Abstract
Temporary but substantial reductions in voluntary food intake routinely accompany parasite infection in hosts ranging from insects to humans. This “parasite-mediated anorexia” drives dynamic nutrient-dependent feedbacks within and among hosts, which should alter the fitness of both hosts and parasites. Yet, few studies have examined the evolutionary and epidemiological consequences of this ubiquitous but overlooked component of infection. Moreover, numerous biomedical, veterinary, and farming practices (e.g., rapid biomass production via high-calorie or high-fat diets, low-level antibiotics to promote growth, nutritional supplementation, nonsteroidal anti-inflammatory drugs like Ibuprofen) directly or indirectly alter the magnitude of host anorexia—while also controlling host diet and therefore the nutrients available to hosts and parasites. Here, we show that anorexia can enhance or diminish disease severity, depending on whether the current dietary context provides nutrients that bolster or inhibit immune function. Feedbacks driven by nutrition-mediated competition between host immune function and parasite production can create a unimodal relationship between anorexia and parasite fitness. Subsequently, depending on the host’s diet, medical or husbandry practices that suppress anorexia could backfire, and inadvertently select for more virulent parasites and larger epidemics. These findings carry implications for the development of integrated treatment programs that consider links between host feeding behavior, nutrition, and disease severity.
Introduction
Parasite-mediated anorexia routinely accompanies infections in hosts ranging from insects to humans (Hart 1988; Adelman and Martin 2009; Bernardo and Singer 2017). These substantial but temporary reductions in food intake are typically considered immunopathology. In fact, many standard medical and veterinary practices (e.g., drugs, vaccines, steroids, antibiotic or diet use for rapid growth, and nutritional supplementation) directly or indirectly subvert anorexia (Plata-Salaman 1996; Fox et al. 2002; Pecchi et al. 2009). For instance, non-steroidal anti-inflammatory drugs such as Ibuprofen work, in part, by suppressing “sickness behaviors” like fever—and anorexia. Such practices clearly do not follow the axiom “feed a cold, starve a fever.”
On the other hand, studies focused on calorie restriction indicate that reduced food intake during illness can improve host health and recovery (Wang et al. 2016; Cheng et al. 2017). These findings—along with countless fad diets extolling the health benefits of intermittent fasting or specific macronutrient ratios—are beginning to change perspectives on the role of diet and food intake on managing disease (Bashir-Tanoli and Tinsley 2014; Kyriazakis 2014; Grant 2017). In fact, novel approaches to disease management are emerging that focus on prescribed calorie restriction, guided fasting, targeting the signaling pathways that control appetite, or even selecting for genetic lines with specific appetite characteristics in livestock (Kyriazakis and Doeschl-Wilson 2009; Longo and Panda 2016; Cheng et al. 2017). These interventions appear to have benefits at the individual level, at least over the short term.
However, none of these host-centric perspectives consider how changes in the host’s nutritional resources (i.e., via anorexia or calorie restriction) might affect parasite fitness. Yet, parasites, by definition, steal resources from their hosts and some nutrients fuel parasite growth and development more than others (Hall 2009a; Vale et al. 2013). Anorexia, therefore, could play a role similar to fever, sequestering crucial nutrients (e.g., iron) away from parasites (Sutak et al. 2008; Lin et al. 2014; Sylvia and Demas 2017). Importantly, such changes to the within-host nutritional environment can alter selective pressures facing parasites with important implications for the evolution of virulence (parasite-induced harm to hosts; Gandon et al. 2001). Such within-host interactions, however, remain overlooked and therefore, poorly resolved. See Box 1 for an overview of this evolutionary puzzle.
Box 1 Overview of parasite-mediated anorexia. The challenges with understanding this ubiquitous behavior, and the approach we use in this study.
Here, we extend previous theory to examine when, why, and how reduced food intake can enhance or diminish disease severity at both the individual- and population-level. More specifically, we develop a general, yet mechanistic, model that integrates theory from disease ecology (Hall et al. 2007) and evolutionary epidemiology (Anderson and May 1992; Gandon et al. 2001) with key findings from empirical studies. Taken together, these studies indicate that parasite-mediated anorexia (or other forms of calorie restriction) may strongly impact the fitness of both hosts and parasites through at least three, potentially overlapping, pathways: (1) altering the quantity and quality of resources available to support host immune functions (Adamo et al. 2010; Cotter et al. 2011, 2019; Bashir-Tanoli and Tinsley 2014; Povey et al. 2014), (2) altering the quantity and quality of resources available to fuel parasite growth and development (Hall et al. 2007, 2009a, b; Civitello et al. 2018), which in turn, affects (3) shedding into the environment and transmission to new susceptible hosts (Chin et al. 2004; Rao et al. 2017). Thus, unlike classical models (Anderson and May 1992; Gandon et al. 2001), the model here explicitly accounts for the dynamic and resource-dependent feedbacks between multiple host and parasite traits.
Our results suggest that parasite-mediated anorexia could strongly alter the evolution of virulence with important consequences for the size and severity of epidemics. Importantly, we find that outcomes depend sensitively on dietary context; interventions that alter the quality and quantity of nutritional resource conditions within hosts through changes in host diet or nutritional intake could powerfully reduce disease severity—or they could backfire, inadvertently selecting for more harmful pathogen strains and driving larger and more severe epidemics. Thus, integrating previous empirical findings into a theoretical framework helps reconcile the mixed findings of previous studies on parasite-mediated anorexia and helps pinpoint key focal areas for future empirical studies. Additionally, while this model is motivated by the puzzle of parasite-mediated anorexia, the model more generally highlights the importance of accounting for links between infectious disease and changes in nutritional resources (e.g., via anthropogenic subsidies, ecological or livestock management, anorexia, calorie restriction).
Materials and methods
Linking anorexia to parasite fitness: theoretical framework and results
To examine links between parasite-mediated anorexia and evolutionary epidemiology under various dietary contexts, we use an adaptive dynamics framework (Diekmann et al. 2010). This model examines the relative costs and benefits of anorexia which ultimately, depend on tension between within-host nutrients fueling: (1) basic host physiology and defense mechanisms versus (2) parasite development, growth, and transmission. This model therefore, tracks the outcomes of multiple resource-dependent processes, which may act simultaneously and exert contrasting effects on host and parasite fitness.
We begin by investigating connections between anorexia and epidemiological dynamics in the absence of evolution. The model tracks changes in the density of susceptible () and infected () hosts, and a free-living parasite () through time () using a set of ordinary differential equations, ODEs:
| (1) |
| (2) |
| (3) |
In this model, all hosts are born susceptible and birth rate is density-dependent, but identical for both susceptible (S) and infected (I) hosts. For simplicity, we assume that both the per-capita birth rate and strength of density-dependence are independent of within-host nutrient levels,. Note that, for this model, represents dietary quantity; the quality of the diet, from an epidemiological perspective, is given by the effect of on epidemiological processes like virulence and recovery (expanded details below: Equations 5–7). As such, can be thought of as the level of within-host nutrient levels in infected hosts, and thus reflects the strength of infection-induced anorexia; infected hosts that exhibit strong anorexia would have low within-host nutrients, whereas infected hosts exhibiting weak anorexia would have high within-host nutrients. Background mortality rate () is independent of density and nutrients. Hosts become infected through contact with free-living parasites () at rate, .
Infected hosts die due to infection at rate ; thus, parasite-induced mortality (i.e., virulence) depends on both a parasite exploitation trait ( and within-host nutrients (). In other words, we make the assumption that the exploitation parameter ( is a measure of the harm a parasite can potentially cause—all else equal, a parasite with higher exploitation will have higher production (i.e., load within an individual host) and therefore virulence (), inducing greater host mortality. With this assumption, we will refer to parasites as more or less harmful on the basis of their exploitation rate. The effect of nutritional resources on virulence is more contingent: for example, if within-host parasite growth and replication are highly sensitive to specific nutrients, virulence will be an increasing function of within-host nutrients; on the contrary, virulence will be a decreasing function of within-host nutrients if resources are used by the host to tolerate infection. This latter possibility would require additional analyses beyond the scope of this current study. However, we discuss potential links between nutrients and host tolerance in more detail in the “Discussion” section and focus here on the case where virulence is an increasing function of resources.
Infected hosts shed free-living parasites into the environment at the rate , whereby shedding depends on both parasite exploitation and nutrient-driven changes to parasite load within hosts. This relationship captures important and biologically-relevant feedbacks between within-host nutrients which can not only fuel immune functions but also fuel parasite growth within hosts (“load”) and therefore, shedding into the environment and transmission to new hosts.
Note, while we do not explore the consequences of anorexia for parasite ingestion (critical for parasites that are transmitted orally) here, this model structure could readily be extended to accommodate that assumption. Free-living pathogens die at a background rate, . Infected hosts recover at rate,, which may increase or decrease with increasing nutrients.
Again, while anorexia alters many aspects of within-host physiology, we focus here on how anorexia simultaneously affects parasite growth and host immune functions. For simplicity, we assume that virulence is linked to parasite exploitation and within-host nutrients via a linear relationship, . We assume that shedding is linked to exploitation and nutrients via a saturating relationship, which captures an empirically-derived link between pathogen load, pathogen shedding, and virulence (Hall et al. 2009a,b; Civitello et al. 2018). Given that interpretation, represents the dependence of parasite burden on exploitation , and within-host nutrients , with the per-parasite virulence. captures the dependence of shedding on parasite load (which depends on the parasite’s level of resource exploitation).
Mounting evidence highlights that specific macronutrients can either promote or inhibit host immune function (Adamo et al. 2010; Cotter et al. 2011, 2019; Bashir-Tanoli and Tinsley 2014; Povey et al. 2014). Thus, we considered three different relationships between nutrients and immune function which in turn, alters the host’s ability to clear infection and recover. As a first pass, we represent these admittedly complex interactions using simple functional forms:
| (4) |
| (5) |
| (6) |
We studied the model using both analytical and simulation-based approaches in Mathematica 11.1 (“Mathematica 11.1” 2017). We used the next-generation matrix theorem (Hurford et al. 2010) to quantify how changing within-host nutrients affect the parasite’s fitness and ability to cause an epidemic:
| (7) |
Biologically, represents the ratio of gains (from transmission and shedding) to losses (from host recovery or mortality). It can be understood as the expected number of new infections produced by a single free-living parasite. The first term quantifies the probability that the free-living parasite encounters a susceptible host. Here, is the equilibrium density of susceptible hosts in the absence of disease (i.e., at the disease-free boundary): . The second term of is the expected number of secondary infections produced by an infected host, which depends on the shedding rate and on the duration of infection where recovery, decreases the length of infection. Parasite fitness is increased by increasing transmission or prolonging the infection by reducing host recovery or host mortality.
The model indicates that regardless of dietary context, the relationship between anorexia and parasite fitness, () is unimodal (hump-shaped). With diets that either weakly or strongly promote host recovery (Equation 5, Fig. 1A and Equation 6; Fig. 1B, respectively), anorexia (low within-host nutrients) drives higher parasite fitness. In these cases, suppressing anorexia (increasing within-host nutrients) reduces parasite fitness and can even drive the parasite extinct ( < 1).
Fig. 1.

Changes in parasite-mediated anorexia alter parasite fitness (R0) in the absence of evolution. The figure illustrates how food intake and dietary context affects parasite strains that differ in virulence levels: calorie intake increases host immune function and promotes recovery either (A) “weakly” (N) or (B) “strongly” () or (C) calorie intake interferes with host recovery (). Parasites can invade above the solid horizontal line (i.e., R0 > 1).
However, with immunosuppressive diets, strong anorexia benefits more virulent parasite strains whereas weak anorexia strongly favors less virulent strains but leads to relatively high parasite fitness (compare Fig. 1C to Fig. 1A, B) and larger epidemics. Notably, this model suggests that during illness, higher consumption of immunosuppressive diets can lead to larger epidemics but less virulent parasites—at least with parasite or pathogens that do not evolve to the within-host conditions of their hosts. These patterns also suggest that with immunosuppressive diets and highly virulent parasites, selection would favor hosts that exhibit weak anorexia and parasites that induce anorexia. In other words, to maximize fitness, more virulent parasites would need to induce stronger anorexic responses. These conditions highlight that anorexia is likely modulated by both hosts and parasites. These results underscore the need for studies that link changes in anorexia to the traits of both hosts and parasites.
The evolution of parasite virulence: theoretical framework and results
Next, we used an evolutionary invasion analysis (Otto and Day 2007; Diekmann et al. 2010) to more formally access the consequences of anorexia on the evolution of parasite virulence. This approach evaluates whether a rare mutant parasite () with novel exploitation () and shedding ()) can invade a system where susceptible hosts (), infected hosts (), and free-living parasites () are at equilibrium. The expanded model, including hosts infected with the mutant parasite (Im) is:
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
As above, we derived the invasion fitness of the mutant parasite () using the next generation theorem (see Online Appendix for full decomposition), which approximates the total number of new parasites produced by a mutant:
| (13) |
where is the number of hosts left uninfected by the resident parasite. Any mutant parasite with > 1 will increase from rarity and is assumed to outcompete and replace the resident parasite. The goal is to find evolutionarily stable (ES) values of the evolving trait, exploitation . At such ES values, the selection gradient (the derivative of with respect to the evolving trait ) vanishes and invasion fitness is maximized. In the Online Appendix, we show that any root of the selection gradient will be ES if the costs of exploitation increase faster than the benefits (e.g., and ). Such roots will satisfy the following:
| (14) |
A more biologically meaningful way to express this condition, using the biological intuition behind the terms of the invasion fitness expression, is that the ES exploitation rate will satisfy:
| (15) |
In other words, at the ES exploitation rate, the relative increase in shedding from increasing is perfectly balanced by an equal decrease in infection duration (Online Appendix). This balance depends on tension between nutrients fueling host recovery versus nutrients fueling parasite production and consequentially, shedding and transmission.
We evaluated the consequences of changes in the magnitude of anorexia (through, for example, medical or husbandry interventions), on the evolution of parasite virulence by implicitly differentiating the fitness gradient expression (Equation 14) with respect to . In other words, we explored how increasing within-host nutrients affected . A sign-equivalent expression for is:
| (16) |
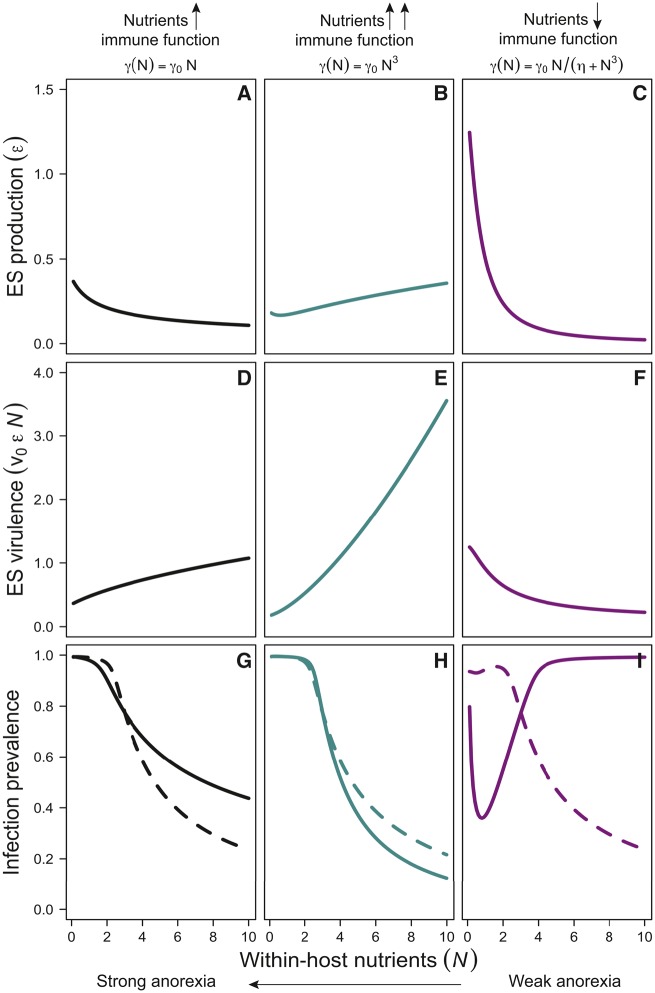
Importantly, this complex expression captures the tension between the effects of nutrients on recovery , shedding , infection duration , and virulence . See Online Appendix for expanded explanations. Existing theory predicts that any factor that shortens infection duration (e.g., increased recovery) should promote the evolution of increased virulence (van Baalen 1998; Gandon et al. 2001; Choo et al. 2003). Yet, our results (Equation 16) indicate that even if nutrients increase recovery rate , ES exploitation may actually decrease, depending on the strength of the relationship between parasite shedding and nutrients. For example, given our functions for virulence and shedding, suppressing anorexia (by increasing within-host nutrients), drives a decrease in the ES exploitation either when nutrients weakly promote host recovery (N, Fig 2A, D), or when nutrients inhibit host recovery (, Fig. 2C, F). However, under dietary contexts where nutritional resources strongly promote host recovery (), suppressing anorexia drives an increase in ES exploitation (and, in this case virulence) that is, (Fig. 2B, E).
Fig. 2.
Evolutionary and epidemiological consequences of changing the magnitude of parasite-mediated anorexia under different dietary contexts. Anorexia alters within-host nutrients which drives subsequent changes in (A–C) parasite exploitation (the parasite’s ability to steal resources from the host) and (D–E) virulence (parasite-induced harm to the host). (G–I) Together, changes in exploitation and virulence alter the size of epidemics (infection prevalence). Solid versus dashed lines illustrate how accounting for nutrition-mediated changes to parasite evolution (solid lines) can alter predictions for infection prevalence relative to predictions that overlook parasite evolution (dashed lines).
In addition to altering parasite evolution, changes to the magnitude of anorexia and within-host nutrients also altered the size of epidemics (disease prevalence). When nutrients increase host recovery in a linear or higher order fashion ( or ), suppressing anorexia or overfeeding (increasing within-host nutrients) decreased infection prevalence (Fig. 2G, H). However, with immunosuppressive nutrients, suppressing anorexia led to higher infection prevalence (Fig. 2I), but this result depended critically on whether the epidemiological dynamics account for parasite evolution or not (Fig. 2G–I: solid vs. dashed lines illustrate dynamics with and without parasite evolution, respectively).
Taken together, these results highlight that parasite-mediated anorexia may have contrasting results at the individual- and population-level (Fig. 2A–F vs. 2G–I). For instance, when resources moderately promote host recovery (; Fig. 2, left column), anorexia leads to slow host recovery and a longer infection, which reduces the fitness benefit of high virulence. Thus, parasite exploitation, production, and consequentially, virulence all decline. However, because the infectious period is longer, shedding and transmission increase, leading to a larger epidemic. These patterns result in less virulent but more abundant parasites. Hence, if the diet moderately promotes recovery, anorexia leads to less virulent parasites but increases infection prevalence.
Under dietary contexts where resources strongly promote host recovery (; Fig. 2, middle column), anorexia, by greatly reducing host recovery, leads to the evolution of much lower exploitation (compared to the moderate recovery case), leading to reductions in production and shedding. These two effects (reduced recovery and reduced shedding) balance each other out to cause little change in infection prevalence. However, weak anorexia (overfeeding and overnutrition) leads to much higher virulence levels (relative to the other two dietary contexts). In this case, higher within-host nutrients lead to much faster recovery and a very short infection period, prompting parasites to increase exploitation and production. Yet, because the infectious period is short, shedding, and therefore transmission and epidemic size, remains relatively low.
These results contrast with the immunosuppressive diet (; Fig. 2, right column), which is a lose–lose scenario. Here, not eating leads to higher exploitation and virulence and a relatively large epidemic, except over a small subset of parameter space. This small dip in infection prevalence arises when not eating maximizes recovery and perfectly matches parasite exploitation, production, and shedding (Fig. 2; see Online Appendix for expanded results). In this case, overeating leads to low virulence but larger epidemics—at least when accounting for parasite evolution in response to changing conditions within the host (Fig. 2I, dotted lines represent infection prevalence without parasite evolution; solid line illustrates results with evolution). Thus, with immunosuppressive diets, not eating could lead to chronic infections (i.e., longer but less severe) and still relatively large epidemics. Eating, on the contrary, leads to more acute infections (severe but of short duration) but larger epidemics. Immunosuppressive diets (e.g., high fat meals; Adamo et al. 2010), therefore, appear generally better for the parasite than the host.
Discussion
We illustrate that parasite-mediated anorexia could alter the evolution of virulence and the size and severity of epidemics. Our findings caution that, depending on dietary context, interventions that alter host appetite and resource intake could powerfully reduce disease severity—or backfire, inadvertently selecting for more harmful pathogen strains and driving larger and more severe epidemics (Figs. 1 and 2). At first glance, this result is unsurprising; any factor that shortens infection duration or reduces parasite-driven mortality (e.g., increased recovery due to vaccination) should promote the evolution of increased virulence (van Baalen 1998; Gandon et al. 2001; Choo et al. 2003). However, these previous approaches rarely consider how host nutrition, physiology, or energetic condition might jointly affect infection duration or harm (i.e., the host’s ability to recover from infection). Our results (Equation 16) indicate that even if nutrients increase recovery rate , ES exploitation may still decrease, depending on the strength of the relationship between parasite shedding, host recovery, and nutrients.
These predictions highlight key areas for future empirical studies. Mounting evidence highlights that not all nutrients improve the host’s ability to fend off disease (through either resistance or tolerance mechanisms); certain micronutrients and macronutrients can either enhance or inhibit immune function (Raubenheimer et al. 2009; Adamo et al. 2010; Cotter et al. 2011, 2019; Mason et al. 2014; Povey et al. 2014). In other words, not all nutritional resources are created equal; some resources can interfere with defense mechanisms (Buck et al. 2017; Cotter et al. 2019). For example, in insects, the protein apolipoprotein III is involved in both lipid transport and immune functioning. This dual-role means that a high fat meal hinders the host’s ability to bind bacteria (because the protein is preoccupied with transporting lipids for digestion), consequentially reducing their ability to resist disease (Adamo et al. 2010).
Yet, while strong patterns emerge within specific systems, it remains difficult to delineate clear patterns across either diseases or specific nutritional resources. Proteins appear generally important building blocks of immune defenses (Buck et al. 2017; Cotter et al. 2019), whereas lipids and carbohydrates can have beneficial, immunosuppressive, or prophylactic effects on immune functioning (van Heugten et al. 1996; Lee et al. 2006; Raubenheimer et al. 2009; Adamo et al. 2010; Povey et al. 2014; Wang et al. 2016). Still, these results all suggest that anorexia may function, in part, to alter both the quantity and quality of nutrients in order to optimize immune functioning (Adamo et al. 2010; Cotter et al. 2011, 2019). Such patterns challenge canonical assumptions of treating anorexia as an immunopathological side-effect.
Previous studies on anorexia have greatly advanced our understanding of interactions between nutritional resources and host defense. Still, scaling up what we know at the molecular and cellular level to the individual or population level remains challenging. The model predictions here underscore that a focus solely on host health only captures part of the equation; fully implementing these findings into an epidemiological framework requires accounting for changes in parasite traits. To date, however, such relationships prove challenging to glean from empirical data (reviewed by Hite et al., manuscript in revision). The model here represents a first pass at examining which parameters and functional responses represent key focal areas for future empirical studies.
Our results underscore that links between nutritional resources and host resistance mechanisms can drive divergent outcomes across individual-level and population-level processes (Mideo et al. 2008; Cressler et al. 2014; Greenspoon et al. 2018). For instance, with chronic cases of diseases (i.e., less severe but longer infections), anorexia may carry population-level benefits by reducing overall infection prevalence and disease severity—even though individual-level recovery may be prolonged over extended time frames (Fig. 2). Such cross-scale connections remain poorly resolved for most diseases (Handel and Rohani 2015) and represent an important avenue for future empirical and theoretical investigations.
We focused here on host resistance mechanisms. However, hosts also differ in their ability to tolerate infections by reducing the negative fitness costs associated with infection without affecting parasite load. Not surprisingly, nutrients can also mediate host tolerance (Howick and Lazzaro 2014; Clough et al. 2016; Budischak and Cressler 2018; Cumnock et al. 2018; Miller and Cotter 2018). Accounting for such interactions can diminish negative relationships between host and parasite fitness (e.g., those examined in the model here), with important implications for evolutionary epidemiology (Rao et al. 2017; reviewed by Budischak and Cressler 2018) and host–parasite co-evolution (J.L. Hite et al., manuscript under review). These interactions are beyond the scope of this study but represent crucial directions for future studies.
From an applied perspective, the approach used here provides a straightforward way to develop a priori predictions to examine how medical or veterinary interventions that alter host food intake and diet could affect the outcomes of disease at both the individual and population level. For instance, current farming practices intensively select for growth (through both genetic selection and diet) and these lines often experience more severe infection characteristics—and anorexia—relative to animals from slower-growing lines (Kyriazakis and Doeschl-Wilson 2009; Zaralis et al. 2008). Moreover, because many practices emphasize rapid biomass production and growth, diet choice and treatments that directly or indirectly alter host anorexia, could backfire; overfeeding hosts could improve individual-level recovery but select for more virulent parasites, subsequently driving the evolution of higher infection prevalence and more severe disease at the population level (Figs. 1 and 2). We propose that theory from ecology and evolutionary biology provides a powerful method to test these predictions. Such interdisciplinary approaches could provide key insight into disease dynamics and facilitate the use of more forward-thinking strategies to manage disease (Longo and Panda 2016; Grant 2017; Wale et al. 2017).
While dietary protocols are peripherally incorporated into many medical treatments and farming practices, we are far from a consensus on what combination of nutritional resources or calorie intake would optimize host recovery. Moreover, current efforts examining diet and disease have largely focused on metabolic diseases (e.g., Type 11 diabetes) or cancer (Longo and Panda 2016). Few studies consider diet-disease links that involve infectious diseases (i.e., those caused by parasites and pathogens). The few studies that do so are largely host-centric, overlooking how such changes might impact parasite evolution (reviewed by Adelman and Martin 2009; Pecchi et al. 2009). To be fair, this gap remains, in part, because such complex and dynamic feedbacks are challenging to disentangle and measure empirically (Cressler et al. 2014; Greenspoon et al. 2018). Nonetheless, our results join others indicating that these nutrient-mediated interactions within-hosts may have unintended epidemiological and evolutionary consequences that warrant serious consideration (Longo and Panda 2016; Wang et al. 2016; Rao et al. 2017; Wale et al. 2017; Cumnock et al. 2018).
Authors’ contributions
J.L.H. was supported by the National Institute Of General Medical Sciences of the National Institutes of Health under Award Number F32GM128246. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.L.H. and C.E.C. conceived and implemented the study. J.L.H. wrote the first draft of the manuscript. C.E.C. contributed to revisions.
Supplementary Material
Acknowledgments
The authors thank the Division of Ecoimmunology and Disease Ecology, Division of Comparative Endocrinology, Division of Animal Behavior, and Division of Ecology and Evolution of the Society for Integrative and Comparative Biology as well as the Macroecology of Infectious Disease Research Coordination Network funded by the National Science Foundation [NSF DEB 1316223] for supporting the symposium “The Scale of Sickness: How Immune Variation across Space and Species Affects Infectious Disease Dynamics” financially.
Funding
This work was funded by the National Institutes of Health [F32GM12846].
Supplementary data
Supplementary data available at ICB online.
References
- Adamo SA, Bartlett A, Le J, Spencer N, Sullivan K.. 2010. Illness-induced anorexia may reduce trade-offs between digestion and immune function. Anim Behav 79:3–10. [Google Scholar]
- Adelman JS, Martin LB.. 2009. Vertebrate sickness behaviors: adaptive and integrated neuroendocrine immune responses. Integr Comp Biol 49:202–14. [DOI] [PubMed] [Google Scholar]
- Anderson RM, May RM.. 1992. Infectious diseases of humans: dynamics and control. New York (NY): Oxford University Press.
- Bashir-Tanoli S, Tinsley MC.. 2014. Immune response costs are associated with changes in resource acquisition and not resource reallocation. Funct Ecol 28:1011–9. [Google Scholar]
- Bernardo MA, Singer MS.. 2017. Parasite-altered feeding behavior in insects: integrating functional and mechanistic research frontiers. J Exp Biol 220:2848–57. [DOI] [PubMed] [Google Scholar]
- Buck MD, Sowell RT, Kaech SM, Pearce EL.. 2017. Metabolic Instruction of Immunity. Cell 169:570–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budischak SA, Cressler CE.. 2018. Fueling defense: effects of resources on the ecology and evolution of tolerance to parasite infection. Front Immunol 9:2453.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C-W, Villani V, Buono R, Wei M, Kumar S, Yilmaz OH, Cohen P, Sneddon JB, Perin L, Longo VD.. 2017. Fasting-mimicking diet promotes Ngn3-driven beta-cell regeneration to reverse diabetes. Cell 168:775–88.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A, Guo FC, Bernier NJ, Woo PTK.. 2004. Effect of Cryptobia salmositica-induced anorexia on feeding behavior and immune response in juvenile rainbow trout Oncorhynchus mykiss. Dis Aquat Organ 58:17–26. [DOI] [PubMed] [Google Scholar]
- Choo K, Williams PD, Day T.. 2003. Host mortality, predation, and the evolution of parasite virulence. Ecol Lett 6:310–5. [Google Scholar]
- Civitello DJ, Fatima H, Johnson LR, Nisbet RM, Rohr JR.. 2018. Bioenergetic theory predicts infection dynamics of human schistosomes in intermediate host snails across ecological gradients. Ecol Lett 21:692–701. [DOI] [PubMed] [Google Scholar]
- Clough D, Prykhodko O, Råberg L.. 2016. Effects of protein malnutrition on tolerance to helminth infection. Biol Lett 12:20160189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter SC, Reavey CE, Tummala Y, Randall JL, Holdbrook R, Ponton F, Simpson SJ, Smith JA, Wilson K.. 2019. Diet modulates the relationship between immune gene expression and functional immune responses. Insect Biochem Mol Biol 109:128–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter SC, Simpson SJ, Raubenheimer D, Wilson K.. 2011. Macronutrient balance mediates trade-offs between immune function and life history traits. Funct Ecol 25:186–98. [Google Scholar]
- Cressler CE, Nelson WA, Day T, Mccauley E.. 2014. Disentangling the interaction among host resources, the immune system and pathogens. Ecol Lett 17:284–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumnock K, Gupta AS, Lissner M, Chevee V, Davis NM, Schneider DS, Cumnock K, Gupta AS, Lissner M, Chevee V, et al. 2018. Host energy source is important for disease tolerance to malaria. Curr Biol 28:1–8. [DOI] [PubMed] [Google Scholar]
- Diekmann O, Heesterbeek JA, Roberts MG.. 2010. The construction of next-generation matrices for compartmental epidemic models. J R Soc Interface 7:873–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MT, Uche UE, Vaillant C, Ganabadi S, Calam J.. 2002. Effects of Ostertagia ostertagi and omeprazole treatment on feed intake and gastrin-related responses in the calf. Veterinary Parasitology 105:285–301. [DOI] [PubMed] [Google Scholar]
- Gandon S, Mackinnon MJ, Nee S, Read AF.. 2001. Imperfect vaccines and the evolution of pathogen virulence. Nature 414:751–6. [DOI] [PubMed] [Google Scholar]
- Grant B. 2017. Running on empty: fasting diets are increasingly popular, but do they really work? The Scientist. p. 1–15. [Google Scholar]
- Greenspoon PB, Banton S, Mideo N.. 2018. Immune system handling time may alter the outcome of competition between pathogens and the immune system. J Theor Biol 447:25–31. [DOI] [PubMed] [Google Scholar]
- Hall SR, Knight CJ, Becker CR, Duffy MA, Tessier AJ, Cáceres CE.. 2009a. Quality matters: resource quality for hosts and the timing of epidemics. Ecol Lett 12:118–28. [DOI] [PubMed] [Google Scholar]
- Hall SR, Simonis JL, Nisbet RM, Tessier AJ, Cáceres CE.. 2009b. Resource ecology of virulence in a planktonic host-parasite system: an explanation using dynamic energy budgets. Am Nat 174:149–62. [DOI] [PubMed] [Google Scholar]
- Hall SR, Sivars-Becker L, Becker C, Duffy MA, Tessier AJ, Cáceres CE.. 2007. Eating yourself sick: transmission of disease as a function of foraging ecology. Ecol Lett 10:207–18. [DOI] [PubMed] [Google Scholar]
- Handel A, Rohani P.. 2015. Crossing the scale from within-host infection dynamics to between-host transmission fitness : a discussion of current assumptions and knowledge. Philos Trans R Soc Lond B Biol Sci. 2015; 370:20140302. (doi: 10.1098/rstb.2014.0302). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. 1988. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12:123–37. [DOI] [PubMed] [Google Scholar]
- Howick VM, Lazzaro BP.. 2014. Genotype and diet shape resistance and tolerance across distinct phases of bacterial infection. BMC Evol Biol 14:56.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurford A, Cownden D, Day T.. 2010. Next-generation tools for evolutionary invasion analyses. J R Soc Interface 7:561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriazakis I. 2014. Pathogen-induced anorexia: a herbivore strategy or an unavoidable consequence of infection? Anim Prod Sci 54:1190–7. [Google Scholar]
- Kyriazakis I, Doeschl-Wilson A.. 2009. Anorexia during infection in mammals: variation and its sources In: Torrallardona D, Roura E, editors. Voluntary feed intake in pigs. Wageningen: Wageningen Academic Publishers; p. 307–21. [Google Scholar]
- Lee KP, Cory JS, Wilson K, Raubenheimer D, Simpson SJ.. 2006. Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc Biol Sci 273:823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Pantapalangkoor P, Tan B, Bruhn KW, Ho T, Nielsen T, Skaar EP, Zhang Y, Bai R, Wang A, et al. 2014. Transferrin iron starvation therapy for lethal bacterial and fungal infections. J Infect Dis 210:254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Panda S.. 2016. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab 23:1048–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason AP, Smilanich AM, Singer MS.. 2014. Reduced consumption of protein-rich foods follows immune challenge in a polyphagous caterpillar. J Exp Biol 217:2250–60. [DOI] [PubMed] [Google Scholar]
- Mideo N, Alizon S, Day T.. 2008. Linking within- and between-host dynamics in the evolutionary epidemiology of infectious diseases. Trends Ecol Evol 23:511–7. [DOI] [PubMed] [Google Scholar]
- Miller CVL, Cotter SC.. 2018. Resistance and tolerance: the role of nutrients on pathogen dynamics and infection outcomes in an insect host. J Anim Ecol 87:500–10. [DOI] [PubMed] [Google Scholar]
- Otto SP, Day T.. 2007. A biologist’s guide to mathematical modeling in ecology and evolution Princeton (NJ: ): Princeton University Press. [Google Scholar]
- Pecchi E, Dallaporta M, Jean A, Thirion S, Troadec J-D.. 2009. Prostaglandins and sickness behavior: old story, new insights. Physiol Behav 97:279–92. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR. 1996. Anorexia during acute and chronic disease. Nutrition 12:69–78. [DOI] [PubMed] [Google Scholar]
- Povey S, Cotter SC, Simpson SJ, Wilson K.. 2014. Dynamics of macronutrient self-medication and illness-induced anorexia in virally infected insects. J Anim Ecol 83:245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raubenheimer D, Simpson SJ, Mayntz D.. 2009. Nutrition, ecology and nutritional ecology: toward an integrated framework. Functional Ecology, 23:4–16. (doi:10.1111/j.1365-2435.2009.01522.x). [Google Scholar]
- Rao S, Schieber AMP, O’Connor CP, Leblanc M, Michel D, Ayres JS.. 2017. Pathogen-mediated inhibition of anorexia promotes host survival and transmission. Cell 168:503–16.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutak R, Lesuisse E, Tachezy J, Richardson DR.. 2008. Crusade for iron: iron uptake in unicellular eukaryotes and its significance for virulence. Trends Microbiol 16:261–8. [DOI] [PubMed] [Google Scholar]
- Sylvia KE, Demas GE.. 2017. A return to wisdom: using sickness behaviors to integrate ecological and translational research. Integr Comp Biol 57:1204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale PF, Choisy M, Little TJ.. 2013. Host nutrition alters the variance in parasite transmission potential. Biol Lett 9:20121145 (http://dx.doi.org/10.1098/rsbl.2012.1145). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Baalen M. 1998. Coevolution of recovery ability and virulence. Proc Biol Sci 265:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heugten E, Coffey MT, Spears JW.. 1996. Effects of immune challenge, dietary energy density, and source of energy on performance and immunity in weanling pigs. J Anim Sci 74:2431–40. [DOI] [PubMed] [Google Scholar]
- Wale N, Sim DG, Jones MJ, Salathe R, Day T, Read AF.. 2017. Resource limitation prevents the emergence of drug resistance by intensifying within-host competition. Proc Natl Acad Sci U S A 2017;114:13774–13779; (DOI:10.1073/pnas.1715874115). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot J-D, Booth CJ, Medzhitov R.. 2016. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell 166:1512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaralis K, Tolkamp BJ, Houdijk JGM, Wylie ARG, Kyriazakis I.. 2008. Consequences of protein supplementation for anorexia, expression of immunity and plasma leptin concentrations in parasitized ewes of two breeds. Br J Nutr 101:499–509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.