Abstract
Antenatal corticosteroids are often administered to women at risk of preterm birth to accelerate fetal lung development; however, there is evidence that this treatment may adversely affect placental function in some fetuses. Our group has recently demonstrated that wave reflections in the umbilical artery (UA), measured using high-frequency ultrasound, are sensitive to placental vascular abnormalities. In the present study, we used this approach to investigate the effect of maternal administration of betamethasone, a clinically relevant corticosteroid, on the feto-placental vasculature of the mouse. Fetuses were assessed at embryonic day (E)15.5 and E17.5 in C57BL6/J mice. At both gestational ages, the UA diameter, UA blood flow, and the wave reflection coefficient were significantly elevated in the betamethasone-treated mice compared to vehicle-treated controls. These observations support the interpretation that placental vascular resistance dropped with betamethasone treatment to an extent that could not be explained by vasodilation of the UA alone. Consistent with clinical studies, the effect of betamethasone on UA end-diastolic velocity was heterogeneous. Our results suggest that UA wave reflections are more sensitive to acute changes in placental vascular resistance compared with the UA pulsatility index, and this technique may have clinical application to identify a favorable placental vascular response to fetal therapies such as antenatal corticosteroids, where the fetal heart rate is likely to vary.
Keywords: corticosteroids, mouse, placenta, ultrasound, umbilical artery, wave reflection
Summary Sentence Antenatal betamethasone administration in healthy pregnant mice resulted in decreased placental vascular resistance, altering wave reflection parameters that are sensitive to these acute changes in vascular tone.
Introduction
Maternally administered corticosteroids have been used for over 40 years to accelerate fetal lung development and improve neonatal outcomes in pregnancies at risk of preterm birth [1, 2]. Their use has been associated with reduced occurrence of respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, and neonatal death [3]. Despite these observed benefits, corticosteroids have been shown to exert transient adverse effects on measures of fetal well-being in healthy preterm fetuses, including decreased fetal breathing and body movements [4–6] and reduced fetal heart rate variability [5, 7, 8]. The effect of antenatal corticosteroid administration on feto-placental vascular resistance has been studied extensively; however, the clinical findings are heterogeneous. In fetuses with normal umbilical artery (UA) diastolic blood flow (in both appropriate growth for gestational age and growth-restricted fetuses), past studies have reported no change in UA pulsatility index (PI) following corticosteroid administration [5, 9–13]. In contrast, in pregnancies complicated by increased placental vascular resistance, as evidenced by absent or reversed end-diastolic velocity (AREDV) in the UA, administration of corticosteroids can result in a transient return of end-diastolic velocity in approximately two-thirds of pregnancies [14–22]. There are, however, some studies reporting no improvement in UA PI in pregnancies with AREDV after corticosteroids [23, 24]. It has also been suggested that persistent AREDV after corticosteroid administration may represent a subset of fetuses that are at higher risk for perinatal complications [18, 19, 21]. Understanding the physiological basis of the placental response to corticosteroids could lead to better tailored administration of corticosteroids in high-risk fetuses and/or improved surveillance following their administration.
While the UA PI is currently used as a surrogate for placental vascular resistance, as a waveform ratio, it is thus affected by multiple additional factors, including the baseline fetal heart rate [25, 26]. To isolate the downstream placental-associated signal from the observed UA Doppler waveforms, our group has developed a noninvasive ultrasound methodology to measure pulse pressure waves that propagate both with and counter to the direction of blood flow along the UA, and has demonstrated its utility in mice [27] and humans [28]. The relative amplitude of these forward and backward propagating waves, termed the reflection coefficient, is proposed as a more specific measure of downstream placental vascular resistance than UA PI.
In the present study, we investigate the changes in UA wave reflection metrics in healthy, pregnant C57BL6/J mice treated with betamethasone, a corticosteroid clinically relevant to human pregnancies. The effect of corticosteroids on lung and brain function and fetal growth has been studied in several animal models including sheep [29–36], rabbits [37–39], and guinea pigs [40]. Administration of betamethasone to pregnant mice has also been shown to accelerate fetal lung maturation [41] and to delay parturition in an endotoxin-induced prematurity model [42]. Here, dams were imaged using high-frequency ultrasound at two time points during late gestation to detect changes in the feto-placental vascular resistance after maternal administration of betamethasone. In addition, having previously established that wave reflection metrics are sensitive to structural changes in the placental vasculature [43], we investigated the effect of betamethasone-induced acute changes in vascular tone on wave reflections.
Materials and methods
Mice
Healthy adult C57BL6/J mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). In house, after virgin females (age 7–13 weeks) were mated with males, the morning that a vaginal plug was detected was designated as embryonic day (E)0.5. Pregnant mice were randomly assigned to either a treatment group that received a single subcutaneous injection of 0.1 mg betamethasone (BMZ) in sterile saline (Celestone Soluspan, Merck Canada Inc., Kirkland, QC, Canada) [42, 44] or a control group that received an equivalent volume of sterile saline. This dose of betamethasone has been shown experimentally to be equivalent to the 24 mg dose exposure over 24 h that is recommended clinically in humans [45]. The dams were imaged using ultrasound biomicroscopy at baseline and 4 h postinjection at either E15.5 (BMZ: 25 fetuses from 8 litters, control: 19 fetuses from 6 litters) or E17.5 (BMZ: 25 fetuses from 9 litters, control: 25 fetuses from 10 litters). Full term for C57BL6/J mice is 18.5 days, and therefore, 4 h postinjection is equivalent to approximately 2 days in humans. All animal experiments were approved by the Animal Care Committee at The Centre for Phenogenomics and conducted in accordance with guidelines established by the Canadian Council on Animal Care.
Ultrasound biomicroscopy
As described previously, the fetal end of the UA was imaged using a high-frequency ultrasound system with a 40-MHz linear array transducer (Vevo 2100, VisualSonics, Toronto, ON, Canada) [27, 43]. Briefly, dams were anesthetized with isoflurane (4% for induction and 2.5% for maintenance in 21% O2) and body temperature was maintained at 35–37 °C using a temperature-regulated platform. M-mode and pulsed wave Doppler recordings were made at approximately the same location in the UA, with the ultrasound beam perpendicular to the vessel for the M-mode recording and the narrowest angle of insonation (<60°) for the Doppler measurement. All UAs from fetuses that were in a favorable spatial orientation for both M-mode and Doppler measurements were imaged (2–4 per dam). Fetuses were chosen from both the right and left uterine horn and typically were located in the lower abdomen (to avoid vessel motion from maternal breathing). One BMZ dataset (M-mode and Doppler) at 4 h postinjection had to be excluded because shadowing from internal organs made it difficult to visualize the UA. One control M-mode dataset at 4 h postinjection had to be excluded because of failure to find a well-defined M-mode trace in the correct location.
Ultrasound image analysis
The ultrasound image analysis was performed as described previously [27, 43]. Briefly, the M-mode time series showing the two walls of the UA were automatically traced and the luminal outline was smoothed using a low-pass, second-order Butterworth filter. The smoothed waveforms were separated into individual cardiac cycles based on the onset of systole. Assuming a circular cross section, the diameter estimates as a function of time were converted to area. The individual UA area waveforms were then temporally aligned and averaged together. Similarly, the mean velocity waveforms were automatically outlined, smoothed, separated into individual cardiac cycles, temporally aligned, and averaged. Datasets were excluded if high levels of maternal gasping or fetal movements were present. Following wave reflection analysis, further postprocessing criteria were applied to the M-mode traces: datasets were excluded: (a) if >35% of the total aligned waveforms were outside bands representing ±20% of the average area change or (b) if there were insufficient individual area traces to average (<4 area traces).
The average area and velocity waveforms were multiplied to calculate the average flow waveform. The pulse wave velocity (PWV) was calculated by plotting flow versus area and fitting a line to 20–80% of the maximum systolic flow region. Using the PWV, the observed flow waveform was decomposed into its forward and reflected components. The reflected waveforms were summarized in terms of reflection coefficient, time delay, and dispersion. The reflection coefficient is defined as the ratio of the peak-to-peak amplitude between the reflected and forward waves. The time delay is the time difference between the peak of the backward and forward waves, and the dispersion is the difference in the full-width at half-maximum of the backward and forward waves. The PI was calculated as the difference between peak systolic and end-diastolic velocities, divided by the mean velocity over the fetal cardiac cycle.
Statistical analysis
All statistical tests were conducted using the R statistical software package (www.r-project.org). The maternal and placental parameters (maternal weight and umbilical cord diameter), physiological parameters (fetal heart rate, UA blood flow, and PWV), wave reflection metrics (reflection coefficient, time delay, and dispersion) and UA PI were analyzed using a linear mixed effect model with group (BMZ treatment, control) and gestational age (E15.5, E17.5) as fixed effects and litter as a random effect. Three-way ANOVAs were used to assess the variance in the change in the reflection coefficient due to group, gestational age, and either the fetal heart rate or the change in UA diameter. All data are reported as means with 95% parametric confidence intervals (CIs). A value of P < 0.05 was taken to be significant.
Results
Data quality
One M-data dataset was excluded because of severe fetal arrhythmia, 34 of the datasets (18%) did not meet the preprocessing acceptance criteria (the UA wall signal was nonphysiological due to maternal gasping or fetal movements) and additional 15 datasets (8%) were excluded following the postprocessing quality control (>35% of the total aligned waveforms were outside bands representing ±20% of the average area change or there were <4 area traces to average). Therefore, after application of the preprocessing and postprocessing steps to the M-mode images, 72% of the UA scans met the quality control criteria and were included in the wave decomposition analysis (baseline: 37 BMZ treated and 28 control; 4 h postinjection: 42 BMZ treated and 29 control). This is notably higher than our previous work, where only 46–51% of the UA scans met the acceptance criteria [27, 43]. Of the total datasets, 30% had a successful scan at both baseline and 4 h postinjection (E15.5: 18 BMZ treated and 11 vehicle treated; E17.5: 16 BMZ treated and 12 vehicle treated).
Baseline characteristics
There was no difference in mean maternal weight prior to mice being randomly assigned to either the betamethasone or control group (E15.5: BMZ 34 g [CI %: 32–36] vs. control 33 g [CI %: 31–35]; E17.5: BMZ 35 g [CI %: 32–38] vs. control 36 g [CI %: 34–38]). Prior to treatment, there were no differences between groups in the mean UA diameter, UA blood flow, reflection coefficient, and UA PI. The gestational age had a significant effect on the UA diameter, UA blood flow and the reflection coefficient, while the UA PI did not show an effect of gestational age (Figure 1). Table 1 summarizes additional physiological and wave reflection metrics at baseline, with no effect of group or gestational age.
Figure 1.
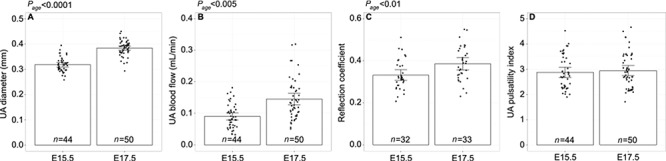
Ultrasound parameters prior to treatment at E15.5 and E17.5. (A) UA diameter, (B) UA blood flow, (C) reflection coefficient, and (D) UA pulsatility index at gestational age E15.5 and E17.5. Main effect of gestational age as determined by a two-way ANOVA is noted as Page. Data shown as means ±95% confidence intervals and n refers to the number of fetuses.
Table 1.
Physiological and hemodynamic parameters prior to treatment at E15.5 and E17.5.
| Gestational age | Fetal heart rate (BPM) | PWV (m/s) | Time delay (s) | Dispersion |
|---|---|---|---|---|
| E15.5 | 156 (147–165) | 2.7 (1.8–3.6) | 0.07 (0.06–0.08) | 0.06 (0.04–0.08) |
| E17.5 | 169 (158–180) | 3.1 (2.1–4.1) | 0.07 (0.06–0.08) | 0.07 (0.02–0.12) |
Data are presented as means (95% confidence intervals).
Effects of betamethasone
Comparing 4 h postinjection to baseline, the UA diameter, UA blood flow, and the reflection coefficient showed a significant group effect (Figure 2). Following administration of betamethasone, the UA blood flow was significantly increased by 27% (CI %: −7–61) at E15.5 and 54% (CI %: 25–83) at E17.5. The increased flow was partially achieved by vasodilation with an increase in the UA diameter of 12% (CI %: 7–17) at E15.5 and 14% (CI %: 8–20) at E17.5. The reflection coefficient significantly increased by 32% (CI %: 16–48) at E15.5 and 34% (CI %: 16–52) at E17.5. The change in UA PI did not show an effect of group or gestational age. In addition, there was no group or gestational age effect on the change in fetal heart rate, time delay, or dispersion. Interestingly, there was a trend toward a group effect on the change in PWV (P = 0.1), with increasing PWV in the betamethasone group.
Figure 2.
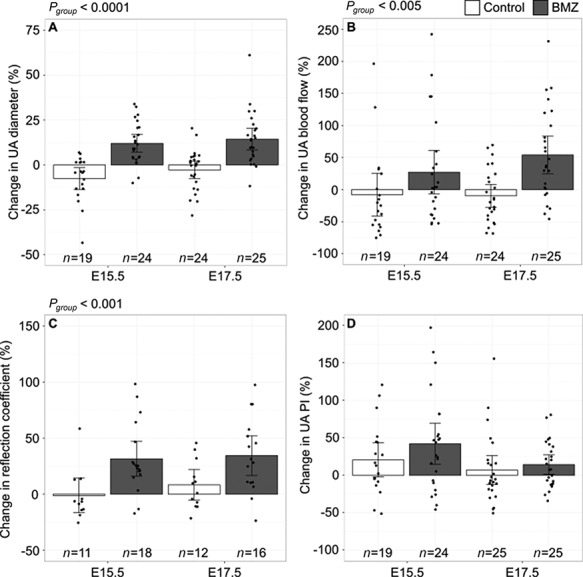
Change in ultrasound parameters with betamethasone treatment. Change in (A) UA diameter, (B) UA blood flow, (C) reflection coefficient, and (D) UA pulsatility index for betamethasone (BMZ)-treated mice (filled bars) compared to vehicle treated (open bars). Main effect of group as determined by a two-way ANOVA are noted as Pgroup. Data shown as means ±95% confidence intervals and n refers to the number of fetuses.
The control group did not show significant changes in the wave reflection metrics between the two scans, providing an estimate of the reproducibility of the wave reflection methodology. Measurement of the reflection coefficient during two separate scanning sessions showed little variability with coefficients of variation of <11%.
There was no correlation between the reflection coefficient and the fetal heart rate (P = 0.6); however, consistent with our previous findings [27, 43], there was a negative correlation between the UA PI and the fetal heart rate (P = 0.03). Finally, there was no correlation between the change in the reflection coefficient and the change in the UA diameter (P = 0.4).
Qualitatively, at baseline, evidence of AREDV (an indicator of high placental vascular resistance) was present in 28 of the 44 waveforms at E15.5 (64%) and 23 of the 50 waveforms at E17.5 (46%). For all cases with end-diastolic velocity present at baseline at E15.5, the end-diastolic velocity became absent or reversed after betamethasone administration (Figure 3A and B); however, at E17.5, the end-diastolic velocity remained positive after betamethasone administration in all but one case (Figure 3C and D). Of the cases at both gestational ages with AREDV at baseline, administration of betamethasone resulted in the return of end-diastolic flow only at E17.5 (in 84% of these mice) (Figure 3E and F). Figure 4 shows how the effect of betamethasone administration on the UA diameter and reflection coefficient differed based on the changes in end-diastolic velocity. It should be noted that for the vehicle-treated group, the UA end-diastolic velocity, whether present or absent/reversed, did not change between time points in 40 of the 44 cases (91%).
Figure 3.
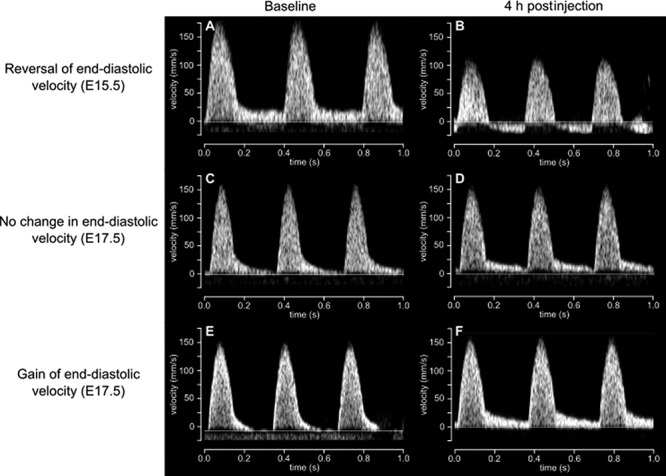
Representative UA Doppler flow waveforms at (A, C, E) baseline and (B, D, F) 4 h after betamethasone administration. The effect of betamethasone on end-diastolic velocity was varied but was strongly influenced by gestational age.
Figure 4.
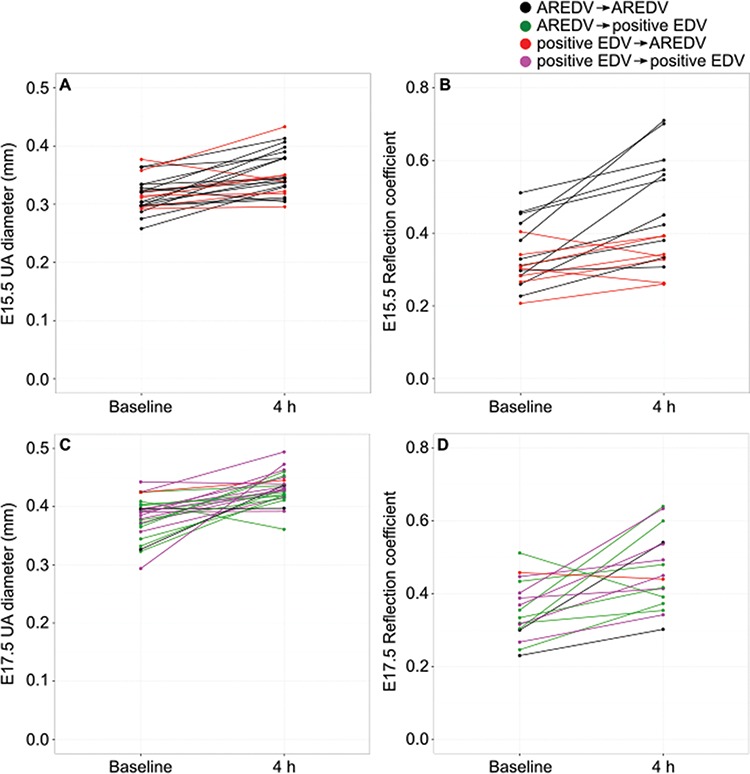
Multiple scatter graphs showing UA diameter and reflection coefficient at baseline and 4 h postinjection of betamethasone for (A and B) E15.5 and (C and D) E17.5. Note the differences based on the changes in UA end-diastolic velocity.
Discussion
In the present study, we determined that ultrasonographic wave reflection measurements in the UA of healthy, pregnant mice are altered by the administration of a corticosteroid during late gestation. At both E15.5 and E17.5, there was a significant increase in the UA reflection coefficient 4 h postinjection of betamethasone. On that timescale, we would not expect the number or diameter of feto-placental vessels to significantly change due to normal maturation, and this conclusion is supported by our results in the vehicle-injected control group. Corticosteroids may induce either vasoconstriction or vasodilation depending on the type of vessel examined [46, 47]. Here, betamethasone administration resulted in vasodilation in the UA and an associated increase in blood flow, consistent with a decrease in feto-placental vascular resistance. This vasodilatory response has also been observed in vitro in the human UA and the placental vascular bed following infusion of the alternate antenatal corticosteroid, dexamethasone [48]. Despite the significant vasodilation of the UA with betamethasone, we do not believe the decrease in placental vascular resistance is explained by vasodilation of the cord artery alone. Previous estimates based on computational flow modeling attributed only 13% of total feto-placental vascular resistance to the UA [49], suggesting that no amount of vasodilation of the UA alone would be sufficient to fully explain the observed flow increases.
In this context, additional information can be gained beyond measuring total UA flow and pulsatility. Unlike the UA PI, the reflection coefficient is not affected by changes in the fetal heart rate and therefore has important advantages over UA PI in detecting dynamic changes in the placental vasculature. It should also be noted that the reflection coefficient is explicitly normalized with respect to the forward flow waveform that is driven by fetal cardiac output. Therefore, the reflection coefficient is not likely to be influenced by transient changes in cardiac output due to fetal heart rate variations noted in the third trimester human fetus. The absence of a correlation between the change in reflection coefficient and the change in UA diameter suggests that the observed reflections originate from beyond the insertion point of the umbilical cord into the placenta. Previous work suggests that approximately 35% of the variation in reflection coefficient is explained by initial branching of the UA on the chorionic plate [43]. It has been proposed that reflections arise from bifurcations where the combined area of the downstream branches differs from that of the parent vessel. In a resistance bed such as the placenta, these area ratios are >1.0 such that blood decelerates as it reaches the smallest vessels. Diffuse vasodilation could alter these ratios throughout the vascular bed and provides a plausible mechanism for the observed increases in wave reflection with corticosteroid treatment. We have previously demonstrated that the reflection coefficient (measured using a similar methodology [50]) increases in the adult mouse cerebral circulation in response to vasodilation from a hypercapnic challenge, concordant with the results presented here for the placental vasculature and further supporting the interpretation that vasodilation is driving the observed changes in the wave reflection coefficient.
Between E15.5 and E17.5, there were no significant differences in the increase in UA blood flow or reflection coefficient following betamethasone administration. Interestingly, the effect on end-diastolic velocity differed by gestational age. Unlike in humans where UA end-diastolic velocities are normally present after 18 weeks of gestation [51], we only observed positive end-diastolic velocities in 36 and 54% of datasets at E15.5 and E17.5 respectively. This increase in the detection rate of positive end-diastolic velocity is consistent with UA waveforms reported for CD-1 mice [52]. At E17.5, the effect of betamethasone on end-diastolic velocities is consistent with clinical findings in human fetuses with fetal growth restriction; in cases with AREDV at baseline, there was a return of end-diastolic velocity and when there was a positive end-diastolic velocity at baseline, it remained positive [5, 9–18]. In contrast, at E15.5, positive end-diastolic velocities changed to AREDV (red, Figure 4A and B) and appear to have a smaller increase in UA diameter (6% [CI %: −3–15] vs. 14% [CI %: 8–20]) and reflection coefficient (12% [CI %: −5–29] vs. 44% [CI %: 23–65]) compared to cases where AREDV did not change (black, Figure 4A and B). At E17.5, the change from AREDV to positive end-diastolic velocity (green, Figure 4C and D) appears to be associated with larger changes in UA diameter and reflection coefficient. These data suggest that despite these fetuses being from healthy pregnancies, there may be occult placental vascular abnormalities present that make them unable to respond by vasodilation to sufficiently decrease the placental vascular resistance following corticosteroid administration. This hypothesis has been suggested for growth-restricted human fetuses that do not show improved UA PI after corticosteroid administration [18, 19, 21]. Future studies using a mouse model of placentally mediated fetal growth restriction will allow us to test this hypothesis.
The effect of corticosteroids on birthweights has been controversial with both animal and clinical human studies reporting either no effect [32, 33, 53, 54] or decreased birthweight [31, 34–36, 39, 55–58] following corticosteroid administration. Here, the observed elevation of umbilical perfusion could potentially maintain fetal weights by providing adequate delivery of oxygen. However, even if placental function is not impaired, corticosteroid administration may have a direct effect on fetal tissues [59]. For example, corticosteroids inhibit DNA synthesis in cultured liver cells and slow organ growth in rats [60].
The mechanisms responsible for the changes observed in this study remain unclear. Corticotropin-releasing hormone is thought to be an important regulator of feto-placental blood flow [61], and Marinoni et al. showed increased secretion of placental corticotropin-releasing hormone following betamethasone administration, causing nitric-oxide-mediated vasodilation of the UA [62]. Mechanisms that can decrease vascular resistance include vascular remodeling, angiogenesis, and decreases in vascular tone. A decrease in placental vascular resistance may also be due to increases in systemic arterial blood pressure. A previous study following betamethasone administration in sheep reported an increase in blood pressure mediated by an increase in fetal cardiac output and an increase in fetal total peripheral vascular resistance [30]. While we did not find an increase in fetal heart rate following betamethasone administration, we did not measure cardiac output, which is a limitation of the study.
The present study had several additional limitations. Clinically in humans, a course of betamethasone to promote fetal lung maturity is given to women via two injections over a 24 h period; however, the short gestation of the mouse meant this schedule was not feasible, and a single dose was deemed equivalent for study purposes. In addition, the low success rate of the M-mode datasets that met the quality criteria makes a longitudinal study challenging and restricted our study design to only one time point postinjection. Another limitation is that the sex of the fetus was not determined, and therefore, it is possible that some of the variability in our measurements could be the result of sex differences. Placental structure and function is known to be sexually dimorphic [63], and fetal sex has been shown in animal and clinical studies to influence the risk of adverse outcome in response to corticosteroid administration [64, 65].
The wave reflection methodology showed high reproducibility, supporting its potential for future clinical translation. While the UA waveforms of mice and humans have similar shape and show similar changes during gestation [52], it is important to consider the differences in placental structure between the two species. In the context of wave reflection, the large difference in size of the placenta between mice and humans has important hemodynamic implications. In particular, a larger placenta will increase the time delay for the arrival of the reflection and the larger number of branches in the placental vascular network creates more potential sources of reflection. Also, the mechanical properties of the vessels may differ, with the human placenta having more generations of muscular arteries that could vasodilate and alter reflections in the UA.
In summary, a longitudinal study design allowed us to investigate whether wave reflection parameters are sensitive to acute changes in vascular tone in the murine placenta. In healthy, pregnant C57BL6/J mice, antenatal betamethasone administration resulted in an increase in the UA diameter, UA blood flow, and reflection coefficient, suggesting a significant decrease in placental vascular resistance. The effect of betamethasone on UA end-diastolic velocity was heterogeneous, consistent with clinical findings. The study of UA wave reflections, combined with mouse models of placental insufficiency, creates new opportunities to understand the mechanisms of placental response to therapies such as corticosteroids.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1. Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972; 50:515–525. [PubMed] [Google Scholar]
- 2. Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2017; 3:CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crowley P, Chalmers I, Keirse MJ. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. Br J Obstet Gynaecol 1990; 97:11–25. [DOI] [PubMed] [Google Scholar]
- 4. Mulder EJ, Derks JB, Visser GH. Antenatal corticosteroid therapy and fetal behaviour: a randomised study of the effects of betamethasone and dexamethasone. Br J Obstet Gynaecol 1997; 104:1239–1247. [DOI] [PubMed] [Google Scholar]
- 5. Rotmensch S, Liberati M, Celentano C, Efrat Z, Bar-Hava I, Kovo M, Golan A, Moravski G, Ben-Rafael Z. The effect of betamethasone on fetal biophysical activities and Doppler velocimetry of umbilical and middle cerebral arteries. Acta Obstet Gynecol Scand 1999; 78:768–773. [PubMed] [Google Scholar]
- 6. Kelly MK, Schneider EP, Petrikovsky BM, Lesser ML. Effect of antenatal steroid administration on the fetal biophysical profile. J Clin Ultrasound 2000; 28:224–226. [DOI] [PubMed] [Google Scholar]
- 7. Multon O, Senat MV, Minoui S, Hue MV, Frydman R, Ville Y. Effect of antenatal betamethasone and dexamethasone administration on fetal heart rate variability in growth-retarded fetuses. Fetal Diagn Ther 1997; 12:170–177. [DOI] [PubMed] [Google Scholar]
- 8. Magee LA, Dawes GS, Moulden M, CWG R. A randomised controlled comparison of betamethasone with dexamethasone: effect on the antenatal fetal heart rate. Br J Obstet Gynaecol 1997; 104:1233–1238. [DOI] [PubMed] [Google Scholar]
- 9. Cohlen BJ, Stigter RH, Derks JB, Mulder EJ, Visser GH. Absence of significant hemodynamic changes in the fetus following maternal betamethasone administration. Ultrasound Obstet Gynecol 1996; 8:252–255. [DOI] [PubMed] [Google Scholar]
- 10. Senat MV, Ville Y. Effect of steroids on arterial Doppler in intrauterine growth retardation fetuses. Fetal Diagn Ther 2000; 15:36–40. [DOI] [PubMed] [Google Scholar]
- 11. Piazze JJ, Anceschi MM, La Torre R, Amici F, Maranghi L, Cosmi EV. Effect of antenatal betamethasone therapy on maternal-fetal Doppler velocimetry. Early Hum Dev 2001; 60:225–232. [DOI] [PubMed] [Google Scholar]
- 12. Deren O, Karaer C, Onderoglu L, Yigit N, Durukan T, Bahado-Singh RO. The effect of steroids on the biophysical profile and Doppler indices of umbilical and middle cerebral arteries in healthy preterm fetuses. Eur J Obstet Gynecol Reprod Biol 2001; 99:72–76. [DOI] [PubMed] [Google Scholar]
- 13. Urban R, Lemancewicz A, Przepieść J, Urban J, Kretowska M. Antenatal corticosteroid therapy: a comparative study of dexamethasone and betamethasone effects on fetal Doppler flow velocity waveforms. Eur J Obstet Gynecol Reprod Biol 2005; 120:170–174. [DOI] [PubMed] [Google Scholar]
- 14. Wallace EM, Baker LS. Effect of antenatal betamethasone administration on placental vascular resistance. Lancet 1999; 353:1404–1407. [DOI] [PubMed] [Google Scholar]
- 15. Edwards A, Baker LS, Wallace EM. Changes in fetoplacental vessel flow velocity waveforms following maternal administration of betamethasone. Ultrasound Obstet Gynecol 2002; 20:240–244. [DOI] [PubMed] [Google Scholar]
- 16. Edwards A, Baker LS, Wallace EM. Changes in umbilical artery flow velocity waveforms following maternal administration of betamethasone. Placenta 2003; 24:12–16. [DOI] [PubMed] [Google Scholar]
- 17. Barkehall-Thomas A, Thompson M, Baker LS, Edwards A, Wallace EM. Betamethasone associated changes in umbilical artery flow velocity waveforms in multiple pregnancies with umbilical artery absent end diastolic flow. Aust NZ J Obstet Gynaecol 2003; 43:360–363. [DOI] [PubMed] [Google Scholar]
- 18. Simchen MJ, Alkazaleh F, Adamson SL, Windrim R, Telford J, Beyene J, Kingdom J. The fetal cardiovascular response to antenatal steroids in severe early-onset intrauterine growth restriction. Am J Obstet Gynecol 2004; 190:296–304. [DOI] [PubMed] [Google Scholar]
- 19. Robertson MC, Murila F, Tong S, Baker LS, Yu VY, Wallace EM. Predicting perinatal outcome through changes in umbilical artery Doppler studies after antenatal corticosteroids in the growth-restricted fetus. Obstet Gynecol 2009; 113:636–640. [DOI] [PubMed] [Google Scholar]
- 20. Thuring A, Malcus P, Maršál K. Effect of maternal betamethasone on fetal and uteroplacental blood flow velocity waveforms. Ultrasound Obstet Gynecol 2011; 37:668–672. [DOI] [PubMed] [Google Scholar]
- 21. Piazze J, Dillon KC, Cerekja A. Betamethasone effects on umbilical arteries and ductus venosus Doppler velocity waveforms in growth-restricted fetuses. J Matern Fetal Neonatal Med 2012; 25:1179–1182. [DOI] [PubMed] [Google Scholar]
- 22. Niroomanesh S, Shojaei K, Moghadam SF, Mohammadi N, Rahimi Z, RezaeiKeyhanaei K. Effect of prenatal betamethasone on fetal, uteroplacental, and maternal blood flow velocity in pregnancies complicated by fetal growth restriction. Int J Gynaecol Obstet 2015; 130:270–273. [DOI] [PubMed] [Google Scholar]
- 23. Müller T, Nanan R, Dietl J. Effect of antenatal corticosteroid administration on Doppler flow velocity parameters in pregnancies with absent or reverse end-diastolic flow in the umbilical artery. Acta Obstet Gynecol Scand 2003; 82:794–796. [PubMed] [Google Scholar]
- 24. Wijnberger LD, Bilardo CM, Hecher K, Stigter RH, Visser GH. Effect of antenatal glucocorticoid therapy on arterial and venous blood flow velocity waveforms in severely growth-restricted fetuses. Ultrasound Obstet Gynecol 2004; 23:584–589. [DOI] [PubMed] [Google Scholar]
- 25. Adamson SL, Langille BL. Factors determining aortic and umbilical blood flow pulsatility in fetal sheep. Ultrasound Med Biol 1992; 18:255–266. [DOI] [PubMed] [Google Scholar]
- 26. Adamson SL. Arterial pressure, vascular input impedance, and resistance as determinants of pulsatile blood flow in the umbilical artery. Eur J Obstet Gynecol Reprod Biol 1999; 84:119–125. [DOI] [PubMed] [Google Scholar]
- 27. Rahman A, Zhou YQ, Yee Y, Dazai J, Cahill LS, Kingdom J, Macgowan CK, Sled JG. Ultrasound detection of altered placental vascular morphology based on hemodynamic pulse wave reflection. Am J Physiol Heart Circ Physiol 2017; 312:H1021–H1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sled JG, Stortz G, Cahill LS, Milligan N, Ayyathurai V, Serghides L, Morgen E, Seravalli V, Delp C, McShane C, Baschat AA, Kingdom J, et al. . Reflected hemodynamic waves influence the pattern of Doppler ultrasound waveforms along the umbilical arteries Am J Physiol Heart Circ Physiol 2019; 316:H1105–H1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ballard PL, Ning Y, Polk D, Ikegami M, Jobe AH. Glucocorticoid regulation of surfactant components in immature lambs. Am J Physiol 1997; 273:L1048–L1057. [DOI] [PubMed] [Google Scholar]
- 30. Derks JB, Giussani DA, Jenkins SL, Wentworth RA, Visser GH, Padbury JF, Nathanielsz PW. A comparative study of cardiovascular, endocrine and behavioural effects of betamethasone and dexamethasone administration to fetal sheep. J Physiol 1997; 499:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ikegami M, Jobe AH, Newnham J, Polk DH, Willet KE, Sly P. Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. Am J Respir Crit Care Med 1997; 156:178–184. [DOI] [PubMed] [Google Scholar]
- 32. Polk DH, Ikegami M, Jobe AH, Sly P, Kohan R, Newham J. Preterm lung function after retreatment with antenatal betamethasone in preterm lambs. Am J Obstet Gynecol 1997; 176:308–315. [DOI] [PubMed] [Google Scholar]
- 33. Dunlop SA, Archer MA, Quinlivan JA, Beazley LD, Newham JP. Repeated prenatal corticosteroids delay myelination in the ovine central nervous system. J Matern Fetal Med 1997; 6:309–313. [DOI] [PubMed] [Google Scholar]
- 34. Jobe AH, Wada N, Berry LM, Ikegami M, Ervin MG. Single and repetitive maternal glucocorticoid exposures reduce fetal growth in sheep. Am J Obstet Gynecol 1998; 178:880–885. [DOI] [PubMed] [Google Scholar]
- 35. Quinlivan JA, Archer MA, Dunlop SA, Evans SF, Beazley LD, Newnham JP. Fetal growth retardation, particularly within lymphoid organs, following repeated maternal injections of betamethasone in sheep. J Obstet Gynaecol Res 1998; 24:173–182. [DOI] [PubMed] [Google Scholar]
- 36. Newnham JP, Evans SF, Godfrey ME, W H, Ikegami M, Jobe AH. Maternal, but not fetal, administration of corticosteroids restricts fetal growth. J Matern Fetal Med 1999; 8:81–87. [DOI] [PubMed] [Google Scholar]
- 37. Tabor BL, Rider ED, Ikegami M, Jobe AH, Lewis JF. Dose effects of antenatal corticosteroids for induction of lung maturation in preterm rabbits. Am J Obstet Gynecol 1991; 164:675–681. [DOI] [PubMed] [Google Scholar]
- 38. Sun BO, Jobe A, Rider E, Ikegami M. Single dose versus two doses of betamethasone for lung maturation in preterm rabbits. Pediatr Res 1993; 33:257–260. [DOI] [PubMed] [Google Scholar]
- 39. Pratt L, Magness RR, Phernetton T, Hendricks SK, Abbott DH, Bird IM. Repeated use of betamethasone in rabbits: effects of treatment variation on adrenal suppression, pulmonary maturation, and pregnancy outcome. Am J Obstet Gynecol 1999; 180:995–1005. [DOI] [PubMed] [Google Scholar]
- 40. Banjanin S, Kapoor A, Matthews SG. Prenatal glucocorticoid exposure alters hypothalamic-pituitary-adrenal function and blood pressure in mature male Guinea pigs. J Physiol 2004; 558:305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Christensen H, Sienko A, Rayburn W, Gonzalez C, Coleman T. Selection of a clinically relevant dose of corticosteroids to enhance fetal lung maturity and their effect on fetal growth and development. Teratology 1996; 53:98. [Google Scholar]
- 42. Schwartz WJ, Christensen HD, Carey JC, Rayburn WF, Gonzalez C. Systemic administration of betamethasone delays endotoxin-induced preterm labor in the murine model. Am J Obstet Gynecol 2003; 188:439–443. [DOI] [PubMed] [Google Scholar]
- 43. Cahill LS, Zhou YQ, Hoggarth J, Yu LX, Rahman A, Stortz G, Whitehead CL, Baschat AA, Kingdom JC, Macgowan CK, Sled JG. Placental vascular abnormalities in the mouse alters umbilical artery wave reflections. Am J Physiol Heart Circ Physiol 2019; 316:H664–H672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ozdemir H, Guvenal T, Cetin M, Kaya T, Cetin A. A placebo-controlled comparison of effects of repetitive doses of betamethasone and dexamethasone on lung maturation and lung, liver, and body weights of mouse pups. Pediatr Res 2003; 53:98–103. [DOI] [PubMed] [Google Scholar]
- 45. Christensen HD, Sienko AE, Rayburn WF, Gonzalez CL, Coleman FH. A placebo-controlled, blinded comparison between betamethasone and dexamethasone to enhance lung maturation in the fetal mouse. J Soc Gynecol Investig 1997; 4:130–134. [DOI] [PubMed] [Google Scholar]
- 46. Yang S, Zhang L. Glucocorticoids and vascular reactivity. Curr Vasc Pharmacol 2004; 2:1–12. [DOI] [PubMed] [Google Scholar]
- 47. Horvath G, Wanner A. Inhaled corticosteroids: effects on the airway vasculature in bronchial asthma. Eur Respir J 2006; 27:172–187. [DOI] [PubMed] [Google Scholar]
- 48. Clifton VL, Wallace EM, Smith R. Short-term effects of glucocorticoids in the human fetal-placental circulation in vitro. J Clin Endocrinol Metab 2002; 87:2838–2842. [DOI] [PubMed] [Google Scholar]
- 49. Rennie MY, Cahilll LS, Adamson SL, Sled JG. Arterio-venous fetoplacental vascular geometry and hemodynamics in the mouse placenta. Placenta 2017; 58:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Macgowan CK, Stoops SJ, Zhou YQ, Cahill LS, Sled JG. Evaluation of cerebrovascular impedance and wave reflection in mouse by ultrasound. J Cereb Blood Flow Metab 2015; 35:521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guzman ER, Schulman H, Karmel B, Higgins P. Umbilical artery Doppler velocimetry in pregnancies of less than 21 weeks’ duration. J Ultrasound Med 1990; 9:655–659. [DOI] [PubMed] [Google Scholar]
- 52. Mu J, Adamson SL. Developmental changes in hemodynamics of uterine artery, utero-and umbilicoplacental, and vitelline circulations in mouse throughout gestation. Am J Physiol Heart Circ Physiol 2006; 291:H1421–H1428. [DOI] [PubMed] [Google Scholar]
- 53. Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. Lancet 2006; 367:1913–1919. [DOI] [PubMed] [Google Scholar]
- 54. Wapner RJ, Sorokin Y, Thom EA, Johnson F, Dudley DJ, Spong CY, Peaceman AM, Leveno KJ, Harper M, Caritis SN, Miodovnik M, Mercer B, et al. . Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am J Obstet Gynecol 2006; 195:633–642. [DOI] [PubMed] [Google Scholar]
- 55. Stewart JD, Gonzalez CL, Christensen HD, Rayburn WF. Impact of multiple antenatal doses of betamethasone on growth and development of mice offspring. Am J Obstet Gynecol 1997; 1138–1144. [DOI] [PubMed] [Google Scholar]
- 56. Stewart JD, Sienko AE, Gonzalez CL, Christensen H, Rayburn WF. Placebo-controlled comparison between a single dose and a multidose of betamethasone in accelerating lung maturation of mice offspring. Am J Obstet Gynecol 1998; 1241–1247. [DOI] [PubMed] [Google Scholar]
- 57. Murphy KE, Hannah ME, Willan AR, Hewson SA, Ohlsson A, Kelly EN, Matthews SG, Saigal S, Asztalos E, Ross S, Delisle MF, Amankwah K, et al. . Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet 2008; 372:2143–2151. [DOI] [PubMed] [Google Scholar]
- 58. Murphy KE, Willan AR, Hannah ME, Ohlsson A, Kelly EN, Matthews SG, Saigal S, Asztalos E, Ross S, Delisle MF, Amankwah K, Guselle P,et al. . Effect of antenatal corticosteroids on fetal growth and gestational age at birth. Obstet Gynecol 2012; 119:917–923. [DOI] [PubMed] [Google Scholar]
- 59. Avery ME. Pharmacological approaches to the acceleration of fetal lung maturation. Br Med Bull 1975; 13–17. [PubMed] [Google Scholar]
- 60. Loeb JN. Corticosteroids and growth. N Engl J Med 1975; 295:547–552. [DOI] [PubMed] [Google Scholar]
- 61. Giles WB, McLean M, Davies JJ, Smith R. Abnormal umbilical artery Doppler waveforms and cord blood corticotropin-releasing hormone. Obstet Gynecol 1996; 87:107–111. [DOI] [PubMed] [Google Scholar]
- 62. Marinoni E, Korebrits C, Di Iorio R, Cosmi EV, Challis JR. Effect of betamethasone in vivo on placental corticotropin-releasing hormone in human pregnancy. Am J Obstet Gynecol 1998; 178:770–778. [DOI] [PubMed] [Google Scholar]
- 63. Clifton VL. Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta 2010; 31 (suppl):S33–9. [DOI] [PubMed] [Google Scholar]
- 64. Willet KE, Jobe AH, Ikegami M, Polk D, Newnham J, Kohan R, Gurrin L, Sly PD. Postnatal lung function after prenatal steroid treatment in sheep: effect of gender. Pediatr Res 1997; 42:885–892. [DOI] [PubMed] [Google Scholar]
- 65. Anadkat JS, Kuzniewicz MW, Chaudhari BP, Cole FS, Hamvas A. Increased risk for respiratory distress among white, male, late preterm and term infants. J Perinatol 2012; 32:780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]


