Abstract
Retinoblastoma tumor (RB) is one of the most prevalent ocular cancers among children. RB may be caused by inherited mutations in RB1 gene as well as some environmental risk factors. Human papillomaviruses (HPV) are suspected as a risk factor of RB due to their pRb inactivating protein. This study evaluated the molecular prevalence of HPV among the RB tumor specimens in Iran. The RB tumor samples were tested for detection of HPV-L1 gene using a nested-PCR approach, and then followed by sequencing and phylogenetic analysis to reveal HPV types. Overall, there were 61 RB tumor samples; 54/61 (88.5%) had unilateral and 7/61 (11.5%) bilateral RB; 55/61 cases (90.2%) had sporadic non-familial RB tumor. HPV-DNA was detected in 6/61 (9.8%) of patients’ tumors; the HPV positive RB cases all had unilateral and unfamiliar sporadic RB tumor. HPV type 16 was the most prevalent type identified across the RB tumor samples (3/61, 4.9%). The rate of detected HPV among the RB specimens seems to be considerable. Further investigations are required to elucidate the exact association between HPV and progression to RB.
Electronic supplementary material
The online version of this article (10.1007/s13337-019-00540-7) contains supplementary material, which is available to authorized users.
Keywords: Retinoblastoma tumor, RB, Human papillomavirus, HPV, Iran
Introduction
Retinoblastoma (RB) is the most common ocular tumor which affects the retina mostly in children. The incidence of RB is estimated one per 15,000–18,000 live births [26], and occur approximately 4/million among children lower than 15 years old [43]. The RB1 gene located on the short arm of chromosome 13 encodes the retinoblastoma protein (pRb), a major human tumor suppressor. Mutations that lead to inactivation or absence of pRb cause development of RB tumor [45]. The pRb has significant functions in the control of cell cycle, and its suppression has a major impact in development of several human cancers [27].
In the etiology of RB, there is a two hit-run hypothesis [7]. A child with an inherited allele mutated in RB1 gene may be later affected with alternative risk factors such as infections, chemicals, etc., which lead to other somatic mutations and complete inactivation of pRb activity [34]. Most cases of sporadic RB have been reported from less developed countries and poor regions of the world such as Asia (mostly from India), Latin America, and Africa [4]. This suggests association of RB with environmental and behavioral factors with low levels of healthcare economy and culture [41].
Human papillomavirus (HPV) is a dsDNA virus which infects epithelial cells of different tissues, and has a distinct replication cycle, which strictly synchronized with the life cycle of host cell [32]. HPV can be transmitted and infect genital tract of both genders, and is the main cause of cervical cancer [38, 39]. There are more than 200 well established HPV types; mucosotropic ones classified into low- and high-risk types according to their oncogenic potential [37, 44]. High risk types of HPV, as the most common oncogenic DNA virus, encode proteins that deactivate and/or interfere with natural human proteins with some anti-tumor functions [10]. For example, HPV-E6 binds to p53 protein, and promotes formation of tumor via down regulation and ubiquitin degradation of p53. Another HPV encoded protein, E7, has the capability of binding to pRb anti-tumor family proteins including pRb, cyclin A, p130, and p107, thereby suppressing their anti-tumor functions [15].
These molecular and epidemiological evidence suggests a possible association between the incidences of non-familial sporadic RB and oncogenic pRb-inactivating viruses such as HPV as an environmental cofactor. Recent findings around the world have introduced a hypothesis on the etiology of RB tumor which suggests a presumptive association between HPV and RB. However, there is not any data around the frequency of HPV across the RB tumor in Iran. So, this research aimed to evaluate the molecular presence and typing of HPV in tumor samples among Iranian children with retinoblastoma.
Materials and methods
Patients and samples
This cross-sectional study was approved in the ethics committee of the research deputy of Iran University of Medical Sciences (ethics code: IR.IUMS.REC 1395.95-04-30-28743). The ophthalmology surgery department in the Rasul Akram hospital, affiliated with IUMS, is a major eye surgery department in Iran, and most of the enucleation procedures are performed in this center. In this eye surgery center, formalin-fixed paraffin-embedded (FFPE) samples confirmed histologically as RB tumor were collected from 2013 to 2018. All of the patients’ demographic and histo-clinical information were also recorded.
Extraction of nucleic acids
The FFPE samples were stored in individual boxes in a cold room until practicing the molecular procedures. Then, for each paraffin block, 20 μm thick sections were taken using separate disposable blades. Care was taken to avoid contamination with other specimens; blade keeper and other attachments of the microtome were cleansed by xylene and ethanol before and after cutting the blocks.
For each sample, 2 sections underwent paraffin removal with a xylene-ethanol protocol. Then, the samples were transferred to a 1.5 µl eppendorf tube for DNA isolation using a tissue DNA extraction kit (MN, Macherey–Nagel GmbH & Co. KG) according to the kit instructions with minor modification. The purity of the isolated DNA was assessed by a BioPhotometer (Nanodrop ND-1000 Spectrophotometer) in line with observance at 260/280 nm. The extraction procedure was confirmed by a PCR for beta-globin gene, performed as described previously [18].
Detection of HPV-DNA
For detection of HPV-DNA, a nested-PCR approach was applied using commonly used MY and GP primers (Table 1), as described previously [17, 29]. In the first step, about 25–50 ng of the extracted DNA was used as template in a reaction including 12.5 µl of 2X-PCR master mix (Red, Amplicon, Denmark), 10 pmol/mL of each outer primers (MY09 and MY11), and then brought to 25 µl using sterile ddH2O. The thermal program started with 95 °C for 10 min, then followed by 35 cycles of 94 °C for 1 min, 55 °C for 30 s, and 72 °C for 45 s, next finalized with 10 min at 72 °C for production of full-length strands. The product was a 452 base pair (bp) fragment of HPV-L1 gene, which was ready for nested PCR. The product of MY09/MY11 was used as template DNA for nested stage with inner primers (GP5+ and GP6+) under reaction conditions as with the first stage. The thermal program initiated with a pre-denaturation step (95 °C for 5 min) followed by three steps including 30 s at 94 °C, 20 s at 46 °C, and 20 s at 72 °C repeated in 35 cycles. The product underwent gel electrophoresis using 2% agarose gel, which demonstrated a 142 bp band. In each run, the extract of HeLa cells was used as the positive control, while sterile water along with a confirmed negative sample were used as negative controls. The stages of DNA extraction, PCR preparation, and post-PCR were individually performed in separate rooms.
Table 1.
The sequences of primers used for detection of HPV-L1, and human β globin
| Primer | Sequence | Target | Product size |
|---|---|---|---|
| MY11 | GCMCAGGGWCATAAYAATGG* | HPV-L1 | 452 |
| MY09 | CGTCCMARRGGAWACTGATC | HPV-L1 | |
| GP5+ | TTTGTTACTGTGGTAGATAC | HPV-L1 | 142 |
| GP6+ | GAAAAATAAACTGTAAATCA | HPV-L1 | |
| Bet1 | TCAACCCTACAGTCACCCAT | β-Globin | 501 |
| Bet2 | CTAACAATTACGAACAGCAATGAG | β-Globin |
*Degenerate nucleotides: M (A, C), W (A, T), Y (C, T), R (A, G)
Genotyping of HPV
The PCR product of GP5+/6+ primers was purified and underwent for direct sequencing (BigDye Terminator V3.1 on the ABI 3730XL DNA Analyzer, BIONEER CO). The obtained sequences were aligned in the online BLAST tool in the database of NCBI to elicit HPV types. Again, the sequences were aligned with attributed type specific reference sequences in BioEdit software. The alignment was introduced in MEGA6 software and prepared for a phylogenetic analysis. The evolutionary history was deduced using the Maximum Likelihood method based on the Tamura 3-parameter model. The bootstrap consensus tree inferred from 1000 replicates to represent the evolutionary history of the taxa analyzed. Initial tree for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. A discrete Gamma distribution was employed to model the evolutionary rate among differences sites [21].
Statistical analysis
All demographic, clinical and virological information was introduced and analyzed in SPSS package version 25. Descriptive analysis was used for comparing continuous variables between the groups. Fisher exact test and Chi square tests were utilized to elicit the association between HPV and tumor as well as clinical status.
Result
Baseline demographic and clinical information
Overall, there were 61 FFPE samples from 61 patients diagnosed as RB tumor. Among the patients, 86.9% were from urban and 13.1% were from rural regions. In terms of gender, 32/61 (52.5%) were male and the rest were female. The mean age of the patients was 28.6 ± 17.3 months at the age of enucleation (ranged 2–70 months). Among the RB tumors, 88.2% were unilateral (54% left eye and 35.3% right eye), while 11.7% of patients had bilateral RB. The mean age of enucleation for unilateral was 30.2 ± 17.5 months, while this was 16.3 ± 18.6 for bilateral cases. The majority of patients had no evidence of familial history of RB tumor (90.2%). The mean lag time was 2.9 ± 7.2 months ranging from zero to 50 months. Among the symptoms, the most recorded sign was leucocoria (23.5%), followed by strabism, red eye, decreased vision, and proptosis which reported in 17%, 11.5%, 7.5% and 4% of the patients, respectively.
Prevalence of HPV-DNA
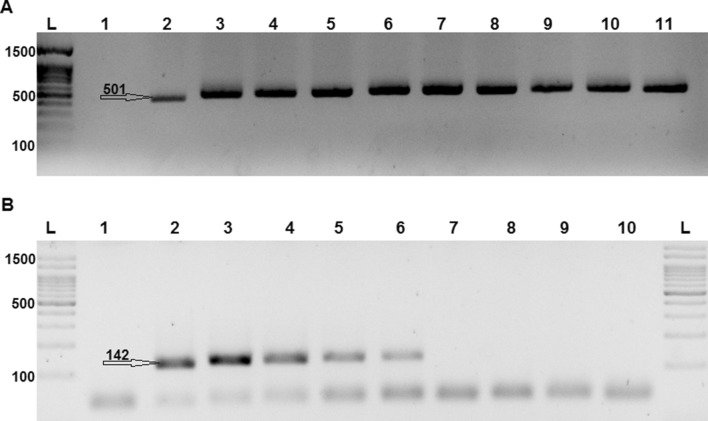
All the Samples were positive for β-globin PCR (Fig. 1), and thus followed by molecular testing of HPV. The product of nested-PCR (GP 5+/6+ primers) was run in the agarose gel, whereby the intended band indicated positive samples (Fig. 1). Overall, there were 6 samples positive for HPV-DNA (9.8%). The mean age of enucleation among HPV positive cases was 27.6 ± 15 months, but among HPV negative cases, it was 29 ± 18 months (p value = 0.646). The mean lag time for HPV positive and negative cases was 3.4 ± 4.8 and 2.8 ± 7.4, months respectively (p value = 0.231). The pathological and clinical characteristics of HPV-positive and -negative RB cases are summarized in Table 2. The prevalence of HPV-DNA among males and females was 6.3%, and 13.8%, respectively (p value = 0.285).
Fig. 1.
a A 501 bp band showing the replication of β-globin gene and efficient DNA extraction; L: 1500 bp. DNA ladder, lane 1: negative control, lane 2: positive control, and lane 3–11: samples. b The PCR products of GP primers loaded in agarose gel representing a 142 bp band: L: 1500 bp DNA ladder, lane 1: negative control, lane 2: positive control, and lane 3–6: positive samples, and lane 7–10 negative samples
Table 2.
Clinical, phenotypic characteristics and status of HPV-DNA among the RB cases
| Characteristic | Number (%) | HPV positive N (%) | HPV negative N (%) | p value |
|---|---|---|---|---|
| Gender | ||||
| Male | 32 (52.5) | 2 (6.3) | 30 (93.7) | 0.285 |
| Female | 29 (47.5) | 4 (13.8) | 25 (86.2) | |
| Laterality | ||||
| Unilateral | 54 (88.5) | 6 (11.1) | 48 (88.8) | 0.045a |
| Bilateral | 7 (11.5) | 0 | 7 (100) | |
| Habitat | ||||
| Urban | 53 (86.9) | 6 (11.3) | 47 (88.7) | 0.125b |
| Rural | 8 (13.1) | 0 | 8 (100) | |
| Tumor differentiation | ||||
| Moderate differentiated | 5 (8.2) | 1 (20) | 4 (80) | 0.654 |
| Poorly differentiated | 3 (4.9) | 0 | 3 (100) | |
| Well differentiated | 33 (54.1) | 2 (6) | 31 (93.9) | |
| Un-known | 18 (29.5) | 3 (16.6) | 15 (83.3) | |
| Tumor extension | ||||
| Intra-ocular | 56 (91.8) | 5 (8.9) | 51 (91.1) | 0.585 |
| Extra-ocular | 5 (8.2) | 1 (20) | 4 (80) | |
aThe prevalence of HPV among unilateral was significantly higher than bilateral
bHPV among urban cases was insignificantly higher than rural ones
Genotyping of HPV
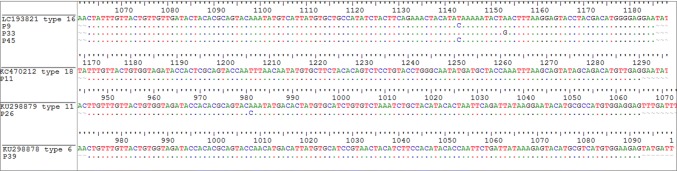
The sequencing was performed successfully; the obtained sequence data were aligned with the reference sequences (Fig. 2) and deposited to the database of gene bank after trimming (Accession numbers: MH539879-MH539884). Phylogenetic analysis was conducted with maximum likelihood program in MEGA6 and represented in a phylogenetic tree, illustrating the distance between the isolates of the current project with the reference sequence of each HPV type (Fig. 3). The result of sequencing and blast revealed that among 6 HPV positive RB cases, 3 cases belonged to HPV type 16 (4.9% of total), with one case for each type of 18, 6, and 11. Overall, the prevalence of high-risk HPV types was 4/61 (6.5%), comprising 66.6% of the HPV-positive cases. There were 2/61 (3.3%) positive for low risk types, comprising 33.3% of the positive cases.
Fig. 2.
Alignment of studied sequences in accordance with reference sequence of each HPV-type
Fig. 3.

Molecular phylogenetic analysis by Maximum Likelihood method: the accession number and HPV types of reference sequences are given in the front of branches. Green circle labels are isolates of the current study. Bovine papillomavirus (AF486184) was used to root the tree (colour figure online)
Discussion
Several viral proteins are inhibitors of pRb, such as SV40-large T antigen, HPV-E7 and adenovirus-E1A [30]. HPV is suspected as a cofactor of RB because of sharing risk factors with RB distribution, furthermore the probability of maternal genital transmission of HPV to the infants has been identified so far [3, 22, 42]. Besides, the risk of RB has been estimated to been protected by cesarean section, and use of condoms in the year prior to pregnancy [9, 16]. There are evidence of presence of HPV in non-epithelial cells [12], it has been also repeatedly isolated from head and neck squamous cell carcinoma [28] and even in a variety of non-RB lesions of eye [11]. Source of detected HPV from non-epithelial cells may be from surrounding HPV infected tissues. Although, HPV-E6 and viral DNA could be synthesized by neurons [11], since transcription factors such as AP-1, Sp1, and NFI are active in neurons and glial cells that are sufficient for early viral protein expression [13]. Since the definitive link between this virus and some cancers has already been demonstrated, assessment for possible virologic impress at retinoblastoma tumor is suggested.
This was the first report investigating HPV in RB tumor in Iran. Molecular assessment of RB samples revealed that 9.8% were positive for HPV-L1 DNA. Around the world, there are several studies that are in line with this study and mostly indicate higher prevalence than ours (Supplementary Table 1). Most reports are from Asia, particular from India, as RB accounts for one of the five most prevalent cancers among children in India. In a most recent report from India, 25.6% of 39 RB cases were positive for HPV [25]. There are also other reports from India with higher rates of HPV; 24% [1], 47% [23], 57.14% [3], and 69.7%, all of which were conducted on FFPE and/or fresh frozen RB samples [41].
There are a few reports from other continents. In two studies from Brazil, prevalence of HPV among 153 FFPE tumor samples was reported as 4.6% [2], and 27.9% among 35 RB cases [31]. In Mexico, the HPV rate among the 39 RB cases was 36% [30], even though, another study from Mexico revealed 82.3% HPV positive out of 51 RB tumor cases tested [24]. These variations in the rate of HPV among different studies may be mostly attributed to the socio-economic level of the population studied, region and race of population, and even technical approaches utilized. In contrast, the prevalence of HPV was zero in the following reports: an investigation from North America did not find any case positive for HPV [14]. Another study from South Korea did not report any HPV positive RB case [33]. These negative results can be explained by the diversity in geography, ethnicity, number of participants, and the assay used. This negative correlation has been also reported in a more recent study, although they used real-time PCR assay that is less sensitive than conventional nested-PCR [36]. In a recent study in India, detection strategies of HPV has been investigated among 20 RB cases, and interestingly detection rate was zero among different assays used (PCR, in situ hybridization and immunohistochemistry) [6]. In the current research, a nested-PCR approach was used, which increased the sensitivity of the test. Also, sequencing was performed to confirm positive cases. The prevalence rate in this study is far less than the rates previously reported, this can be due to regional distribution of HPV, cervical carcinoma and overlapping risk factors of HPV and RB. The prevalence of HPV is so high in developing countries such as Mexico and India, and thus cervical carcinoma is the most common cancer among women in India (30.7 per 100,000 in India, which is highest in south central Asia) [5]. Nevertheless, this figure estimated to 6 per 100,000 in Iran [20].
Another finding of this study, HPV type 16 was the most prevalent type, and in overall 4/61 (6.5%) of RB cases had high-risk types of HPV. In most of other studies, type 16 has also been the prevalent type among the RB cases (Supplementary Table 1) [1, 3, 23, 31]. The dominance of HPV type 16 in the current study and most of previous literature is notable. The burden of cervical atypical squamous lesions and cervical cancer is mostly attributed to this type [8]. HPV type 16 is more likely to integrate into the host chromosome [19, 40], leading to a persistent infection and increasing the level of E6/E7, thereby causing cancer progression.
An interesting finding, the HPV positive RB cases of the current study all had unilateral and unfamiliar sporadic RB tumor, while the rate of HPV was zero among the bilateral cases. Although bilateral RB tumor is mostly caused by germ-line mutations in RB1 gene, meanwhile it has been determined that 17–77% of bilateral ones did not have germ-line mutations in RB1 gene [30]. Again, in regions with high incidence of RB such as India, most of RB occur as non-familial or sporadic cases and familial disease is seen rarely in just 1.7% of cases [35]. In this study, there was not any HPV positive bilateral disease, this finding is in line with previous studies that reported much lower rate of HPV amongst the bilateral RB cases [30].
These findings may suggest a presumptive link between infection with HPV and incidence of sporadic RB. It would be much convenient to conclude if there was a normal control group or neighboring normal tissues for comparing them with RB. Thus, it needs further comprehensive case–control study, and also analysis of RB1 mutations which will be done in future works.
Epidemiological studies on this title have been mostly focused in Asia, and it needs further investigations in different regions of the globe to obtain more conclusive evidence. Despite demonstration of HPV-DNA in RB tumors in the current project and previous studies, further molecular-functional investigations are required to determine the possible involved pathways.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflict of interest
All the authors have stated to have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anand B, Ramesh C, Appaji L, Kumari BSA, Shenoy AM, Nanjundappa, et al. Prevalence of high-risk human papillomavirus genotypes in retinoblastoma. Br J Ophthalmol. 2011;95(7):1014–1018. doi: 10.1136/bjo.2010.199802. [DOI] [PubMed] [Google Scholar]
- 2.Antoneli CBG, Ribeiro KB, Sredni ST, Arias VEA, Andreoli MA, de Camargo B, et al. Low prevalence of HPV in Brazilian children with retinoblastoma. J Med Virol. 2011;83(1):115–118. doi: 10.1002/jmv.21925. [DOI] [PubMed] [Google Scholar]
- 3.Bhuvaneswari A, Pallavi VR, Jayshree RS, Kumar RV. Maternal transmission of human papillomavirus in retinoblastoma: a possible route of transfer. Indian J Med Paediatr Oncol. 2012;33(4):210–215. doi: 10.4103/0971-5851.107080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunin GR, Meadows AT, Emanuel BS, Buckley JD, Woods WG, Hammond GD. Pre-and postconception factors associated with sporadic heritable and nonheritable retinoblastoma. Cancer Res. 1989;49(20):5730–5735. [PubMed] [Google Scholar]
- 5.Chandana V, Gaguturu RB. Prevalence and determinants of high risk human papilloma virus in Hyderabad, India. Int J Reprod Contracept Obstet Gynecol. 2018;7(3):1012–1018. [Google Scholar]
- 6.Chauhan S, Sen S, Singh N, Sharma A, Chawla B, Kashyap S. Human papillomavirus detection strategies in retinoblastoma. Pathol Oncol Res. 2019;26:1–4. doi: 10.1007/s12253-018-00577-x. [DOI] [PubMed] [Google Scholar]
- 7.Chintagumpala M, Chevez-Barrios P, Paysse EA, Plon SE, Hurwitz R. Retinoblastoma: review of current management. Oncologist. 2007;12(10):1237–1246. doi: 10.1634/theoncologist.12-10-1237. [DOI] [PubMed] [Google Scholar]
- 8.de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Paula Silva N, de Souza Reis R, Cunha RG, Oliveira JF, de Oliveira Santos M, Pombo-de-Oliveira MS, de Camargo B. Maternal and birth characteristics and childhood embryonal solid tumors: a population-based report from Brazil. PLoS ONE. 2016;11(10):e0164398. doi: 10.1371/journal.pone.0164398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Villiers E-M, Fauquet C, Broker TR, Bernard H-U, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324(1):17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 11.Di Girolamo N. Association of human papilloma virus with pterygia and ocular-surface squamous neoplasia. Eye. 2011;26:202. doi: 10.1038/eye.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Füle T, Máthé M, Suba Z, Csapó Z, Szarvas T, Tátrai P, et al. The presence of human papillomavirus 16 in neural structures and vascular endothelial cells. Virology. 2006;348(2):289–296. doi: 10.1016/j.virol.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 13.Füle T, Máthé M, Suba Z, Csapó Z, Szarvas T, Tátrai P, Paku S, Kovalszky I. The presence of human papillomavirus 16 in neural structures and vascular endothelial cells. Virology. 2006;348(2):289–296. doi: 10.1016/j.virol.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 14.Gillison ML, Chen R, Goshu E, Rushlow D, Chen N, Banister C, et al. Human retinoblastoma is not caused by known pRb-inactivating human DNA tumor viruses. Int J Cancer. 2007;120(7):1482–1490. doi: 10.1002/ijc.22516. [DOI] [PubMed] [Google Scholar]
- 15.Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000;92(9):690–698. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- 16.Heck JE, Omidakhsh N, Azary S, Ritz B, von Ehrenstein OS, Bunin GR, Ganguly A. A case–control study of sporadic retinoblastoma in relation to maternal health conditions and reproductive factors: a report from the Children’s Oncology group. BMC Cancer. 2015;15(1):735. doi: 10.1186/s12885-015-1773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Javanmard D, Namaei MH, Haghighi F, Ziaee M, Behravan M, Mirzaei J, et al. The frequency and typing of human Papilloma virus among women with normal and abnormal cytology in Southern Khorasan, Eastern Iran. Jundishapur J Microbiol. 2017;10(4):e43213. [Google Scholar]
- 18.Javanmard D, Behravan M, Ghannadkafi M, Salehabadi A, Ziaee M, Namaei MH. Detection of Chlamydia trachomatis in pap smear samples from South Khorasan Province of Iran. Int J Fertil Steril. 2018;12(1):31. doi: 10.22074/ijfs.2018.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karbalaie Niya MH, Keyvani H, Safarnezhad Tameshkel F, Salehi-Vaziri M, Teaghinezhad-S S, Bokharaei Salim F, et al. Human papillomavirus type 16 integration analysis by real-time PCR assay in associated cancers. Transl Oncol. 2018;11(3):593–598. doi: 10.1016/j.tranon.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khorasanizadeh F, Hassanloo J, Khaksar N, Mohammad Taheri S, Marzaban M, H Rashidi B, et al. Epidemiology of cervical cancer and human papilloma virus infection among Iranian women—analyses of national data and systematic review of the literature. Gynecol Oncol. 2013;128(2):277–281. doi: 10.1016/j.ygyno.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 70 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mammas IN, Sourvinos G, Spandidos DA. The ‘Trojan horse’oncogenic strategy of HPVs in childhood. Future Virol. 2013;8(8):801–808. [Google Scholar]
- 23.Mohan A, Venkatesan N, Kandalam M, Pasricha G, Acharya P, Khetan V, et al. Detection of human papillomavirus DNA in retinoblastoma samples: a preliminary study. J Pediatr Hematol Oncol. 2009;31(1):8–13. doi: 10.1097/MPH.0b013e31818b373b. [DOI] [PubMed] [Google Scholar]
- 24.Montoya-Fuentes H, de la Paz Ramirez-Munoz M, Villar-Calvo V, Suarez-Rincon AE, Ornelas-Aguirre JM, Vazquez-Camacho G, et al. Identification of DNA sequences and viral proteins of 6 human papillomavirus types in retinoblastoma tissue. Anticancer Res. 2003;23(3c):2853–2862. [PubMed] [Google Scholar]
- 25.Naru J, Aggarwal R, Singh U, Kakkar N, Bansal D. HPV-16 detected in one-fourth eyes with retinoblastoma: a prospective case-control study from North India. J Pediatr Hematol Oncol. 2016;38(5):367–371. doi: 10.1097/MPH.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 26.Naseripour M, Nazari H, Bakhtiari P, Modarres-zadeh M, Vosough P, Ausari M. Retinoblastoma in Iran: outcomes in terms of patients’ survival and globe survival. Br J Ophthalmol. 2009;93(1):28–32. doi: 10.1136/bjo.2008.139410. [DOI] [PubMed] [Google Scholar]
- 27.Nevins JR. The Rb/E2F pathway and cancer. Hum Mol Genet. 2001;10(7):699–703. doi: 10.1093/hmg/10.7.699. [DOI] [PubMed] [Google Scholar]
- 28.Niya MHK, Tameshkel FS, Panahi M, Salim FB, Monavari SHR, Keyvani H. Human papillomavirus investigation in head and neck squamous cell carcinoma: initial report from the low risk HPV types associations. Asian Pac J Cancer Prev. 2017;18(9):2573. doi: 10.22034/APJCP.2017.18.9.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nobre RJ, de Almeida LP, Martins TC. Complete genotyping of mucosal human papillomavirus using a restriction fragment length polymorphism analysis and an original typing algorithm. J Clin Virol. 2008;42(1):13–21. doi: 10.1016/j.jcv.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 30.Orjuela M, Castaneda VP, Ridaura C, Lecona E, Leal C, Abramson DH, et al. Presence of human papilloma virus in tumor tissue from children with retinoblastoma: an alternative mechanism for tumor development. Clin Cancer Res. 2000;6(10):4010–4016. [PubMed] [Google Scholar]
- 31.Palazzi MA, Yunes JA, Cardinalli IA, Stangenhaus GP, Brandalise SR, Ferreira SA, et al. Detection of oncogenic human papillomavirus in sporadic retinoblastoma. Acta Ophthalmol Scand. 2003;81(4):396–398. doi: 10.1034/j.1600-0420.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 32.Pizzol D, Putoto G, Chhaganlal KD. Human papillomavirus (HPV) infection: a Mozambique overview. Virusdisease. 2016;27(2):116–122. doi: 10.1007/s13337-016-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryoo N-K, Kim J-E, Choung H-K, Kim N, Lee M-J, Khwarg S-I. Human papilloma virus in retinoblastoma tissues from Korean patients. Korean J Ophthalmol. 2013;27(5):368–371. doi: 10.3341/kjo.2013.27.5.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sagi M, Frenkel A, Eilat A, Weinberg N, Frenkel S, Peer J, et al. Genetic screening in patients with Retinoblastoma in Israel. Fam Cancer. 2015;14(3):471–480. doi: 10.1007/s10689-015-9794-z. [DOI] [PubMed] [Google Scholar]
- 35.Sahu S, Banavali S, Pai S, Nair C, Kurkure P, Motwani S, et al. Retinoblastoma: problems and perspectives from India. Pediatr Hematol Oncol. 1998;15(6):501–508. doi: 10.3109/08880019809018311. [DOI] [PubMed] [Google Scholar]
- 36.Saktanasate J, Saksiriwutto P, Uiprasertkul M, Horthongkham N, Trinavarat A, Atchaneeyasakul L. Human papillomavirus DNA in paraffin-embedded retinoblastoma. J Med Assoc Thai. 2018;101:229–231. [Google Scholar]
- 37.Salehi-Vaziri M, Sadeghi F, Bokharaei-Salim F, Younesi S, Alinaghi S, Monavari SH, et al. The prevalence and genotype distribution of human papillomavirus in the genital tract of males in Iran. Jundishapur J Microbiol. 2015;8(12):e21912. doi: 10.5812/jjm.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salehi-Vaziri M, Sadeghi F, Alamsi-Hashiani A, Haeri H, Monavari SH, Keyvani H. Merkel cell polyomavirus and human papillomavirus infections in cervical disease in Iranian women. Adv Virol. 2015;160(5):1181–1187. doi: 10.1007/s00705-015-2368-4. [DOI] [PubMed] [Google Scholar]
- 39.Salehi-Vaziri M, Sadeghi F, Hashemi FS, Haeri H, Bokharaei-Salim F, Monavari SH, et al. Distribution of human papillomavirus genotypes in Iranian women according to the severity of the cervical lesion. Iran Red Crescent Med J. 2016;18(4):e24458. doi: 10.5812/ircmj.24458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitz M, Driesch C, Jansen L, Runnebaum IB, Dürst M. Non-random integration of the HPV genome in cervical cancer. PLoS ONE. 2012;7(6):e39632. doi: 10.1371/journal.pone.0039632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shetty OA, Naresh KN, Banavali SD, Shet T, Joshi R, Qureshi S, et al. Evidence for the presence of high risk human papillomavirus in retinoblastoma tissue from nonfamilial retinoblastoma in developing countries. Pediatr Blood Cancer. 2012;58(2):185–190. doi: 10.1002/pbc.23346. [DOI] [PubMed] [Google Scholar]
- 42.Skoczyński M, Goździcka-Józefiak A, Kwaśniewska A. Risk factors of the vertical transmission of human papilloma virus in newborns from singleton pregnancy—preliminary report. J Matern Fetal Neonatal Med. 2014;27(3):239–242. doi: 10.3109/14767058.2013.807238. [DOI] [PubMed] [Google Scholar]
- 43.Walter SD, Harbour JW. Molecular biology of retinoblastoma. In: Francis J, Abramson D, editors. Recent advances in retinoblastoma treatment. Essentials in Ophthalmology. Cham: Springer; 2015. pp. 1–13. [Google Scholar]
- 44.Yahyapour Y, Shamsi-Shahrabadi M, Mahmoudi M, Motevallian A, Siadati S, Shefaii S, et al. High-risk and low-risk human papillomavirus in esophageal squamous cell carcinoma at Mazandaran, Northern Iran. Pathol Oncol Res. 2013;19(3):385–391. doi: 10.1007/s12253-012-9590-0. [DOI] [PubMed] [Google Scholar]
- 45.Yun J, Li Y, Xu C-T, Pan B-R. Epidemiology and Rb1 gene of retinoblastoma. Int J Ophthalmol. 2011;4(1):103. doi: 10.3980/j.issn.2222-3959.2011.01.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.