Highlights
-
•
The endophytic Bacillus megaterium isolated from Hybrid Pennisetum is promising isolate for Cd bioremediation.
-
•
The mutated strain BM18-2 showed higher capacity to resist Cd until 70 μM and improving plant growth.
-
•
Six different genes of BM18-2 are involved in Cd resistance mechanism.
-
•
Hybrid Pennisetum inoculated with BM18-2 showed higher amount of growth and toleranc to Cd toxicity than uninoculated plants.
Keywords: Bacillus megaterium, Bioremediation, PGP, Hybrid Pennisetum, Endophytes, Cadmium
Abstract
Hybrid Pennisetum (Pennisetum americanum × P. purpureum Schumach L.) is a tall and rapidly growing perennial C4 bunch grass. It has been considered as a promising plant for phytoremediation of heavy metal-contaminated soil due to its high biomass, high resistance to environmental stress, pests and diseases. Heavy metal bioavailability level is the most important parameter for measurement of the phytoremediation efficiency. Endophytic bacteria were used to further enhance phytoremediation of heavy metals through bioaccumulation or bioabsorption process. In the present study, the endophytic Bacillus megaterium strain ‘BM18’ isolated from hybrid Pennisetum was screened under 10-70 μM cadmium (Cd) stress for Cd-resistant mutant colonies. And one such mutant colony‘BM18-2’ was obtained from the screen. Comparably, ‘BM18-2’ was more Cd-tolerant and had higher Cd removal ability than the original strain‘BM18’. The amount of IAA and ammonia production, and phosphate solubilization were 1.09, 1.23 and 1.24 times in ‘BM18-2’ than those of ‘BM18’, respectively. Full genome sequencing of these two strains revealed 6 different genes: BM18GM000901, BM18GM005669 and BM18GM005870 encoding heavy metal efflux pumps, BM18GM003487 and BM18GM005818 encoding transcriptional regulators for metal stress biosensor and BM18GM001335 encoding a replication protein. Inoculation with ‘BM18-2’ or ‘BM18’ both significantly reduced the toxic effect of Cd on hybrid Pennisetum, while the effect of ‘BM18-2’ on plant growth promotion in the presence of Cd was significantly better that of ‘BM18’. Therefore, the mutated strain ‘BM18-2’ could be used as a potential agent for Cd bioremediation, improving growth and Cd absorption of hybrid Pennisetum in Cd contaminated soil.
1. Introduction
Discharges of heavy metals into environment due to industrial revolution, use of different fungicides and land chemical fertilizers, wastewater irrigation and sewage sludge impose considerable health threat worldwide [1,2]. Heavy metals cannot break down to non-toxic forms, and therefore have long-lasting effects on the ecosystem. Many of them are toxic even at very low concentrations, e.g. arsenic, cadmium (Cd), chromium, copper, lead, mercury, nickel, selenium, and silver, are not only cytotoxic but also carcinogenic and mutagenic in nature [3,4,5]. Cd is the most dangerous metal ion characterized by high stability and toxicity [6]. Cd is known to bind with essential respiratory enzymes such as oxidases [6,7] causing oxidative stress and cancer [8, 9]. Cd is highly corrosion resistant and is widely used to plate metal parts in general industrial hardware as well as in automobiles, electronics, marine and aerospace industries [10]. It is urgent to find cost-effective method for remediation of heavy metal contamination and protect the environment from their toxic effects [11].
Remediation of heavy metal polluted soils through phytoremediation has received a wide attention for its cost-effective and eco-friendly in situ remediation nature [4]. Hybrid Pennisetum (Pennisetum americanum × P. purpureum Schumach L.) is a tall and fast-growing perennial C4 bunch grass, with high biomass productivity, adaptability to environmental stress condition, quick regeneration capacity, free from pests and diseases [12,13]. Therefore, hybrid Pennisetum is an ideal plant species for phytoremediation. However, low metal bioavailability was limited by the uptake of heavy metal ions from soil or water by plant roots and subsequent translocation and accumulation in shoots [4]. Alternatively, phytoremediation of heavy metal ions in the environment by microorganisms, especially by endophytic plant growth-promoting bacteria (PGPB), has acquired great attention [14,15].These bacteria greatly affect plant growth and development due to their production of plant-growth-promoting compounds, such as indole-3-acetic acid (IAA), siderophores, and 1-aminocyclopropane-1-carboxylate (ACC) deaminase [16], improve plant resistance to various biotic and abiotic stresses [17,18], and prevent or reduce the toxicity of heavy metals in plants by producing biosurfactants and extracellular polymeric substances [19,20]. Bacteria have been used for the effective removal of heavy metals through biosorption and bioaccumulation processes [21,22].
Bacillus megaterium, generally considered a soil microbe, is gram-positive and has great potential for phytoremediation of metal-polluted sites [23]. Li et al. (2017) demonstrated that hybrid Pennisetum with endophytic B. megaterium H3 may be utilized for biomass production and Cd phytostabilization in different levels of Cd-contaminated aquatic environments [24]. And it was found that B. megaterium could enhance Cd desorption from soil and increase Cd accumulation in plants by decreasing the negative effects of the heavy metals [23].
Previously, our group isolated a B. megaterium strain ‘BM18’ from a hybrid Pennisetum inflorescence. ‘BM18’ can only grow in LB medium with ≤ 40 μM Cd. By inducing in increasing cadmium stress step by step, we obtained a mutated strain ‘BM18-2’ with higher cadmium resistance ability to 70 μM Cd.
The objective of this study was to compare the Cd removal capability of ‘BM18-2’ and the original strain ‘BM18’ at different concentrations of Cd, to investigate their properties in promoting plant growth and Cd accumulation as well as differences in their genome sequences.
2. Material and methods
2.1. Source of entophytic bacteria ‘BM18’ and ‘BM18-2’
The wild type ‘BM18’: The bacterial isolate used in the present study was a Patent (publication number PCT/ CN2018/ 119676) which isolated from the regenerated plants cultured from young spikes of hybrid Pennisetum in vitro and was identified as Bacillus megaterium.
Bacterial mutation ‘BM18-2’: The ‘BM18’ were activated and cultured in LB broth comprising of 10 g tryptone, 5 g yeast extract, 5 g NaCl per liter. The pH of the medium was adjusted to 7.0–7.2. ‘BM18’ stored at -80 °C were activated in tubes with 5 ml LB broth on a rotating shaker (200 rpm) for 16 h, then 50 μl activated ‘BM18’ solution was transferred to LB solid medium supplemented with different concentrations of Cd and cultured for 24 h at 30 °C. Then ‘BM18’ was screened in solid medium with increasing Cd (CdCl2.5/2 H2O) step by step until a separable mutant strain was obtained at 70 μM Cd. And then, the mutant strain was subcultured for 10 generations in LB liquid medium without Cd stress for 24 h at 30 °C. The selected single colonies were subcultured for 10 generations in LB solid medium without Cd stress for 24 h at 30 °C. The last selected single colony was verified in LB solid medium with 70 μM Cd and finally stored at -80 °C as the mutant strain ‘BM18-2’.
2.2. Measurement of the plant growth promoting (PGP) characteristics of ‘BM18-2’and ‘BM18’
The bacterial ability to produce IAA was determined by the Salkowski method [25]. The ‘BM18-2’and ‘BM18’ were inoculated in liquid LB medium then incubated at 30 °C under 200 rpm agitation for 16 h, respectively. The absorbance of bacterial solution was adjusted to OD600 = 0.8. 0.2 ml ‘BM18-2’ or ‘BM18’ was inoculated in 8 ml 100 mg/L tryptophan medium on a rotating shaker (200 rpm) at 30 °C. After incubation for 48 h, bacteria solutions were transferred to Eppendorf tubes and centrifuged at 10,000 rpm for 5 min respectively. 2 ml supernatant was added to 4 ml of Salkowski coloring solution (10 ml 0.5 mol·L−1 FeCl3 mixed with 500 ml 35% perchloric acid). After incubating in the dark for 30 min, the absorbance of the solution was measured at 540 nm. The amount of IAA produced by bacteria was determined by using standard curve plotted for OD540 according to standard IAA solution at 0.5, 1.0, 5.0, 10.0, 20.0 and 25.0 mg·L−1. The production of ammonia of ‘BM18-2’ and ‘BM18’ were tested in peptone water. 10 μl fresh ‘BM18-2’ or ‘BM18’ (OD600 = 0.3) was added into tubes containing 10 ml peptone broth and was incubated at 30 °C. After incubation for 72 h Nessler’s reagent was added into the tube and the development of faint yellow to dark brown color was considered as a positive result for ammonia production and the absorbance was measured at a wavelength of 420 nm [26]. The phosphate solubilization efficiency of ‘BM18-2’ and ‘BM18’ were detected using Pikovskaya medium [27]. 2 μl fresh ‘BM18-2’ or ‘BM18’ (OD600 = 0.3) was inoculated onto Pikovskaya agar plate containing inorganic phosphate [Ca3(PO4)2 5.0 g/L] and incubated at 30 °C for 5 d. Formation of a clear zone around the bacterial colony was considered as an index of solubilization of mineral phosphate. Index = the diameter of clear zone/ the diameter of bacterial colony.
2.3. Comparison of Cd resistance ability of‘BM18-2’ and ‘BM18’
The growth rates and resistance of ‘BM18-2’and ‘BM18’ were compared in LB broth with different Cd concentration. ‘BM18-2’ and ‘BM18’ were activated in tubes with 5 ml LB broth on a rotating shaker (200 rpm) for 16 h at 30 °C, then 10 μl activated ‘BM18-2’ and ‘BM18’ (OD600 = 0.3) were transferred to 100-ml tubes containing 10 ml LB broth supplemented with 10, 20, 30, 40, 50, and 60 μM Cd respectively. After incubation at 30 °C, the optical density OD600 was measured at different time using spectrophotometer (UV-1800 spectrophotometer, Mapada 131000 C) to assess the effect of Cd on the growth of ‘BM18-2’ and ‘BM18’.
2.4. Comparison of Cd enrichment capacity (Cd removal capability) of ‘BM18-2’ and ‘BM18’
The Cd removal capacity of ‘BM18-2’ and ‘BM18’ was assessed based on measuring the difference of the concentration changes of Cd in incubation medium in the presence and absence of ‘BM18-2’ or ‘BM18’, respectively. The incubation mediums were LB medium supplemented with different concentrations of Cd (40, 50 and 60 μM CdCl2.). One hundred μl of ‘BM18-2’ or ‘BM18’ was inoculated in 10 ml incubation medium on a rotating shaker (200 rpm) at 30 °C. The incubation medium without inoculation bacteria was as control. At initial 0 h and final 48 h after incubation, the bacteria solutions were transferred to Eppendorf tubes and centrifuged at 3,000 rpm for 20 min respectively. The pH and Cd concentrations of upper supernatant were monitored. The Cd concentrations were determined using atomic absorption spectroscopy [28]. The experiment was repeated triplicate.
2.5. Comparison of DNA sequences for ‘BM18-2’ and ‘BM18’
Isolation of genomic DNA for ‘BM18-2’ and ‘BM18’ was carried out using SDS method. The quality of total DNA obtained was investigated using agarose gel electrophoresis and evaluated by Qubit. The bacterial genome was sequenced with MPS (massively parallel sequencing) Illumina technology. The DNA library was constructed: a paired-end library with an insert size of 350 bp. An illumina HiSeq4000 using PE150strategy was applied for sequencing the 350 bp library. Library construction and sequencing was carried out at the Beijing Novogene Bioinformatics Technology Co., Ltd. Additionally, Using in-house program the quality control of paired-end reads was performed.
The sequence comparison is the basis of the resequencing analysis. The variation information of samples and the reference is obtained by aligning the sample reads with the designated reference. Burrows-Wheeler Aligner (BWA) software was used for mapping the sample reads to the reference sequence, while the SAMTOOLS software was applied to calculate the similarity of the reads to the reference sequence and to understand the alignment results. The unigenes’ functions were predicted according to the databases of GO and KEGG.
The unigenes (≥ 200 bp) assembled by the SOAP de novo program were analyzed by Blastx alignment search (E-value ≤ 10−5) against protein databases including the Nr, Swiss-Prot, GO, KEGG. The best aligning results were used to determine the sequence direction of the unigenes. To identify the best Blastx hits from the alignments, putative gene names and predicted proteins of the corresponding assembled sequences were produced. The orientation of sequences was also derived from Blastx annotations. Functional categorization by gene ontology (GO) terms was performed based on the best Blastx hits from the Nr database using the Blast 2GO program according to molecular function, biological process and cellular component ontology.
2.6. Effect of ‘BM18-2’ and ‘BM18’ on plant growth of Hybrid Pennisetum in the presence of Cd in pot experiment
Seeds of Hybrid Pennisetum were firstly germinated in soil for 7 d, then the roots of seedlings were immersed in 10 mL fresh ‘BM18-2’ or ‘BM18’ (108CFU·mL−1) for 2 h, and then seedlings were transplanted in plastic pots (7 cm diameter and 10 cm high) containing 40 μM Cd in sand. Plants planted without treatment with 40 μM Cd and bacterial set as the control 1 (CK1), and plants planted with treatment with 40 μM Cd but without bacterial set as the control 2 (CK2). Pots were placed in a controlled growth room (16 h photoperiod, 28–30 º C temperature range) and watered daily. Plants were harvested after transplanting two weeks, and subdivided into roots and shoots and washed with deionized water, and then the fresh weight and the length of shoot and root were measured.
2.7. Effect of ‘BM18-2’ and ‘BM18’ on plant growth and Cd accumulation in Hybrid Pennisetum in Cd contaminated field
Hybrid pennisetum was subjected to field trials in Cd-contaminated fields in 2018. The plants were cultivated in the same experimental field. The primary pollutants in the soil are Cd, with concentrations of 0.50 mg·kg−1. The field experiment consisted 9 plots (each is 4 m long and 4 m wide) arranged in completely random plot design with 3 replicates for control (without ‘BM18-2’ inoculation) and treatment (with ‘BM18-2’ or ‘BM18’ inoculation), respectively. The root divisions of HP in a seedling bedded on 15 April 2018. The seedlings were transplanted to fields on 29 May 2018 after their roots soaking in ‘BM18-2’ or ‘BM18’ suspension (OD600 = 0.2) for 2–3 h. Seedlings without bacterial soaking were control. The planting density was 40 cm × 40 cm. 225 kg ha−1 nitrogen fertilizer (urea) were applied during the whole growth period and the application was 33.3% on the 7th day after transplanting, 66.7% on the 30th day after transplanting. All plots were managed using the same field management. The plants were harvested on 30 October 2018. Plants were harvested 5–7 cm above the ground and divided into 4–5 cm leaves and stems (including leaf sheath). One whole plant was randomly selected from each plot, and a total of 3 plants were selected for each treatment to sample and weigh the fresh leaves and stems. After that, leaves and stems sample were dried (30 min at 105 °C and then dried at 70 °C to constant weight), weighed and finely ground to pass through 40-mesh sieve. 200 mg of plant sample and 5 mL HNO3 and 1 mL HClO4 were added into a glass tube and let the tube sit overnight with cover; on the second day, remove the cap and heat the tube at 90 °C for 30 min and then at 180 °C to yield a clear white or yellow solution, then at 220 °C until the bottom of the tube was nearly dry; Add 1 ml HCl solution (HCl: deionized water 1: 1, v/v) to dissolve the residue after tube cooling, and then diluted with deionized water to 50 mL, filtered through a filter membrane (0.45 μm). The total Cd concentration of plant samples was determined using an atomic absorption spectrophotometer (AAS-Perkin Elmer, PinAAcle 900T). All samples were analyzed in triplicates and a reagent blank (without sample) was carried out through the complete digestion process. Certified materials (GBW07405 and GBW07401) from the National Research Center for Standards, China, were used for quality assurance.
2.8. Statistical analysis
The results obtained in the present study were subjected to analyses using the IPM SPSS20 statistics software. The data were analyzed by the mean, standard deviation and one-way analysis of variance (two-way ANOVA), followed by the Duncan’s multiple range test (DMRT) to determine significant differences among the data.
3. Result
3.1. PGP characteristics of ‘BM18-2’ and ‘BM18’
‘BM18-2’ and ‘BM18’ were examined for their plant-growth promotion (PGP) properties (Table 1). The results revealed that both ‘BM18-2’ and ‘BM18’ were able to produce NH3 and IAA. And ‘BM18-2’ produced significantly more NH3 and IAA than ‘BM18’ (p < 0.05), and the same was true for its higher phosphate solubilization ability (p < 0.05).
Table 1.
Plant-growth-promoting (PGP) characteristics of ‘BM18-2’ and ‘BM18’.
| Bacterial isolate |
NH3 production (OD420) |
IAA (mg·L-1) |
Phosphate solubilization |
|---|---|---|---|
| ‘BM18-2’ | 0.42 ± 0.04a | 3.00 ± 0.25a | 1.56 ± 0.15a |
| ‘BM18’ | 0.34 ± 0.03b | 2.76 ± 0.36b | 1.26 ± 0.09b |
Note: ‘BM18-2’, Bacillus megaterium strain 18–2; ‘BM18’, Bacillus megaterium strain 18; all data are the means of three independent biological replicates and are expressed as means ± standard deviation. Different small letters within rows indicate significant differences at p <0.05 between ‘BM18-2’ and ‘BM18’.
3.2. Cd resistance ability of ‘BM18-2’ and ‘BM18’
Cd resistance abilities of ‘BM18-2’ and ‘BM18’ were investigated in 10-60 μM Cd solutions. The results indicated that ‘BM18-2’ tolerated 60 μM Cd and grew normally while ‘BM18’ only just grew normally in solution with Cd less than 40 μM.
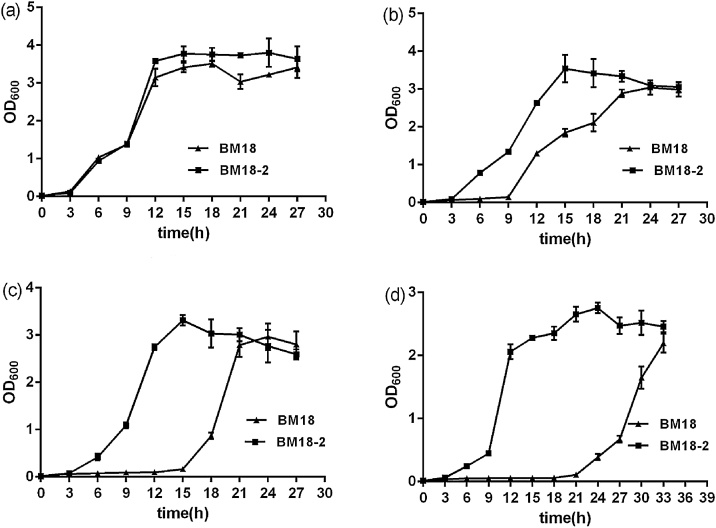
The growth rate and bacteria concentration of ‘BM18-2’ and ‘BM18’ were measured at 10-40 μM Cd stress. The result showed that growth rates of both ‘BM18-2’ and ‘BM18’ decreased gradually with increasing Cd concentration in solution (Fig. 1a). Yet, significant difference in their growth was observed even when the growth medium was supplemented with 10 μM Cd, and the difference between their growth increased with higher concentration of Cd (Fig. 1). For example, the lag phase where the bacteria started to grow in the medium took only 3 h after inoculation for ‘BM18-2’ at 20–40 μM Cd but in case of BM18, the lag phase delayed to 9 h, 15 h, and 21 h after inoculation in growth medium with 20 μM, 30 μM, and 40 μM Cd,respectively (Fig.1 b–d).
Fig. 1.
Effect of Cadmium on the growth rate of ‘BM18-2’ and ‘BM18’. (a) 10 μM Cd, (b) 20 μM Cd, (c) 30 μM Cd, (d) 40 μM Cd.
3.3. Cadmium removal capacity of ‘BM18-2’ and ‘BM18’
The Cd removal capacity of ‘BM18-2’ and ‘BM18’ was showed in Table 2. In general, it was indicated that the Cd removal ability of ‘BM18-2’ was higher than that of ‘BM18’ in all cases (p < 0.01). With increased Cd concentration, the Cd removal ability of ‘BM18-2’ and ‘BM18’ increased at the beginning and then decreased: in growth medium with 40–60 μM Cd, the Cd removal percentage was 8.75%–14.55% of ‘BM18’, while those by‘BM18-2’ was 18.22%–57.55%. The maximal Cd removal ability of ‘BM18-2’ reached 57.56% at 50 μM Cd (Table 2).
Table 2.
Cadmium accumulation by ‘BM18-2’ and ‘BM18’ grown in LB medium supplemented with different Cd concentration.
| Bacterial isolate | Initial Cd concentration in solution (μM) | Absorbed Cd concentration in solution (μΜ) | Cd removal percentage |
|---|---|---|---|
| 40 | 16.85 ± 0.08bB | 42.13% | |
| ‘BM18-2’ | 50 | 28.78 ± 2.44aA | 57.55% |
| 60 | 10.93 ± 0.57cC | 18.22% | |
| 40 | 3.50 ± 0.10eD | 8.75% | |
| ‘BM18’ | 50 | 7.28 ± 4.10dCD | 14.55% |
| 60 | 6.15 ± 0.31deCD | 10.25% |
Note: The different small letters indicate significant differences at p <0.05 and upper letters indicate significant differences at p <0.01 among different bacteria and different Cd concentration according to DMRT.
3.4. Comparison of DNA sequences for ‘BM18-2’ and ‘BM18’
Whole genome of‘BM18-2’ and ‘BM18’ were sequenced using illumina HiSeq4000 high throughout method. The genomic data of two genomes were compared with NCBI genomes database, and the Bacillus megaterium ‘QM B1551’ (NC_014019.1) was the maximum similarity (84.25%). Then taking QM B1551 as a reference, the genomic data of ‘BM18-2’ and ‘BM18’ were de novo assembled and annotated.
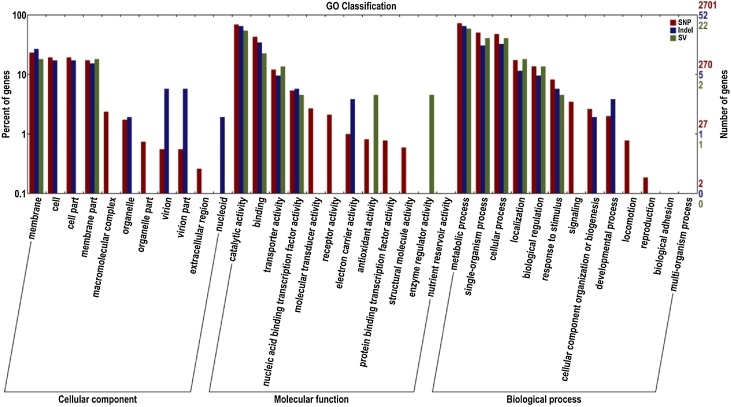
Genome of the original strain ‘BM18’ has a total length of 5,749,928 bp with an average G + C content of 37.61%, while that of the mutant strain ‘BM18-2’ has 5,751,931bp length and average G + C content of 37.61% (Table 3). Total numbers of predicated genes were 6,126 and 6,139 in ‘BM18’ and ‘BM18-2’, respectively (Table 3). All predicted genes were classified into GO (Gene Ontology) families composed of 36 categories. Biological roles were assigned to 49.5% genes of the predicted CDS based on similarity searches with BWA. However, 31.11% and 19.31% genes were attributed for molecular function and cellular component, respectively.
Table 3.
General characters of ‘BM18’ and ‘BM18-2’ genome.
| Character | ‘BM18’ | ‘BM18-2’ |
|---|---|---|
| Total number(#) | 34 | 37 |
| Total length (bp) | 5,749,928 | 5,751,931 |
| Average length (bp) | 169115.53 | 155457.59 |
| N50 length (bp) | 942,386 | 942,494 |
| N90 length (bp) | 82,892 | 83,041 |
| Maximum length (bp) | 2,385,191 | 2,385,104 |
| Mininum length (bp) | 545 | 518 |
| GC content | 37.61% | 37.61% |
| Genes predicted | 6,126 | 6,139 |
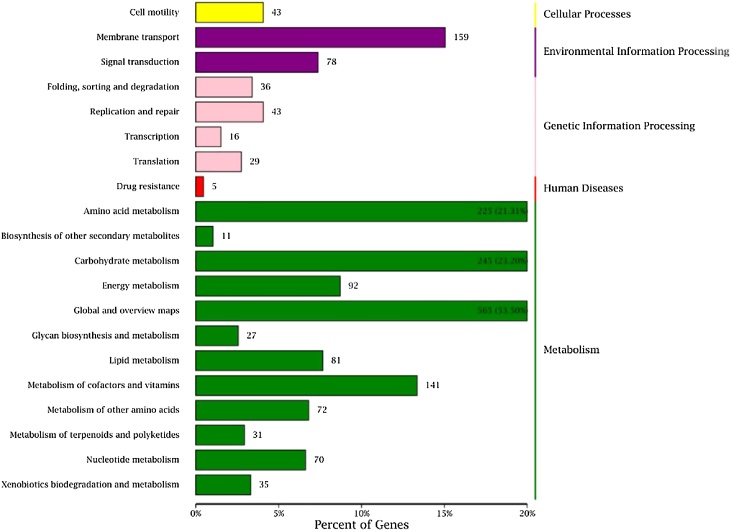
Gene Ontology (GO) and KEGG pathway analyses were conducted (Fig. 2, Fig. 3). The sequences comparison of annotated genes between‘BM18-2’ and ‘BM18’ revealed that there were six different genes, including BM18GM003487, BM18GM000901, BM18GM005669, BM18GM001335, BM18GM005870 and BM18GM005818, among which BM18GM003487 and BM18GM005818 had 99% similarities to the transcription regulator protein in Bacillus sp.; BM18GM001335 gene had 91% similarity to the replication, recombination and repair protein of Bacillus sp.; while BM18GM000901, BM18GM005669 and BM18GM005870 had 98%, 97% and 95% similarity to a hypothetical protein encoded in Bacillus sp., respectively.
Fig. 2.
GO enrichment classification of annotated functional genes in Bacillus megaterium ‘BM18-2’ and ‘BM18’.
Fig. 3.
KEGG enrichment classification of annotated functional genes in Bacillus megaterium ‘BM18-2’ and ‘BM18’.
3.5. Effect of ‘BM18-2’ and ‘BM18’ inoculation on plant growth
Effects of BM8-2 and ‘BM18’ on growth and Cd accumulation of hybrid Pennisetum were studied (Table 4). Treatment with 40 μM Cd significantly decreased the growth of hybrid Pennisetum and biomass accumulation in both aboveground (shoot) and belowground (root). Yet, inoculation with ‘BM18’ or ‘BM18-2’ both significantly alleviated the growth inhibition effect imposed by Cd in the soil. Moreover, inoculation of ‘BM18-2’ increased fresh shoot and whole plant biomass by 105.2% and 83.1% while the original strain ‘BM18’ improved fresh shoot and whole plant biomass by 64.9% and 68.1% in the presence of 40 μM Cd. ‘BM18-2’ promoted plant growth slightly stronger than BM18 (Table 4).
Table 4.
Root and shoot properties of hybrid Pennisetum seedling grown in Cd contaminated sand with and without endophytic bacterial inoculation.
| Treatment | CK1 | CK2 | ‘BM18-2’ | ‘BM18’ |
|---|---|---|---|---|
| Cd concentration (μM) | 0 | 40 | 40 | 40 |
| Fresh shoot biomass (mg·plant−1) | 36.38 ± 9.83aA | 17.40 ± 4.43bA | 35.70 ± 5.64abA | 28.70 ± 5.02abA |
| Fresh root biomass (mg·plant−1) | 7.87 ± 1.93bA | 12.47 ± 4.34abA | 18.97 ± 9.11abA | 21.50 ± 6.22aA |
| Fresh whole plant biomass (mg·plant−1) | 44.25 ± 10.42abA | 29.87 ± 8.18bA | 54.70 ± 13.44aA | 50.20 ± 10.95abA |
| Shoot length (cm) | 8.43 ± 1.93aA | 4.63 ± 0.35cB | 5.96 ± 0.72bcAB | 6.93 ± 0.47abAB |
| Root length (cm) | 3.10 ± 1.35aA | 2.17 ± 0.67aA | 2.53 ± 1.09aA | 2.63 ± 0.40aA |
Note: CK1, Hybrid pennisetum planted without Cd or bacterial treatment; CK2, Hybrid pennisetum planted with 40 μM Cd but without the bacteria; ‘BM18-2’, Hybrid pennisetum planted with 40 μM Cd and ‘BM18-2’; ‘BM18’, Hybrid pennisetum planted with 40 μM Cd and ‘BM18’. All data are the means of 3 independent biological replicates and are expressed as means ± standard deviation. Different small letters within rows indicate significant differences at p < 0.05 and upper letters indicate significant differences at p < 0.01.
3.6. Effect of ‘BM18-2’ and ‘BM18’ on plant growth and Cd accumulation in Hybrid Pennisetum in Cd contaminated field
The Effects of BM8-2 and ‘BM18’ on growth and Cd accumulation of Hybrid Pennisetum in field were also studied (Table 5). Comparing with control, the dry matter yield of above part of plant inoculated with ‘BM18-2’ increase by 8.1% while that of plant inoculated with ‘BM18’ decrease by 28.6%. The highest Cd accumulation in the above part of plant was obtained in plants inoculated with ‘BM18-2’ (572 μg plant−1), which increased by 28.6% than control. However, the lowest Cd accumulation (345 μg plant−1) was in plants inoculated with BM18.
Table 5.
Cadmium phytoremediation performance of Hybrid pennisetum with or without endophytic bacterial inoculation.
| Items | HP(control) | HP-‘BM18-2’ | HP-‘BM18’ | |
|---|---|---|---|---|
| Dry matter yield (g·plant−1) | leave | 154±35aA | 152 ± 11aA | 168 ± 8Aa |
| stem | 429±49bA | 478 ± 26aA | 248 ± 33bB | |
| above part | 583 ± 80aA | 630 ± 40aA | 416 ± 30bB | |
| Cd concentration (mg·kg−1) | leave | 0.79 ± 0.05aA | 0.61 ± 0.03bB | 0.66 ± 0.02bB |
| stem | 0.77 ± 0.02cC | 1.00 ± 0.01aA | 0.94 ±v0.03bB | |
| Cd content (μg·plant−1) | leave | 121 ± 14.37aA | 93 ± 4.89bB | 112 ± 13.21aA |
| stem | 332 ± 6.73bB | 479 ± 2.60aA | 233 ± 7.71cC | |
| above part | 454 ± 6.67bB | 572 ± 4.74aA | 345 ± 9.43cC | |
Note: The data in the table is the Dry matter yield and Cd accumulation data with Hybrid pennisetum planted for one season in field with 0.5 mg·kg−1 Cd. HP, Hybrid pennisetum; HP-’BM18-2’, Hybrid pennisetum inoculated with Bacillus megaterium strain ‘BM18-2’; HP-‘BM18’, Hybrid pennisetum inoculated with Bacillus megaterium strain ‘BM18’; All data are the means of 3 independent biological replicates and are expressed as means ± standard deviation. Different small letters within rows indicate significant differences at at p < 0.05 and upper letters indicate significant differences at p < 0.01.
4. Discussion
Our results of bacterial resistance to different Cd concentrations proved the ability of mutated strain ‘BM18-2’ to tolerate and adapt rapidly with metal toxicity. The differences in the bacterial growth manner could be related to the bioavailability and toxic effect of metals [29]. Higher amount of Cd accumulation in the mutated strain ‘BM18-2’ could be associated with Cd tolerance due to persistent exposure of the bacteria to Cd, leading to acquire a strong resistance to the metal toxicity through genetic variation in the population [30,31]. The metal resistance ability may be correlated with production of additional proteins in the bacterial cell. Some of these are enzymes used for conversion of metals to harmless forms or used for sequestration of metals by intracellular metal binding proteins such as metallothioneins (MT) and phytochelatins along with compounds such as bacterial siderophores which are mostly catecholates. Cd efflux or Cd binding with extracellular polymeric substances in the cell wall was alternative mechanism to decrease the intracellular Cd concentration [32,33]. On other hands, the decrease in removal percentage with increasing Cd concentrations is indication of Cd toxicity which negatively affects the bacterial growth and was directly related to the amount of Cd removed by the biomass [34]. Bacterial strain like Escherichia coli and Moreaxella sp. secreting cell surface phytochelatin 20 have been appeared to collect 25 times more Cd than the wild-type strains [35,36]. In this study, ‘BM18-2’ reduced the toxic effect of Cd on plants and promoted Cd accumulation in the above part of Hybrid Pennisetum in the Cd contaminated field.
The PGPB can be applied not only in agricultural soils for food production, but also in stressful environments for phytoremediation purposes [37]. Their effectiveness for promoting plant growth depends on the intimate interaction with their host plant and soil characteristics besides their inherent capabilities [38,39]. The mutated strain ‘BM18-2’ showed capability of IAA and ammonia production and phosphate mobilization, which, in turn, promoted plant growth. The bacterial IAA plays a very important role in improving the absorption of minerals and nutrients uptake therefore the enhancement of lateral and adventitious rooting, leading, and inducing a bacterial proliferation on the roots by root exudation [1]. It has been reported that many endophytes including Enterobacter, Azotobacter, Serratia, Klebsiella produced IAA which stimulated plant growth [40,41]. Moreover, production of ammonia can be taken up by plants as a source of nitrogen [42]. Ullah et al. [43] reported the ability of four endophytic bacterial isolates to produce ammonia. Additionally, Phosphorus is essential element for plants growth and development, but its present in agriculture soils in low amount [44,45]. Endophytes are known to improve plant growth by the phosphate solubilization [46,47]. In the study conducted by Ullah et al. [43] all tested endophytic bacterial strains were found to have the potential to solubilize phosphorous.
With the annotated genome sequence of ‘BM18-2’, many biological pathways can be predicted. We reported redundant heavy metal resistance related genes (BM18GM003487 and BM18GM005818) encoding heavy metal-sensing transcriptional regulators protein, genes BM18GM000901, BM18GM005669 and BM18GM005870 encoding heavy metal efflux pump and BM18GM001335 which predicted to induce the replication protein in the complete genome sequence of ‘BM18-2’ isolate. By comparing six gene sequences of ‘BM18-2’ with those from other bacterial genomes, we demonstrated that each gene located in the chromosome or plasmid of ‘BM18-2’ cells are similar to those of various near or distant microbes, suggesting the role of evolutionary trajectories of redundant heavy metal resistance genes. Exposure of bacteria to heavy metals leads to altered expression of genes involved in metal transport as well as other stress responses such as heat shock and oxidative stress [48,49]. As a rule, bacteria affected by one stress even at low levels can stimulate a consequent increase in resistance to the same (adaptive) or different (cross-protection) stress [49,50].
Microbial Cd resistance was demonstrated in about six distinct mechanisms. These include toxic metal accumulation in the cell wall, changed collection of the toxic compound and modification of the cell wall plasma membrane complex [49,51]. Cd can get into bacterial cells through different mechanisms, such as divalent cation uptake systems such as Mn or Zn, active Cd efflux pump and improved transcription of metallothionein genes [49,52,53]. The bacterial cells can develop resistance mechanisms against toxic substances which disturb membranes such as solvent efflux pumps [54]. For example, numerous bacteria showed ability to resist Cd through metal efflux systems involving ATPases that encoded by plasmid and require energy in addition to chemiosmotic ion/proton pumps [55,56].
ATPase genes were found to associate with Cd tolerance and involved in the ATP pumping mechanism in Bacillus Cereus S5 strain [57]. Muneer et al. [58] detected the presence of different molecular weight proteins in supernatant as well as in the pellet of Pseudomonas aeruginosa EP-Cd1 under Cd stress. While in other strain P. aeruginosa gene (czcABC) for Cd resistance was detected [59]. This gene encodes ion transporters proteins which were used to pump metal ions out of cytoplasm [60]. Chakraborty and Das [59] demonstrated the Cd resistance phenomena depending on the efflux mechanism as well as Cd binding to its biofilm EPS.
Several studies have demonstrated the ability of metal-resistant plant growth-promoting endophytic bacteria to increase plant growth and phytoremediation potential in metal-contaminated soils [61,62]. Afzal et al. (2017) indicated that inoculation of switchgrass with endophytic root bacteria protected plants from the inhibitory effects of Cd, improves plant growth and decreases Cd concentration in plant [63]. Furthermore, Rice-isolated metal-resistant methylotrophic bacteria were shown to decrease the Cd accumulation and enhance tomato plant growth [64]. Additionally, cadmium-tolerant bacterial strains isolated from the root zone of Indian mustard (Brassica juncea) seedlings were capable of improving the development of B. juncea seedlings in the presence of toxic Cd concentrations [65].
In the present study, the growth of Hybrid pennisetum in Cd contaminated soil greatly enhanced after inoculation with ‘BM18-2’ which reflects the ability of mutant strain to improve plant growth and plant resistance to Cd toxicity. Plant growth promoting endophytic bacteria can improve the growth of the plants by reducing the toxic effects of metals or by producing the phytohormones [66]. Depending on the PGP traits which play a key role in promoting plant development besides reducing the degree of toxicity or damage to plants exposed to stress generated by different heavy metals. The phosphate solubilization ability of tested bacterial isolates may play important role in enhancing the plant uptake of soil minerals such as P in metal contaminated soils [67]. The enhanced biomass growth of the plant grown in heavy metals contaminated soils after inoculation with endophytic bacteria may also attributed to the phytohormone production by bacteria such as auxin IAA [68]. Therefore, Different studies reported the ability of heavy metals resistant bacteria for producing phytohormones even under stress conditions. Dell'Amico et al. [69] reported that the ability of endophytic bacteria to reduce the inhibitory effects of Cd and improve the growth of canola (Brassica napus) under Cd toxicity may be attributed to the bacterial production of IAA and siderophores. The phytoremediation of Cd contaminated soil by Sulla Coronaria and plant growth was greatly enhanced after inoculation with heavy metals resistant PGPR due to their production of PGP compounds [70].
5. Conclusion
The mutated endophytic B. megaterium isolate ‘BM18-2’ had significant improved ability for reducing Cd toxicity with plant growth-promoting characteristics, such as IAA and ammonium production, and phosphate solubilization. ‘BM18-2’ inoculation significantly improved Cd tolerance and promoted plant growth of hybrid Pennisetum grown in Cd contaminated soil. The endophytic bacteria ‘BM18-2’ could be used as one effective means in Cd phytoremediation.
Funding
This research was supported by Exploratory and Disruptive Innovation Program of <g1>Jiangsu Academy of Agricultural Sciences TD</g1> (17) 2017, the <g2>Jiangsu Agriculture Science and Technology Innovation Fund</g2> (JASTIF) (CX (19) 1005).
Declaration of Competing Interest
None.
Contributor Information
Juanzi Wu, Email: jzwu2014@jaas.ac.cn.
Nehal Kamal, Email: nero_micro@yahoo.com.
Huanhuan Hao, Email: 865135808@qq.com.
Chen Qian, Email: Chenqian611@aliyun.com.
Zhiwei Liu, Email: liuzhiweiwin2008@126.com.
Yuke Shao, Email: 393574563@qq.com.
Xiaoxian Zhong, Email: xiaoxian@jaas.ac.cn.
Bin Xu, Email: binxu@njau.edu.cn.
References
- 1.Jadia C.D., Fulekar M.H. Phytoremediation of heavy metals: recent techniques. Afr. J. Biotechnol. 2009;8:921–928. [Google Scholar]
- 2.Akcil A., Erust C., Ozdemiroglu S., Fonti V., Beolchini F. A review of approaches and techniques used in aquatic contaminated sediments: metal removal and stabilization by chemical and biotechnological processes. J. Clean. Prod. 2015;86:24–26. [Google Scholar]
- 3.Salem H.M., Eweida E.A., Farag A. ICEHM: Cairo University; Giza, Egypt: 2000. Heavy metals in drinking water and their environmental impact on human health; pp. 542–556. [Google Scholar]
- 4.Dixit R., Wasiullah E., Malaviya D., Pandiyan K., Singh U., Sahu A. Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability. 2015;7(2):2189–2212. [Google Scholar]
- 5.Mukhtar B., Malik M.F., Shah S.H., Azzam A., Slahuddin, Liaqat I. Heavy metal bioremediation in soil: key species and strategies involved in the process. Int. J. Appl. Biol. Foren. 2017;1(2):5–15. [Google Scholar]
- 6.Akhavan S.A., Sharifian S., Zolfaghari M.R., Khalily Dermany M., Rashedi H. Study on heavy metal resistant fecal Coliforms isolated from industrial, urban wastewater in Arak. Iran. Int. J. Environ. Res. 2015;9(43):1217–1224. [Google Scholar]
- 7.Nies D.H. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 2003;27:313–339. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 8.Banjerdkij P., Vattanaviboon P., Mongkolsuk S. Exposure to cadmium elevates expression of genes in the oxy R and Ohr R regulons and induces cross resistance to peroxide killing treatment in Xanthomonas campestris. Appl. Environ. Microbial. 2005;71(4):1843–1849. doi: 10.1128/AEM.71.4.1843-1849.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satarug S., Vesey D.A., Gobe G.C. Kidney cadmium toxicity, diabetes and high blood pressure: The Perfect Storm. Tohoku. J. Exp. Med. 2017;241(1):65–87. doi: 10.1620/tjem.241.65. [DOI] [PubMed] [Google Scholar]
- 10.Herrero R., Lodeiro P., Rey-Castro C., Vilariño T., Sastre de Vicente M.E. Removal of inorganic mercury from aqueous solutions by biomass of the marine macroalga cystoseira baccata. Water Res. 2005;39:3199–3210. doi: 10.1016/j.watres.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 11.Glick B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010;28:367–374. doi: 10.1016/j.biotechadv.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Premaratne S., Premalal G.G.C. Hybrid Napier (Pennisetum perpureum × Pennisetum americarnum) var CO-3: a resourceful fodder grass for dairy development in Sri Lanka. J. Agric. Sci. 2006;2:22–33. [Google Scholar]
- 13.Li X., Geng X., Xie R., Fu L., Jiang J., Gao L., Sun J. The endophytic bacteria isolated from elephant grass (Pennisetum purpureum Schumach) promote plant growth and enhance salt tolerance of Hybrid Pennisetum. Biotechnol. Biofuels. 2016;9(1):190–202. doi: 10.1186/s13068-016-0592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun L.N., Zhang Y.F., He L.Y., Chen Z.J., Wang Q.Y., Qian M. Genetic diversity and characterization of heavy metal-resistant endophytic bacteria from two copper tolerant plant species on coppermine wasteland. Bioresour. Technol. 2010;101:501–509. doi: 10.1016/j.biortech.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y., Oliveira R.S., Wu L., Luo Y., Rajkumar M., Rocha I. Inoculation with metal-mobilizing plant-growth-promoting rhizobacterium Bacillus sp. SC2b and its role in rhizoremediation. J. Toxicol. Environ. Health A. 2015;78:931–944. doi: 10.1080/15287394.2015.1051205. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari S., Lata C., Chauhan P.S., Nautiyal C.S. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. During drought stress and recovery. Plant Physiol. Biochem. 2016;99:108–117. doi: 10.1016/j.plaphy.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Bottini R., Cassan F., Piccoli P. Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl. Microbiol. Biotechnol. 2004;65:497–503. doi: 10.1007/s00253-004-1696-1. [DOI] [PubMed] [Google Scholar]
- 18.Taghavi S., van der Lelie D., Hoffman A., Zhang Y.B., Walla M.D., Vangronsveld J., Newman L., Monchy S. Genome sequence of the plant growth promoting endophytic bacterium Enterobacter sp. 638. PLoS Genet. 2010;6(5) doi: 10.1371/journal.pgen.1000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajkumar M., Ae N., Freitas H. Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere. 2009;77(2):153–160. doi: 10.1016/j.chemosphere.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 20.Weyens N., Truyens S., Dupae J., Newman L., Taghavi S., van der Lelie D., Carleer R., Vangronsveld J. Potential of the TCE-degrading endophyte Pseudomonas putida W619-TCE to improve plant growth and reduce TCE phytotoxicity and evapotranspiration in poplar cuttings. Environ. Pollut. 2010;158(9):2915–2919. doi: 10.1016/j.envpol.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Chojnacka K. Biosorption and bioaccumulation—the prospects for practical applications. Environ. Int. 2010;36(3):299–307. doi: 10.1016/j.envint.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Joutey N.T., Sayel H., Bahafid W., El Ghachtouli N. Mechanisms of hexavalent chromium resistance and removal by microorganisms. Rev. Environ. Contam. Toxicol. 2015;233:45–69. doi: 10.1007/978-3-319-10479-9_2. [DOI] [PubMed] [Google Scholar]
- 23.Esringüa A., Turanb M., Güneşc A., Rüştü Karaman M. Roles of Bacillus megaterium in remediation of Boron, Lead, and Cadmium from contaminated soil. Commun. Soil Sci. Plant Anal. 2014;45:1–19. [Google Scholar]
- 24.Saleem M., Arshad M., Hussain S., Bhatti A.S. Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J. Ind. Microbiol. Biotech. 2007;34:635–648. doi: 10.1007/s10295-007-0240-6. [DOI] [PubMed] [Google Scholar]
- 25.Li Y., Han H., He L.Y., Wang Q., Sheng X.F. Inoculation with endophytic Bacillus megaterium H3 increases Cd phytostabilization and alleviates Cd toxicity to Hybrid pennisetum in Cd-contaminated aquatic environments. Environ. Sci. Poll. Res. 2017;24(2):1416–1423. doi: 10.1007/s11356-016-7930-4. [DOI] [PubMed] [Google Scholar]
- 26.Cappuccino J., Sherman N. Negative staining. Microbiol. Lab. Manual. 1992:27–28. [Google Scholar]
- 27.Pikovskaya R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiol. 1948;17:362–370. [Google Scholar]
- 28.Luo S.L., Chen L., Chen J.L., Xiao X., Xu T.Y., Wan Y., Rao C., Liu C.B., Liu Y.T., Lai C., Zeng G.M. Analysis and characterization of cultivable heavy metal resistant bacterial endophytes isolated from Cd hyperaccumulator Solanum nigrum L. and their potential use for phytoremediation. Chemosphere. 2011;85(7):1130–1138. doi: 10.1016/j.chemosphere.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 29.Govarthanan M., Park S.H., Park Y.J., Myung H., Krishnamurthy R.R., Lee S.H., Lovanh N., Kamala-Kannan S., Oh B.T. Lead biotransformation potential of allochthonous Bacillus sp. SKK11 with sesame oil cake extract in mine soil. RSC Adv. 2015;5:54564–54570. [Google Scholar]
- 30.Yang X.E., Long X.X., Ni W.Z. Physiological and molecular mechanisms of heavy metal uptake by hyperaccumulating plant species. J. Plant. Nutr. Fert. 2002;8:8–15. [Google Scholar]
- 31.Singh R.P., Jha P., Jha P.N. The plant-growth-promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J. Plant Physiol. 2015;184:57–67. doi: 10.1016/j.jplph.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Vivas A., Biró B., Ruíz-Lozano J.M., Barea J.M., Azcón R. Two bacterial strains isolated from a Zn-polluted soil enhance plant growth and mycorrhizal efficiency under Zn-toxicity. Chemosphere. 2006;62:1523–1533. doi: 10.1016/j.chemosphere.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 33.Jan A.T., Azam M., Ali A., Haq Q.M.R. Prospects for exploiting bacteria for bioremediation of metal pollution. Crit. Rev. Environ. Sci. Technol. 2014;44:519–560. [Google Scholar]
- 34.Jabbari Nezhad Kermani A., Faezi Ghasemi M., Khosravan A., Farahmand A., Shakibaie M.R. Cadmium bioremediation by metal – resistant mutated bacteria isolated from active sludge of industrial waste effluent. Iran. J. Environ. Health Sci. Eng. 2010;7(4):279–286. [Google Scholar]
- 35.Bae W., Mehra R.K., Mulchandani A., Chen W. Genetic engineering of Escherichia coli for enhanced uptake and bioaccumulation of mercury. Appl. Environ. Microbiol. 2001;67:5335–5338. doi: 10.1128/AEM.67.11.5335-5338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bae W., Wu C.H., Kostal J., Mulchandani A., Chen W. Enhanced mercury biosorption by bacterial cells with surface-displayed MerR. Appl. Environ. Microbiol. 2003;69:3176–3180. doi: 10.1128/AEM.69.6.3176-3180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong Z., Glick B.R., Duan J., Ding S., Tian J., McConkey B.J., Wei G. Effects of 1-aminocyclopropane-1-carboxylate (ACC) deaminase-overproducing Sinorhizobium meliloti on plant growth and copper tolerance of Medicago lupulina. Plant Soil. 2015;391:383–398. [Google Scholar]
- 38.Gamalero E., Berta G., Massa N., Glick B.R., Lingua G. Interactions between Pseudomonas putida UW4 and Gigaspora rosea BEG9 and their consequences on the growth of cucumber under salt stress conditions. J. Appl. Microbiol. 2010;108:236–245. doi: 10.1111/j.1365-2672.2009.04414.x. [DOI] [PubMed] [Google Scholar]
- 39.Nadeem S.M., Ahmad M., Zahir Z.A., Javaid A., Ashraf M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014;32:429–448. doi: 10.1016/j.biotechadv.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Lambrecht M., Okon Y., Vande Broek A., Vanderleyden J. Indole-3-acetic acid: a reciprocal signalling molecule in bacteria-plant interactions. Trends Microbiol. 2017;8:298–300. doi: 10.1016/s0966-842x(00)01732-7. [DOI] [PubMed] [Google Scholar]
- 41.Spaepen S., Vanderleyden J., Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007;31:425–448. doi: 10.1111/j.1574-6976.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- 42.Deepa C., Dastager S.G., Pandey A. Isolation and characterization of plant growth promoting bacteria from non-rhizospheric soil and their effect on cowpea (Vigna unguiculata (L.) Walp.) seedling growth. World J. Microbiol. Biotechnol. 2010;26:1233–1240. doi: 10.1007/s11274-009-0293-y. [DOI] [PubMed] [Google Scholar]
- 43.Ullah A., Mushtaq H., Ali U., Hakim Ali E., Mubeen S. Screening, isolation, biochemical and plant growth promoting characterization of endophytic bacteria. Microbiol. Curr. Res. 2018;2(3):62–68. [Google Scholar]
- 44.Fernández V.P., Guzman C.A., Peirce T.M., McBeath M., Khayet L., McLaughlin M.J. Effect of wheat phosphorus status on leaf surface properties and permeability to foliar-applied phosphorus. Plant Soil. 2014;384:7–20. [Google Scholar]
- 45.Shen Q., Wen Z., Dong Y., Li H., Miao Y., Shen J. The responses of root morphology and phosphorus-mobilizing exudations in wheat to increasing shoot phosphorus concentration. AoB Plants. 2018;10(5):1–11. doi: 10.1093/aobpla/ply054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodríguez H., Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999;17(4-5):319–339. doi: 10.1016/s0734-9750(99)00014-2. [DOI] [PubMed] [Google Scholar]
- 47.Matos A.D.M., Gomes I.C.P., Nietsche S., Xavter A.A., Gomes W.S., Dos Santos J.A.N., Pereira M.C.T. Phosphate solubilization by endophytic bacteria isolated from banana trees. Ann. Acad. Bras. Cienc. 2017;89(4):2945–2954. doi: 10.1590/0001-3765201720160111. [DOI] [PubMed] [Google Scholar]
- 48.Blom A., Harder W., Matin A. Unique and overlapping pollutant stress proteins of Escherichia coli. Appl. Environ. Microbiol. 1992;58:331–334. doi: 10.1128/aem.58.1.331-334.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chellaiah E.R. Cadmium (heavy metals) bioremediation by Pseudomonas aeruginosa: a minireview. Appl. Water Sci. 2018;8:154–164. [Google Scholar]
- 50.Mongkolsuk S., Vattanaviboon P., Praitaum W. Induced adaptive and cross-protection responses against oxidative stress killing in a bacterial phytopathogen, Xanthomonas oryzae pv. oryzae. FEMS Microbiol. Lett. 1997;146:217–221. [Google Scholar]
- Mitra R.S., Bernstein I.A. Nature of the repair process associated with the recovery of Escherichia coli after exposure to Cd2+ Biochem. Biophy. Res. Commun. 1977;21:1450–1455. doi: 10.1016/0006-291x(77)90604-0. [DOI] [PubMed] [Google Scholar]
- 52.Tynecka Z., Gos Z., Zajac J. Energy-dependent efflux of cadmium coded by a plasmid resistance determinant in Staphylococcus aureus. J. Bacteriol. 1981;147:313–319. doi: 10.1128/jb.147.2.313-319.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee J.D., Woodrow J.R., Quirk A.V. Investigation of cadmium resistance in an Alcaligenes sp. Appl. Environ. Microbiol. 1986;51:515–520. doi: 10.1128/aem.51.3.515-520.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramos J.L., Sol Cuenca M., Molina-Santiago C., Segura A., Duque E., Gomez-Garcia M.R., Udaondo Z., Roca A. Mechanisms of solvent resistance mediated by interplay of cellular factors in Pseudomonas putida. FEMS Microbiol. Rev. 2015;39(4):555–566. doi: 10.1093/femsre/fuv006. [DOI] [PubMed] [Google Scholar]
- 55.Roane T.M., Pepper I.L. Microorganisms and metal pollution. In: Maier I.L., Pepper C.B., editors. Environmental Microbiology. Gerba, Academic Press; London, UK: 2000. p. 55. [Google Scholar]
- 56.Ahemad M. A review: remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: paradigms and prospects. Arab. J. Chem. 2014:1–13. [Google Scholar]
- 57.Wu H., Wu Q., Wu G., Gu Q., Wei L. Cd-resistant strains of B. Cereus S5 with endurance capacity and their capacities for cadmium removal from cadmium-polluted water. PLoS One. 2016;11(4):1–25. doi: 10.1371/journal.pone.0151479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muneer B., Iqbal M.J., Shakoori F.R., Shakoori A.R. Isolation, identifcation and cadmium processing of Pseudomonas aeruginosa (EP-Cd1) isolated from soil contaminated with electroplating industrial wastewater. Pak. J. Zoo. 2016;48(5):1495–1501. [Google Scholar]
- 59.Chakraborty J., Das S. Characterization and cadmium-resistant gene expression of biofilm-forming marine bacterium Pseudomonas aeruginosa JP-11. Environ. Sci. Pollut. Res. 2014;21:14188–14201. doi: 10.1007/s11356-014-3308-7. [DOI] [PubMed] [Google Scholar]
- 60.Nies D.H. Resistance to cadmium, cobalt, zinc, and nickel in microbes. Plasmid. 1992;27:17–28. doi: 10.1016/0147-619x(92)90003-s. [DOI] [PubMed] [Google Scholar]
- 61.Gao Y., Miao C., Mao L., Zhou P., Jin Z., Shi W. Improvement of phytoextraction and antioxidative defense in Solanum nigrum L. under cadmium stress by application of cadmium- resistant strain and citric acid. J. Hazard. Mater. 2010;181:771–777. doi: 10.1016/j.jhazmat.2010.05.080. [DOI] [PubMed] [Google Scholar]
- 62.Ma Y., Prasad M., Rajkumar M., Freitas H. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol. Adv. 2011;29:248–258. doi: 10.1016/j.biotechadv.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Afzal S.N., Begum N., Zhao H.Z., Fang Z., Lou L., Cai Q. Influence of endophytic root bacteria on the growth, cadmium tolerance and uptake of switchgrass (Panicum virgatum L.) J. Appl. Microbiol. 2017;123:498–510. doi: 10.1111/jam.13505. [DOI] [PubMed] [Google Scholar]
- 64.Madhaiyan M., Poonguzhali S., Sa T. Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.) Chemosphere. 2007;69:220–228. doi: 10.1016/j.chemosphere.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 65.Belimov A., Hontzeas N., Safronova V., Demchinskaya S., Piluzza G., Bullitta S., Glick B. Cadmium tolerant plant growth-promoting bacteria associated with the roots of indian mustard (Brassica juncea L. Czern.) Soil Biol. Biochem. 2005;37:241–250. [Google Scholar]
- 66.Mallick I., Bhattacharyya C., Mukherji S., Dey D., Sarkar S.C., Mukhopadhyay U.K. Effective rhizoinoculation and biofilmformation by arsenic immobilizing halophilic plant growth promoting bacteria (PGPB) isolated from mangrove rhizosphere: a step towards arsenic rhizoremediation. Sci. Total Environ. 2018;610–611:1239–1250. doi: 10.1016/j.scitotenv.2017.07.234. [DOI] [PubMed] [Google Scholar]
- 67.Zaidi S., Usmani S., Singh B.R., Musarrat J. Significance of Bacillus subtilis strain SJ 101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere. 2006;64:991–997. doi: 10.1016/j.chemosphere.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 68.Kuklinsky Sobral J., Araújo W.L., Mendes R., Geraldi I.O., Pizzirani Kleiner A.A., Azevedo J.L. Isolation and characterization of soybean associated bacteria and their potential for plant growth promotion. Environ. Microbiol. 2004;6:1244–1251. doi: 10.1111/j.1462-2920.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- 69.Dell’Amico E., Cavalca L., Andreoni V. Improvement of Brassica napus growth under cadmium stress by cadmium-resistant rhizobacteria. Soil Biol. Biochem. 2008;40:74–84. [Google Scholar]
- 70.Chiboub M., Saadani O., Challougui Fatnassi I., Abdelkrim S., Abid G., Jebara M., Harzalli Jebara S. Characterization of efficient plant growth-promoting bacteria isolated from Sulla coronaria resistant to cadmium and to other heavy metals. C. R. Biol. 2016;339:391–398. doi: 10.1016/j.crvi.2016.04.015. [DOI] [PubMed] [Google Scholar]