Abstract
The hypothesis that ‘microplastic will transfer hazardous hydrophobic organic chemicals (HOC) to marine animals’ has been central to the perceived hazard and risk of plastic in the marine environment. The hypothesis is often cited and has gained momentum, turning it into paradigm status. We provide a critical evaluation of the scientific literature regarding this hypothesis. Using new calculations based on published studies, we explain the sometimes contrasting views and unify them in one interpretive framework. One explanation for the contrasting views among studies is that they test different hypotheses. When reframed in the context of the above hypothesis, the available data become consistent. We show that HOC microplastic-water partitioning can be assumed to be at equilibrium for most microplastic residing in the oceans. We calculate the fraction of total HOC sorbed by plastics to be small compared to that sorbed by other media in the ocean. We further demonstrate consistency among (a) measured HOC transfer from microplastic to organisms in the laboratory, (b) measured HOC desorption rates for polymers in artificial gut fluids (c) simulations by plastic-inclusive bioaccumulation models and (d) HOC desorption rates for polymers inferred from first principles. We conclude that overall the flux of HOCs bioaccumulated from natural prey overwhelms the flux from ingested microplastic for most habitats, which implies that microplastic ingestion is not likely to increase the exposure to and thus risks of HOCs in the marine environment.
Introduction
The contamination of the environment with plastic is considered highly undesirable for ethical and esthetical reasons and is generally considered to be a major threat for the health of aquatic ecosystems.1 It has been demonstrated that numerous species ingest plastic debris or become entangled by it.2−5 The physical damage to wildlife caused by larger forms of plastic (>5 mm in size) is also well documented, although clear indications of harm at the level of populations or communities have not been convincingly demonstrated. Still, the impact of microplastics (<5 mm) remains under investigation.6−8
Because the research examining microplastics is still in its infancy, studies measuring their fate and effects use widely different methods and approaches. As such, they provide fragmentary information and often use high microplastic concentrations and/or other nonenvironmentally relevant conditions to test for effects. Although this has resulted in several recent publications demonstrating new evidence of adverse effects, the body of evidence emphasizes the need to harmonize methodologies and design tests using environmentally relevant conditions.7 Addressing environmentally relevant conditions is a key prerequisite for a scientifically sound assessment of the hazard and risks of microplastics in the environment, which is essential to really understanding the possible risks of microplastics at the level of populations or communities.
One potential hazard stems from the fact that plastic particles contain or efficiently absorb hydrophobic organic chemicals (HOCs), which includes noncovalently bound additive ingredients.9−11 This has sparked hypotheses (1) on how microplastic contributes to the transport of HOCs, and (2) whether the ingestion of microplastic contributes to the bioaccumulation of HOCs by marine animals.12−18 Both ideas have led to considerable attention and debate. The ability of polymers to act as a source of HOCs to organisms has been recognized for a long time as it is the essence of the passive dosing approaches used in ecotoxicology.19,20 The past years this has been reconfirmed in the context of marine plastic debris by a series of laboratory studies that used microplastic as the sole source of HOCs.21−23 The studies in general support the above hypotheses, that is, that microplastics are a vector and source of HOCs to marine organisms.
However, the debate has formed from questions regarding whether plastic is a substantial source of these toxic chemicals to aquatic organisms relative to other sources in the environment. After all, given the low abundance of plastic particles relative to other media present in the oceans, exposure to these HOCs via plastic is probably of less importance than via natural pathways.7,24−28 Some recent papers argue that plastic is (potentially) an important exposure route because the affinity of HOCs for plastic is high.12,21 This is likely overruled when considering that the importance of plastic as a carrier is also dependent on the abundance of plastic compared to that of other carrier media such as water, suspended organic particulates or natural diet and prey items many of which have partition coefficients that are similar to that of plastic.29 Laboratory dietary exposures that demonstrate that plastic debris can be a vector of HOCs to marine organisms have not truly tested hypotheses regarding the relative importance of microplastics in comparison to other sources occurring in real environments. Several recent quantitative assessments, modeling studies and reports have concluded that the contribution of plastic to chemical fate and transport of HOCs in the oceans, and to bioaccumulation of these chemicals by marine organisms is probably small.24−26,28,30 This perceived dichotomy in the discussions of various published studies is confusing and hampers progress toward scientific consensus regarding the actual risks of microplastics. In turn, this may hamper policy development and the prioritization of research needs and remediation measures.
The aim of this paper is to critically review and synthesize the literature concerning the role of plastic as a carrier/vector of chemicals. This includes reviewing empirical and modeling studies, as well as incorporating the lessons learned from the behavior of sorbents other than plastic (e.g., organic matter, black carbon, activated carbon, carbon nanomaterials, and passive dosing or sampling polymer materials) that share the same principles with respect to the binding of HOCs. We also apply models to reinterpret laboratory studies in an attempt to unify various study results into a unified interpretation framework.
To reach our objective, we first discuss the state of (non)equilibrium of the binding of HOCs to microplastics in the marine environment. Plastic particles can only constitute an ongoing source of HOCs to the water or to aquatic organisms if the HOC concentration in these particles is sufficiently high (i.e., higher than equilibrium) compared to that in the water or in the organisms. This calls for a comparison of HOC (de)sorption half-lives and of the residence times and age of plastic particles in the oceans. As far as we know, no such assessment has been performed yet. Second, we discuss the average distribution of HOCs across media in the ocean, a distribution that can be assessed by accounting for (1) the relative abundances of these media, and (2) the relative affinities of HOCs for these media. Assessment of this distribution under environmentally realistic conditions is crucial in order to understand the relative importance of plastic (compared to other media) in the transport of HOCs or in the bioaccumulation of HOCs by marine organisms. A few earlier studies have provided a similar distribution analysis,24,30 however, without taking into account all known media, such as dissolved organic carbon, colloidal carbon, black carbon, detritus and plankton. Third, we discuss recent studies that document transfer of HOCs from plastic to marine organisms or vice versa, and provide novel model calibrations using data from these studies. This provides a mechanistic interpretation of the results of these previous empirical studies, unifies empirical and model-based approaches regarding the same research questions, and allows for extrapolations to natural conditions in case these empirical studies were not fully mimicking natural conditions. Furthermore, past literature is evaluated for environmental relevance, that is, extent of using realistic concentration ranges and completeness with respect to covering processes that are known to occur in the field. Finally, we discuss the implications of our analysis for risk assessment and suggest priorities for future research.
The State of Equilibrium of HOC Partitioning in the Oceans
Equilibrium Sorption of HOCs to Plastics
HOCs are subject to partitioning across environmental media such as water, sediment, biota, air, and since the 1950s: plastic.31 The equilibrium partitioning coefficient for sorption to plastics KPL [L/kg], is defined as12,32
| 1 |
where CPL [μg/kg] and CW [μg/L] are the concentrations in plastic and water, respectively. eq 1 is important to address the question whether HOCs are sorbed to microplastic or are released by microplastic, because the spontaneous transfer of HOCs always occurs in such a direction that the actual concentration ratio (CPL/CW) approaches the value of KPL.15 For instance, if CPL/CW > KPL, then desorption from the plastic to water takes place and vice versa. The time needed to reach equilibrium depends on the molecular properties of the HOC, the properties of the seawater and the microplastic, as well as on the volumes of these compartments. The kinetics of sorption to microplastic is beyond the scope of this review, but it is obvious that sorption equilibrium may exist for microplastics that reside in the ocean for already a long time, whereas equilibrium may not exist for microplastics that were released recently. This means that two aspects need to be compared (1) the age distribution of microplastics currently present in the oceans, and (2) the sorption equilibration times of representative HOCs.
Estimating the Age Distribution of Microplastics in the Oceans
Annual world production data for plastics are well-known and show a gradual increase from 1.7 million tonnes in 1950 to about 299 million tonnes per year in 2013 (Figure S1, data from31). The curve can be smoothed using a second order polynomial (Figure S1), which accurately captures the trend but averages some small fluctuations caused by stagnations in the world economy in the 1970s, 1980s, and recently in 2007. It is commonly assumed that a more or less constant fraction of the world plastic production ends up in the oceans.33 The few million tonnes of plastic emitted in the first years of production have an “environmental age” of about 60 years, whereas the production of 299 million tonnes in 2014 has by definition an age of less than a year. By combining the annually emitted volumes per year with the age of these yearly volumes (i.e., present year minus year of emission) an age distribution for the cumulative quantity of emitted plastic can be calculated (Figure 1). Because a more or less constant fraction of the yearly world production ends up in the oceans, Figure 1 also represents the expected age distribution for all plastic in the oceans. The calculation (for 2015) shows that about 50% of the plastic has been present in the oceans for more than 13 years, whereas 80% and 90% of all the plastic is older than 4 and 2 year, respectively (Figure 1). If we use European production data31 the age distribution is similar, however, it shows that 50% and 90% of the produced plastics have been present in the European seas for more than 17 years and 3 years, respectively (Figure 1). In reality, the oceans do not represent one uniform compartment.34 However, mixing within areas or in gyres can be considered more homogeneous. Furthermore, sources and types of plastics do not substantially differ across the globe. Microplastics are considered ubiquitous global contaminants, whereas transport and mixing causes the spreading of microplastics in the oceans, with contamination of even very remote areas as a result.34−38 Coastal areas may contain relatively “young” plastic particles, yet these areas also receive aged plastics from remote areas. Indeed beached plastic has been shown to also come from remote sources. Remote areas like the Arctic35 or deep sea sediments39 are further away from anthropogenic sources, implying that they may have a higher share of older microplastics. We conclude that the age distribution of microplastic in a given area probably does not show a strong spatial heterogeneity and that the age distribution as given in Figure 1 is roughly uniform across the different major oceanic regions.
Figure 1.
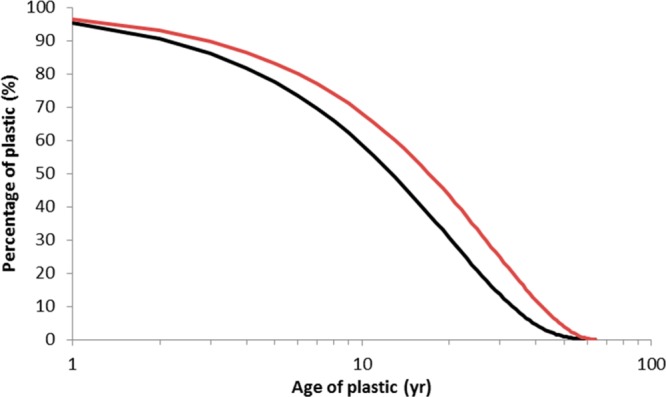
Cumulative age distribution of plastic in the oceans based on world plastic production data (black curve). For about 90% and 50% of the world-produced plastics, the residence time at sea is more than 2 y and 13 y, respectively. The red curve represents the cumulative age distribution based on plastic production in Europe. Using the European production data, the 90% and 50% cut offs relate to residence times at sea of more than 3 and 17 years, respectively.
Comparing HOC Equilibration Times for Microplastic with the Age of Plastic in the Oceans
Several studies have addressed the sorption kinetics under realistic field conditions and by using field-relevant types of microplastics. These studies generally reported desorption half-lives of weeks to 1–2 year for many types of 0.5–5 mm sized microplastic for the most hydrophobic classes of HOCs, like PCBs and PAHs,40−42 with shorter half-lives for the smaller microplastics. Sorption of more hydrophilic compounds generally will be faster, which means that the vast majority of HOCs will be at equilibrium after 2 years, whereas for some very hydrophobic HOCs it may take longer to fully reach equilibrium, especially for the larger macroplastic particles (i.e., > 5 mm). These sorption equilibration times can now be compared to the ages of plastic in the oceans. It follows from the age distribution (Figure 1) that 80–90% of the plastic is older than 2–4 years and therefore will be at or close to sorption equilibrium for all HOCs to be considered, including additives and plasticizers. Virtually all plastic will be at equilibrium for the majority of the HOCs with half-lives in the order of months. The remaining cases, that is, the very hydrophobic HOCs sorbing to the fraction of larger “young microplastic” particles, are in an intermediate state with 50% of equilibrium reached as the best estimated intermediate value. The above estimation most probably under-predicts the magnitude of equilibrium for aged and free-floating microplastics. After all, the above sorption half-lives have been assessed for microplastics in a rather pristine state, based on exposures of up to a year at most. It is known that over longer time scales, embrittlement and abrasion lead to the formation of much smaller particles, cracks and pores37 which increases the rates of sorption due to larger surface area and shorter intrapolymer diffusion paths.32,42 The slow fragmentation of plastic implies that generally the older plastic also will be the smaller and thus more equilibrated microplastic, which (because of its smaller size) also is likely to be bioavailable for a wider range of aquatic organisms. Furthermore, part of the aforementioned sorption half-lives were measured for microplastics enclosed in bags with 1.3 and 10 mm mesh,41 which probably reduced hydrodynamic flow around the particles. This means that the sorption kinetics might have been faster when the plastic particles would have been floating freely. A recent paper measured concentrations of PAHs in both the plastic and water phase in the North Pacific gyre and found that partitioning of PAHs between plastic and water was virtually at equilibrium.43 In summary, we conclude that with respect to sorption of HOCs, sorption equilibrium is a valid general assumption for the majority of microplastics currently present in the oceans.
The Multimedia Distribution of Chemicals in the Oceans
The Need for Calculating the Distribution of HOCs Across Environmental Media
Many papers argue that plastic is highly abundant in the oceans and has very strong binding properties for HOCs, such that plastic probably plays an important role in the transport and transfer of HOCs in the oceans, and in the bioaccumulation of HOCs by marine biota.12,13,17,21−23 This reasoning links the potential risk and harm of plastic to its presumed role as a carrier of chemicals. This role, however, is also played by all other environmental media such as water, air,30,32 and—for HOCs—other carbon-based media such as dissolved organic carbon, organic colloids, black carbon, and biota.24,32,44−46 Therefore, it is important to know the relative quantity of chemicals sorbed by plastics in the oceans, compared to the quantity held by these other media. Marine organisms ingest plastic together with regular prey items, including the abundant detritus fraction, and phyto- and zooplankton species. Exposure to HOCs by the ingestion of plastic may be substantial if the mass of HOC in the plastic is sufficiently large compared to these other “diet” components. This constitutes another reason to assess the relative quantity of HOCs in plastics compared to other solid phases. This relative quantity is governed by repartitioning phenomena among environmental media, including plastic. These media thus act as communicating vessels for chemical transfer.
Plastics and Other Environmental Media As Communicating Vessels
The principles of partitioning of HOCs across environmental media have been assessed extensively in literature,32,47,48 including repartitioning upon addition or removal of an environmental sorbent or anthropogenic sorbents49,50 For instance, addition of sediment in a laboratory bioaccumulation test system reduced chlorobenzene uptake by fish. This reduction could be fully explained by the quantity of sediment added and the chlorobenzene KP for the sediment.51 Three decades ago, the effect of activated carbon addition on PCB bioaccumulation by goldfish was tested in the laboratory, resulting in a 70.9–99.9% reduction in concentrations of PCBs in fish.52 Adding activated carbon as a remediation method for sediments was also tested in the field, showing a 20-fold reduction in PCB bioaccumulation in golden orfe.53 Similarly, plastics have been studied as cleaning materials for the remediation of soils and sediments.54 Such applications require a sufficient quantity of sorbent material in order to cause significant repartitioning and immobilization of HOCs. However, in other applications plastics also have been applied in quantities that are deliberately low compared to those of other environmental media. Polymers like LDPE, POM or silicone rubbers have been applied as negligible depletion passive samplers,9,55,56 where the ability of plastic to reach an HOC equilibrium state in weeks or months (see previous section) is used to infer freely dissolved HOC concentrations in water.
These are just a few examples of a vast body of literature showing the reversibility of HOC partitioning among media including polymers, where the direction of transfer is determined by a concentration ratio being higher or lower than the equilibrium partition coefficient. Some recent studies have reconfirmed these phenomena in the context of marine debris. For instance, the addition of (clean) plastic to laboratory systems with a finite quantity of HOC was observed to cause a decrease in the HOC concentration in the marine organisms being tested.12,13,22 These authors, however, acknowledged that the abundance of plastic in the marine environment would be too low to cause a similar cleaning effect in situ, which also is likely for the opposite process. Below we discuss studies that have addressed this question and provide an update of such calculations based on most recent data.
The Distribution of HOC Across Environmental Media in the Ocean
The relative role of an environmental compartment in the oceans in transport or transfer can be assessed by calculating the quantity of HOC held by that compartment relative to the other compartments.24,30,32 The mass of HOC in a medium is likely to be high if that medium is abundant. Likewise, the mass of HOC in a medium is high if the affinity of the HOC as quantified by the partition coefficient KP is high for that medium. The total mass of a HOC in a volume within a gyre, sea or ocean (QT) can be expressed as
| 2 |
The first term represents the HOC mass in the water with VW and CW being the volume of water and the HOC concentration in water, respectively. The second term accounts for the masses in “n” nonaqueous compartments like dissolved organic carbon, organic colloids, black carbon, detritus, phytoplankton and zooplankton (in this case n = 6). Biota at higher trophic levels could be included but, even though they may have higher levels of HOCs due to biomagnification, are not accounted for here because of their negligible mass compared to that of the other solid phases present.57 In this term, Mi represents the mass of the compartment in the ocean and KP,i the partition coefficient for sorption of the HOC to solid phase “i”, based on the assumption of sorption equilibrium as motivated in the previous section. Similarly, the last term accounts for the mass of HOC present in plastic, with MPL the mass of plastic in the ocean compartment. In essence, the products M × KP determine the relevance of a phase. For instance, although the affinity of a HOC for plastic could be very high, the role of plastic in transfer and accumulation would still be negligible overall, if the abundance of plastic is low relative to the abundance of other media present. The fraction of the mass of HOC in plastic can be expressed as a fraction of the total mass of HOC as (CW cancels out):
| 3 |
Using this type of calculations, Zarfl and Matthies30 calculated mass fluxes of PCBs, PBDEs, and perfluorooctanoic acid (PFOA) to the Arctic. Fluxes for transport of water, plastic and air, were combined with concentrations of HOCs in these media (either estimated from partitioning data, or measured), in order to obtain fluxes of the HOCs. They calculated that the fluxes mediated by plastic were 4–6 orders of magnitude lower than those mediated by oceanic currents and air. Similarly, Gouin et al.24 defined a representative coastal marine ecosystem in which plastic was present. Using the abundances of media and a wide range of HOC partitioning coefficients, they showed that sorption to polyethylene (PE) would occur for a negligible <0.1% of the mass of the chemicals. This means that the plastic abundance currently present in coastal waters is insufficient to cause a meaningful redistribution of HOCs from the oceanic environment to the plastic.
Recently, new data on the abundance of plastics suggest that there is currently 268 940 tonnes of plastic floating in (all) oceans, of which 35 540 tonnes are <4.75 mm microplastics.58 Using these data we calculated the overall average distribution of HOCs across media in the oceans. This update also included media that were thus far not accounted for, like organic colloids, black carbon and zooplankton, next to the common phases water, dissolved organic carbon (DOC) and phytoplankton. Several studies have highlighted the sources and abundance of black carbon in the oceans.59−62 For the present calculations, abundances of black carbon were estimated using data provided by Pohl et al.,62 and for the other organic carbon phases data were taken from Couwet (1978)63 (Figure 2A). The abundances of these phases range from 2.7 × 108 kg for plastic58 to 1.4 × 1021 kg (or ∼ L) for water64 (Figure 2A). This implies that an estimate of the average “whole ocean” concentration of plastic would equate to ∼2 ng/L. We used one of the highest values for KPL measured for a HOC on microplastic; 107 L/kg.10 Several studies have shown that the suspended sediment partition coefficients KP,i on an organic carbon basis are similar10 or higher29 than those reported for microplastic. Nevertheless, following Zarfl and Matthies,30 the organic carbon partition coefficients KP,i were set at a conservative value of 0.01 × KPL, whereas for black carbon they were set at a conservative 100 × KPL.45,55,65 This implies that although the masses of plastic and black carbon are estimated to be about equal, the mass of HOC sorbed to black carbon would be about 2 orders of magnitude higher than to plastic in the ocean (Figure 2B). The distribution calculated using these data and eq 3 shows that most HOCs are present in the water (Figure 2B, Table SI-1), which implies that oceanic water currents constitute the main transport medium for HOCs across the ocean. Plastic binds 1.93 × 10–4 % of this model HOC (Table SI-1), a percentage that will even be smaller for other combinations of HOC and plastic because we used a worst-case calculation (i.e., highest possible KP for plastic, low estimates for KP for the other carbon phases). So, although plastic concentrates HOCs by factors up to 107 from the water, plastic is still irrelevant as a carrier phase because the mass of water is about a factor of 1013 larger than that of plastic. Even if plastic concentrations (locally) would be orders of magnitude higher than the “whole ocean” average used in our calculation, this would not change the large excess of other media compared to plastic.
Figure 2.
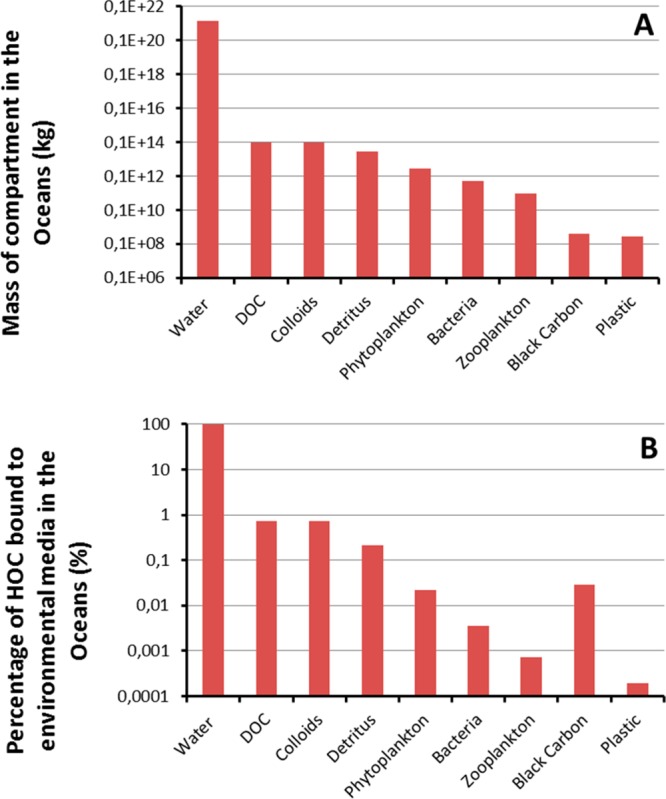
Abundances of environmental media in the oceans (Panel A), and distribution of HOC across these environmental media calculated using eqs 2 and 3 (Panel B). Water dominates the HOC distribution holding 98.3% of HOC whereas plastic holds 0.0002% of HOC (Panel B). The calculations use highest reported KP values for plastic and low estimated KP values for organic carbon phases.
Solid particles, however, may be more important for the vertical transport of HOCs because of their tendency to settle. However, a similar calculation excluding the nonsettling water and DOC shows that colloids, detritus and black carbon nanoparticles would still dominate the solid phase speciation, with only 2 × 10–2 % of HOC bound to plastic (Table SI-1). In summary, these simple calculations confirm the results of earlier studies,24,30 showing that the fraction of HOC held by plastic is negligible compared to that held by other media, which implies that plastic-mediated transport is generally unimportant in terms of HOC masses. As discussed in the previous section, most plastic particles containing additives at higher than equilibrium concentrations will reach an equilibrium, given their long transport and residence times in the oceans, rendering these calculations also applicable to these chemicals.
The Role of Microplastic in Bioaccumulation of HOCs to Marine Aquatic Organisms
Processes Determining the Relative Importance of Microplastic As a Carrier of HOCs under Environmentally Relevant Conditions
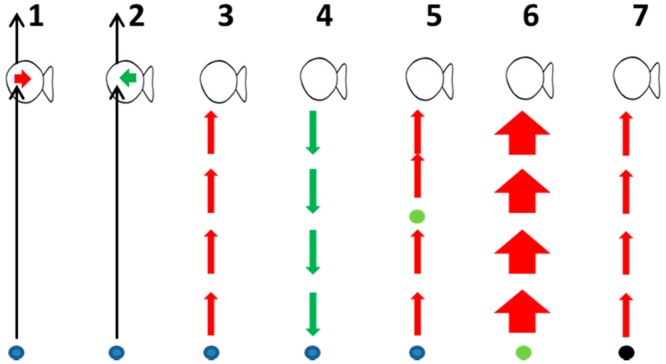
The mechanisms that explain the effect of microplastic on bioaccumulation in marine organisms have been summarized in several recent reviews,13−16,24−26,28,46 whereas numerous papers address these processes separately or in detail. Here we give a brief summary of these isolated mechanisms that affect the role of microplastic as a carrier of HOCs (Figure 3), and provide selected references supporting the occurrence of these mechanisms:
-
1.
Plastic being ingested leading to HOC transfer from the plastic to the organism (“absorption”).21−23,25,66
-
2.
Plastic being ingested leading to increased excretion of HOC from the organism (“cleaning”).24,25,28,67
-
3.
Plastic acting as a source of HOCs in the environment, which subsequently are available for dermal uptake or uptake by the gills (“source”).13,19,20,24,25
-
4.
Plastic accumulating HOCs from the seawater and organisms (“sink”).12,22,24,25,54
-
5.
Desorption of HOCs from plastic followed by uptake by natural organic particles or prey, followed by ingestion of prey (“indirect source, dietary”).44,46,68
-
6.
Uptake of HOC by ingestion of regular (i.e., nonplastic) prey items (“dietary”).44,46,68
-
7.
Uptake of HOC by dermal transfer or transfer across gills from other sources than the plastic (“other source dermal”).44,46,68
Figure 3.
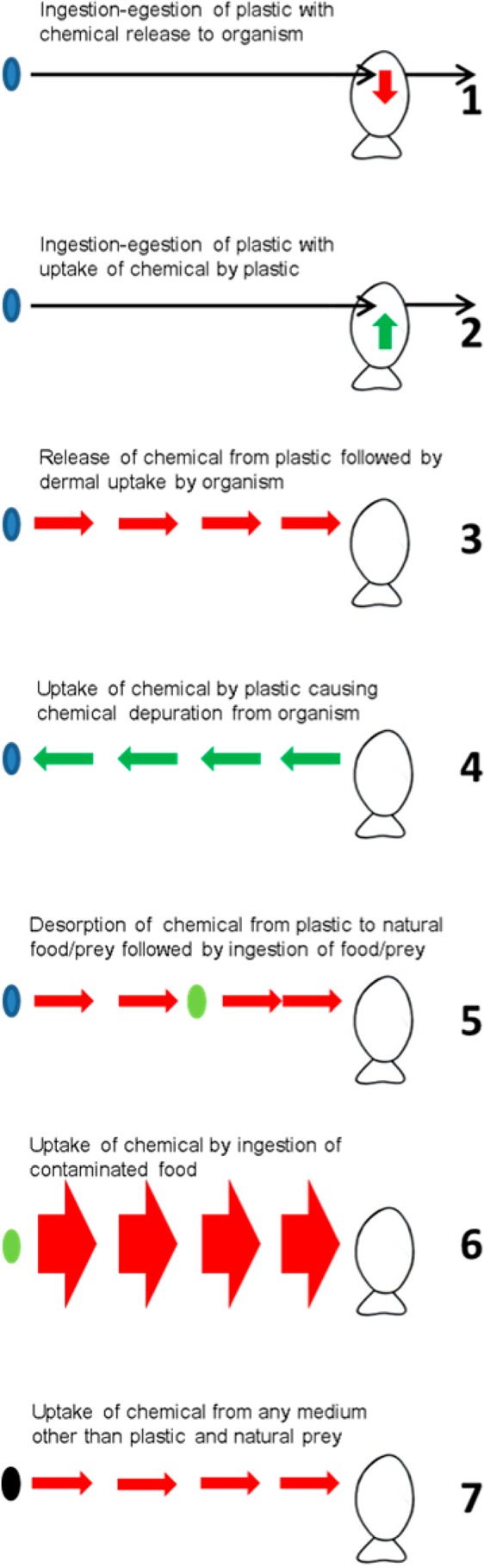
Simultaneous processes affecting the relative importance of microplastics acting as a vector of HOC to aquatic organisms, that is, fish. Blue oval = Microplastic. Green oval = Natural prey item. Black oval = Other source than microplastic. Black arrows indicate transfer of microplastic. Red arrows indicate HOC transfer to organism. Green arrows indicate HOC transfer from organism. Arrow widths represent qualitative indication of relative importance of the pathway. Processes 1–5 involve a role of microplastic. Processes 5 and 6 involve natural uptake paths. Process 7 considers dermal exposure from other sources than microplastic. Per fish individual, processes occur simultaneously for same as well as different HOC, complicating interpretation of field data. Per fish individual, processes increasing or decreasing body burdens occur simultaneously for different HOC. Per HOC, uptake from natural path (6) plus nonmicroplastic source (7) generally overwhelms uptake from microplastic ingestion (1).
Mechanism 1–4 are common for all nondigestible sorbents and determine the net uptake flux from the plastic. Studies that consider the mechanisms 1–4 can provide “proof of principle” or can provide mechanistically relevant knowledge regarding the uptake of HOCs from plastic. Processes 1–4 are supported by several empirical and modeling studies as indicated. Mechanism 2 is less well-recognized in the literature on plastic debris and may need further experimental validation. However, the process is generally well-known for animals and humans as it is the essence of medical treatment after acute poisoning with HOCs.69 Just like plastic,67 liquid paraffin,70 olestra69,71 or activated carbon72 are nondigestible, nonabsorbable lipophilic polymeric phases, which have been shown to increase the excretion and decrease the body burden of HOCs.24 Processes 5 and 6 relate to natural pathways of HOC uptake by organisms yet they are crucial to assess the relative importance of processes 1–4 under environmentally realistic conditions. Process 7 recognizes that HOCs originating from other sources than plastic may be taken up from the water. A recent study for instance, showed that plastic is an important yet not the only source of bisphenol A (BPA) from waste-handling facilities, and that BPA in the leachate from the facilities was freely dissolved and not bound to microplastics.73 All of these studies have helped to further understand the isolated processes. However, to answer the question whether plastic is a relevant carrier of HOCs in the natural environment, we have to evaluate all processes 1–7 occurring in realistic environments. This also implies that studies that do not explicitly consider the processes under 5–7, or that neglect the cleaning mechanisms related to plastic (2 and 4) are inherently less informative for answering that question.
Overview and Critical Evaluation of Research Approaches
Studies that assessed the role of plastic as a mechanism for bioaccumulation in the field have been reviewed recently.15,16 We are aware of 13 studies (excluding seabirds) that somehow addressed the role of plastic in the bioaccumulation of HOCs in the context of pollution with marine debris (Table SI-2). All these studies provide relevant information concerning this question; however, they differ in their research approach and in the extent to which they reflect environmental realism. An overview of these studies that characterizes the extent to which various processes and natural conditions are accounted for, is provided as Supporting Information (Table SI-2). We divide these studies in three main categories each having their specific merits and flaws, and discuss these hereunder.
Laboratory Studies
A first category of empirical studies has provided evidence for the transfer of HOCs from microplastic to biota under controlled laboratory conditions21−23,66,67,74 and has been reviewed before.12,14,16 One study74 was performed under environmentally relevant conditions with all exposure pathways accounted for, and reported an increase in accumulation of ∑PCBs in lugworms of 29%. This percentage, however, decreased at a higher plastic dose, and it was concluded that the effect could not be attributed to chemical uptake from ingested microplastic.25,74 In several other studies,21−23,66 clean or relatively clean organisms were exposed to rather high quantities of HOC spiked microplastics, which forces transfer of the HOCs to the organism. Such experiments can be viewed as chemical bioaccumulation or toxicity tests where plastic acts as vector for administering the contaminants to the test systems, invoking effects of these chemicals once toxicity thresholds are exceeded. These nonequilibrium test designs confirm earlier work showing that polymers will act as a source or carrier material for HOCs toward media with lower than equilibrium fugacity. For instance, passive dosing is a technique increasingly being used in ecotoxicology to control aqueous phase exposure concentrations during toxicity tests.19,20 The main merit of these nonequilibrium set-ups is that HOC transfer from plastic to biota is studied at a maximum possible HOC gradient between plastic and organism, with a limited or negligible role of other uptake pathways like uptake from water or food. This potentially allows the quantification of the parameters that drive chemical transfer from the plastics in the gut, parameters that are urgently needed.24,25,28 Because the aforementioned laboratory studies observed bioaccumulation or even adverse effects of chemical exposure, they concluded that ingestion of microplastic potentially constitutes a hazard in the oceans. This reasoning, however, is not without problems. First, use of freshly spiked or field-contaminated plastics in clean water leads to chemical desorption to the water, leading to dermal uptake. Therefore, to confirm that the bioaccumulation was from ingestion, zero aqueous phase concentrations need to be demonstrated, these data are, however, not provided in these studies (e.g, refs (21−23, 66, and 74)). Second, the argument that ingested plastics will act as a carrier is correct for chemicals that reside in the plastic at higher than equilibrium fugacity (like additives), which however was shown to occur for only a minor fraction of the plastics in the marine environment, based on desorption half-lives (see above). Third, for chemicals that occur in plastic at lower than equilibrium fugacities, plastic would have an opposite, that is, beneficial effect. This effect would decrease the hazard due to plastic and usually is not addressed in these studies. Fourth, some studies did not consider uptake from natural exposure routes.21−23 In most environmentally realistic settings, the concentrations of plastic would be far lower than those used in most of these studies. These studies therefore underestimate the role of natural routes. For instance, the first mentioned experiment74 was performed under realistic conditions yet the authors could not clearly show that plastic acted as a carrier for HOCs. Fifth, to confirm the hypothesis that plastic acts as a carrier of toxic chemicals upon ingestion, ingestion of the plastic should be experimentally confirmed, which however was not the case for some studies (Table SI-2).
Model Studies
A second category of studies has applied models to interpret transfer from plastic in a scenario analysis that includes all the other chemical uptake pathways (Figure 3) as well.24−26,28,75,76 These studies thus provide an environmentally relevant quantitative assessment of the relative importance of plastic as a carrier of chemicals and have been reviewed recently.7,15 They combine empirically validated models for bioaccumulation from regular prey with bioaccumulation from ingested plastic. Comparison of the HOC fluxes bioaccumulated from ingested prey with those of ingested plastic, generally showed small to negligible contributions of plastic to bioaccumulation by the various marine species like lugworm, fish, and seabirds.25,26,28,75,76 These models accounted for cleaning effects due to plastic ingestion,24−26,28 were used for worst case scenarios setting the uptake from plastic at maximum values (assuming 100% absorption),28 or accounted for uncertainties in parameters and input variables by using probabilistic approaches.26 The main merit of these studies is that they provide a mechanistic basis for understanding plastic-inclusive bioaccumulation, which assists in data interpretation of empirical studies and experimental designs. Furthermore, they allow for environmentally realistic scenario studies and extrapolations to low plastic concentrations that occur in the environment, or to higher concentrations in the future. The models that were applied are valid in terms of their agreement to first-principles and accordance with design criteria.77 However, they can only provide indirect evidence, and lack of validation against empirical data sometimes limits the credibility associated with these modeling studies. We are aware of only two studies that compared model calculations with empirical data,25,75 which implies that further validation is recommended (see next section).
Field Studies
A third category of studies proposes to use the observed co-occurrences or correlations among field data on plastic densities or chemical concentrations in plastic, with chemical concentrations in organisms, as evidence supporting the hypothesis of plastic transferring HOCs to organisms.38,78,79 The main merit of these field observations is that they represent the ultimate reality of nature, which is the aim of this papers’ research question. The main challenge in observational field research, however, is proving causality because any observed phenomenon can in theory be explained by many different mechanisms.80 The aforementioned correlations can be explained by several simultaneously acting processes, for instance process 1–5 (Figure 3), or from any combination of these. The problem of multiple causality through parallel uptake pathways means that it is difficult to unambiguously and causally link bioaccumulation to ingestion of plastic alone. There is no reason to deny that bioaccumulation of some HOCs can be linked to a high abundance of plastics that may act as a source of these HOCs38 (Figure 3, processes 3 and 5), however, the relative importance of plastic ingestion is hard to disentangle. A final challenge associated with field studies is that if statistical rigor is required, sufficient gradient in chemical concentrations, plastic abundance, extent of plastic ingestion and mixing ratio with regular food is required. Such gradients are, however, difficult to find on the scale of the oceans.38
We conclude that three categories of studies have discussed the role and importance of microplastic ingestion. They seem to reach different conclusions because they address different hypotheses, different exposure scenarios and have different limitations based on the type of study (i.e., modeling, laboratory, or field observation), which are not always clearly discussed. In summary; laboratory studies that use high doses of only plastic tend to find an effect of ingestion on HOC accumulation. Studies aiming at environmentally realism (either lab or model) by accounting for parallel uptake pathways tend to conclude that there is no (or a negligible) effect. Field studies struggle with the problems of multiple causation, lack of gradient and environmental variability, which limits their use to detect the contribution of plastic ingestion to bioaccumulation.
Unifying Empirical Studies, Modeling Studies, And Theory
To date, most model scenario studies were prospective studies,24−26,28 with only two studies also comparing model simulation results with empirical data.25,75 These scenario studies aimed at addressing environmentally realistic settings by accounting for chemical bioaccumulation from water, natural diet and ingested microplastic. In this section we provide a synthesis of four categories of information: (a) three published laboratory studies that provided evidence for transfer of HOCs from microplastic to biota, (b) published model frameworks that include microplastic as a component of the diet, (c) HOC release rate constants from microplastic measured under gut mimicking conditions, and (d) theoretical estimations for these release rate constants based on first principles. This way, the current model frameworks are further validated as they are tested against published empirical data. The validity of the models was assessed based on whether calibrated parameters align to independently measured parameter values, or whether they agree to values that follow from first principles. In turn, inferences from empirical studies that only addressed the release of HOCs from plastic in artificial gut fluids or that applied plastic-only or plastic-dominated exposure conditions, can be extrapolated to natural conditions by using the validated parameters. To that end, we parametrized a previously published bioaccumulation model that includes plastic as one of the components in the diet and that dynamically models HOC transfer in the gut25 (provided as Supporting Information, Table SI-3). The parametrizations and boundary conditions were set to match the experimental designs and data from the studies by Browne et al.,21 Rochman et al.,66 and Chua et al.22 The main optimization parameter is the rate constant for chemical desorption from plastic in the organisms’ gut15,25,27 (k1G, d–1, see previously published model provided as Supporting Information), which also is the parameter providing the “common currency” for the four categories of information mentioned above. The calibrated k1G parameters were compared (a) with ranges for this parameter that were determined experimentally by Teuten et al.12 and Bakir et al.,81 or recalculated from Tanaka et al.82 (see Table SI-4) and (b) with parameter values inferred from first-principles (i.e., plastic particles sizes and HOC intrapolymer diffusivities). For the definition of the previously published model and its parameters, and for the current new calculations the reader is referred to the Supporting Information.
Comparison of k1G Values Estimated from Modeling Studies, Bioaccumulation Studies, Desorption Studies and First-Principles
Comparison of the magnitude of the rate constant for desorption of HOCs from microplastics in the gut (k1G) across different microplastic types and sizes, organisms and chemicals, and obtained with different methods, has a range of about 2 orders of magnitude (Table SI-5). The 10–90% inter quantile range PR10–90% for the data in Table SI-5, however, is only 0.3–9.8 d–1 with a median of 2.1 d–1. For the separate categories of studies the median values as well as the PR10–90% are virtually identical (Figure 4). This implies that there is a striking consistency among the data obtained for these microplastics of different sizes and polymer types, chemicals and methodologies. The estimates from bioaccumulation studies, laboratory desorption studies and first-principles provide very similar ranges for the “common currency” desorption rate parameter k1G. For PAH desorbing from PE and PVC the range is only about 1–4 d–1 accounting for the bioaccumulation data from Browne et al.21 and Rochman et al.,66 the direct desorption measurements of Teuten et al.12 and Bakir et al.81 as well as the theoretical values provided here (Table SI-5). The PCB k1G value estimated from the data provided by Rochman agrees well with values based on the theoretical principles (Table SI-5).
Figure 4.
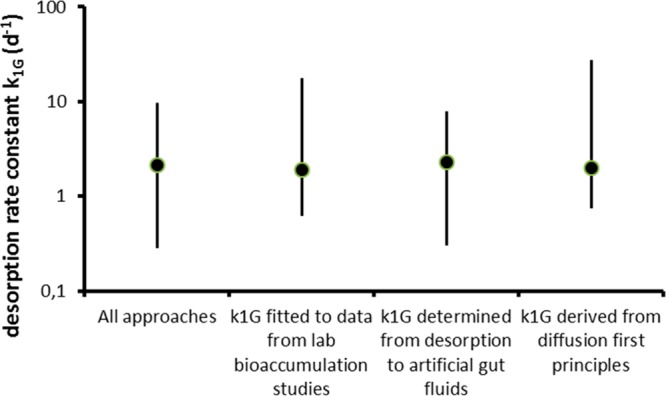
Similarity in median value and range (10–90% inter quantile ranges; PR10–90%) of the rate constants (k1G, d–1) for desorption of HOC from plastic in the gut of marine biota, as calculated using data from all study approaches, laboratory bioaccumulation studies, desorption studies using artificial gut fluids, and diffusion principles. Detailed calculation of the ranges is provided as Supporting Information.
Summarizing Discussion
The Role of Microplastic in the Transport of HOCs
It has been shown that plastic is ubiquitous in the marine environment.3,4,7 Still, on average the present mass of plastic is negligible compared to that of other media that transfer HOCs across the oceans. We applied a “back of the envelope” calculation that showed that on average the fraction of HOCs sorbed by plastic also is negligible compared to the fraction held by other media (Figure 2). The fraction held by plastic is so small that even if we would underestimate the abundance of plastic by orders of magnitude, plastic still would be unimportant as a transfer pathway for HOCs. The same reasoning holds with respect to other uncertainties. The calculations used here were based on the assumption of equilibrium partitioning for the main portion of microplastic in the oceans. The outcome reflects the current state of plastic pollution in the oceans, which may be valid for several more years. However, the yearly production of plastic has increased over the years and can be expected to further increase. This implies that the fraction of “nonequilibrium plastic” will increase, and thus that the nonequilibrium fraction will become increasingly important. Nonequilibrium may increase the potential role of plastic as a carrier for additives and plasticizers, and decrease its role as a carrier for POPs.76 Still, given the abundance of other media that carry the same chemicals, a substantial role of plastic in the transport of chemicals is not likely.
The Role of Microplastic for the Bioaccumulation of POPs, Additives or Plasticizers in the Marine Environment
The previous sections provided evidence that showed transfer of HOCs from plastic to organisms or vice versa, dependent on the fugacity gradient that was used in the various study designs. We provided a synthesis that showed that laboratory exposure studies, model studies and field studies all align and can be interpreted with existing theory of bioaccumulation and partitioning of chemicals to hydrophobic phases such as sediment organic matter and plastics. We demonstrated that the uptake of HOCs from plastics by marine biota can be explained from the principles of polymer diffusion. In turn, the diffusion rates explain the release rates observed in the desorption experiments,12,81 which in turn were shown to be able to explain the observed bioaccumulation in experiments in which uncontaminated test animals were fed contaminated plastic.21,22 We showed that experiments or field studies that also account for uptake from natural pathways are inherently less sensitive to detect an effect of plastic, which simply represents the situation in nature and is explained from the theory presented here. Indeed, parameters estimated from such an experiment66 were consistent with those obtained from laboratory desorption experiments and theoretical principles. The above model calibrations and agreement of its parameters with values independently obtained from experiments, further supports the credibility of the models that have been used to infer the role of plastic as a carrier of HOCs to marine biota under natural conditions.24,25,28,75,76 Based on the synthesis we provided here, we suggest that the scientific evidence is consistent, yet that the dichotomy in study outcomes is perceived and probably reflects and is related to different exposure scenarios used in these different studies.
A central question we addressed is ‘to what extent plastic ingestion leads to increased uptake of chemicals in nature’. Here we briefly reflect on the studies that specifically aimed at analyzing such conditions, taking the aforementioned credibility of validated modeling approaches into account. Gouin et al.24 used a model proposed by Arnot and Gobas83 and modeled HOC uptake from a 10% plastic diet. Such a percentage probably is very high for the vast majority of marine organisms living in diverse habitats,26,84 and instantaneous equilibrium in the gut was assumed. This implies that a worst case was calculated, because plastic might not fully equilibrate during gut passage. The biomagnification of HOCs was calculated to decline up to 20% upon ingestion of plastic, for chemicals with a log KOW between 6.5 and 7.5.24 In the laboratory, Rochman et al.66 exposed Medaka to a diet with 10% plastic, and observed increased uptake of HOCs (∑PAH) up to a factor 2.4. The 10% of plastic in the diet as used in the studies by Gouin24 and Rochman66 is quite high compared to conditions in many aquatic habitats26 and thus can be considered to represent a worst case scenario. Besseling et al.74 investigated the effects of plastic ingestion on PCB bioaccumulation by A. marina, taking all uptake pathways into account. A factor 1.3 increase in bioaccumulation of ∑PCB was found, which however decreased with increasing plastic concentration in the sediment. The increase was ascribed to physical effects of the plastic ingestion and not to transfer of the chemicals from the plastic. Using the data from that study, Koelmans et al.25 simulated the experiment and calculated a negligible contribution of plastic in the transfer of PCBs compared to natural pathways. For an open ocean scenario, plastic was calculated to attenuate biomagnification due to its fugacity being lower than that of biota lipids. It was concluded that bioaccumulation due to ingestion by microplastic would be negligible at plastic concentrations presently occurring in oceans sediments,25 a conclusion that also was drawn for the modeled leaching of additives and subsequent uptake by lugworms or cod.26 In the previous section we showed that the parameters that were used in these model studies align with values independently obtained from desorption studies, as well as with values inferred from studies that only accounted for transfer from plastic. A fourth model study was published recently by Bakir et al.,28 who quantified the relative importance of microplastics as a pathway for the transfer of HOCs to marine biota. First, the model was validated using bioaccumulation data without plastic. Measured desorption rates (k1G) from microplastics as in Table SI-5 were used and a plastic-inclusive model was implemented for lugworm, fish and seabirds, with all uptake pathways accounted for. Their simulations showed that plastic did not increase bioaccumulation for the lugworm, and decreased bioaccumulation in fish and seabirds due to the “cleaning” effect. However, these differences were marginal at the microplastic concentration up to 5% dw of the diet. Only at a very high plastic concentration of 50%, accumulation was predicted to increase (lugworm) or decrease (seabird and fish), the latter decrease confirming the analyses by Gouin et al.24 and Koelmans et al.25
We argue that these empirical laboratory studies and model studies agree that up to realistic as well as at very high concentrations of about 1 to 10% of plastic in the sediment or in the diet, about a factor two change of bioaccumulation in either direction may occur. It has been argued recently, that the unrealistic high microplastic exposure concentrations as used in many studies do not provide any information on the current risks to marine ecosystems.85 Instead, microplastic effect assessments should address more realistic, that is, lower and chronic exposure conditions in sediments,85 as well as in the pelagic zone.86 Under such more realistic environmental conditions, organisms may simply ingest not enough microplastic particles compared to natural prey, rendering the effect on bioaccumulation to be even below a 10–20% difference in either direction.
As for field studies, we are not aware of reports that unambiguously quantify the quantity of HOCs accumulated by marine aquatic organisms from ingested plastic, compared to natural pathways. The variability in in situ bioaccumulation data has been analyzed recently and was shown to be between one to 2 orders of magnitude.68 This implies that a factor of 2 variation in either direction that can be seen under ideal conditions like in model studies or in the laboratory, will be practically impossible to infer from field data. Effects of plastic ingestion can be concluded to be smaller than the biological variability in bioaccumulation data.68 This implies that small effects of microplastic on bioaccumulation of HOCs can be observed under artificial laboratory conditions, but in nature will be overwhelmed by natural variability and by bioaccumulation from natural exposure routes.
Implications for Risk Assessment
Here we address the question whether microplastic ingestion leads to increased risks of chemicals under realistic environmental conditions. The available data suggest that the effects of microplastic ingestion on bioaccumulation probably stay within a factor of 2, which is within typical ranges of biological variability among individuals. For the majority of habitats, bioaccumulation of HOCs from microplastic is probably overwhelmed by uptake via natural pathways, a conclusion that also has been reached recently by the GESAMP WG40 working group.7 It has been argued that such a carrier effect of microplastic probably also is of limited importance for the risk assessment of HOCs,7,25 where assessment factors of 10–1000 usually are applied to account for variability and uncertainty in the effect assessment. Furthermore, increased bioaccumulation or magnification (including secondary poisoning) only implies an increase in risk if the effect thresholds are exceeded, and such a formal risk assessment to date has not been performed. To date, most studies aim at identifying a hazard of microplastic ingestion by searching for potentially increased bioaccumulation. However, it also has been argued15 that microplastic ingestion may increase bioaccumulation for some chemicals in the mixture (additives, plasticizers) yet decrease the body burden of other chemicals at the same time (POPs), in case these chemicals have opposing fugacity gradients between plastic and biota lipids.24,25,28 For a balanced risk assessment aimed at protection of populations or habitats, both effects should be considered, in relation to known effect thresholds.
Our assessments used average and present oceanic conditions, which in reality will show variation.34 Given the high calculated factors between the current microplastic concentrations and the microplastic concentrations required to cause an effect on chemical transport and bioaccumulation, our assessment is rather robust with respect to such uncertainties. Still, risk assessment should always consider local conditions where needed, and reapply tests and models for new cases. The same holds for prospective assessments that have to consider increased emissions of plastic. Nanoplastic constitutes another uncertain factor, because the abundance as well as the potential hazards of nanoplastic have not been addressed.18,87 Our suggestion that the effects of ingestion of microplastic on bioaccumulation most probably is limited for most marine habitats and therefore hard to confirm by field data does not imply that plastics do not have deleterious effects on marine life.88 To answer this ultimate question, more environmentally relevant, long-term effect studies with various species are needed.
Acknowledgments
We thank Chelsea Rochman for valuable discussions and comments on earlier versions of this manuscript. We thank Hideshige Takada for providing the data on the desorption of BDE209 to fish oil.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.5b06069.
Text, figures and tables addressing the plastic-inclusive bioaccumulation model description, model parameters, model simulation results. Modeling kinetic rate constants for desorption to artificial gut fluids. Calculation of the distribution of HOC across environmental media. Overview of studies that addressed the role of plastic in bioaccumulation of HOCs (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Thompson R. C.; Swan S. H.; Moore C. J.; vom Saal F. S. Our plastic age. Philos. Trans. R. Soc., B 2009, 364, 1973–1976. 10.1098/rstb.2009.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory M. R. Environmental implications of plastic debris in marine settings - entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc., B 2009, 364, 2013–2025. 10.1098/rstb.2008.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusher A.Microplastics in the marine environment: distribution, interactions and effects. In Marine Anthropogenic Litter; Bergmann M, Gutow L, Klages M, Eds.; Springer International Publishing: Cham, 2015; pp 245–307. [Google Scholar]
- Kühn S.; Bravo Rebolledo E. L.; van Franeker J. A.. Deleterious effects of litter on marine life. In Marine Anthropogenic Litter; Bergmann M, Gutow L, Klages M, Eds.; Springer International Publishing: Cham, 2015; pp 75–116. [Google Scholar]
- Herta Lwanga E.; Gertsen H.; Gooren H.; Peters P.; Salánki T.; van der Ploeg M.; Besseling E.; Koelmans A. A.; Geissen V. Microplastics in the terrestrial ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 2016, 50, 2685–2691. 10.1021/acs.est.5b05478. [DOI] [PubMed] [Google Scholar]
- State of Europe’s Seas, EEA Report No 2/2015; European Environment Agency, 2015; ISBN 978-92-9213-652-9, DOI: 10.2800/64016. [DOI] [Google Scholar]
- GESAMP. Sources, fate and effects of microplastics in the marine environment: a global assessment. Kershaw P. J., (ed.). (IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection). Rep. Stud. 2015, GESAMP No. 90, 96 p.
- Browne M. A.; Underwood A. J.; Chapman M. G.; Williams R.; Thompson R. C.; Van Franeker J. A. Linking effects of anthropogenic debris to ecological impacts. Proc. R. Soc. London, Ser. B 2015, 282, 2014–2929. 10.1098/rspb.2014.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann R. Critical Review of low-density polyethylene’s partitioning and diffusion coefficients for trace organic contaminants and implications for its use as a passive sampler. Environ. Sci. Technol. 2012, 46, 606–618. 10.1021/es202702y. [DOI] [PubMed] [Google Scholar]
- Velzeboer I.; Kwadijk C. J. A. F.; Koelmans A. A. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes and fullerenes. Environ. Sci. Technol. 2014, 48, 4869–4876. 10.1021/es405721v. [DOI] [PubMed] [Google Scholar]
- Andrady A. L.Plastics and Environmental Sustainability; Wiley, 2015. [Google Scholar]
- Teuten E. L.; Rowland S. J.; Galloway T. S.; Thompson R. C. Potential for plastics to transport hydrophobic contaminants. Environ. Sci. Technol. 2007, 41 (22), 7759–7764. 10.1021/es071737s. [DOI] [PubMed] [Google Scholar]
- Teuten E. L.; Saquing J. M.; Knappe D. R. U.; Barlaz M. A.; Jonsson S.; Björn A.; Rowland S. J.; Thompson R. C.; Galloway T. S.; Yamashita R.; Ochi D.; Watanuki Y.; Moore C.; Viet P. H.; Tana T. S.; Prudente M.; Boonyatumanond R.; Zakaria M. P.; Akkhavong K.; Ogata Y.; Hirai H.; Iwasa S.; Mizukawa K.; Hagino Y.; Imamura A.; Saha M.; Takada H. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc., B 2009, 364 (1526), 2027–2045. 10.1098/rstb.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler R. E. The Complex Interaction between Marine Debris and Toxic Chemicals in the Ocean. Environ. Sci. Technol. 2012, 46, 12302–12315. 10.1021/es3027105. [DOI] [PubMed] [Google Scholar]
- Koelmans A. A.Modeling the role of microplastics in Bioaccumulation of organic chemicals to marine aquatic organisms. A Critical Review. In Marine Anthropogenic Litter; Bergmann M, Gutow L, Klages M, Eds.; Springer: Berlin, 2015; ISBN 978-3-319-16510-3, p 309–324. [Google Scholar]
- Rochman C. M.The complex mixture, fate and toxicity of chemicals associated with plastic debris in the marine environment. In Marine Anthropogenic Litter; Bergmann M, Gutow L, Klages M, Eds.; Springer International Publishing: Cham, 2015; pp 117–140. [Google Scholar]
- Katsnelson A. News Feature: Microplastics present pollution puzzle. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 5547–5549. 10.1073/pnas.1504135112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester H.; Hollman P. C. H.; Peters R. J. B. Potential health impact of environmentally released micro- and nanoplastics in the human food production chain: experiences from nanotoxicology. Environ. Sci. Technol. 2015, 49, 8932–8947. 10.1021/acs.est.5b01090. [DOI] [PubMed] [Google Scholar]
- Mayer P.; Wernsing J.; Tolls J.; De Maagd P.G.-J.; Sijm D. T. H. M. Establishing and controlling dissolved concentrations of hydrophobic organics by partitioning from a solid phase. Environ. Sci. Technol. 1999, 33, 2284–2290. 10.1021/es9808898. [DOI] [Google Scholar]
- Claessens M.; Monteyne E.; Wille K.; Vanhaecke L.; Roose P.; Janssen C. R. Passive sampling reversed: Coupling passive field sampling with passive lab dosing to assess the ecotoxicity of mixtures present in the marine environment. Mar. Pollut. Bull. 2015, 93, 9–19. 10.1016/j.marpolbul.2015.02.028. [DOI] [PubMed] [Google Scholar]
- Browne M. A.; Niven S. J.; Galloway T. S.; Rowland S. J.; Thompson R. C. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr. Biol. 2013, 23, 2388–2392. 10.1016/j.cub.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Chua E. M.; Shimeta J.; Nugegoda D.; Morrison P. D.; Clarke B. O. Assimilation of Polybrominated Diphenyl Ethers from Microplastics by the Marine Amphipod, Allorchestes Compressa. Environ. Sci. Technol. 2014, 48, 8127–8134. 10.1021/es405717z. [DOI] [PubMed] [Google Scholar]
- Avio C. G.; Gorbi S.; Milan M.; Benedetti M.; Fattorini D.; d’Errico G.; Pauletto M.; Bargelloni L.; Regoli F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. 10.1016/j.envpol.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Gouin T.; Roche N.; Lohmann R.; Hodges G. A Thermodynamic Approach for Assessing the Environmental Exposure of Chemicals Absorbed to Microplastic. Environ. Sci. Technol. 2011, 45, 1466–1472. 10.1021/es1032025. [DOI] [PubMed] [Google Scholar]
- Koelmans A. A.; Besseling E.; Wegner A.; Foekema E. M. Plastic as a carrier of POPs to aquatic organisms. A model analysis. Environ. Sci. Technol. 2013, 47, 7812–7820. 10.1021/es401169n. [DOI] [PubMed] [Google Scholar]
- Koelmans A. A.; Besseling E.; Wegner A.; Foekema E. M. Correction to plastic as a carrier of POPs to aquatic organisms. A model analysis. Environ. Sci. Technol. 2013, 47, 8992–8993. 10.1021/es403018h. [DOI] [PubMed] [Google Scholar]
- Koelmans A. A.; Besseling E.; Foekema E.M. Leaching of Plastic Additives to Marine Organisms. Environ. Pollut. 2014, 187, 49–54. 10.1016/j.envpol.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Bakir A.; O’Connor I. A.; Rowland S. J.; Hendriks A. J.; Thompson R. C.. Relative importance of microplastics as a pathway for the transfer of persistent organic pollutants to marine life. Chapter 3. In Modelling the Oral Uptake of Chemicals: The Role of Plastic, Passive Diffusion and Transport Proteins. PhD thesis; O’Connor I. A.; Radboud University Nijmegen: the Netherlands, 2014; ISBN: 978-94-6259-386-2. [Google Scholar]
- Mato Y.; Isobe T.; Takada H.; Kanehiro H.; Ohtake C.; Kaminuma T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001, 35, 318–324. 10.1021/es0010498. [DOI] [PubMed] [Google Scholar]
- Zarfl C.; Matthies M. Are marine plastic particles transport vectors for organic pollutants to the Arctic?. Mar. Pollut. Bull. 2010, 60, 1810–1840. 10.1016/j.marpolbul.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Plastics – the Facts, An Analysis of European Latest Plastics Production, Demand and Waste Data; PlasticsEurope, 2013. [Google Scholar]
- Schwarzenbach R. P.; Gschwend P. M.; Imboden D.M... Environmental Organic Chemistry, 2nd ed.; Wiley-Interscience, 2003. [Google Scholar]
- Jambeck J. R.; Geyer R.; Wilcox C.; Siegler T. R.; Perryman M.; Andrady A.; Narayan R.; Law K. L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- van Sebille E.; England M. H.; Froyland G.. Origin, dynamics and evolution of ocean garbage patches from observed surface drifters. Environ. Res. Lett. 2012, 7, 044040, DOI: 10.1088/1748-9326/7/4/044040. [DOI] [Google Scholar]
- Obbard R. W.; Sadri S.; Wong Y. Q.; Khitun A. A.; Baker I.; Thompson R. C. Global warming releases microplastic legacy frozen in Arctic Sea ice. Earth's Future 2014, 2, 315–320. 10.1002/2014EF000240. [DOI] [Google Scholar]
- Ye S.; Andrady A.L. Fouling of floating plastic debris under biscayne bay exposure conditions. Mar. Pollut. Bull. 1991, 22 (12), 608–613. 10.1016/0025-326X(91)90249-R. [DOI] [Google Scholar]
- Andrady A. L.Persistence of Plastic Litter in the Oceans. In Marine Anthropogenic Litter; Bergmann M.et al. , Eds.; Springer, 2015; DOI 10.1007/978-3-319-16510-3_3. [DOI] [Google Scholar]
- Rochman C. M.; Lewison R. L.; Eriksen M.; Allen H.; Cook A. M.; The S. J. Polybrominated diphenyl ethers (PBDEs) in fish tissue may be an indicator of plastic contamination in marine habitats. Sci. Total Environ. 2014, 476, 622–633. 10.1016/j.scitotenv.2014.01.058. [DOI] [PubMed] [Google Scholar]
- Van Cauwenberghe L.; Vanreusel A.; Mees J.; Janssen C. R. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499. 10.1016/j.envpol.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Karapanagioti H. K.; Ogata Y.; Takada H. Eroded plastic pellets as monitoring tools for polycyclic aromatic hydrocarbons (PAH): Laboratory and field studies. Global NEST J. 2010, 12, 327–334. [Google Scholar]
- Rochman C. M.; Hoh E.; Hentschel B. T.; Kaye S. Long-Term Field Measurement of Sorption of Organic Contaminants to Five Types of Plastic Pellets: Implications for Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 1646–1654. 10.1021/es303700s. [DOI] [PubMed] [Google Scholar]
- Endo S.; Yuyama M.; Takada H. Desorption kinetics of hydrophobic organic contaminants from marine plastic pellets. Mar. Pollut. Bull. 2013, 74, 125–131. 10.1016/j.marpolbul.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Rioz Mendoza L. M.; Jones P. R. Characterisation of microplastics and toxic chemicals extracted from microplastic samples from the North Pacific Gyre. Environ. Chem. 2015, 12, 611–617. 10.1071/EN14236. [DOI] [Google Scholar]
- van der Oost R.; Beyer J.; Vermeulen N. P. E. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. 10.1016/S1382-6689(02)00126-6. [DOI] [PubMed] [Google Scholar]
- Cornelissen G.; Gustafsson Ö.; Bucheli T. D.; Jonker M. T. O.; Koelmans A. A.; van Noort P. C. M. Critical Review. Extensive Sorption of Organic Compounds to Black Carbon, Coal, and Kerogen in Sediments and Soils: Mechanisms and Consequences for Distribution, Bioaccumulation and Biodegradation. Environ. Sci. Technol. 2005, 39, 6881–6895. 10.1021/es050191b. [DOI] [PubMed] [Google Scholar]
- Kelly B. C.; Gobas F. A. P. C.; McLachlan M. S. Intestinal absorption and biomagnification of organic contaminants in fish, wildlife, and humans. Environ. Toxicol. Chem. 2004, 23, 2324–2336. 10.1897/03-545. [DOI] [PubMed] [Google Scholar]
- Karickhoff S. Organic Pollutant Sorption in Aquatic Systems. J. Hydraul. Eng. 1984, 110, 707–735. 10.1061/(ASCE)0733-9429(1984)110:6(707). [DOI] [Google Scholar]
- Luthy R. G.; Aiken G. R.; Brusseau M. L.; Cunningham S. D.; Gschwend P. M.; Pignatello J. J.; Reinhard M.; Traina S. J.; Weber W. J. Jr.; Westall J. C. Sequestration of hydrophobic organic contaminants by geosorbents. Environ. Sci. Technol. 1997, 31, 3341–3347. 10.1021/es970512m. [DOI] [Google Scholar]
- Ghosh U.; Luthy R. G.; Cornelissen G.; Werner D.; Menzie C. A. In situ sorbent amendments: A new direction in contaminated sediment management. Environ. Sci. Technol. 2011, 45, 1163–1168. 10.1021/es102694h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupryianchyk D.; Rakowska M. I.; Reible D.; Harmsen J.; Cornelissen G.; van Veggel M.; Hale S.; Grotenhuis J. T. C.; Koelmans A. A. Positioning activated carbon amendment technologies in a novel framework for sediment management. Integr. Environ. Assess. Manage. 2015, 11, 221–234. 10.1002/ieam.1606. [DOI] [PubMed] [Google Scholar]
- Schrap S. M.; Opperhuizen A. Relationship between bioavailability and hydrophobicity: Reduction of the uptake of organic chemicals by fish due to the sorption on particles. Environ. Toxicol. Chem. 1990, 9, 715–724. 10.1002/etc.5620090604. [DOI] [Google Scholar]
- Shea P. J.; Strek H. J.; Weber J. B. Polychlorinated biphenyls: Absorption and bioaccumulation by goldfish (Carassius auratus) and inactivation by activated carbon. Chemosphere 1980, 9, 157–164. 10.1016/0045-6535(80)90087-9. [DOI] [Google Scholar]
- Kupryianchyk D.; Rakowska M. I.; Roessink I.; Reichman E. P.; Grotenhuis J. T. C.; Koelmans A. A. In situ activated carbon amendment reduces bioaccumulation in aquatic food chains. Environ. Sci. Technol. 2013, 47 (9), 4563–4571. 10.1021/es305265x. [DOI] [PubMed] [Google Scholar]
- Tomei M. C.; Mosca Angelucci D.; Ademollo N.; Daugulis A. J. Rapid and effective decontamination of chlorophenol-contaminated soil by sorption into commercial polymers: Concept demonstration and process modelling. J. Environ. Manage. 2015, 150, 81–91. 10.1016/j.jenvman.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Jonker M. T. O.; Koelmans A. A. Polyoxymethylene solid phase extraction as a partitioning method for hydrophobic organic chemicals in sediment and soot. Environ. Sci. Technol. 2001, 35, 3742–3748. 10.1021/es0100470. [DOI] [PubMed] [Google Scholar]
- Rusina T.; Smedes F.; Koblizkova M.; Klanova J. Calibration of Silicone Rubber Passive Samplers: Experimental and Modeled Relations between Sampling Rate and Compound Properties. Environ. Sci. Technol. 2010, 44, 362–367. 10.1021/es900938r. [DOI] [PubMed] [Google Scholar]
- Odum E. P.Fundamentals of Ecology. 3rd ed.; W.B. Saunders Company: Philadelphia, 1971. [Google Scholar]
- Eriksen M.; Lebreton L. C.; Carson H. S.; Thiel M.; Moore C. J.; Borerro J. C.; Galgani F.; Ryan P. G.; Reisser J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS One 2014, 9 (12), e111913. 10.1371/journal.pone.0111913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiello C. A.; Druffel E. R. M. Black carbon in deep-sea sediments. Science 1998, 280, 1911–1913. 10.1126/science.280.5371.1911. [DOI] [PubMed] [Google Scholar]
- Middelburg J. J.; Nieuwenhuize J.; Van Breugel P. Black carbon in marine sediments. Mar. Chem. 1999, 65, 245–252. 10.1016/S0304-4203(99)00005-5. [DOI] [Google Scholar]
- Mannino A.; Harvey H. R. Black carbon in estuarine and coastal ocean dissolved organic matter. Limnol. Oceanogr. 2004, 49, 735–740. 10.4319/lo.2004.49.3.0735. [DOI] [Google Scholar]
- Pohl K.; Cantwell M.; Herckes P.; Lohmann R. Black carbon concentrations and sources in the marine boundary layer of the tropical Atlantic Ocean using four methodologies. Atmos. Chem. Phys. 2014, 14, 7431–7443. 10.5194/acp-14-7431-2014. [DOI] [Google Scholar]
- Cauwet G. Organic chemistry of sea water particulates. Concepts and developments. Mar. Chem. 1978, 1, 99–105. 10.1016/0304-4203(77)90040-8. [DOI] [Google Scholar]
- Garrison T. S.Oceanography: An Invitation to Marine Science; Thompson Brooks/Cole, 2005; p 4. [Google Scholar]
- Jonker M. T. O.; Koelmans A. A. Sorption of polycyclic aromatic hydrocarbons and polychlorinated biphenyls to soot and soot-like materials in the aqueous environment. Mechanistic Considerations. Environ. Sci. Technol. 2002, 36, 3725–3734. 10.1021/es020019x. [DOI] [PubMed] [Google Scholar]
- Rochman C. M.; Hoh E.; Kurobe T.; Teh S. J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3 (3263), 1–7. 10.1038/srep03263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotterman M.; Larsen B. K.; Sloth J. J.; Rasmussen R. R.; Kwadijk C.; Granby K.. Transfer and accumulation of organic pollutants in Atlantic salmon; Effect of incorporation of plastic particles in feed. In Proceedings, SETAC Annual Meeting, Barcelona, Spain, May 2015.
- Selck H.; Drouillard K.; Eisenreich K.; Koelmans A. A.; Palmqvist A.; Ruus A.; Salvito D.; Schultz I.; Stewart R.; Weisbrod A.; van den Brink N. W.; van den Heuvel-Greve M. 2012. Explaining differences between bioaccumulation measurements in laboratory and field data using a probabilistic modeling approach. Integr. Environ. Assess. Manage. 2012, 8, 42–63. 10.1002/ieam.217. [DOI] [PubMed] [Google Scholar]
- Geusau A.; Tschachler E.; Meixner M.; Sandermann S.; Päpke O.; Wolf C.; Valic E.; Stingl G.; McLachlan M. Olestra increases faecal excretion of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Lancet 1999, 354, 1266–1267. 10.1016/S0140-6736(99)04271-3. [DOI] [PubMed] [Google Scholar]
- Richter E.; Lay J. P.; Klein W.; Korte F. 1977. Enhanced elimination of hexachlorobenzene in rats by light liquid paraffin. Chemosphere 1977, 6, 357–369. 10.1016/0045-6535(77)90101-1. [DOI] [Google Scholar]
- Moser G. A.; McLachlan M. S. A non-absorbable dietary fat substitute enhances elimination of persistent lipophilic contaminants in humans. Chemosphere 1999, 39, 1513–1521. 10.1016/S0045-6535(99)00219-2. [DOI] [PubMed] [Google Scholar]
- Yoshimura H.; Kamimura H.; Oguri K.; Honda Y.; Nakano M. Stimulating effect of activated charcoal beads on fecal excretion of 2,3,4,7,8-pentachlorodibenzofuran in rats. Chemosphere 1986, 15, 219–227. 10.1016/0045-6535(86)90017-2. [DOI] [Google Scholar]
- Morin N.; Arp H. P. H.; Hale S. E. Bisphenol A in Solid Waste Materials, Leachate Water, and Air Particles from Norwegian Waste-Handling Facilities: Presence and Partitioning Behavior. Environ. Sci. Technol. 2015, 49, 7675–7683. 10.1021/acs.est.5b01307. [DOI] [PubMed] [Google Scholar]
- Besseling E.; Wegner A.; Foekema E. M.; van den Heuvel-Greve M. J.; Koelmans A. A. Effects of microplastic on performance and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ. Sci. Technol. 2013, 47 (1), 593–600. 10.1021/es302763x. [DOI] [PubMed] [Google Scholar]
- Herzke D.; Anker-Nilssen T.; Haugdahl T.; Nøst T.; Götsch A.; Christensen-Dalsgaard S.; Langset M.; Fangel K.; Koelmans A. A. Negligible Impact of ingested microplastics on tissue concentrations of persistent organic pollutants in Northern Fulmars off coastal Norway. Environ. Sci. Technol. 2016, 50, 1924–1933. 10.1021/acs.est.5b04663. [DOI] [PubMed] [Google Scholar]
- Van Cauwenberghe L., 2015. Occurrence, effects and risks of marine microplastics. Thesis submitted in fulfilment of the requirements for the degree of Doctor (PhD) in Applied Biological Sciences.
- Rykiel E. J. Testing ecological models: the meaning of validation. Ecol. Modell. 1996, 90, 229–244. 10.1016/0304-3800(95)00152-2. [DOI] [Google Scholar]
- Fossi M. C.; Panti C.; Guerranti C.; Coppola D.; Giannetti M.; Marsili L. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Mar. Pollut. Bull. 2012, 64, 2374–9. 10.1016/j.marpolbul.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Tanaka K.; Takada H.; Yamashita R.; Mizukawa K.; Fukuwaka M.; Watanuki Y. Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar. Pollut. Bull. 2013, 69, 219–22. 10.1016/j.marpolbul.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Scheffer M.; Beets J. Ecological models and the pitfalls of causality. Hydrobiologia 1994, 275/276, 115–124. 10.1007/BF00026704. [DOI] [Google Scholar]
- Bakir A.; Rowland S. J.; Thompson R. C. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014, 185, 16–23. 10.1016/j.envpol.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Tanaka K.; Takada H.; Yamashita R.; Mizukawa K.; Fukuwaka M.; Watanuki Y. Facilitated Leaching of Additive-Derived PBDEs from Plastic by Seabirds’ Stomach Oil and Accumulation in Tissues. Environ. Sci. Technol. 2015, 49, 11799–11807. 10.1021/acs.est.5b01376. [DOI] [PubMed] [Google Scholar]
- Arnot J. A.; Gobas F. A. P. C. A food web bioaccumulation model for organic chemicals in aquatic ecosystems. Environ. Toxicol. Chem. 2004, 23, 2343–2355. 10.1897/03-438. [DOI] [PubMed] [Google Scholar]
- Van Cauwenberghe L.; Claessens M.; Vandegehuchte M. B.; Janssen C. R. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut. 2015, 199, 10–17. 10.1016/j.envpol.2015.01.008. [DOI] [PubMed] [Google Scholar]
- Van Cauwenberghe L.; Devriese L.; Galgani F.; Robbens J.; Janssen C. R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. 10.1016/j.marenvres.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Burton G. A. Losing Sight of Science in the Regulatory Push to Ban Microbeads from Consumer Products and Industrial Use. Integr. Environ. Assess. Manage. 2015, 11, 346–347. 10.1002/ieam.1645. [DOI] [PubMed] [Google Scholar]
- Koelmans A. A.; Besseling E.; Shim W. J.. Nanoplastics in the aquatic environment. Critical Review. In Marine Anthropogenic Litter; Bergmann M, Gutow L, Klages M, Eds.; Springer: Berlin, 2015; ISBN 978-3-319-16510-3, p 325–340. [Google Scholar]
- Marine Anthropogenic Litter; Bergmann M, Gutow L, Klages M, Eds.; Springer International Publishing: Cham, 2015; pp 245–307. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


