Abstract
Osteosarcoma (OSA) is the most common bone tumor in dogs. Protein phosphatase 2A (PP2A), an evolutionary conserved serine/threonine protein phosphatase, is a crucial tumor suppressor. SET is a PP2A inhibitory protein that directly interacts with PP2A and suppresses its phosphatase activity. SET has been reported as a contributor of wide range of human and dog tumor malignancies. However, the role of SET in canine OSA (cOSA) remains unknown. In this study, we investigated the role of SET in cOSA by using 2 cOSA cell lines: POS (primary origin) and HM-POS (metastatic origin). Knockdown (KD) of SET expression was noted to slightly suppress POS cell proliferation only. Furthermore, SET KD effectively suppressed colony formation ability of both POS and HM-POS cells. SET KD was observed to repress ERK1/2, mTOR, E2F1, and NF-κB signaling in HM-POS cells, whereas it inhibited only ERK1/2 signaling in POS. Further, it was observed that SET-targeting drug, FTY720, exerted anti-cancer effects in both POS and HM-POS cells. Moreover, the drug also enhanced the anti-cancer effect of cisplatin. The data suggested that a combination therapy, based on SET targeting drugs and cisplatin, could be a potent strategy for cOSA.
Keywords: canine, osteosarcoma, protein phosphatase 2A (PP2A), SET
Osteosarcoma (OSA) is a predominant bone tumor diagnosis in dogs. Although, any dog could potentially develop OSA, but dog breeds with body weight above 40 kg are at an increased risk of developing OSA [16, 23]. Surgery, limb amputation and limb salvage/sparing, constitutes the first choice of treatment, however, more than 90% of the patients possess micro-metastasis [16, 23]. Therefore, adjuvant and non-adjuvant chemotherapy is important to improve the survival rate. In canine OSA (cOSA), adjuvant chemotherapy with cisplatin, carboplatin, or doxorubicin have been associated with increased survival rates over amputation alone [1, 20]. OSA is a rare cancer in humans, whereas its incidence rate in dogs is 27-times higher [22], suggesting cOSA as a good model for human disease. The standard adjuvant chemotherapeutic approach in human OSA comprises of a combination of cisplatin, methotrexate, and doxorubicin, and has remained relatively unaltered for years [25, 26]. Prognosis in both the species is poor and the survival rate has not improved in decades. Therefore, establishment of novel anti-cancer drugs is urgently required.
Protein phosphatase 2A (PP2A) is an evolutionally conserved serine/threonine phosphatase that suppresses the activity of multiple intracellular signals such as Akt, ERK1/2, and mTOR signaling which regulates cancer cell growth, stemness, and survival [12, 18, 19]. PP2A is reported to negatively regulate the activity of transcription factors such as c-Myc, E2F1, and NF-κB, that mediates tumor cell stemness and the cell cycle [9, 12, 19]. Inhibition of PP2A activity is required for tumor transformation and increased expression of endogenous PP2A inhibitory proteins (such as SET, CIP2A, and PME-1) contributes to lower PP2A activity in cancer cells [5, 21]. We have previously reported that SET contributes to tumor malignancy in dogs including lymphoma, melanoma, and mammary tumor [8, 10, 12]. In these cancers, SET-targeting drugs, such as FTY720 and OP449; dissociates SET from PP2A and exhibits anti-cancer effects via PP2A reactivation. Therefore, SET is considered to be an attractive target for anti-cancer drug.
Here, we demonstrated that SET contributes to an increase in phosphorylation/activity of ERK1/2, mTOR, E2F1, c-Myc, and NF-κB in cOSA cell lines. Moreover, FTY720 displayed anti-cancer effects and enhanced cisplatin’s effect in cOSA cell lines. The results suggested the potential clinical application of SET inhibitors for cOSA.
MATERIALS AND METHODS
Cell culture
Canine osteosarcoma cell lines, POS and HM-POS [2, 11] were cultured in RPMI 1640 containing 10% fetal bovine serum (FBS) and 1 ×antibiotic/antimycotic (Life Technologies, Carlsbad, CA, U.S.A.).
Antibodies
Antibodies were obtained from the indicated supplier: anti-SET (sc-133138), anti-p70S6K (sc-230), anti-E2F1 (sc-193), anti-NF-κB p65 (sc-372), anti-c-Myc (sc-764) (Santa Cruz Biotech, Santa Cruz, CA, U.S.A.), anti-phospho-ERK p42/p44 (9101), anti-ERK p42/p44 (9107), anti-MEK1/2 (4694), anti-phospho-MEK1/2 (9154), anti-phospho-Thr389 p70S6K (9234), anti-phospho-Ser473 Akt1 (4060), anti-phospho-Thr308 Akt1 (13038), anti-Akt (2920), anti-phospho-Ser536 NF-κB p65 (3033) (Cell Signaling, Danvers, MA, U.S.A.), anti-PP2Ac (07-324) (Merck Millipore, Burlington, MA, U.S.A.), anti-p97/VCP (GTX113030) (Gene Tex, Hsinchu City, Taiwan), anti-phospho-Ser62 c-Myc (71–161) (BioAcademia, Osaka, Japan), anti-phospho-Ser364 E2F1 (D246-3) (MBL, Nagoya, Japan).
hRNA and lentivirus production
hRNA sequences and procedure for lentivirus production was previously described [8]. Briefly, to produce lentiviruses, 3 µg of pLVSIN, 2.3 µg of a packaging plasmid (psPAX2) and 1.3 µg of a coat-protein plasmid expressing vesicular stomatitis virus G protein (pMD2.G) were transfected into Lenti-X 293T cells (Takara Bio, Kusatsu, Japan) cultured in 60-mm dishes using PEI Max (Polysciences, Warrington, PA, U.S.A.) according to the manufacturer’s instruction. Viral supernatants were collected after 48 hr, and after filtering (0.22 µm), were added to cells for 12 hr.
Immunoblotting
Proteins were extracted from 1.0 × 106 cells that were cultured in RPMI 1640 containing 10% FBS and 1× antibiotic/antimycotic on 6 cm dish for 24 hr. Immunoblotting was performed as previously described [8]. Briefly, cells were lysed in a buffer containing 50 mM Tris-HCl (pH 8.0), 5 mM EDTA, 5 mM EGTA, 1% Triton X100, 1 mM Na3VO4, 20 mM sodium pyrophosphate and Roche’s complete protease inhibitor cocktail. The proteins were separated by SDS-PAGE and transferred onto nitrocellulose membrane (Wako, Osaka, Japan). Membranes were blocked with 0.5% skim milk and treated with primary antibodies. Immunoreactive bands were detected using Western Lightning ECL Pro (PerkinElmer, Freiburg, Germany) and visualized using a LAS-3000 luminescent image analyzer (Fujifilm, Tokyo, Japan).
Cell proliferation and cell viability assay
A seeding density of 1.0 × 104 cells in 24-well plates were cultured in RPMI 1640 containing 10% FBS and 1 × antibiotic/antimycotic for 4 days. Cell Counting Kit-8 (CCK8, Dojindo, Kumamoto, Japan) was used to analyze cell proliferation according to the manufacturer’s instruction. For cell viability analysis, 1.0 × 104 cells were seeded on 24-well plate, and drugs were added to the medium after 24 hr. After additional 72 hr, CCK8 was used to analyze cell viability.
Colony formation assay
A seeding density of 1.0 × 102 cells in 6-well plates were cultured in RPMI 1640 containing 10% FBS and 1 × antibiotic/antimycotic. After 10 days, cells were fixed with 99.5% ethanol, colonies were stained with Giemsa solution, and the number of colonies was counted.
Statistical analysis
Statistical analysis was performed using SigmaPlot (HULINKS). The results are expressed as means ± S.E. Student’s t test was used for comparison between two groups. Groups more than three were compared using one-way analysis of variance, after which Fisher LSD test was used. For all analyses, a probability value of P<0.05 was considered statistically significant.
RESULTS
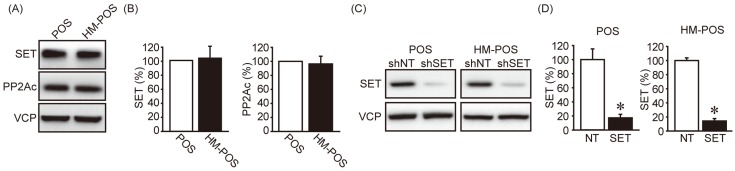
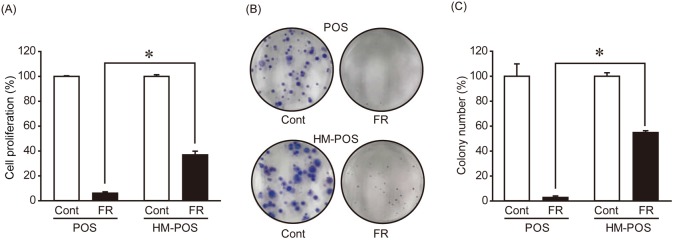
To analyze the role of SET in cOSA, 2 cOSA cell lines, POS (primary origin) and HM-POS (established from nude mice lung metastatic site of POS cells) were used [2, 11]. The protein levels of SET and PP2A were not significantly different between the POS and HM-POS cells (Fig. 1A and 1B). For suppressing SET expression, non-target shRNA (shNT) and SET-targeting shRNA (shSET) were stably expressed in the cOSA cell lines. SET protein expressions were observed as effectively suppressed by shSET as compared to shNT (Fig. 1C and 1D). Furthermore, the effect of SET knockdown (KD) on tumorigenic phenotype of cOSA cell lines was analyzed. SET KD was noted to slightly suppress POS’s cell proliferation only (Fig. 2A). On the other hand, SET KD effectively suppressed the colony formation ability of both POS and HM-POS cells (Fig. 2B and 2C). The data suggested that SET plays an important role in tumor malignancy of cOSA.
Fig. 1.
Expression of SET protein in canine osteosarcoma cell lines. (A, B) SET and PP2Ac protein levels were analyzed by immunoblotting. Representative images (A) and quantitative data (B) from three independent experiments are shown. VCP was used as a loading control. The protein levels were normalized to POS as 100%. (C, D) The effect of non-targeting shRNA (shNT) and SET targeting shRNA (shSET) on SET protein level was determined by immunoblotting. Representative images (C) and quantitative data (D) from three independent experiments are shown. The SET protein levels were normalized to that of shNT as 100%. *P<0.05 vs. shNT.
Fig. 2.
SET knockdown exerts anti-tumor effects on canine osteosarcoma cell lines. (A) Non-target shRNA (shNT) and SET-targeting shRNA (shSET) were stably expressed in POS and HM-POS cells. Cell proliferation was analyzed by Cell Counting Kit-8 (CCK8). Quantitative data from three independent experiments with biological duplicates are shown. The cell proliferation rates are normalized to that at Day 1 of shNT as 100%. *P<0.05 vs. shNT. (B, C) Colony formation ability of POS and HM-POS cells expressing shNT and shSET was analyzed. Representative image (B) and quantitative data (C) from three independent experiments with biological duplicates are shown. *P<0.05 vs. shNT.
Further, the effects of SET KD on cell signaling were analyzed. SET KD has been reported to suppress multiple cellular signaling including Akt, ERK1/2, and mTOR and also the activity of transcriptional factors like E2F1, NF-κB, and c-Myc in PP2A dependent manner [9, 12, 14, 19]. We observed that SET KD suppressed ERK1/2 phosphorylation in both POS and HM-POS cells, without altering the activity of an upstream kinase, MEK1/2 (Fig. 3A–D). The data indicated that SET inhibits PP2A complex, which is responsible for ERK1/2 dephosphorylation. A decreased in p70S6K (mTOR complex 1 (mTORC1) signaling activity marker) phosphorylation in HM-POS cells was also observed (Fig. 3E–F). PDK1 and mTORC2 mediate the phosphorylation of Akt’s Thr308 and Ser473, respectively [15]. SET KD, however, did not affect Akt phosphorylation in both the cell lines (Fig. 3G–H).
Fig. 3.
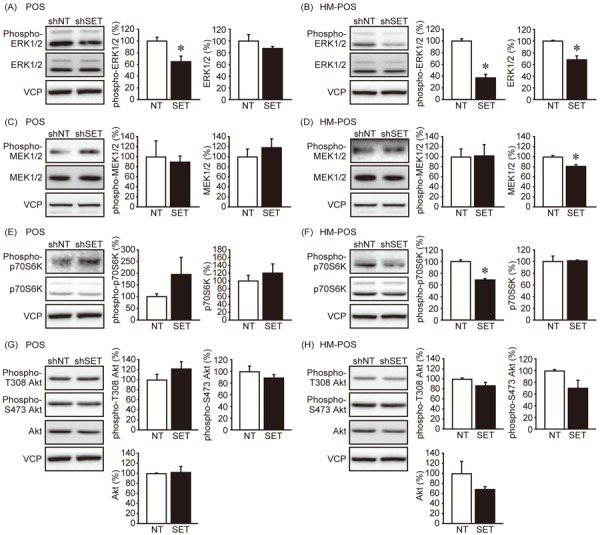
The effect of SET knockdown on cell signaling. The effect of SET knockdown on the phosphorylation and protein levels of ERK1/2 (A, B), MEK1/2 (C, D), p70S6K (E, F), and Akt (G, H) was analyzed by immunoblotting. Representative image and quantitative data from three independent experiments are shown. Band densities were normalized to that of shNT as 100%. *P<0.05 vs. shNT.
Furthermore, we analyzed the effect of SET KD on the phosphorylation levels of transcriptional factors E2F1, NF-κB, and c-Myc (Fig. 4A–F). In POS cells, the phosphorylation and protein levels of these transcriptional factors were not affected. Whereas, SET KD suppressed E2F1 and NF-κB p65 phosphorylation in HM-POS cells and the sites were associated with protein stability and activity, respectively. Interestingly, SET KD increased c-Myc protein level in HM-POS cells. The results suggested that enhanced activity of ERK1/2, mTORC1, E2F1, and NF-κB signaling might be associated with SET-mediated cOSA malignancy.
Fig. 4.

The effect of SET knockdown on the transcriptional factor activity. The effect of SET knockdown on the phosphorylation and protein levels of E2F1 (A, B), NF-κB (C, D), and c-Myc (E, F) was analyzed by immunoblotting. Representative image and quantitative data from three independent experiments are shown. Band densities were normalized to that of shNT as 100%. *P<0.05 vs. shNT.
Although SET KD decreased ERK1/2 phosphorylation levels in both POS and HM-POS cells, it suppressed cell proliferation only in POS cells. To clarify this discrepancy, we analyzed the effects of ERK1/2 inhibitor, FR180204, on cell proliferation and colony formation ability. FR180204 suppressed cell proliferation and colony formation in both cell lines, and POS cells showed significantly higher sensitivity for this drug (Fig. 5A–C). These data indicate that tumorigenic potential of POS cells strongly depend on ERK1/2 activation.
Fig. 5.
The effect of ERK1/2 inhibitor on cell proliferation and colony formation ability of canine osteosarcoma cell lines. (A) POS and HM-POS cells were cultured without (Cont) or with 100 µM of FR180204 (FR) for 72 hr, and the cell viability was analyzed by CCK8. *P<0.05. (B, C) Colony formation ability of POS and HM-POS cells treated with 100 µM of FR for 10 days was analyzed. Representative image (B) and quantitative data (C) from three independent experiments performed biological duplicate are shown. *P<0.05.
FTY720 (Fingolimod), a sphingosine analog, is used as an immunosuppressant in human multiple sclerosis patients. FTY720 has been reported to directly interact with SET and restore PP2A activity [17]. We have previously reported that FTY720 selectively exerts anti-cancer effects on SET-KD-sensitive canine tumor cell lines [12]. The anti-cancer effect of FTY720 on cOSA (Fig. 6A) was analyzed to reveal that it affects both POS and HM-POS cells in a dose-dependent manner. The anti-cancer effect of FTY720 was observed as almost same between 2 cell lines. Cisplatin is one of the most commonly used drugs for cOSA treatment. We analyzed if its combination with FTY720 exerts any additional anti-cancer effects on cOSA cells (Fig. 6B). Co-treatment with FTY720 and cisplatin exhibited effectively suppressed cOSA cell survival rate than FTY720 or cisplatin treatment alone. The result suggested that the combination therapy, based on SET targeting drugs and cisplatin, could act as a potent strategy for cOSA.
Fig. 6.
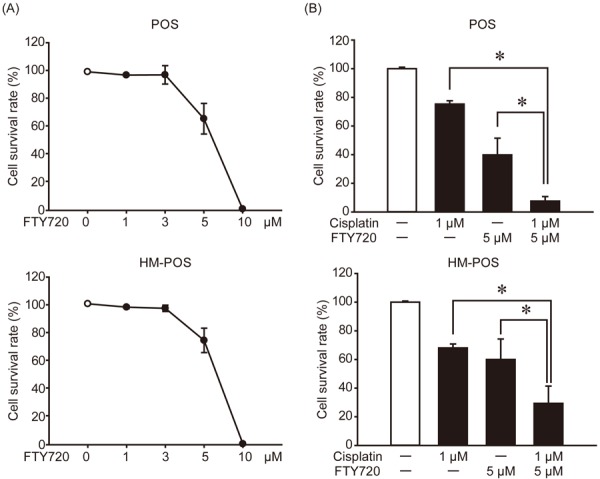
FTY720 exerts anti-cancer effect on cOSA cells and enhances the effects of cisplatin. (A) POS and HM-POS cells were treated with indicated dose of FTY720 for 72 hr, and the cell viability was analyzed by CCK8. (B) POS and HM-POS cells were treated with or without FTY720 (5 µM) and/or cisplatin (1 µM). After 72 hr, the cell viability was analyzed by CCK8. Cell viability was normalized to that without treatment as 100%. Quantitative data from two independent experiments with biological duplicates are shown. *P<0.05.
DISCUSSION
In this study, SET was suggested as a novel anti-cancer target for cOSA. Consistent with our previous report in human cancer cell lines [9], SET KD affects on colony formation ability, a marker for cancer cell stemness, than cell proliferation. It has been reported previously that SET expression level is related to PP2A inhibitory ability and tumor malignancy [6]. The protein expression levels of SET and PP2A are almost similar between POS and HM-POS cells. The observed effects of SET KD and SET-targeting drug on tumorigenic ability were also not much different between these 2 cell lines. However, a difference in the effect of SET KD on cell signaling was noted. SET KD was observed to suppress ERK1/2 signaling in both cell lines, whereas it also suppressed mTORC1, E2F1, and NF-κB signaling in HM-POS cell.
Abnormal activation of ERK1/2 signaling has been implicated as tumor proliferation, migration, and metastasis in human OSA [4]. Our data suggests that ERK1/2 activation plays an important role in SET-mediated cOSA tumor malignancy. ERK1/2 inhibitor suppressed cell proliferation and colony formation of both cell lines, but the sensitivity for the drug is much higher in POS cells compared with HM-POS cells, may be a reason for that only POS cells showed decreased cell proliferation by SET KD. ERK1/2 has been reported as a substrate of PP2A [24]. It was observed that MEK1/2 (an upstream kinase of ERK1/2 phosphorylation) activity was not affected by SET KD, suggesting that re-activation of PP2A was involved in decreased ERK1/2 phosphorylation by SET KD.
It remains unclear why SET KD affects multiple cellular signaling only in HM-POS, even though SET protein levels were almost same in both the cell lines. In humans, 2 highly homological SET isoforms (SETα and SETβ) exists and high expression ratio of SETα/SETβ is observed to be associated with poor prognosis of chronic lymphocytic leukemia patient [3]. We have previously reported in human gastric cancer cell lines that SET KD-induced suppression of tumorigenic potential was rescued by SETα, but not by SETβ [9]. Therefore, expression ratio of SET isoforms might be an important parameter in understanding the role of SET in tumor cell. In canine, at least 4 SET isoforms are reported [27]. Canine SETα protein has approximately 94% homology with human SETα. In this study, we utilized shRNA targeting all the canine SET isoforms. Therefore, difference in expression pattern of SET isoforms between the cell lines might affect the outcome of SET KD.
Drug sensitivity screening using HeLa cells revealed that among PP2A inhibitory proteins, SET depletion was most effective to result in cells sensitivity against anti-cancer drugs [13]. In this study, SET-targeting drug FTY720 was revealed to enhance the anti-cancer effects of cisplatin on cOSA cell lines. Cisplatin induces cancer cell death by preventing DNA replication and suspending cell division [7]. We have previously reported in human cell lines, that SET KD effectively suppresses cancer cell stemness over cell proliferation [9]. Consistent with this, SET KD effectively decreased colony formation ability of cOSA cells, but only slightly affected cell proliferation. Thus, SET is an effective target for combination chemotherapy for cOSA due to the difference in molecular mechanisms between SET-targeting drug and cisplatin, inhibition of stemness and cell cycle, respectively.
Acknowledgments
This work is partly supported by JSPS KAKENHI (Grant Number 17H03915 and 18J13124). The funding source had no role in the study design; collection, analysis or interpretation of data; in the writing of the manuscript; or the decision to submit the manuscript for publication.
REFERENCES
- 1.Alvarez F. J., Kisseberth W., Hosoya K., Lara-Garcia A., Kosarek C., Murahari S., Au J. L. S., Wientjes M. G., Couto J., Couto G.2014. Postoperative adjuvant combination therapy with doxorubicin and noncytotoxic suramin in dogs with appendicular osteosarcoma. J. Am. Anim. Hosp. Assoc. 50: 12–18. doi: 10.5326/JAAHA-MS-5958 [DOI] [PubMed] [Google Scholar]
- 2.Barroga E. F., Kadosawa T., Okumura M., Fujinaga T.1999. Establishment and characterization of the growth and pulmonary metastasis of a highly lung metastasizing cell line from canine osteosarcoma in nude mice. J. Vet. Med. Sci. 61: 361–367. doi: 10.1292/jvms.61.361 [DOI] [PubMed] [Google Scholar]
- 3.Brander D. M., Friedman D. R., Volkheimer A. D., Christensen D. J., Rassenti L. Z., Kipps T. J., Guadalupe E., Chen Y., Zhang D., Wang X., Davis C., Owzar K., Weinberg J. B.2019. SET alpha and SET beta mRNA isoforms in chronic lymphocytic leukaemia. Br. J. Haematol. 184: 605–615. doi: 10.1111/bjh.15677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandhanayingyong C., Kim Y., Staples J. R., Hahn C., Lee F. Y.2012. MAPK/ERK Signaling in Osteosarcomas, Ewing Sarcomas and Chondrosarcomas: Therapeutic Implications and Future Directions. Sarcoma 2012: 404810. doi: 10.1155/2012/404810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W., Possemato R., Campbell K. T., Plattner C. A., Pallas D. C., Hahn W. C.2004. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell 5: 127–136. doi: 10.1016/S1535-6108(04)00026-1 [DOI] [PubMed] [Google Scholar]
- 6.Christensen D. J., Chen Y., Oddo J., Matta K. M., Neil J., Davis E. D., Volkheimer A. D., Lanasa M. C., Friedman D. R., Goodman B. K., Gockerman J. P., Diehl L. F., de Castro C. M., Moore J. O., Vitek M. P., Weinberg J. B.2011. SET oncoprotein overexpression in B-cell chronic lymphocytic leukemia and non-Hodgkin lymphoma: a predictor of aggressive disease and a new treatment target. Blood 118: 4150–4158. doi: 10.1182/blood-2011-04-351072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dasari S., Tchounwou P. B.2014. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 740: 364–378. doi: 10.1016/j.ejphar.2014.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enjoji S., Yabe R., Fujiwara N., Tsuji S., Vitek M. P., Mizuno T., Nakagawa T., Usui T., Ohama T., Sato K.2015. The therapeutic effects of SET/I2PP2A inhibitors on canine melanoma. J. Vet. Med. Sci. 77: 1451–1456. doi: 10.1292/jvms.15-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enjoji S., Yabe R., Tsuji S., Yoshimura K., Kawasaki H., Sakurai M., Sakai Y., Takenouchi H., Yoshino S., Hazama S., Nagano H., Oshima H., Oshima M., Vitek M. P., Matsuura T., Hippo Y., Usui T., Ohama T., Sato K.2018. Stemness Is Enhanced in Gastric Cancer by a SET/PP2A/E2F1 Axis. Mol. Cancer Res. 16: 554–563. doi: 10.1158/1541-7786.MCR-17-0393 [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara N., Kawasaki H., Yabe R., Christensen D. J., Vitek M. P., Mizuno T., Sato K., Ohama T.2013. A potential therapeutic application of SET/I2PP2A inhibitor OP449 for canine T-cell lymphoma. J. Vet. Med. Sci. 75: 349–354. doi: 10.1292/jvms.12-0366 [DOI] [PubMed] [Google Scholar]
- 11.Kadosawa T., Nozaki K., Sasaki N., Takeuchi A.1994. Establishment and characterization of a new cell line from a canine osteosarcoma. J. Vet. Med. Sci. 56: 1167–1169. doi: 10.1292/jvms.56.1167 [DOI] [PubMed] [Google Scholar]
- 12.Kake S., Tsuji S., Enjoji S., Hanasaki S., Hayase H., Yabe R., Tanaka Y., Nakagawa T., Liu H. P., Chang S. C., Usui T., Ohama T., Sato K.2017. The role of SET/I2PP2A in canine mammary tumors. Sci. Rep. 7: 4279. doi: 10.1038/s41598-017-04291-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kauko O., Imanishi S. Y., Kulesskiy E., Laajala T. D., Yetukuri L., Laine A., Jumppanen M., Haapaniemi P., Ruan L., Yadav B., Suni V., Varila T., Corthals G., Reimand J., Wennerberg K., Aittokallio T., Westermarck J.2018. Rules for PP2A-controlled phosphosignalling and drug responses. bioRxiv: 271841. [Google Scholar]
- 14.Kauko O., Westermarck J.2018. Non-genomic mechanisms of protein phosphatase 2A (PP2A) regulation in cancer. Int. J. Biochem. Cell Biol. 96: 157–164. doi: 10.1016/j.biocel.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 15.Manning B. D., Toker A.2017. AKT/PKB signaling: navigating the network. Cell 169: 381–405. doi: 10.1016/j.cell.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller F., Fuchs B., Kaser-Hotz B.2007. Comparative biology of human and canine osteosarcoma. Anticancer Res. 271A: 155–164. [PubMed] [Google Scholar]
- 17.Neviani P., Harb J. G., Oaks J. J., Santhanam R., Walker C. J., Ellis J. J., Ferenchak G., Dorrance A. M., Paisie C. A., Eiring A. M., Ma Y., Mao H. C., Zhang B., Wunderlich M., May P. C., Sun C., Saddoughi S. A., Bielawski J., Blum W., Klisovic R. B., Solt J. A., Byrd J. C., Volinia S., Cortes J., Huettner C. S., Koschmieder S., Holyoake T. L., Devine S., Caligiuri M. A., Croce C. M., Garzon R., Ogretmen B., Arlinghaus R. B., Chen C. S., Bittman R., Hokland P., Roy D. C., Milojkovic D., Apperley J., Goldman J. M., Reid A., Mulloy J. C., Bhatia R., Marcucci G., Perrotti D.2013. PP2A-activating drugs selectively eradicate TKI-resistant chronic myeloid leukemic stem cells. J. Clin. Invest. 123: 4144–4157. doi: 10.1172/JCI68951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nitulescu G. M., Van De Venter M., Nitulescu G., Ungurianu A., Juzenas P., Peng Q., Olaru O. T., Grădinaru D., Tsatsakis A., Tsoukalas D., Spandidos D. A., Margina D.2018. The Akt pathway in oncology therapy and beyond (Review). Int. J. Oncol. 53: 2319–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrotti D., Neviani P.2013. Protein phosphatase 2A: a target for anticancer therapy. Lancet Oncol. 14: e229–e238. doi: 10.1016/S1470-2045(12)70558-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips B., Powers B. E., Dernell W. S., Straw R. C., Khanna C., Hogge G. S., Vail D. M.2009. Use of single-agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. J. Am. Anim. Hosp. Assoc. 45: 33–38. doi: 10.5326/0450033 [DOI] [PubMed] [Google Scholar]
- 21.Sangodkar J., Farrington C. C., McClinch K., Galsky M. D., Kastrinsky D. B., Narla G.2016. All roads lead to PP2A: exploiting the therapeutic potential of this phosphatase. FEBS J. 283: 1004–1024. doi: 10.1111/febs.13573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson S., Dunning M. D., de Brot S., Grau-Roma L., Mongan N. P., Rutland C. S.2017. Comparative review of human and canine osteosarcoma: morphology, epidemiology, prognosis, treatment and genetics. Acta Vet. Scand. 59: 71. doi: 10.1186/s13028-017-0341-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szewczyk M., Lechowski R., Zabielska K.2015. What do we know about canine osteosarcoma treatment? Review. Vet. Res. Commun. 39: 61–67. doi: 10.1007/s11259-014-9623-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Kanegan M. J., Adams D. G., Wadzinski B. E., Strack S.2005. Distinct protein phosphatase 2A heterotrimers modulate growth factor signaling to extracellular signal-regulated kinases and Akt. J. Biol. Chem. 280: 36029–36036. doi: 10.1074/jbc.M506986200 [DOI] [PubMed] [Google Scholar]
- 25.Wang W. G., Wan C., Liao G. J.2015. The efficacy of high-dose versus moderate-dose chemotherapy in treating osteosarcoma: a systematic review and meta-analysis. Int. J. Clin. Exp. Med. 8: 15967–15974. [PMC free article] [PubMed] [Google Scholar]
- 26.Winkler K., Bielack S. S., Delling G., Jürgens H., Kotz R., Salzer-Kuntschik M.1993. Treatment of osteosarcoma: experience of the Cooperative Osteosarcoma Study Group (COSS). Cancer Treat. Res. 62: 269–277. doi: 10.1007/978-1-4615-3518-8_32 [DOI] [PubMed] [Google Scholar]
- 27.Yabe R., Fujiwara N., Mizuno T., Usui T., Ohama T., Sato K.2014. Characterization of SET/I2PP2A isoforms in dogs. J. Vet. Med. Sci. 76: 1235–1240. doi: 10.1292/jvms.14-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]