Abstract
We conducted a records-based cohort study of patients who initiated pre-exposure prophylaxis (PrEP) at a large federally qualified health center in Los Angeles, CA to characterize patterns of PrEP use, identify correlates of PrEP discontinuation, and calculate HIV incidence. Of 3,121 individuals initiating PrEP between 2014–2017, 42% (n=1,314) were active (i.e., had a current PrEP prescription) in April 2018. HIV incidence was 0.1/100 person-years among active PrEP patients, compared to 2.1/100 person-years among patients who discontinued. Compared to patients accessing PrEP through government programs with no prescription copay, risk of discontinuation was higher among those with private insurance (ARR = 1.4, 95% CI: 1.2, 1.7), or no insurance (ARR = 4.5, 95% CI 3.2, 6.4). Sixty-three percent of active PrEP patients had gaps between PrEP prescriptions, averaging one gap per year (median length = 65 days). Increasing access to free or low-cost PrEP can improve PrEP continuity.
RESUMEN
Llevamos a cabo un estudio de cohorte basada en registros de pacientes quienes iniciaron profilaxis preexposición (PrEP) en un centro de salud grande y federalmente calificado en Los Ángeles, CA para caracterizar patrones del uso de PrEP, identificar correlaciones de la discontinuación de PrEP y calcular la incidencia de VIH. De los 3,121 individuos quienes iniciaron PrEP entre los años 2014–2017, 42% (n=1,314) fueron activos (i.e. actualmente tenían una receta para PrEP) en abril 2018. La incidencia de VIH fue 0.1/100 persona-años entre los pacientes activos con PrEP, comparada a 2.1/100 personas-años entre los pacientes quienes lo dejaron de usar. Comparado a los pacientes accediendo a PrEP a través de programas gubernamentales sin copago para las recetas, el riesgo de discontinuación de PrEP fue más alto entre los con seguro de salud privado (RRA = 1.4, 95% CI: 1.2, 1.7) o los que no tienen seguro de salud (RRA = 4.5, 95% CI: 3.2, 6.4). Sesenta y tres por ciento de los pacientes activos de PrEP tenían lapsos sin recetas de PrEP, con un promedio de uno lapso por año (duración del lapso mediano = 65 días). Ampliando el acceso a PrEP gratis o con bajo costo puede mejorar la continuidad de tomar PrEP.
BACKGROUND
In 2012, tenofovir/emtricitabine (TDF/FTC) was approved for use as oral daily HIV pre-exposure prophylaxis (PrEP) by the Food and Drug Administration (FDA) after clinical trials demonstrated it to be over 90% efficacious when taken daily.(1, 2) The Centers for Disease Control and Prevention (CDC) recommend PrEP for HIV negative people at elevated risk for acquiring HIV through sexual or intravenous exposure. In recent years in the United States, approximately 40,000 new HIV cases were diagnosed annually, disproportionately among Black and Latino gay, bisexual, and other men who have sex with men (MSM); transgender women; and people aged 13–29.(3, 4) Modeling studies have demonstrated that making oral PrEP available for communities most affected by HIV could substantially reduce transmission and contribute to ending the epidemic.(5, 6)
Similar to the HIV treatment cascade, several PrEP cascades have been proposed to evaluate the effectiveness of PrEP in community settings.(7–10) Studies of the PrEP cascade outside of clinical trials have identified discontinuation as a major concern, as findings repeatedly demonstrate that about a quarter or more of individuals prescribed PrEP discontinue within the first few months.(11–17) A study in a large, private integrated health system that used prescription refill data to measure PrEP coverage found that overall 22.5% of patients had discontinued PrEP by the end of the study period, and increased risk of discontinuation was observed among females and those with a history of drug/alcohol abuse.(13) A study of clients prescribed PrEP at a large LGBT-focused clinic in Chicago found that individuals with comorbidities had lower discontinuation (as measured by appointment attendance), while those without insurance had higher discontinuation.(16) Clients with comorbidities had more reasons to attend the clinic for other medical care, and authors theorized that these additional appointments provided opportunities for incidental PrEP continuation (e.g. getting a prescription renewal while visiting for another primary complaint). Additionally, many patients did not consistently attend quarterly follow-up appointments, potentially leading to gaps in PrEP prescription coverage.(16) The measure of a successful PrEP intervention is not typically lifelong adherence and may instead be PrEP use during “seasons of risk”.(18, 19) Thus, studies evaluating PrEP in community settings lack both the proscribed endpoints of a clinical prevention trial and the clear implications of non-retention in HIV treatment. Importantly, gaps in PrEP care present opportunities for HIV infection, particularly when individuals start taking PrEP again (or start taking it consistently) only after re-engaging in sexual risk behaviors.(11)
This study used data from the Los Angeles LGBT Center (the Center) – a large federally qualified health center (FQHC) in Los Angeles, California – to examine how demographic and health services factors influence HIV incidence, use of PrEP over time, and gaps in prescription among patients prescribed PrEP. A cross-sectional study at the Center previously identified racial and ethnic disparities in PrEP uptake similar to those observed in a large study of Medi-Cal (California’s expanded Medicaid) beneficiaries.(20, 21) Black and Latino men who have sex with men (MSM) were less likely than White MSM to initiate PrEP. Additionally, the study found that younger people had significantly lower odds of using PrEP compared to older people, despite higher odds of being eligible based on sexual risk behavior.(20)
Studies conducted in community settings can reveal opportunities to improve implementation of effective interventions like PrEP. By examining patterns in PrEP use in this real-world clinical setting, we endeavored to identify how health services factors and patient factors may influence several goals of PrEP retention efforts: ensuring continued access to PrEP services, facilitating consistent PrEP coverage necessary to achieve adherence, and ultimately helping people remain HIV negative. The aims of this analysis were three-fold: 1) characterize longitudinal engagement in PrEP services in an administrative cohort of patients at a large, federally qualified health center, 2) compare risk of HIV seroconversion among active and discontinued clients, 3) identify demographic and health services correlates of PrEP discontinuation and gaps in PrEP prescriptions.
METHODS
Setting
The Los Angeles LGBT Center provided primary care, HIV care, and sexual health services to over 19,000 unique clients annually during the study period.(22) The Center also offered social services, mental healthcare, legal services, and variety of programming tailored to address needs of LGBT people ranging from teens to seniors. In January 2014, the Center started providing PrEP through its primary care clinic in the Hollywood neighborhood of Los Angeles, and expanded PrEP services in October 2015 to its sexual health clinic in West Hollywood. The annual incidence of new HIV infections has averaged 1.5% in recent years.(20, 22)
A 2016 cost analysis of Covered California plans available in Los Angeles found that an individual may expect to pay approximately $50 per month for PrEP medication on a Gold or Silver plan (or $0 with the manufacturer’s copay card), while those on a high-deductible Bronze plan may pay $500 per month (reduced to $200 per month with the copay card).(23) Individuals enrolled in Medi-Cal, California’s Medicaid which was expanded through the ACA, have no copay for PrEP medication. At the Center, both the Los Angeles Department of Public Health Division of HIV and STD Prevention PrEP program and a grant from the California Office of AIDS provide PrEP with no copay to eligible individuals who are uninsured or underinsured and do not qualify for Medi-Cal.
Data Description
Medical records were abstracted for patients 18 and older prescribed TDF/FTC as PrEP at either of the Center’s clinics between January 1, 2014 and December 31, 2017. Follow-up data was available through April 30, 2018. When a provider had an encounter with a patient or opened a patient’s chart for administrative review, a record was created in the clinic’s electronic medical record (EMR). The extracted data only included a record if the provider completed the chart sign-off. Records arising from administrative tasks rather than encounters with patients, (e.g., a provider opened the EMR to cancel a prescription in between a patient’s visits) would ordinarily not be included in the extracted data because such updates do not require a chart sign-off. Data from the EMR were extracted via Structured Query Language (SQL) queries written by the Center’s epidemiology and database management teams.
Demographic variables collected as part of the clinic’s online registration process included age, gender, race/ethnicity, sexual orientation, and housing status. Clinical variables including laboratory results, visit dates, prescriptions, and insurance details were obtained from information that providers entered into the EMR as part of patient care. Insurance payer billed at first visit was categorized as private, government (including Medi-Cal and programs through the local and state public health department that provide PrEP medication at no cost), and none (uninsured/out of pocket). If patients became HIV positive while they had an active PrEP prescription, their charts were reviewed manually to assess reported adherence to PrEP in the time leading up to the diagnosis.
Prescription order date, quantity, and refills were used to estimate time on PrEP, and pattern of PrEP use. Medical visits were counted as PrEP visits if the following criteria were met: 1) A prescription was ordered for TDF/FTC as “Truvada PrEP” or “Truvada” 2) diagnosis codes at the time of prescription did not include International Classification of Diseases, Ninth Revision (ICD-9) codes for HIV disease (V08, 042), 3) the visit was not recorded as an encounter for post-exposure prophylaxis (PEP). For patients whose first PrEP visit was not billed as PrEP Intake, charts were reviewed to verify start date. Patients were followed through the earliest of HIV diagnosis, loss to follow-up, or administrative censoring at the end of the study period.
There were three outcomes of interest: 1) HIV seroconversion, 2) status on PrEP at the end of the study period, 3) pattern of PrEP use. Seroconversion—change in HIV status from negative to positive—was measured as date of first HIV positive test result, following negative result at PrEP intake appointment. HIV incidence rates to compare between current and former PrEP clients were calculated by dividing the number of cases of HIV in each group by person-time in the group. Person-time on PrEP for current PrEP clients was calculated from the date of first PrEP prescription through 21 days after the last day of the most recent prescription, regardless of gaps. The 21-day grace period was chosen as a conservative estimate of the number of days a patient could maintain a protective dose of four pills per week with a thirty-day prescription.(24, 25) Discontinued person-time for former PrEP clients was counted from 22 days after the end of most recent prescription. PrEP status at the end of the study was assessed as active, discontinued, or lost to follow-up as defined below.
Active:
Patient had a current prescription for TDF/FTC at the Center through April 30, 2018 (with grace period, April 9, 2018).
Discontinued:
Patient did not have a prescription for TDF/FTC at the Center that was current as of April 9, 2018, but had at least one medical visit after the last day of most recent TDF/FTC prescription.
Lost to Follow-up:
Patient did not have a prescription for TDF/FTC at the Center on April 9, 2018, and has no medical visit after the last day of most recent TDF/FTC prescription.
A patient’s pattern of PrEP use was classified as “continuous” if they never had more than a 21-day gap between the last day of the previous prescription and the next appointment. Patients had an “episodic” pattern if they ever had gap(s) of 22 days or more between last pill and next appointment.
Statistical Methods
Risk of seroconversion, excluding those who tested positive on the day of first PrEP appointment, by status (active versus discontinued at time of seroconversion) was calculated and compared using a one-tailed Fisher’s exact test due to small number of outcomes. Patients who were lost to follow-up contributed person-time to the time on PrEP through last date of prescription. Differences in PrEP status by baseline characteristics were first assessed using bivariate multinomial regression models (alpha = 0.05). A multivariable multinomial logistic regression model of PrEP status was then specified. Differences in pattern of PrEP access by baseline characteristics were assessed using bivariate logistic regression models (alpha = 0.05). A multivariable logistic regression equation was then specified to model factors associated with episodic (versus continuous) PrEP access. Variables with large p-values (>0.1) in bivariate models were not included in the multivariable models. Because only 50 people were prescribed PrEP in 2014, year of PrEP start was categorized as 2014–2015, 2016, or 2017. An “other genders” category included cisgender women, transgender men, and genderqueer people. For the multivariable models, reference groups were those with the greatest proportion active, except when category was less than 10% of sample, in which case the reference group was the category with greatest proportion active that included at least 10% of the sample. All analyses were performed using SAS 9.4 (Cary, N.C.) and Stata 12 (College Station, T.X.).
To validate our approach to extracting data from the EMR, we conducted a quality check of 60 charts (comprising 241 visits). Using the EMR as the gold standard, we measured how many visits appeared in the dataset but did not correspond to patient encounters in the EMR (12 visits, 5%) and how many patient encounters appeared in the EMR but not the dataset (1 visit, 0.4%). Visits appeared in the dataset but not the EMR if the provider signed-off a chart during an administrative task. Visits were missing from the dataset if the provider did not sign off a chart for a patient encounter. None of the errors misclassified PrEP status or PrEP pattern. However, errors from visits added due to administrative task artificially lengthened person-time on PrEP by an average of 73 days. We conducted a sensitivity analysis to assess how the visits generated due to administrative tasks rather than patient encounters would potentially affect the HIV incidence rates.
RESULTS
Overall, 3,121 unique patients initiated PrEP at the Center during the study period and contributed 3,246 person-years of follow-up. Though the sample primarily consisted of gay cisgender men, PrEP patients were diverse in terms of age and race/ethnicity. Thirty percent had Medi-Cal or Medicare insurance at baseline and 22% received PrEP through one of two government programs (Los Angeles County Division of HIV and STD Prevention program, or California Office of AIDS grant). Thirty-eight percent used private insurance. At baseline, 10% were homeless or unstably housed (Table 1).
Table 1.
Characteristics of clients receiving PrEP at the Los Angeles LGBT Center, n=3,121 starting between January 1, 2014 and December 31, 2017
| Total | ||
|---|---|---|
| n | %g | |
| No. individuals | 3,121 | 100% |
| Person-years (mean/patient, range) | 3,246 (1.0, 0.0–4.0) | |
| Mean time to first discontinuation, person-years (SD, range) | 0.5 (0.5, 0.0–3.2) | |
| Status at end of follow-up | ||
| Current PrEP prescription | 1,314 | 42% |
| Discontinued PrEPa | 762 | 24% |
| Lost to follow-upb | 1,045 | 33% |
| Pattern of PrEP use | ||
| Continuous | 1,692 | 54% |
| Episodic | 1,429 | 46% |
| HIV rate (cases/100 person-years) | ||
| Current PrEP prescription at time of diagnosis | 0.1 (3/2,624) | |
| Discontinued PrEP before time of diagnosis | 2.1 (10/478) | |
| Days between last PrEP pill and HIV diagnosis, mean (SD, range) | 150 (130, 0–294) | |
| Age, mean (SD, range) | 33.3 (8.9, 18.1–75.8) | |
| Age | ||
| 18–24 | 445 | 14% |
| 25–30 | 1,046 | 34% |
| 31–40 | 1,063 | 34% |
| 41–50 | 375 | 12% |
| 51–76 | 192 | 6% |
| Gender | ||
| Cisgender man | 2,941 | 94% |
| Transgender woman | 111 | 4% |
| Cisgender woman | 26 | 1% |
| Genderqueer person | 23 | 1% |
| Transgender man | 18 | 1% |
| Sexual Orientation | ||
| Gay | 2,566 | 82% |
| Bisexual | 323 | 10% |
| Other | 136 | 4% |
| Unknown | 96 | 3% |
| Race/Ethnicity | ||
| Asian/Pacific Islander | 253 | 8% |
| Black or African American | 242 | 8% |
| Hispanic/Latino | 954 | 31% |
| Other | 219 | 7% |
| White | 1,353 | 43% |
| Unknown | 100 | 3% |
| Type of insurance at baseline | ||
| Medicaid | 931 | 30% |
| Government PrEP program | 677 | 22% |
| Private | 1,181 | 38% |
| None/out of pocket | 329 | 11% |
| Unknown | 3 | 0% |
| Baseline housing status | ||
| Homeless | 325 | 10% |
| Not homeless | 2,785 | 89% |
| Unknown | 11 | 0.4% |
| Year started PrEP | ||
| 2014 | 50 | 2% |
| 2015 | 411 | 13% |
| 2016 | 1,148 | 37% |
| 2017 | 1,512 | 48% |
No current PrEP Rx at the end of the study period, but returned to the clinic after most recent PrEP Rx ended
No current PrEP Rx at the end of the study period, and did not return to the clinic after most recent PrEP Rx ended
At the end of the analysis period, 42% (n = 1,314) of patients who started PrEP were active – that is, had a current PrEP prescription. Twenty-four percent (n=762) had discontinued receiving PrEP, and 33% (n=1,045) were lost to follow-up. Just over half of patients were on PrEP continuously (no gaps in prescription coverage between PrEP appointments), while 46% (n=1,429) had one or more gaps of at least 21 days between prescriptions. Average time to first discontinuation was six months (SD: six months, range: 0–38.4).
HIV Incidence
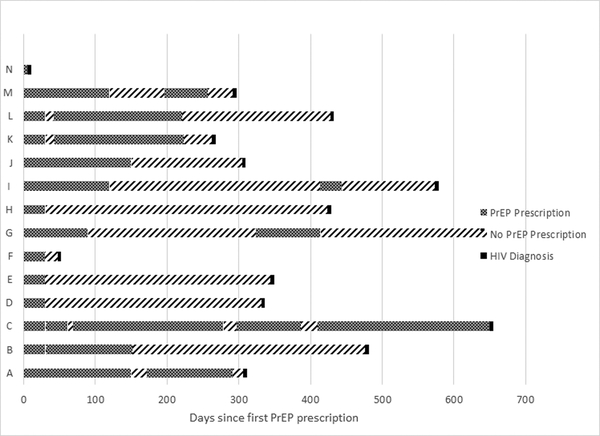
Fourteen patients who had a PrEP intake appointment were diagnosed with HIV during or after their first PrEP visit (Figure 1). One tested HIV positive at their first PrEP appointment and were linked to HIV care (Client N). Ten had discontinued PrEP before time of diagnosis, and three were active PrEP clients at time of HIV diagnosis. Incidence in the discontinued group was significantly higher at 2.1 cases/100 person-years, compared to 0.1 cases/100 person-years in the active group (incidence rate ratio = 21, 95% CI: 5.8, 76.3), one-tailed Fisher’s exact test = 37, p<0.001. A 5% error rate of an average of 73 days in person-time on PrEP across the sample would lead to HIV rates of 1.7 cases/100 person-years in the discontinued group versus 0.1 cases/100 person-years in the active group (one-tailed Fisher’s exact test = 27, p<0.001). The findings were thus robust to the level of errors found in the data audit.
Figure 1.
PrEP Prescription Coverage before HIV Diagnosis
Excluding the client who tested positive at first visit, median time between last day of PrEP prescription and date of HIV diagnosis was 153 days (mean = 150, SD=130, range: 0–294). One patient tested positive for acute HIV infection at the first PrEP follow-up appointment, 48 days after PrEP was first prescribed (Client F). This timeline was consistent with infection prior to starting PrEP. Another patient tested positive for acute HIV infection at 14 days after most recent PrEP prescription ended (Client A). Notes in the EMR indicated the patient had reported missing seven or more doses in a row. A third patient (Client C) tested positive while they had an active Truvada prescription. Based on the provider notes, it appeared that Client C had an acute infection that occurred during a period where the client did not have PrEP, and due to irregularities in HIV testing procedures, the client was not diagnosed until months later.
Current PrEP Status
In bivariate models, PrEP status differed significantly by gender, age group, sexual orientation, type of insurance, baseline housing status, and year of PrEP start but not by race/ethnicity (Table 2). Age group, insurance type, baseline housing status, and year of PrEP start remained significantly associated with PrEP status in the multivariable model. Compared to those aged 41–50, risk of discontinuation was significantly increased among younger people, including those aged 18–24 (ARR = 2.8, CI: 1.9, 4.0) and 25–30 (ARR = 1.6, 95% CI: 1.2, 2.2). Risk of loss to follow up was similarly increased among patients aged 18–24 (ARR = 1.8, 95% CI: 1.3, 2.6) and 25–30 (ARR = 1.8, 95% CI: 1.0, 1.7). Compared to those accessing PrEP through Medi-Cal or another government program, individuals with private insurance had higher risk of discontinuation (ARR = 1.4, 95% CI: 1.2, 1.7) or loss to follow-up (ARR = 1.7, 95% CI: 1.4, 2.0), as did those with no insurance at baseline (discontinuation ARR = 4.5, 95% CI: 3.2, 6.4, loss to follow-up ARR = 3.4, 95% CI: 2.4, 4.8). Patients who were homeless or unstably housed at baseline had higher risk of discontinuation compared to those who had stable housing at baseline (ARR = 2.1, 95% CI: 1.5, 2.8). Those who started PrEP in 2016 and earlier had higher risk of discontinuation compared to those who started in 2017.
Table 2.
Unadjusted and adjusted risk ratios of PrEP discontinuation and loss to follow up among clients receiving PrEP at the Center, n=3,121 starting between January 1, 2014 and December 31, 2017
| Discontinued | Lost to Follow Up | |||||||
|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | aRR* | 95% CI | RR | 95% CI | aRR* | 95% CI | |
| Age | ||||||||
| 18–24 | 2.4 | (1.7, 3.4) | 2.8 | (1.9, 4.0) | 1.7 | (1.3, 2.4) | 1.8 | (1.3, 2.6) |
| 25–30 | 1.5 | (1.1, 2.0) | 1.6 | (1.2, 2.2) | 1.2 | (0.9, 1.6) | 1.3 | (1.0, 1.7) |
| 31–40 | 1.3 | (0.9, 1.7) | 1.3 | (1.0, 1.9) | 1.1 | (0.8, 1.4) | 1.1 | (0.8, 1.4) |
| 41–50 (ref) | 1.0 | -- | 1.0 | -- | 1.0 | -- | 1.0 | -- |
| 51–76 | 1.4 | (0.9, 2.1) | 1.4 | (0.9, 2.2) | 0.8 | (0.5, 1.2) | 0.8 | (0.5, 1.2) |
| Gender | ||||||||
| Cis man (ref) | 1.0 | -- | 1.0 | -- | 1.0 | -- | 1.0 | -- |
| Trans woman | 1.5 | (1.0, 2.4) | 0.8 | (0.4, 1.5) | 0.7 | (0.4, 1.1) | 0.6 | (0.3, 1.1) |
| Other gender | 1.3 | (0.7, 2.5) | 1 | (0.5, 2.1) | 1.2 | (0.7, 2.5) | 1.4 | (0.7, 2.6) |
| Race/ethnicity | ||||||||
| White (ref) | 1.0 | -- | -- | -- | 1.0 | -- | -- | -- |
| Asian/PI | 0.7 | (0.5, 1.0) | -- | -- | 1.0 | (0.7, 1.3) | -- | -- |
| Black | 1.0 | (0.7, 1.4) | -- | -- | 0.9 | (0.6, 1.2) | -- | -- |
| Hispanic/Latino | 1.0 | (0.8, 1.2) | -- | -- | 0.9 | (0.7, 1.0) | -- | -- |
| Other | 0.8 | (0.6, 1.2) | -- | -- | 1.1 | (0.8, 1.5) | -- | -- |
| Sexual orientation | ||||||||
| Gay (ref) | 1.0 | -- | 1.0 | 1.0 | -- | 1.0 | ||
| Bisexual | 1.0 | (0.8, 1.4) | 1.0 | (0.7, 1.3) | 0.9 | (0.7, 1.2) | 0.8 | (0.6, 1.1) |
| Other | 1.5 | (1.0, 2.3) | 1.5 | (0.9, 2.5) | 0.7 | (0.4, 1.0) | 0.7 | (0.4, 1.3) |
| Baseline insurance | ||||||||
| Medi-Cal, or other government PrEP program (ref) | 1.0 | -- | 1.0 | -- | 1.0 | -- | 1.0 | -- |
| Private | 1.4 | (1.2, 1.7) | 1.4 | (1.2, 1.7) | 1.7 | (1.4, 2.0) | 1.7 | (1.4, 2.0) |
| None | 5.5 | (4.0, 7.7) | 4.5 | (3.2, 6.4) | 3.4 | (2.5, 4.7) | 3.4 | (2.4, 4.8) |
| Baseline housing status | ||||||||
| Not homeless (ref) | 1.0 | -- | 1.0 | -- | 1.0 | -- | 1.0 | -- |
| Homeless | 2.1 | (1.6, 2.7) | 2.1 | (1.5, 2.8) | 1.2 | (0.9, 1.6) | 1.4 | (1.0, 1.9) |
| PrEP start year | ||||||||
| 2014–2015 | 2.7 | (2.1, 3.5) | 2.6 | (2.0, 3.5) | 1.1 | (0.6, 1.4) | 1.1 | (0.6, 1.4) |
| 2016 | 2.4 | (2.0, 2.9) | 2.1 | (1.7, 2.6) | 1.5 | (1.3, 1.8) | 1.4 | (1.1, 1.7) |
166 results were omitted due to missing values; effective sample size was 2,955
Among the 1,314 patients who had a current PrEP prescription at the end of the study period, 37% had been on PrEP continuously since their first prescription, while 63% had at least one gap of 22 days or more between PrEP prescriptions. Demographic and health services correlates of episodic PrEP prescriptions included earlier start, not having insurance at baseline, and Hispanic or Black race/ethnicity (Table 3). Overall, active PrEP patients with episodic PrEP prescriptions had an average of one gap (of at least 22 days) per person-year at risk, and these gaps were a median length of 65 days (interquartile range 37 – 129) between the end of one prescription and the start of the next.
Table 3.
Predictors of episodic PrEP coverage among active PrEP patients at the Los Angeles LGBT Center, n=1,314
| Risk ratio of episodic use (vs. continuous) | ||||
|---|---|---|---|---|
| Unadjusted RR | 95% CI | Adjusted RR* | 95% CI | |
| Age | ||||
| 18–24 | 1.4 | (0.9, 2.2) | -- | -- |
| 25–30 | 1.1 | (0.8, 1.6) | -- | -- |
| 31–40 | 1.1 | (0.8, 1.5) | -- | -- |
| 41–50 (ref) | 1.0 | -- | -- | -- |
| 51–76 | 1.2 | (0.7, 2.1) | -- | -- |
| Gender | ||||
| Cis man (ref) | 1.0 | -- | -- | -- |
| Trans woman | 1.5 | (0.8, 2.9) | -- | -- |
| Other gender | 1.3 | (0.5, 3.0) | -- | -- |
| Race/ethnicity | ||||
| White (ref) | 1.0 | -- | 1.0 | -- |
| Asian/PI | 0.7 | (0.5, 1.0) | 0.8 | (0.5, 1.3) |
| Black | 1.6 | (1.0, 2.6) | 1.6 | (1.0, 2.7) |
| Hispanic/Latino | 1.3 | (1.0, 1.7) | 1.4 | (1.1, 1.9) |
| Other | 1.2 | (0.8, 1.9) | 1.3 | (0.8, 2.2) |
| Sexual orientation | ||||
| Gay (ref) | 1.0 | -- | -- | -- |
| Bisexual | 1.4 | (0.9, 2.0) | -- | -- |
| Other | 1.1 | (0.6, 1.9) | -- | -- |
| Baseline insurance | ||||
| Medi-Cal, or other government PrEP program (ref) | 1.0 | -- | 1.0 | -- |
| Private | 1.0 | (0.8, 1.2) | 0.9 | (0.7, 1.2) |
| None | 2.4 | (1.3, 4.7) | 2.0 | (1.0, 4.0) |
| Baseline housing status | ||||
| Not homeless (ref) | 1.0 | -- | 1.0 | -- |
| Homeless | 1.5 | (0.9, 2.2) | 1.3 | (0.8, 2.1) |
| PrEP start year | ||||
| 2014–2015 | 8.2 | (5.0, 13.4) | 8.2 | (5.0, 13.5) |
| 2016 | 4.4 | (3.3, 5.8) | 4.3 | (3.2, 5.8) |
36 observations were removed due to missing values; effective sample size was 1,278
DISCUSSION
This longitudinal study of PrEP delivery at a community clinic with a broad age range and ethnically diverse population provides important data about PrEP delivery outside of clinical trial contexts. The substantially higher HIV incidence rate in patients who discontinued PrEP (2.1 cases/person-year), compared to those who had a current PrEP prescription (0.1 case/person-year), adds evidence to PrEP’s effectiveness in community settings. By comparison, between 2014 and 2017, the annual incidence of HIV among all patients testing at the Center declined from 2.8% to 1.3%. The differential risk of seroconversion by PrEP status (active vs. discontinued) highlights the role that efforts to improve retention in PrEP programs can play in reducing HIV transmission.
That patients with access to PrEP at a lower cost (in this case through government programs) have better retention is consistent with qualitative findings that cite lack of health insurance and cost of medication as barriers to PrEP initiation and continuation.(14) This finding supports continued or increased allocation of resources to programs that provide consistent, low cost PrEP services, which are also an opportunity for linkage to primary care and other health services.(26)
Increased risk of discontinuation among those aged 18–24 is concerning given the elevated HIV incidence and reduced PrEP uptake observed in this age group.(4, 27) Coupled with declining levels of PrEP adherence observed in the Adolescent Trials Network (ATN) 110 cohort (which enrolled 18–22 year-olds), this reinforces the need for targeted strategies to meet the needs of young PrEP users. In the ATN 110 cohort, participants’ main reasons for non-adherence included forgetting to take the pills (29%), being away from home (27%), or being too busy to take the pills (27%).(27) Less common reasons included avoiding side effects (4.5%), not wanting others to see them taking the pills (2.5%), or belief that the pill was harmful (2%).(27) These factors may influence attendance at PrEP appointments along with medication adherence, and strategies such as discreet reminders between appointments, continued education on how PrEP works, and flexible scheduling may simultaneously address adherence and retention challenges. Future studies should examine these and other potential barriers for younger PrEP users. Additionally, the association between unstable housing at baseline and increased PrEP discontinuation emphasizes the role that integrated social services can play in improving PrEP’s effectiveness in community settings.
The finding that most active PrEP users had episodic prescription coverage demonstrates that measuring time to first discontinuation, or measuring retention at three or six months, may not precisely capture the ways people use PrEP. Moreover, quarterly monitoring visits may be a barrier to sustained PrEP use.(16) Solutions that allow patients to complete lab work without having an appointment with a clinician – such as fast track lab-only visits, or mail-in STI/HIV testing – could reduce the burden of PrEP follow-up.
This study adds to the understanding of PrEP in community settings by linking aspects of PrEP retention examined in prior studies. Previously, a large analysis at an LGBT-serving FQHC in Chicago described episodic patterns of visit attendance but not HIV incidence among PrEP clients.(16) Studies from a variety of community-clinic settings in the United States have reported HIV incidence among current or former PrEP clients with limited context on clients’ full histories with PrEP prior to seroconversion.(13–15, 17) To our knowledge, our study is the first to describe prescription coverage preceding seroconversion in a community-based sample. The findings about episodic PrEP coverage were similar to those observed among research participants in Atlanta, though the HIV incidence was lower than in the Atlanta cohort.(11)
That three seroconversions among active PrEP clients occurred in patients with a history of episodic use reinforces the need for adherence support and for long-acting PrEP modalities that reduce the need for follow-up visits and prescription refills. Such steps may be particularly helpful to ensure consistent PrEP coverage in communities most affected by HIV. Though studies at this clinic and in other populations have observed racial/ethnic disparities in PrEP uptake, we did not find an association between race/ethnicity and PrEP discontinuation or loss to follow-up. However, we did observe a slightly increased risk of episodic PrEP coverage among Black and Hispanic/Latino patients. To address challenges in reducing HIV incidence in communities of color, providers need to focus on both motivating clients’ initial uptake and also assuring consistent access to PrEP services and medication following that uptake.
Strengths
The study had several key strengths, including longitudinal design, substantial sample size in a community-based sample, and ability to characterize patterns in PrEP use over time. A longitudinal design made it possible to assess risk of HIV infection, risk of discontinuation, and risk of loss to follow-up. The use of clinical care data from a sample of patients receiving PrEP in a primary care and sexual health setting may render findings more generalizable to other clinical settings where patients start PrEP outside of research studies. Using medical records data addressed generalizability problems inherent in clinical research where selected participants may not be representative of the broader patient population.
Limitations
Use of the EMR data and a community-based sample brought strengths, but also introduced some limitations. Though our findings were robust to the level of error identified in the data audit, the finding that 5% of visits in the dataset may have represented not patient encounters but administrative chart updates highlights the challenges of doing research with EMR data. Though using prescription data allowed us to estimate gaps in PrEP coverage, some time periods may be misclassified because it was not possible to assess when or whether patients picked up the prescriptions. Without data on dosing pattern (e.g., event-driven use), we also could not precisely estimate how long an individual might stretch a PrEP prescription. Because data on PrEP adherence was not available, we could not precisely estimate the degree to which returning for PrEP appointments reflected ongoing PrEP use.
HIV incidence in the discontinued group may be underestimated if some patients who discontinued PrEP did not later return to the Center for HIV testing. It was not possible to assess HIV incidence in the lost to follow-up group. Additionally, it was not possible to determine the specific reasons people stopped coming to the Center for PrEP. Our clinical data did not contain information on changed HIV risk or “seasons of risk” that could lead to PrEP discontinuation – for example, a monogamous relationship with an HIV-negative partner, a period of sexual abstinence, or increased condom use. Furthermore, we could not distinguish between those who stopped using PrEP entirely and those who changed to a different provider. Because continuous attendance at PrEP appointments depended on insurance status, we expect that some of those with private insurance who stopped getting PrEP at the Center may have found a cheaper or more convenient way to obtain PrEP through new insurance or a different provider. Based on this study’s initial findings, the Center undertook a quality-improvement project to survey former PrEP clients about why they had stopped and invite them to re-engage in PrEP services. The Center also hired dedicated PrEP-retention linkage to care staff. Future work evaluating whether these efforts and parallel efforts at other clinics improve PrEP retention will provide valuable information about how to address the discontinuation issues identified in this and other community-based studies. (16, 17)
Conclusions
Patterns of episodic PrEP prescription coverage preceding seroconversion underscore the need to reduce barriers to continued access to PrEP medication. Findings from this study indicate a relationship between robust insurance coverage for PrEP and long-term PrEP use. Increased risk of discontinuation among younger people suggests a need for continued efforts to provide biomedical HIV prevention services for youth. Increasing access to free or low-cost PrEP, reducing frequency of required monitoring visits to avoid gaps in prescriptions, developing long-acting PrEP modalities, and providing social services may contribute to making PrEP effective in community settings.
ACKNOWLEDGMENTS
The authors wish to thank the PrEP navigation team, counselors, and clinicians at the Los Angeles LGBT Center. CLS was supported by the National Institute on Drug Abuse of the National Institutes of Health under award number T32DA035165. SS was supported by the National Institute of Mental Health under award number P30 MH058107. MJ was supported by the National Institutes of Health and National Institute of Allergy and Infectious Disease, grant number K01AI091861. MRB was supported by the UCLA Postdoctoral Fellowship Training Program in Global HIV Prevention Research (Currier and Gorbach, PIs); T32MH080634. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. There are no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
ETHICS
The study was approved by the Institutional Review Board at the University of California, Los Angeles (IRB#17–000717).
Contributor Information
Chelsea L. Shover, Department of Epidemiology, University of California, Los Angeles; Los Angeles, California, USA; Department of Health and Mental Health Services, Los Angeles LGBT Center; Los Angeles, California, USA.
Steven Shoptaw, Departments of Family Medicine and Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA.
Marjan Javanbakht, Department of Epidemiology, Fielding School of Public Health, University of California Los Angeles, Los Angeles, CA.
Sung-Jae Lee, Department of Epidemiology, Fielding School of Public Health, University of California Los Angeles, Los Angeles, CA; Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA.
Robert K. Bolan, Department of Health and Mental Health Services, Los Angeles LGBT Center; Los Angeles, California, USA.
Nicole J. Cunningham, Department of Health and Mental Health Services, Los Angeles LGBT Center; Los Angeles, California, USA.
Matthew R. Beymer, Department of Health and Mental Health Services, Los Angeles LGBT Center; Los Angeles, California, USA; Division of Infectious Diseases, Department of Medicine, University of California, Los Angeles; Los Angeles, California, USA.
Michelle A. DeVost, Department of Health and Mental Health Services, Los Angeles LGBT Center; Los Angeles, California, USA.
Pamina M. Gorbach, Department of Epidemiology, University of California, Los Angeles; Los Angeles, California, USA; Division of Infectious Diseases, Department of Medicine, University of California, Los Angeles; Los Angeles, California, USA.
REFERENCES
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. New Engl J Med. 2010;363(27):2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoots BE, Finlayson T, Nerlander L, Paz-Bailey G, National HIVBSSG. Willingness to Take, Use of, and Indications for Pre-exposure Prophylaxis Among Men Who Have Sex With Men-20 US Cities, 2014. Clin Infect Dis. 2016;63(5):672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ocfemia MCB, Dunville R, Zhang TC, Barrios LC, Oster AM. HIV Diagnoses Among Persons Aged 13–29 Years - United States, 2010–2014. Mmwr-Morbidity and Mortality Weekly Report. 2018;67(7):212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. HIV Surveillance Report, 2016. 2017;28. [Google Scholar]
- 5.Jenness SM, Goodreau SM, Rosenberg E, Beylerian EN, Hoover KW, Smith DK, et al. Impact of the Centers for Disease Control’s HIV Preexposure Prophylaxis Guidelines for Men Who Have Sex With Men in the United States. J Infect Dis. 2016;214(12):1800–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodreau SM, Hamilton DT, Jenness SM, Sullivan PS, Valencia RK, Wang LY, et al. Targeting Human Immunodeficiency Virus Pre-Exposure Prophylaxis to Adolescent Sexual Minority Males in Higher Prevalence Areas of the United States: A Modeling Study. J Adolesc Health. 2018;62(3):311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley CF, Kahle E, Siegler A, Sanchez T, del Rio C, Sullivan PS, et al. Applying a PrEP Continuum of Care for Men Who Have Sex With Men in Atlanta, Georgia. Clinical Infectious Diseases. 2015;61(10):1590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu ACG, Cohen S, Bacon O, Kolber M, Amico KR, Mugavero M, Grant R, Buchbinder S editors. , editor The spectrum of engagement in HIV prevention: proposal for a PrEP cascade. 7th International conference on HIV treatment and prevention adherence; 2012; Florida: Miami Beach. [Google Scholar]
- 9.Parsons JT, Rendina HJ, Lassiter JM, Whitfield TH, Starks TJ, Grov C. Uptake of HIV Pre-Exposure Prophylaxis (PrEP) in a National Cohort of Gay and Bisexual Men in the United States. J Acquir Immune Defic Syndr. 2017;74(3):285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nunn AS, Brinkley-Rubinstein L, Oldenburg CE, Mayer KH, Mimiaga M, Patel R, et al. Defining the HIV pre-exposure prophylaxis care continuum. AIDS. 2017;31(5):731–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serota DP, Rosenberg ES, Lockard AM, Rolle CM, Luisi N, Cutro S, et al. Beyond the Biomedical: Preexposure Prophylaxis Failures in a Cohort of Young Black Men Who Have Sex With Men in Atlanta, Georgia. Clin Infect Dis. 2018;67(6):965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan PA, Mena L, Patel R, Oldenburg CE, Beauchamps L, Perez-Brumer AG, et al. Retention in care outcomes for HIV pre-exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int Aids Soc. 2016;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcus JL, Hurley LB, Hare CB, Nguyen DP, Phengrasamy T, Silverberg MJ, et al. Preexposure Prophylaxis for HIV Prevention in a Large Integrated Health Care System: Adherence, Renal Safety, and Discontinuation. Jaids-J Acq Imm Def. 2016;73(5):540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold T, Brinkley-Rubinstein L, Chan PA, Perez-Brumer A, Bologna ES, Beauchamps L, et al. Social, structural, behavioral and clinical factors influencing retention in Pre-Exposure Prophylaxis (PrEP) care in Mississippi. Plos One. 2017;12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackstock OJ, Patel VV, Felsen U, Park C, Jain S. Pre-exposure prophylaxis prescribing and retention in care among heterosexual women at a community-based comprehensive sexual health clinic. Aids Care. 2017;29(7):866–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rusie LK, Orengo C, Burrell D, Ramachandran A, Houlberg M, Keglovitz K, et al. Preexposure Prophylaxis Initiation and Retention in Care Over 5 Years, 2012–2017: Are Quarterly Visits Too Much? Clin Infect Dis. 2018;67(2):283–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hojilla JC, Vlahov D, Crouch PC, Dawson-Rose C, Freeborn K, Carrico A. HIV Pre-exposure Prophylaxis (PrEP) Uptake and Retention Among Men Who Have Sex with Men in a Community-Based Sexual Health Clinic. Aids Behav. 2018;22(4):1096–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haberer JE, Bangsberg DR, Baeten JM, Curran K, Koechlin F, Amico KR, et al. Defining success with HIV pre-exposure prophylaxis: a prevention-effective adherence paradigm. Aids. 2015;29(11):1277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pines HA, Gorbach PM, Weiss RE, Shoptaw S, Landovitz RJ, Javanbakht M, et al. Sexual Risk Trajectories Among MSM in the United States: Implications for Pre-exposure Prophylaxis Delivery. Jaids-J Acq Imm Def. 2014;65(5):579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shover CL, Javanbakht M, Shoptaw S, Bolan RK, Lee S-J, Parsons JT, et al. HIV Preexposure Prophylaxis Initiation at a Large Community Clinic: Differences Between Eligibility, Awareness, and Uptake. American Journal of Public Health.0(0):e1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harawa NM S; Leibowitz A; Pulsipher C; Holloway I. Examining PrEP Uptake among Medi-Cal Beneficiaries in California: Differences by Age, Gender, Race/Ethnicity and Geographic Region. California HIV/AIDS Policy Research Centers; 2018. [Google Scholar]
- 22.Shover CL, DeVost MA, Beymer MR, Gorbach PM, Flynn RP, Bolan RK. Using Sexual Orientation and Gender Identity to Monitor Disparities in HIV, Sexually Transmitted Infections, and Viral Hepatitis. American Journal of Public Health. 2018;108(S4):S277–S83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King A, Pulsipher CA, Holloway IW. PrEP Cost Analysis for Covered California Health Plans. alifornia HIV/AIDS Policy Research Centers; 2016. [Google Scholar]
- 24.Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med. 2015;373(23):2237–46. [DOI] [PubMed] [Google Scholar]
- 25.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-Tenofovir Concentrations and Pre-Exposure Prophylaxis Efficacy in Men Who Have Sex with Men. Sci Transl Med. 2012;4(151). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcus JL, Levine K, Grasso C, Krakower DS, Powell V, Bernstein KT, et al. HIV Preexposure Prophylaxis as a Gateway to Primary Care. Am J Public Health. 2018;108(10):1418–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosek SG, Rudy B, Landovitz R, Kapogiannis B, Siberry G, Rutledge B, et al. An HIV Preexposure Prophylaxis Demonstration Project and Safety Study for Young MSM. Jaids-J Acq Imm Def. 2017;74(1):21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]