Abstract
Transmissible gastroenteritis (TGE), caused by transmissible gastroenteritis virus (TGEV), is a highly infectious disease in pigs. Vaccination is an effective approach to prevent TGEV infection. Here, we evaluated the potential of TGEV S1 as a DNA vaccine and porcine interleukin (pIL)-12 as an adjuvant in a mouse model. A DNA vaccine was constructed with the TGEV S1 gene to induce immune response in an experimental mouse model; pIL-12 was chosen as the immunological adjuvant within this DNA vaccine. The pVAX1-(TGEV-S1) and pVAX1-(pIL-12) vectors were transfected into BHK-21 cells and expressed in vitro. Experimental mice were separately immunized with each of the recombinant plasmids and controls through the intramuscular route. The lymphocytes isolated from the blood and spleen were analyzed for proliferation, cytotoxic activities, and populations of CD4+ and CD8+ cells. The titers of TGEV S1 in an enzyme-linked immunosorbent assay (ELISA) and TGEV neutralizing antibodies and the concentrations of interferon (IFN)-γ and IL-4 were also analyzed in the serum. The plasmids pVAX1-(TGEV-S1) and pVAX1-(pIL-12) could be expressed in BHK-21 cells, and the combination of pVAX1-(TGEV-S1) and pVAX1-(pIL-12) could induce a significant increase in all markers. pIL-12 could act as an immunological adjuvant in the DNA vaccine for TGEV-S1. Furthermore, the DNA vaccine prepared using TGEV-S1 and porcine IL-12 could induce excellent humoral and cellular immune responses.
Keywords: adjuvant, DNA vaccine, porcine interleukin-12 gene, spike protein, transmissible gastroenteritis virus
Transmissible gastroenteritis is a highly infectious and acute digestive disease caused by transmissible gastroenteritis virus (TGEV) infection. The classical symptoms of this disease include severe diarrhea, vomiting, and dehydration. Swine of different ages and breeds are susceptible to TGEV infection. TGEV is often isolated in cases of poly-infections involving other pathogens and mainly infects piglets. The mortality rate can be as high as 100% and may decline as the animal gets older; however, the decrease in the feed utilization of infected animals may have huge economic consequences [11, 16].
TGEV is a coronavirus and contains a positive-sense single-stranded RNA genome [28], which encodes four structural proteins and three non-structural proteins. Spike (S) protein is the major component of the virion surface and possesses four antigen sites, which may induce production of neutralizing antibodies. We chose the TGEV S1 gene in the present study, as it includes these antigen sites [20, 24].
To date, the most effective strategy to prevent TGEV infection is vaccination, and both inactivated and attenuated vaccines have been widely used in China. However, the limitations of traditional vaccines, including poor safety and protection efficacy, have necessitated the development of an effective vaccine to protect swine from TGEV infection [1].
Eukaryotic expression plasmids are able to induce a robust immune response via endocytosis and antigen-presenting cells [14]. DNA vaccines offer advantages, as they contain no actual infectious agents, can be easily transported, and may induce effective cellular and humoral immune responses. Therefore, nucleic acid vaccines could serve as effective alternatives for diseases control. Several reports have suggested the addition of a cytokine as an adjuvant to increase vaccine efficacy. Interleukin (IL)-12 shows a central function in initiating and regulating cellular immune responses, and many studies have analyzed the use of IL-12 as a potential vaccine adjuvant [2, 17].
In the present study, we constructed eukaryotic expression plasmids encoding the N-terminal fragment (2,106 bp) of the TGEV S protein (S1) and the porcine IL-12 (pIL-12) protein. We aimed to evaluate the potential of TGEV S1 as a DNA vaccine and pIL-12 as an adjuvant in a mouse model [3, 10]. Our results may lay the foundation for further studies on nucleic acid vaccines and immunological adjuvants.
MATERIALS AND METHODS
Plasmid construction
The full-length S gene of TGEV strain PUR46 (GenBank: M94101.1) was used as a polymerase chain reaction (PCR) template. PCR for TGEV S1 gene amplification was performed using the forward primer (P1) (5′-GGGGAAGCTTATGAAAAAACTATTTGTG-3′) and reverse primer (P2) (5′-CCCCGAATTCTTAGTTAGTTTGTCTAATA-3′) carrying a HindIII (P1) and an EcoRI (P2) restriction enzyme site (underlined), respectively. The PCR parameters for TGEV-S1 gene amplification (primers P1/P2) were as follows: 95°C for 5 min, 30 cycles of 94°C for 1 min, 45.7°C for 1 min, and 72°C for 2 min, followed by a final extension of 72°C for 10 min. Products were purified and subjected to restriction enzyme digestion, followed by ligation into the appropriate pVAX1 (Invitrogen, Carlsbad, CA, U.S.A.) eukaryotic expression vector. A recombinant plasmid expressing the entire pIL-12, comprising p40 and p35 genes, was constructed by overlap extension PCR. The plasmid was previously constructed in our laboratory [13]. Clones were picked and identified by PCR amplification, and then named as pVAX1-(TGEV-S1) and pVAX1-(pIL-12).
In vitro expression of pVAX1-(TGEV-S1) and pVAX1-(pIL-12)
All assays were performed using the previously described methods [13, 19] with some modifications. pVAX1-(TGEV-S1), pVAX1-(pIL-12), and pVAX1 were resuspended in 0.1 M phosphate-buffered saline (PBS) at a concentration of 1 µg/µl, and 1 µl of each plasmid was transfected with Lipofectamine 2000 (Invitrogen) into BHK-21 cells at about 90% confluence. The cells were cultured at 37°C for about 24 hr, fixed with 4% (w/v) paraformaldehyde, and incubated with anti-TGEV-S1 or anti-pIL-12 (p40) antibody (1:200 diluted in 1% bovine serum albumin [BSA]). The samples were incubated in the dark with a fluorescein isothiocyanate (FITC)-labeled secondary antibody (1:200 dilution with 1% BSA). The green fluorescence signals were detected using a fluorescence microscope (Leica, Wetzlar, Germany).
Immunization of mice with pVAX1-(TGEV-S1) and pVAX1-(pIL-12)
Six-week-old Kunming mice (Harbin Veterinary Research Institute) were divided into six groups (Table 1) and injected with 50 µl 0.8% lidocaine hydrochloride (27 gauge needles) in the medial vastus muscle. After 15 min, the mice were immunized with 100 µg of pVAX1-(TGEV-S1), pVAX1-(pIL-12), pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) (100 µg each), inactivated TGEV vaccine (vaccine control group; produced by Chengdu TECBOND Biological Products Co., Ltd., Chengdu, China), pVAX1 (empty vector control), or PBS (no treatment control). A total of three doses of immunization were given to each mouse from all groups at 2-week intervals. Four mice per group were euthanized at 0, 7, 14, 21, 28, 35, and 42 days after first immunization. Serum samples were collected, and mononuclear cells (MCs) were isolated from the blood and spleen of the euthanized animals [12]. The animal study was performed in compliance with institutional guidelines and animal welfare. All animal experiments were approved by the Animal Ethics Committee of Northeast Agricultural University.
Table 1. Levels of circulating interferon (IFN)-γ and Interleukin (IL)-4 in immunized mice (¯XA −¯XB ± SE).
| Group | Number of mice | Vaccine category | Immunizing dose (µg) | Number of treatments | IFN-γ (pg/ml) | IL-4 (pg/ml) |
|---|---|---|---|---|---|---|
| A | 28 | pVAX1-(pIL-12) | 100 | 3 | 519.73 ± 70.52 | 150.81 ± 21.36 |
| B | 28 | pVAX1-(TGEV-S1) | 100 | 3 | 1,222.32 ± 129.37a) | 467.26 ± 70.14 |
| C | 28 | pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) | 100 | 3 | 128.84 ± 19.05 | 956.84 ± 122.06a) |
| D | 28 | Diluted inactivated TGEV vaccine | 100 | 3 | 198.09 ± 32.95 | 244.48 ± 36.43 |
| E | 28 | PBS | 100 | 3 | 483.97 ± 60.34 | 67.66 ± 15.78 |
| F | 28 | pVAX1 | 100 | 3 | 328.58 ± 56.87 | 97.58 ± 27.50 |
Experimental groups and cytokine levels. The six groups include pVAX1-(pIL-12) (A), pVAX1-(TGEV-S1) (B), pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) (C), inactivated TGEV vaccine (D), PBS (E), and pVAX1 (F). Immunizing dose, vaccine category, and number of mice are indicated. The levels of IFN-γ and IL-4 were detected at 42 dpi with ELISA kits, a) P<0.01 (highly significant) as compared with other groups.
Analysis of T lymphocytes
Lymphocytes were collected from the peripheral blood or spleen of the experimental animals, and lymphocyte proliferation assays were performed using isolated MCs from the blood and spleen, as previously described [5, 10, 27]. The MCs were diluted to 105 cells/50 µl Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, Waltham, MA, U.S.A.) and added to each well of 96-well plates. Concanavalin A (Sigma, Munich, Germany) and purified recombinant TGEV-S1 protein (20 µg/ml) were added as stimulating agents for 72 hr. The cells were treated with methylthiazolyldiphenyl-tetrazolium bromide (MTT, 10 µl/well) for 4 hr and the reaction was terminated with dimethyl sulfoxide (DMSO). The viability of cells was calculated by reading the absorbance at 490 nm wavelength.
Peripheral blood MCs (PBMCs) and splenic MCs (SMCs) were resuspended at 1 × 107 cells/ml and incubated with either phycoerythrin (PE)-labeled anti-porcine CD8+ or fluorescein isothiocyanate (FITC)-labeled anti-porcine CD4+ antibodies (1:1,000 diluted in PBS; Zhongshan, Guangdong, China). The frequencies of CD4+ and CD8+ cells were quantified with flow cytometric analysis (BD, Franklin Lakes, NJ, U.S.A.).
Detection of TGEV antibody
The TGEV-specific antibody from the serum of vaccinated mice was detected with an enzyme-linked immunosorbent assay (ELISA) using recombinant TGEV S1 protein (50 µg/ml diluted with 0.05 M sodium bicarbonate [NaHCO3]) [18]. The absorbance was detected at 490 nm wavelength.
To determine the efficacy of TGEV-neutralizing antibodies from the serum of vaccinated mice [21], mouse sera (1:10 doubling dilution) were diluted with a diluent (200 µl) of TGEV (3.16 × 106 TCID50/ml) at 37°C for 1 hr. Swine testis (ST) cells were inoculated with the pretreated viruses in 24-well plates (37°C, 5% CO2), and the TGEV-specific cytopathic effects were observed at 48–60 hr post-infection. Based on the cytopathic effect (CPE) of the control, the inhibition rate (%) of TGEV focus-forming unit (FFU) were calculated as follows: [1−(FFU in the test serum/FFU in negative control serum) × 100]. The neutralizing antibody titer was the serum dilution corresponding to a TGEV inhibition rate of 50% and was calculated with the Reed-Muench method as follows: [10^{logarithm of serum dilution (inhibition rate <50%)−(inhibition rate (>50%)−50%)/(inhibition rate (>50%)−inhibition rate (<50%)) × (logarithm of serum dilution (inhibition rate <50%)−logarithm of serum dilution (inhibition rate >50%))}].
Detection of cytokines
Interferon (IFN)-γ and IL-4 test kits (ExCell Bio., Shanghai, China) were used for detecting cytokine expression levels, as per the manufacturer’s instructions [6, 9]. To obtain a standard curve, the standard solution of IFN-γ and IL-4 were diluted from 2,000 to 31.3 pg/ml and 500 to 7.8 pg/ml, respectively. The standard solutions and serum samples were incubated overnight at 37°C on ELISA plates. The serum (1:100) from 42 days post-immunization (dpi) mice was used to evaluate IFN-γ and IL-4 responses. Horseradish peroxidase-labeled goat-anti mouse immunoglobulin (IgG, 1:2,000) was added to each well, and IFN-γ and IL-4 levels were detected at 490 nm wavelength. The concentrations of IFN-γ and IL-4 in the serum were determined according to the standard curve.
Cytotoxicity assay
A lactate dehydrogenase (LDH) test kit was used to analyze cytotoxicity. The test was conducted according to the manufacturer’s instructions (Jiancheng, Nanjing, Jiangsu, China). The effector cells were obtained from the blood and spleen lymphocytes of 42 dpi mice [4, 8], and the target ST cells were infected with TGEV (70 PFU) for 36 hr (37°C, 5% CO2). The effector cells and target cells were mixed at a proportion of 25:1 and incubated in wells of 96-well plates for 6 hr (37°C, 5% CO2). The supernatants (100 µl) were mixed with LDH assay reagent (100 µl/well) for 15 min (37°C) in 96-well plates and the results were analyzed at 490 nm wavelength. The maximum release of LDH was that the total of target cells were dissolved in Triton X-100 (1% v/v), and the spontaneous LDH release was that the target cells exist single. Cytotoxic T lymphocytes (CTLs) were calculated as ([experimental release−spontaneous release]/[maximum release−spontaneous release]) ×100% [3]. All detections were performed in triplicates.
Statistical analysis
All analyzes were performed using SPSS V13.0 software; P<0.01 and P<0.05 were defined as statistically very significant and significant, respectively. One-way analysis of variance (ANOVA) and multiple comparison statistical test were also used.
RESULTS
In vitro expression of pVAX1-(TGEV-S1) and pVAX1-(pIL-12)
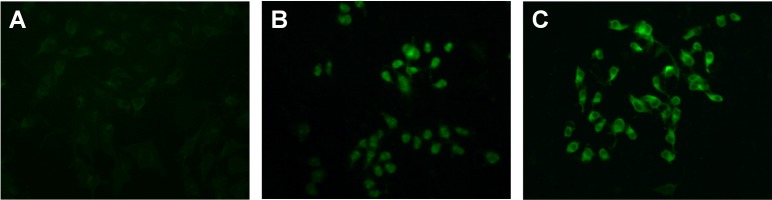
The sequences of pVAX1-(TGEV-S1) and pVAX1-(pIL-12) were analyzed from GenBank, and the results of nucleotide sequence comparison revealed 100 and 99.8% similarity with the strain accession numbers M94101 (PUR46-MAD) (S1) and NM_214013.1 (pIL-12), respectively. The pVAX1-(TGEV-S1) and pVAX1-(pIL-12) plasmids were successfully transfected and expressed in BHK-21 cells, as indicated from the results of the immunofluorescence assay (Fig. 1).
Fig. 1.
Immunofluorescence analysis of BHK-21 cells transfected with recombinant plasmids. BHK-21 cells were transfected with pVAX1-(TGEV-S1) and pVAX1-(pIL-12). (A) pVAX1 vector control, (B) pVAX1-(pIL-12)-expressing cells, (C) pVAX1-(TGEV-S1)-expressing cells. Transient expression of proteins was detected with anti-transmissible gastroenteritis virus (TGEV)-S1 antibody or anti-porcine interleukin (pIL)-12 antibody. The green signals indicate positive protein expression.
Lymphocyte proliferation
As shown in Fig. 2A, T lymphocyte proliferation was significantly upregulated at 14, 28, and 42 dpi in pVAX1-(TGEV-S1) and pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) groups compared to that in the control groups (P<0.01). At 14 and 28 dpi, the pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) group showed a significant upregulation in T lymphocyte proliferation level as compared with the groups treated with the other construct (P<0.01).
Fig. 2.
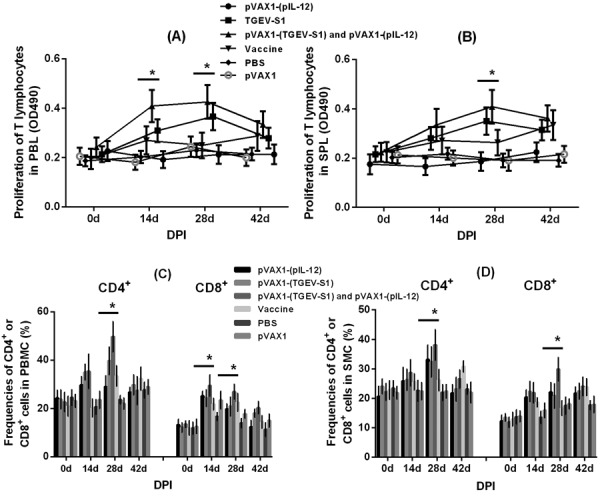
The proliferation of T lymphocytes and changes in the frequencies of CD4+ and CD8+ cells in the peripheral blood and spleen. T lymphocytes from the peripheral blood (PBL) (A) and spleen (SPL) (B) of mice were analyzed with MTT assay. The y-coordinate represents the lymphocyte proliferate index (OD 490 nm) in the peripheral blood or spleen. Mononuclear cells (MCs) from the peripheral blood and spleen of mice immunized with pVAX1-(TGEV-S1) and pVAX1-(pIL-12) were collected and subjected to flow cytometry analysis to evaluate the proportions of CD4+ and CD8+ cells in peripheral blood MCs (PBMCs) (C) and SMCs (D). The y-coordinate represents the changes in the frequencies of CD4+ and CD8+ cells in the peripheral blood or spleen. *P<0.01 (highly significant) versus other groups. DPI, day post-immunization.
The proliferation of spleen T lymphocytes is shown in Fig. 2B. At 28 dpi, the pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) group showed much higher upregulation than any other groups (P<0.01). At 14 and 42 dpi, pVAX1-(TGEV-S1) group also showed a significant upregulation (P<0.01) in spleen T lymphocyte proliferation as compared to the control groups.
Changes in the frequencies of CD4+ and CD8+ cells in PBMCs and SMCs
Changes in the frequencies of CD4+ and CD8+ cells in the blood and spleen from experimental animals were evaluated using flow cytometry. The number of CD4+ and CD8+ cells in the peripheral blood is shown in Fig. 2C. The number of CD8+ cells in the pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) group was significantly higher (P<0.01) than that in the other groups at 14 and 28 dpi. The number of CD4+ cells in the peripheral blood at 28 dpi was significantly higher (P<0.01) in pVAX1-(TGEV-S1) and pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) groups than in the control groups. A similar trend was observed in the number of CD4+ and CD8+ cells in the spleen, as shown in Fig. 2D. At 28 dpi, the pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) group had significantly higher (P<0.01) number of CD4+ and CD8+ cells than other groups.
TGEV antibody detection
The levels of TGEV-specific antibodies in the serum of immunized mice were examined using an indirect ELISA (Fig. 3A). In general, starting from 21 dpi, the pVAX1-(TGEV-S1), pVAX1-(TGEV-S1) plus pVAX1-(pIL-12), and vaccine-treated groups had a higher proportion of antibodies than the control groups (P<0.01). Antibody production reached its peak at 35 dpi. The pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) group induced higher IgG response than other groups except the vaccine group.
Fig. 3.
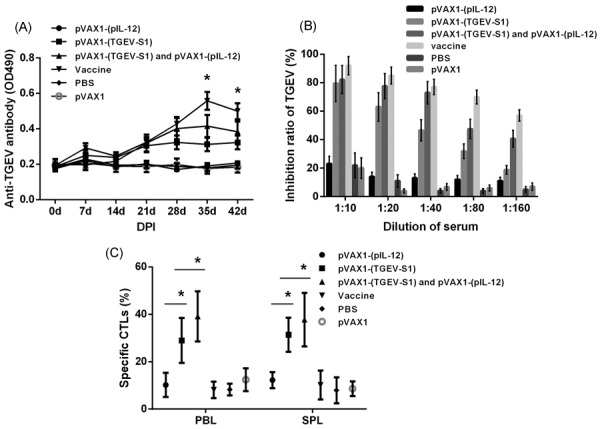
The specific antibody concentration and activity of cytotoxicity T lymphocytes. (A) The levels of anti-TGEV antibody in serum samples were detected with indirect ELISA at OD490nm. *P<0.01 (highly significant) as compared with PBS and pVAX1 groups. (B) The production of neutralizing antibodies in the serum at 35 days post-immunization (DPI) was evaluated with virus neutralizing assays. (C) Cytotoxic T lymphocytes (CTLs) were determined with the lactate dehydrogenase (LDH) release assay, which is used to evaluate the activity of cytotoxic T lymphocytes at 42 dpi. *P<0.01 (highly significant) versus other groups.
Virus neutralizing assay results revealed the levels of neutralizing antibodies in the serum of immunized mice. pVAX1-(TGEV-S1), pVAX1-(TGEV-S1) plus pVAX1-(pIL-12), and inactivated vaccine groups showed better antibody production than the control groups (P<0.01). The serum inhibition rates of TGEV FFU were higher for pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) and vaccine groups than for other groups at any serum dilution (Fig. 3B). The titer of neutralizing antibody was 1:35, 1:75, and 1:195 for pVAX1-(TGEV-S1), pVAX1-(TGEV-S1) plus pVAX1-(pIL-12), and inactivated vaccine groups, respectively.
Detection of IFN-γ and IL-4 levels
IFN-γ and IL-4 levels in different sera were analyzed with ELISA. As shown in Table 1, IFN-γ production was 1,222.32 ± 129.37 pg/ml for pVAX1-(TGEV-S1) group, while IL-4 level was 956.84 ± 122.06 pg/ml for pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) group, which were significantly different from those reported for the control group (P<0.01). The concentration of IL-4 in pVAX1-(TGEV-S1) immunized groups was higher than that in other groups except pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) group. The inactivated vaccine failed to induce IFN-γ and IL-4 production.
Activity of CTLs
Evaluation of the activity of CTLs was performed with the LDH release assay. Figure 3C shows that the CTL activities in the spleen and peripheral blood of the mice treated with pVAX1-(TGEV-S1) and pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) were significantly different from activities in the spleen and peripheral blood of the mice from control groups (P<0.01). The pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) group showed the highest activity of CTLs both in the blood and spleen.
DISCUSSION
The S protein of TGEV near the N terminal portion (S1) has four antigenic epitopes [26]. Researchers have reported that the S1 gene could induce better immune responses than the full-length TGEV S gene in mice [24]. Furthermore, the cytokine IL-12 could not only initiate the proliferation of active T cells but also increase the activity of CTLs and natural killer cells [15, 22]. IL-12 has been widely used as an immunological adjuvant in nucleic acid vaccine research [3, 17, 23]. DNA vaccines are safer and more effective than traditional vaccines [25]. Herein, we assessed the influence of IL-12 as an immunological adjuvant in combination with a nucleic acid vaccine comprising the TGEV S gene.
T lymphocytes are significant effectors of the immune response and are vital for the activation of T helper cells (Th), cytotoxic T cells (Tc), and effector T cells (Te) among others. These cells participate and induce cellular and humoral immune responses within most organisms. In the present study, T lymphocytes showed a significant change, and CD4+ and CD8+ lymphocytes significantly increased in the blood and spleen of mice from pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) group; a significant difference was observed compared to other groups. These cells reflect the proliferation of lymphocytes stimulated with antigens at 14 and 28 dpi. Thus, these plasmids could stimulate the cellular immunity, while pIL-12 exerted a promotional effect. However, all groups showed a decrease in immune response at 42 dpi; this observation indicates that the animals were entering exhaustion or that the immune effect had worn-off in the absence of stimulation.
Th cells can differentiate into Th1 and Th2 through the secretion of different cytokines. Th1 cells are mostly influenced by IL-2 and IFN-γ and regulate cellular immune responses, while Th2 cells are mainly controlled by IL-10 and IL-4 and activate the humoral immune responses. The analyzes of specific antibodies and neutralizing antibodies revealed that pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) and inactivated vaccine had similar effects on humoral immune response and developed a much stronger response than other treatments, including pVAX1-(TGEV-S1). The high titer of neutralizing antibody observed in the inactivated vaccine group was due to its more complex components. Interestingly, the pVAX1-(TGEV-S1) and pVAX1-(TGEV-S1) plus pVAX1-(pIL-12) immunized groups had different IFN-γ and IL-4 responses, suggesting that they activate different arms of the immune system [29].
CTLs directly destroy infected cells, and recognize the major histocompatibility complex I/antigen complexes. CTLs lyse target cells through the release of cytolytic granules, and the surfaces of these cells express the Fas ligand [7]. CTL activity is assessed using the change in LDH levels [4, 8]. CTLs and neutralizing antibodies directly act on viruses and protect the host from viral infection [25]. In the present study, pVAX1-(TGEV-S1) combined with pVAX1-(pIL-12) exerted positive effects on the activation of CTLs both in the blood and spleen and showed a marked advantage over pVAX1-(TGEV-S1) alone, especially in the peripheral blood.
In summary, we report that pIL-12 could act as an immunological adjuvant in the DNA vaccine for TGEV-S1. DNA vaccine developed using TGEV-S1 and pIL-12 could induce excellent activation of both the humoral and cellular immune responses. This study may provide a useful strategy to develop DNA vaccines for the prevention of TGEV in swine. The next step will be to assess its activity in a porcine model.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Acknowledgments
Supported by National Natural Science Foundation of China (No.31372438; 31201911).
REFERENCES
- 1.Babiuk L. A., Gerdts V.2012. Future vaccines for a globalized world. Emerg. Microbes Infect. 1: e4. doi: 10.1038/emi.2012.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charerntantanakul W., Platt R., Johnson W., Roof M., Vaughn E., Roth J. A.2006. Immune responses and protection by vaccine and various vaccine adjuvant candidates to virulent porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 109: 99–115. doi: 10.1016/j.vetimm.2005.07.026 [DOI] [PubMed] [Google Scholar]
- 3.Chen H. W., Pan C. H., Huan H. W., Liau M. Y., Chiang J. R., Tao M. H.2001. Suppression of immune response and protective immunity to a Japanese encephalitis virus DNA vaccine by coadministration of an IL-12-expressing plasmid. J. Immunol. 166: 7419–7426. doi: 10.4049/jimmunol.166.12.7419 [DOI] [PubMed] [Google Scholar]
- 4.Choi Y., Lee H. W., Lee J., Jeon Y. H.2013. The combination of ANT2 shRNA and hNIS radioiodine gene therapy increases CTL cytotoxic activity through the phenotypic modulation of cancer cells: combination treatment with ANT2 shRNA and I-131. BMC Cancer 13: 143. doi: 10.1186/1471-2407-13-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong B., Feng J., Lin H., Li L., Su D., Tu D., Zhu W., Yang Q., Ren X.2013. Immune responses of mice immunized by DNA plasmids encoding PCV2 ORF 2 gene, porcine IL-15 or the both. Vaccine 31: 5736–5744. doi: 10.1016/j.vaccine.2013.09.035 [DOI] [PubMed] [Google Scholar]
- 6.Fernández-Ruiz M., Humar A., Baluch A., Keshwani S., Husain S., Kumar D.2015. Baseline serum interleukin-6 to interleukin-2 ratio is associated with the response to seasonal trivalent influenza vaccine in solid organ transplant recipients. Vaccine 33: 7176–7182. doi: 10.1016/j.vaccine.2015.10.134 [DOI] [PubMed] [Google Scholar]
- 7.He J. S., Ostergaard H. L.2007. CTLs contain and use intracellular stores of FasL distinct from cytolytic granules. J. Immunol. 179: 2339–2348. doi: 10.4049/jimmunol.179.4.2339 [DOI] [PubMed] [Google Scholar]
- 8.Ju H., Xing W., Yang J., Zheng Y., Jia X., Zhang B., Ren H.2016. An effective cytokine adjuvant vaccine induces autologous T-cell response against colon cancer in an animal model. BMC Immunol. 17: 31. doi: 10.1186/s12865-016-0172-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khademi B., Tajvarpour M., Mojtahedi Z., Haghshenas M. R., Erfani N.2016. T-helper Type 1 and 2 Cytokine Levels in Patients with Benign and Malignant Salivary Gland Tumors. Iran. J. Immunol. 13: 9–15. [PubMed] [Google Scholar]
- 10.Li G., Shi N., Suo S., Cui J., Zarlenga D., Ren X.2012. Vaccination of mice with ORF5 plasmid DNA of PRRSV; enhanced effects by co-immunizing with porcine IL-15. Immunol. Invest. 41: 231–248. doi: 10.3109/08820139.2011.614306 [DOI] [PubMed] [Google Scholar]
- 11.Li P., Ren X.2011. Reverse transcription loop-mediated isothermal amplification for rapid detection of transmissible gastroenteritis virus. Curr. Microbiol. 62: 1074–1080. doi: 10.1007/s00284-010-9825-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W., Ye L., Carrion R., Jr, Mohan G. S., Nunneley J., Staples H., Ticer A., Patterson J. L., Compans R. W., Yang C.2015. Characterization of Immune Responses Induced by Ebola Virus Glycoprotein (GP) and Truncated GP Isoform DNA Vaccines and Protection Against Lethal Ebola Virus Challenge in Mice. J. Infect. Dis. 212Suppl 2: S398–S403. doi: 10.1093/infdis/jiv186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X., Ren X.2010. Antibody against biologically active p40 subunit of porcine interleukin-12 expressed in Escherichia coli. Hybridoma (Larchmt) 29: 489–494. doi: 10.1089/hyb.2010.0074 [DOI] [PubMed] [Google Scholar]
- 14.Ma D., Ma C., Pan L., Li G., Yang J., Hong J., Cai H., Ren X.2011. Vaccination of chickens with DNA vaccine encoding Eimeria acervulina 3-1E and chicken IL-15 offers protection against homologous challenge. Exp. Parasitol. 127: 208–214. doi: 10.1016/j.exppara.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 15.Maeto C., Rodríguez A. M., Holgado M. P., Falivene J., Gherardi M. M.2014. Novel mucosal DNA-MVA HIV vaccination in which DNA-IL-12 plus cholera toxin B subunit (CTB) cooperates to enhance cellular systemic and mucosal genital tract immunity. PLoS One 9: e107524. doi: 10.1371/journal.pone.0107524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martins A. M. C. R. P. F., Bersano J. G., Ogata R., Amante G., Nastari B. D. B., Catroxo M. H. B.2013. Diagnosis to Detect Porcine Transmissible Gastroenteritis Virus (TGEV) by Optical and Transmission Electron Microscopy Techniques. Int. J. Morphol. 31: 706–715. doi: 10.4067/S0717-95022013000200059 [DOI] [Google Scholar]
- 17.Mealey R. H., Stone D. M., Hines M. T., Alperin D. C., Littke M. H., Leib S. R., Leach S. E., Hines S. A.2007. Experimental Rhodococcus equi and equine infectious anemia virus DNA vaccination in adult and neonatal horses: effect of IL-12, dose, and route. Vaccine 25: 7582–7597. doi: 10.1016/j.vaccine.2007.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng F., Ren Y., Suo S., Sun X., Li X., Li P., Yang W., Li G., Li L., Schwegmann-Wessels C., Herrler G., Ren X.2013. Evaluation on the efficacy and immunogenicity of recombinant DNA plasmids expressing spike genes from porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus. PLoS One 8: e57468. doi: 10.1371/journal.pone.0057468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng F., Yin J., Li X., Yang W., Li G., Ren X.2010. Production and characterization of a monoclonal antibody against spike protein of transmissible gastroenteritis virus. Hybridoma (Larchmt) 29: 345–350. doi: 10.1089/hyb.2010.0009 [DOI] [PubMed] [Google Scholar]
- 20.Meng F., Zhao Z., Li G., Suo S., Shi N., Yin J., Zarlenga D., Ren X.2011. Bacterial expression of antigenic sites A and D in the spike protein of transmissible gastroenteritis virus and evaluation of their inhibitory effects on viral infection. Virus Genes 43: 335–341. doi: 10.1007/s11262-011-0637-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyauchi K., Sugimoto-Ishige A., Harada Y., Adachi Y., Usami Y., Kaji T., Inoue K., Hasegawa H., Watanabe T., Hijikata A., Fukuyama S., Maemura T., Okada-Hatakeyama M., Ohara O., Kawaoka Y., Takahashi Y., Takemori T., Kubo M.2016. Protective neutralizing influenza antibody response in the absence of T follicular helper cells. Nat. Immunol. 17: 1447–1458. doi: 10.1038/ni.3563 [DOI] [PubMed] [Google Scholar]
- 22.Noble A., Mehta H., Lovell A., Papaioannou E., Fairbanks L.2016. IL-12 and IL-4 activate a CD39-dependent intrinsic peripheral tolerance mechanism in CD8(+) T cells. Eur. J. Immunol. 46: 1438–1448. doi: 10.1002/eji.201545939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng X., Fang X., Li J., Kong L., Li B., Ding X.2016. Enhancing immune responses of EV71 VP1 DNA vaccine by co-inoculating plasmid IL-12 or GM-CSF expressing vector in mice. Cell. Mol. Biol. 62: 35–41. [PubMed] [Google Scholar]
- 24.Ren X. F.2010. Monoclonal Antibody Against TGEV S 7F9. Hybridoma (Larchmt) 29: 379–379. doi: 10.1089/hyb.2010.0028.MAb [DOI] [Google Scholar]
- 25.Sarwar U. N., Costner P., Enama M. E., Berkowitz N., Hu Z., Hendel C. S., Sitar S., Plummer S., Mulangu S., Bailer R. T., Koup R. A., Mascola J. R., Nabel G. J., Sullivan N. J., Graham B. S., Ledgerwood J. E., VRC 206 Study Team2015. Safety and immunogenicity of DNA vaccines encoding Ebolavirus and Marburgvirus wild-type glycoproteins in a phase I clinical trial. J. Infect. Dis. 211: 549–557. doi: 10.1093/infdis/jiu511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suo S., Wang X., Zarlenga D., Bu R. E., Ren Y., Ren X.2015. Phage display for identifying peptides that bind the spike protein of transmissible gastroenteritis virus and possess diagnostic potential. Virus Genes 51: 51–56. doi: 10.1007/s11262-015-1208-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suo S., Ren Y., Li G., Zarlenga D., Bu R. E., Su D., Li X., Li P., Meng F., Wang C., Ren X.2012. Immune responses induced by DNA vaccines bearing Spike gene of PEDV combined with porcine IL-18. Virus Res. 167: 259–266. doi: 10.1016/j.virusres.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trincone A., Schwegmann-Weßels C.2015. Looking for a needle in a haystack: Cellular proteins that may interact with the tyrosine-based sorting signal of the TGEV S protein. Virus Res. 202: 3–11. doi: 10.1016/j.virusres.2014.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q., Li M., Xia L. C., Wen G., Zu H., Gao M.2013. Genetic analysis of differentiation of T-helper lymphocytes. Genet. Mol. Res. 12: 972–987. doi: 10.4238/2013.April.2.13 [DOI] [PubMed] [Google Scholar]