Abstract
Introduction
Plaque incision and Grafting (PIG) for Peyronie’s disease (PD) is not devoid of complications such as erectile dysfunction and penile sensory changes.
Aims
The aim of this study was to define the rate and chronology of penile sensation loss after PIG surgery and to define predictors of such.
Methods
The study population consisted of patients with PD associated penile curvature who underwent PIG surgery and with at least 6 months of follow-up. Demographics and PD factors were recorded. Patient had preoperative assessment of penile sensation and deformity. Postoperative follow-up occurred at 1 week, 1 month, 6 months and 1 year after surgery. Neurovascular bundle elevation was conducted with loupe magnification.
Main outcomes measures
Penile sensation was evaluated with a biothesiometer and graded on a patient reported visual analog scale (0–10) where 0 defined a completely numb area and 10 perfect sensation. The degree of sensation loss was defined as extensive (any single area >5cm), major (2–5 cm) and minor (≤2cm). The penile sensation loss distribution was defined as focal (single site) or diffuse (>1 site).
Results
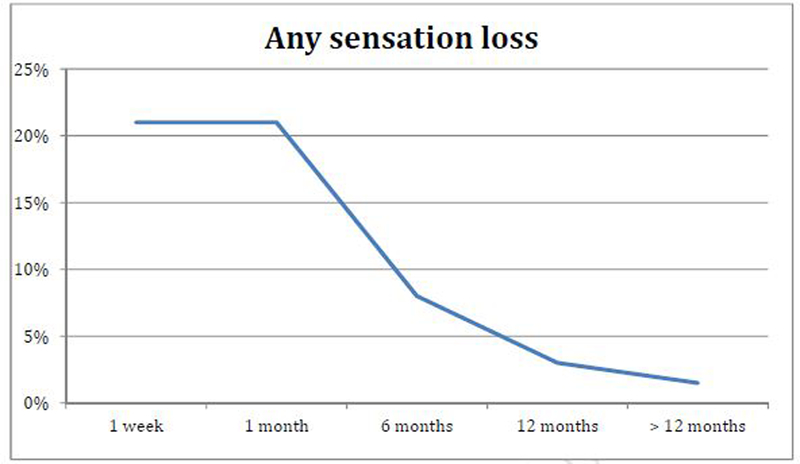
63 patients were analyzed. Mean age was 56±10 years. Mean duration of PD at the time of PIG was 15±7 (12–38) months. 75% had curvature alone, 25% had hourglass/Indentation deformities. Mean primary curvature was 64±28°. The mean operation duration was 3.5±1.8 hours. 21% had some degree of sensation loss at one week, 21% at one month, 8% at 6 months, 3% at 12 months. Only a single patient (1.5%) at 2 years continued to have extensive sensation loss on the glans and distal shaft with a very elevated sensitivity threshold. Using multivariable analysis the only predictor of penile sensation loss ≥ 6 months was duration of operation >4 hours, OR 2.1, 95% CI 1.2–3.0 (p<0.01).
Conclusions
Sensation loss is not uncommon after PIG surgery. It decreases in frequency and severity with time with only rare cases occurring beyond 12 months. Longer operations appeared to be more likely associated with sensation loss.
Keywords: Peyronie’s disease, plaque Incision, Grafting, Penile Induration, Surgical Treatment, Complications, Sensory
Introduction
Peyronie’s disease (PD) is a fibrotic condition of the tunica albuginea that is associated with penile pain, deformity and erectile dysfunction (ED)1.
The prevalence of PD in the general population ranges from 0.3–9% with an incidence of2–5 up to 20% in diabetics patients6. The primary etiologic factor is not clear, numerous etiologic theories exist including: Genital Trauma, genetic predisposition, autoimmune disorder, collagen alterations, over expression of pro inflammatory cytokines7.
Surgical procedures remain the gold standard for definitive deformity correction. 8 The primary goal of surgery is to ensure the patient has a functional erection. Plaque incision and grafting has been used since 19509 and represents a surgical option, typically reserved for men with severe curvature, complex deformities and those who have an hour glass deformity.
However, Plaque incision and Grafting (PIG) is not devoid of complications as postoperative ED rates range from 0–67%1, 10–13. The issue of rates of postoperative penile sensory had been cited from 0–20%1, 14–16. To date, there is little detailed information on the nature or chronology of this sensation loss.
The aim of this study was to define the rate and chronology of penile sensation loss after PIG and furthermore to define predictors of such sensory loss.
Methods
Patient Population:
Patients who had underwent PIG surgery for dorsal or lateral deformity and at least 6 months of follow-up were included in this analysis. Demographics and PD factors were recorded. Patient had a preoperative assessment which included penile sensation and curvature assessment. They had postoperative follow-up at 1 week, 1 month, 6 months and 1 year after surgery.
Curvature Assessment:
All curvature assessments were done after an intracavernosal injection (ICI). The injection agent used was trimix (papaverine/phentolamine/prostaglandin E1) with redosing of vasoactive agent used to induce a rigid erection. The degree of curvature was measured with a goniometer17. The center of the goniometer was positioned over the point of maximum curvature. Stretched flaccid penile length was also measured.
Penile Sensation Assessment:
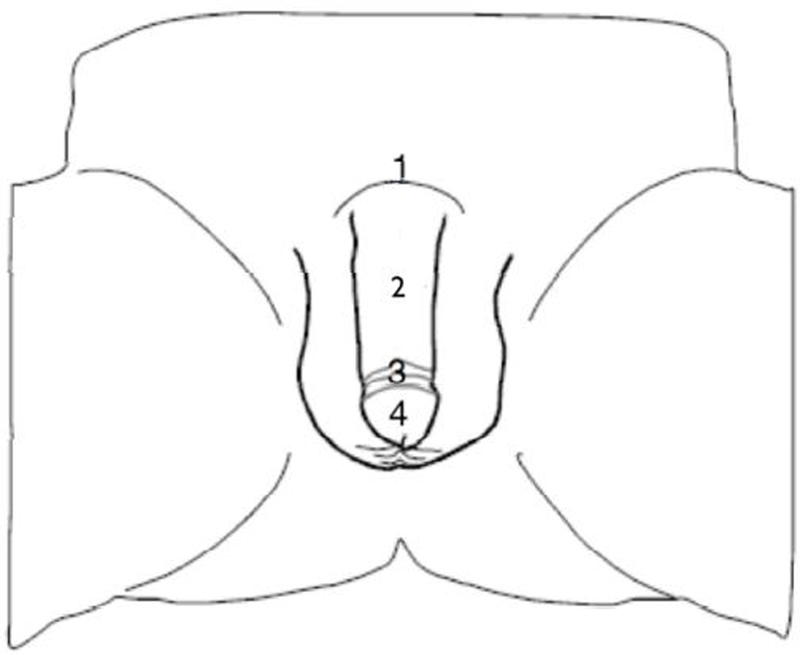
A biothesiometer (Biomedical Instruments, Newsbury, OH, USA) was used for vibratory sensation assessment (Set at 120Hz). This instrument is designed to measure simply and accurately the threshold of appreciation of vibration in human subjects. It is used in many neurological diseases such as, neuropathy in diabetes mellitus18. Previous studies have reported biothesiometry, as a reliable method to measure penile sensory loss 19–21. During testing, participants were asked to lie on a bed in a relaxed position. Two cycles were performed in an “ascending-descending” order. The ascending phase began with the lowest level of stimulation increased until perceived; the descending phase began two levels above the previously detected threshold, and decreased until no longer perceived. The average of the 2 phases was recorded as the detection threshold for each point of stimulation. In each case, the stimulus was applied for 1.5 seconds followed by a 5-second delay to eliminate possible carry-over effects between stimuli. Values for vibration were expressed in volts (V) The procedure consisted of measuring the detection thresholds vibration of 6 body locations illustrated in figure 1 which includes; the penile dorsal base, the middle of dorsal penile shaft, the corona of the glans, the middle of the glans, the frenulum, the penile ventral base. Studies have defined a Score ≤ 7V as normal sensitivity20, 22. A nomogram is available to define normalcy of vibration thresholds that has been shown to be superior to the use of a tuning fork in accuracy20.
Figure 1.
Location of biothesiometry assessment (dorsum). 1 = base of penis, dorsal shaft, foreskin retracted; 2 = mid-shaft dorsum; 3 = coronal sulcus, dorsum of the glans; 4 = mid-glans, dorsum.
We also used a patient reported penile sensation visual analog scale (0–10) where 0 defined a completely numb area and 10 a perfect sensation. The grade of loss sensation was defined as extensive (any single area >5cm), major (2–5 cm) and minor (≤2cm). The penile sensation loss distribution was focal (single site) or diffuse (>1 site).
Surgical Procedure:
A single experienced surgeon did all of the procedures over a 6 years period 2004–2010. Under general anesthesia, the penis was degloved. The neurovascular bundle NVB was elevated under loupe assistance (3.5×). Prior to 2006 the procedure used an H-type incision, after this date the Egydio geometric incision has been used23. Grafting was performed predominantly with cadaveric pericardium ( Tutoplast, Coloplast, Mineapolis, MN, USA), although a small number of procedures were conducted using intestinal submucosa (SIS® surgisis, Cook Urological Incorporated) or dorsal/saphenous vein. Following incision of the tunica and straightening of the deformity, the graft was measured and sutured in place using 4/0 PDS suture. We never used a tourniquet during neurovascular bundle dissection or during plaque incision.
We always prescribe “penile rehabilitation” following surgery including PDE5i’s and traction therapy commencing one week after surgery for a 3 month period after the PIG surgery.
Statistical Analysis:
Means (± standard deviations) and percentages are used to describe the study sample. Percentages are reported to outline the incidence of penile sensation loss, and correlation coefficients are used to determine the relationship between the visual analog score and the penile vibrotactile sensitivity thresholds. Logistic regression was used in multivariable analysis to define predictors of sensory loss. Factors entered into the model were patient age, presence of diabetes mellitus, Peyronie’s disease duration, and operation duration. All statistical analysis were performed in SPSS (SPSS, Inc, Chicago, IL, USA)
Results
Patient Population:
63 patients were included in this analysis. Mean patient age was 56±10 years. Mean duration of PD at the time of PIG was 15±7 (12–38) months, the mean follow-up was 14±12 months and self reported duration of stability was eight months. 10% had diabetes preoperatively with a mean HBA1C =7.2±1.8%. 75% had a curvature alone, 25% had associated hourglass/Indentation deformities. Mean primary curvature was 64±28°. 53 patients had grafting with human cadaveric pericardium, 6 with vein and 4 with intestinal submucosa. 38% (24/63) of the PIG used a H-incision and 62% (39/63) used the Egydio geometric incision. The mean operation duration was 3.5±1.8 (2.5–5.5) hours. 80% had a preoperative and postoperative biothesiometry. Subject characteristics are presented in Table 1.
Table 1.
Subject Characteristics
| Variable | Result (±SD) |
|---|---|
| N | 63 patients |
| Mean Age (Years) | 56±10 |
| Diabetes | 10% |
| Mean Follow-up (Months) | 14±12 |
| Mean PD duration (Months) | 15±7 (12–38) |
| Mean duration of self-reported stable PD (Months) | 8 |
| Curvature alone (%) | 75% |
| Indentations/HGD (%) | 25% |
| Mean primary curvature (Degrees) | 64±28 |
Penile Sensation:
21% had any sensation loss at one week, 21% at one month, 8% at 6 month, 3% at 12 months and only a single patient (1.5%) at 2 years continued to have extensive sensation loss on the glans and distal shaft (Figure 2). The severity and the distribution of the penile sensation loss are presented in Table 2. For the entire study group, the median biothesiometry score stayed ≤7 during all of the follow-up. The median biothesiometry scores were higher than 7 for all patients with loss of sensation. (Table 3). The single patient who continued to have a sensation loss after 12 months had persistently very elevated biothesiometry thresholds at 25. There was no difference in rates of nerve injury with the surgical technique (H-type incision or the Egydio geometric incision) or among graft type used.
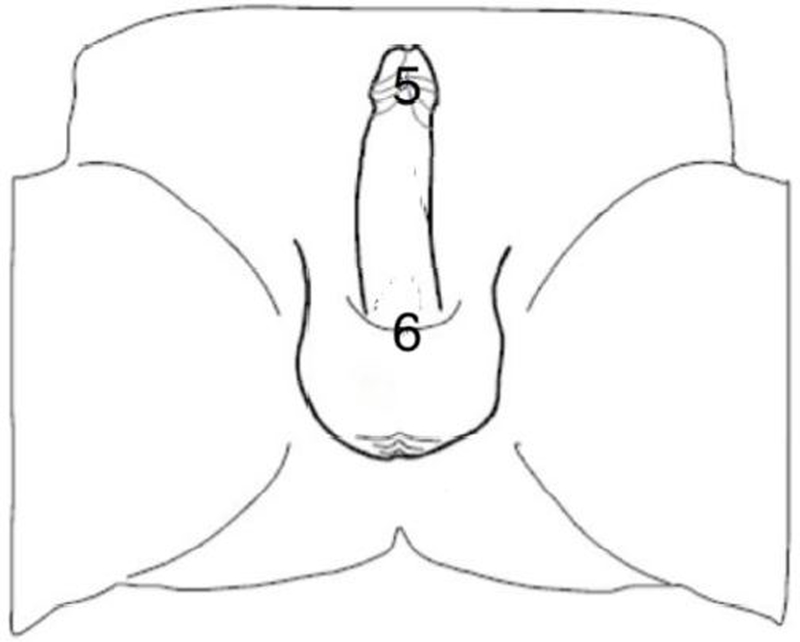
Figure 2.
Location of biothesiometry assessment (ventrum). 5 = Frenulum, foreskin retracted; 6 = base, ventrum.
Table 2.
Sensation Loss Parameters
| Parameter | 1 Month (n=13) | 6 Months (n=5) | 12 Months (n=1) |
|---|---|---|---|
| Mean VAS* score | 3±2 | 5±3 | 7±3 |
| Grade | |||
| Extensive (>5cm) | N=1 (8%) | N=1 (20%) | N=1 (100%) |
| Major (2–5cm) | N=4 (31%) | N=2 (40%) | 0 |
| Minor (<2 cm) | N=8 (61%) | N=2 (40%) | 0 |
| Distribution | |||
| Focal (1 site) | N=3 (23%) | N=6 (60%) | N=1 (100%) |
| Multi-focal (>1 site) | N=10 (77%) | N=4 (40%) | 0 |
visual analog scale
Table 3.
Median Biothesiometry Score (Inter Quartile range). Score ≤7 defined as normal sensitivity
| Parameter | Baseline | 1 Month | 6Months | 12 Months |
|---|---|---|---|---|
| All patients (frenulum) | 3 (2,4) | 5 (2,22) | 5(2,17) | 4(2,6) |
| Patient with loss (site of worst loss) | 3 (2,4) | 11 (8,27)* | 10 (8,11)** | 25*** |
n=13,
n=5,
n=1
Of the 63 patients, 20 had a significant decrease in their erectile rigidity after PIG surgery, 11 using PDE5i to aid in generating a penetration rigidity erection, 9 needing intracavernosal injections.
Using the multivariable analysis, only the duration of operation was a predictor of loss sensation at 6 months postoperatively, duration >4 hours being predictive, OR 2.1, 95% CI 1.2–3.0 (p<0.01).
Discussion
Surgical management is the gold standard for the definitive treatment in patients with stable PD. Many techniques have been described for the surgical correction of PD such as tunical plication or the placement of an inflatable prosthesis. PIG is recommended for patients with complex penile curvature deformities >60°, and/or short penile length, with no preoperative Erectile dysfunction and normal penile hemodynamic evaluation8.
This study was designed to evaluate the chronology, severity and the long-term resolution of penile sensory changes after PIG. 21% had some sensory loss at one week, 21% at one month, 8% at 6 months and 3% at 12 months. Only a single patient (1.5%) at 2 years continued to have sensation loss. In the literature, penile sensation loss after PIG has been cited as 0–20%1, 14–16. However, in most series sensation loss has been evaluated solely by patient self-report12, 14–16, 24–34 The major limitation of the aforementioned studies is the absence of a preoperative sensation assessment and the failure to use a objective assessment such as, biothesiometry.
Overall, complete penile loss sensation has not been mentioned in the literature and in most cases penile sensation was reported as recovering within a few months12, 14–16, 24–34 Taylor et al evaluated sensation changes in patients who underwent PIG (n=81) or plication (n=61) surgery with a mean follow-up of 58 months in the PIG group24. Sensation was assessed only by patient report. The authors found that 31% of patients had diminished sensation in both PIG and plication groups, but 90% of patients were capable to achieve an orgasm in the PIG group versus 98% in the plication group. Conversely 26% of plication group reported new onset “delayed orgasm” versus 23% of PIG patients24. There was no analysis of the chronology of sensation recovery but the authors mentioned a recovery ranging from immediately to 9 months postoperatively24.
More recently, Wimpissinger in a small series (n=30) reported the influence of comorbidities on outcomes and satisfaction after PIG16 with follow-up of more than 10 years. 20% of patients reported penile sensation loss on the glans after the operation. Many studies have reported a significant correlation between penile sensory thresholds and age, penile sensation diminishing with age35–39. Another hypothesis for the high rate in this latter series might be the higher rate of diabetes mellitus, 43% in Wimpissinger versus 10% in our study, but only a single man with diabetes experienced sensation loss in Wimpissinger study16.
Knoll et al reported 17% (27/162) of their patients reported temporary penile sensation loss after PIG with small intestinal submucosa33 (SIS® surgisis, Cook Urological Incorporated). These data are consistent with ours; the mean time of the penile sensation loss was 3 (2–12) months. Knoll et al were the only authors who used a validated instrument to measure penile sensation pre and post surgery. Once penile changes sensation resolved, they mentioned that the post-operative sensory thresholds, obtained with the biothesiometer, were the same from baseline assessment, but no detailed biothesiometry data were presented in the paper.
The biothesiometer has several advantages which include simplicity of usage, cost effectiveness and non-invasiveness. Bemelmans et al reported its excellent intra-individual repeatability39, a fixed frequency is set and then the operator tests variable amplitude, expressed in volts20, 39. However the reproducibility of biothesiometer had been discussed in the literature40, 41, it might be explained by the variability of the loading that is applied by hand to the penis, by the variability of the bodily location and the attentiveness of the patients during assessment. Breda et al reported an age dependency of penile sensory thresholds and developed a nomogram to define normalcy of penile sensory thresholds20.
On multivariable analysis, the single predictor of penile sensory loss at ≥6 months post-PIG surgery was an operation duration >4 hours OR 2.1, 95% CI 1.2–3.0 (p<0.01). The mean operative time was 3.5±1.8 (2.5–5.5) hours, consistent with previous PIG studies14, 15. Although this is at odds some more recent literature42, 43, intra-operatively we devote long periods of time to maximizing the health of the NVB in its separation from the plaque dorsally, as well as ensuring a watertight closure of the graft requiring a long period of time suturing. This finding is not surprising, as neurovascular bundle (NVB) dissection and elevation is laborious and time consuming. Furthermore, prolonged periods of NVB traction can lead to an increased risk of neuropraxia. We failed to show a positive correlation with others factors: patient age, presence of diabetes mellitus, Peyronie’s disease duration. These findings are not consistent with previous studies where age and diabetes were associated with higher penile sensation thresholds than in population control35–39, however the diabetic patient numbers in our study were probably too small to demonstrate a significant correlation. The duration of disease was not correlated with penile sensation loss, this is consistent with our experience, as we have not observed more difficult NVB dissection based on PD duration.
This study has some important clinical implications and contributes to our basic understanding of how PIG affects penile sensation. We believe the study highlights the need during patient consent to discuss penile sensation loss. Patients should be informed that rates of penile sensation loss ranges from 2–30% and most patients will have complete resolution of any sensation loss within one year of follow-up.
Furthermore, our study demonstrates the utility of biothesiometry in measuring penile sensation before and after PIG.
There is a paucity of well-designed studies and limited data concerning penile sensitivity following PIG. To our knowledge a single study reported on sensation evaluation with a validated instrument33 and no other studies have described the chronology and severity of penile sensation following PIG. Currently no gold standard assessment exists for penile sensation; however, the biothesiometer is the most widely used quantitative somatosensory test when evaluating penile sensation 21, 37–39.44–54.
However this study is not devoid of complications. First, quantifying the severity of sensation loss is a clinical challenge, and with vibration threshold assessment (biothesiometry) we can only evaluate the integrity of Pacini’s and Meissner’s corpuscle55. A complete sensation assessment would also need to include light touch using the validated Semmens-Weinstein monofilaments (Meissner corpuscle), pressure using the vulvalgesiometer (Merkel Disc), and temperature and pain for free nerve (pain receptors)55. Assessing all the sensation modalities would take an estimated 30–60 minutes and is not practical in routine clinical practice. For this reason we chose to limit our assessment to biothesiometry because of its simplicity of use and low cost. Second, we considered a cutoff for normalcy of 7 Volts, as reported in literature20, 22. This cutoff may misclassify old or diabetic patients and might not be capable of detecting early reductions in vibration perception thresholds. Third, number of patients and absence of control group represent a limitation, but to our knowledge this is the biggest series specifically focused on penile sensation assessment following PD surgery with biothesiometry published data. Finally, 20% of our patients did not have the biothesiometry conducted prior to their PIG surgery although 100% had postoperative evaluation.
Conclusions
Sensation loss is not uncommon after PIG surgery. It decreases in frequency and severity with time with only rare cases occurring beyond 12 months. Longer operations appear to be more likely associated with sensation loss.
Figure 3.
Chronology of Sensation loss
Acknowledgments
Supported by: the Sidney Kimmel Center for Prostate and Urologic Cancers and the National Institutes of Health/National Cancer Institute to Memorial Sloan Kettering Cancer Center through the Cancer Center Support Grant, award number P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Flores S, Choi J, Alex B, Mulhall JP. Erectile dysfunction after plaque incision and grafting: short-term assessment of incidence and predictors. J Sex Med 2011;8: 2031–7. [DOI] [PubMed] [Google Scholar]
- [2].Schwarzer U, Sommer F, Klotz T, Braun M, Reifenrath B, Engelmann U. The prevalence of Peyronie’s disease: results of a large survey. BJU international. 2001;88: 727–30. [DOI] [PubMed] [Google Scholar]
- [3].La Pera G, Pescatori ES, Calabrese M, et al. Peyronie’s disease: prevalence and association with cigarette smoking. A multicenter population-based study in men aged 50–69 years. Eur Urol 2001;40: 525–30. [DOI] [PubMed] [Google Scholar]
- [4].Mulhall JP, Creech SD, Boorjian SA, et al. Subjective and objective analysis of the prevalence of Peyronie’s disease in a population of men presenting for prostate cancer screening. The Journal of urology. 2004;171: 2350–3. [DOI] [PubMed] [Google Scholar]
- [5].Ventimiglia E, Capogrosso P, Colicchia M, et al. Peyronie’s disease and autoimmunity-a real-life clinical study and comprehensive review. J Sex Med 2015;12: 1062–9. [DOI] [PubMed] [Google Scholar]
- [6].Arafa M, Eid H, El-Badry A, Ezz-Eldine K, Shamloul R. The prevalence of Peyronie’s disease in diabetic patients with erectile dysfunction. International journal of impotence research. 2007;19: 213–7. [DOI] [PubMed] [Google Scholar]
- [7].Mulhall JP. Expanding the paradigm for plaque development in Peyronie’s disease. International journal of impotence research. 2003;15 Suppl 5: S93–102. [DOI] [PubMed] [Google Scholar]
- [8].Hatzimouratidis K, Eardley I, Giuliano F, et al. EAU guidelines on penile curvature. Eur Urol 2012;62: 543–52. [DOI] [PubMed] [Google Scholar]
- [9].Lowsley OS, Boyce WH. Further experiences with an operation for the cure of peyronie’s disease. The Journal of urology. 1950;63: 888–902. [DOI] [PubMed] [Google Scholar]
- [10].Akkus E, Ozkara H, Alici B, et al. Incision and venous patch graft in the surgical treatment of penile curvature in Peyronie’s disease. Eur Urol 2001;40: 531–6; discussion 37. [DOI] [PubMed] [Google Scholar]
- [11].Egydio PH, Lucon AM, Arap S. Treatment of Peyronie’s disease by incomplete circumferential incision of the tunica albuginea and plaque with bovine pericardium graft. Urology. 2002;59: 570–4. [DOI] [PubMed] [Google Scholar]
- [12].Levine LA, Estrada CR. Human cadaveric pericardial graft for the surgical correction of Peyronie’s disease. J Urol 2003;170: 2359–62. [DOI] [PubMed] [Google Scholar]
- [13].Carson CC, Levine LA. Outcomes of surgical treatment of Peyronie’s disease. BJU international. 2014;113: 704–13. [DOI] [PubMed] [Google Scholar]
- [14].Kim DH, Lesser TF, Aboseif SR. Subjective patient-reported experiences after surgery for Peyronie’s disease: corporeal plication versus plaque incision with vein graft. Urology. 2008;71: 698–702. [DOI] [PubMed] [Google Scholar]
- [15].Montorsi F, Salonia A, Maga T, et al. Evidence based assessment of long-term results of plaque incision and vein grafting for Peyronie’s disease. The Journal of urology. 2000;163: 1704–8. [PubMed] [Google Scholar]
- [16].Wimpissinger F, Parnham A, Gutjahr G, Maksys S, Baierlein M, Stackl W. 10 Years’ Plaque Incision and Vein Grafting for Peyronie’s Disease: Does Time Matter? J Sex Med 2016;13: 120–8. [DOI] [PubMed] [Google Scholar]
- [17].Ohebshalom M, Mulhall J, Guhring P, Parker M. Measurement of penile curvature in Peyronie’s disease patients: comparison of three methods. The journal of sexual medicine. 2007;4: 199–203. [DOI] [PubMed] [Google Scholar]
- [18].Salvotelli L, Stoico V, Perrone F, et al. Prevalence of neuropathy in type 2 diabetic patients and its association with other diabetes complications: The Verona Diabetic Foot Screening Program. Journal of diabetes and its complications. 2015;29: 1066–70. [DOI] [PubMed] [Google Scholar]
- [19].Schrader SM, Breitenstein MJ, Lowe BD. Cutting off the nose to save the penis. J Sex Med 2008;5: 1932–40. [DOI] [PubMed] [Google Scholar]
- [20].Breda G, Xausa D, Giunta A, Tamai A, Silvestre P, Gherardi L. Nomogram for penile biothesiometry. European urology. 1991;20: 67–9. [DOI] [PubMed] [Google Scholar]
- [21].Hill BJ, Janssen E, Kvam P, Amick EE, Sanders SA. The effect of condoms on penile vibrotactile sensitivity thresholds in young, heterosexual men. The journal of sexual medicine. 2014;11: 102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Padma-Nathan H. Neurologic evaluation of erectile dysfunction. The Urologic clinics of North America. 1988;15: 77–80. [PubMed] [Google Scholar]
- [23].Egydio PH. Surgical treatment of Peyronie’s disease: choosing the best approach to improve patient satisfaction. Asian journal of andrology. 2008;10: 158–66. [DOI] [PubMed] [Google Scholar]
- [24].Taylor FL, Levine LA. Surgical correction of Peyronie’s disease via tunica albuginea plication or partial plaque excision with pericardial graft: long-term follow up. J Sex Med 2008;5: 2221–8; discussion 29–30. [DOI] [PubMed] [Google Scholar]
- [25].Adeniyi AA, Goorney SR, Pryor JP, Ralph DJ. The Lue procedure: an analysis of the outcome in Peyronie’s disease. BJU Int 2002;89: 404–8. [DOI] [PubMed] [Google Scholar]
- [26].Horstmann M, Kwol M, Amend B, Hennenlotter J, Stenzl A. A self-reported long-term follow-up of patients operated with either shortening techniques or a TachoSil grafting procedure. Asian J Androl 2011;13: 326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chung E, Clendinning E, Lessard L, Brock G. Five-year follow-up of Peyronie’s graft surgery: outcomes and patient satisfaction. J Sex Med 2011;8: 594–600. [DOI] [PubMed] [Google Scholar]
- [28].O’Donnell PD. Results of surgical management of Peyronie’s disease. J Urol 1992;148: 1184–7. [DOI] [PubMed] [Google Scholar]
- [29].Goyal NK, Kumar A, Das SK, et al. Experience with plaque excision and dermal grafting in the surgical treatment of Peyronie’s disease. Singapore Med J. 2008;49: 805–8. [PubMed] [Google Scholar]
- [30].El-Sakka AI, Rashwan HM, Lue TF. Venous patch graft for Peyronie’s disease. Part II: outcome analysis. J Urol 1998;160: 2050–3. [DOI] [PubMed] [Google Scholar]
- [31].Kalsi JS, Christopher N, Ralph DJ, Minhas S. Plaque incision and fascia lata grafting in the surgical management of Peyronie’s disease. BJU Int 2006;98: 110–4; discussion 14–5. [DOI] [PubMed] [Google Scholar]
- [32].Kovac JR, Brock GB. Surgical outcomes and patient satisfaction after dermal, pericardial, and small intestinal submucosal grafting for Peyronie’s disease. J Sex Med 2007;4: 1500–8. [DOI] [PubMed] [Google Scholar]
- [33].Knoll LD. Use of small intestinal submucosa graft for the surgical management of Peyronie’s disease. J Urol 2007;178: 2474–8; discussion 78. [DOI] [PubMed] [Google Scholar]
- [34].Licht MR, Lewis RW. Modified Nesbit procedure for the treatment of Peyronie’s disease: a comparative outcome analysis. J Urol 1997;158: 460–3. [PubMed] [Google Scholar]
- [35].Newman HF. Vibratory sensitivity of the penis. Fertility and sterility. 1970;21: 791–3. [DOI] [PubMed] [Google Scholar]
- [36].Edwards AE, Husted JR. Penile sensitivity, age, and sexual behavior. Journal of clinical psychology. 1976;32: 697–700. [DOI] [PubMed] [Google Scholar]
- [37].Rowland DL, Greenleaf W, Mas M, Myers L, Davidson JM. Penile and finger sensory thresholds in young, aging, and diabetic males. Archives of sexual behavior. 1989;18: 1–12. [DOI] [PubMed] [Google Scholar]
- [38].Rowland DL, Greenleaf WJ, Dorfman LJ, Davidson JM. Aging and sexual function in men. Archives of sexual behavior. 1993;22: 545–57. [DOI] [PubMed] [Google Scholar]
- [39].Bemelmans BL, Hendrikx LB, Koldewijn EL, Lemmens WA, Debruyne FM, Meuleman EJ. Comparison of biothesiometry and neuro-urophysiological investigations for the clinical evaluation of patients with erectile dysfunction. J Urol 1995;153: 1483–6. [PubMed] [Google Scholar]
- [40].Aaserud O, Juntunen J, Matikainen E. Vibration sensitivity thresholds: methodological considerations. Acta neurologica Scandinavica. 1990;82: 277–83. [DOI] [PubMed] [Google Scholar]
- [41].Fagius J, Wahren LK. Variability of sensory threshold determination in clinical use. Journal of the neurological sciences. 1981;51: 11–27. [DOI] [PubMed] [Google Scholar]
- [42].Salem EA, Elkady EH, Sakr A, Maarouf AM, Bendary L, Khalil S, et al. Lingual mucosal graft in treatment of Peyronie disease. Urology 2014;84:1374–7. [DOI] [PubMed] [Google Scholar]
- [43].Hatzichristodoulou G, Gschwend JE, Lahme S. Surgical therapy of Peyronie’s disease by partial plaque excision and grafting with collagen fleece: feasibility study of a new technique. Int J Impot Res 2013;25:183–7. [DOI] [PubMed] [Google Scholar]
- [44].Rowland DL, Haensel SM, Blom JH, Slob AK. Penile sensitivity in men with premature ejaculation and erectile dysfunction. Journal of sex & marital therapy. 1993;19: 189–97. [DOI] [PubMed] [Google Scholar]
- [45].Rowland DL, Leentvaar EJ, Blom JH, Slob AK. Changes in penile sensitivity following papaverine-induced erection in sexually functional and dysfunctional men. The Journal of urology. 1991;146: 1018–21. [DOI] [PubMed] [Google Scholar]
- [46].Xin ZC, Chung WS, Choi YD, Seong DH, Choi YJ, Choi HK. Penile sensitivity in patients with primary premature ejaculation. J Urol 1996;156: 979–81. [PubMed] [Google Scholar]
- [47].Paick JS, Jeong H, Park MS. Penile sensitivity in men with premature ejaculation. International journal of impotence research. 1998;10: 247–50. [DOI] [PubMed] [Google Scholar]
- [48].Lefaucheur JP, Yiou R, Colombel M, Chopin DK, Abbou CC. Relationship between penile thermal sensory threshold measurement and electrophysiologic tests to assess neurogenic impotence. Urology. 2001;57: 306–9. [DOI] [PubMed] [Google Scholar]
- [49].Bleustein CB, Eckholdt H, Arezzo JC, Melman A. Quantitative somatosensory testing of the penis: optimizing the clinical neurological examination. The Journal of urology. 2003;169: 2266–9. [DOI] [PubMed] [Google Scholar]
- [50].Bleustein CB, Arezzo JC, Eckholdt H, Melman A. The neuropathy of erectile dysfunction. International journal of impotence research. 2002;14: 433–9. [DOI] [PubMed] [Google Scholar]
- [51].Bleustein CB, Fogarty JD, Eckholdt H, Arezzo JC, Melman A. Effect of neonatal circumcision on penile neurologic sensation. Urology. 2005;65: 773–7. [DOI] [PubMed] [Google Scholar]
- [52].Vanden Broucke H, Everaert K, Peersman W, Claes H, Vanderschueren D, Van Kampen M. Ejaculation latency times and their relationship to penile sensitivity in men with normal sexual function. The Journal of urology. 2007;177: 237–40. [DOI] [PubMed] [Google Scholar]
- [53].Jiao C, Knight PK, Weerakoon P, McCann BD, Turman AB. Effects of sexual arousal on vibrotactile detection thresholds in aged men with and without erectile dysfunction. Sexual health. 2008;5: 347–52. [DOI] [PubMed] [Google Scholar]
- [54].Rajmil O, Arrus J, Fernandez M, Sarquella J, Ruiz-Castane E, Blasco A. Sensory changes after surgical correction of penile curvature. International journal of impotence research. 2009;21: 366–71. [DOI] [PubMed] [Google Scholar]
- [55].Cordeau D, Belanger M, Beaulieu-Prevost D, Courtois F. The assessment of sensory detection thresholds on the perineum and breast compared with control body sites. The journal of sexual medicine. 2014;11: 1741–8. [DOI] [PubMed] [Google Scholar]