Abstract
Background:
The role of coronary microvascular disease and its impact on functional and energetic reserve in HFpEF remains unclear. We hypothesized that in response to sub-maximal pharmacologic stress (dobutamine), HFpEF patients have impairment in left ventricular (LV) myocardial mechanical (external work, EW), energetic (myocardial oxygen consumption, MVO2) and myocardial blood flow (MBF) reserve. We further assessed if coupling of MBF to EW is impaired in HFpEF and associated with compensatory increases or pathologic decreases in myocardial O2-extraction. Lastly, we assessed if coupling of MVO2 to EW (mechanical efficiency) was impaired in HFpEF.
Methods and Results:
In prospectively-enrolled HFpEF patients (n=19) and age/sex-matched healthy controls (n=19), we performed 11C-acetate positron emission tomography assessing MVO2 and MBF at rest and during dobutamine infusion. EW was calculated as stroke volume (echo) x end-systolic pressure x heart rate. At rest, compared to Controls, HFpEF patients had higher LV EW, MVO2 and MBF. With dobutamine, LV EW, MVO2, and MBF increased in both HFpEF and Controls; however, the magnitude of increases were significantly smaller in HFpEF. In both groups, MBF increased in relation to EW but in HFpEF, the slope of the relationship was significantly smaller than in Controls. Myocardial O2-extraction was increased in HFpEF. Mechanical efficiency was similar in HFpEF and Controls. In a post-hoc analysis, HFpEF patients with LV hypertrophy (n=10) had significant reductions in LV mechanical efficiency relative to Controls.
Conclusions:
In HFpEF during submaximal dobutamine stress, there is myocardial mechanical-, energetic- and flow-reserve dysfunction with impaired coupling of blood flow to demand and slight increases in myocardial O2-extraction. These findings provide evidence that coronary microvascular dysfunction is present in HFpEF, limits O2-supply relative to demand and is associated with reserve dysfunction.
Keywords: heart failure, nuclear cardiology and PET, pathophysiology, metabolism, hypertrophy, clinical studies
Heart failure (HF) with preserved ejection fraction (HFpEF) is a major public health problem without proven effective therapy.1 Myocardial energetics and mechanoenergetic coupling are impaired in HF with reduced EF (HFrEF). Therapies such as β–blockers and cardiac resynchronization therapy improve energetics, efficiency and outcomes in HFrEF.2,3 Myocardial energetics may represent novel therapeutic targets in HFpEF,4,5 but have not been well studied.
Patients with HFpEF have reduced left ventricular (LV) systolic and diastolic reserve during exercise or inotropic infusions.6,7 Coronary microvascular density and myocardial flow reserve in response to maximal pharmacologic vasodilation are reduced in HFpEF.8–11 Whether coronary microvascular dysfunction limits myocardial blood flow (MBF) and oxygen (O2) delivery relative to demand (external work, EW) and contributes to systolic or diastolic reserve dysfunction during sub-maximal stress is unclear.
Ischemia due to epicardial coronary disease results in compensatory increases in myocardial O2-extraction. It has recently been hypothesized that heterogeneity in myocardial O2-tension associated with coronary microvascular dysfunction could result in blood shunting from areas of low-flow/high O2-extraction to areas of high-flow/low O2-extraction and reduce overall myocardial O2-extraction.12 LV hypertrophy (LVH) and other comorbidities common in HFpEF have been associated with alterations in substrate utilization13,14 which may impact myocardial O2 consumption (MVO2) beyond load, contractile state, heart rate (HR) and perfusion and affect mechanical efficiency, the total LV MVO2 needed to produce a given amount of EW.2
We hypothesized that HFpEF patients have impairment in myocardial mechanical (EW), energetic (MVO2) and MBF reserve functions. We further assessed if coupling of MBF to EW is impaired in HFpEF and associated with compensatory increases or pathologic decreases in myocardial O2-extraction. Lastly, we sought to determine if coupling of MVO2 to EW (mechanical efficiency) was altered in HFpEF. Accordingly, we used echocardiography and 11C-acetate PET imaging to simultaneously measure EW, MVO2 and MBF in prospectively-enrolled HFpEF and age/sex-matched healthy Control cohorts, at rest and under sub-maximal pharmacologic increases in demand (dobutamine).
METHODS
The authors declare that all supporting data are available within the article and its online supplementary files.
Study Population
Nineteen patients >50 years old with a diagnosis of HF confirmed by Framingham criteria, NYHA functional class II-IIIb symptoms, and EF ≥50% were studied in an outpatient, compensated state. Exclusion criteria included a previous EF <50%, hemodynamically significant valvular disease, pericardial disease, clinically significant coronary disease (myocardial infarction, unstable angina, revascularization within 60 days of or anticipating revascularization at time of consent), pacemaker dependence, unstable ventricular arrhythmias, infiltrative or hypertrophic cardiomyopathy, clinically significant lung disease, severe renal dysfunction (estimated GFR <20 mL/min/1.73 m2 by modified MDRD equation), primary hepatic disease, hemoglobin <9 g/dL and weight >450 lb (PET scanner table weight limit). Nineteen age- and sex-matched healthy Controls without cardiopulmonary or kidney disease, any cardiovascular risk factors or treatment with cardiovascular medications were recruited by advertisement. Control patients with milder (BMI <35 kg/m2) obesity were not excluded. The Institutional Review Board of the Mayo Foundation approved this study. Participants provided written informed consent.
Study Protocol
Subjects were studied in the fasting state at rest, allowed to eat a small snack thereafter and then fasted again (at least four hours) prior to stress imaging. All vasoactive medications were withheld for at least 12 hours before study. Long-acting β–blockers and non-dihydropyridine calcium channel-blockers were held 24 and 30 hours prior to rest and stress studies, respectively. Their shorter acting formulations were held 12 and 18 hours prior to rest and stress studies, respectively. The study protocol is illustrated in Supplemental Figure 1. Subjects underwent comprehensive transthoracic echocardiography including stroke volume (SV) assessment followed by intravenous 11C-acetate injection and dynamic-PET acquisition at rest. Six hours later, dobutamine infusion was initiated. After 15 minutes of continuous dobutamine, SV was again assessed by echo, followed by a second dose of intravenous 11C-acetate and dynamic-PET acquisition during continued dobutamine infusion. The first 9 subjects (n=5 Control, n=4 HFpEF) received 5 ug/kg/min dobutamine and the remaining subjects (n=14 Control, n=15 HFpEF) received 8 ug/kg/min. The entire duration of the dobutamine infusion was approximately 45 minutes for all subjects. This dose range revealed differential changes in contractile reserve in HFpEF and Controls in a previous study.7 During dobutamine infusion, patients were continuously monitored by electrocardiography. Heart rates and brachial blood pressures were measured every 5 minutes and averaged over the 30-minute PET acquisition period.
Transthoracic Echocardiography
Echo-Doppler measurements represent the mean of ≥3 beats. LV mass was calculated from 2D-measurements of wall thickness and chamber dimension using the cube formula.15 Relative wall thickness was calculated using the sum of the septal- and posterior-wall diastolic thickness divided by LV diastolic diameter. The EF was calculated with Simpson’s biplane method.15 The SV was determined from the LV outflow dimension and pulse-wave Doppler. Arterial elastance was calculated as end-systolic pressure (ESP)/SV and a single-beat estimate of end-systolic elastance (Ees [determined from BP, SV, EF, and pre-ejection and systolic ejection time intervals from LV outflow Doppler]) was calculated.16 ESP was estimated as 0.9*systolic blood pressure as previously validated.17 Systolic LV wall stress was assessed by Quinones’ formula.18 Diastolic function was characterized by assessment of mitral inflow pulsed-wave Doppler (E velocity and deceleration time), medial diastolic annular tissue Doppler velocity (e’) and the E/e’ ratio. Left atrial volume was measured by the biplane method and indexed to BSA. Right ventricular systolic pressure was estimated from the tricuspid regurgitation velocity and inferior vena cava dimensions (rest and sniff).
11C-Acetate PET Imaging
A Discovery 710 PET/CT system (General Electric Medical Systems, Waukesha, WI, USA) with an axial field of view of 15.6 cm was utilized. 11C-acetate was produced by an on-site PET trace cyclotron (General Electric Medical Systems) using the technique described by Pike et al.19 CT scout and transmission images were acquired to locate the heart within the field of view, and for attenuation and model-based scatter corrections, respectively. Subsequently, 14±20% mCi of 11C-acetate was injected intravenously over 30 seconds and a 30-minute dynamic-PET acquisition (10 framesx10s, 1×60s, 5×100s, 3×180s, 2×300s) was performed. PET images were generated (128×128 matrix, voxel size of 3.3mm3) using a fully 3D-iterative reconstruction algorithm. Standard corrections for attenuation, scatter, random coincidences, dead-time and decay were applied. Reconstructed dynamic-PET data was analyzed with PMOD software (PMOD Technologies, Zurich, Switzerland) by generating time-activity curves from regions of interest superimposed over the entire LV myocardium in short-axis planes (Supplemental Figure 2A).
A monoexponential function was fit to the early portion of the ‘wash-out’ phase of myocardial time-activity data (Supplemental Figure 2B) to determine the clearance rate constant (Kmono; min−1)-a surrogate for MVO2- as previously described.20 Using the ‘wash-in’ frames of the myocardium time-activity curve (Supplemental Figure 2C) and an input function derived from the LV blood pool, a differential equation was used to solve for MBF (ml/g/min), as previously described.21
Energetics Analyses
External work (EW) was calculated as the product of ESP, SV and HR (mmHg*ml/min). EW was converted to Joules (J, per minute) by multiplying by 1.33*10−4 as previously described.2 Kmono was converted to MVO2 in ml/100g/min as (Kmono-0.0197)/0.0027 as previously described22 and converted to ml/min by multiplying by LV mass (g)/100. MVO2 was converted to J (per min) by multiplying by 20 J/1 ml MVO2 as previously described.2
LV MBF, MVO2 and EW per minute were assessed during rest and dobutamine. Mechanical efficiency was assessed at rest and during dobutamine as the simple quotient of total LV EW/MVO2, both expressed in J/min. Further, the relationship between MVO2 and EW was displayed across the range of values observed during rest and dobutamine and analyzed by Group using least-squares regression as an additional index of mechanical efficiency.
Coupling of Myocardial Blood Flow and External Work
We assessed the relationship between MBF and EW and the potential for Group differences in the slope or position of this relationship as a measure of appropriate coupling of MBF to demand (EW).
Assessment of Myocardial Oxygen Extraction
According to Fick’s law,23 MVO2=MBF*([O2]ART - [O2]CS); where [O2]ART is the O2-content of arterial blood and [O2]CS is the O2-content of coronary sinus (CS) blood. [O2]ART and [O2]CS are determined by the hemoglobin (Hgb), it’s O2-carrying capacity (1.36ml O2/g Hgb) and the O2-saturation (O2Sat) of the arterial and CS blood respectively (ignoring the very small contribution of dissolved O2). Thus, MVO2 ∝ MBF*Hgb*(O2SatART - O2SatCS). Accordingly, total MVO2 will be higher for any given MBF and Hgb if (O2SatART - O2SatCS) is increased due to a lower O2SatCS relative to O2SatART (increased O2-extraction). Similarly, MVO2 will be lower for any given MBF and Hgb if (O2SatART - O2SatCS) is decreased due to a higher O2SatCS relative to O2SatART (decreased O2-extraction). We assessed the relationship between MVO2 and MBF, Hgb and Group across the range of resting and stress values in HFpEF and Controls to determine if there were Group differences in O2-extraction in HFpEF. To graphically depict Group differences in O2-extraction, we calculated the total O2 delivered to the myocardium from O2SatART, Hgb and MBF, and examined the relationship between MVO2 and delivered O2 by Group.
Effect of LV Hypertrophy
Given previous studies describing variable impact of LV hypertrophy (LVH) on myocardial efficiency,2 a post-hoc analysis compared energetics and MBF in HFpEF subjects with LVH (LV mass/Height2.7>44 in women or >48 in men)24 versus Controls.
Statistical Analysis
All data (irrespective of distribution) in tables are presented as medians (interquartile ranges) or proportions. Simple between-group comparisons were performed on log transformed data and used Student t-test if the data were normally distributed (Shapiro Wilk p value ≥ 0.05) or the Wilcoxon rank sum test if data were not normally distributed (as indicated in table legends). The Pearson Chi-square test was used to test differences in proportions. Paired log transformed data (rest-stress) were compared by paired t-test after confirmation that the distribution of the differences (stress-rest) was normal. By design, the Control cohort was age/sex-matched and thus data were not adjusted for age/sex.
Comparison of Group differences in reserve function and variables during dobutamine used multivariable least-squares regression to adjust for the dose of dobutamine administered (5 vs 8 ug/kg/min). A separate analysis compared Group differences in reserve function adjusting for use of β–blocker therapy. Analysis of the output for these analyses confirmed that the key assumptions of the analytic technique were met.
Group differences in associations between variables (MBF and EW, O2-delivery and MVO2, MVO2 and EW) were assessed across the values observed during rest and dobutamine using multivariable least-squares regression. Between Group slope differences were assessed by adding an interaction term for the independent variables. If no significant difference in slope was present (interaction term p>0.05), potential Group differences due to parallel shifts in the relationship were assessed from the model without the interaction term. Assessment of Group differences in O2-extraction used multivariable least-squares regression to examine determinates of MVO2 (MBF, Hgb, Group) with Group differences interpreted as changes in extraction as outlined above.
In a post-hoc analysis, we also assessed mechanical efficiency and energetics in HFpEF patients with LVH (HFpEF+LVH) vs Control using methods as above. Two-sided p-values <0.05 were considered statistically significant. Statistical analyses were performed with JMP Pro software, version 13 and with support of the Mayo Clinic Center for Translational Science Activities.
RESULTS
Baseline Characteristics
By design, age- and sex-distribution were not significantly different between HFpEF and Controls (Table 1). Comorbidities [hypertension, diabetes, non-obstructive coronary artery disease (CAD), atrial fibrillation and obstructive sleep apnea] and use of cardiovascular medications were common in HFpEF (Table 1). Obesity was present in 5 (26%) Control and 12 (63%) HFpEF subjects. At enrollment, 3 (16%), 11 (58%) and 5 (26%) HFpEF patients described NYHA class II, II-III or III symptoms, respectively. As compared to Controls, HFpEF patients had worse renal function and higher uric acid levels but hemoglobin was not significantly different (Table 1). Of the HFpEF patients, 7 had prior negative stress testing without previous angiography, 8 had either no or non-obstructive (≤50% stenosis) CAD on previous angiography, 2 had previous surgical revascularization and subsequent negative stress perfusion/imaging, 1 had a 50–60% stenosis with negative stress perfusion imaging and 1 patient had an occluded distal right coronary artery with no other significant disease.
Table 1:
Baseline Characteristics
| Controls (n=19) | HFpEF (n=19) | p value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 66 (62–70) | 70 (67–78) | 0.13 |
| Female sex | 12 (63%) | 13 (68%) | 0.73 |
| BMI, kg/m2 | 26.7 (23.2–30.8) | 35.0 (26.4–39.4) | 0.002 |
| BSA, m2 | 1.84 (1.66–2.04) | 1.92 (1.85–2.24) | 0.04 |
| HF hospitalization in last year, n | 0 | 5 (19%) | 0.006 |
| Comorbidities | |||
| Obesity (BMI≥30 kg/m2) | 5 (26%) | 12 (63%) | 0.02 |
| Hypertension | 0 (0%) | 18 (95%) | <0.001 |
| Diabetes | 0 (0%) | 5 (26%) | 0.02 |
| Coronary artery disease* | 0 (0%) | 11 (58%) | <0.001 |
| History of AFib | 0 (0%) | 11 (58%) | <0.001 |
| AFib present at enrollment | 0 (0%) | 6 (32%) | 0.008 |
| COPD | 0 (0%) | 6 (32%) | 0.008 |
| Obstructive sleep apnea | 0 (0%) | 14 (74%) | <0.001 |
| Medications | |||
| ACE inhibitor or ARB | 0 (0%) | 14 (74%) | <0.001 |
| Aldosterone antagonist | 0 (0%) | 3 (16%) | 0.07 |
| Beta-blocker | 0 (0%) | 14 (74%) | <0.001 |
| Non-dihydropyridine CCB | 0 (0%) | 1 (5%) | 0.31 |
| Loop diuretic | 0 (0%) | 13 (68%) | <0.001 |
| Any diuretic | 0 (0%) | 16 (84%) | <0.001 |
| Statin | 0 (0%) | 12 (63%) | <0.001 |
| Laboratory Data | |||
| Creatinine, mg/dL | 0.8 (0.7–0.9) | 1.1 (0.9–1.3) | <0.001 |
| eGFR, mL/min/1.73m2 | 81 (77–88) | 58 (46–73) | <0.001‡ |
| Hemoglobin, mg/dL | 13.3 (12.2–13.7) | 13.1 (11.8–13.6) | 0.28 |
| Uric acid, mg/dL | 4.5 (3.8–5.5) | 6.5 (5.8–7.9) | <0.001 |
Data are number (%) or median (interquartile range).
See text for full details between group statistical comparisons by Student t-test except as indicated.
Statistical comparison by Wilcoxan rank sum test.
Abbreviations: BMI, Body Mass Index; BSA, Body Surface Area; HF, Heart Failure; AFib, Atrial Fibrillation; COPD, Chronic Obstructive Pulmonary Disease; ACE, Angiotensin Converting Enzyme; ARB, Angiotensin Receptor Blocker; CCB, Calcium Channel Blocker; eGFR, Estimated Glomerular Filtration Rate; HFpEF, heart failure with preserved ejection fraction.
Resting Cardiac Structure and Function
As compared to Controls, EF was not significantly different but arterial and LV Ees were significantly higher in HFpEF (Table 2). HFpEF patients had higher LV mass indexed to height2.7. LV systolic wall stress was not significantly different in HFpEF and Control patients. HFpEF patients had more impaired relaxation (lower e’), higher filling pressures (higher E/e’), evidence of restrictive physiology (shorter mitral deceleration time), larger left atrial size and higher pulmonary artery systolic pressures as compared to Controls.
Table 2:
Cardiac Structure and Function
| Controls (n=19) | HFpEF (n=19) | p value | |
| Ejection fraction, % | 63 (58–68) | 65 (62–68) | 0.23 |
| Arterial elastance, mmHg/ml | 1.31 (1.06–1.67) | 1.56 (1.31–1.89) | 0.03 |
| End-systolic elastance, mmHg/mL | 3.3 (2.9–4.6) | 3.9 (3.6–5.3) | 0.01 |
| LVEDd, mm | 47 (44–48) | 49 (47–53) | 0.09 |
| Relative wall thickness | 0.40 (0.36–0.43) | 0.43 (0.39–0.50) | 0.15 |
| LV mass, g/m2.7 | 37.3 (33.4–40.1) | 44.4 (40.1–63.2) | 0.002 |
| LV systolic wall stress (x 103 dyn/cm2) | 204 (175–224) | 212 (186–247) | 0.57 |
| E/A ratio | 1.2 (1.0–1.5) | 1.2 (1.0–2.4) | 0.37* |
| Medial e’, m/sec | 0.07 (0.06–0.10) | 0.06 (0.05–0.07) | 0.01* |
| Medial E/e | 10 (8–10) | 18 (14–24) | <0.001 |
| Deceleration time, ms | 202 (182–237) | 176 (132–191) | 0.006* |
| LA volume/BSA, mL/m2 | 30 (25–34) | 38 (32–47) | 0.006 |
| PASP, mmHg | 25 (22–28) | 42 (34–48) | <0.001 |
Data are median (interquartile range). Between group statistical comparisons by Student t-test except as indicated.
Statistical comparison by Wilcoxan rank sum test.
Abbreviations: BSA, Body Surface Area; LVEDd, Left Ventricular End-diastolic Dimension; LV, Left Ventricular; LA, Left Atrial; PASP, Pulmonary Artery Systolic Pressure
Rest and Pharmacologic Stress Myocardial Energetics and Blood Flow
At rest, HR, ESP and LV mass were greater in HFpEF than Controls while SV and Kmono were not significantly different in the two groups (Table 3). The resting total LV EW, MVO2 and MBF per minute were all greater in HFpEF (Table 3). With dobutamine, HR, ESP, EW, MVO2 and MBF all increased relative to baseline values in both Controls and HFpEF (Table 3). However, while SV increased with dobutamine in Controls, SV did not change relative to baseline in HFpEF. During dobutamine infusion and adjusting for dobutamine dose, as compared to Controls, HR and ESP were greater while SV was smaller in HFpEF (Table 3). Total EW, Kmono, MVO2 and MBF during dobutamine infusion were not significantly different in HFpEF versus Controls.
Table 3:
Rest and Stress Myocardial Energetics
| Control (n=19) | HFpEF (n=19) | T-test P value vs Control | HFpEF + LVH (n=10) | T-test P value vs Control | |
|---|---|---|---|---|---|
| REST | |||||
| HR, bpm | 58 (49–66) | 73 (60–79) | 0.004 | 77 (62–79) | <0.001 |
| ESP, mmHg | 108 (96–120) | 122 (110–148) | 0.007 | 137 (118–155) | 0.003 |
| SV, ml | 88 (71–100) | 80 (65–108) | 0.70 | 89 (67–111) | 0.71 |
| LV mass, g | 153 (116–170) | 175 (142–261) | 0.007 | 254 (188–318) | <0.001 |
| Kmono, min−1 | 0.070 (0.059–0.080) | 0.073 (0.060–0.084) | 0.37 | 0.070 (0.060–0.077) | 0.95 |
| EW, mmHg*ml/min*105 | 5.0 (4.4–6.6) | 6.5 (5.2–10.5) | 0.009 | 9.1 (6.4–11.5) | 0.005 |
| MVO2, ml/min | 25.2 (20.0–30.7) | 36.9 (30.3–47.0) | 0.001 | 46.7 (36.8–52.3) | <0.001 |
| MBF, ml/min | 126 (110–147) | 186 (124–260) | 0.01 | 256 (208–334) | <0.001 |
| Mechanical efficiency, % | 14.2 (10.0–17.1) | 12.5 (10.7–16.1) | 0.52 | 12.0 (10.2–17.2) | 0.64 |
| DOBUTAMINE | Dose-adjusted P‡ | Dose-adjusted P‡ | |||
| HR, bpm | 86 (73–92)* | 109 (71–129)* | 0.03 | 112 (70–131)* | 0.02 |
| ESP, mmHg | 120 (115–127)* | 136 (117–163)† | 0.009 | 134 (120–172) | 0.008 |
| SV, ml | 104 (90–123)* | 82 (72–107) | 0.01 | 83 (71–114) | 0.11 |
| Kmono, min−1 | 0.118 (0.097–0.130)* | 0.109 (0.094–0.120)* | 0.13 | 0.112 (0.084–0.122)* | 0.20 |
| EW, mmHg*ml/min*105 | 10.0 (8.0–11.6)* | 10.8 (9.5–11.6)* | 0.59 | 11.0 (9.5–16.1)* | 0.14 |
| MVO2, ml/min | 46.8 (39.8–64.0)* | 58.5 (44.9–68.2)* | 0.13 | 66.7 (58.4–104.9)* | 0.002 |
| MBF, ml/min | 316 (206–363)* | 332 (230–486)* | 0.25 | 450 (321–519)* | 0.007 |
| Mechanical efficiency, % | 13.5 (10.8–17.1) | 11.7 (9.9–14.6) | 0.23 | 10.9 (8.8–11.6)* | 0.008 |
Data are median (interquartile range)
p<0.05
p<0.10 vs rest by paired t-test.
Multivariable least squares regression analysis. Abbreviations: HR, Heart Rate; ESP, End-Systolic Pressure; LV, Left Ventricular; SV, Stroke Volume; EW, External Work; MBF, Myocardial Blood Flow; MVO2, myocardial oxygen consumption; HFpEF, heart failure with preserved ejection fraction and LVH, left ventricular hypertrophy
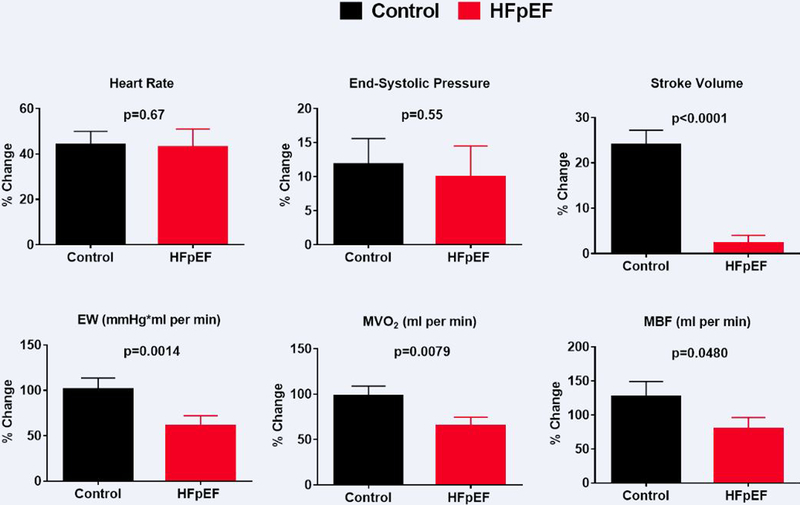
Adjusting for dobutamine dose, the percent change in HR and ESP with dobutamine did not differ between groups while the percent change in SV, EW, MVO2 and MBF were all lower in HFpEF (Figure 1). These differences persisted after adjustment for β–blocker therapy, and β–blocker therapy itself did not significantly impact reserve function variables (Supplemental Table 1 and Supplemental Figure 3). In contrast, in the analysis above, while Group impacted reserve function, dobutamine dose also significantly affected the percent change in HR, ESP, EW, MVO2 and MBF (Supplemental Table 1 and Supplemental Figure 4).
Figure 1. Myocardial Reserve Dysfunction in HFpEF.
The percent (%) increase with dobutamine relative to rest in parameters which influence external work (EW) including heart rate, end-systolic pressure and stroke volume and the % increase in EW, myocardial oxygen consumption (MVO2) and myocardial blood flow (MBF) in Controls (black) and heart failure with preserved ejection fraction (HFpEF; red) is shown. Data are mean (SEM). P values are for Group difference, adjusted for dobutamine dose by multivariable least squares regression analysis.
In summary, in HFpEF, there was impairment in myocardial mechanical (EW), energetic (MVO2) and blood flow reserve functions, consistent with our hypothesis. Reserve function was sensitive to dobutamine dose but not β–blocker therapy (with the β–blocker dose holding protocol used in the study).
Coupling of Myocardial Blood Flow to External Work and Myocardial Oxygen Extraction
In HFpEF, MBF increased less robustly with increases in LV EW (Figure 2) consistent with limited flow reserve relative to demand, even with sub-maximal pharmacologic stress. As expected, total LV MVO2 varied with MBF and Hgb with high R2 values for the model (Table 4). For any given MBF and Hgb, MVO2 was higher in HFpEF, consistent with enhanced myocardial O2-extraction in HFpEF (Table 4). Accordingly, in HFpEF, MVO2 was slightly but significantly higher for any delivered O2 (Figure 3).
Figure 2. Coupling of Myocardial Blood Flow to External Work in HFpEF.
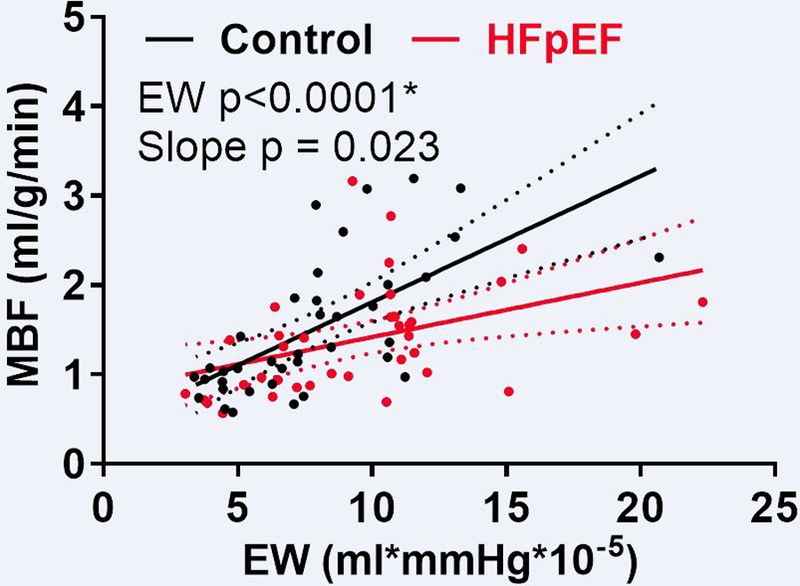
The relationship between myocardial blood flow (MBF) and external work (EW) at rest and during dobutamine infusion in heart failure with preserved ejection fraction (HFpEF; red) and Controls (black) is shown. * in both Controls and HFpEF
Table 4.
Relationship Between Myocardial Oxygen Consumption and Myocardial Blood Flow, Hemoglobin and Group (Myocardial Oxygen Extraction)
| HFpEF vs Control | ||
|---|---|---|
| Model R2 = 0.77 | Parameter Est (SE)* | p value |
| MBF, per 10 ml/min | 1.4 (0.1) | <0.0001 |
| Hgb, per g/dL | 3.6 (1.0) | 0.0007 |
| Group, HFpEF vs Control | 5.7 (2.6) | 0.03 |
Determinates of MVO2 (ml/min) across the range of resting and stress values in HFpEF and Controls. Data reflect multivariable least squares regression analysis. Abbreviations: MBF, Myocardial Blood Flow; MVO2, myocardial oxygen consumption; HFpEF, heart failure with preserved ejection fraction and Hgb, hemoglobin.
The change in MVO2 per unit of MBF, Hgb or Group.
Figure 3. Myocardial Oxygen Extraction in HFpEF.

The relationship between myocardial oxygen consumption (MVO2) and total oxygen delivery at rest and during dobutamine infusion in heart failure with preserved ejection fraction (HFpEF; red) and Controls (black) is shown. MVO2 is higher for any delivered O2 in HFpEF, consistent with enhanced myocardial O2-extraction. * in both Controls and HFpEF, †where slope not different
Mechanoenergetic Coupling
Mechanical efficiency as assessed by total LV EW/MVO2 was numerically but not significantly lower in HFpEF at rest and with dobutamine (Table 3). Across the full range of EW (rest and dobutamine), MVO2 tended to be higher for any given EW but neither the slope nor position of the relationship varied significantly in HFpEF and Controls (Figure 4A). Thus, contrary to our hypothesis, mechanical efficiency was not significantly altered in HFpEF. However, in a post-hoc analysis, in HFpEF+LVH versus Controls, the ratio of EW/MVO2 was significantly lower during stress but not at rest (Table 3). Further, there was significant upward shift in the MVO2/EW relationship (higher MVO2 for any given EW, decreased mechanical efficiency; Figure 4B), suggesting mechanoenergetic uncoupling in HFpEF+LVH which was revealed during pharmacologic stress.
Figure 4. Mechanical Efficiency in HFpEF.
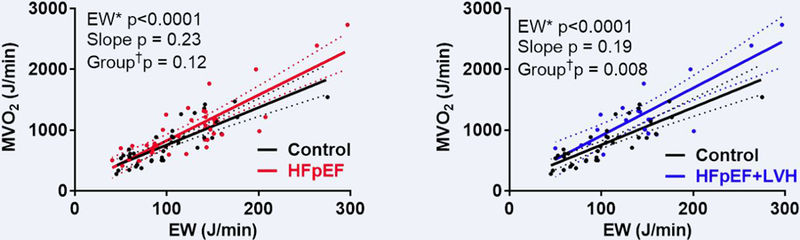
The relationship between total left ventricular myocardial oxygen consumption (MVO2) and external work (EW) at rest and during dobutamine infusion in heart failure with preserved ejection fraction (HFpEF; red) and Controls (black) (left panel) and in HFpEF patients with left ventricular hypertrophy (HFpEF+LVH, blue) and Controls (black) (right panel). * in both Controls and HFpEF, †where slope not different
DISCUSSION
In this study, we simultaneously assessed myocardial mechanical-, flow- and energetic-reserve functions during sub-maximal pharmacologic inotropic stress in prospectively-enrolled Control and HFpEF patients. As compared to Controls, HFpEF patients displayed higher resting total LV EW, MVO2 and MBF. Sub-maximal pharmacologic stress elicited blunted increases (percent change) in EW, MVO2 and MBF in HFpEF patients and the coupling of MBF to EW was impaired in HFpEF. Myocardial O2-extraction was increased in HFpEF vs Controls. Mechanical efficiency was not significantly reduced in HFpEF overall; however, in a post-hoc analysis, mechanical efficiency was reduced in HFpEF patients with LVH, particularly during dobutamine infusion. The current findings provide further evidence that coronary microvascular dysfunction limits O2-supply and contributes to reserve dysfunction in HFpEF.
Reserve Impairment and Microvascular Dysfunction in HFpEF
Previous studies have demonstrated that indices of systolic and diastolic myocardial function do not increase normally in HFpEF patients during exercise25–28 or pharmacologic inotropic stress.7 Coronary microvascular rarefaction has been described in autopsy specimens from HFpEF patients9 and reduced myocardial flow reserve with maximal pharmacologic coronary vasodilatation has been reported in HFpEF.8,10,11 In patients undergoing perfusion imaging for suspected epicardial CAD, those who had impaired myocardial flow reserve and diastolic dysfunction were more likely to have future HFpEF hospitalizations than patients with diastolic dysfunction or impaired coronary flow reserve alone.29 However, impaired myocardial flow reserve has also been demonstrated in patients with cardiovascular risk factors without HF and can be impaired due to increased resting MBF with normal stress MBF.30,31 Thus, whether impairment in myocardial flow reserve contributes to or is merely a marker of intrinsic myocardial reserve dysfunction in HFpEF is unclear. Here we used PET and echo techniques to simultaneously examine myocardial flow, function and energetic reserve during pharmacologic stress in HFpEF patients to gain insight into the functional significance of MBF impairment, both in terms of reserve function and energetics.
Myocardial flow reserve was reduced in HFpEF with sub-maximal pharmacologic inotropic stress, both in terms of percent change in MBF and importantly, in the coupling of MBF to EW. Our findings are consistent with an invasive study by Obokata et al32 where aortic pressure waveform systolic pressure-time integral was used to assess myocardial O2-demand and aortic – pulmonary artery wedge pressure waveform diastolic pressure-time integrals were used to reflect myocardial O2-supply at rest and with supine bicycle exercise. In this invasive study, during exercise, estimated myocardial O2-supply was decreased in HFpEF relative to Controls, associated with systolic and diastolic reserve dysfunction and greater increases in high-sensitivity troponin T, suggesting myocardial injury.
In the current study, HR and ESP were higher at rest in HFpEF, but with sub-maximal pharmacologic stress, the percent changes in HR and ESP were not significantly different in HFpEF and Controls whereas percent increases in SV, EW, MVO2 and MBF were blunted in HFpEF. While chronotropic incompetence has been demonstrated with sub-maximal and maximal exercise stress in HFpEF,27,33 another study administering a similar range of dobutamine doses in HFpEF and Controls also demonstrated quantitatively similar increases in HR and systolic blood pressure in HFpEF and Controls but blunted increases in EF and LV systolic and diastolic strain in HFpEF with dobutamine.7 These findings are akin to ours, where blunted increases in SV are likely mediated by blunted enhancement of systolic and diastolic myocardial function with dobutamine.
Myocardial Oxygen Extraction in HFpEF
Myocardial O2-extraction is much higher than in the periphery and changes in myocardial O2-delivery are thought to be primarily mediated by increases in MBF. However, increases in extraction occur with ischemia related to epicardial coronary artery stenosis. The impact of microvascular dysfunction on O2-extraction is less clear. It has recently been hypothesized that microvascular dysfunction could lead to heterogeneous areas of low-flow/high-extraction next to regions with high-flow/low-extraction and that this could result in functional blood shunting to the high-flow/low-extraction areas resulting in overall reduced O2-extraction.12 However, we found evidence of small but statistically significant increases in myocardial O2-extraction in HFpEF. This is in contrast to a study by van Empel et al which reported blunted increases in transcardiac O2-content gradient with maximal exercise in HFpEF.34 In addition to differences in measurement methods (MBF and MVO2 were not measured to calculate true O2-extraction) there are potentially critical differences in the level of stress (submaximal vs maximal) and type of stimulus (dobutamine infusion vs physiological exercise). It is possible that with a longer duration of stress and/or with a greater magnitude of stress, both of which occur during maximal exercise, the ability of O2-extraction to compensate for reduced O2 delivery could be ‘outstripped’ and become measurably lower than healthy controls. Indeed, this phenomenon has been observed for peripheral oxygen extraction (A-VO2 difference) measured invasively during maximal upright exercise in HFpEF35,36 and may occur in the myocardium as well.34
There is skeletal muscle microvascular dysfunction in HFpEF37 but studies have varied as to whether peripheral O2-extraction is increased38–40 or decreased35,41–43 in HFpEF. Highly endurance-trained athletes have increased myocardial vascular resistance resulting in increased myocardial blood transit time and myocardial O2-extraction which helps to preserve MVO2 despite reduced MBF with exercise.44 Whether the modest increases in O2-extraction we observed during sub-maximal pharmacologic stress could be reversed with maximal exercise is unclear. We used dobutamine infusion to assess the key factors of MBF, MVO2, and O2-extraction during stress because it is not feasible to perform these procedures during physical exercise, which is when chronic, stable HFpEF patients typically experience their symptoms. But in addition to being a submaximal stress, dobutamine differs in several key characteristics from exercise which involves a complex interplay of not only adrenergic stimulation but also of sympathetic withdrawal, increases in venous return (pre-load), and increased afterload, as well as engagement of peripheral vascular and skeletal muscle function which along with cardiac function help enable exercise. Thus, our findings should be interpreted in this context.
Myocardial Efficiency in HFpEF
The myocardium relies on aerobic oxidation of substrates for energy generation and thus, MVO2 is closely related to determinates of energy utilization including load, HR and contractile state.2 Only part of the energy generated from oxidative metabolism is converted to EW, and the relationship between MVO2 and EW is termed mechanical efficiency. Impaired mechanical efficiency is thought to contribute to HFrEF pathophysiology via chronic energy deficits relative to demand.
Reduced mechanical efficiency in HFrEF has been attributed to changes in substrate utilization with shifts from glucose to fatty-acid oxidation which results in greater O2-consumption per unit of ATP produced.45 Oxidative stress and reduced nitric oxide (NO) signaling also contribute to reduced mechanical efficiency in HFrEF via loss of favorable effects of NO on autonomic signaling, calcium cycling and energy consumption.46 In subjects without HF, obesity and insulin resistance may also result in cardiac preference for fatty-acids as the primary fuel source but associated effects on mechanical efficiency appear to vary by sex.13,47 In animal studies, LVH is associated with reversion to a fetal metabolic profile with preference for glucose and lactate over fatty-acids, a change which would favor enhanced efficiency.14 However, in humans with essential hypertension (but not HF), the presence of LVH was associated with reduced mechanical efficiency.48 In LVH associated with aortic stenosis, efficiency was reduced only in the presence of systolic dysfunction.49
Here, across the range of EW observed at rest and with pharmacologic stress, we did not find significant impairment in mechanical efficiency in HFpEF as compared to Controls but in our post-hoc analysis, we did see reduced mechanical efficiency in the subgroup of HFpEF subjects with LVH. Notably, impairments in efficiency in LVH were revealed by stress as efficiency was not significantly different at rest but significantly lower during dobutamine infusion. The impact of stress was also reflected by the difference in slopes when all values (rest and stress MVO2 versus EW) were analyzed by linear regression. Our findings are in contrast to several studies in HFrEF, where dobutamine enhanced efficiency.50 These data underscore the value of stressing the system when assessing differences in myocardial properties in HFpEF and suggest that the hypertrophic HFpEF phenotype has more severe mechanoenergetic uncoupling.
The mechanism whereby greater MVO2 is needed to produce EW in HFpEF patients with LVH cannot be ascertained from the current study. As above, differences in substrate utilization, particularly during stress, may contribute. Impaired NO-cGMP signaling is postulated to contribute to HFpEF pathophysiology and to promote hypertrophic remodeling51 and therefore may contribute to reduced efficiency in HFpEF patients with LVH. Alternatively, novel mechanisms, including impairment in calcium-cycling proteins such as S100 A1 which increase mechanical efficiency may play a role52 in, and potentially interact with, the above factors to impact mechanical efficiency.
Limitations
While this study evaluated a relatively modest number of participants, it is one of the larger 11C-acetate PET studies in HF and unique in HFpEF. Study design and sample size limit more extensive subgroup analysis (obesity) and potentially, the ability to demonstrate significance of more subtle differences with pharmacologic stress. The median age of controls was four years lower than that of HF subjects. While not statistically significant, this age difference could influence our findings. Additional comparator groups such as non-HF hypertensive or obese controls would have added significantly to the study. The complexity of study-flow precluded measurements such as myocardial strain during stress. We did not assess changes in substrate utilization. Isoproterenol is traditionally used to assess cardiac reserve in physiologic protocols; however, dobutamine was used here given its safety profile and previous data in HFpEF.7 Reduction in catecholamine sensitivity may occur in HFpEF53 and may partly explain the reserve dysfunction observed, although sensitivity to dobutamine dose was preserved and the blunted relationship between MBF and EW observed suggests additional mechanisms. The analysis of mechanical efficiency in LVH was a post-hoc analysis and is to be interpreted in that context. Finally, controversy exists over the correct formulas to convert Kmono to MVO22 which may affect the absolute value of, but not group differences in, mechanical efficiency and O2-extraction.
CONCLUSIONS
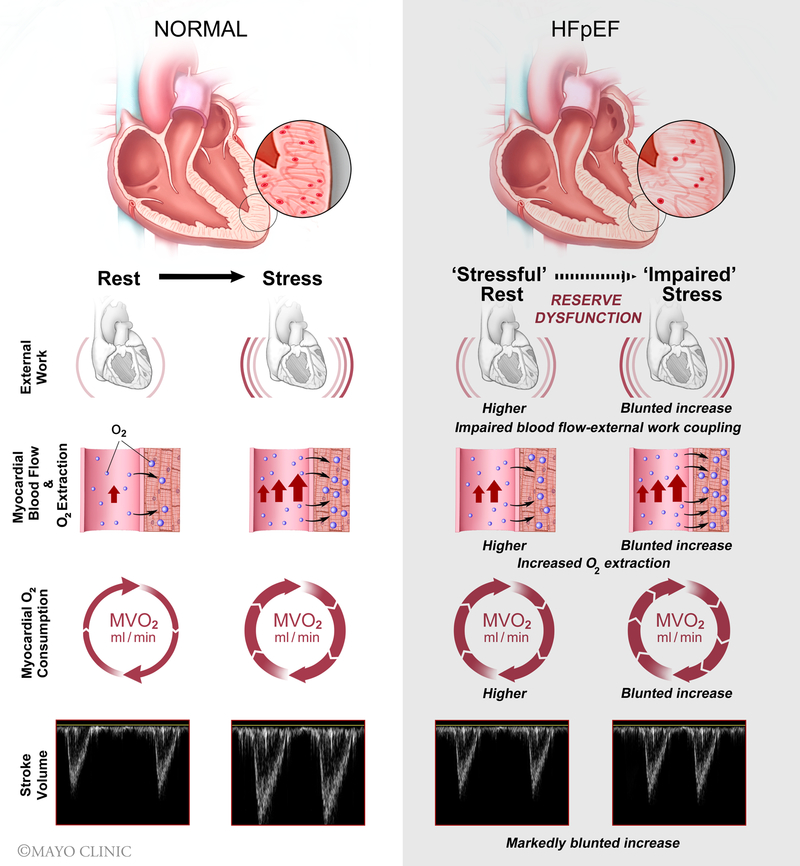
In HFpEF, there is myocardial mechanical-, energetic- and flow-reserve dysfunction in response to sub-maximal pharmacologic inotropic stress and impaired coupling of MBF to EW with small but significant increases in myocardial O2-extraction (Figure 5). Mechanical efficiency was not different in HFpEF overall but was reduced during dobutamine in HFpEF patients with LVH. The current findings provide evidence that coronary microvascular dysfunction is present in HFpEF, limits O2-supply relative to demand and is directly associated with reserve dysfunction.
Figure 5.
Central Illustration: In HFpEF during submaximal dobutamine stress, there is myocardial mechanical-, energetic-, and flow-reserve dysfunction with impaired coupling of blood flow to demand and slight increases in myocardial O2 extraction. HFpEF, heart failure with preserved ejection fraction
Supplementary Material
What’s new?
We simultaneously assessed myocardial mechanical-, flow- and energetic-reserve functions during sub-maximal pharmacologic inotropic stress in Control and HFpEF patients.
Compared to controls, HFpEF displayed higher resting LV external work, oxygen consumption and blood flow with blunted increases in each with stress
Coupling of myocardial blood flow to external work was impaired and myocardial O2 extraction was increased in HFpEF vs controls
Mechanical efficiency was not significantly reduced in HFpEF; however, in post-hoc analyses, mechanical efficiency was reduced in HFpEF patients with LVH.
The current findings provide further evidence that coronary microvascular dysfunction limits O2-supply and contributes to reserve dysfunction in HFpEF.
What are the clinical implications?
Heart failure (HF) with preserved ejection fraction (HFpEF) is a major public health problem without proven effective therapy.
There is a pressing need to rigorously define HFpEF pathophysiology to ultimately identify novel therapeutic targets.
Our findings support investigation of therapies targeting improvement in myocardial flow in HFpEF and investigation of therapies to enhance energetics in HFpEF patients with LV hypertrophy.
AKNOWLEDGMENTS
The authors are greatly indebted to the staff of the Mayo Clinic Nuclear Cardiology and Echocardiography Laboratories for making this work possible.
Sources of external funding: None
Footnotes
Disclosures: None.
References
- 1.Redfield MM. Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2017;376:897. [DOI] [PubMed] [Google Scholar]
- 2.Knaapen P, Germans T, Knuuti J, Paulus WJ, Dijkmans PA, Allaart CP, Lammertsma AA and Visser FC. Myocardial energetics and efficiency: current status of the noninvasive approach. Circulation. 2007;115:918–27. [DOI] [PubMed] [Google Scholar]
- 3.Eichhorn EJ and Bristow MR. Medical therapy can improve the biological properties of the chronically failing heart: a new era in the treatment of heart failure. Circulation. 1996;94:2285–2296. [DOI] [PubMed] [Google Scholar]
- 4.Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A and Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009;54:402–9. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Beadle R, Cameron D, Rudd A, Bruce M, Jagpal B, Schwarz K, Brindley G, McKiddie F, Lang C, Dawson D and Frenneaux M. Randomized double-blind placebo-controlled trial of perhexiline in heart failure with preserved ejection fraction syndrome. Future Cardiol. 2014;10:693–8. [DOI] [PubMed] [Google Scholar]
- 6.Pandey A, Khera R, Park B, Haykowsky M, Borlaug BA, Lewis GD, Kitzman DW, Butler J and Berry JD. Relative Impairments in Hemodynamic Exercise Reserve Parameters in Heart Failure With Preserved Ejection Fraction: A Study-Level Pooled Analysis. JACC Heart Fail. 2018;6:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norman HS, Oujiri J, Larue SJ, Chapman CB, Margulies KB and Sweitzer NK. Decreased cardiac functional reserve in heart failure with preserved systolic function. J Card Fail. 2011;17:301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato S, Saito N, Kirigaya H, Gyotoku D, Iinuma N, Kusakawa Y, Iguchi K, Nakachi T, Fukui K, Futaki M, Iwasawa T, Kimura K and Umemura S. Impairment of Coronary Flow Reserve Evaluated by Phase Contrast Cine-Magnetic Resonance Imaging in Patients With Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc. 2016;5(2):doi: 10.1161/JAHA.115.002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ and Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivaratharajah K, Coutinho T, deKemp R, Liu P, Haddad H, Stadnick E, Davies RA, Chih S, Dwivedi G, Guo A, Wells GA, Bernick J, Beanlands R and Mielniczuk LM. Reduced Myocardial Flow in Heart Failure Patients With Preserved Ejection Fraction. Circ Heart Fail. 2016;9(7):doi: 10.1161/CIRCHEARTFAILURE.115.002562. [DOI] [PubMed] [Google Scholar]
- 11.Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink-Nelson L, Fermer ML, Broberg MA, Gan LM and Lund LH. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J. 2018;39:3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pries AR and Reglin B. Coronary microcirculatory pathophysiology: can we afford it to remain a black box? Eur Heart J. 2017;38:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson LR, Herrero P, Coggan AR, Kisrieva-Ware Z, Saeed I, Dence C, Koudelis D, McGill JB, Lyons MR, Novak E, Davila-Roman VG, Waggoner AD and Gropler RJ. Type 2 diabetes, obesity, and sex difference affect the fate of glucose in the human heart. Am J Physiol Heart Circ Physiol. 2015;308:H1510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolwicz SC Jr. and Tian R Glucose metabolism and cardiac hypertrophy. Cardiovasc Res. 2011;90:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W and Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 16.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M and Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–34. [DOI] [PubMed] [Google Scholar]
- 17.Kelly RP, Ting C-T, Yang T-M, Liu C-P, Maughan WL, Chang M-S and Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. [DOI] [PubMed] [Google Scholar]
- 18.Quinones MA, Mokotoff DM, Nouri S, Winters WL Jr. and Miller RR. Noninvasive quantification of left ventricular wall stress. Validation of method and application to assessment of chronic pressure overload. Am J Cardiol. 1980;45:782–90. [DOI] [PubMed] [Google Scholar]
- 19.Pike VW, Eakins MN, Allan RM and Selwyn AP. Preparation of [1–11C]acetate--an agent for the study of myocardial metabolism by positron emission tomography. Int J Appl Radiat Isot. 1982;33:505–12. [DOI] [PubMed] [Google Scholar]
- 20.Armbrecht JJ, Buxton DB, Brunken RC, Phelps ME and Schelbert HR. Regional myocardial oxygen consumption determined noninvasively in humans with [1–11C]acetate and dynamic positron tomography. Circulation. 1989;80:863–72. [DOI] [PubMed] [Google Scholar]
- 21.van den Hoff J, Burchert W, Borner AR, Fricke H, Kuhnel G, Meyer GJ, Otto D, Weckesser E, Wolpers HG and Knapp WH. [1-(11)C]Acetate as a quantitative perfusion tracer in myocardial PET. J Nucl Med. 2001;42:1174–82. [PubMed] [Google Scholar]
- 22.Sun KT, Yeatman LA, Buxton DB, Chen K, Johnson JA, Huang SC, Kofoed KF, Weismueller S, Czernin J, Phelps ME and Schelbert HR. Simultaneous measurement of myocardial oxygen consumption and blood flow using [1-carbon-11]acetate. J Nucl Med. 1998;39:272–80. [PubMed] [Google Scholar]
- 23.Fick A On liquid diffusion. Journal of Membrane Science. 1995;100:33–38. [Google Scholar]
- 24.Marwick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, Galderisi M, Gottdiener J, Haluska B, Ofili E, Segers P, Senior R, Tapp RJ and Zamorano JL. Recommendations on the Use of Echocardiography in Adult Hypertension: A Report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J Am Soc Echocardiogr. 2015;28:727–54. [DOI] [PubMed] [Google Scholar]
- 25.Borlaug BA. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction. Circ J. 2014;78:20–32. [DOI] [PubMed] [Google Scholar]
- 26.Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM and Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97:964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC and Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–47. [DOI] [PubMed] [Google Scholar]
- 28.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD and Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R and Di Carli MF. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gould KL and Johnson NP. Coronary Physiology Beyond Coronary Flow Reserve in Microvascular Angina: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:2642–2662. [DOI] [PubMed] [Google Scholar]
- 31.Chareonthaitawee P, Kaufmann PA, Rimoldi O and Camici PG. Heterogeneity of resting and hyperemic myocardial blood flow in healthy humans. Cardiovasc Res. 2001;50:151–61. [DOI] [PubMed] [Google Scholar]
- 32.Obokata M, Reddy YNV, Melenovsky V, Kane GC, Olson TP, Jarolim P and Borlaug BA. Myocardial Injury and Cardiac Reserve in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2018;72:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B and Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26:86–9. [DOI] [PubMed] [Google Scholar]
- 34.van Empel VP, Mariani J, Borlaug BA and Kaye DM. Impaired myocardial oxygen availability contributes to abnormal exercise hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc. 2014;3:e001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS and Lewis GD. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circulation Heart failure. 2015;8:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH and Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: Failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. [DOI] [PubMed] [Google Scholar]
- 37.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM and Haykowsky M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. American journal of physiology Heart and circulatory physiology. 2014;306:H1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeder MT, Thompson BR, Brunner-La Rocca HP and Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–63. [DOI] [PubMed] [Google Scholar]
- 39.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD and Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15(7):776–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nanayakkara S, Haykowsky M, Mariani J, Van Empel V, Maeder MT, Vizi D and Kaye DM. Hemodynamic Profile of Patients With Heart Failure and Preserved Ejection Fraction Vary by Age. J Am Heart Assoc. 2017;6:e005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI and Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol (1985). 2015;119:739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR and Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss K, Schär M, Panjrath GS, Zhang Y, Sharma K, Bottomley PA, Golozar A, Steinberg A, Gerstenblith G, Russell SD and Weiss RG. Fatigability, Exercise Intolerance, and Abnormal Skeletal Muscle Energetics in Heart Failure. Circulation Heart failure. 2017;10:e004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heinonen I, Kudomi N, Kemppainen J, Kiviniemi A, Noponen T, Luotolahti M, Luoto P, Oikonen V, Sipila HT, Kopra J, Mononen I, Duncker DJ, Knuuti J and Kalliokoski KK. Myocardial blood flow and its transit time, oxygen utilization, and efficiency of highly endurance-trained human heart. Basic Res Cardiol. 2014;109:413. [DOI] [PubMed] [Google Scholar]
- 45.Beadle RM and Frenneaux M. Modification of myocardial substrate utilisation: a new therapeutic paradigm in cardiovascular disease. Heart. 2010;96:824–30. [DOI] [PubMed] [Google Scholar]
- 46.Saavedra WF, Paolocci N, St John ME, Skaf MW, Stewart GC, Xie JS, Harrison RW, Zeichner J, Mudrick D, Marban E, Kass DA and Hare JM. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ Res. 2002;90:297–304. [DOI] [PubMed] [Google Scholar]
- 47.Peterson LR, Saeed IM, McGill JB, Herrero P, Schechtman KB, Gunawardena R, Recklein CL, Coggan AR, DeMoss AJ, Dence CS and Gropler RJ. Sex and type 2 diabetes: obesity-independent effects on left ventricular substrate metabolism and relaxation in humans. Obesity (Silver Spring). 2012;20:802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laine H, Katoh C, Luotolahti M, Yki-Jarvinen H, Kantola I, Jula A, Takala TO, Ruotsalainen U, Iida H, Haaparanta M, Nuutila P and Knuuti J. Myocardial oxygen consumption is unchanged but efficiency is reduced in patients with essential hypertension and left ventricular hypertrophy. Circulation. 1999;100:2425–30. [DOI] [PubMed] [Google Scholar]
- 49.Hansson NH, Sorensen J, Harms HJ, Kim WY, Nielsen R, Tolbod LP, Frokiaer J, Bouchelouche K, Dodt KK, Sihm I, Poulsen SH and Wiggers H. Myocardial Oxygen Consumption and Efficiency in Aortic Valve Stenosis Patients With and Without Heart Failure. J Am Heart Assoc. 2017;6(2):doi: 10.1161/JAHA.116.004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beanlands RS, Bach DS, Raylman R, Armstrong WF, Wilson V, Montieth M, Moore CK, Bates E and Schwaiger M. Acute effects of dobutamine on myocardial oxygen consumption and cardiac efficiency measured using carbon-11 acetate kinetics in patients with dilated cardiomyopathy. J Am Coll Cardiol. 1993;22:1389–98. [DOI] [PubMed] [Google Scholar]
- 51.Paulus WJ and Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 52.Brinks H, Rohde D, Voelkers M, Qiu G, Pleger ST, Herzog N, Rabinowitz J, Ruhparwar A, Silvestry S, Lerchenmuller C, Mather PJ, Eckhart AD, Katus HA, Carrel T, Koch WJ and Most P. S100A1 genetically targeted therapy reverses dysfunction of human failing cardiomyocytes. J Am Coll Cardiol. 2011;58:966–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM and Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.