Abstract
Different amplification options are available for listeners with congenital unilateral conductive hearing loss (UCHL). For example, bone-conduction devices (BCDs) and middle ear implants. The present study investigated whether intervention with an active BCD, the Bonebridge, or a middle ear implant, the Vibrant Soundbridge (VSB), affected sound-localization performance of listeners with congenital UCHL. Listening with a Bonebridge or VSB might provide access to binaural cues. However, when fitted with the Bonebridge, but not with a VSB, binaural processing might be affected through cross stimulation of the contralateral normal hearing ear, and could interfere with processing of binaural cues. In the present study twenty-three listeners with congenital UCHL were included. To assess processing of binaural cues, we investigated localization abilities of broadband (BB, 0.5–20 kHz) filtered noise presented at varying sound levels. Sound localization abilities were analyzed separately for stimuli presented at the side of the normal-hearing ear, and for stimuli presented at the side of the hearing-impaired ear. Twenty-six normal hearing children and young adults were tested as control listeners. Sound localization abilities were measured under open-loop conditions by recording head-movement responses. We demonstrate improved sound localization abilities of children with congenital UCHL, when listening with a Bone-bridge or VSB, predominantly for stimuli presented at the impaired (aided) side. Our results suggest that the improvement is not related to accurate processing of binaural cues. When listening with the Bonebridge, despite cross stimulation of the contralateral cochlea, localization performance was not deteriorated compared to listening with a VSB.
Keywords: Bonebridge, Bone-conduction device, Binaural hearing, Sound-localization, Unilateral aural atresia, Vibrant soundbridge
1. Introduction
1.1. Benefit of sound amplification in children with congenital unilateral conductive hearing loss
An estimated 1 in 10.000 children is born with aural atresia, two thirds of them suffering from the unilateral form. Aural atresia is defined as a congenital condition where the external ear canal is either abnormally developed or absent and is commonly accompanied by microtia, which is a congenital malformation of the pinna. In the majority of cases the inner ear structures are not affected (Declau et al., 1999; Kountakis, 2013). Depending on the severity of atresia it causes a conductive hearing loss of up to 60 dB HL (e.g. Siegert et al., 2007). Unilateral conductive hearing loss (UCHL) leads to asymmetric hearing and consequently, to deteriorated processing of interaural level differences (ILDs), and interaural time differences (ITDs), which enable sound localization in the horizontal plane. This might cause safety risks, for example in traffic, and feelings of discomfort. In addition, UCHL leads to problems with understanding speech in noisy environments, for example in a classroom.
Several studies have reported that children with unilateral hearing loss lack behind their peers in terms of speech and language development, academic achievement and their psychosocial development (Bess et al., 1984; Lieu et al., 2012; Kesser et al., 2013; Lieu, 2013; Sangen et al., 2017). However, the reported effects are small, the number of studies investigating this topic is limited, and researchers are facing several challenges in providing the optimal treatment (Van Wieringen et al., 2018).
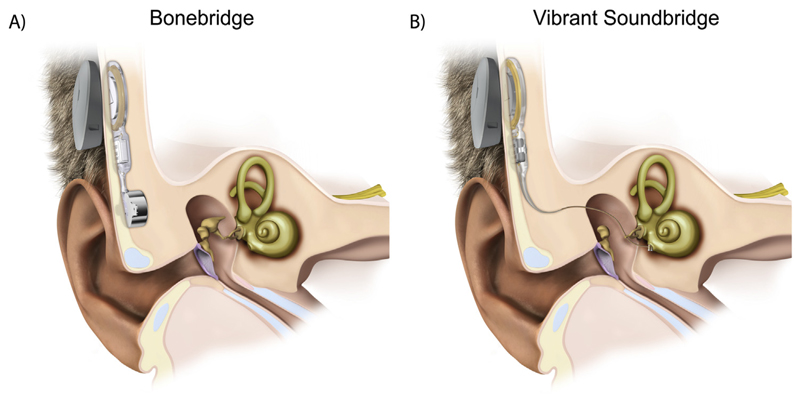
Hearing rehabilitation options for patients with congenital unilateral aural atresia are: reconstructive surgery, a powerful airconduction hearing aid, a bone-conduction device (BCD) or middle ear implant (Vibrant Soundbridge; Agterberg et al., 2014; Snik, 2018). Reconstructive surgery in well-selected patients with a mild form of congenital aural atresia can yield promising outcomes, but rate of revision surgery is up to 26% (Declau et al., 1999; Nadaraja et al., 2013). Therefore, Declau et al. (1999) and Nadaraja et al. (2013) concluded that implantation with a percutaneous BCD (BAHA® or Ponto®) is likely the better option. The Bonebridge® (Med-El, Innsbruck, Austria; Fig. 1A), a direct-driven active transcutaneous BCD, was introduced as an alternative choice of amplification in the pediatric population. Unlike the percutaneous BCD the Bonebridge does not penetrate the skin and leaves it intact. It consists of an external audio processor and an implanted part. Sound waves are captured by the microphones and converted into electrical signals in the processor, which are then transcutaneously transmitted to the implant. Next, the so-called bone conduction-floating mass transducer (BC-FMT) vibrates transmitting the signal to the bone, which conducts it to the inner ear.
Fig. 1.
Illustration of the implanted Bonebridge (A) and implanted VSB (B). Here, the floating-mass transducer (FMT) of the VSB is coupled directly to the round window. Figures are provided by MED-EL.
Typically, children are not implanted with a BCD before the age of 5 years because of the limited thickness of the skull (Snik et al., 2005). With a Vibrant Soundbridge® (VSB; Med-El, Innsbruck, Austria; Fig. 1B) implantation is already possible at the age of 2 years. The VSB, based on the same functional principal (vibratory device) as the Bonebridge, stimulates the cochlea via a much smaller FMT (weight = 25 mg, in comparison BC-FMT: weight = 10 g). In patients with aural atresia the FMT is commonly coupled to the stapes, ossicular chain or directly to the cochlea (i.e. round window), depending on the anatomy of the patient (Frenzel et al., 2009). Still most clinics are conservative in terms of early implantation because it has not been shown yet that early implantation with a BCD, Bonebridge or VSB results in better binaural processing.
Application of percutaneous BCD's has shown to improve speech recognition in noise and directional hearing, in both patients with congenital and acquired UCHL (e.g. Kunst et al., 2008; Agterberg et al., 2011, 2012). Similar results have been demonstrated for the Bonebridge (Hassepass et al., 2015; Rahne et al., 2015; Vyskocil et al., 2017). Concerning the VSB, Frenzel et al. (2009, 2012, 2015) showed that hearing and speech recognition of the impaired ear of children with unilateral aural atresia improved significantly, however, binaural hearing was not tested.
1.2. Cross-hearing
Because of the limited transcranial attenuation of bone-conducted sound (Stenfelt, 2012), any BCD, including the Bonebridge, stimulate not only the ipsilateral ear but also the contralateral cochlea through so-called cross hearing. That might lead to an interfering input to the normal-hearing ear. Therefore, in UCHL, it might be expected that the VSB, which stimulates only the ipsilateral cochlea, provides a better input for sound localization than the Bonebridge.
1.3. Aim of the study
The aim of the presented study is to demonstrate whether unilateral amplification with a VSB or a Bonebridge in children with UCHL result in accurate processing of binaural cues (i.e. accurate sound localization). Furthermore, we aim to demonstrate whether patients implanted with a device free of cross stimulation, the VSB, are performing better than patients implanted with a Bonebridge.
2. Materials and methods
2.1. UCHL listeners and control listeners
Children and adolescences (n = 23) diagnosed with a bony congenital unilateral aural atresia, were implanted with either a direct bone-conduction device (Bonebridge; Med-El; Insbruck, Austria; n = 9; mean age at activation of the implant 11.3y) or an active middle ear implant (Vibrant Soundbridge (VSB); Med-El, Innsbruck, Austria; n = 14; mean age at activation of the implant 7.6y). Patients were implanted at the Department of Otorhinolaryngology and Plastic Operations, University Hospital Schleswig-Holstein, Lübeck, Germany between June 2010 and April 2016. Processors fitted were either the Amade (n = 11) or the Samba (n = 12). Mean age when measuring the patients was 11.7 years and ranged from 4 to 19 years. All listeners were evaluated at least 4 months after fitting.
Characteristics of the patients are listed in Table 1. Patient P2 was excluded due to difficulties in understanding the tasks, because German was not the native language. Also P11 was excluded, because of the air-conduction threshold of the normal-hearing exceeding 20 dB HL. The remaining of the children had a normal-hearing ear on the contralateral side (≤20 dB HL).
Table 1.
Information of congenital UCHL listener.
| UCHL listener | Device | Processor | Implanted side | Age at activation (y) | Age at testing (y) | Years of usage (y) | AC imp.e. (dB HL) | BC imp.e. (dB HL) | Sound levels presented |
|---|---|---|---|---|---|---|---|---|---|
| P1 | BonB | Samba | Right | 3.5 | 4.9 | 1.4 | 64.5 | 20.0 | 1 level |
| P2‡ | BonB | Samba | Right | 4.6 | 5.3 | 0.7 | 72.3 | 22.8 | 1 level |
| P3 | BonB | Amade | Right | 7.1 | 8.9 | 1.8 | 74.3 | 11.8 | 1 level |
| P4 | BonB | Amade | Right | 10.6 | 12.5 | 1.9 | 65.8 | 17.3 | 3 levels |
| P5 | BonB | Amade | Right | 11.2 | 13.1 | 1.9 | 60.3 | 19.3 | 3 levels |
| P6 | BonB | Samba | Right | 14.9 | 15.5 | 0.6 | 78.5 | 16.5 | 3 levels |
| P7 | BonB | Samba | Left | 15.0 | 15.6 | 0.6 | 68.0 | 17.0 | 3 levels |
| P8 | BonB | Samba | Right | 17.0 | 17.4 | 0.4 | 94.5 | 23.8 | 3 levels |
| P9 | BonB | Samba | Right | 17.9 | 19.2 | 1.3 | 72.8 | 10.0 | 3 levels |
| P10 | VSB | Samba | Right | 3.7 | 8.6 | 4.9 | 75.5 | 13.8 | 3 levels |
| P11‡ | VSB | Amade | Right | 4.6 | 6.5 | 1.9 | 76.5 | 26.5 | 3 levels |
| P12 | VSB | Samba | Right | 4.9 | 6.7 | 1.8 | 68.8 | 11.3 | 3 levels |
| P13 | VSB | Amade | Right | 5.4 | 7.8 | 2.4 | 74.3 | 5.3 | 3 levels |
| P14 | VSB | Samba | Left | 5.9 | 7.9 | 3.8 | 79.8 | 23.5 | 3 levels |
| P15 | VSB | Samba | Right | 6.2 | 9.6 | 3.4 | 61.0 | 7.3 | 3 levels |
| P16 | VSB | Samba | Left | 6.4 | 7.3 | 0.9 | 70.0 | 21.5 | 3 levels |
| P17 | VSB | Amade | Right | 8.0 | 14.2 | 6.2 | 77.0 | 16.3 | 3 levels |
| P18 | VSB | Amade | Left | 8.3 | 12.9 | 4.7 | 61.8 | 12.3 | 3 levels |
| P19 | VSB | Amade | Right | 10.0 | 16.4 | 6.2 | 59.5 | 19.3 | 3 levels |
| P20 | VSB | Amade | Left | 10.4 | 14.2 | 3.8 | 66.8 | 13.3 | 3 levels |
| P21 | VSB | Amade | Right | 10.5 | 14.1 | 3.5 | 71.0 | 27.8 | 3 levels |
| P22 | VSB | Amade | Right | 10.5 | 16.2 | 5.6 | 70.0 | 17.3 | 3 levels |
| P23 | VSB | Samba | Right | 11.3 | 14.6 | 3.3 | 85.8 | 22.3 | 3 levels |
AC = air-conduction threshold, BC = bone-conduction threshold, BonB = Bonebridge, imp.e, = impaired ear, Sound levels presented = indicate how many different sound levels were presented, VSB = Vibrant Soundbridge, 1 level = 55 dBA only, 3 levels = 45, 55, 65 dBA
Listener was excluded from analysis.
For reference purpose, 26 normal-hearing listeners were tested (mean age: 12.7 years, range 6–18y). Additionally, all control listeners participated in a unilaterally plugged condition to mimic a UCHL. This was done by inserting an earplug in the ear canal (younger listeners (6–10y): OHROPAX Mini SOFT, SNR 35 dB; older listeners (16–18y): EAR Soft FX, SNR 39 dB) and covering this ear with a muff (younger listener: Kid Peltor (3M, Maplewood, USA); older listener: Optime III Peltor (3M, Maplewood, USA)).
2.2. Conditions
Devices were fitted using the fitting software provided by the manufacturer and fine-tuned according to the listener's wishes. For the measurement the processor settings were set on omnidirectional mode. Listeners were measured with their hearing devices on (aided condition) and off (unaided condition). Half of the time the measurement began with the device turned on and vice versa.
2.3. Set-up
Measurements were carried out in a sound-isolated anechoic mobile auditory laboratory. The walls were covered with absorbing materials. Twenty-four loudspeakers (Genelec 8010, Genelec Oy, Iisalmi, Finland) were positioned within the horizontal (±70°) and vertical (+40/-30°) range. Speakers were covered by a black, soundemitting curtain. Matlab (The MathWorks, Natick, USA) was utilized to control a sound card with 24 analog output channels (MOTU 24Ao, MOTU, Cambridge, USA), and an electronic board (Arduino Uno, Arduino, Somerville, USA), which triggered the fixation LED located at the center of the speaker array. During the task, patients sat comfortably in a chair located 1.2 m from the speakers. Horizontal and vertical head movements were recorded via infrared cameras (Smarttrack, ART, Munich, Germany).
2.4. Stimuli
To test directional hearing broadband (BB, 0.5–20 kHz) Gaussian noise bursts were presented at three different sound levels (45–65 dB, A-weighted (dBA), in 10 dB increments), which were played randomly interleaved. Stimulus duration was 150 ms with a 10 ms on-/offset ramping. For three listeners (P1, P2 and P3) experimental time needed to be limited and, therefore, BB stimuli were presented at just one sound level (55 dBA). The same number of stimuli was presented from the left and from the right hemifield.
2.5. Calibration and instruction
Before calibration the height of the chair was adjusted so the head of the listener was at the same level as the loudspeakers (elevation: 0°). As part of the calibration procedure, listeners were instructed to look at the fixation LED (azimuth: 0°; elevation: 0°) followed by a head movement towards a visual target at + 73° azimuth. To become familiar with the sound localization test, 12 BB stimuli were presented and listeners were monitored on the correct execution of the task. For the experiment, listeners were instructed to look at the fixation LED again. After pressing a button, the fixation LED was turned off, and a stimulus was presented within a variable delay between 200 and 300 ms. Head movement was recorded with an acquisition time of 1.5 s. After data acquisition, the fixation LED was automatically turned on again which initiated the new trial. All listeners were instructed to localize stimuli as fast and as accurate as possible.
2.6. Data analysis
The best linear fit of target-response relationship was computed (equation (1)):
| (1) |
with αRESP being the response azimuth (in degrees), αTARG the target azimuth (in degrees), b the response bias (in degrees) and g response gain (dimensionless). The coefficient of determination (r2) as well as the mean absolute error (MAE, equation (2)) was computed.
| (2) |
2.7. Analysis of binaural hearing
In order to analyze the ability to process binaural cues, localization data was further analyzed by calculating the MAE of the hearing-impaired (plugged ear for normal-hearing listeners) and the healthy side separately. This was done to disentangle possible different localization behaviors on each side for the unaided and aided condition. Therefore, all head movements made for stimuli presented at the right hemifield (positive angle) were analyzed separately from stimuli presented at the left hemifield. For group comparison the impaired side was defined as the right side for all listeners, which required inverting the data sets of P7, P14, P16, P18 and P20, because their impaired side was left. Thus in this analysis, the left side is by definition the healthy side. P1 and P3 were not analyzed due to a lack of a sufficient amount of data points on each side.
3. Results
3.1. Improved sound localization with a Bonebridge and VSB
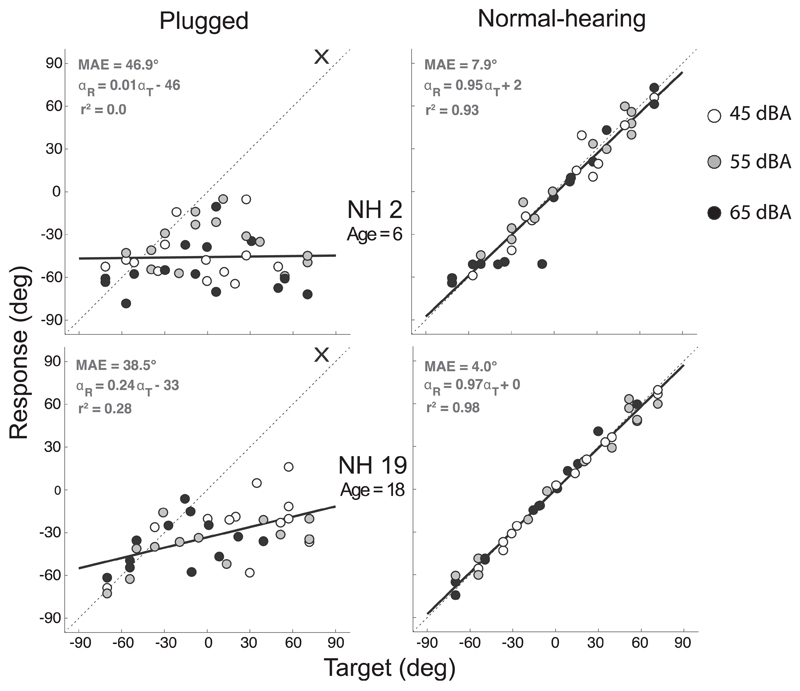
In Fig. 2 individual target-response plots are shown for two normal-hearing listeners (NH2 and NH19). In the right column the normal-hearing condition is shown and in the left column the plugged condition (the right side is plugged - indicated by a cross). Each data point represents an endpoint of a head movement toward a stimulus (response), plotted against the target location in degrees. All data points are responses toward BB noise bursts and shaded (white: 45 dBA, grey: 55 dBA, black: 65 dBA) to indicate the sound level they were presented at. The black regression line represents the best linear fit.
Fig. 2.
Target-response plots of two normal-hearing children (NH2, NH19). In the right column the normal-hearing condition is shown and in the left column the plugged condition (the right side is plugged - indicated by a cross). Data points represent listeners' localization response to a stimulus (white: 45 dBA, grey: 55 dBA, black: 65 dBA). Best-fit linear regression is indicated by a black line.
For both listeners all data points in the normal-hearing condition fall along the dotted diagonal line, indicating good sound source localization abilities (gain close to 1, MAE <10°). Listener NH2 (gain = 0.95) displays a greater variation in the normalhearing condition compared to listener NH19 (gain = 0.97), which is reflected by the smaller r2 (0.93 compared to 0.98) and a marginal greater MAE (7.9° compared to 4.0°). In the plugged listening condition both listeners perceived almost all stimuli as being on the non-occluded side (left; bias = −46°/-33° resp., MAE > 38°).
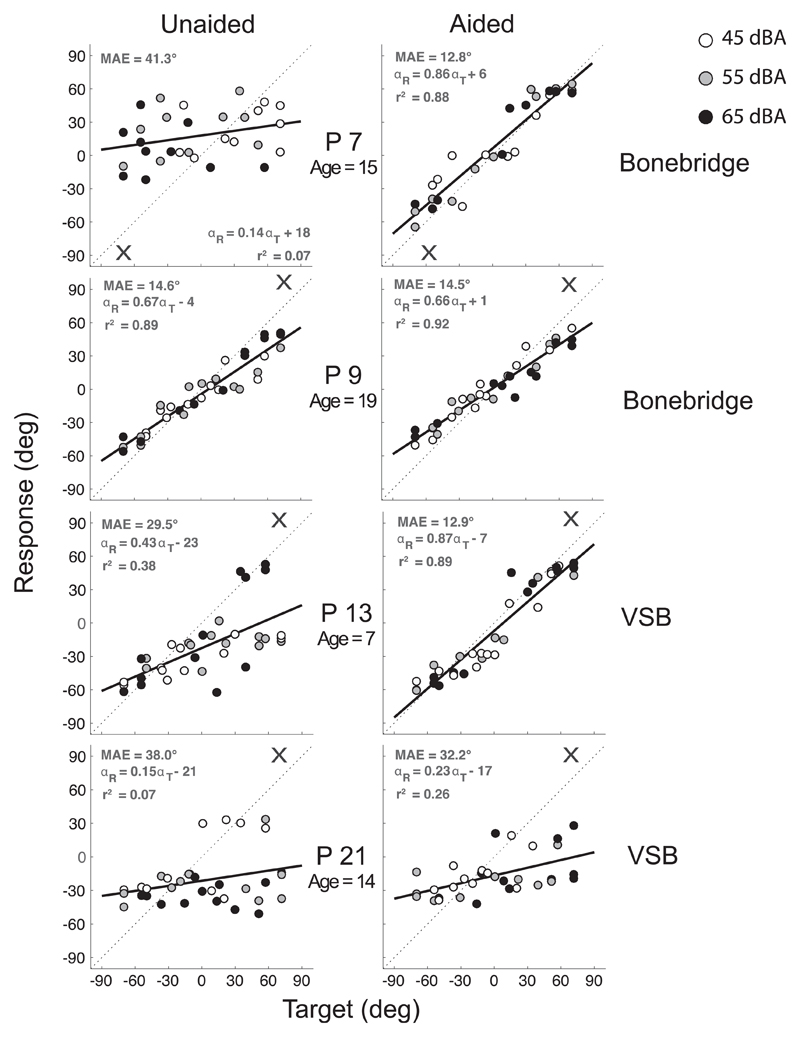
In Fig. 3 individual target-response plots of four patients (P7, P9, P13 and P21) are shown. The different sound levels (white: 45 dBA, grey: 55 dBA, black: 65 dBA) are indicated. In the left column responses are plotted for the unaided (without device) condition and in the right column for the aided (with device) condition. Listeners P9, P13 and P21 were hearing impaired on their right ear and P7 on the left ear. P7 and P9 were fitted with a Bonebridge and P13 and P21 with a VSB. In the unaided condition a great behavioral variation between the four listeners is observed. P7, P13 and P21 perceived the majority of stimuli on the side of their better-hearing ear (P7: bias = 18°; P13: bias = −23°; P21: bias = −21°) indicating poor localization abilities. P9 demonstrated relatively good localization performance in the unaided condition (gain = 0.67, MAE = 14.6°, bias = −4°). When fitted with their device P7 and P13 improved (P7: Δgain = 0.72, ΔMAE = 28.5°; P13: Δgain = 0.44, DMAE = 16.6°), but P9 and P21 showed hardly any improvement (P9: Δgain = 0.01, ΔMAE = 0.1°; P21: Δgain = 0.08, ΔMAE = 5.8°). P21's poor localization performance in the unaided condition (gain = 0.15, MAE = 38°, bias = −21°) remained poor when using the device (gain = 0.23, MAE = 32.2°, bias = −17°), and P9 demonstrated already a good localization performance in the unaided condition and, therefore, did not improve when using the Bonebridge.
Fig. 3.
Target-response plots of four patients (P7, P9, P13, P21). In the right column the aided condition is shown and in the left column the unaided condition. P7 and P9 were fitted with a Bonebridge and P13 and P21 with a VSB. Data points represent listeners' localization response to a target (white: 45 dBA, grey: 55 dBA, black: 65 dBA). Best-fit linear regression is indicated by a black line. The cross (X) indicates the side of the hearing-impaired ear.
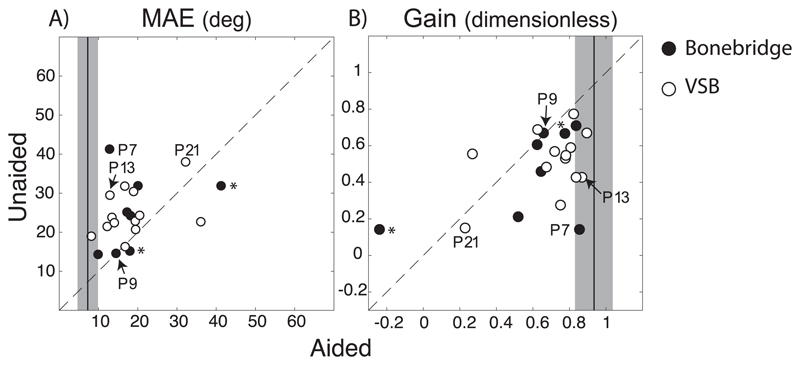
MAE (Fig. 4A) and response gain (Fig. 4B) of the unaided condition is plotted against those of the aided condition. Each data point represents one listener, when fitted with a Bonebridge circles are illustrated in black and with a VSB in white. The depicted examples from Fig. 2 are indicated. Note that two patients (P1, P3) localized BB stimuli presented at only one sound level (55 dBA, indicated by an asterisk (*)). The mean MAE (7.3°) and mean gain (0.93) of the normal-hearing children are indicated by the black vertical line (+/− two standard deviation). The distance between a data point to the diagonal line represents the difference between the two listening conditions.
Fig. 4.
A) MAE and B) gain of aided condition (x-axis) are plotted against those of unaided condition. Black data points indicate Bonebridge listeners and white data points VSB listeners. The four listeners depicted in Fig. 3 are marked (P7, P9, P13, P21). The black vertical lines represents the mean MAE (7.3°) and mean gain (0.93) of the 26 normal-hearing listeners. The grey shaded box is the width of ± two standard deviations. Listeners marked with an asterisk (*) performed sound localization with stimuli presented at 55 dBA only (P1, P3).
The majority of data points fall above the diagonal for Fig. 4A and below the diagonal for Fig. 4B. This demonstrates an improvement in sound localization in the aided condition compared to the unaided condition for this pediatric population (paired t-tests, MAE/gain: p-values < 0.01). However, several data points fall close to the diagonal, indicating a marginal to no change in localization behavior when listening with the device. The majority of patients performed poorer than normal-hearing children (i.e. data points are not close to the scores of normal-hearing controls). All individual scores are indicated in Table 2.
Table 2.
The response gain, r2, bias and mean absolute error (MAE) of all UCHL listeners (except P2 and P11) for the Bonebridge/VSB off (unaided) and Bonebridge/VSB on (aided) conditions.
| UCHL listener | Bonebridge/VSB off (unaided) |
Bonebridge/VSB on (aided) |
||||||
|---|---|---|---|---|---|---|---|---|
| Gain | r2 | Bias | MAE | Gain | r2 | Bias | MAE | |
| P1* | 0.67 | 0.80 | −13 | 15.2 | 0.78 | 0.46 | −8 | 18.0 |
| P3* | 0.14 | 0.04 | −1 | 31.9 | −0.24 | 0.05 | −1 | 41.2 |
| P4 | 0.71 | 0.90 | −9 | 14.3 | 0.84 | 0.96 | −5 | 9.9 |
| P5 | 0.46 | 0.72 | −20 | 24.2 | 0.65 | 0.74 | −6 | 17.9 |
| P6 | 0.61 | 0.72 | −19 | 24.6 | 0.62 | 0.88 | −11 | 17.8 |
| P7 | 0.14 | 0.07 | 18 | 41.3 | 0.86 | 0.88 | 6 | 12.8 |
| P8 | 0.21 | 0.53 | −23 | 32.0 | 0.52 | 0.75 | −11 | 20.1 |
| P9 | 0.67 | 0.89 | −4 | 14.6 | 0.66 | 0.92 | 1 | 14.5 |
| P10 | 0.57 | 0.64 | −14 | 21.9 | 0.72 | 0.67 | −3 | 18.0 |
| P12 | 0.55 | 0.75 | −13 | 20.7 | 0.78 | 0.91 | −7 | 12.2 |
| P13 | 0.43 | 0.38 | −23 | 29.5 | 0.87 | 0.89 | −7 | 12.9 |
| P14 | 0.28 | 0.23 | 18 | 30.4 | 0.75 | 0.71 | 3 | 18.9 |
| P15 | 0.67 | 0.82 | −15 | 19.0 | 0.89 | 0.96 | −3 | 8.2 |
| P16 | 0.43 | 0.31 | 32 | 31.8 | 0.84 | 0.75 | 3 | 16.7 |
| P17 | 0.59 | 0.72 | −20 | 23.8 | 0.81 | 0.89 | −7 | 13.4 |
| P18 | 0.53 | 0.69 | 11 | 21.5 | 0.78 | 0.90 | 5 | 12.2 |
| P19 | 0.69 | 0.67 | −14 | 20.7 | 0.63 | 0.77 | −11 | 19.5 |
| P20 | 0.55 | 0.59 | 10 | 22.7 | 0.27 | 0.38 | 26 | 36.1 |
| P21 | 0.15 | 0.07 | −21 | 38.0 | 0.23 | 0.26 | −17 | 32.2 |
| P22 | 0.78 | 0.80 | −7 | 16.3 | 0.82 | 0.76 | 5 | 16.8 |
| P23 | 0.48 | 0.61 | −10 | 22.9 | 0.67 | 0.69 | −8 | 19.4 |
UCHL = unilateral conductive hearing loss, MAE = mean absolute error.
= P1 and P3 only listened to BB stimuli with 55 dBA.
3.2. Improved localization because of binaural hearing?
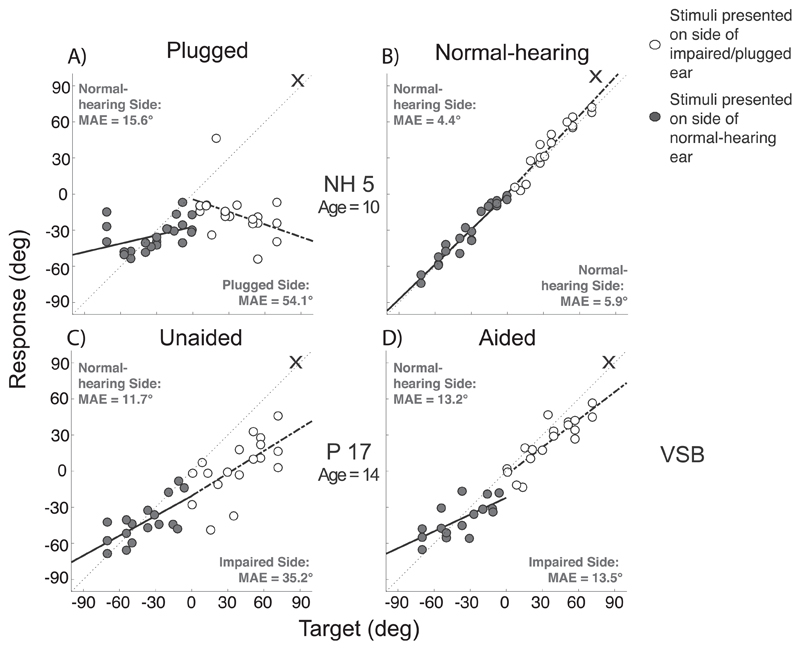
The localization data was further analyzed to investigate sound localization performance on each side independently. Stimuli presented at the side of the normal hearing ear (hearing side) and stimuli presented at the side of the impaired ear (or the plugged side) were analyzed separately. In normal-hearing plugged listeners both sound localization performance of stimuli presented at the side of the open ear as well as stimuli presented at the side of the plugged ear deteriorated (Fig. 5A; listener NH5). The data of patients, however, showed that the localization of stimuli presented at the side of the normal hearing ear was hardly affected when listening with the device on, as compared to listening with the device off. For P17 (VSB) stimuli presented at the hearing side in the unaided condition (Fig. 5C, gray symbols) yields an MAE similar to that in the aided condition (11.7° compared to 13.2°). In contrast, the MAE for stimuli presented at the hearing-impaired side was significant higher in the unaided condition (Fig. 5C, white symbols; 35.2°) as compared to the MAE (Fig. 5D, 13.5°) in the aided condition (i.e. an improvement in localization when listening with the device). The same results were obtained when data was analyzed per sound level (data not shown).
Fig. 5.
Target-response plots of a normal-hearing (NH5) and an UCHL listener (P17). In the right column the aided/normal-hearing condition (B, D) is shown and in the left column the unaided/plugged (A, C) condition. Grey data points represent stimuli presented on the normal-hearing side (left) and white on the impaired/plugged side (right – indicated by cross). Analysis was done for each side separately.
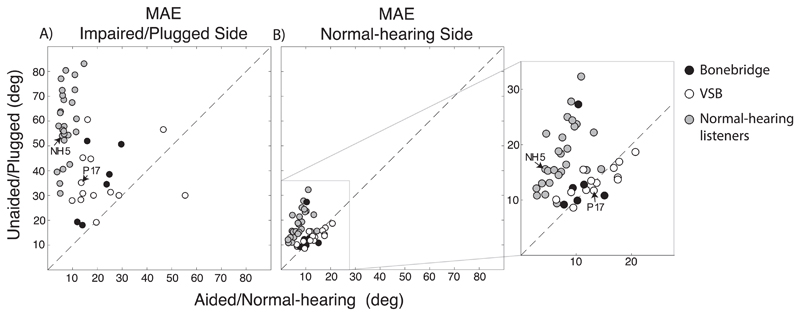
A comparison of the whole pediatric population shows that the responses to sounds presented at the hearing-impaired side are improving (Fig. 6A) while responses to sound presented at the side of the hearing ear are hardly affected when listening with a device. Note that in the unaided condition sound localization on the healthy side was for the majority of patients already quite good, as seen by the small MAE (Fig. 6B, see Table 3). Localization of stimuli presented at the impaired side (Fig. 6A) improved for both listeners with the VSB (paired t-test, p-value = 0.01) and with the Bonebridge (paired t-test, p-value = 0.02) by around 17°. All data points in Fig. 6B are rather close to the diagonal indicating no or minor change in the MAE (mean 2.8°) when listening in the aided compared to the unaided condition. If sound localization is improved by the Bonebridge and VSB based on processing of binaural cues, an improvement would be expected on both sides – stimuli presented at the hearing-impaired as well as the hearing side. Such an expected pattern is only visible in the normal-hearing control listeners (grey data points, MAE values see Table 4), but not in the patient group. A unilateral plug perturbs the processing of ITDs and ILDs, affecting both the localization of stimuli presented at the non-occluded ear (Fig. 6B) and the side of the plugged ear (Fig. 6A).
Fig. 6.
Mean absolute error (MAE) of all patients and normal-hearing listeners for aided condition (x-axis) is plotted against unaided condition for A) impaired/plugged side and B) normal-hearing side. Black data points indicate Bonebridge, white VSB and grey the control listeners. The patient and normal-hearing listener depicted in Fig. 5 are indicated (NH5, P17). In B) a zoomed in window magnifies the location of the data points.
Table 3.
The mean absolute errors (MAE) of all UCHL listeners (except P1 and P3) for stimuli presented on the side of the hearing-impaired ear and on the side of the normal-hearing ear for unaided and aided condition.
| UCHL listener | Side of hearing-impaired ear |
Side of normal-hearing ear |
||
|---|---|---|---|---|
| MAE unaided | MAE aided | MAE unaided | MAE aided | |
| P4 | 19.4 | 11.9 | 9.2 | 7.8 |
| P5 | 38.5 | 24.8 | 9.9 | 10.2 |
| P6 | 34.6 | 23.8 | 12.8 | 11.4 |
| P7 | 52.0 | 15.9 | 27.3 | 10.4 |
| P8 | 50.6 | 29.7 | 12.2 | 9.4 |
| P9 | 18.0 | 14.0 | 10.8 | 15.1 |
| P10 | 30.1 | 18.5 | 13.7 | 17.5 |
| P12 | 30.9 | 14.2 | 8.6 | 9.5 |
| P13 | 45.3 | 14.1 | 11.8 | 11.8 |
| P14 | 44.7 | 17.3 | 18.7 | 20.7 |
| P15 | 27.9 | 9.8 | 10.1 | 6.4 |
| P16 | 60.6 | 16.0 | 14.1 | 17.5 |
| P17 | 35.2 | 13.5 | 11.7 | 13.2 |
| P18 | 28.3 | 13.3 | 15.5 | 11.3 |
| P19 | 30.0 | 28.7 | 11.4 | 9.1 |
| P20 | 30.0 | 55.4 | 15.8 | 16.8 |
| P21 | 56.6 | 46.6 | 16.9 | 17.8 |
| P22 | 19.2 | 19.6 | 13.1 | 13.7 |
| P23 | 31.3 | 25.4 | 13.5 | 12.7 |
UCHL = unilateral conductive hearing loss, MAE = mean absolute error.
Table 4.
The mean absolute errors (MAE) of all control listeners for stimuli presented on the side of the normal-hearing (plugged) ear and on the side of the normal-hearing (non-plugged) ear for normal-hearing and plugged condition.
| Control listener | Side of normal-hearing (plugged) ear |
Side of normal-hearing (non-plugged) ear |
||
|---|---|---|---|---|
| MAE plugged | MAE normal-hearing | MAE plugged | MAE normal-hearing | |
| NH1 | 83.2 | 14.6 | 22.2 | 13.2 |
| NH2 | 80.4 | 7.3 | 19.3 | 8.4 |
| NH3 | 63.8 | 5.2 | 13.1 | 5.2 |
| NH4 | 42.6 | 8.8 | 32.3 | 10.9 |
| NH5 | 54.1 | 5.9 | 15.6 | 4.4 |
| NH6 | 54.4 | 8.3 | 23.7 | 10.1 |
| NH7 | 78.7 | 10.9 | 15.4 | 11.9 |
| NH8 | 52.2 | 6.6 | 21.3 | 7.5 |
| NH9 | 70.4 | 6.0 | 18.4 | 7.2 |
| NH10 | 60.9 | 11.6 | 11.0 | 4.4 |
| NH11 | 67.5 | 9.8 | 15.6 | 14.5 |
| NH12 | 72.6 | 11.0 | 24.4 | 9.3 |
| NH13 | 55.6 | 11.4 | 25.0 | 8.4 |
| NH14 | 68.5 | 6.4 | 23.3 | 9.7 |
| NH15 | 40.6 | 6.2 | 15.3 | 4.9 |
| NH16 | 77.0 | 5.3 | 22.0 | 4.6 |
| NH17 | 58.0 | 4.4 | 15.4 | 6.0 |
| NH18 | 57.8 | 6.6 | 16.4 | 8.2 |
| NH19 | 63.3 | 5.0 | 10.8 | 2.9 |
| NH20 | 54.8 | 6.7 | 15.1 | 7.3 |
| NH21 | 56.5 | 6.0 | 13.0 | 3.8 |
| NH22 | 39.5 | 3.7 | 27.8 | 9.1 |
| NH23 | 34.8 | 4.8 | 9.4 | 6.5 |
| NH24 | 30.9 | 5.1 | 18.8 | 6.8 |
| NH25 | 55.9 | 6.5 | 12.1 | 2.8 |
| NH26 | 72.4 | 5.8 | 14.5 | 6.8 |
MAE = mean absolute error.
3.3. Effect of cross-hearing on sound localization abilities
The Bonebridge stimulate both the ipsilateral and contralateral ear owing to the limited transcranial attenuations of bone-conducted sound propagating through the skull (Stenfelt, 2012). Therefore, cross-stimulation is expected to affect sound localization of Bonebridge listeners, but not that of VSB listeners. The data, however, do not show an effect of cross stimulation on localization performance between the two devices, either overall (Fig. 3, two-sample t-test, MAE/gain: p-value > 0.05), or on the healthy side (Fig. 6B, two-sample t-test, p-value = 0.1). Patients aided with a Bonebridge demonstrate the same improvement as patients aided with a VSB, supporting earlier results comparing a VSB with a percutaneous BCD (Agterberg et al., 2014).
4. Discussion
The present study demonstrates improved sound localization abilities for both patients fitted with a Bonebridge and a VSB. However, some patients showed only a marginal improvement compared to the unaided condition, mainly because of relatively good localization abilities in the unaided condition (For example P9 in Fig. 3).
The most important observation in the present study is that the improved localization when listening with a Bonebridge or VSB, seems not to be based on processing of binaural cues. Both the Bonebridge and the VSB did not affect localization of stimuli presented at the side of the normal-hearing ear (Figs. 5 and 6). Improved localization performance was only observed at the hearing-impaired side. Comparable results have been demonstrated for patients fitted with a Bonebridge recently (Vyskocil et al., 2017).
That binaural hearing is not achieved might be a consequence of the processing time delay, which is > 3 ms in the Bonebridge and VSB. Interaural time differences in the range of 20–600 μs are detectable for normal-hearing listeners and it is not known how a hard-wired system would adapt to these long processing time delays (Portfors and Gersdorff, 2013). Modern devices are often applied in a directional and/or adaptive mode, resulting in inconsistent stimulation, which might limit the success of the devices in terms of spatial hearing. Patients with UCHL, especially children, might need access to constant and reliable cues regarding spatial hearing to benefit maximally from their device. The pediatric population studied here had one normal-hearing ear (PTA < 20 dB HL) and due to insufficient amplification by the Bonebridge or VSB (i.e. hearing asymmetry because of poor aided thresholds) accurate processing of binaural cues is unlikely.
Most listeners with congenital UCHL might have adapted to their hearing impairment while they listened without amplification, as they learned to rely on monaural cues provided by the intact ear (Kumpik et al., 2010; Keating and King, 2013; Keating et al., 2016). In agreement with the present findings the use of monaural cues might lead to reasonable good sound localization abilities in the unaided listening condition (Slattery and Middlebrooks, 1994; Van Wanrooij and Van Opstal, 2007; Agterberg et al., 2014), leaving little room for improvement for such patients when fitted with a hearing device.
Another important aspect is that it is not entirely clear whether or not a critical period exists for the development of binaural hearing in listeners with UCHL. Animal studies have shown that an induced unilateral hearing loss weakens neural representations during development of the affected ear and, therefore, affects binaural integration (Clopton and Silverman, 1977; Brugge et al., 1985; Popescu and Polley, 2010). Hence, there seems to be a consensus that early hearing rehabilitation might be better than later, although later implantation might still be beneficial for patients with congenital UCHL, because the adult brain remains plastic (Keating and King, 2013). Adaptation to induced asymmetric hearing concerning spatial hearing (adaptive cue reweighting) has been found in adults (Kumpik et al., 2010; Irving and Moore, 2011; Keating et al., 2016). However, amplification provided to older (>6y) patients with acquired UCHL most likely holds a greater chance of achieving binaural hearing, because they have a fully maturated auditory system (Moore et al., 1989).
Further, in this study no correlation between patient characteristics and outcome was found. Thus research is needed to investigate the optimal moment of implantation and to investigate whether implantation in patients with acquired UCHL is more successful compared to implantation in patients with congenital UCHL. Whether early implantation results in accurate processing of binaural cues, when processing time delays are eliminated and amplification is optimal, still needs to be studied. However, to be able to fully answer these questions and to formulate clinical guidelines on treatment a larger cohort of UCHL patients is needed, which could be achieved by utilizing uniform experimental protocols in all clinics and research institutes making it possible to pool data.
Acknowledgements
The authors thank Chris-Jan Beerendonk and Ruurd Lof for their technical support. We thank Lara Klunder, Cris Lanting, Rick van Ruler and Ilse Slootweg for testing half of the normal-hearing listeners. This research was funded by: (1) The William Demants og Hustru Ida Emilie's Fond (16-0042). (2) The Donders Institute for Brain, Cognition and Behaviour. (3) The FP7-PEOPLE-2013-ITN Marie Curie Initial Training Network - iCare (FP7-607139). (4) The European Union Horizon-2020 ERC Advanced Grant 2016 - ORIENT (693400).
Abbreviations
- BB
broadband
- BCD
bone-conduction device
- MAE
mean absolute error
- UCHL
unilateral conductive hearing loss
- ILDs
interaural level differences
- ITDs
interaural time differences
- VSB
Vibrant Soundbridge
References
- Agterberg MJH, Snik AFM, Hol MKS, Van Esch TEM, Cremers CWRJ, Van Wanrooij MM, Van Opstal AJ. Improved horizontal directional hearing in bone conduction device users with acquired unilateral conductive hearing loss. J Assoc Res Otolaryngol. 2011;12:1–11. doi: 10.1007/s10162-010-0235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agterberg MJH, Snik AFM, Hol MKS, Van Wanrooij MM, Van Opstal AJ. Contribution of monaural and binaural cues to sound localization in listeners with acquired unilateral conductive hearing loss: improved directional hearing with a bone-conduction device. Hear Res. 2012;286:9–18. doi: 10.1016/j.heares.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Agterberg MJH, Frenzel H, Wollenberg B, Somers T, Cremers CWRJ, Snik AFM. Amplification options in unilateral aural atresia: an active middle ear implant or a bone conduction device? Otol Neurotol. 2014;35:129–135. doi: 10.1097/MAO.0b013e31829b579f. [DOI] [PubMed] [Google Scholar]
- Bess FH, Tharpe AM. Unilateral hearing impairment in children. Pediatrics. 1984;74:206–216. [PubMed] [Google Scholar]
- Brugge JF, Orman SS, Coleman JR, Chan JC, Phillips DP. Binaural interactions in cortical area AI of cats reared with unilateral atresia of the external ear canal. Hear Res. 1985;20:275–287. doi: 10.1016/0378-5955(85)90032-2. [DOI] [PubMed] [Google Scholar]
- Clopton BM, Silverman MS. Plasticity of binaural interaction. II. Critical period and changes in midline response. J Neurophysiol. 1977;40:1275–1280. doi: 10.1152/jn.1977.40.6.1275. [DOI] [PubMed] [Google Scholar]
- Declau F, Cremers C, Van de Heyning P. Diagnosis and management strategies in congenital atresia of the external auditory canal. Br J Audiol. 1999;33:313–327. doi: 10.3109/03005369909090115. [DOI] [PubMed] [Google Scholar]
- Frenzel H, Hanke F, Beltrame M, Steffen A, Schönweiler R, Wollenberg B. Application of the Vibrant Soundbridge to unilateral osseous atresia cases. Laryngoscope. 2009;119:67–74. doi: 10.1002/lary.20036. [DOI] [PubMed] [Google Scholar]
- Frenzel H, Schönweiler R, Hanke F, Steffen A, Wollenberg B. The Lübeck flowchart for functional and aesthetic rehabilitation of aural atresia and microtia. Otol Neurotol. 2012;33:1363–1367. doi: 10.1097/MAO.0b013e3182659adf. [DOI] [PubMed] [Google Scholar]
- Frenzel H, Sprinzl G, Streitberger C, Stark T, Wollenberg B, Wolf-Magele A, Giarbini N, Strenger T, Müller J, Hempel J-M. The Vibrant Soundbridge in children and adolescents: preliminary european mulitcenter results. Otol Neurotol. 2015;36:1216–1222. doi: 10.1097/MAO.0000000000000796. [DOI] [PubMed] [Google Scholar]
- Hassepass F, Bulla S, Aschendorff A, Maier W, Traser L, Steinmetz C, Wesarg T, Arndt S. The Bonebridge as a transcutaneous bone conduction hearing system: preliminary surgical and audiological results in children and adolescents. Eur Arch Oto-Rhino-Laryngol. 2015;272:2235–2241. doi: 10.1007/s00405-014-3137-9. [DOI] [PubMed] [Google Scholar]
- Irving S, Moore DR. Training sound localization in normal hearing listeners with and without a unilateral ear plug. Hear Res. 2011;280:100–108. doi: 10.1016/j.heares.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Keating P, King AJ. Developmental plasticity of spatial hearing following asymmetric hearing loss: context-dependent cue integration and its clinical implications. Front Syst Neurosci. 2013;7:1–20. doi: 10.3389/fnsys.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating P, Rosenior-Patten O, Dahmen JC, Bell O, King AJ. Behavioral training promotes multiple adaptive processes following acute hearing loss. eLife. 2016;5:12264. doi: 10.7554/eLife.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesser BW, Krook K, Gray LC. Impact of unilateral conductive hearing loss due to aural atresia on academic performance in children. Laryngoscope. 2013;123:2270–2275. doi: 10.1002/lary.24055. [DOI] [PubMed] [Google Scholar]
- Kountakis SE. Encyclopedia of Otolaryngology, Head and Neck Surgery. Springer; 2013. pp. 2627–2628. [Google Scholar]
- Kumpik DP, Kacelnik O, King AJ. Adaptive reweighting of auditory localization cues in response to chronic unilateral earplugging in humans. J Neurosci. 2010;30:4883–4894. doi: 10.1523/JNEUROSCI.5488-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst SJ, Leijendeckers JM, Mylanus EA, Hol MKS, Snik AFM, Cremers CWRJ. Bone-anchored hearing aid system application for unilateral congenital conductive hearing impairment: audiometric results. Otol Neurotol. 2008;29:2–7. doi: 10.1097/mao.0b013e31815ee29a. [DOI] [PubMed] [Google Scholar]
- Lieu JEC, Tye-Murray N, Fu Q. Longitudinal study of children with unilateral hearing loss. Laryngoscope. 2012;122:2088–2095. doi: 10.1002/lary.23454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu JEC. Unilateral hearing loss in children: speech-language and school performance. B-ENT. 2013;21:107–115. [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Hutchings ME, King AJ, Kowalchuk NE. Auditory brain stem of the ferret: some effects of rearing with a unilateral ear plug on the cochlea, cochlear nucleus, and projections to the inferior colliculus. J Neurosci. 1989;9:1213–1222. doi: 10.1523/JNEUROSCI.09-04-01213.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadaraja GS, Gurgel RK, Kim J, Chang KW. Hearing outcomes of atresia surgery versus osseointegrated bone conduction device in patients with congenital aural atresia: a systematic review. Otol Neurotol. 2013;34:1394–1399. doi: 10.1097/MAO.0b013e3182a36065. [DOI] [PubMed] [Google Scholar]
- Popescu MV, Polley DB. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron. 2010;65:718–731. doi: 10.1016/j.neuron.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV, Von Gersdorff H. Macrocircuits for sound localization use leaky coincidence detectors and specialized synapses. Neuron. 2013;78:755–757. doi: 10.1016/j.neuron.2013.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahne T, Seiwerth I, Götze G, Heider C, Radetzki F, Herzog M, Plontke SK. Functional results after Bonebridge implantation in adults and children with conductive and mixed hearing loss. Eur Arch Oto-Rhino-Laryngol. 2015;272:3263–3269. doi: 10.1007/s00405-014-3403-x. [DOI] [PubMed] [Google Scholar]
- Sangen A, Royackers L, Desloovere C, Wouters J, van Wieringen A. Single-sided deafness affects language and auditory development - a case-control study. Clin Otolaryngol. 2017;42:979–987. doi: 10.1111/coa.12826. [DOI] [PubMed] [Google Scholar]
- Siegert R, Mattheis S, Kasic J. Fully implantable hearing aids in patients with congenital auricular atresia. Laryngoscope. 2007;117:336–340. doi: 10.1097/MLG.0b013e31802b6561. [DOI] [PubMed] [Google Scholar]
- Slattery WH, 3rd, Middlebrooks JC. Monaural sound localization: acute versus chronic unilateral impairment. Hear Res. 1994;75:38–46. doi: 10.1016/0378-5955(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Snik AFM, Mylanus EAM, Proops DW, Wolfaardt JF, Hodgetts WE, Somers T, Niparko JK, Wazen JJ, Sterkers O, Cremers CWRJ, Tjellström A. Consensus statements on the BAHA system: where do we stand at presents? Ann Otol Rhinol Laryngol. 2005;112:1–12. doi: 10.1177/0003489405114s1201. [DOI] [PubMed] [Google Scholar]
- Snik AFM. Auditory Implants. Where do we Stand at Present. 2018 http://www.snikimplants.nl.
- Stenfelt S. Transcranial attenuation of bone-conducted sound when stimulation is at the mastoid and at the bone conduction hearing aid position. Otol Neurotol. 2012;33:105–114. doi: 10.1097/MAO.0b013e31823e28ab. [DOI] [PubMed] [Google Scholar]
- Van Wanrooij MM, Van Opstal AJ. Sound localization under perturbed binaural hearing sound localization under perturbed binaural hearing. J Neurophysiol. 2007;97:715–726. doi: 10.1152/jn.00260.2006. [DOI] [PubMed] [Google Scholar]
- Van Wieringen A, Boudewyns A, Sangen A, Wouters J, Desloovere C. Unilateral congenital hearing loss in children: challenges and potentials. Hear Res. 2018 doi: 10.1016/j.heares.2018.01.010. (in press) [DOI] [PubMed] [Google Scholar]
- Vyskocil E, Liepins R, Kaider A, Blineder M, Hamzavi S. Sound localization in patients with congenital unilateral conductive hearing loss with a transcutaneous bone conduction implant. Otol Neurotol. 2017;38:318–324. doi: 10.1097/MAO.0000000000001328. [DOI] [PubMed] [Google Scholar]