Abstract
In this study, Matrix Assisted Laser Desorption Ionization-Time-of-Flight (MALDI-TOF) mass spectrometry was used to identify Mycobacterium bovis from cattle and buffalo tissue isolates from the North and South regions of Brazil, grown in solid medium and previously identified by Polymerase Chain Reaction (PCR) based on Region of Difference 4 (RD4), sequencing and spoligotyping. For this purpose, the protein extraction protocol and the mass spectra reference database were optimized for the identification of 80 clinical isolates of mycobacteria. As a result of this optimization, it was possible to identify and differentiate M. bovis from other members of the Mycobacterium tuberculosis complex with 100% specificity, 90.91% sensitivity and 91.25% reliability. MALDI-TOF MS methodology described herein provides successful identification of M. bovis within bovine/bubaline clinical samples, demonstrating its usefulness for bovine tuberculosis diagnosis in the future.
Keywords: bovine tuberculosis, Matrix Assisted Laser Desorption Ionization-Time-of-Flight (MALDI-TOF) mass spectrometry, Mycobacterium bovis, Mycobacterium tuberculosis complex
Mycobacterium bovis (M. bovis) is an intracellular bacterium, belonging to the Mycobacterium tuberculosis complex (MTC), the etiologic agent of bovine tuberculosis (TB) [20, 22, 23].
TB is a threat to public health, mainly by ingestion of unpasteurized dairy products contaminated with M. bovis [5, 13]. In addition, TB causes considerable economic losses arising from the reduction of animal productivity and restrictions on international trade [12, 20].
In Brazil, the control of bovine/bubaline tuberculosis is regulated by the Brazilian National Program for the Control and Eradication of Animal Brucellosis and Tuberculosis (PNCEBT) [7]. There is an increasing pressure from beef import markets for a definitive detection of M. bovis in lesions suggestive of tuberculosis [6].
The diagnosis of TB in cattle consists of the isolation of the etiological agent by microbiological culture, considered the gold standard technique [3]. However, after isolation there is a need for etiological confirmation of the agent, which can be performed by biochemical methods or molecular tests [9, 23, 25, 29]. In Brazil, there are no records of other species of MTC other than M. bovis infecting cattle, although infections with non-tuberculous mycobacteria have been reported in cattle [1, 16].
Specific identification of an isolate as M. bovis can be made using Polymerase Chain Reaction (PCR) targeting the presence/absence of RDs (Regions of Difference) [22]. Region of Difference 4 (RD4) is a ~13 kb fragment absent in M. bovis and present in the other MTC species [8, 14, 26, 28, 29].
Microorganism identification based on mass spectrometry (MS) by MALDI-TOF (Matrix Assisted Laser Desorption Ionization–Time-of-Flight) has been integrated into the microbiology laboratory workflow due to its speed, reliability, cost-effectiveness and high yield, and has enabled efficient identification of environmental and pathogenic mycobacteria, such as those belonging to MTC [21, 30].
MALDI-TOF MS results can be obtained faster than the time spent to obtain PCR and DNA sequencing results [17, 27]. Although the initial investments for the acquisition of the MALDI-TOF MS equipment and the database are relatively high, these investments can be recovered in a few years, due to the economy generated by the routine use of this technique, when compared to molecular methods [2, 17, 27].
In this context, the aim of this study was to analyze a modified method for extracting proteins from M. bovis, in comparison with conventional method, and to evaluate an updated database with proper reference spectra, for identification of M. bovis in bovine/bubaline clinical samples, cultured in solid medium.
MATERIALS AND METHODS
Samples
Eighty tissue samples from cattle (n=35) and buffalo (n=21), naturally infected with or without lesions suggestive of tuberculosis, were obtained at North (n=30) (Pará and Amazonas states) and South (n=26) (Rio Grande do Sul state) regions of Brazil, from sanitary slaughters or sanitary inspection. The tissues collected were: lymph nodes (retropharyngeal, tracheal, mesenteric, pulmonary, pre-scapular), lung, mammary, liver and spleen tissue, and the number of tissues per animal ranged from one to nine. The samples were placed in sealed plastic bags, frozen and sent under refrigeration in a thermal insulation box to the Embrapa Animal Immunology Laboratory, Campo Grande, MS, Brazil. After microbiological culture, 77 samples showed growth of colonies suggestive of M. bovis and three isolates showed morphology not compatible with M. bovis. These isolates were submitted to identification as described below. The virulent strain H37Rv of M. tuberculosis, provided by the Central Laboratory of Public Health (LACEN), Campo Grande, MS, Brazil, and the 08-08BF2 strain of M. bovis (NCBI Biosample SAMN02902642) were used as negative and positive controls for Mb400 PCR (RD4 flanking region), respectively.
Isolation of Mycobacterium bovis by microbiological culture
Tissue fragments (between 10 and 25 mg) were macerated with 1.5 ml of sterile distilled water in Magna Lyser apparatus (Roche Life Science, Penzberg, Germany), followed by decontamination by Petroff method [19, 24] and cultured in Stonebrink medium at 37°C with weekly evaluations for 90 days.
PCR, sequencing, and spoligotyping
For Mb400 PCR, primers Mb400 F (5′AACGCGACGACCTCATATTC3′) and Mb400 R (5′AAGGCGAACAGATTC AGCAT3′), flanking the RD4, were used. As RD4 is deleted in M. bovis, the Mb400 PCR results in the amplification of a fragment of 400 base pairs (bp) [29]. The Mb400 positive isolates were further genotyped by spoligotyping [15]. For characterization of the Mb400 negative isolates, the partial sequence of the hsp65 gene of Mycobacterium sp. was amplified [31]. In order to remove excess primers and unincorporated nucleotides, the products of the hsp65 PCRs were purified with the enzymes exonuclease I and shrimp alkaline phosphatase [4], and sequenced with the BigDye Terminator cycle sequencing kit version 3.1 (Applied Biosystems, Foster City, CA, U.S.A.).
Protein extraction and MALDI-TOF analysis
In previous studies in our laboratory, a protein extraction method called MycoLyser was developed using the M. tuberculosis strain. This protocol is a modification of the recommended MycoEx method (Bruker Daltonics, Bremen, Germany) for mycobacterial protein extraction, and includes homogenization in tissue macerator rather than vortex. In a direct comparison between those two methods, MycoLyser showed higher scores and identification consistency with BiotyperTM system (Bruker Daltonics, Bremen, Germany), as well the best results regarding reliability for M. tuberculosis identification (data not shown). In view of the good results already obtained with the MycoLyser method, this was employed in this study to identify M. bovis. Protein profiles were obtained in triplicate from bacterial cultures. Briefly, the equivalent of two loops of mycobacteria was collected at 200 µl of ultrapure type I water and fully suspended using a vortex mixer. For inactivation, the bacterial suspension was incubated in a thermo-block at 95°C for 45 min, and, after cooling at room temperature, 700 µl of absolute ethanol were added. For cellular disruption, 0.5 mm diameter silica/zirconia beads were added, followed by homogenization in MagNA Lyser apparatus for three cycles of 30 sec at 5,000 rpm. The supernatant was collected and transferred to another tube, followed by centrifugation at 14,000 g for 5 min. The supernatant was discarded and the pellet incubated for three min at room temperature to dry the remaining alcohol. Then, 10 µl of formic acid and equal volume of acetonitrile were added, followed by vortexing for 15 sec and centrifugation for two min at 14,000 g. Subsequently, 1 µl of the supernatant was applied to a MTP 384 ground steel MALDI-TOF target (Bruker Daltonics), allowed to air-dry at room temperature, and 1 µl of α-cyano-4-hydroxy-cinnamic acid (5 mg/ml) was added in a solution containing 50% acetonitrile and 2.5% trifluoroacetic acid (v/v). After drying the solvents, the crystallized mixture was analyzed using the Autoflex III Smartbeam mass spectrometer (Bruker Daltonics), at mass range of 2,000 to 20,000 Daltons and external calibration with Bacterial Test Standard (Bruker Daltonics). The mass spectra acquisition parameters used were: IS1 20 kV, IS2 18.55 kV, lens voltage 8.80 kV and ion extraction delay time of 240 nsec. Random laser shots were performed with sampling rate of 0,5GS/sec, totalizing 2,000 spectra which were summed and processed by the centroid of the peak with FlexControl 3.3 software (Bruker Daltonics), generating the raw spectrum of each isolate.
Optimization of the identification of M. bovis by MALDI BiotyperTM
The raw spectra were processed using the Bruker MALDI BiotyperTM 3.1 software, with default settings. BiotyperTM creates a list of the most significant peaks in the raw spectrum (m/z values and respective intensity, with a signal-to-noise ratio greater than 3, a minimum threshold of 0.1% of the highest peak and a maximum of 100 peaks). Thus, for identification of clinical isolates, this generated peak list containing the mass signals and their intensities was compared, by means of the BiotyperTM algorithm, with Bruker Daltonics mass spectra libraries BDAL-containing 7,311 Mass Spectra Profiles (MSP) for referenced bacteria−and Myco v.5.0−containing 912 MSP for referenced mycobacteria.
The BiotyperTM score value classification used, as proposed by the manufacturer, was: a score between 2.30 and 3.00 indicates the reliable identification of species; a score between 2.00 and 2.29 indicates reliable genus identification and probable species identification; a score between 1.70 and 1.99 indicates likely genus identification; and a score below 1.70 indicates that there is no reliable identification. Still, according to Bruker Daltonics, the reliability of high, medium or low identification can also be evaluated by the consistency of the three highest scores regarding the identification of the same species/genus. In this work, the reliability of the identification was evaluated as follows: 1) Consistency of species−in which the reliability of identification for species is high. In this case, we consider that the three highest scores should present the same result of genus and species identification; 2) Genus consistency−in which only the reliability of identification for genus is high, when the three highest scores present the same result of identification for genus; and 3) Absence of consistency for species or genus−in which there is no reliability of identification for genus or species, because the previous criteria are not met.
The MSP for M. bovis, made up with four clinical isolates from the North (n=3) and South (n=1) regions of Brazil after Mb400 PCR and spoligotyping (SB0121 and SB0822) identification, and for the M. tuberculosis H37Rv reference strain, were generated from the processing of 12 and 18 spectra replicates of each species, respectively, resulting in a consensus of the most reproducible spectrum peaks, which reflects the typical protein pattern of those microorganisms. To create the MSP peak lists of the above bacterial references, BiotyperTM was set for using 80 independent peaks with no less than 50% minimal frequency. The MSP were added to the BiotyperTM reference library, totaling 8,225 distinct MSP which were utilized to identify the 80 clinical isolates. The three highest scores found for each isolate were considered for BiotyperTM identification. Wilcoxon test was used to perform the statistics of the M. bovis identification scores with and without the inclusion of the obtained MSP to the reference database. ClinProTools v.3.0 (Bruker Daltonics) and GraphPad Prism v.8 (GraphPad Software LLC, San Diego, CA, U.S.A.) softwares were used for statistical analysis of the mass spectra and for the production of the graphs, respectively. Comparisons between MALDI-TOF MS and PCR were evaluated using Fischer’s exact test.
RESULTS
Cultures of all 80 bovine and buffalo tissue samples showed bacterial growth in Stonebrink medium. In the analysis by conventional PCR using Mb400 primers flanking RD4, amplification was observed in 77 samples (96.25%). The three negative samples (3.75%) were submitted to PCR for the hsp65 gene and DNA sequencing. Two isolates of Gordonia sp. and one isolate of Mycobacterium nonchromogenicum were identified (Table 1).
Table 1. Species identification of tuberculosis positive cattle and buffalo tissue isolates by Mb400 PCR (Region of Difference 4 flanking region), spoligotyping and Matrix Assisted Laser Desorption Ionization-Time-of-Flight (MALDI-TOF) mass spectrometry + BiotyperTM, with scores after inclusion of the MycoLyser Mycobacterium bovis and M. tuberculosis Mass Spectra Profiles.
| Isolate | Mb400 PCR | Spoligotyping | MALDI-TOF Biotyper |
Score | Isolate | Mb400 PCR | Spoligotyping | MALDI-TOF Biotyper |
Score |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M. bovis | SB0295 | M. bovis | 2.19 | 41 | M. bovis | SB0121 | UN | ≤1.59 |
| 2 | M. bovis | SB0295 | M. bovis | 2.42 | 42 | M. bovis | SB0121 | M. bovis | 1.99 |
| 3 | M. bovis | SB0295 | M. bovis | 2.48 | 43 | M. bovis | SB0121 | M. bovis | 2.59 |
| 4 | M. bovis | SB0295 | M. bovis | 2.30 | 44 | M. bovis | SB0121 | M. bovis | 1.90 |
| 5 | M. bovis | SB0295 | M. bovis | 2.30 | 44 | M. bovis | SB0121 | M. bovis | 2.62 |
| 6 | M. bovis | SB0121 | UN | ≤1.59 | 46 | M. bovis | SB0121 | M. bovis | 2.68 |
| 7 | M. bovis | SB0295 | M. bovis | 2.31 | 47 | M. bovis | SB0121 | M. bovis | 2.45 |
| 8 | M. bovis | SB0295 | M. bovis | 2.61 | 48 | M. bovis | SB0121 | UN | ≤1.59 |
| 9 | M. bovis | SB0121 | M. bovis | 2.49 | 49 | M. bovis | SB0822 | UN | ≤1.59 |
| 10 | M. bovis | SB0121 | M. bovis | 2.51 | 50 | M. bovis | SB0822 | M. bovis | 2.59 |
| 11 | M. bovis | SB0121 | M. bovis | 2.56 | 51 | M. bovis | SB0295 | M. bovis | 2.47 |
| 12 | M. bovis | SB0121 | M. bovis | 2.34 | 52 | M. bovis | SB0295 | M. bovis | 2.55 |
| 13 | M. bovis | SB0121 | M. bovis | 2.15 | 53 | M. bovis | SB0295 | M. bovis | 2.61 |
| 14 | M. bovis | SB0121 | M. bovis | 2.22 | 54 | M. bovis | SB0822 | M. bovis | 2.75 |
| 15 | M. bovis | SB0121 | M. bovis | 2.54 | 55 | M. bovis | SB0822 | M. bovis | 2.47 |
| 16 | M. bovis | SB0121 | M. bovis | 2.63 | 56 | M. bovis | SB1869 | M. bovis | 2.65 |
| 17 | M. bovis | SB0295 | UN | ≤1.59 | 57 | M. bovis | SB1869 | M. bovis | 2.62 |
| 18 | M. bovis | SB0121 | M. bovis | 2.45 | 58 | M. bovis | SB1800 | M. bovis | 2.47 |
| 19 | M. bovis | SB0121 | M. bovis | 2.35 | 59 | M. bovis | SB0822 | M. bovis | 2.73 |
| 20 | M. bovis | SB0121 | M. bovis | 2.39 | 60 | M. bovis | SB0822 | M. bovis | 2.61 |
| 21 | M. bovis | SB0121 | M. bovis | 2.33 | 61 | M. bovis | SB0295 | M. bovis | 2.43 |
| 22 | M. bovis | SB0121 | M. bovis | 2.26 | 62 | M. bovis | SB0822 | M. bovis | 2.64 |
| 23 | M. bovis | SB0121 | M. bovis | 2.46 | 63 | Negative | NA | Gordonia sputi* | 1.98 |
| 24 | M. bovis | SB0121 | M. bovis | 2.41 | 64 | Negative | NA | Gordonia sputi* | 1.61 |
| 25 | M. bovis | SB0121 | M. bovis | 2.26 | 65 | M. bovis | SB0295 | M. bovis | 2.61 |
| 26 | M. bovis | SB0121 | M. bovis | 2.41 | 66 | M. bovis | SB0822 | M. bovis | 2.52 |
| 27 | M. bovis | SB0121 | M. bovis | 2.14 | 67 | M. bovis | SB0822 | M. bovis | 2.66 |
| 28 | M. bovis | SB0121 | M. bovis | 2.49 | 68 | M. bovis | SB0822 | M. bovis | 2.72 |
| 29 | Negative | NA | M. nonchromogenicuma) | 1.89 | 69 | M. bovis | SB0121 | M. bovis | 2.32 |
| 30 | M. bovis | SB0121 | M. bovis | 2.15 | 70 | M. bovis | SB0822 | M. bovis | 2.48 |
| 31 | M. bovis | SB0121 | M. bovis | 2.10 | 71 | M. bovis | SB0295 | M. bovis | 2.34 |
| 32 | M. bovis | SB0121 | M. bovis | 2.11 | 72 | M. bovis | SB1869 | M. bovis | 2.44 |
| 33 | M. bovis | SB0121 | M. bovis | 2.29 | 73 | M. bovis | SB1869 | M. bovis | 2.62 |
| 34 | M. bovis | SB0121 | UN | ≤1.59 | 74 | M. bovis | SB0295 | M. bovis | 2.41 |
| 35 | M. bovis | SB0121 | M. bovis | 2.43 | 75 | M. bovis | SB0121 | M. bovis | 2.62 |
| 36 | M. bovis | SB0121 | UN | ≤1.59 | 76 | M. bovis | SB0822 | M. bovis | 2.51 |
| 37 | M. bovis | SB0121 | M. bovis | 2.47 | 77 | M. bovis | SB0822 | M. bovis | 2.53 |
| 38 | M. bovis | SB0121 | M. bovis | 2.59 | 78 | M. bovis | SB0822 | M. bovis | 2.56 |
| 39 | M. bovis | SB0121 | M. bovis | 2.22 | 79 | M. bovis | SB1608 | M. bovis | 2.61 |
| 40 | M. bovis | SB0121 | M. bovis | 2.56 | 80 | M. bovis | SB0295 | M. bovis | 2.35 |
UN: Unidentified. a) identified by the partial sequencing of the hsp65 gene. NA: Not applicable.
The 77 isolates positive for RD4 flanking region were genotyped by spoligotyping, resulting in six M. bovis genotypes: SB0121 (41 isolates), SB0295 (16 isolates), SB0822 (14 isolates), SB1869 (4 isolates), SB1800 (1 isolate) and SB1608 (1 isolate).
Mass spectra were successfully acquired for all clinical isolates of M. bovis and compared to 7,311 profiles of different genera and species of bacteria (BDAL library) and 912 profiles of mycobacteria species (Myco v.5.0 library).
Since the Bruker libraries are not annotated to differentiate MTC species, the analysis of the spectra in face of the BiotyperTM spectra banks, in its latest versions available, indeed did not allow accurate classification of M. bovis for the isolates in this study. Thus, the classification of the isolates by MALDI-TOF MS, prior addition of any reference spectra from M. bovis and M. tuberculosis to BiotyperTM database, was as follows: 70 (87.50%) were classified as belonging to the M. tuberculosis complex or M. tuberculosis, with scores ranging from ≥1.75 to ≤2.31; two isolates (2.50%) were identified as Gordonia sputi, with a score of 1.61 and 1.98; one isolate (1.25%) as M. nonchromogenicum, with a score of 1.89; and seven (8.75%) isolates were not identified (scores lower than 1.59). As a conclusion, we did not achieve the identification of M. bovis in the condition of analysis with the most current reference spectra available by BiotyperTM database, suggesting that the isolates studied here could present significant profile differences in relation to the reference spectra found in Bruker spectra libraries. However clearly distinct MSP were observed between our M. bovis clinical isolates and M. tuberculosis, suggesting that these species could be distinguished by the MycoLyser methodology established here (Fig. 1).
Fig. 1.
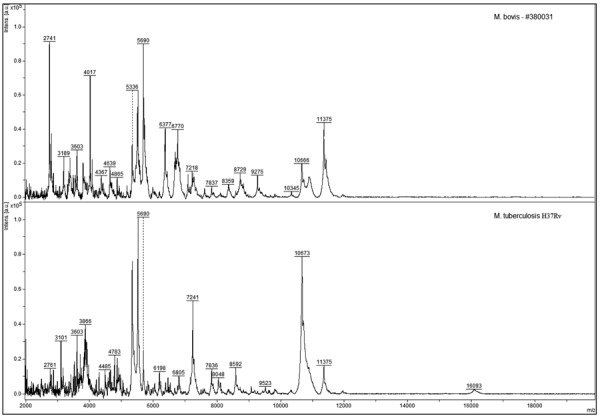
Representative mass spectra for Mycobacterium bovis (isolate 380031) and Mycobacterium tuberculosis (H37Rv strain) obtained after MycoLyser extraction method.
We then decided to generate our own reference spectrum in order to test its applicability in the identification of the M. bovis isolates studied herein. Comparison by BiotyperTM of our MycoLyser M. bovis MSP, generated with four clinical isolates, with 912 MSP for Mycobacterium species at Myco v.5.0 Bruker library showed that the most similar identified MSP are all annotated as MTC species. Also, M. bovis MSP obtained by MycoLyser method presented a relative degree of divergence to the MSP annotated as MTC species and for M. tuberculosis (H37Rv strain) MSP (Fig. 2). The profile similarity between the MycoLyser M. bovis MSP and the eight most similar MSP found by BiotyperTM in the Bruker library may be estimated as to 40% in distance level, as depicted by the dendrogram in Fig. 2. On the other hand, the similarity between the MycoLyser M. bovis MSP and M. tuberculosis H37Rv MSP, generated in the same conditions, was not higher than 60% in distance level.
Fig. 2.
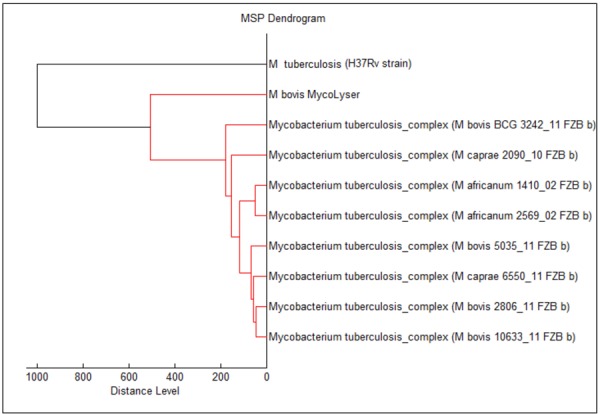
Classification of the MycoLyser Mycobacterium bovis Mass Spectra Profiles (MSP) with BiotyperTM. The dendrogram shows the relatedness of the MycoLyser M. bovis MSP with the 8 most similar MSP out of 912 present in Bruker Myco v.5.0 library. M. tuberculosis (H37Rv strain) MSP, obtained under the same extraction conditions as MycoLyser M. bovis MSP, was used as control and outgroups the clades.
Further analysis with ClinProTools software revealed at least two peaks that statistically could separate the M. bovis and M. tuberculosis species, as shown in Fig. 3A and 3B. According to principal component analysis (PCA), the variance of m/z 4,367 and m/z 4,784 within the spectra used for MSP generation is statistically different (Fig. 3A), which would allow discrimination of M. bovis and M. tuberculosis at species level (Fig. 3B). Interestingly, peak at m/z 4,367 was present in all M. bovis mass spectra and quite absent in M. tuberculosis H37Rv strain, while the opposite was observed for peak at m/z 4,784. With a ClinProTools model using the genetic algorithm and 5 highly discriminatory peaks (AUC=1.0: m/z 4,367, m/z 4,655, m/z 4,784, m/z 4,911 and m/z 6,460) we reached 100% correct classification in an external validation test, thus demonstrating the feasibility of the method for M. bovis identification.
Fig. 3.
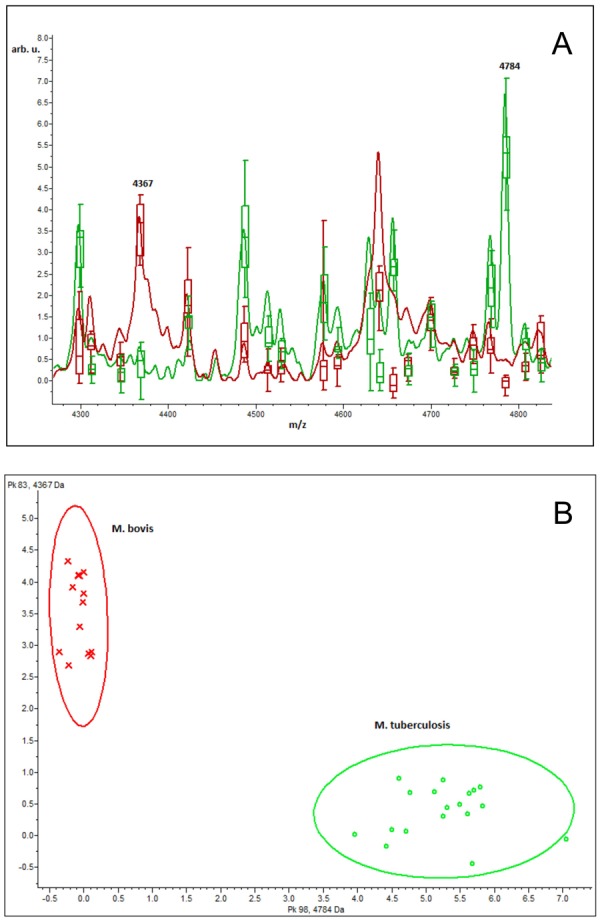
ClinProTools analysis of Mycobacterium bovis and Mycobacterium tuberculosis MycoLyser Mass Spectra Profiles (MSP) illustrating the discriminatory power of peaks at m/z 4,367 and 4,784. (A) Average mass spectra and peak variance (box-plot); (B) conventional PCA ordination plot of the M. bovis (red line) and H37Rv M. tuberculosis strain (green line) MSP.
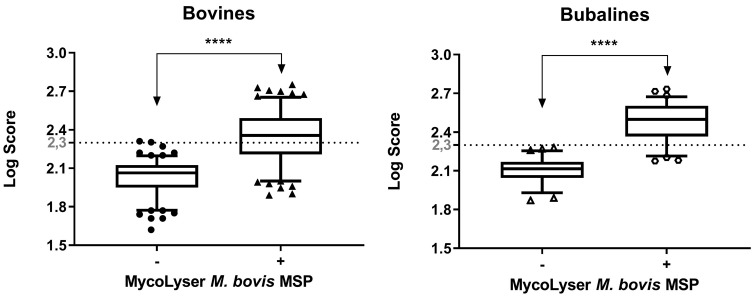
After the addition of the MycoLyser M. bovis MSP to the BiotyperTM commercial database there was a significant increase of the log score median for both cattle and buffalo isolates (P<0.0001) (Fig. 4). The identification score values were far above 1.80, which is the Bruker Daltonics suggested cut-off for reliable mycobacteria identification at species level. Remarkably, BiotyperTM results with MycoLyser M. bovis MSP presented scores often higher than 2.30, which in turn is the usual cut-off value for reliable species level identification of common bacteria, denoting the improvement of our method for mycobacterial identification (Fig. 4). Besides that, there was no change in MALDI-TOF MS scoring profiles for bovine isolates when stratified according to its genotypes (Fig. 5). Moreover, consistency analysis of the MALDI-TOF results for M. bovis identification significantly showed that 64 (92.90%) of the isolates presented consistency at species level, six (7.10%) had consistency for genus and no isolates showed no consistency.
Fig. 4.
Matrix Assisted Laser Desorption Ionization-Time-of-Flight (MALDI-TOF) mass spectrometry identification of Mycobacterium bovis in 70 clinical isolates obtained from cattle and buffalo tissue lesions. Box-plot for the log score of the clinical isolates of cattle (n=48) and buffaloes (n=22) after analyzing the mass spectra in BiotyperTM with 8,225 Mass Spectra Profiles (MSP) (BDAL + Myco v.5.0 libraries + H37Rv M. tuberculosis strain MSP), in the absence or presence of MycoLyser M. bovis MSP − **** indicates significant difference with P<0.0001 (exact value, two-tailed) after Wilcoxon test.
Fig. 5.
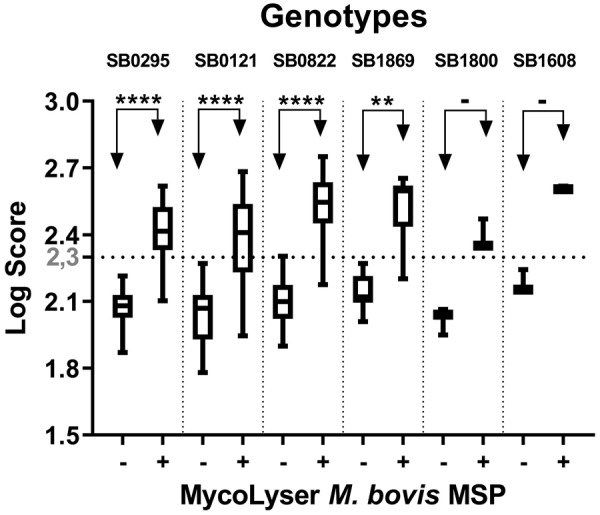
Matrix Assisted Laser Desorption Ionization-Time-of-Flight (MALDI-TOF) mass spectrometry identification of 70 clinical isolates of Mycobacterium bovis cultured from cattle and buffalo tissues, ranked by spoligotyping. Box-plot for the log score of the clinical isolates of cattle (n=48) and buffaloes (n=22) after analyzing the mass spectra in BiotyperTM with 8,225 Mass Spectra Profiles (MSP) (BDAL + Myco v.5.0 libraries + H37Rv M. tuberculosis strain MSP), in the absence or presence of MycoLyser M. bovis MSP. Wilcoxon test (exact, two-tailed P value) was applied: **** indicates significant difference with P<0.0001; ** indicates significant difference with P=0.0039; −indicates not significant difference (n=1).
Regarding species identification, after the inclusion of MycoLyser M. bovis MSP to the BiotyperTM library 70/80 (87.50%) of the isolates were identified by MALDI-TOF MS as M. bovis (Table 1). Fifty-seven out of the 70 isolates positively identified as M. bovis had a MALDI-TOF score greater than 2.30, 11 isolates presented scores between 2.00 and 2.30 and only two isolates had a score lower than 2.00. With respect to the other 10 isolates, not identified as M. bovis by MALDI-TOF, two (2.50%) were identified as Gordonia sputi, with scores of 1.61 and 1.98, one (1.25%) as M. nonchromogenicum, with a score of 1.89 and seven (8.75%) isolates were not identified, presenting mass spectrum scores lower than 1.59 (Table 1).
Mb400 PCR and MALDI-TOF methods detected three non-M. bovis isolates. These three isolates, Mb400 PCR negative, were identified by MALDI-TOF MS as Gordonia sp. and Mycobacterium nonchromogenicum, which proved to be correct after hsp65 gene sequencing. Thus, MALDI-TOF and Mb400 PCR were equally specific and 100% specificity can be ascribed for both methods. In contrast, for the 77 isolates identified as M. bovis by Mb400 PCR and spoligotyping, only 70 were positive by MALDI-TOF MS, resulting 90.91% sensitivity for the proteomic method. As all the M. bovis isolates (77) were positive in the Mb400 PCR, sensitivity of the PCR method (100%) was significantly superior than MALDI-TOF MS (P=0.0207).
DISCUSSION
In this study, the identification of M. bovis in samples of naturally infected cattle and buffalo tissues, isolated in solid medium, previously identified by conventional PCR based on the region flanking RD4 and spoligotyping, was performed using MALDI-TOF MS, including modifications to the Bruker Daltonics’ MycoEx method. Once cell disruption was previously described as critical for the release of mycobacterial proteins, after the addition of the 0.5 mm diameter silica/zirconia beads, a step of homogenizing the samples was performed in MagNA Lyser apparatus [18], and with this modification, it was possible to obtain spectra with better quality, thus confirming the efficacy of the new method of protein extraction. Also, log score median value showed a 10.00% increase upon inclusion of the MagNA Lyser step, which is significantly higher than that obtained with Bruker’s MycoEx method (data not shown).
Although the spectra obtained by the MycoLyser method have presented better quality, differentiation of M. bovis from other members of the MTC was only possible after M. bovis and M. tuberculosis specific reference spectra inclusion to the BiotyperTM database. After that, 87.50% of the isolates were identified as M. bovis, thus enabling M. bovis differentiation from the other MTC members. The inclusion of these spectra in the analysis resulted in the identification scores being higher than even the generic threshold of 2.30 proposed by Bruker Daltonics, above which the reliability for bacterial identification at species level is high. This result demonstrates that the methodology proposed here to identify mycobacteria allows us to use the same reliability threshold of the MALDI-TOF BiotyperTM technique commonly accepted for the identification of the great majority of bacteria.
The addition of self-referenced samples has already been described previously and has helped to improve the identification and differentiation of mycobacteria species due to the incompleteness of commercial databases [11, 17, 27].
The genotyping by spoligotyping of the isolates studied here revealed the presence of six different M. bovis genotypes. The SB0822 genotype is atypical in Brazil, and was previously described only in France, Spain, Portugal and Colombia. Genotypes SB0121 and SB0295 have higher prevalence in Brazil, as opposed to SB1869, SB1608 and SB1800 less frequent, however, described in Argentina, Spain, Venezuela, Mexico, Portugal and France. Although genotypic diversity demonstrated here was high, it is emphasized that the identification by MALDI BiotyperTM was not influenced when isolates were subdivided by genotypes demonstrating the ability of MALDI-TOF MS to identify M. bovis of these genotypes.
With regards to the seven samples not identified in the MALDI-TOF MS (score below 1.59), this fact may be related to the poor quality of the samples, which is often a result of the sampling process, either by culture medium contamination or insufficient culture, thereby generating poor quality spectra and not allowing identification by this method [17]. Conversely, even though the PCR identified a larger number of samples as M. bovis, when compared to MALDI-TOF MS, the latter allowed the precise differentiation of MTC members from non-tuberculous mycobacteria and other actinobacteria without the need for additional methods.
In the present study, MALDI-TOF MS results were obtained on average in 45 min, four times faster than the time spent to obtain PCR-only results for Mb400 PCR (RD4 flanking region). An additional three days were required to obtain the identification by sequencing the negative samples in the same PCR, beyond different oligonucleotide primers and genomic sequencing requirement. Another advantage in the use of MS technique was the fact that it did not demand different disposable consumables and materials, such as those consumed by molecular methods. The cost savings of MALDI-TOF MS is considerable (one euro/sample) compared to other molecular methods, which can be 40 times more expensive [10, 17].
We were unable to obtain isolates from other MTC members in addition to M. bovis and M. tuberculosis, and this was a limitation of this study. However, with the exception of M. pinnipedii, up to date, there are no reports of isolates of those MTC members in Latin America [32]. We also did not evaluate spectra of M. bovis BCG, since the scope of the study was restricted to bovine/bubaline tuberculosis. However, MALDI-TOF MS analysis for the identification of MTC isolates obtained from humans needs to be performed.
The increase of specific spectral pattern number within the databases may allow a rapid and accurate diagnosis of bovine/bubaline TB by MALDI-TOF MS in the future. Further steps to this work should include the validation of our findings using a larger sample size and the evaluation of the cost-effectiveness of a two-step procedure: MALDI-TOF MS as a high-throughput screening method for identification of M. bovis after tissue culture followed by Mb400 PCR for MALDI-TOF MS negative samples.
In conclusion, the use of the MycoLyser method associated with the expansion of the database with proper reference mass spectra resulted in the successful identification of M. bovis in bovine/bubaline clinical samples cultured in solid medium. MALDI-TOF MS provides a short-term and low-cost method for M. bovis identification and may become a useful tool for bovine tuberculosis diagnosis in the future.
Acknowledgments
The authors are grateful to the support of Bruker do Brasil analyst team, namely Wellington Conde, Diego Assis and Luis Santos. To Dr. Carlos Bloch Jr (principal investigator) and Dr. Daniel Sifuentes (analyst), from the Laboratory of Mass Spectrometry at Embrapa Genetic Resources and Biotechnology, by equipment usage support. To Gisele Olivas de Campos Leguizamon, from the Laboratory of Animal Immunology at Embrapa Beef Cattle, by technical support.
This study was supported by the following grants: Embrapa 02.13.10.008.00.00 and 03.13.10.008.00.00, Fundect 085/2015, 59/300, 121/2015 and 071/2017 and CNPq 407826/2018-1 and 443235/2014-7.
REFERENCES
- 1.Araújo C. P., Leite C. Q., Prince K. A., Jorge K. S., Osório A. L.2005. Mycobacterium bovis identification by a molecular method from post-mortem inspected cattle obtained in abattoirs of Mato Grosso do Sul, Brazil. Mem. Inst. Oswaldo Cruz 100: 749–752. doi: 10.1590/S0074-02762005000700013 [DOI] [PubMed] [Google Scholar]
- 2.Asmar S., Chatellier S., Mirande C., van Belkum A., Canard I., Raoult D., Drancourt M.2015. A novel solid medium for culturing Mycobacterium tuberculosis isolates from clinical specimens. J. Clin. Microbiol. 53: 2566–2569. doi: 10.1128/JCM.01149-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbier E., Rochelet M., Gal L., Boschiroli M. L., Hartmann A.2017. Impact of temperature and soil type on Mycobacterium bovis survival in the environment. PLoS One 12: e0176315. doi: 10.1371/journal.pone.0176315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell J.2008. A simple way to treat PCR products prior to sequencing using ExoSAP-IT. Biotechniques 44: 834. doi: 10.2144/000112890 [DOI] [PubMed] [Google Scholar]
- 5.Ben Kahla I., Boschiroli M. L., Souissi F., Cherif N., Benzarti M., Boukadida J., Hammami S.2011. Isolation and molecular characterisation of Mycobacterium bovis from raw milk in Tunisia. Afr. Health Sci. 11Suppl 1: S2–S5. doi: 10.4314/ahs.v11i3.70032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brasil Internal Standard S. D. A. nº02. 2012. Ministry of Agriculture, Livestock and Supply. Secretary of Agriculture Defense. https://www.agricultura.rs.gov.br/upload/arquivos/201612/16165912-pncebt-norma-interna-02-sda-2012.pdf [accessed on July 11, 2019].
- 7.Brasil Normative Instruction S. D. A. nº10. 2017. Ministry of Agriculture, Livestock and Supply. Secretary of Agriculture Defense. http://www.in.gov.br/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/19124587/do1-2017–06-20-instrucao-normativa-n-10-de-3-de-marco-de-2017–19124353 [accessed on July 11, 2019].
- 8.Brosch R., Gordon S. V., Marmiesse M., Brodin P., Buchrieser C., Eiglmeier K., Garnier T., Gutierrez C., Hewinson G., Kremer K., Parsons L. M., Pym A. S., Samper S., van Soolingen D., Cole S. T.2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. U.S.A. 99: 3684–3689. doi: 10.1073/pnas.052548299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunne W. M., Jr, Doing K., Miller E., Miller E., Moreno E., Baghli M., Mailler S., Girard V., van Belkum A., Deol P.2014. Rapid inactivation of Mycobacterium and nocardia species before identification using matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 52: 3654–3659. doi: 10.1128/JCM.01728-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dušková M., Šedo O., Kšicová K., Zdráhal Z., Karpíšková R.2012. Identification of lactobacilli isolated from food by genotypic methods and MALDI-TOF MS. Int. J. Food Microbiol. 159: 107–114. doi: 10.1016/j.ijfoodmicro.2012.07.029 [DOI] [PubMed] [Google Scholar]
- 11.El Khéchine A., Couderc C., Flaudrops C., Raoult D., Drancourt M.2011. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry identification of mycobacteria in routine clinical practice. PLoS One 6: e24720. doi: 10.1371/journal.pone.0024720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ereqat S., Nasereddin A., Levine H., Azmi K., Al-Jawabreh A., Greenblatt C. L., Abdeen Z., Bar-Gal G. K.2013. First-time detection of Mycobacterium bovis in livestock tissues and milk in the West Bank, Palestinian Territories. PLoS Negl. Trop. Dis. 7: e2417. doi: 10.1371/journal.pntd.0002417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghavidel M., Mansury D., Nourian K., Ghazvini K.2018. The most common spoligotype of Mycobacterium bovis isolated in the world and the recommended loci for VNTR typing; A systematic review. Microb. Pathog. 118: 310–315. doi: 10.1016/j.micpath.2018.03.036 [DOI] [PubMed] [Google Scholar]
- 14.Huard R. C., Lazzarini L. C., Butler W. R., van Soolingen D., Ho J. L.2003. PCR-based method to differentiate the subspecies of the Mycobacterium tuberculosis complex on the basis of genomic deletions. J. Clin. Microbiol. 41: 1637–1650. doi: 10.1128/JCM.41.4.1637-1650.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamerbeek J., Schouls L., Kolk A., van Agterveld M., van Soolingen D., Kuijper S., Bunschoten A., Molhuizen H., Shaw R., Goyal M., van Embden J.1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leite C. Q., Anno I. S., Leite S. R., Roxo E., Morlock G. P., Cooksey R. C.2003. Isolation and identification of mycobacteria from livestock specimens and milk obtained in Brazil. Mem. Inst. Oswaldo Cruz 98: 319–323. doi: 10.1590/S0074-02762003000300005 [DOI] [PubMed] [Google Scholar]
- 17.Lotz A., Ferroni A., Beretti J. L., Dauphin B., Carbonnelle E., Guet-Revillet H., Veziris N., Heym B., Jarlier V., Gaillard J. L., Pierre-Audigier C., Frapy E., Berche P., Nassif X., Bille E.2010. Rapid identification of mycobacterial whole cells in solid and liquid culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 48: 4481–4486. doi: 10.1128/JCM.01397-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machen A., Kobayashi M., Connelly M. R., Wang Y. F. W.2013. Comparison of heat inactivation and cell disruption protocols for identification of mycobacteria from solid culture media by use of vitek matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 51: 4226–4229. doi: 10.1128/JCM.02612-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makovcova J., Babak V., Slany M., Slana I.2015. Comparison of methods for the isolation of mycobacteria from water treatment plant sludge. Antonie van Leeuwenhoek 107: 1165–1179. doi: 10.1007/s10482-015-0408-4 [DOI] [PubMed] [Google Scholar]
- 20.Michel A. L., Müller B., van Helden P. D.2010. Mycobacterium bovis at the animal-human interface: a problem, or not? Vet. Microbiol. 140: 371–381. doi: 10.1016/j.vetmic.2009.08.029 [DOI] [PubMed] [Google Scholar]
- 21.Murugaiyan J., Lewin A., Kamal E., Bakuła Z., van Ingen J., Ulmann V., Unzaga Barañano M. J., Humięcka J., Safianowska A., Roesler U. H., Jagielski T.2018. MALDI spectra database for rapid discrimination and subtyping of Mycobacterium kansasii. Front. Microbiol. 9: 587. doi: 10.3389/fmicb.2018.00587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals2018. Bovine Tuberculosis. Chapter 3.4.6. http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.04.06_BOVINE_TB.pdf [accessed on July 11, 2019].
- 23.Padya L., Chin’ombe N., Magwenzi M., Mbanga J., Ruhanya V., Nziramasanga P.2015. Molecular identification of Mycobacterium species of public health importance in cattle in Zimbabwe by 16S rRNA gene sequencing. Open Microbiol. J. 9: 38–42. doi: 10.2174/1874285801509010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petroff S. A.1915. A new and rapid method for the isolation and cultivation of tubercle bacilli directly from the sputum and feces. J. Exp. Med. 21: 38–42. doi: 10.1084/jem.21.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramos D. F., Silva P. E. A., Dellagostin O. A.2015. Diagnosis of bovine tuberculosis: review of main techniques. Braz. J. Biol. 75: 830–837. doi: 10.1590/1519-6984.23613 [DOI] [PubMed] [Google Scholar]
- 26.Ru H., Liu X., Lin C., Yang J., Chen F., Sun R., Zhang L., Liu J.2017. The impact of genome region of Difference 4 (RD4) on mycobacterial virulence and BCG efficacy. Front. Cell. Infect. Microbiol. 7: 239. doi: 10.3389/fcimb.2017.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saleeb P. G., Drake S. K., Murray P. R., Zelazny A. M.2011. Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 49: 1790–1794. doi: 10.1128/JCM.02135-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sales M. L., Fonseca A. A., Jr, Orzil L., Alencar A. P., Hodon M. A., Issa M. A., Soares Filho P. M., Silva M. R., Lage A. P., Heinemann M. B.2014. Validation of two real-time PCRs targeting the PE-PGRS 20 gene and the region of difference 4 for the characterization of Mycobacterium bovis isolates. Genet. Mol. Res. 13: 4607–4616. doi: 10.4238/2014.June.18.3 [DOI] [PubMed] [Google Scholar]
- 29.Sales M. L., Fonseca A. A. J., Jr, Sales E. B., Cottorello A. C., Issa M. A., Hodon M. A., Soares Filho P. M., Ramalho A. K., Silva M. R., Lage A. P., Heinemann M. B.2014. Evaluation of molecular markers for the diagnosis of Mycobacterium bovis. Folia Microbiol. (Praha) 59: 433–438. doi: 10.1007/s12223-014-0317-3 [DOI] [PubMed] [Google Scholar]
- 30.Shitikov E., Ilina E., Chernousova L., Borovskaya A., Rukin I., Afanas’ev M., Smirnova T., Vorobyeva A., Larionova E., Andreevskaya S., Kostrzewa M., Govorun V.2012. Mass spectrometry based methods for the discrimination and typing of mycobacteria. Infect. Genet. Evol. 12: 838–845. doi: 10.1016/j.meegid.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 31.Telenti A., Marchesi F., Balz M., Bally F., Böttger E. C., Bodmer T.1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31: 175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zumárraga M. J., Arriaga C., Barandiaran S., Cobos-Marín L., de Waard J., Estrada-Garcia I., Figueiredo T., Figueroa A., Giménez F., Gomes H. M., Gonzalez-Y-Merchand J. A., Macías A., Milián-Suazo F., Rodríguez C. A., Santillán M. A., Suffys P. N., Trangoni M. D., Zárraga A. M., Cataldi A.2013. Understanding the relationship between Mycobacterium bovis spoligotypes from cattle in Latin American countries. Res. Vet. Sci. 94: 9–21. doi: 10.1016/j.rvsc.2012.07.012 [DOI] [PubMed] [Google Scholar]