Abstract
Chikungunya is a mosquito-borne viral illness associated with chronic arthritic symptoms that persist for months. The IgM antibody appears within a week post any infection and declines at 2–3 months. The present study was aimed to demonstrate the presence of specific IgM antibody among chikungunya confirmed cases. Blood samples were collected from chikungunya PCR positive patients at the time of diagnosis, at 1-week, 1, 8, 10 and 12 months post infection. All acute and follow-up serum samples were evaluated for chikungunya virus-specific IgM antibodies using ELISA technique. Our findings indicate the persistence of anti-chikungunya IgM up to 10-months post-infection in a majority of chikungunya virus infected persons. Interpretation of results should be carefully done as only IgM ELISA is used to diagnose acute infection, especially post chikungunya outbreak. The presence of IgM antibody does not rule out the absence of any other diagnosis due to its persistence. Thus, we hypothesize that real-time PCR is more reliable for the detection of acute chikungunya cases in endemic areas while IgM detection may be useful in identifying exposure to this disease.
Keywords: Chikungunya, Follow-up, Persisting antibodies, Neutralising antibodies
Chikungunya virus (CHIKV) disease is an arthropod-borne alphavirus infection transmitted by the bite of an infected mosquito (Aedes aegypti or Aedes albopictus) [2]. The virus causes an acute febrile illness with appreciable body ache and arthralgia [13]. The disease resolves in 7 days, however, chronic arthralgia and arthritis can persist for months post-chikungunya infection [7] and it can be associated with the persisting virus and a high level of viremia resulting in chronic infection [6]. The IgM being the first antibody to appear in the blood marks the acute infection. It is produced within 3–8 days and declines within 2–3 months. IgM antibody persistence was long reported for viruses like West Nile virus and dengue virus [3, 8, 12]. There are on-going speculations regarding the persistence of antibodies in arboviruses, since, the vector for transmission are similar. Few studies in the past showed the presence of IgM against chikungunya virus for an extended period [1, 5, 9–11]. Here a prospective study was designed to check for persisting CHIKV-specific IgM antibodies in chikungunya positive patient’s serum sample up to a year.
Study participants were identified through hospital-based surveillance for Acute Febrile Illness in India conducted at Manipal Centre for Virus Research (MCVR), NVBDCP Apex Referral Laboratory for arboviral diseases. All chikungunya positive patients enrolled within a period of 1 year, i.e., July 2016 to July 2017 were selected. A total of 69 participants with known chikungunya PCR positive cases from Krishnagiri district, Tamil Nadu, India were registered and a follow-up blood collection was conducted to check for anti-chikungunya IgM persistence at 1, 8, 10 and 12 months post-acute infection. Informed consent was obtained from individual participants. We were able to obtain all samples for 21 patients, which became the final study population. All acute and follow-up serum samples were tested for anti-chikungunya virus-specific IgM antibodies using ELISA technique. The protocol NIV CHIK MAC ELISA and chikungunya IgM Standard Diagnostics (SD BIOLINE, Korea) kits at 1:100 dilution of samples were used for presumptive laboratory diagnosis of chikungunya [14]. All anti-chikungunya IgM positive serum samples at 1:100 dilution were sequentially four-fold diluted in phosphate buffered saline (PBS) (from 1:100 to 1:400, 1:1600, 1:6400, and 1:25,600) and an ELISA was performed using NIV MAC ELISA kit protocol.
Vero cells (African green monkey kidney cells, Cercopithecus aethiops) obtained from American Type Culture Collection (ATCC) was used to prepare virus stock and conduct neutralisation experiments. Minimal essential medium (MEM) with l-glutamine (Gibco™, Thermo Fisher Scientific, USA) was used to culture the cells. Chikungunya virus strain kindly provided by the National Institute of Mental Health and Neurosciences (NIMHANS, Bangalore, India) was used to prepare stock virus to perform CHIKV neutralisation assay. Vero cells were seeded into 96 Well TC-Treated Microplates (Nunc™, Thermo Fisher Scientific, USA) by adding 5000 cells/100 µl in each well. The next day serial dilutions of patient’s serum inactivated at 56 °C were incubated with a known concentration of virus (prepared by diluting 1 µl of virus (100 TCID50 = 1995.26) in 1.9 ml media) at 37 °C for 1 h. This antigen–antibody conjugate was transferred to microplates containing a monolayer of VERO cells to observe the effect.
The statistical analysis was done using IBM SPSS 20.0 software (IBM Corp, 2011, Armonk, NY) and GraphPad Prism 7 (Graph Pad Software, San Diego, CA, USA) was used to design the graphs.
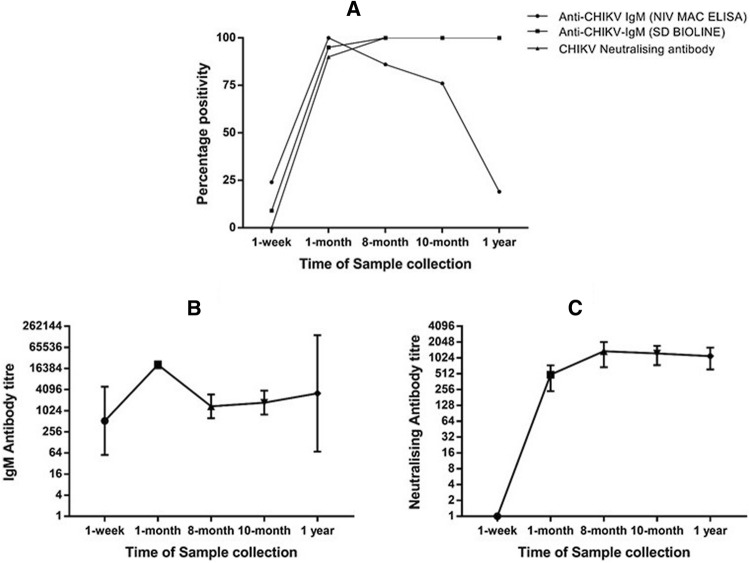
We were able to determine the presence of anti-chikungunya IgM for ten months post infection in the majority of patients (76%). Also, 19% of the cases had detectable IgM at one-year post infection (Fig. 1). The median age of the study population was 26 years (IQR: 12–42.5) at the time of acute infection. Out of the 21 participants, 16 were females (76.2%) and 5 were males (23.8%). Among 21 cases during the acute phase, the majority of them had a fever, headache, chills/rigor, joint pain, malaise, myalgia, retro-orbital pain, and photophobia (Table 1). At follow-up, patients were asked to discuss clinical symptoms throughout the year. While some patients were free of arthralgia within a week, it was observed that 7 (33.3%) out of 21 cases were still complaining about persisting arthralgia up to a year while 1 (4.7%) patient had arthralgia for 4 months. Almost 62% of the patients had chronic arthralgia between the period of 4–12 months. The disease resolved in a few days for males while females had persisting arthralgia and belonged to the age group of 27–50 years. All the 21 cases showed a significant rise in neutralising antibody titer with respect to time of sample collection indicating persistently high antibody response. Neutralising antibody positive samples had a titre between 40 and 5120 units (Fig. 1). Patients had a titre between 40–2560, 160–5120, 80–5120, and 160–5120 units during 1, 8, 10 months and 1 year post-infection (Table 2).
Fig. 1.
Kinetics of antibody response (a) a graph showing variation in percentage of cases with detectable anti-chikungunya IgM by NIV MAC ELISA, Pune and SD BOLINE, Korea and neutralising antibody by CHIKV micro-neutralisation assay; b, c a plot of geometric mean titre (95% confidence interval) of 21 patients at follow up showing anti-chikungunya IgM persistently high neutralising antibody respectively
Table 1.
Demographic details and distribution of clinical symptoms among patients of the study population during the acute phase (n = 21)
| Variables | Study participants n = 21 N(%) |
|---|---|
| Gender | |
| Male | 5 (23.8) |
| Female | 16 (76.2) |
| Clinical features | |
| Fever | 21 (100) |
| Chills/Rigors | 17 (81) |
| Night sweats | 10 (47.6) |
| Coryza | 5 (23.8) |
| Cough | 6 (28.6) |
| Headache | 19 (90.5) |
| Photophobia | 10 (47.6) |
| Retro-orbital pain | 15 (71.4) |
| Myalgia | 17 (81) |
| Joint pain | 18 (85.7) |
| Malaise | 16 (76.2) |
| Prostration | 7 (33.3) |
| Abdominal pain | 8 (38.1) |
| Nausea | 8 (38.1) |
| Vomiting | 8 (38.1) |
| Diarrhea | 8 (38.1) |
| Burning micturition | 6 (28.6) |
| Neck stiffness | 8 (38.1) |
| Median age (IQR) (in years) | 26 (12–42.5) |
Table 2.
Antibody titres of 21 patients at follow-up
| Cases (n = 21) | Signs of chronic arthralgia | Anti-chikungunya IgM antibody titre | Neutralising antibody titre | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-week | 1-month | 8-month | 10-month | 1 year | 1-week | 1-month | 8-month | 10-month | 1 year | ||
| Case 1 | Yes | 0 | > 25,600 | 1600 | 1600 | 400 | 0 | 160 | 160 | 160 | 160 |
| Case 2 | No | 0 | > 25,600 | 400 | 1600 | 0 | 0 | 320 | 320 | 640 | 320 |
| Case 3 | No | 0 | > 25,600 | 6400 | 6400 | 0 | 0 | 320 | 320 | 640 | 1280 |
| Case 4 | No | 0 | 6400 | 0 | 0 | 0 | 0 | 320 | 320 | 1280 | 1280 |
| Case 5 | No | 6400 | > 25,600 | > 25,600 | > 25,600 | > 25,600 | 0 | 160 | 640 | 80 | 160 |
| Case 6 | No | 1600 | > 25,600 | 6400 | 1600 | 0 | 0 | 40 | 1280 | 2560 | 640 |
| Case 7 | Yes | 0 | > 25,600 | 400 | 400 | 0 | 0 | 40 | 320 | 640 | 640 |
| Case 8 | Yes | 0 | > 25,600 | 1600 | 6400 | 400 | 0 | 640 | 2560 | 1280 | 1280 |
| Case 9 | Yes | 0 | > 25,600 | 6400 | 6400 | 0 | 0 | 320 | 320 | 640 | 1280 |
| Case 10 | Yes | 0 | > 25,600 | 400 | 1600 | 0 | 0 | 640 | 2560 | 1280 | 1280 |
| Case 11 | No | 100 | > 25,600 | 100 | 0 | 0 | 0 | 640 | 2560 | 1280 | 1280 |
| Case 12 | No | 0 | > 25,600 | 6400 | 6400 | > 25,600 | 0 | 320 | 160 | 640 | 640 |
| Case 13 | Yes | 0 | 6400 | 100 | 0 | 0 | 0 | 320 | 2560 | 1280 | 320 |
| Case 14 | No | 0 | > 25,600 | 1600 | 400 | 0 | 0 | 1280 | 5120 | 2560 | 2560 |
| Case 15 | No | 0 | 6400 | 400 | 100 | 0 | 0 | 2560 | 640 | 1280 | 320 |
| Case 16 | No | 0 | > 25,600 | 0 | 0 | 0 | 0 | 320 | 5120 | 5120 | 1280 |
| Case 17 | No | 0 | > 25,600 | 0 | 0 | 0 | 0 | 160 | 320 | 320 | 320 |
| Case 18 | Yes | 0 | > 25,600 | 6400 | 6400 | 0 | 0 | 640 | 1280 | 1280 | 5120 |
| Case 19 | No | 400 | > 25,600 | 400 | 400 | 0 | 0 | 640 | 320 | 1280 | 1280 |
| Case 20 | No | 0 | > 25,600 | 1600 | 400 | 0 | 0 | 160 | 640 | 640 | 640 |
| Case 21 | No | 100 | > 25,600 | 1600 | 1600 | 0 | 0 | 320 | 1280 | 1280 | 1280 |
Two different IgM ELISA kits were used to check persisting antibodies in patients. The difference in the percentage of cases with detectable IgM corresponds to variation in sensitivity of the kits. While NIV MAC ELISA kit could differentiate between the presence of persisting antibodies, SD BIOLINE kit showed almost all samples positive for persisting anti-chikungunya IgM (Fig. 1). As a result, existing kits should be tested of their detecting capabilities; this will help in accurate diagnosis of persistent infection post-outbreak scenario. To rule out the sheer possibility of a recurrent CHIKV infection, a PCR was performed on follow-up samples to check the presence of viral RNA. All were negative, which suggest that RNA was not detectable in blood.
Few studies documented showed the presence of IgM against chikungunya virus for a longer period. According to a study in Italy, about 17% of patients with acute CHIKV infection showed the presence of IgM antibodies up to 12–13 months post-infection [11]. In a survey conducted in La Reunion (2005–2006), IgM was still present between 2 and 18 months after the disease onset [5]. A case with a travel history to India was reported in Japan. The patient was positive for chikungunya IgM on day 108 but was negative on day 137, thus, indicating that IgM lasts for 3–4 months [1]. Post-2006 CHIKV outbreak in India, a study conducted in Bavi village of Maharashtra indicated the presence of IgM antibody in infected patients up to 6 months. Also, people with no history of CHIKV illness showed the presence of IgM antibody indicating sub-clinical infections [4]. Considerate levels of IgM and IgG antibody was detected for a longer duration among healthcare workers in Lagos and Osun. Asymptomatic cases went unnoticed [9]. Persistence of IgM as a part of the previous transmission cycle may misinterpret the existence of an acute infection, thus, giving false results. Thus, IgM and its diagnostic utility must be obtained in endemic areas. Reporting of recurrent CHIKV infection based on the presence of IgM by the clinicians should be cautiously done for high-risk patients as the disease progresses to neurological complications. The relation between the persisting virus and antibody response can be further evaluated. The neutralising activity of IgM can be assessed, and a purified immunoglobulin preparation of specific neutralising antibodies can be used to treat pregnant women to prevent the chances of mother to child transmission. Testing for the presence of IgM would help in monitoring and provide supportive treatment to the patient. The disappearance of IgM antibody may indicate clinical cure. It is well known that few viral infections can lead to rheumatoid arthritis. Whether immune response generated is as a result of the persistence of the virus in small joints or it is induced as a result of inflammatory response produced against the virus is still debatable. One cannot state the persistence of virus completely based on the presence of antibodies. Post-viral chronic joint pain raises questions whether underlying causes, age-related chronic pain or cross-reactivity with other illnesses provoke persisting symptoms. Further studies have to be done to understand the course of the immune response against chikungunya virus.
Our study poses some limitations. A sample size of 21 patients might not adequately represent the entire population. We were able to follow-up only 30% of all CHIKV positive cases from the hospital and this low follow-up rates can lead to bias. The cases were not completely evaluated for underlying conditions.
In conclusion, this study showed that up to 10-months after acute chikungunya virus infection, a majority of the chikungunya virus-infected persons had persisting anti-chikungunya IgM in their blood. Also, there was a significant increase in neutralising antibody levels. Additional research is needed to understand the human immune response to chikungunya infection. Further, results should be interpreted carefully when only IgM ELISA is used to diagnose current chikungunya infection, particularly the year following a chikungunya outbreak.
Funding
The study was partially supported by CDC Cooperative Agreement 5U01GH001051-05 and Indian Council of Medical Research Grant File No. (5/8/7/15/2010/ECD-I).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aoyama I, Uno K, Yumisashi T, Takasaki T, Lim C-K, Kurane I, et al. A case of chikungunya fever imported from India to Japan, follow-up of specific IgM and IgG antibodies over a 6-month period. Jpn J Infect Dis. 2010;63(1):65–66. [PubMed] [Google Scholar]
- 2.Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet Lond Engl. 2012;379:662–671. doi: 10.1016/S0140-6736(11)60281-X. [DOI] [PubMed] [Google Scholar]
- 3.Busch MP, Kleinman SH, Tobler LH, Kamel HT, Norris PJ, Walsh I, et al. Virus and antibody dynamics in acute West Nile virus infection. J Infect Dis. 2008;198:984–993. doi: 10.1086/591467. [DOI] [PubMed] [Google Scholar]
- 4.Chopra A, Anuradha V, Ghorpade R, Saluja M. Acute Chikungunya and persistent musculoskeletal pain following the 2006 Indian epidemic: a 2-year prospective rural community study. Epidemiol Infect. 2012;140:842–850. doi: 10.1017/S0950268811001300. [DOI] [PubMed] [Google Scholar]
- 5.Grivard P, Le Roux K, Laurent P, Fianu A, Perrau J, Gigan J, et al. Molecular and serological diagnosis of chikungunya virus infection. Pathol Biol. 2007;55:490–494. doi: 10.1016/j.patbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest. 2010;120:894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markoff L. 153—alphaviruses A2—Bennett, John E. In: Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennetts principles and practice of infectious disease. 8. Philadelphia: Content Repository Only; 2015. pp. 1865–1874.e2. [Google Scholar]
- 8.Murray KO, Garcia MN, Yan C, Gorchakov R. Persistence of detectable immunoglobulin M antibodies up to 8 years after infection with West Nile virus. Am J Trop Med Hyg. 2013;89:996–1000. doi: 10.4269/ajtmh.13-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olajiga OM, Adesoye OE, Emilolorun AP, Adeyemi AJ, Adeyefa EO, Aderibigbe IA, et al. Chikungunya virus seroprevalence and associated factors among hospital attendees in two states of southwest Nigeria: a preliminary assessment. Immunol Invest. 2017;46:552–565. doi: 10.1080/08820139.2017.1319383. [DOI] [PubMed] [Google Scholar]
- 10.Oliver M, Grandadam M, Marimoutou C, Rogier C, Botelho-Nevers E, Tolou H, et al. Persisting Mixed Cryoglobulinemia in Chikungunya Infection. PLoS Negl Trop Dis. 2009;3:e374. doi: 10.1371/journal.pntd.0000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierro A, Rossini G, Gaibani P, Finarelli AC, Moro ML, Landini MP, et al. Persistence of anti–chikungunya virus–specific antibodies in a cohort of patients followed from the acute phase of infection after the 2007 outbreak in Italy. New Microbes New Infect. 2015;7:23–25. doi: 10.1016/j.nmni.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prince HE, Matud JL. Estimation of dengue virus IgM persistence using regression analysis. Clin Vaccine Immunol. 2011;18:2183–2185. doi: 10.1128/CVI.05425-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon F, Javelle E, Cabie A, Bouquillard E, Troisgros O, Gentile G, et al. French guidelines for the management of chikungunya (acute and persistent presentations). November 2014. Méd Mal Infect. 2015;45:243–263. doi: 10.1016/j.medmal.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization, Regional Office for South-East Asia . Guidelines for prevention and control of chikungunya fever. New Delhi: World Health Organization, Regional Office for South-East Asia; 2009. [Google Scholar]