Abstract
In recent years, rigid analogues of phenylalkylamine hallucinogens have appeared as recreational drugs. Examples include 2-(8-bromo-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b′]difuran-4-yl)ethan-1-amine (2C-B-FLY) and 1-(8-bromobenzo[1,2-b;4,5-b ’]difuran-4-yl)-2-aminopropane (Bromo-DragonFLY, DOB-DFLY). Although some rigid compounds such as DOB-DFLY reportedly have higher potency than their non-rigid counterparts, it is not clear whether the same is true for 2C-B-FLY and other tetrahydrobenzodifurans. In the present study, the head twitch response (HTR), a 5-HT2A receptor-mediated behavior induced by serotonergic hallucinogens, was used to assess the effects of 2,5-dimethoxy-4-bromoamphetamine (DOB) and its α-desmethyl homologue 2,5-dimethoxy-4-bromophenethylamine (2C-B), as well as their benzodifuranyl and tetrahydrobenzodifuranyl analogs, in C57BL/6J mice. DOB (ED50 = 0.75 μmol/kg) and 2C-B (ED50 = 2.43 μmol/kg) induced the HTR. The benzodifurans DOB-DFLY (ED50 = 0.20 μmol/kg) and 2C-B-DFLY (ED50 = 1.07 μmol/kg) had significantly higher potency than DOB and 2C-B, respectively. The tetrahydrobenzodifurans DOB-FLY (ED50 = 0.67 μmol/kg) and 2C-B-FLY (ED50 = 1.79 μmol/kg), by contrast, were approximately equipotent with their non-rigid counterparts. Three novel tetrahydrobenzodifurans (2C-I-FLY, 2C-E-FLY and 2C-EF-FLY) were also active in the HTR assay but had relatively low potency. In summary, the in vivo potency of 2,5-dimethoxyphenylalkylamines is enhanced when the 2- and 5-methoxy groups are incorporated into aromatic furan rings, whereas potency is not altered if the methoxy groups are incorporated into dihydrofuran rings. The potency relationships for these compounds in mice closely parallel the human hallucinogenic data. The high potency of DOB-DFLY is probably linked to the presence of two structural features (a benzodifuran nucleus and an α-methyl group) known to enhance the potency of phenylalkylamine hallucinogens.
Keywords: 5-HT2A receptor, psychedelics, head twitch, new psychoactive substance, NPS, 2C-B-DragonFLY, 3C-B-FLY
1. INTRODUCTION
Serotonergic hallucinogens induce profound alterations of perception, mood, and cognition (Preller and Vollenweider 2018). One group of hallucinogens are based on the phenylalkylamine structural template, including phenylethylamines such as mescaline and 2,5-dimethoxy-4-bromophenylethylamine (2C-B), and amphetamines such as 2,5-dimethoxy-4-bromoamphetamine (DOB) and 2,5-dimethoxy-4-methylamphetamine (DOM). Examples of compounds from these classes are shown in Figure 1. Analogs of phenylalkylamine hallucinogens have also been synthesized where the methoxy groups are incorporated into rigid dihydrofuran or furan rings (Monte et al. 1996; Parker et al. 1998; Chambers et al. 2001). Serotonergic hallucinogens containing 2,3,6,7-tetrahydrobenzo[1,2-b;4,5-b′]difuran or benzo[1,2-b;4,5-b′]difuran ring systems are commonly designated by the code-names “FLY” and “DragonFLY”, respectively, because of their structural resemblance to winged insects (Trachsel et al. 2013). These compounds were originally developed to probe the active binding conformation of phenylalkylamine hallucinogens at the 5-HT2A receptor, which is believed to be the primary site of action of (+)-lysergic acid diethylamide (LSD) and other serotonergic hallucinogens (Vollenweider et al. 1998; Kometer et al. 2013; Valle et al. 2016; Kraehenmann et al. 2017; Preller et al. 2017). In recent years, several of these rigid phenylalkylamines have appeared as recreational drugs. According to the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), the European Union Early-Warning System reported the first detection of Bromo-DragonFLY (DOB-DFLY) and 2C-B-FLY in 2006 and 2007, respectively (EMCDDA 2007,2008; King 2014). DOB-DFLY was also identified in drug exhibits seized in Oregon in 2007 and Australia in 2008 (Anonymous 2007,2008). Recreational use of DOB-DFLY has been linked to multiple fatalities as well as severe adverse reactions including seizures and limb ischemia (Personne and Hultén 2008; Thorlacius et al. 2008; Andreasen et al. 2009; Wood et al. 2009; Nielsen et al. 2010; Corazza et al. 2011; Chavarin et al. 2013; Iwersen-Bergmann et al. 2018).
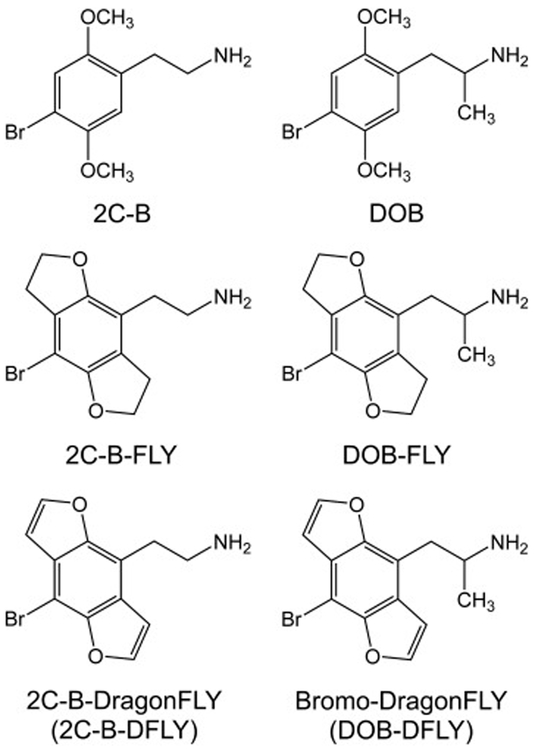
Figure 1.
Chemical structures of the phenylalkylamine hallucinogens 2C-B and DOB, as well as their tetrahydrobenzodifuran and benzodifuran analogs. Abbreviations: 2C-B (2,5-dimethoxy-4-bromophenethylamine), DOB (2,5-dimethoxy-4-bromoamphetamine), 2C-B-FLY (2-(8-bromo-tetrahydrobenzo[1,2-b:4,5-b′]difuran-4-yl)ethan-1-amine), DOB-FLY (1-(8-bromo-tetrahydrobenzo[1,2-b:4,5-b′]difuran-4-yl)propan-2-amine ), 2C-B-DFLY (2-(8-bromobenzo [1,2-b:4,5-b′]difuran-4-yl)ethan-1-amine), DOB-DFLY (1-(8-bromobenzo[1,2-b:4,5-b′]difuran-4-yl)propan-2-amine ).
DOB-DFLY is a highly potent compound. DOB-DFLY has sub-nanomolar affinity for the 5-HT2A receptor, with a Ki value of 0.04 nM for the cloned human receptor labeled with [125I]DOI (Parker et al. 1998). DOB-DFLY is reportedly active orally in humans in the range of 0.2–0.8 mg (Trachsel et al. 2013) and has a slow onset and long duration of action, potentially lasting for up to three days. In comparison, LSD has a typical dosage range of 60–200 μg p.o. (Shulgin and Shulgin 1997). DOB-DFLY also shows rather potent activity in rats trained to discriminate LSD from saline (ED50 = 22 nmol/kg) (Parker et al. 1998) and is more potent than DOB (ED50 = 1.06 μmol/kg) (Chambers et al. 2003). In contrast to DOB-DFLY, relatively little is known about the activity of other hallucinogens containing a benzodifuran ring system.
Tethering the methoxy groups of phenylalkylamine hallucinogens into a tetrahydrobenzodifuran nucleus has little effect on 5-HT2A affinity. For example, DOB (Ki = 22 nM) and DOB-FLY (Ki = 18 nM) have equivalent affinities for 5-HT2A receptors labeled with [3H]ketanserin in rat cortical homogenates (Monte et al. 1996). 2C-B (Ki = 8.6 nM) and 2C-B-FLY (Ki = 11 nM) have similar affinities for cloned human 5-HT2A receptors using [3H]ketanserin as the radioligand (Rickli et al. 2015a; Rickli et al. 2015b). 2C-B and 2C-B-FLY are also essentially equipotent in humans; 2C-B-FLY is active at doses of 10–20 mg orally (Hanna et al. 2008; Green 2013) and 2C-B is active at 12–24 mg (Shulgin and Shulgin 1991). Although little is known about the human psychopharmacology of DOB-FLY, it reportedly produces hallucinogenic effects at 1 mg orally (Shulgin et al. 2011), which overlaps with the 1–3 mg effective dosage range for DOB (Shulgin and Shulgin 1991). Although the presence of a tetrahydrobenzodifuran functionality does not appreciably alter the 5-HT2A receptor affinity or human potency of phenylalkylamine hallucinogens, it does increase their potency in the drug discrimination paradigm. Both DOB and DOB-FLY produce full substitution in rats trained to discriminate 0.08 mg/kg LSD from saline but the latter compound has 18-fold higher potency (Nichols et al. 1994; Monte et al. 1996). Likewise, 2C-B-FLY has almost fourfold higher potency than 2C-B in LSD-trained rats (Monte et al. 1996; Juncosa et al. 2013).
The conflicting data described above led us to compare the potencies of 2C-B and DOB relative to their tetrahydrobenzodifuranyl and benzodifuranyl analogs using the head twitch response (HTR). Serotonergic hallucinogens induce the HTR, a brief paroxysmal head rotation, in rats and mice via activation of the 5-HT2A receptor (Schreiber et al. 1995; Halberstadt et al. 2011; Canal and Morgan 2012; Halberstadt and Geyer 2014). The HTR is commonly used as a behavioral proxy in rodents for human hallucinogenic effects because it can reliably distinguish hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists (Gonzalez-Maeso et al. 2007). Similar to the discriminative stimulus effects of hallucinogens, the HTR serves as a behavioral readout of 5-HT2A activation and can be used to compare the in vivo potencies of 5-HT2A receptor agonists (Halberstadt and Geyer 2014; Nichols et al. 2015; Brandt et al. 2016; Brandt et al. 2017; Halberstadt et al. 2018; Klein et al. 2018). Although DOB is known to produce head twitches in rodents (Wieland et al. 1990; Gonzalez-Maeso et al. 2007; Moya et al. 2007), it is not yet clear whether 2C-B and other phenylethylamine hallucinogens can reliably induce the behavior (c.f., Moya et al. 2007). In the present investigation, mouse HTR studies were conducted with 2C-B and DOB, their benzodifuranyl and tetrahydrobenzodifuranyl analogs, as well as three novel phenethylamines with rigidified methoxy groups (2C-I-FLY, 2C-E-FLY, and 2C-EF-FLY). The results confirmed that 2C-B and DOB are active in the HTR assay. Furthermore, although the in vivo potency of 2C-B and DOB was unaffected by incorporating their 2- and 5-methoxy groups into dihydrofuran rings, incorporating the methoxy groups into fully aromatic furan rings resulted in a significant enhancement of potency.
2. MATERIALS AND METHODS
2.1. Animals
Male C57BL/6J mice (6-8 weeks old) obtained from Jackson Laboratories (Bar Harbor, ME, USA) were housed in a vivarium at the University of California San Diego, an AAALAC-approved animal facility that meets all Federal and State requirements for care and treatment of laboratory animals. Mice were housed up to four per cage in a climate-controlled room on a reverse-light cycle (lights on at 1900 h, off at 0700 h) and were provided with ad libitum access to food and water, except during behavioral testing. Testing was conducted between 1000 and 1800 h. All animal experiments were carried out in accordance with NIH guidelines and were approved by the UCSD animal care committee.
2.2. Drugs
4-Bromo-2,5-dimethoxyphenethylamine (2C-B) hydrochloride, 4-bromo-2,5-dimethoxyamphetamine (DOB) hydrochloride, 1-(8-Bromo-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b′]difuran-4-yl)propan-2-amine (DOB-FLY) hydrochloride, and 1-(8-bromobenzo[1,2-b:4,5-b′]difuran-4-yl)propan-2-amine (DOB-DFLY) hydrochloride were obtained from Cayman Chemical (Ann Arbor, MI, USA). 2-(8-Bromobenzo[1,2-b:4,5-b′]difuran-4-yl)ethan-1-amine (2C-B-DFLY) hydrochloride was obtained from Lipomed Inc. (Arlesheim, Switzerland). 2-(8-Bromo-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b′]difuran-4-yl)ethan-1-amine (2C-B-FLY) hydrochloride, 2-(8-iodo-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b′]difuran-4-yl)ethan-1-amine (2C-I-FLY) hydrochloride, 2-(8-ethyl-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b′]difuran-4-yl)ethan-1-amine (2C-E-FLY) hydrochloride, and 2-[8-(2-fluoroethyl)-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b′]difuran-4-yl]ethan-1-amine (2C-EF-FLY) hydrochloride were obtained from Synex Synthetics BV (Maastricht, Netherlands). Test substances were dissolved in isotonic saline and injected intraperiotoneally (IP) at a volume of 5 mL/kg.
2.3. Head Twitch Response Studies
The head twitch response (HTR) was assessed using a head-mounted magnet and a magnetometer detection coil (Halberstadt and Geyer 2013,2014; Nichols et al. 2015; Klein et al. 2018). Briefly, mice were anesthetized, a small incision was made in the scalp, and a small neodymium magnet was attached to the dorsal surface of the cranium using dental cement. Following a two-week recovery period, HTR experiments were carried out in a well-lit room with at least 7 days between sessions to avoid carryover effects. According to experiments performed in our laboratory (data not shown) and literature reports (Nagayama and Lu 1996), the level of illumination does not influence the magnitude of the HTR induced by hallucinogens. Test compounds were injected immediately prior to testing. Mice (n = 5–7/group) were injected with drug or vehicle and then HTR activity was recorded in a glass cylinder surrounded by a magnetometer coil for 30 min. Coil voltage was low-pass filtered (2–10 kHz cutoff frequency), amplified, and digitized (20 kHz sampling rate) using a Powerlab/8SP with LabChart v 7.3.2 (ADInstruments, Colorado Springs, CO, USA), then filtered off-line (40–200 Hz band-pass). Head twitches were identified manually based on the following criteria: 1) sinusoidal wavelets; 2) evidence of at least three sequential head movements (usually exhibited as bipolar peaks) with frequency ≥ 40 Hz; 3) amplitude exceeding the level of background noise; 4) duration < 0.15 s; and 5) stable coil voltage immediately preceding and following each response.
After magnet implantation, mice were tested in multiple HTR experiments, for up to 4–5 months. Repeated administration of hallucinogens at weekly intervals does not produce tolerance in the HTR paradigm (Gewirtz and Marek 2000; Rangel-Barajas et al. 2014; Smith et al. 2014). We have confirmed that experimental results obtained using these procedures are stable and replicable over time, both within single cohorts of mice and across multiple independent cohorts. Individual experiments were performed between-subjects, with pseudorandomized group assignments, which further reduces the likelihood of carryover effects.
2.4. Data Analysis
The entire 30-min recordings were examined for head twitches, but in some cases a shorter block of time was used for analysis to accommodate compounds with a brief duration-of-action since potency calculations can be confounded by extended periods of inactivity. Head twitch counts were analyzed using one-way analyses of variance (ANOVA). Post hoc pairwise comparisons between selected groups were performed using Tukey's studentized range method. Significance was demonstrated by surpassing an α-level of 0.05.
Median effective doses (ED50 values) and 95% confidence intervals (95% CI) for HTR dose-response experiments were calculated by nonlinear regression (Prism 7.00, GraphPad Software, San Diego, CA, USA). A Gaussian distribution (Christopoulos et al. 2001) was used to fit biphasic HTR dose-response data:
In these equations, E is the drug effect, Baseline is the response in the control group, Range is the distance from Baseline to the top of the curve, [A] is the dose of the drug, and midA is the logarithm of the dose corresponding to the top of the curve. To determine whether potency differences exist between individual compounds, ED50 values were compared using an extra-sum-of-squares F-test. Significance was demonstrated by surpassing an α-level of 0.05.
3. RESULTS
The HTR data are presented in Table 1. 2C-B and DOB, as well as their tetrahydrobenzodifuranyl analogs 2C-B-FLY and DOB-FLY, induced the HTR in mice. 2C-B (ED50 = 2.43 μmol/kg) and 2C-B-FLY (ED50 = 1.79 μmol/kg) were approximately equipotent (F(1,55)=1.16, NS). The same was observed for DOB (ED50 = 0.75 μmol/kg) and DOB-FLY (ED50 = 0.67 μmol/kg)(F(1,53)=0.17, NS). Based on these results, tethering the methoxy groups of 2,5-dimethoxyphenylalkylamines into a rigid tetrahydrobenzodifuran ring system does not appear to alter their behavioral potency in mice.
Table 1.
Summary of the head twitch response (HTR) data for the compounds shown in Figure 1.
| Compound | One-way ANOVA | Time (min) |
Dose (mg/kg) |
N | HTR Counts (mean ± SEM) |
ED50mg/kg (95% CI) |
ED50μmol/kg (95% CI) |
|---|---|---|---|---|---|---|---|
| 2C-B | F(5,25)=17.60, p<0.0001 | 30 | 0 | 6 | 11.8 ± 2.8 | 0.72 (0.48–1.09) |
2.43 (1.62–3.66) |
| 0.1 | 5 | 23.8 ± 6.5 | |||||
| 0.3 | 5 | 21.6 ± 4.9 | |||||
| 1 | 5 | 54.6 ± 5.9 * | |||||
| 3 | 5 | 79.2 ± 9.3 * | |||||
| 10 | 5 | 55.0 ± 7.2 * | |||||
| DOB | F(5,27)=28.79, p<0.0001 | 30 | 0 | 7 | 9.1 ± 1.3 | 0.23 (0.17–0.32) |
0.75 (0.55–1.03) |
| 0.03 | 5 | 16.4 ± 4.1 | |||||
| 0.1 | 5 | 29.4 ± 3.5 | |||||
| 0.3 | 5 | 75.8 ± 8.6 * | |||||
| 1 | 6 | 118.8 ± 15.4 * | |||||
| 3 | 5 | 85.4 ± 8.2 * | |||||
| 2C-B-FLY | F(5,24)=21.22, p<0.0001 | 30 | 0 | 5 | 4.2 ± 0.6 | 0.57 (0.40–0.81) |
1.79 (1.26–2.54) |
| 0.1 | 5 | 8.8 ± 1.9 | |||||
| 0.3 | 5 | 19.6 ± 4.2 | |||||
| 1 | 5 | 61.0 ± 5.5 * | |||||
| 3 | 5 | 75.2 ± 9.7 * | |||||
| 10 | 5 | 45.0 ± 9.8 * | |||||
| DOB-FLY | F(4,21)=15.61, p<0.0001 | 30 | 0 | 6 | 4.3 ±0.7 | 0.22 (0.14–0.35) |
0.67 (0.43–1.04) |
| 0.1 | 5 | 36.4 ± 3.7 | |||||
| 0.3 | 5 | 101.8 ± 10.3 * | |||||
| 1 | 5 | 160.2 ± 17.6 * | |||||
| 3 | 5 | 116.2 ± 31.6 * | |||||
| 2C-B-DFLY | F(5,28)=12.88, p<0.0001 | 30 | 0 | 6 | 5.0 ± 0.3 | 0.34 (0.22–0.52) |
1.07 (0.70–1.66) |
| 0.1 | 5 | 13.0 ± 7.2 | |||||
| 0.3 | 6 | 41.7 ± 6.8 | |||||
| 1 | 6 | 77.5 ± 11.5 * | |||||
| 3 | 6 | 77.5 ± 12.9 * | |||||
| 10 | 5 | 33.6 ± 5.9 | |||||
| DOB-DFLY | F(4,23)=24.62, p<0.0001 | 30 | 0 | 6 | 6.2 ± 1.6 | 0.068 (0.036–0.126) |
0.20 (0.11–0.38) |
| 0.03 | 5 | 39.6 ± 1.8 | |||||
| 0.1 | 5 | 80.4 ± 16.6 * | |||||
| 0.3 | 6 | 118.8 ± 7.6 * | |||||
| 1 | 6 | 124.5 ± 15.2 * |
p < 0.01, significant difference from the vehicle control group (Tukey's test).
In addition to examining the tetrahydrobenzodifuranyl analogs of 2C-B and DOB, the fully aromatic benzodifuranyl analogs were also evaluated. As shown in Table 1, DOB-DFLY (the benzodifuranyl analog of DOB) induced the HTR with an ED50 of 204.8 nmol/kg, making it significantly more potent than DOB (F(1,29)=24.89, p<0.0001). In comparison, LSD had an ED50 of 132.8 nmol/kg under equivalent conditions (Halberstadt and Geyer 2013), meaning that DOB-DFLY induces the HTR with 65% of the molar potency of LSD. 2C-B-DragonFLY (2C-B-DFLY), the benzodifuranyl analog of 2C-B, was also active in the HTR assay (ED50 = 1.07 μmol/kg) and was more potent than 2C-B (F(1,59)=5.08, p=0.0279).
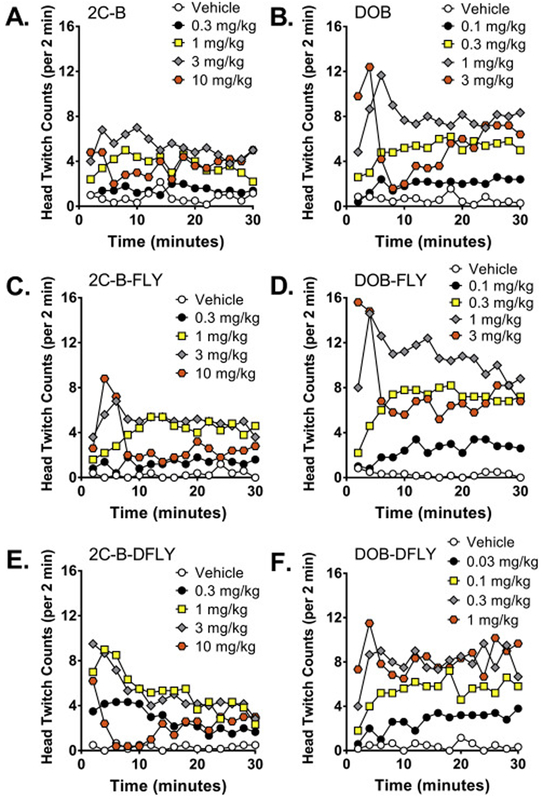
HTR counts were also analyzed in 2-min bins to examine the time-course of the responses. There was no interaction between drug and time for 2C-B (F(70,350)=1.10, NS). Similar to 2C-I (Halberstadt and Geyer 2014), 2C-B induced a relatively constant level of responding during the 30-min test session (Fig. 2A). By contrast, time was a factor in the responses produced by 2C-B-FLY (Drug × Time: F(70,336)=3.48, p<0.0001), 2C-B-DFLY (Drug × Time: F(70,392)=2.78, p<0.0001), DOB (Drug × Time: F(70,378)=5.64, p<0.0001), DOB-FLY (Drug × Time: F(56,294)=6.12, p<0.0001), and DOB-DFLY (Drug × Time: F(56,322)=1.45, p=0.0266). For the latter compounds, the responses typically peaked during the first 10 min of the assessment and then plateaued, with the interval between injection and maximal effect being inversely proportional to the dosage (Fig. 2B-F).
Figure 2.
Time-course of the head twitch response induced by 2C-B (A), DOB (B), 2C-B-FLY (C), DOB-FLY (D), 2C-B-DFLY (E), and DOB-DFLY (F). Data are presented as group means in 2-min blocks. Groups receiving low doses were sometimes omitted from the graphs for clarity.
Several other phenylethylamines with rigidified methoxy groups were also tested in mice (see Fig. 3). As shown in Table 2, 2C-I-FLY, 2C-E-FLY, and 2C-EF-FLY were active in the HTR paradigm. 2C-E-FLY (ED50 = 2.10 μmol/kg) was almost as potent as 2C-B-FLY, whereas 2C-EF-FLY (ED50 = 4.37 μmol/kg) and 2C-I-FLY (ED50 = 5.12 μmol/kg) induced the HTR with 2- and 3-fold lower potency, respectively.
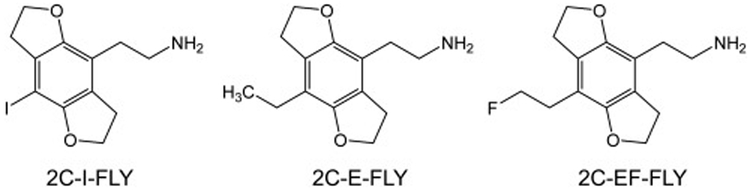
Figure 3.
Chemical structures of novel tetrahydrobenzodifuran hallucinogens. Abbreviations: 2C-I-FLY (2-(8-iodo-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b′]difuran-4-yl)ethan-1-amine), 2C-E-FLY (2-(8-ethyl-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b′]difuran-4-yl)ethan-1-amine), and 2C-EF-FLY (2-[8-(2-fluoroethyl)-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b′]difuran-4-yl]ethan-1-amine).
Table 2.
Summary of the head twitch response (HTR) data for the compounds shown in Figure 3.
| Compound | One-way ANOVA | Time (min) |
Dose (mg/kg) |
N | HTR Counts (mean ± SEM) |
ED50mg/kg (95% CI) |
ED50μmol/kg (95% CI) |
|---|---|---|---|---|---|---|---|
| 2C-E-FLY | F(4,20)=9.29, p=0.0002 | 30 | 0 | 5 | 5.6 ± 0.9 | 0.57 (0.32–1.02) |
2.10 (1.17–3.77) |
| 0.3 | 5 | 16.2 ± 5.0 | |||||
| 1 | 5 | 90.0 ± 14.2 * | |||||
| 3 | 5 | 89.4 ± 14.4 * | |||||
| 10 | 5 | 55.6 ± 20.3 | |||||
| 2C-EF-FLY | F(5,25)=17.55, p<0.0001 | 30 | 0 | 6 | 5.7 ± 0.6 | 1.26 (0.90–1.76) |
4.37 (3.13–6.11) |
| 0.1 | 5 | 9.2 ± 2.8 | |||||
| 0.3 | 5 | 12.4 ± 1.8 | |||||
| 1 | 5 | 31.0 ± 6.5 | |||||
| 3 | 5 | 75.8 ± 10.1 * | |||||
| 10 | 5 | 50.2 ± 11.1 * | |||||
| 2C-I-FLY | F(5,26)=26.48, p<0.0001 | 30 | 0 | 6 | 7.0 ± 1.4 | 1.88 (1.42–2.50) |
5.12 (3.87–6.78) |
| 0.3 | 5 | 10.8 ± 1.9 | |||||
| 1 | 5 | 26.8 ± 3.9 | |||||
| 3 | 6 | 64.3 ± 5.3 * | |||||
| 10 | 5 | 72.8 ± 8.2 * | |||||
| 30 | 5 | 14.0 ± 9.3 |
p < 0.01, significant difference from the vehicle control group (Tukey's test).
In order to investigate the influence of α-methyl substitution on the response to phenylalkylamine hallucinogens, we compared the potencies of the amphetamines and their α-desmethyl (i.e., phenylethylamine) homologues. As noted above, DOB has a threefold higher potency than 2C-B, which is a statistically significant difference (F(1,58)=14.54, p=0.0003). Likewise, DOB-FLY was significantly more potent than 2C-B-FLY (F(1,50)=12.32, p=0.001) and DOB-DFLY was significantly more potent than 2C-B-DFLY (F(1,30)=19.00, p=0.0001).
4. DISCUSSION
The present investigation was conducted to assess the behavioral effects of the phenylalkylamines 2C-B and DOB, as well as several analogs incorporating tetrahydrobenzodifuran and benzodifuran ring systems. Consistent with their activity as serotonergic hallucinogens, 2C-B and DOB induced the HTR in mice. DOB was previously shown to produce head twitches (Wieland et al. 1990; Benneyworth et al. 2007; Gonzalez-Maeso et al. 2007; Moya et al. 2007), but as far as we are aware this is the first evidence that 2C-B is active in the HTR assay. The ability of these phenylalkylamines to induce the HTR is consistent with their 5-HT2A agonist activity (Chambers et al. 2001; Chambers et al. 2003; Rickli et al. 2015a). Incorporating the 2- and 5-methoxy groups of 2C-B and DOB into aromatic furan rings resulted in a significant increase in potency in the HTR assay. Conversely, incorporating the methoxy groups into dihydrofuran rings did not alter the potency of 2C-B or DOB to an appreciable degree. These potency differences in mice are consistent with the limited human data reported to date. Our findings provide additional evidence that the mouse HTR assay can be used to investigate the SAR of serotonergic hallucinogens.
The absence of a potency difference between 2C-B-FLY and DOB-FLY and their non-rigid counterparts (2C-B and DOB, respectively) is noteworthy. Based on these results, the tetrahydrobenzodifuran nucleus present in the FLY compounds likely matches the active conformation of the methoxy groups in 2,5-dimethoxyphenylalkylamines. As was previously noted by Nichols and colleagues (Monte et al. 1996), when 2,5-dimethoxyphenylalkylamines bind to the 5-HT2A receptor, the oxygen lone pairs of the 2-methoxy group are likely oriented syn to the aminoalkyl side-chain, whereas the lone pairs in the 5-methoxy group are likely oriented anti to the side-chain. These findings are consistent with the 5-HT2A receptor model proposed by Westkaemper and Glennon (1994) where two serine residues on opposite sides of the binding pocket donate hydrogen-bonds to the methoxy groups seen in 2,5-dimethoxyphenylalkylamines. Subsequent mutagenesis and homology modelling studies identified Ser-159 and Ser-239 as likely candidates to engage the 2- and 5-methoxy groups, respectively (Braden and Nichols 2007; Isberg et al. 2011).
In contrast to the tetrahydrobenzodifurans, the aromatic benzodifurans had significantly higher potency than their non-rigid counterparts. Indeed, DOB-DFLY has about five times the potency of DOB in humans (Trachsel et al. 2013) and also possesses considerably higher affinity for the 5-HT2A receptor than DOB (Parker et al. 1998). With the exception of DOB-DFLY, little is known about the human psychopharmacology of hallucinogens with a benzodifuranyl structure; however, based on the present findings, it is reasonable to anticipate that 2C-B-DFLY would have significantly higher potency than 2C-B and 2C-B-FLY in humans. The relatively high affinity of benzodifuran hallucinogens at 5-HT2A receptors is somewhat unexpected because aromatization of the tetrahydrobenzodifuran ring system should reduce the hydrogen-bonding capacity of its oxygen atoms. There are at least two potential explanations for the affinity increase. First, the orthosteric 5-HT2A binding pocket is a relatively hydrophobic environment, so any detrimental effect of aromatization on hydrogen-bonding may be offset by the increased hydrophobicity of a benzodifuran ring system. Second, the extended tricyclic aromatic ring system in the benzodifurans could increase 5-HT2A affinity by enhancing the Van der Waals interaction known to occur between the aromatic ring of phenylalkylamine hallucinogens and Phe-340 in the binding pocket (Parrish et al. 2005; Braden et al. 2006; Isberg et al. 2011).
In addition to their 5-HT2A affinity, another factor potentially contributing to the relatively high in vivo potency of the benzodifurans might be their reported metabolic stability. According to Noble et al. (2018), no metabolism occurred when DOB-DFLY was incubated with pooled human liver microsomes (HLM), pooled human liver cytosol (HLC), monoamine oxidase (MAO), flavin-containing monooxygenase (FMO) or cytochrome P450 (CYP) isoenzymes. By contrast, DOB, DOI, DOM, and 2C-B-FLY are metabolized by CYP2D6 (Ewald and Maurer 2008; Noble et al. 2018). The benzodifuran nucleus in DOB-DFLY and other DragonFLY compounds may be resistant to hepatic metabolism, potentially resulting in prolonged bioavailability and toxicity.
In contrast to our results, 2C-B failed to provoke the HTR in rats when tested in a previous study (Moya et al. 2007). The failure of 2C-B to induce the HTR is surprising because it acts as a 5-HT2A agonist (Parrish et al. 2005; McLean et al. 2006; Rickli et al. 2015b), fully substitutes in rats trained to discriminate LSD and DOM (Glennon et al. 1988; Juncosa et al. 2013), and produces hallucinogenic effects in humans (Shulgin and Shulgin 1991; Caudevilla-Galligo et al. 2012; Papaseit et al. 2018). Furthermore, several other phenylethylamine hallucinogens, including mescaline (Corne and Pickering 1967; Silva and Calil 1975; Yamamoto et al. 1983), 2C-I (Halberstadt and Geyer 2014; Elmore et al. 2018), 2C-C (Elmore et al. 2018), and 2C-T-7 (Fantegrossi et al. 2005) are known to induce head twitches. Differences in experimental design could have played a role in these discrepant findings. For example, Moya et al. assessed head twitches during the light phase of the 24-h light/dark cycle, whereas our experiments were conducted during the dark phase of the circadian cycle. The magnitude of the HTR is known to vary over the light/dark cycle (Singleton and Marsden 1981; Moser and Redfern 1985; Nagayama and Lu 1996; Darmani 1998). Based on our findings, 2C-B and other phenylethylamine hallucinogens clearly induce head twitches when administered to mice and therefore do not produce false negative results in this behavioral assay.
An additional goal of the present investigation was to compare the in vivo potencies of amphetamine hallucinogens and their α-desmethyl homologues. Amphetamine-based hallucinogens are typically more potent than phenylethylamines in vivo. For example, DOB is about ten times more potent than 2C-B in humans (Shulgin and Shulgin 1991). Similarly, 3,4,5-trimethoxyamphetamine (TMA) is twice as potent as mescaline (Shulgin et al. 1961). Only a few preclinical studies have investigated the influence of an α-methyl group on the behavioral potency of phenylalkylamine hallucinogens. In drug discrimination studies in rats, TMA is more potent than mescaline (Glennon and Young 1982), DOM has higher potency than its α-desmethyl homologue (Glennon et al. 1983), and DOB exceeds the potency of 2C-B (Glennon et al. 1988). Likewise, DOB-FLY has about four times the potency of 2C-B-FLY in rats trained with LSD (Monte et al. 1996). Our experiments confirmed that DOB, DOB-FLY, and DOB-DFLY have two- to threefold greater potency than their phenylethylamine homologues in mice. The presence of an α-methyl group has little effect on the 5-HT2A affinity of phenylethylamines (Johnson et al. 1990; Glennon et al. 1992; Parrish et al. 2005), so there must be another explanation for these potency differences. The presence of an α-methyl group may increase potency by reducing the metabolic lability of the amine side-chain (Nichols et al. 1991). Deamination by MAO is the primary route of metabolism for phenylethylamines such as 2C-B (Theobald and Maurer 2007; Kanamori et al. 2013) but plays only a minor role in the metabolism of amphetamines (Ho et al. 1971; Ewald et al. 2007; Ewald et al. 2008; Noble et al. 2018). DOB-DFLY was found to be a potent inhibitor of MAOA (IC50 = 0.54 μM) (Noble et al. 2018). 2C-B-FLY, on the other hand, was reported to have a significantly weaker effect on the activity of this enzyme, with IC50 values ranging from 19 μM (Wagmann et al., manuscript submitted) to 27.7 μM (Noble et al. 2018). The enhanced lipophilicity due to the α-methyl group could also potentially enhance CNS penetration. These two factors, either alone or in combination, likely explain why amphetamines have higher potency than phenylethylamines in our HTR studies.
In addition to the potency differences between amphetamines and phenylethylamines in the HTR assay, the amphetamines also induced a greater maximal number of head twitches compared to their phenylethylamine counterparts. For example, as shown in Figure 2, the response to DOB-FLY peaked at 15.6±0.9 (mean±SEM) head twitches/2 min, whereas 2C-B-FLY produced a maximum of 8.8±2.3 head twitches/2 min. In a previous study that examined the relationship between 5-HT2A activation and head twitch for a series of 5-HT2 ligands, intrinsic efficacy at 5-HT2A was significantly correlated with the maximum number of head twitches but did not influence potency in the HTR assay (Vickers et al. 2001). Importantly, compared to phenylethylamines, amphetamines have higher intrinsic efficacy at cloned 5-HT2A receptors (Nichols et al. 1994; Parrish et al. 2005; Moya et al. 2007). The presence of an α-methyl group reportedly strengthens the interaction of phenylalkylamines with Phe-340 in the 5-HT2A binding pocket via a van der Waals interaction (Parrish et al. 2005), potentially helping to stabilize the receptor in the active conformation. Hence, differences in the magnitude of the HTR produced by amphetamines and phenylethylamines likely reflect differences in their agonist activity at the 5-HT2A receptor, although additional work is necessary to confirm this explanation.
As noted in Section 1, DOB-DFLY is a potent hallucinogen, active in sub-milligram dosages and only marginally less potent than the prototypical agent LSD. The recreational use of DOB-DFLY has resulted in toxicity and fatalities (Personne and Hultén 2008; Thorlacius et al. 2008; Andreasen et al. 2009; Wood et al. 2009; Nielsen et al. 2010; Corazza et al. 2011; Chavarin et al. 2013; Iwersen-Bergmann et al. 2018). As shown by the HTR data, DOB-DFLY is also highly potent in mice, which is not surprising given that it contains two structural features (a benzodifuran ring system and an α-methyl group) capable of enhancing the potency of phenylalkylamine hallucinogens. 2C-B-FLY, by contrast, represents the fully saturated counterpart and does not contain the α-methyl group, and is therefore observed to be considerably less potent than DOB-DFLY. Similar to DOB-DFLY, the recreational use of 2C-B-FLY and other phenethylamine hallucinogens has been linked to adverse reactions. Although some of these cases occurred due to adulteration of samples with a high potency compound such as DOB-DFLY (Chavarin et al. 2013; Iwersen-Bergmann et al. 2018), in other cases the phenylethylamines themselves were the causative agents (Curtis et al. 2003; Drees et al. 2009; Topeff et al. 2011; Sacks et al. 2012; Bosak et al. 2013; Van Vrancken et al. 2013; Stoller et al. 2017). In addition to 2C-B and 2C-I, which are popular phenethylamine hallucinogens (de Boer and Bosman 2004; Caudevilla-Galligo et al. 2012; Burns et al. 2014), 4-ethyl-2,5-dimethoxyphenethylamine (2C-E) is also used recreationally (Topeff et al. 2011; Sacks et al. 2012; Van Vrancken et al. 2013; Woo and Hanley 2013). According to Shulgin and Shulgin (1991), 2C-E is active at doses of 10–25 mg. 4-(2-Fluoroethyl)-2,5-dimethoxyphenethylamine (2C-EF) also reportedly acts as a hallucinogen and is orally active in humans at doses of 6–12 mg with a duration of 12 hours (Shulgin et al. 2011), although information about the extent of recreational use of this substance appears to be lacking. Based on our HTR data, the tetrahydrobenzofurans 2C-I-FLY, 2C-E-FLY and 2C-EF-FLY tested in this study for the first time are likely to have LSD-like psychopharmacology. These compounds may appear as new recreational drugs in the future but would likely exhibit relatively low potency compared to DOB-DFLY.
To summarize our findings, 2C-B, DOB, and several structurally rigid analogues induced the HTR in mice. The potency of 2C-B and DOB was increased by incorporating the 2- and 5-methoxy groups into aromatic furan rings, whereas potency was unaffected when the methoxy groups were incorporated into dihydrofuran rings. Indeed, the benzodifuran DOB-DFLY is known to be a highly potent hallucinogen in humans, whereas the tetrahydrobenzodifuran 2C-B-FLY is about as potent as its non-rigid analog 2C-B. In general, the potencies of these compounds in the mouse HTR assay parallel the human hallucinogenic data as well as the established structure-activity relationships for binding to the 5-HT2A receptor. Although head twitches likely have limited value as a model of hallucinogenesis (Canal and Morgan 2012), the HTR assay may be useful for investigations of the in vivo potency relationships of hallucinogens. Additional studies, however, are necessary to evaluate the predictive and translational value of ED50 values generated using this behavioral paradigm.
The hallucinogens 2C-B and DOB induced head twitches in mice
Tethering the methoxy groups into a benzodifuran nucleus increased potency
Tethering the methoxy groups into a tetrahydrobenzodifuran nucleus did not alter potency
The benzodifuran hallucinogen Bromo-DragonFLY was highly potent in mice
5. ACKNOWLEDGMENTS
These studies were supported by an award from NIDA (R01 DA041336), as well as by the Veteran's Administration VISN 22 Mental Illness Research, Education, and Clinical Center. The authors would like to thank Mark A. Geyer, Ph.D., for his assistance with this project.
6. ROLE OF THE FUNDING SOURCE
The sponsors of this research had no involvement in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, or in the decision to submit the article for publication.
Footnotes
DECLARATIONS OF INTEREST: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andreasen MF, Telving R, Birkler RI, Schumacher B, Johannsen M (2009) A fatal poisoning involving Bromo-Dragonfly. Forensic Sci Int 183: 91–6. [DOI] [PubMed] [Google Scholar]
- Anonymous (2007) "Bromo DragonFLY" (bromo-benzodifuranyl-isopropylamine) in Ashland, Oregon. Microgram Bull 40: 78–79. [Google Scholar]
- Anonymous (2008) "Bromo-DragonFLY" in Queensland, Australia. Microgram Bull 41: 16–17. [Google Scholar]
- Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E (2007) A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol 72: 477–84. [DOI] [PubMed] [Google Scholar]
- Bosak A, LoVecchio F, Levine M (2013) Recurrent seizures and serotonin syndrome following "2C-I" ingestion. J Med Toxicol 9: 196–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden MR, Nichols DE (2007) Assessment of the roles of serines 5.43(239) and 5.46(242) for binding and potency of agonist ligands at the human serotonin 5-HT2A receptor. Mol Pharmacol 72: 1200–1209. [DOI] [PubMed] [Google Scholar]
- Braden MR, Parrish JC, Naylor JC, Nichols DE (2006) Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol Pharmacol 70: 1956–64. [DOI] [PubMed] [Google Scholar]
- Brandt SD, Kavanagh PV, Westphal F, Elliott SP, Wallach J, Colestock T, Burrow TE, Chapman SJ, Stratford A, Nichols DE, Halberstadt AL (2017) Return of the lysergamides. Part II: Analytical and behavioural characterization of N6 -allyl-6-norlysergic acid diethylamide (AL-LAD) and (2'S,4'S)-lysergic acid 2,4-dimethylazetidide (LSZ). Drug Test Anal 9: 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, Kavanagh PV, Westphal F, Stratford A, Elliott SP, Hoang K, Wallach J, Halberstadt AL (2016) Return of the lysergamides. Part I: Analytical and behavioural characterization of 1-propionyl-d-lysergic acid diethylamide (1P-LSD). Drug Test Anal 8: 891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns L, Roxburgh A, Matthews A, Bruno R, Lenton S, Van Buskirk J (2014) The rise of new psychoactive substance use in Australia. Drug Test Anal 6: 846–9. [DOI] [PubMed] [Google Scholar]
- Canal CE, Morgan D (2012) Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal 4: 556–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudevilla-Galligo F, Riba J, Ventura M, Gonzalez D, Farre M, Barbanoj MJ, Bouso JC (2012) 4-Bromo-2,5-dimethoxyphenethylamine (2C-B): presence in the recreational drug market in Spain, pattern of use and subjective effects. J Psychopharmacol 26: 1026–35. [DOI] [PubMed] [Google Scholar]
- Chambers JJ, Kurrasch-Orbaugh DM, Parker MA, Nichols DE (2001) Enantiospecific synthesis and pharmacological evaluation of a series of super-potent, conformationally restricted 5-HT(2A/2C) receptor agonists. J Med Chem 44: 1003–10. [DOI] [PubMed] [Google Scholar]
- Chambers JJ, Parrish JC, Jensen NH, Kurrasch-Orbaugh DM, Marona-Lewicka D, Nichols DE (2003) Synthesis and pharmacological characterization of a series of geometrically constrained 5-HT(2A/2C) receptor ligands. J Med Chem 46: 3526–35. [DOI] [PubMed] [Google Scholar]
- Chavarin A, Nogue S, Castaneda-Pomeda M, Gil V (2013) The dangers of buying "research chemicals" online, bromo-dragonfly mislabelled as 2C-B Fly: a confirmed case report, and its follow up in "research chemical" specific social media. Clin Toxicol 51: 347. [Google Scholar]
- Christopoulos A, Grant MK, Ayoubzadeh N, Kim ON, Sauerberg P, Jeppesen L, El-Fakahany EE (2001) Synthesis and pharmacological evaluation of dimeric muscarinic acetylcholine receptor agonists. J Pharmacol Exp Ther 298: 1260–8. [PubMed] [Google Scholar]
- Corazza O, Schifano F, Farre M, Deluca P, Davey Z, Torrens M, Demetrovics Z, Di Furia L, Flesland L, Siemann H, Skutle A, Van Der Kreeft P, Scherbaum N (2011) Designer drugs on the internet: a phenomenon out-of-control? the emergence of hallucinogenic drug Bromo-Dragonfly. Curr Clin Pharmacol 6: 125–9. [DOI] [PubMed] [Google Scholar]
- Corne SJ, Pickering RW (1967) A possible correlation between drug-induced hallucinations in man and a behavioural response in mice. Psychopharmacologia 11: 65–78. [DOI] [PubMed] [Google Scholar]
- Curtis B, Kemp P, Harty L, Choi C, Christensen D (2003) Postmortem identification and quantitation of 2,5-dimethoxy-4-n-propylthiophenethylamine using GC-MSD and GC-NPD. J Anal Toxicol 27: 493–8. [DOI] [PubMed] [Google Scholar]
- Darmani NA (1998) The silent and selective 5-HT1A antagonist, WAY 100635, produces via an indirect mechanism, a 5-HT2A receptor-mediated behaviour in mice during the day but not at night. Short communication. J Neural Transm (Vienna) 105: 635–43. [DOI] [PubMed] [Google Scholar]
- de Boer D, Bosman I (2004) A new trend in drugs-of-abuse; the 2C-series of phenethylamine designer drugs. Pharm World Sci 26: 110–3. [DOI] [PubMed] [Google Scholar]
- Drees JC, Stone JA, Wu AH (2009) Morbidity involving the hallucinogenic designer amines MDA and 2C-I. J Forensic Sci 54: 1485–7. [DOI] [PubMed] [Google Scholar]
- Elmore JS, Decker AM, Sulima A, Rice KC, Partilla JS, Blough BE, Baumann MH (2018) Comparative neuropharmacology of N-(2-methoxybenzyl)-2,5-dimethoxyphenethylamine (NBOMe) hallucinogens and their 2C counterparts in male rats. Neuropharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMCDDA (2007) EMCDDA-Europol 2006 Annual Report on the Implementation of Council Decision 2005/387/JHA. Publications Office of the European Union, Lisbon. [Google Scholar]
- EMCDDA (2008) EMCDDA-Europol 2007 Annual Report on the Implementation of Council Decision 2005/387/JHA. Publications Office of the European Union, Lisbon. [Google Scholar]
- Ewald AH, Ehlers D, Maurer HH (2008) Metabolism and toxicological detection of the designer drug 4-chloro-2,5-dimethoxyamphetamine in rat urine using gas chromatography-mass spectrometry. Anal Bioanal Chem 390: 1837–42. [DOI] [PubMed] [Google Scholar]
- Ewald AH, Fritschi G, Maurer HH (2007) Metabolism and toxicological detection of the designer drug 4-iodo-2,5-dimethoxy-amphetamine (DOI) in rat urine using gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 857: 170–4. [DOI] [PubMed] [Google Scholar]
- Ewald AH, Maurer HH (2008) 2,5-Dimethoxyamphetamine-derived designer drugs: studies on the identification of cytochrome P450 (CYP) isoenzymes involved in formation of their main metabolites and on their capability to inhibit CYP2D6. Toxicol Lett 183: 52–7. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH (2005) Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology (Berl) 181: 496–503. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ (2000) Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology 23: 569–76. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Raghupathi R, Bartyzel P, Teitler M, Leonhardt S (1992) Binding of phenylalkylamine derivatives at 5-HT1C and 5-HT2 serotonin receptors: evidence for a lack of selectivity. J Med Chem 35: 734–40. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, Lyon RA (1988) A preliminary investigation of the psychoactive agent 4-bromo-2,5-dimethoxyphenethylamine: a potential drug of abuse. Pharmacol Biochem Behav 30: 597–601. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R (1982) Comparison of behavioral properties of di- and trimethoxyphenylisopropylamines. Pharmacol Biochem Behav 17: 603–7. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Jacyno JM (1983) Indolealkylamine and phenalkylamine hallucinogens. Effect of alpha-methyl and N-methyl substituents on behavioral activity. Biochem Pharmacol 32: 1267–73. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53: 439–52. [DOI] [PubMed] [Google Scholar]
- Green SL (2013) Benzofurans and benzodifurans In: Dargan PI, Wood DM (eds) Novel Psychoactive Substances: Classification, Pharmacology and Toxicology. Academic Press, London, pp 383–392 [Google Scholar]
- Halberstadt AL, Geyer MA (2013) Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology (Berl) 227: 727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA (2014) Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology 77: 200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Klein LM, Chatha M, Valenzuela LB, Stratford A, Wallach J, Nichols DE, Brandt SD (2018) Pharmacological characterization of the LSD analog N-ethyl-N-cyclopropyl lysergamide (ECPLA). Psychopharmacology (Berl) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Koedood L, Powell SB, Geyer MA (2011) Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J Psychopharmacol 25: 1548–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Erowid E, Erowid F (2008) Survey provides new 2C-B-FLY data. Erowid Extracts 15: 3–5. [Google Scholar]
- Ho BT, Estevez V, Tansey LW, Englert LF, Creaven PJ, McIsaac WM (1971) Analogs of amphetamine. 5. Studies of excretory metabolites of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM) in rats. J Med Chem 14: 158–60. [DOI] [PubMed] [Google Scholar]
- Isberg V, Balle T, Sander T, Jorgensen FS, Gloriam DE (2011) G protein- and agonist-bound serotonin 5-HT2A receptor model activated by steered molecular dynamics simulations. J Chem Inf Model 51: 315–25. [DOI] [PubMed] [Google Scholar]
- Iwersen-Bergmann S, Lehman S, Heinemann A, Schroder C, Muller A, Jungen H, Andresen-Streichert H, Pueschel K, Vidal C, Mercer-Chalmers-Bender K (2018) Mass poisoning with NPS: 2C-E and Bromo-DragonFly. Int J Legal Med. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Mathis CA, Shulgin AT, Hoffman AJ, Nichols DE (1990) [125I]-2-(2,5-dimethoxy-4-iodophenyl)aminoethane ([125I]-2C-I) as a label for the 5-HT2 receptor in rat frontal cortex. Pharmacol Biochem Behav 35: 211–7. [DOI] [PubMed] [Google Scholar]
- Juncosa JI Jr., Hansen M, Bonner LA, Cueva JP, Maglathlin R, McCorvy JD, Marona-Lewicka D, Lill MA, Nichols DE (2013) Extensive rigid analogue design maps the binding conformation of potent N-benzylphenethylamine 5-HT2A serotonin receptor agonist ligands. ACS Chem Neurosci 4: 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori T, Nagasawa K, Kuwayama K, Tsujikawa K, Iwata YT, Inoue H (2013) Analysis of 4-bromo-2,5-dimethoxyphenethylamine abuser's urine: identification and quantitation of urinary metabolites. J Forensic Sci 58: 279–87. [DOI] [PubMed] [Google Scholar]
- King LA (2014) New phenethylamines in Europe. Drug Test Anal 6: 808–18. [DOI] [PubMed] [Google Scholar]
- Klein LM, Cozzi NV, Daley PF, Brandt SD, Halberstadt AL (2018) Receptor binding profiles and behavioral pharmacology of ring-substituted N,N-diallyltryptamine analogs. Neuropharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kometer M, Schmidt A, Jancke L, Vollenweider FX (2013) Activation of serotonin 2A receptors underlies the psilocybin-induced effects on alpha oscillations, N170 visual-evoked potentials, and visual hallucinations. J Neurosci 33: 10544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenmann R, Pokorny D, Vollenweider L, Preller KH, Pokorny T, Seifritz E, Vollenweider FX (2017) Dreamlike effects of LSD on waking imagery in humans depend on serotonin 2A receptor activation. Psychopharmacology (Berl). [DOI] [PubMed] [Google Scholar]
- McLean TH, Parrish JC, Braden MR, Marona-Lewicka D, Gallardo-Godoy A, Nichols DE (2006) 1-Aminomethylbenzocycloalkanes: conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists. J Med Chem 49: 5794–803. [DOI] [PubMed] [Google Scholar]
- Monte AP, Marona-Lewicka D, Parker MA, Wainscott DB, Nelson DL, Nichols DE (1996) Dihydrobenzofuran analogues of hallucinogens. 3. Models of 4-substituted (2,5-dimethoxyphenyl)alkylamine derivatives with rigidified methoxy groups. J Med Chem 39: 2953–61. [DOI] [PubMed] [Google Scholar]
- Moser PC, Redfern PH (1985) Circadian variation in behavioural responses to central 5-HT receptor stimulation in the mouse. Psychopharmacology (Berl) 86: 223–7. [DOI] [PubMed] [Google Scholar]
- Moya PR, Berg KA, Gutierrez-Hernandez MA, Saez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP (2007) Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Pharmacol Exp Ther 321: 1054–61. [DOI] [PubMed] [Google Scholar]
- Nagayama H, Lu JQ (1996) Circadian rhythm in the responsiveness of central 5-HT2A receptor to DOI in rats. Psychopharmacology (Berl) 127: 113–6. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Frescas S, Marona-Lewicka D, Huang X, Roth BL, Gudelsky GA, Nash JF (1994) 1-(2,5-Dimethoxy-4-(trifluoromethyl)phenyl)-2-aminopropane: a potent serotonin 5-HT2A/2C agonist. J Med Chem 37: 4346–51. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Oberlender R, McKenna DJ (1991) Stereochemical aspects of hallucinogenesis In: Watson RR (ed) Biochemistry and Physiology of Substance Abuse, Volume 3 CRC Press, Boca Raton, FL, pp 1–39 [Google Scholar]
- Nichols DE, Sassano MF, Halberstadt AL, Klein LM, Brandt SD, Elliott SP, Fiedler WJ (2015) N-Benzyl-5-methoxytryptamines as Potent Serotonin 5-HT2 Receptor Family Agonists and Comparison with a Series of Phenethylamine Analogues. ACS Chem Neurosci 6: 1165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen VT, Hogberg LC, Behrens JK (2010) [Bromo-Dragonfly poisoning of 18-year-old male]. Ugeskr Laeger 172: 1461–2. [PubMed] [Google Scholar]
- Noble C, Holm NB, Mardal M, Linnet K (2018) Bromo-dragonfly, a psychoactive benzodifuran, is resistant to hepatic metabolism and potently inhibits monoamine oxidase A. Toxicol Lett. [DOI] [PubMed] [Google Scholar]
- Papaseit E, Farre M, Perez-Mana C, Torrens M, Ventura M, Pujadas M, De la Torre R, Gonzalez D (2018) Acute pharmacological effects of 2C-B in humans: an observational study. Front Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MA, Marona-Lewicka D, Lucaites VL, Nelson DL, Nichols DE (1998) A novel (benzodifuranyl)aminoalkane with extremely potent activity at the 5-HT2A receptor. J Med Chem 41: 5148–9. [DOI] [PubMed] [Google Scholar]
- Parrish JC, Braden MR, Gundy E, Nichols DE (2005) Differential phospholipase C activation by phenylalkylamine serotonin 5-HT 2A receptor agonists. J Neurochem 95: 1575–84. [DOI] [PubMed] [Google Scholar]
- Personne M, Hultén P (2008) Bromo-dragonfly, a life threatening designer drug. Clin Toxicol 46: 379–380. [Google Scholar]
- Preller KH, Herdener M, Pokorny T, Planzer A, Kraehenmann R, Stampfli P, Liechti ME, Seifritz E, Vollenweider FX (2017) The Fabric of Meaning and Subjective Effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr Biol 27: 451–457. [DOI] [PubMed] [Google Scholar]
- Preller KH, Vollenweider FX (2018) Phenomenology, Structure, and Dynamic of Psychedelic States. Curr Top Behav Neurosci 36: 221–256. [DOI] [PubMed] [Google Scholar]
- Rangel-Barajas C, Malik M, Vangveravong S, Mach RH, Luedtke RR (2014) Pharmacological modulation of abnormal involuntary DOI-induced head twitch response in male DBA/2J mice: I. Effects of D2/D3 and D2 dopamine receptor selective compounds. Neuropharmacology 83: 18–27. [DOI] [PubMed] [Google Scholar]
- Rickli A, Kopf S, Hoener MC, Liechti ME (2015a) Pharmacological profile of novel psychoactive benzofurans. Br J Pharmacol 172: 3412–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickli A, Luethi D, Reinisch J, Buchy D, Hoener MC, Liechti ME (2015b) Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs). Neuropharmacology 99: 546–53. [DOI] [PubMed] [Google Scholar]
- Sacks J, Ray MJ, Williams S, Opatowsky MJ (2012) Fatal toxic leukoencephalopathy secondary to overdose of a new psychoactive designer drug 2C-E ("Europa"). Proc (Bayl Univ Med Cent) 25: 374–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ (1995) (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther 273: 101–12. [PubMed] [Google Scholar]
- Shulgin AT, Bunnell S, Sargent T (1961) The psychotomimetic properties of 3,4,5-trimethoxyamphetamine. Nature 189: 1011–1012. [Google Scholar]
- Shulgin AT, Manning T, Daley PF (2011) The Shulgin Index, Vol. 1, Psychedelic Phenethylamines and Related Compounds. Transform Press, Berkeley, CA [Google Scholar]
- Shulgin AT, Shulgin A (1991) PiHKAL: A Chemical Love Story. Transform Press, Berkeley, CA [Google Scholar]
- Shulgin AT, Shulgin A (1997) TIHKAL: the Continuation. Transform Press, Berkeley [Google Scholar]
- Silva MT, Calil HM (1975) Screening hallucinogenic drugs: systematic study of three behavioral tests. Psychopharmacologia 42: 163–71. [DOI] [PubMed] [Google Scholar]
- Singleton C, Marsden CA (1981) Circadian variation in the head twitch response produced by 5-methoxy-N1,N1-dimethyltryptamine and p-chloroamphetamine in the mouse. Psychopharmacology (Berl) 74: 173–6. [DOI] [PubMed] [Google Scholar]
- Smith DA, Bailey JM, Williams D, Fantegrossi WE (2014) Tolerance and cross-tolerance to head twitch behavior elicited by phenethylamine- and tryptamine-derived hallucinogens in mice. J Pharmacol Exp Ther 351: 485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller A, Dolder PC, Bodmer M, Hammann F, Rentsch KM, Exadaktylos AK, Liechti ME, Liakoni E (2017) Mistaking 2C-P for 2C-B: What a Difference a Letter Makes. J Anal Toxicol 41: 77–79. [DOI] [PubMed] [Google Scholar]
- Theobald DS, Maurer HH (2007) Identification of monoamine oxidase and cytochrome P450 isoenzymes involved in the deamination of phenethylamine-derived designer drugs (2C-series). Biochem Pharmacol 73: 287–97. [DOI] [PubMed] [Google Scholar]
- Thorlacius K, Borna C, Personne M (2008) [Bromo-dragon fly--life-threatening drug. Can cause tissue necrosis as demonstrated by the first described case]. Lakartidningen 105: 1199–200. [PubMed] [Google Scholar]
- Topeff JM, Ellsworth H, Willhite LA, Bangh SA, Edwards EM, Cole JB (2011) A series of symptomatic patients including one fataility, following 2C-E exposure. Clin Toxicol: 526. [Google Scholar]
- Trachsel D, Lehmann D, Enzensperger C (2013) Phenethylamine: Von der Struktur zur Funktion. Nachtschatten, Solothurn [Google Scholar]
- Valle M, Maqueda AE, Rabella M, Rodriguez-Pujadas A, Antonijoan RM, Romero S, Alonso JF, Mananas MA, Barker S, Friedlander P, Feilding A, Riba J (2016) Inhibition of alpha oscillations through serotonin-2A receptor activation underlies the visual effects of ayahuasca in humans. Eur Neuropsychopharmacol 26: 1161–75. [DOI] [PubMed] [Google Scholar]
- Van Vrancken MJ, Benavides R, Wians FH Jr. (2013) Identification of designer drug 2C-E (4-ethyl-2, 5-dimethoxy-phenethylamine) in urine following a drug overdose. Proc (Bayl Univ Med Cent) 26: 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, Kennett GA (2001) Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists. Pharmacol Biochem Behav 69: 643–52. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9: 3897–902. [DOI] [PubMed] [Google Scholar]
- Westkaemper RB, Glennon RA (1994) Molecular modeling of the interaction of LSD and other hallucinogens with 5-HT2 receptors. NIDA Res Monogr 146: 263–83. [PubMed] [Google Scholar]
- Wieland S, Kreider MS, McGonigle P, Lucki I (1990) Destruction of the nucleus raphe obscurus and potentiation of serotonin-mediated behaviors following administration of the neurotoxin 3-acetylpyridine. Brain Res 520: 291–302. [DOI] [PubMed] [Google Scholar]
- Woo TM, Hanley JR (2013) "How high do they look?": identification and treatment of common ingestions in adolescents. J Pediatr Health Care 27: 135–44. [DOI] [PubMed] [Google Scholar]
- Wood DM, Looker JJ, Shaikh L, Button J, Puchnarewicz M, Davies S, Lidder S, Ramsey J, Holt DW, Dargan PI (2009) Delayed onset of seizures and toxicity associated with recreational use of Bromo-dragonFLY. J Med Toxicol 5: 226–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Tazoe N, Ueki S, Shimomura K, Satoh H, Mori J (1983) Effect of zotepine on head-twitch induced by L-5-hydroxytryptophan, mescaline and 2,5-dimethoxy-4-methylamphetamine in mice and rats. Jpn J Pharmacol 33: 319–25. [DOI] [PubMed] [Google Scholar]