Abstract
Actin-based protrusions driving cell migration are reinforced through positive feedback, but it is unclear how the cell restricts the eventual size of protrusions or limits positive signals to allow them to split or retract. We have identified an evolutionarily conserved regulator of the protrusion machinery, which we name CYRI (CYFIP-related Rac interactor). CYRI binds specifically to activated Rac1 via a common motif that is also found in CYFIP, the Domain of Unknown Function DUF1394; we demonstrate that DUF1394 is a new class of Rac1 binding module. CYRI-depleted cells have broad lamellipodia enriched in Scar/WAVE, but exhibit reduced protrusion-retraction dynamics. Pseudopods induced by optogenetic Rac1 activation are larger and longer-lived in the absence of CYRI. Conversely, CYRI overexpression suppresses recruitment of active Scar/WAVE complex to the cell edge, resulting in short-lived, unproductive protrusions. CYRI’s role in cell behaviour is therefore to focus positive protrusion signals and regulate pseudopod complexity and dynamics by inhibiting Scar/WAVE induced actin. As such it behaves like a “local inhibitor” predicted and described in widely accepted mathematical models, but not previously identified in living cells. CYRI is important for biological processes requiring polarity and plasticity of protrusions, including directional migration and polarization of epithelial cysts.
Introduction
Cell migration is an ancient and fundamental mechanism by which cells interact with their environment - from seeking nutrients to organizing into specialized tissues. Motile cells have the intrinsic ability to polymerise actin and dozens of proteins regulate the spatio-temporal dynamics of actin, enabling the cytoskeleton to perform complex and specialised behaviours. Protrusion formation is an indispensable process driving cell migration1. However, the relationship between de novo pseudopod generation, pseudopod splitting and retraction during cell translocation is an area of active debate2.
The Scar/WAVE complex is the main driver of Arp2/3 complex-induced branched actin networks underlying pseudopod generation. The complex consists of five subunits CYFIP, NCKAP1, Scar/WAVE, ABI, HSPC300 (see Supplementary Table 1 for nomenclature). The main Arp2/3 activating subunit, Scar/WAVE, is normally autoinhibited until activation signals cause a conformational change, leading to interaction with and activation of Arp2/3 complex 3, 4. The Scar/WAVE complex is also thought to be recruited to the plasma membrane via a patch of basic charges that are electrostatically attracted to acidic phospholipids. 3 The small GTPase Rac1 interacts with Scar/WAVE complex in vitro3, but this interaction has not yet been demonstrated in live cells. Many open questions remain about how Rac1 regulates Scar/WAVE complex and live cell studies have revealed faster dynamics of Scar/WAVE recruitment and turnover at the leading edge than Rac1 5–7.
While protrusion and actin assembly are crucial for migration, turnover of actin networks is also centrally important for migration. A wide range of cells, including fibroblasts and Dictyostelium, steer by splitting pseudopods into two or more daughters. Controlling which daughter pseudopods are maintained and which are retracted then provides a directional bias that steers cells up chemotactic gradients8. Actin and associated signal transduction networks have been described as excitable systems that propagate in waves and self-limit to drive responsive and dynamic protrusions1, 9, 10. Descriptions of the underlying mechanisms may involve actin and associated cytoskeletal components as controllers of their own excitability. Alternatively, they invoke excitable networks of upstream regulators such as signaling lipids, but it is clear that dynamic interplay between “on” and “off” signals is essential for migration to be plastic and responsive.
How this activation mechanism is modulated or attenuated is not known, but it is clear that the Scar/WAVE complex is part of a multicomponent network of regulation, in which we only currently understand a small fraction of the players. At least three negative regulators of Arp2/3 complex have been described, including Gadkin, which sequesters Arp2/3 at the trans Golgi network and endosomes11. Another trans-Golgi localized Arp2/3 inhibitor is PICK1, whose role as an Arp2/3 inhibitor is still debated 12, 13 Finally, Arpin mimics the tail of WASP proteins but inhibits rather than stimulates the Arp2/3 complex 14. Here, we describe the first negative regulator that acts at the level of the Scar/WAVE complex, CYRI (encoded by the FAM49 gene), an evolutionarily conserved protein that mimics the Rac1 interaction domain of CYFIP and acts to promote dynamic splitting of pseudopods to provide plasticity during motility.
Results
CYRI is an evolutionarily conserved N-myristoylated protein with homology to CYFIP
We sought new interactors of the Scar/WAVE complex by pulldown of GFP-fused NAP1 (the largest subunit of the Scar/WAVE complex15, Supplementary Table 1) stably expressed in napA knockout Dictyostelium cells. Reversible formaldehyde crosslinking in cellulo16 stabilised transient interactions and subsequent GFP-Trap immunocapture recovered Scar/WAVE complex members Scar/WAVE, ABI, HSPC300 and PIR121 (homologue of mammalian CYFIP protein). Among other interactors, we noticed an associated uncharacterized protein with homology to human FAM49 (FAMily of unknown function 49; Fig 1a and Supplementary Table 2). Even though subsequent attempts to immunoprecipitate FAM49 with the Scar/WAVE complex in the absence of crosslinking showed that this interaction was likely indirect, FAM49 caught our attention for two reasons. Firstly, it is highly conserved across evolution, hinting at a fundamental function. FAM49 is present in all of the major eukaryotic superfamilies (as defined by Keeling et al.17)- unikonts, chromalveolates, excavates and at least one example in plants and is roughly co-conserved with the Scar/WAVE complex18 (Supplementary Fig.1a). Secondly, both Pfam and InterPro identified FAM49 as uniquely sharing a DUF1394 domain with CYFIP proteins (Fig. 1b and Supplementary Fig.1b) suggesting a functional linkage with the Scar/WAVE complex. FAM49 proteins contain little more than DUF1394, while CYFIP proteins also have a cytoplasmic fragile X interaction domain, previously implicated in neuronal dendritic spine function 19 (Fig. 1b). At this point, we renamed FAM49 to a more functional name, CYRI for CYFIP-related Rac1 Interactor, due to the functions described below. In mammals, the two isoforms are CYRI-A (FAM49-A) and CYRI-B (FAM49-B). We henceforth refer to the FAM49 protein and gene as CYRI; this study focuses on the characterization of mammalian CYRI-B and the unique Dictyostelium CYRI.
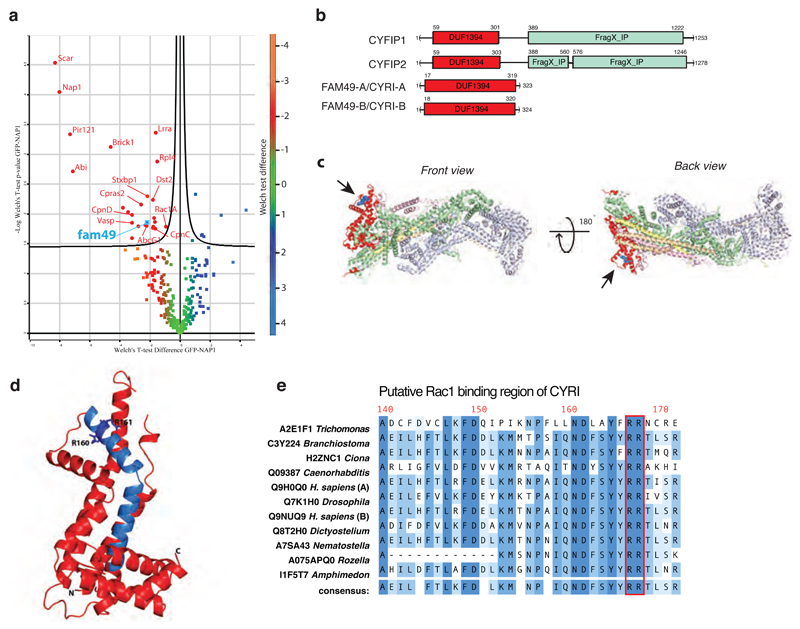
Figure 1. CYRI (Fam49) proteins show homology to CYFIP and contain a putative Rac1 interaction motif.
a – Volcano plot illustrating pooled results from four LC-MS/MS experiments showing comparison of formaldehyde crosslinked proteins co-immunoprecipitating with GFP or GFP-NAP1 in Dictyostelium napA knockout cells. Color-coding based on Welch test difference. Curved line is 5% false discovery rate. Identified interactors are labeled with gene symbols and presented in Table S2 with additional information.
b – Schematic of human CYFIP1/2 and CYRI-A/B showing amino acid numbers and domains. Common DUF1394 domain (Pfam PF07159) in red and CYFIP1/2 C-terminal cytoplasmic Fragile X Mental Retardation FMR1-interacting domain (FragX-IP, Pfam PF05994) in light green.
c - Two views of ribbon crystal structure of the Scar/WAVE complex (PDB 3P8C)2. NCKAP1 in lilac, CYFIP1 in light green and red, Scar/WAVE in peach, HSPC300 in yellow and ABI1 in orange. DUF1394 is red, with putative Rac1 interaction residues in blue and highlighted by arrows.
d – Phyre prediction of structure of the DUF1394 domain of CYRI-B. The putative Rac1-binding domain of CYRI is blue with Arg160 and Arg161 indicated as a stick representation.
e - Sequence alignment of the putative Rac1-binding domain of CYRI in different organisms. The CYFIP Lys189 and Arg190 equivalent residues are well conserved in CYRI (Arg160 and Arg161) and are highlighted in red.
Based on the published structure of the Scar/WAVE complex, we highlighted the DUF1394 region of sequence homology on CYFIP of the Scar/WAVE complex (PDB 3P8C) (Red - Fig. 1c). The DUF1394 domain of CYFIP partly overlaps with the Rac1 interaction site on Scar/WAVE complex, in particular Arg190 in CYFIP1, which was demonstrated to be important for Rac1 binding3 (Fig. 1c, Black arrow and blue balls). As expected from the domain resemblance, modeling the structure of the DUF1394 of CYRI-B using the protein homology/analogy recognition engine Phyre2, shows structural similarities with the published structure of CYFIP (Fig. 1d). The analogous Arg 161 of CYRI (Fig 1d blue sidechains, and e red box) is part of a highly conserved 33-amino acid stretch showing sequence similarity >75% across diverse phyla (Supplementary Fig. 1b-c). Furthermore, we noticed that Arg 160 is also conserved in CYRI but replaced by a lysine in CYFIP (Fig. 1d-e and Supplementary Fig. 1b-c), keeping similar biochemical properties.
Previous unbiased mass spectrometry analyses had identified lipid modification of CYRI 20–22. The N-termini of CYRI proteins show conservation of a putative myristoylation site at glycine 2 (Supplementary Fig. 1d), which is not conserved in CYFIP. We confirmed the myristoylation of CYRI-B by assessing the incorporation of myristate analogue (C14:0-azide) onto the glycine using CLICK chemistry in cellulo. Site-directed mutagenesis of this glycine to alanine abolishes the CLICK signal to a level similar to the GFP negative control (Supplementary Fig. 1 e-f).
In summary, we have defined CYRI, a DUF1394 containing protein, as a highly conserved protein with a possible conserved binding site for Rac1. Furthermore, myristoylation at position 2 implies a role for CYRI cycling on and off of the plasma membrane23, where active Rac1 stimulates the Scar/WAVE complex to catalyse actin polymerization and lamellipodial growth.
CYRI interacts directly with activated Rac1 in vitro
The provocative homology of CYRI with CYFIP, in a region that is key for interaction with active Rac1 (Supplementary Fig. 1b), led us to investigate binding between CYRI-B and active Rac1. A blind yeast two-hybrid screen using active Rac1G12V as bait retrieved CYRI-B from various mouse and human cDNA libraries (Supplementary Fig. 2a). The core interacting sequence of CYRI-B was mapped to a central domain encompassing amino acids 30-236 (hereafter referred to as Rac Binding Domain or RBD), (Supplementary Fig. 2b-c) and tested for Rac1 interaction. GFP-RBD expressed in CHL-1 human melanoma cells interacted selectively with purified recombinant active GST-Rac1Q61L but not with GST-Rac1WT. Mutation of CYRI-B Arg160 or Arg161 (in GFP-RBD) to an acidic amino acid abrogated this interaction (Fig. 2a-c). Interaction of CYRI-B with Rac1 was further confirmed by an alternative in vitro pulldown assay using GST-CYRI-B RBD and MBP-Rac1 and gave very similar result (Supplementary Fig. 2d-f). In this assay, CYRI does not co-precipitate with Rac1T17N, Rac1G12V, or Rac1 WT, likely due to the low affinity of CYRI-B for Rac1. However, the double mutant Rac1 P29S/Q61L, recently described by Chen and colleagues as having a high affinity for the Scar/WAVE complex24, gave an enhanced binding to CYRI-B RBD (~3-3.5-fold increase) over Rac1Q61L (Fig. 2d-f and Supplementary Fig. 2 d-f). Moreover, this mutant did not show increased binding to Pak1-CRIB (Supplementary Fig. 2 d-f). We also tested the Rac1/CYRI-B RBD interaction directly by surface plasmon resonance using purified recombinant Rac1 and CYRI-B RBD. Immobilised CYRI-B RBD specifically bound to purified Rac1 Q61L with a Kd of 27 μM and the reverse assay, with Rac1 Q61L immobilised returned a similar Kd of 22 μM (Fig. 2g). We conclude that Rac1 interacts directly with the CYRI-RBD.
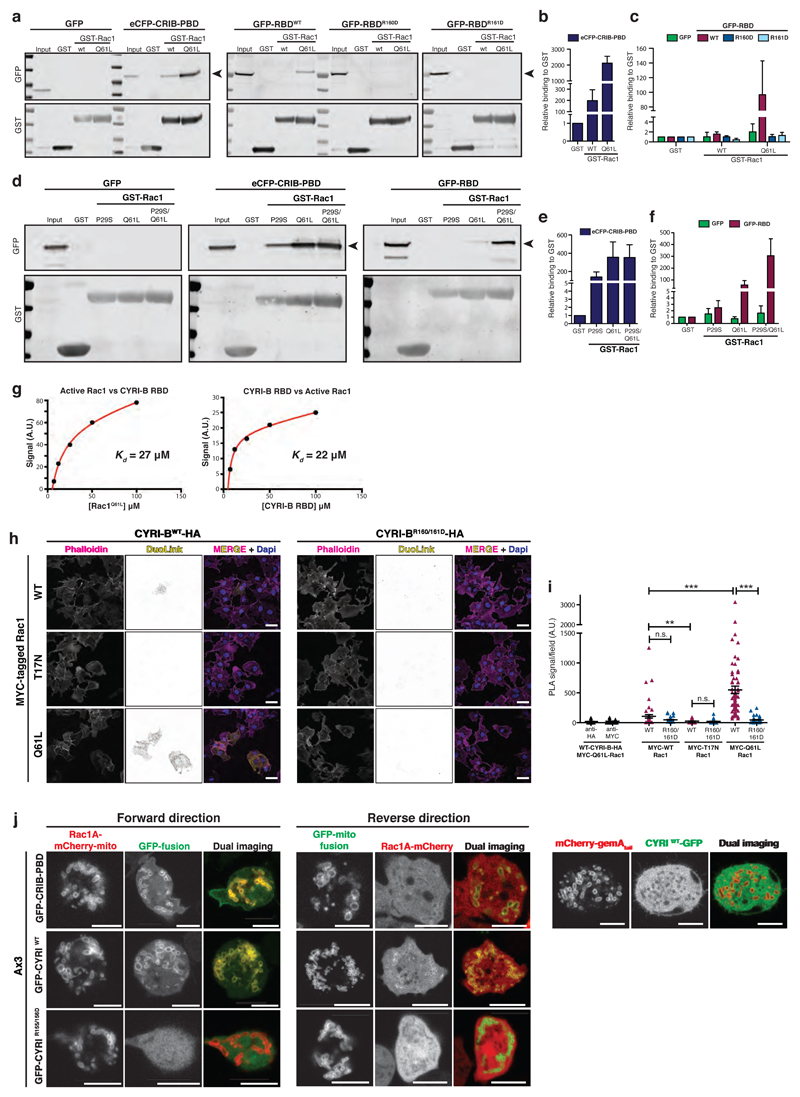
Figure 2. CYRI proteins interact with active Rac1.
a-c - Western blot images from pulldown of GST control, GST-Rac1WT or GST-Rac1Q61L immobilized on beads, mixed with cell lysate expressing either GFP alone, positive control PAK1 eCFP-CRIB-PBD, GFP-RBDWT, GFP-RBDR160D or GFP-RBDR161D (a). Binding relative to GST was quantified by densitometry (b-c).
d-f - Western blot images from pulldown of GST control, GST-Rac1P29S or GST-Rac1Q61L or the double mutant GST-Rac1P29S/Q61L immobilized on beads, mixed with cell lysate expressing either GFP alone, eCFP-CRIB-PBD, GFP-RBDWT (d). Relative binding to GST was quantified by densitometry (e-f).
g – Summary of steady state surface plasmon resonance (SPR) binding curves between Rac1Q61L and CYRI-B-RBD. Left: GST-CYRI-B was immobilized on anti-GST surface vs increasing concentrations of Rac1Q61L. Right: His-Rac1 was immobilized on NTA surface vs increasing concentrations of CYRI-B RBD. Biacore evaluation software 3.0 was used to fit a simple 1:1 binding model. Kd = equilibrium dissociation constant, A.U. = arbitrary units.
h-i Proximity ligation assay was performed on COS-7 cells plated on laminin and co-expressing either CYRI-B-HA or CYRI-BR160/161D-HA and MYC-tagged Rac1 constructs as indicated. PLA signal (yellow), F-actin (magenta) and nuclei (blue). See Supplementary Fig. 2 for negative controls. Quantification in (i). One-way ANOVA with Dunn’s post-test was performed between CYRI-BWT and the different MYC-Rac1 constructs. Mann Whitney test was tested between CYRI-BWT and CYRI-BR160/161D for each MYC-Rac1 construct. n.s. p> 0.05, ** p≤0.01, *** p≤0.001. (Cell counts: anti-HA n=55 ; anti-Myc n=54 ; Myc-WT/WT-HA n=55 ; Myc-WT/R160/161D-HA n=55 ; Myc-T17N/WT-HA n=63 ; Myc-T17N/R160/161D-HA n=84 ; Myc-Q61L/WT-HA n=69 ; Myc-Q61L/R160/161D-HA n=65) Scale bar = 50 μm.
j - Still pictures from mitochondrial recruitment of CYRI (Forward) or Rac1AP29S/Q61L (Reverse) in Ax3 D. discoideum. Quantification was performed using the co-localisation tool of Imaris. The Pearson’s coefficient of correlation for co-localisation (and standard deviation SD) at the mitochondria for Rac1A-mCherry-mito and the GFP-fusions were: CRIB-PBD 0.80 (SD: 0.20) ; CYRIWT 0.77 (SD: 0.21) ; CYRIR155/156D 0.05 (SD: 0.12). The correlation coefficients for Rac1A-mCherry and the GFP-CYRI-mito fusions were: CRIB-PBD 0.33 (SD: 0.12) ; CYRIWT 0.44 (SD: 0.19) ; CYRIR155/156D -0.23 (SD: 0.05).
Cells co-expressing a mitochondrial reporter (mCherry-gemA tail) and CYRI-GFP were also imaged (right panel), confirming the absence of mitochondrial localisation of CYRI. The correlation coefficient was -0.06 (SD: 0.15). (n=4-5 cells containing >300 mitochondria/cell).
Scale bar = 5 μm
All data presented are representative of at least 3 biologically independent experiments. Bar and scatter plots show data points with mean and S.E.M. except if stated otherwise.
As CYRI-RBD shows no homology to CRIB (Cdc42 Rac interaction binding) motifs, which can interact with both Rac1 and Cdc42, we probed the specificity of the interaction of CYRI between Rac1, RhoA and Cdc42. Once again, CYRI-RBD showed clear interaction with Rac1Q61L but did not interact with constitutively active RhoAQ63L or Cdc42Q61L (Fig. 2d and Supplementary Fig. 2g-h).
Thus CYRI-B RBD interacts specifically with active Rac1. Two conserved basic residues in the DUF1394 (of both CYFIPs and Fam49s) are important for this interaction. This suggests a signal-regulated interaction between active Rac1 and CYRI, in the same way that Rac1 interacts with Scar/WAVE complex, implying that DUF1394 is a new class of Rac1 mediator.
CYRI interacts with active Rac1 in cells
Having established biochemically that CYRI interacts specifically and directly with active Rac1, we explored the nature of this interaction in cells. Proximity ligation25 revealed an interaction between Rac1WT and CYRI-B in COS-7 cells, as well as a stronger interaction between Rac1Q61L (Fig. 2h-i and Supplementary Figure 2i-l). Mutation of key arginines in the CYRI-B (CYRI-BR160/161-HA) abolished this interaction (Fig. 2h-i and Supplementary Figure 2i-l) and no interaction occurred with Rac1T17N dominant negative mutant (Fig. 2 h-I and Supplementary Fig. 2i-l). We further queried the interaction between CYRI and Rac1A in Dictyostelium cells, using a method similar to the knocksideways method of Robinson et al.26, but by constitutively targeting either CYRI or Rac1A to mitochondria and assessing the co-recruitment of the other protein (Figure 2j). Using both forward and reverse recruitment strategies, we find that Dictyostelium CYRIWT, but not CYRI mutated for the analogous arginines R155/156D, strongly co-recruits with active Rac1A P29S/Q61L in cells. We quantified this interaction by measuring the Pearson’s coefficient of fluorescence correlation (PCC) within the cell (see Methods). The correlation for Rac1A-mCherry-mito and the GFP-fusions revealed a coefficient of 0.77 for CYRIWT; and 0.05 for CYRIR155/156D, where 1 = perfect correlation, 0= no correlation and -1 = excluded. The correlation coefficients for Rac1A-mCherry and the GFP-mitofusions were: CYRI WT 0.44 and CYRI R155/156D -0.23. Importantly, we could not see any co-localisation between CYRI-GFP and a mitochondrial reporter (PCC = -0.06). Thus, CYRI interacts with Rac1, with a preference for the activated state, mediated by the key conserved arginines, in both mammalian and Dictyostelium cells.
CYRI opposes recruitment of the Scar/WAVE complex to lamellipodia
Knockdown or knockout of CYRI-B by siRNA or CRISPR in COS-7 or CHL-1 cells did not affect growth rate in culture (not shown), but it promoted unusually large and broad lamellipodia highly enriched in WAVE2 (Fig. 3a-b and Supplementary Fig. 3a-g). Cells spread over a larger area and adopted a “fried-egg” phenotype, correlating with an increase in circularity (Fig. 3c-d, Supplementary Fig. 3e-g). Expression levels of Scar/WAVE complex subunits are not obviously altered in cyri-b knockout cells (Supplementary Fig. 3h), suggesting that loss of CYRI-B does not drive lamellipodia by increasing overall levels or stability of the Scar/WAVE complex. Cell area and circularity were both rescued by re-expression of untagged CYRI-BWT, but not the R160/161D mutant that was impaired in Rac1 binding (Fig. 3e-f, Supplementary Figure 3i-k). The CYRI-BG2A mutant that cannot be N-myristoylated failed to rescue the phenotype (Fig. 3g-h and Supplementary 3l-m), suggesting that lipid modification promoting targeting to membranes is crucial for CYRI-B functions. cyri knockout Dictyostelium cells also showed a recruitment of the Scar/WAVE complex (as reported by the GFP-HSPC300 reporter) to a much broader leading edge (Supplementary Fig. 3n – yellow dotted line and Supplementary Movie 1), similar to mammalian cells. Moreover, Scar/WAVE patches in cyri knockout cells are ill-defined but longer-lived, suggesting CYRI’s ability to suppress Scar/WAVE complex activity outside of active protrusions. (Supplementary Fig. 3n, heat map). We conclude that CYRI, via its interaction with active Rac1 and its recruitment to the membrane by lipid modification, opposes active Scar/WAVE complex recruitment to the plasma membrane and drives the formation of more focussed and sharper lamellipodial areas at the cell periphery.
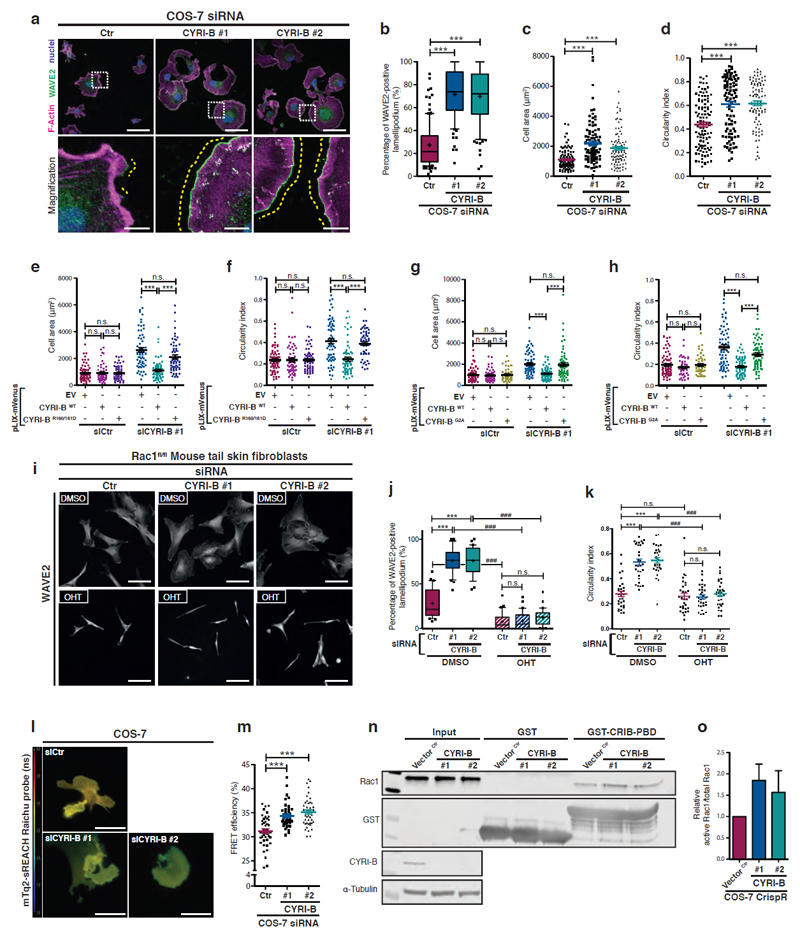
Figure 3. Loss of CYRI-B increases Rac1-mediated Scar/WAVE localisation to lamellipodia.
a-d - Immunofluorescence of control (Ctr) or cyri-b knockdown (siRNA #1 and 2) COS-7 cells plated on laminin for only 1h and stained for WAVE2 (green), nuclei (blue) and F-actin (magenta). Scale bar = 50 μm. Box insets show zoom, Scale bar = 10 μm.
The ratio of extension of WAVE2 staining (yellow dotted line) vs the total cell perimeter shown in (b) is a read-out of the extent of the cell edge devoted to lamellipodium. Manual quantification of cell area in (c) and circularity (d). One-way ANOVA with Dunn’s post-test n.s. p> 0.05, *** p≤0.001.
(a-c: Scramble n=111 ; #1 n=95 ; #2 n=96 – d: Scramble n=115 ; #1 n=92 ; #2 n=98 cells)
e-f – Rescue experiments showing COS-7 cells on laminin treated with siRNA as above and transfected with pLIX-mVenus plasmid co-expressing an siRNA resistant untagged CYRI-B (WT or R160/161D mutant) or control empty vector (EV). (see Supplementary Fig. 3l). Quantification of the cell area (e) and circularity (f) is shown. One-way ANOVA with Dunn’s post-test n.s. p> 0.05, *** p≤0.001. (Scramble/EV n=78 ; Scramble/WT n=58 ; Scramble/R160/161D n=66 ; #1/EV n=66 ; #1/WT n=64 ; #1/R160/161D n=60 cells).
g-h – Control or cyri-b knockdown COS-7 cells obtained by siRNA were transfected with the mVenus reporter plasmid (pLIX-mVenus) co-expressing a siRNA resistant untagged CYRI-B (WT or G2A mutant) or control empty vector (EV). Quantification of the cell area (g) and circularity (h). One-way ANOVA with Dunn’s post-test n.s. p> 0.05, *** p≤0.001. (Scramble/EV n=70 ; Scramble/WT n=52 ; Scramble/G2A n=46 ; #1/EV n=63 ; #1/WT n=64 ; #1/G2A n=65 cells)
i-k - Control (DMSO) or rac1 knockout (OHT) mouse tail fibroblasts treated with Scramble (siCtr) or mouse specific Cyri-B siRNA, plated on laminin and stained for WAVE2 (i). Scale bar = 50 μm. Quantification of the WAVE2 staining at the lamellipodium (j) and circularity (k) are displayed. One-way ANOVA with Dunn’s post-test *** p≤0.001. Mann Whitney test was performed between same sample treated or not with OHT. ### p≤0.001. (30 cells/conditions).
l-m - FLIM/FRET experiment with control (siCtr) or cyri-b knockdown (siCYRI-B #1 and #2) COS-7 cells transfected with mTq2-sREACH and plated on laminin. The jet2 color code (left colour bar) shows average lifetime of the probe, spanning 1-4 ns (blue to red) (l). Quantification of the FRET efficiency shown in (m). One-way ANOVA with Dunn’s post-test. *** p≤0.001. (Scramble n=61 ; #1 n=61, #2 n=63 cells)
Scale bar = 50 μm
n-o - Active Rac1 pulldown comparing control CrispR (Vector Ctr) or 2 independent cyri-b CrispR knockout (#1 and #2) COS-7 cell lines. Lysate was incubated with GST or GST-CRIB-PBD beads and western blot probed as indicated. Relative active Rac1 was quantified by densitometry (o).
All data presented are representative of at least 3 biologically independent experiments.
Bar and scatter plots show data points with mean and S.E.M.
Whisker plots show 10-90 percentile, median (bar) and mean (cross).
To determine whether Rac1 was required for the exaggerated lamellipodial phenotype of cyri-b knockout cells, we co-depleted Rac1 and CYRI-B from mouse tail skin fibroblasts cultured from a conditional inducible Rac1 knockout mouse 27. We used ROSA26-Cre::ERT2+;p16Ink4a-/-, Rac1fl/fl mouse tail skin fibroblasts, treated with hydroxytamoxifen (OHT, to induce deletion of Rac1) and then with siRNA against Cyri-b (Supplementary Fig. 3o). Deletion of Rac1 led to a spindle-shaped morphology and a loss of lamellipodia as previously described 28–30. Loss of CYRI-B did not cause excessive lamellipodia or altered circularity in Rac-deleted cells (Fig. 3i-k). Thus, Rac1 is absolutely required for CYRI-B driven actin reorganisation.
The increased circularity of cyri-b depleted cells is reminiscent of the fried-egg shape observed following Rac1 hyperactivation31, suggesting that CYRI-B might buffer Rac1 activity. Indeed, a dark acceptor mTq2-sREACH Raichu FRET probe 32, 33 showed a modest but consistent increase in Rac1 signaling activity in CYRI-B depleted cells, as measured by FRET efficiency in both COS-7 (Fig. 3l-m) and CHL-1 cells (Supplementary Figure 3p-q), which was confirmed by biochemical pulldown using cyri-b CrispR knockout COS-7 cells (Fig. 3n-o). Together, these data indicate an increase in Rac1 signaling activity in CYRI-B depleted cells. Conversely, inducible overexpression of untagged CYRI-B (Supplementary Fig. 4a-b) drove lamellipodia to assume a more fractal appearance, decreasing WAVE2 recruitment, cell area and circularity (Fig. 4a-d, Supplementary Fig. 4c-f – Vehicle-treated control cells). In parallel, overexpression of CYRI-B also drove a decrease in the Rac1 activity signal of the Raichu FRET probe (Fig. 4e-f) which was fully reversed by mutation of both Arg160 and Arg161 (Fig. 4g). Thus, CYRI-B opposes Rac1-Scar/WAVE mediated expansion of lamellipodia protrusions. Ideally, we would like to follow the dynamic recruitment, but adding a GFP-tag to either end of CYRI-B interfered with its function. However, CYRI-B-FLAG showed significant enrichment at pseudopods in fixed cells, similar to WAVE2 enrichment (Supplementary Fig. 4g-h). This supports our model for CYRI as co-accumulating in an overlapping region with WAVE2 at the leading edge of a lamellipod. Localisation of endogenous protein awaits better antibodies, as available antibodies to CYRI-B (FAM49B) did not reveal any specific staining by immunofluorescence.
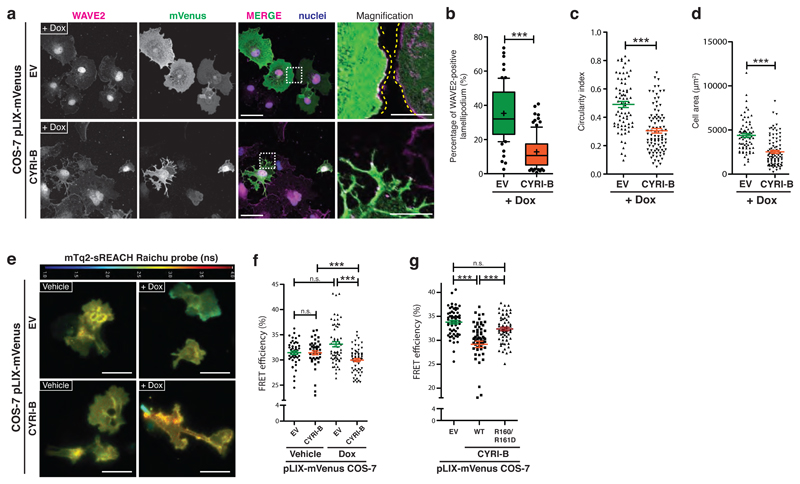
Figure 4. Overexpression of CYRI-B opposes Rac1-mediated Scar/WAVE recruitment to the leading edge.
a-d - Immunofluorescence of doxycycline-induced control empty vector (EV) or CYRI-B overexpression in COS-7 cells and fixed after a longer time (4h of spreading) and stained for WAVE2 (magenta), nuclei (blue) and GFP (green). Scale bar = 50 μm. Insets show zoom of white dashed field. Scale bar = 10 μm (a). WAVE2 ratio and circularity were measured and reported in (b) and (c) respectively. Cell area quantification was based on phalloidin staining (d). Mann-Whitney test *** p≤0.001. (Dox/EV n=73 ; Dox/CYRI-B n=93 cells)
e-f - FLIM/FRET experiment with mTq2-sREACH Raichu Rac1 showing vehicle or doxycycline-treated COS-7 cells expressing a control empty vector (EV) or CYRI-B. The jet2 color code (bar at top) shows the average lifetime of the probe, spanning 1-4 ns (blue to red) (e). Quantification of the FRET efficiency (f) Mann-Whitney test n.s. p> 0.05, *** p≤0.001. (Veh/EV n=47 ; Veh/CYRI-B n=46 ; Dox/EV n=62 ; Dox/CYRI-B n=62 cells)
Scale bar = 50 μm.
g - FRET efficiency obtained from control (EV) or COS-7 cells overexpressing CYRIWT or CYRI-BR160/161D after doxycycline induction. One-way ANOVA with Dunn’s post-test was performed. n.s. p> 0.05, *** p≤0.001. (EV n=59 ; WT n=62 ; R160/161D n=63 cells).
Data represent at least 3 biologically independent experiments.
Bar and scatter plots show data points with mean and S.E.M.
Whisker plots show 10-90 percentile, median (bar) and mean (cross).
Overall, cyri-b knockout cells show broader Scar/WAVE driven lamellipodia and increased Rac1 activation, leading to an increase in circularity and spread area. This implies that the negative feedback loops controlling Rac1 signaling to lamellipodia are negatively regulated by CYRI-B. Our data support a role for CYRI-B as a buffer of Rac1 activity at the leading edges of cells, regulating activation of the Scar/WAVE complex.
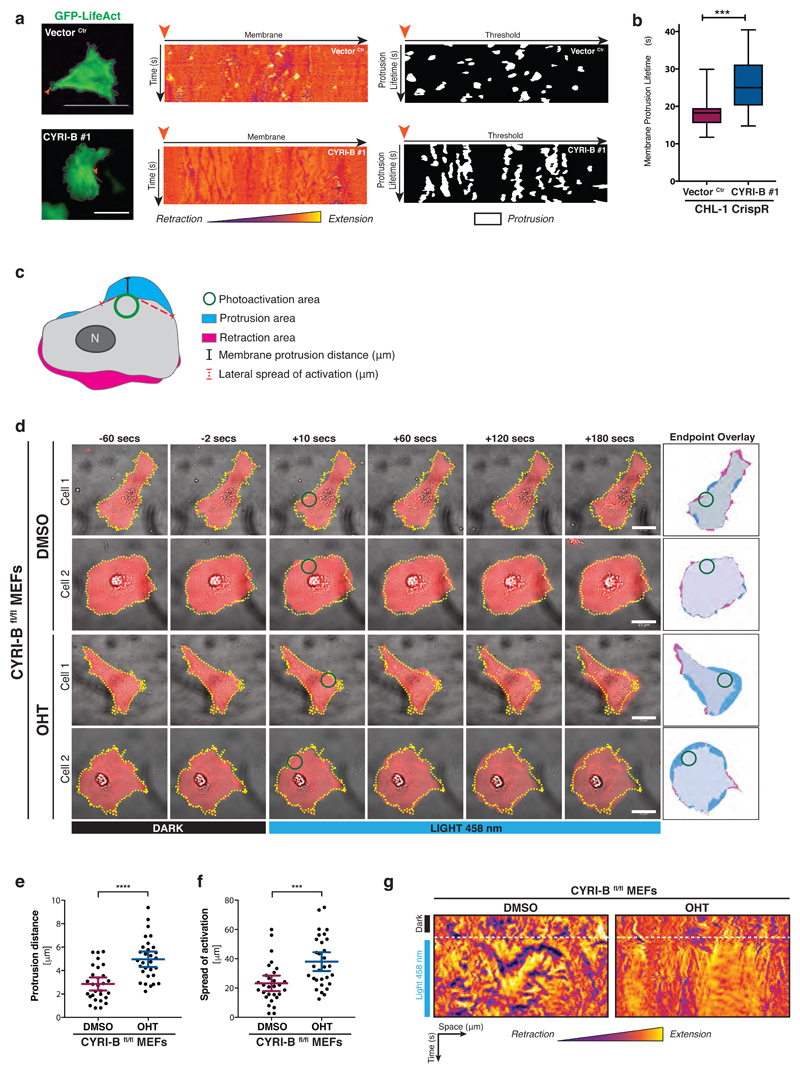
CYRI regulates the duration and extent of protrusions
Since loss of CYRI affected cell shape and recruitment of the Scar/WAVE complex to the cell leading edge, we next sought to determine the consequences of CYRI-B depletion for lamellipodial actin dynamics. First, we observed actin dynamics live using fast frame-rate videos in CHL-1 cells expressing GFP-Lifeact (Fig. 5a – Left panel and Supplementary Movie 2). We tracked the cell edge and used unwrapped (polar) kymographs to visualise and measure the area of protrusion (Figure 5a, yellow colour) versus retraction (Figure 5a, purple colour) over time. From the polar kymographs (Middle panel), which represent the evolution of the cell’s edge over time, it is apparent that control cells showed small but rapid bursts of actin-based protrusion (yellow patches on kymograph), while cyri-b knockouts had longer-lived and less dynamic responses (Fig. 5a - Right panel and Fig. 5b). If CYRI-B buffers Rac1 at the lamellipodium, we speculated that cyri-b knockout cells would struggle to restrain protrusion formation upon Rac1 activation. To investigate this, we used the Rac1-LOV optogenetic probe, which allows activation of Rac1 with blue light 34. Rac1 activation was performed using pulses of blue light in a discrete area on the cell periphery and the lamellipodial response was measured over 180sec (Fig. 5c-d and Supplementary Movie 3). While protrusions were elicited in both the wild-type and knockout cells in response to blue light, cyri-b knockout cells showed a more sustained and extensive protrusion response and increased peripheral propagation of the lamellipodial response (Fig. 5e-g). Thus, we demonstrate using optogenetic Rac1 activation that CYRI-B limits Rac1 mediated activation of the Scar/WAVE complex and it provides the cell with increased plasticity to allow more rapid turning off of Rac1 signals.
Figure 5. CYRI-B controls the duration and extent of Rac1-mediated protrusions.
a - Control (Vector Ctr) and cyri-b CrispR knockout CHL-1 cells on laminin expressing GFP-LifeAct, recorded for 3 minutes at 1 frame/sec. The cell periphery (magenta) is tracked using the GFP-LifeAct signal (green) (Left panel). The membrane is unravelled from the orange arrow and a representative polar kymograph of the changes in membrane dynamics over time between control (Vector Ctr - Top) and cyri-b CrispR knockout (Bottom) CHL-1 cells is shown. Membrane extensions (positive values) are visualised in yellow through to orange, while retractions (negative values) are purple-blue (Middle panel). Thresholding of the kymograph to remove noise (values ≥ + 0.6) reveals protrusions over time (white signal – Right panel)
Still from movie S2. Scale bar = 25 μm.
b - Box plot representing the distribution of the average protrusion lifetime for each individual cell. Error bars represent S.D. Mann Whitney test was performed. *** p≤0.001. (20 cells/condition)
c - Schematic representation showing protruding (blue) and retracting (magenta) area following photoactivation of Rac1-LOV probe. Photo activation area (green circle) was used as the origin to measure the maximal protrusion distance (outward - black line) and the longest uninterrupted lateral spread of the protrusion (red dotted line)
d - Still pictures from videos of photoactivation time course showing selected cells from DMSO (Control) or OHT-treated (knockout) immortalized CRE-ERT2+ Cyri-Bfl/fl MEFs on fibronectin. Endpoint overlay as from schematic (c). Scale bar = 25 μm.
e-f - Quantification of the protrusion distance (e) and the spread of activation (f) between control (DMSO) or cyri-b knockout (OHT) MEFs.
Error bars represent 95% CI. Unpaired two-tailed t-test (e) and Mann-Whitney test (f). *** p≤0.001, **** p≤0.0001. (DMSO n=29 ; OHT n=30 cells).
g - Kymograph representation before and after photo activation. Membrane extensions are visualised in yellow through to orange, while retractions are observed in purple-blue. Time of photoactivation is highlighted by a white dotted line.
All data presented are representative of at least 3 biologically independent experiments.
CYRI focuses actin assembly in leading pseudopods to promote plasticity of migration
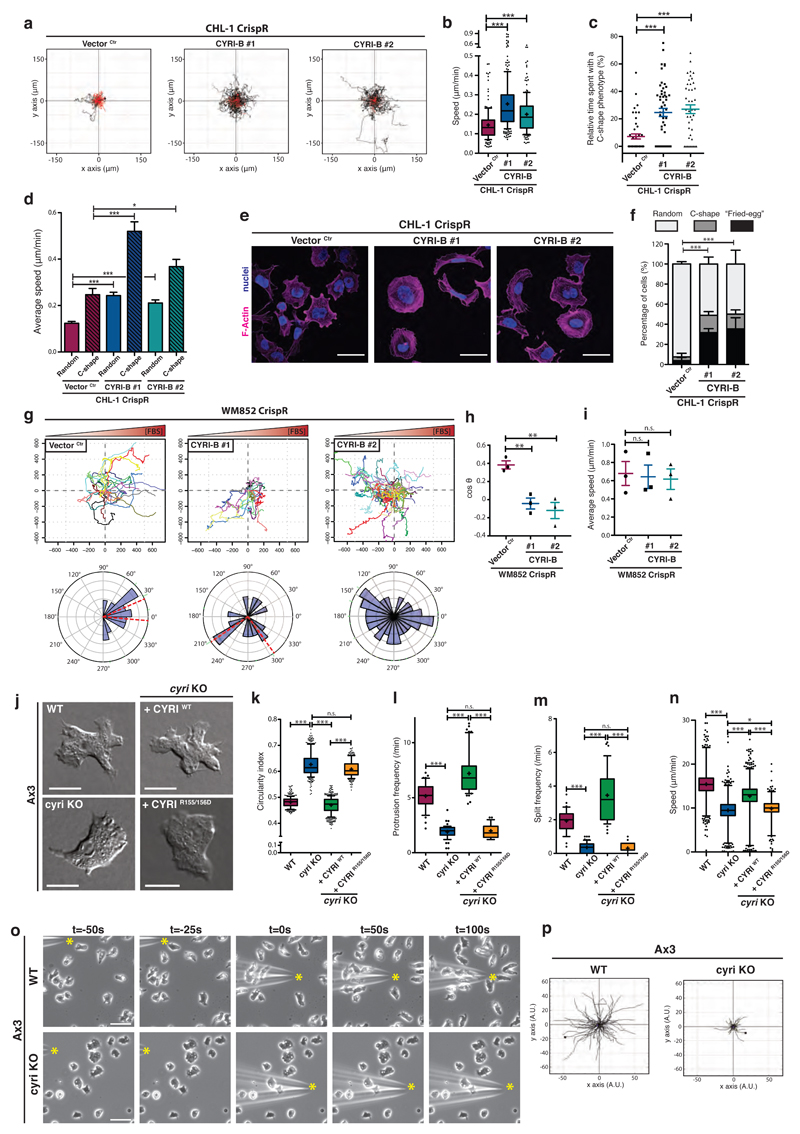
We examined the role of CYRI in cell migration and chemotaxis. CHL-1 melanoma cells are normally nearly static when seeded at low density in 2D-culture, but cyri-b knockout cells gained the ability to migrate over longer distances and with a 1.5 to 2-fold increase in speed (Fig. 6a-b and Supplementary Movie 4). We noticed that cyri-b knockout cells frequently (40-50% of the time) assumed either a rounded “fried-egg” shape or C-shape, with a broad spread lamellipodium spanning approximately the front half of the cell perimeter and a convex rear (Supplementary Fig. 5a yellow arrows, Supplementary Movie 4). cyri-b knockout cells spent on average 25% of their time in a C-shape (Fig. 6c), which resembled the fast-moving goldfish keratocyte 35 and moved faster than the other common shapes (Fig. 6d). Migration speed correlated positively with the fraction of time spent as a C-shape (Fig. 6e-f and Supplementary Fig. 5b-c). Lamellipodia need to be polarized and dynamic for efficient cell migration30, 36. Therefore, we hypothesise that cyri-b knockout cells show enhanced Rac1-Scar/WAVE activation at the periphery and if this becomes polarized toward one half of the cell, they assume a more motile C-shape. Normally CHL-1 cells assemble short lived protrusions and hardly move, but polarized protrusion leads to persistent migration, as dynamic behavior is reduced without CYRI.
Figure 6. CYRI proteins mediate plasticity of protrusions needed for directional migration.
a-b - Spider plots of control (Vector Ctr) or cyri-b CrispR knockout (#1 and #2) CHL-1 cells on collagen-I-coated dishes and recorded for 17h (a) (See movie S4). Black and red lines represent tracks with a travelled distance greater or shorter than 100 μm respectively. Average speed is plotted in (b). Whisker plots show 10-90 percentile and mean (cross). One-way ANOVA with Dunn’s post-test *** p≤0.001. (Ctr n=161 ; #1 n=228, #2 n=178 cells).
c - Relative time spent with a fast moving C-shape phenotype between control (Vector Ctr) and cyri-b CrispR knockout (#1 and #2) CHL-1 cells. One-way ANOVA with Dunn’s post-test *** p≤0.001. (Ctr n=45 ; #1 n=53, #2 n=42 cells).
d - Speed of control (Vector Ctr) or cyri-b CrispR knockout (#1 and #2) CHL-1 cells displaying a random or a C-shape. One-way ANOVA with Dunn’s post-test n.s. p>0.05, * p≤0.05, *** p≤0.001. (Ctr n=45 ; #1 n=53, #2 n=42 cells)
e-f - Immunofluorescence of control (Vector Ctr) or cyri-b CrispR knockout (#1 and #2) CHL-1 cells on collagen and stained for F-actin (magenta) and nuclei (blue) (e). Scale bar = 50 μm. Cells were classified into 3 categories according to their morphology (Fried-egg, C-shape, Random) and the percentage of each population is shown in (f). Two-tailed Chi-square test (95% confidence). *** p≤0.001. (Ctr n=276 ; #1 n=216, #2 n=210 cells)
g-i - Spider plots and rose plots representing the chemotactic ability of control (Vector Ctr) or cyri-b knockout WM852 cells migrating toward 10% FBS (g) (see movie S5). Red-dashed lines represent a 95% confidence interval for the mean direction. Cosθ (chemotactic index) (h) and average speed (i). Two-tailed unpaired t-test. n.s. p>0.05, ** p≤0.01. (Ctr n=129 ; #1 n=132, #2 n=151 cells).
j-n - Representative DIC pictures from an under agarose chemotaxis assay of Ax3 (WT) and Ax3-derived cell lines (j) (see movie S6). Scale bar = 10 μm. Automatic cell segmentation and tracking used to quantify cell circularity (k), protrusions and split frequency (l and m respectively), and speed (n). Whisker plots show 10-90 percentile (k-m) and 1-99 percentile (n) with median (bar) and mean (cross). One-way ANOVA with Dunn’s post-test was performed. n.s. p>0.05, * p≤0.05, *** p≤0.001. (Cell counts: k: WT n=360 ; cyri KO n=352 ; cyri KO + CYRI WT n=480 ; cyri KO + CYRI R155/156 n=240 - l: WT n=45 ; cyri KO n=57 ; cyri KO + CYRI WT n=53 ; cyri KO + CYRI R155/156 n=31 - m: WT n=42 ; cyri KO n=62 ; cyri KO + CYRI WT n=46 ; cyri KO + CYRI R155/156 n=33 - n: WT n=2389 ; cyri KO n=2460 ; cyri KO + CYRI WT n=3024 ; cyri KO + CYRI R155/156 n=1169)
o-p - Needle assay using WT or cyri knockout Ax3 cells migrating toward cAMP. Time-lapse phase contrast movies (see movie S8) of cells responding to cAMP (yellow start). Scale bar = 25 μm (o). Spider plots obtained from manual tracking of the first 100 seconds following needle re-orientation (p). (WT n=86 ; cyri KO n=79 cells)
All data presented are representative of at least 3 biologically independent experiments.
Bar and scatter plots show data points with mean and S.E.M.
Since cells need to maintain plasticity of their lamellipodia to respond effectively to chemoattractant cues37, we predicted that depletion of CYRI-B would affect chemotactic migration. CHL-1 cells are not chemotactic to serum, but WM852 melanoma cells were previously reported as highly chemotactic38. Loss of CYRI-B (Supplementary Fig. 5d-e) severely affected chemotaxis of these cells towards serum with no effect on their basal speed (Fig. 6g-i and Supplementary Movie 5). Plots of cell tracks (normalized so each cell starts at the origin – “spider plots”) revealed that cyri-b knockouts often migrated very long distances in the opposite direction to the chemoattractant gradient, having lost the plasticity enabling them to reorient toward the source of serum lysophosphatidic acid. Thus, CYRI-B loss has a strong and penetrant effect on how cells polarize their lamellipodia, their ability to remodel protrusions and thus reorient during directed migration.
CYRI promotes pseudopod splitting and opposes persistent migration in Dictyostelium
We examined Dictyostelium cells (Ax3, cyri knockout and rescue - Supplementary Fig. 5f) migrating under agarose up self-generated gradients of the chemoattractant folate39 (Supplementary Fig. 5g). Similar to CHL-1 cells, cyri knockout cells were rounder, with blunted pseudopods (Fig. 6j-k, Supplementary Movies 6-7). Dictyostelium cells, like many others, primarily turn by splitting their leading pseudopod into differently-oriented daughters8; cyri knockouts showed a decreased rate of generation of new protrusions by splitting (from ~5/min to ~2/min, Fig. 6l). Protrusions in cyri knockouts thus persisted for longer (Fig. 6m). Slower forward protrusion led to a decrease in speed (Fig. 6n). Cells could still perceive and orient towards the folate gradient, but their less efficient turning is clearly reflected by a smaller angle of turn between steps (Supplementary Fig. 5h). Thus, CYRI promotes pseudopod splitting in Dictyostelium cells, which is dispensible for the ability to sense a folate gradient, but compromises the speed of migration and the ability to change direction while steering.
We rescued Dictyostelium cyri knockouts with CYRIWT or CYRIR155/156D as stable, single-copy transfectants40 under an actin15 promoter (Fig. 6 j-n, Supplementary Movies 6-7). CYRIWT expressing cells showed increased complexity at their leading edges over wild-type cells, exhibiting more numerous fractal and branched pseudopods (Fig. 6j-k). As expected, expression of CYRIWT decreased the circularity and enhanced the frequency of protrusion generation and the rate of pseudopod splitting (Fig. 6l-m) even over WT cells. Rescue with CYRIWT also restored cells’ ability to turn during chemotaxis (Supplementary Fig. 5h).
Another widely- used chemotaxis assay involves a chemoattractant-filled microneedle introduced just next to Dictyostelium cells. The resulting steep attractant gradient can induce new pseudopods directly toward the needle, and consequently reorient the cells. When cyclic-AMP (cAMP)-sensitive cyri knockout or rescue cells were challenged with cAMP in a needle assay, the cyri knockouts were initially unable to form new pseudopods (Fig. 6o), while CYRIWT cells rapidly protruded a pseudopod and reoriented toward the needle (Figure 6o-p and Supplementary Movie 8). As the assay went on, cyri knockouts slowly became elongated and streamed toward the needle, but they maintained their resistance to new pseudopod formation and rapid reorientation. Thus, cells that lack CYRI can still sense an attractant gradient, but their broad and unfocussed protrusions split rarely, and their diminished ability to generate new pseudopods makes them respond inefficiently to changes in the gradient.
Modeling CYRI’s role in pseudopod plasticity
Since CYRI affects plasticity of pseudopod dynamics, we likened its activity to the mathematical model of Meinhardt 41, where local inhibitors are required to limit the amount of cell edge devoted to pseudopods. Actin assembly pathways are not linear cascades, but rather feedback loops where positive stimulation is self-reinforcing and causes further activation until overcome by negative feedback1, 10. In models of migration based around positive feedback, a locally-acting inhibitor is also needed to destabilise existing pseudopods, so the cell can change direction. Without this, cells polarize, but cannot turn to migrate toward an attractant. We used a modified version of a published simulation42 based on the Meinhardt model41 to visualise the concentrations of the activator and the local inhibitor at the cell edge (Supplementary Fig. 5i and Supplementary Movie 9), to provide a clear illustration of the proposed role for CYRI-B in regulation of Rac1 and Scar/WAVE signaling. A peak in the activator (which represents, for example, active Rac and Scar/WAVE) results in the formation of a new pseudopod. The peak also causes an increase in the concentration of the local inhibitor, which is smaller and thus diffuses faster. This causes two changes – initially, it limits the lateral spread of the pseudopod (Supplementary Fig. 5i, panel 1); later, levels of inhibitor rise in the middle of the pseudopod, destabilizing it and causing it to split (Supplementary Fig. 5i, panel 2). The weaker of the pseudopods then retracts and the stronger is reinforced until the cycle of inhibition catches up with it and starts the splitting all over again (Supplementary Fig. 5i, panels 3-4). The local inhibitor thus increases both the morphological complexity of the cell and the competition between pseudopods. This is supported by the lack of pseudopod splitting in Dictyostelium and our optogenetic data showing that protrusions in cyri knockout cells are more long-lived and spread laterally to a greater extent. Thus, Meinhardt’s model offers insight into the role of CYRI proteins as local inhibitors, which perhaps counterintuitively enhance leading edge dynamics and add plasticity to the positive feedback loops driving migration.
CYRI-B regulates epithelial polarity via a Rac1-dependent mechanism
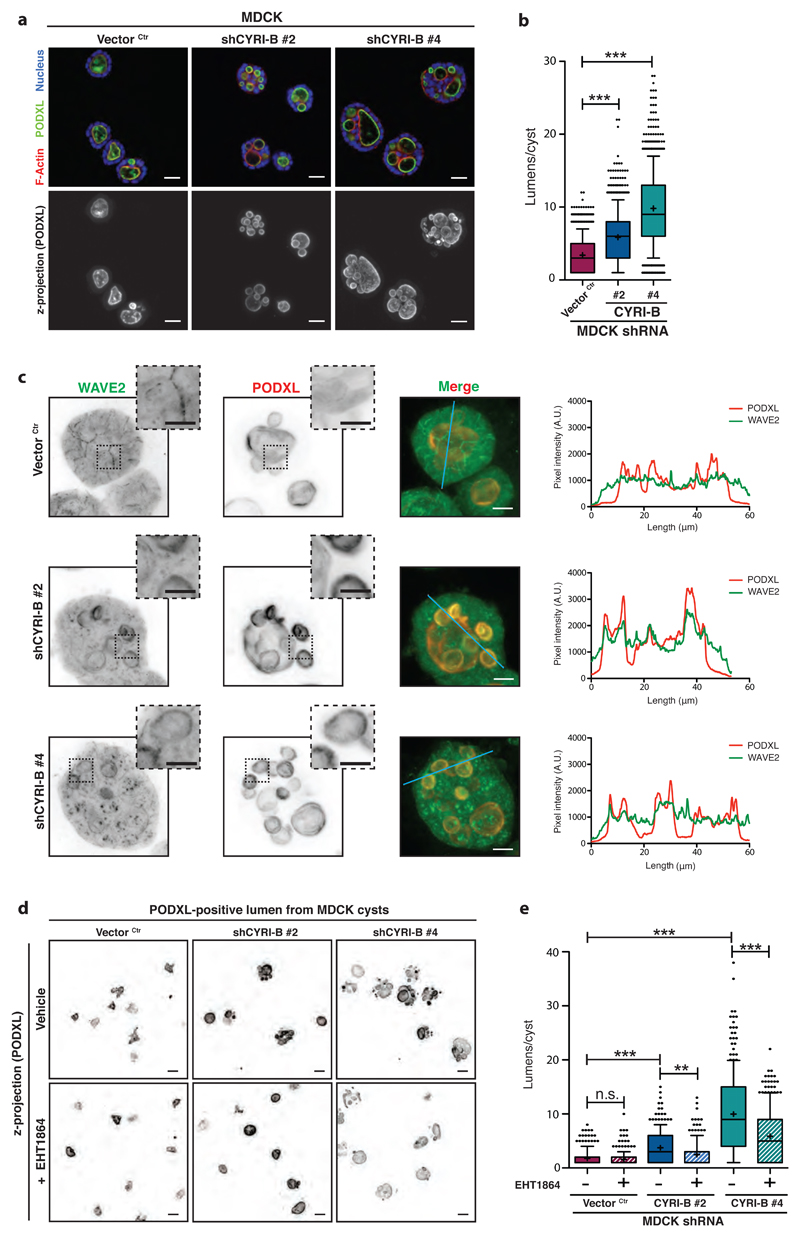
Finally, we tested a role for CYRI-B in another system where the Scar/WAVE complex is controlled by Rac1 activation gradients, the polarized epithelial cyst 43, 44. Cysts form from single cells that divide and establish a lumen via selective membrane trafficking and polarized recruitment and activation of cytoskeletal components45. Specific spatial regulation is dependent on matrix and adhesions, but elegant studies showed that Rac1 activation is important for lumen formation46 and is tightly regulated by differential recruitment of the GEF TIAM1 at each side of the cyst leading to an apico-basal gradient of activation43. We hypothesized that CYRI-B might play a role in regulating the maintenance of high Rac1 levels at the basolateral surfaces, allowing Scar/WAVE complex recruitment and activation to be spatially controlled during cyst formation. Indeed, knockdown of CYRI-B using shRNA in MDCK cells (Supplementary Fig. 6a-b) led to a multilumen phenotype during cyst formation, similar to that previously described for deregulation of active Rac1 (Figure 7a-b, 45). In order to investigate whether Scar/WAVE complex localization was also affected in this system, we performed immunofluorescence staining for WAVE2. In normal MDCK cysts, WAVE2 was prominently localized to the basolateral surfaces, but mostly absent from the luminal surface, as marked by podocalyxin (PODXL) (Fig. 7c). Traces of pixel intensity showed no correlation between the lumen and high WAVE2 localisation. However, when CYRI-B was depleted, WAVE2 staining was considerably increased at the luminal periphery and coincided with PODXL staining (Fig. 7c) suggesting aberrant recruitment of WAVE2 to the lumen. Mislocalisation of the actin cytoskeleton machinery to cyst luminal surfaces results in aberrant orientation of the mitotic cleavage plane during polarized cell division, which we observed in the cyri-b knockdown cysts (Supplementary Fig. 6c-e). If this multilumen phenotype was due to inappropriate Rac1 activation, we reasoned that it might be possible to at least partly rescue it by titrating in Rac1 inhibitors. Use of moderate concentrations of either EHT1864 (Fig. 7d-e) or NSC23766 (Supplementary Fig. 6f) provided a substantial rescue of the multilumen phenotype. Thus, loss of CYRI-B destabilised epithelial polarity during the formation of epithelial cell cysts by allowing inappropriate Rac1-mediated recruitment of WAVE2 to the nascent luminal surface. This provides further evidence of the importance of CYRI-B in maintenance of spatial regulation of activation of the Scar/WAVE complex by dynamic negative regulation and buffering of Rac1 activation of the Scar/WAVE complex at the apical side.
Figure 7. CYRI-B regulates Rac1-dependent recruitment of Scar/WAVE complex during epithelial cystogenesis.
a-b – Immunofluorescence of control (Vector Ctr) or cyri-b shRNA knockdown (#1 and #2) MDCK cysts fixed after 5 days of culture and stained for Podocalyxin (PODXL) (green), F-actin (red) and nuclei (blue). Top row is a confocal section and bottom row represents Z-maximal projection intensity of PODXL staining. Scale bar = 50 μm (a). Quantification of lumens in (b). One-way ANOVA with Dunn’s post-test. *** p<0.001. (Ctr n=1000, #1 n=1000, #2 n=800 cysts).
c - Immunofluorescence of control (Vector Ctr) or cyri-b shRNA knockdown (#1 and #2) MDCK cysts stained for WAVE2 (green) and Podocalyxin (PODXL) (red) after 5 days of culture. Inverted LUT images, merge and representative surface profile plots shown. PODXL (red) and WAVE2 (green) staining intensity was measured along the blue line. Scale bar = 9 μm. Insets provide a magnified view of the dotted square area. Scale bar = 5 μm.
d-e – Immunofluorescence of control (Vector Ctr) or cyri-b shRNA knockdown (#1 and #2) MDCK cysts grown during 5 days, treated or not with 50 nM EHT1864 and stained for Podocalyxin (PODXL). Pictures represent the Z-maximal projection intensity from a representative z-stack running across the entire cyst volume. Scale bar = 50 μm (d). Number of lumens per cyst was quantified for vehicle or EHT1864-treated cysts and plotted in (e). One-way ANOVA with Dunn’s post-test was performed between control (Vector Ctr), shCYRI-B #1 and shCYRI-B #2 whereas unpaired two-tailed t-test was applied between vehicle and drug-treated cyst. n.s. p>0.05, ** p≤0.01 *** p≤0.001. (250 cysts/condition)
All data presented are representative of at least 3 biologically independent experiments.
Bar and scatter plots show data points with mean and S.E.M.
Whisker plots show 10-90 percentile, median (bar) and mean (cross).
Discussion
CYRI is highly conserved and DUF1394 represents a Rac1 interaction module
CYRI proteins are highly conserved in eukaryotes and we propose that they constitute a Rac1 interaction module that directly limits lamellipodia extension in response to activation of Rac1. The DUF1394 domain of CYRI is shared with CYFIP proteins of the Scar/WAVE complex and we find that interaction with activated Rac1 is conserved between CYRI and CYFIP and involves two highly conserved arginine/lysine residues, previously described as part of the Rac1 interaction site on CYFIP13. Our data show that, like CYFIP1, CYRI is specific for activated Rac1 over RhoA and Cdc42. We implicate glycine 2 of CYRI as a myristoylation site and also show that it is required for efficient rescue of the CYRI knockout effect on cell shape. We thus envisage CYRI as a dynamic regulator of the activation of Scar/WAVE complex by Rac1 at the plasma membrane. Myristoylation may target CYRI and allow recycling of CYRI between active pseudopods and the cytoplasm or membrane vesicles 47. The Rac1-interacting formin FMNL2 is also myristoylated48, potentially providing a common mechanism to recruit specific actin cytoskeletal regulators to local regions of plasma membrane protrusions. CYRI does not show any homology to GTPase activating proteins (GAPs), so it is unlikely that CYRI directly affects the nucleotide bound state of Rac1, but rather it likely competes with CYFIP1 of the Scar/WAVE complex for active Rac1. Pulldown of CYRI-B-GFP using GFP-Trap and analysis by mass spectrometry did not reveal interactions with Rac GEFs or GAPs (unpublished), but a detailed analysis of whether CYRI interacts with other regulators of the Rac1 signaling pathways awaits future work. Why would a cell need CYRI if it has Rac-GAPs? In our opinion, CYRI could give the cell a specific buffer for Scar/WAVE-driven lamellipodia plasticity, rather than a general protein to turn off Rac1. Recently, it was shown that the Scar/WAVE complex contains a second Rac1-binding site (“D-site”), at the interface between NCKAP1 and CYFIP124. It would be particularly interesting to know if CYRI competes with both sites or specifically for the lower affinity “A-site” containing the homologous helicies with conserved basic residues24.
CYRI opposes recruitment of active Scar/WAVE complex to leading edges and promotes plasticity
Depletion of CYRI from melanoma cells, fibroblasts or Dictyostelium cells resulted in more circular shaped cells with blunted wide pseudopod protrusions. Loss of CYRI also increased recruitment of the Scar/WAVE complex to the leading edges of cells and increased the width of areas of cell protrusion. In general, cells lacking CYRI showed longer-lived protrusions that advanced forward and spread out laterally along the cell periphery more extensively. Thus, CYRI promotes dynamic pseudopod behavior, reducing the duration and extent of the cell’s response to a Rac1 activation signal.
Modulating the levels of CYRI had different effects on cell speed in different cell types: Dictyostelium cyri KO were modestly slower, CHL-1 melanoma moved modestly faster and WM852 cyri-b KO melanoma moved with similar speed. While this may seem paradoxical, the basal speeds of these cell types and modes by which they migrate are different. Furthermore, migration speed is the result of a combination of protrusion, adhesion and directionality/persistence, so is a very complex output reflecting many parameters. Yamada and colleagues previously showed that optimal Rac1 activity level can control persistence and speed of migration of fibroblasts and that higher Rac1 activity could be as paralyzing as low Rac136. CYRI modulates Rac-induced lamellipodia and makes them more dynamic. In cells like Dictyostelium, which are optimized by nature to be fast-moving and relatively non-adhesive, nearly any change in this programme will result in slower migration. In contrast, the speed of adhesive slow-moving cancer cell lines may benefit from removing the brakes on Rac1 activity. Future investigations will reveal how CYRI modulates adhesion, interplay with other Rac1 targets, such as FMNL formins and whether CYRI is itself regulated by signals other than the lipid modifications described here.
Negative regulators of Arp2/3 complex have been described 11, 12, 14, but to our knowledge, CYRI is the first characterized negative regulator of the Scar/WAVE complex. Importantly, it is widely conserved in evolution along with the Scar/WAVE complex, so is a universal negative regulator. CYRI and CYFIP likely resulted from an ancient gene duplication and have now co-evolved for long enough to be just recognizable as homologues. However, both proteins have retained the Rac1 binding function, so that they can be recruited by the same signals and thus CYRI is most likely a local inhibitor as proposed by Meinhardt41. While we don’t yet know the concentration of CYRI in the cell types used in our study, a recent quantitative mass spectrometry study estimated concentrations of CYRI-B to be 4-fold higher in protein copy number than Scar/WAVE complex 49 in 3 of 4 cell lines (A549 4-fold, HepG2 5-fold, PC3 4.4-fold and U87 0.53-fold, based on comparison with CYFIP1). Thus, there is likely enough CYRI-B in cells to compete with the Scar/WAVE complex for Rac1 binding.
CYRI provides spatiotemporal regulation of the connection between Rac1 and Scar/WAVE complex
Cell migration involves cycles of protrusion and retraction coupled with adhesion to produce forward locomotion 50. Cells with wild-type levels of CYRI showed rapid protrusion-retraction dynamics indicative of transient activation of the Scar/WAVE complex followed by inactivation (e.g. kymograph Fig. 5a). cyri knockouts showed broader and more sustained lamellipodia and increased Scar/WAVE recruitment both at steady state and initiated in response to optogenetic Rac1 activation. This places CYRI as a key part of the feedback loop controlling leading edge actin dynamics, in line with Arpin, a negative regulator of the Arp2/3 complex 14 and coronin, which positively regulates Rac1 activation 51, 52,1. Breaking the feedback loop by deleting CYRI not only affected recruitment of Scar/WAVE complex to the leading edge, but also affected Rac1 activity. Thus, the actin machinery feeds back to Rac1 as well as Rac1 feeding forward to actin assembly. Cells need to adapt motility when changing direction, for example, to follow a chemotactic gradient. Melanoma cells lacking CYRI were less efficient than wild type cells at chemotaxis up a serum gradient. Dictyostelium cells, which move extremely rapidly, could chemotax up folate gradients, but were seriously compromised in their ability to respond to rapidly changing cAMP gradients. Thus, CYRI enhances cells’ ability to create and suppress dynamic protrusions and change their motility decisions in response to environmental stimuli.
CYRI also regulates polarized function of Rac1-Scar/WAVE complex in epithelial cells in 3D. Epithelial cells specify apical lumen formation and maintain polarity using differential trafficking and asymmetric positioning of mitotic spindle orientation. It was recently shown that epithelial cells establish a Rac1 gradient that helps establish and maintain polarity by asymmetric distribution of β2-syntrophin and Par3 43. Par3, localized apically, inhibits the Rac-GEF TIAM1, while β2-syntrophin, localized basally, activates TIAM1. This gradient helps establish and maintain apical-basolateral polarity and is required for proper luminogenesis. In keeping with its role as a modulator of the Rac1- Scar/WAVE connection, we also found that CYRI contributes to epithelial localization of Scar/WAVE complex to basolateral surfaces and away from the apical lumen. CYRI-B promotes correct mitotic spindle orientation and helps direct formation of a single polarized lumen. This effect requires Rac1 activity, as we could partially rescue the phenotype with Rac1 inhibitors. It would be interesting to investigate preferential recruitment of CYRI to the apical surface via selective trafficking due to its lipid modification 45 or to other signals.
It has been asserted for many years that cell migration is the outcome of feedback loops that control the dynamics of cell shapes 10, 41, 53–55. Travelling and spreading wave patterns (for example 10, 53) are frequently seen in actin-based protrusions, which strongly imply the presence of positive feedback loops. However, positive feedback must be controlled with negative feedback 41 to prevent pathways being universally activated. Attempts to identify feedback loops in migration have generally fallen into two classes. In one, actin and actin-binding proteins themselves make up an excitable system 10, 54. In the other class, actin polymerization and pseudopods are seen as simple readouts of excitable signaling systems involving small GTPases, lipid kinases and associated proteins e.g.9. Our data imply that CYRI acts at the interface; by competing with Scar/WAVE (an actin-nucleating complex) for Rac1 (a small GTPase that takes part in many signaling pathways) it helps connect signaling and actin polymerization, allowing excitable behaviours in either to be moderated.
In conclusion, we propose that CYRI is a universal regulator of the dynamics of the Rac1 – Scar/WAVE pathway, providing plasticity and adding complexity to leading edge dynamics.
Supplementary Material
Acknowledgements
We thank Margaret O’Prey and the BAIR imaging facility for help with microscopy, Chloe Tesniere for careful work with CYRI overexpression plasmids, Michael Mcilwraith for help with protein purification, Benjamin Tyrell for isolation of the inducible Rac fl/fl fibroblasts, Klemens Rottners and Matthias Schaks for technical advice and discussion, Roland Wedlich-Söldner for the GFP-Lifeact construct. We thank CRUK for core funding to L.M.M. (grant A15673), R.H.I. (grant A19257) and S.Z. (C596/A12935), BBSRC for funding to L.H.C and N.C.O.T (BB/L022087/1), and NIH for funding to G.S.M (NIH RO1 EY025205).
Footnotes
Author contributions
R.H.I. and J.B. conceived and carried out the initial screen and recognized the similarity of CYRI to CYFIP. L.F. designed and carried out the majority of the experiments on mammalian CYRI-B. L.M.M., R.H.I. and L.F. conceived the study and wrote the paper. P.A.T. designed and constructed the mitochondrial relocalisation tools and carried out the Dictyostelium experiments in Figs 2 and 6. K.M. and K.I.A. designed the Raichu FRET probe and with L.F. carried out the FRET experiments. P.B. and L.F. carried out the surface plasmon resonance experiments. J.G., N.C.O.T., and L.C. synthesized probes for, advised on and carried out the myristoylation experiments with L.F. S.L. and S.Z. carried out and analysed the mass spectrometry with L.F. and J.B. P.A.T., G.S.M., J.A.W., H.J.S., L.T and S.I. provided essential advice, carried out experiments and analysis of data. M.N. and R.H.I. constructed the model and advised on its use.
References
- 1.Krause M, Gautreau A. Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat Rev Mol Cell Biol. 2014;15:577–590. doi: 10.1038/nrm3861. [DOI] [PubMed] [Google Scholar]
- 2.Insall R. The interaction between pseudopods and extracellular signalling during chemotaxis and directed migration. Curr Opin Cell Biol. 2013;25:526–531. doi: 10.1016/j.ceb.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, et al. Structure and control of the actin regulatory WAVE complex. Nature. 2010;468:533–538. doi: 10.1038/nature09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson AJ, Insall RH. Actin-based motility: WAVE regulatory complex structure reopens old SCARs. Curr Biol. 2011;21:R66–68. doi: 10.1016/j.cub.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Hoeller O, et al. Gbeta Regulates Coupling between Actin Oscillators for Cell Polarity and Directional Migration. PLoS Biol. 2016;14:e1002381. doi: 10.1371/journal.pbio.1002381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veltman DM, King JS, Machesky LM, Insall RH. SCAR knockouts in Dictyostelium: WASP assumes SCAR's position and upstream regulators in pseudopods. J Cell Biol. 2012;198:501–508. doi: 10.1083/jcb.201205058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner OD, et al. Hem-1 complexes are essential for Rac activation, actin polymerization, and myosin regulation during neutrophil chemotaxis. PLoS Biol. 2006;4:e38. doi: 10.1371/journal.pbio.0040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrew N, Insall RH. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat Cell Biol. 2007;9:193–200. doi: 10.1038/ncb1536. [DOI] [PubMed] [Google Scholar]
- 9.Devreotes PN, et al. Excitable Signal Transduction Networks in Directed Cell Migration. Annu Rev Cell Dev Biol. 2017;33:103–125. doi: 10.1146/annurev-cellbio-100616-060739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graziano BR, Weiner OD. Self-organization of protrusions and polarity during eukaryotic chemotaxis. Curr Opin Cell Biol. 2014;30:60–67. doi: 10.1016/j.ceb.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maritzen T, et al. Gadkin negatively regulates cell spreading and motility via sequestration of the actin-nucleating ARP2/3 complex. Proc Natl Acad Sci U S A. 2012;109:10382–10387. doi: 10.1073/pnas.1206468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madasu Y, et al. PICK1 is implicated in organelle motility in an Arp2/3 complex-independent manner. Mol Biol Cell. 2015;26:1308–1322. doi: 10.1091/mbc.E14-10-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocca DL, Martin S, Jenkins EL, Hanley JG. Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis. Nat Cell Biol. 2008;10:259–271. doi: 10.1038/ncb1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dang I, et al. Inhibitory signalling to the Arp2/3 complex steers cell migration. Nature. 2013;503:281–284. doi: 10.1038/nature12611. [DOI] [PubMed] [Google Scholar]
- 15.Ibarra N, Blagg SL, Vazquez F, Insall RH. Nap1 regulates Dictyostelium cell motility and adhesion through SCAR-dependent and - independent pathways. Curr Biol. 2006;16:717–722. doi: 10.1016/j.cub.2006.02.068. [DOI] [PubMed] [Google Scholar]
- 16.Sobczyk GJ, Wang J, Weijer CJ. SILAC-based proteomic quantification of chemoattractant-induced cytoskeleton dynamics on a second to minute timescale. Nat Commun. 2014;5:3319. doi: 10.1038/ncomms4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keeling PJ, et al. The tree of eukaryotes. Trends Ecol Evol. 2005;20:670–676. doi: 10.1016/j.tree.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Veltman DM, Insall RH. WASP family proteins: their evolution and its physiological implications. Mol Biol Cell. 2010;21:2880–2893. doi: 10.1091/mbc.E10-04-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bramham CR, Jensen KB, Proud CG. Tuning Specific Translation in Cancer Metastasis and Synaptic Memory: Control at the MNK-eIF4E Axis. Trends Biochem Sci. 2016;41:847–858. doi: 10.1016/j.tibs.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Bienvenut WV, et al. Comparative large scale characterization of plant versus mammal proteins reveals similar and idiosyncratic N-alpha-acetylation features. Mol Cell Proteomics. 2012;11 doi: 10.1074/mcp.M111.015131. M111 015131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broncel M, et al. Multifunctional reagents for quantitative proteome-wide analysis of protein modification in human cells and dynamic profiling of protein lipidation during vertebrate development. Angew Chem Int Ed Engl. 2015;54:5948–5951. doi: 10.1002/anie.201500342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang W, Di Vizio D, Kirchner M, Steen H, Freeman MR. Proteome scale characterization of human S-acylated proteins in lipid raft-enriched and non-raft membranes. Mol Cell Proteomics. 2010;9:54–70. doi: 10.1074/mcp.M800448-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanyon-Hogg T, Faronato M, Serwa RA, Tate EW. Dynamic Protein Acylation: New Substrates, Mechanisms, and Drug Targets. Trends Biochem Sci. 2017 doi: 10.1016/j.tibs.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Chen B, et al. Rac1 GTPase activates the WAVE regulatory complex through two distinct binding sites. Elife. 2017;6 doi: 10.7554/eLife.29795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soderberg O, et al. Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods. 2008;45:227–232. doi: 10.1016/j.ymeth.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Robinson MS, Sahlender DA, Foster SD. Rapid inactivation of proteins by rapamycin-induced rerouting to mitochondria. Dev Cell. 2010;18:324–331. doi: 10.1016/j.devcel.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walmsley MJ, et al. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459–462. doi: 10.1126/science.1089709. [DOI] [PubMed] [Google Scholar]
- 28.Li A, et al. Activated mutant NRas(Q61K) drives aberrant melanocyte signaling, survival, and invasiveness via a Rac1-dependent mechanism. J Invest Dermatol. 2012;132:2610–2621. doi: 10.1038/jid.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li A, et al. Rac1 drives melanoblast organization during mouse development by orchestrating pseudopod-driven motility and cell-cycle progression. Dev Cell. 2011;21:722–734. doi: 10.1016/j.devcel.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steffen A, et al. Rac function is crucial for cell migration but is not required for spreading and focal adhesion formation. J Cell Sci. 2013;126:4572–4588. doi: 10.1242/jcs.118232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 32.Martin KJ, et al. Accepting from the best donor; analysis of long-lifetime donor fluorescent protein pairings to optimise dynamic flim-based FRET experiments. PLoS One. 2017 doi: 10.1371/journal.pone.0183585. In revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura T, Kurokawa K, Kiyokawa E, Matsuda M. Analysis of the spatiotemporal activation of rho GTPases using Raichu probes. Methods Enzymol. 2006;406:315–332. doi: 10.1016/S0076-6879(06)06023-X. [DOI] [PubMed] [Google Scholar]
- 34.Wu YI, et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keren K, Yam PT, Kinkhabwala A, Mogilner A, Theriot JA. Intracellular fluid flow in rapidly moving cells. Nat Cell Biol. 2009;11:1219–1224. doi: 10.1038/ncb1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pankov R, et al. A Rac switch regulates random versus directionally persistent cell migration. J Cell Biol. 2005;170:793–802. doi: 10.1083/jcb.200503152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Insall R, Andrew N. Chemotaxis in Dictyostelium: how to walk straight using parallel pathways. Curr Opin Microbiol. 2007;10:578–581. doi: 10.1016/j.mib.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Muinonen-Martin AJ, et al. Melanoma cells break down LPA to establish local gradients that drive chemotactic dispersal. PLoS Biol. 2014;12:e1001966. doi: 10.1371/journal.pbio.1001966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tweedy L, Knecht DA, Mackay GM, Insall RH. Self-Generated Chemoattractant Gradients: Attractant Depletion Extends the Range and Robustness of Chemotaxis. PLoS Biol. 2016;14:e1002404. doi: 10.1371/journal.pbio.1002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuspa A, Loomis WF. Tagging developmental genes in Dictyostelium by restriction enzyme-mediated integration of plasmid DNA. Proc Natl Acad Sci U S A. 1992;89:8803–8807. doi: 10.1073/pnas.89.18.8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meinhardt H. Orientation of chemotactic cells and growth cones: models and mechanisms. J Cell Sci. 1999;112(Pt 17):2867–2874. doi: 10.1242/jcs.112.17.2867. [DOI] [PubMed] [Google Scholar]
- 42.Neilson MP, Mackenzie JA, Webb SD, Insall RH. Use of the parameterised finite element method to robustly and efficiently evolve the edge of a moving cell. Integr Biol (Camb) 2010;2:687–695. doi: 10.1039/c0ib00047g. [DOI] [PubMed] [Google Scholar]
- 43.Mack NA, et al. beta2-syntrophin and Par-3 promote an apicobasal Rac activity gradient at cell-cell junctions by differentially regulating Tiam1 activity. Nat Cell Biol. 2012;14:1169–1180. doi: 10.1038/ncb2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mangan AJ, et al. Cingulin and actin mediate midbody-dependent apical lumen formation during polarization of epithelial cells. Nat Commun. 2016;7 doi: 10.1038/ncomms12426. 12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Overeem AW, Bryant DM, van ISC. Mechanisms of apical-basal axis orientation and epithelial lumen positioning. Trends Cell Biol. 2015;25:476–485. doi: 10.1016/j.tcb.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Yagi S, Matsuda M, Kiyokawa E. Suppression of Rac1 activity at the apical membrane of MDCK cells is essential for cyst structure maintenance. EMBO Rep. 2012;13:237–243. doi: 10.1038/embor.2011.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang H, et al. Protein Lipidation: Occurrence, Mechanisms, Biological Functions, and Enabling Technologies. Chem Rev. 2018;118:919–988. doi: 10.1021/acs.chemrev.6b00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grobe H, Wustenhagen A, Baarlink C, Grosse R, Grikscheit K. A Rac1-FMNL2 signaling module affects cell-cell contact formation independent of Cdc42 and membrane protrusions. PLoS One. 2018;13:e0194716. doi: 10.1371/journal.pone.0194716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wisniewski JR, Hein MY, Cox J, Mann M. A "proteomic ruler" for protein copy number and concentration estimation without spike-in standards. Mol Cell Proteomics. 2014;13:3497–3506. doi: 10.1074/mcp.M113.037309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meacci G, et al. alpha-Actinin links extracellular matrix rigidity-sensing contractile units with periodic cell-edge retractions. Mol Biol Cell. 2016;27:3471–3479. doi: 10.1091/mbc.E16-02-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swaminathan K, Muller-Taubenberger A, Faix J, Rivero F, Noegel AA. A Cdc42- and Rac-interactive binding (CRIB) domain mediates functions of coronin. Proc Natl Acad Sci U S A. 2014;111:E25–33. doi: 10.1073/pnas.1315368111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swaminathan K, et al. Coronin7 regulates WASP and SCAR through CRIB mediated interaction with Rac proteins. Sci Rep. 2015;5 doi: 10.1038/srep14437. 14437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerisch G, et al. Mobile actin clusters and traveling waves in cells recovering from actin depolymerization. Biophys J. 2004;87:3493–3503. doi: 10.1529/biophysj.104.047589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Killich T, et al. The locomotion, shape and pseudopodial dynamics of unstimulated Dictyostelium cells are not random. J Cell Sci. 1993;106(Pt 4):1005–1013. doi: 10.1242/jcs.106.4.1005. [DOI] [PubMed] [Google Scholar]
- 55.Tweedy L, Susanto O, Insall RH. Self-generated chemotactic gradients-cells steering themselves. Curr Opin Cell Biol. 2016;42:46–51. doi: 10.1016/j.ceb.2016.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.