Abstract
The prognostic significance of tumor-infiltrating lymphocytes has been determined in cancers of the lung, colon and breast, though there is no standardized method for using this prognostic indicator for lung cancer. We applied a modified version of the method proposed by the International Immuno-Oncology Biomarkers Working Group to primary lung adenocarcinoma, which uses histologic findings of hematoxylin and eosin sections. The study included a total cohort of 146 lung adenocarcinoma patients who underwent lobectomy with lymph node dissection at two hospitals between 2008 and 2012. The full-face sections of hematoxylin and eosin-stained slides were reviewed, and we evaluated the level of tumor-infiltrating lymphocytes as a percentage of the area occupied out of the total intra-tumoral stromal area. Histopathologic factors include histologic grade, necrosis, extracellular mucin, lymphovascular invasion, lymph node metastasis, level of tumor infiltrating lymphocytes, tertiary lymphoid structures around the tumor, and the presence of a germinal center in tertiary lymphoid structures. The high level of tumor-infiltrating lymphocytes was found to be significantly correlated with the histologic grade (p = 0.023), necrosis (p = 0.042), abundance of tertiary lymphoid structures(p<0.001) and presence of a germinal center in tertiary lymphoid structures (p = 0.004). A high level of tumor-infiltrating lymphocytes was associated with better progression-free survival (p = 0.011) as well as overall survival (p = 0.049). On multivariable analysis, high tumor-infiltrating lymphocyte levels were a good independent prognostic factor for progression-free survival (Hazard ratio: 0.389, 95% confidence interval: 0.161–0.941, p = 0.036). Histologic evaluation of tumor-infiltrating lymphocytes level in lung adenocarcinoma with H&E sections therefore has prognostic value in routine surgical pathology.
Introduction
Remarkable advances in immunotherapy have resulted in recent increased interest in cancer immunology. The immune system is now believed to have an important role in cancer development through “cancer immunoediting”, encompassing three processes: elimination, equilibrium, and escape [1, 2], and various studies support the role of immunosurveillance in lung cancer. Immune-mediated paraneoplastic syndromes in malignant tumors occur most frequently in lung cancer [3]. Organ transplant recipients, who are immunosuppressed, have a higher risk of developing non-small cell lung cancers [4]. Furthermore, Ichiki et al. reported that in the case of lung cancer, the immune system spontaneously recognized the tumor-associated antigens [5]. Also, the immune microenvironment in NSCLC is known to have a strong prognostic impact [6].
The prognostic significance of tumor-infiltrating lymphocytes (TILs) has been determined in various tumors, including cutaneous melanoma, breast cancer, and colon cancer. In addition, there are standardized methods for evaluating TILs in colon cancer and breast cancer [7, 8]. In colon cancer, the consensus Immunoscore is a reliable estimate of the risk of recurrence, which is a scoring system that represents the density of CD3+ and CD8+ T cells within the tumor along with the invasive margin [8]. The mean of the four percentiles obtained for each marker at either the tumor center or at invasive margins was calculated and translated into the Immunoscore scoring system. The final immunoscore was subdivided into 3 groups based on mean percentile: Immunoscore Low (0–25%), Intermediate (25–70%) and High (70–100%). The International TILs Working Group made a recommendation for evaluation of TILs in breast cancer. In brief, TILs should be estimated as a percentage for the stromal compartment within the tumor border [7]. All mononuclear cells encompassing lymphocytes and plasma cells should be scored, and granulocytes and other polymorphonuclear leukocytes should be excluded. Immunohistochemistry to assess the subtyping lymphocytes is not recommended outside of a research setting. The cutoff of stromal lymphocytes for lymphocyte predominant breast cancer is about 50–60% of the tumor stromal area [7].
Though many studies tried to delineate the prognostic significance of TILs in lung cancer, there had been no standardized consensus for TILs evaluation. In this background, the International Immuno-Oncology Biomarkers Working Group proposed the standardized method for TILs assessment in NSCLC [9].
In this study, we modified the TILs evaluation method proposed by the International Immuno-Oncology Biomarkers Working Group, then applied it to primary lung adenocarcinomas to evaluate its prognostic impact.
Materials and methods
Patients and tissue specimens
This study was performed retrospectively and included patients with primary lung adenocarcinoma who underwent resection with lymph node dissection between 2008 and 2012 at Pusan National University Hospital and Yangsan Pusan National University Hospital. Patients who had pre-operative chemo- or radiotherapy history were excluded, so all subjects were all pre-operatively chemo- and radiotherapy naïve. The final cohort was composed of 146 patients. Surgically resected specimens were cut along the bronchus and fixed in 10% buffered formalin overnight. For tumors which were grossly 3.0 cm or less in size whole tumor tissue was embedded for H&E sections, and in cases of those larger than 3.0 cm, 1 section per extra 1 cm was made additionally. All available hematoxylin and eosin (H&E) stained slides, which were made at the time of diagnosis, were used. Clinicopathological data were retrieved from the electric medical records and pathologic reports. Exemption from informed consent after de-identification of the patients’ information was approved by the Institutional Review Board of Pusan National University Hospital (H-1802-018-064) and Pusan National University Yangsan Hospital (05-2019-127).
Histologic evaluation
All available full-face H&E sections were used. Two pathologists (A Kim and DH Shin) reviewed the H&E sections using a multi-head microscope to result in consensus. Histopathological factors included histologic grade, necrosis, extracellular mucin production, lymphovascular invasion, lymph node metastasis, tertiary lymphoid structures (TLSs) around the tumor, a germinal center in TLSs, and TILs level. The histologic grade was based on the predominant subtype among five histological patterns, including lepidic, acinar (including cribriform), papillary, solid and micropapillary patterns [10, 11].
The method of TILs evaluation was primarily based on the guidelines from International Immuno-Oncology Biomarkers Working Group [9]. We assessed TILs level within the borders of the invasive tumor (in other words, within the stromal-tumor borderline), and areas within the tumor and particular area within the tumor was excluded in TILs evaluation. Area with necrosis, crush artifacts, fibrosis, and prior biopsy sites were excluded for TILs evaluation. Also, the peri-bronchial area was avoided in evaluating TILs, because bronchus-associated lymphoid tissue (BALT) originally resides in close proximity to the basal side of the bronchial epithelium [12]. Also, areas with aerogenic spread, which has no desmoplasia, and areas showing pure lepidic or intra-alveolar growth patterns without desmoplastic reaction were not included. However, stroma in fibrovascular cores of papillary structures were included. The invasive margin and the center of tumor were not separately considered and TILs within the tumor border (stromal-tumor borderline) were evaluated. A full assessment of average TILs level within the tumor border using all available full-face H&E sections was performed, so that the hotspots were included but not focused. TILs were reported for the stromal compartment within the tumor border. However, intra-tumoral TILs, which are TILs in tumor nests showing cell-to-cell contact with no intervening stroma and directly contacting tumor cells, were not considered. For the percentage of stromal TILs the area of stromal tissue, not the number of stromal cells, was used as a denominator. The level of TILs was calculated according to the percentage of stroma in the invasive area and was evaluated as a percentage in 10% increments. In cases of TILs level of less than 10%, a 1% or 5% criteria was applied (Fig 1). All mononuclear cells including lymphocytes and plasma cells were scored. Also, granulocytes and other polymorphonuclear leukocytes, especially intra-alveolar inflammatory cells including alveolar macrophages, were excluded from the TILs evaluation.
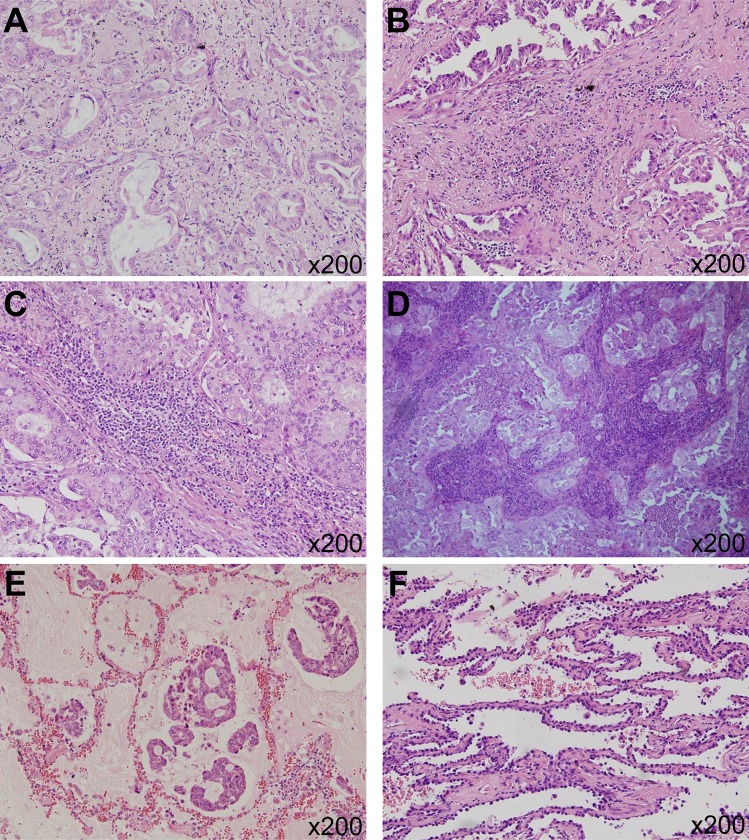
Fig 1. Assessment of tumor-infiltrating lymphocytes (TILs) level.
(A) 5% of TILs (x200) (B) 20% of TILs (x200) (C) 60% of TILs (x200) (D) 80% of TILs (x200) (E) Area with aerosol spread was excluded. (x200) (F) Area with lepidic growth pattern was not included in assessment of TILs. (x200).
TLS is a lymph node-like structure that is ectopic aggregation of lymphoid cells with specialized high endothelial venules (Fig 2). We evaluated the amount of TLSs as a percentage of the total circumference of the tumor front (stromal-tumor borderline) [13].
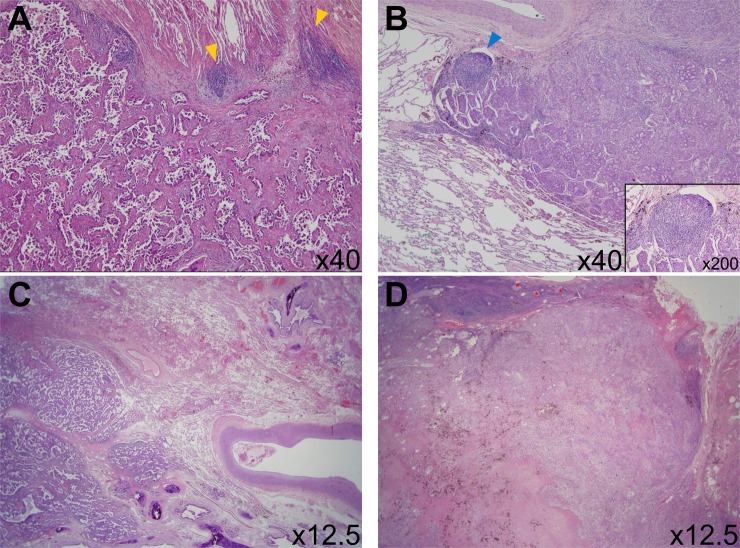
Fig 2. Assessment of tertiary lymphoid structures (TLSs).
(A) Yellow colored arrow head indicates TLSs without germinal center around invasive tumor. (x40) (B) Blue colored arrow head indicates TLS with germinal center around invasive tumor (x40) and inset shows the magnified image of TLS (x200) (C) Absence of TLSs around invasive tumor (x12.5) (D) Abundant TLSs with germinal center (red asterick) around invasive tumor (x12.5).
The cutoff for lymphocyte-predominant breast cancer is about 50–60%, but there is no standardized cutoff for lymphocyte-predominant lung cancer [7]. For statistical analysis, patients were subdivided into 2 categories (≥ 50% and <50%) according to arbitrary TILs level of 50%. The level of TLSs was categorized into 2 subgroups (low and high) based on the mean value.
Statistical analysis
SPSS statistical software (version19: IBM, Armonk, NY, USA) was utilized for all statistical analyses. The Chi-square test, two-tailed test, Fisher’s exact test, Spearman rank correlation test, Cox proportional hazards regression model, log-rank test and Kaplan-Meier survival analyses were used as appropriate. Overall survival (OS) was defined as the time from the day of diagnosis until the death of the patient as determined by Kaplan-Meier survival curves. Progression-free survival (PFS) was defined as the time from the day of diagnosis until any event including additional metastasis, recurrence or an increase in the size of pre-existing lesion, and death by Kaplan-Meier survival curves. For all tests, a p-value of less than 0.05 was considered to be statistically significant.
Results
Clinicopathologic characteristics of primary lung adenocarcinomas
A cohort of 146 patients with primary lung adenocarcinoma was included, composed of 77 females and 69 male patients. Patient age ranged from 35 to 85 years (mean ± standard deviation: 63.73 ± 10.176 years) and the size of the lung adenocarcinoma ranged from 0.4 to 7.2 cm (mean ± standard deviation: 2.82 ± 1.319cm). One hundred twenty patients (82.2%) had early stage (stage I and II) lung cancer, and the other 26 patients (17.5%) had lung cancer of advanced stage (stage III and IV). Thirty-three patients (22.6%) were categorized as high TILs level, whereas the other 113 patients (77.4) had low TILs level. The amount of TLSs ranged from 0% to 60% (mean ± standard deviation: 4.68 ± 9.197%). Forty patients (27.4%) had high TLSs around tumor and 106 patients (72.6%) had low TLSs around the tumor. Also, 17 patients (11.6%) had a germinal center in TLSs and 129 patients (88.4%) had no germinal center in TLSs (Table 1).
Table 1. Basic data of primary lung adenocarcinoma.
| Characteristic | Number (%)* |
|---|---|
| Age, mean ± SD | 63.730 ± 10.176 years |
| Size, mean ± SD | 2.820 ± 1.319 cm |
| Smoking history | |
| Never-smoker | 85 (58.2) |
| Smoker | 61 (41.8) |
| Histologic grade | |
| Well differentiated | 111 (76.0) |
| Moderately differentiated | 27 (18.5) |
| Poorly differentiated | 8 (5.5) |
| Necrosis in tumor | |
| Absent | 109 (74.7) |
| Present | 37 (25.3) |
| Extracellular production | |
| Absent | 123 (84.2) |
| Present | 23 (15.8) |
| Lymphovascular invasion | |
| Absent | 121 (82.9) |
| Present | 25 (17.1) |
| Lymph node metastasis | |
| Absent | 111 (76.0) |
| Present | 35 (24.0) |
| Stage (AJCC 7th edition) | |
| I | 95 (65.1) |
| II | 25 (17.1) |
| III | 13 (8.9) |
| IV | 13 (18.9) |
| Tumor infiltrating lymphocytes | |
| Low (<50%) | 113 (77.4) |
| High (≥ 50%) | 33 (22.6) |
| Tertiary lymphoid structures around tumor | |
| Absent | 89 (61.0) |
| Present | 57 (39.0) |
| Tertiary lymphoid structures around tumor | |
| Low | 106 (72.6) |
| High | 40 (27.4) |
| Germinal center in tertiary lymphoid structures | |
| Absent | 129 (88.4) |
| Present | (11.6) |
* except for age and sizes. SD, standard deviation.
Association between TILs level of lung adenocarcinomas and various histopathologic factors
The high level of TILs was significantly associated with a high histologic grade (p = 0.014), the presence of necrosis in tumor (p = 0.042), the presence of TLSs around tumor (p<0.001), the abundance of TLSs around tumor (p<0.001) and the presence of germinal center in TLSs (p = 0.004). However, the presence of a germinal center in TLSs was not correlated with high TILs level among the 57 patients with TLSs (data not shown). Additionally, the high TILs level correlated with absence of lymphovascular invasion, though this was not statistically significant (p = 0.067) (Table 2). In correlation analysis, the TILs level and TLSs abundance showed a positive association (rho = 0.438, p<0.01).
Table 2. Clinicopathologic correlation according to level of TILs+.
| Low TILs level (<50%) Number (%)* |
High TILs level (≥50%) Number (%)* |
P-value | |
|---|---|---|---|
| Age** | 64.170±10.350 | 62.210±9.552 | 0.333 |
| Size (cm) ** | 2.872±1.352 | 2.672±1.207 | 0.446 |
| Smoking history | 0.231 | ||
| Never-smoker | 69 (61.1%) | 16 (48.5%) | |
| Smoker | 44 (38.9%) | 17(51.5%) | |
| Histologic grade | 0.014 | ||
| Well differentiated | 91 (80.5%) | 20 (60.6%) | |
| Moderately differentiated | 18 (15.9%) | 9 (27.3%) | |
| Poorly differentiated | 4 (3.5%) | 4 (12.1%) | |
| Necrosis | 0.042 | ||
| Absent | 89 (78.8%) | 20 (60.6%) | |
| Present | 24 (21.2%) | 13 (39.4%) | |
| Extracellular mucin production | 0.786 | ||
| Absent | 96 (85.0%) | 27 (81.8%) | |
| Present | 17 (15.0%) | 6 (18.2%) | |
| Lymphovascular invasion | 0.067 | ||
| Absent | 90 (79.6%) | 31 (93.9%) | |
| Present | 23 (20.4%) | 2 (6.1) | |
| Lymph node metastasis | 0.103 | ||
| Absent | 82 (72.6%) | 29 (87.9%) | |
| Present | 31 (27.4%) | 4 (12.1%) | |
| Stage | 0.196 | ||
| Early (stage I and II) | 90 (79.6%) | 30 (90.9%) | |
| Advanced (stage III and IV) | 23 (20.4%) | 3 (9.1%) | |
| TLSs++ around tumor | <0.001 | ||
| Absence | 81 (71.7) | 8 (24.2) | |
| Presence | 32 (28.3) | 25 (75.8) | |
| TLSs around tumor | <0.001 | ||
| Low | 97 (85.8%) | 9 (27.3%) | |
| High | 16 (14.2%) | 24 (72.7%) | |
| Germinal center in TLSs | 0.004 | ||
| Absent | 105 (92.9%) | 24 (72.7%) | |
| Present | 8 (7.1%) | 9 (27.3%) | |
| EGFR+++ mutation | |||
| No data | 6 | 5 | |
| Absent | 48 (44.9%) | 14 (50.0%) | 0.627 |
| Present | 59 (55.1%) | 14 (50.0%) | |
| KRAS mutation | |||
| No data | 7 | 5 | |
| Absent | 92 (86.8%) | 24 (85.7%) | 0.868 |
| Present | 14 (13.1%) | 4 (14.3%) |
* except for age and sizes.
** t-test was performed and the equality of variances was assumed. SD, standard deviation. The Pearson’s Chi-square test or Fisher’s exact test was used as appropriate for the other variables. EGFR and KRAS mutation was detected by pyrosequencing.
+TILs, tumor-infiltrating lymphocytes
++TLSs, tertiary lymphoid structures
+++EGFR, epidermal growth factor receptor
Prognostic significance of TILs in lung adenocarcinomas
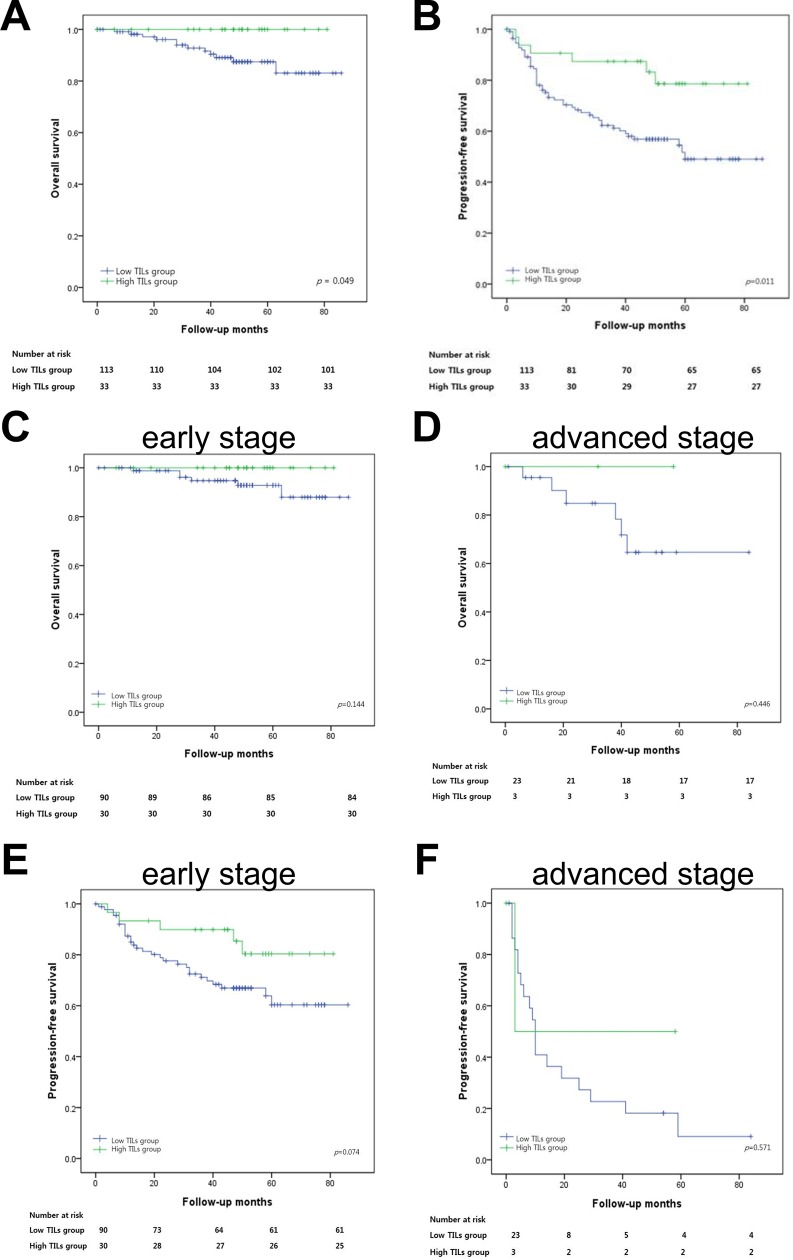
The median follow-up was 48 months (0–86), and twelve patients died during the follow-up period. Fifty-two patients were found to have either metastasis or recurrence. According to Kaplan-Meier survival analysis, a high level of TILs in lung adenocarcinoma was significantly associated with a better progression-free survival (p = 0.011) and overall survival (p = 0.049). In early stage disease, high TILs level tended to associate with better progression-free survival, though it was not statistically significant (p = 0.078) (Fig 3). On multivariable analysis, high TILs level remained a good prognostic factor for progression-free survival after correcting for factors which were significant on univariate analysis (Hazard ratio: 0.389, 95% confidence interval: 0.161–0.941, p = 0.036, Table 3).
Fig 3. Survival analysis in resected lung adenocarcinoma.
(A) Overall survival according to tumor-infiltrating lymphocytes (TILs) level (B) Progression-free survival according to TILs level (C) Overall survival according to TILs level in early stage (stage I and II) disease (D) Overall survival according to TILs level in advanced stage (stage III and IV) disease (E) Progression-free survival according to TILs level in early stage (stage I and II) disease (F) Progression-free survival according to TILs level in advanced stage (stage III and IV) disease.
Table 3. Univariate and multivariable analyses of overall survival and progression free survival.
| Overall survival | Progression free survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Univariate analysis | Multivariable analysis* | |||||||
| HR**,+ | 95% CI++ | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (≥64 yeas vs <64 years) |
1.381 | 0.438–4.355 | 0.581 | 1.443 | 0.843–2.470 | 0.181 | |||
| Histologic grade (moderately & poorly vs well) |
0.773 | 0.168–3.550 | 0.741 | 2.286 | 1.301–4.018 | 0.004 | 2.034 | 1.105–3.743 | 0.023 |
| Lymphovascular invasion (present vs absent) |
5.300 | 1.652–17.004 | 0.005 | 4.901 | 2.767–8.681 | <0.001 | 2.752 | 1.369–5.531 | 0.004 |
| Stage (III & IV vs I & II) |
7.009 | 2.210–22.228 | 0.001 | 5.197 | 2.970–9.094. | <0.001 | 2.627 | 1.323–5.216 | 0.006 |
| TILs+++ (≥50% vs <50%) |
0.032 | 0.000–8.784 | 0.230 | 0.353 | 0.151–0.825 | 0.006 | 0.389 | 0.161–0.941 | 0.036 |
*Multivariable analysis was performed with covariables showing p-value of less than 0.10 in the univariate analyses for multivariable analysis.
**The likelihood ratio test was done to ensure the proportional hazard assumptions in the Cox regression model.
+HR, hazard ratio
++CI, confidence interval
+++TILSs, tumor-infiltrating lymphocytes
Discussion
There have been various studies to determine the significance of the immune microenvironment in NSCLCs. Most of these have focused on the subpopulation of TILs using immunohistochemistry for various subtypes of immune cells [14–20]. High levels of CD8 positive T-cell infiltration showed positive effects on prognosis in many reports [14, 16, 18, 20], however these studies used different cutoffs to define low and high groups, and some used manual counting while others used digital image analysis. Although TILs assessment with routine H&E sections can be easily applied to the routine workflow of pathology and can facilitate rapid accumulation of knowledge of TILs, there is only limited data addressing generalized TILs based on H&E section evaluation. Kilic et al. reported that a high level of TILs was significantly associated with lower disease-recurrence in large sized NSCLCs [21]. Horne et al. showed that patients with a high intra-tumoral TILs level had better recurrence-free survival in stage IA NSCLCs [22]. Also, intense lymphocytic infiltration was associated with better overall survival and disease-free survival in the discovery and validation set of resected NSCLCs [23]. Feng et al. also showed that a higher density of TILs was correlated with a better postoperative survival time in completely resected stage IIIA NSCLCs using the method previously proposed by Kilic and Horne [24]. However, the methodology for TILs evaluation was briefly described in these studies, although they showed consistent positive impacts. Recently, Rakaee et al. reported that a high level of stromal TILs was associated with better disease-specific survival, overall survival, and disease-free survival [25]. Also, they suggested the methodology of TILs assessment, adapted from the recommendations by an International TILs Working Group of breast cancer, could be applied to NSCLCs.
We evaluated TILs level primarily based on a standardized method from the International Immuno-Oncology Biomarkers Working Group, which also chiefly referred to the International TILs Working Group recommendation. The method from the International Immuno-Oncology Biomarkers Working Group precisely considered the structures and growth patterns of the lung adenocarcinoma including lepidic growth, aerogenous spread, and papillary structures.
A high TILs level was associated with a high histologic grade (p = 0.014) in this study. This may be due to a small number of high histologic grades and uneven distribution of histologic grades in our cohort. High level of TILs was an independent good prognostic factor on progression-free survival, however, multivariable analysis for overall survival was not possible, because there were only 12 deaths in our cohort.
We have solely evaluated the prognostic significance of histologic TILs level in lung adenocarcinoma, while others have included NSCLCs including squamous cell carcinoma and the like. Also, this study has additionally avoided the peri-bronchial area in evaluation of TILs to exclude BALTs in this study. These are found in the lungs of fetus and disappear in normal adults [26], though BALTs were found in a small subset of adults in autopsy series [27]. They are also significantly more common in smokers than non-smokers [28].
Considering the prognostic significance of TILs, a better understanding of TILs and the mechanism of TILs recruitment into the tumor is critical in the development of effective immunotherapy. TLS mature dendritic cells are suggested to have a major role in the shaping of the tumor immune contexture in NSCLCs [29, 30]. The density of mature dendritic cells, which are specific markers of TLSs, was positively correlated with the density of TILs, especially CD4 positive and T-bet positive Th1 cells in early stage NSCLCs [29]. Also, the density of dendritic cells was significantly associated with strong infiltration of T cells and high density of mature dendritic cells associated with long-term survival in completely resected NSCLCs [18]. Taken together, these data suggest the role of TLSs in T-cell recruitment in NSCLCs. This is in accordance with our data which shows that TILs level and TLSs abundance have positive association (rho = 0.438, p<0.01).
Although two pathologists reviewed the slides, we used the multi-head microscope to reach a consensus. We could not assess the inter-observer variability or reproducibility in this study. Also, comparison with morphometric analysis of histologic TILs level should be performed in future studies.
Conclusion
Histologic assessment of TILs with H&E section in lung adenocarcinoma has prognostic value. The level of TILs was significantly associated with abundance of TLSs around the invasive tumor. Also, the standardized method proposed by the International Immuno-Oncology Biomarkers Working Group could be useful in routine surgical pathologic practice.
Acknowledgments
This study was supported by Biomedical Research Institute Grant (2017–029), Pusan National University Hospital.
Data Availability
All relevant data are within the manuscript and Supporting Information files. The biospecimens and data used for this study were provided by the Biobank of Pusan National University Hospital, a member of the Korea Biobank Network.
Funding Statement
This study was supported by Biomedical Research Institute Grant (2017-029), Pusan National University Hospital. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. 10.1016/j.cell.2011.02.013 . [DOI] [PubMed] [Google Scholar]
- 2.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70. 10.1126/science.1203486 . [DOI] [PubMed] [Google Scholar]
- 3.Richardson GE, Johnson BE. Paraneoplastic syndromes in lung cancer. Curr Opin Oncol. 1992;4(2):323–33. 10.1097/00001622-199204000-00014 . [DOI] [PubMed] [Google Scholar]
- 4.Engels EA, Pfeiffer RM, Fraumeni JF, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891–901. 10.1001/jama.2011.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ichiki Y, Takenoyama M, Mizukami M, So T, Sugaya M, Yasuda M, et al. Simultaneous cellular and humoral immune response against mutated p53 in a patient with lung cancer. J Immunol. 2004;172(8):4844–50. 10.4049/jimmunol.172.8.4844 . [DOI] [PubMed] [Google Scholar]
- 6.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. Epub 2012/03/15. 10.1038/nrc3245 . [DOI] [PubMed] [Google Scholar]
- 7.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–71. Epub 2014/09/13. 10.1093/annonc/mdu450 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128–39. Epub 2018/05/10. 10.1016/S0140-6736(18)30789-X . [DOI] [PubMed] [Google Scholar]
- 9.Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-Small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. 10.1097/PAP.0000000000000161 2017;24(6):311–35. PubMed Central PMCID: PMC5638696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc. 2011;8(5):381–5. 10.1513/pats.201107-042ST . [DOI] [PubMed] [Google Scholar]
- 11.Tsuta K, Kawago M, Inoue E, Yoshida A, Takahashi F, Sakurai H, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer. 2013;81(3):371–6. Epub 2013/07/26. 10.1016/j.lungcan.2013.06.012 . [DOI] [PubMed] [Google Scholar]
- 12.Hwang JY, Randall TD, Silva-Sanchez A. Inducible Bronchus-Associated Lymphoid Tissue: Taming Inflammation in the Lung. Front Immunol. 2016;7:258 Epub 2016/06/30. 10.3389/fimmu.2016.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SJ, Hwang CS, Kim YK, Lee HJ, Ahn SJ, Shin N, et al. Expression of Myxovirus Resistance A (MxA) is Associated with Tumor-Infiltrating Lymphocytes (TILs) in Human Epidermal Growth Factor Receptor 2(HER2)-Positive Breast Cancers. Cancer Res Treat. 2016. 10.4143/crt.2016.098 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14(16):5220–7. 10.1158/1078-0432.CCR-08-0133 . [DOI] [PubMed] [Google Scholar]
- 15.Al-Shibli K, Al-Saad S, Donnem T, Persson M, Bremnes RM, Busund LT. The prognostic value of intraepithelial and stromal innate immune system cells in non-small cell lung carcinoma. Histopathology. 2009;55(3):301–12. 10.1111/j.1365-2559.2009.03379.x . [DOI] [PubMed] [Google Scholar]
- 16.Donnem T, Hald SM, Paulsen EE, Richardsen E, Al-Saad S, Kilvaer TK, et al. Stromal CD8+ T-cell Density—A Promising Supplement to TNM Staging in Non-Small Cell Lung Cancer. Clin Cancer Res. 2015;21(11):2635–43. Epub 2015/02/13. 10.1158/1078-0432.CCR-14-1905 . [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K, Kadota K, Sima CS, Nitadori J, Rusch VW, Travis WD, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor β2 (IL-12Rβ2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. 2013;31(4):490–8. Epub 2012/12/26. 10.1200/JCO.2012.45.2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74(3):705–15. Epub 2013/12/23. 10.1158/0008-5472.CAN-13-1342 . [DOI] [PubMed] [Google Scholar]
- 19.Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpréville V, et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194(7):3475–86. Epub 2015/02/27. 10.4049/jimmunol.1402711 . [DOI] [PubMed] [Google Scholar]
- 20.Geng Y, Shao Y, He W, Hu W, Xu Y, Chen J, et al. Prognostic Role of Tumor-Infiltrating Lymphocytes in Lung Cancer: a Meta-Analysis. Cell Physiol Biochem. 2015;37(4):1560–71. Epub 2015/10/30. 10.1159/000438523 . [DOI] [PubMed] [Google Scholar]
- 21.Kilic A, Landreneau RJ, Luketich JD, Pennathur A, Schuchert MJ. Density of tumor-infiltrating lymphocytes correlates with disease recurrence and survival in patients with large non-small-cell lung cancer tumors. J Surg Res. 2011;167(2):207–10. Epub 2009/09/23. 10.1016/j.jss.2009.08.029 . [DOI] [PubMed] [Google Scholar]
- 22.Horne ZD, Jack R, Gray ZT, Siegfried JM, Wilson DO, Yousem SA, et al. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res. 2011;171(1):1–5. Epub 2011/04/22. 10.1016/j.jss.2011.03.068 . [DOI] [PubMed] [Google Scholar]
- 23.Brambilla E, Le Teuff G, Marguet S, Lantuejoul S, Dunant A, Graziano S, et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non-Small-Cell Lung Cancer. J Clin Oncol. 2016;34(11):1223–30. Epub 2016/02/01. 10.1200/JCO.2015.63.0970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng W, Li Y, Shen L, Cai XW, Zhu ZF, Chang JH, et al. Prognostic value of tumor-infiltrating lymphocytes for patients with completely resected stage IIIA(N2) non-small cell lung cancer. Oncotarget. 2016;7(6):7227–40. 10.18632/oncotarget.6979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakaee M, Kilvaer TK, Dalen SM, Richardsen E, Paulsen EE, Hald SM, et al. Evaluation of tumor-infiltrating lymphocytes using routine H&E slides predicts patient survival in resected non-small cell lung cancer. Hum Pathol. 2018. Epub 2018/06/06. 10.1016/j.humpath.2018.05.017 . [DOI] [PubMed] [Google Scholar]
- 26.Tschernig T, Pabst R. Bronchus-associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology. 2000;68(1):1–8. 10.1159/000028109 . [DOI] [PubMed] [Google Scholar]
- 27.Hiller AS, Tschernig T, Kleemann WJ, Pabst R. Bronchus-associated lymphoid tissue (BALT) and larynx-associated lymphoid tissue (LALT) are found at different frequencies in children, adolescents and adults. Scand J Immunol. 1998;47(2):159–62. 10.1046/j.1365-3083.1998.00276.x . [DOI] [PubMed] [Google Scholar]
- 28.Richmond I, Pritchard GE, Ashcroft T, Avery A, Corris PA, Walters EH. Bronchus associated lymphoid tissue (BALT) in human lung: its distribution in smokers and non-smokers. Thorax. 1993;48(11):1130–4. 10.1136/thx.48.11.1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26(27):4410–7. 10.1200/JCO.2007.15.0284 . [DOI] [PubMed] [Google Scholar]
- 30.Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74(3):705–15. Epub 2013/12/25. 10.1158/0008-5472.CAN-13-1342 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript and Supporting Information files. The biospecimens and data used for this study were provided by the Biobank of Pusan National University Hospital, a member of the Korea Biobank Network.