, related to Figure 3. Batch-corrected gene expression data combining samples from this study, Gury-BenAri et al., and Shih et al.
Supplemental Data S2, related to Figure 3. Up-regulated and down-regulated ILC subset gene signatures (see Methods).
Supplemental Data S3, related to Figure 3. ∆ATAC prior matrix.
Supplemental Data S4, related to Figure 3. The ILC TRN.
Highlights:
RNA- and ATAC-seq integration yields genome-scale networks of gene regulation in ILCs
Transcription-factor activators and repressors for each ILC subset are identified
c-MAF and BCL6 regulate the balance of ILC1-to-ILC3 in the intestine
BCL6 suppresses ILC3 genes and promotes NK gene programs in ILC subsets
Acknowledgements
We thank the Flatiron Institute Scientific Computing Core (I. Fisk) and the NYU School of Medicine Genome Technology Core (GTC) (A. Heguy and P. Zappile). This work was supported by the Cincinnati Children’s Research Foundation (Trustee Award to E.R.M., Research Innovation Award to S.N.W.; Research In Residency Award to N.S.C.; and Arnold P. Strauss Fellowship to D.O.), the Simons Foundation (E.R.M., A.W., N.D., N.C., R.B.), U.S. National Institute of Health (5T32AI100853 to M.P.; R01-DK103358-01 to R.B. and D.R.L.; DA038017 to S.N.W.; and R01-GM112192-01 to R.B., T32 CA009161 (Levy) to J.A.H.), Howard Hughes Medical Institute (D.R.L.), Damon Runyon Cancer Research Foundation (Dale and Betty Frey Fellowship to J.A.H.) and the Colton Center for Autoimmunity (D.R.L). The NYU GTC was supported by the Cancer Center Support Grant P30CA016087 at the Laura and Isaac Perlmutter Cancer Center. Cell sorting and flow cytometric data acquired at Cincinnati Children’s relied on equipment maintained by the Research Flow Cytometry Core, which is supported in part by NIH grants AR47363, DK78392 and DK90971.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations
The authors declare no competing interests.
DATA AND SOFTWARE AVAILABILITY
The raw and processed RNA-seq and ATAC-seq data have been deposited in the NCBI Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE116093.
ADDITIONAL RESOURCES
Networks were visualized using a newly designed interactive interface, based on iPython and packages: igraph, numpy and scipy (https://github.com/simonsfoundation/jp_gene_viz). ILC TRNs and heatmaps of gene expression are available from the following link: https://github.com/flatironinstitute/ILCnetworks.
References
- Adams NM, Lau CM, Fan X, Rapp M, Geary CD, Weizman O-E, Diaz-Salazar C, and Sun JC (2018). Transcription Factor IRF8 Orchestrates the Adaptive Natural Killer Cell Response. Immunity [DOI] [PMC free article] [PubMed]
- Anders S, Pyl PT, and Huber W (2015). HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta-Ortiz ML, Hafemeister C, Bate AR, Chu T, Greenfield A, Shuster B, Barry SN, Gallitto M, Liu B, Kacmarczyk T, et al. (2015). An experimentally supported model of the Bacillus subtilis global transcriptional regulatory network. Mol. Syst. Biol 11, 839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aust JG, Gays F, Mickiewicz KM, Buchanan E, and Brooks CG (2009). The Expression and Function of the NKRP1 Receptor Family in C57BL/6 Mice. J. Immunol 183, 106 LP-116. [DOI] [PubMed] [Google Scholar]
- Bernink JH, Peters CP, Munneke M, Te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, and Buskens CJ (2013). Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat. Immunol 14, 221. [DOI] [PubMed] [Google Scholar]
- Bernink JH, Krabbendam L, Germar K, de Jong E, Gronke K, Kofoed-Nielsen M, Munneke JM, Hazenberg MD, Villaudy J, and Buskens CJ (2015). Interleukin-12 and-23 control plasticity of CD127+ group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 43, 146–160. [DOI] [PubMed] [Google Scholar]
- Björklund AK, Forkel M, Picelli S, Konya V, Theorell J, Friberg D, Sandberg R, and Mjösberg J (2016). The heterogeneity of human CD127+ innate lymphoid cells revealed by single-cell RNA sequencing. Nat. Immunol 17, 451–460. [DOI] [PubMed] [Google Scholar]
- Blatti C, Kazemian M, Wolfe S, Brodsky M, and Sinha S (2015). Integrating motif, DNA accessibility and gene expression data to build regulatory maps in an organism. Nucleic Acids Res 43, 3998–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau R, Reiss DJ, Shannon P, Facciotti M, Hood L, Baliga NS, and Thorsson V (2006). The Inferelator: an algorithm for learning parsimonious regulatory networks from systems-biology data sets de novo. Genome Biol 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Giresi PG, Zaba LC, Chang HY, and Greenleaf WJ (2013). Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyle JR, Mesci A, Ljutic B, Belanger S, Tai L-H, Rousselle E, Troke AD, Proteau M-F, and Makrigiannis AP (2006). Molecular and Genetic Basis for Strain-Dependent NK1.1 Alloreactivity of Mouse NK Cells. J. Immunol 176, 7511 LP-7524. [DOI] [PubMed] [Google Scholar]
- Chang CC, Ye BH, Chaganti RS, and Dalla-Favera R (1996). BCL-6, a POZ/zinc-finger protein, is a sequence-specific transcriptional repressor. Proc. Natl. Acad. Sci 93, 6947 LP-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier M, Ohnmacht C, Cording S, and Eberl G (2012). Development and function of intestinal innate lymphoid cells. Curr. Opin. Immunol 24, 277–283. [DOI] [PubMed] [Google Scholar]
- Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, Agarwal A, Huang W, Parkurst CN, Muratet M, et al. (2012). A validated regulatory network for Th17 cell specification. Cell 151, 289–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides MG, McDonald BD, Verhoef PA, and Bendelac A (2014). A committed precursor to innate lymphoid cells. Nature 508, 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides MG, Gudjonson H, McDonald BD, Ishizuka IE, Verhoef PA, Dinner AR, and Bendelac A (2015). PLZF expression maps the early stages of ILC1 lineage development. Proc. Natl. Acad. Sci 112, 5123 LP-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Johnston RJ, and Schoenberger SP (2010). Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat. Immunol 11, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G, Colonna M, Di Santo JP, and McKenzie ANJ (2015). Innate lymphoid cells: A new paradigm in immunology. Science (80-). 348, aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, and Zhu J (2017). Dynamic balance between master transcription factors determines the fates and functions of CD4 T cell and innate lymphoid cell subsets. J. Exp. Med jem-20170494. [DOI] [PMC free article] [PubMed]
- Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, Lindsten T, and Reiner SL (2012). The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity 36, 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J, Oehrle K, Worthen G, Alenghat T, Whitsett J, and Deshmukh H (2017). Intestinal commensal bacteria mediate lung mucosal immunity and promote resistance of newborn mice to infection. Sci. Transl. Med 9, eaaf9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gury-BenAri M, Thaiss CA, Serafini N, Winter DR, Giladi A, Lara-Astiaso D, Levy M, Salame TM, Weiner A, David E, et al. (2016). The Spectrum and Regulatory Landscape of Intestinal Innate Lymphoid Cells Are Shaped by the Microbiome. Cell 166, 1231–1246.e13. [DOI] [PubMed] [Google Scholar]
- Gustafsson M, Gawel DR, Alfredsson L, Baranzini S, Bjorkander J, Blomgran R, Hellberg S, Eklund D, Ernerudh J, Kockum I, et al. (2015). A validated gene regulatory network and GWAS identifies early regulators of T cell-associated diseases. Sci. Transl. Med 7, 1–10. [DOI] [PubMed] [Google Scholar]
- Hatzi K, Jiang Y, Huang C, Garrett-Bakelman F, Gearhart MD, Giannopoulou EG, Zumbo P, Kirouac K, Bhaskara S, Polo JM, et al. (2013). A hybrid mechanism of action for BCL6 in B-cells defined by formation of functionally distinct complexes at enhancers and promoters. Cell Rep 4, 10.1016/j.celrep.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, and Crotty S (2015a). BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J. Exp. Med 212, 539—553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzi K, Nance JP, Kroenke MA, Bothwell M, Haddad EK, Melnick A, and Crotty S (2015b). BCL6 orchestrates Tfh cell differentiation via multiple distinct mechanisms. J. Exp. Med 212, 539—553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held W, Clevers H, and Grosschedl R (2003). Redundant functions of TCF-1 and LEF-1 during T and NK cell development, but unique role of TCF-1 for Ly49 NK cell receptor acquisition. Eur. J. Immunol 33, 1393–1398. [DOI] [PubMed] [Google Scholar]
- Hepworth MR, Monticelli LA, Fung TC, Ziegler CGK, Grunberg S, Sinha R, Mantegazza AR, Ma H-L, Crawford A, and Angelosanto JM (2013). Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 498, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth MR, Fung TC, Masur SH, Kelsen JR, McConnell FM, Dubrot J, Withers DR, Hugues S, Farrar MA, and Reith W (2015). Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria–specific CD4+ T cells. Science (80-). 348, 1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, et al. (2011). Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol 12, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister K, Kusam S, Wu H, Clegg N, Mondal A, Sawant DV, and Dent AL (2013). Insights into the Role of Bcl6 in Follicular Th Cells Using a New Conditional Mutant Mouse Model. J. Immunol 191, 3705 LP-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyler T, Klose CSN, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M, and Diefenbach A (2012). The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity 37, 634–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Li C, and Rabinovic A (2007). Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8, 118–127. [DOI] [PubMed] [Google Scholar]
- Kallies A, Carotta S, Huntington ND, Bernard NJ, Tarlinton DM, Smyth MJ, and Nutt SL (2011). A role for Blimp1 in the transcriptional network controlling natural killer cell maturation. Blood 117, 1869 LP-1879. [DOI] [PubMed] [Google Scholar]
- Karwacz K, Miraldi ER, Pokrovskii M, Madi A, Yosef N, Wortman I, Chen X, Watters A, Carriero N, Awasthi A, et al. (2017). Critical role of IRF1 and BATF in forming chromatin landscape during type 1 regulatory cell differentiation. Nat. Immunol 18, 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradpour P, and Kellis M (2014). Systematic discovery and characterization of regulatory motifs in ENCODE TF binding experiments. Nucleic Acids Res 42, 2976–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose CSN, Kiss EA, Schwierzeck V, Ebert K, Hoyler T, d’Hargues Y, Göppert N, Croxford AL, Waisman A, and Tanriver Y (2013). A T-bet gradient controls the fate and function of CCR6− RORγt+ innate lymphoid cells. Nature 494, 261. [DOI] [PubMed] [Google Scholar]
- Klose CSN, Flach M, Möhle L, Rogell L, Hoyler T, Ebert K, Fabiunke C, Pfeifer D, Sexl V, and Fonseca-Pereira D (2014). Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 157, 340–356. [DOI] [PubMed] [Google Scholar]
- Koues OI, Collins PL, Cella M, Robinette ML, Porter SI, Pyfrom SC, Payton JE, Colonna M, and Oltz EM (2016). Distinct Gene Regulatory Pathways for Human Innate versus Adaptive Lymphoid Cells. Cell 165, 1134–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, and Salzberg SL (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AI, Menegatti S, Bustamante J, Le Bourhis L, Allez M, Rogge L, Casanova J-L, Yssel H, and Di Santo JP (2016). IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J. Exp. Med jem-20151750. [DOI] [PMC free article] [PubMed]
- Lim AI, Verrier T, Vosshenrich CAJ, and Di Santo JP (2017). Developmental options and functional plasticity of innate lymphoid cells. Curr. Opin. Immunol 44, 61–68. [DOI] [PubMed] [Google Scholar]
- Liu H, Roeder K, and Wasserman L (2010). Stability Approach to Regularization Selection (StARS) for High Dimensional Graphical Models. In Advances in Neural Information Processing Systems 23, Lafferty JD, Williams CKI, Shawe-Taylor J, Zemel RS, and Culotta A, eds. (Curran Associates, Inc.), pp. 1432–1440. [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace EM, Bigley V, Gunesch JT, Chinn IK, Angelo LS, Care MA, Maisuria S, Keller MD, Togi S, and Watkin LB (2017). Biallelic mutations in IRF8 impair human NK cell maturation and function. J. Clin. Invest 127, 306–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MT, and O’Callaghan CA (2014). PeaKDEck: a kernel density estimator-based peak calling program for DNaseI-seq data. Bioinformatics 30, 1302–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo-Gonzalez F, and Hepworth MR (2017). Functional and phenotypic heterogeneity of group 3 innate lymphoid cells. Immunology 150, 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke LA, Groom JR, Rankin LC, Seillet C, Masson F, Putoczki T, and Belz GT (2013). TCF-1 Controls ILC2 and NKp46<sup>+</sup>RORγt<sup>+</sup> Innate Lymphocyte Differentiation and Protection in Intestinal Inflammation. J. Immunol 191, 4383 LP-4391. [DOI] [PubMed] [Google Scholar]
- Miraldi ER, Pokrovskii M, Waters A, Castro DM, De Veaux N, Hall J, Lee J-Y, Ciofani M, Madar A, and Carriero N (2019). Leveraging chromatin accessibility for transcriptional regulatory network inference in T Helper 17 Cells. Genome Res 29, 449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narni-Mancinelli E, Chaix J, Fenis A, Kerdiles YM, Yessaad N, Reynders A, Gregoire C, Luche H, Ugolini S, and Tomasello E (2011). Fate mapping analysis of lymphoid cells expressing the NKp46 cell surface receptor. Proc. Natl. Acad. Sci 108, 18324–18329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohne Y, Silver JS, Thompson-Snipes L, Collet MA, Blanck JP, Cantarel BL, Copenhaver AM, Humbles AA, and Liu Y-J (2016). IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat. Immunol 17, 646. [DOI] [PubMed] [Google Scholar]
- van de Pavert SA, and Vivier E (2015). Differentiation and function of group 3 innate lymphoid cells, from embryo to adult. Int. Immunol 28, 35–42. [DOI] [PubMed] [Google Scholar]
- Pikovskaya O, Chaix J, Rothman NJ, Collins A, Chen Y-H, Scipioni AM, Vivier E, and Reiner SL (2016). Cutting edge: eomesodermin is sufficient to direct type 1 innate lymphocyte development into the conventional NK lineage. J. Immunol 1502396. [DOI] [PMC free article] [PubMed]
- Pique-Regi R, Degner JF, Pai AA, Gaffney DJ, Gilad Y, and Pritchard JK (2011). Accurate inference of transcription factor binding from DNA sequence and chromatin accessibility data. Genome Res 21, 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope SD, and Medzhitov R (2018). Emerging Principles of Gene Expression Programs and Their Regulation. Mol. Cell 71, 389–397. [DOI] [PubMed] [Google Scholar]
- Powell N, Walker AW, Stolarczyk E, Canavan JB, Gökmen MR, Marks E, Jackson I, Hashim A, Curtis MA, and Jenner RG (2012). The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity 37, 674–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, and Hall IM (2010). BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabacal W, Pabbisetty SK, Hoek KL, Cendron D, Guo Y, Maseda D, and Sebzda E (2016). Transcription factor KLF2 regulates homeostatic NK cell proliferation and survival. Proc. Natl. Acad. Sci 113, 5370 LP-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinette ML, Fuchs A, Cortez VS, Lee JS, Wang Y, Durum SK, Gilfillan S, and Colonna M (2015). Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat. Immunol 1, 45–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz S, Noubade R, Eidenschenk C, Ota N, Zeng W, Zheng Y, Hackney J, Ding J, Singh H, and Ouyang W (2011). Transcription factor c-Maf mediates the TGF-β-dependent suppression of IL-22 production in TH17 cells. Nat. Immunol 12, 1238. [DOI] [PubMed] [Google Scholar]
- Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, and Hogenesch JB (2004). A Functional Genomics Strategy Reveals Rora as a Component of the Mammalian Circadian Clock. Neuron 43, 527–537. [DOI] [PubMed] [Google Scholar]
- Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, and Eberl G (2010). Lineage Relationship Analysis of RORγt<sup>+</sup> Innate Lymphoid Cells. Science (80-). 330, 665 LP-669. [DOI] [PubMed] [Google Scholar]
- Schlenner SM, Madan V, Busch K, Tietz A, Läufle C, Costa C, Blum C, Fehling HJ, and Rodewald H-R (2010). Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity 32, 426–436. [DOI] [PubMed] [Google Scholar]
- Sciumé G, Hirahara K, Takahashi H, Laurence A, Villarino AV, Singleton KL, Spencer SP, Wilhelm C, Poholek AC, Vahedi G, et al. (2012). Distinct requirements for T-bet in gut innate lymphoid cells. J. Exp. Med 209, 2331 LP-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini N, Vosshenrich CAJ, and Di Santo JP (2015). Transcriptional regulation of innate lymphoid cell fate. Nat. Rev. Immunol 15, 415. [DOI] [PubMed] [Google Scholar]
- Shih HY, Sciumè G, Mikami Y, Guo L, Sun HW, Brooks SR, Urban JF, Davis FP, Kanno Y, and O’Shea JJ (2016). Developmental Acquisition of Regulomes Underlies Innate Lymphoid Cell Functionality. Cell 165, 1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Maurin M, Cho H Il, Becknell B, Freud AG, Yu J, Wei S, Djeu J, Celis E, Caligiuri MA, et al. (2010). PRDM1/Blimp-1 Controls Effector Cytokine Production in Human NK Cells. J. Immunol 185, 6058 LP-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, and Artis D (2015). Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat. Med 21, 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, and Cupedo T (2012). Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol 30, 647–675. [DOI] [PubMed] [Google Scholar]
- Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, and Mebius RE (2013). Innate lymphoid cells—a proposal for uniform nomenclature. Nat. Rev. Immunol 13, 145. [DOI] [PubMed] [Google Scholar]
- Studham ME, Tjärnberg A, Nordling TEM, Nelander S, and Sonnhammer ELL (2014). Functional association networks as priors for gene regulatory network inference. Bioinformatics 30, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait Wojno ED, and Artis D (2012). Innate lymphoid cells: balancing immunity, inflammation, and tissue repair in the intestine. Cell Host Microbe 12, 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait Wojno ED, and Artis D (2016). Emerging concepts and future challenges in innate lymphoid cell biology. J. Exp. Med 213, 2229–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, and Glimcher LH (2004). T-bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity 20, 477–494. [DOI] [PubMed] [Google Scholar]
- Verrier T, Satoh-Takayama N, Serafini N, Marie S, Di Santo JP, and Vosshenrich CAJ (2016). Phenotypic and functional plasticity of murine intestinal NKp46+ group 3 innate lymphoid cells. J. Immunol 1502673. [DOI] [PubMed]
- Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, et al. (2018). Innate Lymphoid Cells: 10 Years On. Cell 174, 1054–1066. [DOI] [PubMed] [Google Scholar]
- Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, and Hölscher C (2010). Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt+ innate lymphocytes. Immunity 33, 736–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JA, Barlow JL, and McKenzie ANJ (2013). Innate lymphoid cells-how did we miss them? Nat. Rev. Immunol 13, 75–87. [DOI] [PubMed] [Google Scholar]
- Weirauch MT, Yang A, Albu M, Cote AG, Montenegro-Montero A, Drewe P, Najafabadi HS, Lambert SA, Mann I, Cook K, et al. (2014). Determination and Inference of Eukaryotic Transcription Factor Sequence Specificity. Cell 158, 1431–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wende H, Lechner SG, Cheret C, Bourane S, Kolanczyk ME, Pattyn A, Reuter K, Munier FL, Carroll P, Lewin GR, et al. (2012). The Transcription Factor c-Maf Controls Touch Receptor Development and Function. Science (80-). 335, 1373 LP-1376. [DOI] [PubMed] [Google Scholar]
- Xu M, Pokrovskii M, Ding Y, Yi R, Au C, Harrison OJ, Galan C, Belkaid Y, Bonneau R, and Littman DR (2018). c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature 554, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosef N, Shalek AK, Gaublomme JT, Jin H, Lee Y, Awasthi A, Wu C, Karwacz K, Xiao S, Jorgolli M, et al. (2013). Dynamic regulatory network controlling TH17 cell differentiation. Nature 496, 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Freud AG, and Caligiuri MA (2013). Location and cellular stages of natural killer cell development. Trends Immunol 34, 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, and Hogenesch JB (2014). A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci 111, 16219 LP-16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data S1, related to Figure 3. Batch-corrected gene expression data combining samples from this study, Gury-BenAri et al., and Shih et al.
Supplemental Data S2, related to Figure 3. Up-regulated and down-regulated ILC subset gene signatures (see Methods).
Supplemental Data S3, related to Figure 3. ∆ATAC prior matrix.
Supplemental Data S4, related to Figure 3. The ILC TRN.