Abstract
Methyl jasmonate (MeJA) is widely used as a model chemical to study hypersensitive responses to biotic stress impacts in plants. Elevated levels of methyl jasmonate induce jasmonate-dependent defense responses, associated with a decline in primary metabolism and enhancement of secondary metabolism of plants. However, there is no information of how stress resistance of plants, and accordingly the sensitivity to exogenous MeJA can be decreased by endophytic plant growth promoting rhizobacteria (PGPR) harboring ACC (1-aminocyclopropane-1-carboxylate) deaminase. In this study, we estimated stress alleviating potential of endophytic PGPR against MeJA-induced plant perturbations through assessing photosynthetic traits and stress volatile emissions. We used mild (5 mM) to severe (20 mM) MeJA and endophytic plant growth promoting rhizobacteria Burkholderia vietnamiensis CBMB40 and studied how MeJA and B. vietnamiensis treatments influenced temporal changes in photosynthetic characteristics and stress volatile emissions. Separate application of MeJA markedly decreased photosynthetic characteristics and increased lipoxygenase pathway (LOX) volatiles, volatile isoprenoids, saturated aldehydes, lightweight oxygenated compounds (LOC), geranyl-geranyl diphosphate pathway (GGDP) volatiles, and benzenoids. However, MeJA-treated leaves inoculated by endophytic bacteria B. vietnamiensis had substantially increased photosynthetic characteristics and decreased emissions of LOX, volatile isoprenoids and other stress volatiles compared with non-inoculated MeJA treatments, especially at later stages of recovery. In addition, analysis of leaf terpenoid contents demonstrated that several mono- and sesquiterpenes were de novo synthesized upon MeJA and B. vietnamiensis applications. This study demonstrates that foliar application of endophytic bacteria B. vietnamiensis can potentially enhance resistance to biotic stresses and contribute to the maintenance of the integrity of plant metabolic activity
Keywords: Eucalyptus grandis, isoprene, methyl jasmonate, monoterpenes, photosynthesis, plant growth promoting rhizobacteria
1. Introduction
Plants produce an array of volatiles either constitutively or in response to direct impacts of different stresses and in response to airborne stress signals (Niinemets, 2010). Jasmonates (JA) are lipid-derived hormones, acting as key plant growth regulators and plant immunizing agents (Katsir et al., 2008; Lalel et al., 2003). Generally, abiotic and biotic stresses modulate endogenous levels of jasmonic acid and its methylated volatile ester, methyl jasmonate (MeJA) (Jiang et al., 2017a). Elevated levels of MeJA released into the air from plants acts as a signaling cue for triggering jasmonate-dependent defense pathways in undamaged parts of the affected plants or adjacent plants (Heil and Ton, 2008; Jiang et al., 2017a; Tamogami et al., 2008). Activation of jasmonate-triggered signalling will often lead to modulation of the activity of secondary metabolic pathways, including changes in constitutive defences and activation of stress-elicited defences including release of stress volatiles (Repka et al., 2013; Zhang and Xing, 2008).
Time-courses of volatile emissions can be diagnostic clues for estimation of the rate of priming and acclimation responses during and/or after stress-impacts (Kanagendran et al., 2017; Kanagendran et al., 2018a; Niinemets, 2010; Timmusk et al., 2014). Exogenous MeJA treatments often activate volatile biosynthesis pathways, particularly LOX (lipoxygenase), MEP/DOXP (2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate), MVA (mevalonate) and shikimate pathways, eventually leading to enhanced emission rates of LOX pathway volatiles and volatile isoprenoids (Jiang et al., 2017a; Mäntylä et al., 2014; Semiz et al., 2012). In fact, the emission of lipoxygenase pathway volatiles (LOX pathway volatiles, also called green leaf volatiles, GLV) is considered as a characteristic early stress response, followed by the emission of volatile isoprenoids: isoprene, monoterpenes, and sesquiterpenes (Kanagendran et al., 2018b; Kanagendran et al., 2018a). However, after the initial rapid emission, there is a second or third elicitation response corresponding to endogenous activation of JA pathway in 10-30 h since the initial stress impact (Jiang et al., 2017a; Li et al., 2017). These emissions of LOX pathway volatiles do reflect the stress status in the plant and are also involved in attracting herbivore enemies and priming adjacent plants (Copolovici et al., 2014a; Copolovici et al., 2011; Frost et al., 2008).
Generally, biotic stresses result in enhanced emission rates of LOX pathway volatiles, volatile isoprenoids, and volatile benzenoids (Heil and Ton, 2008; Kappers et al., 2010). In biotic stress studies, the emission rates of stress-induced volatiles are often positively correlated with the severity of biotic stress, e.g., with the percentage of leaf area eaten, the degree of fungus and bacterial infection, and the number of feeding larvae. Nevertheless, quantitative scaling of biotic stresses in plants is often difficult due to their complex localized spread and temporal impacts (Copolovici and Niinemets, 2016; Jiang et al., 2017a; Niinemets et al., 2013). Given that MeJA activates jasmonate-dependent defense pathways, exogenous application of MeJA with precisely defined doses is often employed to mimic biotic stress (Jiang et al., 2017a; Shi et al., 2015; Suh et al., 2013). In fact, previous studies revealed that a blend of volatiles induced by MeJA is almost similar to the emission blend elicited by herbivores, including geometrid moth Cabera pusaria feeding on European alder Alnus glutinosa (Copolovici et al., 2011), Spodoptera exigua caterpillars feeding on tomato Solanum lycopersicum (Thaler et al., 2002), gypsy moth (Lymantria dispar) caterpillars attack of blueberry Vaccinium corymbosum (Rodriguez-Saona et al., 2009), herbivores Pieris rapae and Plutella xylostella feeding on cabbage Brassica oleracea (Bruinsma et al., 2009), and pathogens, including infection by leaf rust of Salix spp. (Toome et al., 2010), by oak powdery mildew (Erysiphe alphitoides) of pedunculate oak (Quercus robur) leaves (Copolovici et al., 2014b) (Brüggemann and Schnitzler, 2001), by Ceratocystis fagacearum fungus of live oak (Q. fusiformis) plants (Anderson et al., 2000), by oak gall wasps of Quercus robur leaves (Jiang et al., 2017b) and by Fusarium poae fungus of perennial ryegrass (Lolium perenne) (Pańka et al., 2013). In addition, MeJA response is dose specific and higher doses of MeJA are known to cause hypersensitive reactions and programmed cell death similarly to necrotic lesion formation in response to insect and pathogen elicitors and severe abiotic stresses such as ozone stress (Jiang et al., 2017a; Repka et al., 2013; Zhang and Xing, 2008). Furthermore, similar to real biotic stresses, the volatiles induced by exogenous MeJA applications demonstrated attractiveness to herbivore enemies and repellency to herbivores (Bruinsma et al., 2009; Moraes et al., 2009; Ozawa et al., 2008). Taken together, these reports collectively underscore the biological significance of MeJA as a model for biotic stress impacts and chemical signaling.
In agriculture, there is a variety of strategies employed to enhance plant stress resistance, including breeding for stress resistance, developing transgenic plants, and using plant-growth-promoting-rhizobacteria (PGPR) (Chatterjee et al., 2018a; Koevoets et al., 2016; Mickelbart et al., 2015). Several studies have indicated that application of PGPR successfully alleviated the severity of biotic (Jain and Choudhary, 2014; Kadaikunnan et al., 2015; Nguyen and Kim, 2015) and abiotic stresses (Abd El-Daim et al., 2014; Chatterjee et al., 2017). Therefore, inoculating plants by PGPR is a highly effective, an economically feasible, and a relatively simple approach to cope with many negative effects of stresses in plants (Timmusk et al., 2014; Yang et al., 2009). A vast amount of literature demonstrates a positive impact of PGPR on plant growth, development and enhanced stress tolerance upon seed or root inoculation by PGPR (Abd El-Daim et al., 2014; Figueiredo et al., 2008; Koevoets et al., 2016; Lee et al., 2006; Onofre-Lemus et al., 2009; Timmusk et al., 2014; Yang et al., 2009; Zahir et al., 2003), but there is a scarcity of information about the changes in stress resistance due to inoculation of leaves by PGPR. Very few studies have demonstrated a significant outcome in plant growth and development in response to foliage inoculation by PGPR (Esitken et al., 2002; Esitken et al., 2003; Esitken et al., 2006; Esitken et al., 2010).
So far, there is no clear-cut mechanism of how PGPR can affect MeJA-induced plant stress responses. However, there are many explanations emphasizing the possible mechanisms of stress resistance enhancement by PGPR, including: (1) PGPR biosynthesize 1-amino cyclopropane-1-carboxylate deaminase (ACC deaminase) that catalyzes the formation of α-ketobutyrate and ammonia from ethylene precursor; as the result, the hypersensitive ethylene-induced stress response was curbed (Chatterjee et al., 2018a; Chatterjee et al., 2017; Chatterjee et al., 2018b); (2) PGPR quantitatively increased ROS-scavenging enzymes and in turn, alleviated the effects of oxidative stress in plants (Mastouri et al., 2012; Wang et al., 2012); (3) inoculation by PGPR led to enhanced biosynthesis of plant growth regulators IAA, gibberellic acid, cytokinins, and auxin, ultimately augmenting primary and secondary metabolism in plants (Figueiredo et al., 2008); and (4) PGPR biosynthesized certain antioxidants, i.e catalase, that increased induced-systemic resistance in plants (Yang et al., 2009). There is very limited information about the impact of PGPR on photosynthetic traits and volatile emission responses in plants under stress (Chatterjee et al., 2018a). Therefore, the main goal of this work was to estimate the stress tolerance potential of endophytic PGPR in response to foliage inoculation through assessing volatile emissions and photosynthetic traits.
In the present study, we used flooded gum (Eucalyptus grandis), a model plant frequently used in isoprenoid studies (Henery et al., 2008; Myburg et al., 2014; Oates et al., 2015). Eucalyptus species and subspecies are endemic to Australian continent, but many Eucalyptus spp., including E. grandis, are extensively grown in tropical, subtropical, and Mediterranean and warm temperate climates due to their high adaptability and rapid growth rate in a wide range of climatic conditions and soil types (Külheim et al., 2015). In addition, eucalypts are strong constitutive-isoprenoid emitters and therefore often used as potential model species in volatile isoprenoid emission studies (Funk et al., 2006; Guenther et al., 1991; He et al., 2000; Kanagendran et al., 2018a; Street et al., 1997; Winters et al., 2009). A distinctive morphological feature of eucalypt leaves is the presence of numerous and relatively large sub-dermal secretory cavities (~200 μm diameter) and oil-glands, where volatile and non-volatile terpenoids are stored (Goodger et al., 2016; Külheim et al., 2015). As a common phenomenon in terpene-storing plant species including eucalypts, terpenes are slowly diffusing out of storage pools in a temperature-dependent manner (Grote and Niinemets, 2008; Kanagendran et al., 2018a). In addition, several storage emitters also synthesize mono- and sesquiterpenes in a light and temperature-dependent manner in mesophyll cells either constitutively or during stress (Grote et al., 2013; Holzke et al., 2006). Ultimately, the sum of storage and de novo emissions of mono- and sesquiterpenes makes up the total volatile emission blend of a given species under given set of environmental conditions (Copolovici and Niinemets, 2016). However, there is still a lack of information about the regulation volatile emissions upon biotic and abiotic stresses in eucalypts.
In this study, we used Burkholderia vietnamiensis CBMB40 (GenBank Accession No: AY683043) as plant growth promoting rhizobacteria. Burkholderia vietnamiensis CBMB40 is a methylotrophic bacterium and an endophytic and nitrogen fixing strain (Madhaiyan et al., 2008). In addition, B. vietnamiensis CBMB40 biosynthesizes indole-3-acetic acid that enhances photosynthetic characteristics in plants (Aldesuquy, 2000). Furthermore, B. vietnamiensis CBMB40 produces ACC deaminase and also expresses a high antagonistic activity against biotic stress caused by phytopathogen Erwinia caratovora subsp. caratovora in plants (Madhaiyan et al., 2008). Our primary study indicated that B. vietnamiensis CBMB40 can grow successfully in a medium containing higher MeJA doses. Furthermore, several Burkholderia species have developed strong beneficial interactions with plants and colonize roots, stems and even leaves (Coenye and Vandamme, 2003; Estrada-De Los Santos et al., 2001).
We applied precisely defined doses (0-20 mM) of MeJA solution to B. vietnamiensis inoculated and un-inoculated leaves of E. grandis and studied time-dependent variations in photosynthetic traits and different classes of stress volatile emission rates after treatments. In addition, we analyzed terpenoid content and composition in untreated E. grandis leaves to estimate the storage capacity for foliage terpenes and differentiate stored and de novo synthesized terpenoids. We soughed to study (1) whether foliage inoculation by endophytic bacteria B. vietnamiensis enhances photosynthetic traits upon subsequent MeJA treatments; (2) how foliage inoculation by B. vietnamiensis impacts foliage volatile emissions in response to subsequent treatments of MeJA; and (3) how bacterial inoculation alters the relationship between photosynthetic traits and volatile emission throughout the stressed-period upon MeJA treatments in inoculated and non-inoculated leaves of E. grandis. We hypothesized that (1) exogenous application of MeJA reduces photosynthetic traits and the degree of reduction scales with MeJA dose; (2) exogenous application of MeJA immediately elicits stress volatile emissions, followed by elicitation of specific isoprenoid and benzenoid emissions and after reaching the maximum values, the emissions decline after stress applications; (3) foliage inoculation by B. vietnamiensis enhances photosynthetic traits and decreases volatile emission rates; and (4) changes in photosynthetic traits and volatile emission upon MeJA treatments in bacteria-inoculated leaves are less than those in non-inoculated leaves, suggesting that B. vietnamiensis inoculation protects plant cells from subsequent oxidative stress. We know of no studies investigating the impact of foliar inoculation of symbiotic methylotrophic bacteria on plant volatiles, and changes in simulated biotic stress.
2. Materials and Methods
2.1. Plants and growth conditions
Three-year-old eucalypt (E. grandis) plants were used in this experiment. The plants were grown in 10 L pots filled with commercial potting mixture (Biolan Oy, Kekkilä group, Finland) and supplemented with essential macronutrients N (100 mg L-1), P (30 mg L-1), and K (200 mg L-1) and micronutrients. The soil water pH in the pots was 6.2. The plants were grown in a plant growth room under the controlled environmental conditions of light intensity of 300 - 400 μmol m-2 s-1 (HPI-T Plus 400 W metal halide lamps, Philips) provided for 12 h photoperiod, day/night temperature of 28/25 °C, ambient CO2 concentration of 380-400 ppm, and relative air humidity of 60-70%. Plants were regularly watered to soil field capacity. Plants were fertilized once a week with a liquid fertilizer (Baltic Agro, Lithuania). The fertilizer included NPK (N: P2O5: K2O - 5:5:6), and micronutrients B (0.01%), Cu (0.03%), Fe (0.06%), Mn (0.028%), and Zn (0.007%), and at each time, each plant was fertilized with 80 mL diluted liquid fertilizer (ca. 0.4% solution) for the optimum plant growth.
2.2. Bacterial strain, culture conditions, media and foliage applications
Burkholderia vietnamiensis CBMB40 was grown in an ammonium mineral salt (AMS) medium supplemented with 0.5 % sodium succinate as a sole carbon source, according to the protocol of Lee et al. (2006). The bacterial culture was allowed to grow up to mid exponential phase in AMS broth, then the culture was centrifuged at 5000 rpm for 5 min, the cell pellets were washed twice, and finally the bacterial suspension was prepared in 0.03 M MgSO4. Our preliminary study indicated that B. vietnamiensis CBMB40 successfully grew in the growth medium that contained up to 50 mM MeJA. The bacterial inoculation in E. grandis leaves was carried out as a foliar spray on both sides of the leaves. One ml of bacterial solution was applied to each leaf. In the case of combined application of bacteria and MeJA (bacteria and MeJA), first the leaves were sprayed with bacteria once in a day for four consecutive days. Thereafter, the plants with leaves inoculated with bacteria were kept in a controlled growth room for 10 days for optimum growth of bacteria and then MeJA applications were carried out.
2.3. MeJA application protocol
A variety of protocols have been used for the preparation and application of MeJA on plants (Heijari et al., 2005; Jiang et al., 2017a; Liang et al., 2006; Loivamäki et al., 2004). A comparison of various protocols suggested that the use of aqueous ethanol (5-20%) as a solvent for MeJA (Sigma-Aldrich, St Louis, MO, USA) was the most suitable method as it was associated with negligible non-specific effects in control (aqueous alcohol) treatments (Jiang et al., 2017a). In our study with thick highly hydrophobic leaves of eucalypt, we used MeJA dissolved in 20% ethanol solution. As in the study of Jiang et al. (2017a), there were no significant differences in photosynthetic characteristics and volatile emissions between the untreated leaves and the leaves treated with 20% ethanol. To construct MeJA dose responses, the following concentrations of 1 ml of MeJA solution were sprayed on both sides of each individual leaf: 0 (control), 5, 10, and 20 mM MeJA. In all cases, a randomly selected branch of the same age and similar biomass, consisting of three fully mature leaves was selected for MeJA treatments, and each different MeJA dose treatment was applied to three individual plants. In the case of combined application of bacteria and MeJA, first the leaves were inoculated with bacteria as described above and then, MeJA was applied as for the treatments with MeJA alone. Immediately after treatments, the plants were kept in the plant growth room under controlled environmental conditions (see “Plants and growth conditions” for details).
2.4. Description of the gas-exchange system and measurement set-up
A custom-made cylindrical glass chamber (1.2 L) was used to measure gas exchange and volatile emission rates. The temperature of the glass chamber was maintained constant at 25 °C by circulating distilled water of 25 °C between the double layers of the glass chamber. The measurement light was provided by four halogen lamps, and its intensity was controlled at 700 - 800 μmol m-2 s-1 at the leaf surface by a dimming regulator. The leaf temperature varied between 27 - 29 °C. The measurement air was taken from outside and purified by passing through a charcoal-filter and a custom-made ozone trap, and humidified by a custom-made humidifier. During the measurements, the ambient CO2 concentration was 390 - 400 ppm and the relative air humidity 60 - 70 % in the chamber. The air temperature inside the glass chamber was measured by a thermistor (NTC thermistor, model ACC-001, RTI Electronics, Inc., St. Anaheim, CA, USA) and the leaf temperature was monitored by a thermocouple. A fan (Sunon Group, Beijing, China) inside the chamber provided high air turbulence, resulting in high leaf boundary layer conductance with no still air pockets. The bottom of the chamber had an opening for the leaf petiole. After the enclosure of the leaf, the chamber opening was sealed with low-emitting modelling clay to ensure there was no air leakage from or into the chamber. All chamber connections were made of stainless steel and all tubing was made of Teflon® to assure low adsorption of volatile on the system surfaces. Reference and measurement modes at the chamber-inlet and outlet ports were used to take gas exchange measurements and volatile sampling.
CO2 and H2O concentrations at reference (ingoing air) and measurement (outgoing air) modes were estimated by an infrared dual-channel gas analyzer (CIRAS II, PP-systems, Amesbury, MA, USA). All measurements were taken when stomata opened and steady chamber CO2 and H2O concentrations were achieved, ca. 15-20 min. after leaf enclosure and then volatiles were collected as explained below. Foliage net assimilation rate (A) and stomatal conductance to water vapor (gs) per projected unit leaf-area were estimated according to von Caemmerer and Farquhar (1981). A detail description of the similar glass chamber set up can be found in Kanagendran et al. (2018a) and Copolovici and Niinemets (2010).
In the case of bacterial inoculation alone, the gas exchange and volatile measurements taken at days 1, 3, and 7 referred to the number of days after the last bacterial inoculations were carried out, whereas in the case of separate MeJA, and combined bacterial and MeJA treatments, the gas exchange and volatile measurements taken at days 1, 3, and 7 referred to the number of days after MeJA treatments were carried out.
2.5. VOC collection and analysis by gas-chromatography/mass spectrometry
Volatiles emitted by the leaves were collected onto stainless steel adsorbent cartridges, filled with three Carbotrap adsorbents with different surface area and mesh size. The three different types of adsorbent materials are carbotrap C 20-40 mesh, carbopack B 40-60 mesh, and carbotrap X 20-40 mesh (Kännaste et al., 2014). An air sample of four liters was sampled through the chamber-outlet onto the adsorbent cartridges, using an air sample portable pump 210-1003MTX (SKC Inc., Houston, TX, USA) operated at a constant suction rate of 0.2 l min-1 for 20 minutes. The cartridges were analyzed for VOC in gas chromatography-mass spectrometry (GC-MS) (Shimadzu TD20 automated cartridge desorber and Shimadzu 2010 Plus GC-MS instrument, Shimadzu Corporation, Kyoto, Japan), with a Zebron ZB-624 fused silica capillary column (0.32 mm i.d., 60 m length, 1.8 μm film thickness, Phenomenex, Torrance, CA, USA).
A two-step desorption method was employed to analyze the stainless steel cartridges in GC-MS. In the first step, He purge flow was set to 40 ml / min, primary desorption temperature to 250 °C, and primary desorption time to 6 min. In the second step, a trap temperature during primary desorption was set to -20 °C, and the second stage trap desorption temperature was set to 280 °C and the withhold time of 6 min (Kännaste et al., 2014).
GC oven program used for separation of the volatile compounds was as follows: 40 °C for 1 min, 9 °C / min to 120 °C, 2 °C / min to 190 °C, 20 °C / min to 250 °C, and eventually maintaining for 250 °C for 5 min. The mass spectrometer of GC-MS instrument was operated in electron-impact mode (ei) at 70 eV in the scan range of m/z 30 - 400. The transfer line temperature was set to 240 °C and ion-source temperature was set to 150 °C. The cartridges were analyzed for qualitative and quantitative estimation of LOX pathway volatiles, volatile isoprenoids, saturated aldehydes, lightweight oxygenated volatile organic compounds (LOC), geranyl-geranyl diphosphate pathway volatiles (GGDP volatiles), and benzenoids. Compounds were identified by mass spectra of national institute of standards and technology (NIST 05) library based on retention time identity and confirmed with the authentic standards (GC purity) (Kännaste et al., 2014). The emission rates of individual volatiles and total emissions for different volatile compound classes per unit leaf area were estimated according to the following equation (Liu et al., 2018; Niinemets et al., 2011).
In this equation, φe is the emission rate of a VOC (nmol m-2 s-1); Pi is its peak area in the chromatogram, F is the rate of air flow (1.4 L min-1) in the glass chamber; C is the calibration factor (nmol-1) calculated by dividing peak area in the chromatogram by the amount of a standard compound for given VOC; S is the enclosed leaf area (m2) in the chamber; and V is the volume (L) of gas collected onto cartridges during 20-min collection. The volatile measurements in the empty chamber were also carried out to estimate the net foliage emission rate of volatiles per unit leaf area. A detailed description of the adsorbent cartridges, and the method of volatile collection and GC-MS analysis is provided in Toome et al. (2010) and Kännaste et al. (2014).
2.6. Measurement of foliage chlorophyll fluorescence and estimation of leaf area
Upon completion of gas-exchange measurements and VOC collection, leaves were darkened for 10 min and dark-adapted maximum chlorophyll fluorescence yield (Fv/Fm) was estimated with an imaging PAM flourometer (Walz IMAG-MIN/B, Walz GmbH, Effeltrich, Germany). First, the minimum fluorescence yield (Fo) was estimated, followed by the application of a 1 s saturating pulse (7000 μmol m-2 s-1) blue light (460 nm) to asses Fm. After these measurements, the leaves were scanned at 300 dpi to estimate the leaf area using a custom-made software tool.
2.7. Quantitative estimation of foliage terpene contents
Terpene contents were quantitatively estimated for untreated leaves of E. grandis after hexane extraction. For the extraction, six fresh leaf samples (120 ± 30 mg) were randomly taken from six mature leaves of individual plants. The samples were transferred in 2 ml tubes with 2.8 mm stainless-steel beads (Bertin Technologies, Aix-en-Provence, France). Tubes containing fresh leaf samples were immediately frozen in liquid N2 and then 1.5 ml GC-grade hexane (Sigma-Aldrich, St. Louis, MO, USA) was added to each tube. The samples were homogenized with a homogenizer (Precellys 24, Bertin Technologies, Aix-en-Provence, France) at 2400 g for 30 seconds at 25°C. Thereafter, the homogenized mixture was incubated in a Thermo-Shaker (TS-100C, Biosan, Riga, Latvia) for 3 h at 1000 rpm at 25°C. After incubation, samples were centrifuged (Universal 320R, Hettich, Tuttlingen, Germany) for 30 sec at 13200 g at 25°C. One point five millilitre of supernatant was transferred to a 2 ml GC-MS glass vial (Sigma-Aldrich, St. Louis, MO, USA) for further GC-MS analysis. One microlitre of sample was injected into GC-MS. The quantitative estimation of foliage terpene contents was estimated as follows,
In this equation, φm is the foliage terpene content (nmol g-1DM), Pi is the peak area of given compound in a chromatogram, C is the calibration factor of a compound (nmol/ peak area), Vf is the final volume (μl) of supernatant in GC-MS vial, m is the dry mass (g) of a fresh leaf sample, Vi is the injected volume (μl) of sample into GC-MS.
2.8. Data analysis
In this study, there were four replicates for control and bacterial treatments, and three replicates for separate MeJA, and combined bacteria and MeJA treatments. All data were checked for normality of distributions and homogeneity of variances according to Shapiro-Wilk and Levene's tests. When appropriate, the data were log-transformed to comply with normality and homoscedasticity assumptions for parametric tests. Generalized Linear Models (GLM) with maximum likelihood model fitting were used to estimate individual and interactive effect of MeJA, bacteria, and time (number of days after treatments) on photosynthetic characteristics, and volatile emission rates. GLM were conducted with SPSS v.24 (IBM SPSS, Chicago, IL, USA). Principal Component Analysis (PCA) was conducted with RStudio (R version 3.5.1) using the package of ‘Factoextra’ (Alboukadel and Fabian, 2017) to test for the efficiency of bacterial and MeJA treatments on VOC emissions. In addition, we performed a partial least squares discriminant analysis (PLS-DA) using the package of ‘DiscriMiner’ (Gaston, 2013) in Rstudio (R version 3.5.1) for various volatile blends upon different treatments at 1, 3, and 7-days after treatments, to identify which VOC-class primarily contributed to the discrimination between treatments using the first two partial least square (PLS) components. All plots were done with SigmaPlot version 12.5 (Systat Software Inc, San Jose, CA, USA), except for PCA and PLS-DA plots that were made with Rstudio. In all cases, the effects were statistically significant at P < 0.05.
3. Results
3.1. Impact of MeJA and bacterial treatments on time-courses of foliage photosynthetic characteristics
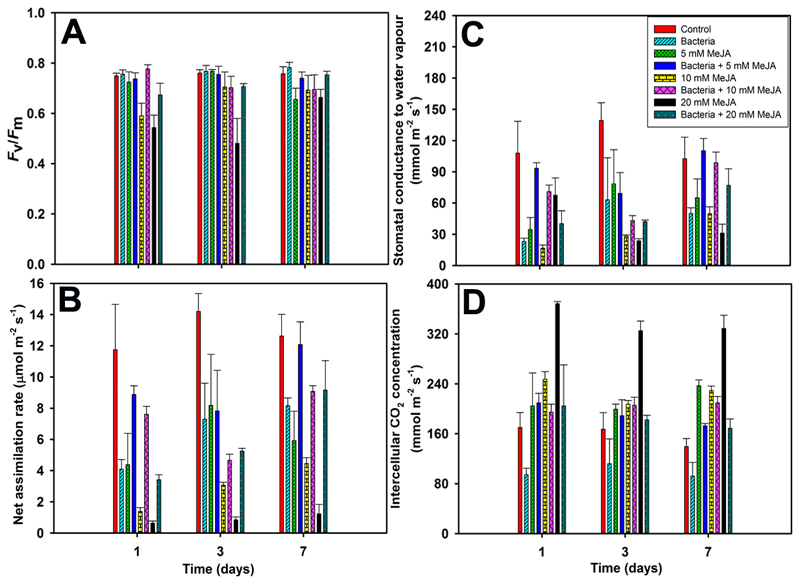
The dark-adapted photosystem II (PS II) quantum yield estimated by chlorophyll fluorescence (Fv/Fm) decreased in MeJA-treated leaves, especially in leaves treated with 10 and 20 mM MeJA (Table 1, Fig. 1A). However, Fv/Fm was not significantly affected by bacterial treatment and by the combined bacteria and MeJA treatments, albeit there was a tendency of lower Fv/Fm in 20 mM bacteria and MeJA treated leaves (Table 1, Fig. 1A). Fv/Fm was fully recovered by day 7 for 10 mM MeJA treatment, but not for 5 mM and 20 mM treatment (Fig. 1A, Table 1).
Table 1.
Output of generalized linear model (GLM) for individual and interactive effects of methyl jasmonate (MeJA) application and bacterial inoculation, and bacteria and MeJA treatments, and effects of time after treatments (1, 3, and 7 days after treatment) on dark-adapted (10-minute darkening) maximum quantum yield of photosystem II (PSII) estimated by chlorophyll fluorescence (Fv/Fm), foliage net assimilation rate, and stomatal conductance to water vapor, and intercellular CO2 concentration in E. grandis leaves. Stress application protocol and the baseline environmental conditions during measurements as in Fig. 1. P values show the significance of wald chi-square (χ2) statistics.
| Physiological characteristics | Effects | |||
|---|---|---|---|---|
| χ2 | df | P value | ||
| Fv/Fm | MeJA | 65.113 | 1 | 0.000*** |
| Bacteria | 1.498 | 1 | 0.221 | |
| Bacteria + MeJA | 3.880 | 1 | 0.049* | |
| Time | 1.757 | 1 | 0.185 | |
| MeJA x Time | 7.261 | 1 | 0.007** | |
| Bacteria x Time | 0.436 | 1 | 0.509 | |
| (Bacteria + MeJA) x Time | 1.879 | 1 | 0.170 | |
| Net assimilation rate (A) | MeJA | 167.047 | 1 | 0.000*** |
| Bacteria | 6.433 | 1 | 0.011* | |
| Bacteria + MeJA | 21.395 | 1 | 0.000*** | |
| Time | 12.813 | 1 | 0.000*** | |
| MeJA x Time | 0.117 | 1 | 0.732 | |
| Bacteria x Time | 0.044 | 1 | 0.833 | |
| (Bacteria + MeJA) x Time | 0.292 | 1 | 0.589 | |
| Stomatal conductance to water vapor (gs) | MeJA | 19.723 | 1 | 0.000*** |
| Bacteria | 16.649 | 1 | 0.000*** | |
| Bacteria + MeJA | 7.860 | 1 | 0.005** | |
| Time | 9.792 | 1 | 0.002** | |
| MeJA x Time | 3.136 | 1 | 0.077 | |
| Bacteria x Time | 0.135 | 1 | 0.713 | |
| (Bacteria + MeJA) x Time | 0.002 | 1 | 0.964 | |
| Intercellular CO2 concentration (Ci) | MeJA | 67.413 | 1 | 0.000*** |
| Bacteria | 72.504 | 1 | 0.000*** | |
| Bacteria + MeJA | 0.066 | 1 | 0.797 | |
| Time | 0.295 | 1 | 0.587 | |
| MeJA x Time | 0.000 | 1 | 0.991 | |
| Bacteria x Time | 0.004 | 1 | 0.948 | |
| (Bacteria + MeJA) x Time | 0.000 | 1 | 0.998 | |
Figure 1.
Representative time-courses (mean + SE) of dark-adapted maximum quantum yield of photosystem II (PS II) estimated by chlorophyll fluorescence (Fv/Fm: A), net assimilation rate (A: B), stomatal conductance to water vapor (gs: C), and intercellular CO2 concentration (Ci: D) of control, endophytic bacteria-inoculated (Burkholderia vietnamiensis CBMB40), MeJA treated (5, 10, and 20 mM MeJA in 20% ethanol), and bacteria and MeJA treated leaves (first inoculated with bacteria, followed by 5, 10, and 20 mM MeJA treatments) at 1, 3, and 7 days after treatments in Eucalyputs grandis. The bacterial inoculation of E. grandis leaves was carried out as foliar spray on both sides of the leaves for four consecutive days. In the case of combined application of MeJA and bacteria, first the leaves were sprayed with bacteria once a day for four consecutive days and then the plants with selected leaves inoculated with bacteria were kept in a controlled growth room for 10 days for optimum growth of bacteria in the leaves before MeJA applications. In fact, there were no significant temporal changes in photosynthetic characteristics between the leaves treated with 20% ethanol and non-treated leaves. In this experiment, mature leaves of three-year-old eucalypt plants were used. Photosynthetic measurements were conducted upon establishing a stable gas flow in the glass chamber. The baseline environmental conditions during measurements were: PPFD of 700-800 μmol m-2 s-1, leaf temperature of 27 - 29 °C, ambient CO2 concentration of 390-400 μmol mol-1, and relative air humidity of 60-70 %. In this study, there were four replicates for control and bacterial treatments, and all other treatments were replicated thrice. The details of statistical analysis as in Table 1.
Bacterial treatment alone resulted in reduced net assimilation rate (A) and stomatal conductance (gs) at day 1, but both traits slightly increased at days 3 and 7 (Fig. 1B and 1C, Table 1). Similarly, A and gs in response to separate MeJA and combination of bacterial and MeJA treatments were slightly increased at days 3 and 7 (P < 0.01, Table 1); however, a sustained decline in both physiological traits was observed for separate MeJA treatments, compared to combined bacterial and MeJA treatments (Fig. 1B and 1C). Overall, the reduction was particularly large at day 1 after the MeJA treatment and then a partial recovery was observed (Fig 1B and 1C, Table 1). In all cases, changes in A and gs were highly correlated throughout the stressed-phase. However, in bacterial treatment alone, intercellular CO2 concentration (Ci) was reduced, but for MeJA treatments, Ci was increased (P < 0.001, Table 1 for separate bacterial and MeJA treatments). In fact, Ci was invariable for bacterial and MeJA treatments (Fig. 1D, P < 0.5, Table 1).
3.2. Temporal changes in constitutive isoprene emissions in dependence on MeJA and bacterial treatments
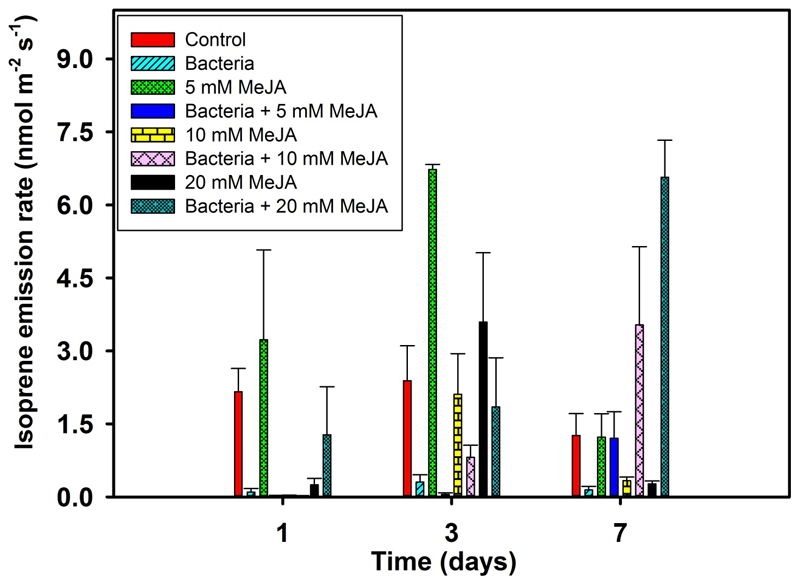
In control leaves, the baseline emission rate of isoprene varied between 1.3 - 2.4 nmol m-2 s-1 (Fig. 2, Table 2). However, surprisingly, isoprene emission was suppressed by bacterial treatment alone (P < 0.001, Table 2), but it was initially elicited to the higher level when the leaves were treated with MeJA alone and with bacterial and MeJA treatments (Fig. 2, Table 2). The highest elicitation of isoprene emission rate varied with the type of treatments; it was at day 3 for separate MeJA treatments and at day 7 for combined bacterial and MeJA treatments (Fig. 2).
Figure 2.
Temporal variation (means + SE) in isoprene emission rate from E. grandis leaves in response to control, bacterial inoculation, MeJA treatments (5, 10, and 20 mM), and bacterial and MeJA treatments (first inoculated with bacteria, followed by 5, 10, and 20 mM MeJA treatments) at 1, 3, and 7 days after treatments. Details of treatments and replications, and standard environmental conditions during measurements as in Fig. 1. Details of statistical analysis as in Table 2.
Table 2.
Output of generalized linear model for individual and interactive effects of MeJA, bacteria, bacteria and MeJA, and time (1, 3, and 7 days after treatment) after treatments on foliage isoprene emission rate in E. grandis. Stress application protocol and the baseline environmental conditions during measurements as in Fig. 1. P values show the significance of wald chi-square (χ2) statistics.
| Compounds | Effects | |||
|---|---|---|---|---|
| χ2 | df | P value | ||
| Isoprene | MeJA | 0.119 | 1 | 0.730 |
| Bacteria | 15.696 | 1 | 0.000*** | |
| Bacteria + MeJA | 0.159 | 1 | 0.690 | |
| Time | 7.573 | 1 | 0.006** | |
| MeJA x Time | 1.369 | 1 | 0.242 | |
| Bacteria x Time | 1.337 | 1 | 0.248 | |
| (Bacteria + MeJA) x Time | 3.002 | 1 | 0.083 | |
3.3. Temporal changes of individual LOX pathway volatiles in response to MeJA and bacterial treatments
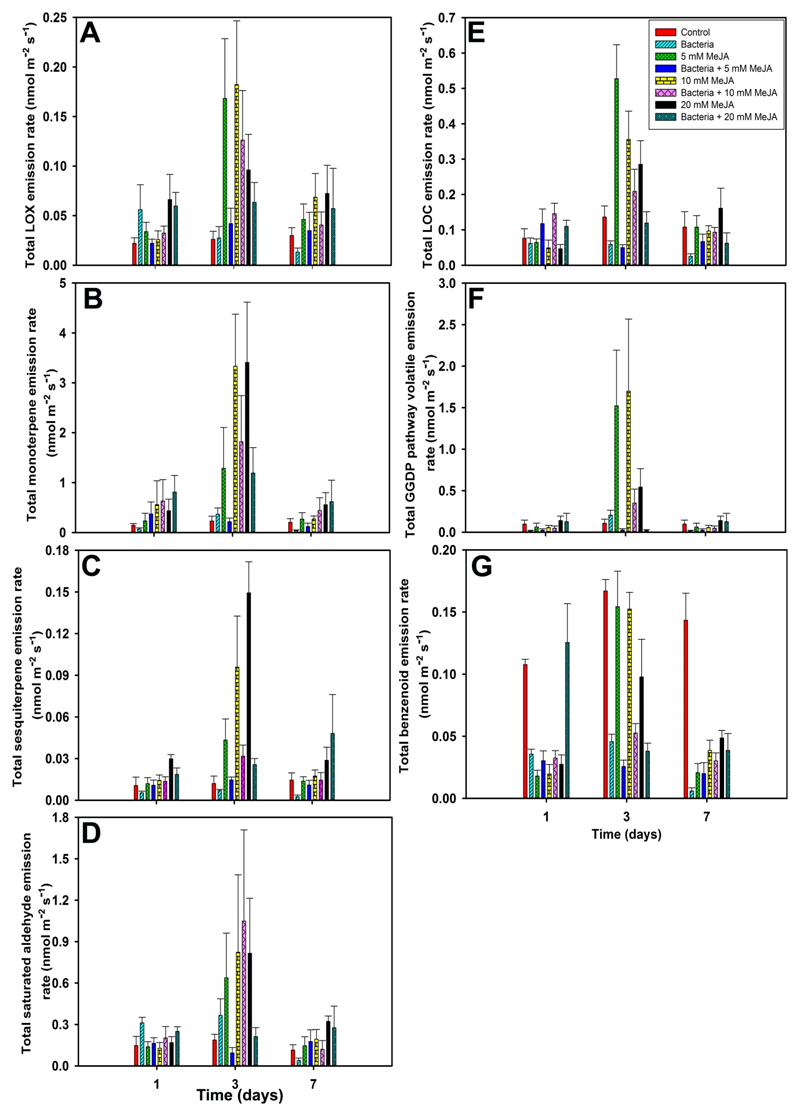
The emission rate of total LOX pathway volatiles in control leaves was in the range of 0.02-0.03 nmol m-2 s-1 (Fig. 3A). However, bacterial inoculation increased total LOX volatile emission at day 1 and then it started decreasing in a time-dependent manner at day 3 and day 7 (Fig. 3A). In addition, the emission rate of total LOX pathway volatiles was exponentially enhanced when the leaves were subjected to separate MeJA (P < 0.001, Table 3), followed by combined bacterial and MeJA applications. At day 1 and 7, total LOX pathway volatile emission scaled positively with MeJA dose (Fig. 3A). Generally, the emission rates of total LOX pathway volatiles were higher at day 3 after stress applications (ca. 5-fold increment for separate MeJA treatments and ca. 2-fold increment for combined bacterial and MeJA treatments, compared to controls), where the highest emission was observed in response to 10 mM MeJA treatment. A greater share of total LOX emission blend remained almost higher at all times after stress application due to the sustained emission rates of hexanal and pentanal (Fig. 3A and Fig. S1).
Figure 3.
Temporal variation in emission rates (Mean + SE) of total lipoxygenase pathway (LOX pathway or GLV, green leaf volatiles) volatiles (A), monoterpenes (B), sesquiterpenes (C), saturated aldehydes (D), light weight oxygenated compounds (LOC) (E), geranyl geranyl diphosphate (GGDP) pathway volatiles (F), and benenoids (G) from E. grandis leaves response to control, bacterial inoculation, MeJA treatments (5, 10, and 20 mM), and bacterial and MeJA treatments (first inoculated with bacteria, followed by 5, 10, and 20 mM MeJA treatments) at 1, 3, and 7 days after treatments. Details of treatments and replications, and standard environmental conditions during measurements as in Fig. 1. Details of statistical analysis as in Table 3.
Table 3.
Output of generalized linear model for individual and interactive effects of MeJA, bacteria, MeJA and bacteria, and time (1, 3, and 7 days after treatment) after treatments on total volatile emission rates of various volatile classes emitted by E. grandis leaves. Stress application protocol and the baseline environmental conditions during measurements as in Fig. 1. P values show the significance of wald chi-square (χ2) statistics.
| Compounds | Effects | |||
|---|---|---|---|---|
| χ2 | df | P value | ||
| Total LOX volatiles | MeJA | 11.559 | 1 | 0.001*** |
| Bacteria | 2.892 | 1 | 0.089 | |
| Bacteria + MeJA | 3.022 | 1 | 0.082 | |
| Time | 0.754 | 1 | 0.385 | |
| MeJA x Time | 1.270 | 1 | 0.260 | |
| Bacteria x Time | 7.431 | 1 | 0.006** | |
| (Bacteria + MeJA) x Time | 2.169 | 1 | 0.141 | |
| Total monoterpenes | MeJA | 12.588 | 1 | 0.000*** |
| Bacteria | 14.183 | 1 | 0.000*** | |
| Bacteria + MeJA | 12.745 | 1 | 0.000*** | |
| Time | 3.718 | 1 | 0.050* | |
| MeJA x Time | 0.264 | 1 | 0.607 | |
| Bacteria x Time | 0.206 | 1 | 0.650 | |
| (Bacteria + MeJA) x Time | 0.476 | 1 | 0.490 | |
| Total sesquiterpenes | MeJA | 49.998 | 1 | 0.000*** |
| Bacteria | 29.431 | 1 | 0.000*** | |
| Bacteria + MeJA | 12.322 | 1 | 0.000*** | |
| Time | 0.221 | 1 | 0.639 | |
| MeJA x Time | 0.485 | 1 | 0.486 | |
| Bacteria x Time | 5.482 | 1 | 0.019* | |
| (Bacteria + MeJA) x Time | 0.030 | 1 | 0.863 | |
| Total saturated aldehydes | MeJA | 6.391 | 1 | 0.011* |
| Bacteria | 0.023 | 1 | 0.878 | |
| Bacteria + MeJA | 2.191 | 1 | 0.139 | |
| Time | 6.186 | 1 | 0.013* | |
| MeJA x Time | 4.719 | 1 | 0.030* | |
| Bacteria x Time | 9.702 | 1 | 0.002** | |
| (Bacteria + MeJA) x Time | 0.074 | 1 | 0.786 | |
| Total light-weight oxygenated volatiles (LOC) | MeJA | 4.239 | 1 | 0.040* |
| Bacteria | 12.426 | 1 | 0.000*** | |
| Bacteria + MeJA | 0.051 | 1 | 0.821 | |
| Time | 0.608 | 1 | 0.436 | |
| MeJA x Time | 6.668 | 1 | 0.010** | |
| Bacteria x Time | 1.669 | 1 | 0.196 | |
| (Bacteria + MeJA) x Time | 1.758 | 1 | 0.185 | |
| Total GGDP volatiles | MeJA | 9.523 | 1 | 0.002** |
| Bacteria | 0.084 | 1 | 0.772 | |
| Bacteria + MeJA | 0.538 | 1 | 0.463 | |
| Time | 6.384 | 1 | 0.012* | |
| MeJA x Time | 0.131 | 1 | 0.717 | |
| Bacteria x Time | 3.841 | 1 | 0.050* | |
| (Bacteria + MeJA) x Time | 0.322 | 1 | 0.571 | |
| Total benzenoids | MeJA | 2.595 | 1 | 0.107 |
| Bacteria | 6.628 | 1 | 0.107 | |
| Bacteria + MeJA | 0.074 | 1 | 0.785 | |
| Time | 9.919 | 1 | 0.002** | |
| MeJA x Time | 8.298 | 1 | 0.004** | |
| Bacteria x Time | 7.108 | 1 | 0.008** | |
| (Bacteria + MeJA) x Time | 1.655 | 1 | 0.198 | |
The blend of LOX pathway volatiles consisted of hexanal, 2-(E)-hexenal, 3-(Z)-hexenol, and pentanal and the emission blend was dominated by pentanal, followed by hexanal upon all treatments (Fig. S1 and Table S1). In addition, separate MeJA treatments led to a stronger LOX volatile emission rates, followed by combined bacterial and MeJA treatments at day 3. However, the emission of classical LOX pathway volatiles, (E)-2-hexenal and (Z)-3-hexenol, tended to peak for 20 mM MeJA treatment at day-1 after treatments and then started declining. The separate application of MeJA had a statistically significant impact on hexanal and pentanal emissions, whereas bacterial treatment alone and combined application of bacteria and MeJA had a statistically significant impact on (E)-2-hexenal and (Z)-3-hexenol emissions (P < 0.05, Table S1). In addition, (Z)-3-hexenyl acetate emission was also observed at trace levels upon separate MeJA treatments (data not shown).
3.4. Time-dependent regulation of foliage mono-and sesquiterpene emissions upon MeJA and bacterial treatments
Eucalyptus grandis is a low-level mono- and sesquiterpene emitter under non-stressed conditions. The total average emission rate of monoterpenes by control leaves was varied between 0.14 - 0.23 nmol m-2 s-1 (Fig. 3B). Generally, bacterial inoculation decreased total mono- and sesquiterpenes in a time-dependent manner, compared to controls (Fig. 3B and 3C, P < 0.001 for bacterial inoculation alone, Table 3). However, the emission rate peaked when leaves were subjected to separate MeJA treatments (ca. 12-fold increment compared to controls for 20 mM MeJA at day 3), followed by combined bacterial and MeJA treatments at day 3 (ca. 6-fold increment compared to controls for a combined application of bacteria and 10 mM MeJA at day 3) (P < 0.001 for separate and combined bacterial and MeJA treatments, Table 3).
Eucalyptus grandis leaves emitted fifteen monoterpenes (Fig. S2). The blend of monoterpenes was dominated by 1,8-cineole (ca. 20 % of total monoterpenes), followed by α-pinene (ca. 13 % of total monoterpenes) and p-cymene (ca. 10 % of total monoterpenes). Generally, the separate MeJA treatments resulted in a greater impact on individual monoterpene emission rates, followed by combined bacterial and MeJA treatments (Fig. S2 and Table S1). In addition, classical stress-induced monoterpenes (E)-β-ocimene, and (Z)-β-ocimene were also observed in this study (Fig. S2J and S2K). In fact, the emission rates of both (E)-β-ocimene, and (Z)-β-ocimene were higher for separate MeJA treatments than for combined bacterial and MeJA treatments (P < 0.001 for separate MeJA, and combined bacterial and MeJA treatments, Table S1). In our study, emission responses of linalool were scattered (emission rate ranging between 3-13 pmol m-2 s-1, data not shown) and detected only from the leaves treated with combined bacterial and MeJA at day 1 after treatments.
Separate and combined MeJA and bacterial treatments significantly impacted the timing of monoterpene emission responses. In general, there were four types of emission kinetics observed for monoterpene emissions. In the case of m-cymene (Fig. S2E), p-cymene (Fig. S2F), limonene (Fig. S2G), the emission responses remained almost the same throughout all times after treatments. For 1,8-cineole (Fig. S2D), 1,3,8-p-menthatriene (Fig. S2H) and β-myrcene (Fig. S2I), the emission rates increased to the maxima at day 1 and day 3, and then decreased to the minima at day 7 after treatments. However, for α-phellandrene (Fig. S2L), the emission rate was the highest at day 1 and day 7, and the lowest at day 3 after treatments. For all other monoterpenes, the emissions achieved the maxima at day 3 and minima at day 1 and 7 after treatments.
The average total sesquiterpene emission of control leaves was 0.010-0.014 nmol m-2 s-1 (Fig. 3C). Similar to the dynamics of total monoterpenes, the emission rate of total sesquiterpene was progressively enhanced by separate MeJA treatments (ca. 8-fold increment compared to controls controls for 20 mM MeJA at day 3), followed by combined bacterial and MeJA treatments at day 3 (ca. 4-fold increment compared to controls a combined application of bacteria and 10 mM MeJA at day 3) (P < 0.001 for separate and combined bacterial and MeJA treatments, Table 3). There were five sesquiterpenes emitted in this study, of which the emission was dominated by α-copaene (ca. 60% of total sesquiterpenes), followed by Δ-cadinene (ca. 30% of total sesquiterpenes) (Fig. S3). Similar to the emission pattern of most of the monoterpenes, the leaves subjected to separate MeJA treatments significantly increased all sesquiterpenes, followed by combined bacterial and MeJA treatments (Fig. S3, and Table S1). However, the elicitation of Δ-cadinene was the highest, followed by α-copaene and longifolene. In addition, the leaves treated with bacteria alone suppressed the emission rates of sesquiterpenes, compared to controls (Fig. 3C). In the case of individual sesquiterpenes, there were two different types of emission kinetics observed. The emission responses remained almost the same throughout stressed-period for Δ-cadinene, β-farnesene, and longifolene (Fig. S3B, S3D, and S3E). In the case of alloaromadendrene and α-copaene (Fig. S3A and S3C), the emissions were peaked at day 3 and remained minimum at day 1 and 7 after treatments. Generally, the emission rates of total mono- and sesquiterpenes were scaled with the severity of stress applications (Fig. 3B and 3C).
3.5. Quantitative estimation of foliage terpene contents
There were 23 monoterpenes and 17 sesquiterpenes were detected in the storage structures of E. grandis leaves (Table 4). The storage pool of monoterpenes was dominated by 1, 8-cineole and the sesquiterpenes were dominated by aromadendrene. The monoterpenes (Z)-allo-ocimene, m-cymene, (E)-β-ocimene, and (Z)-β-ocimene, and the sesquiterpenes (E)-β-farnesene and longifolene were observed in the headspace collection of volatile blends, but not in the foliage storage structures (Fig. S2 and S3, and Table 4).
Table 4.
Output of generalized linear models for individual and interactive effects of MeJA, bacteria, bacteria and MeJA and time (1, 3, and 7 days after treatment) after treatments on individual volatiles emitted by E. grandis leaves.
| Compounds | Effects | |||
|---|---|---|---|---|
| χ2 | df | P value | ||
|
LOX volatiles Hexanal |
MeJA | 4.604 | 1 | 0.032* |
| Bacteria | 8.855 | 1 | 0.003** | |
| Bacteria + MeJA | 0.322 | 1 | 0.571 | |
| Time | 0.730 | 1 | 0.393 | |
| MeJA x Time | 0.232 | 1 | 0.630 | |
| Bacteria x Time | 1.733 | 1 | 0.188 | |
| (Bacteria + MeJA) x Time | 0.652 | 1 | 0.419 | |
| (E)-2-Hexenal | MeJA | 0.197 | 1 | 0.657 |
| Bacteria | 6.056 | 1 | 0.014* | |
| Bacteria + MeJA | 6.725 | 1 | 0.010** | |
| Time | 17.945 | 1 | 0.000*** | |
| MeJA x Time | 1.320 | 1 | 0.251 | |
| Bacteria x Time | 6.981 | 1 | 0.008** | |
| (Bacteria + MeJA) x Time | 6.906 | 1 | 0.009** | |
| (Z)-3-Hexenol | MeJA | 0.011 | 1 | 0.915 |
| Bacteria | 5.375 | 1 | 0.020* | |
| Bacteria + MeJA | 5.837 | 1 | 0.016* | |
| Time | 13.451 | 1 | 0.000*** | |
| MeJA x Time | 0.650 | 1 | 0.420 | |
| Bacteria x Time | 5.944 | 1 | 0.015* | |
| (Bacteria + MeJA) x Time | 7.489 | 1 | 0.006** | |
| Pentanal | MeJA | 9.349 | 1 | 0.002** |
| Bacteria | 2.220 | 1 | 0.136 | |
| Bacteria + MeJA | 3.301 | 1 | 0.069 | |
| Time | 0.151 | 1 | 0.698 | |
| MeJA x Time | 1.564 | 1 | 0.211 | |
| Bacteria x Time | 2.405 | 1 | 0.121 | |
| (Bacteria + MeJA) x Time | 0.655 | 1 | 0.418 | |
|
Monoterpenes (Z)-allo-ocimene |
MeJA | 19.253 | 1 | 0.000*** |
| Bacteria | 11.088 | 1 | 0.001*** | |
| Bacteria + MeJA | 10.542 | 1 | 0.001*** | |
| Time | 9.135 | 1 | 0.003** | |
| MeJA x Time | 1.511 | 1 | 0.219 | |
| Bacteria x Time | 1.010 | 1 | 0.315 | |
| (Bacteria + MeJA) x Time | 4.297 | 1 | 0.038* | |
| Camphene | MeJA | 0.774 | 1 | 0.379 |
| Bacteria | 17.588 | 1 | 0.000*** | |
| Bacteria + MeJA | 0.309 | 1 | 0.579 | |
| Time | 7.340 | 1 | 0.007** | |
| MeJA x Time | 4.059 | 1 | 0.044* | |
| Bacteria x Time | 16.045 | 1 | 0.000*** | |
| (Bacteria + MeJA) x Time | 2.901 | 1 | 0.089 | |
| Δ3-Carene | MeJA | 4.137 | 1 | 0.042* |
| Bacteria | 0.513 | 1 | 0.474 | |
| Bacteria + MeJA | 0.465 | 1 | 0.495 | |
| Time | 2.753 | 1 | 0.097 | |
| MeJA x Time | 2.269 | 1 | 0.132 | |
| Bacteria x Time | 0.696 | 1 | 0.404 | |
| (Bacteria + MeJA) x Time | 6.376 | 1 | 0.012* | |
| 1,8-Cineole | MeJA | 5.391 | 1 | 0.020* |
| Bacteria | 3.841 | 1 | 0.050* | |
| Bacteria + MeJA | 3.174 | 1 | 0.075 | |
| Time | 0.120 | 1 | 0.729 | |
| MeJA x Time | 0.520 | 1 | 0.471 | |
| Bacteria x Time | 0.007 | 1 | 0.933 | |
| (Bacteria + MeJA) x Time | 2.176 | 1 | 0.140 | |
| m-Cymene | MeJA | 3.291 | 1 | 0.070 |
| Bacteria | 0.616 | 1 | 0.433 | |
| Bacteria + MeJA | 0.288 | 1 | 0.591 | |
| Time | 9.198 | 1 | 0.002** | |
| MeJA x Time | 0.119 | 1 | 0.730 | |
| Bacteria x Time | 2.700 | 1 | 0.100 | |
| (Bacteria + MeJA) x Time | 1.810 | 1 | 0.179 | |
| p-Cymene | MeJA | 0.469 | 1 | 0.493 |
| Bacteria | 14.038 | 1 | 0.000*** | |
| Bacteria + MeJA | 6.576 | 1 | 0.010** | |
| Time | 6.998 | 1 | 0.008** | |
| MeJA x Time | 1.128 | 1 | 0.288 | |
| Bacteria x Time | 0.196 | 1 | 0.658 | |
| (Bacteria + MeJA) x Time | 0.287 | 1 | 0.592 | |
| Limonene | MeJA | 0.592 | 1 | 0.442 |
| Bacteria | 4.406 | 1 | 0.036* | |
| Bacteria + MeJA | 5.034 | 1 | 0.025* | |
| Time | 1.322 | 1 | 0.250 | |
| MeJA x Time | 2.597 | 1 | 0.107 | |
| Bacteria x Time | 2.546 | 1 | 0.111 | |
| (Bacteria + MeJA) x Time | 2.952 | 1 | 0.086 | |
| 1,3,8-p-Menthatriene | MeJA | 32.373 | 1 | 0.000*** |
| Bacteria | 34.725 | 1 | 0.000*** | |
| Bacteria + MeJA | 16.355 | 1 | 0.000*** | |
| Time | 4.986 | 1 | 0.026* | |
| MeJA x Time | 0.004 | 1 | 0.947 | |
| Bacteria x Time | 3.177 | 1 | 0.075 | |
| (Bacteria + MeJA) x Time | 9.673 | 1 | 0.002** | |
| β-Myrcene | MeJA | 2.377 | 1 | 0.123 |
| Bacteria | 2.415 | 1 | 0.120 | |
| Bacteria + MeJA | 9.725 | 1 | 0.002** | |
| Time | 1.157 | 1 | 0.282 | |
| MeJA x Time | 0.342 | 1 | 0.559 | |
| Bacteria x Time | 0.414 | 1 | 0.520 | |
| (Bacteria + MeJA) x Time | 7.148 | 1 | 0.008** | |
| (E)-β-Ocimene | MeJA | 18.737 | 1 | 0.000*** |
| Bacteria | 8.560 | 1 | 0.003** | |
| Bacteria + MeJA | 26.540 | 1 | 0.000*** | |
| Time | 0.228 | 1 | 0.633 | |
| MeJA x Time | 0.798 | 1 | 0.372 | |
| Bacteria x Time | 0.085 | 1 | 0.771 | |
| (Bacteria + MeJA) x Time | 0.375 | 1 | 0.540 | |
| (Z)-β-Ocimene | MeJA | 43.197 | 1 | 0.000*** |
| Bacteria | 25.608 | 1 | 0.000*** | |
| Bacteria + MeJA | 23.919 | 1 | 0.000*** | |
| Time | 14.054 | 1 | 0.000*** | |
| MeJA x Time | 0.322 | 1 | 0.570 | |
| Bacteria x Time | 3.265 | 1 | 0.071 | |
| (Bacteria + MeJA) x Time | 2.458 | 1 | 0.117 | |
| α-Phellandrene | MeJA | 8.484 | 1 | 0.004** |
| Bacteria | 4.186 | 1 | 0.041* | |
| Bacteria + MeJA | 3.332 | 1 | 0.068 | |
| Time | 36.873 | 1 | 0.000*** | |
| MeJA x Time | 0.424 | 1 | 0.515 | |
| Bacteria x Time | 0.210 | 1 | 0.647 | |
| (Bacteria + MeJA) x Time | 1.051 | 1 | 0.305 | |
| α-Pinene | MeJA | 2.082 | 1 | 0.149 |
| Bacteria | 2.429 | 1 | 0.119 | |
| Bacteria + MeJA | 0.270 | 1 | 0.603 | |
| Time | 0.344 | 1 | 0.557 | |
| MeJA x Time | 5.868 | 1 | 0.015* | |
| Bacteria x Time | 0.369 | 1 | 0.544 | |
| (Bacteria + MeJA) x Time | 0.694 | 1 | 0.405 | |
| β-Pinene | MeJA | 0.515 | 1 | 0.473 |
| Bacteria | 0.172 | 1 | 0.678 | |
| Bacteria + MeJA | 0.011 | 1 | 0.915 | |
| Time | 1.156 | 1 | 0.282 | |
| MeJA x Time | 7.973 | 1 | 0.005** | |
| Bacteria x Time | 0.503 | 1 | 0.478 | |
| (Bacteria + MeJA) x Time | 0.658 | 1 | 0.417 | |
| γ-Terpinene | MeJA | 5.767 | 1 | 0.016* |
| Bacteria | 2.047 | 1 | 0.152 | |
| Bacteria + MeJA | 11.595 | 1 | 0.001*** | |
| Time | 0.649 | 1 | 0.421 | |
| MeJA x Time | 2.445 | 1 | 0.118 | |
| Bacteria x Time | 0.012 | 1 | 0.912 | |
| (Bacteria + MeJA) x Time | 1.929 | 1 | 0.165 | |
|
Sesquiterpenes Alloaromadendrene |
MeJA | 0.192 | 1 | 0.661 |
| Bacteria | 0.104 | 1 | 0.748 | |
| Bacteria + MeJA | 0.109 | 1 | 0.742 | |
| Time | 0.677 | 1 | 0.411 | |
| MeJA x Time | 1.113 | 1 | 0.291 | |
| Bacteria x Time | 2.051 | 1 | 0.152 | |
| (Bacteria + MeJA) x Time | 0.159 | 1 | 0.690 | |
| Δ-Cadinene | MeJA | 58.936 | 1 | 0.000*** |
| Bacteria | 67.744 | 1 | 0.000*** | |
| Bacteria + MeJA | 40.024 | 1 | 0.000*** | |
| Time | 0.022 | 1 | 0.882 | |
| MeJA x Time | 0.146 | 1 | 0.702 | |
| Bacteria x Time | 0.001 | 1 | 0.971 | |
| (Bacteria + MeJA) x Time | 0.062 | 1 | 0.804 | |
| α-Copaene | MeJA | 3.687 | 1 | 0.055 |
| Bacteria | 29.741 | 1 | 0.000*** | |
| Bacteria + MeJA | 3.217 | 1 | 0.073 | |
| Time | 2.372 | 1 | 0.124 | |
| MeJA x Time | 3.608 | 1 | 0.058 | |
| Bacteria x Time | 1.881 | 1 | 0.170 | |
| (Bacteria + MeJA) x Time | 4.338 | 1 | 0.037* | |
| (E)-β-Farnesene | MeJA | 61.511 | 1 | 0.000*** |
| Bacteria | 57.718 | 1 | 0.000*** | |
| Bacteria + MeJA | 2.777 | 1 | 0.096 | |
| Time | 5.936 | 1 | 0.015* | |
| MeJA x Time | 4.579 | 1 | 0.032* | |
| Bacteria x Time | 6.067 | 1 | 0.014* | |
| (Bacteria + MeJA) x Time | 4.331 | 1 | 0.037* | |
| Longifolene | MeJA | 0.502 | 1 | 0.478 |
| Bacteria | 0.064 | 1 | 0.800 | |
| Bacteria + MeJA | 0.220 | 1 | 0.639 | |
| Time | 0.860 | 1 | 0.354 | |
| MeJA x Time | 0.117 | 1 | 0.733 | |
| Bacteria x Time | 1.735 | 1 | 0.188 | |
| (Bacteria + MeJA) x Time | 3.611 | 1 | 0.057 | |
|
Saturated aldehydes Decanal |
MeJA | 6.478 | 1 | 0.011* |
| Bacteria | 0.201 | 1 | 0.654 | |
| Bacteria + MeJA | 0.499 | 1 | 0.480 | |
| Time | 9.340 | 1 | 0.002** | |
| MeJA x Time | 2.785 | 1 | 0.095 | |
| Bacteria x Time | 7.211 | 1 | 0.007** | |
| (Bacteria + MeJA) x Time | 0.014 | 1 | 0.904 | |
| Heptanal | MeJA | 4.182 | 1 | 0.041* |
| Bacteria | 0.784 | 1 | 0.376 | |
| Bacteria + MeJA | 0.909 | 1 | 0.340 | |
| Time | 2.134 | 1 | 0.144 | |
| MeJA x Time | 4.887 | 1 | 0.027* | |
| Bacteria x Time | 9.048 | 1 | 0.003** | |
| (Bacteria + MeJA) x Time | 2.188 | 1 | 0.139 | |
| Nonanal | MeJA | 6.345 | 1 | 0.012* |
| Bacteria | 0.103 | 1 | 0.748 | |
| Bacteria + MeJA | 4.790 | 1 | 0.029* | |
| Time | 4.797 | 1 | 0.029* | |
| MeJA x Time | 6.617 | 1 | 0.01* | |
| Bacteria x Time | 10.873 | 1 | 0.001** | |
| (Bacteria + MeJA) x Time | 0.154 | 1 | 0.695 | |
| Octanal | MeJA | 3.918 | 1 | 0.048* |
| Bacteria | 0.290 | 1 | 0.590 | |
| Bacteria + MeJA | 1.656 | 1 | 0.198 | |
| Time | 3.046 | 1 | 0.081 | |
| MeJA x Time | 4.445 | 1 | 0.035* | |
| Bacteria x Time | 8.211 | 1 | 0.004** | |
| (Bacteria + MeJA) x Time | 0.436 | 1 | 0.509 | |
|
Lightweight oxygenated compounds (LOC) Acetaldehyde |
MeJA | 2.186 | 1 | 0.139 |
| Bacteria | 22.125 | 1 | 0.000*** | |
| Bacteria + MeJA | 3.283 | 1 | 0.070 | |
| Time | 0.425 | 1 | 0.515 | |
| MeJA x Time | 17.979 | 1 | 0.000*** | |
| Bacteria x Time | 3.358 | 1 | 0.067 | |
| (Bacteria + MeJA) x Time | 8.281 | 1 | 0.004** | |
| Acetone | MeJA | 3.559 | 1 | 0.059 |
| Bacteria | 7.298 | 1 | 0.007** | |
| Bacteria + MeJA | 1.185 | 1 | 0.276 | |
| Time | 0.207 | 1 | 0.649 | |
| MeJA x Time | 2.832 | 1 | 0.092 | |
| Bacteria x Time | 1.224 | 1 | 0.269 | |
| (Bacteria + MeJA) x Time | 0.253 | 1 | 0.615 | |
|
GGDP pathway volatiles (Z)-Geranyl acetone |
MeJA | 10.026 | 1 | 0.002** |
| Bacteria | 1.245 | 1 | 0.265 | |
| Bacteria + MeJA | 0.094 | 1 | 0.760 | |
| Time | 7.960 | 1 | 0.005** | |
| MeJA x Time | 0.852 | 1 | 0.356 | |
| Bacteria x Time | 2.304 | 1 | 0.129 | |
| (Bacteria + MeJA) x Time | 0.218 | 1 | 0.640 | |
| 6-Methyl-5-hepten-2-one | MeJA | 0.008 | 1 | 0.930 |
| Bacteria | 1.137 | 1 | 0.286 | |
| Bacteria + MeJA | 15.766 | 1 | 0.000*** | |
| Time | 0.278 | 1 | 0.598 | |
| MeJA x Time | 0.827 | 1 | 0.363 | |
| Bacteria x Time | 3.728 | 1 | 0.050* | |
| (Bacteria + MeJA) x Time | 10.394 | 1 | 0.001*** | |
|
Benzenoids Benzaldehyde |
MeJA | 12.758 | 1 | 0.000*** |
| Bacteria | 2.394 | 1 | 0.122 | |
| Bacteria + MeJA | 7.300 | 1 | 0.007** | |
| Time | 4.253 | 1 | 0.039* | |
| MeJA x Time | 4.083 | 1 | 0.043* | |
| Bacteria x Time | 12.295 | 1 | 0.000*** | |
| (Bacteria + MeJA) x Time | 0.259 | 1 | 0.611 | |
| o-Xylene | MeJA | 4.074 | 1 | 0.044** |
| Bacteria | 3.325 | 1 | 0.068 | |
| Bacteria + MeJA | 0.051 | 1 | 0.821 | |
| Time | 1.469 | 1 | 0.225 | |
| MeJA x Time | 0.421 | 1 | 0.516 | |
| Bacteria x Time | 3.724 | 1 | 0.050* | |
| (Bacteria + MeJA) x Time | 0.291 | 1 | 0.589 | |
| p-Xylene | MeJA | 2.741 | 1 | 0.098 |
| Bacteria | 0.968 | 1 | 0.325 | |
| Bacteria + MeJA | 10.746 | 1 | 0.001*** | |
| Time | 0.004 | 1 | 0.947 | |
| MeJA x Time | 1.808 | 1 | 0.179 | |
| Bacteria x Time | 0.403 | 1 | 0.525 | |
| (Bacteria + MeJA) x Time | 1.249 | 1 | 0.264 | |
| Toluene | MeJA | 0.627 | 1 | 0.429 |
| Bacteria | 0.647 | 1 | 0.421 | |
| Bacteria + MeJA | 0.154 | 1 | 0.694 | |
| Time | 3.683 | 1 | 0.055 | |
| MeJA x Time | 2.343 | 1 | 0.126 | |
| Bacteria x Time | 1.574 | 1 | 0.210 | |
| (Bacteria + MeJA) x Time | 1.185 | 1 | 0.276 | |
Stress application protocol and the baseline environmental conditions during measurements as in Figure 1. P values show the significance of wald chi-square (χ2) statistics.
3.6. Impact of MeJA and bacterial treatments on temporal emission dynamics of saturated aldehydes
The emission rate of total saturated aldehyde was higher for all treatment effects with a progressive impact on day 3 after separate and combined applications of bacterial and MeJA (Fig. 3D, Table 3). In particular, the emission rate peaked by MeJA treatment alone (P < 0.001 for MeJA treatments, Table 3), followed by combined bacterial and MeJA treatments, and bacterial treatment alone.
In this study, the pool of saturated aldehydes emitted was comprised of decanal, heptanal, nonanal, and octanal (Fig. S4, and Table S1). Similar to the emission kinetics of LOX volatiles, hexanal and pentanal (Fig. S1A and S1D), the inoculation with bacteria enhanced the emission rates of all saturated aldehydes, compared to controls (Fig. S4, and Table S1). However, the separate application of MeJA resulted in the highest emission burst of decanal and combined application of bacteria and MeJA led to the highest emission rates of heptanal, nonanal, and octanal particularly at day 3 after stress applications (P < 0.05 for separate MeJA treatments, Table S1). All saturated aldehydes demonstrated emission maxima at day 3 and minima at day 1 and 7 after treatments.
3.7. Emission trends in LOC, GGDP pathway volatiles, and benzenoids in MeJA and bacterial treatments and at different times after treatments
The emission rates of total LOC, benzenoids, and GGDP pathway volatiles were higher at day 3 after stress applications (Fig. 3E - 3G). In fact, bacterial inoculation decreased total LOC and GGDP pathway volatiles and increased total benzenoid emissions at all recovery phase. However, total LOC, benzenoids, and GGDP pathway volatiles were the highest when leaves were subjected to MeJA treatment alone (P < 0.05, Table 3), followed by combined bacterial and MeJA treatments (Fig. 3E - 3G).
Two LOC volatiles, acetaldehyde and acetone, were observed in the emission blend (Fig. S5A and S5B). The acetone constituted a greater share of LOC (Fig. S5B). The emission rates of acetaldehyde and acetone were decreased in response to bacterial inoculation at all recovery phase, compared to controls (P < 0.01 for bacteria, Table S1). Acetone emission responses tended to peak for separate MeJA treatments, compared to combined bacterial and MeJA treatments (Fig. S5B). In the case of acetaldehyde, surprisingly the emission tended to peak upon combined application of bacteria and MeJA at day 1 (Fig. S5A). Regarding the emission kinetics, the kinetics of acetaldehyde was remained the same through the different times of measurements after treatments, but in the case of acetone, the emission achieved to the maximum at day 3 after measurements and remained minimum at day 1 and 7 after treatments.
There were two compounds detected under the category of GGDP pathway volatiles, (Z)-geranyl acetone and 6-methyl-5-hepten-2-one (Fig. S5C and S5D, Table S1). Inoculation of bacteria alone led to enhanced emission rates of GGDP pathway volatiles till day 3 after stress applications and then to decreased emission rates, compared to controls. In fact, the emission rates of (Z)-geranyl acetone and 6-methyl-5-hepten-2-one were lower in response to combined bacterial and MeJA treatments than separate MeJA treatments at day 3 since treatments (Fig. S5C and S5D, Table S1). The combined application of bacteria and MeJA had a statistically significant impact on the emission rate of 6-methyl-5-hepten-2-one (P < 0.001, Table S1). Regarding the emission kinetics of both cases, the emission achieved to the maxima at day 3 after measurements and then decayed.
The blend of benzenoid was dominated by benzaldehyde, followed by toluene, o-xylene, and p-xylene upon all treatments and through the time of measurements after treatments (Fig. S6). In the case of benzaldehyde, bacterial inoculation increased the emission responses till day 3 and then decreased it, compared to controls (Fig. S6A). In fact, benzenoid emissions were always higher for the separate application of MeJA than combined bacterial and MeJA treatments at day 3 after stress treatments (Fig. S6A, and Table S1). The highest emission of benzaldehyde was observed upon 10 mM MeJA, followed by 5 mM MeJA at day 3. Regarding the emission kinetics of p-xylene, application of bacteria inoculation ceased the emission throughout the stressed-phase (Fig. S6C). In the case of toluene emission, all treatments lowered the emission responses, compared to controls in different times after treatments; however, more severe decline was observed in response to combined bacterial and MeJA treatment than MeJA treatment alone particularly at day 3 after treatments (Fig. S6D).
3.8. Overall changes in the emission blend in response to various treatments
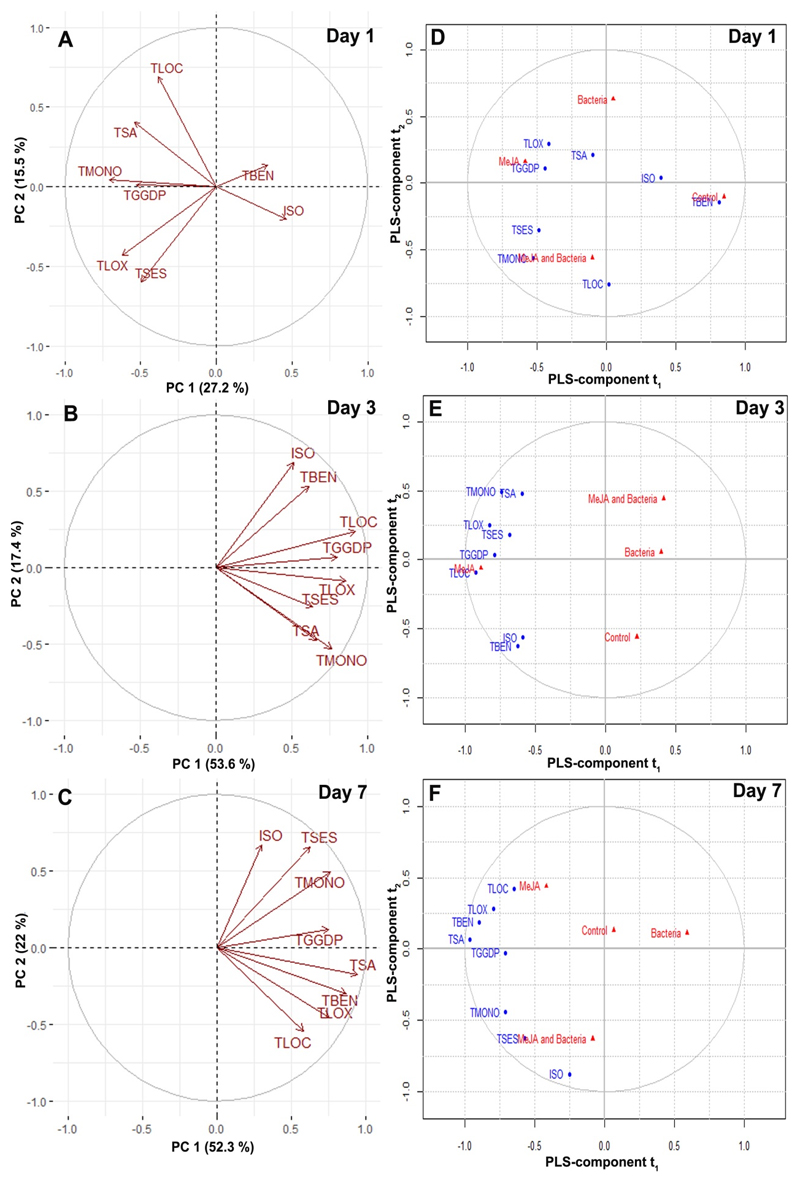
PCA analysis indicated that the first two principal components, PC1 and PC2, explained 40-80 % of the total variation in all cases (Fig. 4A, B, and C). The distribution of volatile compounds in the PCA loading plots from untreated and treated leaves showed highly quantitative variations in the volatile emission blends. In particular, isoprene and total benzenoid emission rates were negatively correlated with all other classes of compound emission at day 1, and this trend was changed in day 3 and day 7 after treatments, where all volatile classes were positively correlated (Fig. 4A, B, and C). In fact, the total monoterpene emission rate was strongly correlated with the emission rate of total GGDP pathway volatiles in day 1; however, it was strongly correlated with the emission rate of total saturated aldehydes in day 3, and total sesquiterpenes in day 7 after treatments. Furthermore, isoprene emission rates were strongly correlated with the total emission rates of benzenoids at day 1 and day 3, but it was strongly correlated with total sesquiterpene in day 7. Furthermore, the discriminant analysis based on PLS further indicates the influence of particular terpenes on the differences in the overall volatile blend upon different treatments (Fig. 4C, D, and E). In fact, MeJA treatment was highly influenced by total LOX, GGDP pathway volatiles, and saturated aldehydes at day 1, and it was highly influenced by total LOC at day 3, and total LOX, LOC, benzenoids, and saturated aldehydes at day 7. Bacterial inoculation was particularly influenced by isoprene at day 1 and none of the specific compounds at day 3 and day 7. However, combined application of MeJA and bacteria was highly influenced by total mono- and sesquiterpenes at day 1 and day 7, and it was not influenced by any compounds at day 3 after treatments. The association of other classes of volatiles to a particular treatment depended on the temporal variation of volatiles upon treatments and various controls (Fig. 4C, D, and E).
Figure 4.
Loading plots (A, B, and C) of principal component analysis (PCA) of emission rates of foliage isoprene and other classes of total VOCs in E. grandis leaves in response to control, bacterial inoculation, MeJA treatments, and bacteria and MeJA treatments (first inoculated with bacteria, followed by MeJA treatments) at 1, 3, and 7 days after treatments. Loading plots (C, D, and E) of partial least square discriminant analysis (PLS-DA) of isoprene and other classes of total VOCs in E. grandis leaves upon control, bacterial inoculation, MeJA treatments, and bacteria and MeJA treatments. The triangles represent the loading score for different treatments. Details of stress applications as in Figure 1. Details of isoprene and total volatile emissions as in Figure 2 and 3. Abbreviations represent different volatile classes as follows: ISO- isoprene, TLOX - total LOX, TMONO - total monoterpenes, TSES - total sesquiterpenes, TSA - total saturated aldehydes, TLOC- total lightweight oxygenated volatile compounds, TGGDP - total GGDP pathway volatiles, and TBEN - total benzenoids.
4. Discussion
4.1. Impact of MeJA and bacterial treatments on time-dependent changes in photosynthetic traits
In the present study, inoculation by endophytic PGPR, B. vietnamiensis, alone reduced stomatal conductance to water vapor by 60-70% compared to controls and in turn, reduced net assimilation rate (Fig. 1B and 1C). The reduction in net assimilation rate is due to the reduction in foliage stomatal conductance upon PGPR inoculation as an adoptive mechanism to increase foliage water-content under stress as reported by Benabdellah et al. (2011). In our study, this phenomenon was further confirmed by the sustained reduction of intercellular CO2 concentration (Fig. 1B - 1D). Therefore, this phenomenon implies that foliage application of B. vietnamiensis would have increased the leaf-water content in E. grandis leaves, compared to non-inoculated leaves. However, exogenous MeJA applications considerably reduced photosynthetic traits, compared to controls and the reductions were quantitatively associated with the severity of stress applications (Fig. 1). Generally, a decline in maximum dark-adapted photosystem II (PSII) quantum yield estimated by chlorophyll florescence (Fv/Fm) upon MeJA treatments is a typical stress response and a sensitive indicator of the stress-induced damage in PSII reaction centers (Wierstra and Kloppstech, 2000). In this work, higher doses of MeJA likely caused hypersensitive reactions and severe stress-induced perturbations in photosynthetic machinery, eventually leading to a sustained decline in photosynthetic characteristics. Our findings are consistent with previous reports stating that energy dissipation activity and PSII efficiency were dramatically decreased upon elevated MeJA applications (Wierstra and Kloppstech, 2000). In addition, elevated MeJA treatments resulted in pronounced degradation of D1 protein synthesis and a subsequent delay in the recovery rate of photosystem II activity (Wierstra and Kloppstech, 2000), ultimately leading to sustained reduction in Fv/Fm and photoinhibition (Fig. 1A and 1B).
In our study, higher doses of MeJA likely caused: (1) irreversible damage in thylakoid membranes as a result of hypersensitive reactions (Kohlmann et al., 1999), (2) downregulation of photosynthetic protein biosynthesis and the expression profiles of nuclear and plastid photosynthetic genes encoding essential photosynthetic proteins (Wierstra and Kloppstech, 2000), and (3) a reduced biosynthesis and activity of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), a key enzyme in Calvin cycle (Rodrigues Cavalcante et al., 1999), eventually leading to a sustained reduction in foliage photosynthesis. Furthermore, acute MeJA treatments generates inhibitory effect on guard cells and reduced stomatal movements due to enhanced production of ROS and abscisic acid, which is also associated with reduced photosynthesis (Zhu et al., 2012) (Fig. 1B and 1C). Thus, these reports conclusively establish that the exposure of elevated MeJA in E. grandis leaves simultaneously triggered multiple biochemical and non-biochemical mechanisms, associated with a progressive reduction in photosynthetic characteristics (Fig. 1). Similar to our findings, biotic stresses reduced photosynthetic traits, including leaf rust infection in willow (Salix burjatica x S. dasyclados) (Toome et al., 2010), oak powdery mildew (Erysiphe alphitoides) infection in pedunculate oak (Quercus robur) leaves (Copolovici et al., 2014b), infection of rust fungus (Melampsora larici-populina) in poplar (Populus balsamifera var. suaveolens) leaves (Jiang et al., 2016), larvae of green alder sawfly (Monsoma pulveratum) feeding on European alder (Alnus glutinosa) leaves.
We demonstrated that inoculation by endophytic bacteria B. vietnamiensis significantly protected the photosynthetic machinery upon subsequent application of MeJA treatments (Fig. 1), possibility due to enhanced production of indole acetic acid associated with increased production of chlorophyll contents as observed by Onofre-Lemus et al. (2009) for tomato plants treated with PGPR B. unamae. In addition, it was also evident that inoculation by a PGPR B. aquimaris upregulated relative water content, accumulation of proline, soluble sugar, activities of superoxide dismutase, catalase, peroxidase, and ascorbate peroxidase in maize leaves, ultimately leading to enhanced protection of photosynthetic machinery from salt stress (Li and Jiang, 2017). Thus, we suggest the similar mechanisms could be associated with B. vietnamiensis to increase the photosynthetic activity upon subsequent MeJA treatments. Importantly, B. vietnamiensis was reported to produce thiol specific antioxidants in plants (http://www.ecogene.org) that protect host-cells from hostile oxidative environments (Hyang et al., 2005). Therefore, it implies that enhanced production of thiol specific antioxidants B. vietnamiensis likely weakened hypersensitive reactions caused by elevated MeJA treatments in E. grandis leaves. However, future research is required to address the question of how B. vietnamiensis inoculation protects the integrity of photosynthetic machinery during higher MeJA treatments.
4.2. Temporal emission dynamics of isoprene upon MeJA and bacteria treatments
Given that isoprene is de novo biosynthesized via MEP pathway, the emission rate of isoprene is ultimately controlled by isoprene synthase gene expression, terminal enzyme activity and availability of substrates (Rasulov et al., 2016). However, stresses can often modify isoprene synthase gene expression, terminal enzyme activity, and the availability of substrates, ultimately leading to modification in isoprene emission responses (Niinemets, 2010). The present study illustrates that inoculation by B. vietnamiensis in E. grandis leaves decreased isoprene emission rate by 88-95 % at all times, implying that bacterial inoculation significantly altered the MEP pathway. In addition, bacterial inoculation decreased net assimilation rate by 20-50% compared to controls (Fig. 1B and 2). Thus, it seems that substrate limitation did not significantly contribute to the lower rate of isoprene emission; instead, bacterial inoculation could have downregulated terminal enzyme activities in the MEP pathway and/or isoprene synthase gene expression levels. Nevertheless, enhancement of other isoprenoid synthesis, e.g. volatile terpenoids and non-volatile terpenoids such as carotenoids can severely suppress isoprene emission because of greater affinity of these branches of terpenoid synthesis pathway to metabolic precursors (Rasulov et al., 2014; Rasulov et al., 2015).
However, bacterial inoculation declined stomatal conductance to water vapour by 60-70% compared to controls (Fig. 1C). In fact, changes in stomatal conductance do not have a significant direct impact on the changes in isoprene emission rate, due to the development of partial pressure of isoprene in leaf intercellular airspaces upon reduced stomatal conductance (Niinemets and Reichstein, 2003). However, isoprene emission can slightly be increased by moderate stomatal closure due to positive influence of enhanced intercellular CO2 concentration on isoprene emission rate (Rasulov et al., 2016; Wilkinson et al., 2009) (Fig. 1D and 2).
In all cases, foliage isoprene emission was increased in response to separate MeJA treatments, but decreased upon combined bacterial and MeJA treatments at day 3 after treatments, compared to controls (Fig. 2A). However, net assimilation rate was significantly decreased for separate MeJA and combined bacterial and MeJA treatments, compared to control. In fact, a previous study revealed that exogenous MeJA treatments did not change isoprene synthase gene expression levels in Populus alba (Sasaki et al., 2005). Therefore, our study suggests that changes in isoprene emission upon separate MeJA and combined bacterial and MeJA treatments were likely associated with the changes in isoprene synthase and other terminal enzyme activities in MEP pathway. In contrast to our findings, biotic stresses reported to downregulate isoprene emission responses. For example, isoprene emission negatively scaled with the percentage of leaf damage upon rust fungus (Melampsora larici-populina) infection of poplar (Populus balsamifera var. suaveolens) leaves (Jiang et al., 2016), oak powdery mildew (Erysiphe alphitoides) infection of pedunculate oak (Quercus robur) leaves (Copolovici et al., 2014b), and leaf rust infection of willow (Salix burjatica x S. dasyclados) (Toome et al., 2010).
Surprisingly, isoprene emission was higher for combined bacterial and MeJA treatments than for separate MeJA treatments at day 7 after treatments. We argue in this particular case that B. vietnamiensis inoculation led to abolishment of hypersensitive reactions and programmed cell death to some extent, caused by lethal doses of MeJA, particularly by 20 mM MeJA (Reinbothe et al., 2009; Zhang and Xing, 2008) in E. grandis leaves. This phenomenon ultimately led to activation of MEP pathway and enhanced isoprene emission at later stages of recovery. This phenomenon was further confirmed by analogous emission rates of total mono- and sesquiterpenes for combined bacterial and 20 mM MeJA treatments at day 7 (Fig. 2, 3B and 3C). However, molecular studies of isoprene biosynthesis are required to understand the clear-cut mechanism of isoprene emission responses in this particular case.
4.3. Elicitation of LOX pathway volatiles under MeJA and bacteria treatments
Generally, separate MeJA and combined bacterial and MeJA treatments substantially elicited LOX pathway volatiles at all times after stress applications (Fig. 3A and Fig. S1). In fact, LOX compounds are biosynthesized in plant cell membranes as a product of the reaction between free polyunsaturated fatty acids and lipoxygenases, which is constitutively active in plant cells (Feussner and Wasternack, 2002; Jiang et al., 2016). Thus, LOX volatiles are rapidly biosynthesized and released upon availability of their substrate, polyunsaturated fatty acids and their emission often last for some minutes to few hours (Jiang et al., 2017a). In the case of biotic stresses, emission of LOX pathway volatiles continues for 2-30 minutes and then it rapidly decays (Jiang et al., 2016; Niinemets et al., 2013; Scala et al., 2013). In fact, higher doses of MeJA lead to excessive production of ROS upon hypersensitive reactions that eventually results in sustained biosynthesis and emission of LOX compounds (Feussner and Wasternack, 2002; Wohlgemuth et al., 2002). Thus, as observed in the present work, the emission of classical LOX volatiles, (E)-2-hexenal and (Z)-3-hexenol, remained for 7 days after stress application implies that an exogenous application of MeJA led to a significantly greater production of ROS and upregulation of LOX pathway gene expression (Martin et al., 2002; Martin et al., 2003) (Fig. S1B and S1C). To further support this suggestion, there was a non-linearity between total and individual LOX emission versus MeJA concentrations in separate MeJA treatments (Fig. 3A and Fig. S1). This could be associated with the late-MeJA responses reflecting the enhanced levels of endogenously synthesized jasmonate (Tamogami et al., 2008) and thus, the higher emission rates of LOX at later stages of recovery reflects the jasmonate-dependent gene expression, not typically associated with dose-dependent constitutive LOX emission, indicated in many studies for lipoxygenase pathway genes (Jiang et al., 2017a; Wasternack and Parthier, 1997).
In this study, the LOX volatile emission was dominated by hexanal. In fact, hexanal emission is a distinctive phenomenon of an oxidative cleavage of membrane lipids by lipoxygenase and hydroperoxide lyase enzymes (Heiden et al., 2003; Kohlmann et al., 1999), demonstrating that leaf oxidative status scaled positively with MeJA treatments in our study. In accordance with our study, exogenous application of MeJA increased hexanal emission in grape berries (Wang et al., 2015) and barley leaves (Kohlmann et al., 1999). Furthermore, past studies indicated that biotic stresses significantly elicited LOX pathway volatiles. For example, leaf rust infection in willow (Salix burjatica x S. dasyclados) (Toome et al., 2010), oak powdery mildew (Erysiphe alphitoides) infection in pedunculate oak (Quercus robur) leaves (Copolovici et al., 2014b), infection of rust fungus (Melampsora larici-populina) in poplar (Populus balsamifera var. suaveolens) leaves (Jiang et al., 2016), larvae of green alder sawfly (Monsoma pulveratum) feeding on European alder (Alnus glutinosa) leaves (Copolovici et al., 2014a).
A substantial reduction of LOX pathway volatiles in response to combined application of bacteria and MeJA, compared to separate MeJA treatments is consistent with the hypothesis that inoculation of endophytic bacteria B. vietnamiensis protects plants cells from subsequent oxidative stress through enhanced biosynthesis of antioxidants and reduced formation of ROS in inoculated plants. However, the exact mechanism of this bacteria-induced enhanced defence process in plants warrants a detailed investigation.
4.4. Temporal emission dynamics of foliage mono- and sesquiterpenes due to MeJA and bacteria treatments
The biosynthesis of mono- and sesquiterpenes occurs via two separate pathways: the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate pathway (MEP/DOXP pathway) in plastids for monoterpenes and the mevalonate pathway (MVA) in cytosol for sesquiterpenes (Pazouki and Niinemets, 2016). A regulation of MEP/DOXP pathway is mainly fueled by the continuous supply of NADPH and ATP produced by photosynthetic electron transport reactions (Niinemets et al., 2002b; Rasulov et al., 2016). Therefore, a strong positive correlation between monoterpene emission and net assimilation rates are generally expected (Niinemets et al., 2002b; Staudt and Lhoutellier, 2011), but such correlation is not expected for the biosynthesis of sesquiterpenes.
Inoculation by B. vietnamiensis of E. grandis leaves decreased total mono-and sesquiterpenes (Fig. 3B), likely associated with enhanced defense responses. In addition, lower emission rates of foliage mono- and sesquiterpenes upon combined application of bacteria and MeJA than that treated with MeJA alone imply that B. vietnamiensis inoculation significantly preserved the stability of plant cell membranes from programmed cell death and necrosis. This suggestion is further supported by the enhancement of photosynthetic traits and decreased LOX volatile emissions in response to combined bacterial and MeJA treatments, compared to separate MeJA treatments (Fig. 1, 3B, and 3C). To some extent, higher emission bursts of mono-and sesquiterpenes were likely caused by severe hypersensitive reactions induced by MeJA treatments in epithelial and epidermal cells of sub-dermal secretory cavities and oil-glands in E. grandis leaves (Reinbothe et al., 2009; Zhang and Xing, 2008) that must have led to severe leakage of mono- and sesquiterpenes out of storage structures (Külheim et al., 2015). Furthermore, the emission of LOX pathway volatiles could have primed for the sustained elicitation of mono- and sesquiterpenes for longer term, not necessarily induced my MeJA per se (Jiang et al., 2017a). In fact, the contrasting changes in net assimilation rate and emission rates of monoterpenes at different times after stress applications collectively imply that a modification in emission blend of monoterpenes was not substantially associated with changes in substrate levels (Fig. 1B, 3B, and Fig. S2). Given that longer term emission kinetics of mono- and sesquiterpenes were observed in this study, we cannot exclude the possibility of upregulation of both mono- and sesquiterpene synthase gene expression and terminal enzyme activities in MEP and MVA pathways, leading to sustained emission rates of mono- and sesquiterpenes. In support of this suggestion, previous studies reported that exogenous applications of MeJA to Norway spruce (Picea abies) trees resulted in enhanced transcript abundance of mono-and diterpenoid synthases in stem tissues (Fäldt et al., 2003) and induced activity of both constitutive and induced terpene synthases in foliage of Norway spruce (Martin et al., 2003). In addition, the higher emission bursts of mono- and sesquiterpenes, particularly at day 3 after stress applications, could also have been induced by jasmonate-dependent gene expression, analogously with the elicitation of LOX emissions (Fig. 3A - 3C, and Fig. S1 - S3).
As sesquiterpene biosynthesis is not dependent on photosynthetic intermediates, the time-dependent modifications of their emission rate was likely regulated by sesquiterpene synthase gene expression and/or sesquiterpene synthase enzyme activity upon separate and combined bacterial and MeJA treatments. Consistent with our suggestions, previous studies reported that application of biotic stresses upregulated the transcript abundance of multiple terpene synthase genes (TPS) (Irmisch et al., 2014). For example, separate white pine weevils (Pissodes strobi) attack and exogenous MeJA treatment on Sitka spruce (Picea sitchensis) stem led to an upregulation of mono-, sesqui- and diterpene synthase gene expression, associated with enhanced terpene emissions in Sitka spruce stem (Miller et al., 2005). Similarly, western balsam poplar (Populus trichocarpa) leaves fed by herbivore gypsy moth (Lymantria dispar) was accumulated with multiple TPS transcripts, eventually leading to higher emission burst of volatile terpenoids (Irmisch et al., 2014).
Present study indicated the total emission rates of mono-and sesquiterpenes quantitatively scaled with the severity of MeJA doses (Fig. 3B and 3C). This is similar to the impact of herbivory feeding on the emission of mono- and sesquiterpenes, where the emission rates are quantitatively related with the percentage of leaf area eaten by geometrid moth Cabera pusaria on Alnus glutinosa (Copolovici et al., 2014a; Copolovici et al., 2011) and with the necrotic area of rust fungus (Melampsora larici-populina) infection in Populus balsamifera var. suaveolens leaves (Jiang et al., 2016). Although minor, we cannot ignore the possibility that the emission rate of oxygenated monoterpenes, β-linalool and 1,8-cineole and a sesquiterpene, β-farnesene are generally regulated by stomatal conductance, whereas the emission rate of other mono- and sesquiterpenes are insensitive to stomatal conductance (Loreto et al., 1996; Niinemets and Reichstein, 2003) (Fig. 1C, and Fig. S2D and S3D).
4.5. Contribution of de novo synthesized and stored terpenes to the overall emission blend of mono-and sesquiterpenes
In our study, the monoterpenes both in emission blend and storage pools were dominated by 1,8-cineole, followed by α-pinene (Fig. S2, and Table 4). This suggests that a greater share of these two abundant monoterpene emissions were primarily released from storage pools. In fact, the emission blend of sesquiterpenes was dominated by α-copaene, followed by Δ-cadinene, but the storage pool of sesquiterpene was dominated by aromadendrene, followed by globulol. Thus, a major proportion of α-copaene and Δ-cadinene emissions were mainly originated from de novo synthesis (Fig. S3, and Table 4). In particular, hexane extract analysis indicated that monoterpenes (Z)-allo-ocimene, m-cymene, (E)-β-ocimene, and (Z)-β-ocimene, and sesquiterpenes (E)-β-farnesene and longifolene were exclusively de novo biosynthesized (Fig. S2 and Table 4). Taken together, temporal variations in the emissions of (Z)-allo-ocimene, m-cymene, (E)-β-ocimene, and (Z)-β-ocimene, (E)-β-farnesene, and longifolene upon foliage inoculation by B. vietnamiensis demonstrated that bacterial inoculation significantly modulated both the MEP and MVA pathways on a time-dependent manner.
4.6. Temporal regulation of other stress volatile emissions in response to MeJA and bacterial inoculation
Application of separate MeJA increased foliage saturated aldehydes, lightweight oxygenated compounds, benzenoids, geranyl-geranyl diphosphate pathway volatiles, compared to controls. In most of the cases, the emission responses were positively correlated with the severity of stress applications (Fig. 3D - 3G, and Fig. S4 - S6).
The exact biosynthesis pathway and mechanism for saturated aldehyde remains speculative; however, lower molecular mass compounds, C5 and C6 might partially share the lipoxygenase or hydroperoxide lyase pathway (Heiden et al., 2003; Wildt et al., 2003). The present work indicated the emission rate of saturated aldehydes observed for 7 days upon separate and combined application of bacteria and MeJA treatments, suggesting that separate and combined application of bacterial and MeJA treatments simulated the gene expression responses for saturated aldehydes (Fig. 3D, and Fig. S4). In addition, the emission rate of saturated aldehydes highly resembled LOX pathway volatiles and their emission rates were ca. 5-6 fold higher than LOX volatiles. Therefore, given that it is a late-induced MeJA response, LOX pathway volatiles could have primed for the emission of saturated aldehydes to some extent as suggested by Wildt et al. (2003) (Fig. 3A and 3D, and Fig. S1 and S4). Similar to our findings, the increased saturated aldehyde emissions were observed for ozone exposure and pathogen attack for six different plant species (Wildt et al., 2003), ozone exposure (Kanagendran et al., 2017), ozone and wounding treatments (Kanagendran et al., 2018a), heat stress (Kask et al., 2016) and MeJA treatment (Jiang et al., 2017a). A significant reduction of saturated aldehydes after combined application of bacteria and MeJA, compared to separate MeJA treatments was likely due to bacteria-mediated abolishment of hypersensitive reactions to some extent.
Acetaldehyde and acetone were the main volatile compounds constituting the blend of light weight oxygenated compounds in our study (Fig. 3E, and Fig. S5A and S5B). Acetaldehyde is produced primarily due to oxidative reaction of lipoxygenase enzyme with C18 fatty acids in cell membranes (Blokhina et al., 2003). Generally, inhibiting mitochondrial respiration and pyruvate transport, which possibly enhance pyruvate concentrations in the cytoplasm, often resulted in induced acetaldehyde emissions (Jardine et al., 2009). Thus, we argue that these reactions could be enhanced upon hypersensitive reactions caused by acute MeJA exposures.
A mechanistic explanation for acetone biosynthesis in plants upon stresses is remained obscure and very few studies were investigated the emission of acetone (Bracho-Nunez et al., 2011; Macdonald and Fall, 1993). However, it was postulated that cyanogenic pathway could be responsible for the formation of acetone (Bracho-Nunez et al., 2011). In fact, we cannot exclude the possibility that acetone can also be formed from acetoacetate metabolism (Macdonald and Fall, 1993). In addition, higher acetone emission is reciprocally related with enhanced need for defense responses (Bracho-Nunez et al., 2011). Thus, in our study, the emission rate of acetone for the combined bacterial and MeJA treatment was lower than separate MeJA treatments (Fig. S5B), suggesting that inoculation by bacteria reduced “the demand” for enhanced plant-defense responses.
GGDP pathway products are biosynthesized in plastids as a product of oxidative cleavage of carotenoids (Goff and Klee, 2006; Memari et al., 2013; Tieman et al., 2006) and thus, the emission pattern of these volatiles likely indicate the carotenoid turnover during plant metabolism (Beisel et al., 2010). There were two key volatile compounds observed under GGDP pathway volatile category: geranyl acetone and 6-methyl-5-hepten-2-one (Fig. S5C and S5D). Geranyl acetone is a C13 acyclic product, probably synthesized from phytoene, phytofluene or δ-carotene (Simkin et al., 2004). In fact, separate and combined application of bacteria and MeJA changed the emission rates of GGDP pathway volatiles and thus, both factors affected the carotenoid turnover upon stress applications. In consistent with our study, acute MeJA treatments increased the emission of GGDP pathway volatiles (Jiang et al., 2017a).
Plant benzenoids are produced in shikimate pathway (Schuurink et al., 2006). The shikimate pathway is coupled with carbohydrate metabolism for the synthesis of aromatic amino acids, including benzenoids (Schuurink et al., 2006). However, benzenoid biosynthesis is primarily regulated by transcriptional control of the shikimate pathway, particularly by benzenoid biosynthesis genes and S-adenosyl-methionine cycle genes (Schuurink et al., 2006). In addition, biosynthesis of benzenoids is inversely controlled by ethylene (Schuurink et al., 2006). There were four benzenoid compounds detected in our study: benzaldehyde, toluene, o-xylene and p-xylene and their emission rates were greatly varied in a time-dependent manner. In fact, there was no relationship between MeJA-dose and emission responses for benzenoid products (Fig. 3G, and Fig. S6). In this study, we did not detect common stress-induced volatile, methyl salicylate (MeSA), the emission of which is often reported upon biotic stress, including phytophagous spider mite (Tetranychus urticae) attack on lima bean (Arimura et al., 2002), soybean aphid (Aphis glycines) feeding on soybean (Zhu and Park, 2005), and phytophagous spider mite (Tetranychus urticae) attack on tomato (Ament et al., 2004). Altogether, our data suggest that endophytic bacteria B. vietnamiensis inoculation primarily regulated the expression of genes in shikimate pathway.
4.7. Modifications in the volatile blend in response to separate and combined applications of MeJA and bacteria
PCA analysis indicated that in all cases, foliage isoprene was differently positioned from the clusters, indicating that it was differentially regulated from other stress volatiles. In addition, higher correlation of volatile emissions occurred at day 3 and day 7 after treatments, indicating that all treatments collectively altered all volatile biosynthesized pathways at day 3 and day 7 after treatments (Fig. 4A, B, and C). PCA further indicates that the correlation between compound classes was varied in each day after treatments. Furthermore, loading plots of PLS-DA illustrated that various treatments were differentially influenced by given volatile compounds, suggesting that there should be a selective advantage of biosynthesizing given volatile classes due to the unique physiological impact of a particular stress application in plants. In this case, further studies are required to understand the clear-cut relationship between the emission of given volatile classes with unique stress application (Fig. 4D, E, and F).
5. Conclusion
In summary, the present study is the first report demonstrating the possibility of foliage application of endophytic bacteria B. vietnamiensis CBMB40 to enhance resistance in E. grandis for MeJA treatments. As reflected in decreased VOC emission and higher photosynthesis rates in E. grandis leaves primed by B. vietnamiensis and subsequently treated with MeJA, bacterial inoculation considerably improved plant stress resistance against acute MeJA treatments. Furthermore, the reduction in stress-induced VOC emission rates and increment in photosynthetic traits upon combined bacterial and MeJA treatments, compared to separate MeJA treatments further imply that substantially lower carbon was consumed for the biosynthesis of VOCs, potentially contributing to a greater productivity in response to elevated MeJA treatments. This adaptive trait will lead to greater carbon assimilation in unaffected leaf area upon herbivore feeding or pathogen infection. However, further molecular level studies are required to understand the mechanism of how foliage spray of B. vietnamiensis improving resistance against MeJA through their effects on jasmonate dependent defense pathways and how it modifies the pool size of ACC, antioxidants, and plant growth regulators in leaves, collectively associated with enhanced stress resistance and increased photosynthesis. Certainly, more relevant PGPR strains and their longer term impact on primary and secondary metabolic activity of plants upon acute MeJA exposures should be tested to gain insight into sensitivity of the suggested method. Furthermore, we showed that estimating the severity of stress by distinctive volatile emission kinetics provides diagnostic information for rapid screening of potential PGPR strains and rate of stress resistance developed over time.
This work further underscores the feasibility of foliage spray of B. vietnamiensis in realistic biological settings in fields that would be possible to cope with biotic stresses, associated with greater economic losses in crops. Previous reports reveal that some PGPR including certain endophytic Burkholderia spp. not only colonize in rhizosphere but also in internal tissues of plants and significantly express plant growth-promoting effects in internal plant tissues, where they colonize (Compant et al., 2010; Compant et al., 2008; Hallmann and Berg, 2006; Sessitsch et al., 2004). Therefore, the mode of B. vietnamiensis bacteria improving stress tolerance in the present observational study may likely be similar to that applied in rhizosphere. However, future research should compare the stress resistance potential of B. vietnamiensis in response to MeJA treatments and real biotic stress episodes.
Supplementary Material
Table 5.
Terpene contents in untreated leaves of E. grandis. Data are averages (± SE) of six independent replicates. The concentration of terpenoid compounds (Mean ± SE) was rounded up to the nearest whole number.
| Foliage terpene contents (nmol g−1 DM) |
||
|---|---|---|
| Monoterpenoids | Mean ± SE | |
| 1 | α-Thujene | 20 ± 10 |
| 2 | α-Pinene | 13600 ± 2700 |
| 3 | α-Fenchene | 40 ± 20 |
| 4 | Camphene | 120 ± 70 |
| 5 | β-Pinene | 80 ± 20 |
| 6 | β-Myrcene | 200 ± 120 |
| 7 | α-Phellendrene | 200 ± 60 |
| 8 | Δ3-Carene | 100 ± 50 |
| 9 | α-Terpinene | 100 ± 70 |
| 10 | p-Cymene | 3200 ± 400 |
| 11 | Limonene | 2300 ± 800 |
| 12 | 1,8-Cineole | 30700 ± 10800 |
| 13 | δ-Terpinene | 10400 ± 4300 |
| 14 | α-Terpinolene | 1700 ± 1000 |
| 15 | Linalool | 3 ± 1 |
| 16 | 6-Camphenol | 20 ± 10 |
| 17 | 1,3,8-p-Menthatriene | 6 ± 4 |
| 18 | β-Fenchyl alcohol | 60 ± 30 |
| 19 | (E)-Pinocarveol | 100 ± 50 |
| 20 | Borneol | 600 ± 200 |
| 21 | Terpinen-4-ol | 1900 ± 800 |
| 22 | α-Terpineol | 1560 ± 360 |
| 23 | (E)-Geraniol | 90 ± 50 |
| 24 | (Z)-Allo-ocimene nd | - |
| 25 | m-Cymene nd | - |
| 26 | (E)-β-Ocimene nd | - |
| 27 | (Z)-β-Ocimene nd | - |
| Sesquiterpenoids | ||
| 1 | Isoledene | 100 ± 60 |
| 2 | α-Copaene | 50 ± 20 |
| 3 | α-Gurjunene | 1050 ± 600 |
| 4 | β-Gurjunene | 140 ± 70 |
| 5 | (E)-β-Caryophyllene | 60 ± 30 |
| 6 | Aromadendrene | 2500 ± 1300 |
| 7 | Alloaromadendrene | 900 ± 500 |
| 8 | (E)-α-Caryophyllene | 70 ± 30 |
| 9 | Viridiflorene | 600 ± 300 |
| 10 | δ-Muurolene | 80 ± 50 |
| 11 | Germacrene D | 530 ± 200 |
| 12 | α-Bulnesene | 50 ± 30 |
| 13 | δ-Gurjunene | 50 ± 30 |
| 14 | Δ-Cadinene | 170 ± 100 |
| 15 | Globulol | 2100 ± 1100 |
| 16 | Epiglobulol | 800 ± 400 |
| 17 | β-Eudesmol | 1000 ± 500 |
| 18 | (E)-β-Farnesene nd | - |
| 19 | Longifolene nd | - |
nd, not detected in storage pools. However, all compounds labelled “nd” in this table were detected in headspace collections.
Funding
This research was supported by the grants from the European Commission through the European Research Council (advanced grant 322603, SIP-VOL+), and the European Regional Development Fund (Centre of Excellence EcolChange) and the Estonian Ministry of Science and Education (institutional grant IUT-8-3), Republic of Estonia, and the basic science research program through the National Research foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2015R1A2A1A05001885), Republic of Korea.
Footnotes
Conflict of Interest
None
References
- Abd El-Daim IA, Bejai S, Meijer J. Improved heat stress tolerance of wheat seedlings by bacterial seed treatment. Plant and Soil. 2014;379(1):337–350. [Google Scholar]
- Alboukadel K, Fabian M. R package version 1.0.5. 2017. Factoextra: extract and visualize the results of multivariate data analyses. [Google Scholar]
- Aldesuquy HS. Effect of Indol-3-yl acetic acid on photosynthetic characteristics of wheat flag leaf during grain filling. Photosynthetica. 2000;38(1):135–141. [Google Scholar]
- Ament K, Kant MR, Sabelis MW, Haring MA, Schuurink RC. Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiology. 2004;135(4):2025–2037. doi: 10.1104/pp.104.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LJ, Harley PC, Monson RK, Jackson RB. Reduction of isoprene emissions from live oak (Quercus fusiformis) with oak wilt. Tree Physiology. 2000;20(17):1199–1203. doi: 10.1093/treephys/20.17.1199. [DOI] [PubMed] [Google Scholar]
- Arimura Gi, Ozawa R, Nishioka T, Boland W, Koch T, Kühnemann F, Takabayashi J. Herbivore-induced volatiles induce the emission of ethylene in neighboring lima bean plants. The Plant Journal. 2002;29(1):87–98. doi: 10.1046/j.1365-313x.2002.01198.x. [DOI] [PubMed] [Google Scholar]
- Beisel K, Jahnke S, Hofmann D, Köppchen S, Schurr U, Matsubara S. Continuous turnover of carotenes and chlorophyll a in mature leaves of arabidopsis revealed by 14CO2 pulse-chase labeling. Plant Physiology. 2010;152:2188–2199. doi: 10.1104/pp.109.151647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabdellah K, Abbas Y, Abourouh M, Aroca R, Azcón R. Influence of two bacterial isolates from degraded and non-degraded soils and arbuscular mycorrhizae fungi isolated from semi-arid zone on the growth of Trifolium repens under drought conditions: mechanisms related to bacterial effectiveness. European Journal of Soil Biology. 2011;47(5):303–309. [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany. 2003;91(2):179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracho-Nunez A, Welter S, Staudt M, Kesselmeier J. Plant-specific volatile organic compound emission rates from young and mature leaves of mediterranean vegetation. Journal of Geophysical Research: Atmospheres. 2011;116(D16) [Google Scholar]
- Brüggemann N, Schnitzler J-P. Influence of powdery mildew (Microsphaera alphitoides) on isoprene biosynthesis and emission of pedunculate oak (Quercus robur L.) leaves. Journal of applied botany - Angewandte Botanik. 2001;75(3/4):91–96. [Google Scholar]
- Bruinsma M, Posthumus MA, Mumm R, Mueller MJ, van Loon JJA, Dicke M. Jasmonic acid-induced volatiles of Brassica oleracea attract parasitoids: effects of time and dose, and comparison with induction by herbivores. Journal of Experimental Botany. 2009;60(9):2575–2587. doi: 10.1093/jxb/erp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P, Kanagendran A, Samaddar S, Pazouki L, Sa T-M, Niinemets Ü. Inoculation of Brevibacterium linens RS16 in Oryza sativa genotypes enhanced salinity resistance: Impacts on photosynthetic traits and foliar volatile emissions. Science of The Total Environment. 2018a;645:721–732. doi: 10.1016/j.scitotenv.2018.07.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P, Samaddar S, Anandham R, Kang Y, Kim K, Selvakumar G, Sa T. Beneficial soil bacterium Pseudomonas frederiksbergensis OS261 augments salt tolerance and promotes red pepper plant growth. Frontiers in Plant Science. 2017;8(705) doi: 10.3389/fpls.2017.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P, Samaddar S, Niinemets Ü, Sa T-M. Brevibacterium linens RS16 confers salt tolerance to Oryza sativa genotypes by regulating antioxidant defense and H+ ATPase activity. Microbiological Research. 2018b;215:89–101. doi: 10.1016/j.micres.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Coenye T, Vandamme P. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environmental Microbiology. 2003;5(9):719–729. doi: 10.1046/j.1462-2920.2003.00471.x. [DOI] [PubMed] [Google Scholar]
- Compant S, Clément C, Sessitsch A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biology and Biochemistry. 2010;42(5):669–678. [Google Scholar]
- Compant S, Kaplan H, Sessitsch A, Nowak J, Ait Barka E, Clément C. Endophytic colonization of Vitis vinifera L. by Burkholderia phytofirmans strain PsJN: from the rhizosphere to inflorescence tissues. FEMS Microbiology Ecology. 2008;63(1):84–93. doi: 10.1111/j.1574-6941.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Remmel T, Niinemets Ü. Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environmental and Experimental Botany. 2014a;100:55–63. doi: 10.1016/j.envexpbot.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Remmel T, Vislap V, Niinemets Ü. Volatile emissions from Alnus glutionosa induced by herbivory are quantitatively related to the extent of damage. Journal of Chemical Ecology. 2011;37(1):18–28. doi: 10.1007/s10886-010-9897-9. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Niinemets Ü. Flooding induced emissions of volatile signalling compounds in three tree species with differing waterlogging tolerance. Plant, Cell & Environment. 2010;33(9):1582–1594. doi: 10.1111/j.1365-3040.2010.02166.x. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Niinemets Ü. Environmental impacts on plant volatile emission. In: Blande JD, Glinwood R, editors. Deciphering Chemical Language of Plant Communication. Springer International Publishing; Cham: 2016. pp. 35–59. [Google Scholar]
- Copolovici L, Väärtnõu F, Estrada MP, Niinemets Ü. Oak powdery mildew (Erysiphe alphitoides) induced volatile emissions scale with the degree of infection in Quercus robur. Tree physiology. 2014b;34(12):1399–1410. doi: 10.1093/treephys/tpu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esitken A, Karlidag H, Ercisli S, Sahin F. Effects of foliar application of Bacillus subtilis OSU142 on the yield, growth and control of shot-hole disease (Coryneum blight) of apricot. Gartenbauwissenschaft. 2002;67(4):139–142. [Google Scholar]
- Esitken A, Karlidag H, Ercisli S, Turan M, Sahin F. The effect of spraying a growth promoting bacterium on the yield, growth and nutrient element composition of leaves of apricot (Prunus armeniaca L. cv. Hacihaliloglu) Australian Journal of Agricultural Research. 2003;54(4):377–380. [Google Scholar]
- Esitken A, Pirlak L, Turan M, Sahin F. Effects of floral and foliar application of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrition of sweet cherry. Scientia Horticulturae. 2006;110(4):324–327. [Google Scholar]
- Esitken A, Yildiz HE, Ercisli S, Figen Donmez M, Turan M, Gunes A. Effects of plant growth promoting bacteria (PGPB) on yield, growth and nutrient contents of organically grown strawberry. Scientia Horticulturae. 2010;124(1):62–66. [Google Scholar]
- Estrada-De Los Santos P, Bustillos-Cristales Ro, Caballero-Mellado J. Burkholderia, a genus rich in plant-associated nitrogen fixers with wide environmental and geographic distribution. Applied and Environmental Microbiology. 2001;67(6):2790–2798. doi: 10.1128/AEM.67.6.2790-2798.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fäldt J, Martin D, Miller B, Rawat S, Bohlmann J. Traumatic resin defense in norway spruce (Picea abies): methyl jasmonate-induced terpene synthase gene expression, and cDNA cloning and functional characterization of (+)-3-carene synthase. Plant Molecular Biology. 2003;51(1):119–133. doi: 10.1023/a:1020714403780. [DOI] [PubMed] [Google Scholar]
- Feussner I, Wasternack C. The lipoxygenase pathway. Annual Review of Plant Biology. 2002;53(1):275–297. doi: 10.1146/annurev.arplant.53.100301.135248. [DOI] [PubMed] [Google Scholar]
- Figueiredo MVB, Burity HA, Martínez CR, Chanway CP. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Applied Soil Ecology. 2008;40(1):182–188. [Google Scholar]
- Frost C, Mescher M, Dervinis C, Davis J, Carlson J, De Moraes C. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytologist. 2008;180(3):722–734. doi: 10.1111/j.1469-8137.2008.02599.x. [DOI] [PubMed] [Google Scholar]
- Funk JL, Giardina CP, Knohl A, Lerdau MT. Influence of nutrient availability, stand age, and canopy structure on isoprene flux in a Eucalyptus saligna experimental forest. Journal of Geophysical Research: Biogeosciences. 2006;111(G2) [Google Scholar]
- Gaston S. R package version 0.1-29. 2013. DiscriMiner: tools of the trade for discriminant analysis. [Google Scholar]
- Goff SA, Klee HJ. Plant volatile compounds: sensory cues for health and nutritional value? Science. 2006;311(5762):815–819. doi: 10.1126/science.1112614. [DOI] [PubMed] [Google Scholar]
- Goodger JQD, Seneratne SL, Nicolle D, Woodrow IE. Foliar essential oil glands of Eucalyptus subgenus Eucalyptus (Myrtaceae) are a rich source of flavonoids and related non-volatile constituents. PLOS ONE. 2016;11(3):e0151432. doi: 10.1371/journal.pone.0151432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote R, Monson RK, Niinemets Ü. Leaf-level models of constitutive and stress-driven volatile organic compound emissions. In: Niinemets Ü, Monson RK, editors. Biology, Controls and Models of Tree Volatile Organic Compound Emissions. Springer Netherlands; Dordrecht: 2013. pp. 315–355. [Google Scholar]
- Grote R, Niinemets Ü. Modeling volatile isoprenoid emissions – a story with split ends. Plant Biology. 2008;10(1):8–28. doi: 10.1055/s-2007-964975. [DOI] [PubMed] [Google Scholar]
- Guenther AB, Monson RK, Fall R. Isoprene and monoterpene emission rate variability: observations with Eucalyptus and emission rate algorithm development. Journal of Geophysical Research: Atmospheres. 1991;96(D6):10799–10808. [Google Scholar]
- Hallmann J, Berg G. Spectrum and population dynamics of bacterial root endophytes. In: Schulz BJE, Boyle CJC, Sieber TN, editors. Microbial Root Endophytes. Springer Berlin Heidelberg; Berlin, Heidelberg: 2006. pp. 15–31. [Google Scholar]
- He C, Murray F, Lyons T. Monoterpene and isoprene emissions from 15 Eucalyptus species in Australia. Atmospheric Environment. 2000;34(4):645–655. [Google Scholar]
- Heiden AC, Kobel K, Langebartels C, Schuh-Thomas G, Wildt J. Emissions of oxygenated volatile organic compounds from plants part I: Emissions from lipoxygenase activity. Journal of Atmospheric Chemistry. 2003;45(2):143–172. [Google Scholar]
- Heijari J, Nerg AM, Kainulainen P, Viiri H, Vuorinen M, Holopainen JK. Application of methyl jasmonate reduces growth but increases chemical defence and resistance against Hylobius abietis in Scots pine seedlings. Entomologia Experimentalis et Applicata. 2005;115(1):117–124. [Google Scholar]
- Heil M, Ton J. Long-distance signalling in plant defence. Trends in Plant Science. 2008;13(6):264–272. doi: 10.1016/j.tplants.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Henery ML, Wallis IR, Stone C, Foley WJ. Methyl jasmonate does not induce changes in Eucalyptus grandis leaves that alter the effect of constitutive defences on larvae of a specialist herbivore. Oecologia. 2008;156(4):847–859. doi: 10.1007/s00442-008-1042-x. [DOI] [PubMed] [Google Scholar]
- Holzke C, Hoffmann T, Jaeger L, Koppmann R, Zimmer W. Diurnal and seasonal variation of monoterpene and sesquiterpene emissions from Scots pine (Pinus sylvestris L.) Atmospheric Environment. 2006;40(17):3174–3185. [Google Scholar]
- Burkholderia vietnamiensis G4 chromosome 2. [Accessed 24 May 2018];2018 http://www.ecogene.org, http://www.ecogene.org/refseq/refseq/NC_009255/searchtool///protein///all, NCBI reference sequence accession: NC 009255.
- Hyang SD, Saem J, Jeong PS, Jung KY, Min AJ, Wankee K, Wonja C. Characterization of thiol-specific antioxidant 1 (TSA1) of Candida albicans. Yeast. 2005;22(11):907–918. doi: 10.1002/yea.1283. [DOI] [PubMed] [Google Scholar]
- Irmisch S, Jiang Y, Chen F, Gershenzon J, Köllner TG. Terpene synthases and their contribution to herbivore-induced volatile emission in western balsam poplar (Populus trichocarpa) BMC Plant Biology. 2014;14:270. doi: 10.1186/s12870-014-0270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Choudhary DK. Induced defense-related proteins in soybean (Glycine max L. Merrill) plants by Carnobacterium sp. SJ-5 upon challenge inoculation of Fusarium oxysporum. Planta. 2014;239(5):1027–1040. doi: 10.1007/s00425-014-2032-3. [DOI] [PubMed] [Google Scholar]
- Jardine K, Karl T, Lerdau M, Harley P, Guenther A, Mak JE. Carbon isotope analysis of acetaldehyde emitted from leaves following mechanical stress and anoxia. Plant Biology. 2009;11(4):591–597. doi: 10.1111/j.1438-8677.2008.00155.x. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Veromann-Jürgenson L-L, Ye J, Niinemets Ü. Oak gall wasp infections of Quercus robur leaves lead to profound modifications in foliage photosynthetic and volatile emission characteristics. Plant, Cell & Environment. 2017b;41(1):160–175. doi: 10.1111/pce.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Ye J, Li S, Niinemets Ü. Methyl jasmonate-induced emission of biogenic volatiles is biphasic in cucumber: a high-resolution analysis of dose dependence. Journal of Experimental Botany. 2017a;68(16):4679–4694. doi: 10.1093/jxb/erx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Ye J, Veromann L-L, Niinemets Ü. Scaling of photosynthesis and constitutive and induced volatile emissions with severity of leaf infection by rust fungus (Melampsora larici-populina) in Populus balsamifera var. suaveolens. Tree Physiology. 2016;36(7):856–872. doi: 10.1093/treephys/tpw035. [DOI] [PubMed] [Google Scholar]
- Kadaikunnan S, Rejiniemon TS, Khaled JM, Alharbi NS, Mothana R. In-vitro antibacterial, antifungal, antioxidant and functional properties of Bacillus amyloliquefaciens. Annals of Clinical Microbiology and Antimicrobials. 2015;14:9. doi: 10.1186/s12941-015-0069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagendran A, Pazouki L, Bichele R, Külheim C, Niinemets Ü. Temporal regulation of terpene synthase gene expression in Eucalyptus globulus leaves upon ozone and wounding stresses: relationships with stomatal ozone uptake and emission responses. Environmental and Experimental Botany. 2018b;155:552–565. doi: 10.1016/j.envexpbot.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagendran A, Pazouki L, Li S, Liu B, Kännaste A, Niinemets Ü. Ozone-triggered surface uptake and stress volatile emissions in Nicotiana tabacum ‘Wisconsin’. Journal of Experimental Botany. 2017;69(3):681–697. doi: 10.1093/jxb/erx431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagendran A, Pazouki L, Niinemets Ü. Differential regulation of volatile emission from Eucalyptus globulus leaves upon single and combined ozone and wounding treatments through recovery and relationships with ozone uptake. Environmental and Experimental Botany. 2018a;145:21–38. doi: 10.1016/j.envexpbot.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kännaste A, Copolovici L, Niinemets Ü. Gas chromatography–mass spectrometry method for determination of biogenic volatile organic compounds emitted by plants. In: Rodríguez-Concepción M, editor. Plant Isoprenoids: Methods and Protocols. Springer New York; New York, NY: 2014. pp. 161–169. [DOI] [PubMed] [Google Scholar]
- Kappers IF, Verstappen FWA, Luckerhoff LLP, Bouwmeester HJ, Dicke M. Genetic variation in jasmonic acid- and spider mite-induced plant volatile emission of cucumber accessions and attraction of the predator phytoseiulus persimilis. Journal of Chemical Ecology. 2010;36(5):500–512. doi: 10.1007/s10886-010-9782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kask K, Kännaste A, Talts E, Copolovici L, Niinemets Ü. How specialized volatiles respond to chronic, and short-term physiological and shock heat stress in Brassica nigra: Responses of Brassica nigra to heat stress. Plant, Cell & Environment. 2016;39(9):2027–2042. doi: 10.1111/pce.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsir L, Chung HS, Koo AJK, Howe GA. Jasmonate signaling: a conserved mechanism of hormone sensing. Current Opinion in Plant Biology. 2008;11(4):428–435. doi: 10.1016/j.pbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koevoets IT, Venema JH, Elzenga JTM, Testerink C. Roots withstanding their environment: exploiting root system architecture responses to abiotic stress to improve crop tolerance. Frontiers in Plant Science. 2016;7(1335) doi: 10.3389/fpls.2016.01335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmann M, Bachmann A, Weichert H, Kolbe A, Balkenhohl T, Wasternack C, Feussner I. Formation of lipoxygenase-pathway-derived aldehydes in barley leaves upon methyl jasmonate treatment. European Journal of Biochemistry. 1999;260(3):885–895. doi: 10.1046/j.1432-1327.1999.00231.x. [DOI] [PubMed] [Google Scholar]
- Külheim C, Padovan A, Hefer C, Krause ST, Köllner TG, Myburg AA, Degenhardt J, Foley WJ. The Eucalyptus terpene synthase gene family. BMC Genomics. 2015;16(1):450. doi: 10.1186/s12864-015-1598-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalel HJD, Singh Z, Tan SC. The role of methyl jasmonate in mango ripening and biosynthesis of aroma volatile compounds. The Journal of Horticultural Science and Biotechnology. 2003;78(4):470–484. [Google Scholar]
- Lee HS, Madhaiyan M, Kim CW, Choi SJ, Chung KY, Sa TM. Physiological enhancement of early growth of rice seedlings (Oryza sativa L.) by production of phytohormone of N2-fixing methylotrophic isolates. Biology and Fertility of Soils. 2006;42(5):402–408. [Google Scholar]
- Li HQ, Jiang XW. Inoculation with plant growth-promoting bacteria (PGPB) improves salt tolerance of maize seedling. Russian Journal of Plant Physiology. 2017;64(2):235–241. [Google Scholar]
- Li S, Harley PC, Niinemets Ü. Ozone-induced foliar damage and release of stress volatiles is highly dependent on stomatal openness and priming by low-level ozone exposure in Phaseolus vulgaris. Plant, Cell & Environment. 2017;40(9):1984–2003. doi: 10.1111/pce.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YS, Kim HK, Lefeber AWM, Erkelens C, Choi YH, Verpoorte R. Identification of phenylpropanoids in methyl jasmonate treated Brassica rapa leaves using two-dimensional nuclear magnetic resonance spectroscopy. Journal of Chromatography A. 2006;1112(1):148–155. doi: 10.1016/j.chroma.2005.11.114. [DOI] [PubMed] [Google Scholar]
- Liu B, Kaurilind E, Jiang Y, Niinemets Ü. Methyl salicylate differently affects benzenoid and terpenoid volatile emissions in Betula pendula. Tree Physiology. 2018;38(10):1513–1525. doi: 10.1093/treephys/tpy050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loivamäki M, Holopainen JK, Nerg A-M. Chemical changes induced by methyl jasmonate in oilseed rape grown in the laboratory and in the field. Journal of Agricultural and Food Chemistry. 2004;52(25):7607–7613. doi: 10.1021/jf049027i. [DOI] [PubMed] [Google Scholar]
- Loreto F, Ciccioli P, Cecinato A, Brancaleoni E, Frattoni M, Tricoli D. Influence of environmental factors and air composition on the emission of α-pinene from Quercus ilex leaves. Plant Physiology. 1996;110(1):267–275. doi: 10.1104/pp.110.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RC, Fall R. Acetone emission from conifer buds. Phytochemistry. 1993;34(4):991–994. [Google Scholar]
- Madhaiyan M, Poonguzhali S, Kwon S-W, Song M-H, Sa T. Molecular characterization of Burkholderia strains isolated from rice cultivars (Oryza sativa L.) for species identification and phylogenetic grouping. J Microbiol Biotechnol. 2008;18(6):1005–1010. [PubMed] [Google Scholar]
- Mäntylä E, Blande JD, Klemola T. Does application of methyl jasmonate to birch mimic herbivory and attract insectivorous birds in nature? Arthropod-Plant Interactions. 2014;8(2):143–153. [Google Scholar]
- Martin D, Tholl D, Gershenzon J, Bohlmann J. Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of norway spruce stems. Plant Physiology. 2002;129(3):1003–1018. doi: 10.1104/pp.011001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DM, Gershenzon J, Bohlmann J. Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of norway spruce. Plant Physiology. 2003;132(3):1586–1599. doi: 10.1104/pp.103.021196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastouri F, Björkman T, Harman GE. Trichoderma harzianum enhances antioxidant defense of tomato seedlings and resistance to water deficit. Molecular Plant-Microbe Interactions. 2012;25(9):1264–1271. doi: 10.1094/MPMI-09-11-0240. [DOI] [PubMed] [Google Scholar]
- Memari HR, Pazouki L, Niinemets Ü. The biochemistry and molecular biology of volatile messengers in trees. In: Niinemets Ü, Monson RK, editors. Biology, Controls and Models of Tree Volatile Organic Compound Emissions. Springer Netherlands; Dordrecht: 2013. pp. 47–93. [Google Scholar]
- Mickelbart MV, Hasegawa PM, Bailey-Serres J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nature Reviews Genetics. 2015;16:237. doi: 10.1038/nrg3901. [DOI] [PubMed] [Google Scholar]
- Miller B, Madilao LL, Ralph S, Bohlmann J. Insect-induced conifer defense. white pine weevil and methyl jasmonate induce traumatic resinosis, de novo formed volatile emissions, and accumulation of terpenoid synthase and putative octadecanoid pathway transcripts in Sitka Spruce. Plant Physiology. 2005;137(1):369–382. doi: 10.1104/pp.104.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes MCB, Laumann RA, Pareja M, Sereno FTPS, Michereff MFF, Birkett MA, Pickett JA, Borges M. Attraction of the stink bug egg parasitoid Telenomus podisi to defence signals from soybean activated by treatment with cis-jasmone. Entomologia Experimentalis et Applicata. 2009;131(2):178–188. [Google Scholar]
- Myburg AA, Grattapaglia D, Tuskan GA, Hellsten U, Hayes RD, Grimwood J, Jenkins J, Lindquist E, Tice H, Bauer D, Goodstein DM, et al. The genome of Eucalyptus grandis. Nature. 2014;510:356. doi: 10.1038/nature13308. [DOI] [PubMed] [Google Scholar]
- Nguyen TM, Kim J. Antifungal and antibacterial activities of Streptomyces polymachus sp. nov. isolated from soil. International Journal of Systematic and Evolutionary Microbiology. 2015;65(8):2385–2390. doi: 10.1099/ijs.0.000268. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. Mild versus severe stress and BVOCs: thresholds, priming and consequences. Trends in Plant Science. 2010;15(3):145–153. doi: 10.1016/j.tplants.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Kännaste A, Copolovici L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Frontiers in Plant Science. 2013;4(262) doi: 10.3389/fpls.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Kuhn U, Harley PC, Staudt M, Arneth A, Cescatti A, Ciccioli P, Copolovici L, Geron C, Guenther A, Kesselmeier J, et al. Estimations of isoprenoid emission capacity from enclosure studies: measurements, data processing, quality and standardized measurement protocols. Biogeosciences. 2011;8(8):2209–2246. [Google Scholar]
- Niinemets Ü, Reichstein M. Controls on the emission of plant volatiles through stomata: differential sensitivity of emission rates to stomatal closure explained. Journal of Geophysical Research: Atmospheres. 2003;108(D7) [Google Scholar]
- Niinemets Ü, Reichstein M, Staudt M, Seufert G, Tenhunen JD. Stomatal constraints may affect emission of oxygenated monoterpenoids from the foliage of Pinus pinea. Plant Physiology. 2002b;130(3):1371–1385. doi: 10.1104/pp.009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates CN, Külheim C, Myburg AA, Slippers B, Naidoo S. The transcriptome and terpene profile of Eucalyptus grandis reveals mechanisms of defense against the insect pest, Leptocybe invasa. Plant and Cell Physiology. 2015;56(7):1418–1428. doi: 10.1093/pcp/pcv064. [DOI] [PubMed] [Google Scholar]
- Onofre-Lemus J, Hernández-Lucas I, Girard L, Caballero-Mellado J. ACC (1-Aminocyclopropane-1-Carboxylate) deaminase activity, a widespread trait in Burkholderia Species, and its growth-promoting effect on tomato plants. Applied and Environmental Microbiology. 2009;75(20):6581–6590. doi: 10.1128/AEM.01240-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa R, Shiojiri K, Sabelis MW, Takabayashi J. Maize plants sprayed with either jasmonic acid or its precursor, methyl linolenate, attract armyworm parasitoids, but the composition of attractants differs. Entomologia Experimentalis et Applicata. 2008;129(2):189–199. [Google Scholar]
- Pańka D, Piesik D, Jeske M, Baturo-Cieśniewska A. Production of phenolics and the emission of volatile organic compounds by perennial ryegrass (Lolium perenne L.)/Neotyphodium lolii association as a response to infection by Fusarium poae. Journal of Plant Physiology. 2013;170(11):1010–1019. doi: 10.1016/j.jplph.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Pazouki L, Niinemets Ü. Multi-substrate terpene synthases: their occurrence and physiological significance. Frontiers in Plant Science. 2016;7:1019. doi: 10.3389/fpls.2016.01019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Bichele I, Laisk A, Niinemets Ü. Competition between isoprene emission and pigment synthesis during leaf development in aspen. Plant, Cell and Environment. 2014;37:724–741. doi: 10.1111/pce.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Talts E, Kännaste A, Niinemets Ü. Bisphosphonate inhibitors reveal a large elasticity of plastidic isoprenoid synthesis pathway in isoprene-emitting hybrid aspen. Plant Physiology. 2015;168:532–548. doi: 10.1104/pp.15.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasulov B, Talts E, Niinemets Ü. Spectacular oscillations in plant isoprene emission under transient conditions explain the enigmatic CO2 response. Plant Physiology. 2016;172(4):2275–2285. doi: 10.1104/pp.16.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbothe C, Springer A, Samol I, Reinbothe S. Plant oxylipins: role of jasmonic acid during programmed cell death, defence and leaf senescence. The FEBS Journal. 2009;276(17):4666–4681. doi: 10.1111/j.1742-4658.2009.07193.x. [DOI] [PubMed] [Google Scholar]
- Repka V, Čarná M, Pavlovkin J. Methyl jasmonate-induced cell death in grapevine requires both lipoxygenase activity and functional octadecanoid biosynthetic pathway. Biologia. 2013;68(5):896–903. [Google Scholar]
- Rodrigues Cavalcante AP, Jacinto T, Machado OLT. Methyl jasmonate changes the levels of rubisco and other leaf proteins in. Ricinus communis. 1999;21(2):161–166. [Google Scholar]
- Rodriguez-Saona CR, Rodriguez-Saona LE, Frost CJ. Herbivore-induced volatiles in the perennial shrub, Vaccinium corymbosum, and their role in inter-branch signaling. Journal of Chemical Ecology. 2009;35(2):163–175. doi: 10.1007/s10886-008-9579-z. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Ohara K, Yazaki K. Gene expression and characterization of isoprene synthase from Populus alba. FEBS Letters. 2005;579(11):2514–2518. doi: 10.1016/j.febslet.2005.03.066. [DOI] [PubMed] [Google Scholar]
- Scala A, Allmann S, Mirabella R, Haring MA, Schuurink RC. Green leaf volatiles: a plant's multifunctional weapon against herbivores and pathogens. Int J Mol Sci. 2013:17781–17811. doi: 10.3390/ijms140917781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurink RC, Haring MA, Clark DG. Regulation of volatile benzenoid biosynthesis in petunia flowers. Trends in Plant Science. 2006;11(1):20–25. doi: 10.1016/j.tplants.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Semiz G, Blande JD, Heijari J, Isik K, Niinemets Ü, Holopainen JK. Manipulation of VOC emissions with methyl jasmonate and carrageenan in the evergreen conifer Pinus sylvestris and evergreen broadleaf Quercus ilex. Plant Biology. 2012;14(s1):57–65. doi: 10.1111/j.1438-8677.2011.00485.x. [DOI] [PubMed] [Google Scholar]
- Sessitsch A, Reiter B, Berg G. Endophytic bacterial communities of field-grown potato plants and their plant-growth-promoting and antagonistic abilities. Canadian Journal of Microbiology. 2004;50(4):239–249. doi: 10.1139/w03-118. [DOI] [PubMed] [Google Scholar]
- Shi J, Ma C, Qi D, Lv H, Yang T, Peng Q, Chen Z, Lin Z. Transcriptional responses and flavor volatiles biosynthesis in methyl jasmonate-treated tea leaves. BMC Plant Biology. 2015;15:233. doi: 10.1186/s12870-015-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin AJ, Schwartz SH, Auldridge M, Taylor MG, Klee HJ. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles β-ionone, pseudoionone, and geranylacetone. The Plant Journal. 2004;40(6):882–892. doi: 10.1111/j.1365-313X.2004.02263.x. [DOI] [PubMed] [Google Scholar]
- Staudt M, Lhoutellier L. Monoterpene and sesquiterpene emissions from Quercus coccifera exhibit interacting responses to light and temperature. Biogeosciences. 2011;8:2757–2771. [Google Scholar]
- Street RA, Hewitt CN, Mennicken S. Isoprene and monoterpene emissions from a Eucalyptus plantation in Portugal. Journal of Geophysical Research: Atmospheres. 1997;102(D13):15875–15887. [Google Scholar]
- Suh HW, Hyun S-H, Kim S-H, Lee S-Y, Choi H-K. Metabolic profiling and enhanced production of phytosterols by elicitation with methyl jasmonate and silver nitrate in whole plant cultures of Lemna paucicostata. Process Biochemistry. 2013;48(10):1581–1586. [Google Scholar]
- Tamogami S, Rakwal R, Agrawal GK. Interplant communication: airborne methyl jasmonate is essentially converted into JA and JA-Ile activating jasmonate signaling pathway and VOCs emission. Biochemical and Biophysical Research Communications. 2008;376(4):723–727. doi: 10.1016/j.bbrc.2008.09.069. [DOI] [PubMed] [Google Scholar]
- Thaler JS, Farag MA, Paré PW, Dicke M. Jasmonate-deficient plants have reduced direct and indirect defences against herbivores. Ecology Letters. 2002;5(6):764–774. [Google Scholar]
- Tieman D, Zeigler M, Schmelz E, Taylor M, Bliss P, Kirst M, J Klee H. Identification of loci affecting flavor volatile emissions in tomato fruits. Journal of Experimental Botany. 2006;57(4):887–896. doi: 10.1093/jxb/erj074. [DOI] [PubMed] [Google Scholar]
- Timmusk S, Abd El-Daim IA, Copolovici L, Tanilas T, Kännaste A, Behers L, Nevo E, Seisenbaeva G, Stenström E, Niinemets Ü. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: enhanced biomass production and reduced emissions of stress volatiles. PLOS ONE. 2014;9(5):e96086. doi: 10.1371/journal.pone.0096086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toome M, Randjärv P, Copolovici L, Niinemets Ü, Heinsoo K, Luik A, Noe SM. Leaf rust induced volatile organic compounds signalling in willow during the infection. Planta. 2010;232(1):235–243. doi: 10.1007/s00425-010-1169-y. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153(4):376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Wang C-J, Yang W, Wang C, Gu C, Niu D-D, Liu H-X, Wang Y-P, Guo J-H. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLOS ONE. 2012;7(12):e52565. doi: 10.1371/journal.pone.0052565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Baldwin EA, Plotto A, Luo W, Raithore S, Yu Z, Bai J. Effect of methyl salicylate and methyl jasmonate pre-treatment on the volatile profile in tomato fruit subjected to chilling temperature. Postharvest Biology and Technology. 2015;108:28–38. [Google Scholar]
- Wasternack C, Parthier B. Jasmonate-signalled plant gene expression. Trends in Plant Science. 1997;2(8):302–307. [Google Scholar]
- Wierstra I, Kloppstech K. Differential effects of methyl jasmonate on the expression of the early light-inducible proteins and other light-regulated genes in barley. Plant Physiology. 2000;124(2):833–844. doi: 10.1104/pp.124.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildt J, Kobel K, Schuh-Thomas G, Heiden AC. Emissions of oxygenated volatile organic compounds from plants part II: emissions of saturated aldehydes. Journal of Atmospheric Chemistry. 2003;45(2):173–196. [Google Scholar]
- Wilkinson MJ, Monson RK, Trahan N, Lee S, Brown E, Jackson RB, Polley HW, Fay PA, Fall R. Leaf isoprene emission rate as a function of atmospheric CO2 concentration. Global Change Biology. 2009;15(5):1189–1200. [Google Scholar]
- Winters AJ, Adams MA, Bleby TM, Rennenberg H, Steigner D, Steinbrecher R, Kreuzwieser J. Emissions of isoprene, monoterpene and short-chained carbonyl compounds from Eucalyptus spp. in southern Australia. Atmospheric Environment. 2009;43(19):3035–3043. [Google Scholar]
- Wohlgemuth H, Mittelstrass K, Kschieschan S, Bender J, Weigel HJ, Overmyer K, Kangasjärvi J, Sandermann H, Langebartels C. Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant, Cell & Environment. 2002;25(6):717–726. [Google Scholar]
- Yang J, Kloepper JW, Ryu C-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends in Plant Science. 2009;14(1):1–4. doi: 10.1016/j.tplants.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Zahir Z, Arshad M, Frankenberger W. Plant growth promoting rhizobacteria: applications and perspectives in agriculture. Advances in Agronomy. 2003;81:97–168. [Google Scholar]
- Zhang L, Xing D. Methyl jasmonate induces production of reactive oxygen species and alterations in mitochondrial dynamics that precede photosynthetic dysfunction and subsequent cell death. Plant and Cell Physiology. 2008;49(7):1092–1111. doi: 10.1093/pcp/pcn086. [DOI] [PubMed] [Google Scholar]
- Zhu J, Park K-C. Methyl salicylate, a soybean aphid-induced plant volatile attractive to the predator Coccinella septempunctata. Journal of Chemical Ecology. 2005;31(8):1733–1746. doi: 10.1007/s10886-005-5923-8. [DOI] [PubMed] [Google Scholar]
- Zhu M, Dai S, Chen S. The stomata frontline of plant interaction with the environment-perspectives from hormone regulation. Frontiers in Biology. 2012;7(2):96–112. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.