Abstract
Ag/Cu2O/ZnO nanoparticles (NPs) were synthesized in situ on wool yarns. The wool yarns were subsequently dyed with the roots and stems of berberis vulgaris. The antibacterial, antioxidant, and dyeing properties of the treated wool yarns were studied. Scanning electron microscopy (SEM), differential scanning calorimeters (DSC), and weight gain (%) analyses were employed for the characterization of the treated samples. The results indicated that the highest color strength (K/S) was obtained at a dye concentration of 50% over the weight of fiber (o.w.f.), temperature 100 °C, time 60 min, and pH 5. Moreover, the antioxidant and antimicrobial activities of the treated samples with NPs, and dyed with roots and stems of berberis vulgaris were excellent (about 100%). The treated samples with Ag NPs and dyed with the natural dyes showed very high antimicrobial activity (> 84%) after 10 repeated washing cycles. Finally, the colorfastness properties of the dyed and treated wool samples against washing and light irradiation were studied. It was concluded that the roots and stems of berberis vulgaris could be considered as suitable natural colorants for dyeing of wool yarns with acceptable colorfastness properties, excellent antimicrobial and antioxidant activities.
Keywords: Nanoparticles, Antimicrobial, Natural dyes, Antioxidant, Wool yarn
Introduction
Microorganisms can grow and infiltrate within natural fibers and eventually spread and transmit various diseases. Wool is a protein-based natural fiber that can biodegrade in contact with microbes and its mechanical properties may be adversely affected accordingly (Ali et al. 2011; Sadeghi-Kiakhani et al. 2018). Antimicrobial agents which classified into three groups of organic, inorganic, and natural, should be able to stop the growth of bacteria efficiently in a short period of time (Annalisa et al. 2016; Zhou and Kan 2014; Bendak et al. 2008). Common antimicrobial agents are metal salts such as Ag and Cu2O (Annalisa et al. 2016; Ghoranneviss and Shahidi 2013), quaternary ammonium compounds (Massi et al. 2009; Chadeau et al. 2012), polyhexamethylene biguanide (Ashraf et al. 2012; Zhao et al. 2010), triclosan (Simoncic and Tomsic 2010; Purwar and Joshi 2004; Orhan et al. 2007), chitosan (Bashar and Khan 2013; Sadeghi-Kiakhani et al. 2013) and some natural and synthetic dyes such as berberine and metal complex dyes (Simoncic and Tomsic 2010).
One of the aims in this domain is to find non-toxic and environmentally-friendly antimicrobial agents for textiles. Some natural dyes show antibacterial activity when applied to textiles and they are good candidates for this purpose. The literature review shows that various natural sources have been used in textile dyeing as the green antibacterial agents (Ghoreishian et al. 2013; Guo et al. 2015; Dashti et al. 2014). Some plants with antimicrobial activities can be cheap and green alternatives for expensive and toxic antimicrobial agents (Vasantharaj et al. 2019a, b; Sathiyavimal et al. 2018). Using these natural plants can also help to minimize waste, water and energy consumptions. The medicinal and dyeing properties of some natural products have long been recognized (Pugazhendhi et al. 2019; Ramkumar et al. 2017a, b).
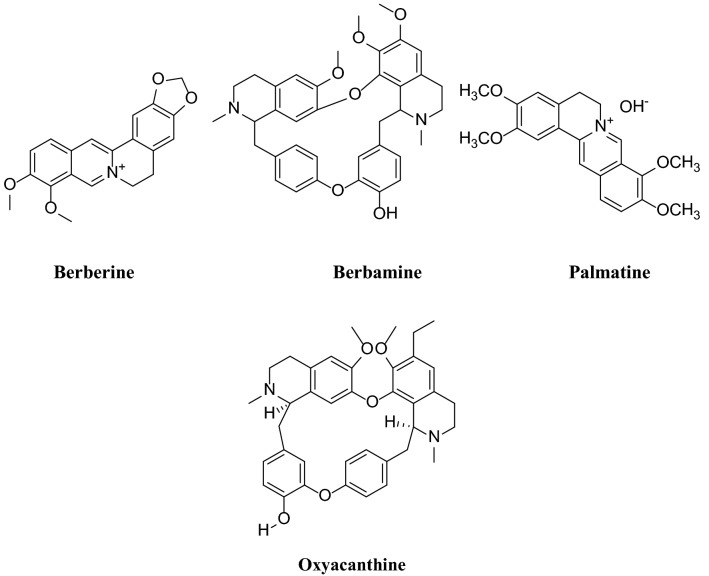
Berberis vulgaris, also known as common barberry, is a native shrub to central and southern Europe, Northwest Africa and Western Asia. About 500 species of berberis are cultivated worldwide, and South Khorasan province in Iran is the largest producer of berberis Vulgaris. The roots and stems of this plant contain different alkaloids (Villinski et al. 2003; Hosseini Hashemi and Aghajani 2017) such as berberine, oxyacanthine, berbamine and palmatine that can be used for dyeing of fibers (Fig. 1). The presence of alkaloids compounds in this plant renders antimicrobial properties (Kamrani Rad et al. 2017; Rounsaville and Ranney 2010).
Fig. 1.
Chemical structure of various colorant compounds in berberis vulgaris
Nanomaterials with antimicrobial activity can also be applied directly or through loading into carrier systems onto textiles. Recently, several nanoparticles (NPs) have been used to antimicrobial finishing of textiles (Ramkumar et al. 2017a, b; Oves et al. 2018). Ag composites as a more famous antibacterial material have been usually applied to increase antimicrobial activities on various surfaces (Shanmuganathan et al. 2018; Jacob et al. 2019, Pugazhendhi et al. 2018). Cellulosic and synthetic fabrics were treated with silver NPs and the antibacterial activity of the samples was preserved after several washing cycles (Lombi et al. 2014). Recently, the production of multifunctional textiles using NPs has attracted the attention of researchers, and it has been proposed that the antimicrobial activity of textiles can be increased by reducing the size of NPs (Zille et al. 2014; Khamseh et al. 2017).
Considering the ecological and economic restrictions imposed on the textile industry, it is required to find environmentally friendly methods to enhance the antimicrobial activity of textiles with lower consumption of silver salts (Kucuk et al. 2016). Another commercial and cost-effective method of antimicrobial finishing of textiles is the use of natural products. The medicinal and dyeing properties of some natural products have long been recognized. Many of the plants used for the dyeing of textiles are also categorized in the medicinal plant groups. Reducing both the costs of production, wasting and saving in consumption of water, energy, chemicals and time are the benefits of natural antimicrobial colorants which are simultaneously practical in dyeing and finishing (Kucuk et al. 2016).
The aim of this study was to develop a viable and environmentally friendly technique for the production of antimicrobial and antioxidant wool yarns using antimicrobial natural dyes and Ag, Cu2O, and ZnO NPs. The antimicrobial activity of treated Ag/Cu2O/ZnO NPs with respect to raw wool was optimized against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) bacteria. Also, dyeing of wool yarn with roots and stems Berberis vulgaris as the natural colorants with high antimicrobial potential was performed, then color strength (K/S), antimicrobial and antioxidant activities of dyed yarns are evaluated. Moreover, washing, rubbing and light fastness properties of the dyed treated wool have been measured.
Materials and methods
Chemicals
The wool yarn (200 Tex/fourfold) was purchased from Azar Barf Yarn. The Stems and roots of Berberis Vulgaris plant were collected from Khorasan Razavi Province, Iran. The collected samples were dried at room temperature and ground to a fine powder.
AgNO3, ZnO, and Cu2O were purchased from Merck (Germany), and other chemicals used in this study were of analytical grade. Milli-Q water (Millipore, Milford, MA, USA) was used to perform all the experiments.
Apparatus
Dynamic light scattering (DLS) technique (Horiba SZ-100, Japan) was used to determine the particle size of NPs, and the intensity of light scattered from a suspension was randomly measured. The thermal stability of samples was studied by differential scanning calorimetric (NETZSCH, Germany) under a constant nitrogen purge. The measurements were carried out by aluminum pan in the thermal range of 0–300 °C, and the scanning rate was 10 °C/min. A scanning electron microscope (LEO 1455VP, UK) was used for morphological analyses of all samples coated with gold (Au) in a high-resolution sputter coater. The colorimetric parameters of all dyed samples were determined by Gretag Macbeth spectrophotometer Color-Eye 7000A, USA) under D65 illumination, and 10° observer.
In situ treatment of wool with prepared Ag/Cu2O/ZnO NPs
The AgNO3, Cu2O, ZnO and bi-component mixtures (1:1) of NPs were prepared as follows; various concentrations of NPs (0.1–0.25% o.w.f.) were poured in 30 mL water, and stirred. The sonication of mixture was performed at 40 °C for 30 min to form a clear solution.
Before treatment, wool yarns were scoured with 5% o.w.f. nonionic detergent at a liquor ratio (L.R) 1:40 at 60 °C for 30 min. Then, wool yarns were immersed in a prepared solution containing citric acid as a crosslinking agent (4% o.w.f.) and sodium hypophosphite (4% o.w.f.) as a catalyst. The mixture was shaken with shaking rate 120 rpm for 2 h. The size of the formed NPs was determined by the DLS equipment. The samples were dried (85 °C, 15 min), cured (110 °C, 3 min), and thoroughly rinsed and air-dried. Then, the weight gain (%) of treated samples was determined using Eq. (1):
| 1 |
where Wfinal, and Winitial are the weight of treated and raw wool samples, respectively.
Dyeing method
Raw and treated wool yarns were dyed with roots and stems of Berberis (5–100% o.w.f.) in a dye bath with different pHs (adjusted with 4% o.w.f. sulfuric acid, 4% o.w.f. acetic acid, and neutral). The samples were placed in the dye bath at 30 °C for 5 min, L.R. 40:1. The temperature of the dye bath was raised from 30 °C to a set temperature in the range of 40–100 °C with a gradient of 2 °C/min. The temperature was then controlled at the set temperature for 90 min and finally cooled to the ambient temperature. The dyed samples were then washed with hot and cold water, and dried at room temperature (25 °C).
The Kubelka–Munk was used to determine the color strength (K/S) of dyed samples (Eq. 2).
| 2 |
where K, S, R are the absorbance coefficient, the scattering coefficient and the reflectance values of dyed samples at the wavelength of maximum absorption (λmax) = 400 nm, respectively.
Fastness properties
ISO 105 C06 C2S:1994 (E) standard test method was used to measure the washing fastness properties of samples. This test was completed at 60 °C for 30 min, and a Gray Scale was used to analyze the color change of the dyed samples and staining on adjacent fabrics. ISO 105 B02:1988 (E) standard test was used for evaluating the light fastness of the samples using a standard Blue Scale.
Antimicrobial test
According to AATCC 100-2004 standard test method, Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) bacteria were used for measuring the antimicrobial activity of the samples. The efficiency of the antimicrobial treatment was determined by comparing the reduction in the number of bacterial colonies of the treated sample with that of the untreated control sample after incubation at 37 ± 2 °C for 18 h. The microbial inhibition was determined by the reduction in the number of colony-forming units (CFU) with respect to the untreated control sample using Eq. (3):
| 3 |
where R is the reduction percentage of the bacterial count; B and A are the surviving cells (CFU/mL) for the flasks containing treated wool samples and the untreated wool as control, respectively.
Antioxidant activity test
1,1-Diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging test was used to evaluate the antioxidant activity of the raw and treated samples (Rather et al. 2017). Briefly, 0.15 mM of DPPH was poured in 40 ml methanol, and 2.5 cm2 of all samples were immersed prepared solution, and incubated at room temperature for 30 min in the dark. The absorbance and color change from violet to pale yellow was measured with a UV–Visible at 517 nm. DPPH radical scavenging activity was calculated using Eq. (4).
| 4 |
D and C are the absorbance values of the treated and raw wool samples, respectively.
Results and discussion
Characterization of treated samples
The difference between the primary and secondary weights of samples can be considered as a criterion for the in situ synthesis of NPs on the surface of wool yarns (Fig. 2). The results showed that the weight differences of the treated samples have increased by of the dosage of NPs, which can be attributed to the formation of two or more separate coordinate bonds between the wool yarn as a ligand and NPs as metal-compounds (Hosseinkhani et al. 2017; Sadeghi-Kiakhani et al. 2019). So, the concentration of 0.15–0.20% o.w.f. for each of nanoparticles was considered as the optimum concentration.
Fig. 2.
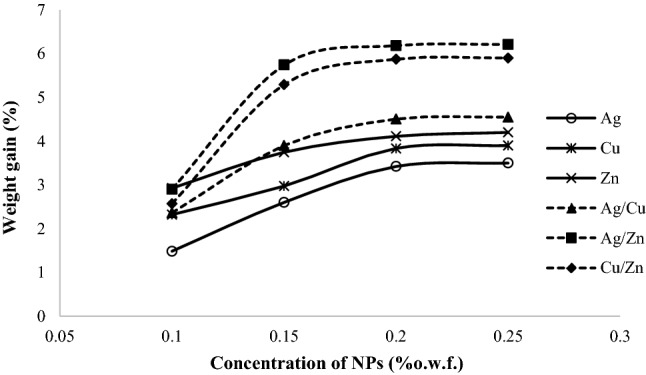
The weight gain (%) of the treated wool yarns with NPs
By dissolving NPs in the aqueous solution, Ag+, Cu+ and Zn+ ions are adsorbed on polar and ionize able functional groups of wool macromolecules by coordination or electrostatic interactions. For the in situ formation of NPs, metal atoms should be reduced. Wool thiol groups are generally formed via degradation of cysteine bonds of wool fibers (–S–S–) → (–SH) under moisture and heat (Hosseinkhani et al. 2017; Yu et al. 2015). It has been suggested that the reduction of metal ions to the in situ formation of metal nanoparticles is occurred in the presence of these groups in the protein chains of wool fibers without further reducing agents (Hosseinkhani et al. 2017; Yu et al. 2015). Here, Ag+ and NO3− and ionic interactions are formed between positive metal ions and thiol groups with a negative charge. Thus, metal ions can be reformed to metal NPs with the available thiol groups in wool fibers. It should also be noted that citric acid improves the levelness of silver ions attraction on wool. The grafting of NPs on the textile substrates can be completed via physical adsorption or chemical bonding. Also, the presence of various functional groups in the protein chains of the wool fibers can improve the NPs grafting on the wool, and consequently the formation of NPs aggregation is diminished.
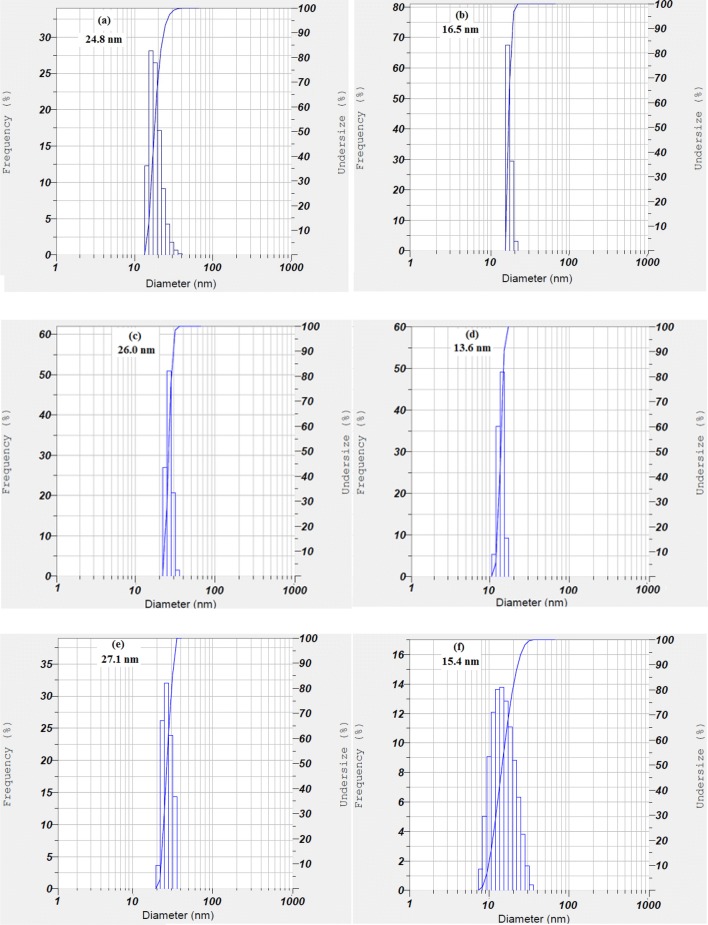
Horiba SZ-100 particle size analyzer was used to measure the size of NPs. The average size obtained by the DLS distribution plot was between 13 and 27 nm (Fig. 3). The average particle sizes of the NPs are significantly diverse and the type of metal salts affects its size (Zille et al. 2014; Kucuk et al. 2016, Sathiyavimal et al. 2018). The average size of NPs obtained from DLS was in a good agreement with the results obtained from SEM.
Fig. 3.
DLS analyses of NPs a Ag, b Cu, c Zn, d Ag/Cu, e Ag/Zn, f Cu/Zn
The morphology and presence of NPs on wool yarns were further confirmed by SEM (Fig. 4). The scales on the surface of raw wool yarn is observable, so that, after treating the wool fibers with inorganic salts, some foreign particles were visible on the surface of the treated fibers and a flat layer covers the surface of the fibers (Hosseinkhani et al. 2017; Yu et al. 2015). These images suggested that wool fibers were successfully and homogeneously treated within NPs.
Fig. 4.
SEM images of a Wool, b Wool-Ag, c Wool-Cu, d Wool-Zn, e Wool-Ag/Cu, f Wool-Ag/Zn and g Wool-Cu/Zn
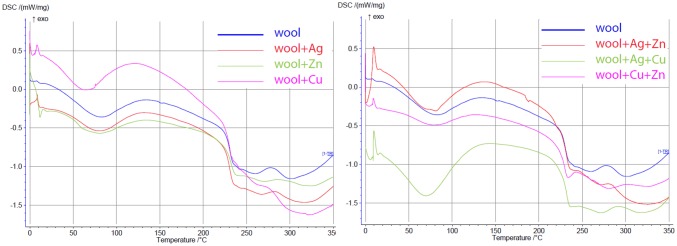
The DSC thermal analysis of treated and untreated wool fibers was provided in (Fig. 5). An endothermic peak is observed in the region of 240 °C for a wool sample, which relates to the thermal decomposition of α-Helix in the protein structure of wool. In the curve of the treated samples, the peak temperature was lowered to (218 °C), which may indicate interactions between NPs and wool structure. The glass transition was observed between 40 and 60 °C, and it is difficult to be recognized due to the overlapping with the initial broad peak corresponding to water evaporation at around 90–100 °C. Also, another peak at around 240 °C is related to the denaturation of the helicoidally material, which results from the melting of the α-form crystallites in wool (Sadeghi-Kiakhani et al. 2013; Hsieh et al. 2004). It was found that the crystallinity of the treated wool was enhanced due to an increase in the melting temperature, and it is reduced with increasing interaction of functional groups of wool with NPs in the amorphous phase.
Fig. 5.
DSC curves of wool treated with various NPs
Dyeing property
Effect of initial natural dye concentration
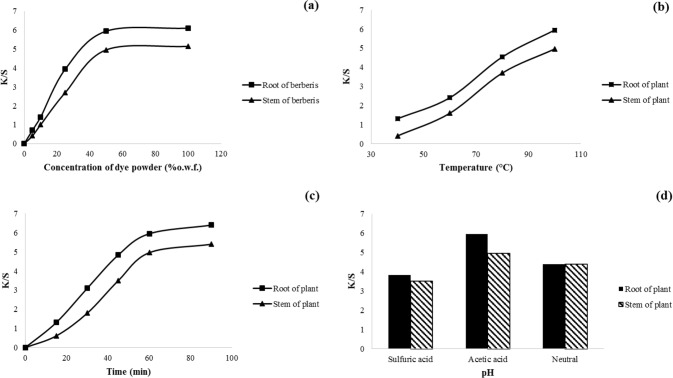
The color strength of two natural dyes in various initial concentrations on wool yarn is shown in Fig. 6a. Results indicated that K/S values were enhanced quickly by increasing of the dye concentration up to 50% o.w.f. By increasing the adsorbed amount of dyes on wool yarn, the antimicrobial activity increases as well. Dye concentration in the dyeing process is an important parameter, so there is an equilibrium concentration for each dye on each yarn (Sadeghi-Kiakhani et al. 2014). In the equilibrium concentration, no more dye molecules are absorbed in the wool yarn and dye remain in the wastewater. However, in lower initial dye concentrations, adsorptive dye sites in the yarn are readily available to dye molecules and more dye absorb at a faster rate. So, Cdye = 50% o.w.f. was chosen as an optimum concentration of both natural dyes for wool dyeing and investigated the effects of other parameters at this fixed dye concentration.
Fig. 6.
Effect of a initial dye concentration, b temperature, c time, and d pH on the color strength of wool yarns. The optimum dyeing conditions were found to be [dye] = 50% o.w.f., 100 °C, pH 5, and for 60 min
Effect of dyeing temperature
It is evident that the absorption of both natural dyes on wool samples steadily increases with temperature (Fig. 6b). It is noteworthy that temperature affects the dyeing system, so that the dissolving of colorants existing in the natural source and the aggregation of dye molecules in the dye bath diminished by the temperature increase. On the other hand, the internal structure of the wool is swelled, and consequently, the diffusion of dye compounds into wool structure becomes easier (Sadeghi-Kiakhani 2015). The mechanical properties of wool were adversely affected at temperature above 100 °C, so the optimal temperature for dyeing of both natural dyes on wool yarns was set at T = 100 °C.
Effect of dyeing time
The natural dyes absorption on wool yarns is investigated at various dyeing time intervals (Fig. 6c). Results indicated that the dye absorption on wool yarns improved with increasing the time of process because the absorption opportunity increases statistically. Also, the levelness of the dyed samples can increase by increasing the dyeing time. Since wool dyeing in the boiling temperature for a long time may decline the physical properties of wool yarns, so the proper time for wool dyeing with suitable dye absorption was 60 min (Zargarkazemi et al. 2015).
Effect of dye bath pH
The pH of the dyeing bath has an essential influence on the chemical property of yarns and dyes. It can also affect the protonation of the dye sites to improve the affinity of dye molecules towards the fibers (Mirnezhad et al. 2017). It was found that the maximum K/S values of both natural dyes on wool samples were observed at pH = 5 (Fig. 4d). It seems that ionic and hydrogen bonds between active groups of dye molecules and functional groups of wool fiber mainly affect the dye absorption. Since, the number of dye adsorption sites of wool samples (i.e., amino and carboxylic acid groups) is more or less the same in weak acidic condition (at pH ~ 5), the dye-wool interaction via hydrogen bonding should be pronounced. Some of positively charged molecules among the extracted dyestuff (alkaloid such as berberine) can also adsorb via electrostatic attraction on wool fibers. The intermolecular interactions between the dye molecules and wool fibers decrease at elevated pH due to lower protonation (Mirnezhad et al. 2017). Therefore, pH 5 was considered as an optimum pH for wool dyeing with both natural dyes.
Effect of metal mordants
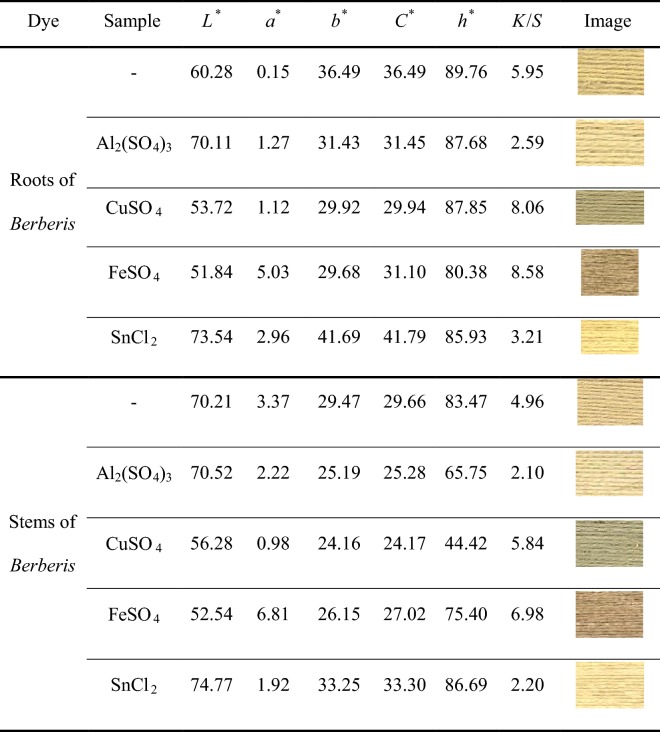
The color fastness properties of natural dyes on natural fibers are generally weak to moderate. So, various metal ions as mordants are utilized in the dyeing process to reach various and deep colors with enhanced colorfastness properties. In this regard, a mordanting pretreatment of wool samples with stannous chloride, aluminum, copper, and iron sulfates was followed. Table 1 shows the colorimetric parameters and images of the dyed samples. Among the mordanted samples, wool mordanted with Al imparted the lowest K/S value probably due to weaker coordination complexes and lower affinity toward natural dyes. On contrary, iron and copper mordants showed strong coordination complexes with wool fibers and dye molecules resulting in higher values of K/S for the dyed samples (Oliveira et al. 2008). So, the order of K/S values of the pre-mordanted wool yarns was: Fe > Cu > Sn > Al.
Table 1.
Effect pre-mordanting of metal salts on colorimetric data of dyed wool yarns (concentration of dye 50% o.w.f., concentration of Al, Cu, Fe 5% o.w.f., and concentration of Sn 2% o.w.f)
Effect of finishing on color data of dyed samples
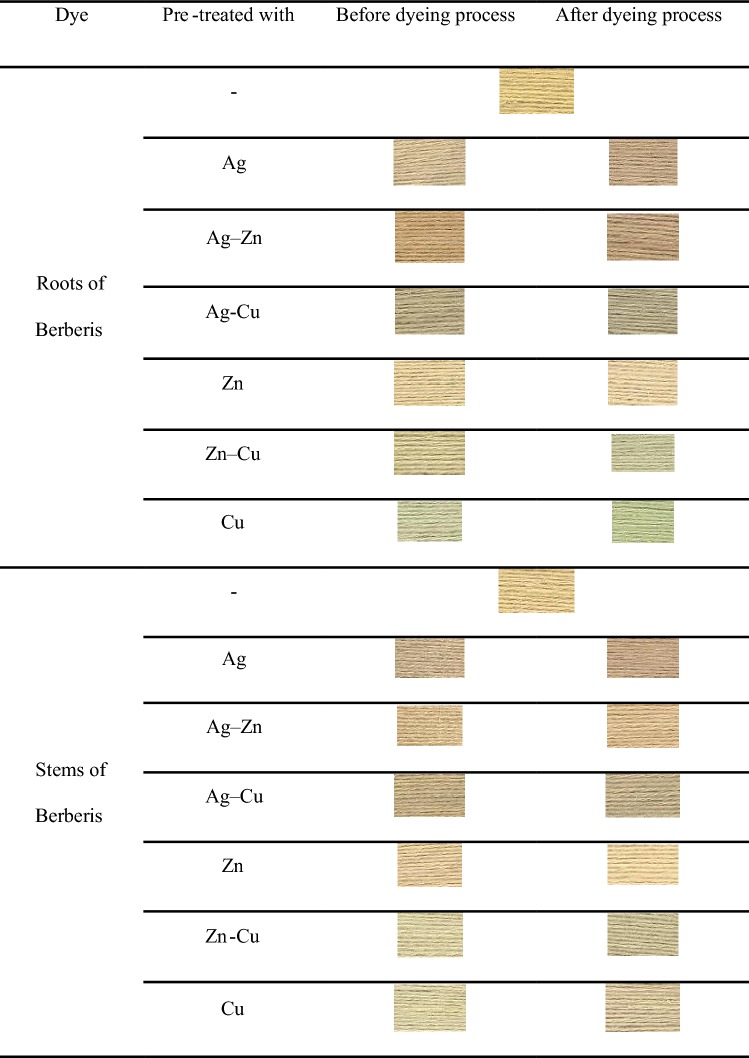
The treatment of wool yarns with NPs was completed before and after the dyeing process, and colorimetric data and images of dyed yarns were provided in Tables 2 and 3. The pretreatment with these inorganic salts (especially with copper salts) had changed the final color which was a sign of dye-inorganic salt coordination. It seems the presence of NPs as mordants on the surface of wool fibers can improve the affinity of the natural dyes. The color strength of the samples reduced when treatment was performed before the dyeing process, which can be attributed to the change in absorption properties of the treated samples.
Table 2.
Colored data values of treated samples (concentration of dye 50% o.w.f. and concentration of NPs 0.15% o.w.f.)
| Dye | Sample | Before dyeing | After dyeing | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | K/S | L* | a* | b* | K/S | ||
| Roots of Berberis | – | 60.28 | 0.15 | 36.496 | 5.95 | 60.28 | 0.15 | 36.49 | 5.95 |
| Ag | 62.67 | 2.31 | 22.8 | 3.37 | 51.17 | 5.82 | 23.09 | 6.45 | |
| Ag–Zn | 54.93 | 7.81 | 29.24 | 6.13 | 48.72 | 6.663 | 22.55 | 7.78 | |
| Ag/Cu | 56.56 | 1.11 | 21.05 | 4.57 | 51.75 | 1.076 | 18.45 | 5.53 | |
| Zn | 67.39 | 1.12 | 25.28 | 2.29 | 63.97 | 2.716 | 23.09 | 2.83 | |
| Zn/Cu | 64.03 | − 0.96 | 24.72 | 3.16 | 59.26 | 1.76 | 17.04 | 4.17 | |
| Cu | 63.47 | − 7.42 | 25.66 | 2.29 | 60.3 | 3.136 | 21.02 | 3.82 | |
| Stems of Berberis | – | 70.21 | 3.37 | 29.47 | 4.96 | 52.84 | 6.30 | 26.46 | 4.96 |
| Ag | 42.75 | 2.99 | 16.76 | 4.89 | 59.73 | 6.833 | 22.10 | 5.43 | |
| Ag/Zn | 61.90 | 4.19 | 25.783 | 4.35 | 41.32 | 5.483 | 24.40 | 4.19 | |
| Ag/Cu | 56.19 | 3.58 | 23.113 | 5.35 | 66.44 | 2.36 | 15.15 | 4.56 | |
| Zn | 63.00 | 2.89 | 23.07 | 3.08 | 64.71 | 3.483 | 25.27 | 2.67 | |
| Zn/Cu | 60.86 | 0.36 | 22.33 | 3.69 | 49.06 | 0.713 | 22.38 | 2.82 | |
| Cu | 62.52 | 2.00 | 21.113 | 3.28 | 68.34 | 0.407 | 16.67 | 2.71 | |
Table 3.
Images of treated and untreated samples before and after dyeing with the natural dyes (concentration of dye 50% o.w.f. and concentration of NPs 0.15% o.w.f.)
Antibacterial activity of natural dyes
Stems and roots of berberis have a promising antimicrobial activity against a number of germs (Kamrani Rad et al. 2017; Rounsaville and Ranney 2010). The antimicrobial activity of the dyed samples before and after the pretreatment with NPs at various initial concentrations has been summarized in Table 4. The raw wool sample did not show any noticeable antimicrobial effect before any pretreatment and dyeing. The antimicrobial activity against E. coli bacteria of the samples dyed with stems and roots of berberis plant increased to a minimum of 30% and 45%, respectively. This can be attributed to the presence of high alkaloids content in the berberis (Kamrani Rad et al. 2017; Rounsaville and Ranney 2010). The results indicated that the antimicrobial activity of wool yarns treated with Ag NPs was the highest among all of the used inorganic salts. The dyeing of wool treated samples with the aforementioned natural dyes enhanced the antimicrobial properties of the samples generally. The high antimicrobial activity of the wool samples treated with silver NPs can be due to a smaller size of NPs (i.e., higher surface area to volume ratio) in contact with microorganisms (Lombi et al. 2014).
Table 4.
Antibacterial properties of the wool samples treated with various NPs and dyed with the natural dyes against E. coli (gram-negative) and S. aureus (gram-positive) bacteria, (concentration of dye 50% o.w.f. and concentration of NPs 0.15% o.w.f.)
| Sample | % (o.w.f.) | Antibacterial activity (%) | |||||
|---|---|---|---|---|---|---|---|
| Un-dyed samples | Roots of berberis | Stems of berberis | |||||
| E. coli | S. aureus | E. coli | S. aureus | E. coli | S. aureus | ||
| Wool-Ag | – | – | – | 30.24 | 27.72 | 45.62 | 39.53 |
| 0.1 | 16.21 | 12.60 | 84.16 | 77.41 | 78.40 | 72.19 | |
| 0.15 | 86.38 | 79.43 | 100 | 100 | 100 | 100 | |
| 0.20 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Wool-Ag/Zn | 0.1 | 14.08 | 10.70 | 82.83 | 75.67 | 74.11 | 70.63 |
| 0.15 | 75.72 | 70.38 | 100 | 100 | 100 | 100 | |
| 0.20 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Wool-Ag/Cu | 0.1 | 15.19 | 13.82 | 83.27 | 80.97 | 75.32 | 69.92 |
| 0.15 | 68.43 | 65.45 | 100 | 100 | 100 | 100 | |
| 0.20 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Wool-Zn | 0.1 | 10.44 | 7.17 | 84.81 | 73.83 | 75.46 | 66.41 |
| 0.15 | 42.36 | 37.92 | 90.59 | 78.31 | 81.80 | 70.44 | |
| 0.20 | 60.82 | 58.43 | 88.73 | 85.20 | 85.05 | 77.84 | |
| Wool-Zn/Cu | 0.1 | 12.73 | 50.27 | 88.07 | 81.37 | 77.20 | 68.05 |
| 0.15 | 62.49 | 58.33 | 100 | 100 | 100 | 100 | |
| 0.20 | 73.52 | 68.73 | 100 | 100 | 100 | 100 | |
| Wool-Cu | 0.1 | 14.26 | 11.28 | 83.13 | 77.10 | 78.72 | 71.69 |
| 0.15 | 65.50 | 61.17 | 100 | 100 | 100 | 100 | |
| 0.20 | 78.19 | 73.46 | 100 | 100 | 100 | 100 | |
By increasing the initial concentrations of inorganic salts during the pretreatment of the wool yarns, the antimicrobial properties of the samples increased. The satisfactory antimicrobial properties of the wool yarns can be achieved by the pretreatment of the samples with 0.15% o.w.f. of silver containing inorganic salts and further dyeing with stems and roots of berberis. The presence of these natural dyes on the surface of the wool fibers can reduce the amount of needed NPs for the pretreatment. This will be a greener and cheaper approach for dyeing and improving the antimicrobial properties of the wool yarns.
The pretreated and dyed wool samples were repeatedly washed according to ISO 6330-1984 standard test method to study the stability of the antimicrobial treatment (Table 5). The washing durability of the antimicrobial function depends on the bonding strength between NPs and wool fibers. The pretreated wool samples with Ag and Cu salts that had also been dyed with stems and roots of berberis showed very good antimicrobial durability after 10 repeated washing cycles (Sadeghi-Kiakhani et al. 2019). Table 4 also showed that the antimicrobial activity of pretreated samples without further dyeing dropped to 12–20% after repeated washing cycles. It can be concluded that dyeing with these natural colorants improved the fixation and stability of bonded nanoparticles against repeated washing cycles. The protection mechanism can be via coordination between the inorganic salts and the natural colorants.
Table 5.
Antimicrobial activity to washing durability of samples (concentration of dye 50% o.w.f. and concentration of NPs 0.15% o.w.f.)
| Sample | Repeated washing cycles | Antibacterial activity (%) | |||
|---|---|---|---|---|---|
| Dyed with roots of Berberis | Dyed with stems of Berberis | ||||
| E. coli | S.aureus | E. coli | S.aureus | ||
| Wool-Ag | – | 100 | 100 | 100 | 100 |
| 5 | 93.07 | 89.45 | 88.71 | 84.51 | |
| 10 | 84.69 | 80.23 | 78.40 | 72.17 | |
| Wool-Ag/Zn | – | 100 | 100 | 100 | 100 |
| 5 | 86.71 | 81.23 | 82.74 | 78.56 | |
| 10 | 80.83 | 75.67 | 74.11 | 70.63 | |
| Wool-Ag/Cu | – | 100 | 100 | 100 | 100 |
| 5 | 89.28 | 89.64 | 89.07 | 88.21 | |
| 10 | 82.27 | 78.97 | 75.32 | 71.93 | |
| Wool-Zn | – | 88.73 | 75.12 | 75.06 | 67.84 |
| 5 | 77.59 | 73.31 | 71.80 | 65.44 | |
| 10 | 73.81 | 68.83 | 67.46 | 61.41 | |
| Wool-Zn/Cu | – | 100 | 100 | 100 | 100 |
| 5 | 88.66 | 85.26 | 84.77 | 80.26 | |
| 10 | 78.07 | 73.37 | 69.31 | 68.49 | |
| Wool-Cu | – | 100 | 100 | 100 | 100 |
| 5 | 92.25 | 89.63 | 88.55 | 84.44 | |
| 10 | 83.13 | 77.10 | 76.69 | 72.71 | |
Table 4 showed that by increasing the washing cycles, the antimicrobial activity of the pretreated samples decreased 16% and 27% when Ag and Zn salts were used, respectively. By repeated washing cycles, the adsorbed inorganic salts can be detached from the wool surface which adversely affects the antimicrobial activity. Some of the pretreated and dyed wool yarns showed very good and decent antimicrobial properties even after 10 repeated washing cycles (e.g., the samples treated with Ag, Ag/Cu, and Cu salts). Such promising antimicrobial activities cannot be achieved without using citric acid as a crosslinking agent. When the samples were treated with the inorganic salts using citric acid, the antimicrobial activities and the washing durability were improved.
The obtained results are strongly correlated with the findings of researchers where, treated samples with Ag NPs showed more antimicrobial activity compared to other NPs and natural dyes (Sadeghi-Kiakhani et al. 2019). With this background, it is considered that Ag based mordants will continue to play an important role as antimicrobial agents in textile finishing. Table 6 shows the antimicrobial activity results for a series of NPs and natural dyes on various textile fibers. The proposed system of “Stems and roots of berberis + Ag/Zn NPs” has shown over 92% antioxidant activity and near-complete antimicrobial activity which is outstanding compared to the results of other reported systems in this table.
Table 6.
Antimicrobial and antioxidant activities for the several NPs and natural dyes on the textiles
| Textile | Natural dyes + NPs | Antioxidant activity (%) | Antimicrobial activity | References |
|---|---|---|---|---|
| Cotton | Leaf extract of Ruellia tuberosa FeONPs | – | E.coli: 14 mm, S.aureous: 12 mm | Vasantharaj et al. (2019a, b) |
| – | Seaweed Padina gymnospora + PVP/PtNPs nanocomposite |
DPPH: 30 Superoxide: 50 |
E.coli: 15.6 mm, S.aureous: 13.6 mm | Ramkumar et al. (2017a, b) |
| Wool and nylon | Conocarpus erectus extract | – | E.coli: 75% | Ramadan et al. (2017) |
| Linen | Henna + Copper sulphate | 92.10 | E.coli: 99.50%, S.aureous: 99.75% | Yadav et al. (2019) |
| Polyamide | Green walnut shells | – |
E.coli: 92 S.aureous: 97.47% |
Mirjalili and Karimi (2013) |
| – | Extract of coleus vettiveroids + silver nanoparticles | 63.88 |
E.coli: 26 mm S.aureous: 29 mm |
Thomas et al. (2018) |
| Silk | Madder dye + Moreinga olefera seed extract |
E.coli: 94% S.aureous: 90% |
Ali et al. (2015) | |
| Polyamide | Violacein | 14 |
E.coli: 55% S.aureous: 45% |
Kanelli et al. (2018) |
| Wool | Stems and roots of berberis + Ag/Zn NPs | Stem of berberis: 96% root of berberis: 92% |
E.coli: 100% S.aureous: 100% |
In this study |
Antioxidant property
DPPH free radical scavenging test was used to investigate the antioxidant activity of the raw, and treated wool samples (Fig. 7). The antioxidant activity of the untreated wool was < 30% while the dyed samples with the stems and roots of berberis showed a minimum off 80% antioxidant activity. This was attributed to the radical scavenging nature of these natural dyes due to the presence of polyphenol compounds and active hydrogen groups. The results clearly showed that the initial concentration of NPs as low as 0.15% o.w.f was suitable to produce antioxidant wool yarns for medical application. The highest antioxidant activity achieved when Ag/Zn nanocomposites were employed for treatment. This antioxidant activity may be due to the capping constituents present in plant extract and on the metal surface. The obtained results in this study were compared with previous publications and are given in Table 6.
Fig. 7.
Antioxidant activity of dyed samples (concentration of dye 50% o.w.f. and concentration of NPs 0.15% o.w.f.)
Mechanism of the in situ formation of metal NPs and natural dyes on the wool
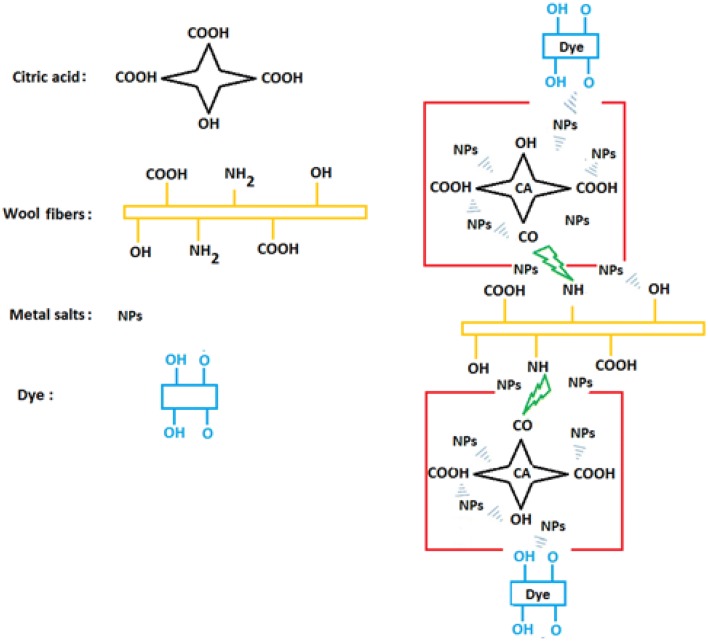
Figure 8 shows the in situ formations of metal NPs on wool fibers schematically. The role of citric acid (CA) as a cross-linking agent is significant for the adsorption and stability of NPs on the surface of wool fibers. The proposed mechanism for the adsorption of NPs on the surface of wool fibers and finally dye absorption on the treated samples is based on the interactions such as covalent: wool–CA, coordination: Wool–NPs–Dye, hydrogen and van der Waals between Wool–CA–NPs–Dye system.
Fig. 8.
In situ formation of metal NPs and natural dyes on the wool
Colorfastness
Light- and washing-fastness properties of wool yarns dyed with stem and root of berberis plant are given in Table 7. The best fastness properties were again obtained for the wool yarns pretreated with silver-containing salts. The untreated dyed wool samples have very good wash fastness with moderate light-fastness. The pretreatment with any of the inorganic salts clearly improved the light-fastness of the dyed samples to 7–8 (very good). This can be attributed to the complex formation and coordination of the natural dyes with the NPs on the fiber surface. The results showed that there are no significant differences in colorfastness properties of treated and untreated wool yarns, and it can be concluded that antimicrobial finishing can be completed before and after the dyeing process and has no adverse effect on the colorfastness of dyed samples.
Table 7.
The effect of various NPs on color fastness properties of dyed samples (concentration of NPs: 0.15% o.w.f.)
| Dye | Sample | Treatment before dyeing | Treatment after dyeing | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Light fastness | Wash fastness | Light fastness | Wash fastness | ||||||
| SC* | SW* | CC* | SC* | SW* | CC* | ||||
| Roots of Berberis | No salt | 7–8 | 2 | 4–5 | 4–5 | 7–8 | 3 | 4–5 | 4–5 |
| Ag | 8 | 2 | 5 | 5 | 7–8 | 3 | 5 | 5 | |
| Ag/Zn | 7–8 | 4–5 | 5 | 5 | 7–8 | 5 | 5 | 5 | |
| Ag/Cu | 8 | 3 | 5 | 5 | 7–8 | 2 | 5 | 5 | |
| Zn | 7–8 | 2 | 5 | 5 | 7–8 | 2 | 5 | 5 | |
| Zn/Cu | 8 | 2 | 5 | 5 | 7–8 | 2 | 5 | 5 | |
| Cu | 7–8 | 2 | 5 | 5 | 7–8 | 2 | 5 | 5 | |
| Stems of Berberis | No salt | 7–8 | 3 | 4–5 | 4–5 | 7–8 | 3 | 4–5 | 4–5 |
| Ag | 8 | 4 | 5 | 5 | 8 | 5 | 5 | 5 | |
| Ag/Zn | 8 | 3 | 5 | 5 | 7–8 | 4–5 | 5 | 5 | |
| Ag/Cu | 7–8 | 4 | 5 | 5 | 7–8 | 5 | 5 | 5 | |
| Zn | 7–8 | 3 | 5 | 5 | 7–8 | 2 | 5 | 5 | |
| Zn/Cu | 7–8 | 2 | 5 | 5 | 7–8 | 2 | 5 | 5 | |
| Cu | 7–8 | 2 | 5 | 5 | 8 | 2 | 5 | 5 | |
Conclusion
In this study, wool yarns were treated with a series of NPs and dyed with stems and roots of beberis (natural dyes). The samples were characterized by various techniques such as SEM and DSC. The results showed that the modification increased the antimicrobial activity of wool yarns noticeably due to the scavenging nature of the natural dyes. The antimicrobial activity of the treated samples was roughly in this order: Ag ≈ Ag/Cu ≈ Ag/Zn > Cu > Cu/Zn > Zn. Moreover, the obtained results exhibited that the stems and roots of berberis plant could be utilized as natural colorants to increase the antimicrobial activity of wool yarns with or without using antimicrobial inorganic salts due to the presence of alkaloid in the chemical structure of the dyes. Also, the roots and stem of berberis plant increased the antimicrobial and antioxidant activities of the treated wool yarns with NPs. The results also indicated that the antimicrobial finishing could be completed before and after the dyeing process without having a negative effect on the dye absorption and the colorfastness properties of the dyed samples. Furthermore, metal salts may only be used to produce various shades. The antimicrobial and antioxidant activities of the wool yarns treated with NPs and dyed with the stems and roots of berberis vulgaris increased noticeably. The proposed method for dyeing and finishing wool is an environmentally friendly approach that enhances the antimicrobial and antioxidant properties of the wool yarns.
References
- Ali NF, Mohamedy RSREL, El-Khatib EM. Antimicrobial activity of wool fabric dyed with natural dyes. Res J Text Appar (RJTA) 2011;15:1–10. [Google Scholar]
- Ali NF, El-Khatib EM, El-Mohamedy RSR. The antimicrobial activity of pretreated silk fabrics dyed with natural dye. Int J Curr Microbiol Appl Sci. 2015;4(6):1166–1173. [Google Scholar]
- Annalisa C, Francesca B, Giulio M, Chiara M, Monica P. DNA-chitosan cross-linking and photografting to cotton fabrics to improve washing fastness of the fire-resistant finishing. Cellulose. 2016;23:3963–3984. [Google Scholar]
- Ashraf S, Akhtar N, Ghauri MA, Rajoka MI, Khalid ZM, Hussain I. Polyhexamethylene biguanide functionalized cationic silver nanoparticles for enhanced antimicrobial activity. Nanoscale Res Lett. 2012;7:267. doi: 10.1186/1556-276X-7-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashar MM, Khan MA. An overview on surface modification of cotton fiber for apparel use. J Polym Environ. 2013;21(1):181–190. [Google Scholar]
- Bendak A, Raslan WM, Salama M. Treatment of wool with metal salts and their effects on its properties. J Nat Fibers. 2008;5:251–269. [Google Scholar]
- Chadeau E, Brunon C, Degraeve P, Leonard D, Grossiord C, Bessueille F, et al. Evaluation of antimicrobial activity of a polyhexamethylene biguanide-coated textile by monitoring both bacterial growth (ISO 20743/2005 standard) and viability (Live/Dead BacLight kit) J Food Saf. 2012;32(2):141–151. [Google Scholar]
- Dashti Z, Shariatifar N, Nafchi AM. Study on antibacterial and antioxidant activity of Berberis vulgaris aqueous extracts from Iran. Int J Pharma Sci Res (IJPSR) 2014;5:705–708. [Google Scholar]
- Ghoranneviss M, Shahidi S. Effect of various metallic salts on antibacterial activity and physical properties of cotton fabrics. J Ind Text. 2013;42:193–203. [Google Scholar]
- Ghoreishian SM, Maleknia L, Mirzapour H, Norouzi M. Antibacterial properties and color fastness of silk fabric dyed with turmeric extract. Fibers Polym. 2013;14:201–207. [Google Scholar]
- Guo N, Zhao X, Li W, Shi C, Meng R, Liu Z, Yu L. The synergy of berberine chloride and totarol against Staphylococcus aureus grown in planktonic and biofilm cultures. J Med Microbiol. 2015;64:891–900. doi: 10.1099/jmm.0.000106. [DOI] [PubMed] [Google Scholar]
- Hosseini Hashemi SK, Aghajani H. Chemical composition and antioxidant capacity of extracts from the wood of Berberis vulgaris stem. Lignocellulose. 2017;6(1):36–47. [Google Scholar]
- Hosseinkhani M, Montazer M, Harifi T. Protein and silver nitrate interaction during finer wool production: enhancing tensile properties along with synthesis of nano silver. J Text Inst. 2017;108:78–83. [Google Scholar]
- Hsieh SH, Huang ZK, Huang ZZ, Tseng ZS. Antimicrobial and physical properties of woolen fabrics cured with citric acid and chitosan. J Appl Polym Sci. 2004;94:1999–2007. [Google Scholar]
- Jacob JM, John MS, Jacob A, Abitha P, Kumar SS, Rajan R, Natarajan S, Pugazhendhi A. Bactericidal coating of paper towels via sustainable biosynthesis of silver nanoparticles using Ocimum sanctum leaf extract. Mater Res Express. 2019;6:1–12. [Google Scholar]
- Kamrani Rad SZ, Rameshrad M, Hosseinzadeh H. Toxicology effects of Berberis vulgaris (barberry) and its active constituent, berberine: a review. Iran J Basic Med Sci. 2017;20(5):516–529. doi: 10.22038/IJBMS.2017.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanelli M, Mandic M, Kalakona M, Vasilakos S, Kekos D, Nikodinovic-Runic J, Topakas E. Microbial production of violacein and process optimization for dyeing polyamide fabrics with acquired antimicrobial properties. Front Microbiol. 2018;9:1–13. doi: 10.3389/fmicb.2018.01495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamseh S, Tekieh Fatemia SM, Koozegar Kaleji B, Sadeghi-Kiakhani M. Investigations on sputter-coated cotton fabric with regard to their microstructure, antibacterial, hydrophobic properties and thermal stability. J Text I. 2017;108:2184–2190. [Google Scholar]
- Kucuk D, Balci O, Tutak M. In-situ coated of Ag, ZnO, Ag/ZnO composite nanoparticles to the technical fiber by hydrothermal method. Int J Cloth Sci Technol. 2016;28:340–367. [Google Scholar]
- Lombi E, Donner E, Scheckel KG, Sekine R, Lorenz C, Goetz NV, Nowack B. Silver speciation and release in commercial antimicrobial textiles as influenced by washing. Chemosphere. 2014;111:352–358. doi: 10.1016/j.chemosphere.2014.03.116. [DOI] [PubMed] [Google Scholar]
- Massi L, Guittard F, Levy R, Gêribaldi S. Enhanced activity of fluorinated quaternary ammonium surfactants against Pseudomonas aeruginosa. Eur J Med Chem. 2009;44:1615–1622. doi: 10.1016/j.ejmech.2008.07.032. [DOI] [PubMed] [Google Scholar]
- Mirjalili M, Karimi L. Extraction and characterization of natural dye from green walnut shells and its use in dyeing polyamide: focus on antibacterial properties. J Chem. 2013;2013:1–9. [Google Scholar]
- Mirnezhad S, Safapour S, Sadeghi-Kiakhani M. Dual-mode adsorption of cochineal natural dye on wool fibers: kinetic, equilibrium, and thermodynamic studies. Fiber Polym. 2017;18:1134–1145. [Google Scholar]
- Oliveira I, Sousa A, Ferreira ICFR, Bento A, Estevinho L, Pereira JA. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem Toxicol. 2008;46:2326–2331. doi: 10.1016/j.fct.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Orhan M, Kut D, Gunesoglu C. Use of triclosan as antibacterial agent in textiles. Indian J Fibre Text Res. 2007;32:114–118. [Google Scholar]
- Oves M, Aslam M, Rauf MA, Qayyum S, Qari HA, Khan MS, Alam MZ, Tabrez S, Pugazhendhi A, Ismail IMI. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mater Sci Eng C Mater Biol Appl. 2018;89:429–443. doi: 10.1016/j.msec.2018.03.035. [DOI] [PubMed] [Google Scholar]
- Pugazhendhi A, Jebakumar TN, Edison I, Karuppusamy I, Kathirvel B. Inorganic nanoparticles: a potential cancer therapy for human welfare. Int J Pharm. 2018;539:104–111. doi: 10.1016/j.ijpharm.2018.01.034. [DOI] [PubMed] [Google Scholar]
- Pugazhendhi A, Prabhu R, Muruganantham K, Shanmuganathan R, Natarajan S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J Photochem Photobiol B. 2019;190:86–97. doi: 10.1016/j.jphotobiol.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Purwar R, Joshi M. Recent developments in antimicrobial finishing of textiles—a review. AATCC Rev. 2004;4:22–26. [Google Scholar]
- Ramadan MA, Nassar SH, Aty AAAE, Nassar MI, Elshamy AI, Montaser AS, Kantouch F. Antimicrobial fabrics using Conocarpus erectus aqueous extract. Egypt J Chem. 2017;60:1111–1121. [Google Scholar]
- Ramkumar VS, Pugazhendhi A, Gopalakrishnan K, Sivagurunathan P, Saratale GD, Dung TNB, Kannapiran E. Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed Enteromorpha compressa and its biomedical properties. Biotechnol Rep. 2017;14:1–7. doi: 10.1016/j.btre.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkumar VS, Pugazhendhi A, Prakash S, Ahila NK, Vinoj G, Selvam S, Kumar G, Kannapiran E, Rajendran RB. Synthesis of platinum nanoparticles using seaweed Padina gymnospora and their catalytic activity as PVP/PtNPs nanocomposite towards biological applications. Biomed Pharmacother. 2017;92:479–490. doi: 10.1016/j.biopha.2017.05.076. [DOI] [PubMed] [Google Scholar]
- Rather LJ, Akhter S, Padder RA, Hassan QP, Hussain M, Khan MA, Mohammad F. Colorful and semi durable antioxidant finish of woolen yarn with tannin-rich extract of Acacia nilotica natural dye. Dyes Pigments. 2017;139:812–819. [Google Scholar]
- Rounsaville TJ, Ranney TG. Ploidy levels and genome sizes of Berberis L. and Mahonia Nutt. species, hybrids, and cultivars. HortScience. 2010;45:1029–1033. [Google Scholar]
- Sadeghi-Kiakhani M. Eco-friendly dyeing of wool and nylon using madder as a natural dye: kinetic and adsorption isotherm studies. Int J Environ Sci Technol. 2015;12:2363–2370. [Google Scholar]
- Sadeghi-Kiakhani M, Arami M, Gharanjig K. Application of a biopolymer chitosan-poly(propylene)imine dendrimer hybrid as an antimicrobial agent on the wool fabrics. Iran Polym J. 2013;22:931–940. [Google Scholar]
- Sadeghi-Kiakhani M, Gharanjig K, Arami M. Study on dyeing and fastness properties of wool-polyester blend fabrics using novel mono azonaphthalimide dyes. J Text I. 2014;105:52–58. [Google Scholar]
- Sadeghi-Kiakhani M, Tehrani-Bagha AR, Safapour S. Enhanced anti-microbial, anti-creasing and dye absorption properties of cotton fabric treated with Chitosan-Cyanuric Chloride hybrid. Cellulose. 2018;25:883–893. [Google Scholar]
- Sadeghi-Kiakhani M, Tehrani-Bagha AR, Gharanjig K, Hashemi E. Use of pomegranate peels and walnut green husks as the green antimicrobial agents to reduce the consumption of inorganic nanoparticles on wool yarns. J Clean Prod. 2019;231:1463–1473. [Google Scholar]
- Sathiyavimal S, Vasantharaj S, Bharathi D, Saravanan M, Manikandan E, Kumar SS, Pugazhendhi A. Biogenesis of copper oxide nanoparticles (CuONPs) using Sida acuta and their incorporation over cotton fabrics to prevent the pathogenicity of Gram negative and Gram positive bacteria. J Photochem Photobiol B. 2018;188:126–134. doi: 10.1016/j.jphotobiol.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Shanmuganathan R, Ali DM, Prabakar D, Muthukumar H, Thajuddin N, Kumar SS, Pugazhendhi A. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: green approach. Environ Sci Pollut Res. 2018;25:10362–10370. doi: 10.1007/s11356-017-9367-9. [DOI] [PubMed] [Google Scholar]
- Simoncic B, Tomsic B. Structures of novel antimicrobial agents for textiles: a review. Text Res J. 2010;80:1721–1737. [Google Scholar]
- Thomas B, Prasad AA, Vithiya SM. Evaluation of antioxidant, antibacterial and photo catalytic effect of silver nanoparticles from methanolic extract of Coleus vettiveroids—an endemic species. J Nanostruct. 2018;8(2):179–190. [Google Scholar]
- Vasantharaj S, Sathiyavimal S, Saravanan M, Senthilkumar P, Gnanasekaran K, Shanmugavel M, Manikandan E, Pugazhendhi A. Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: characterization of antibacterial activity and dye degradation potential. J Photochem Photobiol B. 2019;191:143–149. doi: 10.1016/j.jphotobiol.2018.12.026. [DOI] [PubMed] [Google Scholar]
- Vasantharaj S, Sathiyavimal S, Senthilkumar P, Oscar FL, Pugazhendhi A. Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: antimicrobial properties and their applications in photocatalytic degradation. J Photochem Photobiol B. 2019;192:74–82. doi: 10.1016/j.jphotobiol.2018.12.025. [DOI] [PubMed] [Google Scholar]
- Villinski J, Dumas E, Chai HB, Pezzuto J, Angerhofer C, Gafner S. Antibacterial activity and alkaloid content of Berberis thunbergii, Berberis vulgaris and hydrastis canadensis. Pharm Biol. 2003;41:551–557. [Google Scholar]
- Yadav R, Anubhav Mathur P, Sheikh J. Antibacterial, UV protective and antioxidant linen obtained by natural dyeing with Henna. Cellulose Chem Technol. 2019;53(3–4):357–362. [Google Scholar]
- Yu D, Tian W, Sun B, Li Y, Wang W, Tian W. Preparation of silver-plated wool fabric with antibacterial and anti-mold properties. Mater Lett. 2015;151:1–4. [Google Scholar]
- Zargarkazemi A, Sadeghi-Kiakhani M, Arami M, Bahrami SH. Modification of wool fabric using prepared chitosan-cyanuric chloride hybrid. J Text I. 2015;106:80–89. [Google Scholar]
- Zhao X, Qiao ZZ, He JX. Preparation of chitosan biguanidine hydrochloride and application in antimicrobial finish of wool fabric. J Eng Fiber Fabr. 2010;5:16–24. [Google Scholar]
- Zhou CE, Kan CW. Plasma-assisted regenerable chitosan antimicrobial finishing for cotton. Cellulose. 2014;21:2951–2962. [Google Scholar]
- Zille A, Almeida L, Amorim T, Carneiro N, Esteves MF, Silva CJ, Souto AP. Application of nanotechnology in antimicrobial finishing of biomedical textiles. Mater Res Express. 2014;1:1–38. [Google Scholar]