Abstract
Trichinella spiralis (T. spiralis) is widely distributed throughout the world and can cause serious zoonotic parasitic diseases. Serine protease inhibitors (SPIs) have unique enzyme inhibitory activity and occupy an important position in the interaction between parasites and hosts. In order to further understand the immunoprotective effect of SPIs on T. spiralis invasion in vivo, the Kazal and Serpin type SPI of T. spiralis (TsKaSPI and TsAdSPI) were mixed with Freund’s adjuvant in equal volume to immunize mice. The results showed that the expression of IgG1 and IgG2a in serum, the proliferation of spleen cells, and the expression level of cytokines were all increased. The results of flow cytometry showed that the expression of CD4+CD25+Foxp3+ Tregs, CD8+CD28− T cells, CD19+CD5+CD1dhi Bregs in spleen were also increased. Therefore, both TsKaSPI and TsAdSPI could induce strong humoral and cellular immune responses. And the results of adult reduction rate and pathological changes of intestine after adult invasion also indicated that both TsKaSPI and TsAdSPI could prevent T. spiralis from invading intestine. To explore the regulatory effects of TsKaSPI and TsAdSPI on the immune function of macrophage, the results of ELISA showed that the expression of cytokines in cell supernatant were increased. And the results of Western blot showed that both TsKaSPI and TsAdSPI could induce phosphorylation of JAK2 and STAT3 receptors, thereby affecting the signal transduction of macrophages. This experiment demonstrated that SPIs could act as effector molecules affecting the immune function of host when infected with T. spiralis.
Subject terms: Autoimmunity, Inflammation
Introduction
Trichinella spiralis (T. spiralis) is a nematode that establishes persistent infection in the muscle of mammals and can cause very serious zoonotic parasitic diseases1,2. It is one of the most widely distributed zoonotic pathogens in the world. T. spiralis has a wide range of hosts in nature, and ability to complete parasitism in a variety of animals. Moreover, the different developmental phase of T.spialis all occurs in a single host, causing severe damage of the host, so its mechanism of evading the host’s immune system has attracted extensive attention. When T. spiralis establishes a parasitic relationship with the host, it will generate various immune evasion mechanisms, so that it can successfully parasitize and minimize the damage of the host. In the early phase of T. spiralis infection, it can induce Th1/Th2 mixed immune response in the host, and mainly based on Th2 type3. The main manifestations are increased IgG and cytokines level, as well as increased eosinophils and basophils, which can help the host to resist infection. For a long time, scholars have been studying the key components of T. spiralis that play an important role in immune evasion, and T. spiralis serine protease inhibitors (SPIs) can inhibit a variety of intestinal digestive enzymes of the host, it has been identified as the major regulatory antigen in the process of T. spiralis invading the host4,5. Therefore, the study on its structure and function is of great significance.
SPI is an enzyme activity regulator with conserved amino acid sequence and special spatial structure. It can inhibit target enzymes by changing its own conformation, and involved in many basic life activities, such as cell migration, tumor inhibition, inflammatory reaction, protein folding, cell matrix reconstruction6,7. Studies have shown that parasite SPI has unique enzyme inhibitory activity, which can protect the parasite against the digestion of the host’s digestive enzymes, and provide favorable conditions for the parasite to survive, develop, migrate and settle in the host, help the parasite to resist the host’s immune response8–11.
Our laboratory has obtained active recombinant T. spiralis SPIs (TsKaSPI, TsAdSPI) by prokaryotic expression. Ma et al.12 found that TsKaSPI can inhibit the activity of trypsin, elastase, chymotrypsin, cathepsin G, thrombin and granulase B. TsAdSPI also inhibited the activity of trypsin, elastase and chymotrypsin13. Some researchers believe that in the intestinal phase of T. spiralis infection, SPI will not induce the host to produce specific antibodies, but will rapidly bind to multiple proteases in the intestine, the autoimmune sites can be quickly masked, thereby reducing the responsiveness of the intestinal phase and playing a role in immune evasion14,15. In parenteral phase, the antigen sites of SPI exposed, and play a role in immune evasion by regulating multiple molecules of the immune system. The regulation of the immune system by T. spiralis SPI is gradually being revealed, and the purpose of this study is to investigate its regulatory effect on host’s immune system during T. spiralis invasion.
Result
Spleen cells proliferation
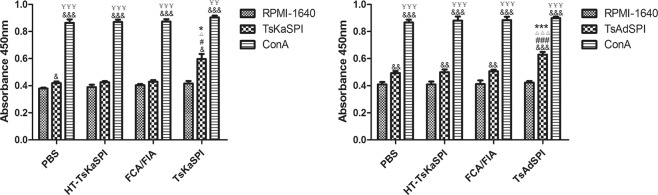
ConA has a potent effect on promoting mitosis and lymphocyte transformation, so stimulated with ConA in vitro was selected as positive control. The results showed that the number of spleen cells extracted from the PBS, HT-TsKaSPI, FCA/FIA, TsKaSPI and TsAdSPI group were significantly increased after ConA stimulation, compared with RPMI-1640, TsKaSPI and TsAdAPI stimulation (P < 0.001). The spleen cells extracted from the TsKaSPI (P < 0.05) and TsAdSPI group (P < 0.01) were stimulated with the corresponding proteins in vitro, and the number of spleen cells in the two groups were significantly higher than that in the PBS, HT-TsKaSPI and FCA/FIA group (Fig. 1).
Figure 1.
Proliferation of spleen cells extracted from each group after ConA, RPMI-1640, recombinant protein stimulation in vitro. Data are shown as mean ± SD of 3 mice per group. #P < 0.05, ##P < 0.01, ###P < 0.001 versus PBS group; △P < 0.05, △△P < 0.01, △△△P < 0.001 versus HT-TsKaSPI group; *P < 0.05, **P < 0.01, ***P < 0.001 versus FCA/FIA group; §P < 0.05, §§P < 0.01, §§§P < 0.001 for TsKaSPI vs TsAdSPI; &P < 0.05, &&P < 0.01, &&&P < 0.001 for ConA, TsKaSPI/TsAdSPI vs RPMI-1640 in the same group; ¥P < 0.05, ¥¥P < 0.01, ¥¥¥P < 0.001 for ConA vs TsKaSPI/TsAdSPI in the same group.
Changes of expression of CD4+CD25+Foxp3+ Treg cells, CD8+CD28− T cells and CD19+CD5+CD1dhi Breg cells in spleen
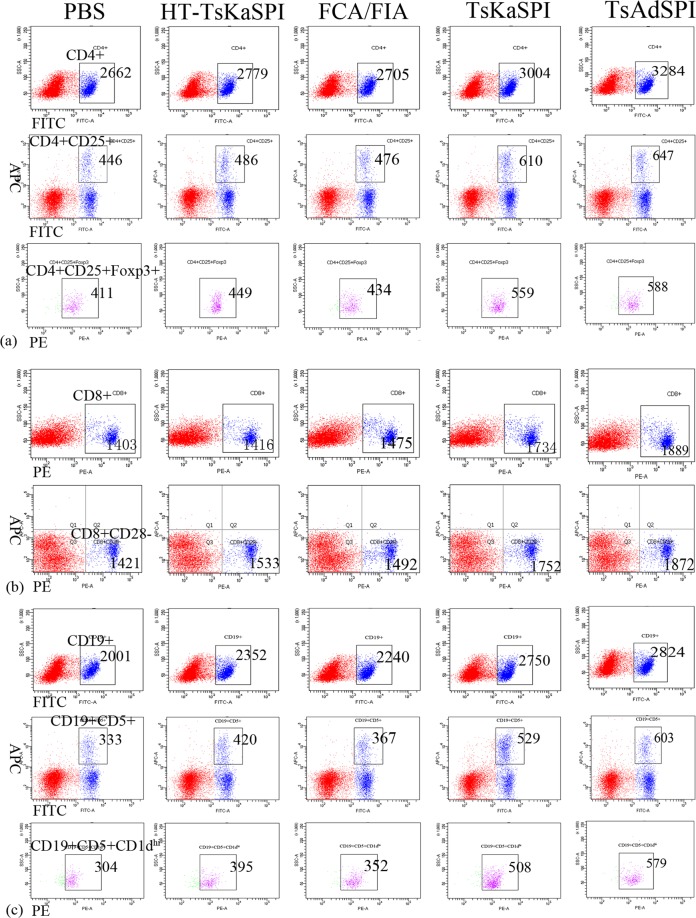
Mice were sacrificed 7 days after the third immunization, and prepared spleen lymphocyte single cell suspension. Flow cytometry was performed to detect CD4+CD25+Foxp3+ Treg cells (Fig. 2(a)), CD8+CD28− T cells (Fig. 2(b)) and CD19+CD5+CD1dhi Breg cells (Fig. 2(c)) in the spleen of each group. And the ratio of CD4+/CD8+ T cells in each group was calculated.
Figure 2.
Demonstration of the gating strategy for the flow cytometric analysis of mouse CD4 CD25+Foxp3+ Treg (a), CD8+CD28− T cell (b), CD19+CD5+CD1dhi Breg (c) from spleen. In this experiment a single cell suspension was prepared from the spleen of each group and stained with CD4 (FITC), CD25 (APC), Foxp3 (PE), CD8 (PE), CD28 (APC), CD19 (FITC), CD5 (APC), CD1d (PE) based on surface and intracellular staining protocols, respectively. Data were collected with FACSDiva flow cytometer and analyzed. Lymphocytes are identified by their scatter properties (FSC-A × SSC-A plot).
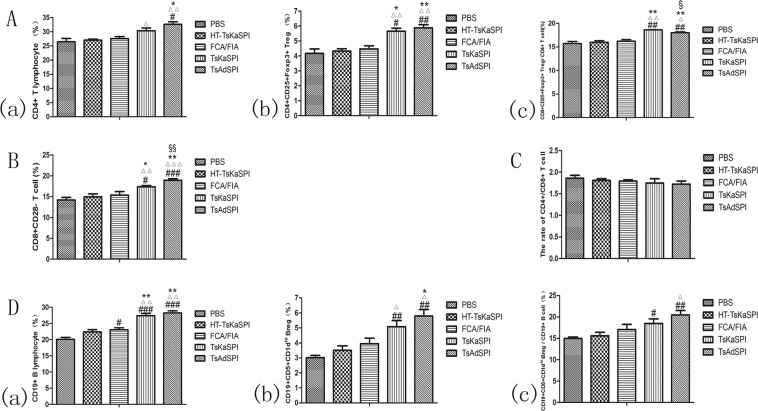
The results showed that the percentage of CD4+ T lymphocytes in the total number of cells in the gate in the TsAdSPI group (32.62 ± 1.58) was significantly higher than that in the PBS group (26.45 ± 2.09) (P < 0.05), HT-TsKaSPI group (27.05 ± 0.68) (P < 0.01) and FCA/FIA group (27.58 ± 1.22) (P < 0.05). While there was no significant difference compared the TsKaSPI group with PBS group and FCA/FIA group (Fig. 3A(a)); The percentage of CD4+CD25+Foxp3+ Treg cells in the total number of cells in the gate was significantly higher in the TsKaSPI group (5.66 ± 0.33) and TsAdSPI group (5.88 ± 0.37) than that in the PBS group (4.17 ± 0.51), HT-TsKaSPI group (4.33 ± 0.26) and FCA/FIA group (4.47 ± 0.36) (Fig. 3A(b)); At the same time, the percentage of CD4+CD25+Foxp3+ Treg in CD4+ T lymphocytes was calculated. The results showed that it was significantly higher in the TsKaSPI group (18.64 ± 0.04) and TsAdSPI group (18.03 ± 0.27) than that in the PBS group (15.73 ± 0.73), HT-TsKaSPI group (15.99 ± 0.57) and FCA/FIA group (16.21 ± 0.58), and the percentage of TsKaSPI group was significantly higher than that of TsAdSPI group (P < 0.05) (Fig. 3A(c)); For CD8+CD28− T cells, the percentage of it in total lymphocytes in the gate in the TsAdSPI group (18.95 ± 0.38) was significantly higher than the PBS group (14.21 ± 0.62) (P < 0.001), HT-TsKaSPI group (14.97 ± 0.72) (P < 0.001), FCA/FIA group (15.37 ± 0.87) (P < 0.01) and TsKaSPI group (17.37 ± 0.30) (P < 0.01), and the TsKaSPI group was significantly higher than the PBS group (P < 0.05), HT-TsKaSPI group (P < 0.01) and FCA/FIA group (P < 0.05) (Fig. 3B); The ratio of CD4+/CD8+ T cells was found to be lower in the TsKaSPI group (1.75 ± 0.10) and TsAdSPI group (1.72 ± 0.07) than that in the PBS group (1.86 ± 0.07), but there was no significant difference between the groups (Fig. 3C);
Figure 3.
The percentage of CD4+ T lymphocytes in the total lymphocytes in the gate (a), the percentage of CD4+CD25+Foxp3+ Tregs in the total lymphocytes in the gate (b) and the percentage of CD4+ T lymphocytes in CD4+CD25+Foxp3+ Tregs (c) in spleens of five groups were showed in (A); the percentage of CD8+CD28− T cells in the total lymphocytes in the gate were showed in (B); the rate of CD4+/CD8+ T cells in spleens of five groups were showed in (C); and the percentage of CD19+ B lymphocytes in the total lymphocytes in the gate (a), the percentage of CD19+CD5+CD1dhi+ Bregs in the total lymphocytes in the gate (b) and the percentage of CD19+ B lymphocytes in CD19+CD5+CD1dhi Bregs (c) in spleens of five groups were showed in (D). Data are shown as mean ± SD of 3 mice per group. #P < 0.05, ##P < 0.01, ###P < 0.001 versus PBS group; △P < 0.05, △△P < 0.01, △△△P < 0.001 versus HT-TsKaSPI group; *P < 0.05, **P < 0.01, ***P < 0.001 versus FCA/FIA group; §P < 0.05, §§P < 0.01, §§§P < 0.001 for TsKaSPI vs TsAdSPI.
The percentage of CD19+ B lymphocytes in total lymphocytes was significantly higher in the TsKaSPI group (27.39 ± 1.15) and TsAdSPI group (28.21 ± 1.20) than that in the PBS group (20.17 ± 1.08) (P < 0.001), HT-TsKaSPI group (22.37 ± 1.25) (P < 0.01) and FCA/FIA group (23.03 ± 1.05) (P < 0.01), and there was no significant difference between the two groups (Fig. 3D(a)); The percentage of CD19+CD5+CD1dhi Breg in the TsKaSPI group (5.08 ± 0.71) and TsAdSPI group (5.79 ± 0.77) was significantly higher than that in the PBS group (3.01 ± 0.26) (P < 0.01) and HT-TsKaSPI group (3.51 ± 0.51) (P < 0.05), and the percentage in the TsAdSPI group was significantly higher than that in the FCA/FIA group (3.95 ± 0.64) (P < 0.05) (Fig. 3D(b)); Therefore, the percentage of CD19+CD5+CD1dhi Breg in CD19+ B lymphocytes was calculated, and the results showed that it was significantly higher in the TsKaSPI group (18.50 ± 1.82) (P < 0.05) and TsAdSPI group (20.46 ± 1.84) (P < 0.01) than PBS group (14.99 ± 0.53), and that in the TsAdSPI group was significantly higher than the HT-TsKaSPI group (15.62 ± 1.41) (P < 0.05), but there was no significant difference among other groups (Fig. 3D(c)).
Effects of two recombinant proteins on IgG subtypes
Mice were sacrificed 7 days after the third immunization, and serum samples of each group were collected to analyze the changes of IgG subtypes: IgG1 and IgG2a. The results were shown in Fig. 4. The expression level of IgG1 in the TsKaSPI group (0.94 ± 0.04) and TsAdSPI group (0.87 ± 0.03) were significantly higher than that in the PBS group (0.05 ± 0.02), HT-TsKaSPI group (0.16 ± 0.05) and FCA/FIA group (0.35 ± 0.05) (P < 0.001), and the TsAdSPI group was significantly higher than the TsKaSPI group (P < 0.01); Meanwhile the expression level of IgG2a in each group was similar to IgG1; however, there was significantly difference between IgG1 and IgG2a in FCA/FIA group and TsAdSPI group (P < 0.05).
Figure 4.
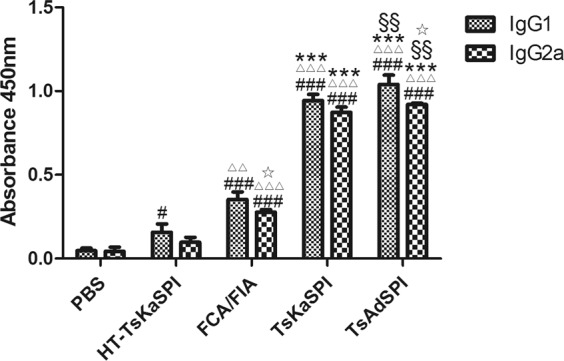
Analyze changes of IgG subtypes: IgG1 and IgG2a. Data are shown as mean ± SD of 3 mice per group. #P < 0.05, ##P < 0.01, ###P < 0.001 versus PBS group; △P < 0.05, △△P < 0.01, △△△P < 0.001 versus HT-TsKaSPI group; *P < 0.05, **P < 0.01, ***P < 0.001 versus FCA/FIA group; §P < 0.05, §§P < 0.01, §§§P < 0.001 for TsKaSPI vs TsAdSPI; ☆P < 0.05, ☆☆P < 0.01, ☆☆☆P < 0.001 for IgG1 vs IgG2a in the same group.
Changes in the expression of cytokine
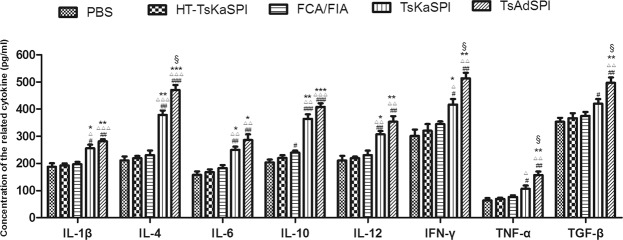
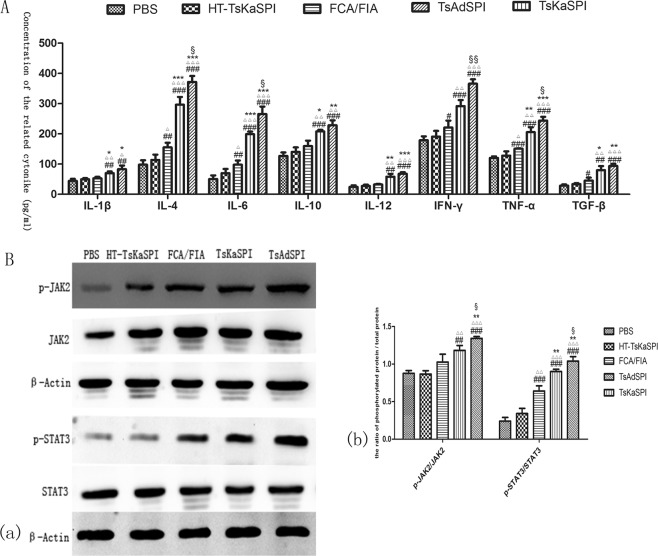
The serum of each group was collected and the expression of IL-1β, IL-4, IL-6, IL-10, IL-12, IFN-γ, TNF-α and TGF-β were detected by ELISA. The expression levels of the above cytokines in the TsKaSPI group and TsAdSPI group were significantly higher than those in the PBS group, HT-TsKaSPI group and FCA/FIA group, however, the changes of IL-4, IL-6, IL-10 and IL-12 were significantly higher than IL-1β, IL-4, IL-6, IL-10, IL-12, IFN-γ, TNF-α and TGF-β. And the expression levels of IL-4, IFN-γ, TNF-α, and TGF-β in the TsAdSPI group were significantly higher than those in the TsKaSPI group. But there was no significant difference in the expression of IL-1β, IL-6, IL-10 and IL-12 between the TsKaSPI group and TsAdSPI group (P > 0.05) (Fig. 5).
Figure 5.
The expression changes of IL-1β, IL-4, IL-6, IL-10, IL-12, IFN-γ, TNF-α and TGF-β were detected by ELISA. Data are shown as mean ± SD of 3 mice per group. #P < 0.05, ##P < 0.01, ###P < 0.001 versus PBS group; △P < 0.05, △△P < 0.01, △△△P < 0.001 versus HT-TsKaSPI group; *P < 0.05, **P < 0.01, ***P < 0.001 versus FCA/FIA group; §P < 0.05, §§P < 0.01, §§§P < 0.001 for TsKaSPI vs TsAdSPI.
Adult reduction rate
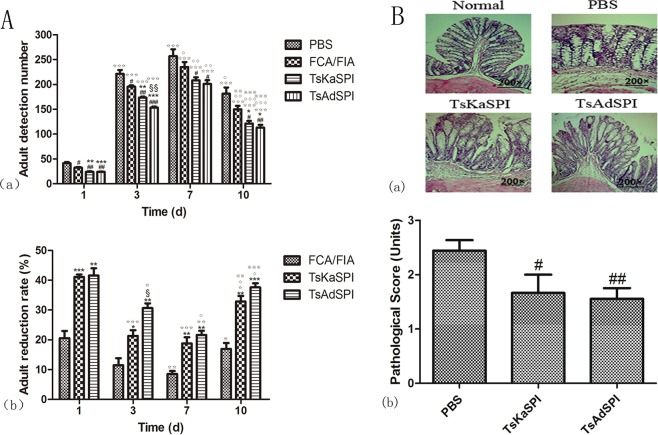
Mice in each group were infected with 500 T. spiralis orally 7 days after the third immunization, and adults were counted on the 1st, 3rd, 7th, and 10th day after infection, and the adult reduction rate was calculated. On the 1st, 3rd, 7th, and 10th day after infection, the number of adults detected in the TsKaSPI group and TsAdSPI group was significantly higher than that in the PBS group and FCA/FIA group, and there was a significant difference between the TsKaSPI group and TsAdSPI group only on the 3rd day (P < 0.01), and there was also a significant difference in the number of adults detected in the same group on different days (Fig. 6A(a)).
Figure 6.
The number of adults detected and the adult reduction rate were showed in (A). Data are shown as mean ± SD of 3 mice per group. #P < 0.05, ##P < 0.01, ###P < 0.001 versus PBS group; *P < 0.05, **P < 0.01, ***P < 0.001 versus FCA/FIA group; §P < 0.05, §§P < 0.0, §§§P < 0.001 for TsKaSPI vs TsAdSPI; ☆P < 0.05, ☆☆P < 0.01, ☆☆☆P < 0.001 for each group on the 3rd, 7th, 10th day vs the corresponding group on the 1st day; ◇P < 0.05, ◇◇P < 0.01, ◇◇◇P < 0.001 for each group on the 7th, 10th day vs the corresponding group on the 3rd day; ※P < 0.05, ※※P < 0.01, ※※※P < 0.001 for each group on the 10th day vs the corresponding group on the 7th day. Light micrograph of HE-stained colonic section were showed in (B) (a). Scale bar represents 200 μm. And the pathological score were showed in (B) (b). TsKaSPI group and TsAdSPI group showed significant improvement than the PBS group.
The adult reduction rate was calculated according to the formula. The results showed that the adult reduction rate of the TsKaSPI group and the TsAdSPI group was significantly higher than that of the FCA/FIA group on the 1st, 3rd, 7th, and 10th day, and there was no significant difference between the TsKaSPI group and TsAdSPI group. At the same time, the adult reduction rate of the same group on different days was also different (Fig. 6A(b)).
Effect of recombinant protein on intestinal pathological changes
After 5 days of infection, the mice were sacrificed and the intestine was collected for pathological sections, H.E staining was performed. The intestinal pathological changes of mice in each group were observed under the microscope. As shown in the Fig. 6B(a), intestinal wall edema, intestinal epithelial villus rupture, more bleeding spots, inflammatory cell infiltration in the mucosa and submucosa, and uneven arrangement of glands were observed in the PBS group. And the TsKaSPI group and TsAdSPI group showed significant improvement, the mild intestinal wall edema, less bleeding points and cell infiltration were observed. The pathological score which showed in Fig. 6B(b) were used to explain the pathological changes in term of quantitation. From the figure, we observed that in the PBS group (2.44 ± 0.20) was significantly higher than the TsKaSPI group (1.67 ± 0.34) (P < 0.05) and TsAdSPI group (1.56 ± 0.20) (P < 0.01).
The expression level of cytokines in cell supernatant
ELISA was used to detect the expression of IL-1β, IL-4, IL-6, IL-10, IL-12, IFN-γ, TNF-α and TGF-β in mouse peritoneal macrophages. The expression levels of the above cytokines in the TsKaSPI group and TsAdSPI group were significantly higher than those in the PBS group, HT-TsKaSPI group and FCA/FIA group. Meanwhile compared the TsKaSPI group with the TsAdSPI group, only the concentration of IL-6, IFN-γ, TNF-α were significantly different (P < 0.05) (Fig. 7A).
Figure 7.
The expression changes of IL-1β, IL-4, IL-6, IL-10, IL-12, IFN-γ, TNF-α and TGF-β in the cell supernatant were showed in (A), and the representative gel of the Western Blot were shown in (B) (a), and the graph of the quantified band density were also shown in (B) (b). Data are shown as mean ± SD of 3 mice per group. #P < 0.05, ##P < 0.01, ###P < 0.001 versus PBS group; △P < 0.05, △△P < 0.01, △△△P < 0.001 versus HT-TsKaSPI group; *P < 0.05, **P < 0.01, ***P < 0.001 versus FCA/FIA group; §P < 0.05, §§P < 0.01, §§§P < 0.001 for TsKaSPI vs TsAdSPI.
The effects of two recombinant proteins on JAK2/STAT3 signaling pathway
Total protein from the five groups were extracted and used Western blot to analyze the phosphorylation of JAK2/STAT3. Compared the TsKaSPI (1.18 ± 0.07) and TsAdSPI group (1.34 ± 0.03) with the PBS (0.88 ± 0.04) and HT-TsKaSPI group (0.87 ± 0.05), the results showed that the ratio of p-JAK2 to JAK2 were significantly higher in the front two groups, and the TsAdSPI group was significantly different from the FCA/FIA group (1.03 ± 0.11) (P < 0.01). Meanwhile the phosphorylation of STAT3 were significantly higher in the TsKaSPI (0.90 ± 0.03) and TsAdSPI group (1.04 ± 0.06) than that in the PBS (0.24 ± 0.05) (P < 0.001), HT-TsKaSPI (0.34 ± 0.07) (P < 0.001) and FCA/FIA group (0.64 ± 0.07) (P < 0.01). At the same time, the TsKaSPI group was significantly different from the TsAdSPI group (P < 0.05). The results showed that both TsKaSPI and TsAdSPI could induce the phosphorylation of JAK2/STAT3 (Fig. 7B) and the complete protein band of p-JAK2, JAK2, p-STAT3, STAT3 were shown in supplemental file.
Discussion
Some scholars have confirmed that T. spiralis infection will produce an immune response characterized by Th1 and Th2 type16. Since IgG1 and IgG2a represent Th2 type and Th1 type immune responses, respectively17, therefore, we measured the changes of the expression levels of IgG1 and IgG2a in the serum of mice immunized with TsKaSPI and TsAdSPI. The results showed that intraperitoneal injection TsKaSPI and TsAdSPI could increased the concentration of IgG1 and IgG2a in serum of mice, and IgG1 dominated, so we comfirmed that both TsKaSPI and TsAdSPI could induce a mixed Th1/Th2 immune response18 with Th2 dominated, it is consistent with expected. Spleen cells proliferation experiments showed that both TsKaSPI and TsAdSPI could induce a strong cellular immune response in the host, so that the body could quickly induce humoral immune and cellular immune response, accelerating the discharge of worms and killing them.
Cytokine is a kind of regulatory protein that regulate immune response19. Bojalil et al.20 showed that IFN-γ acted as a pro-inflammatory cytokine involved in killing T. spiralis newbron larvae; Patel et al.21 demonstrated that IL-4 played an important role in host resistance to T.s infection; Beiting et al.22 showed that IL-10 could inhibit local inflammation in the early phase of T. spiralis infection; And the elevated levels of pro-inflammatory cytokines in serum of rats which infected with T. spiralis were confirmed by Farid23. In this experiment, we measured the expression levels of IL-1β, IL-4, IL-6, IL-10, IL-12, IFN-γ, TNF-α and TGF-β in the serum of mice immunized with TsKaSPI and TsAdSPI, and it was found that the expression of the above cytokines were increased, but with different degrees. This indicated that cytokines have a vital role in regulating the immune response to T. spiralis invasion the hosts, and the mixed internal environment formed by pro-inflammatory and anti-inflammatory factors was conducive to protect host from inflammatory damage and inhibited T. spiralis parasitization.
T lymphocytes are a major group of immunocompetent cells, which are divided into CD4+ (TH) and CD8+ (TS/TC) cell subsets according to their functions24. The subsets are interrelated, mutually promoting and mutually restricting. Ahn et al.25 found that the activated CD4+CD25+Foxp3+ Tregs in mice which infected with T. spiralis was higher than the control group. Ivanoska et al.26 found that T. spiralis could inhibit the pig’s immune function through CD8+ T cells and the inhibitors they secreted, thus evading the attack of host’s immune system. The results of our experiment showed that immunization with TsKaSPI and TsAdSPI could increased the CD4+ T cells and CD4+CD25+Foxp3+ Tregs significantly. Similarly, the CD8+CD28− T cells was also significantly higher. Therefore, it was speculated that the increased number of CD4+ T cells, expression of Foxp3 and number of CD8+CD28− T cells played a critical role in regulating host immune response. Barriga et al.27 found that the excretory secretion antigen of T. spiralis could cause an increase of IL-2, which leaded to an increase of CD8+ cells, resulting in the ratio of CD 4+/CD8+ decreased and inhibited the immune system of host. Our results showed that the CD4+/CD8+ ratio decreased by TsKaSPI and TsAdSPI, but compared with the PBS group, the difference was not significantly. Therefore, the decreased CD4+/CD8+ ratio had little effect on the immunomodulation during T. spiralis infection.
Bregs are a group of important immune cells that exert negative immunoregulatory function in vivo. Studies have confirmed that Leishmania major and Schistosoma infection could induce Breg cell production. Breg can exert its immunosuppressive function independent of the release of cytokines28. CD19+CD5+CD1dhi Breg is currently the most reported type in mouse. However, there are still few reports on whether T. spiralis infection can also cause changes of the number of Bregs. Therefore, this experiment analyzes the change of the number of Bregs in the spleen by FCM. The results showed that both TsKaSPI and TsAdSPI significantly increased the CD19+ B cells and CD19+CD5+CD1dhi Breg cells. It has been proved that Breg has a regulatory function on host immune imbalance, but the specific mechanism remains to be further studied.
T. spiralis parasitise the intestine in the early phase of infection. Adults feed on the intestinal villi. The larvae penetrate the intestinal villus, and after undergoing four molts, become adult worms29. Finally, intestinal tissue would damage and necrosis, hemorrhage and edema, then form ulcers. Calculated the adult reduction rate and compared intestinal pathological changes, the results indicated that TsKaSPI and TsAdSPI might resisted T. spiralis invasion and alleviated the pathological damage by regulating the intestinal mucosal immune system.
It is generally believed that the excretory secretion of parasites directly regulates antigen-presenting cells (APC) including macrophages30. This study aimed to analyze the effects of TsKaSPI and TsAdSPI on the activity of macrophages in vivo. Our study found that both TsKaSPI and TsAdSPI could significantly increase the expression and secretion of IL-1β, IL-4, IL-6, IL-10, IL-12, IFN-γ, TNF-α and TGF-β of mouse macrophages. It indicated that TsKaSPI and TsAdSPI possibly affect the balance between host inflammatory factors by regulating macrophages express and secret cytokines, so as to regulate the host immune response and create an environment conducive to the survival and development of T. spiralis in host.
JAK2/STAT3 signaling pathway plays a key role in immune regulation31. Some researchers used gene knockout technology to knock out the STAT3 gene in mice, and found that the pro-inflammatory cytokines secreted by macrophages in mice were significantly increased, suggesting that this signaling pathway has a critical role in inducing the transformation of macrophages to alternative activated phenotype32. In the process of parasite infection, the JAK2/STAT3 signaling pathway also has a vital role. Trichinella pseudospiralis serine protease inhibitors can activate phosphorylation of JAK2/STAT3 in host, and induce macrophage to an alternative activation phenotype to regulates the dynamic balance between pro-inflammatory and anti-inflammatory cytokines33. In this study, Western blot was used to detected the effects of TsKaSPI and TsAdSPI on JAK2/STAT3 signaling pathway. The results showed that both TsKaSPI and TsAdSPI could induce the phosphorylation of JAK2/STAT3, thereby transmitting intracellular and extracellular signals and regulating the function of immune cells. We preliminarily speculated that JAK2/STAT3 is one of the signaling pathways in the regulation of host immune responses by T. spiralis, which is correlated with the immune escape of T. spiralis to some extent.
In order to rule out the effect of LPS components in the recombinant protein on the experimental results, we boiled the recombinant protein for ten minutes, in which the protein component was inactivated, but LPS was still actived. The experimental results showed that the effect of the HT-TsKaSPI group on the experimental results was slightly. This experiment analyzed the effects of TsKaSPI and TsAdSPI on the immune system from various aspects, provided clues for elucidating the immune escape mechanism of T. spiralis, and also provided a certain experimental basis for the prevention and treatment of Trichinosis. Therefore, it has certain scientific value. However, for the complex and diverse immuneregulation mechanism remains to be further studied.
Methods
Animal and Recombinant protein
Male BALB/c mice (SPF) aged 6–8 weeks were purchased from the Animal Center of Harbin Medical University. The study protocol was approved by Northeast Agriculture University Veterinary Research Ethics Committee. And all procedures were strictly in accordance with the guidelines of the Chinese National Institute of Health Guide for the Care and Use of Laboratory Animals. During the whole experiment, the mice were guaranteed to eat and drink freely, and the bedding materials were changed regularly to ensure the excellent feeding environment. And mice were sacrificed using cervical dislocation to alleviate their pain. T. spiralis Kazal-type serine protease inhibitors (TsKaSPI) and T. spiralis adult serine protease inhibitors (TsAdSPI) recombinant protein were preparation followed the previous method34 and stored at −80 °C (Fig. 8).
Figure 8.
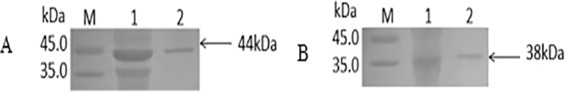
SDS-PAGE analysis of the expression products of before and after purification. M: Protein Marker; 1: Unpurified Protein; 2: Purified Protein.
Experimental grouping
The laboratory carried out the immune dose optimization experiment, mice were immunized with 20 ug, 50 ug, 100 ug recombinant protein respectively by intraperitoneal injection, the results showed that immunized with 50 ug or 100 ug recombinant protein could promote the concentration of specific IgG in the serum of mice, and there was no significant difference between the 50 ug and 100 ug, so this experiment used 50 ug recombinant protein to immunize mice. We also selected the most commonly used Freund’s adjuvant.
In this experiment, 15 mice were divided into 5 groups: (1) PBS group, immunized mice with 200 ul PBS; (2) HT-TsKaSPI group, the TsKaSPI recombinant protein was diluted to 0.5 ug/ul, then boiled 10 minutes, immunized mice with 100 ul HT-TsKaSPI mixed with 100 ul Freund’s adjuvant; (3) FCA/FIA group, immunized mice with 100 ul PBS and 100 ul Freund’s adjuvant; (4) TsKaSPI group, the TsKaSPI recombinant protein was diluted to 0.5 ug/ul, and immunized mice with 100 ul TsKaSPI mixed with 100 ul Freund’s adjuvant. (5) TsAdSPI group, the TsAdSPI recombinant protein was diluted to 0.5 ug/ul, and immunized mice with 100 ul TsAdSPI mixed with 100 ul Freund’s adjuvant. The mice were immunized three times, each interval of 2 weeks, the first immunization used Freund’s complete adjuvant, the second and third used Freund’s incomplete adjuvant, 7 days after the last immunization, the mice were sacrificed for testing.
Detection of spleen cells proliferation by CCK-8 method
Prepared the spleen lymphocyte single-cell suspension and inoculated the cell suspension in 96-well plate (100 ul/well), then the plate was pre-incubated in an incubator for 24 hours (37 °C, 5% CO2). 10 ul 0.5 ug/ul recombinant protein, ConA and RPMI 1640 were added separately, and stimulated for 48 h in the incubator. Then added 10 ul CCK-8 reaction solution (Beyotime Biotechnology) into each well, and measured the absorbance at 450 nm after incubation for 4 hours in the incubator.
Flow cytometry detection of CD4+CD25+Foxp3+ Treg cells, CD8+CD28− T cells and CD19+CD5+CD1dhi Breg cells
Prepared the spleen lymphocyte single-cell suspension, each sample was divided into three tubes with 1 × 106 cells per tube. One tube were resuspended in PBS and incubated for 30 min in the dark at 4 °C with FITC anti-mouse CD4 and APC anti-mouse CD25 (Sungene Biotech). Added fixation/permeabilization solution (Invitrogen, USA) to resuspend cells, then incubated for 30–60 min, and washed again. PE anti-mouse Foxp3 (Sungene Biotech) was added after resuspension and incubated for 20 min, washed, then resuspended again for FCM34; One tube was resuspended in PBS, added with PE anti-mouse CD8, APC anti-mouse CD28, and incubated for 30 min. Then washed and resuspended for FCM; Other tube was resuspended in PBS, added with FITC anti-mouse CD19, APC anti-mouse CD5 and PE anti-mouse CD1d and then incubated for 30 min. Then washed and resuspended for FCM.
Detection of IgG subtypes by indirect ELISA
The concentration of recombinant protein was diluted to 10 ug/ml with coating solution, added to the ELISA plate at 100 ul/well, overnight at 4 °C. Washed, then added 100 ul diluted serum sample to the above reaction well, incubated at 37 °C for 1 hour, then washed. Added 100 ul diluted IgG 1 and IgG 2a, incubated for 1 hour. After washing, TMB chromogen solution (Alphabiotech) was added, and added stop solution after 10 min, then measured the absorbance at 450 nm.
The expression of Th1 and Th2 cytokines detected by ELISA
The serum of mice in each group was collected and cytokines were detected according to the ELISA kit (Alphabiotech) manual.
Effect of recombinant protein on the adult reduction rate
One week after the third immunization, mice in each group were orally infected with 500 T. spiralis, and the mice were sacrificed on the 1st, 3rd, 7th, and 10th day after infection, opened the small intestine longitudinally, removed the contents, placed in normal saline. Then incubated for 3–4 hours at 37 °C, counted the adults under a microscope, and calculated the adult reduction rate according to the formula. The formula is as follows: Adult worm reduction rate (%) = (1 − average adult detection number in the experimental group/average adult detection number in the PBS group) × 100%.
Effect of recombinant protein on intestinal changes
The intestinal specimens were fixed in 10% paraformaldehyde for several hours and embedded in paraffin sections. Then performed Hematoxylin and eosin (H.E) staining. Histological lesions were assessed using Wallace and Keenan criteria35.
Extraction and purification of peritoneal macrophages
The mice were sacrificed by cervical dislocation, and immersed in 75% alcohol for 3–5 seconds. then placed the mice on dissecting table, and 5 ml pre-cooled PBS was injected into the abdominal cavity along the midline of the abdomen. At the same time, pressed the peritoneal wall for 2–3 minutes. Under sterile conditions, absorbed the liquid in the abdominal cavity to the centrifuge tube. Rinsed the abdominal cavity with the same volume of PBS for 2–4 times until the rinse solution became clear. Centrifuged at 4 °C 250 × g for 10 min, then removed supernatant. The cells concentration adjusted to 5 × 106 cells/ml with RPMI-1640 which containing 10% fetal bovine serum, 100 U/ml penicillin, 10 μg/ml streptomycin. And cultured for 3 h in a 37 °C, 5% CO2 incubator. Then washed the cell culture flask 2–3 times with RPMI-1640 medium pre-warmed at 37 °C to removed unattached cells, thus obtaining purified peritoneal macrophage.
Secretion of cytokines in macrophages detected by ELISA
Cultured the purified peritoneal macrophages for 24 hours. The cell culture supernatant was collected, centrifuged at 2000–3000 r/min for 20 min, and collected the supernatant, then detected the expression of cytokines according to the ELISA kit (Alphabiotech) manual.
Western blot analysis of the phosphorylation of JAK2/STAT3 pathway in macrophages
Added lysate to fully lyse tissue samples, then centrifuged to obtain the supernatant. Used SDS-PAGE to separated the total protein and blotted the target protein onto a NC membrane. The membrane was blocked for 2 h. Subsequently, incubated with anti-p-JAK2, anti-JAK2, anti-p-STAT3, anti-STAT3 and anti-β-actin (1:4,000 dilution, Bioss) at 4 °C overnight. After washing, incubated with a peroxidase-conjugated secondary antibody (1:5,000 dilution, Bioss) for 1 h. Washed again, then dropped ultrasensitive ECL chemiluminescence reagent (Sangon Biotech) and exposed. Image J software were used to analyze the bands.
Statistical analysis
All results were expressed as the mean ± standard error. All statistical analysis were performed using GraphPad Prism software and SPSS 13.0 software. P < 0.05 was considered statistically significant.
Supplementary information
Acknowledgements
We would like to thank all of the staff members who provided laboratory assistance. This work was supported by the National Natural Science Foundation of China (31372427), Heilongjiang Province Natural Science Foundation of China (C2016030) and the National Key Research and Development Program of China (2017YFD0501200).
Author contributions
Jingyun Xu was the first author to designed the experiment, conducted most of the experiment, analyzed the data and written the article, Pengcheng Yu, Lijia Wu, Mingxu Liu and Yixin Lu helped throughout the experiment.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-52624-5.
References
- 1.Cui J, et al. Survey of Trichinella infections in domestic pigs from northern and eastern Henan, China. Vet Parasitol. 2013;194:133–135. doi: 10.1016/j.vetpar.2013.01.038. [DOI] [PubMed] [Google Scholar]
- 2.Zocevic A, et al. Identification of Trichinella spiralis early antigens at the pre-adult and adult stages. Parasitology. 2011;138:463–471. doi: 10.1017/S0031182010001526. [DOI] [PubMed] [Google Scholar]
- 3.Ding J, et al. Immune Cell Responses and Cytokine Profile in Intestines of Mice Infected with Trichinella spiralis. Front Microbiol. 2017;8:2069. doi: 10.3389/fmicb.2017.02069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J, et al. Molecular characterization of Trichinella spiralis galectin and its participation in larval invasion of host’s intestinal epithelial cells. Vet Res. 2018;49:79. doi: 10.1186/s13567-018-0573-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song YY, et al. Characterization of a serine protease inhibitor from Trichinella spiralis and its participation in larval invasion of host’s intestinal epithelial cells. Parasit Vectors. 2018;11:499. doi: 10.1186/s13071-018-3074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molehin AJ, Gobert GN, McManus DP. Serine protease inhibitors of parasitic helminths. Parasitology. 2012;139:681–695. doi: 10.1017/S0031182011002435. [DOI] [PubMed] [Google Scholar]
- 7.Gettins PG. Serpin structure, mechanism, and function. Chem Rev. 2002;102:4751–4804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- 8.Maizels RM, et al. Immune evasion genes from filarial nematodes. Int J Parasitol. 2001;31:889–898. doi: 10.1016/S0020-7519(01)00213-2. [DOI] [PubMed] [Google Scholar]
- 9.Cao JX, et al. A male reproduction-related Kazal-type peptidase inhibitor gene in the prawn, Macrobrachium rosenbergii: molecular characterization and expression patterns. Mar Biotechnol. 2007;9:45–55. doi: 10.1007/s10126-006-6026-4. [DOI] [PubMed] [Google Scholar]
- 10.Zang. X, Maizels RM. Serine proteinase inhibitors from nematodes and the arms race between host and pathogen. Trends Biochem Sci. 2001;26:191–197. doi: 10.1016/S0968-0004(00)01761-8. [DOI] [PubMed] [Google Scholar]
- 11.Chandrashekar. R, Ogunrinade AF, Weil GJ. Use of recombinant Onchocerca volvulus antigens for diagnosis and surveillance of human onchocerciasis. Trop Med Int Health. 1996;1:575–580. doi: 10.1111/j.1365-3156.1996.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, et al. Expression and immunological correlation of Kazal-type serine protease inhibitor of Trichinella spiralis. Chinese. Journal of Veterinary Science. 2015;35:1284–1289. [Google Scholar]
- 13.Zhang ZX, et al. High-level expression and characterization of two serine protease inhibitors from Trichinella spiralis. Vet Parasitol. 2016;219:34–39. doi: 10.1016/j.vetpar.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Yepez-Mulia L, Ortega-Pierres MG. Current aspects of trichinosis diagnosis. Rev Latinoam Microbiol. 1994;36:127–138. [PubMed] [Google Scholar]
- 15.Van Loveren H, et al. Detection of IgA Antibodies and Quantification of IgA Antibody-Producing Cells Specific for Ovalbumin or Trichinella spiralis in the Rat. Scandinavian Journal of Immunology. 1988;28:377–381. doi: 10.1111/j.1365-3083.1988.tb01463.x. [DOI] [PubMed] [Google Scholar]
- 16.Else KJ. Have gastrointestinal nematode outwitted the immune system? Parasite Immunol. 2005;27:407–415. doi: 10.1111/j.1365-3024.2005.00788.x. [DOI] [PubMed] [Google Scholar]
- 17.Crawley A, Raymond C, Wilkie BN. Control of immunoglobulin isotype production by porcine B-cells cultured with cytokines. Vet Immunol Immunopathol. 2003;91:141–154. doi: 10.1016/S0165-2427(02)00293-3. [DOI] [PubMed] [Google Scholar]
- 18.Ilic N, et al. Trichinella spiralis antigens prime mixed Th1/Th2 response but do not induce de novo generation of Foxp3+ T cells in vitro. Parasite Immunol. 2011;33:572–582. doi: 10.1111/j.1365-3024.2011.01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puech C, et al. Design and evaluation of a unique SYBR Green real-time RT-PCR assay for quantification of five major cytokines in cattle, sheep and goats. BMC Vet Res. 2015;17:65. doi: 10.1186/s12917-015-0382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bojalil R, et al. Thymus-related cellular immune mechanisms in sex-associated resistance to experimental murine cysticercosis (Taenia crassiceps) J Parasitol. 1993;79:384–389. doi: 10.2307/3283574. [DOI] [PubMed] [Google Scholar]
- 21.Patel N, et al. Characterisation of effector mechanisms at the host: parasite interface during the immune response to tissue-dwelling intestinal nematode parasites. Int J Parasitol. 2009;39:13–21. doi: 10.1016/j.ijpara.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beiting DP, et al. Interleukin-10 Limits Local and Body Cavity Inflammation during Infection with Muscle-Stage Trichinella spiralis. Infect and Immun. 2004;72:3129–3137. doi: 10.1128/IAI.72.6.3129-3137.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farid AS, et al. Paraoxonase-1 activity is related to Trichinella spiralis-induced hepatitis in rats. Eur J Clin Invest. 2017;47:250–261. doi: 10.1111/eci.12731. [DOI] [PubMed] [Google Scholar]
- 24.Janeway CA., Jr. The T cell receptor as a multicomponent signaling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immuno. 1990;10:645–74. doi: 10.1146/annurev.iy.10.040192.003241. [DOI] [PubMed] [Google Scholar]
- 25.Ahn JB, et al. Activation and Recruitment of Regulatory T Cells via Chemokine Receptor Activation in Trichinella spiralis-Infected Mice. Korean J Parasitol. 2016;54:163–171. doi: 10.3347/kjp.2016.54.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivanoska D, Cuperlovic K, Lunney JK. Peripheral blood mononuclear cell subsets during Trichinella spiralis infection in pigs. Res Vet Sci. 1990;49:92–97. doi: 10.1016/S0034-5288(18)31054-3. [DOI] [PubMed] [Google Scholar]
- 27.Barriga OO. Responses of B-cell to mitogens and antigen in mice receiving isogenic splenocytes from animals treated with Trichinella extracts. J Parasitol. 1980;66:730–734. doi: 10.2307/3280661. [DOI] [PubMed] [Google Scholar]
- 28.Lu FT, et al. Thymic B cells promote thymus-derived regulatory T cell development and proliferation. J Autoimmun. 2015;61:62–72. doi: 10.1016/j.jaut.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Liu RD, et al. Analysis of differentially expressed genes of Trichinella spiralis larvae activated by bile and cultured with intestinal epithelial cells using real-time PCR. Parasitol Res. 2013;112:4113–4120. doi: 10.1007/s00436-013-3602-1. [DOI] [PubMed] [Google Scholar]
- 30.Goodridge HS, et al. Modulation of Macrophage Cytokine Production by ES-62, a Secreted Product of the Filarial Nematode Acanthocheilonema viteae. J Immuno. 2001;167:940–945. doi: 10.4049/jimmunol.167.2.940. [DOI] [PubMed] [Google Scholar]
- 31.Yoshikawa T, et al. JAK2/STAT3 pathway as a therapeutic target in ovarian cancers. Oncol Lett. 2018;15:5772–5780. doi: 10.3892/ol.2018.8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mills CD, et al. M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J Immuno. 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [Google Scholar]
- 33.Song YY, et al. The immune protection induced by a serine protease inhibitor from Trichinella spiralis. Front Microbiol. 2018;9:1544. doi: 10.3389/fmicb.2018.01544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, et al. Effect of two recombinant Trichinella spiralis serine protease inhibitors on TNBS-induced experimental colitis of mice. Clin Exp Immunol. 2018;194:400–413. doi: 10.1111/cei.13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace JL, et al. An orally active inhibitor of leukotriene synthesis accelerates healing in a rat model of colitis. Am J Physiol. 1990;258(4 Pt 1):G527–534. doi: 10.1152/ajpgi.1990.258.4.G527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.