Abstract
Background:
An emphasis on precision health (PH) has stimulated precision medicine studies to focus on the interplay of biological, behavioral, and environmental factors with disease risks, treatments, prognoses, and outcomes affecting health disparities. It is imperative, as well, that improving health equity among underserved populations remains central to the efforts and aims of PH.
Objectives:
Apply the transdisciplinary ConNECT Framework: A model for advancing behavioral medicine science and practice to foster health equity to PH by integrating a population health agenda for reducing health disparities.
Methods:
Describe the ConNECT principles: (a) integrating context; (b) fostering a norm of inclusion; (c) ensuring equitable diffusion of innovations; (d) harnessing communication technology; and (e) prioritizing specialized training as an organizing framework to PH, including examples of how to integrate behavioral and socioecological determinants to better understand the contexts of individuals, systems and place to design targeted treatments and interventions.
Results:
We describe proactive, actionable strategies for the systematic application of ConNECT Framework principles to address health equity via the PH initiative. Context and implications for nursing research and practice are also described.
Discussion:
The ConNECT Framework emphasizes that diversity inclusion is imperative for true population health benefit from PH, broadly in public health, behavioral medicine, medicine and nursing, to equip health researchers and practitioners to account for contextual socio-ecologic data that can be aligned with biologic data for more population responsive and individually tailored interventions to prevent, diagnose and treat diseases.
Precision health (PH) is revolutionizing how we prevent, diagnose, and treat diseases. In this paper, the term precision health encompasses the broader context of this burgeoning science. In contrast to one-size-fits-all approaches to healthcare, PH has the potential to offer improved care by selecting prevention and medical treatments based on an individual’s characteristics, therefore optimizing clinical and quality of life outcomes (McNeil, 2015). To achieve the greatest benefit, however, precision treatments of disease and strategies for risk reduction practices must be rooted in data from diverse patient populations, including historically underserved and underrepresented populations, and must be accessible to all communities so as not to contribute toward perpetuating or exacerbating health inequities.
Nursing science can advance population health by engaging in all phases of scientific inquiry (Eckardt et al., 2017). Additionally, the recent report of the science committee of the Council for the Advancement of Nursing Science established four priority areas for nursing science; one of which is precision science (Eckardt et al., 2017). As such the application of the model described in this paper aligns well with current strategic directions in nursing research.
We previously developed the transdisciplinary ConNECT Framework: A model for advancing behavioral medicine science and practice to foster health equity (Alcaraz et al., 2017) to provide an actionable approach to help achieve health equity and eliminate health disparities. ConNECT comprises five principles intended to broaden the context through which PH can enrich and inform our science:
integrating context;
fostering a norm of inclusion;
ensuring equitable diffusion of innovations;
harnessing communication technology; and
prioritizing specialized training.
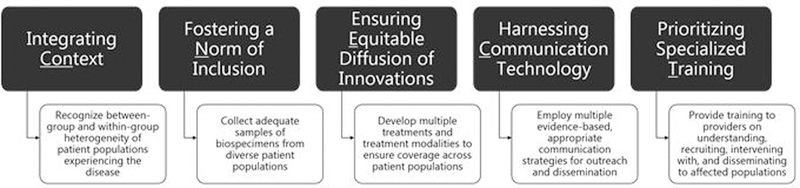
The purpose of this paper is to discuss the five ConNECT principles in the context of PH and describe application strategies to achieve health equity in PH (Fig.1). Although the ConNECT Framework was developed for behavioral medicine in general, the principles described and strategies detailed are relevant to nursing research in most any field or topic. Nurse researchers have maintained a commitment to health disparities and health equity, and as such, may find these principles useful for studies on genomics or systems research or informatics—all of which will ultimately affect patient health.
FIGURE 1: Application of the ConNECT Framework to Precision Health.
The figure depicts examples of how the ConNECT Framework can be applied across various stages of the research process in precision health studies.
Principle One: Integrating Context
ConNECT’s first principle, integrating context, calls for elucidating social and contextual influences on health (Alcaraz et al., 2017). With increasing population diversity, physical and socioecological environmental factors, genetic mutations, and molecular and genomic factors, all contribute to disease development and prognosis, treatment response, morbidity, mortality, and survival. Therefore, disease risk factors, therapeutic selection, and outcomes can vary by population and patient characteristics. Attention to social determinants provides the platform that links PH with population medicine. However, current efforts have concentrated on molecular medicine, whereby informative genomic, genetic, and molecular tests have been used to enable more precise therapeutics at the individual level. The hope and promise of population-centered PH is that examining and understanding this level of complexity will: (a) reveal specific socioecological contexts of the health of different underserved groups; and (b) illuminate the homogeneity and heterogeneity that exists within groups.
A socio-ecological approach emphasizes the need to understand social and contextual influences on health that to date have been vastly understudied, despite the well-documented role of the social and built environment on biological and genetic processes (Edwards & Di Ruggiero, 2011). Structural factors are important contextual health determinants that influence the health of persons and communities (Marmot, 2008), and include the sociopolitical, place and geography, demographic, and socioeconomic context (Bilheimer, 2010). In current research, aspects of socioeconomic status (SES) or socioeconomic position (SEP) have been used to explain and account for health outcomes, including health inequalities (Galobardes, Lynch, & Smith, 2007). For instance, monozygotic and dizygotic twins who as children were raised in one household but had different SES or SEP as adults report health status differences. Specifically, twins with lower SES or SEP had poorer health status than their higher SEP twin counterparts (Krieger, Chen, Waterman, Rehkopf, & Subramanian, 2005). Other aspects of social position yet to be thoroughly explored include socially derived political status among underserved communities (e.g., leadership positions within faith communities).
Social position strongly influences proximal health factors. Proximal factors include the cultural (Ruetter & Kushner, 2010), psychosocial (Bilheimer, 2010; Parrish, 2010), behavioral (Bilheimer, 2010; CDC, 2017), environmental (Bilheimer, 2010; Jakubowski & Frumkin, 2010), biological, and health system contexts (Bilheimer, 2010; Kottke & Isham, 2010) which are expected to have direct influences on specific disease risks and outcomes. For example, residing in a certain zip code can shift life expectancy by 15 years (Chetty, Hendren, & Katz, 2015). In addition, being a member of certain faith communities influences health practices and cancer mortality (e.g., Seventh-Day Adventists and dietary behaviors) (Koenig, 2015). Proximal factors are themselves multidimensional. For instance, the psychosocial context contains social networks, psychological status, and family stability. Both structural and proximal determinants are posited to independently and directly influence health outcomes, as well as work synergistically to affect health and disease outcomes. Therefore, integrating both structural and proximal factors into PH research will serve to advance the mission of achieving health equity.
Opportunities exist for considering and integrating context in PH. For example, systematically collecting behavioral and social data on patients would facilitate a more holistic approach to healthcare. Such information—which could be incorporated into electronic health records (EHR)—could provide important insights needed to prescribe care that is not only individualized, but also acceptable and feasible for a given patient. Relatedly, researchers have begun to examine contextual, community-level data by capturing geocoded community “vital signs” including the built environment (e.g., number of fast food restaurants, liquor stores, population density), environmental exposures (e.g., air quality, median housing structure age), neighborhood economic conditions (e.g., percent of foreclosures, vacant addresses), neighborhood race/ethnic composition (e.g., residential segregation), neighborhood resources (e.g., number of recreation facilities, number of healthy food stores), and neighborhood socioeconomic composition (e.g., number of people with bachelor’s degree or higher, median household income). These community vital signs can be incorporated into the EHR for clinician use. (Bazemore et al., 2016). Further, opportunities exist for designing and implementing more focused interdisciplinary research on context. Biobehavioral studies (multilevel studies that simultaneously examine genetic, social, and geospatial determinants) and studies employing lifecourse perspectives, such as social epigenetics, seem particularly promising. A better understanding of context could help inform and, more importantly, optimize PH in the years ahead.
Principle Two: Fostering a Norm of Inclusion
The second principle of ConNECT, fostering a norm of inclusion, emphasizes the planned inclusion or involvement of individuals from diverse backgrounds to achieve health equity (Alcaraz et al., 2017). Recognizing that inclusion is an important underpinning to the generalizability of research or clinical data, we argue for inclusion of diverse and historically underrepresented groups in PH research for the purpose of maximizing understanding of all persons.
The existing literature lacks comprehensive data across diverse populations. This principle is particularly important in PH because clinical decisions are often based on research conducted with individuals’ health information, including their biospecimens. Prior genetic studies have revealed the need for heterogeneity in samples due to variation in mutations (Spratt et al., 2016). Also, it is imperative that biobanks are applicable to all persons for PH advancement, particularly for underserved populations. For example, the majority of collected cancer-related biospecimen samples (e.g., tissue, blood) are from non-Hispanic White cancer patients (Spratt et al., 2016; Popejoy & Fullerton, 2016). Also, underrepresentation of biospecimen samples from diverse populations is evident in other types of diseases/health domains, as well such as kidney disease and congenital heart defects (Kraus et al., 2015; Gelb, 2016).
Lack of adequate representation of diverse groups in research—specifically PH research—means that genetic variability of mutations may remain undetected and fuller understanding of important commonalities and differences continues to be curtailed. This is also important in populations characterized by high levels of within-group genetic diversity (Cohn, Henderson, & Appelbaum, 2016) (e.g., individuals of African descent). For example, treatments that would be efficacious for treating cancer for all persons may go undiscovered because of the low numbers of tissue banked from racial/ethnic minorities, especially from Asian and Hispanic Americans (Spratt et al., 2016; Popejoy & Fullerton, 2016). In another compelling example, a clinical trial of gefitinib revealed a survival benefit for Asians with advanced lung cancer whereas no benefit was found in the remainder of the cohort, which was 77% White (Spratt et al., 2016). These examples underscore the importance of considering race and ethnicity to provide equitable precision genomic medicine and to ultimately reduce disparities in mortality (Wu et al., 2017). However, attention to nongenetic, social determinants of health is equally important. Race/ethnicity is a social construct that influences genetic expression and mutation. For instance, gender, SES, sexual orientation, religiosity/spirituality, disability status, geographical location, access to care, and other characteristics should be considered in PH (Koenig, 2015; Legato, Johnson, & Manson, 2016; Adams & Petersen, 2016;).
A challenge described by Cohn and colleagues (2016) is how to identify and select appropriate criteria for individual inclusion. Current approaches to inclusion primarily center on the use of self-reported race and ethnicity (Cohn, Henderson, & Appelbaum, 2016). However, improving genetic diversity requires having genomic information (e.g., genetic and genomic markers considering ancestry and admixture information). Even today, such gene-level analyses are often not addressed or occur after enrollment in selected studies (Cohn, Henderson, & Appelbaum, 2016). Here, we underscore diversity inclusion that integrates self-reported race/ethnicity and place-based (i.e., immigration, where persons live, work and play) information with genetic markers of ancestry in conducting population PH.
Strategies to promote diversity in research include: (a) targeted outreach strategies; (b) appropriate and targeted recruitment and study materials, including culturally and linguistically relevant materials that are transcreated rather than simply translated; (c) oversampling of groups and subgroups when conducting research (including bio specimen collection [Alcaraz et al., 2017; Cohn, Henderson & Appelbaum, 2016]), and proportional representation according to burden of disease or population characteristics (Hawk et al., 2014); and, (d) use of community-based participatory research to engage underserved communities as research partners.
To illustrate, Dang et al. (2014) described three studies that highlighted the importance of conducting formative work through community-based participatory approaches to successfully engage community members from diverse racial/ethnic backgrounds (i.e., African American, Asian American, Hispanic, and White) in PH. Methods included the use of community advisory boards, conduct of focus groups and interviews with key informants, and /or the development of language-specific materials/educational classes. These studies—conducted in three geographically dispersed regions of the U.S. (Northwest, West, and Southeast) —emphasized the value of taking the time to develop relationships with community members to promote trust, colearning, and clear communications. As a result, a series of biobanking educational tools (PowerPoints, DVDs, brochures) were created based on these participatory/inclusionary processes to enhance awareness of PH (Meade et at., 2015). Similarly, work by McElfish and colleauges (2017) illustrates participatory approaches utilized with Pacific Islander groups (such as the Marshallese) that can be used as examples for others who wish to use collaborative approaches to ensure inclusion in PH research. In order to foster inclusiveness in a diabetes prevention program with individuals from the Marshallese community across four states, researchers highlighted the importance of colearning and collaboration (McElfish et al., 2018). Furthermore, educational information was developed with input from Marshallese coinvestigators and delivered by lay health educators at Marshallese churches (McElfish et al., 2018). At study conclusion, research results will be disseminated to the community through town hall meetings and informational sheets (McElfish et al., 2018). Similar participatory approaches can be used to ensure that all subgroups within racial/ethnic categories are included in PH research.
Clearly, the field of PH is accelerating at a rapid rate. Fostering a norm of inclusion requires a mindset of inclusivity and a carefully thought-out plan to involve individuals from diverse backgrounds to achieve health equity. As such, researchers have a moral, ethical, and scientific obligation to use a variety of recruitment, communication, partnerships. and outreach strategies to engage diverse population groups.
Principle Three: Ensuring Equitable Diffusion of Innovations
ConNECT’s third principle focuses on ensuring that diverse groups equitably benefit from scientific and clinical advances, that is, ensuring equitable diffusion of innovations (Alcaraz et al., 2017). The dissemination and implementation of PH on a population level presents considerable challenges to researchers and program developers as discussed in Principle Two. If underserved populations are not enrolled in research, it can be difficult to disseminate innovations to them that are evidence based. For example, in a national sample of African-American adults, only one third indicated that they were very or somewhat likely to participate in a government-sponsored research study that involved providing a biospecimen and generating data that could be shared with other researchers to conduct future studies (Hawk et al., 2014). Further, members of racial/ethnic minority groups are less likely than others to agree to molecular testing for cancer treatment, or may be offered fewer such prospects, thereby missing opportunities to provide precise data that would facilitate treatment decision processes (Yusef et al., 2014). The reluctance of participation may result from lack of trust in the medical system and in research study procedures rooted in institutional and interpersonal discrimination (e.g., research misconduct in the Tuskegee trials, health provider bias and discrimination) or worried about exploitation of their biospecimen (Buseh, Underwood, Stevens, Townsend, & Kelber, 2013). These factors may significantly affect recruitment and participation of minority groups in PH studies.
The Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework (Gaglio, Shoup, & Galsgow, 2013) and the Pragmatic-Explanatory Continuum Indicator Summary (PRECIS) (Thorpe et al., 2009) are two models that provide useful domains and principles to guide science translation in PH studies. In RE-AIM, reach is the number, proportion, and representativeness of people willing to participate in an initiative. Researchers may consider conducting ongoing process evaluations and adapt strategies to improve ‘reach’ as needed. The PRECIS model describes 10 different domains that researchers may use to differentiate between pragmatic and explanatory trials to maximize the diversity of the study population reached (Thorpe et al., 2009). For example, input on the first domain of participant eligibility criteria from members of the target population and community advisors may be helpful in maximizing reach and ultimately the generalizability of study findings.
Strategies to enhance engagement of minorities or other underrepresented groups include collaborating with key influencers, such as physicians who have an established relationship with patients, are willing to endorse and are enthusiastic about PH (Beckie et al., 2009), and local community facilitators or health workers who are able to engage in tailored outreach and communication efforts based on their understanding of the target group’s language and customs (Shankar et al., 2009). Dissemination and implementation science can play a vital role in the context of PH to ensure that interdisciplinary collaborations are developed and maintained, and facilitate efforts to guarantee that research findings and treatment innovations reach the entire population. Such work can contribute to the development of culturally appropriate targeted prevention and intervention efforts to improve health (Khoury & Galea, 2016).
To maximize the demand and spread of evidence-based PH interventions, stakeholders— including members of racial/ethnic minority groups—need to be involved in the research process from the beginning. However, a survey of 266 researchers indicated that only one third reported always or usually involving stakeholders in this process (Brownson, Jacobs, Tabak, Hoehner, & Stamatakis, 2013). Below, we outline five key steps to integrate community-engaged research methodology to maximize dissemination and implementation potential in PH studies. The first three steps set the foundation for successful dissemination and implementation by describing key processes for community and stakeholder engagement.
Step 1.
Understand motivating and decision-making factors and barriers to participating in research studies among underrepresented populations. Identifying motivation for participation among underrepresented groups may help minimize barriers to recruitment and participation. For instance, participants may enroll in research studies to help generate a knowledge base specific to their community or population (Butrick et al., 2014). To assist researchers and communities in promoting research literacy and participation among understudied and/or underserved populations, the Intervention and Research Readiness Engagement and Assessment of Community Health Care (I-RREACH) measure was developed to promote dialogue and ensure that the multiple stakeholders are on the same page (Maar et al., 2015).
Step 2.
Identify community partners and obtain buy-in. To communicate with potential community partners, one may consider using lay language and graphs to present data (findings), address their key concerns, and share testimonials (via engaging narrative format, if possible) associated with data/findings to demonstrate how the products may potentially benefit the community and reduce their concerns. Cost/resource is usually a concern for implementation, especially for PH where the tests can be costly and not always covered by insurance, thus creating barriers to access (Smith et al., 2016). Moreover, it is important to keep in mind that community partners who work closely with researchers may not necessarily be the decision makers in dissemination and implementation. Hence, preparing and training community partners to communicate with decision makers (e.g., assisting community partners in preparing presentation or reports, conducting mock presentations, and providing feedback) may facilitate dissemination and implementation processes.
Step 3.
Apply principles and methods of community-based participatory research. Reciprocal trust, clear communication, respect, and being patient and persistent are critical when building community-academic partnerships (Israel, Schulz, Parker, & Becker, 2008). While community partners often understand the importance of dissemination and implementation to affect wide scale change, devoting time, money, and effort for planning and activities required for successful dissemination and implementation may not be their top or immediate priority. Strategies to maximize early and continuing involvement, and to minimize undue burden are critical, such as train-the-trainer models, online training, providing certification, or continued education credits, etc. It is critically important to work with community partners to identify feasible, effective strategies that are minimally burdensome to staff and mutually beneficial.
Step 4.
Discuss goals, strategies, and challenges for dissemination and implementation. In many cases, one may suggest dissemination or implementation strategies to the decision maker(s) who may or may not be familiar with clinical practice but are instead focused on meeting goals. To make potentially meaningful suggestions, researchers can work closely with community partners who serve clients to formulate plans that may be less costly or burdensome to staff working in clinical settings, and plan for expanded stakeholder and personnel buy-in.
Step 5.
Build a dissemination and implementation support system. Research indicates that to optimize uptake and adoption of innovations, dissemination should be more demand-driven (rather than evidence-driven); establishing a dissemination support system that yields practice-ready program and products is crucial; and specialists (not researchers) are better suited to promote and support the spread of innovations (Dearing & Krueter, 2010). Such specialists may include community leaders and members who understand and identify with the target population. The following three components make up a dissemination support system: user review panels, design and marketing teams, and dissemination field agents (Krueter & Wang, 2015).
User review panels comprise key stakeholders and decision makers (in this case, community members and leaders) who review and provide feedback on innovations. Findings from such panels can highlight participants’ preferences of a certain procedure or product and reasons for these preferences. Community partners can assist design and marketing teams to understand needs of various user subgroups, refine and package intervention materials (e.g., resources, brochures, posters, curriculum, ads) for use outside of the research setting, building partnerships, establishing a distribution system, providing training and technical assistance, and creating incentives for adoption (Krueter & Burnhardt, 2009). Finally, dissemination field agents can be used to consistently integrate evidence-based and practice-ready PH interventions that are in demand within the target population. For example, training selected community representatives (e.g., community leaders, change agents) can be used to promote awareness and participation in PH studies as well as to disseminate any findings back to those communities.
Principle Four: Harnessing Communication Technology
The fourth ConNECT principle emphasizes the utility of communication technology platforms as tool for achieving parity in health outcomes (Alcaraz et al., 2017). The application of communication technologies to healthcare delivery is often referred to as eHealth, mHealth, or connected health (Kumar et al., 2013). Communication technologies include the use of phone-based counseling to internet-enabled technologies that can facilitate patient surveillance, data collection, health promotion, and decision support systems (Carter, Burley, Nykjaer, & Cade, 2013; Mohr, Burns, Schueller, Clark, & Klinkman, 2013).
Communication technologies also provide an opportunity to disseminate data or feedback that can shape health outcomes. For example, the Silent Spring Institute (Silent Spring Institute, 2015) provides kits for testing levels of bodily chemical exposure and personalized reports to facilitate interpretations of the test results. Additionally, when communication technologies are integrated within diverse communities and populations, they can play a critical role in reducing health disparities.
Such technologies may play an important role in connecting researchers and practitioners with historically underserved populations. In a PH era, communication technologies can enable more rapid, widespread, and thorough data collection and interventions with minimal human support that are not biased by laboratory constraints (Kumar et al., 2013).
Opportunities exist to leverage communication technology to reach underserved populations. For example, the percentage of Hispanic adults who report using the internet has increased from 64% in 2009 to 84% in 2015 (Pew Research Center Internet and Broadband Fact Sheet, 2018). Hispanics and African Americans are more likely to use smartphones to obtain information about health conditions when compared to non-Hispanic Whites (73% Hispanics, 67% African American, 58% non-Hispanic Whites [PRC, 2018]). Despite these promising trends and the potential of communication technologies to increase healthy behaviors and improve outcomes, the majority of communication technology research has not fully reached members of underrepresented groups in the U.S. This results in missed opportunities to engage people from many backgrounds and contributes to a lack of generalizability in collected data.
Given the increasing use of smartphones among racial/ethnic minority groups in the U.S., they may be particularly poised to benefit from technology-assisted access to healthcare. Communication technologies offer several advantages when working with minority and underserved patients. Technology-assisted methods for collecting data and providing healthcare are feasible among diverse and underserved minority groups; enhance accessibility, scalability, and adaptability; and have the potential to be low-cost relative to more traditional, in-person methods (Muñoz et al., 2015; Yanez et al., 2015).
It is important to keep in mind that not all populations access and use communication technologies in the same way (PRC, 2018). For example, Hispanics are more likely to access the Internet from a mobile device than a computer and unlike non-Hispanic Whites, Hispanics, and Blacks have remained stagnant in their uptake of broadband internet (PRC, 2018). In addition to variations in methods of accessing the internet, cultural variations in engagement with technology also vary. For example, some Hispanics may prefer human interactions to augment the use of the communication technology (Victorson et al., 2014); other groups living in areas with poor wireless signal are disadvantaged from taking part in internet-based research. These examples underscore the importance of tailoring technology-assisted research to specific populations and communities.
Additionally, for populations and communities that have been historically marginalized, the use of communication technologies, in place of human interactions to collect data and deliver interventions, may exacerbate concerns about medical mistrust. Therefore, providing assurances regarding issues of confidentiality and the use of data are key to the success of using communication technologies to collect data and implement interventions among diverse populations. Large-scale cohort studies that collect data and deliver interventions in a uniform manner may miss key population segments, leading to an unrepresentative sample.
Despite the promise of communication technologies to reach and engage underserved or underrepresented populations, most technology-assisted interventions lack cultural tailoring (Gibsons et al., 2011). For instance, few technology-based interventions are designed with both the appropriate cultural and linguistic approaches that can facilitate their uptake and effects among racial/ethnic minority and other underserved populations (Montague & Perchonok, 2012). Communication technologies used for large-scale studies in the U.S., such as the All of Us Research Program (National Institutes of Health All Of Us Research Program [NIH], n.d.), often target the greatest common denominators in populations, as opposed to providing tailored delivery of technology, and therefore can lead to access and use barriers for ethnic minority and other underserved populations. In order to promote the uptake of communication technologies in PH among diverse groups, it is imperative that researchers and practitioners (a) leverage multiple communication technology platforms (e.g., mobile devices, Internet enabled devices) to reach diverse groups of individuals across multiple geographic regions and (b) tailor the content within and delivery of communication technologies to meet the unique cultural and language needs of specific communities and populations.
Principle Five: Prioritizing Specialized Training
In the context of PH, the fifth ConNECT principle underscores the need to develop a health workforce with the appropriate education, training, and mentoring (Specialized Training) necessary to engage in research and practice that contributes to achieving health equity and improve the health of the diverse U.S. population (Haspel & Saffitz, 2014; Alcaraz et al., 2017). As such, the workforce needs to have the skills and experiences necessary to conduct PH research and develop, implement, and disseminate interventions aimed at improving population health and reducing health disparities. Congruent with the first principle of the ConNECT Framework, the training of researchers, practitioners and community partners should emphasize endorsing a socioecological perspective that considers multiple contexts and factors that influence individual and population health (Khoury & Galea, 2016). Current empirical evidence supports the idea that behavioral, social, and environmental contextual factors have stronger associations with health than genotypes (Buseh, Underwood, Stevens, Townsend, & Kelber, 2013).
Education and training should focus on providing researchers and practitioners with: (a) knowledge about genetics and biological factors (McGrath & Ghersi, 2016); (b) an understanding the interactive role of behavioral, social, and environmental factors on biological and genetic processes (NIH, n.d.); and (c) a grounding on multidisciplinary research and methods aimed at identifying and developing interventions seeking to achieve health equity. (Khoury & Galea, 2016).
Importantly, advancing efforts to achieve health equity necessitates the development of a diverse workforce that mirrors the full representation and all dimensions of population diversity. Diversity in the workforce has been shown to contribute to greater innovation, creativity, and capacity to engage in complex problem-solving (Valantine & Collins, 2015), improve access to healthcare for ethnic/racial minority groups, and increase care that is culturally and contextually appropriate (Cohen, Gabriel, & Terrell, 2002). Unfortunately, in the U.S., a current problem in the biomedical work force is the underrepresentation of racial/ethnic minorities (i.e., African Americans, American Indians, Alaska Natives, Hispanic/Latinos, and U.S. Pacific Islanders) and underrepresented groups (i.e., individuals with disabilities, low-income individuals, women) as scientists and practitioners in the biomedical and behavioral field. Increasing representation of these groups will require greater investments from multiple sectors (i.e., government, education) to recruit, retain, and graduate members of these groups at all levels of education in the biomedical and behavioral field (Committee on Underrepresented Groups and the Expansion of the Science and Engineering Workforce Pipeline, 2011). Further, once members of these groups enter the field, there is a need for eliminating institutional and individual barriers to career success and retention of members of diverse groups (Valantine & Collins, 2015).
Another important step in in bolstering efforts to achieve health equity involves diversifying the pool of funded researchers in biomedical and behavioral fields. This entails mentoring that may facilitate the transition from training to independent research and practice, increasing support for investigators from underrepresented backgrounds. Unfortunately, a study investigated the association between NIH R01 applicants’ self-identified race or ethnicity and the probability of receiving an award, and found that applications from African Americans, Asian, and Hispanic/Latinos investigators were 13.1, 5.4, and 2.7, respectively, percentage points less likely to be awarded funding than white investigators (Ginther, Kahn, & Schaffer, 2016). Strategies to counter such bias, whether conscious or unconscious, include enhancing review panels’ training and diversity promises to facilitate the biomedical scientific workforce and therefore the diversity of those engaged in PH science.
In addition, without investments in training on genetics, the behavioral, social and contextual determinants of health (Khoury & Galea, 2016), equitable outreach and inclusion, and dissemination and implementation efforts, PH is likely to fall short of its potential for contributing to improvements in population health (Haspel & Saffitz, 2014; Khoury & Galea, 2016) and to efforts to achieve health equity in the U.S.
Lastly, education efforts should be extended by providing training to community partners, community leaders, and decision makers on social determinants or health, precision medicine, and research. Bolstering their knowledge and training in these areas can help increase the reach of PH to populations and geographic locations that are underrepresented in current research. Further, providing these partners with training in communication and dissemination of information can increase the inclusion of these underrepresented groups in research and intervention efforts.
Exemplar Study: Specific Application of ConNECT Principles to Precision Health
Consider a multinational study that aims to enroll 100,000 participants into a database that will allow for researchers from across the country and the world to access people and data for analysis of various health conditions. Eligibility criteria are only that one gives consent; resides in the U.S., Canada, or Mexico; and is aged 21 or older. A core set of measures and biospecimens will be collected from each participant, with additional data to be collected at various intervals. Below we describe how the five ConNECT principles may be applied in the process of designing and implementing this study. While this exemplar is by no means a complete and conclusive list of strategies to be included for each principle, it does serve to provide a snapshot of how to consider application to a study setting.
Principle 1: Integrating Context
It is critical that core measures include not just simple demographics by key determinants from a socio-ecological context. Because this is a multinational study, key determinants of health may also need to conclude contextual information to better assemble information on environmental factors such as place of residence and exposures, etc. Contextual factors include, for example, proximal variables (e.g., culture, health system interactions), which could influence participation, and must be integrated into the design both for informing data to be collected and recruitment purposes.
Principle 2: Fostering a Norm of Inclusion
Here we refer to the planned engagement of traditionally underrepresented individuals (e.g., racial/ethnic minorities, people with disabilities, sexual minorities, poor people or those not integrated in to the health care system, to name a few). Deliberate engagement of people representing such groups (proportionate to disease burden) is imperative in all aspects of the study from design to dissemination. Because of the vast diversity of such groups, engagement efforts too must vary from focus groups and town halls to advisory boards, and participant representation at protocol governance levels.
Principle 3: Ensuring Equitable Diffusion of Innovations:
It may be too late to design dissemination and implementation of findings after results are obtained. Such efforts, especially among disadvantaged groups may be seen as an afterthought, leading to lack of engagement with innovations. Similar to engagement of diverse groups for design and recruitment, a specific effort to understand not only how to share findings at the population level, but also how to determine that there is equitable access to innovative treatments and other results from different studies is a key component of the design of the study. By involving stakeholders and potential participant representatives in the planning phase, investigators will have planned for, or at the very least, begun consideration of how to ensure maximum benefit at the population level from results.
Principle 4: Harnessing Communication Technology
With the growing dependence on technology in healthcare, we must be careful that innovations from studies conducted on data collected from the cohort, do not leave out or leave behind populations unable to utilize such technology. Lack of use may be attributed to multiple factors (e.g., access, knowledge, fear, literacy), however, planning with community engagement to develop and incorporate user-friendly and accessible systems can go a long way towards not exacerbating the digital divide.
Principle 5: Prioritizing Specialized Training
Lastly, study of this nature could be a living laboratory for practical training experiences for young investigators or postdocs. Summer institutes could engage high school students across all aspects of the research process, while researchers may be trained to recognize and integrate the socioecological perspectives that are critical to achieving health equity in precision health advances.
Summary
The ConNECT Framework: A Model for Advancing Behavioral Medicine Science and Practice to Foster Health Equity can guide how PH researchers can comprehensively study population disease risk and develop effective therapeutics towards remedying health inequities. Population-centered PH has the potential to open exciting new opportunities for the integration of behavioral, contextual and place-based determinants of health to more precisely understand and improve population, family, and individual health. The ConNECT Framework requires engaging multisectoral stakeholders from academia, community, practice, and research in the design, implementation and evaluation of the PH Initiative. Further, scientists, providers, and policy makers must attend to broader contextual factors including structural determinants, cultural norms, spiritual and religious tenets, and medical truth-telling that influence persons’ acceptability, participation, and expectation of PH science and practice.
For practicing nurses and nurse scientists, in particular, there will an increased demand for use of genomic information in clinical patient care based on rapid technological advances in whole-genome sequencing. There will be growing need to use genetic and genomic information to optimize quality. This shift will have a profound effect on disease prevention, screening, diagnosis, prognosis, treatment selection, and treatment efficacy. Nurse clinicians, scientists, and educators in the 21 century will be challenged to become proficient in genetic competencies to provide the best available evidenced-based care to patients (Calzone et al., 2010)
The ConNECT Framework emphasizes that diversity inclusion within PH studies is required for true population health benefit from PH. Specifically, the ConNECT Framework articulates how diversity inclusion in PH is necessary to necessary to equip health researchers, and practitioners—within broad fields including public health, behavioral medicine, medicine and nursing—with contextual socio-ecologic data that can be aligned with biologic data for more population responsive and individually tailored interventions to prevent, diagnose, and treat diseases. Our recommendations support the inclusion of advocates across disciplines to stimulate public engagement in large PH consortium studies and policy guidelines; advocate for diversity inclusion in PH science, citizen science, and policy makers; and activate understanding and addressing the lived experiences (e.g., poverty, racism, discrimination stress, housing, environmental exposure) of communities to best tackle and eradicate disparities in health.
Conclusion
The ConNECT Framework health equity implications in PH are relevant across broad health sectors. Practice: Increase access and benefit of PH research studies and treatments, especially among diverse and historically underrepresented populations Policy: Support policies for PH advances to reach underserved and medically vulnerable populations. Research: Maximize inclusion in study population and appropriate diversity representation in research to meaningfully reflect the disparate burden of disease or inequities under study. Support research funding that targets—along with the biological bases—the complex interaction of behavioral and social determinants in health and health inequalities.
Acknowledgement:
Research reported in this publication was supported, in part, by the National Institutes of Health’s National Cancer Institute, Grant Numbers U54CA203000, R25CA090314 and NIH/OD Grant Number UG3 OD023171. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors gratefully acknowledge Dianna Candito, Program Coordinator and Jennifer Hulbert, Administrative Assistant for their editorial assistance.
The authors acknowledge research reported in this publication was supported, in part, by the National Institutes of Health’s National Cancer Institute, Grant Numbers U54CA203000, R25CA090314 and NIH/OD Grant Number UG3 OD023171. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors would like to thank Dianna Candito, Program Coordinator, and Jennifer Hulbert, Administrative Assistant, for their editorial assistance.
This report is a commentary and review of the literature. No human subjects were involved in this report, and it is not based on findings from a clinical trial.
Footnotes
All authors declare that they have no conflict of interest.
Ethical Conduct of Research: This report is a commentary and review of the literature. No human subjects were involved in this report.
The authors have no conflict of interest to report.
Clinical Trial Registration:
This report is not on findings from a clinical trial.
Contributor Information
Usha Menon, University of South Florida College of Nursing, Tampa, FL.
Kimlin Ashing, Center of Community Alliance for Research Education, Beckman Research Institute, City of Hope Medical Center, Duarte, CA; Department of Population Sciences, Beckman Research Institute, City of Hope Medical Center, Duarte, CA.
Mei Wei Chang, The Ohio State University, College of Nursing, Columbus, OH.
Shannon M. Christy, Department of Health Outcomes and Behavior, Division of Population Science, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Florida 33612.
Katarina Friberg-Felsted, College of Nursing, University of Utah, Salt Lake City, UT.
Virginia Gil Rivas, Department of Psychological Science, University of North Carolina at Charlotte, Charlotte, NC.
Clement K. Gwede, Division of Population Science, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL.
Qian Lu, Department of Psychology, University of Houston, Houston, TX, Department of Health Disparities Research, MD Anderson Cancer Center, Houston, TX.
Cathy D. Meade, Division of Population Science, H. Lee Moffitt Cancer Center and Research Institute & Department of Oncological Sciences, University of South Florida, College of Medicine, Tampa, FL.
Jamila Sly, Department of Oncology Sciences, Icahn School of Medicine at Mount Sinai, New York, NY.
Monica Wang, Department of Community Health Sciences, Boston University School of Public Health and Department of Social and Behavioral Sciences, and Harvard T.H. Chan School of Public Health, Boston, MA.
Betina Yanez, Department of Medical Social Sciences and Robert H. Lurie Comprehensive Cancer Center at Northwestern University Feinberg School of Medicine, Chicago, IL.
Karen Yeary, Fay W. Boozman College of Public Health, University of Arkansas for Medical Sciences, Little Rock, AR.
Jean C Yi, Biobehavioral Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA.
Kassandra I. Alcaraz, Behavioral Research Center, American Cancer Society, Atlanta, GA.
References
- Adams SA, & Petersen CJ (2016). Precision medicine: Opportunities, possibilities, and challenges for patients and providers. Journal of the American Medical Informatics Association, 23, 787–790. doi: 10.1093/jamia/ocv215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz KI, Sly J, Ashing K, Fleisher L, Gil-Rivas V, Ford S, . . . Gwede CK. (2017). The ConNECT Framework: A model for advancing behavioral medicine science and practice to foster health equity. Journal of Behavioral Medicine, 40, 23–38. doi: 10.1007/s10865-016-9780-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazemore AW, Cottrell EK, Gold R, Hughes LS, Phillips RL, Angier H, . . . Devoe JE (2016). “Community vital signs”: Incorporating geocoded social determinants into electronic records to promote patient and population health. Journal of the American Medical Informatics Association, 23, 407–412. doi: 10.1093/jamia/ocv088 [DOI] [PubMed] [Google Scholar]
- Beckie TM, Mendonca MA, Fletcher GF, Schocken DD, Evans ME, & Banks SM (2009). Examining the challenges of recruiting women into a cardiac rehabilitation clinical trial. Journal of Cardiopulmonary Rehabilitation and Prevention, 29, 22–23. doi: 10.1097/HCR.0b013e31819276cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer LT (2010). Evaluating metrics to improve population health. Preventing Chronic Disease, 7, A69. [PMC free article] [PubMed] [Google Scholar]
- Brownson RC, Jacobs JA, Tabak RG, Hoehner CM, & Stamatakis KA (2013). Designing for dissemination among public health researchers: Findings from a national survey in the United States. American Journal of Public Health, 103, 1693–1699. doi: 10.2105/ajph.2012.301165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buseh AG, Underwood SM, Stevens PE, Townsend L, & Kelber ST (2013). Black African immigrant community leaders’ views on participation in genomics research and DNA biobanking. Nursing Outlook, 61, 196–204. doi: 10.1016/j.outlook.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Butrick MN, Vanhusen L, Leventhal K, Hooker GW, Nusbaum R, Peshkin BN, . . . Graves KD (2014). Discussing race-related limitations of genomic testing for colon cancer risk: Implications for education and counseling. Social Science & Medicine, 114, 26–37. doi: 10.1016/j.socscimed.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzone KA, Cashion A, Feetham S, Jenkins J, Prows CA, Williams JK, & Wung S (2010). Nurses transforming health care using genetics and genomics. Nursing Outlook, 58, 26–35. doi: 10.1016/j.outlook.2009.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter MC, Burley VJ, Nykjaer C, & Cade JE (2013). Adherence to a smartphone application for weight loss compared to website and paper diary: Pilot randomized controlled trial. Journal of Medical Internet Research, 15, e32. doi: 10.2196/jmir.2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty R, Hendren N, & Katz LF (2015). The effects of exposure to better neighborhoods on children: New evidence from the moving to opportunity experiment. Harvard University and NBER. doi: 10.3386/w21156 [DOI] [PubMed] [Google Scholar]
- Cohen JJ, Gabriel BA, & Terrell C (2002). The case for diversity in the health care workforce. Health Affairs, 21, 90–102. doi: 10.1377/hlthaff.21.5.90 [DOI] [PubMed] [Google Scholar]
- Cohn EG, Henderson GE, & Appelbaum PS (2016). Distributive justice, diversity, and inclusion in precision medicine: What will success look like? Genetics in Medicine, 19, 157–159. doi: 10.1038/gim.2016.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Underrepresented Groups and the Expansion of the Science and Engineering Workforce Pipeline. (2011). Expanding underrepresented minority participation: America’s science and technology talent at the crossroads. Washington, DC: National Academies Press. doi: 10.17226/112984 [DOI] [Google Scholar]
- Dang JHT, Rodriguez EM, Luque JS, Erwin DO, Meade CD, & Chen MS Jr. (2014). Engaging diverse populations about biospecimen donation for cancer research. Journal of Community Genetics, 5, 313–327. doi: 10.1007/s12687-014-0186-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearing JW, & Kreuter MW (2010). Designing for diffusion: How can we increase uptake of cancer communication innovations? Patient Education and Counseling, 81, doi: 10.1016/j.pec.2010.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt P, Culley JM, Corwin E, Richmond T, Dougherty C, Pickler RH, . . . DeVon HA (2017). National nursing science priorities: Creating a shared vision. Nursing Outlook, 65, 726–736. doi: 10.1016/j.outlook.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Edwards N, & Di Ruggiero E (2011). Exploring which context matters in the study of health inequities and their mitigation. Scandinavian Journal of Public Health, 39, 43–49. doi: 10.1177/1403494810393558 [DOI] [PubMed] [Google Scholar]
- Gaglio B, Shoup JA, & Glasgow RE (2013). The RE-AIM framework: A systematic review of use over time. American Journal of Public Health, 103, e38–e46. doi: 10.2105/ajph.2013.301299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galobardes B, Lynch J, & Smith GD (2007). Measuring socioeconomic position in health research. British Medical Bulletin, 81, 21–37. doi: 10.1093/bmb/ldm001, [DOI] [PubMed] [Google Scholar]
- Gelb BD (2016). Genetic discovery for congenital heart defects. In O. Nakanishi, Markwald RR, Baldwin HS, Keller BB, D. Srivastava, & H. Yamagishi (Eds.), Etiology and morphogenesis of congenital heart disease (pp. 355–360). Tokoyo: Springer. doi: 10.1007/978-4-431-54628-3_51 [DOI] [PubMed] [Google Scholar]
- Gibbons MC, Fleisher L, Slamon RE, Bass S, Kandadai V, & Beck JR (2011). Exploring the potential of Web 2.0 to address health disparities. Journal of Health Communication, 16, 77–89. doi: 10.1080/10810730.2011.596916 [DOI] [PubMed] [Google Scholar]
- Ginther DK, Kahn S, & Schaffer WT (2016). Gender, race/ethnicity, and National Institutes of Health R01 research awards: Is there evidence of a double bind for women of color? Academic Medicine: Journal of the Association of American Medical Colleges, 91, 1098–1107. doi: 10.1097/acm.0000000000001278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel RL, & Saffitz JE (2014). Genomic oncology education. Cancer Journal, 20, 91–95. doi: 10.1097/ppo.0000000000000015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk ET, Habermann EB, Ford JG, Wenzel JA, Brahmer JR, Chen MS Jr . . . Vickers SM (2014). Five National Cancer Institute-designated cancer centers’ data collection on racial/ethnic minority participation in therapeutic trials. Cancer, 120, 1113–1121. doi: 10.1002/cncr.28571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel BA, Schulz AJ, Parker EA, & Becker AB (2008). Critical issues in developing and following CBPR principles In Minkler M & Wallerstein N (Eds.), Community-based participatory research for health: From process to outcomes (2nd ed) (pp. 47–66). San Francisco, CA: Jossey-Bass. [Google Scholar]
- Jakubowski B, & Frumkin H (2010). Environmental metrics for community health improvement. Preventing Chronic Disease, 7, A76. [PMC free article] [PubMed] [Google Scholar]
- Khoury MJ, & Galea S (2016). Will precision medicine improve population ,health? JAMA, 316, 1357. doi: 10.1001/jama.2016.12260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig HG (2015). Religion, spirituality, and health: A review and update. Advanced Mind-Body Medicine, 29, 19–26. [PubMed] [Google Scholar]
- Kottke TE, & Isham GJ (2010). Measuring health care access and quality to improve health in populations. Preventing Chronic Disease, 7, A73. [PMC free article] [PubMed] [Google Scholar]
- Kraus WE, Granger CB, Sketch MH Jr Donahue MP, Ginsburg GS, Hauser ER, . . . Shah SH (2015). A guide for a cardiovascular genomics biorepository: The CATHGEN experience. Journal of Cardiovascular Translational Research, 8, 449–457. doi: 10.1007/s12265-015-9648-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter MW, & Bernhardt JM (2009). Reframing the dissemination challenge: A marketing and distribution perspective. American Journal of Public Health, 99, 2123–2127. doi: 10.2105/ajph.2008.155218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter MW, & Wang ML (2015). From evidence to impact: Recommendations for a dissemination support system. New Directions for Child and Adolescent Development, 149, 11–23. doi: 10.1002/cad.20110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Chen JT, Waterman PD, Rehkopf DH, & Subramanian SV (2005). Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: The Public Health Disparities Geocoding Project. American Journal of Public Health, 95, 312–323. doi: 10.2105/ajph.2003.032482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Nilsen WJ, Abernethy A, Atienza A, Patrick K, Pavel M, . . . Swendeman D (2013). Mobile health technology evaluation: The mHealth evidence workshop. American Journal of Preventive Medicine, 45, 228–236. doi: 10.1016/j.amepre.2013.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legato MJ, Johnson PA, & Manson JE (2016). Consideration of sex differences in medicine to improve health care and patient outcomes. JAMA, 316, 1865–1866. doi: 10.1001/jama.2016.13995 [DOI] [PubMed] [Google Scholar]
- Maar M, Yeates K, Barron M, Hua D, Liu P, Lum-Kwong MM, . . . Tobe SW (2015). I-RREACH: An engagement and assessment tool for improving implementation readiness of researchers, organizations and communities in complex interventions. Implementation Science, 10, 64. doi: 10.1186/s13012-015-0257-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot M (2008). Social resources and health. In Kessle S, Rosenfeld P, & Anderson N (Eds.), Interdisciplinary research:Case studies from health and social science (pp. 292–321). New York, NY: Oxford University Press. doi: 10.1093/acprof:oso/9780195324273.003.0019 [DOI] [Google Scholar]
- McElfish PA, Goulden PA, Bursac Z, Hudson J, Purvis RS, Yeary KHK, . . . Kohler PO (2017). Engagement practices that join scientific methods with community wisdom: Designing a patient-centered, randomized control trial with a Pacific Islander community. Nursing Inquiry, 24, e12141. doi: 10.1111/nin.12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElfish PA, Long CR, Kaholokula JKA, Aitaoto N, Bursac Z, Capelle L, . . . Yeary KH. (2018). Design of a comparative effectiveness randomized controlled trial testing a faith-based Diabetes Prevention Program (WORD DPP) vs. a Pacific culturally adapted Diabetes Prevention Program (PILI DPP) for Marshallese in the United States. Medicine, 97, e0677. doi: 10.1097/md.0000000000010677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath S, & Ghersi D (2016). Building towards precision medicine: Empowering medical professionals for the next revolution. BMC Medical Genomics, 9, 23. doi: 10.1186/s12920-016-0183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil C (2015). NCI-MATCH launch highlights new trial design in precision-medicine era. Journal of the National Cancer Institute, 107, djv193. doi: 10.1093/jnci/djv193 [DOI] [PubMed] [Google Scholar]
- Meade CD, Rodriguez EM, Arevalo M, Luque JS, Harris N, San Miguel G, . . . Erwin DO. (2015). Introducing biospecimen science to communities: Tools from two cities. Progress in Community Health Partnerships: Research, Education, and Action, 9, 51–59. doi: 10.1353/cpr.2015.0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr DC, Burns MN, Schueller SM, Clarke G, & Klinkman M (2013). Behavioral intervention technologies: Evidence review and recommendations for future research in mental health. General Hospital Psychiatry, 35, 332–338. doi: 10.1016/j.genhosppsych.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague E, & Perchonok J (2012). Health and wellness technology use by historically underserved health consumers: Systematic review. Journal of Medical Internet Research, 14, e78. doi: 10.2196/jmir.2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz RF, Bunge EL, Chen K, Schueller SM, Bravin JI, Shaughnessy EA, & Perez-Stable EJ (2015). Massive open online interventions: A novel model for delivering behavioral-health services worldwide. Clinical Psychological Science, 4, 194–205. doi: 10.1177/2167702615583840 [DOI] [Google Scholar]
- National Institutes of Health All Of Us Research Program. (n.d.). All of Us Research Program. Retrieved September 20, 2017, from https://allofus.nih.gov/
- Parrish RG. Measuring population health outcomes. (2010). Prev Chronic Dis. 7(4):A71. Retrieved November 19, 2018 from http://www.cdc.gov/pcd/issues/2010/jul/10_0005.htm. [PMC free article] [PubMed] [Google Scholar]
- Pew Research Center Internet and Broadband Fact Sheet. (2018). Retrieved August 30, 2018, from http://www.pewinternet.org/fact-sheet/internet-broadband/
- Popejoy AB, & Fullerton SM (2016). Genomics is failing on diversity. Nature, 538, 161–164. doi: 10.1038/538161a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutter L, & Kushner KE (2010). ‘Health equity through action on the social determinants of health’: Taking up the challenge in nursing. Nursing Inquiry, 17, 269–280. doi: 10.1111/j.1440-1800.2010.00500.x [DOI] [PubMed] [Google Scholar]
- Shankar AV, Asrilla Z, Kadha JK, Sebayang S, Apriatni M, Sulastri A, . . . Shankar AH (2009). Programmatic effects of a large-scale multiple-micronutrient supplementation trial in Indonesia: Using community facilitators as intermediaries for behavior change. Food and Nutrition Bulletin, 30, S207–S214. doi: 10.1177/15648265090302s204 [DOI] [PubMed] [Google Scholar]
- Silent Spring Institute. (2015). Our Tools. Retrieved September 10, 2018, from https://silentspring.org/our-tools
- Smith CE, Fullerton SM, Dookeran KA, Hampel H, Tin A, Maruthur NM, . . . Ordovás JM (2016). Using genetic technologies to reduce, rather than widen, health disparities. Health Affairs, 35, 1367–1373. doi: 10.1377/hlthaff.2015.1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt DE, Chan T, Waldron L, Speers C, Feng FY, Ogunwobi OO, & Osborne JR (2016). Racial/ethnic disparities in genomic sequencing. JAMA Oncology, 2, 1070–1074. doi: 10.1001/jamaoncol.2016.1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, . . . Chalkidou K (2009). A pragmatic-explanatory continuum indicator summary (PRECIS): A tool to help trial designers. Journal of Clinical Epidemiology, 62, 464–475. doi: 10.1016/j.jclinepi.2008.12.011 [DOI] [PubMed] [Google Scholar]
- Valantine HA, & Collins FS (2015). National Institutes of Health addresses the science of diversity. Proceedings of the National Academy of Sciences, 112, 12240–12242. doi: 10.1073/pnas.1515612112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victorson D, Banas J, Smith J, Languido L, Shen E, Gutierrez S, . . . Flores L,(2014). eSalud: Designing and implementing culturally competent ehealth research with Latino patient populations. American Journal of Public Health, 104, 2259–2265. doi: 10.2105/ajph.2014.302187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P-Y, Cheng C-W, Kaddi CD, Venugopalan J, Hoffman R, & Wang MD (2017). Omic and electronic health record big data analytics for pecision medicine. IEEE Transactions on Biomedical Engineering, 64, 263–273. doi: 10.1109/tbme.2016.2573285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez B, McGinty HL, Mohr DC, Begale MJ, Dahn JR, Flury SC, . . . Penedo FJ (2015). Feasibility, acceptability, and preliminary efficacy of a technology-assisted psychosocial intervention for racially diverse men with advanced prostate cancer. Cancer, 121, 4407–4415. doi: 10.1002/cncr.29658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf RA, Rogith D, Hovick SR, Peterson SK, Burton-Chase AM, Fellman BM . . . Meric-Bernstam F (2014). Attitudes toward molecular testing for personalized cancer therapy. Cancer, 121, 243–250. doi: 10.1002/cncr.28966 [DOI] [PMC free article] [PubMed] [Google Scholar]