Abstract
Purpose
To describe the epidemiology of intra-abdominal infection in an international cohort of ICU patients according to a new system that classifies cases according to setting of infection acquisition (community-acquired, early onset hospital-acquired, and late-onset hospital-acquired), anatomical disruption (absent or present with localized or diffuse peritonitis), and severity of disease expression (infection, sepsis, and septic shock).
Methods
We performed a multicenter (n = 309), observational, epidemiological study including adult ICU patients diagnosed with intra-abdominal infection. Risk factors for mortality were assessed by logistic regression analysis.
Results
The cohort included 2621 patients. Setting of infection acquisition was community-acquired in 31.6%, early onset hospital-acquired in 25%, and late-onset hospital-acquired in 43.4% of patients. Overall prevalence of antimicrobial resistance was 26.3% and difficult-to-treat resistant Gram-negative bacteria 4.3%, with great variation according to geographic region. No difference in prevalence of antimicrobial resistance was observed according to setting of infection acquisition. Overall mortality was 29.1%. Independent risk factors for mortality included late-onset hospital-acquired infection, diffuse peritonitis, sepsis, septic shock, older age, malnutrition, liver failure, congestive heart failure, antimicrobial resistance (either methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, extended-spectrum beta-lactamase-producing Gram-negative bacteria, or carbapenem-resistant Gram-negative bacteria) and source control failure evidenced by either the need for surgical revision or persistent inflammation.
Conclusion
This multinational, heterogeneous cohort of ICU patients with intra-abdominal infection revealed that setting of infection acquisition, anatomical disruption, and severity of disease expression are disease-specific phenotypic characteristics associated with outcome, irrespective of the type of infection. Antimicrobial resistance is equally common in community-acquired as in hospital-acquired infection.
Electronic supplementary material
The online version of this article (10.1007/s00134-019-05819-3) contains supplementary material, which is available to authorized users.
Keywords: Intra-abdominal infection, Peritonitis, Sepsis, Intensive care, Multidrug resistance, Mortality
Key message
|
A multinational epidemiological study on intra-abdominal infection in ICU patients revealed that setting of infection acquisition, anatomical barrier disruption, and severity of disease expression are disease-specific phenotypic characteristics associated with mortality. Antibiotic resistance appeared equally in community-acquired as in hospital-acquired infection. |
Introduction
Severe intra-abdominal infections are a frequent and important issue in intensive care (ICU). According to international literature, the abdomen often ranks first or second among the sources of infection or sepsis [1–3].
Intra-abdominal infections pose several particular clinical challenges. First, there is a large span of disease severity ranging from uncomplicated cases to fulminant septic shock and multi-organ dysfunction. Second, there is the broad spectrum of pathogens including Gram-positive and Gram-negative aerobic bacteria, anaerobes, and fungi [4]. Third, the contribution of microbiological diagnosis is not straightforward as cultures cannot always readily discriminate true pathogens from harmless micro-organisms [5, 6]. Furthermore, source control encompassing all interventions to eradicate the source of infection, control on-going contamination, and to restore anatomic derangements and physiologic function, is key to clinical management and success, but often difficult to achieve [5, 7, 8]. Finally, there is the wide variety of clinical entities within intra-abdominal infections. Besides local abscess formation or solid organ infection (e.g., liver abscesses and infected pancreatic necrosis), a classic approach recognizes three types of peritonitis: i.e., primary peritonitis (peritoneal dialysis-related or spontaneous bacterial peritonitis), secondary peritonitis (following anatomical disruption of the GI tract), or tertiary peritonitis (persistent infection despite adequate source control intervention). In addition, cases of intra-abdominal infection are often classified as uncomplicated or complicated. Complicated describes extension of infection from their source into the peritoneal cavity.
Because of this heterogeneity, the intra-abdominal infections are difficult to study [9]. To bring more clarity in the terminology, an alternative classification for intra-abdominal infections has been proposed [10]. This system classifies intra-abdominal infections according to their setting of acquisition (community-acquired, healthcare-associated or early onset hospital-acquired, or late-onset hospital-acquired), presence of anatomical disruption (either absent or present resulting in localized or diffuse peritonitis), and severity of disease expression (infection, sepsis, or septic shock). This classification defines different phenotypes of the same disease (e.g., diverticulitis) by covering aspects of (i) the extent of intra-abdominal contamination reflecting the complexity of source control, (ii) level of associated organ failure indicating sense of urgency and prognosis, and (iii) likelihood of antimicrobial resistant micro-organisms or otherwise important pathogens which may require broader antimicrobial coverage (enterococci, Candida spp.).
The objective of the study was to describe the epidemiology of intra-abdominal infection in an international cohort of ICU patients and to validate the predictive value for mortality of an alternative classification system.
Methods
A complete version of the Methods is in Supplement-1. AbSeS was an international, multicenter, prospective observational cohort study conducted between January and December 2016. Consecutive, adult ICU patients diagnosed with intra-abdominal infection, either as primary diagnosis leading to ICU admission or as a complication occurring during the ICU course, were eligible for inclusion. Overall, approval by established national, regional, or local institutional review boards was expedited and granted. The study is registered at ClinicalTrials.gov (number NCT03270345).
Data recorded and definitions
We obtained data describing the hospital and intensive-care facility through a center report form. Anonymous patient data were collected through the case report form. Examples of the center and case report forms are in Supplement-2. Type of intra-abdominal infection was defined according to the International Sepsis Forum Consensus Conference Definitions [11]. Intra-abdominal infections were classified according to setting of infection acquisition, anatomical barrier disruption, and severity of disease expression [10]. Setting is community-acquired, healthcare-associated and/or early onset hospital-acquired (≤ 7 days of hospital admission), or late-onset hospital-acquired (> 7 days of hospital admission [12]). Healthcare-associated onset is defined by at least one of the following risk factors for multidrug-resistant pathogens: nursing home resident, out-of-hospital parenteral nutrition or vascular access, chronic dialysis, recent hospital admission (< 6 months), or recent antimicrobial therapy (< 6 months). For convenience sake, ‘healthcare-associated and/or early-onset hospital-acquired’ cases are designated ‘early-onset hospital-acquired’. Intra-abdominal infections were classified as either without anatomical disruption, or with anatomical disruption resulting in localized or diffuse peritonitis (i.e., contamination spread to entire abdominal cavity). Severity of disease expression is defined as either infection, sepsis, or septic shock [13]. Microbiological assessment was left at the discretion of the physician. Eligible cultures included intra-operative cultures, trans-abdominal fine-needle aspiration, blood cultures presumably related to the intra-abdominal infection, and cultures from abdominal drains sampled ≤ 24 h post-surgery. Thresholds for resistance were those as reported by The European Committee on Antimicrobial Susceptibility Testing (EUCAST) [14]. Antimicrobial resistance was defined as methicillin resistance for Staphylococcus aureus, vancomycin resistance for enterococci, and for Gram-negative bacteria either production of extended-spectrum beta-lactamase (ESBL), carbapenem resistance, or fluoroquinolone resistance (resistance against ciprofloxacin, levofloxacin, or moxifloxacin). To assess relationships between resistance and mortality, we also used the definition of “difficult-to-treat” resistance for Gram-negative bacteria. This combines resistance to all tested carbapenem, beta-lactam, and fluoroquinolone agents, and is associated with worse clinical outcomes in bloodstream infection [15, 16]. We deviated from this definition, however, using ESBL production as a proxy for resistance against penicillins, cephalosporins, and monobactams. For reporting microbiological results, the number of patients with cultures sampled is used as denominator. Data on anti-infective management included antimicrobial therapy and source control. Antimicrobial coverage of empiric therapy was evaluated for basic coverage (i.e., coverage of Gram-positive, Gram-negative, and anaerobic bacteria), and the association of an antimicrobial agent or initial choice with potential clinical activity against Pseudomonas aeruginosa, methicillin-resistant S. aureus (MRSA), enterococci, vancomycin-resistant enterococci (VRE), and Candida. In this regard, coverage of enterococci targets Enterococcus faecalis [6]. Outcome data included source control assessment 7 days post-diagnosis or earlier if the patient died within that time window. Source control was judged as either successful or having failed. Failure represented either persistent inflammation (clinical evidence of a remaining source of infection) or the necessity of re-intervention following the initial approach (conservative management or source control intervention). Main outcome is ICU mortality with a minimum of 28 days of observation.
Data management and statistical analyses
Simple descriptive statistics were used to characterize the study population; continuous data were summarized by median and interquartile range, categorical data as n (%). Logistic regression analysis was used to assess relationships with mortality. Details on the regression models are in Supplement-1. It can be considered inappropriate to include ‘source control achievement at day 7’ in the model as this covariate is instrumental to the biological pathway between infection onset and mortality. Therefore, we report a logistic regression model with and without source control achievement.
Results
During the study period, 2850 patients were included; 229 were excluded, because essential data were missing. As such, 2621 patients from 309 ICUs from 42 countries were entered for analysis. Most patients were included in various European regions (n = 1830; 69.8%), followed by Middle & South America (n = 366; 14.0%), North Africa & Middle-East (n = 214; 8.8%), Asia-Pacific (n = 174; 6.6%), North America (n = 29; 1.1%), and Sub-Saharan Africa (n = 8; 0.3%) (Supplement-3).
Characteristics of the study cohort according to setting of infection acquisition are reported in Table 1. Setting of infection acquisition was community-acquired in 828 patients (31.6%), early onset hospital-acquired in 656 patients (25.0%), and late-onset hospital-acquired in 1137 patients (43.4%). Underlying conditions were more frequently observed in cases with healthcare-associated or hospital-acquired infection. Cases with hospital-acquired infection had higher SOFA scores and more often septic shock.
Table 1.
Patient characteristics of intensive-care unit patients with intra-abdominal infection/sepsis according to setting of infection acquisition
| Characteristic | Total cohort (n = 2621) | Community-acquired (n = 828) | Early onset hospital-acquired (n = 656) | Late-onset hospital-acquired (n = 1137) | p* |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 66 (54–75) | 67 (52–77) | 66 (54–77) | 66 (55–74) | 0.213 |
| Sex, male | 1488/2615 (56.9) | 452 (54.6) | 364 (55.5) | 672 (59.1) | 0.133 |
| Type of ICU admission | 2592** | 799** | 656 | 1137 | |
| Medical | 472 (18.2) | 109 (13.7) | 131 (20.0) | 232 (20.4) | <0.001 |
| Surgical, non-emergency | 233 (9.0) | 19 (2.4) | 39 (5.9) | 175 (15.4) | < 0.001 |
| Surgical, emergency | 1847 (71.3) | 660 (82.6) | 478 (72.9) | 709 (62.4) | < 0.001 |
| Trauma | 40 (1.5) | 11 (1.4) | 8 (1.2) | 21 (1.8) | 0.496 |
| ICU stay, days | 9 (4-18) | 9 (4–18) | 9 (4–17) | 10 (5–19) | 0.183 |
| Underlying conditions*** | |||||
| Chronic pulmonary disease | 342 (13.0) | 96 (11.6) | 90 (13.7) | 156 (13.7) | 0.324 |
| AIDS | 14 (0.5) | 6 (0.7) | 3 (0.5) | 5 (0.4) | 0.661 |
| Malignancy | 699 (26.7) | 116 (14.0) | 170 (25.9) | 413 (36.3) | < 0.001 |
| Neurologic disease | 165 (6.3) | 42 (5.1) | 60 (9.1) | 75 (6.6) | 0.008 |
| Peptic ulcer disease | 176 (6.7) | 57 (6.9) | 52 (7.9) | 67 (5.9) | 0.246 |
| Liver disease | 127 (4.8) | 24 (1.5) | 44 (6.7) | 59 (5.2) | 0.002 |
| Chronic renal failure | 282 (10.8) | 57 (6.9) | 100 (15.2) | 125 (11.0) | < 0.001 |
| Myocardial infarction | 188 (7.2) | 48 (5.8) | 57 (8.7) | 83 (7.3) | 0.098 |
| Chronic heart failure (NY Heart Association class IV) | 184 (7.0) | 36 (4.3) | 64 (9.8) | 84 (7.4) | < 0.001 |
| Peripheral vascular disease | 169 (6.4) | 34 (4.1) | 48 (7.3) | 87 (7.7) | 0.004 |
| Diabetes mellitus | 488 (18.6) | 116 (14.0) | 141 (21.5) | 231 (20.3) | < 0.001 |
| Immunosuppression | 253 (9.7) | 47 (5.7) | 83 (12.7) | 123 (10.8) | < 0.001 |
| Lifestyle risk factors | 1363 (52.0) | 413 (49.9) | 355 (54.1) | 595 (52.3) | 0.257 |
| Malnutrition (body mass index < 20) | 177 (6.8) | 46 (5.6) | 53 (8.1) | 78 (6.9) | 0.154 |
| Obesity (body mass index ≥ 30) | 735 (28.0) | 236 (28.5) | 197 (30.0) | 302 (26.6) | 0.271 |
| Tobacco use (> 20 pack years) | 446 (17.0) | 127 (7.1) | 106 (16.2) | 213 (18.7) | 0.113 |
| Alcohol abuse (> 10 g alcohol/day) | 196 (7.5) | 59 (7.1) | 49 (7.5) | 88 (7.7) | 0.261 |
| IV drug abuse | 17 (0.6) | 8 (1.0) | 3 (0.5) | 6 (0.5) | – |
| Severity of acute illness | |||||
| SAPS II score at time of ICU admission | 49 (39–60) | 48 (38–59) | 49 (39–61) | 49 (38–60) | 0.183 |
| SOFA score at diagnosis | 6 (3–10) | 5 (3–9) | 7 (3–10) | 6 (3–10) | < 0.001 |
| Severity of disease expression | |||||
| Infection without sepsis | 164 (6.3) | 51 (6.2) | 42 (6.4) | 71 (6.2) | 0.981 |
| Sepsis | 1590 (60.7) | 528 (63.8) | 399 (60.8) | 663 (58.3) | 0.050 |
| Septic shock | 867 (33.1) | 249 (30.1) | 215 (32.8) | 403 (35.4) | 0.043 |
| Anatomical disruption | |||||
| Not present | 615 (23.5) | 186 (22.5) | 166 (25.3) | 263 (23.1) | 0.413 |
| Yes, with localized peritonitis | 981 (37.4) | 342 (41.3) | 256 (39.0) | 383 (33.7) | 0.002 |
| Yes, with diffuse peritonitis | 1025 (39.1) | 300 (36.2) | 234 (35.7) | 491 (43.2) | 0.001 |
Data are reported as n (%) or median (1st–3rd quartile)
SAPS simplified acute physiology score, SOFA sequential organ failure assessment
*p value indicates differences between patients with community-acquired infection, healthcare-associated infection or early onset hospital-acquired infection, and late-onset hospital-acquired infection
**Data missing from 29 patients
***More details regarding underlying conditions are reported in Supplement–4
The vast majority of cases involved secondary peritonitis (68.4%), followed by biliary tract infection (12.2%), intra-abdominal abscess (6.9%), and pancreatic infection (6.3%). Primary peritonitis, toxic megacolon, peritoneal dialysis-related peritonitis, and typhlitis were less frequent (< 4%). Details on the distribution according to setting of infection acquisition are reported in Table 2.
Table 2.
Proportion of types of intra-abdominal infection and distribution according to origin of infection acquisition
| Type of abdominal sepsis | Total n (%)* | Community-acquired n (%)** | Early onset hospital-acquired n (%)** | Late-onset hospital-acquired n (%)** |
|---|---|---|---|---|
| Primary peritonitis | 103 (3.9) | 33 (32) | 28 (27.2) | 42 (40.8) |
| Secondary and tertiary peritonitis | 1794 (68.4) | 588 (32.8) | 431 (24) | 775 (43.2) |
| PD-related peritonitis | 9 (0.3) | 0 | 2 (20) | 7 (70) |
| Intra-abdominal abscess | 180 (6.9) | 36 (20) | 49 (27.2) | 95 (52.8) |
| Biliary tract infection | 319 (12.2) | 117 (36.7) | 95 (29.8) | 107 (33.5) |
| Pancreatic infection | 165 (6.3) | 45 (27.3) | 33 (20) | 87 (52.7) |
| Typhlitis | 9 (0.3) | 0 | 3 (33.3) | 6 (66.6) |
| Toxic megacolon | 42 (1.6) | 9 (21.4) | 15 (35.7) | 18 (42.9) |
PD-related peritoneal dialysis-related
*% Within column; **% within row
Microbiology
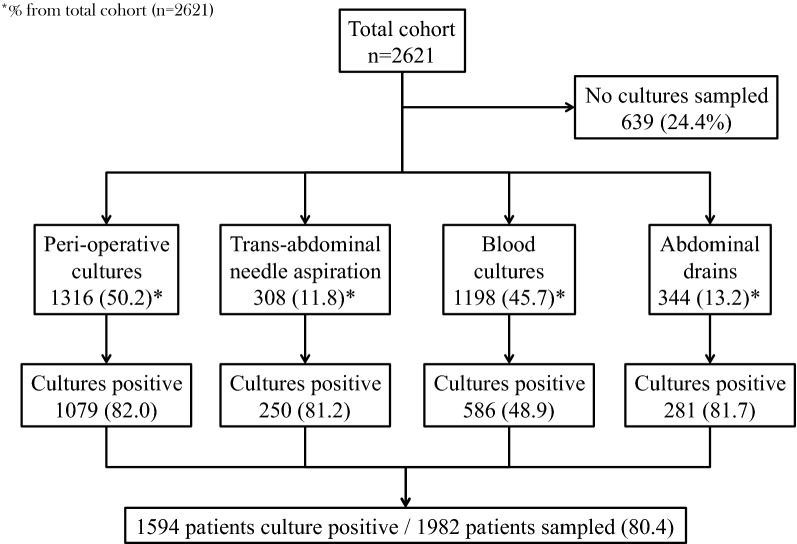
Microbiological samples were obtained in 1982 patients (75.6%). In 80.4% of these patients, at least one culture was found positive (n = 1594). Figure 1 reports the type of samples obtained with their respective proportion of culture positivity. Gram-negative bacteria were most frequently isolated (58.6%) with Enterobacterales as predominant family (51.7%) and Escherichia coli as most common pathogen (36.8%). Gram-positive aerobic bacteria were isolated in 39.4% of patients with enterococci as most prevalent species (25.9%). Furthermore, anaerobic bacteria and fungi were isolated in 11.7% and 13.0% of patients, respectively. Detailed results on isolated micro-organisms are reported in Table 3. Multidrug-resistant micro-organisms were isolated from 522 patients (26.3%). Antimicrobial resistance rates were not different among community-acquired (26.5%), early onset hospital-acquired (29.0%), and late-onset hospital-acquired infection (24.6%) (p = 0.215). There was also no difference in antimicrobial resistance among patients with infection (27.6%), sepsis (26.9%), and septic shock (25.0%) (p = 0.449). Antimicrobial resistance is mainly a matter of Gram-negatives, but variations according to geographic region are substantial (Table 4). Regions of particular concern include Eastern- and South-East Europe, North Africa and the Middle-East, and Latin America as > 35% of patients are infected by at least one antimicrobial resistant micro-organism. Antimicrobial resistance rates according to setting of infection acquisition and region are reported in Supplement-5.
Fig. 1.
Types of microbiological cultures sampled and culture-positive rate in patients with intra-abdominal infection
Table 3.
Micro-organisms isolated from cultures sampled in patients with intra-abdominal infection
| Micro-organism | Total cohort (n = 1982) | Setting of infection acquisition | ||
|---|---|---|---|---|
| Community-acquired (n = 664) | Early onset hospital-acquired (n = 482) | Late-onset hospital-acquired (n = 836) | ||
| Gram-negative bacteria | 1161 (58.6) | 385 (58) | 287 (59.5) | 498 (58.5) |
| Enterobacterales | 1024 (51.7) | 344 (51.8) | 247 (51.2) | 433 (51.8) |
| Citrobacter sp. | 21 (1.1) | 6 (0.9) | 8 (1.7) | 7 (0.8) |
| Citrobacter freundii | 18 (0.9) | 6 (0.9) | 3 (0.6) | 9 (0.9) |
| Escherichia coli | 729 (36.8) | 252 (38) | 172 (35.7) | 304 (36.4) |
| Enterobacter aerogenes | 37 (1.9) | 15 (2.3) | 6 (1.2) | 16 (1.9) |
| Enterobacter cloacae | 80 (4) | 31 (4.7) | 16 (3.3) | 34 (4.1) |
| Hafnia alvei | 8 (0.4) | 3 (0.5) | 2 (0.4) | 3 (0.4) |
| Morganella morganii | 25 (1.3) | 10 (1.5) | 5 (1) | 10 (1.2) |
| Klebsiella sp. | 51 (2.6) | 22 (3.3) | 12 (2.5) | 17 (2) |
| Klebsiella oxytoca* | 44 (2.2) | 23 (3.5) | 11 (2.3) | 10 (1.2) |
| Klebsiella pneumoniae | 170 (8.6) | 57 (8.6) | 37 (7.7) | 76 (9.1) |
| Proteus sp. | 23 (1.2) | 9 (1.4) | 7 (1.5) | 7 (0.8) |
| Proteus mirabilis | 63 (3.2) | 28 (4.2) | 15 (3.1) | 20 (2.4) |
| Providencia sp. | 3 (0.2) | 0 | 1 (0.2) | 2 (0.2) |
| Salmonella enterica | 4 (0.2) | 2 (0.3) | 2 (0.4) | 0 |
| Serratia marcescens | 12 (0.6) | 2 (0.3) | 4 (0.8) | 6 (0.7) |
| Enterobacterales, other | 24 (1.2) | 7 (1.1) | 5 (1) | 12 (1.4) |
| Non-fermenting bacteria | 233 (11.8) | 72 (10.8) | 66 (13.7) | 95 (11.4) |
| Pseudomonas aeruginosa | 131 (6.6) | 41 (6.2) | 34 (7.1) | 56 (6.7) |
| Pseudomonas sp. (other or NI) | 15 (0.8) | 3 (0.5) | 4 (0.8) | 8 (1) |
| Stenotrophomonas maltophilia | 11 (0.6) | 5 (0.8) | 2 (0.4) | 4 (0.5) |
| Acinetobacter baumannii | 61 (6.2) | 18 (2.7) | 22 (4.6) | 21 (2.5) |
| Acinetobacter sp. (other or NI) | 32 (1.6) | 8 (1.2) | 12 (2.5) | 12 (1.4) |
| Other Gram-negative bacteria | ||||
| Haemophilus influenzae | 4 (0.2) | 2 (0.3) | 0 | 2 (0.2) |
| Gram-positive bacteria | 781 (39.4) | 274 (41.3) | 187 (38.8) | 320 (38.3) |
| Staphylococci | 195 (9.8) | 69 (10.4) | 44 (9.1) | 82 (9.8) |
| Staphylococcus aureus | 64 (3.2) | 23 (3.5) | 19 (3.9) | 22 (2.6) |
| Coagulase-negative staphylococci | 100 (5) | 37 (5.6) | 23 (4.8) | 40 (4.8) |
| Staphylococcus sp. (other or NI) | 37 (1.9) | 11 (1.7) | 5 (1) | 21 (2.5) |
| Enterococci | 513 (25.9) | 173 (26.1) | 121 (25.1) | 219 (26.2) |
| Enterococcus faecalis | 257 (13) | 83 (12.5) | 59 (12.2) | 115 (13.8) |
| Enterococcus faecium | 216 (10.9) | 70 (10.5) | 46 (9.5) | 100 (12) |
| Enterococcus sp. (other or NI) | 77 (3.9) | 33 (5) | 18 (3.7) | 26 (3.1) |
| Other Gram-positive bacteria | ||||
| Streptococcus Group A, B, C, G | 117 (5.9) | 44 (6.6) | 27 (5.6) | 46 (5.5) |
| Streptococcus pneumoniae | 9 (0.5) | 4 (0.6) | 2 (0.4) | 3 (0.4) |
| Streptococcus viridans | 33 (1.7) | 13 (2) | 7 (1.5) | 13 (1.6) |
| Corynebacterium | 8 (0.4) | 1 (0.2) | 3 (0.6) | 4 (0.5) |
| Anaerobe bacteria | 231 (11.7) | 83 (12.5) | 45 (9.3) | 103 (12.3) |
| Clostridium perfringens | 21 (1.1) | 7 (1.1) | 3 (0.6) | 11 (1.3) |
| Peptostreptococcus sp. | 4 (0.2) | 1 (0.2) | 2 (0.4) | 1 (0.1) |
| Actinomyces sp. | 2 (0.1) | 1 (0.2) | 0 | 1 (0.1) |
| Gram-positive anaerobe sp. (other or NI) | 53 (2.7) | 17 (2.6) | 12 (2.5) | 24 (2.9) |
| Clostridium difficile | 8 (0.4) | 3 (0.5) | 1 (0.2) | 4 (0.5) |
| Bacteroides sp.* | 103 (5.2) | 46 (6.9) | 17 (3.5) | 40 (4.8) |
| Porphyromonas sp. | 2 (0.1) | 0 | 2 (0.4) | 0 |
| Prevotella sp. | 5 (0.3) | 3 (0.5) | 0 | 2 (0.2) |
| Fusobacterium sp. | 9 (0.5) | 7 (1.1) | 0 | 2 (0.2) |
| Gram-negative anaerobe sp. (other or NI) | 66 (3.3) | 20 (3) | 13 (2.7) | 33 (3.9) |
| Fungi | 258 (13) | 80 (12) | 71 (14.7) | 107 (12.8) |
| Aspergillus sp. | 3 (0.2) | 0 | 2 (0.4) | 1 (0.1) |
| Candida sp. | 257 (13) | 81 (12.2) | 69 (14.3) | 107 (12.8) |
| Candida albicans | 173 (8.7) | 56 (8.4) | 50 (10.4) | 67 (8) |
| Candida glabrata | 35 (1.8) | 10 (1.5) | 9 (1.9) | 16 (1.9) |
| Candida krusei | 3 (0.2) | 2 (0.3) | 0 | 1 (0.1) |
| Candida parapsilosis | 9 (0.5) | 4 (0.6) | 1 (0.2) | 4 (0.5) |
| Candida tropicalis | 16 (0.8) | 6 (0.9) | 2 (0.4) | 8 (1) |
| Candida sp. (other or NI) | 20 (1) | 2 (0.3) | 7 (1.5) | 11 (1.3) |
Table reports n patients positive (% of total number of patients with cultures sampled)
NI not identified
*p < 0.05 for differences between setting of infection acquisition
Table 4.
Rates of antimicrobial resistance in intra-abdominal infections according to geographic region
| Antibiotic-resistant pathogen | Total cohort (n = 1982) | Geographic region | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Western Europe (n = 601) | Southern Europe (n = 558) | Eastern and South-East Europe (n = 151) | Central Europe (n = 99) | North Africa and Middle-East (n = 172) | Latin America (n = 249) | North America (n = 22) | Asia–Pacific (n = 123) | ||
| Difficult-to-treat resistant Gram-negative bacteria | 85 (4.3) | 2 (0.3) | 38 (6.8) | 9 (6) | 0 | 15 (8.7) | 16 (6.4) | 0 | 5 (4.1) |
| Any resistant Gram-negative bacteria* | 480 (24.2) | 54 (9) | 140 (25.1) | 59 (39.1) | 20 (20.2) | 82 (47.7) | 90 (36.1) | 7 (31.8) | 26 (21.1) |
| ESBL-producing Gram-negative bacteria | 326 (16.4) | 37 (6.2) | 81 (14.5) | 37 (24.5) | 9 (9.1) | 65 (37.8) | 69 (27.7) | 7 (31.8) | 20 (16.3) |
| Carbapenem-resistant Gram-negative bacteria | 145 (7.3) | 3 (0.5) | 61 (10.9) | 23 (15.2) | 1 (1) | 23 (13.4) | 25 (10) | 0 | 9 (7.3) |
| Fluoroquinolone-resistant Gram-negative bacteria | 339 (17.1) | 29 (4.8) | 108 (19.4) | 37 (24.5) | 18 (18.2) | 57 (33.1) | 69 (27.7) | 3 (13.6) | 17 (13.8) |
| MRSA | 20 (1) | 1 (0.2) | 5 (0.9) | 5 (3.3) | 0 | 5 (2.9) | 3 (1.2) | 0 | 1 (0.8) |
| VRE | 56 (2.8) | 11 (1.8) | 15 (2.7) | 5 (3.3) | 2 (2) | 9 (5.2) | 11 (4.4) | 1 (4.5) | 2 (1.6) |
| Antimicrobial resistance** (total) | 153 (7.7) | 14 (2.3) | 57 (10.2) | 16 (10.6) | 2 (2) | 29 (16.9) | 27 (10.8) | 1 (4.5) | 7 (5.7) |
| Antimicrobial resistance*** (total) | 522 (26.3) | 63 (10.5) | 152 (27.2) | 65 (43) | 21 (21.2) | 87 (50.6) | 96 (38.6) | 8 (36.4) | 28 (22.8) |
% Represent proportion per column; Resistance rates reflect proportion of patients in which resistant strains are isolated (e.g., n MRSA/total n patients) and do not represent proportion of resistance within particular pathogens (e.g., n MRSA/total S. aureus isolates)
Denominator for microbiological data includes only patients in which cultures were sampled (data from South Africa are excluded as they included only seven patients)
ESBL extended-spectrum beta-lactamase-producing, MRSA methicillin-resistant Staphylococcus aureus, VRE vancomycin-resistant enterococci
*Gram-negative bacteria that are either ESBL-producing, or carbapenem-resistant, or fluoroquinolone-resistant
**Total rates of multidrug resistance considering difficult-to-treat resistant Gram-negative bacteria, MRSA, and VRE
***Total rates of multidrug resistance considering any type of Gram-negative resistance (either ESBL-producing, or carbapenem-resistant, or fluoroquinolone-resistant bacteria), MRSA, and VRE
Antimicrobial therapy
Data on the first-line empiric antimicrobial therapy was available from 2427 patients (92.6%). A basic schedule covering aerobic Gram-positive, Gram-negative, and anaerobic bacteria was prescribed in 2291 patients (94.4%). An anti-pseudomonal agent was prescribed in 1978 patients (81.8%). Empiric coverage of MRSA and VRE was added in, respectively, 647 patients (26.7%) and 140 patients (5.8%). An antifungal agent was associated in 436 patients (18%). In 365 patients, two agents with anti-anaerobic activity were prescribed (15%). Double anti-anaerobic coverage was more frequently prescribed in hospital-acquired cases (18.2%) compared with community-acquired cases (14.2%). No other differences in antimicrobial coverage according to setting of infection acquisition were observed (Supplement-6).
Source control
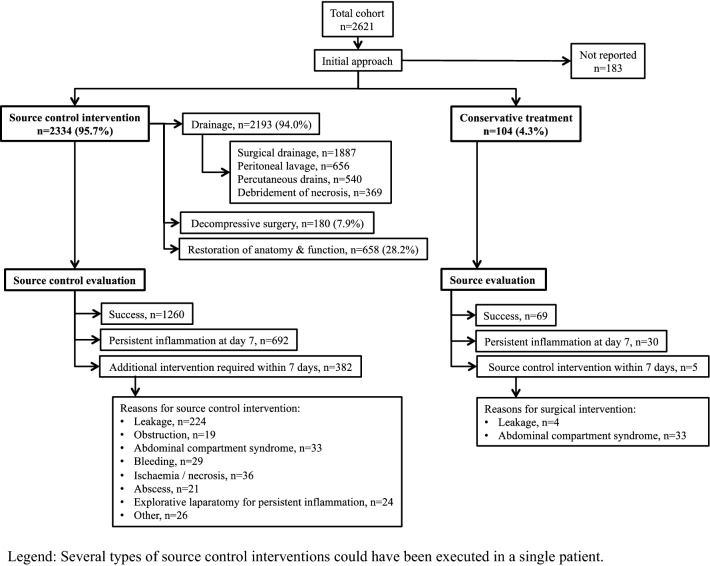
Data on the initial approach to control the infection are reported in 2438 patients. A source control intervention was carried out in 2334 patients (95.7%), and included drainage (94.0%), decompressive surgery (7.9%), and restoration of anatomy and function (28.2%). Among patients undergoing source control, persistent inflammation at day 7 was reported in 692 patients (29.6%). An additional intervention was deemed necessary in 382 patients (16.4%). Among patients with an initial conservative approach (n = 104), 30 patients experienced persistent inflammation (28.8%), and a source control intervention was performed in 5 patients (4.8%). More details on source control interventions and evaluations are summarized in Fig. 2.
Fig. 2.
Initial approach to control the source of infection. Several types of source control interventions could have been executed in a single patient
Mortality
Overall mortality was 29.1% (752/2588). Univariate relationships with mortality are reported in Supplement-7. Mortality stepwise increased with ascending SOFA scores (Supplement-8). Achievement of source control at day 7 was associated with lower mortality (248/1438, 17.2%) compared with cases with persistent inflammation (367/761, 51.8%) and those requiring surgical revision (110/389, 28.3%) (p < 0.001). We reported mortality according to setting of infection acquisition, anatomical disruption, and severity of disease expression. Mortality was 23.7% in community-acquired cases, 27.3% in early onset hospital-acquired cases, and 33.9% in late-onset hospital-acquired cases (p < 0.001). Regarding anatomical disruption, no difference in mortality was observed between patients without anatomical disruption and those with localized peritonitis (respectively, 25.0% and 24.2%, p = 0.135). Mortality in patients with diffuse peritonitis (36.0%) was higher compared with the former categories (p < 0.001). Finally, mortality stepwise increased with greater severity of disease expression: 12.8% in infected patients without sepsis, 24.5% in septic patients, and 40.3% in patients with septic shock (p < 0.001). Table 5 reports mortality rates for all different phenotypes of intra-abdominal infection according to setting of infection acquisition, anatomical disruption, and severity of disease expression. The grid describes a stepwise increase in mortality along with combinations including septic shock, diffuse peritonitis, and late-onset hospital-acquired infection.
Table 5.
Mortality according to alternative classification of intra-abdominal infection
| Severity of disease expression | Setting of infection acquisition | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Community-acquired | Early onset hospital-acquired | Late-onset hospital-acquired | |||||||
| Septic shock |
18/64 28.1% |
25/83 30.1% |
48/101 47.5% |
21/63 33.3% |
13/61 21.3% |
37/91 40.7% |
45/103 43.7% |
48/110 43.6% |
94/190 49.5% |
| Sepsis |
13/116 11.2% |
42/221 19% |
37/174 21.3% |
27/90 30% |
33/170 19.4% |
43/128 33.6% |
26/147 17.7% |
62/237 26.2% |
99/275 36% |
| Infection |
1/7 14.3% |
3/22 13.6% |
4/22 18.2% |
0/7 0% |
0/21 0% |
2/14 14.3% |
1/12 8.3% |
8/36 22.2% |
2/23 8.7% |
| No | Yes, with localized peritonitis | Yes, with diffuse peritonitis | No | Yes, with localized peritonitis | Yes, with diffuse peritonitis | No | Yes, with localized peritonitis | Yes, with diffuse peritonitis | |
| Anatomical disruption | Anatomical disruption | Anatomical disruption | |||||||
Logistic regression analysis identified late-onset hospital-acquired infection, diffuse peritonitis, sepsis and septic shock, older age, malnutrition, diabetes mellitus, liver failure, and congestive heart failure as independent risk factors for death (Table 6). The association of an anti-MRSA agent in the empiric antimicrobial scheme was associated with decreased risk of death. Antimicrobial resistance defined as MRSA, VRE, or difficult-to-treat resistant Gram-negatives did not reached the final models. However, when antimicrobial resistance in Gram-negative bacteria was defined as either ESBL production or carbapenem resistance, this covariate became significantly associated with mortality (Supplement-9).
Table 6.
Independent relationships with mortality in critically ill patients with intra-abdominal infection
| Variable | Model with source control achievement* OR (95% CI) |
Model without source control achievement** OR (95% CI) |
|---|---|---|
| Setting of infection acquisition | ||
| Community-acquired infection | Reference | Reference |
| Early onset hospital-acquired infection (≤ 7 days) | 1.15 (0.84–1.58) | 1.18 (0.88–1.59) |
| Late-onset hospital-acquired infection (> 7 days) | 1.76 (1.34–2.32) | 1.76 (1.36–2.30) |
| Anatomical disruption | ||
| No anatomical barrier disruption | Reference | Reference |
| Anatomical disruption with localized peritonitis | 1.28 (0.95–1.75) | 1.26 (0.95–1.69) |
| Anatomical disruption with diffuse peritonitis | 1.99 (1.49–2.67) | 2.04 (1.55–2.70) |
| Severity of disease expression | ||
| Infection | Reference | Reference |
| Sepsis | 2.44 (1.37–4.66) | 2.28 (1.31–4.28) |
| Septic shock | 5.22 (2.91–10) | 4.93 (2.80–9.30) |
| Age (per year increase) | 1.03 (1.02–1.04) | 1.03 (1.03–1.04) |
| Underlying conditions | ||
| Malnutrition (body mass index < 20) | 2.07 (1.34–3.17) | 2.15 (1.43–3.21) |
| Diabetes mellitus | 1.31 (0.99–1.73) | 1.32 (1.01–1.72) |
| Liver failure | 2.03 (1.23–3.33) | 2.50 (1.55–4.02) |
| Congestive heart failure | 1.86 (1.24–2.81) | 1.92 (1.31–2.81) |
| Empiric antimicrobial coverage | ||
| Anti-MRSA agent | 0.77 (0.59–1) | 0.77 (0.59–0.98) |
| Double anaerobe coverage | – | 1.28 (0.97–1.71) |
| Source control achievement at day 7 | ||
| Success | Reference | – |
| Failure, persistent signs of inflammation | 4.85 (3.79–6.22) | – |
| Failure, additional intervention required following initial approach | 1.93 (1.41–2.65) | – |
The variable “antimicrobial resistance” defined as either MRSA, vancomycin-resistant enterococci (VRE), or difficult-to-treat resistant Gram-negative bacteria did not achieve the final regression model. Supplement-9 reports the results of the logistic regression models with antibiotic resistance defined as either MRSA, VRE, ESBL-producing, or carbapenem-resistant Gram-negative bacteria. In these logistic regression models, antibiotic resistance was associated with increased risk of mortality, while other covariates remained stable
OR odds ratio, CI confidence interval, MRSA methicillin-resistant Staphylococcus aureus
*Area under the receiver-operating curve characteristic: 0.778; **Area under the receiver-operating curve characteristic: 0.689
Discussion
This multicenter observational study provided epidemiological insights in critically ill patients with intra-abdominal infection. The multicentre input of sequential cases of intra-abdominal infection offers a global view of the case mix of different presentations of intra-abdominal infection requiring ICU admission or occurring within the framework of an ICU stay. In spite of clinical heterogeneity, the core characteristics of intra-abdominal infection are quite generic including anatomical disruption and polymicrobial infection. Because of the broad variety in intra-abdominal infections, data were described according to a new classification based on setting of acquisition, presence of anatomical disruption, and severity of disease. Irrespective of type of intra-abdominal infection, mortality was higher in late-onset hospital-acquired cases with diffuse peritonitis and septic shock. This classification allows comparison across a spectrum of intra-abdominal infections and might be used for including patients in future clinical trials.
There were no differences in the prevalence of antimicrobial resistance in microbiological cultures sampled in community-acquired vs. early onset vs. late-onset hospital-acquired infection. This may be explained at least in part by the spread of resistance clones/genes into the community, as is the case for ESBL-producing or carbapenem-resistant Enterobacterales (formerly known as Enterobacteriaceae). This is certainly the case for risk regions such as Eastern and South-East Europe, the Middle-East, and Latin America, and matches with the results of a global point prevalence study on antimicrobial consumption and resistance [17]. This confirms the trend that classic risk factors for antimicrobial resistance involvement are losing predictive value as illustrated in a multicenter study reporting antimicrobial resistance in 39% of infections in patients without an obvious risk profile as evidenced by prior antibiotic exposure and/or hospitalisation [18]. This observation is highly relevant as it might stress the need for last-line antimicrobial therapy in community-acquired infection in selected regions. Considering local ecology together with the individual patient profile, and disease severity remains essential. However, antimicrobial resistance in key-pathogens isolated in intra-abdominal infection does not seem to be associated with increased virulence, as it occurred at similar rates in infection, sepsis, and septic shock. Overall prevalence of enterococci was 26% and thereby substantially higher as previously reported [19–22]. This trend can be attributed to the steadily emergence of enterococci in acute care settings or to the particular composition of a cohort of exclusively critically ill patients [23].
No differences in empiric antibacterial regimens were observed according to setting of infection acquisition. Anti-pseudomonal coverage was provided up-front in not only late-onset cases, a supposed classic risk factor for antimicrobial resistant infection, including P. aeruginosa strains, but also in community-acquired or early onset hospital-acquired infections. This is probably triggered by a safety-reflex in physicians, not to miss any potential pathogen, especially P. aeruginosa strains. Thus, the risk factor-based antibiotic strategy that appears in all guidelines seems not to be implemented in a large real-life sample of intra-abdominal infection in the ICU, reflecting response to severity.
It is reassuring that the vast majority of intra-abdominal infections in the ICU were approached by an early source control intervention. It has been established that surgery needs to be performed after hemodynamic stabilization, but nevertheless should be performed as early as possible aiming at damage control [24]. The importance of source is evidenced by the increased mortality among patients with persistent inflammation or need for additional surgical intervention.
Late-onset hospital-acquired infection, diffuse peritonitis, and septic shock were identified as independent risk factors for mortality, and confirm the robustness of the new classification system for risk stratification. Antimicrobial resistance defined as either MRSA, VRE, ESBL-producing, or carbapenem-resistant Gram-negative bacteria was independently associated with increased mortality (Supplement-9). Surprisingly, however, the more strict definition of either MRSA, VRE, or difficult-to-treat resistant Gram-negative bacteria was not associated with increased mortality. Probably, the cohort lacked sufficient power as in only 85 patients, difficult-to-treat Gram-negatives were involved vs. 341 ESBL-producing or carbapenem-resistant Gram-negative bacteria. We have no explanation for the favorable association with anti-MRSA agents. This can hardly be due to the anti-MRSA activity as such, since MRSA was isolated in only 20 patients. The advantageous association might be due to the anti-enterococcal activity of these agents. Yet, enterococcal coverage as such (not necessarily covering MRSA) was not retained in the final regression model assessing relationships with mortality. Hence, this observation might just be an incidental finding. On the other hand, the absence of an association between empiric antifungal therapy and outcome seems consistent with the finding of other cohort studies and randomized-controlled trials that did not demonstrate the effect of empirical Candida coverage and favorable outcome [25, 26].
This study has limitations. This is an observational cohort study disposed to confounding. Some geographic regions are poorly represented obstructing conclusive results. Evaluation of source control achievement remains a subjective appreciation performed by the attending physician; given the study scale, it was not feasible to establish an independent panel for in-depth evaluation of source control as previously reported [27]. At the same line, given the observational study design, there was no predefined approach to source control [7]. In addition, with this paper, we intended to provide a general epidemiological snapshot. Therefore, detailed country-specific or disease-specific analyses fell outside the scope of this report. Finally, we could not report the proportion of ICU patients with intra-abdominal infection/sepsis as the total number of admissions during the inclusion of cases was not recorded.
In conclusion, this multinational cohort of ICU patients with intra-abdominal infection revealed that late-onset healthcare-associated infection, diffuse peritonitis, and sepsis or septic shock are independent risk factors for mortality. Therefore, setting of infection acquisition, anatomical disruption, and severity of disease expression are disease-specific phenotypic characteristics associated with outcome, irrespective of the type of intra-abdominal infection. Antimicrobial resistance is mainly an issue of Gram-negatives and a particular concern in specific geographic areas and associated with worse outcome as was failure of source control.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Collaborators AbSeS study: National Coordinators: Algeria: Amin Lamrous (CHU Alger), Argentina: Cecilia Pereyra (Hospital Interzonal Agudos Prof Dr Luis Guemes, Buenos Aires), Fernando Lipovestky (Universidad Abierta Interamericana Hospital, Buenos Aires); Australia: Despoina Koulenti (UQCCR, Faculty of Medicine, The University of Queensland, Brisbane); Belgium: Jan De Waele (Ghent University Hospital, Ghent); Canada: Joao Rezende-Neto (St Michael’s Hospital, Toronto); (Colombia: Yenny Cardenas (Hospital Universitario Fundación Santa Fe, Bogotá); Czech Republic: Tomas Vymazal (Motol University Hospital, Prague); Denmark: Hans Fjeldsoee-Nielsen (Fjeldsoee-Nielsen (Nykoebing Falster Hospital, Nykoebing Falster); France: Philippe Montravers (CHU Bichat Claude Bernard, Paris); Germany: Matthias Kott (Universitätsklinikum, Schleswig–Holstein, Kiel); Greece: Arvaniti Kostoula (Papageorgiou General Hospital, Thessaloniki); India: Yash Javeri (Nayati Healthcare, Delhi); Italy: Massimo Girardis (University Hospital of Modena, Modena); Israel: Sharon Einav (Shaare Zedek Medical Centre, Jerusalem); Netherlands: Dylan de Lange (University Medical Center, Utrecht); Peru: Luis Daniel Umezawa Makikado (Clínica Ricardo Palma, Lima); Poland: Adam Mikstacki (Regional Hospital, Poznan); Portugal: José-Artur Paiva (Centro Hospitalar Universitário Sao João, Porto); Romania: Dana Tomescu (Fundeni Clinical Institute, Bucharest); Russian Federation: Alexey Gritsan (Krasnoyarsk State Medical University, Krasnoyarsk Regional Clinical Hospital, Krasnoryarsk); Serbia; Bojan Jovanovic (Clinical Center of Serbia, Belgrade); Singapore: Kumaresh Venkatesan (Khoo Teck Puat Hospital, Singapore); Slovenia: Tomislav Mirkovic (University Medical Centre, Ljubljana); Spain: Emilio Maseda (Hospital Universitario La Paz, Madrid); Turkey: Yalim Dikmen (Istanbul University-Cerrahpasa, Cerrahpasa Medical School, Istanbul); United Kingdom: Benedict Creagh-Brown (Royal Surrey County Hospital NHS Foundation Trust, Guilford); Investigators: ALGERIA: CHU (Algiers): Amin Lamrous; ARGENTINA: Sanatorio Güemes (Buenos Aires): Monica Emmerich, Mariana Canale; Sanatorio de la Trinidad Mitre (Buenos Aires): Lorena Silvina Dietz, Santiago Ilutovich; Hospital General de Agudos “Dr. Teodoro Alvarez” (Buenos Aires): John Thomas Sanchez Miñope, Ramona Baldomera Silva; Hospital Militar Central (Buenos Aires): Martin Alexis Montenegro, Patricio Martin; Policlinico Central Union Obrera Metalurgica (Buenos Aires): Pablo Saul, Viviana Chediack; Sanatorio San José (Buenos Aires): Giselle Sutton, Rocio Couce; Hospital General de Agudos “Dr. Ignacio Pirovano” (Buenos Aires): Carina Balasini, Susana Gonzalez; Hospital Britanico (Buenos Aires): Florencia Maria Lascar, Emiliano Jorge Descotte; CMPF Churruca-Visca (Buenos Aires): Natalia Soledad Gumiela, Carina Alejandra Pino; Clinica San Camilo (Buenos Aires): Cristian Cesio, Emanuel Valgolio; Hospital Francisco Javier Muñiz (Buenos Aires): Eleonora Cunto, Cecilia Dominguez; Universidad Abierta Interamericana Hospital (Buenos Aires): Fernando Lipovestky; Hospital Alberto Balestrini (Buenos Aires): Nydia Funes Nelson, Esteban Martin Abegao; Hospital Interzonal Agudos Prof Dr Luis Güemes (Buenos Aires): Cecilia Pereyra, Norberto Christian Pozo; Hospital Español (Buenos Aires): Luciana Bianchi, Enrique Correger; Clinica Zabala (Caba): Maria Laura Pastorino, Erica Aurora Miyazaki; Hospital César Milstein (Caba): Norberto Christian Pozo, Nicolas Grubissich; Hospital Regional Victor Sanguinetti (Comodoro): Mariel Garcia, Natalia Bonetto; Hospital Municipal de Urgencias (Cordoba): Noelia Elizabeth Quevedo, Cristina Delia Gomez; Hospital Manuel B Cabrera (Coronel Pringles): Felipe Queti, Luis Gonzalez Estevarena; Hospital Español de Mendoza (Mendoza,Godoy Cruz: Ruben Fernandez, Ignacio Santolaya; H.I.G.A. Prof. Dr. Luis Güemes (Haedo): Norberto Christian Pozo; Hospital Municipa Doctor Carlos Macias (Mar de Ajo): Sergio Hugo Grangeat, Juan Doglia; Hospital Luis C. Lagomaggiore (Mendoza): Graciela Zakalik, Carlos Pellegrini; Hospital Nacional Profesor Alejandro Posadas (Moron): Maria Monserrat Lloria, Mercedes Esteban Chacon; Hospital Provincial de Neuquen (Neuquen): Mariela Fumale; Clinica Modelo S.A (Paraná): Mariela Leguizamon; Sanatorio de la Ciudad (Puerto Madryn): Irene Beatriz Hidalgo, Roberto Julian Tiranti; Sanatorio Nosti (Rafaela): Paola Capponi, Agustin Tita; Hospital Provincial del Centenario (Rosario): Luis Cardonnet, Lisandro Bettini; Sanatorio Parque (Rosario): Agñel Ramos, Luciano Lovesio; Hospital Papa Francisco (Salta): Edith Miriam Miranda, Angelica Beatriz Farfan; Hospital San Juan Bautista (San Fernando del Valle de Catamarca): Carina Tolosa, Lise Segura; Hospital Central San Isidro Dr Melchor A. Posse (San Isidro-Buenos Aires): Adelina Bellocchio, Brian Alvarez; Hospital Guillermo Rawson (San Juan): Adriana Manzur, Rodolfo Lujan; Establecimiento Asistencial Dr Lucio Molas (Santa Rosa): Natalia Fernandez, Nahuel Scarone; Clínica de Especialidades (Villa María): Alan Zazu, Carina Groh; AUSTRALIA: The Bendigo Hospital (Bendigo): Jason Fletcher, Julie Smith; Coffs Harbour Health Campus (Coffs Harbour): Raman Azad, Nitin Chavan; Concord Hospital (Concord): Helen Wong; Mark Kol; Royal Darwin Hospital (Darwin): Lewis Campbell; Royal Brisbane and Women’s Hospital (Herston, Brisbane): Despoina Koulenti, Therese Starr; Sir Charles Gairdner Hospital (Nedlands): Brigit Roberts, Bradley Wibrow; Redcliffe Hospital (Redcliffe): Timothy Warhurst; St Vincent’s Hospital (Toowommba): Meher Chinthamuneedi, Bernal Buitrago Ferney; BELGIUM: Cliniques du Sud Luxembourg (CSL)-Hôpital Saint-Joseph (Arlon): Marc Simon; Chirec Hospital (Braine-l’Alleud): Daniel De Backer; Cliniques Universitaires St Luc (Brussels): Xavier Wittebole; Brugmann University Hospital (Brussels): David De Bels, Cliniques de l’Europe - St-Michel (Brussels): Vincent Collin; University Hospital Antwerp (Edegem): Karolien Dams, Philippe Jorens; Ghent University Hospital (Ghent): Jan De Waele; Jessa Ziekenhuis (Hasselt): Jasperina Dubois; University Hospitals Leuven (Leuven): Jan Gunst; CHU Ambroise Paré (Mons): Lionel Haentjens; Clinique Saint-Pierre (Ottignies): Nicolas De Schryver, Thierry Dugernier; CANADA: St. Michael’s Hospital (Toronto): Joao Rezende-Neto, Sandro Rizoli; CHILE: Hospital Clinico Viña del Mar (Viña del Mar): Paul Santillan; CHINA: Jiangsu Province Hospital (Nanjing): Yi Han; Yangpu Hospital of Tongji University (Shanghai): Ewelina Biskup, Changjing Qu; Urumqi General Hospital (Urumqi): Xinyu Li, Wannan Medical College First Affiliated Hospital, Yijishan Hospital (Wuhu): Tao Yu, Lu Weihua; COLOMBIA: Clinica Universitaria Colombia (Bogota): Daniel Molano-Franco, José Rojas, Mederi Hospital (Bogota): Juan Mauricio Pardo Oviedo; Dario Pinilla; Hospital Universitario Fundación Santa Fe (Bogota): Yenny Cardenas, Edgar Celis; Clinica Santa Gracia (Popayan): Mario Arias; CROATIA: Opća bolnica Dubrovnik (Dubrovnik): Anita Vukovic, Maja Vudrag; General Hospital Karlovac (Karlovac): Matija Belavic, Josip Zunic; Clinical Hospital Center Rijeka (Rijeka): Janja Kuharic, Irena Bozanic Kricka; University Hospital Center of Zagreb (Zagreb): Ina Filipovic-Grcic, Boris Tomasevic; University Hospital Center Sestre Milosrdnice (Zagreb): Melanija Obraz, Bruna Bodulica; CZECH REPUBLIC: Nemocnice Břeclav (Břeclav): Martin Dohnal; University Hospital Brno (Brno): Jan Malaska, Milan Kratochvil; Municipal Hospital (Havirov): Igor Satinsky, Peter Schwarz; Hospital Karlovy Vary (Karlovy Vary): Zdenek Kos; University Hospital Olomouc (Olomouc): Ladislav Blahut; University Hospital of Ostrava (Ostrava): Jan Maca; Institute for Clinical and Experimental Medicine (Prague): Marek Protus, Eva Kieslichová; DENMARK: Odense University Hospital (Odense): Louise Gramstrup Nielsen, Birgitte Marianne Krogh; ECUADOR: San Vicente de Paúl Hospital (Ibarra): Francisco Rivadeneira; Hospital Oncologico “Dr. Julio Villacreses Colmont” SOLCA (Portoviejo): Freddy Morales, José Mora; Hospital General Puyo (Puyo): Alexandra Saraguro Orozco; Hospital de Especialidades “Eugenio Espejo” (Quito): Diego Rolando MorochoTutillo, Nelson Remache Vargas; Clinica La Merced (Quito): Estuardo Salgado Yepez; Hospital Militar (Quito): Boris Villamagua; EGYPT: Kasr El AINI Hospital, Cairo University (Cairo): Adel Alsisi, Abdelraouf Fahmy; FRANCE: CHU Amiens (Amiens): Hervé Dupont; CHU Angers (Angers): Sigismond Lasocki; Hôpital Beaujon (Clichy): Catherine Paugam-Burtz, Arnaud Foucrier; Centre Hospitalier Compiegne Noyon (Compiègne): Alexandru Nica, Geneviève Barjon; Centre Hospitalier de Lens (Lens): Jihad Mallat; Hôpital Edouard Herriot (Lyon): Guillaume Marcotte; Hôpital Nord (Marseille): Marc Leone, Gary Duclos; Clinique du Millénaire (Montpellier): Philippe Burtin; CHU Bichat Claude Bernard (Paris): Philippe Montravers, Enora Atchade; Groupe Hospitalier Paris Saint-Joseph (Paris): Yazine Mahjoub, Benoît Misset; Hôpital Bichat (Paris): Jean-François Timsit, Claire Dupuis; CHU de Rouen, Hôpital Charles Nicolle (Rouen): Benoît Veber; Centre Hospitalier Yves le Foll (Saint-Brieuc): Matthieu Debarre; Hôpitaux Universitaires de Strasbourg, NHC -Nouvel Hôpital Civil (Strasbourg): Oliver Collange; Hôpitaux Universitaires de Strasbourg, Hôpital de Hautepierre (Strasbourg): Julien Pottecher, Stephane Hecketsweiler; Hôpital Cochin (Paris): Mélanie Fromentin, Antoine Tesnière; GERMANY: University Hospital Giessen (Giessen): Christian Koch, Michael Sander; Universitätsklinikum Schleswig–Holstein (Kiel): Matthias Kott, Gunnar Elke; University Hospital of Leipzig (Leipzig): Hermann Wrigge, Philipp Simon; GREECE: General Hospital of Agios Nikolaos (Agios Nikolados): Anthoula Chalkiadaki, Charalampos Tzanidakis; Democritus University of Thrace (Alexandroupolis): Ioannis Pneumatikos, Eleni Sertaridou; Evangelismos Hospital (Athens): Zafiria Mastora, Ioannis Pantazopoulos; Hippocrateion General Hospital of Athens (Athens): Metaxia Papanikolaou, Theonymfi Papavasilopoulou; General Hospital Laiko (Athens): John Floros, Virginia Kolonia; University Hospital Attikon (Athens): George Dimopoulos, Chryssa Diakaki; General Hospital Asklepieio Voulas (Athens): Michael Rallis, Alexandra Paridou; General Hospital G. Gennimatas (Athens): Alexandros Kalogeromitros, Vasiliki Romanou; Konstantopouleio Hospital (Athens): Charikleia Nikolaou, Katerina Kounougeri; Agioi Anargiroi General Oncological Hospital of Kifissia (Athens): Evdoxia Tsigou, Vasiliki Psallida; Red Cross Hospital (Athens): Niki Karampela, Konstantinos Mandragos; General Hospital St George (Chania): Eftychia Kontoudaki, Alexandra Pentheroudaki; Thriassio General Hospital of Eleusis (Eleusis): Christos Farazi-Chongouki, Agathi Karakosta; Giannitsa General Hospital (Giannitsa): Isaac Chouris, Vasiliki Radu; University Hospital Heraklion (Heraklion): Polychronis Malliotakis, Sofia Kokkini; Venizelio General Hospital of Heraklion (Heraklion): Eliana Charalambous, Aikaterini Kyritsi; University Hospital of Ioannina (Ioannina): Vasilios Koulouras, Georgios Papathanakos; General Hospital Kavala (Kavala): Eva Nagky, Clairi Lampiri; Lamia General Hospital (Lamia): Fotios Tsimpoukas, Ioannis Sarakatsanos; Agios Andrea’s General Hospital of Patras (Patras): Panagiotis Georgakopoulos, Ifigeneia Ravani; Tzaneio General Hospital (Pireaus): Athanasios Prekates, Konstantinos Sakellaridis; General Hospital of Pyrgos (Pyrgos Hleias): Christos Christopoulos, Efstratia Vrettou; General Hospotal of Rethymnon (Rethymnon): Konstantinos Stokkos, Anastasia Pentari; Papageorgiou Hospital (Thessaloniki): Kostoula Arvaniti, Kyriaki Marmanidou; Hippokration Hospial (Thessaloniki): Christina Kydona, Georgios Tsoumaropoulos; G. Papanikolaou General Hospital (Thessaloniki): Militisa Bitzani, Paschalina Kontou; Agios Pavlos Hospital (Thessaloniki): Antonios Voudouris, Elli-Nikki, Flioni; General Hospital of Thessaloniki G.Gennimatas (Thessaloniki): Elli Antypa, Eleftheria Chasou; Theagenio Anticancer Hospital (Thessaloniki): Souzana Anisoglou, Eirini Papageorgiou; General Hospital of Trikala (Trikala): Theoniki Paraforou, Agoritsa Tsioka; Achillopoyleio General Hospital Volos (Volos): Antigoni Karathanou; Xanthi General Hospital (Xanthi): Aristeidis Vakalos; INDIA: CIMS Hospital (Ahmedabad): Bhagyesh Shah, Chirag Thakkar; CHL Hospitals (Indore): Nikhilesh Jain; Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS) (Lucknow): Mohan Gurjar, Arvind Baronia; Ruby Hall Clinic (Pune): Prachee Sathe, Shilpa Kulkarni; Jubilee Mission Medical College & Research Institute (Thrissur): Cherish Paul, John Paul; IRAN: Nemazi Hospital (Shiraz): Mansoor Masjedi; Anesthesiology and Critical Care Research Center, Shiraz University of Medical Sciences (Shiraz): Reza Nikandish, Farid Zand; Shiraz Trauma Hospital (Shiraz): Golnar Sabetian; Shohada Hospital (Tabriz): Ata Mahmoodpoor; Masih Daneshvari Hospital (NRITLD (Tehran): Seyed Mohammadreza Hashemian; ISRAEL: Hadassah Hebrew University Medical Center (Jerusalem): Miklosh Bala; ITALY: Cardarelli Ospedale (Campobasso): Romeo Flocco, Sergio Torrente; PinetaGrande Private Hospital (Castel Volturno): Vincenzo Pota; Arcispedale Sant’Anna (Ferrara): Savino Spadaro, Carlo Volta; University Hospital of Modena (Modena): Massimo Girardis, Giulia Serafini; Ospedale S.Antonio (Padova): Sabrina Boraso, Ivo Tiberio; Azienda Ospedaliera Universitaria Policlinico Paolo Giaccone (Palermo): Andrea Cortegiani, Giovanni Misseri; Azienda Ospedaliero-Universitaria di Parma (Parma): Maria Barbagallo, Davide Nicolotti; Azienda Ospedaliero-Universitaria Pisana (Pisa): Francesco Forfori, Francesco Corradi; Fondazione Policlinico Universitario A.Gemelli IRCCS (Roma): Massimo Antonelli, Gennaro De Pascale; Regina Elena National Cancer Institute of Rome (Roma): Lorella Pelagalli; Azienda Ospedaliero-Universitaria Citta della Salute e della Scienza di Torino, Presidio Ospedaliero Molinette (Torino): Luca Brazzi, Ferdinando Giorgio Vittone; Policlinico Universitario GB Rossi (Verona): Alessandro Russo, Davide Simion; University-Hospital of Foggia (Foggia): Antonella Cotoia, Gilda Cinnella; JAMAICA: University Hospital of the West Indies (Kingston): Patrick Toppin, Roxanne Johnson-Jackson; JAPAN: Kameda General Hospital (Kamogawa): Yoshiro Hayashi, Ryohei Yamamoto; Japanese Red Cross Musashino Hospital (Tokyo): Hideto Yasuda, Yuki Kishihara; Okinawa Prectural Chube Hospital (Uruma, Okinawa): Junji Shiotsuka; MEXICO: UMAE Hospital Especialidades Antonio Fraga Mouret-Centro Medico Nacional La Raza IMSS (Mexico City): Luis Alejandro Sanchez-Hurtado, Brigitte Tejeda-Huezo; Hospital Juárez de Mexico (Mexico City): Luis Gorordo; Instituto Nacional de Cancerologia (Mexico City): Silvio A. Ñamendys-Silva, Francisco J. Garcia-Guillen; Hospital general # 5 IMSS (Nogales, Sonora): Manuel Martinez; Hospital Regional de Alta Especialidad de la Península de Yucatán (Merida, Yacatan): Erick Romero-Meja, Ever Colorado-Dominguez; NETHERLANDS: Deventer Hospital (Deventer): Huub van den Oever, Karel Martijn Kalff; Medisch Spectrum Twente (Enschede): Wytze Vermeijden, Alexander Daniel Cornet; Tjongerschans Hospital (Heerenveen): Oliver Beck, Nedim Cimic; Zuyderland Medisch Centrum (Heerlen): Tom Dormans, Laura Bormans; Erasmus MC University Medical Center (Rotterdam): Jan Bakker, Ditty Van Duijn; Elisabeth-TweeSteden Ziekenhuis (Tilburg): Gerrit Bosman, Piet Vos; University Medical Center (Utrecht): Dylan de Lange, Jozef Kesecioglu; Diakonessenhuis (Utrecht): Lenneke Haas; OMAN: Khoula Hospital (Muscat): Akram Henein; PARAGUAY: Hospital Regional de Luque (Luque): Ariel M Miranda; PERU: Clínica Ricardo Palma (Lima): Luis Daniel Umezawa Makikado, Gonzalo Ernesto Gianella Malca; Victor Lazarte Echegaray Hospital (Trujillo): Abel Arroyo-Sanchez; POLAND: Silesian Hospital Cieszyn (Cieszyn): Agnieszka Misiewska-Kaczur; Wojewodzki Szpital Zesoloby w Koninie (Konin): Frisch Akinyi; First Public Teaching Hospital (Lublin): Miroslaw Czuczwar; Szpital Wojewodzki w Opolu SPZOZ (Opole): Karolina Luczak; SPZZOZ w Ostrowi Mazowieckiej (Ostrow Mazowiecka): Wiktor Sulkowski; Poznan University of Medical Sciences, Regional Hospital in Poznan (Poznan): Barbara Tamowicz, Adam Mikstacki; Centrum Medyczne (Poznan): Beata Swit, Bronisław Baranowski; University Hospital (Poznan): Piotr Smuszkiewicz, Iwona Trojanowska; WSM im. J. Strusia (Poznan): Stanislaw Rzymski; Niepubliczny Zakład Opieki Zdrowotnej Szpital w Puszczykowie im. prof. Stefana Tytusa Dąbrowskiego (Puszczykowo): Mariusz Sawinski, Marta Trosiak; Infant Jesus Teaching Hospital of Warsaw Medical University (Warsaw): Malgorzata Mikaszewska-Sokolewicz; PORTUGAL: Hospital de Braga (Braga): Ricardo Alves, Dina Leal; Centro Hospitalar Algarve (Faro): Andriy Krystopchuk, Pedro Muguel Hilario Mendonca; Centro Hospitalar Universitário Lisboa Central - Hospital Curry Cabral (Lisboa): Rui Antunes Pereira; Centro Hospitalar Universitário Lisboa Norte - Hospital de Santa Maria (Lisboa): Maria Raquel Lopes Marques de Carvalho, Carlos Candeias; Hospital Pedro Hispano (Matosinhos): Elena Molinos, Amélia Ferreira; Centro Hospitalar Sao Joao - Serviço Medicina Intensiva - UCIPU (Porto): Guiomar Castro, José-Artur Paiva; Centro Hospitalar Sao Joao - Serviço Medicina Intensiva - UCIPG (Porto):José-Manuel Pereira; Centro Hospitalar Sao Joao - Infectious Diseases ICU (Porto): Lurdes Santos, Alcina Ferreira; Hospital do Litoral Alentejano (Santiago do Cacém): Dulce Pascoalinho; São Bernardo - Centro Hospitalar Setubal (Setubal): Rosa Ribeiro, Guilherme Domingos; Hospital Vila Franca de Xira (Vila Franca de Xira): Pedro Gomes, David Nora; Centro Hospitalar de Trás-os-Montes e Alto Douro (Vila Real): Rui Pedro Costa, Anabela Santos; QATAR: Hamad Medical Corporation (Doha): Ahmed Subhy Alsheikhly; ROMANIA: Fundeni Clinical Institute (Bucharest): Dana Tomescu, Mihai Popescu; Regional Institute of Oncology (lasi): Ioana Grigoras, Emilia Patrascanu; RUSSIAN FEDERATION: Krasnodar Regional Hospital #2 (Krasnodar): Igor Zabolotskikh, Tatiana Musaeva; Krasnoyarsk State Medical University, Krasnoyarsk Regional Clinical Hospital (Krasnoyarsk): Alexey Gritsan, Denis Gaigolnik; Vishnevsky Institute of Surgery (Moscow): Vladimir Kulabukhov; Privolzhskiy District Medical Center (Nizhniy Novgorod): Vladislav Belskiy; Clinical Hospital # 4 (Perm): Nadezhda Zubareva, Maxim Tribulev; SAUDI ARABIA: International Medical Center (Jeddah): Ahmed Abdelsalam, Ayman Aldarsani; King Faisal Specialist Hospital & Research Centre (Riyadh): Muhammad Al-Khalid; PSMMC (Riyadh): Ghaleb Almekhlafi, Yasser Mandourah; SERBIA: Clinical Centre of Serbia (Belgrade): Bojan Jovanovic, Krstina Doklestic; Clinic for Digestive Surgery (Belgrade): Jelena Velickovic, Dejan Velickovic; Clinical Center Nis, (Nis): Radmilo Jankovic, Anita Vukovic; Oncology Institute of Vojvodina (Sremska Kamenica): Svetlana Skoric-Jokic, Dragana Radovanovic; SOUTH AFRICA: Charlotte Maxeke Johannesburg Academic Hospital (Johannesburg): Guy Richards, Ahmad Alli; SPAIN: Complejo Hospitalario Universitario de Albacete (Albacete): Maria del Carmen Cordoba Nielfa, Rafael Sánchez Iniesta; Parc de Salut Mar (Barcelona): Adela Benítez-Cano Martínez, Carlos Garcia Bernedo; Hospital Delfos (Barcelona): Santiago Alberto Picos Gil; Vall d’Hebron University Hospital (Barcelona): Xavier Nuvials, Jordi Rello; Hospital Universitario de Basurto (Bilbao): Joseba Gonzalez Garcia, Jose Manuel Garcia Peña; Hospital General Universitario Santa Lucia (Cartagena): Roberto Jimenez, Luis Herrera; Hospital General Universitari de Castelló (Castelló): Laura Galarza Barrachina, Ignacio Catalan Monzon; Hospital General Universitario de Ciudad Real (Ciudad Real): Francisco Javier Redondo, Ruben Villazala; Hospital Costa de la Luz (Huelva): Diego Fernando Matallana Zapata, Isabel Maria Villa Lopez; Hospital Universitari de Bellvitge (L´Hospitalet de Llobregat): Gabriel Moreno-Gonzalez, Juan Carlos Lopez-Delgado; Hospital Universitario de Canarias (La Laguna): Jorge Solera Marin; Hospital Universitario Severo Ochoa (Léganes): Purificacion Sanchez-Zamora; Hospital Universitari Arnau de Vilanova (Lleida): Montserrat Vallverdú Vidal; Hospital Quirón Campo de Gibraltar (Cádiz): Jesús Flores González; Hospital Universitarion del Henares (Madrid): Irene Salinas, Cecilia Hermosa; Hospital Universitario La Paz (Madrid): Emilio Maseda; Hospital Clinico San Carlos (Madrid): Fernando Martinez-Sagasti, Sara Domingo-Marín; Central de la Defensa Gomez Ulla (Madrid): Johanna Abril Victorino; Hospital 12 de Octubre (Madrid): Raquel Garcia-Alvarez, Pablo López-Arcas Calleja; Hospital Universitario de Malaga (Malaga): Maria-Victoria de la Torre-Prados; CHU Ourense (Ourense): Pablo Vidal-Cortes, Lorena del Río-Carbajo; Complejo Hospitalario de Navarra (Pamplona): Javier Izura, Victoria Minguez; Hospital Universitari Mutua Terrassa (Terrassa): Josep Trenado Alvarez, Anna Parera Prous; Complejo Hospitalario de Toledo (Toledo): Daniel Paz; Hospital Verge de la Cinta (Tortosa): Ferran Roche-Campo; Hospital Clínico Universitario de Valencia (Valencia): Gerardo Aguilar, Javier Belda; Rio Hortega University Hospital (Valladolid): Jesus Rico-Feijoo, Cesat Aldecoa; Hospital Clinico Universitario Lozano Blesa (Zaragoza): Begoña Zalba-Etayo; SWITZERLAND: Kantonsspital Frauenfeld (Frauenfeld): Martin Lang; Alexander Dullenkopf; THAILAND: Faculty of Medicine Vajira Hospital, Navamindradhiraj University (Bangkok): Konlawij Trongtrakul; Anusang Chtsomkasem; TURKEY: Düzce University Hospital (Duzce): Türkay Akbaş; Ankara University School of Medicine (Ankara): Mustafa Necmettin Unal, Menekse Ozcelik; Akdeniz University Medical School (Antalya): Ayca Gumus, Atilla Ramazanoglu; Trakya University Medical Faculty (Edirne): Dilek Memis, Inal Mehmet; Istanbul University-Cerrahpasa, Cerrahpasa Medical School (Istanbul): Yalim Dikmen, Seval Urkmez; Haydarpaşa Numune Training and Research Hospital (Istanbul): Asu Ozgultekin; Istanbul University Cerrahpasa Medical School Hospital (Istanbul): Oktay Demirkiran; Medipol Mega Hospitals Complex (Istanbul): Nesrin Ahu Aslan, Deniz Kizilaslan; Uludag University, School of Medicine (Nilüfer/Bursa): Ferda Kahveci, Nurdan Ünlü; Elazig Training & Research Hospital (Elazig): Zeynep Ozkan; UNITED KINGDOM: Aberdeen Royal Infirmary (Aberdeen): Callum Kaye, Jan Jansen; Antrim Area Hospital (Antrim): Orla O’Neill, Christopher Nutt; Barnet General Hospital, RFL NHS FT (Barnet): Rajeev Jha, Nicolas Hooker; Basingsoke & North Hampshire Hospital (Basingstoke): Irina Grecu, Christina Petridou; Royal Victoria Hospital (Belfast): Murali Shyamsundar, Lia McNamee; Ulster Hospital (Belfast): John Trinder, Samantha Hagan; Belfast City Hospital (Belfast): Catriona Kelly, Jonathon Silversides; Brighton and Sussex University Hospitals (Brighton): Casiano Barrera Groba, Owen Boyd; West Suffolk Hospital NHS Foundation Trust (Bury St Edmunds): Kaushik Bhowmick, Sally Humphreys; Cambridge University Hospitals NHS Foundation Trust and University of Cambridge (Cambridge): Charlotte Summers, Petra Polgarova; Western Sussex NHS Foundation Trust, St Richard’s Hospital (Chichester, West Sussex): Michael Margarson, Justin Dickens; Colchester General Hospital (Colchester): Suzanne Pearson, Elaine Chinery; Altnagelvin Hospital (Derry): Noel Hemmings, Sinead O’Kane; Ninewells Hospital (Dundee): Pauline Austin, Stephen Cole; Medway NHS Foundation Trust (Gillingham): Catherine Plowright, Roberta Box; Queen Elizabeth University Hospital (Glasgow): Christopher Wright, Lorna Young; Royal Surrey County Hospital (Guildford): Ben Creagh-Brown, Laura Montague; Aintree University Hospital (Liverpool): Robert Parker; Ben Morton; Guy’s and St Thomas Hospitals (London): Marlies Ostermann, Julia Bilinska; University Hospital Lewisham (London): Bernd Oliver Rose, Rosie Reece-Anthony; St Georges University Hospitals NHS Foundation Trust (London): Christine Ryan, Mark Hamilton; King’s College Hospital (London): Philip Hopkins, Julia Wendon; Luton and Dunstable Hospital (Luton): Giovanni Brescia, Nazia Ijaz; Maidstone and Tunbridge Wells NHS Trust Hospital (Maidstone): James Wood, Michelle George; Prince Charles Hospital (Merthyr Tydfil): Piroska Toth-Tarsoly; Northumbria Specialist Emergency Care Hospital (Newcastle Upon Tyne): Bryan Yates, Maureen Armstrong; Royal Victoria Infirmary (Newcastle Upon Tyne): Carmen Scott, Christine Boyd; Royal Gwent Hospital (Newport): Tamas Szakmany, David Rees; Kings Mill Hospital (Nottingham): Paul Pulak, Mandy Coggon; Royal Oldham Hospital (Oldham): Bhaskar Saha, Linda Kent; Royal Glamorgan Hospital (Pontyclun): Bethan Gibson; Poole Hospital NHS FT (Poole): Julie Camsooksai, Henrik Reschreiter; East Surrey Hospital (Redhill): Pat Morgan, Sivatharshini Sangaralingham; Conquest Hospital (St Leonards-on-sea): Alastair Lowe, Petr Vondras; Lister Hospital (Stevenage): Sunil Jamadarkhana, Carina Cruz; University Hospital of North Tees (Stockton-on-Tees): Rakesh Bhandary; Sunderland Royal Hospital (Sunderland): Peter Hersey, Julie Furneval; Musgrove Park Hospital (Taunton): Richard Innes, Patricia Doble; Warwick Hospital (Warwick): Ben Attwood, Penny Parsons; Watford General Hospital (Watford): Valerie Page, Xiaobei Zhao; Royal Hampshire County Hospital (Winchester): Irina Grecu, Julian Dalton; UNITED ARAB EMIRATES: Sheikh Khalifa Medical City (Abu Dhabi): Mohammed Hegazy, Yasser Awad; UNITED STATES: Cleveland Clinic (Cleveland): Douglas Naylor, Amanda Naylor; Detroit Medical Center (Detroit): Sarah Lee; University of South Alabama Medical Center (Mobile, AL): Sidney Brevard, Noelle Davis.
Funding
AbSeS is a Trials Group Study of the European Society of Intensive Care Medicine. The study was supported by a Pfizer investigator-initiated research grant.
Compliance with ethical standards
Conflicts of interest
Received grants related to the submitted work: S. Blot (Pfizer). Received honoraria or grants outside the submitted work: M. Antonelli (Fresenius, Pfizer, Toray); J. De Waele (Research Foundation Flanders, Pfizer, Bayer, MSD); C. Eckmann (Merck, Pfizer); J. Lipman (MSD, Pfizer); E. Maseda (Astellas Pharma, Pfizer, MSD); All other authors: no conflict of interest.
Footnotes
The members of the Abdominal Sepsis Study (AbSeS) group for the Trials Group of the European Society of Intensive Care Medicine have been given in the Acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Stijn Blot, Email: stijn.blot@UGent.be.
the Abdominal Sepsis Study (AbSeS) group on behalf of the Trials Group of the European Society of Intensive Care Medicine:
Amin Lamrous, Cecilia Pereyra, Fernando Lipovestky, Despoina Koulenti, Jan De Waele, Joao Rezende-Neto, Yenny Cardenas, Tomas Vymazal, Hans Fjeldsoee-Nielsen, Philippe Montravers, Matthias Kott, Arvaniti Kostoula, Yash Javeri, Massimo Girardis, Sharon Einav, Dylan de Lange, Luis Daniel Umezawa Makikado, Adam Mikstacki, José-Artur Paiva, Dana Tomescu, Alexey Gritsan, Bojan Jovanovic, Kumaresh Venkatesan, Tomislav Mirkovic, Emilio Maseda, Yalim Dikmen, Benedict Creagh-Brown, Amin Lamrous, Monica Emmerich, Mariana Canale, Lorena Silvina Dietz, Santiago Ilutovich, John Thomas Sanchez Miñope, Ramona Baldomera Silva, Martin Alexis Montenegro, Patricio Martin, Pablo Saul, Viviana Chediack, Giselle Sutton, Rocio Couce, Carina Balasini, Susana Gonzalez, Florencia Maria Lascar, Emiliano Jorge Descotte, Natalia Soledad Gumiela, Carina Alejandra Pino, Cristian Cesio, Emanuel Valgolio, Eleonora Cunto, Cecilia Dominguez, Fernando Lipovestky, Nydia Funes Nelson, Esteban Martin Abegao, Cecilia Pereyra, Norberto Christian Pozo, Luciana Bianchi, Enrique Correger, Maria Laura Pastorino, Erica Aurora Miyazaki, Norberto Christian Pozo, Nicolas Grubissich, Mariel Garcia, Natalia Bonetto, Noelia Elizabeth Quevedo, Cristina Delia Gomez, Felipe Queti, Luis Gonzalez Estevarena, Ruben Fernandez, Ignacio Santolaya, Norberto Christian Pozo, Sergio Hugo Grangeat, Juan Doglia, Graciela Zakalik, Carlos Pellegrini, Maria Monserrat Lloria, Mercedes Esteban Chacon, Mariela Fumale, Mariela Leguizamon, Irene Beatriz Hidalgo, Roberto Julian Tiranti, Paola Capponi, Agustin Tita, Luis Cardonnet, Lisandro Bettini, Agñel Ramos, Luciano Lovesio, Edith Miriam Miranda, Angelica Beatriz Farfan, Carina Tolosa, Lise Segura, Adelina Bellocchio, Brian Alvarez, Adriana Manzur, Rodolfo Lujan, Natalia Fernandez, Nahuel Scarone, Alan Zazu, Carina Groh, Jason Fletcher, Julie Smith, Raman Azad, Nitin Chavan, Helen Wong, Mark Kol, Lewis Campbell, Despoina Koulenti, Therese Starr, Brigit Roberts, Bradley Wibrow, Timothy Warhurst, Meher Chinthamuneedi, Bernal Buitrago Ferney, Marc Simon, Daniel De Backer, Xavier Wittebole, David De Bels, Vincent Collin, Karolien Dams, Philippe Jorens, Jan De Waele, Jasperina Dubois, Jan Gunst, Lionel Haentjens, Nicolas De Schryver, Thierry Dugernier, Joao Rezende-Neto, Sandro Rizoli, Paul Santillan, Yi Han, Ewelina Biskup, Changjing Qu, Xinyu Li, Tao Yu, Lu Weihua, Daniel Molano-Franco, José Rojas, Juan Mauricio Pardo Oviedo, Dario Pinilla, Yenny Cardenas, Edgar Celis, Mario Arias, Anita Vukovic, Maja Vudrag, Matija Belavic, Josip Zunic, Janja Kuharic, Irena Bozanic Kricka, Ina Filipovic-Grcic, Boris Tomasevic, Melanija Obraz, Bruna Bodulica, Martin Dohnal, Jan Malaska, Milan Kratochvil, Igor Satinsky, Peter Schwarz, Zdenek Kos, Ladislav Blahut, Jan Maca, Marek Protus, Eva Kieslichová, Louise Gramstrup Nielsen, Birgitte Marianne Krogh, Francisco Rivadeneira, Freddy Morales, José Mora, Alexandra Saraguro Orozco, Diego Rolando MorochoTutillo, Nelson Remache Vargas, Estuardo Salgado Yepez, Boris Villamagua, Adel Alsisi, Abdelraouf Fahmy, Hervé Dupont, Sigismond Lasocki, Catherine Paugam-Burtz, Arnaud Foucrier, Alexandru Nica, Geneviève Barjon, Jihad Mallat, Guillaume Marcotte, Marc Leone, Gary Duclos, Philippe Burtin, Philippe Montravers, Enora Atchade, Yazine Mahjoub, Benoît Misset, Jean-François Timsit, Claire Dupuis, Benoît Veber, Matthieu Debarre, Oliver Collange, Julien Pottecher, Stephane Hecketsweiler, Mélanie Fromentin, Antoine Tesnière, Christian Koch, Michael Sander, Matthias Kott, Gunnar Elke, Hermann Wrigge, Philipp Simon, Anthoula Chalkiadaki, Charalampos Tzanidakis, Ioannis Pneumatikos, Eleni Sertaridou, Zafiria Mastora, Ioannis Pantazopoulos, Metaxia Papanikolaou, Theonymfi Papavasilopoulou, John Floros, Virginia Kolonia, George Dimopoulos, Chryssa Diakaki, Michael Rallis, Alexandra Paridou, Alexandros Kalogeromitros, Vasiliki Romanou, Charikleia Nikolaou, Katerina Kounougeri, Evdoxia Tsigou, Vasiliki Psallida, Niki Karampela, Konstantinos Mandragos, Eftychia Kontoudaki, Alexandra Pentheroudaki, Christos Farazi-Chongouki, Agathi Karakosta, Isaac Chouris, Vasiliki Radu, Polychronis Malliotakis, Sofia Kokkini, Eliana Charalambous, Aikaterini Kyritsi, Vasilios Koulouras, Georgios Papathanakos, Eva Nagky, Clairi Lampiri, Fotios Tsimpoukas, Ioannis Sarakatsanos, Panagiotis Georgakopoulos, Ifigeneia Ravani, Athanasios Prekates, Konstantinos Sakellaridis, Christos Christopoulos, Efstratia Vrettou, Konstantinos Stokkos, Anastasia Pentari, Kostoula Arvaniti, Kyriaki Marmanidou, Christina Kydona, Georgios Tsoumaropoulos, Militisa Bitzani, Paschalina Kontou, Antonios Voudouris, Elli-Nikki, Flioni, Elli Antypa, Eleftheria Chasou, Souzana Anisoglou, Eirini Papageorgiou, Theoniki Paraforou, Agoritsa Tsioka, Antigoni Karathanou, Aristeidis Vakalos, Bhagyesh Shah, Chirag Thakkar, Nikhilesh Jain, Mohan Gurjar, Arvind Baronia, Prachee Sathe, Shilpa Kulkarni, Cherish Paul, John Paul, Mansoor Masjedi, Reza Nikandish, Farid Zand, Golnar Sabetian, Ata Mahmoodpoor, Seyed Mohammadreza Hashemian, Miklosh Bala, Romeo Flocco, Sergio Torrente, Vincenzo Pota, Savino Spadaro, Carlo Volta, Massimo Girardis, Giulia Serafini, Sabrina Boraso, Ivo Tiberio, Andrea Cortegiani, Giovanni Misseri, Maria Barbagallo, Davide Nicolotti, Francesco Forfori, Francesco Corradi, Massimo Antonelli, Gennaro De Pascale, Lorella Pelagalli, Luca Brazzi, Ferdinando Giorgio Vittone, Alessandro Russo, Davide Simion, Antonella Cotoia, Gilda Cinnella, Patrick Toppin, Roxanne Johnson-Jackson, Yoshiro Hayashi, Ryohei Yamamoto, Hideto Yasuda, Yuki Kishihara, Junji Shiotsuka, Luis Alejandro Sanchez-Hurtado, Brigitte Tejeda-Huezo, Luis Gorordo, Silvio A. Ñamendys-Silva, Francisco J. Garcia-Guillen, Manuel Martinez, Erick Romero-Meja, Ever Colorado-Dominguez, Huub van den Oever, Karel Martijn Kalff, Wytze Vermeijden, Alexander Daniel Cornet, Oliver Beck, Nedim Cimic, Tom Dormans, Laura Bormans, Jan Bakker, Ditty Van Duijn, Gerrit Bosman, Piet Vos, Dylan de Lange, Lenneke Haas, Akram Henein, Ariel M. Miranda, Luis Daniel Umezawa Makikado, Gonzalo Ernesto Gianella Malca, Abel Arroyo-Sanchez, Agnieszka Misiewska-Kaczur, Frisch Akinyi, Miroslaw Czuczwar, Karolina Luczak, Wiktor Sulkowski, Barbara Tamowicz, Adam Mikstacki, Beata Swit, Bronisław Baranowski, Piotr Smuszkiewicz, Iwona Trojanowska, Stanislaw Rzymski, Mariusz Sawinski, Marta Trosiak, Malgorzata Mikaszewska-Sokolewicz, Ricardo Alves, Dina Leal, Andriy Krystopchuk, Pedro Muguel Hilario Mendonca, Rui Antunes Pereira, Maria Raquel Lopes Marques de Carvalho, Carlos Candeias, Elena Molinos, Amélia Ferreira, Guiomar Castro, José-Artur Paiva, José-Manuel Pereira, Lurdes Santos, Alcina Ferreira, Dulce Pascoalinho, Rosa Ribeiro, Guilherme Domingos, Pedro Gomes, David Nora, Rui Pedro Costa, Anabela Santos, Ahmed Subhy Alsheikhly, Dana Tomescu, Mihai Popescu, Ioana Grigoras, Emilia Patrascanu, Igor Zabolotskikh, Tatiana Musaeva, Alexey Gritsan, Denis Gaigolnik, Vladimir Kulabukhov, Vladislav Belskiy, Nadezhda Zubareva, Maxim Tribulev, Ahmed Abdelsalam, Ayman Aldarsani, Muhammad Al-Khalid, Ghaleb Almekhlafi, Yasser Mandourah, Bojan Jovanovic, Krstina Doklestic, Jelena Velickovic, Dejan Velickovic, Radmilo Jankovic, Anita Vukovic, Svetlana Skoric-Jokic, Dragana Radovanovic, Guy Richards, Ahmad Alli, Maria del Carmen Cordoba Nielfa, Rafael Sánchez Iniesta, Adela Benítez-Cano Martínez, Carlos Garcia Bernedo, Santiago Alberto Picos Gil, Xavier Nuvials, Jordi Rello, Joseba Gonzalez Garcia, Jose Manuel Garcia Peña, Roberto Jimenez, Luis Herrera, Laura Galarza Barrachina, Ignacio Catalan Monzon, Francisco Javier Redondo, Ruben Villazala, Diego Fernando Matallana Zapata, Isabel Maria Villa Lopez, Gabriel Moreno-Gonzalez, Juan Carlos Lopez-Delgado, Jorge Solera Marin, Purificacion Sanchez-Zamora, Montserrat Vallverdú Vidal, Jesús Flores González, Irene Salinas, Cecilia Hermosa, Emilio Maseda, Fernando Martinez-Sagasti, Sara Domingo-Marín, Johanna Abril Victorino, Raquel Garcia-Alvarez, Pablo López-Arcas Calleja, Maria-Victoria de la Torre-Prados, Pablo Vidal-Cortes, Lorena del Río-Carbajo, Javier Izura, Victoria Minguez, Josep Trenado Alvarez, Anna Parera Prous, Daniel Paz, Ferran Roche-Campo, Gerardo Aguilar, Javier Belda, Jesus Rico-Feijoo, Cesat Aldecoa, Begoña Zalba-Etayo, Martin Lang, Alexander Dullenkopf, Konlawij Trongtrakul, Anusang Chtsomkasem, Türkay Akbaş, Mustafa Necmettin Unal, Menekse Ozcelik, Ayca Gumus, Atilla Ramazanoglu, Dilek Memis, Inal Mehmet, Yalim Dikmen, Seval Urkmez, Asu Ozgultekin, Oktay Demirkiran, Nesrin Ahu Aslan, Deniz Kizilaslan, Ferda Kahveci, Nurdan Ünlü, Zeynep Ozkan, Callum Kaye, Jan Jansen, Orla O’Neill, Christopher Nutt, Rajeev Jha, Nicolas Hooker, Irina Grecu, Christina Petridou, Murali Shyamsundar, Lia McNamee, John Trinder, Samantha Hagan, Catriona Kelly, Jonathon Silversides, Casiano Barrera Groba, Owen Boyd, Kaushik Bhowmick, Sally Humphreys, Charlotte Summers, Petra Polgarova, Michael Margarson, Justin Dickens, Suzanne Pearson, Elaine Chinery, Noel Hemmings, Sinead O’Kane, Pauline Austin, Stephen Cole, Catherine Plowright, Roberta Box, Christopher Wright, Lorna Young, Ben Creagh-Brown, Laura Montague, Robert Parker, Ben Morton, Marlies Ostermann, Julia Bilinska, Bernd Oliver Rose, Rosie Reece-Anthony, Christine Ryan, Mark Hamilton, Philip Hopkins, Julia Wendon, Giovanni Brescia, Nazia Ijaz, James Wood, Michelle George, Piroska Toth-Tarsoly, Bryan Yates, Maureen Armstrong, Carmen Scott, Christine Boyd, Tamas Szakmany, David Rees, Paul Pulak, Mandy Coggon, Bhaskar Saha, Linda Kent, Bethan Gibson, Julie Camsooksai, Henrik Reschreiter, Pat Morgan, Sivatharshini Sangaralingham, Alastair Lowe, Petr Vondras, Sunil Jamadarkhana, Carina Cruz, Rakesh Bhandary, Peter Hersey, Julie Furneval, Richard Innes, Patricia Doble, Ben Attwood, Penny Parsons, Valerie Page, Xiaobei Zhao, Irina Grecu, Julian Dalton, Mohammed Hegazy, Yasser Awad, Douglas Naylor, Amanda Naylor, Sarah Lee, Sidney Brevard, and Noelle Davis
References
- 1.Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 2.Kübler A, Adamik B, Ciszewicz-Adamiczka B, Ostrowska E. Severe sepsis in intensive care units in Poland—a point prevalence study in 2012 and 2013. Anaesthesiol Intensive Ther. 2015;47:315–319. doi: 10.5603/AIT.2015.0047. [DOI] [PubMed] [Google Scholar]
- 3.Sakr Y, Jaschinski U, Wittebole X, et al. Sepsis in intensive care unit patients: worldwide data from the intensive care over nations audit. Open Forum Infect Dis. 2018;5:ofy313. doi: 10.1093/ofid/ofy313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blot S, De Waele JJ. Critical issues in the clinical management of complicated intra-abdominal infections. Drugs. 2005;65:1611–1620. doi: 10.2165/00003495-200565120-00002. [DOI] [PubMed] [Google Scholar]
- 5.Montravers P, Blot S, Dimopoulos G, et al. Therapeutic management of peritonitis: a comprehensive guide for intensivists. Intensive Care Med. 2016;42:1234–1247. doi: 10.1007/s00134-016-4307-6. [DOI] [PubMed] [Google Scholar]
- 6.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010;11:79–109. doi: 10.1089/sur.2009.9930. [DOI] [PubMed] [Google Scholar]
- 7.Azuhata T, Kinoshita K, Kawano D, et al. Time from admission to initiation of surgery for source control is a critical determinant of survival in patients with gastrointestinal perforation with associated septic shock. Crit Care. 2014;18:R87. doi: 10.1186/cc13854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sartelli M, Chichom-Mefire A, Labricciosa FM, et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12:29. doi: 10.1186/s13017-017-0141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tolonen M, Coccolini F, Ansaloni L, et al. Getting the invite list right: a discussion of sepsis severity scoring systems in severe complicated intra-abdominal sepsis and randomized trial inclusion criteria. World J Emerg Surg. 2018;13:17. doi: 10.1186/s13017-018-0177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blot S, De Waele JJ, Vogelaers D. Essentials for selecting antimicrobial therapy for intra-abdominal infections. Drugs. 2012;72:e17–e32. doi: 10.2165/11599800-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calandra Thierry, Cohen Jonathan. The International Sepsis Forum Consensus Conference on Definitions of Infection in the Intensive Care Unit. Critical Care Medicine. 2005;33(7):1538–1548. doi: 10.1097/01.CCM.0000168253.91200.83. [DOI] [PubMed] [Google Scholar]
- 12.Trouillet JL, Chastre J, Vuagnat A, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998;157:531–539. doi: 10.1164/ajrccm.157.2.9705064. [DOI] [PubMed] [Google Scholar]
- 13.Singer Mervyn, Deutschman Clifford S., Seymour Christopher Warren, Shankar-Hari Manu, Annane Djillali, Bauer Michael, Bellomo Rinaldo, Bernard Gordon R., Chiche Jean-Daniel, Coopersmith Craig M., Hotchkiss Richard S., Levy Mitchell M., Marshall John C., Martin Greg S., Opal Steven M., Rubenfeld Gordon D., van der Poll Tom, Vincent Jean-Louis, Angus Derek C. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 5.0, 2015. http://www.eucast.org. Accessed 31 Mar 2014
- 15.Kadri SS, Adjemian J, Lai YL, et al. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis. 2018;67:1803–1814. doi: 10.1093/cid/ciy378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadri SS, Lai YLE, Ricotta EE, et al. External validation of difficult-to-treat resistance prevalence and mortality risk in gram-negative bloodstream infection using electronic health record data from 140 US hospitals. Open Forum Infect Dis. 2019;6:ofz110. doi: 10.1093/ofid/ofz110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Versporten A, Zarb P, Caniaux I, et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. 2018;6:e619–e629. doi: 10.1016/S2214-109X(18)30186-4. [DOI] [PubMed] [Google Scholar]
- 18.Vogelaers D, De Bels D, Forêt F, et al. Patterns of antimicrobial therapy in severe nosocomial infections: empiric choices, proportion of appropriate therapy, and adaptation rates—a multicentre, observational survey in critically ill patients. Int J Antimicrob Agents. 2010;35:375–381. doi: 10.1016/j.ijantimicag.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Solomkin JS, Yellin AE, Rotstein OD, et al. Ertapenem versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections: results of a double-blind, randomized comparative phase III trial. Ann Surg. 2003;237:235–245. doi: 10.1097/01.SLA.0000048551.32606.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solomkin JS, Wilson SE, Christou NV, et al. Results of a clinical trial of clinafloxacin versus imipenem/cilastatin for intraabdominal infections. Ann Surg. 2001;233:79–87. doi: 10.1097/00000658-200101000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomkin JS, Reinhart HH, Dellinger EP, et al. Results of a randomized trial comparing sequential intravenous/oral treatment with ciprofloxacin plus metronidazole to imipenem/cilastatin for intra-abdominal infections. The Intra-Abdominal Infection Study Group. Ann Surg. 1996;223:303–315. doi: 10.1097/00000658-199603000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Ruiter J, Weel J, Manusama E, et al. The epidemiology of intra-abdominal flora in critically ill patients with secondary and tertiary abdominal sepsis. Infection. 2009;37:522–527. doi: 10.1007/s15010-009-8249-6. [DOI] [PubMed] [Google Scholar]
- 23.Blot K, Hammami N, Blot S, et al. Increasing burden of Escherichia coli, Klebsiella pneumoniae, and Enterococcus faecium in hospital-acquired bloodstream infections (2000–2014): a national dynamic cohort study. Infect Control Hosp Epidemiol. 2019;40:705–709. doi: 10.1017/ice.2019.59. [DOI] [PubMed] [Google Scholar]
- 24.Martínez ML, Ferrer R, Torrents E, et al. Impact of source control in patients with severe sepsis and septic shock. Crit Care Med. 2017;45:11–19. doi: 10.1097/CCM.0000000000002011. [DOI] [PubMed] [Google Scholar]
- 25.Montravers P, Dupont H, Gauzit R, et al. Candida as a risk factor for mortality in peritonitis. Crit Care Med. 2006;34:646–652. doi: 10.1097/01.CCM.0000201889.39443.D2. [DOI] [PubMed] [Google Scholar]
- 26.Knitsch W, Vincent JL, Utzolino S, et al. A randomized, placebo-controlled trial of preemptive antifungal therapy for the prevention of invasive candidiasis following gastrointestinal surgery for intra-abdominal infections. Clin Infect Dis. 2015;61:1671–1678. doi: 10.1093/cid/civ707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van de Groep K, Verhoeff TL, Verboom DM, et al. Epidemiology and outcomes of source control procedures in critically ill patients with intra-abdominal infection. J Crit Care. 2019;52:258–264. doi: 10.1016/j.jcrc.2019.02.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.