Abstract
Mental disorders merge highly with thyroid diseases. Because of its regulatory effects on serotonin and noradrenalin, T3 has been linked closely to depression and anxiety. It has known that in many cases, the mental symptoms persist even after normalization of thyroid function by treatment. Psychosocial factors including stress have been associated with mental symptoms even after thyroid function normalization in Graves’ disease and a combination of mental disorders have been related to the exacerbation of hyperthyroidism. These findings suggest that psychosomatic approaches based on the bio-psycho-social medical model are important for the treatment of mental disorders associated with Graves’ disease.
Keywords: Graves’ disease, Mental disorder, Depressive disorder, Anxiety disorder, Eating disorder, Stress
Introduction
As the terms “Basedow psychosis” and “myxoedema psychosis” indicate, it is known that mental disorders merge highly with thyroid diseases [1]. These conditions are equivalent to the “symptomatic psychosis” indicated in psychiatry. However, many biases exist, because only patients with potential mental conditions and who were introduced to psychiatry were previously targeted.
It has been recently shown that mental symptoms accompany all inpatient and outpatient medical diseases. Previous research investigating the association of depression with medical conditions have indicated that hypothyroidism and hyperthyroidism show a 56% and 31% merging rate, respectively [2]. On the other hand, it has been shown that abnormalities in the thyroid test values occur during depression, indicating a merge with the hypothalamus pituitary thyroid axis (HPT axis), and are correlated with the state of the illness. We also found that a depressed merger was related to the aggravation of Graves’ hyperthyroidism. Bidirectional causality is thought to be present between depression and thyroid.
Many researchers have studied the causality between thyroid and mental disorders, focusing on depression and anxiety. However, almost all of these studies were based on the biomedical model that considers only the biological factors associated with the thyroid hormones. In this review article, the etiology and treatment of mental disorders associated with Graves’ disease (GD) using the bio-psycho-social medical model are outlined [3].
Thyroid hormones and their influence on mood
The mechanisms associated with the development of mental disorders are still unclear, even though both psychosocial factors and biological factors are thought to participate in their etiology [4].
It is known that various psychosocial factors such as traumas, life events, daily life stressors, social support, and various personality traits affect the occurrence and aggravation of mental disorders in many studies. On the other hand, the strongest hypothesis is the monoamine hypothesis [5] which correlates mental disorders with the activity of monoamine neurotransmitters from the view point of biological factors. These substances have been studied widely in the field of pathophysiology, leading to the development of novel medications including selective serotonin reuptake inhibitor (SSRI), selective serotonin noradrenalin reuptake inhibitor (SNRI) etc., which are effective against depressive disorders and anxiety disorders. With regards to the relationship with thyroid hormones, it is known that triiodothyronine (T3) controls the level and the action of serotonin [6] and noradrenalin [7]. A decline in serotonin and noradrenalin is related to depression and anxiety and a decline in T3 can cause depressive and anxiety disorders. According to a meta-analysis, the administration of T3 in addition to tricyclic antidepressants in refractory depression was effective in 25% of cases [8]. Serotonin and noradrenalin are enhanced by the effect of T3.
During depression, a decrease in serotonin in the brain stimulates thyrotropin-releasing hormone (TRH) secretion by the hypothalamus. This is thought to be because TRH is inhibited by serotonin, resulting in the secretion of thyrotropin (TSH) s by the pituitary and the subsequent production of thyroxine (T4) and T3 by thyroid gland. It’s known that in depressed patients without thyroid diseases, FT4 is the normal upper limit often merge, and these values return to normal as the symptoms of depression improve [6]. The mechanism of such abnormalities in the thyroid test values may be explained by the above mentioned relationship between T4 and serotonin. TSH secretion can also be stimulated and the production of T4 and T3 can be increased by the decrease of CSF somatostatin, which prevents the TSH secretion, that occurs during depression [9].
The HPT axis and mental disorders [10]
When considering the relationship between thyroid diseases and mental disorders, it is important to also consider thyroid hormone metabolism in the brain. The HPT axis includes the complicated interaction between several factors (thyroid hormones, deiodization enzymes, transportation proteins and receptors). A thorough understanding of each of these factors may be useful to clarify the etiology of mental diseases as well as their treatment.
The secretion of thyroid hormones is regulated by pituitary TSH, which is in turn stimulated by hypothalamic TRH and down regulated by thyroid hormones. Twenty percent of T3 in the cerebral cortex is secreted directly from the thyroid, while the remaining 80% is derived from the conversion of T4. Most of the T4 enters the brain via large transporters including transthyretin (TTR), a thyroid hormone transport protein synthesized by the colloid plexus and secreted into the cerebrospinal fluid. Deiodization occurs in glial cells and T4 must enter these cells through specialized plasma membrane carrier proteins including the organic anion transporter polypeptide 1 (OATP1C1) and monocarboxylase transporter 8 (MCT8). While the former preferentially transports T4 and 3′3′5-triiodothyronine (rT3), the latter is more specific for T3 transport. In giant cells, T4 is converted to T3 by the deiodization enzyme type 2 (D2), while it is inactivated to rT3 in neuronal cells by the deiodization enzyme type 3 (D3). The latter also deiodinates T3 into its inactive form, T2. The action of T3 is mediated by binding to the thyroid hormone nuclear receptor (THRs). THR-α is highly expressed in the adult brain and constitutes 70–80% of the THR distribution. Thus, the HPT axis includes several complex pathways, and impairment in its components has been linked in some studies to behavior changes. Cortisol, which is stimulated by the hypothalamus pituitary adrenal axis (HPA axis), stress, and depression, inhibits TRH and TSH [11]. A blunted reaction of TSH to a TRH load stimulation has been reported in depressed patients without thyroid diseases. This response normalizes upon treatment of depression. It is thought as a mechanism that cortisol increased by the HPA axis influence the HPT axis. As a result, cortisol elevation results in a decrease in T3 fall in the brain via inhibition of D2 [12].
A previous study indicated that the T4 transporter TTR, which is present in the cereblospainal fluid, was significantly lower in eight subjects with refractory depression compared with nine neurological patients without depression [13]. These results suggest a potential mechanism for “brain hypothyroidism” with normal thyroid concentrations in depression.
GD and mental disorders [14]
Parry [15] first described a female patient who experienced hyperthyroidism together with panic attacks while upon falling out of a wheelchair in 1825. When Graves described a similar syndrome in 1835 [16], he focused on the neurosis which suggested a relationship between the thyroid and the hysteria spherical syndrome. Basedow described a case that merged mental disorders with thyroid dysfunction in 1840 [17].
“Irritability”, “anxiety”, “emotional instability”, “sleeplessness”, “restlessness”, “sensitive”, “easy of anger”, etc. are all mental symptoms associated with hyperthyroidism. All activity is accelerated seemingly in patients with hyperthyroidism, resulting in a state akin to mania called “manic veneer”. Therefore, mania or a manic-depressive psychosis (=bipolar disorder) can be related to hyperthyroidism. Conversely, it is thought that depression is closely related to hypothyroidism. However, anxiety and depression may be mental symptoms of GD patients according to research conducted using objective indexes [18] (Table 1). Several studies have shown that the mental symptoms improve after thyroid function is normalized [19], [20], [21], [22], but it is also known that the mental symptoms remain in many patients after thyroid function is normalized [29], [30], [31]. Some studies suggest that bipolar disorder is related to GD [32], [33]. However, in these studies the correlation between the symptoms and thyroid function were not investigated, therefore the association between mental disorders and hyperthyroidism are unclear. Lee et al. [34] reported that in patients with hyperthyroidism, anxiety, depression and stressful life events were more than in those with normal thyroid function, but there was no correlation between these psychological disorders and thyroid function in subjects with hyperthyroidism. In Hunt’s study in Norway, a large-scale study involving more than 30,000 people, there were no differences in the frequency of depression and anxiety between patients with thyroid abnormalities and healthy controls [35]. Interestingly, in this study there were more episodes of depression and anxiety in patients with a thyroid condition than in the group who discovered a thyroid function abnormality for the first time during the study. These findings were not related to thyroid function. This result suggests that patients who are aware of thyroid diseases complain of mental symptoms more frequently [36]. It is thought that after the treatment of hyperthyroidism, mental disorders were in the exhaustion state [37] from the view point of biomedical model [37]. But next psychosomatic study [38] in Japan explained successfully about the relationship between GD and mental disorders from the view point of bio-psycho-social medical model.
Table 1.
Overt hyperthyroidism and mental disorders (modified from reference [18]).
| Study author and year | Study method | n | Mean Age (years) | Duration of follow up | Results |
|---|---|---|---|---|---|
| Kathol (1986) [19] | Questionnaire Case series |
33 with newly diagnosed untreated hyperthyroidism | 47 | – | High levels of anxiety and depression found using a structured questionnaire. |
| Trzepacz (1988) [21] | Questionnaire Case series |
13 with untreated newly diagnosed Graves’ disease | 38.9 | – | High levels of anxiety and depression as well as mild deficits in attention, memory and complex problem solving were found on neurophysical testing. |
| Gulseren (2006) [23] | Questionnaire Case control |
160 51 with overt hyperthyroidism |
Mean 42.3 in overt hyperthyroidism | – | Anxiety and depressive symptoms were more severe in patients with overt hyperthyroidism. Psychological symptoms improved with treatment. |
| Fornaro (2010) [24] | Interview Case series |
218 women with endocrine disorders, 42 with hyperthyroidism | 46.7 | 12 weeks | Women with hyperthyroidism were more likely to exhibit anxiety and panic disorders. |
| Chattopadhyay (2012) [25] | Interview Case control |
36 with newly diagnosed Graves’ disease 30 controls |
35.8 | 1 year | Greater frequency of anxiety disorders in patients with Graves’ disease. Improvement with anti-thyroid treatment. |
| Hu (2013) [26] | Interview Longitudinal |
21, 574 with hyperthyroidism 21, 574 controls |
41 (median) | 5.98 (median) | Increased incidence of bipolar disorder in hyperthyroid group. |
| Wu (2013) [27] | Interview Longitudinal |
761, 834 from general population database. 4593 with depressive disorder |
≧18 | 5 | Greater prevalence and incidence of hyperthyroidism in patients with depressive disorder. |
| Brandr (2014) [28] | Interview Longitudinal |
2631 with hyperthyroidism 10, 524 controls Subgroup of 375 same-sex twins |
67 | 6 years | Increased risk of being treated with antipsychotics, antidepressants and anxiolytics prior to diagnosis of hyperthyroidism. Increased risk of hospitalization with psychiatric diagnoses and being treated with psychotropic medications after diagnosis of hyperthyroidism. Increased psychiatric morbidity in hyperthyroid compared to euthyroid twins. |
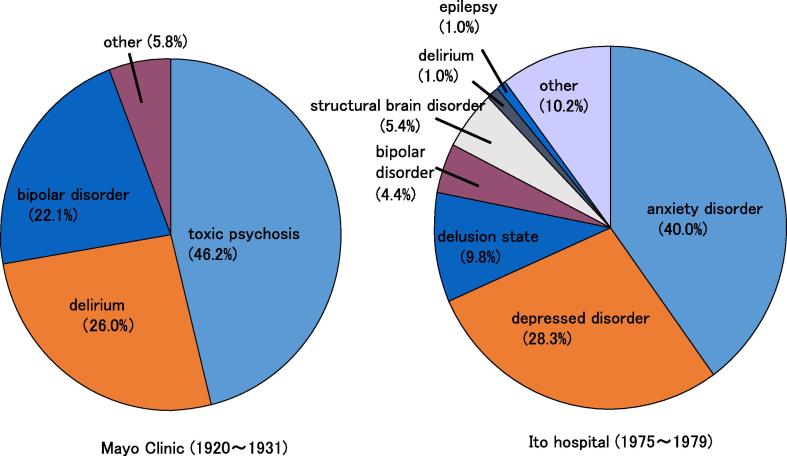
According to a report by Fujinami, a long-time psychiatrist who works at Ito hospital, the distribution of thyroid diseases in 325 patients with psychiatric issues included 205 suffering from GD (63.1%) and 34 patients with Hashimoto’s disease (HD) (10.5%). Therefore, an abnormality on thyroid function seems to be associated with mental abnormalities. However, when the thyroid function of 220 patients with mental abnormalities was analyzed, 123(55.9%) showed no abnormality in thyroid function. Mental abnormality cannot be explained solely by thyroid function. This study also included 82 cases of anxiety disorder (40.0%), 58 of depression (28.3%), 20 of hallucination delusion state (9.8%) and 9 of bipolar disorder (4.4%) at Ito hospital during the period spanning from 1975 to 1979. The same study also reported 16 cases of anxiety disorder (47.1%), 10 caseswith depression disorder (29.4%), 3 cases of hallucination delusion state (8.8%) and a case of bipolar disorder (2.9%) in 34 cases of HD with mental abnormality. Interestingly, the distribution of the psychiatric conditions included mainly anxiety disorder and depression disorder for both diseases, and hallucination delusion states and bipolar disorder known as “Basedow psychosis [1]” and “myxoedema psychosis [39]” were few. When the psychiatric outlook of “Basedow psychosis” was compared with a report [40] from Mayo Clinic spanning from 1920 to 1931, there were clear differences (Fig. 1). It was the order of the toxic psychosis (46.2%), the delirium (26.0%) and the bipolar disorder (22.1%) in the latter study. The therapy of GD was only surgical operation and thyroid storms with severe mental disorders often might occur in the latter study. So Fujinami suggested that mental disorders in thyroid diseases became mild by the progress of medicine of recent years as the reason that this difference has formed, and various factors including psychogenesis, the character, developmental history, living circumstances, treatment and concurrent diseases besides the thyroid function abnormality affect as its cause.
Fig. 1.
Comparison of the psychiatric outlook of “Basedow psychosis” between Mayo Clinic (1920–1931) and Ito hospital (1975–1979). Cited from reference [38].
Emotional stress and the onset of GD (Table 2)
Table 2.
Case control studies about the role of emotional stresses on the onset of GD.
| Author (year) | Relationship | Study method | Subjects (Number; Male, Female) | Period of stress evaluation/Thyroid status at the time of study |
|---|---|---|---|---|
| Gray et al. (1985) [49] | No | Interview Retrospective |
Thyrotoxicosis (50; M39, F11) Non-toxic goiter (50; M45, F5) |
6 months prior to the first symptoms Untreated patients |
| Winsa et al. (1991) [42] | Yes | Questionnaire Retrospective |
Graves’ disease (208; M37, F171) Control (3 7 2) |
1 year prior to diagnosis Less than 2 weeks after diagnosis |
| Sonin et al. (1993) [43] | Yes | Interview Retrospective |
Graves’ disease (70; M12, F58) Control (70) |
1 year prior to first signs Euthyroid after treatment |
| Kun (1995) [44] | Yes | Questionnaire Retrospective |
Graves’ disease (95; M5, F80) Control (95) |
1 year prior to diagnosis Untreated patients |
| Radosaljevic et al. (1996) [45] | Yes | Interview Retrospective |
Graves’ disease (100; M7, F93) Control (1 0 0) |
1 year prior to diagnosis Not available |
| Yoshiuchi et al. (1998) [46] | Yes | Questionnaire Retrospective |
Graves’ disease (228; M46, F182) Control (2 2 8) |
1 year prior to diagnosis Within 1 month of diagnosis |
| Martin-du Pan (1998) [50] | No | Graves’ disease (98; M12, F86) Hashimoto (95; M4, 女F91) Benign nodule (97; F97) |
||
| Chiovato et al. (1998) [51] | No | Thyroid function Prospective |
Panic disorder (87; M17, F70) Control (2 6 2) |
1–30 years No Graves’ disease |
| Matos-Santos et al. (2001) [47] | Yes | Interview Retrospective |
Graves’ disease (31; M9, F22) Toxic nodular goiter (31) Control (31) |
I year prior to first symptoms Euthyroid after treatment |
| Effraimidis et al. (2011) [52] | No | Questionnaire Prospective |
Euthyroid women who were 1st and 2nd degree relatives of AITD patients (521; F521) | 2.7 ± 1.5 years Untreated patients |
| Topcu et al. (2012) [48] | Yes | Interview Retrospective |
Graves’ disease (45; M12, F33) Toxic nodular goiter (24; M7, F17) Control (36) |
6 months prior to the first symptoms Or euthyroid after one year treatment |
Recent studies [41], [42] suggest that psychosocial factors including emotional stress are related to the onset of GD. Winsa et al. [43] reported the first large population-based case-control study demonstrating a relationship between stress and GD. 208 (95%) of 219 eligible patients with newly diagnosed GD and 372 (80%) of all selected matched controls answered an identical mailed questionnaire concerning marital status, occupation, drinking and smoking habits, physical activity, familial occurrence of thyroid disease, life events, social support and personality. Compared with controls, GD patients claimed to have had more negative life events in the 12 months preceding the diagnosis, and negative life-event scores were also significantly higher (odds ratio 6·3, 95% confidence interval 2·7-14·7, for the category with the highest negative score). When the results were adjusted for possible confounding factors in a multivariate analysis, the risk estimates were almost unchanged. After this report, many case control studies were reported. Sonino et al. [44] reported via a structured interview that 70 GD patients had reported significantly more life events compared to 70 controls. They also have had more independent events on thyrotoxicosis that had an objective negative impact according to an independent rater, unaware whether the events had occurred in patients or controls. Kun [45] reported using questionnaires that 95 GD patients had reported more daily hassles as well as negative life events compared to 95 controls. Radosaljevic et al. [46] reported by a structured interview that 100 GD patients had reported more independent life events and potentially dependent life events associated with illness compared to 100 controls. Yoshiuchi et al. [47] reported by questionnaires that 182 female GD patients had reported more life events compared to 228 controls but daily hassles were not significantly different. Matos-Santos et al. [48] reported by a structured interview that 31 GD patients had reported more stressful life events compared to 30 toxic nodular goiter (Plummer’s disease) patients and 31 controls, and no significant differences were found between toxic nodular goiter patients and controls. Topc et al. [49] reported by a structured interview that according to a stressful life events scale, 45 GD patients had significant differences with respect to 36 healthy controls when negative events number and impact were considered. These retrospective data suggest a positive relationship between stress and the onset of GD.
Conversely, some authors obtained contradictory findings. Gray and Hoffenberg [50] found no association between stressful life events in 50 thyrotoxic patients by a structured interview. However, this study had some methodological problems including an uncertain date of onset of any symptoms, the thyrotoxic patients included GD and toxic nodules and 50 control subjects were not healthy subjects but suffered from non-toxic goiter. Chiovato et al. [51] found that endogenous stress did not precipitate GD in a series of 87 patients with panic disorders, encompassing a total of 478 patient-years of exposure to stress. Martin-du Pan [52] evaluated the role of major stress and pregnancy in triggering autoimmune thyroid diseases in 98 GD patients and 97 patients with benign thyroid nodules. There were no significant differences of stress factors between the two groups, and generally the role of stress in triggering GD seemed weak and dubious compared to the role of pregnancy and the postpartum period. Effraimidis et al. [53] reported a prospective cohort study on the association between stress and the onset of autoimmune thyroid disease (AITD) in 521 euthyroid women who were 1st or 2nd degree relatives of AITD patients. They could not find that stress factors (stressful life events, daily hassles and negative feeling) were involved in the onset of GD including the development of TPOAb and hyperthyroidism.
Some criticisms of case-control studies were proposed [54]. There are some general methodological problems and limitations in studies dealing with stress, especially concering retrospective studies based on the assessment of life events preceding thyrotoxicosis or the diagnosis of GD. Firstly, the main scientific problem is the difficulty in defining “stress” and objectively quantifying individual stressors. Second, recall bias cannot be avoided in retrospective studies. GD patients may be more prone to recall stressful life events than healthy controls. Third, it is impossible to precisely date the onset of GD. Therefore stressful life events may occur after the onset of GD. Some studies investigated life events in the 12 months before diagnosis, rather than before the first symptoms or signs. However, some events could have occurred between the onset and diagnosis. Finally, thyrotoxicosis itself can cause psychological disturbances and behavioral changes such as anxiety and depression, which may have an effect on life events. Therefore, some stressful life events may be the consequence rather than the trigger for disease development. Although each of the above-mentioned studies were carefully designed, some problems remained. Therefore, the role of stress on the onset of GD is still controversial.
Emotional stress on the clinical course of GD (Table 3, Table 4)
Table 3.
Case control studies about the role of emotional stressors on the clinical course of GD.
| Author (year) | Relationship | Study method | Subjects (Number; Male, Female) | Period of stress evaluation/Thyroid status at the time of study |
|---|---|---|---|---|
| Yoshiuchi et al. (1998) [56] | Yes | Questionnaire Prospective |
Graves’ disease (230; M48, F182) | 1 year after treatment |
| Fukao et al. (2003) [57] | Yes | Questionnaire Prospective |
Graves’ disease (69; M4, F65) Control (32; M1, F31) |
1 year after the cessation of ATD/Euthyroid after 2–5 years treatment |
| Vita et al. (2014) [58] | Yes | Interview Retrospective |
Graves’ disease (58; M22, F36) | After ATD treatment for 1–5 years |
Table 4.
Case control studies about the role of personalities and psychological states on the clinical course of GD.
| Author (year) | Relationship | Study method | Subjects (Number; Male, Female) | Period of prognosis evaluation/Personality evaluated |
|---|---|---|---|---|
| Fukao et al. (2002) [59] | Yes | Questionnaire Prospective |
Graves’ disease (73; M11, F62) | 3 years after ATD treatment Ego state, depression, alexithymia |
| Fukao et al. (2003) [57] | Yes | Questionnaire Prospective |
Graves’ disease (69; M4, F65) Control (32; M1, F31) |
1 year after the cessation of ATD treatment hypochondriasis, depression, psychasthenia |
| Fukao et al. (2011) [60] | Yes | Questionnaire Prospective |
Graves’ disease (48; M6, F42) | 4 years after ATD treatment hypochondriasis, depression, psychasthenia |
On the other hand, there are case reports in which emotional stress induces an exacerbation and relapse of hyperthyroidism. Ferguson-Rayport [55] reported that the course of thyrotoxicosis in 20 patients during antithyroid drug (ATD) treatment seemed to be related to the patients’ ability to cope with life stress, especially when confronted with a loss or bereavement. If successful solutions were found, the illness subsided; if not, the exacerbation progressed. Voth et al. [56] reported that among 239 women the hyper functioning regions on thyroid scintiscans appeared to rise and fall in a direct relationship with life stress followed for 12 years, and some women developed clinical thyrotoxicosis during conditions of severe or prolonged life strain. Yoshiuchi et al. [57] investigated the association between the short term outcome of 230 newly diagnosed GD patients, assessed 12 months after the ATD therapy, and stressful life events. They reported that daily hassles at 6 months after the initiation of the therapy were associated with a continued hyperthyroid state 12 months later in female patients. Vita et al. [59] reported that some GD patients are prone to develop hyperthyroidism when exposed to stressful life events. Vita et al. [62] also reported that certain HLA alleles were associated with stress-triggered GD and influenced its course.
The authors [60] carried out a prospective study to investigate the relationship between ego states of GD patients evaluated by The Tokyo University Egogram including terms of adult (A), showing the ability for rational consideration, free child (FC), showing an ability to describe feelings, and adapted child (AC), showing a tendency to suppress feelings, and the prognosis of hyperthyroidism. Seventy-three GD patients were divided into two groups; a high A group (44 patients) whose A at the euthyroid state after ATD treatment were greater than the 50th percentile and a low A group (29 patients) whose A were lower than the 50th percentile. The relationships between the ego states of these groups and the prognosis of disease at three years were investigated. Additionally, similar relationships were investigated in another two groups; the FC predominant group (40 patients) whose FC were greater than AC and the AC predominant group (33 patients) whose AC were greater than FC conversely. Age, sex, rate of smoking, serum FT4, FT3 concentrations, serum TRAb, TSAb activities, 131I-uptake, goiter size before treatment were not significantly different between each group. Serum TRAb activity and the diameter of the thyroid during treatment were significantly higher in the low A group than in the high A group and the remission rate at three years were significantly lower in the low A group than in the high A group (10% vs 41%). The remission rate at three years was also significantly lower in the AC predominant group than in the FC predominant group (18% vs 40%).
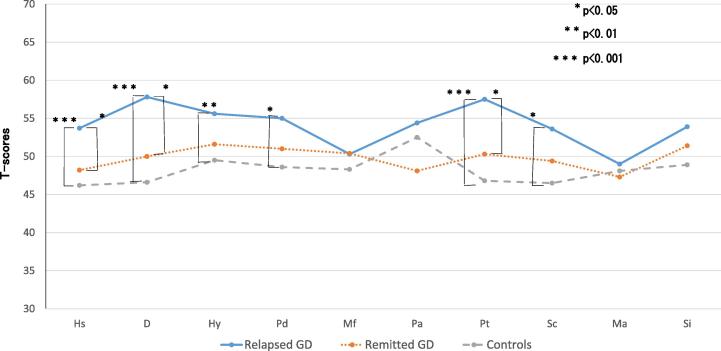
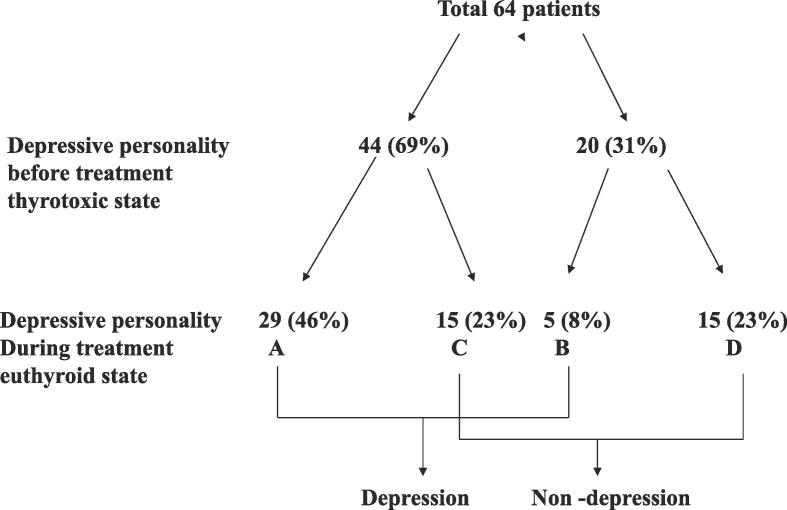
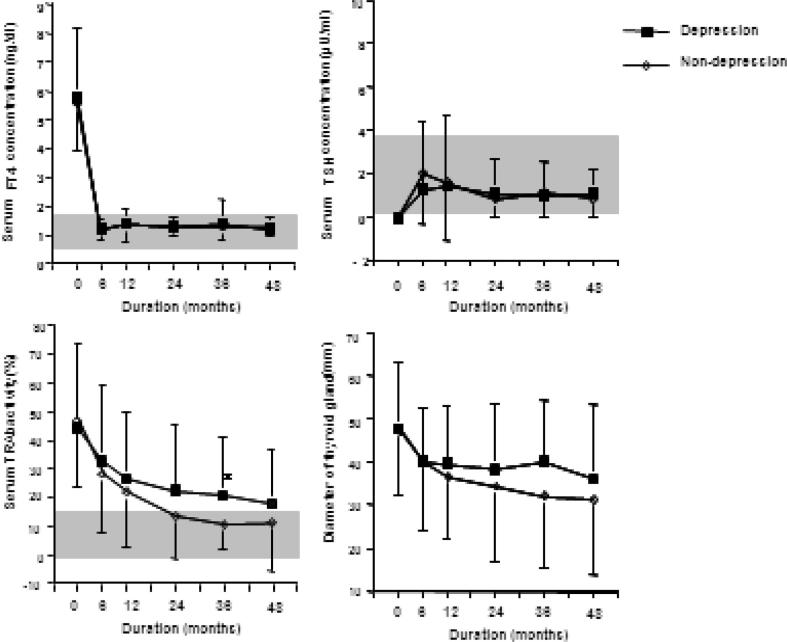
The authors [58] also examined the relationship between the prognosis, psychological stress and personality traits using the Minnesota Multiphasic Personality Inventory (MMPI) and stress questionnaires. The authors found that the depressive tendency (hypochondria, depression and nervous breakdown, by the linear measure, high price) remain in many patients even if thyroid function becomes normal after ATD treatment, and that depressive tendencies and daily hassles were significantly higher in the relapsed group compared with the remitted group and the healthy control group (Fig. 2). These results suggest that the depressed state which remains after treatment of GD relates more closely to psychological stress than thyroid function and relates to the exacerbation of hyperthyroidism The authors conducted a prospective study [61] involving 64 cases of patients undergoing pretreatment to better clarify this relationship. Forty-four cases of depressed personality (hypochondria, depression and nervous breakdown, with a T score (deviation score) of more than 60) were admitted (69%) before treatment (Fig. 3). Of these, 15 (23%) had scores decreased to a normal range after treatment (group C). However, in the remaining 29 patients (46%), the depressive personality persisted after treatment (group A). Normal scores in the untreated state were found in 20 patients (31%), and the scores were continuously normal for 15 patients (23%) (group D), while the remaining 5 patients (8%) had a higher depressive personality score after treatment (group B). A significant difference in thyroid function and the degree of illness was not identified in the 4 groups (A to D). On the other hand, even in the euthyroid state (group A and B) patients with a depressive personality had significantly lower daily uplifts (positive experiences) than the remaining patients (group C and D). These findings suggest that daily life stress is related to the depressed state. Furthermore, the rate of remission at 4 years after treatment was significantly lower in the depression group than in non-depression group (22% vs 52%) (Fig. 4).
Fig. 2.
Comparison of clinical scales of MMPI among the three groups of subjects. The data are shown as mean of T-scores in MMPI. T-scores (deviation scores) express the psychiatric tendency by each clinical scales. Table cited from reference [58] was modified to figure. Hs (hypochondriasis), D (depression), Hy (conversion hysteria), Pd (psychopathic deviation), Mf (masculity and feminity), Pa (paranoia), Pt (psychastenia), Sc (schizophrenia), Ma (hypomania), Si (social introversion).
Fig. 3.
Changes in the depressive personality of Graves’ disease patients before and during treatment. Depressive personality patients show T-scores (deviation scores) for hypochondriasis, depression or psychasthenia greater than 60 points in the MMPI. GroupA: depressive personality was present before and persisted after treatment. GroupB: depressive personality scores became higher after treatment. GroupC: depressive personality was present before treatment and decreased to within the normal range after treatment GroupD: depressive personality did not appear either before or after treatment. Cited from reference [61].
Fig. 4.
Comparison of the prognosis of hyperthyroidism between the depression and non-depression groups. The data from each group are shown as mean ± SD. The gray zone expresses the normal range. Significant difference: *P < 0.05 using a Student t-test. Remission rate: depressive group 22% (5/23) vs non-depressive group 52% (13/25) (P < 0.05 using the chi-square test). Cited from reference [61].
Eating disorder associated with GD
Eating disorder is also an important mental disorder associated with thyroid diseases in particular in GD patients [63]. The frequency is unclear but it is clear that eating disorders such as anorexia nervosa in particular, coupled with hyperthyroidism may have a significant impact. Many patients stop ATD from fear of obesity due to a decrease in metabolism. Therefore it is necessary to consider eating disorders when treating thyroid disorders. But the convalescence tends to be better for eating disorders coupled with GD than the independent cases of eating disorders, as suggested by Tanaka’s and other studies [64].
Treatment
It is important to normalize thyroid function to remove the influence of thyroid hormones for the treatment of symptomatic psychosis in GD. The normalization of thyroid function by antithyroid drugs, surgery or radioactive iodine tends to improve mental symptoms by hyperthyroidism. However, when the mental symptoms persist even after thyroid function normalization, it is necessary to consider mental disorder or existence of psychosocial factors.
Benzodiazepines are useful for anxiety. A retrospective study [65] and a case report [66] suggested that benzodiazepine prevents the recurrence of GD during ATD treatment. However benzodiazepine dependency should be noted. SSRI and SNRI should be used in the hyperthyroid state because tri-cyclic antidepressants or tetracyclic antidepressants may reinforce the anti-cholinergic action in thyrotoxicosis that influences the circulatory system. The authors [67] encountered 3 cases of first remission after long term ATD treatment together with antidepressants (paroxetine) in 3 GD patients with depression. Chinese medical treatment (Shakannzoutou, Keishikaryuukotsuboreitou and Hangekoubokutou) is often also useful for Basedow psychosis [68]. Psychotherapy may also be effective. Tanaka et al. [69] reported that the remission rate of ATD treated GD patients was significantly higher in patients with longer (over than 31 times) psychotherapy treatment than in patients with a shorter (less than 30 times) psychotherapy treatment regimen.
In eating disorder patients merged with GD, continuation of ATD treatment may create a fear of obesity and remission becomes difficult. Therefore, it is effective to normalize thyroid function soon by surgical treatment or radioactive iodine after psychological education regarding the clinical condition for the patients and their families. The authors experienced a case in which the eating disorder as well as the hyperthyroidism improved following surgical operation [70].
Finally, psychosomatic approaches should be introduced to specialists as well. It should be introduced to the department of psychosomatic internal medicine (“Shinryo Naika” in Japanese) or clinical psychology for cases with anxiety disorder, depressive disorder or eating disorder especially in persistent cases or in cases exacerbated by stress. On the other hand, psychiatic treatment should be introduced for all psychiatric symptoms including patients suffering from hallucinations, delusion and the behavioral abnormalities including a strong suicidal desire or a desire for self-injury.
Conclusion
Mental disorders, depression and anxiety often merge with GD. Psychosocial factors including stress and disease awareness as well as biological factors including the effects of thyroid hormones may influence the course of a disease. Psychosomatic approaches including antipsychotic drugs and psychotherapies based on the bio-psycho-social medical model are thought to be useful in GD patients with mental symptoms together with hyperthyroidism.
Conflict of interest
The authors declare they have no conflicts of interest.
References
- 1.Bauer M., Samuels M.H., Whybrow P.C. Behavioral and psychiatric aspects of thyrotoxicosis. In: Braverman L.E., Daniel D.S., editors. Werner and Ingbar’s the thyroid: a fundamental and clinical text. 10th ed. Lippincott-Raven Publishers; Philadelphia: 2013. pp. 475–480. [Google Scholar]
- 2.Rouchell A.M., Pouns R., Tierney J.G. Depression. In: Wise M.G., Rundell J.R., editors. Textbook of consultation-liaison psychiatry. Psychiatry in the medically ill. 2nd ed. American Psychiatric Publishing; Washington, DC: 2002. pp. 307–338. [Google Scholar]
- 3.Engel G.L. The need for new medical model: a challenge for biomedicine. Science. 1977;196(4286):129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 4.Kendler K.S., Gardner C.O. Depressive vulnerability, stressful life events and episode onset of Major depression: a longitudinal model. Psychol Med. 2016;46(9):1865–1874. doi: 10.1017/S0033291716000349. Epub 2016 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Den Boe J.A. Looking beyond the monoamine hypothesis. Eur Neurol Rev. 2006;6:87–92. [Google Scholar]
- 6.Kirkegaard C., Faber J. The role of thyroid hormones in depression. Eur J Endocrinol. 1998;138:1–9. doi: 10.1530/eje.0.1380001. [DOI] [PubMed] [Google Scholar]
- 7.Mason G.A., Walker C.H., Plange A.J., Jr L-triiodothyronine:is this peripheral hormone a central neurotransmitter? Neuropsychopharmacology. 1993;8:253–257. doi: 10.1038/npp.1993.28. [DOI] [PubMed] [Google Scholar]
- 8.Aronson R., Offman H.J., Joffe R.T. Triiodothyronine augmentation in the treatment of refractory depression: a meta-analysis. Arch Gen Psychiatry. 1996;53:842–848. doi: 10.1001/archpsyc.1996.01830090090013. [DOI] [PubMed] [Google Scholar]
- 9.Rubinow D.R., Gold P.W., Post R.M., Ballenger J.C., Cowdry R., Bollinger J. CSF somatostatin in affective illness. Arch Gen Psychiatry. 1983;40:377–386. doi: 10.1001/archpsyc.1983.01790040063009. [DOI] [PubMed] [Google Scholar]
- 10.Hage M.P., Azar S.T. The link between thyroid function and depression. J Thyroid Res. 2012;2012:1–8. doi: 10.1155/2012/590648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson I. The thyroid axis and depression. Thyroid. 1998:951–956. doi: 10.1089/thy.1998.8.951. [DOI] [PubMed] [Google Scholar]
- 12.Hindal J.T., Kaplan M.M. Inhibition of thyroxine 5’-deiodination Type Ⅱ in cultured human placental cells by cortisol, insulin, 3,5’-cyclic adenosine monophosphate and butyrate. Metab Clin Exp. 1988;37:664–668. doi: 10.1016/0026-0495(88)90087-x. [DOI] [PubMed] [Google Scholar]
- 13.Hatterer J.A., Herbert J., Hidaka C., Roose S.P., Gorman J.M. CSF tranthyretin in patients with depression. Am J Psychiat. 1993;150:813–815. doi: 10.1176/ajp.150.5.813. [DOI] [PubMed] [Google Scholar]
- 14.Fukao A., Takamatsu J., Ito M., Arishima T., Yokoyama H., Tanaka M. Thyroid hormone and mental diseases. J Jpn Thyroid Assoc. 2017 [Google Scholar]
- 15.Parry C.H. Vol. 2. Underwoods; London: 1825. (Collections from the unpublished writings of the late C.H.Parry). [Google Scholar]
- 16.Graves R.J. Newly observed affection of the thyroid gland in females. Lond Med Surg J. 1835;7:516. [Google Scholar]
- 17.Major R.H. Charles C Thomas; Splingfield, IL: 1959. Classic descriptions of disease; p. 45. [Google Scholar]
- 18.Ritchie M., Yeap B.B. Thyroid hormone: Influences on mood and cognition in adults. Maturitas. 2015;81(2):266–275. doi: 10.1016/j.maturitas.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Kathol R.G., Delahunt J.W. The relationship of anxiety and depression to symptoms of hyperthyroidism using operational criteria. Gen Hosp Psychiatry. 1986 Jan;8(1):23–28. doi: 10.1016/0163-8343(86)90060-5. [DOI] [PubMed] [Google Scholar]
- 20.Katohl R.G., Turner R., Delahunt J. Depression and anxiety associated with hyperthyroidism:response to antithyroid therapy. Psychosomatics. 1986;27:501–505. doi: 10.1016/S0033-3182(86)72656-X. [DOI] [PubMed] [Google Scholar]
- 21.Trzepacz P.T., Klein I., Roberts M., Greenhouse J., Levey G.S. Graves’ disease: an analysis of thyroid hormone levels and hyperthyroid signs and symptoms. Am J Med. 1989 Nov;87(5):558–561. doi: 10.1016/s0002-9343(89)80614-x. [DOI] [PubMed] [Google Scholar]
- 22.Trzepacz P.T., McCue M., Klein I., Greenhouse J., Levey G.S. Psychiatric and neuropsychological response to propranolol in Graves’ disease. Biol Psychiatry. 1988;23(7):678–688. doi: 10.1016/0006-3223(88)90051-0. [DOI] [PubMed] [Google Scholar]
- 23.Gulseren S., Gulseren L., Hekimsoy Z., Cetinay P., Ozen C., Tokatlioglu B. Depression, anxiety, health-related quality of life, and disability in patients with overt and subclinical thyroid dysfunction. Arch Med Res. 2006;37(1):133–139. doi: 10.1016/j.arcmed.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Fornaro M., Iovieno N., Clementi N., Boscaro M., Paggi F., Balercia G. Diagnosis of co-morbid axis-I psychiatric disorders among women with newly diagnosed, untreated endocrine disorders. World J Biol Psychiatry. 2010;11(8):991–996. doi: 10.3109/15622975.2010.491126. Epub 2010 Jun 22. [DOI] [PubMed] [Google Scholar]
- 25.Chattopadhyay C., Chakrabarti N., Ghosh S. An assessment of psychiatric disturbances in Graves’ disease in a medical college in eastern India. Niger J Clin Pract. 2012;15(3):276. doi: 10.4103/1119-3077.100620. [DOI] [PubMed] [Google Scholar]
- 26.Hu L.Y., Shen C.C., Hu Y.W., Chen M.H., Tsai C.F., Chiang H.L. Hyperthyroidism and risk for bipolar disorders: a nationwide population-based study. PLoS One. 2013;8(8):e73057. doi: 10.1371/journal.pone.0073057. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu E.L., Chien I.C., Lin C.H., Chou Y.J., Chou P. Increased risk of hypothyroidism and hyperthyroidism in patients with major depressive disorder: a population-based study. J Psychosom Res. 2013 Mar;74(3):233–237. doi: 10.1016/j.jpsychores.2012.12.016. Epub 2013 Jan 22. [DOI] [PubMed] [Google Scholar]
- 28.Brandt F., Thvilum M., Almind D., Christensen K., Green A., Hegedüs L. Hyperthyroidism and psychiatric morbidity: evidence from a Danish nationwide register study. Eur J Endocrinol. 2013;170(2):341–348. doi: 10.1530/EJE-13-0708. Print 2014 Feb. [DOI] [PubMed] [Google Scholar]
- 29.Stern R.A., Robinson B., Thorner A.R., Arruda J.E., Prohaska M.L., Prange A.J., Jr. A survey study of neuropsychiatric complaints in patients with Graves’ disease. J Neuropsychiatry Clin Neurosci. 1996;8(2):181–185. doi: 10.1176/jnp.8.2.181. [DOI] [PubMed] [Google Scholar]
- 30.Fahrenfort J.J., Wi1terdink A.M.L., van der Veen E.A. Long-term residual complaints and psychological sequate after remission of hyperthyroidism. Psychoneuroimmunology. 2000;25:201–211. doi: 10.1016/s0306-4530(99)00050-5. [DOI] [PubMed] [Google Scholar]
- 31.Elberling T.V., Rasmussen A.K., Feldt-Rasmussen U., Hørding M., Perrild H., Waldemar G. Impaired health-related quality of life in Graves’ disease. A prospective study. Eur J Endocrinol. 2004 Nov;151(5):549–555. doi: 10.1530/eje.0.1510549. [DOI] [PubMed] [Google Scholar]
- 32.Thomsen A.F., Kvist T.K., Andersen P.K., Kessing L.V. Increased risk of affective disorder following hospitalisation with hyperthyroidism - a register-based study. Eur J Endocrinol. 2005 Apr;152(4):535–543. doi: 10.1530/eje.1.01894. [DOI] [PubMed] [Google Scholar]
- 33.Thomsen A.F., Kessing L.V. Increased risk of hyperthyroidism among patients hospitalized with bipolar disorder. Bipolar Disord. 2005 Aug;7(4):351–357. doi: 10.1111/j.1399-5618.2005.00205.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee I.T., Sheu W.H., Liau Y.J., Lin S.Y., Lee W.J., Lin C.C. Relationship of stressful life events, anxiety and depression to hyperthyroidism in an Asian population. Horm Res. 2003;60(5):247–251. doi: 10.1159/000074039. [DOI] [PubMed] [Google Scholar]
- 35.Engum A., Bjøro T., Mykletun A. Dahl AA An association between depression, anxiety and thyroid function-a clinical fact or an artifact? Arch Psychiatr Scand. 2002;106:27–34. doi: 10.1034/j.1600-0447.2002.01250.x. [DOI] [PubMed] [Google Scholar]
- 36.Panicker V., Evans J., Bjøro T., Asvold B.O., Dayan C.M., Bjerkeset O. A paradoxical difference in relationship between anxiety, depression and thyroid function in subjets on and not on T4: findings from the HUNT study. Clin Endocrinol (Oxf) 2009;70:484–492. doi: 10.1111/j.1365-2265.2008.03521.x. [DOI] [PubMed] [Google Scholar]
- 37.Bunevicius R., Prange A.J., Jr Thyroid disease and mental disorders: cause and effect or only comorbidity? Curr Opin Psychiatry. 2010;23:363–368. doi: 10.1097/YCO.0b013e3283387b50. [DOI] [PubMed] [Google Scholar]
- 38.Fujinami S. Thyroid function abnormality and mental symptoms. Igaku no Ayumi. 1991;157:67–71. (written by Japanese) [Google Scholar]
- 39.Schuff K.G., Samuels M.H., Whybrow P.C. Psychiatric and cognitive effects of hypothyroidism. In: Braverman L.E., Daniel D.S., editors. Werner and Ingbar’s the thyroid: a fundamental and clinical text. 10th ed. Lippincott-Raven Publishers; Philadelphia: 2013. pp. 596–600. [Google Scholar]
- 40.Dunlap H.F., Moersch F.P. Psychiatric manifestations associated with hyperthyroidism. Am J Psychiatry. 1935;91:1215–1238. [Google Scholar]
- 41.Fukao A., Takamatsu J., Miyauchi A., Hanafusa T. Endocrine diseases. iConcept Press; 2014. Stress and thyroid disease. ISBN: 978-1-922227-78-2. [Google Scholar]
- 42.Mizokami T., Wu Li A., El-Kaissi S., Wall J.R. Stress and thyroid autoimmunity. Thyroid. 2004;14:1047–1055. doi: 10.1089/thy.2004.14.1047. [DOI] [PubMed] [Google Scholar]
- 43.Winsa B., Adami H.O., Bergström R., Gamstedt A., Dahlberg P.A., Adamson U. Stressful life events and Graves’ disease. Lancet. 1991;338:1475–1479. doi: 10.1016/0140-6736(91)92298-g. [DOI] [PubMed] [Google Scholar]
- 44.Sonino N., Girelli M.E., Boscaro M., Fallo F., Busnardo B., Fava G.A. Life events in the pathogenesis of Graves’ disease. A controlled study. Acta Endocrinol. 1993;128:293–296. doi: 10.1530/acta.0.1280293. [DOI] [PubMed] [Google Scholar]
- 45.Kun A.W.C. Life events, daily stresses and coping in patients with Graves’ disease. Clin Endocrinol (Oxf) 1995;42:303–308. doi: 10.1111/j.1365-2265.1995.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 46.Radosaljevic V.R., Jankovic S.M., Marinkovic J.M. Stressful life events in the pathogenesis of Graves’ disease. Eur J Endocrinol (Oxf) 1996;134:699–701. doi: 10.1530/eje.0.1340699. [DOI] [PubMed] [Google Scholar]
- 47.Yoshiuchi K., Kumano H., Nomura S., Yoshimura H., Ito K., Kanaji Y. Stressful life events and smoking were associated with Graves’ disease in women, but not in men. Psychosom Med. 1998;60:182–185. doi: 10.1097/00006842-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Matos-Santos A., Nobre E.L., Costa J.G., Nogueira P.J., Macedo A., Galvão-Teles A. Relationship between the number and impact of stressful life events and the onset of Graves’ disease and toxic nodular goiter. Clin Endocrinol (Oxf) 2001;55:15–19. doi: 10.1046/j.1365-2265.2001.01332.x. [DOI] [PubMed] [Google Scholar]
- 49.Topcu C.B., Celik O., Tasan E. Effect of stressful life events on the initiation of Graves’ disease. Int J Psychiatry Clin Pract. 2012;16(4):307–311. doi: 10.3109/13651501.2011.631016. Epub 2011 Dec 5. [DOI] [PubMed] [Google Scholar]
- 50.Gray J., Hoffenberg R. Thyrotoxicosis and stress. Quart J Med, New series. 1985;54:153–160. [PubMed] [Google Scholar]
- 51.Chiovato L., Marino M., Perugi G. Chronic recurrent stress due to panic disorder dose not precipitate Graves’ disease. J Endocrinol Invest. 1998;21:758–764. doi: 10.1007/BF03348042. [DOI] [PubMed] [Google Scholar]
- 52.Martin-du Pan R.C. Triggering role of stress and pregnancy in the occurrence of 98cases of Graves’ disease compared to 95cases of Hashimoto thyroiditis and 97cases of thyroid nodules. Ann Endocrinol (Paris) 1998;59:107–112. [PubMed] [Google Scholar]
- 53.Effraimidis G., Tijssen J.G., Brosschot J.F., Wiersinga W.M. Psychoneuroendocrinology. 2012 Aug;37(8):1191–1198. doi: 10.1016/j.psyneuen.2011.12.009. Epub 2012 Jan 5. [DOI] [PubMed] [Google Scholar]
- 54.Chiovato L., Pinchera A. Stressful life events and Graves’ disease. Eur J Endocrinol. 1996;134:680–682. doi: 10.1530/eje.0.1340680. [DOI] [PubMed] [Google Scholar]
- 55.Ferguson-Rayport S.M. The relation of emotional factors to recurrence of thyrotoxicosis. Can Med Assoc. 1956;15:993–1000. [PMC free article] [PubMed] [Google Scholar]
- 56.Voth H.M., Holman P.S., Katz J.B., Wallerstein R.S. Thyroid “hot spots”: their relationship to life stress. Psychosom Med. 1970;32:561–568. doi: 10.1097/00006842-197011000-00001. [DOI] [PubMed] [Google Scholar]
- 57.Yoshiuchi K., Kumano H., Nomura S., Yoshimura H., Ito K., Kanaji Y. Psychosocial factors influencing the short term outocome of antithyroid drug therapy in Graves’ disease. Psychosom Med. 1998;60:592–596. doi: 10.1097/00006842-199809000-00014. [DOI] [PubMed] [Google Scholar]
- 58.Fukao A., Takamatsu J., Murakami Y., Sakane S., Miyauchi A., Kuma K. The relationship of psychological factors to the prognosis of hyperthyroidism in antithyroid drug-treated patients with Graves’ disease. Clin Endocrinol (Oxf) 2003;58:550–555. doi: 10.1046/j.1365-2265.2003.01625.x. [DOI] [PubMed] [Google Scholar]
- 59.Vita R., Lapa D., Trimarchi F., Benvenga S. Stress triggers the onset and the recurrences of hyperthyroidism in patients with Graves’ disease. Endocrine. 2015 Feb;48(1):254–263. doi: 10.1007/s12020-014-0289-8. Epub 2014 May 23. [DOI] [PubMed] [Google Scholar]
- 60.Fukao A., Takamatsu J. The role of psychological factors on the onset and clinical course of hyperthyroid Graves’ disease. Recent Res Dev Endocrinol. 2002;3:369–376. [Google Scholar]
- 61.Fukao A., Takamatsu J., Kubota S., Miyauchi A., Hanafusa T. The thyroid function of Graves’ disease patients is aggravated by depressive personality during antithyroid drug treatment. Bio Psycho Social Med. 2011;5:9. doi: 10.1186/1751-0759-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vita R., Lapa D., Trimarchi F., Vita G., Fallahi P., Antonelli A. Certain HLA alleles are associated with stress-triggered Graves’ disease and influence its course. Endocrine. 2017 Jan;55(1):93–100. doi: 10.1007/s12020-016-0909-6. Epub 2016 Mar 7. [DOI] [PubMed] [Google Scholar]
- 63.Tiller J., Macrae A., Schmidt U., Bloom S., Treasure J. The prevalence of eating disorders in thyroid diseases: a pilot study. J Psychosom Res. 1994;18:609–616. doi: 10.1016/0022-3999(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka M., Kawai T., Kanayama Y., Kuwabara H. The miscellany of the 26th Annual Meeting of The Association of Japanese Clinical Psychology. 2007:89. [Google Scholar]
- 65.Benvenga S. Benzodiazepine and remission of Graves’ disease. Thyroid. 1996;6:659–660. doi: 10.1089/thy.1996.6.659. [DOI] [PubMed] [Google Scholar]
- 66.Vita R., Lapa D., Vita G., Trimarchi F., Benvenga S. A patient with stress-related onset and exacerbations of Graves disease. Nat Clin Pract Endocrinol Metab. 2009 Jan;5(1):55–61. doi: 10.1038/ncpendmet1006. Epub 2008 Nov 25. [DOI] [PubMed] [Google Scholar]
- 67.Fukao A., Takamatsu J., Tsujimoto N. Three cases of Graves’ disease patients with depression successfully treated by antidepressant (Abstr) Endocrine J. 2010;57(suppl.2) [Google Scholar]
- 68.Arishima T., Fukao A. Kampo therapy for Graves’ psychosis. J Jpn Assoc Orient Psychosomatic Med. 2011;26(1):240–245. (written by Japanese with English abstract) [Google Scholar]
- 69.Tanaka M., Kanayama Y., Kawai T., Kuwabara H., Kubota S., Fukao A. On the features of psychotherapy with Graves’ disease: from the experiences in a thyroid hospital. Jpn J Psychosom Int Med. 2013;17:174–179. (written in Japanese with English abstract) [Google Scholar]
- 70.Fukao A. The relationship between mind and body in successful treatment by psychosomatic medicine in two female patients with Graves’ disease. J JSPOG. 2003;8:137–143. (written by Japanese with English abstract) [Google Scholar]