Abstract
Large numbers of bioactive natural products from plant species such as alkaloids, phenolics, terpenoids etc. are remaining unexplored for their potential as plant protective agents as inhibitors for viral and other pathogenic infections of plant. Myzus aphids are important plant pests and vectors for several plant viruses. Cauliflower mosaic virus (CaMV) belongs to the plant virus family Caulimoviridae which is transmitted “non-circulative” from plant to plant through an interaction with aphid insect vectors. This viral transmission process most likely involves a protein–protein binding interaction between aphid stylet receptor cuticular protein and viral proteins namely, CaMV aphid transmission Helper Component protein and virion associated protein. Aphid stylets are made of cuticle and little is known about the structure of cuticle protein of this insect group. The present study reports the molecular modeling of the structures of Myzus persicae aphid stylet’s cuticular protein (MpsCP) and cauliflower mosaic virus aphid transmission Helper component protein (CaMV HCP). Protein–protein docking studies and molecular dynamics simulations are performed to establish the mode of binding of MpsCP with CaMV HCP. Molecular docking and molecular dynamics investigations of terpenoids Annosquamosin-A from Annona squamosa complex with CaMV transmitting aphid M. persicae stylet’s cuticular protein revealed their means of interaction perhaps relates to restrain viral binding and transmission. QM/MM optimization of mesoporous silica nanopores composite with Annosquamosin-A for smart and safe delivery of bioactive is carried out to study their electronic parameters such as heat of formation, total energy, electronic energy, Ionization potential, Highest Occupied Molecular Orbital, Lowest Un-occupied Molecular Orbital and energy gaps.
Keywords: Molecular modeling, Molecular dynamics, Myzus persicae stylet’s cuticle protein, Cauliflower mosaic virus aphid transmission, Annosquamosin-A, Nano-porous silica
Introduction
Cauliflower mosaic virus (CaMV) is a plant virus belongs to the family of Caulimoviridae. CaMV is transmitted from plant to plant through an interaction with aphid insect vectors [18]. Aphids are the most common arthropod vectors which widely adopted the noncirculative transmission strategy for virus–vector interaction. Hundreds of plant virus species transmitted by insect vectors is categorized as “noncirculative” when the virus is absorbed from an infected plant by a feeding vector, taken up on the cuticle layer of the inner component of the feeding device known as stylet, and subsequently released to new host plant for inoculation. This process involves an aphid stylet receptor (ASR) and two viral proteins namely, CaMV aphid transmission Helper Component protein (HC-P2) and virion associated protein (P3). HC-P2 binds to both the aphid stylet receptor (ASR) and P3, itself firmly combined with the virus particle, with the assembly making a contagious viral complex. One of the reported characteristics of aphid stylet receptor (ASR) is to be a deeply embedded cuticular protein. The 3D atomic structure of aphid stylet cuticular protein (ASR) is not yet resolved due to the technical complications associated in extracting cuticle components and biochemical examination of live insects.
Viral putative receptor possibly the cuticle protein in the stylet of aphid plays an important role in aphid acquisition of plant virus [23]. The non-persistent plant viruses are attached in the food canal site [42]. Therefore, the mechanism of association between non-persistent plant viruses and their vector may be explored by shedding light on the 3D structural knowledge of the composition of aphid cuticle proteins and the concerning role of CaMV in transmission [3]. In vitro association between aphid cuticle protein and the potyviral transmission HC-Protein is reported by Dombrovsky et al. [9], however, there is a need for extensive structural elucidation of aphid stylet cuticle proteins and their binding with viral transmission HC-Protein to shed additional light on the plant viral transmission process. The structural dynamics insight will be helpful in elucidating the 3D structural models of CaMV HC-Protein P2 and Aphid stylet’s cuticular protein. The protein–protein docking of Aphid stylet’s cuticular protein with CaMV aphid transmission helper component protein (HCP) P2 is essential to obtain more detailed structural information on the protein components, binding mode of interactions and conformation that probably occur during binding, which would be highly valuable for improved knowledge of plant-aphid-virus interactions.
Natural products from plant species such as alkaloids, phenolics, terpenoids etc. are phytochemicals known for their protective role against viruses, bacteria, fungus, insects and other pathogens [25]. Huge numbers of plant product compounds remain unexplored for their potential as plant protective agents as inhibitors for viral and other pathogenic infections of plant. Antiviral chemicals have been used to protect plants against some viruses transmitted by aphids [34]. The limonoid class of phytochemicals segregated from Munronia unifoliola Oliv. and beta-carboline alkaloids and quassinoid seperated from the wood of Picrasma quassioides Bennhave in MeOH extort been reported showing Tobacco Mosaic antiviral activity [6, 16]. Proteins interact in complicated ways because their shapes are vastly complex [13] and bioinformatics tools are effective in characterizing the binding sites of protein interaction. Annosquamosin-A is a diterpenoid from Annona squamosa [43] commonly known as sugar apple that is widely used for many applications including pest management [24]. Natural biocompatible nano-particulate based smart delivery systems [21] might aid the safe release kinetics of antiviral small molecules without which the bioactive molecules may suffer disintegration due to their sensitivity to UV radiation and other abiotic factors. The purpose of nanotechnology in delivery of pesticide aims to decrease the unsystematic use of conventional pesticides and guarantee their secure use [28]. Green Synthezised mesoporous silica with nanopores produced from sugarcane waste ash [1, 29] can be considered for further composite with Annosquamosin-A for the reason that not only for the safe and smart delivery of the bioactive to restrain CaMV aphid transmission which is a multilayered interaction [7] but also due to the additional plant protection benefit of mesoporous bio-silica (SiO2, Silicon dioxide) in amorphous form providing a structural barrier to pathogens [11, 32]. Mesoporous Silica nanomaterials (MSNs) were widely utilized to deliver biomolecules and biopesticides as “nanocarriers”, as it is promising to make uniform, dispersible and porous nanoparticles with self-assembly induced by evaporation and colloidal chemistry. However, the modern agricultural investigators established the prospective applications of nanotechnology employing MSNs only in most recent years as they have several exclusive properties, such as a huge surface area, a tunable pore size for high loading ability, biocompatibility and an ability to control bioactive pesticide release that could have an advantageous effect on environmental safety and reduce non-target insect contact to pesticides such as Pyrimethanil [46].
The present study aims to perform the following computational studies using bioinformatics tools: (1) Molecular modeling of Myzus persicae aphid stylet’s cuticular protein (MpsCP) and CaMV aphid transmission Helper Component protein (CaMV HCP) (2) Determining the mode of biding of MpsCP complex with CaMV HCP using protein–protein docking interaction studies (3) Computational docking and screening of natural product terpenoids against MpsCP binding with CaMV HCP. (4) Molecular dynamics of MpsCP-annosquamosin-A complex (5) Designing of mesoporous Silica nano-pores as carrier for smart delivery of promising diterpenoid annosquamosin-A using QM/MM optimization.
Materials and methods
Molecular modeling
Molecular modeling of aphid M. persicae stylet’s cuticle protein (MpsCP) and cauliflower mosaic virus aphid transmission Helper Component protein (CaMV HCP) were performed using computational threading of macromolecular structure prediction of proteins. The 3D structure of MpsCP with 226 amino acids {Uniprot KB: Q95V16} and CaMV HCP with 159 amino acids {SWISS PROT ID: P03548} were computed based on threading approach by I-TASSAR server [30, 44, 45] and the binding site residues were predicted. The mode of biding of MpsCP complex with CaMV HCP is determined using protein–protein docking interaction studies. Natural product plant protective agents such as terpenoids, phenols and alkaloids were collected from phytochemical structure databases such as chEBI, chEMBL and PubChem.
Protein structure preparation
The MspCP and CaMV HCP structures were preprocessed individually using the protein preparation wizard present in Maestro v9.2 to refine the protein structures. The molecular mechanics force field OPLS 2005 (optimized potentials for liquid simulations) was employed with RMSD cut-off of 0.3 Å in the impref minimization. Minimization is carried out for assigning the bond order, adding hydrogen atoms and amendment of ionization and tautomerization state with pH 7.0. A receptor grid generation was performed surround the binding site of MpsCP utilizing Glide v5.7 [14]. This binding site is a region on MpsCP that binds to other proteins or ligands.
Ligand preparation
The terpenoid structures collected from different phytochemical structure databases were converted from 2D to 3D and prepared using LigPrep tool. LigPrep constructs 3D structures corresponding to low energy besides accurate chiralities for each accomplished input ligands. LigPrep generates number of conformation from every structure with diverse ring conformations, stereochemistries, tautomers and ionization states using Epik [36] besides removes the molecules found on unfit functional group and molecular weight. The minimum energy conformations of terpenoids were established by a short conformational search performed in the energy minimization incorporating Macromodel of Schrödinger suit using OPLS 2005 force field. OPLS 2005 is compatible for aqueous environment besides ab initio calculations [35].
Glide docking
Molecular docking is the procedure which calculates the stable complex bound to form in favored orientation of one molecule to the other [4, 22]. Docking analysis was performed to know the binding modes of terpenoids against MpsCP protein as well as CaMV aphid transmission HC protein. The binding manner of protein–ligand complex can be calculated using the docking tool Glide. Hierarchical sequence of filters is employed in Glide that glance the binding pocket of the protein for probable binding sites of ligands. The receptor grid of 20 × 20 × 20 Å was created surround the binding site of M. persicae stylet’s cuticular protein (MpsCP) protein employing glide v5.7 [14]. Extra precision (XP) docking and Standard precision (SP) docking were the two types of docking precision incorporated in the present work. Screening compounds of indefinite quality in huge amount is achieved by SP while XP docking is to a large extent potent and sensitive procedure, which needs longer duration to run than SP. Ligand poses that have a high score with SP docking is further subjected to XP docking procedure which is accurate and generates 10,000 poses for each ligand and the best is pose predicted based on Emodel. XPGscore (extra precision glide score) of each ligand is accounted for ranking the best poses. Good quality binding affinity towards protein is indicated by the least XPGscore for a ligand [15].
Molecular dynamics of protein–ligand complex
Molecular dynamic (MD) simulation for the MpsCP–Annosquamosin-A complex was carried out for 60 ns (nanosecond) production run incorporating the OPLS-2005 force field in TIP3P water model aqueous environment [20] using Desmond v3.2 [33] software. Classical physics concepts of molecular dynamic is integrated in Desmond computer simulation program besides compatible with the following biomolecular force fields: CHARMM, GROMOS, OPLS-AA, AMBER and MMFF, in which the sum of bonded and non-bonded interactions contribute to the computation of total potential energy (E) [5]. However, Desmond is also suitable for a longer time steps of 2 fs used in the present molecular dynamics. The MpsCP–Annosquamosin-A complex was solvated in a 61 Å × 62 Å × 88 Å size box of orthorhombic shape which makes sure the solvent molecule enfold the entire complex. Counter ions Na+ and Cl− were added to balance the net charge of the system and the whole system was neutralized. Approximately 33,421 atoms were present in total in the system simulated by Desmond with periodic boundary conditions. The MpsCP–Annosquamosin-A complex system is subjected to a sequence of restrained minimizations and MD simulations in which the coordinate of the protein molecule were not much deviating from the initial structure. NVT (constant number of atoms N, volume V and temperature T) ensemble was maintained with the relaxed system for 12 ps (picoseconds). During MD simulation, Long-Range electrostatic interactions [12, 39] were computed using particle-mesh Ewald (PME) method and the cut-off of 9 Å was fixed for van der waals (VDW) force. The temperature and pressure was maintained at 300 K and 1.01325 bar respectively. The geometry constraints of hydrogen atoms in covalent bonds were assured by shake algorithm at every time step [31] during simulation. The simulation of whole system was carried out to ascertain the stability of MpsCP–Annosquamosin-A complex. The fluctuations in Energy profile and RMSD for the heavy atom and backbone of the complex were analyzed through MD simulation trajectory. The hydrogen bond interactions of intermolecular MpsCP–Annosquamosin-A complex were evaluated for consistency. The conformational stability of amino acid residues of MpsCP–Annosquamosin-A complex during MD trajectory runs were examined by calculating the dihedral angle Phi (Φ) and Psi (ψ) of the backbone atom and verified by Ramachandran plots.
Semi-empirical quantum mechanics optimization
QM/MM optimization of silica nanopores conjugated with annosquamosin-A is carried out using Molecular Orbital Package [38] and Argus Lab (“Thompson, ACS Meeting, 2004”) which are popular tools to compute the QM/MM output that includes the effect of composite nanomaterials—bioactive structure on its energy, heat of formation, charge distribution, dipole moments and their significant electronic parameters resulting in electronic energy, Ionization potential, Highest Occupied Molecular Orbital, (HOMO), energy gap, Lowest Un-occupied Molecular Orbital (LUMO), and Molecular Electrostatic potential (ESP) maps that are reported.
Results
Molecular modeling of aphid M. persicae stylet’s cuticle protein
The amino acid sequence of cuticular protein from M. persicae aphid stylet (226aa) was downloaded from uniprot entry: Q95V16 in FASTA format and representative PDB structure library is used for first threading to search for the possible folds by Hidden Markov Model, Profile- Profile Alignment (PPA), PSI-BLAST profiles, Smith-Waterman and Needleman-Wunsch alignment algorithms. The top 10 alignments are included from the threading programs as follow: MUSTER, SPARKS-X, Neff-PPAS and PROSPECT2. The MpsCP structure was modeled using the PDB ID: 4NL6A as template as it had the best Z-score using all the ten algorithms. Table 1 represents the top 10 proteins from the PDB that have the closest structural similarity to the predicted MpsCP model. Out of the 5 models predicted from I-TASSER server in which the best model with C-Score of − 2.41 was selected with estimated accuracy of 0.43 ± 0.14 (i.e., TM-Score of 0.57). C-score is a score of confidence calculated to estimate the quality of predicted models based on the convergence parameters of the structure assembly simulations besides the significance of threading template alignments by I-TASSER. The binding site residues predicted in the 3D structure of MpsCP were SER11, TYR74, TYR100, TYR105, SER106, VAL108, ARG115, VAL117, TYR119, TYR124, ALA129, ALA149, ALA151, ALA154, PRO155, ALA159, TYR 157, TYR 196, SER197 and TYR 206.
Table 1.
Top PDB structural analogs Identified by I-Tasser to compute protein structure of Myzus persicae stylet’s cuticle protein and cauliflower mosaic virus aphid transmission Helper Component Protein
| MpsCP | CaMV HCP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | PDB hit | TM-score | RMSD | Identity | Coverage | Rank | PDB hit | TM-score | RMSD | Identity | Coverage |
| 1 | 4NL6A | 0.897 | 2.21 | 0.066 | 0.978 | 1 | 4PU3A2 | 0.562 | 4.34 | 0.032 | 0.862 |
| 2 | 2R7TA | 0.445 | 5.92 | 0.063 | 0.774 | 2 | 3P8CA2 | 0.555 | 4.29 | 0.107 | 0.830 |
| 3 | 1BXRA | 0.439 | 5.76 | 0.041 | 0.757 | 3 | 3FBRA | 0.554 | 4.49 | 0.014 | 0.855 |
| 4 | 1LSHA | 0.438 | 6.19 | 0.057 | 0.796 | 4 | 1ST6A | 0.533 | 3.62 | 0.075 | 0.723 |
| 5 | 3S1SA | 0.426 | 5.93 | 0.014 | 0.739 | 5 | 4IGGA | 0.532 | 3.74 | 0.055 | 0.717 |
| 6 | 3UOXA | 0.425 | 5.88 | 0.046 | 0.735 | 6 | 4U94A1 | 0.532 | 3.88 | 0.085 | 0.774 |
| 7 | 1B25A | 0.420 | 5.88 | 0.059 | 0.735 | 7 | 4BY6A | 0.524 | 4.78 | 0.099 | 0.868 |
| 8 | 1T3TA | 0.419 | 5.93 | 0.066 | 0.739 | 8 | 3AKJA | 0.523 | 4.62 | 0.054 | 0.811 |
| 9 | 2ZE9A | 0.418 | 5.82 | 0.054 | 0.704 | 9 | 3U24A | 0.522 | 4.35 | 0.078 | 0.767 |
| 10 | 1V0TA | 0.417 | 5.79 | 0.073 | 0.708 | 10 | 3G15A2 | 0.520 | 3.99 | 0.082 | 0.736 |
Molecular modeling of cauliflower mosaic virus aphid transmission Helper component protein
The amino acid sequence of cauliflower mosaic virus (strain Strasbourg) aphid transmission helper component protein P2 (159aa) was downloaded from uniprot entry: P03548 in FASTA format and threaded using a PDB structure library to search for the probable folds. The PDB ID: 4PU3A had been predicted with the best structural resemblance Z-score using all the structural alignment algorithms and was used for modeling CaMV HCP2 protein structure. The top 10 proteins from the PDB that have the closest structural similarity to the predicted CaMV HCP2 protein model are listed in Table 1. The model with best C-Score of − 4.09 estimated by I-TASSER server was selected. The binding site residues predicted in the 3D model structure of CaMV HCP protein were ASN64, ASP72, GLU75, ASN113, ILE116, LYS117, GLU 120, LYS137, GLU138, GLU139, LYS141, GLU142, LYS144, GLU145, ASN148, SER149, ILE150, GLY153 and ASN156.
Mode of binding of M. persicae stylet’s cuticle protein complex with cauliflower mosaic virus aphid transmission helper component protein by protein–protein docking and molecular dynamics
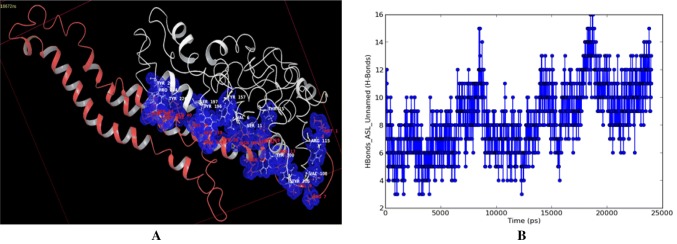
Molecular docking simulation as well as molecular dynamics study of protein–protein interaction of aphid M. persicae stylet’s cuticle protein (MpsCP) and cauliflower mosaic virus aphid transmission Helper Component Protein (CaMV HCP) revealed their mode of binding (Fig. 1) and atomic level intermolecular hydrogen bonding interactions (Table 3). The binding site residues GLU138, GLU139, GLU145 and ASN148 of CaMV HCP and counterpart binding site residues SER11, TYR100, TYR105, VAL108, ARG115, TYR 157, TYR196, SER197 and TYR 206 of MpsCP were involved in interacting through direct hydrogen bonds and water bridges in the CaMV HCP-MpsCP complex throughout the MD simulation.
Fig. 1.
a Modeling of Protein–protein docked complex of aphid Myzus persicae stylet’s cuticle protein (in white) and CaMV transmission HC Protein (in red) and b Hydrogen Bond profile (in blue) of the complex during MD simulation trajectory (color figure online)
Table 3.
Intermolecular hydrogen bonding observed during the protein–protein interaction of CaMV aphid transmission HCP protein complex with Myzus persicae stylet’s cuticle protein (MpsCP)
| Sl. no. | Myzus persicae cuticle protein | CaMV transmission helper component protein P2 | Distance Å | ||
|---|---|---|---|---|---|
| Residues | Atom | Residues | Atom | ||
| 1 | PRO:224 | O | ASN:44 | HD22 | 2.18 |
| 2 | TYR:226 | O | GLN:40 | HE22 | 2.17 |
| 3 | TYR:206 | O | ASN:148 | HD22 | 1.86 |
| 4 | TYR:206 | HH | GLU:145 | OE2 | 1.67 |
| 5 | VAL:108 | H | PRO:7 | O | 2.03 |
| 6 | TYR:105 | O | ARG:17 | HH21 | 1.79 |
| 7 | SER:11 | H | TYR:30 | O | 1.97 |
| 8 | TYR:196 | HH | GLU:138 | OE1 | 1.71 |
| 9 | THR:15 | OG1 | LYS:19 | HZ1 | 1.96 |
| 10 | VAL:6 | H | ASN:26 | OD1 | 2.02 |
| 11 | SER:197 | OG | LYS:36 | HZ1 | 1.72 |
| 12 | TYR:157 | HH | GLU:139 | OE1 | 1.89 |
| 13 | SER:11 | HG | SER:22 | OG | 2.46 |
| 14 | TYR:100 | OH | ASN:24 | HD22 | 1.72 |
| 15 | SER:11 | H | TYR:30 | O | 1.97 |
| 16 | ARG:115 | H | MET:1 | O | 2.11 |
Molecular docking screen of natural product terpenoids against the viral binding with aphid stylet’s receptor
The structural details of following terpenoids (Table 2) were collected from PubChem and HTVS (High Throughput Virtual Screening, SP and XP docking) was computed using Software Schrodinger’s Glide to complex with the refined structural model of MpsCP for assessing their binding interactions: Annosquamosin-A, Bulleyanin, Ruvoside, 2′r,3r,4a′r,5r,5′r,8a′s)-5-(furan-3-yl)-2′,5′-dimethyloctahydro-2′h-spiro[furan-3,1′-naphthalene]-2,7′(3′h)-dione, 4-Carvomenthenol alpa-Terpineol, Nopol, 3D- Bulleyanin, Dihydronopol, Phenol, Gefarnate, Limonene, Bornyl Chloride, Di-epi-alpha-cedrene, Strobane and Myrcenyl Acetate. Annosquamosin-A is found to be a suitable ligand that can bind with CaMV transmitting aphid M. persicae Stylet’s CP and may restrain the binding of CaMV virion transmission HCP (p2) Protein.
Table 2.
Docking studies of terpenoids against cauliflower mosaic virus aphid transmission
| S. no. | Compound name | Pub Chem ID | Myzus persicae stylet’s CP | CaMV transmission HCP Protein | ||
|---|---|---|---|---|---|---|
| Docking XPG SCORE | Docking energy kcal/mol | Docking XPG SCORE | Docking energy kcal/mol | |||
| 1 | Annosquamosin A | 177318 | − 5.47 | − 34.17 | − 4.66 | − 42.95 |
| 2 | Bulleyanin | 494286 | − 4.30 | − 35.46 | − 3.40 | − 42.56 |
| 3 | Ruvoside | 23292 | − 4.09 | − 32.32 | − 4.93 | − 42.14 |
| 4 | Crotonin (terpene) | 186030 | − 4.095 | − 29.40 | − 3.03 | − 28.54 |
| 5 | Gefarnate | 5282182 | − 2.90 | − 34.07 | − 3.54 | − 38.04 |
| 6 | 4-Carvomenthenol | 11230 | − 3.87 | − 16.81 | − 2.78 | − 15.84 |
| 7 | Nopol | 31408 | − 3.62 | − 18.00 | − 2.91 | − 20.26 |
| 8 | Alpa-terpineol | 17100 | − 3.79 | − 22.67 | − 2.75 | − 17.82 |
| 9 | Dihydronopol | 107330 | − 3.23 | − 13.27 | − 2.41 | − 17.83 |
| 10 | Phenol | 996 | − 3.16 | − 14.68 | – | – |
| 11 | Limonene | 22311 | − 2.80 | − 16.79 | − 2.31 | − 17.69 |
| 12 | Bornyl Chloride | 10048 | − 2.22 | − 14.96 | − 2.18 | − 16.20 |
| 13 | Di-epi-alpha-cedrene | 521207 | − 2.18 | − 17.21 | − 2.66 | − 19.69 |
| 14 | Strobane | 22833294 | − 2.08 | − 21.21 | − 2.01 | − 21.29 |
| 15 | Myrcenyl Acetate | 14235 | − 1.35 | − 20.56 | – | – |
| 16 | 3D- Bulleyanin | 338942 | − 3.59 | − 35.71 | − 0.72 | − 33.49 |
Docking of terpenoids against M. persicae stylet’s cuticle protein
The di-terpenoid Annosquamosin-A has the lowest XPG scoring of − 5.47 and glide energy of − 34.17 kcal/mol. The bound terpenoid interrelate to MpsCP with the following two hydrogen bondings: the oxygen atom (O) intermingled not only with atom H of non-polar side chain residue MET:4 with a intermolecular hydrogen bond distance of 2.161 Å but also with hydrogen atom (H) of polar residue TYR:3 with a distance of 2.272 Å. Bulleyanin has the XPG score of − 4.30 and glide energy of − 35.46 kcal/mol when bound to MpsCP with the following two hydrogen bonds: the hydrogen atom (H) interacted with oxygen atom (O)of non-polar side chain residue MET:4 with an inter atomic distance of 1.749 Å and oxygen atom (O) interacted with atom H of non-polar side chain residue MET:4 with an inter atomic distance of 2.079 Å. Ruvoside exhibits a XPG score of − 4.09 and glide energy of − 32.32 kcal/mol when bound to MpsCP with the following hydrogen bond: the hydrogen atom (H) interrelated with hydroxyl group (OH) of polar side chain residue TYR:3 with an inter atomic bond distance of 2.108 Å. Crotonin/2′r,3r,4a′r,5r,5′r,8a′s)-5-(furan-3-yl)-2′,5′-dimethyloctahydro-2′h-spiro[furan-3,1′-naphthalene]-2,7′(3′h)-dione shows a XPG scoring of − 4.09 and glide energy of − 29.40 kcal/mol when complex with MpsCP interrelating as tag along hydrogen bonds: the oxygen atom (O) intermingled with hydrogen atom (H) of non-polar side chain residue MET:4 with an inter atomic bond distance of 2.262 Å. XPG score of − 3.87 and glide energy of − 16.81 kcal/mol was recorded for 4-Carvomenthenol complex with MpsCP with a hydrogen bond length of 2.043 Å between the atom (H) of 4-Carvomenthenol with oxygen atom (O) of non-polar side chain amino acid ALA:146.
The alpa-Terpineol, Nopol, Dihydronopol and Phenol when complex with MpsCP show XPG scores of − 3.79, − 3.62, − 3.23 and − 3.16 respectively and glide energy of − 22.67, − 18.00, − 13.27 and − 14.68 kcal/mol respectively. The atoms (H) of alpa-Terpineol and Nopol interrelated with atom (O) of polar side chain residue SER:11 via an intermolecular hydrogen bond of lengths 1.863 Å and 1.738 Å respectively. The atoms (H) of dihydronopol and phenol each formed an intermolecular hydrogen bond of lengths 1.824 Å and 1.833 Å respectively with atoms (O) of non-polar side chain residues ALA:146 and ALA:154 respectively.
Docking of terpenoids against cauliflower mosaic virus aphid transmission Helper component protein
The terpenoid Ruvoside has the lowest XPG scoring of − 4.93 and glide energy value of − 42.14 kcal/mol when bound to cauliflower mosaic virus aphid transmission helper component protein P2. Annosquamosin A, Bulleyanin, Gefarnate and Crotonin when complex with CaMV HCP2 show the XPG scores of − 4.66, − 3.40, − 3.54 and − 3.03 respectively and glide energy of − 42.95, − 42.56, − 38.04 and − 28.54 kcal/mol respectively.
Molecular docking screen studies of High troughput virtual screening (HTVS), XP and SP of phytochemicals terpenoids to complex with CaMV transmitting aphid M. persicae stylet’s cuticle protein (MpsCP) and CaMV HCP proteins revealed that Annosquamosin-A (a diterpenoid) from A. squamosa (Custard apple fruits) would be a suitable ligand with lowest docking energy and scoring that can be considered for further bio-conjugation with silica nanoporous materials for smart delivery to restrain the binding of CaMV HCP protein with M. persicae stylet’s cuticle proteins.
Molecular dynamics studies of M. persicae stylet’s cuticle protein complex with Annosquamosin-A
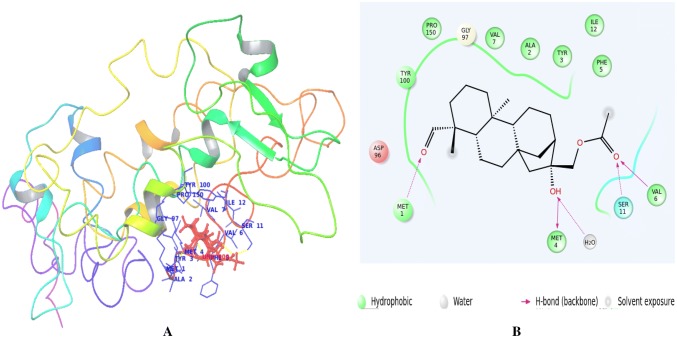
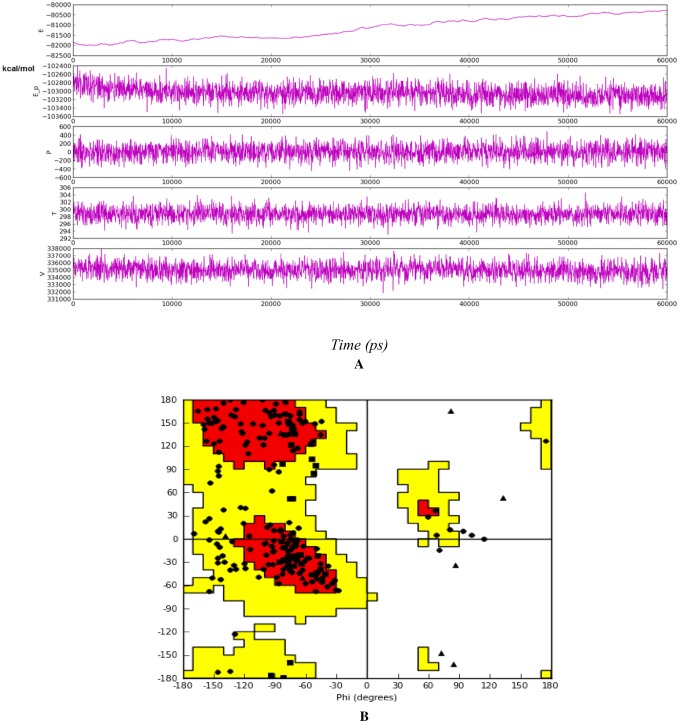
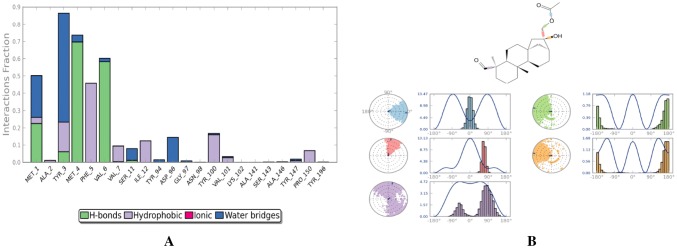
The mode of binding of annosquamosin A with M. persicae Stylet’s CP and stability of the complex is studied by molecular dynamics analysis for 60 ns (20 ns X 3). The intermolecular hydrogen bonds, water bridges and hydrophobic interactions observed during the MD trajectory of protein–ligand complex (Fig. 2a) suggest that the protein–ligand complex is stable. The MD trajectory of protein–protein interaction of aphid M. persicae stylet’s cuticle protein (MpsCP) and CaMV transmission HC Protein complex was analysed to determine the time dependent interactions and release using molecular dynamics simulations. A diagram of comprehensive ligand atom contacts with the protein residues interactions in the selected trajectory that occur more than 30.0% of the simulation time (0.00 ns through 20.00 ns X 3), are shown in Fig. 2b. Protein secondary structure elements (SSE) such as alpha helices and beta strands are observed throughout the MD simulation of protein–ligand interaction of aphid M. persicae stylet’s cuticle protein (MpsCP) complex with annosquamosin A. The total SSE distribution by residue index is computed as 12.78% for throughout the MpsCP protein structure which is a sum of 11.34% for alpha-helices and 1.44% for beta-strands. MD energy profile in Fig. 3a and Ramachandran map in Fig. 3b for the Protein–ligand complex suggest the structural intactness of the complex.
Fig. 2.
a, b Binding site view of Myzus persicae Stylet’s cuticle protein complex with Annosqumosin-A complex during Molecular Dynamics trajectory
Fig. 3.
a MD energy profile in 60 ns trajectroy; b Ramachandran Plot for the Myzus persicae Stylet’s cuticle protein complex with annosquamosin-A after 60 ns
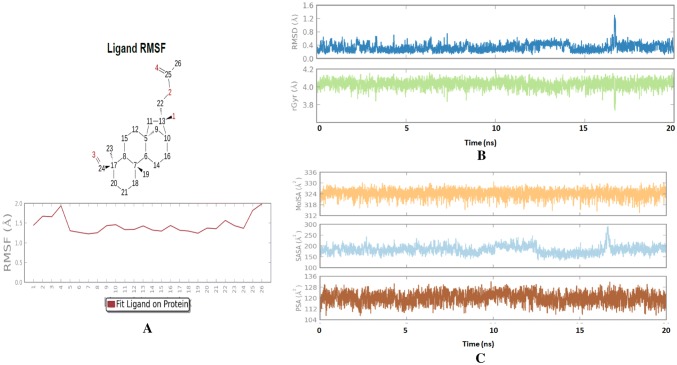
The protein–ligand contact (Fig. 4a) and ligand torsion profile (Fig. 4b) of annosquamosin-A into the binding site of MpsCP records the allowed rotations of ligand bonds in the binding site during molecular dynamics. The kinetics of annosquamosin-A and stable intactness of the ligand pertain to the binding pocket in protein structure during MD trajectory of protein–ligand interaction of aphid M. persicae stylet’s cuticle protein (MpsCP) complex were obtained as follows: Ligand RMSD and Radius of Gyration (rGyr) that measures the ‘extendedness’ of a ligand. The ligand properties of annosquamosin-A during the equilibrated MD trajectory of protein–ligand interaction of aphid M. persicae stylet’s cuticle protein (MpsCP) complex was analysed (Fig. 5a–c) to determine the following: Ligand RMSD, Radius of Gyration (rGyr) that measures the ‘extendedness’ of a ligand, Molecular Surface Area, Polar Surface Area and Solvent Accessible Surface Area. Molecular Surface Area (MolSA) of the ligand corresponds to a van der Waals surface area, Solvent Accessible Surface Area (SASA) is denoting accessible region by a water molecule and Polar Surface Area (PSA) in a molecule is contributed only by nitrogen and oxygen atoms. The Ligand Root Mean Square Fluctuation (L-RMSF) is useful for describing variations in the ligand atom positions. The ligand RMSF gives the insights on how ligand fragments interact with the protein and their entropic role in the binding event. The ligand RMSF of annosquamosin-A during the two phases of MD trajectory of protein–ligand interaction of aphid M. persicae stylet’s cuticle protein (MpsCP) complex were analysed and found to be stable.
Fig. 4.
a Protein–Ligand contact and b Ligand torsion profile of Annosquamosin-A into the binding site of Myzus persicae stylet’s cuticle protein during Molecular Dynamics trajectory of 60 ns
Fig. 5.
a RMSF, b RMSD and rGyr, c PSA, SASA and MolSA of Annosquamosin A into the binding site of MpsCP during equilibrated final 20 ns MD simulation
Quantum mechanics/molecular mechanics optimization of mesoporous silica nanopores composite with Annosquamosin-A for smart delivery
A hybrid quantum mechanics/molecular mechanics (QM/MM) approach is a potent modeling procedure that allows one to determine the electronic state density of state (DOS) structure and associated electrostatic effects of the complex inhomogeneous nanomaterials such as bioactives composite of meso-porous silica on the reactants such as bioactive compounds, enzymes, chromophore, etc. The QM/MM approach is mainly useful when less expensive implicit solvation models are inappropriate. The complexation mechanism of Annosquamosin-A with nano-porous silica are investigated through the electronic structure estimation by semi-empirical ZINDO quantum mechanical approach besides UFF molecular mechanics method was employed to examine their geometry using Argus Lab. Molecular Orbital Package (MOPAC) is a popular tool to compute the QM/MM output that includes the effect of composite nanomaterial structure on its energy, heat of formation, charge distribution and dipole moments. Molecular modeling and dynamics investigations of a diterpenoid Annosquamosin-A from A. squamosa (Custard apple fruits) complex with cauliflower mosaic virus (CaMV) transmitting aphid M. persicae stylet’s cuticle protein (MpsCP) revealed the mode of binding of annosquamosin-A as an appropriate ligand for MpsCP that can be considered for further bio-conjugation with silica nanomaterials for smart delivery. QM/MM optimization of silica nanopores conjugated with annosquamosin (A) is carried out and their significant electronic parameters resulting in heat of formation (enthalpy), total energy, electronic energy, Ionization potential, HOMO, LUMO, energy gap, and ESP Map are reported. Band gap change is observed with respect to the variation in pore size of the silica nanomaterials conjugated annosquamosin-A (Fig. 6a, b). UFF MM Geometric Optimization of Annosquamosin-A loaded mesoporous silica nanopore composite is carried out with convergence energy gradient of 0.1 kcal/mol/Ang. BFGS line search protocol is operated and the Total Energy output is − 10,580.22 kcal/mol. ZINDO QM electronic structure determination is carried out for the molecular orbitals of Annosquamosin-A loaded mesoporous silica nanopore consisting of a set of (984) valance electrons in which HOMO (492) = − 9.1685 eV; LUMO (493) = 1.1042 eV and ESP Map shown in (Fig. 6c–e).
Fig. 6.
a Annosquamosin-A composite with mesoporous Silica nanopore (one layer of silica is shown); b Narrowing of Band Gap Energy with different nanopore size; c HOMO; d LUMO; e ESP Map
The band gap narrowing signifies the increase in oxygen atoms in the composite pore that is responsible for the holding of bioconjugate in the composite and releasing when dissolved. However, there is a saturation in decrease in band gap is observed indicating the minimum optimized pore size (> 6 nm) of the silica nanomaterial composite with annosquamosin (A). The band gap narrowing signifies the increase in oxygen atoms in the composite pore that is responsible for the holding of bioconjugate in the composite and releasing when dissolved.
Discussion
The binding site residues predicted in the present model structure of CaMV HCP protein were ASN64, ASP72, GLU75, ASN113, ILE116, LYS117, GLU 120, LYS137, GLU138, GLU139, LYS141, GLU142, LYS144, GLU145, ASN148, SER149, ILE150, GLY153 and ASN156 in which the amino acids ASN64, ASP72 and GLU75 were not only associated with vector binding [26, 41] but also ASN113, ILE116, LYS117 and GLU 120 belong to helix α1 and LYS137, GLU138, GLU139, LYS141, GLU142, LYS144, GLU145, ASN148, SER149, ILE150, GLY153 and ASN156 belong to helix α2 where as these helices are responsible for self association of virion binding [17]. Out of the twelve binding site residues predicted by present threading algorithms [30, 44, 45] used for the 3D structure of MysCP, the TYR105, SER106, VAL117, TYR119 and ALA129 belong to the conserved amino-acids of ‘hard’ cuticle cross-linking [10, 19].
The present protein–protein docking and molecular dynamics study of CaMV HCP complex with MpsCP disclosed that not only the involvement of predicted binding site residues but also the following N-terminus amino acid residues from vector binding region [41] of CaMV HCP such as PRO:7, ARG:17, LYS:19, SER:22, ASN:24, ASN:26,TYR:30, LYS:36, GLN:40 and ASN:44 are seen actively engaged in direct hydrogen bond interaction with the following counterpart residues of MpsCP such as VAL:108, TYR:105, THR:15, SER:11, TYR:100, VAL:6, SER:11, SER:197, TYR:226 and PRO:224 respectively along with water mediated hydrogen bond (Table 3).
The molecular dynamics structural investigation of diterpenoids [40] modeled into the binding site of target proteins received considerable attention to signify the stability of the complex. Molecular modeling and dynamics investigations of Annosquamosin-A complex with CaMV transmitting aphid M. persicae stylet’s cuticle protein (MpsCP) revealed the mode of binding of Annosquamosin-A as an appropriate ligand for restraining the CaMV transmission binding with MpsCP and Annosquamosin-A-MpsCP complex is stable throughout the 60 ns MD simulations. Present target to restrain the interaction between CaMV HCP protein and MpsCP using Annosquamosin-A is similar as referred earlier as bioactive small molecules from botanical sources and their analogues could be able to modulate the protein–protein interaction interface [2, 27, 37]. QM/MM computation to optimize the electronic parameters of silica nanopores composite with current annosquamosin-A revealed a band gap narrowing that signifies the increase in oxygen atoms in the composite pore that is responsible for the holding of bioactive in the composite and releasing when dissolved.
Molecular Modeling and molecular dynamics of protein–protein interaction of aphid M. persicae stylet’s cuticle protein (MpsCP) complex with cauliflower mosaic virus aphid transmission Helper Component Protein (P2) revealed their mode of binding and atomic level intermolecular interactions. Molecular docking screen and molecular dynamic studies of terpenoids to complex with CaMV transmitting aphid M. persicae stylet’s cuticle protein (MpsCP) revealed that Annosquamosin-A would be a suitable ligand that can be considered for restraining CaMV aphid transmission. The MD trajectory analysis for 60 ns validated the stability of Annosquamosin-A complex with M. persicae stylet’s cuticle protein (MpsCP). QM/MM computation of Annosquamosin-A composite with bio-synthesized mesoporous silica nanopore propose the possibility of smart delivery of bioactive Annosquamosin-A that relates to the group of natural terpenes [8] probably helpful for restraining aphid viral transmission in plants.
Acknowledgements
Tamil Nadu Agricultural University is acknowledged for the sanctioned URP No. (NRM/CBE/NST/2015/003) and the authors acknowledge the computational facility by Science and Engineering Research Board, DST (SERB) Ref No. (SR/FT/LS-157/2009), Government of India, New Delhi.
Compliance with ethical standards
Conflict of interest
All the authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alves RH, Reis TVS, Rovani S, Fungaro DA. Green synthesis and characterization of biosilica produced from sugarcane waste ash. J Chem. 2017 doi: 10.1155/2017/6129035. [DOI] [Google Scholar]
- 2.Arkin MR, Tang Y, Wells JA. Small-molecule inhibitors of protein–protein interactions: progressing toward the reality. Chem Biol. 2014;21(9):1102–1114. doi: 10.1016/j.chembiol.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bak A, Gargani D, Macia JL, Malouvet E, Vernerey MS, Blanc S, et al. Virus factories of Cauliflower mosaic virus are virion reservoirs that engage actively in vector transmission. J Virol. 2013;87:12207–12215. doi: 10.1128/JVI.01883-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blessy JJ, Sharmila DJS. Molecular modeling of methyl-α-Neu5Ac analogues docked against cholera toxin—a molecular dynamics study. Glycoconj J. 2015;32:49–67. doi: 10.1007/s10719-014-9570-6. [DOI] [PubMed] [Google Scholar]
- 5.Bowers KJ, Chow E, Xu H, Dror RO, Eastwood MP, Gregersen BA, Klepeis JL, Kolossvary I, Moraes MA, Sacerdoti FD, Salmon JK, Shan Y, Shaw DE. Scalable algorithms for molecular dynamics simulations on commodity clusters. In: SC 2006 conference, proceedings of the ACM/IEEE; 2006. p. 43–3.
- 6.Chen J, Yan XH, Dong JH, Sang P, Fang X, et al. Tobacco mosaic virus (TMV) inhibitors from Picrasma quassioides benn. J Agric Food Chem. 2009;57:6590–6595. doi: 10.1021/jf901632j. [DOI] [PubMed] [Google Scholar]
- 7.Dáder B, Then C, Berthelot E, Ducousso M, Ng JCK, Drucker M. Insect transmission of plant viruses: multilayered interactions optimize viral propagation. Insect Sci. 2017;24(6):929–946. doi: 10.1111/1744-7917.12470. [DOI] [PubMed] [Google Scholar]
- 8.Dambolena JS, Zunino MP, Herrera JH, Pizzolitto RP, Areco VA, Zygadlo JA. Terpenes: natural products for controlling insects of importance to human health—a structure-activity relationship study. Psyche A J Entomol. 2016 doi: 10.1155/2016/4595823. [DOI] [Google Scholar]
- 9.Dombrovsky A, Gollop N, Chen S, Chejanovsky N, Raccah B. In vitro association between the helper component-proteinase of zucchini yellow mosaic virus and cuticle proteins of Myzus persicae. J Gen Virol. 2007;88:1602–1610. doi: 10.1099/vir.0.82769-0. [DOI] [PubMed] [Google Scholar]
- 10.Dombrovsky A, Huet H, Zhang H, Chejanovsky N, Raccah B. Comparison of newly isolated cuticular protein genes from six aphid species. Insect Biochem Mol Biol. 2003;33:709–715. doi: 10.1016/S0965-1748(03)00065-1. [DOI] [PubMed] [Google Scholar]
- 11.Elsharkawy MM, Mousa KM. Induction of systemic resistance against Papaya ring spot virus (PRSV) and its vector Myzus persicae by Penicillium simplicissimum GP17-2 and silica (SiO2) nanopowder. Int J Pest Manag. 2015;61(4):353–358. doi: 10.1080/09670874.2015.1070930. [DOI] [Google Scholar]
- 12.Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG. A smooth particle mesh Ewald method. J Chem Phys. 1995;103:8577. doi: 10.1063/1.470117. [DOI] [Google Scholar]
- 13.Ferrari S, Pellati F, Costi MP. Protein–protein interaction inhibitors: case studies on small molecules and natural compounds. In: Mangani S, editor. Disruption of protein–protein interfaces. Berlin: Springer; 2013. pp. 31–60. [Google Scholar]
- 14.Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 15.Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein–ligand complexes. J Med Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 16.Ge YH, Liu KX, Zhang JX, Mu SZ, Hao XJ. The limonoids and their antitobacco mosaic virus (TMV) activities from Munronia unifoliolata Oliv. J Agric Food Chem. 2012;60:4289–4295. doi: 10.1021/jf205362d. [DOI] [PubMed] [Google Scholar]
- 17.Hebrard E, Drucker M, Leclerc D, Hohn T, Uzest M, Froissart R, Strub JM, Sanglier S, van Dorsselaer A, Padilla A, Labesse G, Blanc S. Biochemical characterization of the helper component of Cauliflower mosaic virus. J Virol. 2001;75(18):8538–8546. doi: 10.1128/JVI.75.18.8538-8546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoh F, Uzest M, Drucker M, Plisson-Chastang C, Bron P, Blanc S, Dumas C. Structural insights into the molecular mechanisms of Cauliflower mosaic virus transmission by its insect vector. J Virol. 2010;84(9):4706–4713. doi: 10.1128/JVI.02662-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iconomidou VA, Willis JH, Hamodrakas SJ. Unique features of the structural model of ‘hard’ cuticle proteins: implications for chitin-protein interactions and cross-linking incuticle. Insect Biochem Mol Biol. 2005;35(6):553–560. doi: 10.1016/j.ibmb.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1983;79:926. doi: 10.1063/1.445869. [DOI] [Google Scholar]
- 21.Lembo D, Cavalli R. Nanoparticulate delivery systems for antiviral drugs. Antivir Chem Chemother. 2010;21(2):53–70. doi: 10.3851/IMP1684. [DOI] [PubMed] [Google Scholar]
- 22.Lengauer T, Rarey M. Computational methods for biomolecular docking. Curr Opin Struct Biol. 1996;6:402–406. doi: 10.1016/S0959-440X(96)80061-3. [DOI] [PubMed] [Google Scholar]
- 23.Liang Y, Gao XW. The cuticle protein gene MPCP4 of Myzus persicae (Homoptera: Aphididae) plays a critical role in cucumber mosaic virus acquisition. J Econ Entomol. 2017;110(3):848–853. doi: 10.1093/jee/tox025. [DOI] [PubMed] [Google Scholar]
- 24.Lin CY, Wu DC, Yu JZ, Chen BH, Wang CL, Ko WH. Control of silverleaf whitefly, cotton aphid and kanzawa spider mite with oil and extracts from seeds of sugar apple. Neotrop Entomol. 2009;38(4):531–536. doi: 10.1590/S1519-566X2009000400016. [DOI] [PubMed] [Google Scholar]
- 25.Mann RS, Kaufman PE. Natural product pesticides: their development, delivery and use against insect vectors. Mini-Rev Org Chem. 2012;9(2):185–202. doi: 10.2174/157019312800604733. [DOI] [Google Scholar]
- 26.Moreno A, Hebrard E, Uzest M, Blanc S, Fereres A. A single amino acid position in the helper component of Cauliflower mosaic virus can change the spectrum of transmitting vector species. J Virol. 2005;79:13587–13593. doi: 10.1128/JVI.79.21.13587-13593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullard A. Protein–protein interaction inhibitors get into the groove. Nat Rev Drug Discov. 2012;11(3):173–175. doi: 10.1038/nrd3680. [DOI] [PubMed] [Google Scholar]
- 28.Nuruzzaman M, Rahman MM, Liu Y, Naidu R. Nanoencapsulation, nano-guard for pesticides: a new window for safe application. J Agric Food Chem. 2016;64:1447–1483. doi: 10.1021/acs.jafc.5b05214. [DOI] [PubMed] [Google Scholar]
- 29.Rahman NA, Widhiana I, Juliastuti SR, Setyawan H. Synthesis of mesoporous silica with controlled pore structure from bagasse ash as a silica source. Colloids Surf A Physicochem Eng Asp. 2015;476:1–7. doi: 10.1016/j.colsurfa.2015.03.018. [DOI] [Google Scholar]
- 30.Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryckaert JP, Ciccotti G, Berendsen HJC. Numerical integration of cartesian equations of motion of a system with constraints: molecular dynamics of N-alkanes. J Comput Phys. 1977;23:327–341. doi: 10.1016/0021-9991(77)90098-5. [DOI] [Google Scholar]
- 32.Sakr N. Silicon control of bacterial and viral diseases in plants. J Plant Prot Res. 2016;56(4):331–336. doi: 10.1515/jppr-2016-0052. [DOI] [Google Scholar]
- 33.Shan Y, Kim ET, Eastwood MP, Dror RO, Seeliger MA, Shaw DE. How does a drug molecule find its target binding site? J Am Chem Soc. 2011;133:9181–9183. doi: 10.1021/ja202726y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shanks CH, Chapman RK. The use of antiviral chemicals to protect plants against some viruses transmitted by aphids. Virology. 1965;25:f13–f17. doi: 10.1016/0042-6822(65)90255-2. [DOI] [PubMed] [Google Scholar]
- 35.Sharmila DJS, Blessy JJ. Molecular dynamics of sialic acid analogues complex with cholera toxin and DFT optimization of ethylene glycol-mediated zinc nanocluster conjugation. J Biomol Struct Dyn. 2017;35(1):182–206. doi: 10.1080/07391102.2015.1136689. [DOI] [PubMed] [Google Scholar]
- 36.Shelley JC, Cholleti A, Frye LL, Greenwood JR, Timlin MR, Uchimaya M. Epik: a software program for pK a prediction and protonation state generation for drug-like molecules. J Comput Aided Mol Des. 2007;21:681–691. doi: 10.1007/s10822-007-9133-z. [DOI] [PubMed] [Google Scholar]
- 37.Sheng C, Dong G, Miao Z, Zhang W, Wang W. State-of-the-art strategies for targeting protein–protein interactions by small-molecule inhibitors. Chem Soc Rev. 2015;44(22):8375. doi: 10.1039/c5cs90090e. [DOI] [PubMed] [Google Scholar]
- 38.Stewart JJP. Stewart Computational Chemistry, Colorado Springs, CO, USA, MOPAC 2016; 2016. http://OpenMOPAC.net.
- 39.Strahan GD, Keniry MA, Shafer RH. NMR structure refinement and dynamics of the K+-[d(G3T4G3)]2 quadruplex via particle mesh Ewald molecular dynamics simulations. Biophys J. 1998;75:968–981. doi: 10.1016/S0006-3495(98)77585-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun HD, Huang SX, Han QB. Diterpenoids from Isodon species and their biological activities. Nat Prod Rep. 2006;23(5):673–698. doi: 10.1039/b604174d. [DOI] [PubMed] [Google Scholar]
- 41.Syller J. The roles and mechanisms of helper component proteins encoded by potyviruses and caulimoviruses. Physiol Mol Plant Pathol. 2006;67:119–130. doi: 10.1016/j.pmpp.2005.12.005. [DOI] [Google Scholar]
- 42.Whitfield AE, Falk BW, Rotenberg D. Insect vector-mediated transmission of plant viruses. Virology. 2015;479–480:278–289. doi: 10.1016/j.virol.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 43.Wu YC, Hung YC, Chang FR, Cosentino M, Wang HK, Lee KH. Identification of ent-16 beta, 17-dihydroxykauran-19-oic acid as an anti-HIV principle and isolation of the new diterpenoids annosquamosins A and B from Annona squamosa. J Nat Prod. 1996;59(6):635–637. doi: 10.1021/np960416j. [DOI] [PubMed] [Google Scholar]
- 44.Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. The I-TASSER suite: protein structure and function prediction. Nat Methods. 2015;12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao P, Cao L, Ma D, Zhou Z, Huang Q, Pan C. Synthesis of pyrimethanil-loaded mesoporous silica nanoparticles and its distribution and dissipation in cucumber plants. Molecules. 2017;22(5):817–829. doi: 10.3390/molecules22050817. [DOI] [PMC free article] [PubMed] [Google Scholar]