Abstract
A highly sensitive and ultra-fast high performance liquid chromatography- tandem mass spectrometry (LC–MS/MS) assay is developed and validated for the quantification of Lenalidomide in human plasma. Lenalidomide is extracted from human plasma by Liquid- Liquid Extraction by Ethyl Acetate and analyzed using a reversed phase isocratic elution on a XTerra RP18, (4.6 × 50 mM, 5 µm) column. A 0.1% Formic acid: Methanol (10:90% v/v), is used as mobile phase and detection was performed by Triple quadrupole mass spectrometry LC-MS/MS using electrospray ionization in positive mode. Fluconazole is used as the internal standard. The lower limit of quantification is 9.999 ng/mL for Lenalidomide. The calibration curves are consistently accurate and precise over the concentration range of 9.999 to 1010.011 ng/mL in plasma for Lenalidomide. This novel LC–MS/MS method competes with all the regulatory requirements and shows satisfactory accuracy and precision and is sufficiently sensitive for the performance of pharmacokinetic and bioequivalence studies in humans.
Keywords: Lenalidomide, LC-MS/MS, Validation, Human plasma, Liquid-liquid extraction
1. Introduction
Multiple myeloma (MM) is a hematologic cancer characterized by the accumulation of malignant plasma cells in the bone marrow, which causes bone destruction and marrow failure (National Cancer Institute, 2016). Globally, multiple myeloma affected 488,000 people and resulted in 101,100 deaths in 2015 (GBD, 2015a, GBD, 2015b). This gives a five-year survival rate of about 49% (SEER, 2016). It usually occurs around the age of 61 and is more common in men than women (Raab et al., 2009). Without treatment, typical survival is seven months. Despite the fact that clinical introduction of novel drugs has led to a dramatic improvement of disease, MM ultimately relapses and remains an incurable disease (Mahindra et al., 2012, Fulciniti et al., 2016).
Lenalidomide (LDM), chemically is (RS)-3-(4-amino-1-oxo-3H-isoindol-2-yl) piperidine-2,6-dione is represented in Fig. 1. The Empirical Formula is C13H13N3O3 and the Molecular Weight is 259.261 g/mol (Drug Bank, 2005).
Fig. 1.
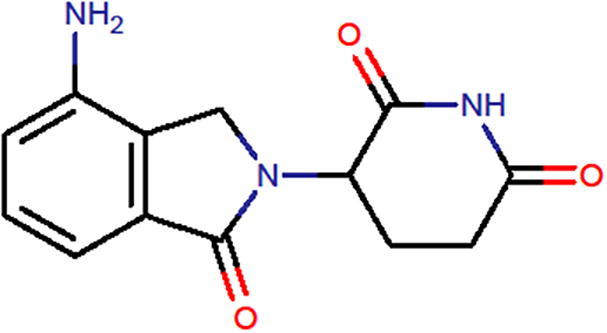
Chemical structure of lenalidomide.
LDM is classified as a thalidomide analogue, it is a novel oral immune modulatory drug with antiangiogenic and antineoplastic effects (Kastritis and Dimopoulos, 2007), exhibits an improved toxicity profile (Mitsiades and Mitsiades, 2004). LDM is used in the treatment of hematological malignancies, particularly multiple myeloma and a myelodysplastic syndrome subset (Rajkumar et al., 2005, Richardson et al., 2006, List et al., 2005, List et al., 2006). It may act by inhibiting the growth of new blood vessels in tumors, enhancing the status of the immune system, or decreasing cytokine and growth factor production.
Several chromatographic methods including liquid chromatography – UV Detector (LC–UV) (Pulla et al., 2011, Alzoman, 2016, Maheswara Reddy et al., 2012), HPLC tandem mass spectrometry (LC-MS/MS) have been developed to measure LDM in biological fluids. All these reported methods are inadequate, considering the need for pharmaco economic cancer treatments, it is essential to develop reliable and sensitive bioanalytical method for LDM determination in human plasma in order to conduct bioequivalence studies of new generic drug products.
2. Materials and methods
2.1. Chemicals and reagents
Lenalidomide working standard was supplied by Natco pharma limited and was certified to contain 100.30% purity for Lenalidomide. Fluconazole Internal standard (IS) was supplied by Tablets India and was certified to contain 98.85% purity for Fluconazole. Both standard and internal standard were used without further purification. The organic solvent methanol of HPLC grade used and was obtained from Merck for standard preparation and further dilution was made by Methanol: Water (80:20 v/v). Ethyl Acetate was of Analytical Grade was obtained from Merck. Water was obtained from a Milli-Q Gradient water purification system (Millipore, Barnstead). Formic acid used in mobile phase preparation was of Guaranteed Reagent (GR) grade obtained from Sigma Aldrich.
2.2. Chromatographic conditions
An Agilent 1260 Infinity II Liquid Chromatography (HPLC) instrument was used in this study. Separation was carried out on a XTerra RP18, (4.6 × 50 mm, 5 µm) column maintained at 35 °C. The LC mobile phase consisted of 0.1% Formic Acid: Methanol (10:90% v/v). The flow rate was 0.400 mL/min. The injection volume was 3.0 μL and the runtime was 2.0 min (mins).
2.3. Mass spectrometry conditions
Detection was carried out by a Agilent 6460 triple quadrupole MS/MS fitted with Agilent Jet Stream Electrospray ionization (AJS-ESI) probe and operated in the positive ion mode. Detection was carried out in multiple reactions monitoring (MRM) mode. Nitrogen 99.999% was used as the collision gas. The optimized conditions were as follows: Nebulizer Pressure, 35 psi; Drying Gas Temperature, 250 °C; Sheath Gas Temperature, 350 °C; Drying Gas Flow, 9 (l/min); Sheath Gas Flow, 12 (l/min); Capillary, 4500 V and Nozzle Voltage, 500 V. The MRM transitions and the related optimized Fragmentor Voltage, collision energy for analyte and IS are shown in Table 1.
Table 1.
Mass spectrometer parameters.
| Parameters | Lenalidomide | Fluconazole |
|---|---|---|
| Molecular weight (Da) | 259.261 | 306.271 |
| Parent mass (m/z) | 260.1 | 307.1 |
| Product mass (m/z) | 148.8 | 238.0 |
| Collision energy | 20 | 24 |
| Fragmentor voltage | 90 | 100 |
2.4. Preparation of standard and sample solutions
Standard stock solutions of Lenalidomide 1 mg/mL (w/v) and Fluconazole (IS) 1 mg/mL (w/v) were separately prepared in 10 mL volumetric flasks with methanol. Working solutions for calibration and controls were prepared from the stock solution by adequate dilution using diluents Methanol: Water (80:20 v/v). The Internal Standard (IS) working solution (50.000 ng/mL) was prepared by diluting the stock solution with diluents. 20 µL of working solutions were added to 980 µL drug free human plasma to obtain Lenalidomide concentration levels of 9.999, 25.250, 50.299, 150.492, 301.993, 503.995, 705.998, 906.990 and 1010.011 ng/mL respectively. Quality control (QC) samples were prepared as a bulk based on an independent weighing of standard drug, at concentrations of 9.999 ng/mL (LLOQ), 26.260 ng/mL (LQC), 323.204 ng/mL (MQC) and 808.009 ng/mL (HQC) as a single batch at each concentration. These samples were divided into aliquots in micro centrifuge tubes (Tarson, 0.250 mL) and stored in the freezer at below −70 °C until analysis.
2.5. Sample preparation
Sample preparation involved a liquid-liquid extraction with Ethyl Acetate. A spiked plasma stability sample of Lenalidomide was removed from the deep freezer maintained at −70 °C and left at room temperature to thaw. The samples were vortexed, mixed adequately and centrifuged before pipetting. As soon as the stability samples were thawed, these samples were aliquoted (0.250 mL) and freshly prepared. Calibration Standards (CS) and Quality Control (QC) samples were spiked with 50 µL IS (50.000 ng/mL) into pre labeled RIA vials to each tube except blank and mixed for 30 s on vortexer. To this 2.5 ml of solvent Ethyl acetate was added to each tube and vortexed in Vibramax for 10 min at 2500 rpm. Then samples were centrifuged at 4500 rpm for 10 min at 5 °C. 1.8 mL of supernatant layer was transferred, separated and evaporated under gentle steam of nitrogen gas pressure at 40 °C up to dryness, reconstituted with 250.00 µL mobile phase and vortexed for 30 s then transferred to a HPLC vials with insert for analysis (Lakshmana Prabu and Suriyaprakash, 2012).
2.6. Method validation
2.6.1. Specificity
To verify the absence of interfering substances around the retention time of analyte, the specificity of the method was investigated by chromatograms obtained from eight different sources of blank plasma one heamolysed and one lipeamic source spiked at LLOQ, ULOQ levels.
2.6.2. Linearity
Calibration curves were constructed using matrix matched calibration standard solutions by plotting the peak area of the quantitative ion of each analyte versus concentrations. Concentration range of Lenalidomide was found to be accurate and precise from 9.999 to 1010.011 ng/mL respectively. Correlation coefficient was greater than 0.99 for Lenalidomide.
2.6.3. Lower limit of detection and quantitation
The lower limit of detection (LLOD) was defined as the lowest concentration of each analyte that could be reliably differentiated from background noise assessed with a blank sample; the lower limit of quantification (LLOQ) was defined as the lowest concentration that could be measured with an interday coefficient of variation (CV) of <20% and accuracy between 80 and 120%.
2.6.4. Accuracy and precision
Intra assay precision and accuracy of Lenalidomide were calculated at lower limit of quantification (LLOQ) (9.999 ng/mL), low quality control (LQC) (26.260 ng/mL), Middle quality control (MQC) (323.204 ng/mL) and high quality control (HQC) (808.009 ng/mL) levels for the six replicates, each of the same analytical run. Inter assay precision and accuracy was calculated after the replicates in three different analytical runs.
2.6.5. Recovery
The recovery (RE) of Lenalidomide was calculated by comparing the peak area of the analyte from the extracted plasma standard with that obtained from an unextracted standard at the same concentration for the QC samples containing 26.260, 323.204, 808.009 ng/mL for Lenalidomide. Internal Standard recovery was tested at 50.000 ng/mL by comparing six extracted and unextracted samples at each concentration.
2.6.6. Matrix effect
The matrix effects (ME) were investigated for eight different lots including one heamolysed and one lipeamic lot of blank K3EDTA human plasma were processed in duplicate and reconstituted with aqueous HQC and LQC samples. Extracted and aqueous samples were then compared to determine the matrix factor for analyte and Internal Standard (IS). IS normalized matrix factor for individual lot is also determined. The calculated matrix factor of all LQC and HQC samples must be within 0.85–1.15 of their nominal concentration. At least 67% of QC samples must fall within the above mentioned criteria at each LQC and HQC levels.
2.6.7. Dilution integrity
The dilution integrity (DI) was calculated by spiking the matrix with an analyte concentration above the ULOQ and diluting the sample with blank matrix. Accuracy and precision should be within ±15%. Dilution integrity should cover the dilution applied to the study samples.
2.6.8. Stability
Exhaustive experiments were performed to assess the stability of Lenalidomide in stock solution and in plasma samples under different conditions, simulating the conditions occurring during the analysis of study samples. Room temperature stability, extracted sample stability (process stability), Auto sampler stability, freeze thaw stability, long term stability of plasma samples, dry extract stability and stock solution stability were performed.
3. Results and discussion
3.1. Method development and optimization
The reliability of the method was assessed on the basis of linearity, sensitivity, selectivity, precision and accuracy, recovery and carry over test. On the basis of the results obtained for the above parameters, the combination of mobile phase, flow rate and pH were selected for validation. Out of several tried combinations, Results suggested a combination of 10:90 ratio of mobile phase (0.1% Formic Acid buffer: Methanol), Flow rate 0.400 mL/min and pH 2.7 which resulted in a retention time of 1.054 for lenalidomide and 1.073 for the IS.
3.2. Method validation
The developed method was validated in terms of mentioned parameters with respect to FDA, Guidance for Industry: Bioanalytical Method Validation (Guidance for Industry Bioanalytical Method Validation, 2013, Guidance for Industry Bioanalytical Method Validation, 2001, Guideline on bioanalytical method validation, 2012, Burhenne, 2012, Sonawane et al., 2014, Whitmire et al., 2011).
3.2.1. Specificity
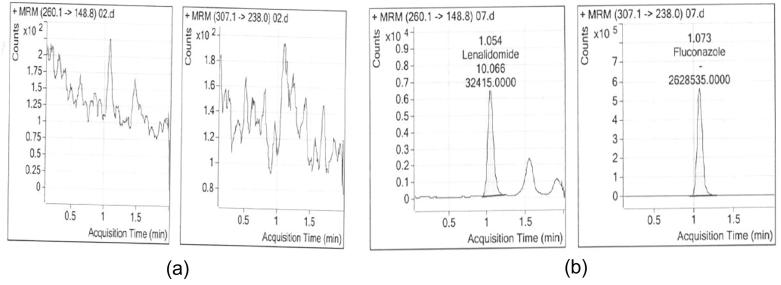
The Specificity was assessed by analyzing extracted samples of analyte at LLOQ and ULOQ without internal standard, internal standard sample at working concentration without analyte and blank sample without analyte and internal standard. The area observed at the retention time of Lenalidomide was found to be less than 20% of the LLOQ area (9.999 ng/mL). It was found that internal standard is not interfering with analyte and vice versa. Representative chromatograms shown in Fig. 2 (Fig. 2A and B). LLOD was 3.000 ng/mL for LDM.
Fig. 2.
Blank chromatograms (2A) and LLOQ level (2B).
3.2.2. Calibration curve regression
The calibration curve regression for lenalidomide was a linear regression (1/concentration2). This gave the best fit and coefficient of determination (r2) for validation and was greater than 0.99 which was in the acceptable range. The average value for r2 was found to be 0.9953.
3.2.3. Accuracy and precision
The Intra batch coefficients of variation ranged from 1.10 to 7.86% and percentage accuracy ranged from 87.81 to 102.53% for Lenalidomide. The results are within ±15% and for LLOQ the results are within ±20%. The Inter batch coefficients of variation ranged from 1.44 to 6.66% and percentage accuracy ranged from 93.98 to 102.24% for Lenalidomide respectively. The Intraday and Interday results are presented in Table 2.
Table 2.
Lenalidomide Intraday and Interday batch precision and accuracy in QC pools.
| QCa Nom.conc(ng/mL) | Mean (ng/mL) |
SDb |
CVc (%) |
Accuracy (%) |
||||
|---|---|---|---|---|---|---|---|---|
| Intra | Inter | Intra | Inter | Intra | Inter | Intra | Inter | |
| LLOQ (9.999) | 10.252 | 10.252 | 0.11 | 0.15 | 1.10 | 1.44 | 102.53 | 102.24 |
| LQC (26.260) | 23.058 | 24.680 | 0.57 | 1.64 | 2.46 | 6.66 | 87.81 | 93.98 |
| MQC (323.204) | 304.094 | 314.073 | 4.74 | 16.73 | 1.56 | 5.33 | 94.09 | 97.17 |
| HQC (808.009) | 804.282 | 811.665 | 63.18 | 46.44 | 7.86 | 5.72 | 99.54 | 100.45 |
Quality control.
Standard deviation.
Coefficient of variation.
3.2.4. Recovery
Recovery of Lenalidomide and the internal standard Fluconazole were evaluated by comparing mean peak response of six extracted low, middle and high level quality control samples to those of six appropriately diluted aqueous solutions. Mean recovery values of Lenalidomide are 92.28, 95.62 and 96.10 Mean recovery values of internal standard Fluconazole are 82.51, 79.28 and 74.68 for low, middle and high quality control levels respectively. Total mean recovery of analyte Lenalidomide and the internal standard Fluconazole are 94.67% and 78.82% respectively. This shows that the method has very good recovery of both analytes and IS.
3.2.5. Matrix effect
Processed and analyzed calibration standards in the same matrix which is to be used during validation experiment and duplicates from eight different lots of plasma at LQC and HQC levels as per the procedure are described in the sample preparation section. The calculated matrix factor of all LQC and HQC samples was within 0.85–1.15 of their nominal concentration. At least 67% of QC samples must fall within the above mentioned criteria at each LQC and HQC levels were met. The above reported method showed that no matrix effect was found for plasma and results shown in Table 3.
Table 3.
Internal standard normalized matrix factor.
| S. No. | Lot ID | Lenalidomide |
|
|---|---|---|---|
| HQC | LQC | ||
| 1 | LS_01 | 1.14 | 1.00 |
| 2 | LS_02 | 1.03 | 1.12 |
| 3 | LS_03 | 1.11 | 1.01 |
| 4 | LS_04 | 0.99 | 0.95 |
| 5 | LS_05 | 0.95 | 1.05 |
| 6 | LS_06 | 1.05 | 0.86 |
| 7 | LS_07(Lipeamic) | 1.01 | 0.96 |
| 8 | LS_08(Hemophilic) | 0.85 | 0.85 |
| Mean | 1.0170 | 0.9760 | |
| SD | 0.0909 | 0.0902 | |
| % CV | 8.94 | 9.24 | |
3.2.6. Dilution integrity
DIQC (Dilution integrity quality control) samples having double the concentration of HQC was diluted 2 and 4 times with screened pooled blank plasma. 6 replicates of each dilution are extracted and analysed. The extracted samples were injected along with calibration curve standards. Percentage accuracy for 1/2 dilution of Lenalidomide was 96.92% and for 1/4 dilution are 97.58% respectively and within ±15% of the nominal concentration, the coefficient of variation for 1/2 dilution are 6.17% and for 1/4 dilution are 4.69% respectively. The dilution integrity was found to be within accepted range.
3.2.7. Stability
The stability of the analyte and IS in human plasma under different temperature and time conditions were evaluated, as well as the stability in stock solution, was also evaluated and follows. All the stability Figure of the studies were carried out at two concentration levels of Lenalidomide (26.260 ng/mL and 808.009 ng/mL) as low and high values with six determinations for each stability test along with a calibration curve standards. The results are shown in Table 4.
Table 4.
Stability results of lenalidomide.
| S. No | Stability experiments | Stability duration and temperature | Nominal sample Conc. (ng/mL) (n = 6) LQC, HQC | Conc. Found (ng/mL) (mean ± S.D.) | % Mean change at QC level Acceptance Limit (85–115%) | %Stability |
|---|---|---|---|---|---|---|
| 1A | Auto sampler Stability-LQC | 24 h 42 m at 10 °C | 26.260 | 27.033 ± 0.448 | 102.94 | 114.39 |
| 1B | Auto sampler Stability-HQC | 24 h 42 m at 10 °C | 808.009 | 776.633 ± 20.196 | 96.12 | 101.65 |
| 2A | Freeze thaw cycles (FT)-LQC | After 5th FT cycle at (−70 °C) | 26.260 | 23.673 ± 0.251 | 90.15 | 100.17 |
| 2B | Freeze thaw cycles (FT)-HQC | After 5th FT cycle at (−70 °C) | 808.009 | 774.987 ± 6.634 | 95.91 | 101.43 |
| 3A | Bench top Stability-LQC | 10 h 27 m at 20 °C | 26.260 | 24.278 ± 1.543 | 92.45 | 102.73 |
| 3B | Bench top Stability-HQC | 10 h 27 m at 20 °C | 808.009 | 782.729 ± 48.290 | 96.87 | 102.45 |
| 4A | Dry extract Stability-LQC | 24 h 08 m at 20 °C | 26.260 | 24.728 ± 0.826 | 94.16 | 104.64 |
| 4B | Dry extract Stability-HQC | 24 h 08 m at 20 °C | 808.009 | 807.456 ± 38.688 | 99.93 | 105.68 |
| 5A | Wet extract Stability-LQC | 19h12m at 2–8 °C | 26.260 | 24.783 ± 0.792 | 94.37 | 104.87 |
| 5B | Wet extract Stability-HQC | 19h12m at 2–8 °C | 808.009 | 817.619 ± 22.802 | 101.19 | 107.01 |
| 6A | Intermediate stability-LQC | 03 days 02 h at −20 °C | 26.260 | 24.278 ± 1.543 | 92.45 | 102.73 |
| 6B | Intermediate stability-HQC | 03 days 02 h at −20 °C | 808.009 | 782.729 ± 48.290 | 96.87 | 102.45 |
For short term stability determination, stored plasma aliquots were thawed and kept at room temperature for a period of time exceeding that was expected to be encountered during the routine sample preparation (around 6 h). These results indicate reliable stability behavior under the experimental conditions of the regular analytical procedure.
The stability of Quality Control (QC) samples kept in the auto sampler for 24 h 42 min was assessed. The results indicate that solutions of Lenalidomide were found to be stable for 24 h 42 min in auto sampler with percentage stability 101.65–114.39%, percentage accuracy 96.12–102.94% respectively. Coefficient of variation was 1.66–2.60% respectively. Lenalidomide and Internal Standard Fluconazole can remain in the auto sampler for at least 24 h 42 min, without showing a significant loss in the quantified values, indicating that processed samples kept in auto sampler should be analysed within this Period.
The Analyte were found to be stable as dry extract for 24 h 08 min, found to be stable as wet extract 19 h 12 min and found to be stable as bench top for 10 h 27 min. The results revealed optimum stability in Plasma samples.
Lenalidomide were found to be stable in human plasma stored at −20 °C for 03 days 02 h and was stable over five freeze and thaw cycles at −70 °C, Results shown in Table 4.
The stability of stock solutions was tested and established at room temperature for 09 h 42 min and under refrigeration (2–8 °C) for 04 days 01 h 8 min. The results revealed optimum stability for the prepared stock solutions throughout the period intended for their daily use.
The Long term matrix stability (LTMS) of Quality Control (QC) samples were stored at −70 °C in the deep freezer for 65 days was assessed. The results indicate that Lenalidomide were found to be stable for 65 days in deep freezer at −70 °C with percentage stability 97.76–99.17%, Coefficient of variation was 3.40–3.41% respectively. These findings indicate that storage of Lenalidomide in plasma samples at below −70 °C is adequate and stable for at least 65 days without showing a significant loss in the quantified values, indicating that stored samples kept in deep freezer should be analysed within this Period and no stability related problems would be expected during routine analysis for pharmacokinetic, bioavailability or bioequivalence studies for this period.
4. Conclusion
The LC-MS/MS method described here is specific, accurate, precise and in accordance with FDA guidelines. Good sensitivity, RE, ME and DI were obtained for the measurement of the analyte. The stability indicating method with less sample volume can facilitate the bio study of LDM with additional time point’s inclusion. The analytical characteristics of this method make it suitable for implementation as a routine technique in the clinical laboratory for pharmacokinetic and bioequivalence studies in humans allowing the assessment of LDM activities in a single analytical run.
Declaration of interest
There authors confirm that there is no conflicts of interests and are also liable for the content writing of this article.
Acknowledgments
The authors are mostly thankful to the principal, Sri Venkateshwara College of pharmacy, chitoor, Andhrapradesh for providing necessary facilities to carry out the work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alzoman Nourah Z. A validated stability-indicating and stereoselective HPLC method for the determination of lenalidomide enantiomers in bulk form and capsules. J. Chrom. Sci. 2016;54(5):730–735. doi: 10.1093/chromsci/bmv247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhenne, Jurgen, 2012. Bioanalytical Method Validation. J. Anal. Bioanal. Tech. 3, 7.
- Drug Bank Online. <https://www.drugbank.ca/drugs/DB00480/> (accessed December 2005).
- Fulciniti Mariateresa, Amodio Nicola, Cea Michele, Maiso Patricia, Azab Abdel Kareem. Biological insights into myeloma and other B cell malignancies. Biomed. Res. Int. 2016 doi: 10.1155/2016/5218093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD Mortality and causes of death collaborators. Lancet. 2015;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD Disease and injury incidence and prevalence collaborators. Lancet. 2015;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidance for Industry Bioanalytical Method Validation, 2001.
- Guidance for Industry Bioanalytical Method Validation, 2013.
- Guideline on bioanalytical method validation. 2012. European Medicines Agency, 44. [DOI] [PubMed]
- Kastritis E., Dimopoulos M.A. The evolving role of lenalidomide in the treatment of hematologic malignancies. Exp. Opin. Pharmacother. 2007;8(4):497–509. doi: 10.1517/14656566.8.4.497. [DOI] [PubMed] [Google Scholar]
- Lakshmana Prabu S., Suriyaprakash T.N.K. Extraction of drug from the biological matrix: a review. Appl. Biol. Eng. – Prin. Pract. 2012;(March):479–506. [Google Scholar]
- List Alan, Dewald Gordon, Bennett John, Giagounidis Aristotle, Raza Azra, Feldman Eric. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N. Engl. J. Med. 2006;355(14):1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- List A., Kurtin S., Roe D.J., Buresh A., Mahadevan D., Fuchs D. Efficacy of lenalidomide in myelodysplastic syndromes. N. Engl. J. Med. 2005;352(6):549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- Maheswara Reddy L., Janardhan Reddy K., Bhaskar Reddy I., Raveendra reddy P. Development of a rapid and sensitive HPLC assay method for lenalidomide capsules and its related substances. E. J. Chem. 2012;9(3):1165–1174. [Google Scholar]
- Mahindra A., Laubach J., Raje N., Munshi N., Richardson P.G., Anderson K. Latest advances and current challenges in the treatment of multiple myeloma. Nat. Rev. Clin. Oncol. 2012;9(3):135–143. doi: 10.1038/nrclinonc.2012.15. [DOI] [PubMed] [Google Scholar]
- Mitsiades C.S., Mitsiades N. CC-5013 Celgene. Curr. Opin. Investig. Drugs. 2004;5:635–647. [PubMed] [Google Scholar]
- National Cancer Institute Online, <https://www.cancer.gov/types/myeloma/hp/myeloma-treatment-pdq/> (accessed. July 2016).
- Pulla Ravi Pratap, Sastry B.S., Rajendra Prasad Y., Appala Rajuy N. Estimation of lenalidomide in capsules dosage forms by RP-HPLC. J. Pharm. Res. 2011;4(4):1199–1200. [Google Scholar]
- Raab M.S., Podar K., Breitkreutz I., Richardson P.G., Anderson K.C. Multiple myeloma. Lancet. 2009;374(9686):324–339. doi: 10.1016/S0140-6736(09)60221-X. [DOI] [PubMed] [Google Scholar]
- Rajkumar S.V., Hayman S.R., Lacy M.Q., Dispenzieri A., Geyer S.M., Kabat B. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106(13):4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P.G., Blood E., Mitsiades C.S., Jagannath S., Zeldenrust S.R., Alsina M. A Randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108(10):3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEER Stat Fact Sheets Myeloma, National Cancer Institute Online. <https://seer.cancer.gov/statfacts/html/mulmy.html/> (accessed August 2016).
- Sonawane Lalit V., Poul Bhagwat N., Usnale Sharad V., Waghmare Pradeepkumar V., Surwase Laxman H. Bioanalytical method validation and its pharmaceutical application- a review. Pharm. Anal. Acta. 2014;5:3. [Google Scholar]
- Whitmire Monica, Ammerman Jennifer, de Lisio Patricia, Killmer Jacqueline, Kyle Devon, Mainstone Emily, Porter Lynann, Zhang Tianyi. LC-MS/MS bioanalysis method development, validation, and sample analysis: points to consider when conducting nonclinical and clinicalstudies in accordance with current regulatory guidances. J. Anal. Bioanal. Tech. 2011;4:1–10. [Google Scholar]