Abstract
The chromobox homolog 2 (CBX2) was found to be important for human testis development, but its role in the human ovary remains elusive. We conducted a genome-wide analysis based on DNA adenine methyltransferase identification (DamID) and RNA sequencing strategies to investigate CBX2 in the human granulosa cells. Functional analysis revealed that CBX2 was upstream of genes contributing to ovarian function like folliculogenesis and steroidogenesis (i.e. ESR1, NRG1, AKR1C1, PTGER2, BMP15, BMP2, FSHR and NTRK1/2). We identified CBX2 regulated genes associated with polycystic ovary syndrome (PCOS) such as TGFβ, MAP3K15 and DKK1, as well as genes implicated in premature ovarian failure (POF) (i.e. POF1B, BMP15 and HOXA13) and the pituitary deficiency (i.e. LHX4 and KISS1). Our study provided an excellent opportunity to identify genes surrounding CBX2 in the ovary and might contribute to the understanding of ovarian physiopathology causing infertility in women.
Subject terms: Developmental biology, Genetics, Molecular biology, Endocrinology, Molecular medicine
Introduction
The ovarian development depends on a highly orchestrated chain of genetic events involving multiple transcription factors and genetic circuits. Disruption of this orchestrated network can lead to many clinical syndromes, including POF, polycystic ovarian syndrome (PCOS), ovarian hyperstimulation syndrome, ovulation defects, poor ovarian reserve, and ovarian cancer1. The genetic regulatory cascade still lacks a master regulator as an equivalent of SRY (Sex-Determining Region Y) gene2 in the male pathway. Genes such as wingless-type MMTV integration site family, member 4 (WNT4)3, R-spondin1 (RSPO1)4 and Forkhead box L2 (FOXL2)5 are female-specific genes governing the ovarian pathway in coordination with other genes to promote and maintain oocytes health during fetal ovary development6. In typical 46, XX female embryonic differentiation, FOXL2 and the β-catenin pathway stimulated by WNT4 and RSPO1, inhibit SOX9 action, blocking the differentiation of cells into Sertoli cells7.
We recently identified CBX2 as being upstream of SRY and essential for male sex development8,9. This homolog of the murine polycomb-like gene M33 is a highly conserved chromatin modifier10,11 and a regulator of homeotic gene expression during early embryogenesis12. In humans, two isoforms of CBX2 have been identified13, the long CBX2 isoform-1 containing a polycomb (Pc) box and the short CBX2.2 isoform lacking the Pc box13. Nonetheless, the individual regulation of these different isoforms remains mainly unknown and require further investigation and clarification. A previous study reported that both isoforms can function as repressors of reporter gene activity when bound proximal from a promoter13. CBX2.2 does not bind to CBX2.1 and was found to be significantly less active than the long isoform10,13. In humans, deficiency in CBX2 represents an autosomal-recessive cause of 46,XY disorders of sex development (DSD)8. The 46,XY DSD CBX2.1 deficient patients had normal female internal and external genitalia and ovarian-like tissue at histology8. More recently, the description of 46,XX DSD patient with gonadal dysgenesis suggested that CBX2.1 is essential for gonad formation in both sexes. Concerning CBX2.2, 46,XY DSD patients carrying genetic variants of CBX2.2 presented severe testicular dysgenesis phenotype9, different from the ovarian‐like gonadal phenotype found in the 46,XY DSD CBX2.1 deficient patient8. In mice, while Sry-positive Cbx2 XY−/− animals showed male-to-female sex reversal14, knock out Cbx2 XX−/− animals exhibited gonadal growth retardation and germ cell loss and a high proportion of oocytes with abnormal synapsis and non-homologous interactions which resulted in small ovaries and infertility14,15.
To provide further enlightenment about the molecular basis relating CBX2 to the ovary, we investigated the whole transcriptome associated with CBX2.1 and CBX2.2, which could advance our understanding of the ovarian development, disease and ultimately promote optimal women's health.
Results
Gene Ontology (GO) analysis of CBX2.1 and CBX2.2 targets
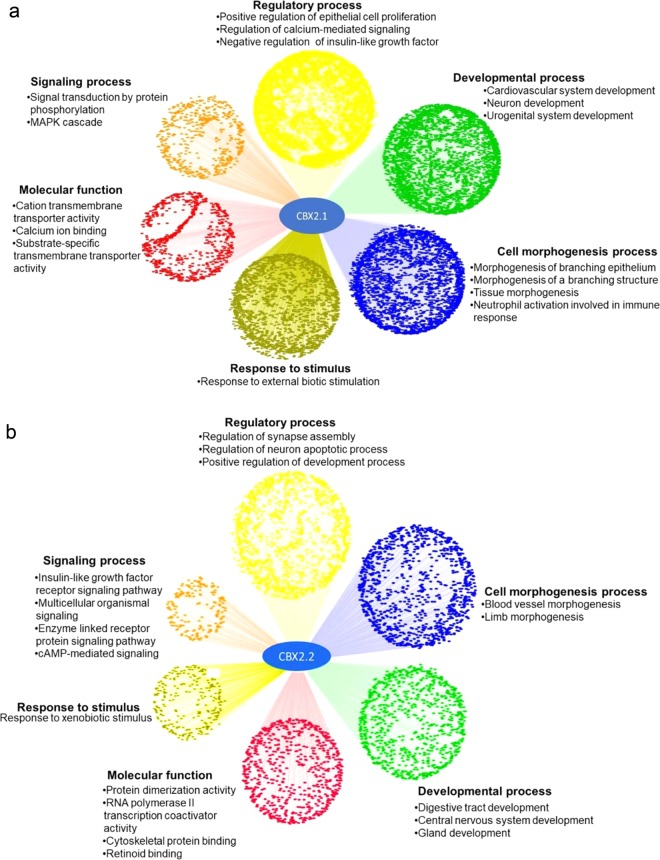
To gain a functional profile of the high-throughput gene sets obtained from DamID and RNA sequencing, unbiased enrichment analysis was classified into sets of genes with over-represented gene ontology terms. We used ToppCluster16 to analyse functional GO-enrichment of CBX2.1 and CBX2.2 downstream genes16. Our enrichment analysis indicated multiple genes of CBX2.1 and CBX2.2 specifically enriched in generic development, morphogenesis and differentiation of the brain, digestive tube, and glands (Fig. 1a,b). We showed that CBX2.1 targets are over-represented for GO-term associated with urogenital system development (Fig. 1a) and that CBX2.1 and CBX2.2 regulate neuronal differentiation by directly interacting with several neuro-associated genes. We identified significant enrichment of genes involved in immune responses through the activation of leukocytes and neutrophils (Fig. 1a). We found a strong enrichment of CBX2.2 related genes involved in the retinoid-binding activity (Fig. 1b) revealed to be crucial during the early female embryonic development17. Other CBX2.2 genes were involved in regulatory and signalling processes (Fig. 1b) mediated cyclic adenosine monophosphate (cAMP). This pathway is one of the multiple pathways modulating the ovarian steroidogenesis by increasing the expression of steroidogenic acute regulatory protein 1 activity (StAR)18.
Figure 1.
(a) Cytoscape representation of GO-enrichment analysis of CBX2.1 targets. Every dot represents a gene related to the enriched GO‐terms. In green are the GO‐terms over-presented in the developmental process. In blue are the GO‐terms involved in morphogenesis process. In red are all GO-terms related to Molecular Function. The orange colour represents the cluster of genes coding for signalling pathways. Some regulatory processes were over-represented by the yellow colour. The genes presented in the mustard colour were over-represented in response to a stimulus. All data is filtered according to p < 0.05. (b) Cytoscape representation of GO-enrichment analysis of CBX2.2 targets. The green colour represents the GO‐terms which are involved in morphogenesis and differentiation process. In the blue cluster, we found GO‐terms involved in the developmental process. The red colour indicates genes responsible for Molecular Function. The yellow colour represents the regulatory processes. The orange colour is the cluster, which contains genes coding for signalling processes. The genes present in the mustard colour cluster were over-represented in response to a stimulus. All data is filtered according to p < 0.05.
Protein/DNA interaction and transcriptome: Crossover
We identified common genes regulated by CBX2.1 and CBX2.2 represented in the Venn diagram (Cytoscape 3.7.1)19 (Supplementary Fig. 1). For the effects of the two isoforms analyses, we used Fold Change> 2 as the criterion for determining the set of the common genes that exhibit differential expression and p-value has been set for all comparisons to be p < 0.05. More specifically, the combination of CBX2.1-DamID targets and CBX2.1-RNA-seq related genes showed in total, 53 common genes (Supplementary Fig. 1). About CBX2.2 genes, the intersection between the groups of regulated targets derived from DamID and RNA-seq resulted in 27 up and downregulated common genes in the intersections A, B and D (A∩B∩D) (Supplementary Fig. 2). We defined A as the intersection between the DamID-overexpression of CBX2.1 or CBX2.2 genes and RNA-seq-knock down of CBX2.1 or CBX2.2 genes. The group of genes B is the intersection between the DamID-overexpression of CBX2.1 or CBX2.2 genes and the RNA-seq-overexpression of CBX2.1 or CBX2.2 related genes. The group of genes C resulted in the combination between the RNA-seq-knock down of CBX2.1 or CBX2.2 regulated genes and the RNA-seq-overexpression CBX2.1 or CBX2.2 regulated genes. Group D: is the intersection between the three sets: A, B and C. There were relatively few differentially expressed genes (95 genes) in common between the CBX2.1 and CBX2.2 direct regulated genes obtained from unbiased DamID data (Supplementary Table 1). We recognized 481 overlapped genes acting in diverse pathways between CBX2.1 and CBX2.2 targets resulted from RNA-seq experiments as indicated in Table 2 of the supplementary. This result suggested overlapping pathways of the two isoforms and indicated that CBX2 isoforms could co-regulate ovary development-specific genes. Thus, we obtained a novel genetic network in which the two isoforms were acting directly or indirectly in the ovary (Fig. 2). The regulated genes have been shown to influence gonad development, apoptosis, proliferation and differentiation processes (Fig. 3). As represented in Table 1, we were able to provide the most up and downregulated genes by CBX2.1 and CBX2.2 which were up to now new in the scene of sex development network.
Table 2.
DamID selected genes related to CBX2.1 and CBX2.2.
| Target name | Function | Sexual dysfunctions | |
|---|---|---|---|
| DamID CBX2.1 | ESR1 | Maintaining the female phenotype of the endocrine somatic cells of the ovary by inhibiting male cell development128. | ERKO mice develop testis-like features. Null ERα mutations in human females exhibit profound estrogen resistance and have features analogous to those in the knock out mouse67. |
| NRG1 | Induced by luteinizing hormone (LH)/hCG to activate the MAKP3/1 pathway to promote GC differentiation and controls ovulation and luteinization related events74. | Not reported | |
| BMP2 | BMP2 with FOXL2 ensure expression follistatin in the developing ovary. It amplifies FSH-induced estradiol production in sheep granulosa cell23. | In mice, BMP2 null mutation is embryonic lethal and foetuses contain a low number of primordial germ cells leading to POF129. | |
| PTGER2 | Regulation of ovulation and luteinization130. | Mice deficient in Ptger2 have ovulatory defects that are related to an abnormality in cumulus expansion131. | |
| SOX9 | Stimulates the differentiation of Sertoli cells132. | In mice, derepression of Sox9 expression in XX gonads leads to testis development. Human mutation: 46, XY-sex reversal8. Duplication: 46,XX DSD. | |
| POF1B | Regulates ovarian function133 | Assumed to be a causative candidate of POF134 | |
| DKK1 | Repress WNT mediated beta-catenin signalling during the developing testis135. | In humans, it is a PCOS risk candidate136. | |
| FZD7 | WNT signalling regulation137 | Not reported | |
| DamID CBX2.2 | AMIGO2 | Potential role in lipid metabolism138 | Not reported |
| TGFα | Stimulate GC proliferation; inhibit follicle stimulating hormone (FSH) receptor (granulosa cell), and LH receptor (thecal cell) expression; inhibit steroidogenesis139. | Not reported | |
| TGFb2 | Follicle growth82 | Polycystic ovary syndrome82 | |
| NTRK2 | Involved in the development and the maturation of the central and peripheral nervous systems140. | In the knock out mice reduced the number of secondary follicles and a decrease in granulosa cell proliferation141. | |
| AKR1C1 | Implicated in the inactivation and formation of male and female sex hormones142. | In Akr1c1 deficient mice high progesterone levels and display a delay in parturition of several days143. | |
| FZD5 | Induce the beta-catenin pathway144 | Not reported | |
| SOX4 | Heart function145 | Reported in human ovarian cancer146 | |
| RSPO3 | Regulation of Wnt/beta-catenin signalling. It has a possible role in folliculogenesis and development of germ cells of fish147. | Not reported |
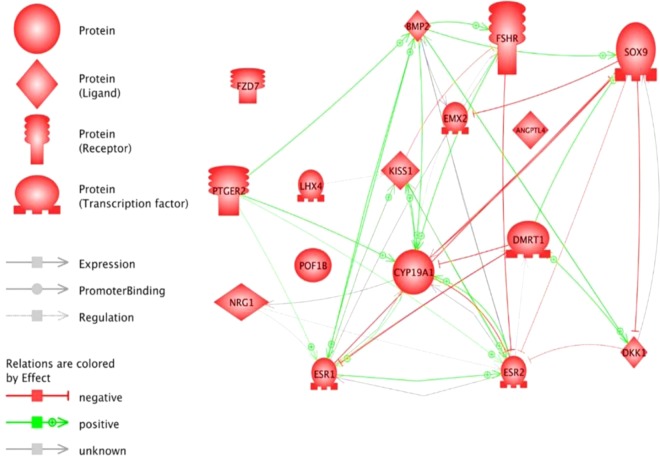
Figure 2.
Regulatory network of downstream targets of CBX2.1 in the ovary. We created by the mean of PathwayStudio 11 a network relating the ovarian targets. The genes were found highly interconnected. CBX2.1 targets are SOX9, POF1B, DKK1, ANGPTL4, CYP19A1, DMRT1, KISS1, EMX2, ESR2, POU4F1, FZD7, ESR1, NRG1, BMP2, PTGER2 and FSHR. The interactions of the genes are represented by diverse arrows. Red arrows (T) indicate negative regulations, the green arrows symbolize positive regulations and grey arrows are undefined effects.
Figure 3.
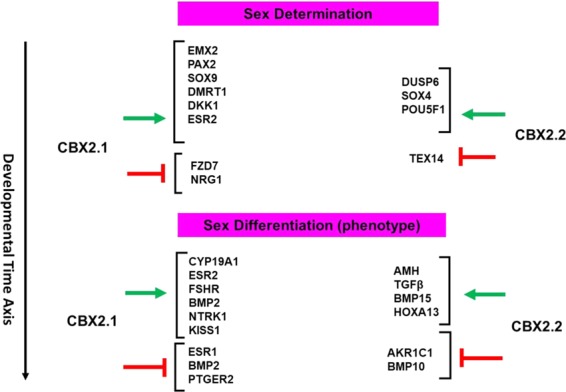
Potential role of CBX2.1 and CBX2.2 in the regulation of sex development. The developmental stages plotted in relation to time are indicated as sex determination. We presented male and female factors involved in gonadal development. Green arrows indicate stimulatory effects of CBX2.1 and CBX2.2 on examined downstream factors; the red arrows (T) indicate inhibitory effects.
Table 1.
Top downstream targets of CBX2.1 and CBX2.2 obtained from DamID and RNA-seq data.
| Gene symbol | −log10(p-value) | Fold enrichment | Gene symbol | log2 Ratio | fdr | p-Value |
|---|---|---|---|---|---|---|
| CBX2.1 DamID | CBX2.1 RNA-seq | |||||
| MYC | 83.89999 | 8.48561 | RNA5-8S5 | -4.289 | 8.71E-07 | 0.001152 |
| CBX2 | 14.52065 | 7.43178 | RNA5-8S5 | -4.074 | 8.63E-07 | 0.001152 |
| PCDH9 | 14.07725 | 8.75539 | CYP19A1 | 2.524 | 3.04E-07 | 0.0005027 |
| MIR1258 | 14.07725 | 8.75539 | ESM1 | 1.448 | 2.83E-07 | 0.0005027 |
| GRHL2 | 14.07725 | 8.75539 | PSMA1 | 2.349 | 2.49E-07 | 0.0005027 |
| LOC100130331 | 14.07725 | 8.75539 | COL3A1 | -1.482 | 1.51E-07 | 0.0004006 |
| TTC39C | 13.25753 | 8.31353 | ACTA2 | -1.296 | 1.50E-07 | 0.0004006 |
| YTHDC1 | 12.90472 | 7.73385 | PLIN2 | 1.423 | 1.05E-07 | 0.0004006 |
| RCBTB2 | 12.64768 | 8.0819 | ANGPTL4 | 3.349 | 1.81E-24 | 1.19E-20 |
| LOC338862 | 12.64768 | 8.0819 | CBX2 | 9.212 | 1.93E-59 | 2.55E-55 |
| CBX2.2 DamID | CBX2.2 RNA-seq | |||||
| LMNB1 | 13.63833 | 8.52171 | MS4A4E | 2.273 | 1.274E-36 | 0.00507 |
| C6orf105 | 13.63833 | 8.52171 | RP11-512M8.5 | 1.945 | 1.534E-21 | 0.003451 |
| ATP6V1H | 13.63833 | 8.52171 | ANGPTL4 | 1.847 | 1.629E-20 | 0.002252 |
| TRPC7 | 13.41263 | 8.24715 | SHANK1 | 5.793 | 1.629E-20 | 0.001976 |
| LOC151162 | 13.36989 | 8.22215 | NYNRIN | -4.972 | 1.629E-20 | 0.001862 |
| PCDH9 | 12.24367 | 7.86619 | HPCAL4 | 5.927 | 1.543E-19 | 0.001322 |
| LPHN2 | 12.24367 | 7.86619 | UQCR11 | 2.075 | 1.543E-19 | 0.001226 |
| EPS8L3 | 12.24367 | 7.86619 | DNASE1L2 | 6.34 | 5.678E-19 | 0.0003375 |
| LOC151658 | 12.24367 | 7.86619 | C4orf48 | 2.908 | 7.747E-19 | 0.0001456 |
| RHO | 12.24367 | 7.86619 | CBX2 | 6.879 | 5.923E-18 | 2.66E-17 |
Identification of CBX2.1 and CBX2.2 genomic direct and indirect targets in KGN cells
Within 72 hours post-transfection of CBX2, pre-granulosa cells or KGN did not exhibit any morphological changes similar to the small and astrocytic shape of the male NT2-D1 cell line morphology20 (data not shown). This suggests that there is no link between CBX2 gene expression and KGN morphological changes. We applied the DamID method that couples whole genome-wide protein-DNA interaction to next‐generation sequencing to gain deeper insights into the function of CBX2 isoforms in granulosa cells (GC). We identified 524 and 835 enriched binding sequences of CBX2.1 and CBX2.2, respectively. We expanded CBX2 transcriptional landscape, by the used of RNA sequencing that identifies, contrary to DamID, also genes that are not necessarily physically bound by CBX2 and can be considered indirect targets. Thus, we found 692 and 668 differently expressed genes, respectively. A larger number of 1167 and 810 genes were significantly up or downregulated by CBX2.1 and CBX2.2 silencing, respectively.
To independently validate the DamID and RNA-seq results, we selected a subset of genes regulated by CBX2.1 and CBX2.2 (as shown in Tables 2 and 3) to evaluate their expression using quantitative real‐time PCR (RT‐qPCR). Genes selection was based on their potential links to sex development, their role in human and animal sexual diseases and their specific expression in tissues involved in sex development (gonads, sex organs, hypothalamus and pituitary).
Table 3.
RNA-seq Genes regulated by CBX2.1 and CBX2.2.
| Genes symbol | Function | Sexual dysfunctions | |
|---|---|---|---|
| CBX2.1 Targets | NTRK1 | Involved in testicular development and spermatogenesis. | Genetic knock out mice resulted in a reduced number of testis cords148. |
| ANGPTL4 | Potential role in lipid metabolism149 | Not reported | |
| CYP19A1 | Converts androstenedione to estrone and testosterone to estradiol. | 46, XX: virilization of external genitalia150 | |
| DMRT1 | Expressed only in the genital ridge. A dose-dependent effect on postnatal testis development. | XY null mice have normal prenatal testis development, but abnormal postnatal testis differentiation. In human: 46, XY testis maldevelopment; 46, XX primary hypogonadism151 | |
| EMX2 | Homeodomain transcription factor EMX2 is critical for the central nervous system and urogenital development. | Emx2 mutant mice died soon after birth because of the absence of kidneys indicating an essential role in the morphogenesis of the urogenital system58. | |
| ESR2 | Nuclear receptor transcription factors have a crucial role in reproductive function. | Subfertility and reduced litter sizes and granulosa cell defect32. | |
| KISS1 | Implicated in the stimulation of gonadotropin-releasing hormone (GnRH)-induced gonadotropin secretion. | KISS1 knock out female mice demonstrated an abnormal reproductive system with abnormal reproductive system phenotype. A clinical case of hypogonadotropic was associated with a loss-of-function of KISS148. | |
| BMP2 | BMP2 with FOXL2 ensure expression follistatin in the developing ovary. It amplifies FSH-induced estradiol production in sheep GC. | In mice, BMP2 null mutation is embryonic lethal and foetuses contain a low number of primordial germ cells leading to POF129 | |
| LHX4 | Acts as a transcriptional regulator that is involved in the control of differentiation and development of the pituitary gland. | In human impaired sexual development, Lhx4−/− double-mutant exhibited a specific abnormal placentas phenotype152. | |
| POF1B | Regulates ovarian function | Assumed to be a causative candidate of POF153 | |
| FSHR | Follicle stimulating hormone receptor | Mutations in the FSHR cause primary ovarian failure in females and impaired spermatogenesis in males50. | |
| CBX2.2 Targets | BMP15 | Stimulation of ovarian granulosa cell growth and proliferation and downregulates FSH receptor expression. | In mice: subfertile, in human: ovarian dysgenesis154. |
| TEX14 | Involvement in spermatogenesis and male fertility. | Male mice infertility96 | |
| BMP10 | Inducer of trophoblast differentiation in human. | Not reported | |
| MAP3K15 | Involved in the adrenal pathway | Not reported | |
| HOXA13 | Required for morphogenesis of terminal gut and urogenital tract, including Müllerian structures. | Mouse: XX null has hypoplasia of the cervix and vagina. 46, XX human mutation: a hand-footgenital syndrome with uterine malformation89. |
CBX2.1 downstream targets
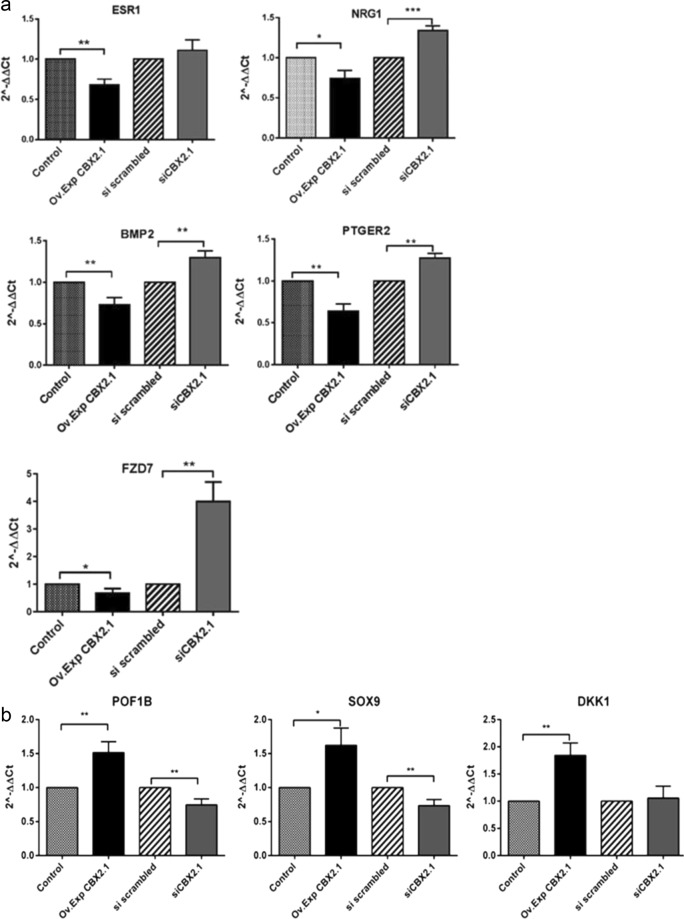
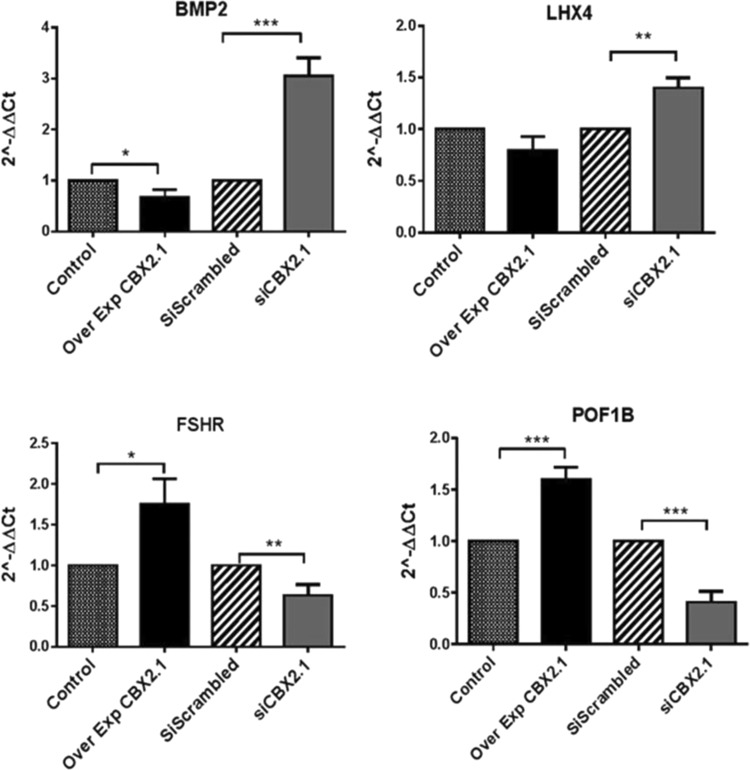
The genes ESR1, NRG1, BMP2, PTGER2, FZD7, POF1B, DKK1 and SOX9 are DamID-CBX2.1 downstream targets. The set of genes, namely ESR1, NRG1, BMP2, PTGER2, FZD7 were found to be negatively regulated by CBX2.1 (0.4-, 0.3-, 0. 35-, 0.3- and 0.44-fold, respectively, compared to the control vector (Fig. 4a). The genes were reported to be implicated in the female sex development and were found to be controlled by the ovarian specific genes FOXL2 and WNT421–26 recently shown to be downregulated by CBX2.1 isoform66. The expression levels of NRG1, BMP2, PTGER2, and FZD7 but not ESR1 were significantly increased after CBX2.1 knocking down (1.33-, 1.29-, 1.27-, and 4-fold, respectively) (Fig. 4a). CBX2.1 activated POF1B, DKK1 and SOX9 gene expressions (1.51-, 1.62- and 1.84-fold, respectively) (Fig. 4b). Of particular interest, SOX9 an essential male-specific gene was demonstrated to be a positive downstream target of CBX2.18,27 in the testis developmental pathway28. Inversely, expression levels of SOX9 and POF1B were shown to be significantly reduced following CBX2.1 silencing (about 0.3- and 0.35-fold, respectively) (Fig. 4b).
Figure 4.
(a) RT-qPCR analysis of CBX2.1 downstream genes identified by DamID. Relative expression levels (2−ΔΔCt) of the genes were determined after normalization to Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH). Following CBX2.1 overexpression (Ov Exp. CBX2.1), ESR1, NRG1, BMP2, PTGER2, and FZD7 were found downregulated by CBX2.1 compared to the control set at 1. After silencing the CBX2.1 (si CBX2.1), genes were significantly upregulated except for ESR1 which showed an effect comparable to scrambled sample. All graphs are the average of three independent experiments, error bars represent the standard deviation (SD) from the mean (SEM) and values are expressed as relative to control =1; ***P < 0.001; **P < 0.01 and *P < 0.05. non-significant differences are not indicated. (b) Relative expression levels (2−ΔΔCt) of CBX2.1 related genes. POF1B, DKK1 and SOX9 were upregulated after CBX2.1 forced expression (Ov. Exp CBX2.1). Whereas, when CBX2.1 was silenced (si CBX2.1), SOX9 and POF1B were significantly downregulated. DKK1 did not show any expression change towards the siCBX2.1. All graphs are the average of three independent experiments, error bars represent SD from the mean (SEM), and values are expressed as relative to control =1; ***P < 0.001; **P < 0.01 and *P < 0.05. non-significant differences are not indicated.
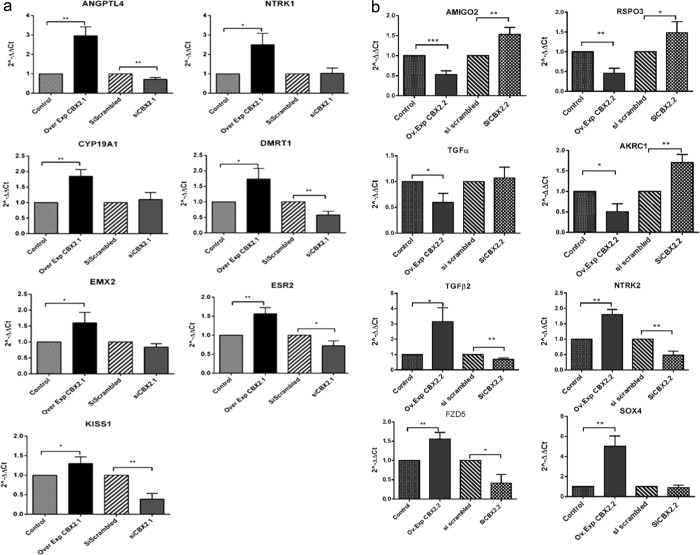
Among the RNA-seq genes, we found that NTRK1, ANGPTL4, CYP19A1, DMRT1, EMX2, ESR2, KISS1, POF1B and FSHR (follicle-stimulating hormone receptor) were significantly increased following CBX2.1 forced expression (2.5-, 3-, 1.8-, 1.7-, 1.5-, 1.5-, 1.3, 3.6- and 1.7-fold, respectively) (Fig. 5a). To prove a CBX2-dependent expression, we tested the gene expression under CBX2.1 silencing. Substantial downregulation affected ANGPTL4, DMRT1, ESR2 and KISS1 genes (0.3-, 0.4-, 0.3-, and 0.58- fold, respectively) (Fig. 5a). However, CYP19A1, EMX2 and NTRK1 seemed not to be affected by CBX2.1 knock down, suggesting their regulation by redundant genetic pathways. We found that CBX2.1 overexpression reduced significantly the relative expression of BMP2 (0.49-, fold), whereas LHX4 gene seemed not to be affected (Fig. 5b). As shown in Fig. 5b, BMP2 and LHX4 genes were found to be remarkably upregulated (3- and 1.4-fold, respectively) after CBX2.1 knocking down.
Figure 5.
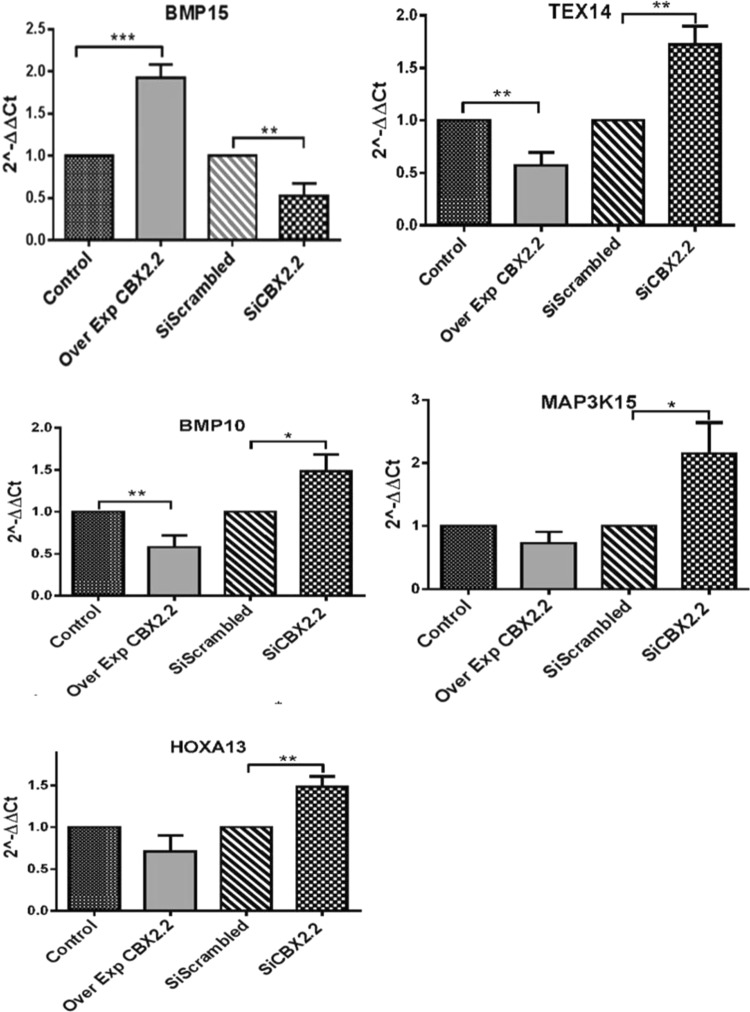
(a) Relative expression levels (2−ΔΔCt) of the RNA-seq of CBX2.1 downstream genes. ANGPTL4, NTRK1, CYP19A1, DMRT1, EMX2, ESR2 and KISS1 were upregulated after CBX2.1 overexpression (Over Exp). CBX2.1 silencing assay (siCBX2.1) reduced significantly the genes: ANGPTL4, DMRT1, ESR2 and KISS1. NTRK1, CYP19A1 and EMX2 gene did not show any expression in response to siCBX2.1. All graphs are the average of three independent experiments, error bars represent SD from the mean (SEM), and values are expressed as relative to control =1; ***P < 0.001; **P < 0.01; *P < 0.05. non-significant differences are not indicated. (b) Effect of CBX2.2 on DamID downstream targets: AKRC1, TGFα, AMIGO2 and RSPO3. Gene expression levels showed a substantial downregulation after CBX2.2 overexpression (Ov. Exp). The silencing of CBX2.2 (si CBX2.2) significantly stimulated the expression of AMIGO2, RSPO3 and AKR1C1 genes compared to scrambled siRNA. TGBβ2, NTRK2, FZD5 and SOX4 genes were significantly upregulated by CBX2.2. In the siCBX2.2 samples, NTRK2, FZD5 and TGFβ2 expression levels were found to be negatively regulated. TGFα and SOX4 expressions showed no effect relative to the scrambled sample. All graphs are the average of three independent experiments, error bars represent SD from the mean (SEM), and values are expressed as relative to control =1; ***P < 0.001; **P < 0.01; *P < 0.05. non-significant differences are not indicated.
CBX2.2 downstream targets
Our result showed that AKR1C1, TGFα, AMIGO2 and RSPO3 DamID-genes were significantly downregulated by CBX2.2 (0.5-, 0.4-, 0.7- and 0.65-fold, respectively) (Fig. 6). The silencing of the CBX2.2 isoform significantly enhanced the expression of AMIGO2, RSPO3 and AKR1C1 genes (2-, 1.5- and 1.7-fold, respectively) compared to the si-scrambled (set at 1). The lack of considerable effects on the expression of TGFα after downregulating CBX2.2 is unclear, but it might be attributed to redundant pathways controlling the expression of this gene. On the other hand, another DamID derived-genes TGBβ2, NTRK2, FZD5 and SOX4 were increased after CBX2.2 forced expression (3-, 1.8-, 1.5- and 5-fold, respectively) whereas, under CBX2.2 downregulation TGFβ2, NTRK2, and FZD5 expression levels were found to be decreased (0.5-, 0.6-, and 0.3-fold, respectively) (Fig. 6). SOX4 expression did not exhibit any expression variation after knocking down CBX2.2.
Figure 6.
RT-qPCR analysis of RNA-seq downstream genes of CBX2.1. After CBX2.1 overexpression (Ov. Exp), the relative expression of BMP2 was downregulated compared to the control (empty vector). CBX2.1 knocking down induced the relative expression of BMP2 and LHX4. However, POF1B and FSHR were significantly decreased. Forced expression of CBX2.1 does not impact LHX4. All graphs are the average of three independent experiments, error bars represent SD from the mean (SEM), and values are expressed as relative to control =1; ***P < 0.001; **P < 0.01; *P < 0.05. non-significant differences are not indicated.
The RT-qPCR analysis showed that the ovarian gene BMP15 was significantly upregulated by 2.2- fold after CBX2.2 forced expression in KGN. Whereas expression of TEX14 and BMP10 was significantly reduced after CBX2.2 overexpression (0.33- and 0.40-fold, respectively) (Fig. 7). Silencing of the CBX2 isoform-2 decreased the expression of BMP15 by 0.62-fold. But, it enhanced expression of TEX14, BMP10, MAP3K15 and HOXA13 (1.7-, 1.4-, 2.4- and 3- and 1.6-fold, respectively) (Fig. 7).
Figure 7.
Relative expression levels (2−ΔΔCt) of the RNA-seq downstream genes of CBX2.2. CBX2.2 forced expression (Over Exp) positively regulated BMP15. Whereas, silencing (si) CBX2.2 (siCBX2.2) resulted in the downregulation of the same gene. Under overexpression (Over Exp) of CBX2.2, the genes TEX14 and BMP10 were significantly downregulated. Expressions of MAP3K15 and HOXA13 were comparable to the control. Silencing CBX2.2 (siCBX2.2) significantly enhanced the genes TEX14, BMP10, MAP3K15 and HOXA13. All graphs are the average of three independent experiments, error bars represent SD from the mean (SEM), and values are expressed as relative to control =1; ***P < 0.001; **P < 0.01; *P < 0.05. non-significant differences are not indicated.
It is important to indicate that RT-qPCR did not show that CBX2.1 and CBX2.2 influenced the expression of each other. This agrees with results published by Völkel et al., showing that long CBX2.1 isoform interacts with the polycomb repressive complex-1 (PRC1) components. In total contrast, none of the PRC1 components was identified with the CBX2.2 short isoform13. According to the same authors, CBX2.2 forms homopolymers in a PRC1-independent way. Unlike CBX2.1, CBX2.2 lacks the Pc domain, essential for the interaction with the PRC1 partners13.
Discussion
In this work, we showed the gene expression landscape of CBX2 isoforms in the ovary based on data-driven from profiling genes and transcriptome data. Unbiased GO analysis data obtained from the whole genome protein/DNA interaction and RNA-seq methods revealed a greatly expanded “atlas” of CBX2 new targets implicated in several developmental and functional pathways in KGN. Of particular interest, we demonstrated that CBX2.1 targets are over-represented for GO-term associated with urogenital system development, thereby supporting the involvement of CBX2.1 in human sex development as has been reported by Biason-Lauber and co-workers8,27. Several genes with diverse functions related to folliculogenesis, steroidogenesis and ovarian disease like PCOS, POF were found to be regulated directly and indirectly by CBX2 isoforms. Multiple categories of CBX2.1 and CBX2.2 related genes are implicated in generic development, morphogenesis and differentiation events. Our findings are in harmony with recent data revealing the implication of murine Cbx2 gene in the central nervous system development in mice29. We showed significant enrichment of genes involved in immune responses, supporting the results of Katoh-Fukui in Cbx2/M33 knock out mice with immunological deficits due to spleen development abnormalities14. Yet, no immunological deficit is found to be influenced by CBX2 mutations in human patients8. This could be explained by the difference between human and mouse phenotypes and the presence of alternative pathways of CBX2 gene in humans. Among CBX2.1 and CBX2.2-regulated genes were factors involved in the regulation of insulin-like growth factor (IGF) receptors, which were reported to be required for sex determination in mice30. In the double knock out insulin-Igf1 receptor null embryos, a delay in ovarian differentiation has been observed, suggesting that in mouse gonads lacking insulin/Igf signalling remain undifferentiated with no clear pathway decision of either testicular or ovarian pathways for several days31.
CBX2.1 related genes were found to be highly interconnected in ovarian developmental processes and supported an active contribution of CBX2.1 in ovarian function and maintenance. Genes regulated by CBX2.1 like CYP19A1, KISS1, and ESR2 were found to work together to determine and maintain the ovary phenotype32–35. Unlike CBX2.1, the CBX2.2 network appeared to be much more limited, most likely due to the novelty of CBX2.2's functions and the lack of animal model studies. Taken together, the network-based transcriptome and profiling data offered a solid starting point for the elucidation of detailed connections of CBX2 isoforms genes in the human ovarian pathway.
A new single-cell RNA-seq analysis using human fetal gonad cells and their neighbouring somatic cells from 15 embryos between 4 and 19 weeks post-fertilization (GEO accession GSE86146)36, demonstrated that the CBX2 transcript is highly expressed in both sexes. The CBX2 expression level was remarkably higher in the female than in the male embryo37. Additionally, human follicular transcriptome data (GSE107746) obtained from various developmental stages of oocytes (primordial, primary, secondary, antral and preovulatory) and the corresponding GC published by Zhang et al., showed that CBX2 expression was consistently high at all stages of follicular development compared with GC38. In mice, a study revealed a spectrum of meiotic abnormalities in Cbx2 deficient oocytes at the diplotene stage with abnormal synapsis and non-homologous chromosome interactions in Cbx2 (XX−/−) mutant oocytes15. This phenotype observed in fetal oocytes lacking Cbx2 might suggest that Cbx2 is expressed in follicles and have a functional role for chromatin remodelling required for the establishment and repair of homologous chromosome pairing.
The gene Cytochrome P450 family 19 subfamily A member 1 (CYP19A1), estrogen receptor 2 (ESR2), (Kisspeptin) KISS1 and FSHR were found to be the upregulated by CBX2.1. The CYP19A1 or aromatase A gene is responsible for the aromatization of androgens into estrogens in many tissues in female and male39. A previous animal study showed that Nr5a1, also called steroidogenic factor-1 (Sf-1), depletion leads to reduced Cyp19a1 expression and low estradiol levels resulting in testis differentiation in the XX gonad40. The gene ESR2 was associated with follicular growth41. Mutations of ESR2 were found to be responsible for 46,XY and 46,XX DSD, both with gonadal dysgenesis42. The genes KISS1 and FSHR were related to ovarian diseases like POF and PCOS43,44. Recent studies have shown that kisspeptin-1 and its receptor are expressed in the mammalian ovary and are critical for initiating puberty and regulating ovulation in sexually mature females via the central control of the hypothalamic-pituitary-gonadal axis45. Data gathered recently suggested a putative role of kisspeptin signalling in follicular development, oocyte maturation, steroidogenesis, and ovulation46. Additionally, loss-of-function of KISS1 is associated with hypogonadotropic hypogonadism leading to reproductive function failure and female infertility47,48. As for FSHR, it plays a major role in the development of follicles and steroidogenesis in the ovary49 and a loss-of-function of FSHR causes ovarian dysgenesis50.
We identified upregulated factors such as SRY-box 9 (SOX9) and doublesex and mab-3 related transcription factor 1 (DMRT1)8,27,51,52 as CBX2.1 downstream masculinizing factors. Heterozygous loss-of-function mutations in human SOX9 cause sex development disorder in XY males53,54 while gain-of-function mutations, such as gene duplication, can lead to XX female DSD55. According to Ledig and co-workers, a partial deletion of DMRT1 causes 46,XY ovotesticular sexual disorder56. The ovotestis formation is caused by the disturbed action of DMRT1 in germ cells as well as in Sertoli cells, causing female reprogramming of the testis56.
Other genes like empty spiracles homeobox 2 also known as EMX2, Dickkopf-related protein 1 (DKK1) and neurotrophic receptor tyrosine kinase 1 (NTRK1) are gonadal dual-functional factors upregulated by CBX2.1 in ovarian GC. It has been reported that EMX2 is indispensable for the formation of the embryonic structures Müllerian and Wolffian ducts in the female and male embryo57,58. A nonsense mutation of EMX2 resulted in uterus didelphysis in Chinese women with incomplete Müllerian fusion59. In agreement with our data, a murine study showed that Emx2 is downregulated in Cbx2-deficient gonads14. In women, genetic variation in DKK1 may result in hyperandrogenism and metabolic dysfunction of PCOS60. Other data suggested that mice Dkk1 plays a backup or fail-safe role in preventing Wnt signalling which is in harmony with the possible antineoplasic role of CBX261. We also found that CBX2.1 stimulated NTRK1 expression, which has been reported to be involved in the assembly of primordial follicles62 to facilitate the progression of follicular development within the ovary62. Together, our data indicate that CBX2.1 might be required within ovarian cells for follicular fate regulation which agrees with previous findings showing Cbx2 mutant female mice with small ovaries and significant germ cell loss11.
We found angiopoietin-like-4 (ANGPTL4), a factor yet novel to the scene of sex development, that we found it upregulated by CBX2.1 in GC. Importantly, murine Angptl4 has been found to play a role in lipid metabolism, which can provide cellular energy and mobilize substrate for progesterone synthesis in breeding females63. Together, we suggested a putative correlation between CBX2.1 and ANGPTL4 to maintain hormone metabolism in the ovary. ANGPTL4 has been also reported to be an apoptosis survival factor capable of preventing metastasis by inhibiting vascular growth and protecting from tumour cell invasion64. The explanation of the role of ANGPTL4 in ovarian physiopathology, if any, seems to be more challenging.
Some of the most important factors negatively regulated by CBX2.1 are estrogen receptor 1 (ESR1), prostaglandin E receptor 2 (PTGER2) and bone morphogenetic protein 2 (BMP2). The genes seem to be interconnected with FOXL265. CBX2.1 was demonstrated to downregulate the female determining factor FOXL2 in testis and ovary gonads27,66. ESR1 was reported to cooperate with FOXL2 to restrain SOX9 in the ovary65. In women, ESR1 deficiency was associated with clinical features of estrogen resistance, including primary amenorrhea, the absence of breast development, a small uterus and enlarged multicystic ovaries67,68. In humans, PTGER2 and BMP2 gene are prerequisite for ovulation and are activated by female factor FOXL221,69. The repressive effect of CBX2.1 on these ovarian factors together with the stimulation of male-typical factors, such as SOX9 and DMRT1, indicate a sort of dual-function of CBX2.1 in the development of the human gonads. It seems not to be an isolated example. Other genes like the WNT4, ESR2 and SF-1 have a necessary role for ovarian and testicular development function70–72 as demonstrated by the fact that genetic variants in these genes cause gonadal dysgenesis in 46,XY individuals70,73,74, with ovarian failure in women and ovotesticular DSD42,70,74. Some authors suggested that sex determination is sensitive to gonad genes dosage at multiple steps in the gonads pathway75, which might be the case of the CBX2 gene. Recent preliminary reports of CBX2.1 genetic variations in 46, XX individuals with gonadal abnormalities lend further weight to an essential role of CBX2 in human ovarian and testicular development76.
There is very little information available about the role of the second isoform CBX2.2 in any process in women. Recently, Sproll et al. showed the existence of two CBX2.2 genetic variants that fail to regulate the expression of genes essential for sexual development, leading to a severe 46,XY DSD defects9.
Among the factors upregulated by CBX2.2, were the bone morphogenetic protein 15 (BMP15) and the transforming growth factor-beta 2 (TGF-β2) genes. Previous studies showed that BMP15 affected the production of estradiol and progesterone77–79. Studies in animal have shown that the activation of the primordial follicles is mediated by bmp1580. In humans, the defected BMP15 in patients was found to cause ovarian failure81. Mounting evidence supported the implication of TGF-β2 in female reproduction and development82. TGF- β superfamily members may play different roles in the development of follicles across the species80. Tgf-β2 knock out mice study showed multiple developmental defects, including cardiopulmonary, skeletal, ocular, and urogenital system defects83. In women, dysregulation of this transforming growth factor circuitry was associated with PCOS84,85 and fertility problems86. These findings may point to potential roles of CBX2.2 in regulating transforming growth factors networks reportedly found crucial during follicular development38,87,88.
In the present study, CBX2.2 upregulated dual-functional factors in gonads like the early expressing gonadal factors, Homeobox protein Hox-A13 (HOXA13) and SRY-box 4 (SOX4). In humans, HOXA13 mutations were found to affect uterine development89,90 and produced hand-foot-genital syndrome in females91 with a decrease in androgen expression in males90. These data imply that CBX2.2 might play a role in the normal expression of HOXA13 in the early developing ovary, mirroring the situation in the human testis92. In our hands, the group C SOX transcription factor SOX4 is downstream of CBX2.2. In mice, Sox4 deficiency results in abnormal gonads of both ovaries and testes93. A recent animal study revealed that Sox4 was among Foxl2 positively regulated genes in the mice ovary94,95 and showed to repress transcription of Sox9 in fetal gonads, raising the possibility that SOX4 may function as a new feminizing C SOX factor in the regulation of the ovarian determination.
The testis expressed-14 gene (TEX14) is a masculinizing factor downregulated by CBX2.2. It has been reported to be required for intercellular bridges in vertebrate germ cells96. In females, these embryonic intercellular bridges have been proposed to have a role in the development of the primordial germ cells96 and Tex14−/− ovaries have fewer oocytes relative to control ovaries in mice97. The human fetal ovary expresses TEX14 after the 12th week of gestation, suggesting that the growth of oogonia may be induced by cellular precursor transport from neighbouring oogonia via the TEX14 channels98.
CBX2.2 negatively regulated dual-functional factors as mitogen-activated protein kinase kinase kinase 15 (MAP3K15) and Aldo-keto reductase family 1 member C1 (AKR1C1). The two genes appear to serve as markers involved in steroidogenesis99,100. Reduced levels of activated mitogen-activated protein kinase (MAPK) contribute to excessive ovarian androgen production in women with PCOS101. Besides, the change of expression patterns of MAPKs in rat ovaries was significantly higher during the secondary and antral follicle stages than those in the primordial follicles, primary follicles and corpora lutea indicating their possible involvement during follicular growth and development102,103. We assume that CBX2.2 could be implicated in the optimal control of the MAPKs signalling pathway in GC during differentiation and proliferation processes. We studied one of the Aldo-keto reductase steroidogenic enzymes, which is the AKR1C1. Recently collected data showed that it is expressed in adrenal tissue and may be involved in the fine regulation of androgen and androgen receptors (AR) availability in adipose tissue in men and women104,105. Aldo-keto reductases (AKR1C1-C4), 5α-reductases and retinol dehydrogenase were found to be expressed in the ovary, indicating that the human ovary might produce dihydrotestosterone via the alternative steroid backdoor pathway106. This pathway seems to be considerably enhanced in the polycystic ovary syndrome104. Little is known about the direct involvement of AR actions in the female. Nonetheless, previous results based on global AR knock out female mice105,107 demonstrated that they are subfertile, have defective folliculogenesis and ultimately develop POF. In the human ovary, androgen precursors are crucial for estrogen synthesis and hyperandrogenism in pathologies such as the polycystic ovary syndrome108. Taken together, our study provides a piece of indirect evidence about the role of CBX2.2 in the regulation of androgen receptor in the ovary that could be through the control of AKR1C1 expression. However, further studies are paramount to show how these new targets fit into the expanding CBX2.2-regulated network and how CBX2.2 activation and suppression can impact our understanding of ovarian functionality in humans.
We concentrated our study on the developmental side of CBX2 since variants of CBX2 in human leads to developmental defects like gonadal dysgenesis in women and men8, Although yet no defect in CBX2 is known in later stages we suggested that CBX2 and some of its targets might be involved in adult ovarian dysfunction such as PCOS and POF. To lend more weight to our hypotheses, we compared our selected CBX2.1 and CBX2.2 targets with the existing RNA-seq datasets of female embryonic and mature gonad cells37.We found that CBX2 is greatly and specifically expressed during all stages of female fetal gonadal cells (FFGC) including mitotic, retinoid acid (RA) responsive, meiotic, oogenesis, endothelial, early granulosa, mural granulosa and late granulosa phases38. Substantially, some of the major CBX2.1 and CBX2.2 downstream targets like SOX4, ANGPTL4, AKR1C1, BMP2, EMX2, DMRT1, CYP19A1, FZD5 and TEX14 showed also high and specific expression patterns in the same fetal stages. In our study, the ANGPTL4, DMRT1, EMX2, CYP19A1 genes were found significantly activated by CBX2.1. Using the same available public dataset, ANGPTL4 and DMRT1 were abundantly expressed during the FFGC and particularly in the mitotic, RA-responsive and meiotic stages. New findings indicated that in mice the lack of Dmrt1 in the fetal ovary resulted in the formation of many fewer primordial follicles in the juvenile ovary109. EMX2 and CYP19A1 showed high expression in the early, mural and late granulosa stages. To the best of our knowledge, Emx2 is one of the genes which was found to be necessary for the survival of the female and male gonads in mice. Nonetheless, the gene was not reported to play a crucial role at various stages during oocyte development110. We showed that CBX2.1 downregulated BMP2 ovarian marker. According to the transcriptome data of Zhang and co-workers38, this gene was found highly expressed during the meiotic, oogenesis, early granulosa, mural granulosa and late granulosa stages. This is not surprising given the well-proven expression of BMP2 in GC and germ cells111. Consistent with this, BMP2 was reported to be important for the follicular development111 and a predictor marker of embryo quality112. We demonstrated that genes like SOX4 and FZD5 are positively regulated by CBX2.2 and seem to have consistent high expressions during all stages of the FFGC. Noteworthy, a recent mice study indicated that Sox4 plays an important role in mouse gonad development by promoting gonad germ cell differentiation93. The AKR1C1 and TEX14 are CBX2.2 downregulated genes and exhibited high expression profiles during all ovarian fetal cells. In the first place, the results indicated that these factors are most likely node genes that establish a robust regulatory network governed by CBX2 gene in the fetal ovary, which may be involved in the control of these genes pathways during fetal germ cells and their neighbouring GC development38. Overall, this data could offer a solid reference dataset for the implication of the CBX2 and the candidate genes in the regulation of follicular development and could be valuable factors that are important in several stages of ovarian life, spanning from development, maintenance, reproductive potential and function.
A limitation of our study is that we did not use primary cultured human GC, but a KGN tumour cell line. Primary GCs can be isolated from follicular fluid during oocyte retrieval procedures. This retrieval takes place after ovulation when GCs undergo a process of luteinization which involves structural and genomic changes that lead to the terminal differentiation of follicular cells with increased progesterone production113. Luteinized GCs stop their proliferation, making the long-term cultivation of GCs primary cells extremely challenging114. Furthermore, the differentiation of GC into luteinized cells has effects on intracellular signalling and cell cycle regulation, rendering these cells not suitable to represent developing GCs. The use of the animal models as an alternative remains problematic37 as many genes are expressed differently and preferentially in human and mice. Also, Homo sapiens seem to be the only mammalian species to have two CBX2 isoforms. We therefore resorted to the use of cell lines. The KGN cell line is very well characterized66,115–119 and represents the most suitable alternative in vitro model and the closest to human ovarian cells to ascertain the role of CBX2.1 and CBX2.2 in human ovary cells.
In all, the combination of different hypothesis-generating NGS-approaches allowed us to shed light on the transcriptionally CBX2-dependent landscape in the ovarian pathway. Certainly, new knowledge in CBX2 network using novel advanced technologies for detecting and sequencing exomes are essential to prove the exact role of the new putative CBX2 regulated genes in the developing ovary network. It would be an important investigation, not only to advance our understanding regarding gonadal development but also to expand our ability to diagnose, counsel and properly accompany patients affected by ovarian dysfunctions like infertility and cancer.
Methods
Cell culture
The human ovarian granulosa-like tumour commercially available cell line or KGN116 has been provided by RIKEN BRC. It has been established from a tumour specimen enucleated from a 63-year-old woman who was diagnosed with a local recurrence of granulosa cell carcinoma after menopause. A portion of the granulosa tumour tissue obtained was used as the source of the cell culture116. We maintained KGN cells in Dulbecco's essential medium/Ham’s F12 medium supplemented with 10% fetal calf serum, 5% Penicillin/Streptomycin at 37 °C in a 5% CO2 as described in Nishi et al. protocol116. Cells were transfected by 2 µg of plasmids encoding for CBX2.1 (SC303599, OriGene Rockville, Maryland, USA), C-Myc-CBX2.2 (RC216313 OriGene Rockville, Maryland, USA) and pCMV6-empty plasmid was used as a control vector (PS100010 OriGene Rockville, Maryland, USA). We transfected cells with Metafectene (Biontex Laboratories, Munich, Germany) with ratio 1:4 (transfection reagent: DNA). siRNA duplexes (purchased from Microsynth) were introduced into cells using Lipofectamine RNAiMAX (Invitrogen) in two consecutive rounds at a final concentration of 40 nM. Experiments were typically performed 48 hours after the first transfection. siRNA duplex for CBX2 isoforms silencing was designed to alter specifically CBX2.1 and CBX2.2 so that siCBX2.1-oligos are not targeting CBX2.2 and vice-versa (si-oligo sequences are available upon request).
DamID
In principle, the DamID technique is based on in vivo expression of a chromatin protein of interest fused to DNA adenine methyltransferase (Dam)120. CBX2-Dam identification was achieved as previously described by Eid et al. (2015). Briefly, human CBX2.1 and CBX2.2 cDNA (OriGene, Rockville, Maryland, USA) were amplified and cloned then recombined into the destination vector to generate Dam-CBX2 construct. DamID was performed using a lentiviral transduction protocol120. Genomic DNA was isolated and used as templates to amplify methylated genomic fragments. DNA libraries were then prepared using the TruSeq DNA LT Sample Prep Kit (Illumina) and the libraries were sequenced on a HiSeq. 2000 sequencer (Illumina).
Illumina RNA sequencing
RNA sequencing provides far higher coverage and greater resolution of the dynamic nature of the transcriptome121. Total RNA was isolated and physical integrity was examined on Agilent Bioanalyser 2100 and Agilent 2200 TapeStation. Three replicates for each sample were performed for transcriptome analysis. RNA library preparation for sequencing was achieved according to the protocols of the functional genomics centre of Zurich (Switzerland)122. Typical cut-offs for candidate selection are Log2Ratio>1 (2x Fold) or Log2Ratio <−1 (2x Fold in the other direction) and an FDR (false discovery rate) of 0,05. The resulting p-values were FDR corrected using the Benjamini-Hochberg method. Genes with FDR values equal or smaller than ≤0,05 were considered differentially expressed.
GO enrichment analysis
GO‐terms with p‐values ≤ 0.05 and more than five target genes associated with the corresponding GO‐term were defined as significant. CBX2.1 and CBX2.2 target genes were clustered depending on GO‐terms and visualized using a spring-embed layout with Cytoscape v3. 3.0123. We used ToppCluster124 to explore the functional significance of the binding patterns of CBX2.1 and CBX2.2. GO‐enrichment permits to analyse functional features of gene sets, clustering them by their involvement in pathways related to Molecular Function, Biological Process and/or Cellular Component. GO-terms were considered as significantly enriched when value equal to or smaller than ≤0,05. Downstream genes were clustered according to their GO-terms. PathwayStudio 11 (Elsevier) is an exhaustive resource of easily searchable data from biology articles describing interactions between molecules, cell processes and diseases125. The platform allowed us to analyse the connection of CBX2 related genes in the human ovary genetic network.
Quantitative RT-PCR
In this study, we performed the RT-PCR as a technical validation approach. Gene expressions were evaluated using KAPA SYBR FAST qPCR Kit (KAPA BIOSYSTEMS). All samples were run in triplicates and the normalized relative expression values (2−ΔΔCt)126 of multiple independent experiments were plotted against the control vector set at 1. Statistical analyses were conducted using GraphPad Prism version 6.07 (Software, La Jolla, California, USA) and data sets were analysed for statistically significant differences using unpaired Student's t-test127 with confidence intervals set at 95%. Our gene-specific primer sequences and RT-qPCR conditions for CBX2 isoforms and selected targets amplification are available upon request.
Supplementary Information
Acknowledgements
This work was supported by the Swiss National Science Foundation Grants: 320030_130645 320030_160334 to A.B.-L.
Author contributions
L.B. conceived and conducted the experiments: KGN cell culture and characterization, transfections assays, RT-PCR experiments, optimization experiments, primers and sequences designing, genes sequencing, RNA sequencing experiments and data analysis, figures design by GraphPad, DamID data analysis, PathwayStudio analysis, all results interpretation and writing the manuscript). W.E. designed the DamID experiments. P.S. designed the Cytoscape and Venn diagrams. A.L.B. director of the whole project. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Patrick Sproll and Wassim Eid.
Supplementary information
is available for this paper at 10.1038/s41598-019-53370-4.
References
- 1.Richards JS, Pangas SA. The ovary: basic biology and clinical implications. The Journal of Clinical Investigation. 2010;120:963–972. doi: 10.1172/JCI41350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berta P, et al. Genetic evidence equating SRY and the testis-determining factor. Nature. 1990;348:448–450. doi: 10.1038/348448a0. [DOI] [PubMed] [Google Scholar]
- 3.Biason-Lauber A, Konrad D, Navratil F, Schoenle EJ. A WNT4 mutation associated with Mullerian-duct regression and virilization in a 46,XX woman. N Engl J Med. 2004;351:792–798. doi: 10.1056/NEJMoa040533. [DOI] [PubMed] [Google Scholar]
- 4.Parma P, et al. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 2006;38:1304–1309. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- 5.Crisponi L, et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet. 2001;27:159–166. doi: 10.1038/84781. [DOI] [PubMed] [Google Scholar]
- 6.Biason-Lauber A. WNT4, RSPO1, and FOXL2 in sex development. Semin Reprod Med. 2012;30:387–395. doi: 10.1055/s-0032-1324722. [DOI] [PubMed] [Google Scholar]
- 7.Yao HH-C. The pathway to femaleness: current knowledge on embryonic development of the ovary. Mol. Cell. Endocrinol. 2005;230:87–93. doi: 10.1016/j.mce.2004.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biason-Lauber A, Konrad D, Meyer M, DeBeaufort C, Schoenle EJ. Ovaries and female phenotype in a girl with 46,XY karyotype and mutations in the CBX2 gene. American journal of human genetics. 2009;84:658–663. doi: 10.1016/j.ajhg.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sproll P, et al. Assembling the jigsaw puzzle: CBX2 isoform 2 and its targets in disorders/differences of sex development. Molecular genetics & genomic medicine. 2018;6:785–795. doi: 10.1002/mgg3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katoh-Fukui Y, et al. Mouse Polycomb M33 is required for splenic vascular and adrenal gland formation through regulating Ad4BP/SF1 expression. Blood. 2005;106:1612–1620. doi: 10.1182/blood-2004-08-3367. [DOI] [PubMed] [Google Scholar]
- 11.Katoh-Fukui Y, et al. Male-to-female sex reversal in M33 mutant mice. Nature. 1998;393:688–692. doi: 10.1038/31482. [DOI] [PubMed] [Google Scholar]
- 12.Bel-Vialar S, et al. Altered retinoic acid sensitivity and temporal expression of Hox genes in polycomb-M33-deficient mice. Developmental biology. 2000;224:238–249. doi: 10.1006/dbio.2000.9791. [DOI] [PubMed] [Google Scholar]
- 13.Volkel P, Le Faou P, Vandamme J, Pira D, Angrand PO. A human Polycomb isoform lacking the Pc box does not participate to PRC1 complexes but forms protein assemblies and represses transcription. Epigenetics. 2012;7:482–491. doi: 10.4161/epi.19741. [DOI] [PubMed] [Google Scholar]
- 14.Katoh-Fukui Y, et al. Cbx2, a polycomb group gene, is required for Sry gene expression in mice. Endocrinology. 2012;153:913–924. doi: 10.1210/en.2011-1055. [DOI] [PubMed] [Google Scholar]
- 15.Baumann C, Fuente DL, Role R. of polycomb group protein cbx2/m33 in meiosis onset and maintenance of chromosome stability in the Mammalian germline. Genes (Basel) 2011;2:59–80. doi: 10.3390/genes2010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaimal V, Bardes EE, Tabar SC, Jegga AG, Aronow BJ. ToppCluster: a multiple gene list feature analyzer for comparative enrichment clustering and network-based dissection of biological systems. Nucleic Acids Res. 2010;38:W96–102. doi: 10.1093/nar/gkq418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustafson A-L, Donovan M, Annerwall E, Dencker L, Eriksson U. Nuclear import of cellular retinoic acid-binding protein type I in mouse embryonic cells. Mechanisms of Development. 1996;58:27–38. doi: 10.1016/S0925-4773(96)00554-0. [DOI] [PubMed] [Google Scholar]
- 18.Christenson LK, Johnson PF, McAllister JM, Strauss JF. CCAAT/Enhancer-binding Proteins Regulate Expression of the Human Steroidogenic Acute Regulatory Protein (StAR) Gene. Journal of Biological Chemistry. 1999;274:26591–26598. doi: 10.1074/jbc.274.37.26591. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Boutros PC. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics. 2011;12:35. doi: 10.1186/1471-2105-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haile Y, et al. Characterization of the NT2-derived neuronal and astrocytic cell lines as alternative in vitro models for primary human neurons and astrocytes. J Neurosci Res. 2014;92:1187–1198. doi: 10.1002/jnr.23399. [DOI] [PubMed] [Google Scholar]
- 21.Georges A, et al. The transcription factor FOXL2 mobilizes estrogen signaling to maintain the identity of ovarian granulosa cells. eLife. 2014;3:e04207. doi: 10.7554/eLife.04207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicol, B. & Yao, H. H. C. Gonadal Identity in the Absence of Pro-Testis Factor SOX9 and Pro-Ovary Factor Beta-Catenin in Mice1. Biology of Reproduction 93, 35, 31-12-35, 31–12, 10.1095/biolreprod.115.131276 (2015). [DOI] [PMC free article] [PubMed]
- 23.Kashimada K, et al. FOXL2 and BMP2 act cooperatively to regulate follistatin gene expression during ovarian development. Endocrinology. 2011;152:272–280. doi: 10.1210/en.2010-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q, et al. WNT4 acts downstream of BMP2 and functions via beta-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology. 2013;154:446–457. doi: 10.1210/en.2012-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Usongo M, Farookhi R. β-catenin/Tcf-signaling appears to establish the murine ovarian surface epithelium (OSE) and remains active in selected postnatal OSE cells. BMC developmental biology. 2012;12:17–17. doi: 10.1186/1471-213X-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Georges A, et al. FOXL2: a central transcription factor of the ovary. 2014;52:R17. doi: 10.1530/jme-13-0159. [DOI] [PubMed] [Google Scholar]
- 27.Eid W, Opitz L, Biason-Lauber A. Genome-wide identification of CBX2 targets: insights in the human sex development network. Mol Endocrinol. 2015;29:247–257. doi: 10.1210/me.2014-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maatouk DM, DiNapoli L, Taketo MM, Capel B. Investigating the Role of Beta-Catenin in Sex Determination. Biology of Reproduction. 2008;78:189–190. doi: 10.1093/biolreprod/78.s1.189c. [DOI] [Google Scholar]
- 29.Gu X, et al. CBX2 Inhibits Neurite Development by Regulating Neuron-Specific Genes Expression. Frontiers in molecular neuroscience. 2018;11:46–46. doi: 10.3389/fnmol.2018.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nef S, et al. Testis determination requires insulin receptor family function in mice. Nature. 2003;426:291–295. doi: 10.1038/nature02059. [DOI] [PubMed] [Google Scholar]
- 31.Griffeth RJ, Bianda V, Nef S. The emerging role of insulin-like growth factors in testis development and function. Basic and clinical andrology. 2014;24:12–12. doi: 10.1186/2051-4190-24-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popesku JT, et al. The goldfish (Carassius auratus) as a model for neuroendocrine signaling. Mol Cell Endocrinol. 2008;293:43–56. doi: 10.1016/j.mce.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Stocco C. Tissue physiology and pathology of aromatase. Steroids. 2012;77:27–35. doi: 10.1016/j.steroids.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang DS, et al. Doublesex- and Mab-3-related transcription factor-1 repression of aromatase transcription, a possible mechanism favoring the male pathway in tilapia. Endocrinology. 2010;151:1331–1340. doi: 10.1210/en.2009-0999. [DOI] [PubMed] [Google Scholar]
- 35.Brock O, Bakker J. The two kisspeptin neuronal populations are differentially organized and activated by estradiol in mice. Endocrinology. 2013;154:2739–2749. doi: 10.1210/en.2013-1120. [DOI] [PubMed] [Google Scholar]
- 36.Guo F, et al. The Transcriptome and DNA Methylome Landscapes of Human Primordial Germ Cells. Cell. 2015;161:1437–1452. doi: 10.1016/j.cell.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Li L, et al. Single-Cell RNA-Seq Analysis Maps Development of Human Germline Cells and Gonadal Niche Interactions. Cell stem cell. 2017;20:858–873.e854. doi: 10.1016/j.stem.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, et al. Transcriptome Landscape of Human Folliculogenesis Reveals Oocyte and Granulosa Cell Interactions. Molecular cell. 2018;72:1021–1034.e1024. doi: 10.1016/j.molcel.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 39.Duffy TA, et al. Ontogenesis of gonadal aromatase gene expression in atlantic silverside (Menidia menidia) populations with genetic and temperature-dependent sex determination. J Exp Zool A Ecol Genet Physiol. 2010;313:421–431. doi: 10.1002/jez.612. [DOI] [PubMed] [Google Scholar]
- 40.Baetens D, et al. NR5A1 is a novel disease gene for 46,XX testicular and ovotesticular disorders of sex development. Genetics in medicine: official journal of the American College of Medical Genetics. 2017;19:367–376. doi: 10.1038/gim.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelletier G, El-Alfy M. Immunocytochemical localization of estrogen receptors alpha and beta in the human reproductive organs. J Clin Endocrinol Metab. 2000;85:4835–4840. doi: 10.1210/jcem.85.12.7029. [DOI] [PubMed] [Google Scholar]
- 42.Lang-Muritano M, et al. Early-Onset Complete Ovarian Failure and Lack of Puberty in a Woman With Mutated Estrogen Receptor beta (ESR2) J Clin Endocrinol Metab. 2018;103:3748–3756. doi: 10.1210/jc.2018-00769. [DOI] [PubMed] [Google Scholar]
- 43.Witchel SF, Tena-Sempere M. The Kiss1 system and polycystic ovary syndrome: lessons from physiology and putative pathophysiologic implications. Fertil Steril. 2013;100:12–22. doi: 10.1016/j.fertnstert.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 44.Bouilly J, et al. R-spondin2, a novel target of NOBOX: identification of variants in a cohort of women with primary ovarian insufficiency. Journal of Ovarian Research. 2017;10:51. doi: 10.1186/s13048-017-0345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu K-L, Zhao H, Chang H-M, Yu Y, Qiao J. Kisspeptin/Kisspeptin Receptor System in the Ovary. Front. Endocrinol. (Lausanne) 2018;8:365–365. doi: 10.3389/fendo.2017.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol. Rev. 2012;92:1235–1316. doi: 10.1152/physrev.00037.2010. [DOI] [PubMed] [Google Scholar]
- 47.Topaloglu AK, et al. Inactivating KISS1 Mutation and Hypogonadotropic Hypogonadism. New England Journal of Medicine. 2012;366:629–635. doi: 10.1056/NEJMoa1111184. [DOI] [PubMed] [Google Scholar]
- 48.de Roux N, et al. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ilgaz NS, et al. Impact of follicle-stimulating hormone receptor variants in female infertility. J Assist Reprod Genet. 2015;32:1659–1668. doi: 10.1007/s10815-015-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aittomaki K, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–968. doi: 10.1016/0092-8674(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 51.Pellegrini M, Pantano S, Lucchini F, Fumi M, Forabosco A. Emx2 developmental expression in the primordia of the reproductive and excretory systems. Anat Embryol (Berl) 1997;196:427–433. doi: 10.1007/s004290050110. [DOI] [PubMed] [Google Scholar]
- 52.Turnbull Clare, Rapley Elizabeth A, Seal Sheila, Pernet David, Renwick Anthony, Hughes Deborah, Ricketts Michelle, Linger Rachel, Nsengimana Jeremie, Deloukas Panagiotis, Huddart Robert A, Bishop D Timothy, Easton Douglas F, Stratton Michael R, Rahman Nazneen. Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. Nature Genetics. 2010;42(7):604–607. doi: 10.1038/ng.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foster JW, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 54.Wagner T, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 55.Huang B, et al. sex reversal caused by duplication of SOX9. Am J Med Genet. 1999;87:349–353. doi: 10.1002/(SICI)1096-8628(19991203)87:4<349::AID-AJMG13>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 56.Murphy MW, et al. An ancient protein-DNA interaction underlying metazoan sex determination. Nat Struct Mol Biol. 2015;22:442–451. doi: 10.1038/nsmb.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hannema SE, Hughes IA. Regulation of Wolffian duct development. Horm Res. 2007;67:142–151. doi: 10.1159/000096644. [DOI] [PubMed] [Google Scholar]
- 58.Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S. Defects of urogenital development in mice lacking Emx2. Development. 1997;124:1653–1664. doi: 10.1242/dev.124.9.1653. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Zhao YY, Qiao J. Obstetric outcome of women with uterine anomalies in China. Chin Med J (Engl) 2010;123:418–422. [PubMed] [Google Scholar]
- 60.Jones MR, et al. Harnessing expression data to identify novel candidate genes in polycystic ovary syndrome. PLoS One. 2011;6:e20120. doi: 10.1371/journal.pone.0020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gecz J, et al. Assignment of a polycomb-like chromobox gene (CBX2) to human chromosome 17q25. Genomics. 1995;26:130–133. doi: 10.1016/0888-7543(95)80091-Y. [DOI] [PubMed] [Google Scholar]
- 62.Kerr B, Garcia-Rudaz C, Dorfman M, Paredes A, Ojeda SR. NTRK1 and NTRK2 receptors facilitate follicle assembly and early follicular development in the mouse ovary. Reproduction. 2009;138:131–140. doi: 10.1530/rep-08-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koster A, et al. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 2005;146:4943–4950. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 64.Galaup A., Cazes A., Le Jan S., Philippe J., Connault E., Le Coz E., Mekid H., Mir L. M., Opolon P., Corvol P., Monnot C., Germain S. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proceedings of the National Academy of Sciences. 2006;103(49):18721–18726. doi: 10.1073/pnas.0609025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uhlenhaut NH, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 66.Leila Bouazzi, M. F., Alexander Maret and Anna Biason-Lauber. CBX2 and the Ovary: Novel Insights into Regulatory Networks in Humans. Gynecology and Reproductive Medicine 2, 1 of 9 (2018).
- 67.Quaynor SD, et al. Delayed puberty and estrogen resistance in a woman with estrogen receptor alpha variant. N Engl J Med. 2013;369:164–171. doi: 10.1056/NEJMoa1303611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bulun SE. Aromatase and estrogen receptor alpha deficiency. Fertil Steril. 2014;101:323–329. doi: 10.1016/j.fertnstert.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yao HH, et al. Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn. 2004;230:210–215. doi: 10.1002/dvdy.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jeays-Ward K, Dandonneau M, Swain A. Wnt4 is required for proper male as well as female sexual development. Developmental biology. 2004;276:431–440. doi: 10.1016/j.ydbio.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 71.Calatayud NE, et al. Ontogeny of the oestrogen receptors ESR1 and ESR2 during gonadal development in the tammar wallaby, Macropus eugenii. Reproduction. 2010;139:599–611. doi: 10.1530/rep-09-0305. [DOI] [PubMed] [Google Scholar]
- 72.Buaas FW, Gardiner JR, Clayton S, Val P, Swain A. In vivo evidence for the crucial role of SF1 in steroid-producing cells of the testis, ovary and adrenal gland. Development. 2012;139:4561–4570. doi: 10.1242/dev.087247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jameson SA, Lin Y-T, Capel B. Testis development requires the repression of Wnt4 by Fgf signaling. Dev. Biol. 2012;370:24–32. doi: 10.1016/j.ydbio.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in Male Physiology. Physiol. Rev. 2017;97:995–1043. doi: 10.1152/physrev.00018.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jordan BK, et al. Up-Regulation of WNT-4 Signaling and Dosage-Sensitive Sex Reversal in Humans. The American Journal of Human Genetics. 2001;68:1102–1109. doi: 10.1086/320125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McElreavey, K. Mutations in CBX2 associated with gonadal anomalies in 46,XY and 46,XX individuals. Human Developmental Genetics I-DSD Symposium Programme (2019).
- 77.Pierre A, et al. The Bone Morphogenetic Protein 15 Up-Regulates the Anti-Mullerian Hormone Receptor Expression in Granulosa Cells. J Clin Endocrinol Metab. 2016;101:2602–2611. doi: 10.1210/jc.2015-4066. [DOI] [PubMed] [Google Scholar]
- 78.Estienne A, et al. Anti-Mullerian hormone regulation by the bone morphogenetic proteins in the sheep ovary: deciphering a direct regulatory pathway. Endocrinology. 2015;156:301–313. doi: 10.1210/en.2014-1551. [DOI] [PubMed] [Google Scholar]
- 79.Prapa E, et al. Effect of Anti-Mullerian hormone (AMH) and bone morphogenetic protein 15 (BMP-15) on steroidogenesis in primary-cultured human luteinizing granulosa cells through Smad5 signalling. J Assist Reprod Genet. 2015;32:1079–1088. doi: 10.1007/s10815-015-0494-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim JY. Control of ovarian primordial follicle activation. Clinical and experimental reproductive medicine. 2012;39:10–14. doi: 10.5653/cerm.2012.39.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Di Pasquale E, Beck-Peccoz P, Persani L. Hypergonadotropic Ovarian Failure Associated with an Inherited Mutation of Human Bone Morphogenetic Protein-15 (BMP15) Gene. American Journal of Human Genetics. 2004;75:106–111. doi: 10.1086/422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Memon MA, Anway MD, Covert TR, Uzumcu M, Skinner MK. Transforming growth factor beta (TGFbeta1, TGFbeta2 and TGFbeta3) null-mutant phenotypes in embryonic gonadal development. Mol Cell Endocrinol. 2008;294:70–80. doi: 10.1016/j.mce.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bartram U, et al. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation. 2001;103:2745–2752. doi: 10.1161/01.CIR.103.22.2745. [DOI] [PubMed] [Google Scholar]
- 84.Raja-Khan N, et al. A variant in the fibrillin-3 gene is associated with TGF-beta and inhibin B levels in women with polycystic ovary syndrome. Fertil Steril. 2010;94:2916–2919. doi: 10.1016/j.fertnstert.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Almahbobi G, Misajon A, Hutchinson P, Lolatgis N, Trounson AO. Hyperexpression of epidermal growth factor receptors in granulosa cells from women with polycystic ovary syndrome. Fertility and sterility. 1998;70:750–758. doi: 10.1016/S0015-0282(98)00252-0. [DOI] [PubMed] [Google Scholar]
- 86.Li Q. Transforming growth factor β signaling in uterine development and function. Journal of Animal Science and Biotechnology. 2014;5:52. doi: 10.1186/2049-1891-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang J, et al. Transforming growth factor-β is involved in maintaining oocyte meiotic arrest by promoting natriuretic peptide type C expression in mouse granulosa cells. Cell Death & Disease. 2019;10:558. doi: 10.1038/s41419-019-1797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chegini N, Flanders KC. Presence of transforming growth factor-beta and their selective cellular localization in human ovarian tissue of various reproductive stages. Endocrinology. 1992;130:1707–1715. doi: 10.1210/endo.130.3.1537318. [DOI] [PubMed] [Google Scholar]
- 89.Goodman FR, et al. Novel HOXA13 mutations and the phenotypic spectrum of hand-foot-genital syndrome. Am J Hum Genet. 2000;67:197–202. doi: 10.1086/302961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morgan EA, Nguyen SB, Scott V, Stadler HS. Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development. 2003;130:3095–3109. doi: 10.1242/dev.00530. [DOI] [PubMed] [Google Scholar]
- 91.Mortlock DP, Innis JW. Mutation of HOXA13 in hand-foot-genital syndrome. Nat Genet. 1997;15:179–180. doi: 10.1038/ng0297-179. [DOI] [PubMed] [Google Scholar]
- 92.Sproll P, et al. Assembling the jigsaw puzzle: CBX2 isoform 2 and its targets in disorders/differences of sex development. Molecular Genetics & Genomic. Medicine. 2018;6:785–795. doi: 10.1002/mgg3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao L, et al. SOX4 regulates gonad morphogenesis and promotes male germ cell differentiation in mice. Dev. Biol. 2017;423:46–56. doi: 10.1016/j.ydbio.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 94.Batista F, Vaiman D, Dausset J, Fellous M, Veitia RA. Potential targets of FOXL2, a transcription factor involved in craniofacial and follicular development, identified by transcriptomics. Proc Natl Acad Sci USA. 2007;104:3330–3335. doi: 10.1073/pnas.0611326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Silva JR, et al. The activin-follistatin system and in vitro early follicle development in goats. J Endocrinol. 2006;189:113–125. doi: 10.1677/joe.1.06487. [DOI] [PubMed] [Google Scholar]
- 96.Greenbaum MP, et al. TEX14 is essential for intercellular bridges and fertility in male mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4982–4987. doi: 10.1073/pnas.0505123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocrine reviews. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kawashima I, Kawamura K. Disorganization of the germ cell pool leads to primary ovarian insufficiency. Reproduction. 2017;153:R205–r213. doi: 10.1530/rep-17-0015. [DOI] [PubMed] [Google Scholar]
- 99.Del Valle I, et al. A genomic atlas of human adrenal and gonad development. Wellcome Open Res. 2017;2:25. doi: 10.12688/wellcomeopenres.11253.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bergman J, et al. The Human Adrenal Gland Proteome Defined by Transcriptomics and Antibody-Based Profiling. Endocrinology. 2017;158:239–251. doi: 10.1210/en.2016-1758. [DOI] [PubMed] [Google Scholar]
- 101.Nelson-Degrave VL, et al. Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol Endocrinol. 2005;19:379–390. doi: 10.1210/me.2004-0178. [DOI] [PubMed] [Google Scholar]
- 102.Hu S, et al. Expression patterns of p38αMAPK during follicular development in the ovaries of neonatal rats. Acta Histochemica. 2017;119:538–542. doi: 10.1016/j.acthis.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 103.Oktem O, Oktay K. The Role of Extracellular Matrix and Activin-A in In Vitro Growth and Survival of Murine Preantral Follicles. Reprod. Sci. 2007;14:358–366. doi: 10.1177/1933719107303397. [DOI] [PubMed] [Google Scholar]
- 104.Marti N, et al. Genes and proteins of the alternative steroid backdoor pathway for dihydrotestosterone synthesis are expressed in the human ovary and seem enhanced in the polycystic ovary syndrome. Molecular and Cellular Endocrinology. 2017;441:116–123. doi: 10.1016/j.mce.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 105.Blouin K, Veilleux A, Luu-The V, Tchernof A. Androgen metabolism in adipose tissue: recent advances. Mol Cell Endocrinol. 2009;301:97–103. doi: 10.1016/j.mce.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 106.Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol. Metab. 2004;15:432–438. doi: 10.1016/j.tem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 107.Shiina H, et al. Premature ovarian failure in androgen receptor-deficient mice. Proc. Natl. Acad. Sci. USA. 2006;103:224–229. doi: 10.1073/pnas.0506736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nelson VL, et al. The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2001;86:5925–5933. doi: 10.1210/jcem.86.12.8088. [DOI] [PubMed] [Google Scholar]
- 109.Krentz AD, et al. DMRT1 promotes oogenesis by transcriptional activation of Stra8 in the mammalian fetal ovary. Developmental biology. 2011;356:63–70. doi: 10.1016/j.ydbio.2011.05.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pangas SA, Rajkovic A. Transcriptional regulation of early oogenesis: in search of masters. Human Reproduction Update. 2005;12:65–76. doi: 10.1093/humupd/dmi033. [DOI] [PubMed] [Google Scholar]
- 111.Demiray SB, et al. Expression of the Bone Morphogenetic Protein-2 (BMP2) in the Human Cumulus Cells as a Biomarker of Oocytes and Embryo Quality. J Hum Reprod Sci. 2017;10:194–200. doi: 10.4103/jhrs.JHRS_21_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fatehi AN, et al. Expression of bone morphogenetic protein2 (BMP2), BMP4 and BMP receptors in the bovine ovary but absence of effects of BMP2 and BMP4 during IVM on bovine oocyte nuclear maturation and subsequent embryo development. Theriogenology. 2005;63:872–889. doi: 10.1016/j.theriogenology.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 113.Alexopoulos E, Shahid J, Ongley HZ, Richardson MC. Luteinized human granulosa cells are associated with endogenous basement membrane-like components in culture. Molecular human reproduction. 2000;6:324–330. doi: 10.1093/molehr/6.4.324. [DOI] [PubMed] [Google Scholar]
- 114.Bruckova L, et al. Proliferative potential and phenotypic analysis of long-term cultivated human granulosa cells initiated by addition of follicular fluid. J Assist Reprod Genet. 2011;28:939–950. doi: 10.1007/s10815-011-9617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu Y, et al. Effects of Smad3 on the proliferation and steroidogenesis in human ovarian luteinized granulosa cells. IUBMB Life. 2014;66:424–437. doi: 10.1002/iub.1280. [DOI] [PubMed] [Google Scholar]
- 116.Nishi Y, et al. Establishment and Characterization of a Steroidogenic Human Granulosa-Like Tumor Cell Line, KGN, That Expresses Functional Follicle-Stimulating Hormone Receptor. Endocrinology. 2001;142:437–445. doi: 10.1210/endo.142.1.7862. [DOI] [PubMed] [Google Scholar]
- 117.Alexiadis M, et al. Transcriptomic analysis of stage 1 versus advanced adult granulosa cell tumors. Oncotarget. 2016;7:14207–14219. doi: 10.18632/oncotarget.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tremblay PG, Sirard MA. Transcriptomic analysis of gene cascades involved in protein kinase A and C signaling in the KGN line of human ovarian granulosa tumor cellsdagger. Biol Reprod. 2017;96:855–865. doi: 10.1093/biolre/iox024. [DOI] [PubMed] [Google Scholar]
- 119.Rosario R, Araki H, Print CG, Shelling AN. The Transcriptional Targets of Mutant FOXL2 in Granulosa Cell Tumours. Plos One. 2012;7:e46270. doi: 10.1371/journal.pone.0046270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Steensel B, Henikoff S. Identification of in vivo DNA targets of chromatin proteins using tethered dam methyltransferase. Nat Biotechnol. 2000;18:424–428. doi: 10.1038/74487. [DOI] [PubMed] [Google Scholar]
- 121.Kukurba KR, Montgomery SB. RNA Sequencing and Analysis. Cold Spring Harbor protocols. 2015;2015:951–969. doi: 10.1101/pdb.top084970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rehrauer H, et al. AGRONOMICS1: a new resource for Arabidopsis transcriptome profiling. Plant Physiol. 2010;152:487–499. doi: 10.1104/pp.109.150185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shannon P, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ashburner M, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nikitin A, Egorov S, Daraselia N, Mazo I. Pathway studio—the analysis and navigation of molecular networks. Bioinformatics. 2003;19:2155–2157. doi: 10.1093/bioinformatics/btg290. [DOI] [PubMed] [Google Scholar]
- 126.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 127.Fabri PJ, Knierim TH. Simple calculation of the unpaired t test. Surg Gynecol Obstet. 1988;167:381–382. [PubMed] [Google Scholar]
- 128.Ditewig AC, Yao HH-C. Organogenesis of the ovary: a comparative review on vertebrate ovary formation. Organogenesis. 2005;2:36–41. doi: 10.4161/org.2.2.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shimada M, Yamashita Y. The Key Signaling Cascades in Granulosa Cells During Follicular Development and Ovulation Process. Journal of Mammalian Ova Research. 2011;28:25–31. doi: 10.1274/jmor.28.25. [DOI] [Google Scholar]
- 130.Kim SO, Harris SM, Duffy DM. Prostaglandin E2 (EP) receptors mediate PGE2-specific events in ovulation and luteinization within primate ovarian follicles. Endocrinology. 2014;155:1466–1475. doi: 10.1210/en.2013-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Richards JS. Genetics of ovulation. Semin Reprod Med. 2007;25:235–242. doi: 10.1055/s-2007-980217. [DOI] [PubMed] [Google Scholar]
- 132.Frojdman K, Harley VR, Pelliniemi LJ. Sox9 protein in rat sertoli cells is age and stage dependent. Histochem Cell Biol. 2000;113:31–36. doi: 10.1007/s004180050004. [DOI] [PubMed] [Google Scholar]
- 133.Padovano V, et al. The POF1B candidate gene for premature ovarian failure regulates epithelial polarity. J Cell Sci. 2011;124:3356–3368. doi: 10.1242/jcs.088237. [DOI] [PubMed] [Google Scholar]
- 134.Lacombe A, et al. Disruption of POF1B binding to nonmuscle actin filaments is associated with premature ovarian failure. Am J Hum Genet. 2006;79:113–119. doi: 10.1086/505406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Combes AN, et al. Expression and functional analysis of Dkk1 during early gonadal development. Sexual development: genetics, molecular biology, evolution. endocrinology, embryology, and pathology of sex determination and differentiation. 2011;5:124–130. doi: 10.1159/000327709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Y, et al. Progesterone inhibition of Wnt/beta-catenin signaling in normal endometrium and endometrial cancer. Clin Cancer Res. 2009;15:5784–5793. doi: 10.1158/1078-0432.ccr-09-0814. [DOI] [PubMed] [Google Scholar]
- 137.Tanaka S, Akiyoshi T, Mori M, Wands JR, Sugimachi K. A novel frizzled gene identified in human esophageal carcinoma mediates APC/beta-catenin signals. Proc Natl Acad Sci USA. 1998;95:10164–10169. doi: 10.1073/pnas.95.17.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 139.Ma YJ, Dissen GA, Merlino G, Coquelin A, Ojeda SR. Overexpression of a human transforming growth factor-alpha (TGF alpha) transgene reveals a dual antagonistic role of TGF alpha in female sexual development. Endocrinology. 1994;135:1392–1400. doi: 10.1210/en.135.4.1392. [DOI] [PubMed] [Google Scholar]
- 140.Klein R, Martin-Zanca D, Barbacid M, Parada LF. Expression of the tyrosine kinase receptor gene trkB is confined to the murine embryonic and adult nervous system. Development. 1990;109:845–850. doi: 10.1242/dev.109.4.845. [DOI] [PubMed] [Google Scholar]
- 141.Dorfman MD, et al. Loss of Ntrk2/Kiss1r Signaling in Oocytes Causes Premature Ovarian Failure. Endocrinology. 2014;155:3098–3111. doi: 10.1210/en.2014-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Penning TM, et al. Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351:67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang L, et al. The expression of sex steroid synthesis and inactivation enzymes in subcutaneous adipose tissue of PCOS patients. J Steroid Biochem Mol Biol. 2012;132:120–126. doi: 10.1016/j.jsbmb.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 144.Carmon KS, Loose DS. Wnt7a interaction with Fzd5 and detection of signaling activation using a split eGFP. Biochemical and biophysical research communications. 2008;368:285–291. doi: 10.1016/j.bbrc.2008.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Paul MH, Harvey RP, Wegner M, Sock E. Cardiac outflow tract development relies on the complex function of Sox4 and Sox11 in multiple cell types. Cell Mol Life Sci. 2014;71:2931–2945. doi: 10.1007/s00018-013-1523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhao H, Li Z, Cooney AJ, Lan ZJ. Orphan nuclear receptor function in the ovary. Front Biosci. 2007;12:3398–3405. doi: 10.2741/2321. [DOI] [PubMed] [Google Scholar]
- 147.Ohkawara B, Glinka A, Niehrs C. Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev Cell. 2011;20:303–314. doi: 10.1016/j.devcel.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 148.Cupp AS, Tessarollo L, Skinner MK. Testis developmental phenotypes in neurotropin receptor trkA and trkC null mutations: role in formation of seminiferous cords and germ cell survival. Biol Reprod. 2002;66:1838–1845. doi: 10.1095/biolreprod66.6.1838. [DOI] [PubMed] [Google Scholar]
- 149.Koliwad SK, et al. Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. J Biol Chem. 2009;284:25593–25601. doi: 10.1074/jbc.M109.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ishikawa T, Glidewell-Kenney C, Jameson JL. Aromatase-independent testosterone conversion into estrogenic steroids is inhibited by a 5 alpha-reductase inhibitor. J Steroid Biochem Mol Biol. 2006;98:133–138. doi: 10.1016/j.jsbmb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 151.Chen JK, Heckert LL. Dmrt1 Expression Is Regulated by Follicle-Stimulating Hormone and Phorbol Esters in Postnatal Sertoli Cells. Endocrinology. 2001;142:1167–1178. doi: 10.1210/endo.142.3.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tian G, et al. Expression and function of the LIM-homeobox containing genes Lhx3 and Lhx4 in the mouse placenta. Developmental dynamics: an official publication of the American Association of Anatomists. 2008;237:1517–1525. doi: 10.1002/dvdy.21546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bione S, Toniolo D. X chromosome genes and premature ovarian failure. Semin Reprod Med. 2000;18:51–57. doi: 10.1055/s-2000-13475. [DOI] [PubMed] [Google Scholar]
- 154.Sproul K, Jones MR, Mathur R, Azziz R, Goodarzi MO. Association study of four key folliculogenesis genes in polycystic ovary syndrome. BJOG: An International Journal of Obstetrics & Gynaecology. 2010;117:756–760. doi: 10.1111/j.1471-0528.2010.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.