Drug resistance is one of the most serious issues in epilepsy. Despite using various appropriate anti-epileptic drugs (AEDs), 30% of epilepsy patients are still drug-resistant. The percentage of resistance in temporal lobe epilepsy (TLE) is even higher [1]. Although epilepsy surgery and deep brain stimulation are emerging as alternative therapeutic strategies, the curative outcome is still unsatisfactory. So far, the precise mechanisms of drug resistance are still “tales from the mist”, which restricts the discovery of optimal targets and the further application of precise treatment. A recent study by Xu et al. [2] first revealed the critical “gating” role of subicular pyramidal neurons in drug-resistant TLE and unveiled the genesis of drug resistance in TLE at the neuronal level.
At present, two major hypotheses of drug resistance in epilepsy have been put forward, although there is ongoing debate [3]: (1) the “drug-transporter hypothesis”, in which AEDs are removed from epileptogenic tissue through the overexpression of multidrug transporters such as P-glycoprotein and multidrug resistance-associated proteins; and (2) the “target hypothesis”, in which drug-target sensitivity declines in epileptogenic brain regions. In this report, Xu et al. first established a classic phenytoin (PHT)-resistant TLE model in male and female Wistar and Sprague-Dawley rats and found that increasing the concentration of PHT in the brain had no therapeutic effects on PHT-resistant rats, which suggested that the drug-transporter hypothesis does not explain drug resistance.
Thus, the authors focused on the target hypothesis. By in vivo neural recording, they showed for the first time that PHT loses its intrinsic inhibitory effect on the firing rate of subicular pyramidal neurons in PHT-resistant rats. This “off-target” phenomenon is site-specific, and is only evident in the subiculum but not in CA1, CA3, dentate gyrus, or entorhinal cortex. These results suggest that the “off-target” effect of PHT specifically in subicular pyramidal neurons is closely involved in PHT resistance.
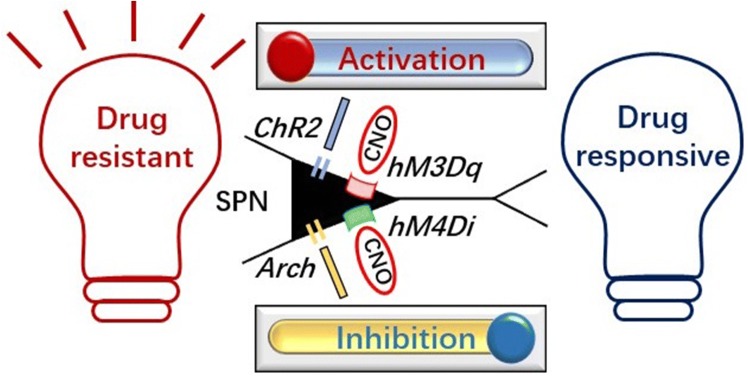
To explore the causality between the “off-target” effect and PHT resistance, they further used optogenetics and chemogenetics to precisely modulate subicular pyramidal neurons. The results showed that the resistance is reversed by selective inhibition of the subicular pyramidal neurons in PHT-resistant rats; while in PHT-responsive rats, the resistance is directly induced by the selective activation of these neurons. More interestingly, in a variety group (randomly PHT-responsive or non-responsive in three PHT screens), rats can be bi-directionally manipulated into becoming PHT-responsive or non-responsive by inhibiting or activating the subicular pyramidal neurons. However, the selective modulation of CA1 pyramidal neurons has no effect on PHT resistance. Therefore, the results provide the first direct in vivo evidence that subicular pyramidal neurons are sufficient and necessary for PHT resistance (Fig. 1), which provides a potential therapeutic target for drug-resistant epilepsy. Certainly, patients with drug-resistant TLE often exhibit a tendency for multidrug resistance. However, only PHT was tested in this report, so it would be valuable to further determine whether other AEDs similarly lack an inhibitory effect on subicular pyramidal neurons.
Fig. 1.
Cartoon of bi-directional manipulation of rats into a PHT-responsive or resistant state by inhibiting or activating subicular pyramidal neurons (SPN). ChR2: channelrhodopsin-2; Acrh: archaerhodopsin; hM3Dq: Gq-coupled human M3 muscarinic receptor; hM4Di: Gi-coupled human M4 muscarinic receptor; CNO: clozapine-N-oxide.
Since the clinical translation of optogenetic manipulation is limited by the invasion of the virus, the authors further tested the translational potential of deep brain stimulation, which is a clinically feasible strategy for epilepsy. In a previous study, it has been reported that low-frequency stimulation (LFS) at the subiculum alleviates epileptogenesis with a wide therapeutic time window in kindling and pilocarpine models [4]. To further test its effect on drug-resistant TLE, the authors delivered LFS to the subiculum in PHT-resistant male and female rats of two strains. They found that long-term LFS of the subiculum directly attenuates the seizure severity in PHT-resistant TLE rats and reverses the resistance through inhibiting subicular pyramidal neurons. This is the first report that long-term LFS at the subiculum not only alleviates seizures but also reverses drug resistance. The importance of these findings lies in providing valuable pre-clinical evidence that can easily be translated to humans. Given that clinical trials of LFS on epileptic patients are proceeding in several epilepsy centers [5], it will be interesting to further study the related translational medicine issues, for example, the efficacy of LFS at the subiculum in treatment of drug resistant epilepsy in patients.
Finally, the authors further explored the mechanism underlying the “off-target” effect of PHT on subicular pyramidal neurons using in vitro electrophysiology. They found that PHT fails to reduce the amplitude of the maximum activated Na+ current in subicular pyramidal neurons in hippocampal slices from PHT-resistant rats, while the reduction is significant in slices from the responsive and variety groups. Interestingly, tetrodotoxin (TTX), which blocks Na+ channel activity extracellularly, blocks the Na+ currents in subicular pyramidal neurons from PHT-resistant rats and intra-subicular injection of TTX reverses the PHT resistance. Despite the fact that the exact mechanism is still unknown, the authors speculate that the reason PHT fails to block Na+ channels might be the intracellular manner of PHT blockade. Furthermore, it is still unclear whether the “off-target” effect of PHT in drug-resistant rats is inborn or modified by disease. Nevertheless, their clinical data provided by high-resolution MRI imaging in a limited number of TLE patients provide a hint that the pathological changes in the subiculum are associated with the use of Na+ channel inhibitors. However, more clinical evidence is needed. Overall, these results suggest that the underlying mechanism of PHT resistance involves Na+ channels in subicular pyramidal neurons. As there are various subtypes of Na+ channel in the brain [6, 7], the characteristics of those in subicular pyramidal neurons, such as the subtypes, protein expression, gene mutations, and functional alterations, are worthy of future study.
The subiculum, which receives neuronal input from the hippocampus and projects to other cortical and subcortical regions, such as the entorhinal cortex, perirhinal cortex, and amygdala, is known to be crucially involved in epileptogenesis and its spread from the hippocampus to other regions through its projecting pyramidal neurons. Accumulating evidence has shown that the subiculum exhibits hyperexcitability and excessive synchrony in both animal models and patients with TLE [8]. Specifically, 1-Hz interictal spikes specifically originate from the subiculum in TLE patients [9]. Their previous report also showed that depolarized GABAergic signaling in subicular microcircuits mediates generalized seizures in TLE [10]. Further, this report extends previous findings and highlights that the subiculum may be a crucial structure closely associated with the genesis of drug resistance. Hence, the subiculum can be considered a potential target for drug-resistant TLE. It will be interesting and valuable to further investigate the important characteristics of the subiculum in other drug-resistant models. Also, the possible downstream circuit of the subiculum involved in drug resistance deserves future study. Furthermore, clinical treatment with deep brain stimulation of the subiculum in epileptic patients can be explored.
Overall, the work in the highlighted study supplements the “target hypothesis” and improves understanding of the mechanism underlying drug resistance in TLE. Subicular pyramidal neurons may be a vital switch for the genesis of drug resistance in TLE and a potential effective target, which could be precisely regulated to control drug-resistant TLE.
References
- 1.Wang Y, Chen Z. An update for epilepsy research and antiepileptic drug development: toward precise circuit therapy. Pharmacol Ther. 2019;201:77–93. doi: 10.1016/j.pharmthera.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Xu C, Wang Y, Zhang S, Nao J, Liu Y, Wang Y, et al. Subicular pyramidal neurons gate drug resistance in temporal lobe epilepsy. Ann Neurol. 2019;86:626–640. doi: 10.1002/ana.25554. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt D, Loscher W. Drug resistance in epilepsy: putative neurobiologic and clinical mechanisms. Epilepsia. 2005;46:858–877. doi: 10.1111/j.1528-1167.2005.54904.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhong K, Wu DC, Jin MM, Xu ZH, Wang Y, Hou WW, et al. Wide therapeutic time-window of low-frequency stimulation at the subiculum for temporal lobe epilepsy treatment in rats. Neurobiol Dis. 2012;48:20–26. doi: 10.1016/j.nbd.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Koubeissi MZ, Kahriman E, Syed TU, Miller J, Durand DM. Low-frequency electrical stimulation of a fiber tract in temporal lobe epilepsy. Ann Neurol. 2013;74:223–231. doi: 10.1002/ana.23915. [DOI] [PubMed] [Google Scholar]
- 6.Wei F, Yan LM, Su T, He N, Lin ZJ, Wang J, et al. Ion channel genes and epilepsy: functional alteration, pathogenic potential, and mechanism of epilepsy. Neurosci Bull. 2017;33:455–477. doi: 10.1007/s12264-017-0134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S, Zhang Z, Shen Y, Zhu Y, Du K, Guo J, et al. SCN9A epileptic encephalopathy mutations display a gain-of-function phenotype and distinct sensitivity to oxcarbazepine. Neurosci Bull. 2019 doi: 10.1007/s12264-019-00413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stafstrom CE. The role of the subiculum in epilepsy and epileptogenesis. Epilepsy Curr. 2005;5:121–129. doi: 10.1111/j.1535-7511.2005.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Xu C, Xu Z, Ji C, Liang J, Wang Y, et al. Depolarized GABAergic signaling in subicular microcircuits mediates generalized seizure in temporal lobe epilepsy. Neuron. 2017;95:1221. doi: 10.1016/j.neuron.2017.08.013. [DOI] [PubMed] [Google Scholar]