Abstract
Methyl salicylate (MeSA) is a long-distance signal transduction chemical that plays an important role in plant responses to abiotic stress and herbivore and pathogen attacks. However, it is unclear how photosynthesis and elicitation of plant volatile organic compounds (VOC) from different metabolic pathways respond to the dose of MeSA. We applied different MeSA concentrations (0-50 mM) to study how exogenous MeSA alters VOC profiles of silver birch (Betula pendula) leaves from application through recovery (0.5-23 h). MeSA application significantly reduced net assimilation rate (A) in 10 mM and 20 mM MeSA treated plants. No significant effects of MeSA were observed on the stomatal conductance at any MeSA concentration. MeSA elicited emissions of benzenoids (BZ), monoterpenes (MT), and fatty acid derived compounds (LOX products). Emission rates of BZ were positively, but emission rates of MT were negatively correlated with MeSA concentration. Total emission of LOX products was not influenced by MeSA concentration. Emission rate of MT was negatively correlated with BZ and the share of MT in the total emission blend decreased and the share of BZ increased with increasing MeSA concentration. Although the share of LOX products was similar across MeSA treatments, some LOX products responded differently to MeSA concentration, ultimately resulting in unique VOC blends. Overall, this study demonstrates inverse responses of MT and BZ to different MeSA doses such that plant defense mechanisms induced by lower MeSA doses mainly lead to enhanced MT synthesis, whereas greater MeSA doses trigger BZ-related defense mechanisms. Our results will contribute to improving the understanding of birch defenses induced upon regular herbivore attacks and pathogen infections in boreal forests.
Keywords: silver birch (Betula pendula), methyl salicylate (MeSA), monoterpene emissions, benzenoid emissions, fatty acid derived compounds, volatile organic compounds (VOC)
Introduction
Salicylic acid (SA) is synthesized from isochorismate through shikimate pathway in plants (Chen et al. 2009), and methyl salicylate (MeSA) is its methylated derivative. As one of the most important phytohormones related to biotic and abiotic stress responses, methylation of SA increases its membrane permeability and volatility, and thus, endogenous MeSA can be emitted from stressed foliage to surrounding atmosphere, allowing for effective long distance transport of the defense signal (War et al. 2012). Thus, MeSA is a well-known airborne signaling molecule involved in both plant-insect and inter- and intra-plant communication, attracting insect predators of herbivores or transmitting stress signals to the neighboring plants (Park et al. 2007; War et al. 2012).
The production of MeSA is a common stress response, and MeSA is released both when plants are exposed to abiotic stresses (Ding et al. 2001; Kask et al. 2016; Li et al. 2017; Munné-Bosch and Peñuelas 2003) and biotic stresses (Copolovici et al. 2014; Jiang et al. 2016, 2017a; Kost and Heil 2006). There is evidence that in SA signaling pathway, MeSA indeed serves as a mobile signal to induce systemic acquired resistance (SAR) in Nicotiana tabacum (Shulaev et al. 1997), Arabidopsis (Dempsey et al. 2011) and Populus (Zhao et al. 2009). During defense responses, MeSA translocated within the plant from the sites of synthesis to target sites or taken up from the ambient atmosphere is converted back in the target tissues to SA to subsequently trigger SAR (Dempsey et al. 2011).
In addition to MeSA release, plants emit a large number of different volatile organic compounds (VOCs) either constitutively or upon exposure to different abiotic and biotic stresses (stress-induced volatiles) (Copolovici and Niinemets 2016; Loreto and Schnitzler 2010; Niinemets et al. 2010). Typically, three major groups of VOC are produced in a wide range of plant species under stresses: monoterpenes from the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway, fatty acid derived compounds from lipoxygenase (LOX) pathway, and benzenoids from shikimate/phenylpropanoid (PAL) pathway (Dudareva et al. 2006; Gershenzon and Dudareva 2007; Loreto and Schnitzler 2010). But little is known of how the rate and composition of VOC emissions scale with the dose of exogenous elicitors. Elicitor dose-dependent scaling of VOC emissions in non-stressed “recipient” plants is plausible because higher elicitor doses are expected to be associated with greater stresses in neighboring “recipient” plants. Thus, provided atmospheric MeSA concentration scales with the stress severity in “donor” plants, higher MeSA concentration are expected to induce stronger VOC emissions from the MeSA “recipient” plants. So far many studies have used JA and its methylated form methyl jasmonate (MeJA) to investigate the regulation role of JA pathway in plant VOC synthesis (Degenhardt et al. 2010; Filella et al. 2006; Jiang et al. 2017b; Menzel et al. 2014), and indeed, a MeJA dose-dependent upregulation of VOC emissions has been observed (Jiang et al. 2017b).
Although SA signaling pathway in plants is well studied (Loake and Grant 2007), few studies have focused on the dose-dependent relationships between exogenous MeSA and induced VOC emissions in stressed plants. Available evidence does indicate that application of MeSA could at least impact emission of VOC synthesized from different synthesis pathways. For example, Peñuelas et al. (2007) demonstrated that concurrent effects of MeSA and increased temperature could result in greater monoterpene emissions in MeSA fumigated holm oak (Quercus ilex) seedlings. Similarly, Tang et al. (2015) found that the release of several fatty acid derived VOCs was significantly induced by MeSA treatments in hybrid poplar (Populus × euramericana) cuttings.
Silver birch (Betula pendula Roth.) is a dominant early-successional species in Eurasian temperate and boreal forests. Although B. pendula is a low-level constitutive VOC emitter (Niinemets et al. 2010), it can become a strong emitter of different VOCs, primarily monoterpenes following stress occurrence (Hakola et al. 2001; Hakola et al. 1999; Pag et al. 2013). Due to the frequent exposure to herbivore attacks and pathogen infections, B. pendula is potentially one of the strongest VOC emitters among the boreal and temperate tree species (Blande et al. 2010; Maja et al. 2015). The emission of MeSA from boreal and temperate forests are projected to continuously increase due to enhanced spread of pathogens and more frequent herbivore attacks in future climates (Maja et al. 2015; Vuorinen et al. 2007). The quantitative relationships between MeSA dose and total emission rates and changes in the share of different VOC groups will provide important input into quantitative modeling of VOC formation above the boreal and temperate forests.
In this study, we used B. pendula to investigate effects of different concentrations of MeSA on leaf photosynthetic characteristics and on elicitation of VOC emissions. We focused on how MeSA applications impact photosynthetic characteristics as well as the emissions of VOC from three main pathways: MEP/DOXP, LOX and shikimate/PAL pathways. We hypothesized that a dose-dependent relationship exists i) between MeSA and photosynthetic characteristics, i.e. net assimilation rate and stomatal conductance; and ii) between MeSA and the induced VOC emissions from different biochemical synthesis pathways. Because MeSA is derived from shikimate, we also hypothesized that the contribution of shikimate/PAL pathway VOCs to total volatile blend increases with increasing applied MeSA concentration.
Material and methods
Plant material
One-year-old B. pendula seedlings were collected from the campus of the Estonian University of Life Sciences (58˚23’34”N, 26˚41’23”E) and transplanted into 2 L plastic pots filled with a 1:1:1 mixture of commercial garden soil (N:P:K=10:8:16, Kekkilä Group, Finland), sand (AS Silicate, Estonia) and vermiculite (Schetelig Group, Finland). All seedlings were kept in a plant growth room at 21°C under 12 h light period with the light intensity of 600 μmol m-2 s-1 at the level of plants (Philips HPI/TPlus 400 W metal halide lamps). Plants were watered twice a week to soil field capacity.
Methyl salicylate (MeSA) treatment of birch leaves
Three replicate B. pendula seedlings were used in every in situ MeSA treatment. From each seedling, one mature fully expanded leaf was chosen and investigated. MeSA (≥99.9%, Sigma-Aldrich, USA) solutions of 0 mM (control), 5 mM, 10 mM, 20 mM and 50 mM were prepared in 5% ethanol. 10 ml of MeSA solution was homogeneously sprayed on both surfaces of the leaf blade. The total spraying procedure took ca. 1-2 min. After spraying with the MeSA solution, the treated leaf was allowed to dry shortly such that no liquid was remaining on leaf surface. After the treated leaf was enclosed in the glass chamber, gas exchange measurements and VOC collections were conducted subsequently at 0.5 h, 4 h, 8 h, 12 h, 20 h and 23 h after the treatments in the following 23 hours. After the last measurements at 23 h, leaves were immediately harvested and scanned. Projected leaf area was measured by a custom-made software.
Gas exchange measurements
A custom-made open gas-exchange system was used to measure photosynthesis rate by analyzing CO2 and H2O concentration changes and to collect VOC samples as described by Copolovici and Niinemets (2010). The system is equipped with a 1.2 L double-walled glass chamber specifically designed for trace gas measurements. The temperature of the chamber was controlled by circulating water from a circulating water bath between the chamber walls. The ambient air was pumped through a 10 L buffer volume and an HCl-activated copper tubing to scrub ozone and humidified to ca. 60% using a custom-made humidifier before passing into the glass chamber. The flow rate to chamber was maintained at 0.027 L s-1, and mixed by a fan installed inside the chamber. The CO2 concentration in the chamber was between 380-400 µmol mol-1, light intensity at the leaf surface was 600 µmol m-2 s-1 and the chamber was kept at 25°C (leaf temperature was within ± 1 °C of chamber temperature). An infra-red dual-channel gas analyzer (CIRAS II, PP-Systems, Amesbury, USA) was used to measure CO2 and H2O concentrations at the chamber in- and outlets. The measurements of net assimilation rate and stomatal conductance were recorded after the gas flows stabilized and the leaf gas exchange rates reached a steady state, typically in 10-20 min after leaf enclosure. Foliage gas-exchange rates were calculated according to von Caemmerer and Farquhar (1981).
Volatile collection and GC-MS analyses
VOC samples were collected simultaneously with gas exchange measurements. At each measurement time, the volatiles were collected onto stainless steel cartridges filled with three different carbon-based (Carbotrap) adsorbents to trap all VOCs between C3-C17 using an air sample pump (210-1003MTX, SKC Inc., USA). The VOC were sampled for 20 min at a constant flow rate of 0.2 L min-1. The cartridges were analyzed with a combined Shimadzu TD20 automated cartridge desorber and a Shimadzu 2010 Plus GC-MS instrument (Shimadzu, Kyoto, Japan) with a Zebron ZB-624 fused silica capillary column (0.32 mm i.d., 60 m length, 1.8 µm film thickness, Phenomenex, USA) according to the protocol described in Kännaste et al. (2014). The VOC were identified by National Institute of Standards and Technology library (NIST 05) and then confirmed and quantified by authentic standards (Sigma-Aldrich, Germany). Background concentrations of VOC collected from empty chambers were subtracted from the measurements with plant leaves. The emission rates were calculated as:
where φi is the emission rate of the given VOC (nmol m-2 s-1); Pi is its peak area in the chromatogram, F is the air flow rate in chamber (0.027 L s-1); C is the calibration factor which is calculated by dividing peak area by the amount of a standard compound (g-1); M is the molar mass of the VOC (g mol-1); S is the enclosed leaf area from each treated leaf (m2) and V is the volume of gas sampled onto the cartridge (L).
Statistical analyses
Data were tested for normality (Shapiro-Wilk test) and equality of variances (Levene´s test). Ln-transformation was applied when necessary. In addition, linear and non-linear regression analyses were conducted to explore the relationships among the MeSA treatment concentration, gas exchange characteristics and different VOC groups (Sigmaplot 11.0, Systat Software GmbH, Germany). Linear mixed models (SPSS 16.0, Chicago, USA) with MeSA treatment (different concentration of MeSA) and recovery time as fixed effects, and tree number as a random effect were used to test for the effects of MeSA treatments on the photosynthetic characteristics and VOC emission rates through the recovery time between control and treated plants. Post-hoc Fisher’s LSD test (one-way ANOVA) was used to test for the differences in emission rates between individual VOCs. Kruskal-Wallis non-parametric one-way ANOVA was used when the normality test failed. Principal component analysis (PCA) was used to evaluate the effects of different concentrations of MeSA on the VOC compositions. Loading and score plots were initially derived after mean-centering and cube root transformation by MetaboAnalyst version 3.0 (Xia et al. 2012, 2015; Xia and Wishart 2016) and then redrawn in OriginLab 8.0 (OriginLab Corporation, USA).
Results
Effects of MeSA treatments on leaf photosynthetic characteristics
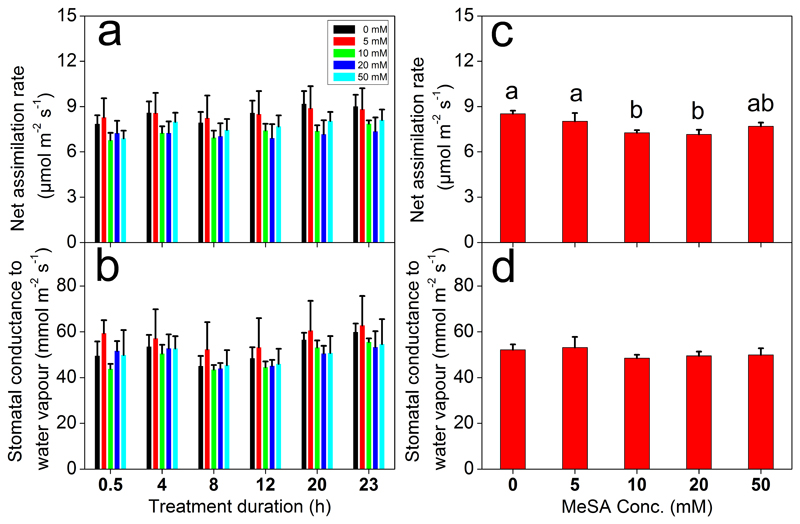
None of the MeSA concentrations led to visible leaf damage by the end of the recovery period, but MeSA treatments significantly reduced net assimilation rate (A) (Fig. 1a; Table 1). The time of recovery did not affect A, but the reduction in A initially increased with increasing MeSA concentration and the decrease leveled off at the higher concentration of MeSA (Fig. 1a, c; Table 1). However, the overall reduction in A was at most ca. 15% in 10 mM and 20 mM MeSA treatments (Fig. 1c). Differently from A, MeSA treatments did not affect stomatal conductance to water vapor (gs) that varied only 4-7% across treatments (Fig. 1b, d; Table 1).
Fig. 1.
Effects of treatment with different concentrations of methyl salicylate (MeSA) at different times since the treatment on average ±SE net assimilation rates (a, c) and stomatal conductance (b, d) of Betula pendula leaves at different times through recovery. Linear mixed models were applied to test the treatment and time of recovery effects. The effects of recovery time and MeSA concentration × time interaction were not significant according to the analysis of linear mixed models (Table 1). Data correspond to three independent biological replicates. Different letters indicate statistically significant differences at P<0.05.
Table 1.
Summary of linear mixed model analysis of effects of applied MeSA concentration and recovery time on gas exchange characteristics and VOC emission rates of Betula pendula leaves. Significant values are shown in bold (P<0.05).
| Variable | MeSA Con. | Time | MeSA Con. × Time | |
|---|---|---|---|---|
| A | numDF | 4 | 5 | 20 |
| denDF | 60 | 60 | 60 | |
| Sig. | 0.009 | 0.597 | 1.000 | |
| gs | numDF | 4 | 5 | 20 |
| denDF | 39 | 59 | 59 | |
| Sig. | 0.053 | 0.124 | 1.000 | |
| BZ emission rates | numDF | 4 | 5 | 20 |
| denDF | 60 | 60 | 60 | |
| Sig. | 0.019 | 0.997 | 0.316 | |
| MT emission rates | numDF | 4 | 5 | 20 |
| denDF | 56 | 59 | 59 | |
| Sig. | <0.001 | 0.953 | 0.991 | |
| LOX emission rates | numDF | 4 | 5 | 20 |
| denDF | 60 | 60 | 60 | |
| Sig. | 0.363 | 0.213 | 0.755 |
MeSA con., methyl salicylate concentration; A, net assimilation rate; gs, stomatal conductance; BZ, benzenoid; MT, monoterpene; LOX, fatty acid derived compounds; numDF and denDF are the numerator and denominator degrees of freedom for the F-tests.
Effects of MeSA treatments on volatile emissions
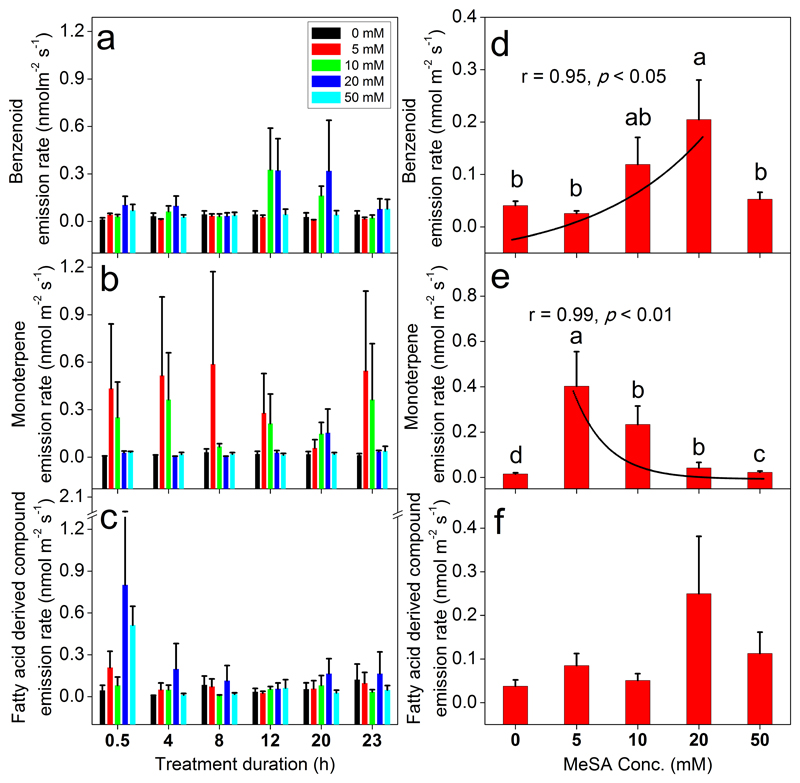
In total 30 VOCs (6 benzenoids, 14 monoterpenes and 10 fatty acid derived compounds) were detected in the emissions of B. pendula leaves after MeSA treatments (Table 2). In control leaves, total benzenoid (BZ, 0.041±0.008 nmol m-2 s-1), monoterpene (MT, 0.015±0.005 nmol m-2 s-1) and total emission of fatty acid derived compounds (LOX, 0.038±0.014 nmol m-2 s-1) were emitted at a low level. Application of MeSA significantly altered the total emissions of BZ and MT (Fig. 2a and 2b; Table 1), while the total emission of LOX compounds was not significantly affected by MeSA treatments (Fig. 2c; Table 1). Similarly to leaf gas exchange characteristics, the time of recovery did not alter total BZ, MT and LOX compound emissions (Fig. 2a-c; Table 1).
Table 2.
Average ± SE emission rates (nmol m-2 s-1) of volatiles released from Betula pendula leaves in response to treatments with different concentrations of methyl salicylate (MeSA; 0 (control), 5 mM, 10 mM, 20 mM and 50 mM)1 and Log2-transformed fold-changes indicated by different colors.
| Compound | 0 mM | 5 mM | 10 mM | 20 mM | 50 mM | No. | Compound | 5 mM | 10 mM | 20 mM | 50 mM | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benzenoids | Benzenoids | |||||||||||||
| Toluene | 0.0236±0.0121 | 0.0099±0.0037 | 0.0341±0.0191 | 0.0671±0.0226 | 0.0347±0.0081 | 1 | Toluene | -1.3 | 0.5 | 1.5 | 0.5 | 10.0 | ||
| Ethylbenzene | 0.0096±0.0038 | 0.0015±0.0009 | 0.0139±0.0096 | 0.0099±0.0058 | 0.0013±0.0004 | 2 | Ethylbenzene | -2.3 | 0.5 | 0.0 | -3.3 | 5.0 | ||
| p-Xylene | 0.0048±0.0031 | 0.0062±0.003 | 0.0303±0.0207 | 0.0219±0.0117 | 0.0032±0.0016 | 3 | p-Xylene | 0.3 | 2.6 | 2.1 | -0.7 | 0.0 | ||
| Benzaldehyde | 0.0071±0.0040 | 0.0045±0.0021 | 0.0275±0.0112 | 0.0585±0.0402 | 0.0085±0.0039 | 4 | Benzaldehyde | -0.8 | 1.9 | 3.1 | 0.2 | -5.0 | ||
| 2-Propenylbenzene | ≤0.00001 | ≤0.00001 | ≤0.00001 | ≤0.00001 | 0.0001±0.0001 | 5 | 2-Propenylbenzene | 0.0 | 0.0 | 0.0 | 3.6 | -10.0 | ||
| Benzyl alcohol | ≤0.00001 | 0.0008±0.0007 | ≤0.00001 | 0.0020±0.0020 | 0.0020±0.0014 | 6 | Benzyl alcohol | 6.6 | 0.0 | 7.6 | 7.6 | |||
| Monoterpenes | Monoterpenes | |||||||||||||
| 3-Thujene | ≤0.00001 | 0.0130±0.0079 | 0.0027±0.0015 | ≤0.00001 | ≤0.00001 | 7 | 3-Thujene | 10.3 | 8.2 | 0.0 | 0.0 | |||
| α-Pinene | 0.0100±0.0039 | 0.0750±0.0302 | 0.0400±0,0150 | 0.0082±0.0040 | 0.0087±0.0030 | 8 | α-Pinene | 2.9 | 2.0 | -0.3 | -0.2 | |||
| Camphene | 0.0013±0.0007 | 0.0139±0.0055 | 0.0141±0.0059 | 0.0022±0.0008 | 0.0025±0.0007 | 9 | Camphene | 3.8 | 3.8 | 1.0 | 1.6 | |||
| Sabinene | ≤0.00001 | 0.0057±0.0051 | 0.0030±0.0030 | ≤0.00001 | ≤0.00001 | 10 | Sabinene | 9.2 | 8.2 | 0.0 | 0.0 | |||
| 2Myrcene | ≤0.00001 b | 0.0038±0.0014 a | 0.0008±0.0005 b | 0.0006±0.0003 b | ≤0.00001 b | 11 | Myrcene | 8.6 | 6.6 | 6.6 | 0.0 | |||
| β-Pinene | ≤0.00001 | 0.0084±0.0041 | 0.0024±0,0014 | 0.0030±0.0020 | ≤0.00001 | 12 | β-Pinene | 9.6 | 7.6 | 0.0 | 0.0 | |||
| 23-Carene | 0.0015±0.0015 b | 0.0027±0.0013 b | 0.0071±0.0020 a | 0.0056±0.0026 a | 0.0015±0.0013 b | 13 | 3-Carene | 1.6 | 2.8 | 2.6 | 0.0 | |||
| β-Ocimeue | ≤0.00001 | ≤0.00001 | ≤0.00001 | 0.0212±0.0173 | 0.0013±0.0008 | 14 | (E)β-Ocimeue | 0.0 | 0.0 | 11.0 | 6.6 | |||
| 4-Carene | ≤0.00001 | 0.0386±0.0203 | 0.0165±0.0108 | ≤0.00001 | ≤0.00001 | 15 | 4-Carene | 11.9 | 10.6 | 0.0 | 0.0 | |||
| 2Limonene | 0.0080±0.0080 c | 0.0269±0.0108 a | 0.0236±0.0067 b | 0.0021±0.0010 d | 0.0036±0.0011 c | 16 | Limonene | 4.8 | 4.6 | 1.0 | 2.0 | |||
| 2p-Cymene | 0.0015±0.0005 b | 0.1527±0.0623 a | 0.1011±0.0385 a | ≤0.00001 c | 0.0033±0.0010 b | 17 | p-Cymene | 6.3 | 5.7 | -7.6 | 0.6 | |||
| β-Phellandrene | ≤0.00001 | 0.0063±0.0029 | 0.0018±0.0010 | ≤0.00001 | ≤0.00001 | 18 | β-Phellandrene | 9.2 | 7.6 | 0.0 | 0.0 | |||
| 1,8-Cineole | ≤0.00001 | 0.0037±0.0018 | 0.0025±0.0011 | 0.0016±0.0013 | 0.0021±0.0010 | 19 | 1,8-Cineole | 8.6 | 7.6 | 7.6 | 7.6 | |||
| γ-Terpinene | ≤0.00001 | 0.0523±0.0260 | 0.0182±0.0110 | ≤0.00001 | ≤0.00001 | 20 | γ-Terpinene | 12.3 | 10.8 | 0.0 | 0.0 | |||
| Fatty acid derived compounds | Fatty acid derived compounds | |||||||||||||
| Hexanal | 0.0043±0.0033 | 0.0062±0.0023 | 0.0046±0.0017 | 0.0117±0.0037 | 0.0108±0.0039 | 21 | Hexanal | 0.6 | 0.3 | 1.6 | 1.5 | |||
| (Z)-3-Hexen-1-ol | 0.0043±0.0037 | 0.0243±0.0188 | 0.0018±0.0016 | 0.0997±0.0989 | 0.0379±0.0237 | 22 | (Z)-3-Hexen-1-ol | 2.6 | -1.0 | 4.6 | 3.2 | |||
| 1-Hexanol | 0.0003±0.0002 | 0.0015±0.0008 | ≤0.00001 | 0.0028±0.0027 | 0.0010±0.0007 | 23 | 1-Hexanol | 1.7 | -4.9 | 3.3 | 1.7 | |||
| 2Heptanal | 0.0003±0.0003 b | 0.0036±0.0016 a | 0.0024±0.0009 a | 0.0101±0.0033 a | 0.0075±0.0029 a | 24 | Heptanal | 3.7 | 2.7 | 5.1 | 4.5 | |||
| 2-Ethyl hexanal | 0.0047±0.0014 | 0.0053±0.0025 | 0.0162±0.0078 | 0.0098±0.0079 | 0.0040±0.0023 | 25 | 2-Ethyl hexanal | 0.0 | 1.7 | 1.0 | -0.3 | |||
| (3Z)-3-Hexenyl acetate | 0.0010±0.0010 | ≤0.00001 | ≤0.00001 | 0.0089±0.0080 | 0.0151±0.0090 | 26 | (3Z)-3-Hexenyl acetate | -6.6 | -6.6 | 3.2 | 3.9 | |||
| Octanal | 0.0044±0.0024 | 0.0100±0.0042 | 0.0052±0.0022 | 0.0262±0.0091 | 0.0121±0.0053 | 27 | Octanal | 1.3 | 0.3 | 2.7 | 1.6 | |||
| 2(E)-2-Nonen-1-ol | ≤0.00001 b | 0.0008±0.0005 b | ≤0.00001 b | 0.0046±0.0017 a | ≤0.00001 b | 28 | (E)-2-Nonen-1-ol | 6.6 | 0.0 | 9.0 | 0.0 | |||
| 21-Octanol | ≤0.00001 c | 0.0040±0.0014 a | 0.0008±0.0004 b | 0.0057±0.0025 a | ≤0.00001 c | 29 | 1-Octanol | 8.6 | 6.6 | 9.2 | 0.0 | |||
| Nonanal | 0.0166±0.0081 | 0.0245±0.0088 | 0.0134±0.0049 | 0.0631±0.0229 | 0.0181±0.0077 | 30 | Nonanal | 0.5 | -0.4 | 1.9 | 0.1 | |||
In all cases, fully-expanded, healthy attached leaves were used. MeSA solutions were prepared in 5% ethanol and 10 ml of the MeSA solution was homogeneously sprayed on both surfaces of the treated leaf. Plants treated with only 5% ethanol were used as controls (0 mM). Volatile samples were collected at 0.5 h, 4 h, 8 h, 12 h, 20 h and 23 h after MeSA treatments and averages across different recovery times are shown. Three independent biological replicates were used for each MeSA concentration treatment. Based on the volatile structure and synthesis pathway, detected volatile compounds were divided into three primary compound groups: benzenoids, monoterpenes and fatty acid derived compounds.
Different letters indicate statistically significant differences (P < 0.05) as tested by one-way ANOVA followed by Fisher’s LSD test.
Fig. 2.
Effects of MeSA treatments on emission rates of total benzenoids (a, d), monoterpenes (b, e) and fatty acid derived compounds (c, f) of Betula pendula leaves. Treatments, number of biological replicates and statistical analyses as in Fig. 1. Effects of MeSA on individual VOCs are shown in Table 2. The effects of time and MeSA concentration × time interaction were not significant (Table 1) according to linear mixed models. Data in (d) and (e) were fitted by non-linear regressions and the corresponding regression equations are y = 0.04e0.09x (d) and y = 0.77e-0.13x (e).
MeSA concentration dependence of total BZ emission rate was a function with a maximum; BZ emissions increased with MeSA concentrations up to 20 mM MeSA (0.21±0.08 nmol m-2 s-1) and further decreased at 50 mM to 0.053±0.013 nmol m-2 s-1 (Fig. 2d). Three BZ compounds, toluene, benzaldehyde and benzyl alcohol were the main compounds reaching the highest emission rate in 20 mM MeSA treated plants (Table 2). However, despite the reduction of total BZ emissions, 2-propenyl benzene was only observed at a detectable level in 50 mM MeSA treatment (Table 2).
In contrast to BZ emissions, total MT emission decreased exponentially with increasing MeSA concentrations from 5 (highest emission of 0.40±0.15 nmol m-2 s-1; 27-fold increase relative to control plants) to 50 mM (0.02±0.01 nmol m-2 s-1; 1.5-fold increase, Fig. 2e). Compared to the control plants, emission rates of three MTs, myrcene, limonene and p-cymene were significantly enhanced and another 7 new MTs were detected at 5 mM MeSA (Table 2). However, (E)-β-ocimene was undetectable until MeSA concentration reached 20 mM, and 3-carene was significantly elicited at 10 mM and 20 mM treatments.
Although total LOX compound emissions were not significantly affected by MeSA treatments (Fig. 2f), emissions of three C7-C9 VOCs, heptanal, (E)-2-nonen-1-ol and 1-octanol were significantly enhanced after the MeSA application (Table 2).
Changes in the bouquets of volatiles upon MeSA treatment
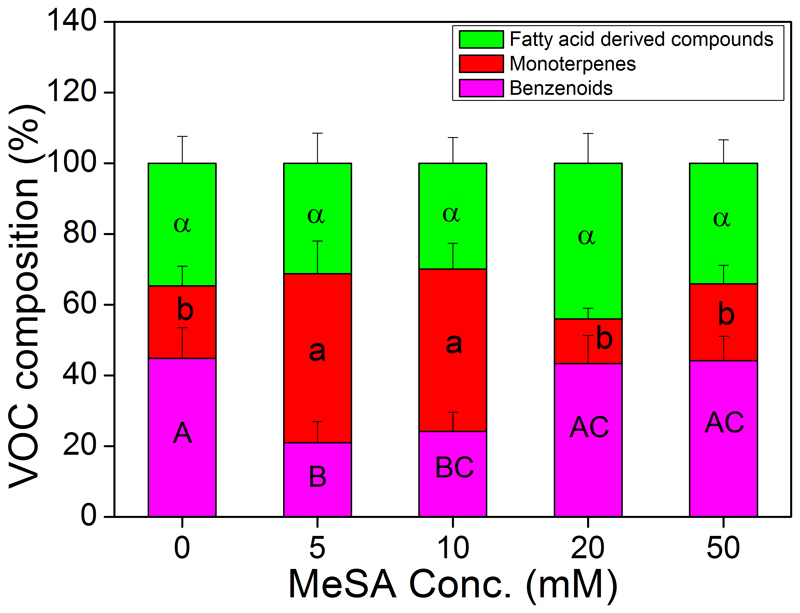
Among all the three VOC groups emitted by B. pendula leaves, significant alternations in VOC compositions were found in 5 mM and 10 mM MeSA treated plants (Fig. 3). The proportion of BZs dropped significantly from 45% in control plants to 21% in 5 mM and to 24% in 10 mM MeSA treatment, whereas the proportion of MTs increased from 21% in control plants to 48% in 5 mM and to 46% in 10 mM MeSA treatment. Higher MeSA concentrations (20 mM and 50 mM) did not affect the BZ proportion (Fig. 3). In contrast to the increased MT proportion in 5 mM and 10 mM MeSA treated plants, MT proportion in 20 mM MeSA treated plants decreased to 13%, i.e. nearly 2-fold reduction compared to control plants. Application of MeSA did not significantly affect the composition of LOX compounds in the total VOC emission (Fig. 3).
Fig. 3.
Effects of MeSA treatments on the distribution of volatiles (average ±SE percentages of total emissions) between three major compound classes: benzenoids, monoterpenes and fatty acid derived compounds in Betula pendula leaves. Data were collected from three independent biological replicates at each MeSA treatments. As indicated in Fig. 2, VOC emission rates of different compound groups did not differ through the recovery period, and thus, emission rates of all recovery times were pooled, and analyzed by one-way ANOVA followed by Fisher’s LSD test. Different letters indicate statistically significant differences at P<0.05.
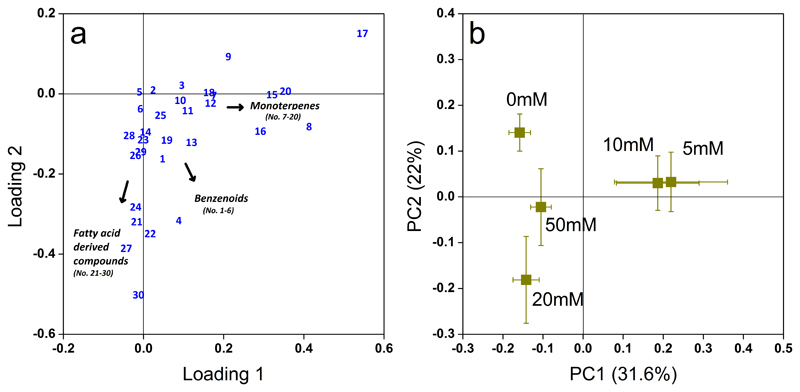
The principal component analysis (PCA) further revealed clear separations in the VOC emission blends induced by different MeSA concentrations (Fig. 4). Two principal components (PC1 and PC2) explained 53.6% of the variation of the observed VOC emissions. All MeSA treatments were clearly separated from the controls due to changes in emission rates of MT, BZ and LOX compounds (Fig. 4). Higher emissions of α-pinene, 4-carene, limonene, p-cymene and γ-terpinene were associated with lower concentrations of MeSA (5 mM and 10 mM). (Table 2, Fig. 4b), whereas the emissions of LOX compounds, hexanal, (Z)-3-hexen-1-ol as well as saturated aldehydes like heptanal, octanal and nonanal constituted the characteristic VOCs that separated 20 mM and 50 mM treatments from others (Table 2; Fig. 4a and b). Notably the BZ compound, benzaldehyde, induced by 20 mM MeSA treatment also significantly contributed to the separation of 20 mM MeSA treatment from other treatments in the loading plot.
Fig. 4.
Loading plot (a) and score plot (b) of the principal component analysis (PCA) of the VOC emission profiles as affected by MeSA treatments in leaves of Betula pendula. Emission rates of all volatiles (Table 2 for compound codes) collected from three biological replicates during 23 hours after application of different concentrations of MeSA were used in the PCA analysis. In the loading plot (a), the impact of volatiles increases with the distance from the origin of the co-ordinate system. In the score plot (b), the mean scores± SE (n =3 for each treatment) of principal components (PC1 and PC2) were calculated by MetaboAnalyst version 3.0 and redrawn in OriginLab 8.0. The variation explained by each PC is shown in both axes.
Correlations among emissions of different volatile groups and photosynthetic characteristics
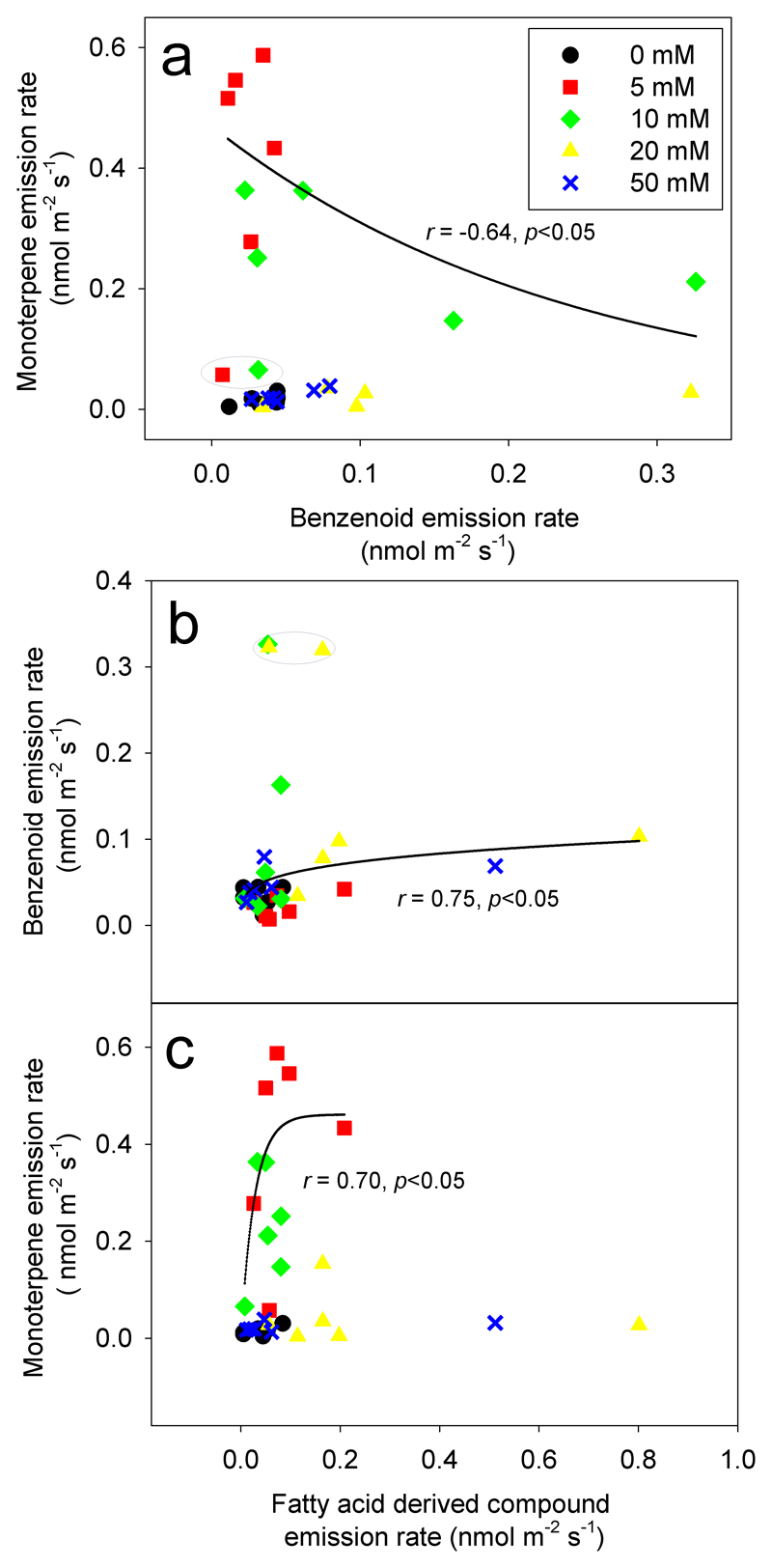
The emission rates of total BZ and MT compounds were negatively correlated with each other for 5 mM and 10 mM MeSA treatments (Fig. 5a). In contrast, positive correlation between total emission rate of the LOX compounds were observed with BZ for 20 mM and 50 mM MeSA treatments (Fig. 5b), and with MT for 5 mM and 10 mM MeSA treatments (Fig. 5c).
Fig. 5.
Correlations among total monoterpene and benzenoid emissions (a), benzenoid and fatty acid derived compound emissions (b) and monoterpene and fatty acid derived compound emissions (c) in MeSA treated leaves of Betula pendula. The emission rates were measured at 0.5 h, 4 h, 8 h, 12 h, 20 h and 23 h after MeSA application, and each symbol corresponds to the mean value of three independent biological replicates at different recovery times for each treatment concentration. Data for 5 mM and 10 mM MeSA treated plants in (a) and (c), and for 20 mM and 50 mM MeSA treated plants in (b) were fitted by non-linear regressions. The corresponding regression equations are y = 0.47e-4.15x (a), y = 20.8x0.23 (b) and y = 461(1-e-0.036x) (c). Outliers were indicated by a dotted ellipse.
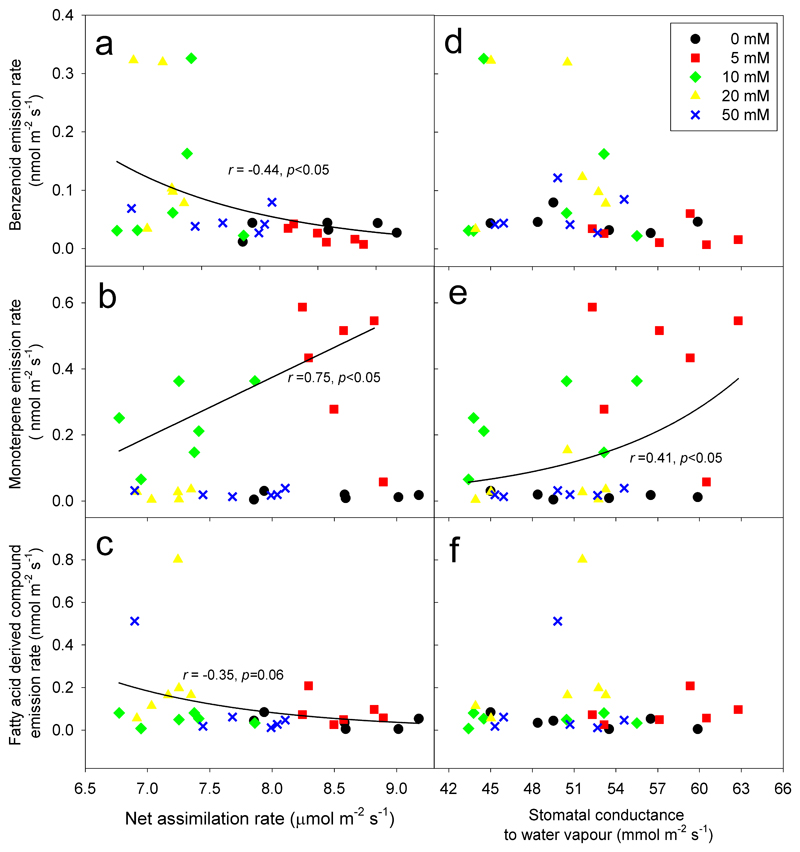
Total emission of BZ compounds was negatively correlated with A across all data (Fig. 6a). In contrast, total emission of MT compounds was positively correlated with A for 5 mM and 10 mM MeSA treated plants (Fig. 6b). In all MeSA treated plants, there was a negative trend between A and fatty acid derived compound emissions (Fig. 6c). Stomatal conductance was poorly related to VOC emission rates, and only a weak positive correlation between gs and MT emission rate was observed across treatments (Fig. 6e).
Fig. 6.
Correlations of VOC emission rates with net assimilation rate (a-c) and stomatal conductance (d-f) in MeSA-treated leaves of Betula pendula. Data presentation as in Fig. 5. Non-linear regressions were fitted to all data in (a), (c) and (e) with the regression equations of y = 24700e-0.75x, y = 52600e-0.81x and y = 0.85(0.16x), respectively. In (b) a linear regression with the equation of y = -1.08 + 0.18x was fitted to date from 5 mM and 10 mM MeSA treated plants.
Discussion
In this study, we demonstrated that manipulating MeSA concentrations affected gas leaf exchange and induced VOC emissions in Betula pendula seedlings. Particularly, we demonstrated quantitative, partly optimum-type dose-dependent relationships between the MeSA concentrations and the magnitude of elicitation of specific VOC groups.
Effects of MeSA treatments on plant photosynthetic performance
Our results indicate that MeSA treatments result in varying effects on foliage photosynthetic characteristics, whereas the net assimilation rate (A) is more strongly affected by MeSA treatments than stomatal conductance (gs) (Fig. 1; Table 1). So far, it is unclear how photosynthesis is regulated in response to MeSA, but our results suggest that MeSA effect on photosynthesis is non-stomatal. The non-stomatal effects of MeSA on photosynthesis can result from changes in cytoplastic and chloroplastic pH as well as due to production of reactive oxygen species (ROS) and concomitant oxidative damage (Fariduddin et al. 2003; Garrido et al. 2009).
Several studies have observed a stronger reduction of photosynthetic characteristics at greater SA concentrations (Janda et al. 2014). Regarding stomatal conductance, for example, in tomato (Lycopersicum esculentum) leaves, application of SA in the range of 0.0001 mM to 0.1 mM did not affect stomatal conductance, but higher SA concentrations up to 1 mM led to stomatal closure (Poór and Tari 2012). Our results indicate that the doses of MeSA employed are non-toxic for the plant leaves. It could be presumed that the endogenous SA concentration derived from the final assimilated MeSA was not high enough in stomatal guard cells and/or in surrounding epidermal cells to affect gs in B. pendula leaves in our study.
Differently from gs, A indeed decreased with increasing MeSA concentration, except for 50 mM MeSA treatment that did not significantly differ from the control treatment and from leaves treated with 10 mM and 20 mM MeSA (Fig. 1c). Such a non-responsiveness might reflect limitations in MeSA uptake, i.e. the maximum uptake capacity of leaf tissues for MeSA might have been already reached before the MeSA concentration increased to 50 mM. This speculation is supported by the stable level of foliar MeSA concentration in holm oak (Quercus ilex) leaves subjected to 24 h constant MeSA fumigation (Llusià et al. 2005). Alternatively, lower response to 50 mM MeSA might also reflect saturation of physiological responses or inhibition of responses. During our study, we did not observe visible damage of leaf tissues by any MeSA concentrations, nor were higher MeSA concentrations lethal. However, different endogenous MeSA concentrations might qualitatively alter the conversion rate of MeSA to SA, as well as alter the cross-talk between SA and JA-dependent signaling, and there is also evidence of certain synergy and antagonism between both signalling pathways (Liu et al. 2016; Mur et al. 2006; Niki et al. 1998). This is relevant as both SA- and JA-dependent signaling pathways are involved in affecting photosynthesis and VOC emissions in plants (Attaran et al. 2014; Nazar et al. 2015; Ozawa et al. 2000). Alternation between SA and JA pathways caused by MeSA might explain the complex dose dependence of net assimilation rate and induced VOCs (see below).
Effects of MeSA treatments on benzenoid emissions
We hypothesized that application of exogenous MeSA enhances SA accumulation in treated leaves and subsequently affects BZ emissions by indirectly mediating the shikimate/PAL pathway activity. Indeed, BZ emission rate was positively correlated with MeSA concentration (Fig. 2d). In particular, the emission rate of the dominant BZ compound, benzaldehyde, increased ca. 4-fold in 10 mM and 8-fold in 20 mM MeSA treated plants (Table 2). Increased emission of benzaldehyde can be interpreted as a direct impact of exogenous MeSA on the PAL pathway, as benzaldehyde is considered one of the SA precursors derived from trans-cinnamic acid in shikimate/PAL pathway (Dempsey et al. 2011; Ibdah et al. 2009). Another BZ compound benzyl alcohol is formed in the same pathway as benzaldehyde. Thus, elicitation of its emission in response to exogenous MeSA suggests that endogenous SA biosynthesis pathway was enhanced in MeSA-treated B. pendula seedlings. Additionally, toluene, p-xylene and ethyl benzene were also detected in the VOC blends (Table 2). The specific biological pathway synthesizing those simple BZ VOCs in plants is still unknown. However, the elicitation of toluene and p-xylene under stresses (e.g. wounding, heat, and herbivory attack) in other species implies their potential roles in indirect defense mechanisms (Heiden et al. 1999; Manninen et al. 2002; Misztal et al. 2015).
Induction of monoterpene emissions upon MeSA treatments
The induced emissions of MT are de novo synthesized MTs and thus, these emissions are closely connected to the photosynthesis characteristics (Loreto et al. 1996). Accordingly, in our study, we found that total emission rates of MTs were positively correlated to net assimilation rate under 5 mM and 10 mM MeSA treatments (Fig. 6b).
In our study, significant emissions of MT were elicited under all MeSA treatments in B. pendula seedlings (Fig.2b, 2e; Table 1). MTs have been found to play a positive role in plant stress resistance in various studies (Llusià et al. 2014; Loreto et al. 2004). MeSA-induced emission of MTs might also have a synergistic effect with MeSA in strengthening SAR in both the MeSA “donor” and “recipient” plants. In Arabidopsis thaliana, both MeSA and α-pinene and β-pinene treatments induced SAR in treated plants and the induction of defense in neighboring plants was associated with presence of α-pinene, β-pinene and camphene emissions in the “donor” plants (Riedlmeier et al. 2017). Indeed, emission rates of most MTs increased under 5 mM and 10 mM MeSA treatments, with most prominent rise in α-pinene (7-fold) and camphene (14-fold) emissions (Table 2).
By externally applying MeSA we demonstrated a negative correlation between exogenous MeSA concentration and the emission rate of induced MTs (Fig. 2e). Specifically, our results indicated that lower exogenous MeSA concentration (5 mM to 10 mM) facilitate, but higher concentrations (20 mM to 50 mM) suppress MT emissions in B. pendula seedlings (Fig. 2e). Of the total induced VOCs, the share of MTs reached up to almost 50% in 5 mM and 10 mM MeSA treatments; whereas the MT share was only 13% in 20 mM and 22% in 50 mM treated plants (Fig. 3). Thus, the relationship between the share of MT in total VOC emission blend and MeSA concentration was not linear in B. pendula leaves. Llusià et al. (2005) fumigating Quercus ilex leaves with MeSA observed uncontrolled ROS accumulation and cell death in treated plants. Therefore, direct effects of MeSA on induction of MT volatiles may be attributed to the production of ROS in leaf tissues. It has been shown that infestation-induced SA accumulation suppresses JA-induced enzyme, (E)-β-ocimene synthase gene expression that catalyzes synthesis of (E)-β-ocimene (Zhang et al. 2009). Interestingly in our study, the emission rate of (E)-β-ocimene, that is often specifically induced by herbivory attack and considered as one of the stress marker MTs (Navia-Giné et al. 2009; Pickett et al. 2003; Shimoda et al. 2012) increased only in 20 mM and 50 mM MeSA treated plants after 4 h of treatment (Table 2; Supplementary Fig. S1). Emission of (E)-β-ocimene emission from 20 mM and 50 mM MeSA treated plants (Table 2; Supplementary Fig. S1) provides some indirect evidence that MeSA induced more strongly SA accumulation in 5 mM and 10 mM than in 20 mM and 50 mM MeSA treated plants, although differential effects on JA-dependent signalling cannot be ruled out.
Effects of MeSA treatments on emissions of fatty acid derived volatiles
Previous studies have shown that transient MeSA treatment triggered oxidative burst and eventually led to hypersensitive cell death in plants (Shulaev et al. 1997; Yun and Chen 2011). Thus, one would expect that a higher concentration of exogenous MeSA would increase endogenous SA concentration and induce cell damage in leaf tissues and subsequently induce emission of LOX compounds. Although we did not observe any visual damage, we observed that A was negatively correlated with the emission rate of LOX compounds (Fig. 6c). Yet, this correlation was relatively weak, as 50 mM MeSA treatment did not significantly affect A (see above). However, the emission rates of all LOX compounds detected in this study, i.e. hexanal, (Z)-3-hexen-1-ol, 1-hexanol, 2-ethyl hexanal, (3Z)-3-hexenyl acetate and (E)-2-nonen-1-ol were in general higher in plants treated with 20 mM and 50 mM MeSA than with 5 mM and 10 mM MeSA (Table 2). In addition to these classic LOX compounds, emission rates of an aliphatic saturated aldehyde heptanal and a linear alcohol 1-octanol were also significantly increased by MeSA treatments (Table 2). These results confirm that MeSA application breaks the homeostasis in leaf tissues and thus, triggers the early stress responses.
How did different MeSA concentrations affect VOC emission rates and overall emission composition?
As a stress elicitor or stress signal transmitter, MeSA could affect VOC emission patterns in B. pendula seedlings by either directly triggering ROS formation or indirectly by affecting the cross-talk between various physiological pathways orchestrating the VOC syntheses. The release of LOX compounds can be considered as a signal to infer damage at cell membrane level in leaf tissues (Niinemets et al. 2013; Spinelli et al. 2011). Although through the entire recovery period, total emission of LOX compounds was not significantly affected by MeSA treatments, increased emissions of several individual LOX compounds in 20 mM and 50 mM MeSA treated plants were indicators for cellular damage or enhanced ROS brought about by MeSA treatments (Table 2). Moreover, emission of LOX compounds played an important role as main components separating the treatments with higher MeSA concentration (20 mM and 50 mM) from the treatments with lower concentration (5 mM and 10 mM) in PCA (Fig. 4). Interestingly, we found that the emission rates of LOX compounds were positively correlated with BZ in 20 mM and 50 mM MeSA treatments, but with MT in 5 mM and 10 mM MeSA treatments (Fig. 5b and 5c). The presence of LOX compound emissions is related to the degree of stress in leaf tissues (Jiang et al. 2017b), and it could be inferred that synthesis and emission of MTs played an important role in plant resistance to moderate stress, while BZ release was associated with severe stress. This is further supported by the evidence that the share of BZs and MTs in the total VOC blend at the lower MeSA treatment concentrations of 5 mM and 10 mM MeSA was shifted to MTs, while the share was shifted to BZs in plants treated with higher MeSA concentrations of 20 mM and 50 mM (Fig. 3). As discussed above, shifts in the activity of SA and JA signaling pathways by MeSA might be responsible for altered BZ and MT emission patterns. In plants, the two pathways responsible for synthesis of BZs and MTs, shikimate/PAL and MEP/DOXP pathways, are both located in plastids and have a common early intermediate, phosphoenolpyruvate (PEP), suggesting that there might be a certain competition between these pathways at substrate level (see the pathway scheme in Niinemets et al. 2013). If this is the case, preferential activation of one pathway by changes in flux controlling enzyme activity can shift the production of end-products by the other pathway if the enzymatic apparatus of the other pathway remains unchanged or increases less. Thus, decreased MT emissions coupled with increased BZ emissions as MeSA treatment concentration increased might indicate preferential activation of the shikimate/PAL pathway (Fig. 3 and 5a).
Conclusions
In this study, we investigated how exogenous MeSA affected leaf gas exchange and VOC emission rates and composition in B. pendula seedlings. Application of MeSA led to reduced net assimilation rate without affecting stomatal conductance. We further demonstrated contrasting dose dependencies among the applied MeSA dose and emissions of different VOC groups. These results reveal the potential role of MeSA in plant-plant stress signal transduction through influencing several secondary metabolism and signaling pathways, including shikimate/PAL pathway, MEP/DOXP pathway and SA/JA signaling pathway. Further studies are needed to gain an insight into how the cross-talk between SA and JA signaling pathways results in qualitatively different VOC responses at different MeSA concentrations.
Supplementary Material
Acknowledgements
The authors wish to thank Rinaldo Anni for assisting in VOC sample analyses, Veronika Sulg for taking care of the plants and Lauri Laanisto for proofreading the manuscript.
Funding
European Commission through the European Regional Fund (the Center of Excellence EcolChange); European Research Council (advanced grant 322603, SIP-VOL+) and the Estonian Ministry of Science and Education (institutional grant IUT-8-3).
References
- Attaran E, Major IT, Cruz JA, Rosa BA, Koo AJ, Chen J, Kramer DM, He SY, Howe GA. Temporal dynamics of growth and photosynthesis suppression in response to jasmonate signaling. Plant physiology. 2014;165:1302–1314. doi: 10.1104/pp.114.239004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blande JD, Korjus M, Holopainen JK. Foliar methyl salicylate emissions indicate prolonged aphid infestation on silver birch and black alder. Tree Physiology. 2010;30:404–416. doi: 10.1093/treephys/tpp124. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zheng Z, Huang J, Lai Z, Fan B. Biosynthesis of salicylic acid in plants. Plant Signaling & Behavior. 2009;4:493–496. doi: 10.4161/psb.4.6.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Remmel T, Niinemets Ü. Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environmental and Experimental Botany. 2014;100:55–63. doi: 10.1016/j.envexpbot.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolovici L, Niinemets Ü. Flooding induced emissions of volatile signaling compounds in three tree species with differing waterlogging tolerance. Plant, Cell & Environment. 2010;33:1582–1594. doi: 10.1111/j.1365-3040.2010.02166.x. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Niinemets Ü. Environmental impacts on plant volatile emission. In: Blande J, Glinwood R, editors. Deciphering Chemical Language of Plant Communication. Springer International Publishing; Berlin: 2016. pp. 35–59. [Google Scholar]
- Degenhardt DC, Refi-Hind S, Stratmann JW, Lincoln DE. Systemin and jasmonic acid regulate constitutive and herbivore-induced systemic volatile emissions in tomato, Solanum lycopersicum. Phytochemistry. 2010;71:2024–2037. doi: 10.1016/j.phytochem.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Dempsey DMA, Vlot AC, Wildermuth MC, Klessig DF. Salicylic acid biosynthesis and metabolism. The Arabidopsis Book. 2011:e0156. doi: 10.1199/tab.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C-K, Wang CY, Gross KC, Smith DL. Reduction of chilling injury and transcript accumulation of heat shock proteins in tomato fruit by methyl jasmonate and methyl salicylate. Plant Science. 2001;161:1153–1159. [Google Scholar]
- Dudareva N, Negre F, Nagegowda DA, Orlova I. Plant volatiles: recent advances and future perspectives. Critical Reviews in Plant Sciences. 2006;25:417–440. [Google Scholar]
- Fariduddin Q, Hayat S, Ahmad A. Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica. 2003;41:281–284. [Google Scholar]
- Filella I, Peñuelas J, Llusià J. Dynamics of the enhanced emissions of monoterpenes and methyl salicylate, and decreased uptake of formaldehyde, by Quercus ilex leaves after application of jasmonic acid. New Phytologist. 2006;169:135–144. doi: 10.1111/j.1469-8137.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- Garrido I, Espinosa F, Álvarez-Tinaut MC. Oxidative defense reactions in sunflower roots induced by methyl-jasmonate and methyl-salicylate and their relation with calcium signalling. Protoplasma. 2009;237:27–39. doi: 10.1007/s00709-009-0069-0. [DOI] [PubMed] [Google Scholar]
- Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nature Chemical Biology. 2007;3:408–414. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- Hakola H, Laurila T, Lindfors V, Hellén H, Gaman A, Rinne J. Variation of the VOC emission rates of birch species during the growing season. Boreal Environment Research. 2001;6:237–249. [Google Scholar]
- Hakola H, Rinne J, Laurila T. The VOC emission rates of boreal deciduous trees. In: Laurila T, Lindfors V, editors. Biogenic VOC emissions and photochemistry in the boreal regions of Europe–Biphorep. European Commission; Brussels: 1999. pp. 21–28. [Google Scholar]
- Heiden A, Kobel K, Komenda M, Koppmann R, Shao M, Wildt J. Toluene emissions from plants. Geophysical Research Letters. 1999;26:1283–1286. [Google Scholar]
- Ibdah M, Chen Y-T, Wilkerson CG, Pichersky E. An aldehyde oxidase in developing seeds of Arabidopsis converts benzaldehyde to benzoic acid. Plant Physiology. 2009;150:416–423. doi: 10.1104/pp.109.135848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda T, Gondor OK, Yordanova R, Szalai G, Pál M. Salicylic acid and photosynthesis: signalling and effects. Acta Physiologiae Plantarum. 2014;36:2537–2546. [Google Scholar]
- Jiang Y, Veromann-Jürgenson LL, Ye J, Niinemets Ü. Oak gall wasp infections of Quercus robur leaves lead to profound modifications in foliage photosynthetic and volatile emission characteristics. Plant, Cell & Environment. 2017a doi: 10.1111/pce.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Ye J, Li S, Niinemets Ü. Methyl jasmonate-induced emission of biogenic volatiles is biphasic in cucumber: a high-resolution analysis of dose dependence. Journal of Experimental Botany. 2017b;68:4679–4694. doi: 10.1093/jxb/erx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Ye J, Veromann L-L, Niinemets Ü. Scaling of photosynthesis and constitutive and induced volatile emissions with severity of leaf infection by rust fungus (Melampsora larici-populina) in Populus balsamifera var. suaveolens. Tree Physiology. 2016;36:856–872. doi: 10.1093/treephys/tpw035. [DOI] [PubMed] [Google Scholar]
- Kännaste A, Copolovici L, Niinemets Ü. Gas chromatography-mass spectrometry method for determination of biogenic volatile organic compounds emitted by plants. Methods in Molecular Biology (Clifton, NJ) 2014;1153:161–169. doi: 10.1007/978-1-4939-0606-2_11. [DOI] [PubMed] [Google Scholar]
- Kask K, Kännaste A, Talts E, Copolovici L, Niinemets Ü. How specialized volatiles respond to chronic and short-term physiological and shock heat stress in Brassica nigra. Plant, Cell & Environment. 2016;39:2027–2042. doi: 10.1111/pce.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost C, Heil M. Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants. Journal of Ecology. 2006;94:619–628. [Google Scholar]
- Li S, Harley PC, Niinemets Ü. Ozone-induced foliar damage and release of stress volatiles is highly dependent on stomatal openness and priming by low-level ozone exposure in Phaseolus vulgaris. Plant, Cell & Environment. 2017;40:1984–2003. doi: 10.1111/pce.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llusià J, Bermejo-Bermejo V, Calvete-Sogo H, Peñuelas J. Decreased rates of terpene emissions in Ornithopus compressus L. and Trifolium striatum L. by ozone exposure and nitrogen fertilization. Environmental pollution. 2014;194:69–77. doi: 10.1016/j.envpol.2014.06.038. [DOI] [PubMed] [Google Scholar]
- Llusià J, Peñuelas J, Munné-Bosch S. Sustained accumulation of methyl salicylate alters antioxidant protection and reduces tolerance of holm oak to heat stress. Physiologia Plantarum. 2005;124:353–361. [Google Scholar]
- Loake G, Grant M. Salicylic acid in plant defence—the players and protagonists. Current Opinion in Plant Biology. 2007;10:466–472. doi: 10.1016/j.pbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Loreto F, Ciccioli P, Cecinato A, Brancaleoni E, Frattoni M, Fabozzi C, Tricoli D. Evidence of the photosynthetic origin of monoterpenes emitted by Quercus ilex L. leaves by 13C labeling. Plant Physiology. 1996;110:1317–1322. doi: 10.1104/pp.110.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Pinelli P, Manes F, Kollist H. Impact of ozone on monoterpene emissions and evidence for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiology. 2004;24:361–367. doi: 10.1093/treephys/24.4.361. [DOI] [PubMed] [Google Scholar]
- Loreto F, Schnitzler J-P. Abiotic stresses and induced BVOCs. Trends in Plant Science. 2010;15:154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Maja MM, Kasurinen A, Holopainen T, Kontunen-Soppela S, Oksanen E, Holopainen JK. Volatile organic compounds emitted from silver birch of different provenances across a latitudinal gradient in Finland. Tree Physiology. 2015;35:975–986. doi: 10.1093/treephys/tpv052. [DOI] [PubMed] [Google Scholar]
- Manninen A-M, Pasanen P, Holopainen JK. Comparing the VOC emissions between air-dried and heat-treated Scots pine wood. Atmospheric Environment. 2002;36:1763–1768. [Google Scholar]
- Menzel TR, Weldegergis BT, David A, Boland W, Gols R, van Loon JJ, Dicke M. Synergism in the effect of prior jasmonic acid application on herbivore-induced volatile emission by Lima bean plants: transcription of a monoterpene synthase gene and volatile emission. Journal of Experimental Botany. 2014;65:4821–4831. doi: 10.1093/jxb/eru242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misztal P, Hewitt C, Wildt J, Blande J, Eller A, Fares S, Gentner D, Gilman J, Graus M, Greenberg J. Atmospheric benzenoid emissions from plants rival those from fossil fuels. Scientific Reports. 2015;5 doi: 10.1038/srep12064. 12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S, Peñuelas J. Photo-and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta. 2003;217:758–766. doi: 10.1007/s00425-003-1037-0. [DOI] [PubMed] [Google Scholar]
- Navia-Giné WG, Yuan JS, Mauromoustakos A, Murphy JB, Chen F, Korth KL. Medicago truncatula (E)-β-ocimene synthase is induced by insect herbivory with corresponding increases in emission of volatile ocimene. Plant Physiology and Biochemistry. 2009;47:416–425. doi: 10.1016/j.plaphy.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Nazar R, Umar S, Khan NA. Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress. Plant signaling & behavior. 2015;10:e1003751. doi: 10.1080/15592324.2014.1003751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Arneth A, Kuhn U, Monson R, Peñuelas J, Staudt M. The emission factor of volatile isoprenoids: stress, acclimation, and developmental responses. Biogeosciences. 2010;7:2203–2223. [Google Scholar]
- Niinemets Ü, Kännaste A, Copolovici L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Frontiers in Plant Science. 2013;4:262. doi: 10.3389/fpls.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa R, Arimura G-i, Takabayashi J, Shimoda T, Nishioka T. Involvement of jasmonate-and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant and Cell Physiology. 2000;41:391–398. doi: 10.1093/pcp/41.4.391. [DOI] [PubMed] [Google Scholar]
- Pag A, Bodescu A, Kännaste A, Tomescu D, Niinemets Ü, Copolovici L. Volatile organic compounds emission from Betula verrucosa under drought stress. Scientific Bulletin of ESCORENA. 2013;8:45–53. [Google Scholar]
- Park S-W, Kaimoyo E, Kumar D, Mosher S, Klessig DF. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science. 2007;318:113–116. doi: 10.1126/science.1147113. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Llusià J, Filella I. Methyl salicylate fumigation increases monoterpene emission rates. Biologia Plantarum. 2007;51:372–376. [Google Scholar]
- Pickett J, Rasmussen H, Woodcock C, Matthes M, Napier J. Plant stress signalling: understanding and exploiting plant–plant interactions. Portland Press Limited; 2003. [DOI] [PubMed] [Google Scholar]
- Poór P, Tari I. Regulation of stomatal movement and photosynthetic activity in guard cells of tomato abaxial epidermal peels by salicylic acid. Functional Plant Biology. 2012;39:1028–1037. doi: 10.1071/FP12187. [DOI] [PubMed] [Google Scholar]
- Riedlmeier M, Ghirardo A, Wenig M, Knappe C, Koch K, Georgii E, Dey S, Parker JE, Schnitzler J-P, Vlot C. Monoterpenes support systemic acquired resistance within and between plants. The Plant Cell. 2017 doi: 10.1105/tpc.16.00898. tpc. 00898.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda T, Nishihara M, Ozawa R, Takabayashi J, Arimura Gi. The effect of genetically enriched (E)-β-ocimene and the role of floral scent in the attraction of the predatory mite Phytoseiulus persimilis to spider mitersimilis floral scent in the attraction of the predatory mite of gen. 2012 doi: 10.1111/j.1469-8137.2011.04018.x. [DOI] [PubMed] [Google Scholar]
- Shulaev V, Silverman P, Raskin I. Airborne signalling by methyl salicylate in plant pathogen resistance. Nature. 1997;385:718–721. [Google Scholar]
- Spinelli F, Cellini A, Marchetti L, Nagesh KM, Piovene C. Emission and function of volatile organic compounds in response to abiotic stress. In: Shanker A, Venkateswarlu B, editors. Abiotic stress in plants-mechanisms and adaptations. InTech; West Palm Beach: 2011. [DOI] [Google Scholar]
- Tang F, Fu Y-Y, Ye J-R. The effect of methyl salicylate on the induction of direct and indirect plant defense mechanisms in poplar (Populus× euramericana ‘Nanlin 895’) Journal of Plant Interactions. 2015;10:93–100. [Google Scholar]
- von Caemmerer Sv, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Vuorinen T, Nerg A-M, Syrjälä L, Peltonen P, Holopainen JK. Epirrita autumnata induced VOC emission of silver birch differ from emission induced by leaf fungal pathogen. Arthropod-Plant Interactions. 2007;1:159–165. [Google Scholar]
- War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC. Mechanisms of plant defense against insect herbivores. Plant Signaling & Behavior. 2012;7:1306–1320. doi: 10.4161/psb.21663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Mandal R, Sinelnikov IV, Broadhurst D, Wishart DS. MetaboAnalyst 2.0—a comprehensive server for metabolomic data analysis. Nucleic Acids Research. 2012;40:W127–W133. doi: 10.1093/nar/gks374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Research. 2015;43:W251–W257. doi: 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Wishart DS. Using metaboanalyst 3.0 for comprehensive metabolomics data analysis. Current Protocols in Bioinformatics. 2016:14.10. 1–14.10. 91. doi: 10.1002/cpbi.11. [DOI] [PubMed] [Google Scholar]
- Yun LJ, Chen WL. SA and ROS are involved in methyl salicylate-induced programmed cell death in Arabidopsis thaliana. Plant Cell Reports. 2011;30:1231–1239. doi: 10.1007/s00299-011-1031-0. [DOI] [PubMed] [Google Scholar]
- Zhang P-J, Zheng S-J, van Loon JJ, Boland W, David A, Mumm R, Dicke M. Whiteflies interfere with indirect plant defense against spider mites in Lima bean. Proceedings of the National Academy of Sciences. 2009;106:21202–21207. doi: 10.1073/pnas.0907890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Guan J, Forouhar F, Tschaplinski TJ, Cheng Z-M, Tong L, Chen F. Two poplar methyl salicylate esterases display comparable biochemical properties but divergent expression patterns. Phytochemistry. 2009;70:32–39. doi: 10.1016/j.phytochem.2008.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.