Abstract
Ocular hypertension due to impaired aqueous humor (AH) drainage through the trabecular meshwork (TM) is a major risk factor for glaucoma, a leading cause of irreversible blindness. However, the etiology of ocular hypertension remains unclear. Although autotaxin, a secreted lysophospholipase D and its catalytic product lysophosphatidic acid (LPA) have been shown to modulate AH drainage through TM, we do not have a complete understanding of their role and regulation in glaucoma patients, TM and AH outflow. This study reports a significant increase in the levels of autotaxin, lysophosphatidylcholine (LPC), LPA and connective tissue growth factor (CTGF) in the AH of Caucasian and African American open angle glaucoma patients relative to age-matched non-glaucoma patients. Treatment of human TM cells with dexamethasone, tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) increased the levels of autotaxin protein, a response that was mitigated by inhibitors of glucocorticoid receptor, NF-kB and SMAD3. Dexamethasone, TNF-α, IL-1β and LPC treatment of TM cells also led to an increase in the levels of CTGF, fibronectin and collagen type 1 in an autotaxin dependent manner. Additionally, in perfused enucleated mouse eyes, autotaxin and LPC were noted to decrease, while inhibition of autotaxin was increased aqueous outflow through the TM. Taken together, these results provide additional evidence for dysregulation of the autotaxin-LPA axis in the AH of glaucoma patients, reveal molecular insights into the regulation of autotaxin expression in TM cells and the consequences of autotaxin inhibitors in suppressing the fibrogenic response and resistance to AH outflow through the TM.
Keywords: Autotaxin, glaucoma, trabecular meshwork, intraocular pressure, aqueous humor
1. INTRODUCTION
Primary open-angle glaucoma, considered one of the leading causes of blindness globally, is associated with elevated intraocular pressure (IOP) [1]. While elevation of IOP in glaucoma patients is known to result from impairment of aqueous humor (AH) drainage through the trabecular pathway, we lack a thorough understanding of the molecular mechanisms regulating AH outflow via the trabecular pathway in both normal and glaucomatous eyes [1–4]. This knowledge is fundamental to the development of targeted and efficacious therapies for lowering IOP in glaucoma patients.
Alterations in the levels of various external factors (e.g. growth factors, hormones, cytokines, corticosteroids and extracellular matrix) and dysregulation of intracellular signaling mechanisms including integrin-dependent, Rho/Rho kinase, SMAD, cellular contractility, cell survival, Yap/Taz, and stress signaling pathways and fibrogenic activity have all been shown to impair AH outflow through the trabecular meshwork (TM) and result in increased IOP [3–6]. Previous studies from our laboratory and others have demonstrated that lipid growth factors including lysophosphatidic acid (LPA) and sphingosine 1-phosphate modulate AH outflow through the trabecular pathway (consisting of trabecular meshwork, juxtacanalicular tissue and Schlemm’s canal) by activating Rho/Rho kinase signaling and contractile activity [6–8]. Although, LPA which is produced primarily extracellularly by autotaxin (ATX), also known as ectonucleotide pyrophosphatase/phosphodiesterase 2, [9, 10] has been shown to decrease AH outflow through the trabecular pathway, little is known about how it is produced in the AH outflow pathway and the molecular and cellular basis for increased resistance to AH outflow exerted by LPA. ATX is a secretory lysophospholipase D (LysoPLD) which catalyzes the production of LPA from lysophosphatidylcholine (LPC) [9, 10]. LPA is capable of interacting with six different G-protein coupled cell surface receptors (LPAR 1–6) to activate intracellular signaling pathways and regulates biological and pathophysiological processes [10–13]. Importantly, dysregulated activation of the ATX-LPA signaling pathway is recognized to be involved in the pathobiology of chronic inflammation, metastatic cancer, fibrosis and other diseases. The ATX-LPA pathway is therefore considered to be a promising therapeutic target for the treatment of various diseases with several ongoing clinical and preclinical studies currently in progress [10, 13–16].
In support of the possible role of the ATX-LPA signaling pathway in the etiology of glaucoma and aqueous humor outflow, our recent studies uncovered ATX as a common AH constituent in human and other species, and demonstrated not only that ATX activity is elevated in the AH of primary open-angle glaucoma (POAG) patients, but also that the pharmacological inhibition of ATX activity lowered IOP in rabbits [17]. Further support for the dysregulation of ATX levels in the AH of glaucoma patients and animal models comes from independent studies performed by other laboratories [18, 19]. Very little is currently known however, regarding the regulation of ATX expression in TM cells and levels of ATX in the AH of glaucoma patients, or the role of ATX in AH outflow/drainage and TM cell physiology [17, 20, 21]. To gain more insights into the role of ATX-LPA signaling pathways in the TM and the etiology of POAG, in this study, we determined the levels of ATX, LPC and LPA in the AH of POAG patients and non-glaucoma controls (cataract patients), investigated the regulation of ATX expression and its role in the fibrogenic response of human TM cells, and evaluated the consequences of increased ATX-LPA activity in AH outflow in enucleated mouse eyes. This study provides conclusive evidence for elevated levels of ATX, LPA and LPC in North American POAG patients (in both Caucasians and African Americans), and offers novel molecular insights into the regulation of ATX expression in TM cells and its role in the fibrotic response and AH outflow.
2. METHODS
2.1. Human study approval
Research involving collection of human AH samples has been approved by the Duke University School of Medicine’s institutional review board (IRB) in compliance with HIPAA (Health Insurance Portability and Accountability Act) guidelines and the tenets of the Declaration of Helsinki. Written informed consent was obtained from patients prior to collection of AH.
2.2. Aqueous humor collection from human patients
Preoperative human AH samples from cataract and primary open angle glaucoma patients were obtained at the initiation of cataract surgery or surgery for glaucoma at the Duke Eye Center. Patients with diabetic retinopathy, uveitis and prior glaucoma surgery were excluded. A cannula attached to a tuberculin syringe with a 30-gauge needle was inserted into the anterior chamber through a limbal paracentesis tract at the start of the surgery and approximately 30–100 μl of AH was slowly aspirated. The AH samples were transferred from the syringe to a 1.5 ml Eppendorf tube, and centrifuged at 1000×g for 10 min at 4°C. The supernatant obtained from AH samples was collected and stored at −80°C until further use.
2.3. Recording of glaucoma human patients IOP, corneal thickness, cup-to-disk ratio of optic nerve head and nerve fiber layer thickness of retina
Intraocular pressure, corneal thickness and nerve fiber layer thickness of retina in glaucoma patients were recorded using Goldmann Applanation tonometer (Haag-Streit Diagnostics, Essex, UK), Pachmate 2 DGH 55 pachymeter (DGH Technology, Exton, PA) and SPECTRALIS OCT (Heidelberg Engineering Inc, Franklin, MA), respectively. Whereas the Cup-to-disk ratio of optic nerve head was recorded by the operating surgeon.
2.4. Animals
All ex-vivo animal experiments described in this study were conducted in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and Association for Research in Vision and Ophthalmology. The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Duke University School of Medicine. Seven to nine month old C57BL/6J mice (male and female) were purchased from the Jackson Laboratory and housed in clear cages, fed ad libitum and kept in housing rooms at 21°C with a 12 h: 12 h light-dark cycle. Mice were anesthetized using isoflurane, followed by decapitation and eyes were enucleated for use in perfusion studies.
2.5. Aqueous outflow facility measurements
Perfusion studies were performed using an iPerfusion system as described by Sherwood et al. [22]. Briefly, freshly enucleated mouse eyes (contralateral pairs) were mounted to a platform with a small amount of Superglue and fully submerged in a bath of PBS containing D-glucose (5.5 mM) maintained at 35.0 ± 0.5 °C throughout the duration of the experiment. Glass needles mounted on micromanipulators were used to cannulate the anterior chamber of the eye under a microscope. A glass microneedle, filled with LPC (5 μM) alone, mouse recombinant ATX (2 μg/ml, obtained from F. Hoffmann-La Roche Ltd. Basel, Switzerland) plus LPC (5 μM), or the ATX inhibitor ATX-A (1 μM) alone, was inserted into the anterior chamber of the eye using a micromanipulator. The contralateral eye was simultaneously perfused with the respective vehicle under similar conditions. Both eyes were perfused at 8 mmHg for at least 45 min to allow acclimatization (preconditioning) and delivered the experimental agents to the outflow pathway. Following this, aqueous outflow was monitored continuously for nearly 90 min with sequential pressure steps of 5, 6.5, 8, 9.5, 11, 12.5, 14, 15.5, and 17 mmHg. Data were analyzed as described previously [22]. A non-linear flow-pressure model was used to account for the pressure dependence of outflow facility in mice, and the reference facility was analyzed at the reference pressure of 8 mmHg [22].
2.5. Histological analysis
After completion of aqueous outflow facility measurements, perfused mouse eyes were removed from the perfusion system and fixed using 4% paraformaldehyde for 24h at 4°C, followed by 2.5% glutaraldehyde in PBS (pH 7.4) for 24h at 4°C. Fixed eyes were bisected at the equator and the anterior segment of the eye was cut into 4 quadrants for embedding in Epon resin. Semi-thin sections of eye anterior segments (0.5 μm) were stained with methylene blue and visualized using light microscopy. Schlemm’s canal tissue area was calculated using ImageJ software (NIH).
2.7. ATX and CTGF measurements in aqueous humor
Human ENPP-2/Autotaxin Quantikine ELISA kit (R&D Systems; C. No. DENP20) and human CTGF/CCN2 ELISA kit (Novus Biologicals; C. No. NBP2–75009) were used to determine the levels of ATX and CTGF, respectively, in human AH. Manufacturer protocols were used for the analyses which included appropriate standards and background controls.
2.8. Quantification of lysophospholipid species by Liquid Chromatography-Mass Spectrometry (LC/MS)
Extraction, analysis and quantification of the different species of lysophospholipids present in human AH, including LPC 16:0 (palmitic), LPC 18:0 (stearic), LPC 18:1 (oleic), total LPC (Σ LPCs), LPA 16:0 (palmitic), LPA 18:0 (stearic), LPA 18:1 (oleic), and total LPA (Σ LPAs) was carried out using LC/MS, as described earlier by us [23]. LPC and LPA reference standards were purchased from Avanti Polar Lipids (Alabaster, AL). The quantification of LPA and LPC species used a weighted linear regression fit of the calibrator standard curve to account for the natural LPA and LPC background of the matrix used to prepare calibration standards as described earlier [23].
2.9. Cell Culture and treatments
Human TM cells were isolated and cultured as we previously described [21, 24]. Briefly, human primary TM cells (HTM) were cultured from TM tissue isolated from freshly obtained donor corneal rings after they had been used for the corneal transplantation at the Duke Ophthalmology clinical service. We used the corneal rings derived from non-glaucoma subjects with no known ocular complications of trauma, retinopathy and uveitis, and diabetes. Initially, the extracted TM tissue was chopped into small pieces in serum, which were then placed under a glass coverslip in six-well plastic culture plates and cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 20% FBS and penicillin (100 U/500 ml)-streptomycin (100 μg/500 ml)-glutamine (4 mM), and incubated in a humidified atmosphere of 5% CO2 at 37°C. Cells derived from the TM tissue were passaged further and cultured in DMEM medium containing 10% fetal bovine serum (FBS), penicillin (100 U/ml) – streptomycin (100 μg/ml) and glutamine (4 mM) as described above. All human TM cell culture experiments were performed using cells between passages 3–6, and derived from 4 different human donors - aged 28, 39, 51 and 88 years. A minimum of two cell strains with duplicate technical repeats were used for each analysis described in this study. All studies using inhibitors and stimulators were performed using cells that had undergone a 24 h period of serum starvation.
To identify external inputs influencing the expression of ATX in human TM cells, we evaluated the effects of physiologic agents including dexamethasone (0.5 μM, added daily for 96 h; Sigma-Aldrich Corp.; C. No. D4902), IL-1β (10 ng/ml for 24 h; R&D systems; C. No. 210-TA), TNF-α (10 ng/ml for 24 h; BD Biosciences; C. No. 554602), TGF-β2 (5 ng/ml for 24 h; Sigma- Aldrich; C. No. T2815), endothelin-1 (2 μM for 24 h; Sigma-Aldrich; C. No. E7764) or angiotensin-2 (1 μM for 24 h; Sigma-Aldrich; C. No. A9525), all of which have been implicated in pathobiology of POAG [4, 25–27]. These reagents were reconstituted per manufacturer’s recommendation, with the respective vehicle being used to treat control cells (ethanol for dexamethasone, 0.1% acetic acid for endothelin-1, distilled water for angiotensin-2, and phosphate buffered saline containing 0.1% bovine serum albumin for TGF-β2, TNFα or IL-1β).
For siRNA based knockdown studies, human TM cells were grown to semi-confluence and the culture media was switched to 1% FBS on the day before transfection. TM cells were transfected with 40 pmoles of silencer select predesigned siRNA against human ATX (Santa Cruz Biotechnology, Inc.; C. No. sc-44906), or with a scrambled control (Santa Cruz Biotechnology, Inc.; C. No. sc-37007) in medium containing 1% FBS. Transfections were performed using Lipofectamine RNAiMAX (Invitrogen; C. No. 13778150) for 48 h, per the manufacturer’s protocol. To determine the role of ATX in fibrogenic response in TM cells, the siRNA transfected cells were harvested at either 48 h (siRNA alone) or were treated with 10 ng/ml TNF-α or 10 ng/ml IL-1β and incubated for an extra 24 h prior to being harvested. Similarly, for dexamethasone (0.5 μM) treatment, siRNA transfected cells were incubated for an extra 72 h with daily addition of dexamethasone (0.5 μM) prior to being harvested.
To determine the role of glucocorticoid receptor (GR), NF-kB, ERK or SMAD3 in the regulation of dexamethasone or TNF-α-induced expression of ATX, serum starved human TM cells were pretreated with mifepristone (1 μM, C. No. 10006317), BAY 11–7082 (5 μM, C. No. 10010266), FR 180204 (10 μM, C. No. 15544) or SIS3 (1 μM, C. No. 15945) obtained from Cayman Chemical (Ann Arbor, MI) for 1 h prior to being stimulated with dexamethasone (0.5 μM) or TNF-α (10 ng/ml). While TNF-α treated cells were harvested after 24 h, the dexamethasone treated cells were maintained for 96 h with daily addition of dexamethasone and repeat addition of mifepristone, BAY 11–7082, FR 180204 or SIS3 at 72 h after the first addition, as described above.
To test whether LPC induces fibrogenic response in an ATX-dependent manner in TM cells, serum starved human TM cells pretreated for 1 h with ATX specific inhibitors (0.1 μM PF8380, Cayman Chemical; C. No. 12018 or 1 μM ATX-A provided by F. Hoffmann-La Roche Ltd) were incubated with LPC (5 μM; 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine, 16:0 Lyso PC; Avanti Polar Lipids, Inc.; C. No. 855675) for 10 h prior to being harvested.
2.10. Immunoblot analysis
Cells exposed to the treatments described above (section 2.6, Cell Culture and treatments) were rinsed twice with cold PBS and lysed in cold hypotonic buffer (10 mM Tris buffer, pH 7.4, containing 0.2 mM MgCl2, 5 mM N-ethylmaleimide, 2 mM Na3VO4, 10 mM NaF, 60 μM phenylmethyl sulfonyl fluoride, 0.4 mM iodoacetamide, and protease and phosphatase inhibitor cocktail (one tablet each/10 ml buffer, Roche Pharmaceuticals; C. No. 11836170001 and 04906837001, respectively), prior to sonication and centrifugation at 800×g for 10 min at 4°C. Supernatants were collected and stored at −20°C until analysis. Simultaneously, the conditioned media was also collected, centrifuged briefly at 4°C to remove cellular debris and concentrated using Millipore Centrifugal Filter units (Regenerated cellulose 10,000 NMWL; Merck Millipore; C. No. UFC801024). Following this, samples were again centrifuged at 2000×g at 4°C for 20–30 min, prior to determination of protein content in cell lysates and conditioned media by the Pierce BCA method (ThermoFisher Scientific, Waltham, MA).
Cell lysates and conditioned media samples were also separated by SDS-PAGE (with varying amounts of acrylamide), and transferred to PVDF or nitrocellulose membranes. After blocking with 5% skim milk, membranes were incubated overnight at 4°C with primary antibodies directed against: ATX (1:1000 dilution, monoclonal antibody obtained from F. Hoffmann-La Roche Ltd. Basel, Switzerland), fibronectin (1:15000 dilution; rabbit polyclonal; Abcam; C. No. ab23750), collagen type I (1:1000 dilution; rabbit polyclonal; Rockland Immunochemicals; C. No. 600-401-103-0-1) or CTGF (1:1000 dilution; rabbit polyclonal; Abcam; C. No. ab6992). After washing, membranes were incubated for 2 h at room temperature with the respective HRP-conjugated secondary antibodies (1:5000 dilution; anti-mouse or anti-rabbit; Jackson Immuno Research; C. No. 115-035-146 or 111-035-144, respectively). Blots were developed by chemiluminescent detection using HRP substrate (GE Healthcare Life Science; C. No. RPN2232) and images acquired using a luminescent image analyzer (Bio-Rad ChemiDoc imaging system). Band intensities for the proteins of interest were normalized against GAPDH (1:10000 dilution, mouse monoclonal; Proteintech Group, Inc.; C. No. 60004-1-g) and quantitated using ImageJ software (NIH).
2.11. Statistical analyses
Statistical analyses were performed using GraphPad Prism version 7. For clinical data analyses, Kolmogorov-Smirnov test was used to check if data were normally distributed. A 2-tailed unpaired t-test or Mann Whitney U-test was used to compare 2 groups. For data comparisons involving 3 or more groups, a One-way ANOVA with Bonferroni’s multiple comparisons test and multiple t-test were performed for comparing data within groups. Pearson correlation analysis was used to analyze the correlation between 2 groups. Fisher’s exact test and Wilcoxon signed rank test was used for comparing two variables. For all immunoblot analyses, data represent the average results from at least 4 independent samples unless otherwise mentioned.
3. RESULTS
3.1. Elevated levels of ATX, LPC and LPA in the aqueous humor of POAG patients
To better understand our previous observations of LPA-induced resistance to AH outflow in ex-vivo porcine eyes and elevated levels of ATX activity in the AH of POAG patients [7, 17], we undertook a holistic evaluation of ATX levels and those of the ATX substrate LPC and product LPA in the AH of POAG patients. To do this, AH samples were collected from POAG and age-matched non-glaucoma (cataract) patients of differing demography and analyzed by ELISA or mass spectrometry-based approaches. Figure 1A shows the demographic details of POAG patients (n=27) and controls (cataract patients; n=27). The cataract and POAG groups in this study were age-matched, with the average age being 69 years and 71 years, respectively (Wilcoxon signed rank test; p<0.001). There was no significant gender difference between the POAG and cataract patient groups based on Fisher’s exact test. There were significantly fewer Caucasians than African Americans (14 verses 22) in the POAG group relative to the cataract group (13 versus 3), respectively. The average pre-surgical IOP in POAG patients was 22.67± 1.67 mm Hg which is well above the normal range (~12 to 14 mm Hg) expected for non-glaucoma patients [1].
Figure 1. Elevated levels of ATX in the AH of POAG patients.
A). Demographic details of the study population. B). ATX protein levels were significantly elevated in the AH of POAG patients (n = 27) compared to cataract (control) patients (n = 27) based on Mann Whitney U test. C). The ATX protein levels in AH samples and preoperative IOP values POAG patients were significantly and positively correlated (Pearson Correlation Coefficient = 0.622, 95% confidence interval = 0.316 to 0.810, p≤0.001). D). ATX protein levels in the AH of POAG patients was significantly elevated in both males (n = 9) and females (n = 18) compared to cataract patients (males, n = 13; females, n = 13) by the multiple t-test. Within the POAG group however, the ATX protein levels did not differ between males and females. E). AH ATX protein levels were significantly elevated in the Caucasian (n = 14) and African American POAG patients (n = 13) compared to cataract patients (Caucasian, n = 22 and African American, n = 3) based on the Multiple t-test. F). No correlation was found between patient age and AH ATX protein level in the POAG group (Pearson Correlation Coefficient = 0.231, 95% CI = 0.562 to 0.164, p = 0.247). Individual dots represent results for individual patients/subjects. Black dots = cataract subjects. Blue dots = POAG patients. Data in panels B, D and E represent the median and interquartile range. Data in C and F panels represent the mean linear regression (black line) and the 95% confidence interval (black dotted line).
Patients with POAG had significantly higher levels of ATX protein in the AH (216.55±15.37 ng/ml), compared to patients with cataract (132.89 ± 6.67 ng/ml; p≤0.001; Fig.1B) and this elevation in ATX protein levels was significantly and positively correlated with IOP readings in POAG patients (p≤0.001; Fig. 1C). Additionally, both male and female POAG patients showed significantly (p≤0.01) higher levels of ATX in the AH compared to the cataract patients (Fig. 1D). Similarly, the average level of ATX was significantly higher (p≤0.001) in both Caucasian (187.64 ± 13.89 ng/ml) and African American (247.62 ± 26.15 ng/ml) POAG patients relative to the respective demographic in the cataract group (Caucasian: 132.36 ± 8.08 ng/ml; African American: 121.67 ± 8.76 ng/ml). ATX protein levels were higher in African American POAG patients (by ~32%) compared to Caucasian POAG patients, this difference however did not rise to the level of statistical significance with the sample numbers in the current study. ATX protein levels in the AH of POAG patients did not exhibit association with patient age. No significant correlations were noted between ATX levels and corneal thickness, and nerve fiber layer thickness of retina (Supplemental material Fig.1S). A significant (p=0.015) but negative correlation was documented between the cup-to-disk ratio and ATX levels in POAG patients. There was no correlation observed between IOP values and corneal thickness, nerve fiber layer thickness of retina and cup-to-disc ratio of optic nerve head in POAG patients (Supplemental material Fig. 1S). Among the POAG patients included in this study, we did not find evidence for significant influence of preoperative use of glaucoma topical medications (including prostaglandin, α2 agonist, β blocker, and carbonic anhydrase inhibitor) on the levels of ATX in AH (Supplemental material Fig. 2S and Table 1S).
The levels (total and subspecies) of ATX substrate LPC and product LPA in AH samples were quantified by mass spectrometry which revealed a significant increase in the levels of both, total LPA (Σ LPA) and LPC (Σ LPC) in the AH of POAG patients compared to cataract subjects (Fig. 2A). There was also a significant and positive correlation among the levels of ATX, LPA and LPC in the AH of POAG patients, as well as a positive correlation between IOP, ATX and total LPA levels in POAG patients (Fig. 2). Additionally, the levels of individual subspecies of LPC including 16:0; 18:0 and 18:1 were significantly elevated in the AH of POAG patients compared to cataract patients (Supplemental material Fig. 3S & Table 2S). Similar significant increases were found in the levels of LPA 16:0 and 18:1 species in the AH of POAG patients compared to cataract patients (Supplemental material Fig. 3S & Table 2S). In these POAG samples, we also evaluated differences in levels of AH LPC between POAG patients with (n=13) and without (n=13) cataract surgery. Although there was an increasing trend in the levels of LPC (all three species analyzed) in the AH of POAG patients with cataract surgery compared to POAG patients without cataract surgery, the difference was not significant.
Figure 2. Increased LPC and LPA levels in the AH of POAG patients and their correlation with preoperative IOP and ATX protein levels.
A). Total LPC (Ʃ LPC) and LPA (Ʃ LPA) levels in the AH of POAG patients were significantly evaluated compared to cataract patients based on multiple t-test. C). Levels of total LPA in AH samples correlated significantly and positively with preoperative IOP readings in POAG patients (Pearson Correlation Coefficient = 0.267, 95% CI = 0.052 to 0.459, p = 0.008) but not between LPC and IOP (B). D & E). Similarly, the levels of AH LPC and LPA showed a positive and significant correlation with the AH ATX protein levels in POAG patients (Pearson Correlation Coefficient = 0.412, 95% CI = 0.212 to 0.578, p≤0.001; 0.369, 95% CI = 0.164 to 0.544, p≤ 0.001, respectively). F). A significant positive correlation was also found between the levels of AH LPC and LPA in POAG patients (Pearson Correlation Coefficient = 0.501, 95% CI = 0.317 to 0.648, p≤0.001).
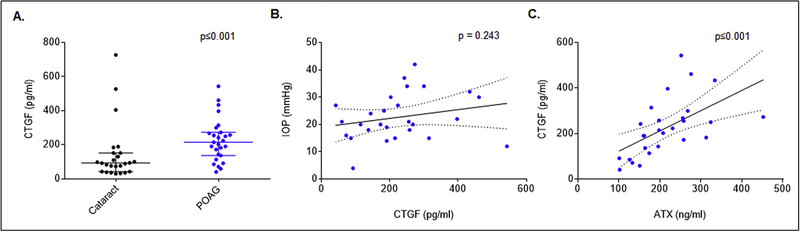
3.2. Elevated levels of CTGF in the AH of POAG patients
There is overwhelming evidence for the involvement of a dysregulated ATX-LPA axis in the etiology of fibrosis in various tissues, with LPA having been demonstrated to stimulate Yap/Taz activation and induce the expression of CTGF and ECM proteins in TM cells [15, 21, 28, 29]. Given the increase in levels of both ATX and LPA observed in AH of POAG patients, we determined the levels of CTGF by ELISA in the same samples (Fig. 3). AH samples from POAG patients exhibited a significant (p≤0.001) increase in CTGF protein levels (276.72 ± 24.04 pg/ml; n=27) compared to AH from cataract patients (142.57 ± 13.97 pg/ml; n=27). Additionally, the elevated levels of CTGF were significantly (p≤0.001) and positively correlated with ATX levels in AH samples from POAG patients (Fig. 3).
Figure 3. Elevated levels of CTGF in the AH of POAG patients.
A). The levels of CTGF (determined by ELISA) were significantly elevated in the AH of POAG patients compared to cataract subjects (n=27) based on the Mann Whitney U test. The data represent the median, 5% and 95% quartile range and each dot represents individual patients/subjects. B). While the correlation between AH CTGF levels and preoperative IOP in POAG patients was not statistically significant (Pearson Correlation Coefficient = 0.233, 95% CI = −0.162 to 0.563, p = 0.243), C). There was a significant correlation between the levels of CTGF and ATX in AH from POAG patients (Pearson Correlation Coefficient = 0.568, 95% CI = 0.240 to 0.780, p≤0.001).
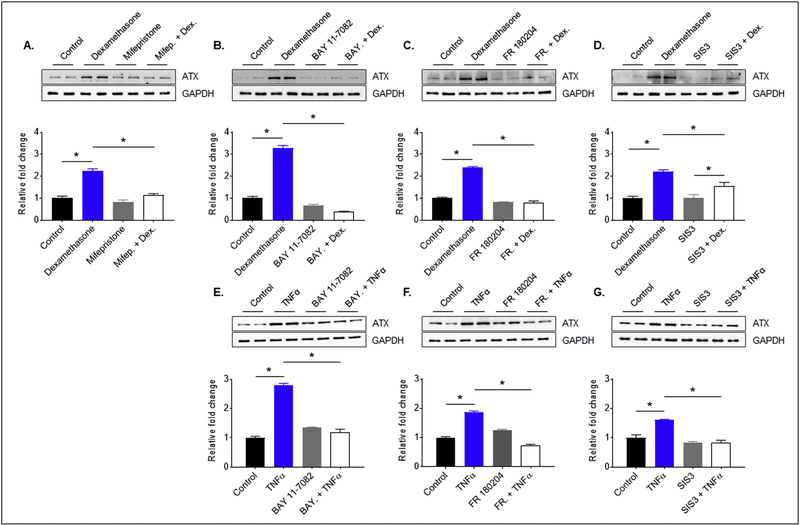
3.3. Regulation of ATX protein levels in TM cells
To evaluate regulation of ATX protein levels in TM cells, we tested the effects of various cytokines, growth factors and dexamethasone in serum starved human TM cells using immunoblotting analysis. In a pilot screen, we noted that treatment of human TM cells with TNF-α (10 ng/ml) and IL-1β (10 ng/ml) for 24 h and dexamethasone (0.5 μM for 96 h) resulted in obvious increases in the levels of ATX protein in both cell lysates and conditioned media, relative to treatment of these cells with TGF-β2 (5 ng/ml, 24 h), endothelin-1 (2 μM, 24 h) or angiotensin-2 (1 μM, 24 h). Having documented these preliminary observations, we focused on characterizing the effects of dexamethasone, TNF-α and IL-1β on ATX protein levels in TM cells. Human TM cells serum starved for 24 h prior to treatment with dexamethasone (0.5 μM for 96 h with daily addition), TNF-α (10 ng/ml for 24 h) or IL-1β (10 μg/ml for 24 h) revealed a significant (p<0.05; n=4) increase in the levels of ATX protein in both cell lysates and conditioned media compared to vehicle-treated control cells. Fig. 4 shows representative immunoblots of two independent samples (both lysates and conditioned media) for each treatment (panels A, B and C) and the densitometry- based quantitative changes in ATX protein levels in the indicated respective samples. GAPDH was immunblotted as loading control for cell lysates. Equal amounts of total protein from conditioned media samples were separated by SDS-PAGE and stained with Coomassie blue. One of the Coomassie-stained protein bands (showed in Fig.4) was then used as loading control.
Figure 4. Regulation of ATX protein levels in human TM cells.
ATX protein levels in serum starved human TM cells treated with dexamethasone (A; 0.5 μM, 96 h, daily addition), TNFα (B; 10 ng/ml, 24 h) or IL-1β (C; 10 ng/ml, 24 h) were significantly increased in both cell lysates and conditioned media compared to control cells (vehicle treated). Histograms represent the fold change in ATX protein levels under the above described conditions, based on densitometry analysis. GAPDH was used as a loading control for the cell lysates. For the conditioned media samples, equal amounts of protein samples were subjected to SDS-PAGE and one of the indicated Coomassie Brilliant blue stained protein bands was used for normalizing protein loads. * p ≤ 0.05. N = 4
We then attempted to gain insights into the possible transcriptional mechanisms underlying induction of ATX expression in TM cells by dexamethasone and TNF-α. Consistent with the previous results (Fig. 4), treatment of serum starved human TM cells with dexamethasone (0.5 μM for 96 h) or TNF-α (10 ng/ml for 24 h) led to a significant increase of ATX protein levels in cell lysates relative to control cells (Fig. 5). However, this effect of dexamethasone and TNF-α was blocked to a significant extent in TM cells pretreated with NK-kB inhibitor (5 μM, Bay11–7082) or ERK inhibitor (10 μM, FR180204). While pretreatment with SMAD3 inhibitor (1 μM) also significantly suppressed the TNF-α-induced ATX expression, it had a modest but not significant effect on dexamethasone-induced ATX expression in human TM cells (Fig. 5). The glucocorticoid receptor antagonist Mifepristone (1 μM) however, significantly suppressed dexamethasone-induced ATX expression in human TM cells compared to the respective controls (Fig. 5). Inhibitors were added twice to cells treated with dexamethasone, at 1 h prior to and 72 h after dexamethasone addition, whereas for cells treated with TNF-α, inhibitors were added only once. Fig. 5 shows representative immunoblots for two individual samples for each of the treatments described above, and densitometry-based quantitative changes in ATX protein levels in human TM cells. Data were normalized to GAPDH. Collectively, these results indicate a definitive role for the glucocorticoid receptors, NF-kB, ERK and SMAD3 in transcriptional regulation of ATX expression in TM cells.
Figure 5. Regulation of ATX expression by intracellular signaling pathways in human TM cells.
Dexamethasone (0.5 μM for 96 h with daily additions) treatment mediated increase in ATX protein levels in human TM cells was significantly inhibited in response to pretreatment with the glucocorticoid receptor antagonist- mifiepristone (1 μM; A), NF-kB inhibitor-BAY 11–7082 (5 μM; B), ERK inhibitor-FR 180204 (10 μM; C) or SMAD2/3 inhibitor SIS3 (1 μM; D). In the case of dexamethasone, inhibitors were added twice (first addition 1 h prior to and the second addition 72 h after initial dexamethasone addition) while all other inhibitors were added 1 h prior to the extracellular factor. Unlike with mifiepristone, BAY 11–7082 and FR 180204 treatments, pretreatment with SIS3, dexamethasone induced ATX levels were still significantly higher compared to cells treated with SIS3 alone. Similarly, the TNFα (10 ng/ml for 24 h)-induced increase in ATX protein levels was significantly inhibited in human TM cells pretreated (1 h) with BAY 11–7082 (E), FR 180204 (F) or SIS3 (G). ATX protein levels were normalized to GAPDH and assessed by densitometry analysis. * p ≤ 0.05. N = 4
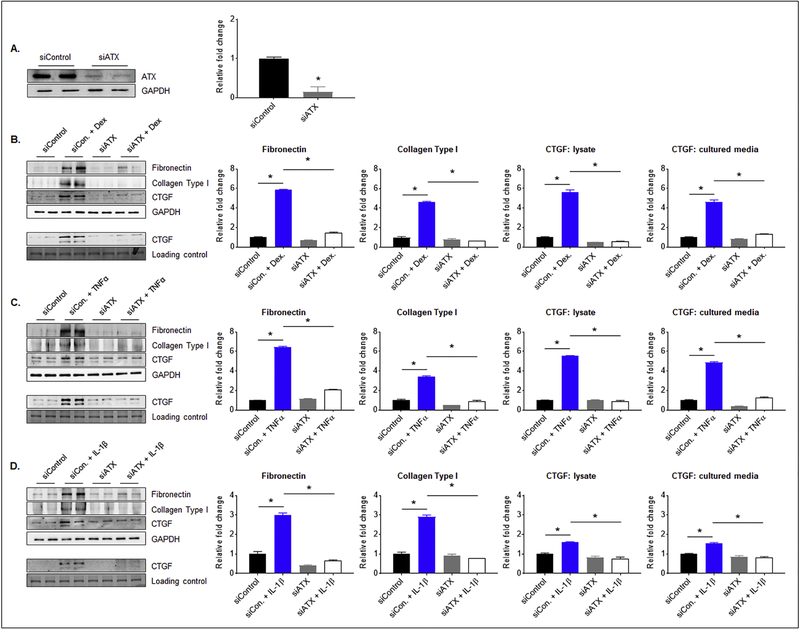
3.4. The role of ATX in dexamethasone-, TNF-α- and IL-1β-induced fibrogenic response in TM cells
In addition to addressing the regulation of ATX expression in TM cells described above, we were also interested in gaining insights into how ATX may be impacting TM cell biology and AH outflow resistance. Since ATX and LPA have been found to involve in the etiology of fibrosis [30, 31], we investigated to determine whether dexamethasone-, TNF-α- and IL-1β-induced ATX expression in TM cells triggers fibrogenic responses. For this, we suppressed ATX expression in human TM cells using ATX-specific siRNA prior to determining the effects of the above described agents on expression of fibronectin, collagen type 1 and CTGF by immunoblotting analysis and densitometric quantification. Human TM cells treated with ATX-specific siRNA (40 pmoles) for 48 h showed a significant (80%) decrease in ATX protein levels relative to cells treated with a control, scrambled siRNA. Fig. 6A shows a representative immunoblot for 2 samples and quantitative changes in ATX protein levels. Human TM cells downregulated in ATX expression were treated with dexamethasone (0.5 μM for 72 h with daily addition), TNF-α (10 ng/ml for 24 h) or IL-1β (10 ng/ml for 24 h) in the presence of 1% FBS and then analyzed for changes in fibronectin and collagen type 1 (using cell lysates), or CTGF (using cell lysates and conditioned media). While addition of dexamethasone, TNF-α or IL-1β to TM cells treated with a scrambled siRNA control resulted in significant increase in the levels of fibronectin, collagen type I and CTGF (in both lysates and conditioned media) relative to cells treated with scrambled siRNA alone, TM cells treated with ATX siRNA failed to show any changes in fibronectin, collagen type 1 or CTGF levels in response to the above agents, as shown in Fig. 6. Treatment with either ATX siRNA or scrambled siRNA alone did not have significant effects on the levels of fibronectin, collagen type 1 or CTGF (Fig. 6). For all the above described analyses, representative immunoblots for two independent samples are shown along with the respective loading controls. Panels B, C & D show densitometry-based quantitative changes in fibronectin, collagen type 1 and CTGF protein levels. *P < 0.05; n=4.
Figure 6. Knockdown of ATX expression decreases fibrogenic activity in human TM cells.
A). Treatment of human TM cells with siRNA specific to ATX (human) for 48 h led to a significant decrease in ATX protein levels (by 80%) relative to the control cells treated with a scrambled siRNA control. The ability of dexamethasone (0.5 μM, 72 h, daily addition); B), TNFα (10 ng/ml, 24 h; or C) IL-1β (10 ng/ml, 24 h; D) to stimulate increases in the levels of fibronectin, collagen type I and CTGF (in both cell lysate and conditioned media) proteins was significantly inhibited in TM cells downregulated for ATX expression, relative to TM cells treated with a scrambled siRNA control. Lower panels in B, C and D panels (immunoblots) show changes in CTGF protein levels in the conditioned media. Changes in protein levels of ATX, fibronectin, collagen type I and CTGF were evaluated by densitometry analysis. Protein loading was normalized against GAPDH (for lysates). Conditioned media protein fraction was separated by SDS-PAGE and one of the stained (Coomassie Brilliant Blue) protein bands was used for normalization. * p ≤ 0.05. N = 4
3.5. Suppression of LPC-induced fibrogenic response by ATX inhibitors in TM cells
Having found that ATX augments the fibrogenic response in TM cells, we asked whether LPC, the substrate of ATX and the precursor of LPA, mediates the effects of ATX on fibrogenic activity in human TM cells. To address this aspect, human TM cells starved of serum for 24 h were treated with LPC (5 μM) in the presence or absence of pharmacological inhibitors of ATX (PF8380 or ATX-A) used at two different concentrations of 0.1 and 1 μM. ATX inhibitors were added to the TM cells one h prior to addition of LPC. After 10 h of LPC treatment, cell lysates were analyzed for changes in the levels of fibronectin, collagen type 1 and CTGF by immunoblotting analysis and densitometric quantification. Treatment of TM cells with LPC alone revealed a consistent and significant (P<0.05; n=4) increase in the levels of fibronectin, collagen type 1 and CTGF compared to control samples (Fig. 7A). This effect of LPC on fibronectin, collagen type 1 and CTGF levels in human TM cells was suppressed completely and significantly (P<0.05; n=4) by 0.1 μM and 1 μM ATX-A, and by 1 μM PF8380 (Fig. 7B). Relative to 0.1 μM ATX-A, 0.1 μM PF8380 did not significantly prevent LPC induced increases in the levels of fibronectin, collagen typ1 I and CTGF in TM cells (Fig. 7). Treatment of TM cells with ATX inhibitors (PF8380 and ATX-A) alone did not significantly influence the fibrogenic response compared to the control cells. GAPDH was immunoblotted as a loading control in these analyses. Representative immunoblots for two independent samples for each of the treatments are shown in Fig. 7.
Figure 7. Suppression of LPC induced fibrogenic activity in human TM cells by inhibition of ATX activity.
Treatment of serum starved human TM cells with LPC (5 μM for 10 h) stimulated significant increase in the levels of fibronectin, collagen type I and CTGF proteins compared to control cells (A & B). These effects of LPC were significantly suppressed in cells pretreated with ATX inhibitor - PF8380 (1 μM) or ATX-A (1 μM) for 1 hr. On a relative basis, while 0.1 μM ATX-A also significantly suppressed LPC induced fibrogenic activity, 0.1 μM PF8380 exhibited only a modest effect on the ability of LPC to stimulated increase in the levels of fibronectin, collagen type 1 and CTGF. GAPDH was used as a loading control for the cell lysates. * p ≤ 0.05. N = 4
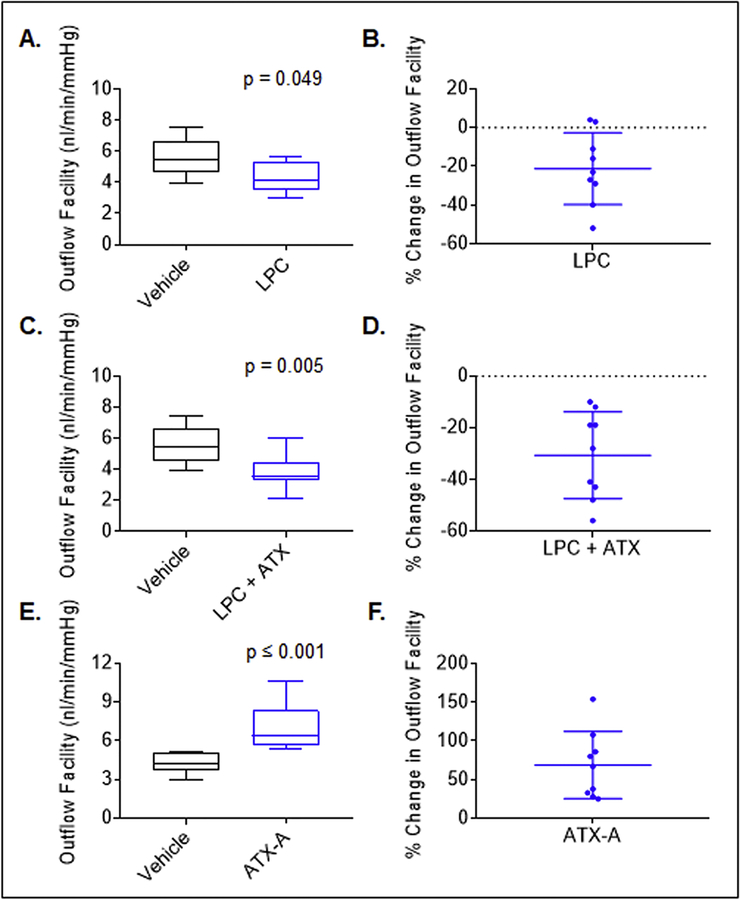
3.6. ATX and LPC decrease aqueous outflow through the trabecular pathway in mouse eye
In our previous study, we had obtained evidence for the ability of ATX inhibition to lower IOP in rabbits [17]. However, it was not clear from that study whether the decrease in IOP stems from increased AH drainage through the trabecular meshwork, and what the direct effects of LPC or ATX were on AH outflow. Therefore, to address these questions, we determined the effects of recombinant ATX (mouse) and ATX substrate LPC alone, or in combination, on AH outflow facility through the trabecular pathway. These experiments were performed using enucleated mouse eyes and an iPerfusion system which is used to quantitate small changes in AH outflow in a short term perfusion model [22]. For this study, we used 7 to 9 month-old male and female mice of C57BL/6J genetic background. Enucleated mouse eyes (left and right) were perfused in pairs (control and test) as described in the Methods section. Enucleated mouse eyes were perfused with LPC (3 μM) or recombinant ATX (2 μg/ml) + LPC (3 μM) or ATX-A (1 μM) alone for 45 min along with their respective control eyes (contralateral) which were perfused with vehicle containing perfusion media. Following this, changes in aqueous outflow were monitored for 90 min and facility was analyzed as described in the Methods section. These studies revealed a significant (by ~20%, P<0.05; n=9) decrease in aqueous outflow facility in eyes perfused with LPC (4.4 ±0.3 nl/min/mmHg) compared to control eyes perfused with vehicle (5.7±0.4 nl/min/mmHg; Fig. 8A &B). In eyes perfused with LPC plus recombinant ATX, the decrease in aqueous outflow facility was not only significant (P<0.005; n=9) but also greater (~35% with outflow facility of 3.8±0.4 nl/min/mmHg) relative to that measured for vehicle perfused control eyes (Fig. 8C&D). The difference in aqueous outflow between LPC perfused eyes and LPC plus ATX perfused eyes was not found to be significant. In clear contrast, eyes perfused with the ATX inhibitor (1 μM ATX-A) exhibited a significant (p<0.01; n=9) increase in aqueous outflow facility (by ~ 60%, 7.1±0.6 nl/min/mmHg) relative to vehicle perfused control eyes (4.3±0.2 nl/min/mmHg). Data in Fig. 8 A, C, and D represent the median with the 5% and 95% quartile range, a Mann Whitney U test was used to assess for statistical significance. Panels B, D, and F in Fig. 8 represent the percentage change (mean ± SD) in aqueous outflow facility under the described perfusion conditions.
Figure 8. Modulation of aqueous outflow facility in ex-vivo mouse eyes perfused with LPC, ATX and ATX inhibitor.
To determine the effects of LPC, ATX and ATX inhibitor on aqueous outflow facility, changes in aqueous outflow facility were assessed in the enucleated mouse eyes perfused with LPC, LPC plus ATX or ATX inhibitor and compared to contralateral eyes perfused with vehicle alone, using an iPerfusion system as described in Methods section. A). Perfusion of eyes with LPC (3 μM) led to a significant decrease of aqueous outflow facility by ~20% (4.4 ±0.3 nl/min/mmHg) compared to eyes perfused with vehicle (5.7±0.4 nl/min/mmHg). C). Eyes perfused with LPC (3 μM) plus ATX (2 μg/ml, recombinant mouse) exhibited a significant and approximately ~30% reduction in aqueous outflow facility (3.8±0.4 nl/min/mmHg) relative to vehicle controls (5.7±0.4 nl/min/mmHg). E). Eyes perfused with ATX inhibitor (ATX-A, 1 μM) alone exhibited a significant increase in aqueous outflow facility (by ~60%; 7.1±0.6 nl/min/mmHg) compared to vehicle controls (4.3±0.2 nl/min/mmHg). Data in panels A, C and E represent the median with the 5% and 95% quartile range, statistical significance was assessed using a Mann Whitney U test. Data in B, D, and F panels represent the percentage change (mean ± SD) in aqueous outflow facility in response to the treatments described. N = 9 for each of the perfusion treatments described including vehicle perfusion.
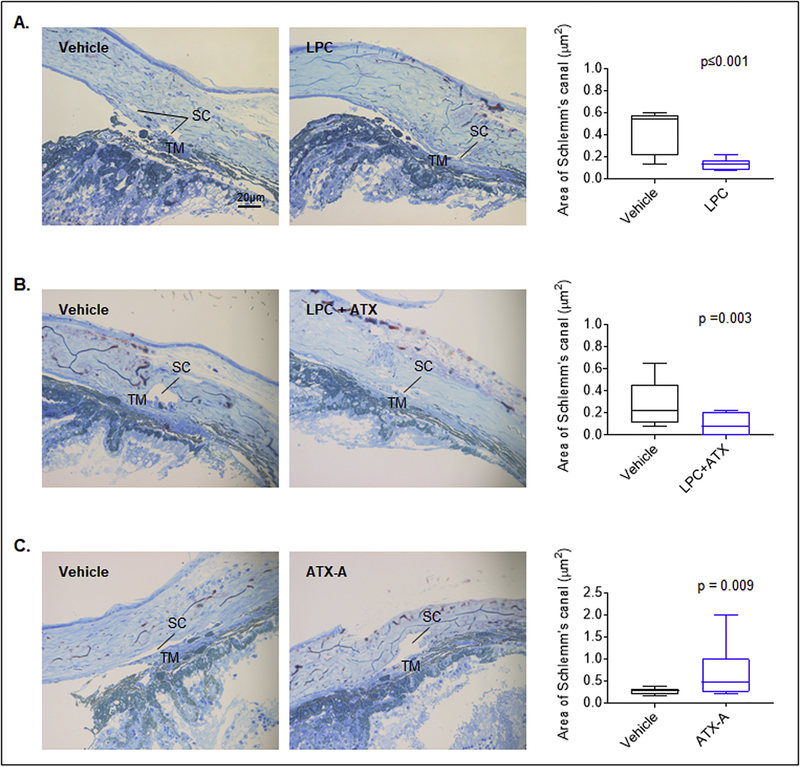
3.7. ATX inhibitor-induced AH outflow facility in the mouse eye is associated with alteration in trabecular pathway morphology
Light microscopy based analysis was performed to gain histological insight into the LPC/ATX and ATX inhibitor-induced changes in aqueous outflow in the perfused enucleated mouse eyes described above. Semi-thin tissue sections of the anterior chambers of eyes perfused with LPC, LPC/ATX and ATX inhibitor stained with methylene blue revealed notable histological changes in the area of Schlemm’s canal relative to control specimens. While the Schlemm’s canal area was significantly reduced in the LPC (0.13 ± 0.02 μm2) and LPC/ATX (0.07± 0.02 μm2) perfused eyes (by ~70 and ~77%, respectively; Fig. 9 A/B), it was significantly increased in eyes perfused with the ATX inhibitor (by ~129%; 0.62±0.13 μm2) relative to the respective vehicle perfused control eyes (LPC; 0.42 ± 0.06 μm2, LPC plus ATX; 0.30 ± 0.06 μm2, ATX-A; 0.27 ± 0.02 μm2, Fig. 9C). These results were based on two independent samples per group with three sections per sample.
Figure 9: LPC, ATX and ATX inhibitor induced changes in Schlemm’s canal histology in ex-vivo perfused mouse eyes.
Semi-thin plastic sections of perfused mouse eye anterior segments stained with methylene blue and examined under light microscopy revealed that the Schlemm’s canal area in eyes perfused with LPC (3 μM; A) and LPC (3 μM) plus ATX (2 μg recombinant mouse; B) was significantly reduced by ~70% (0.13±0.02 μm2) and ~77% (0.07 ± 0.02 μm2) respectively, compared to eyes perfused with vehicle (0.42±0.06 μm2 and 0.30±0.06 μm2, respectively). In contrast, perfusion of eyes with ATX inhibitor (1 μM ATX-A) resulted in a significant increase in the Schlemm’s canal area by ~129% (0.62±0.13 μm2) relative to vehicle perfused eyes (0.27±0.02 μm2). Each treatment included a paired contralateral eye as a control. For each treatment, two eyes were analyzed and 3 semi-thin sections used per specimen to calculate the area of the Schlemm’s canal tissue using ImageJ software.
4. DISCUSSION
Towards understanding the etiological mechanisms involved in ocular hypertension resulting from impaired AH drainage through the trabecular pathway in glaucoma patients, this study reports elevated levels of ATX and its substrate (LPC) and product (LPA) in the AH of POAG patients, and demonstrates that perfusion of ATX and LPC decreases AH outflow through the trabecular pathway while inhibition of ATX increases AH outflow. Additionally, this study identifies external cues and intracellular signaling pathways that regulate ATX expression and provides novel molecular insights into the ability of ATX and LPC to induce markers of the fibrogenic response in human TM cells. Collectively, these clinical, cell culture-based and ex-vivo findings uncover the existence of a dysregulated ATX-LPA signaling pathway in the AH of glaucoma patients, establish its correlation with elevated IOP and induction of fibrogenic activity in the TM, and demonstrate the ability of ATX inhibitors to mitigate these adverse effects by enhancing AH outflow through the trabecular pathway.
Although loss of vision in glaucoma patients is caused due to optic nerve axonal damage and loss of retinal ganglion cells, the main course of treatment available to restore vision in these patients is focused on lowering IOP (in all types of glaucoma patients) either through medical or surgical approaches [1, 2]. It is therefore imperative that we have a thorough understanding of the regulation of AH outflow through the trabecular or conventional pathway which accounts for more than 80% of total outflow, in order to develop efficacious IOP lowering treatments [4]. Various extracellular factors and intracellular molecular mechanisms are recognized to modulate AH outflow through the TM pathway [3, 4, 6, 20]. There exists overwhelming support from both experimental and clinical studies indicating that intracellular molecular pathways controlling TM tissue contractile activity, stiffness, cell adhesion and fibrogenic activity modulate AH outflow through the TM [3, 4, 6]. More recently, Rho kinase inhibitors and nitric oxide donors which have been demonstrated to regulate the contractile activity of TM and Schlemm’s canal were approved for use to lower IOP in glaucoma patients [6, 32–34]. Importantly, this recent clinical advancement has provided impetus for evaluation and identification of extracellular factors that might influence AH outflow through the TM by regulating the contractile activity of TM. In this context, LPA is recognized to serve as a potent inducer of actin stress fibers and contractile activity in various cell types including TM cells, by working via G-protein couple receptors to activate downstream intracellular pathways including Rho/Rho kinase and calcium signaling [6, 7, 35]. Dysregulation of the ATX-LPA pathway is not only recognized to participate in the etiology of various diseases, but targeting of this pathway is considered to have therapeutic significance for the treatment of several diseases [14–16, 36, 37].
Therefore, to gain additional and definitive support for our previous preliminary observations regarding elevated levels of ATX activity in the AH of POAG patients [17], we undertook a comprehensive and quantitative analysis of the levels of ATX and different species of LPC and LPA in a greater number of AH samples obtained from POAG and age-matched non-glaucoma subjects (cataract) by ELISA and LC/MS, respectively. In these new clinical samples, the levels of ATX protein and both LPC and LPA were found to be significantly higher in the AH of POAG patents (Caucasian and African American, both males and females) compared to cataract patients, with the changes exhibiting a positive correlation with elevated IOP in the same patients. After we initiated these analyses in POAG patients, Honjo et. al [19] also recently reported very similar results to ours regarding the levels of ATX, LPA and PLC in the AH of Japanese glaucoma patients, using similar approaches. It is noteworthy that although the Japanese POAG patients also exhibited elevated levels of ATX and LPA in the AH compared to cataract patients, this difference achieved significance only when a much higher sample size was evaluated (n=49) relative to that used in our study (n=27). In a recent study however, the authors of the Japanese study did not find a significant difference in either ATX or LPA levels in the AH of POAG patients relative to cataract patients, when a sample size of n=29 was evaluated [38]. Therefore, dysregulation of ATX and LPA levels in the AH appears to be more prominent in North American patients relative to those noted for the Japanese POAG patients. Despite, these differences, these clinical observations collectively confirm that POAG patients of different race/ethnicities and demography exhibit higher levels of ATX, LPA and LPC in the AH samples relative to non-glaucoma subjects. It is also worth noting that ATX levels in the AH of African American POAG patients (247.62±26.15 ng/ml; n=13) are ~30% higher than those in Caucasian POAG patients (n=14; 187.64±13.89 ng/ml), corroborating data showing that African Americans are known to have a higher incidence of POAG relative to Caucasians [39]. While this difference in ATX levels in AH samples from the two groups did not achieve statistical significance, it is necessary to increase sample size in future studies to critically evaluate the relevance of this difference for regulation of aqueous outflow and IOP. Importantly, pre-surgical use of IOP lowering medications did not appear to have significant effects on levels of ATX, LPC and LPA in the AH of glaucoma patients.
Our attempts to identify extracellular factors that induce ATX expression and secretion by human TM cells revealed that dexamethasone, TNF-α and IL-1β significantly increased the levels of ATX in both cytosol and conditioned media. Consistent with these observations, dexamethasone has been previously reported to induce ATX expression in TM cells [40]. While glucocorticoids including dexamethasone are well recognized to induce ocular hypertension in human subjects, the molecular basis for this effect is not well defined [27, 41]. It is reasonable to speculate that the elevated levels of ATX induced by these external factors could trigger increased actin stress fiber formation, contractile activity and stiffness of TM cells via catalyzing the production of LPA from LPC, ultimately impairing AH outflow through the trabecular pathway. Additionally, our studies to characterize the intracellular mechanisms underlying the dexamethasone-, TNF-α- and IL-1β-induced increases in ATX in TM cells uncovered a definitive role for glucocorticoid receptor, NF-kB and ERK signaling, with a relatively modest involvement of the SMAD3 pathway. Inhibitors of NF-kB, ERK and SMAD3 were all found to cause a significant decrease in the levels of TNF-α-stimulated ATX expression in TM cells, indicating the involvement of multiple transcriptional mechanisms in extracellular factor-mediated regulation of ATX expression in TM cells.
To gain insights into how the dysregulated ATX-LPA pathway might affect TM cell biology and eventually AH outflow through the TM, we focused on the fibrogenic response of TM cells since LPA and ATX have been known to induce fibrotic activity through different mechanisms including activation of Rho/Rho kinase, contractile tension, Yap/Taz transcriptional activity and endothelial to mesenchymal transition (EndMT) [6, 14, 15, 21, 28, 29, 42]. Our studies showed that siRNA-mediated downregulation of ATX expression in human TM cells led to a significant decrease in the levels of CTGF, a well-recognized profibrotic cytokine whose expression is known to be induced by Yap/Taz transcriptional activity in response to mechanical tension and contractile force [29, 43–45]. Significant decreases in the levels of collagen type 1 and fibronectin were also noted under these conditions, uncovering a prominent role for ATX in dexamethasone, TNF-α and IL-1β induced fibrogenic activity in TM cells. This conclusion was further supported by data from our subsequent experiments demonstrating that pharmacological inhibition of ATX suppresses the ability of the ATX substrate LPC to stimulate increases in the levels of CTGF, collagen type 1 and fibronection in TM cells. Increased fibrotic activity in the trabecular pathway has been shown to impair AH outflow [3, 6, 46, 47]. Our studies collectively provide compelling evidence for the involvement of a dysregulated ATX/LPA signaling axis in glaucoma via induction of profibrotic response in TM cells, a process that may be partly mediated through EndMT and activation of Yap/Taz transcriptional response, as previously shown by us in LPA- treated TM cells [21, 42].
Finally, in this study we also evaluated the ability of ATX, LPC and ATX inhibitors to modulate AH outflow through the trabecular pathway. Although we have previously demonstrated that ATX inhibition lowers IOP, the study did not address whether the effect of ATX inhibition on IOP derives from altered AH outflow through the TM [17]. The results from our current study using enucleated mouse eyes indicate that treatment with ATX and LPC decreases AH outflow while treatment with an ATX inhibitor increases AH outflow revealing a definitive role for the ATX-LPA pathway in modulation of AH outflow through the TM and eventually in IOP regulation. The LPA-induced increase in AH outflow resistance in porcine eyes reported previously by us also supports this conclusion [7]. Finally, our data on the histological changes in ATX inhibitor perfused eyes, especially the increase in area/enlargement of the Schlemm’s canal associated with increased AH outflow indicates a direct influence of ATX and ATX inhibitors on the histological integrity of the trabecular pathway.
5. CONCLUSION
This study not only provides conclusive and additional supportive clinical evidence for a dysregulated ATX-LPA signaling axis in the AH of glaucoma patients but also demonstrates that ATX expression in the TM can be regulated by various extracellular factors and transcriptional mechanisms. Importantly, while ATX and its substrate LPC induced a profibrotic response in TM cells and increased resistance to AH outflow through the trabecular pathway, ATX inhibitors abrogated these responses, suggesting that targeting the ATX-LPA axis could be of therapeutic significance for lowering ocular hypertension in glaucoma patients.
Supplementary Material
HIGHLIGHTS.
AH derived from the POAG patients contains elevated levels of ATX, LPA and LPC
Dexamethasone, TNFα and IL-1β increase ATX protein levels in TM cells
ATX plays a crucial role in dexamethasone, TNFα and IL-1β induced fibrogenic response in TM cells
LPC induces fibrogenic response in TM cells in an ATX-dependent manner
ATX inhibitor increases aqueous outflow facility in mouse eyes
6. ACKNOWLEDGEMENTS
This study was funded by the Roche postdoctoral program (LTY Ho) and a National Institutes of Health grant (R01EY018590). We thank Ying Ho for help with histological analysis and Sandra Stinnett, DrPH, for statistical analyses.
Abbreviations:
- POAG
primary open angle glaucoma
- AH
aqueous humor
- IOP
intraocular pressure
- TM
trabecular meshwork
- ATX
autotaxin
- LPA
lysophosphatidic acid
- LPC
lysophosphatidylcholine
- CTGF
connective tissue growth factor
- IL-1β
interleukin-1β
- TNF-α
tumor necrosis factor-alpha
- ERK
extracellular Signal-regulated Kinase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- ELISA
enzyme-linked immunosorbent assay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Anja Osterwald, Iris Ruf and Christoph Ullmer are employees of F. Hoffmann-La Roche Ltd. Basel, Switzerland. Leona Ho, Pratap Challa, Robin Vann and Vasantha Rao declare no conflicts of interest.
Disclosures: ATX inhibitor (ATX-A), recombinant mouse ATX and ATX antibody (MAB5/5) used in this study were provided by F. Hoffmann-La Roche Ltd. Basel, Switzerland.
REFERENCES
- [1].Kwon YH, Fingert JH, Kuehn MH, Alward WL, Primary open-angle glaucoma, The New England journal of medicine, 360 (2009) 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Weinreb RN, Aung T, Medeiros FA, The pathophysiology and treatment of glaucoma: a review, Jama, 311 (2014) 1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tamm ER, Braunger BM, Fuchshofer R, Intraocular Pressure and the Mechanisms Involved in Resistance of the Aqueous Humor Flow in the Trabecular Meshwork Outflow Pathways, Progress in molecular biology and translational science, 134 (2015) 301–314. [DOI] [PubMed] [Google Scholar]
- [4].Stamer WD, Acott TS, Current understanding of conventional outflow dysfunction in glaucoma, Current opinion in ophthalmology, 23 (2012) 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Carreon T, van der Merwe E, Fellman RL, Johnstone M, Bhattacharya SK, Aqueous outflow - A continuum from trabecular meshwork to episcleral veins, Progress in retinal and eye research, 57 (2017) 108–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rao PV, Pattabiraman PP, Kopczynski C, Role of the Rho GTPase/Rho kinase signaling pathway in pathogenesis and treatment of glaucoma: Bench to bedside research, Experimental eye research, 158 (2017) 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mettu PS, Deng PF, Misra UK, Gawdi G, Epstein DL, Rao PV, Role of lysophospholipid growth factors in the modulation of aqueous humor outflow facility, Investigative ophthalmology & visual science, 45 (2004) 2263–2271. [DOI] [PubMed] [Google Scholar]
- [8].Stamer WD, Read AT, Sumida GM, Ethier CR, Sphingosine-1-phosphate effects on the inner wall of Schlemm’s canal and outflow facility in perfused human eyes, Experimental eye research, 89 (2009) 980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H, Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production, The Journal of cell biology, 158 (2002) 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].van Meeteren LA, Moolenaar WH, Regulation and biological activities of the autotaxin-LPA axis, Progress in lipid research, 46 (2007) 145–160. [DOI] [PubMed] [Google Scholar]
- [11].Sheng X, Yung YC, Chen A, Chun J, Lysophosphatidic acid signalling in development, Development, 142 (2015) 1390–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Aikawa S, Hashimoto T, Kano K, Aoki J, Lysophosphatidic acid as a lipid mediator with multiple biological actions, Journal of biochemistry, 157 (2015) 81–89. [DOI] [PubMed] [Google Scholar]
- [13].Kihara Y, Mizuno H, Chun J, Lysophospholipid receptors in drug discovery, Experimental cell research, 333 (2015) 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yung YC, Stoddard NC, Chun J, LPA receptor signaling: pharmacology, physiology, and pathophysiology, Journal of lipid research, 55 (2014) 1192–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ninou I, Magkrioti C, Aidinis V, Autotaxin in Pathophysiology and Pulmonary Fibrosis, Frontiers in medicine, 5 (2018) 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Castagna D, Budd DC, Macdonald SJ, Jamieson C, Watson AJ, Development of Autotaxin Inhibitors: An Overview of the Patent and Primary Literature, Journal of medicinal chemistry, 59 (2016) 5604–5621. [DOI] [PubMed] [Google Scholar]
- [17].Iyer P, Lalane R 3rd, Morris C, Challa P, Vann R, Rao PV, Autotaxin-lysophosphatidic acid axis is a novel molecular target for lowering intraocular pressure, PloS one, 7 (2012) e42627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang H, Edwards G, Garzon C, Piqueras C, Bhattacharya SK, Aqueous humor phospholipids of DBA/2J and DBA/2J-Gpnmb(+)/SjJ mice, Biochimie, 113 (2015) 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Honjo M, Igarashi N, Kurano M, Yatomi Y, Igarashi K, Kano K, Aoki J, Weinreb RN, Aihara M, Autotaxin-Lysophosphatidic Acid Pathway in Intraocular Pressure Regulation and Glaucoma Subtypes, Investigative ophthalmology & visual science, 59 (2018) 693–701. [DOI] [PubMed] [Google Scholar]
- [20].Rao PV, Bioactive lysophospholipids: role in regulation of aqueous humor outflow and intraocular pressure in the context of pathobiology and therapy of glaucoma, J Ocul Pharmacol Ther, 30 (2014) 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ho LTY, Skiba N, Ullmer C, Rao PV, Lysophosphatidic Acid Induces ECM Production via Activation of the Mechanosensitive YAP/TAZ Transcriptional Pathway in Trabecular Meshwork Cells, Investigative ophthalmology & visual science, 59 (2018) 1969–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sherwood JM, Reina-Torres E, Bertrand JA, Rowe B, Overby DR, Measurement of Outflow Facility Using iPerfusion, PloS one, 11 (2016) e0150694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dacheva I, Ullmer C, Ceglowska K, Nogoceke E, Hartmann G, Muller S, Rejdak R, Nowomiejska K, Reich M, Nobl M, Tandogan T, Kretz FT, Auffarth GU, Koss MJ, Lysophosphatidic Acids and Autotaxin in Retinal Vein Occlusion, Retina, 36 (2016) 2311–2318. [DOI] [PubMed] [Google Scholar]
- [24].Pattabiraman PP, Rao PV, Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells, American journal of physiology. Cell physiology, 298 (2010) C749–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sawada H, Fukuchi T, Tanaka T, Abe H, Tumor necrosis factor-alpha concentrations in the aqueous humor of patients with glaucoma, Investigative ophthalmology & visual science, 51 (2010) 903–906. [DOI] [PubMed] [Google Scholar]
- [26].Taurone S, Ripandelli G, Pacella E, Bianchi E, Plateroti AM, De Vito S, Plateroti P, Grippaudo FR, Cavallotti C, Artico M, Potential regulatory molecules in the human trabecular meshwork of patients with glaucoma: immunohistochemical profile of a number of inflammatory cytokines, Molecular medicine reports, 11 (2015) 1384–1390. [DOI] [PubMed] [Google Scholar]
- [27].Clark AF, Wordinger RJ, The role of steroids in outflow resistance, Experimental eye research, 88 (2009) 752–759. [DOI] [PubMed] [Google Scholar]
- [28].Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL, Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling, Cell, 150 (2012) 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL, TEAD mediates YAP-dependent gene induction and growth control, Genes & development, 22 (2008) 1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tager AM, Autotaxin emerges as a therapeutic target for idiopathic pulmonary fibrosis: limiting fibrosis by limiting lysophosphatidic acid synthesis, Am J Respir Cell Mol Biol, 47 (2012) 563–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bain G, Shannon KE, Huang F, Darlington J, Goulet L, Prodanovich P, Ma GL, Santini AM, Stein AJ, Lonergan D, King CD, Calderon I, Lai A, Hutchinson JH, Evans JF, Selective Inhibition of Autotaxin Is Efficacious in Mouse Models of Liver Fibrosis, J Pharmacol Exp Ther, 360 (2017) 1–13. [DOI] [PubMed] [Google Scholar]
- [32].Kopczynski CC, Heah T, Netarsudil ophthalmic solution 0.02% for the treatment of patients with open-angle glaucoma or ocular hypertension, Drugs of today, 54 (2018) 467–478. [DOI] [PubMed] [Google Scholar]
- [33].Inoue T, Tanihara H, Ripasudil hydrochloride hydrate: targeting Rho kinase in the treatment of glaucoma, Expert opinion on pharmacotherapy, 18 (2017) 1669–1673. [DOI] [PubMed] [Google Scholar]
- [34].Weinreb RN, Liebmann JM, Martin KR, Kaufman PL, Vittitow JL, Latanoprostene Bunod 0.024% in Subjects With Open-angle Glaucoma or Ocular Hypertension: Pooled Phase 3 Study Findings, Journal of glaucoma, 27 (2018) 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ridley AJ, Signal transduction through the GTP-binding proteins Rac and Rho, Journal of cell science. Supplement, 18 (1994) 127–131. [DOI] [PubMed] [Google Scholar]
- [36].Zhao Y, Hasse S, Zhao C, Bourgoin SG, Targeting the autotaxin - Lysophosphatidic acid receptor axis in cardiovascular diseases, Biochemical pharmacology, 164 (2019) 74–81. [DOI] [PubMed] [Google Scholar]
- [37].Orosa B, Garcia S, Conde C, The autotaxin-lysophosphatidic acid pathway in pathogenesis of rheumatoid arthritis, European journal of pharmacology, 765 (2015) 228–233. [DOI] [PubMed] [Google Scholar]
- [38].Igarashi N, Honjo M, Kurano M, Yatomi Y, Igarashi K, Kano K, Aoki J, Aihara M, Increased aqueous autotaxin and lysophosphatidic acid levels are potential prognostic factors after trabeculectomy in different types of glaucoma, Scientific reports, 8 (2018) 11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Quigley HA, Vitale S, Models of open-angle glaucoma prevalence and incidence in the United States, Investigative ophthalmology & visual science, 38 (1997) 83–91. [PubMed] [Google Scholar]
- [40].Honjo M, Igarashi N, Nishida J, Kurano M, Yatomi Y, Igarashi K, Kano K, Aoki J, Aihara M, Role of the Autotaxin-LPA Pathway in Dexamethasone-Induced Fibrotic Responses and Extracellular Matrix Production in Human Trabecular Meshwork Cells, Investigative ophthalmology & visual science, 59 (2018) 21–30. [DOI] [PubMed] [Google Scholar]
- [41].Fini ME, Schwartz SG, Gao X, Jeong S, Patel N, Itakura T, Price MO, Price FW Jr., Varma R, Stamer WD, Steroid-induced ocular hypertension/glaucoma: Focus on pharmacogenomics and implications for precision medicine, Progress in retinal and eye research, 56 (2017) 58–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pattabiraman PP, Maddala R, Rao PV, Regulation of plasticity and fibrogenic activity of trabecular meshwork cells by Rho GTPase signaling, Journal of cellular physiology, 229 (2014) 927–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shimomura T, Miyamura N, Hata S, Miura R, Hirayama J, Nishina H, The PDZ-binding motif of Yes-associated protein is required for its co-activation of TEAD-mediated CTGF transcription and oncogenic cell transforming activity, Biochemical and biophysical research communications, 443 (2014) 917–923. [DOI] [PubMed] [Google Scholar]
- [44].Rinschen MM, Grahammer F, Hoppe AK, Kohli P, Hagmann H, Kretz O, Bertsch S, Hohne M, Gobel H, Bartram MP, Gandhirajan RK, Kruger M, Brinkkoetter PT, Huber TB, Kann M, Wickstrom SA, Benzing T, Schermer B, YAP-mediated mechanotransduction determines the podocyte’s response to damage, Science signaling, 10 (2017). [DOI] [PubMed] [Google Scholar]
- [45].Gokey JJ, Sridharan A, Xu Y, Green J, Carraro G, Stripp BR, Perl AT, Whitsett JA, Active epithelial Hippo signaling in idiopathic pulmonary fibrosis, JCI insight, 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wallace DM, Murphy-Ullrich JE, Downs JC, O’Brien CJ, The role of matricellular proteins in glaucoma, Matrix biology: journal of the International Society for Matrix Biology, 37 (2014) 174–182. [DOI] [PubMed] [Google Scholar]
- [47].Pattabiraman PP, Rinkoski T, Poeschla E, Proia A, Challa P, Rao PV, RhoA GTPase-induced ocular hypertension in a rodent model is associated with increased fibrogenic activity in the trabecular meshwork, The American journal of pathology, 185 (2015) 496–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.