Abstract
Porcine reproductive and respiratory syndrome (PRRS) is one of the major swine diseases responsible for a significant challenge in the global swine industry. The current PRRS inactivated vaccine only confers limited protection against PRRSV. Thus, using an appropriate adjuvant via a suitable administration route may help improve vaccine efficacy. In this study, the recombinant B subunit of the Escherichia coli heat-labile enterotoxin rLTB, was highly expressed in Pichia pastoris, through high-density fermentation. rLTB intranasal adjuvant properties were evaluated on an inactivated PRRS antigen in mice. Compared to the group immunized with solely PRRS antigen, a dose of 50 µg rLTB remarkably raised antigen-specific IgA antibodies at mucosal sites, and increased serum IgG antibodies, preferentially the IgG2a and IgG2b subclasses. Further, rLTB induced increases in Th1- (IFN-γ and IL-12) and Th17 (IL-6) cytokine profiles, but had little effect on Th2 cytokine profiles (IL-4 and IL-10). Moreover, there were no overt toxicities associated with intranasal rLTB administration. Our data provide evidence that the rLTB produced by P. pastoris fermentation portrays low toxicity, and its intranasal adjuvant effect involves immune system modulation to a Th1 profile.
Keywords: intranasal adjuvant, porcine reproductive and respiratory syndrome virus antigen, rLTB, Th1
Porcine reproductive and respiratory syndrome (PRRS) caused by the PRRS virus (PRRSV), is one of the most prevalent viral diseases in pigs, causing tremendous financial losses to the global swine industry. In the United States pork industry, PRRS is responsible for a loss of over $664 million, annually [4]. In 2012, the mean economic loss per sow during the 18-week PSSR outbreak in the Netherlands was estimated at approximately €126 [25]. Clinical symptoms include late-term reproductive failure in pregnant sows, and respiratory distress in piglets and growing pigs. PRRSV is a positive-sense, single-stranded RNA virus that belongs to the Arteriviridae and Nidovirales family and order, respectively [4]. It mainly replicates in porcine alveolar macrophages in the lungs and lymphoid organs, and damages the host’ immune system, leading to weak immune responses, and increasing susceptibility to secondary bacterial, and other viral infections [13]. Current vaccines available for PRRSV prevention include the modified live virus and inactivated vaccines. The PRRSV live attenuated vaccine clinically reduces porcine lung lesions and viral shedding, and confers protection against the homologous virus [14, 26], but does not induce adequate heterologous immunity [9, 29]. More importantly, the PRRSV live attenuated vaccine can potentially revert to virulence [30]. The PRRSV inactivated vaccine on the other hand, has a good safety profile, but lacks sufficient immunogenicity [1]. An appropriate immune adjuvant may therefore help to potentiate PRRSV inactivated vaccine efficacy. Given that the respiratory mucosa surface is the primary PRRSV infection site, PRRSV vaccine and adjuvant delivery via the intranasal route to elicit both mucosal and systemic immunity, is ideal for protection against PRRSV.
In animals, the B subunit of the Escherichia coli heat-labile enterotoxin LTB, is known to be an effective and safe adjuvant for various nasal immunization vaccines [12, 17, 24]. Lee et al. demonstrated that LTB increased antigen-specific serum IgG and IgA antibody titers, and promoted splenic IFN-γ and IL-2 expression in mice, when intranasally co-administered with the Naegleria fowleri rNfa1 protein [11]. Furthermore, the rNfa1 protein formulated with LTB successfully rescued 80% mice from Naegleria fowleri fatal infection, with no survivor in the rNfa 1-treated group. In another study, LTB was fused to a multi-antigen chimera composed of 3 Mycoplasma hyopneumoniae antigens (C-terminalportion of P97, heat shock protein P42, and NrdF), as an intranasal adjuvant. Other animal experiments demonstrated that LTB was essential for enhanced IgG and IgA antibody responses in the serum and tracheobronchial lavages of mice and pigs [18]. Moreover, a recombinant fowl cholera outer membrane protein H (rOmpH) was intranasally co-delivered with LTB in chickens, and the results suggested that the LTB supplement conferred 70% protection against challenge, compared with 0% protection in rOmpH-immunized chickens [34]. Nevertheless, LTB preparation remains challenging, owing to its structural complexity, inclusion forms, and recombinant LTB (rLTB) stability [16]. The methylotrophic Pichia pastoris, used as an expression host for producing recombinant proteins, has unique advantages because it has mature posttranslational modifications, is bacterial endotoxin contamination free, and offers low cost fermentation [15, 19]. These advantages are often difficult to achieve by prokaryotic expression systems, but essential for large-scale functional protein production. The recombinant cholera toxin B subunit (rCTB) produced by P. pastoris, has been reported to perform excellent intranasal adjuvant activities [5, 19, 20]. Although the method for rLTB production in eukaryotic expression systems has been studied in the past years, intranasal adjuvant properties of rLTB from P. pastoris are yet to be reported.
In this study, large quantities of rLTB were produced by P. pastoris fermentation in a fed-batch mode, and its mucosal adjuvant activities investigated in mice by intranasal vaccination with a PRRSV antigen. The safety profile of the purified rLTB, as an intranasal adjuvant, was also evaluated. The observations of this study would open room for further porcine research.
MATERIALS AND METHODS
Animals
Female ICR mice aged 6–8 weeks were purchased from the Shanghai Laboratory Animal Center. Animals were housed under specific-pathogen-free conditions and used in accordance with the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal care/use protocols were approved by the Zhejiang Academy of Agricultural Sciences Institutional Animal Care.
Cells and viruses
MARC-145 cells were used to prepare PRRSV stocks and assays. The cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, U.S.A.) containing 10% (v/v) fetal bovine serum (FBS; Gemini Bio-products, Sacramento, CA, U.S.A.), 2 mM L-glutamine, 100 U penicillin/ml, and 100 µg streptomycin/ml, at 37°C in 5% CO2. A type 2 endemic PRRSV isolated in Zhejiang province was propagated, purified, and titrated in MARC-145 cells.
Preparation of the PRRSV antigen
The 10th passage of PRRSV (GenBank accession no. FJ536165) was harvested after 72 hr of infection, and titrated in MARC-145 cells with 1 × 107 TCID50/ml. The virus was then inactivated using 0.05% β-propiolactone (Solarbio Life Sciences, Beijing, China) at 4°C for 12 hr, and another 2 hr at 37°C. Inactivation was confirmed by blindly passaging thrice, in MARC-145 cells. For the enzyme-linked immunosorbent assay (ELISA), the virus was sonicated and quantified using the BCA assay kit (Sangon Biotech, Shanghai, China).
Expression, purification and characterization of rLTB
Briefly, P. pastoris GS115 strains were transformed with the SalI-linearized pPIC9k-LTB plasmid previously constructed [37] by electroporation, following the manufacturer’s instructions (Invitrogen, Carlsbad, CA, U.S.A.). The screening of geneticin G418-resistant P. pastoris transformants was first carried out on an MD plate, and then on YPD plates containing 2 mg/ml geneticin G418. Each single transformant colony was amplified in YPD medium at 30°C with overnight shaking (250 rpm), and then transferred to BMGY growth medium. The culture was then incubated at 30°C with 24 hr shaking (250 rpm), until OD600 reached 6. The entire inoculum was incubated in a fermentor (Bioflo 415; New Brunswick Scientific Co., Edison, NJ, U.S.A.) containing 4 l of basal salts medium and PTM1 trace salts. During fermentation, temperature and pH were controlled at 30°C and pH 6, respectively, by water cooling, and the addition of 28% NH4OH. The stirring speed was set to 200–950 rpm, to maintain airflow at 2–25 l/min. One percent (v/v) methanol containing 1.2% (v/v) PTM1 salts, was initially fed when the glycerol in the medium had been completely exhausted, as indicated by a sudden increase in the dissolved oxygen level. The methanol feeding was controlled at 1.5%, during induction.
The dissolved oxygen level was maintained above 20% during the whole batch time. Culture supernatants were harvested at 24, 48, 72, 96 and 120 hr post methanol initial feeding, and analyzed using 12% SDS-PAGE. Culture supernatants were precipitated with saturated (NH4)2SO4, and purified on a HiTrapTM Ni2+ column (Amersham Biosciences, Sunnyvale, CA, U.S.A.), using an AKTATM FPLC system (Amersham Pharmacia Biotech, Uppsala, Sweden). The GM1-ganglioside binding capability of rLTB was estimated based on ELISA.
GM1–ganglioside binding assay
The GM1-ganglioside binding assay was conducted as previously described [21]. Briefly, a polyvinyl 96-well microtiter plate was coated with 1 µg/ml GM1 (Sigma-Aldrich, St. Louis, MO, U.S.A.) in bicarbonate buffer at 4°C overnight, and blocked with 5% skimmed milk in PBS at 37°C for 2 hr. Serial dilutions of protein samples or PBS were added to the wells and incubated for 2 hr at 37°C. Rabbit anti-CT antibody (1:2,000) was added after 3 washes, and incubated for 2 hr at 37°C. A 1:5,000 dilution of HRP-conjugated goat anti-rabbit IgG was added to each well, and incubated for 1 hr at 37°C. Plates were washed again, and 100 µl of 3,3′,5,5′-tetramethyl benzidine substrate solution (100 µg/ml of 0.1 M citrate-phosphate, pH 5.0) added to each well, and incubated for 15 min. The reaction was stopped with 50 µl of 2 M H2SO4. The ODs of each plate was read at 450 nm, using an ELISA plate reader (Thermo Multiscan MK3; Thermo Scientific, Waltham, MA, U.S.A.).
Intranasal immunization
Thirty-six ICR mice were randomly divided into 6 groups of 6 animals each. Mice were intranasally (50 µl volume) immunized with either saline or solely 5 × 105 TCID50 inactivated PRRSV antigen or antigen admixed with 200 µg alum, or 10, 50, or 100 µg of rLTB on 0, 7, and 21 days, following intraperitoneal anesthetizing (in 0.1 ml volume) with ketamine/xylazine at 0.03/0.015 mg/g body weight.
Mice body weights were monitored on day 0, 7, 14, 21, and 28 post primary immunization. Body weight changes were calculated as percent change in body weight from weight on day 0. On day 28, all mice were euthanized and their blood samples, bronchoalveolar lavage (BAL) fluid, fecal pellets, and vaginal washing collected for antigen-specific antibody assays. The spleens were harvested for determining cytokine production.
Measurement of PRRSV-specific IgA and IgG responses by ELISA
An indirect ELISA was conducted to measure PRRSV-specific IgA, IgG, IgG1, IgG2a, and IgG2b antibodies. Briefly, polyvinyl 96-well microtiter plates were coated with 100 µl of PRRSV antigen (10 µg/ml in 0.05 M carbonate buffer, pH 9.6) at 4°C overnight. The coated plates were washed thrice with PBS+0.05% Tween 20 (PBST) and blocked with 5% skimmed milk in PBS at 37°C for 2 hr. Samples were diluted using 5% skimmed milk in PBS. Aliquots (100 µl/well) of appropriately diluted samples were added to duplicate wells, and the plates incubated at room temperature for 2 hr. The sample dilutions used for the ELISA assays were 1:2 for fecal and BAL IgA, 1:20 for vaginal IgA, and 1:1,000 for serum IgG, IgG1, IgG2a, and IgG2b. After another round of washing, 100 µl of HRP-conjugated goat anti-mouse IgA (1:1,000), IgG (1:5,000), IgG1 (1:2,000), IgG2a (1:2,000), or IgG2b (1:2,000), was added and incubated at room temperature for 1 hr. Plates were washed again, and 100 µl of 3,3′,5,5′-tetramethyl benzidine substrate solution (100 µg/ml of 0.1 M citrate-phosphate, pH 5.0) added to each well, and incubated for 15 min. The reaction was stopped using 50 µl of 2 M H2SO4. The ODs of each plate was read at 450 nm using an ELISA plate reader (Thermo Multiscan MK3; Thermo Scientific).
Measurement of rLTB-specific IgA and IgG responses by ELISA
The rLTB-specific IgA and IgG antibody responses were analyzed by ELISA as described above, except for the changes indicated below. Each well was coated with 100 µl of rLTB (5 µg/ml in 0.05 M carbonate buffer, pH 9.6). To analyze anti-rLTB IgA antibodies, fecal, BAL, and vaginal wash samples were used without dilution. For anti-rLTB IgG antibodies, sera were diluted 1:100.
Cytokine measurement
Splenocytes were prepared as mentioned above, and seeded into a 24-well flat-bottom microtiter plate at 5.0 × 106 cells/ml in 2 ml of RPMI medium (Gibco). Thereafter 20 µg of the PRRSV antigen was added, and incubated at 37°C. After 48 hr treatment, supernatants were collected and measured in duplicates, using the Mouse Magnetic Luminex Screening Assay kit (R&D Systems, Minneapolis, MN, U.S.A.) and a Luminex® 100IS system (Luminex Corp., Austin, TX, U.S.A.).
Blood chemistry analyses
Blood samples were collected on days 2 and 28 post primary immunization. Sera were separated and assayed for the levels of alkaline phosphatase (ALP), blood urea nitrogen (BUN), creatinine, and bilirubin, using the respective assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), following the manufacturer’s instructions.
Statistical analyses
Data are expressed as mean ± standard deviation (SD), and were evaluated using one-way analysis of variance (ANOVA), followed by Dunnet’s test. Differences were considered significant if P was <0.05.
RESULTS
rLTB expression and immunoreactivity
To determine the inducible expression of the rLTB protein, culture supernatants were collected at indicated time points post initial methanol addition, and analyzed using SDS-PAGE and Western blot. SDS-PAGE showed that the target protein (approximately 14 kDa) was successfully secreted into the medium, alongside a few other proteins (Fig. 1A). Twenty four hours post induction, only traces of rLTB could be detected in the medium. There was a clear increase in quantity after induction for 48 hr, which peaked at 96 hr. The purified protein was recognized by anti-CT antibody, as shown on the Western blot (Fig. 1B). No corresponding band was observed in the negative control lane.
Fig. 1.
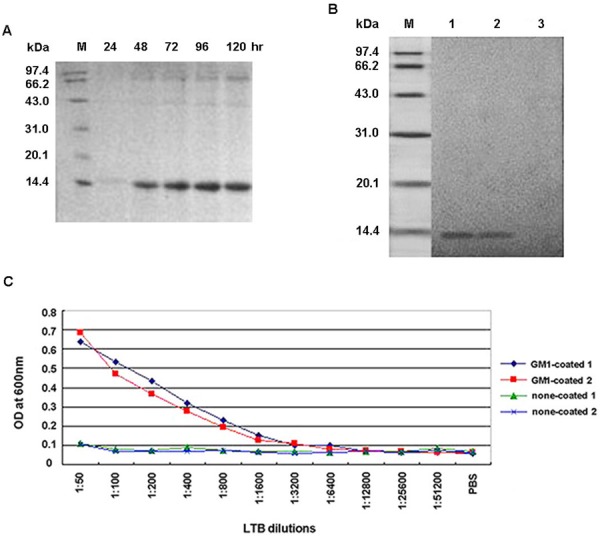
Expression, purification, and characterization of the recombinant B subunit of the Escherichia coli heat-labile enterotoxin (rLTB). (A) Sodium dodecyl sulfate-Polyacrylamide gel electrophoresis (SDS-PAGE) analyses for rLTB expression at indicated time points, post initial methanol addition to culture supernatants. (B) Western blot analyses for rLTB determination. Lanes 1 and 2: purified rLTB obtained from 2 independent experiments cultured in anti-cholera toxin (CT) antibody; Lane 3: purified rLTB cultured in CT-negative serum. (C) Ganglioside M1 (GM1)-ganglioside binding assay using enzyme-linked immunosorbent assay (ELISA). GM1 ganglioside coated or non-coated plates were incubated with purified rLTB, diluted as indicated. Results are from 2 independent experiments.
The purified rLTB was found to bind to the GM1-ganglioside receptor, and the affinity positively correlated with protein concentration, indicating that the purified rLTB was in the correct pentameric form (Fig. 1C).
rLTB enhances PRRSV-specific IgA and IgG antibody responses
Anti-PRRSV IgA and IgG antibodies were measured using indirect ELISA, to evaluate the adjuvant effect of the purified rLTB on humoral-mediated immune responses (Fig. 2). Compared with the PRRSV antigen-only group, alum supplement significantly (P<0.001) increased serum IgG antibodies against the PRRSV antigen, but had little effect on mucosal immune response enhancement. The 50 µg rLTB dose significantly (P<0.01) raised PRRSV-specific serum IgG responses, and increased PRRSV-specific IgA antibodies in BAL fluids, fecal pellets, and vaginal washing samples (P<0.001). Significantly (P<0.05) elevated vaginal and fecal IgA responses were also observed in the PRRSV antigen supplemented with 10 µg rLTB group. However, no enhancement activity was observed in the 100 µg rLTB-adjuvanted group.
Fig. 2.
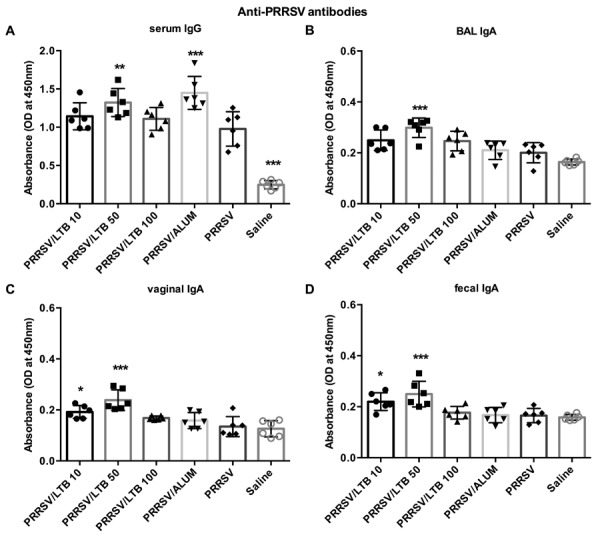
Anti-porcine reproductive and respiratory syndrome virus (PRRSV) antibody responses. Mice (n=6) were intranasally immunized with either saline, PRRSV antigen-only or antigen admixed with 200 µg alum, or 10, 50, or 100 µg rLTB, on days 0, 7, and 21. On day 28, sera were harvested for anti-PRRSV IgG analyses (A), and bronchoalveolar lavage (BAL) fluid (B), vaginal washing (C), and fecal pellets (D) were collected for anti-PRRSV IgA antibody measurements, using indirect ELISA. Data are expressed as mean ± SD. *P<0.05, **P<0.01, and ***P<0.001 versus the PRRSV antigen-only group.
A high rLTB dose elicits self-destructive antibody responses
To determine if antibody responses were elicited against rLTB itself, anti-rLTB IgG and IgA antibody responses were determined using ELISA (Fig. 3). Administering 10 or 50 µg of rLTB to mice, elicited limited levels of anti-rLTB serum IgG and mucosal IgA antibody responses, which were similar with those in the PRRSV-only group. However, the 100 µg rLTB dose significantly raised serum IgG (P<0.001), and BAL (P<0.01), fecal (P<0.001), and vaginal (P<0.05) IgA responses against itself, all significantly higher, compared with those in the PRRSV antigen-only group.
Fig. 3.
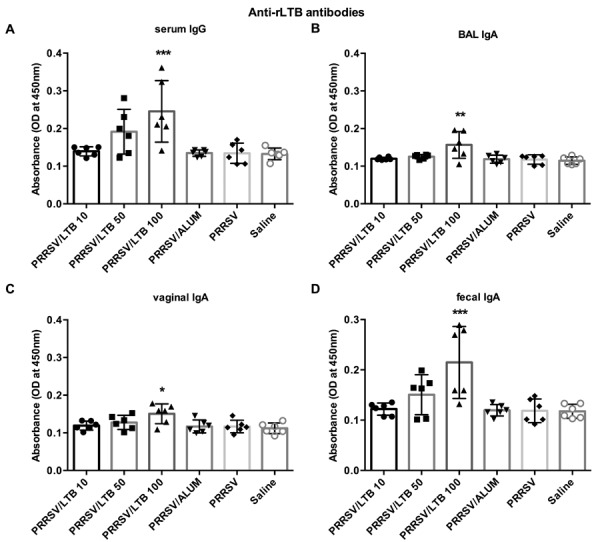
Anti-rLTB IgG and IgA responses. Mice (n=6) were intranasally immunized with either saline, porcine reproductive and respiratory syndrome virus (PRRSV) antigen-only or antigen admixed with 200 µg alum, or 10, 50, or 100 µg rLTB, on days 0, 7, and 21. On day 28, sera (A) were collected for anti-rLTB IgG antibody response analyses. BAL fluid (B), vaginal washing (C), and fecal pellets (D) were collected for measuring anti-rLTB IgA antibody responses. Data are expressed as mean ± SD. *P<0.05, **P<0.01 and ***P<0.001 versus the PRRSV antigen-only group.
rLTB increases PRRSV-specific serum IgG subclass production biased to a Th1-type response
The serum levels of PRRSV-specific IgG antibody subclasses were analyzed to assess rLTB effect on T helper cell differentiation. In mice and pigs, IgG1 is produced predominantly by a Th2-type response, while IgG2a and IgG2b are produced by a Th1-type response [3, 22]. Compared with the PRRSV antigen-only group, alum, known as a Th2-biased adjuvant, significantly (P<0.001) elevated IgG1 antibody titers against the PRRSV antigen, but had negligible effects on IgG2a and IgG2b antibody induction (Fig. 4).Conversely, the 50 µg rLTB supplement elicited significantly (P<0.05) stronger IgG2a and IgG2b responses, compared with those in the PRRSV antigen-only group, but had no effect on IgG1 antibody elicitation, suggesting the recruitment of Th1-type immune responses. There was no significant effect by 10 or 100 µg rLTB on the induction of either IgG1 or IgG2 responses.
Fig. 4.
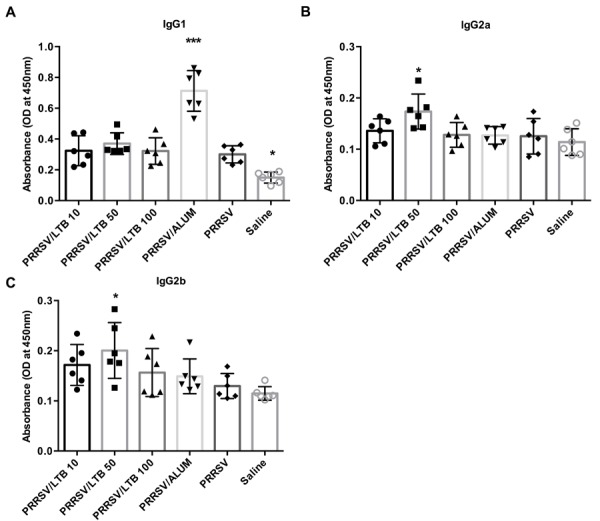
Porcine reproductive and respiratory syndrome virus (PRRSV) specific antibody subclasses. Mice (n=6) were intranasally immunized with either saline, PRRSV antigen-only or antigen admixed with 200 µg alum, or 10, 50, or 100 µg rLTB, on days 0, 7, and 21. On day 28, sera were harvested for IgG1 (A), IgG2a (B), and IgG2b (C) antibody responses, using indirect ELISA. Data are expressed as mean ± SD. *P<0.05 and ***P<0.001 versus the PRRSV antigen-only group.
rLTB promotes Th1- and Th17-type cytokine expression by lymphocytes
After re-stimulating with the PRRSV antigen in vitro, the splenocyte supernatants were collected to determine cytokine production. As shown in Fig. 5, PRRSV antigen admixed with alum, significantly promoted IL-4 (P<0.05), IL-10 (P<0.05), and IL-6 (P<0.01) production, but had no effect on IFN-γ and IL-12 secretion. Conversely, the 50 µg rLTB supplement significantly (P<0.05) up-regulated IFN-γ, IL-12, and IL-6 expression, but did not alter IL-4 and IL-10 production. There was no significant effect by 10 or 100 µg rLTB on the regulation of any cytokine expression.
Fig. 5.
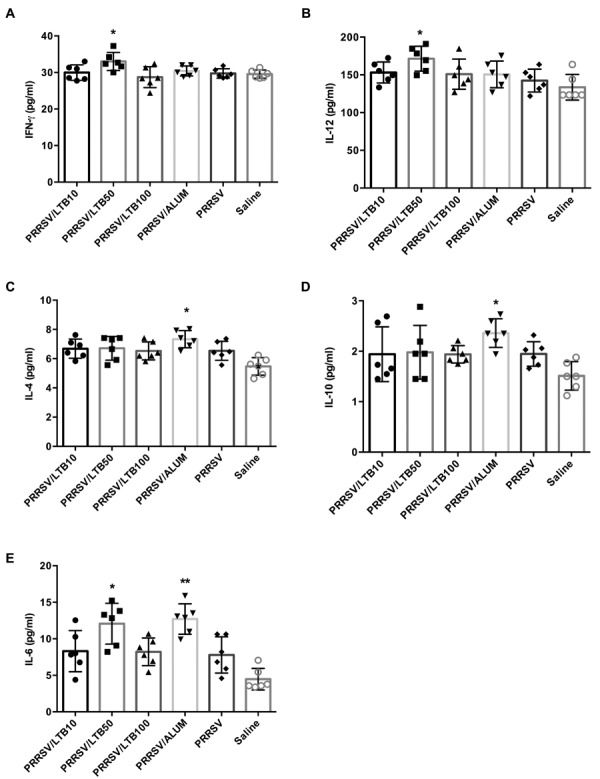
Production of IFN-γ, IL-12, IL-4, IL-10, and IL-6 by splenocytes. Mice (n=6) were intranasally immunized with either saline, porcine reproductive and respiratory syndrome virus (PRRSV) antigen-only or antigen admixed with 200 µg alum, or 10, 50, or 100 µg rLTB, on days 0, 7, and 21. Splenocytes were prepared on day 28 and cultured with PRRSV antigen for 48 hr. The supernatants were tested for IFN-γ (A), IL-12 (B), IL-4 (C), IL-10 (D), and IL-6 (E) analyses, using a Luminex® 100IS system. Data are expressed as mean ± SD. *P<0.05 and **P<0.01 versus the PRRSV antigen-only group.
Safety of rLTB as an intranasal adjuvant
To evaluate the safety of intranasal rLTB delivery, body weight gain was monitored as an indicator of general toxicity. Average mice body weight on day 0 was 21.86 ± 1.14 g. During the vaccination period, the body weight of each adjuvanted group continuously increased, at rates comparable to those in the saline group (Fig. 6). In the PRRSV antigen-only group, mice weights continuously increased on days 7, 14, and 21, but dropped by day 28. The increasing rates in the PRRSV antigen-only group on days 7 (P<0.05), 14 (P<0.01), and 28 (P<0.05), were significantly lower, compared with those in the saline group.
Fig. 6.
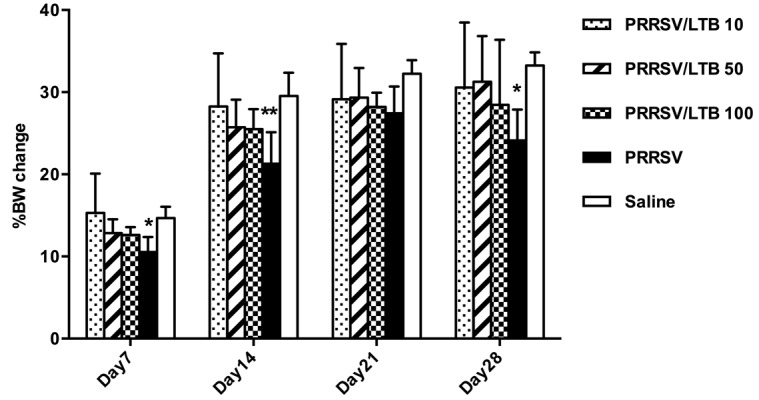
Percent change in mice body weights. Mice (n=6) were intranasally immunized with either saline, porcine reproductive and respiratory syndrome virus (PRRSV) antigen-only or antigen admixed with 10, 50 or 100 µg rLTB, on days 0, 7, and 21. Mice body weights were monitored at days 0, 7, 14, 21, and 28 post primary immunization. The changes in body weight were calculated as percent change in body weight, from weight on day 0. *P<0.05 and **P<0.01 versus the saline group.
Serum levels of ALP, BUN, creatinine, and bilirubin were analyzed on days 2 and 28, as shown in Fig. 7. High ALP, bilirubin, BUN, and creatinine levels clinically indicate hepatic and renal dysfunction. In our results, there were no significant treatment-related differences in ALP, BUN, and bilirubin levels. Significantly (P<0.001) decreased creatinine levels were observed in the 10 and 100 µg rLTB adjuvated and non-adjuvanted groups on day 28, compared with those in the saline group, although this decrease is not clinically logical. Therefore, there were no overall adverse effects on hepatic or renal function.
Fig. 7.
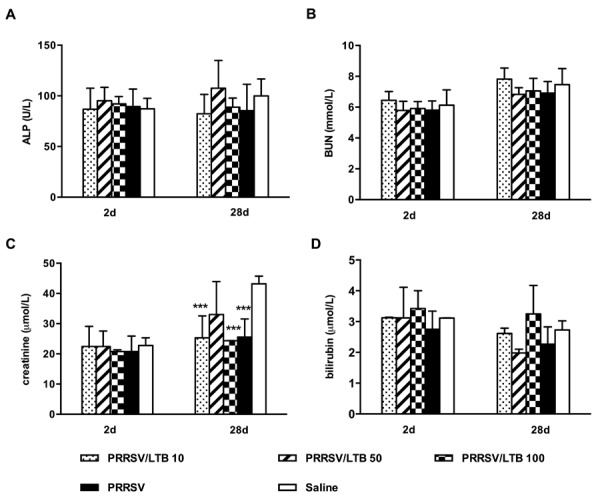
Mice serum levels of alkaline phosphatase (ALP), blood urea nitrogen (BUN), creatinine, and bilirubin. Mice (n=6) were intranasally immunized with either saline, porcine reproductive and respiratory syndrome virus (PRRSV) antigen-only or antigen admixed with 10, 50, or 100 µg rLTB, on days 0, 7, and 21. Sera were collected on days 2 and 28, and ALP (A), BUN (B), creatinine (C), and bilirubin (D) levels detected. Data are expressed as mean ± SD. ***P<0.001 versus the saline group.
DISCUSSION
The preference of the PRRSV inactivated vaccine over the attenuated live vaccine is attributed to safety concerns. However, its poor immunogenicity limits field use. Several reports have demonstrated that PRRSV inactivated vaccine under field situations, failed to induce sufficient specific antibody- [7] and cell-mediated [23, 28] immune responses, and conferred little protection against PRRSV [6]. However, a recent strategy using intranasally delivered poly (lactic-co-glycolic) acid nanoparticle-entrapped inactivated PRRSV vaccine, with the whole cell lysate of Mycobacterium tuberculosis as adjuvant, elicited a broad cross-protective effect against heterogeneous PRRSV strains [23]. This suggests that a suitable immune adjuvant and appropriate immunization route may help enhance PRRSV inactivated vaccine efficacy. The non-toxic LTB is a potent mucosal adjuvant, which has been used in various vaccines. The expression system of the methylotrophic yeast P. pastoris, has been used as an ideal host for large-scale production of recombinant proteins, including rLTB, with many advantages such as high heterologous protein expression, efficient secretion, and high growth density in fermentor cultures [16]. In this study, 49.9 mg/ml rLTB protein was successfully produced from P. pastoris, and purified on a Ni2+ column after concentration, and showed high affinity to the GM1-ganglioside receptor, as shown in Fig. 1. The intact receptor-binding site of the LTB pentamer is necessary for its potent adjuvant nature [16, 17].
Highly specialized innate and adaptive mucosal immune responses at mucosal surfaces are of major importance in host defense against pathogen epithelial extravastation to cause diseases at other tissues [2, 33]. Secretory IgA is the main immunoglobulin isotype mediating humoral immunity at mucosal surfaces. Our antibody results demonstrated that the 50 µg rLTB dose was capable of enhancing systemic and mucosal antibodies to co-delivered antigen, following intranasal immunization. The anti-PRRSV IgA antibodies increased at both the local (BAL fluid) and distal gastrointestinal (fecal pellets) mucosal sites, and urogenital (vaginal wash) tracts in mice, indicating rLTB potential to activate humoral immunity at mucosal sites (Fig. 2). The elicitation of antibody responses against the adjuvant itself can inhibit the immune responses raised against the antigen, especially upon repeated use of the adjuvant for host immunization [27]. In this study, strong antibody response against rLTB itself, was observed in mice administered 100 µg of rLTB, which may lead to the suppression of PRRSV-specific immune responses (Figs. 2 and 3). As a result, the optimal rLTB dose for adjuvant activities appears to be 50 µg.
Immunity to different infectious agents requires distinct types of immune responses. In pigs, the predominant IgG isotype is controlled by Th1/Th2 type cytokines, and associated with resistance to disease. Recent studies have indicated that PRRSV-infected pigs are typically deficient in Th1-type immune responses, which may lead to prolonged immunosuppression, followed by weaker antibody responses, increased viremia, and greater disease severity [10, 31]. Distinct from the alum-induced Th2-biased enhancement effect, the 50 µg rLTB dose only promoted IFN-γ and IL-12 expression, which were associated with predominant IgG2a and IgG2b subclass responses, suggesting the promotion of Th1-biased immune response (Figs. 4 and 5). Furthermore, the 50 µg rLTB dose enhanced lymphocyte IL-6 secretion, in response to PRRSV antigen re-stimulation. IL-6, known as a pleiotropic cytokine, serves as an important mediator regulating the balance between Th17 cells and regulatory T cells (Tregs) [8]. Recent research demonstrated that a strain of the Chinese HP-PRRSV appeared to decrease Th17 cells in the peripheral blood and lungs of pigs, increasing susceptibility to secondary bacterial infections [36]. Moreover, the immunosuppressive responses induced by PRRSV infection are associated with a strong Treg response [32, 35]. Thus, the rLTB-enhanced IL-6 activities may help to trigger Th17 cell differentiation from naïve T cells, and suppress Treg development, ultimately attenuating PRRSV-induced immunosuppressive responses.
There were no vaccination-related overt clinical signs observed in the immunized mice. The PRRSV antigen-only treatment reduced the rates of weight gain on days 7, 14, and 28, and caused approximately a 3.32% decrease in body weight on day 28, compared with that on day 21 (Fig. 6). However, body weight gain in adjuvanted groups increased continuously throughout the vaccination period, comparable with those in the saline group. This suggests that to some extent, rLTB may attenuate the antigen-related weight loss. The detailed blood clinical chemistry indicated no adverse effects on the hepatic or renal function in all the groups (Fig. 7).
Summarily, this study demonstrated the intranasal adjuvant properties of rLTB produced from P. pastoris fermentation. The presence of rLTB, particularly at the 50 µg dose, significantly increased PRRSV-specific IgA and IgG antibodies, remarkably enhanced Th1- and Th17-type cellular immune responses, and showed no potential toxicity concerns associated with intranasal immunization. Our data therefore supports further porcine research.
Acknowledgments
This study was supported by the National Natural Scientific Foundation of China (31802235). We would like to thank Professor Yicheng Wang for his contribution to rLTB preparation.
REFERENCES
- 1.Binjawadagi B., Dwivedi V., Manickam C., Ouyang K., Wu Y., Lee L. J., Torrelles J. B., Renukaradhya G. J.2014. Adjuvanted poly(lactic-co-glycolic) acid nanoparticle-entrapped inactivated porcine reproductive and respiratory syndrome virus vaccine elicits cross-protective immune response in pigs. Int. J. Nanomedicine 9: 679–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouvet J. P., Decroix N., Pamonsinlapatham P.2002. Stimulation of local antibody production: parenteral or mucosal vaccination? Trends Immunol. 23: 209–213. doi: 10.1016/S1471-4906(02)02186-5 [DOI] [PubMed] [Google Scholar]
- 3.Crawley A. M., Mallard B., Wilkie B. N.2005. Genetic selection for high and low immune response in pigs: effects on immunoglobulin isotype expression. Vet. Immunol. Immunopathol. 108: 71–76. doi: 10.1016/j.vetimm.2005.07.006 [DOI] [PubMed] [Google Scholar]
- 4.Evans A. B., Loyd H., Dunkelberger J. R., van Tol S., Bolton M. J., Dorman K. S., Dekkers J. C. M., Carpenter S.2017. Antigenic and Biological Characterization of ORF2-6 variants at early times following PRRSV Infection. Viruses 9: 113. doi: 10.3390/v9050113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harakuni T., Sugawa H., Komesu A., Tadano M., Arakawa T.2005. Heteropentameric cholera toxin B subunit chimeric molecules genetically fused to a vaccine antigen induce systemic and mucosal immune responses: a potential new strategy to target recombinant vaccine antigens to mucosal immune systems. Infect. Immun. 73: 5654–5665. doi: 10.1128/IAI.73.9.5654-5665.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Y., Cong X., Chen L., Qi J., Wu X., Zhou M., Yoo D., Li F., Sun W., Wu J., Zhao X., Chen Z., Yu J., Du Y., Wang J.2016. Synergy of TLR3 and 7 ligands significantly enhances function of DCs to present inactivated PRRSV antigen through TRIF/MyD88-NF-κB signaling pathway. Sci. Rep. 6: 23977. doi: 10.1038/srep23977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H., Kim H. K., Jung J. H., Choi Y. J., Kim J., Um C. G., Hyun S. B., Shin S., Lee B., Jang G., Kang B. K., Moon H. J., Song D. S.2011. The assessment of efficacy of porcine reproductive respiratory syndrome virus inactivated vaccine based on the viral quantity and inactivation methods. Virol. J. 8: 323. doi: 10.1186/1743-422X-8-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura A., Kishimoto T.2010. IL-6: regulator of Treg/Th17 balance. Eur. J. Immunol. 40: 1830–1835. doi: 10.1002/eji.201040391 [DOI] [PubMed] [Google Scholar]
- 9.Labarque G., Van Gucht S., Van Reeth K., Nauwynck H., Pensaert M.2003. Respiratory tract protection upon challenge of pigs vaccinated with attenuated porcine reproductive and respiratory syndrome virus vaccines. Vet. Microbiol. 95: 187–197. doi: 10.1016/S0378-1135(03)00157-3 [DOI] [PubMed] [Google Scholar]
- 10.Lee J., Kim Y. M., Kim J. H., Cho C. W., Jeon J. W., Park J. K., Lee S. H., Jung B. G., Lee B. J.2018. Nasal delivery of chitosan/alginate nanoparticle encapsulated bee (Apis mellifera) venom promotes antibody production and viral clearance during porcine reproductive and respiratory syndrome virus infection by modulating T cell related responses. Vet. Immunol. Immunopathol. 200: 40–51. doi: 10.1016/j.vetimm.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 11.Lee J., Yoo J. K., Sohn H. J., Kang H. K., Kim D., Shin H. J., Kim J. H.2015. Protective immunity against Naegleria fowleri infection on mice immunized with the rNfa1 protein using mucosal adjuvants. Parasitol. Res. 114: 1377–1385. doi: 10.1007/s00436-015-4316-3 [DOI] [PubMed] [Google Scholar]
- 12.Lei H., Peng X., Shu H., Zhao D.2015. Intranasal immunization with live recombinant Lactococcus lactis combined with heat-labile toxin B subunit protects chickens from highly pathogenic avian influenza H5N1 virus. J. Med. Virol. 87: 39–44. doi: 10.1002/jmv.23983 [DOI] [PubMed] [Google Scholar]
- 13.Li Z., He Y., Xu X., Leng X., Li S., Wen Y., Wang F., Xia M., Cheng S., Wu H.2016. Pathological and immunological characteristics of piglets infected experimentally with a HP-PRRSV TJ strain. BMC Vet. Res. 12: 230. doi: 10.1186/s12917-016-0854-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linhares D. C., Cano J. P., Wetzell T., Nerem J., Torremorell M., Dee S. A.2012. Effect of modified-live porcine reproductive and respiratory syndrome virus (PRRSv) vaccine on the shedding of wild-type virus from an infected population of growing pigs. Vaccine 30: 407–413. doi: 10.1016/j.vaccine.2011.10.075 [DOI] [PubMed] [Google Scholar]
- 15.Liu X., Dong Y., Wang J., Li L., Zhong Z., Li Y. P., Chen S. J., Fu Y. C., Xu W. C., Wei C. J.2017. VSV-G Viral Envelope Glycoprotein Prepared from Pichia pastoris Enhances Transfection of DNA into Animal Cells. J. Microbiol. Biotechnol. 27: 1098–1105. doi: 10.4014/jmb.1611.11082 [DOI] [PubMed] [Google Scholar]
- 16.Ma X., Yao B., Zheng W., Li L.2010. Comparative study on characterization of recombinant B subunit of E. coli heat-labile enterotoxin (rLTB) prepared from E. coli and P. patoris. J. Microbiol. Biotechnol. 20: 550–557. [PubMed] [Google Scholar]
- 17.Ma Y.2016. Recent advances in nontoxic Escherichia coli heat-labile toxin and its derivative adjuvants. Expert Rev. Vaccines 15: 1361–1371. doi: 10.1080/14760584.2016.1182868 [DOI] [PubMed] [Google Scholar]
- 18.Marchioro S. B., Fisch A., Gomes C. K., Jorge S., Galli V., Haesebrouck F., Maes D., Dellagostin O., Conceição F. R.2014. Local and systemic immune responses induced by a recombinant chimeric protein containing Mycoplasma hyopneumoniae antigens fused to the B subunit of Escherichia coli heat-labile enterotoxin LTB. Vet. Microbiol. 173: 166–171. doi: 10.1016/j.vetmic.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 19.Miyata T., Harakuni T., Taira T., Matsuzaki G., Arakawa T.2012. Merozoite surface protein-1 of Plasmodium yoelii fused via an oligosaccharide moiety of cholera toxin B subunit glycoprotein expressed in yeast induced protective immunity against lethal malaria infection in mice. Vaccine 30: 948–958. doi: 10.1016/j.vaccine.2011.11.059 [DOI] [PubMed] [Google Scholar]
- 20.Miyata T., Harakuni T., Tsuboi T., Sattabongkot J., Kohama H., Tachibana M., Matsuzaki G., Torii M., Arakawa T.2010. Plasmodium vivax ookinete surface protein Pvs25 linked to cholera toxin B subunit induces potent transmission-blocking immunity by intranasal as well as subcutaneous immunization. Infect. Immun. 78: 3773–3782. doi: 10.1128/IAI.00306-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyata T., Oshiro S., Harakuni T., Taira T., Matsuzaki G., Arakawa T.2012. Physicochemically stable cholera toxin B subunit pentamer created by peripheral molecular constraints imposed by de novo-introduced intersubunit disulfide crosslinks. Vaccine 30: 4225–4232. doi: 10.1016/j.vaccine.2012.04.047 [DOI] [PubMed] [Google Scholar]
- 22.Nagaputra J. C., Rollier C. S., Sadarangani M., Hoe J. C., Mehta O. H., Norheim G., Saleem M., Chan H., Derrick J. P., Feavers I., Pollard A. J., Moxon E. R.2014. Neisseria meningitidis native outer membrane vesicles containing different lipopolysaccharide glycoforms as adjuvants for meningococcal and nonmeningococcal antigens. Clin. Vaccine Immunol. 21: 234–242. doi: 10.1128/CVI.00561-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nan Y., Wu C., Gu G., Sun W., Zhang Y. J., Zhou E. M.2017. Improved Vaccine against PRRSV: Current Progress and Future Perspective. Front. Microbiol. 8: 1635. doi: 10.3389/fmicb.2017.01635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newsted D., Fallahi F., Golshani A., Azizi A.2015. Advances and challenges in mucosal adjuvant technology. Vaccine 33: 2399–2405. doi: 10.1016/j.vaccine.2015.03.096 [DOI] [PubMed] [Google Scholar]
- 25.Nieuwenhuis N., Duinhof T. F., van Nes A.2012. Economic analysis of outbreaks of porcine reproductive and respiratory syndrome virus in nine sow herds. Vet. Rec. 170: 225. doi: 10.1136/vr.100101 [DOI] [PubMed] [Google Scholar]
- 26.Ouyang K., Hiremath J., Binjawadagi B., Shyu D. L., Dhakal S., Arcos J., Schleappi R., Holman L., Roof M., Torrelles J. B., Renukaradhya G. J.2016. Comparative analysis of routes of immunization of a live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in a heterologous virus challenge study. Vet. Res. (Faisalabad) 47: 45. doi: 10.1186/s13567-016-0331-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel G. B., Ponce A., Zhou H., Chen W.2008. Safety of intranasally administered archaeal lipid mucosal vaccine adjuvant and delivery (AMVAD) vaccine in mice. Int. J. Toxicol. 27: 329–339. doi: 10.1080/10915810802352703 [DOI] [PubMed] [Google Scholar]
- 28.Piras F., Bollard S., Laval F., Joisel F., Reynaud G., Charreyre C., Andreoni C., Juillard V.2005. Porcine reproductive and respiratory syndrome (PRRS) virus-specific interferon-gamma+ T-cell responses after PRRS virus infection or vaccination with an inactivated PRRS vaccine. Viral Immunol. 18: 381–389. doi: 10.1089/vim.2005.18.381 [DOI] [PubMed] [Google Scholar]
- 29.Renukaradhya G. J., Meng X. J., Calvert J. G., Roof M., Lager K. M.2015. Live porcine reproductive and respiratory syndrome virus vaccines: Current status and future direction. Vaccine 33: 4069–4080. doi: 10.1016/j.vaccine.2015.06.092 [DOI] [PubMed] [Google Scholar]
- 30.Renukaradhya G. J., Meng X. J., Calvert J. G., Roof M., Lager K. M.2015. Inactivated and subunit vaccines against porcine reproductive and respiratory syndrome: Current status and future direction. Vaccine 33: 3065–3072. doi: 10.1016/j.vaccine.2015.04.102 [DOI] [PubMed] [Google Scholar]
- 31.Shi K. C., Guo X., Ge X. N., Liu Q., Yang H. C.2010. Cytokine mRNA expression profiles in peripheral blood mononuclear cells from piglets experimentally co-infected with porcine reproductive and respiratory syndrome virus and porcine circovirus type 2. Vet. Microbiol. 140: 155–160. doi: 10.1016/j.vetmic.2009.07.021 [DOI] [PubMed] [Google Scholar]
- 32.Silva-Campa E., Flores-Mendoza L., Reséndiz M., Pinelli-Saavedra A., Mata-Haro V., Mwangi W., Hernández J.2009. Induction of T helper 3 regulatory cells by dendritic cells infected with porcine reproductive and respiratory syndrome virus. Virology 387: 373–379. doi: 10.1016/j.virol.2009.02.033 [DOI] [PubMed] [Google Scholar]
- 33.Su F., Patel G. B., Hu S., Chen W.2016. Induction of mucosal immunity through systemic immunization: Phantom or reality? Hum. Vaccin. Immunother. 12: 1070–1079. doi: 10.1080/21645515.2015.1114195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thanasarasakulpong A., Poolperm P., Tankaew P., Sawada T., Sthitmatee N.2015. Protectivity conferred by immunization with intranasal recombinant outer membrane protein H from Pasteurella multocida serovar A:1 in chickens. J. Vet. Med. Sci. 77: 321–326. doi: 10.1292/jvms.14-0532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wongyanin P., Buranapraditkun S., Chokeshai-Usaha K., Thanawonguwech R., Suradhat S.2010. Induction of inducible CD4+CD25+Foxp3+ regulatory T lymphocytes by porcine reproductive and respiratory syndrome virus (PRRSV). Vet. Immunol. Immunopathol. 133: 170–182. doi: 10.1016/j.vetimm.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 36.Zhang L., Zhou L., Ge X., Guo X., Han J., Yang H.2016. The Chinese highly pathogenic porcine reproductive and respiratory syndrome virus infection suppresses Th17 cells response in vivo. Vet. Microbiol. 189: 75–85. doi: 10.1016/j.vetmic.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 37.Zhou X., Wang Y., Jiang P., Yuan X., Li J., Du X.2014. Dynamic and distribution of recombinant E.coli heat-labile enterotoxin B subunit in duck. Huabei Nongxuebao 29: 104–109. [Google Scholar]


