Abstract
Background
The International Liaison Committee on Resuscitation guidelines recommend target temperature management (TTM) between 32 and 36 °C for patients after out-of-hospital cardiac arrest, but did not indicate patient-specific temperatures. The association of serum lactate concentration and neurological outcome in out-of-hospital cardiac arrest patient has been reported. The study aim was to investigate the benefit of 32–34 °C in patients with various degrees of hyperlactatemia compared to 35–36 °C.
Methods
This study was a post hoc analysis of the Japanese Association for Acute Medicine out-of-hospital cardiac arrest registry between June 2014 and December 2015. Patients with complete targeted temperature management and lactate data were eligible. Patients were stratified to mild (< 7 mmol/l), moderate (< 12 mmol/l), or severe (≥ 12 mmol/l) hyperlactatemia group based on lactate concentration after return of spontaneous circulation. They were subdivided into 32–34 °C or 35–36 °C groups. The primary endpoint was an adjusted predicted probability of 30-day favorable neurological outcome, defined as a cerebral performance category score of 1 or 2.
Result
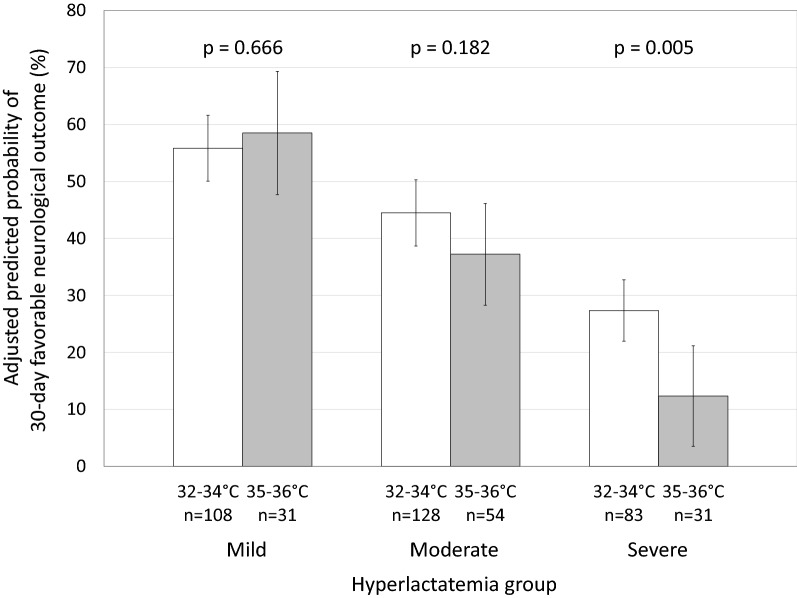
Of 435 patients, 139 had mild, 182 had moderate, and 114 had severe hyperlactatemia. One hundred and eight (78%) with mild, 128 with moderate (70%), and 83 with severe hyperlactatemia (73%) received TTM at 32–34 °C. The adjusted predicted probability of a 30-day favorable neurological outcome following severe hyperlactatemia was significantly greater with 32–34 °C (27.4%, 95% confidence interval: 22.0–32.8%) than 35–36 °C (12.4%, 95% CI 3.5–21.2%; p = 0.005). The differences in outcomes in those with mild and moderate hyperlactatemia were not significant.
Conclusions
In OHCA patients with severe hyperlactatemia, the adjusted predicted probability of 30-day favorable neurological outcome was greater with TTM at 32–34 °C than with TTM at 35–36 °C. Further evaluation is needed to determine whether TTM at 32–34 °C can improve neurological outcomes in patients with severe hyperlactatemia after out-of-hospital cardiac arrest.
Keywords: Out-of-hospital cardiac arrest, Hyperlactatemia, Targeted temperature management
Background
Out-of-hospital cardiac arrest (OHCA) is associated with high mortality and poor neurological outcomes [1]. Targeted temperature management (TTM) is often used to treat comatose OHCA patients after the return of spontaneous circulation (ROSC) [2–4]. The International Liaison Committee on Resuscitation guidelines strongly recommend TTM between 32 and 36 °C [5, 6] but did not provide patient-specific target temperatures. Precision medicine allows mild therapeutic hypothermia or normothermia to be chosen for individual patients, based on the initial brain damage [7]. Specific OHCA patient characteristics that can predict the benefits of 32–34 °C compared with 35–36 °C [8–12] have not been identified.
Serum lactate concentration is a nonspecific marker of tissue hypoxia, aerobic glycolysis, and lactate clearance [13]. The association of serum lactate and neurological outcome has been described in OHCA [14–17] but the differential benefits of TTM at 32–34 °C and TTM at 35–36 °C in patients with different initial serum lactate concentrations have not been reported.
We hypothesized that the effects of TTM at 32–34 °C had different influence according to the severity of hyperlactatemia after ROSC. The study aim was to investigate the benefit of TTM at 32–34 °C in OHCA patients with various degrees of hyperlactatemia compared to TTM at 35–36 °C.
Methods
Study design, setting, and patient selection
This study was a post hoc analysis of patients who were included in the Japanese Association for Acute Medicine out-of-hospital cardiac arrest (JAAM-OHCA) registry between June 2014 and December 2015. The registry is a nationwide, multicenter prospective registry that included 56 institutions in 2014 and 73 in 2015. The JAAM-OHCA registry collected both pre- and post-hospitalization data. Prehospitalization data were obtained from the All Japan Utstein Registry of the Fire and Disaster Management Agency as previously reported [18]. In-hospital data were collected via an Internet-based system by physicians or medical staff at each institution. The JAAM-OHCA registry committee integrated the prehospital and in-hospital data, as previously described [1]. The protocol was approved by the Institutional Review Board of each participating hospital. Comatose adult OHCA patients with TTM treatment after ROSC were included and patients with withdrawn TTM were excluded.
Data collection
Patient age, sex, witness status, presence of dispatcher instruction, presence of a bystander who performed cardiopulmonary resuscitation (CPR), etiology of cardiac arrest (cardiac or not), initial cardiac rhythm, prehospital epinephrine administration, prehospital airway management, prehospital automated external defibrillator (AED) use, response time (time from call to contact with a patient), time from call to hospital arrival, prehospital ROSC, Glasgow Coma Scale score, coronary angiography, use of a mechanical circulatory device (extracorporeal membrane oxygen and/or intra-aortic balloon pumping), door-to-first ABG measurement after ROSC, arterial blood gas measurement data including serum lactate concentration after ROSC, door-to-TTM initiation, target temperature during TTM, and neurological outcome 30 days after cardiac arrest were included in the analysis. The responsibility for setting target temperature was entrusted entirely to each physician or each institution. There were no specific protocols of TTM for the JAAM-OHCA registry.
Primary exposure
Patients were divided by serum lactate concentration after ROSC into mild (< 7 mmol/l), moderate (< 12 mmol/l), or severe (≥ 12 mmol/l) hyperlactatemia group. The cutoff values were chosen for reasons that were previously described [15, 16]. Then, patients in each hyperlactatemia group were subdivided into 32–34 °C and 35–36 °C groups based on their target temperature.
Outcome measures
The primary endpoint was an adjusted predicted probability of 30-day favorable neurological outcome. A favorable neurological outcome was defined as a cerebral performance category (CPC) score of 1 or 2 [19]. The CPC score includes five outcomes, including 1, good cerebral recovery; 2, moderate cerebral disability; 3, severe cerebral disability; 4, coma or vegetative state; and 5, death/brain death [20]. The secondary outcome was an adjusted predicted probability of 30-day survival.
Statistical analysis
To obtain the adjusted predicted probabilities of 30-day favorable neurological outcome and 30-day survival, we used multiple logistic regression model adjusted for age > 65 [21, 22], gender [21, 23], witness [24], dispatcher instruction [25, 26], bystander CPR [24, 27], cardiac etiology [28], initial shockable rhythm [29, 30], prehospital epinephrine administration [31], prehospital advanced airway management [32], time from call to hospital arrival [33, 34], prehospital ROSC [35, 36], Glasgow Outcome Scale score [37], coronary angiography [38], extracorporeal membrane oxygenation (ECMO) and/or intra-aortic balloon pumping (IABP) [22, 39], and PaCO2 30–50 mm Hg [40, 41], as performed in previous studies [23, 42].
We firstly examined the comparison between patients finally analyzed and those excluded. Then, we assessed the association of serum lactate after ROSC and both crude favorable neurological outcomes and the adjusted predicted probability of favorable neurological outcomes in the patients finally analyzed. Furthermore, study participants were divided into 32–34 °C and 35–36 °C groups in each hyperlactatemia category based on primary exposure. We compared the adjusted predicted probabilities of favorable neurological outcomes and survival between 32–34 and 35–36 °C groups in each hyperlactatemia category. We calculated p value for interaction between hyperlactatemia group and TTM (32–34 °C or 35–36 °C) for the adjusted predicted probabilities of 30-day favorable neurological outcome and 30-day survival.
Statistical analysis was performed with EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [43]. Continuous variables were compared with Mann–Whitney U or Kruskal–Wallis tests. Binary variables were compared with Fisher’s exact test. p values < 0.05 were considered statistically significant. Missing data were not replaced or estimated.
Results
Baseline patient characteristics
A total of 12,024 OHCA patients were included in the OHCA registry during the study period. Of 3971 adult patients with ROSC, there were 3262 patients without TTM treatment, 162 with withdrawn TTM, and 112 without lactate concentration after ROSC. Then, 435 were included in the final analysis (Fig. 1). Favorable neurological outcomes were observed in 41.8% of the study patients. Baseline characteristics between the patients excluded and those included are shown in Table 1.
Fig. 1.
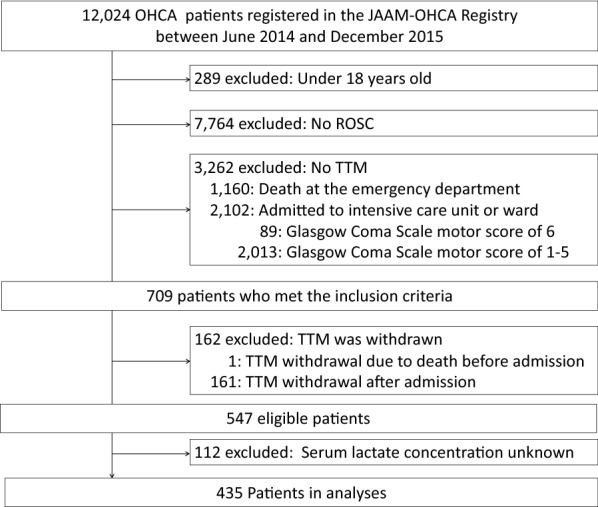
Flow diagram of study
Table 1.
Patient baseline characteristics between inclusion group and three exclusion groups
| Variables | Inclusion n = 435 |
No TTM n = 2013a |
p valuec | TTM withdrawal n = 161b |
p valuec | Unknown lactate concentration n = 112 |
p valuec |
|---|---|---|---|---|---|---|---|
| Age, years | 63 [50, 73] | 75 [6, 83] | < 0.001 | 65 [53, 75] | 0.126 | 63 [51, 72] | 0.881 |
| Age > 65 years | 182 (42) | 1405 (70) | < 0.001 | 78 (48) | 0.163 | 51 (46) | 0.521 |
| Male sex | 338 (78) | 1190 (59) | < 0.001 | 115 (71) | 0.130 | 85 (76) | 0.705 |
| Witness | 348 (80) | 1312 (65) | < 0.001 | 122 (76) | 0.261 | 83 (74) | 0.195 |
| Dispatcher instruction | 198 (46) | 852 (42) | 0.24 | 75 (47) | 0.853 | 45 (40) | 0.338 |
| Bystander-performed CPR | 228 (52) | 839 (42) | < 0.001 | 88 (55) | 0.645 | 50 (45) | 0.168 |
| Cardiac etiology | 332 (76) | 786 (39) | < 0.001 | 128 (80) | 0.443 | 85 (76) | 0.902 |
| Initial shockable rhythm | 235 (54.0) | 218 (11) | < 0.001 | 74 (46) | 0.096 | 54 (48) | 0.290 |
| Prehospital epinephrine administration | 99 (23) | 613 (31) | 0.001 | 57 (35) | 0.001 | 29 (26) | 0.532 |
| Prehospital advanced airway management | 145 (33) | 871 (43) | < 0.001 | 61 (38) | 0.332 | 43 (38) | 0.318 |
| Prehospital AED | 313 (72) | 777 (39) | < 0.001 | 110 (68) | 0.416 | 73 (65) | 0.165 |
| Response time, min | 8 [6, 9] | 8 [7, 10] | < 0.001 | 8[7, 10] | < 0.001 | 8 [6, 10] | 0.197 |
| Time from call to hospital arrival, min | 30 [24, 36] | 32 [27, 40] | < 0.001 | 32 [27, 40] | 0.014 | 30 [24, 36] | 0.942 |
| Prehospital ROSC | 252 (58) | 563 (28) | < 0.001 | 36 (22) | < 0.001 | 28 (25) | < 0.001 |
| Glasgow Coma Scale score | 3 [3, 3] | 3 [3, 3.] | < 0.001 | 3 [3, 3] | < 0.001 | 3 [3, 3] | 0.003 |
| Coronary angiography | 257 (59) | 235 (12) | < 0.001 | 100 (62) | 0.512 | 75 (67) | 0.131 |
| ECMO and/or IABP | 123 (28) | 157 (8) | < 0.001 | 76 (47) | < 0.001 | 54 (48) | < 0.001 |
| Targeted temperature management | NA | 0.198 | 0.005 | ||||
| 32–34 °C | 319 (73) | 0 (0) | 124 (79) | 92 (86) | |||
| 35–36 °C | 116 (27) | 0 (0) | 33 (21) | 15 (14) | |||
| 30-day favorable neurological outcome | 182 (42) | 183 (9) | < 0.001 | 20 (12) | < 0.001 | 32 (29) | 0.012 |
| 30-day survival | 325 (75) | 354 (18) | < 0.001 | 36 (22) | < 0.001 | 67 (60) | 0.003 |
Continuous variables were presented as median [Interquartile range]. Categorical variables were presented as n (%)
TTM targeted temperature management, CPR cardiopulmonary resuscitation, AED automated external defibrillator, ROSC return of spontaneous circulation, ECMO extracorporeal membrane oxygenation, IABP intra-aortic balloon pumping, ABG arterial blood gas
aThere were 1160 patients excluded due to death at the emergency department and 89 patients excluded because they got Glasgow Coma Scale motor score of 6 after return of spontaneous circulation
bThere was one patient excluded due to death at the emergency department
cVs. inclusion group
Association of serum lactate concentrations after ROSC and favorable neurological outcomes
The crude and adjusted predicted probability of a favorable 30-day neurological outcome across the range of serum lactate concentrations after ROSC is shown in Additional file 1: Figure S1. We found a trend toward decreases of both crude and adjusted predicted probability of a favorable 30-day neurological outcome with increasing serum lactate concentration. There were 139 patients in the mild, 182 in the moderate, and 114 in the severe hyperlactatemia groups. Table 2 shows the characteristics of the patients in each hyperlactatemia group. There were significant differences among the three groups in witness, initial shockable rhythm, prehospital epinephrine administration, prehospital advanced airway management, prehospital AED, prehospital ROSC, Glasgow coma scale score, ECMO and/or IABP, door-to-first arterial blood gas measurement after ROSC, pH, PaCO2, HCO3, and base excess. The percentage of patients with favorable neurological outcomes decreased with the worsening of hyperlactatemia: 60.4% with mild, 41.8% with moderate, and 19.3% with severe hyperlactatemia had favorable neurological outcomes.
Table 2.
Comparison between three hyperlactatemia groups
| Variables | Hyperlactatemia classification | p value | ||
|---|---|---|---|---|
| Mild n = 139 |
Moderate n = 182 |
Severe n = 114 |
||
| Age, years | 64 [52, 73] | 64 [50, 75] | 60 [48, 68] | 0.087 |
| Age > 65 years | 63 (45) | 81 (45) | 38 (33) | 0.098 |
| Male sex | 105 (76) | 143 (79) | 90 (79) | 0.757 |
| Witness | 118 (85) | 149 (82) | 81 (71) | 0.017 |
| Dispatcher instruction | 66 (47) | 89 (49) | 43 (38) | 0.149 |
| Bystander-performed CPR | 77 (55) | 100 (55) | 51 (45) | 0.161 |
| Cardiac etiology | 115 (83) | 135 (74) | 82 (72) | 0.089 |
| Initial shockable rhythm | 88 (63) | 100 (55) | 47 (41) | 0.002 |
| Prehospital epinephrine administration | 21 (15) | 41 (23) | 37 (32) | 0.005 |
| Prehospital advanced airway management | 33 (24) | 61 (34) | 51 (45) | 0.002 |
| Prehospital AED | 114 (82) | 132 (73) | 67 (59) | < 0.001 |
| Response time, min | 8 [6, 9] | 8 [6, 9] | 8 [7, 9] | 0.376 |
| Time from call to hospital arrival, min | 32 [25, 39] | 30 [24, 35] | 29 [24, 37] | 0.200 |
| Prehospital ROSC | 102 (73) | 112 (62) | 38 (33) | < 0.001 |
| Glasgow Coma Scale score | 3 [3, 4] | 3 [3, 3] | 3 [3, 3] | < 0.001 |
| Coronary angiography | 85 (61) | 106 (58) | 66 (58) | 0.833 |
| ECMO and/or IABP | 45 (32) | 37 (20) | 41 (36) | 0.006 |
| Door-to-first ABG measurement after ROSC, min | 11 [5, 30] | 9 [5, 18] | 15 [7, 30] | 0.007 |
| pH | 7.28 [7.22, 7.33] | 7.12 [7.02, 7.28] | 6.92 [6.75, 7.03] | < 0.001 |
| PaCO2, mm Hg | 43 [37, 51] | 52 [35, 75] | 70 [43, 97] | < 0.001 |
| PaCO2 30–50 mm Hg | 90 (65) | 63 (35) | 27 (24) | < 0.001 |
| PaO2, mm Hg | 176 [91, 337] | 187 [92, 356] | 150 [79, 346] | 0.760 |
| HCO3, mmol/l | 19.9 [17.1, 21.7] | 16.4 [13.9, 18.6] | 12.9 [10.3, 16.8] | < 0.001 |
| Base excess, mmol/l | − 6.2 [− 9.1, − 4.0] | − 12 [− 14.7, − 9.5] | − 19.4 [− 24.1, − 15.4] | < 0.001 |
| Lactate value, mmol/l | 5.2 [3.7, 6.1] | 9.1 [7.9, 10.2] | 14.9 [13.2, 18.0] | < 0.001 |
| Door-to-TTM initiation, min | 106 [30, 188] | 115 [36, 190] | 136 [46, 225] | 0.324 |
| Targeted temperature management | 0.331 | |||
| 32–34 °C | 108 (78) | 128 (70) | 83 (73) | |
| 35–36 °C | 31 (22) | 54 (30) | 31 (27) | |
| 30-day favorable neurological outcome | 84 (60) | 76 (42) | 22 (19) | < 0.001 |
| 30-day survival | 117 (84) | 144 (79) | 64 (56) | < 0.001 |
Continuous variables were presented as median [Interquartile range]. Categorical variables were presented as n (%)
CPR cardiopulmonary resuscitation, AED automated external defibrillator, ROSC return of spontaneous circulation, ECMO extracorporeal membrane oxygenation, IABP intra-aortic balloon pumping, ABG arterial blood gas
Baseline characteristics of patients stratified by hyperlactatemia and treated with 32–34 °C or 35–36 °C
Comparison of the baseline characteristics of patients with mild, moderate, or severe hyperlactatemia and treated with either 32–34 °C or 35–36 °C are summarized in Table 3. The analysis revealed significant differences in initial shockable rhythm rates and door-to-TTM initiation time among the mild hyperlactatemia group, in ages and door-to-TTM initiation time among the moderate hyperlactatemia group, and door-to-TTM initiation time among the severe hyperlactatemia group.
Table 3.
Comparison between 32–34 and 35–36 °C in each hyperlactatemia group
| Variables | Mild hyperlactatemia | p value | Moderate hyperlactatemia | p value | Severe hyperlactatemia | p value | |||
|---|---|---|---|---|---|---|---|---|---|
| 32–34 °C n = 108 |
35–36 °C n = 31 |
32–34 °C n = 128 |
35–36 °C n = 54 |
32–34 °C n = 83 |
35–36 °C n = 31 |
||||
| Age, years | 64 [52, 72] | 66 [54, 79] | 0.105 | 62 [50, 74] | 71 [51, 80] | 0.036 | 58 [47, 67] | 64 [55, 71] | 0.112 |
| Age > 65 years | 47 (43.5) | 16 (51.6) | 0.540 | 50 (39) | 31 (57) | 0.033 | 24 (28.9) | 14 (45.2) | 0.121 |
| Male sex | 80 (74) | 25 (81) | 0.636 | 101 (79) | 42 (78) | 0.846 | 67 (81) | 23 (74) | 0.449 |
| Witness | 92 (85) | 26 (84) | 0.784 | 106 (83) | 43 (80) | 0.675 | 61 (74) | 20 (65) | 0.361 |
| Dispatcher instruction | 48 (44) | 18 (58) | 0.222 | 63 (49) | 26 (48) | 1.000 | 31 (37) | 12 (39) | 1.000 |
| Bystander-performed CPR | 58 (54) | 19 (61) | 0.540 | 73 (57) | 27 (50) | 0.417 | 39 (47) | 12 (39) | 0.527 |
| Cardiac etiology | 90 (83) | 25 (81) | 0.788 | 98 (77) | 37 (69) | 0.271 | 61 (74) | 21 (68) | 0.640 |
| Initial shockable rhythm | 74 (69) | 14 (45) | 0.021 | 75 (59) | 25 (46) | 0.144 | 36 (43) | 11 (36) | 0.524 |
| Prehospital epinephrine administration | 18 (17) | 3 (10) | 0.409 | 31 (24) | 10 (19) | 0.444 | 27 (33) | 10 (32) | 1.000 |
| Prehospital advanced airway management | 24 (22) | 9 (29) | 0.475 | 41 (32) | 20 (37) | 0.606 | 37 (45) | 14 (45) | 1.000 |
| Prehospital AED | 90 (83) | 24 (77) | 0.437 | 93 (73) | 39 (72) | 1.000 | 50 (60) | 17 (55) | 0.671 |
| Response time, min | 8 [6, 9] | 8 [6, 10] | 0.749 | 7 [6, 9] | 8 [6, 9] | 0.381 | 8 [6, 9] | 8 [7, 11] | 0.329 |
| Time from call to hospital arrival, min | 32 [25, 40] | 31 [23, 33] | 0.283 | 30 [24, 34] | 31 [24, 36] | 0.542 | 29 [25, 37] | 28 [24, 39] | 0.769 |
| Prehospital ROSC | 77 (71) | 25 (81) | 0.362 | 78 (61) | 34 (63) | 0.868 | 31 (37) | 7 (23) | 0.181 |
| Glasgow Coma Scale score | 3 [3, 3] | 3 [3, 6] | 0.097 | 3 [3, 3] | 3 [3, 3] | 0.602 | 3 [3, 3] | 3 [3, 3] | 0.856 |
| Coronary angiography | 66 (61) | 19 (61) | 1.000 | 78 (61) | 28 (52) | 0.324 | 52 (63) | 14 (45) | 0.135 |
| ECMO and/or IABP | 37 (34.3) | 8 (25.8) | 0.514 | 50 (39) | 13 (24) | 0.061 | 32 (39) | 9 (29) | 0.388 |
| Door-to-first ABG measurement after ROSC, min | 11 [5, 32] | 9 [5, 22] | 0.300 | 10 [5, 17] | 9 [6, 18] | 0.784 | 13 [6, 30] | 19 [11, 35] | 0.056 |
| pH | 7.28 [7.22, 7.32] | 7.31 [7.20, 7.37] | 0.107 | 7.14 [7.02, 7.28] | 7.08 [7.01, 7.25] | 0.356 | 6.92 [6.81, 7.03] | 6.91 [6.74, 7.15] | 0.817 |
| PaCO2, mm Hg | 43 [38, 51] | 40 [33, 51] | 0.318 | 47 [34, 75] | 60 [36, 75] | 0.200 | 69 [44, 90] | 72 [31, 108] | 0.873 |
| PaCO2 30–50 mm Hg | 71 (65.7) | 19 (61.3) | 0.674 | 50 (39) | 13 (24) | 0.061 | 23 (28) | 4 (13) | 0.137 |
| PaO2, mm Hg | 170 [91, 350] | 176 [93, 325] | 0.975 | 179 [86, 354] | 195 [113, 350] | 0.449 | 148 [76, 345] | 240 [86, 363] | 0.437 |
| HCO3, mmol/l | 20.0 [16.8, 21.5] | 19.9 [18.0, 22.8] | 0.608 | 16.3 [13.9, 18.5] | 16.6 [13.6, 19.5] | 0.471 | 13.0 [10.4, 17.1] | 12.7 [10.3, 15.1] | 0.530 |
| Base excess, mmol/l | − 6.4 [− 9.3, − 4.0] | − 5.7 [− 7.3, − 4.1] | 0.369 | − 11.9 [− 14.9, − 9.4] | − 12.5 [− 14.0, − 9.9] | 0.905 | − 19.6 [− 23.7, − 16.2] | − 19.4 [− 24.5, − 12.6] | 0.703 |
| Lactate value, mmol/l | 5.1 [3.6, 6, 1] | 5.4 [3.9, 6.5] | 0.185 | 9.1 [7.9, 10.1] | 9.3 [8.1, 10.6] | 0.775 | 14.7 [13.1, 17.3] | 16.2 [14.0, 19.4] | 0.113 |
| Door-to-TTM initiation, min | 82 [20, 174] | 167 [109, 213] | 0.003 | 78 [21, 170] | 164 [103., 218] | < 0.001 | 105 [35, 202] | 198 [109, 261] | 0.002 |
| 30-day favorable neurological outcome | 65 (60) | 19 (61) | 1.000 | 56 (44) | 20 (37) | 0.417 | 18 (22) | 4 (13) | 0.425 |
| 30-day survival | 89 (82) | 28 (90) | 0.406 | 100 (78) | 44 (82) | 0.693 | 48 (58) | 16 (52) | 0.672 |
Continuous variables were presented as median [Interquartile range]. Categorical variables were presented as n (%)
CPR cardiopulmonary resuscitation, AED automated external defibrillator, ROSC return of spontaneous circulation, ECMO extracorporeal membrane oxygenation, IABP intra-aortic balloon pumping, ABG arterial blood gas
Favorable neurological outcomes and survival following 32–34 °C or 35–36 °C in the three hyperlactatemia groups
Multiple logistic regression models to obtain the adjusted predicted probabilities of 30-day favorable neurological outcome showed that age older than 65 years, initial shockable rhythm, prehospital epinephrine administration, prehospital advanced airway management, time from call to hospital arrival, prehospital ROSC, Glasgow Coma Scale score, and PaCO2 30–50 mm Hg were significantly associated with neurological outcome (Additional file 2: Table S1). There were no significant differences in the rates of crude and adjusted predicted 30-day favorable neurological outcomes following 32–34 °C and 35–36 °C in patients with mild or moderate hyperlactatemia. In patients with severe hyperlactatemia, the adjusted predicted probability of a favorable outcome, 27.4% (95% confidence interval (CI) 22.0–32.8%) with 32–34 °C and 12.4% (95% CI 3.5–21.2%) with 35–36 °C, were significantly different (p = 0.005, Fig. 2). The interaction between hyperlactatemia groups and TTM groups was significant (p for interaction = 0.043). Thirty-day survival decreased with the worsening of hyperlactatemia, from 84.2% with mild to 79.1% with moderate and 56.1% with severe hyperlactatemia (Table 2). Multiple logistic regression models to obtain the adjusted predicted probabilities of 30-day survival showed that initial shockable rhythm, prehospital epinephrine administration, time from call to hospital arrival, prehospital ROSC, and Glasgow Coma Scale score were significantly associated with 30-day mortality (Additional file 3: Table S2). Within each hyperlactatemia group, crude 30-day survival and adjusted predicted probability of 30-day survival with 32–34 °C or with 35–36 °C did not differ significantly (Table 3, Additional file 4: Figure S2).
Fig. 2.
Adjusted predicted probability of favorable 30-day neurological outcomes of 32–34 °C and 35–36 °C among patients in the three hyperlactatemia group. Error bars indicate 95% confidence intervals. p for interaction = 0.043
Discussion
This post hoc analysis of a nationwide, multicenter prospective registry found no significant differences in the adjusted predicted probability of favorable neurological outcomes with TTM at 32–34 °C or 35–36 °C in OHCA patients with mild (< 7 mmol/l) or moderate (< 12 mmol/l) hyperlactatemia after ROSC. TTM at 32–34 °C was associated with a greater adjusted predicted probability of a favorable outcome in patients with severe hyperlactatemia than TTM at 35–36 °C.
Two previous retrospective observational studies also reported that TTM at 32–34 °C was beneficial in patients with severe OHCA patients [8, 44]. One was a single-center cohort study including 1200 witnessed OHCA patients [8]. It was found that TTM at 32–34 °C was associated with improved neurological outcomes in patients with relatively longer times from collapse to the start of resuscitation attempts. The second study evaluated 871 witnessed, shockable adult patients prospectively included in a population-based registry in Toronto, Canada [44]. The beneficial effect of TTM at 32–34 °C was more apparent in patients with a prolonged time from collapse to initial defibrillation.
Two retrospective studies reported that lower target temperature improved outcome in OHCA patients with less severe baseline characteristics [11, 12]. One evaluated the outcomes in a cohort of Japanese patients treated with either low (32.0–33.5 °C) or moderate (34.0–35 °C) target temperatures and stratified to a short (< 30 min) or long interval to resuscitation [11]. Improved neurological outcomes were observed only in the short-interval group. However, a target temperature of 34 °C was classified as moderate target temperature group in this study, and more than 75% of patients in the moderate target temperature group had a target temperature of 34 °C. In case of classifying target temperature of 34 °C into low temperature group as in our current study or other studies [8, 12, 44], patient characteristics and main results may be subject to a major change. Thus, it is very difficult to compare this study and our current study at present. Another retrospective observational study included patients with or without early evidence of hypoxic encephalopathy provided by brain computed tomography [12]. Compared to TTM at 35–36 °C, the benefit of TTM at 34 °C was evident only in patients without hypoxic encephalopathy. In that study, the brain damage resulting from hypoxic encephalopathy was considered to be too severe to permit a favorable response to TTM. The limited available data suggest that patients with severe OHCA benefit from TTM at 32–34 °C, as shown in this study, but the effect may not be seen in extremely severe cases.
Serum lactate concentrations reflect not only tissue hypoxia but also aerobic glycolysis and lactate clearance in critically ill patients [13]. Several observational studies have shown that serum lactate concentration in OHCA patients was influenced by factors in addition to the duration of cardiac arrest, including vasopressors, high arterial oxygen partial pressure, and decreased renal function [14, 45]. Serum lactate seems to be a comprehensive marker of severity in OHCA patients [46], and the association of serum lactate concentrations and neurological outcome has been described in many previous studies [14–17, 47, 48]. In this study, both crude and predicted probability of 30-day favorable neurological outcomes decreased with increasing serum lactate concentration after ROSC (Fig. 2). Although serum lactate in OHCA patients does not necessarily reflect brain metabolites [49], it does reflect OHCA severity after ROSC.
The finding that TTM at 32–34 °C was not associated with predicted neurological outcomes in patients with mild or moderate hyperlactatemia was consistent with the results of the TTM trial, which did not show a significant benefit of TTM at 33 °C compared with 36 °C [4]. The lactate concentration of 6.7 ± 4.5 mmol/l in the TTM study population was thought to account for the lack of demonstrated benefit of TTM at 33 °C. The mean lactate concentration in both temperature groups evaluated in the TTM trial was similar to the concentration in the mild to moderate hyperlactatemia patients in our current study. Thus, post hoc analysis of the TTM trial results did not identify a patient subset that benefited from TTM at 33 °C compared with 36 °C [9, 10].
The present study had several limitations. First, this was a post hoc analysis. Although we calculated predicted probability using multiple logistic regression analysis, selection bias and uncontrolled confounding variables could have influenced the results. For example, the target temperature depended upon the physician’s choice, and more than 70% of patients received 32–34 °C (Table 2). The physicians had a tendency to assign patients with unstable hemodynamics to the 35–36 °C group to avoid hypotension and arrhythmia caused by 32–34 °C [50, 51]. However, there were no significant differences in crude survival and predicted probability of 30-day survival. Second, exclusion of the 3262 patients without TTM may affect the reliability of external validity. However, given that, of those, 1160 patients died at the emergency department, 89 patients reached Glasgow Coma Scale motor score of 6 after ROSC, and 2013 comatose admitted patients without TTM had much more severe prehospital characteristics than the patients finally analyzed (Table 1), it seemed reasonable that the physicians did not provide TTM to these patients. However, the responsibility for non-indication of TTM depended on each physician or each institution, so it could cause some selection biases. Third, our current study included only patients who completed TTM, which may have led to additional selection bias. However, 30-day survival rate in patients with TTM withdrawn was not one-third as high as that in those finally analyzed (22% vs 75%, p < 0.001, Additional file 2: Table S1) despite no significant difference in the proportion of TTM at 32–34 °C between two groups (73% vs. 79%, p = 0.198). Adverse events of TTM seemed unlikely to cause this profound difference. We thought that there were so many patients without indication of TTM among these patients. On the other hand, exclusion of the patient with missing lactate concentration may result in selection bias and decreased generalizability. In addition, we divided patients into 32–34 °C or 35–36 °C according to “targeted” temperature declared by each physician or each institute. We did not evaluate actual temperature and how strictly TTM was performed. Patient outcome might have been affected by the time needed to achieve target temperature during the induction phase, temperature fluctuation during the maintenance phase, and the rewarming rate [52]. Lastly, we could not assess long-term neurological outcomes.
Conclusions
In OHCA patients with severe hyperlactatemia, the adjusted predicted probability of 30-day favorable neurological outcome was greater with 32–34 °C than with 35–36 °C. Further study is needed to determine whether 32–34 °C can improve neurological outcome in patients with severe hyperlactatemia.
Supplementary information
Additional file 1: Figure S1. Serum lactate concentration and crude and adjusted predicted probability of favorable 30-day neurological outcomes.
Additional file 2: Table S1. Univariate analysis and multiple logistic regression models to obtain the adjusted predicted probabilities of 30-day favorable neurological outcome.
Additional file 3: Table S2. Univariate analysis and multiple logistic regression models to obtain the adjusted predicted probabilities of 30-day survival.
Additional file 4: Figure S2. Adjusted predicted probability of 30-day survival of 32–34 °C and 35–36 °C among patients in the three hyperlactatemia group.
Acknowledgements
We are so grateful to all members and institutions of the JAAM-OHCA registry for their contribution. The participating institutions of the JAAM-OHCA Registry are listed at the following URL: http://www.jaamohca-web.com/list/.
Abbreviations
- OHCA
out-of-hospital cardiac arrest
- TTM
targeted temperature management
- ROSC
return of spontaneous circulation
- JAAM-OHCA registry
Japanese Association for Acute Medicine out-of-hospital cardiac arrest registry
- CPR
cardiopulmonary resuscitation
- AED
automated external defibrillator
- CPC score
cerebral performance category score
- ECMO
extracorporeal membrane oxygenation
- IABP
intra-aortic balloon pumping
- CI
confidence interval
Authors’ contributions
TO and TH were responsible for the article and drafted and revised the manuscript. KK and YK helped to draft the manuscript. All authors take full responsibility for all aspects of the study. All authors read and approved the final manuscript.
Funding
The JAAM-OHCA registry was supported by research funding from the Japanese Association for Acute Medicine and scientific research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (16K09034 and 15H05006) and the Ministry of Health, Labor, and Welfare of Japan (Grant No. 25112601). The founders of this study had no role in study design, data analysis, data interpretation, or writing of this manuscript.
Availability of data and materials
The data that support the findings of this study are available from the JAAM-OHCA registry committee but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the JAAM-OHCA registry committee.
Ethics approval and consent to participate
The protocol and consent procedures were approved by the Institutional Review Board of each participating hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tomoya Okazaki and Toru Hifumi contributed equally to this work
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13613-019-0603-y.
References
- 1.Kitamura T, Iwami T, Atsumi T, Endo T, Kanna T, Kuroda Y, et al. The profile of Japanese Association for Acute Medicine—out-of-hospital cardiac arrest registry in 2014–2015. Acute Med Surg. 2018;5(3):249–258. doi: 10.1002/ams2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 3.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369(23):2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 5.Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, et al. Part 8: post-cardiac arrest care: 2015 American Heart Association Guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl 2):S465–S482. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VR, Deakin CD, et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation. 2015;95:202–222. doi: 10.1016/j.resuscitation.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Vincent JL. The coming era of precision medicine for intensive care. Crit Care. 2017;21(Suppl 3):314. doi: 10.1186/s13054-017-1910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Testori C, Sterz F, Holzer M, Losert H, Arrich J, Herkner H, et al. The beneficial effect of mild therapeutic hypothermia depends on the time of complete circulatory standstill in patients with cardiac arrest. Resuscitation. 2012;83(5):596–601. doi: 10.1016/j.resuscitation.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Kjaergaard J, Nielsen N, Winther-Jensen M, Wanscher M, Pellis T, Kuiper M, et al. Impact of time to return of spontaneous circulation on neuroprotective effect of targeted temperature management at 33 or 36 degrees in comatose survivors of out-of hospital cardiac arrest. Resuscitation. 2015;96:310–316. doi: 10.1016/j.resuscitation.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Dankiewicz J, Friberg H, Belohlavek J, Walden A, Hassager C, Cronberg T, et al. Time to start of cardiopulmonary resuscitation and the effect of target temperature management at 33 degrees C and 36 degrees C. Resuscitation. 2016;99:44–49. doi: 10.1016/j.resuscitation.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko T, Kasaoka S, Nakahara T, Sawano H, Tahara Y, Hase M, et al. Effectiveness of lower target temperature therapeutic hypothermia in post-cardiac arrest syndrome patients with a resuscitation interval of ≤ 30 min. J Intensive Care. 2015;3(1):28. doi: 10.1186/s40560-015-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishikimi M, Ogura T, Nishida K, Takahashi K, Fukaya K, Liu K, et al. Differential effect of mild therapeutic hypothermia depending on the findings of hypoxic encephalopathy on early CT images in patients with post-cardiac arrest syndrome. Resuscitation. 2018;128:11–15. doi: 10.1016/j.resuscitation.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Kraut JA, Madias NE. Lactic acidosis. N Engl J Med. 2014;371(24):2309–2319. doi: 10.1056/NEJMra1309483. [DOI] [PubMed] [Google Scholar]
- 14.Mullner M, Sterz F, Domanovits H, Behringer W, Binder M, Laggner AN. The association between blood lactate concentration on admission, duration of cardiac arrest, and functional neurological recovery in patients resuscitated from ventricular fibrillation. Intensive Care Med. 1997;23(11):1138–1143. doi: 10.1007/s001340050470. [DOI] [PubMed] [Google Scholar]
- 15.Prause G, Ratzenhofer-Comenda B, Smolle-Juttner F, Heydar-Fadai J, Wildner G, Spernbauer P, et al. Comparison of lactate or BE during out-of-hospital cardiac arrest to determine metabolic acidosis. Resuscitation. 2001;51(3):297–300. doi: 10.1016/S0300-9572(01)00424-5. [DOI] [PubMed] [Google Scholar]
- 16.Shinozaki K, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Watanabe E, et al. Blood ammonia and lactate levels on hospital arrival as a predictive biomarker in patients with out-of-hospital cardiac arrest. Resuscitation. 2011;82(4):404–409. doi: 10.1016/j.resuscitation.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Lee DH, Cho IS, Lee SH, Min YI, Min JH, Kim SH, et al. Correlation between initial serum levels of lactate after return of spontaneous circulation and survival and neurological outcomes in patients who undergo therapeutic hypothermia after cardiac arrest. Resuscitation. 2015;88:143–149. doi: 10.1016/j.resuscitation.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Kitamura T, Iwami T, Kawamura T, Nagao K, Tanaka H, Hiraide A. Nationwide public-access defibrillation in Japan. N Engl J Med. 2010;362(11):994–1004. doi: 10.1056/NEJMoa0906644. [DOI] [PubMed] [Google Scholar]
- 19.Perkins GD, Jacobs IG, Nadkarni VM, Berg RA, Bhanji F, Biarent D, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Circulation. 2015;132(13):1286–1300. doi: 10.1161/CIR.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 20.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–484. doi: 10.1016/S0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 21.Oh SH, Park KN, Lim J, Choi SP, Oh JS, Cho IS, et al. The impact of sex and age on neurological outcomes in out-of-hospital cardiac arrest patients with targeted temperature management. Crit Care. 2017;21(1):272. doi: 10.1186/s13054-017-1860-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue A, Hifumi T, Yonemoto N, Kuroda Y, Kawakita K, Sawano H, et al. The impact of heart rate response during 48-hour rewarming phase of therapeutic hypothermia on neurologic outcomes in out-of-hospital cardiac arrest patients. Crit Care Med. 2018;46(9):e881–e888. doi: 10.1097/CCM.0000000000003254. [DOI] [PubMed] [Google Scholar]
- 23.Malta Hansen C, Kragholm K, Dupre ME, Pearson DA, Tyson C, Monk L, et al. Association of bystander and first-responder efforts and outcomes according to sex: results from the north carolina heart rescue statewide quality improvement initiative. J Am Heart Assoc. 2018;7(18):e009873. doi: 10.1161/JAHA.118.009873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kragholm K, Wissenberg M, Mortensen RN, Fonager K, Jensen SE, Rajan S, et al. Return to work in out-of-hospital cardiac arrest survivors: a nationwide register-based follow-up study. Circulation. 2015;131(19):1682–1690. doi: 10.1161/CIRCULATIONAHA.114.011366. [DOI] [PubMed] [Google Scholar]
- 25.Nikolaou N, Dainty KN, Couper K, Morley P, Tijssen J, Vaillancourt C. A systematic review and meta-analysis of the effect of dispatcher-assisted CPR on outcomes from sudden cardiac arrest in adults and children. Resuscitation. 2019;138:82–105. doi: 10.1016/j.resuscitation.2019.02.035. [DOI] [PubMed] [Google Scholar]
- 26.Ro YS, Shin SD, Lee YJ, Lee SC, Song KJ, Ryoo HW, et al. Effect of dispatcher-assisted cardiopulmonary resuscitation program and location of out-of-hospital cardiac arrest on survival and neurologic outcome. Ann Emerg Med. 2017;69(1):52–61. doi: 10.1016/j.annemergmed.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 27.Nakahara S, Tomio J, Ichikawa M, Nakamura F, Nishida M, Takahashi H, et al. Association of bystander interventions with neurologically intact survival among patients with Bystander-Witnessed Out-of-Hospital Cardiac Arrest in Japan. JAMA. 2015;314(3):247–254. doi: 10.1001/jama.2015.8068. [DOI] [PubMed] [Google Scholar]
- 28.Dumas F, Rea TD. Long-term prognosis following resuscitation from out-of-hospital cardiac arrest: role of aetiology and presenting arrest rhythm. Resuscitation. 2012;83(8):1001–1005. doi: 10.1016/j.resuscitation.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 29.Spaite DW, Bobrow BJ, Stolz U, Berg RA, Sanders AB, Kern KB, et al. Statewide regionalization of postarrest care for out-of-hospital cardiac arrest: association with survival and neurologic outcome. Ann Emerg Med. 2014;64(5):496–506. doi: 10.1016/j.annemergmed.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 30.Grimaldi D, Dumas F, Perier MC, Charpentier J, Varenne O, Zuber B, et al. Short- and long-term outcome in elderly patients after out-of-hospital cardiac arrest: a cohort study. Crit Care Med. 2014;42(11):2350–2357. doi: 10.1097/CCM.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 31.Maria V, Pasquale B, Carmine I, Giuseppe S. Epinephrine for out of hospital cardiac arrest: a systematic review and meta-analysis of randomized controlled trials. Resuscitation. 2019;136:54–60. doi: 10.1016/j.resuscitation.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa K, Hiraide A, Chang Y, Brown DF. Association of prehospital advanced airway management with neurologic outcome and survival in patients with out-of-hospital cardiac arrest. JAMA. 2013;309(3):257–266. doi: 10.1001/jama.2012.187612. [DOI] [PubMed] [Google Scholar]
- 33.Patterson C, Pitts SR, Akhter M. Alternative effects of transportation time on out-of-hospital cardiac arrests. Resuscitation. 2017;117:e5. doi: 10.1016/j.resuscitation.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Cournoyer A, Notebaert E, de Montigny L, Ross D, Cossette S, Londei-Leduc L, et al. Impact of the direct transfer to percutaneous coronary intervention-capable hospitals on survival to hospital discharge for patients with out-of-hospital cardiac arrest. Resuscitation. 2018;125:28–33. doi: 10.1016/j.resuscitation.2018.01.048. [DOI] [PubMed] [Google Scholar]
- 35.Wissenberg M, Folke F, Hansen CM, Lippert FK, Kragholm K, Risgaard B, et al. Survival after out-of-hospital cardiac arrest in relation to age and early identification of patients with minimal chance of long-term survival. Circulation. 2015;131(18):1536–1545. doi: 10.1161/CIRCULATIONAHA.114.013122. [DOI] [PubMed] [Google Scholar]
- 36.Gregers E, Kjaergaard J, Lippert F, Thomsen JH, Kober L, Wanscher M, et al. Refractory out-of-hospital cardiac arrest with ongoing cardiopulmonary resuscitation at hospital arrival-survival and neurological outcome without extracorporeal cardiopulmonary resuscitation. Crit Care. 2018;22(1):242. doi: 10.1186/s13054-018-2176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hifumi T, Kuroda Y, Kawakita K, Sawano H, Tahara Y, Hase M, et al. Effect of admission glasgow coma scale motor score on neurological outcome in out-of-hospital cardiac arrest patients receiving therapeutic hypothermia. Circ J. 2015;79(10):2201–2208. doi: 10.1253/circj.CJ-15-0308. [DOI] [PubMed] [Google Scholar]
- 38.Jaeger D, Dumas F, Escutnaire J, Sadoune S, Lauvray A, Elkhoury C, et al. Benefit of immediate coronary angiography after out-of-hospital cardiac arrest in France: a nationwide propensity score analysis from the ReAC Registry. Resuscitation. 2018;126:90–97. doi: 10.1016/j.resuscitation.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Sakamoto T, Morimura N, Nagao K, Asai Y, Yokota H, Nara S, et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: a prospective observational study. Resuscitation. 2014;85(6):762–768. doi: 10.1016/j.resuscitation.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 40.Roberts BW, Kilgannon JH, Chansky ME, Mittal N, Wooden J, Trzeciak S. Association between postresuscitation partial pressure of arterial carbon dioxide and neurological outcome in patients with post-cardiac arrest syndrome. Circulation. 2013;127(21):2107–2113. doi: 10.1161/CIRCULATIONAHA.112.000168. [DOI] [PubMed] [Google Scholar]
- 41.McKenzie N, Williams TA, Tohira H, Ho KM, Finn J. A systematic review and meta-analysis of the association between arterial carbon dioxide tension and outcomes after cardiac arrest. Resuscitation. 2017;111:116–126. doi: 10.1016/j.resuscitation.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Inoue A, Hifumi T, Kuroda Y, Nishimoto N, Kawakita K, Yamashita S, et al. Mild decrease in heart rate during early phase of targeted temperature management following tachycardia on admission is associated with unfavorable neurological outcomes after severe traumatic brain injury: a post hoc analysis of a multicenter randomized controlled trial. Crit Care. 2018;22(1):352. doi: 10.1186/s13054-018-2276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drennan IR, Lin S, Thorpe KE, Morrison LJ. The effect of time to defibrillation and targeted temperature management on functional survival after out-of-hospital cardiac arrest. Resuscitation. 2014;85(11):1623–1628. doi: 10.1016/j.resuscitation.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 45.Dell’Anna AM, Sandroni C, Lamanna I, Belloni I, Donadello K, Creteur J, et al. Prognostic implications of blood lactate concentrations after cardiac arrest: a retrospective study. Ann Intensive Care. 2017;7(1):101. doi: 10.1186/s13613-017-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bellomo R, Martensson J, Eastwood GM. Metabolic and electrolyte disturbance after cardiac arrest: how to deal with it. Best Pract Res Clin Anaesthesiol. 2015;29(4):471–484. doi: 10.1016/j.bpa.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Orban JC, Novain M, Cattet F, Plattier R, Nefzaoui M, Hyvernat H, et al. Association of serum lactate with outcome after out-of-hospital cardiac arrest treated with therapeutic hypothermia. PLoS ONE. 2017;12(3):e0173239. doi: 10.1371/journal.pone.0173239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaji AH, Hanif AM, Bosson N, Ostermayer D, Niemann JT. Predictors of neurologic outcome in patients resuscitated from out-of-hospital cardiac arrest using classification and regression tree analysis. Am J Cardiol. 2014;114(7):1024–1028. doi: 10.1016/j.amjcard.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 49.Hifumi T, Kawakita K, Yoda T, Okazaki T, Kuroda Y. Association of brain metabolites with blood lactate and glucose levels with respect to neurological outcomes after out-of-hospital cardiac arrest: a preliminary microdialysis study. Resuscitation. 2017;110:26–31. doi: 10.1016/j.resuscitation.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 50.Kim YM, Youn CS, Kim SH, Lee BK, Cho IS, Cho GC, et al. Adverse events associated with poor neurological outcome during targeted temperature management and advanced critical care after out-of-hospital cardiac arrest. Crit Care. 2015;19:283. doi: 10.1186/s13054-015-0991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomsen JH, Kjaergaard J, Graff C, Pehrson S, Erlinge D, Wanscher M, et al. Ventricular ectopic burden in comatose survivors of out-of-hospital cardiac arrest treated with targeted temperature management at 33 degrees C and 36 degrees C. Resuscitation. 2016;102:98–104. doi: 10.1016/j.resuscitation.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 52.Polderman KH, Varon J. How low should we go? Hypothermia or strict normothermia after cardiac arrest? Circulation. 2015;131(7):669–675. doi: 10.1161/CIRCULATIONAHA.114.012165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Serum lactate concentration and crude and adjusted predicted probability of favorable 30-day neurological outcomes.
Additional file 2: Table S1. Univariate analysis and multiple logistic regression models to obtain the adjusted predicted probabilities of 30-day favorable neurological outcome.
Additional file 3: Table S2. Univariate analysis and multiple logistic regression models to obtain the adjusted predicted probabilities of 30-day survival.
Additional file 4: Figure S2. Adjusted predicted probability of 30-day survival of 32–34 °C and 35–36 °C among patients in the three hyperlactatemia group.
Data Availability Statement
The data that support the findings of this study are available from the JAAM-OHCA registry committee but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the JAAM-OHCA registry committee.