Abstract
5′ AMP-activated protein kinase (AMPK), insulin receptors and transporters are distorted in diabetes mellitus. In this study, the effect of Panax ginseng was assessed on glucose manipulating enzymes activities and gene expression of AMPK, IRA and GLUT2 in streptozotocin-induced diabetic male rats. Forty male albino rats were randomly divided to four groups 10 rats of each, group I, normal control group (received saline orally); group II, normal rats received 200 mg/kg of Panax ginseng orally; group III, Streptozotocin (STZ) –induced diabetic rats and group IV, STZ-induced diabetic rats received 200 mg/kg of Panax ginseng orally. The duration of experiment was 30 days. Results showed the ability of Panax ginseng to induce a significant decrease in the blood glucose and increase in the serum insulin levels, hepatic glucokinase (GK), and glycogen synthase (GS) activities with a modulation of lipid profile besides high expression levels of AMPK, insulin receptor A (IRA), glucose transporting protein-2 (GLUT-2) in liver of diabetic rats. In conclusion, the obtained results point to the ability of Panax ginseng to improve the glucose metabolism in diabetic models.
Keywords: AMPK, Diabetes mellitus, Ginseng, GLUT2, Insulin
1. Introduction
Many enzymes are concerned with energy homeostasis among them 5 AMP activated protein kinase (EC 2.7.11.31) that regulates the energy homeostasis inside the cell (Winder and Hardie, 1999). The enzyme has been confirmed to be expressed in liver (Huang et al., 2018), and muscles (Dial et al., 2018). Activation of the enzyme leads to activation of fatty acids oxidations in muscles and liver, ketogenesis, glucose uptake by the cells and inhibition of cholesterol synthesis, lipogenesis, triglycerides synthesis and modulation of insulin secretion by beta cells of pancreas (Winder and Hardie, 1999). Several studies which have been used agents for controlling of Diabetes Mellitus (DM) pointed AMPK as target (Jung et al., 2017, Liu et al., 2018a, Xiong et al., 2018). DM as a chronic metabolic disorder is attractive for many researchers. There is a daily continuous seeking for a modulator or preventer for DM, however the success of the modulator depends on its target inside the body and its relevance to the disorder (Sangeetha et al., 2017). Natural agents’ especially medicinal plants now constitute the major targets used for controlling of DM (Neamsuvan et al., 2015). Panax ginseng is a medicinal plant contributed for the controlling of many disorders (Ru et al., 2015), in the same line, its pharmacological action have been demonstrated in disorders such as cancer including; breast, lung, liver, colon and skin cancer (Majeed et al., 2018), cardiovascular diseases (Zheng et al., 2017), acute menopausal symptoms (Kargozar et al., 2017), acute pancreatitis (Liu et al., 2018b), it has been used for stimulating immune activity (Kang and Min, 2012, Yu et al., 2018), as a neuro-protective agent (Luo et al., 2018), and for its antioxidant activities (Shergis et al., 2014), anti stress (Wang et al., 2018) and anti-aging (Bjorklund et al., 2018) activities. Ginseng now is one of the most famous natural agents used in controlling the metabolic syndromes such as DM and its complications (Deng et al., 2017, Kim et al., 2017, Wang et al., 2017, Xu et al., 2017). Recently, it has been demonstrated that ginseng can improves the glucose intake, attenuates insulin resistance and reduce fat mass in high fat diet-obesity mice (Dai et al., 2018). The matter which gives us the impetus to study the effectiveness of Panax ginseng on enzymes and proteins related to DM. We considered AMPK as a target in the present study. Its mRNA expression levels were measured in the all experimental animals, beside the expression levels of glucose transporter-2 (GLUT-2), Insulin receptor A (IRA) and activities of Glucokinase (GK), Glycogen synthase (GS) in livers of experimental groups. Blood glucose, insulin, total Cholesterol (TC), triacyl-glycerol (TAG), HDL-cholesterol (HDL-c), LDL-cholesterol levels were also determined.
2. Material and methods
2.1. Animals selection and grouping
Forty male albino rats 6 month of age and weighing 120 ± 20 g were obtained from the animal house of Zagazig University, Egypt. The animal were housed in individual suspended stainless steel cages at 22 ± 2 °C with a 12 h light/dark cycle and allowed to acclimatize for period of 7 days before beginning the experiment. Rats were divided randomly into 4 groups (n = 10) and were allowed free access to food and water. Rats were divided randomly into 4 groups. (n = 10) in each. Group I served as non-treated control group, group II received daily dose of ginseng 200 mg/kg Bwt for constitutive 30 days, group III STZ-induced diabetic rats did not receive any type of treatment, group IV STZ-induced diabetic rats received 200 mg/kg Bwt of ginseng for constitutive 30 days.
2.2. Chemicals
Streptozotocin (STZ) (Sigma-Aldrich Co. St. Louis, Missouri, USA). Root powder of Korean ginseng (Panax ginseng C.A. Meyer).
2.3. Ethical statement
All procedures of the current experiment have been approved by the Ethical Committee of the Faculty of Vet. Med. Zagazig University Egypt.
2.4. Induction of experimental diabetes mellitus
Diabetes mellitus was induced by a single intra-peritoneal injection of 100 mg/kg B.wt. of a freshly prepared Streptozotocin powder “STZ” (Sigma-Aldrich Co. St. Louis, Missouri, USA) dissolved in 0.01 M cold sodium citrate buffer (pH 4.5) immediately before use. The rats with STZ were given 5% W/V glucose solution next 24 h to prevent the hypoglycemia. After 72 h rats with fasting blood glucose more than 250 mg/dL had been selected as diabetic rats (Alkaladi et al., 2014).
2.5. Sampling protocol
After 12 h fasting, the blood samples were collected from median canthus of eye and the sera were separated by centrifugation and stored at −20 °C for biochemical determinations. Liver tissues were collected and divided in to two parts. The first part was used to prepare a tissue homogenate for biochemical measurements of enzymes activities and the other part collected on liquid nitrogen, preserved at −80 °C until the extraction of RNA and was used for gene expression investigation.
2.6. Biochemical determinations
The blood glucose concentrations were determined using glucose oxidase method and the kits provided by SPINREACT (Sant Esteva de Bas, Girona, Spain). The insulin levels in serum were estimated using IMMULITE Insulin kits (Catalog Number: LKIN1, Avenue, Silver Spring, MD 20993) following manufacture instructions (Chevenne et al., 1998). Serum total cholesterol (Richmond, 1973), triacyl-glycerols (Fossati and Prencipe, 1982), HDL - cholesterol (Lopes-Virella et al., 1977), and LDL-cholesterol (Glatter, 1984) were determined in the sera of all experimental animals. The activities of hepatic GK (Pakoskey et al., 1965) and GS (Brady, 2003) were determined in the liver tissue homogenates of all experimental rats.
2.7. Molecular biological determinations
2.7.1. RNA extraction and cDNA synthesis
Total RNA was extracted from liver tissue using RNeasy Mini Kit (Qiagen, Cat. No. 74104) and following the manufacturer instructions. The amount of extracted RNA was quantified and qualified by NanoDrop® ND-1000 Spectrophotometer, NanoDrop Technologies, Wilmington, Delaware USA. The first strand cDNA was synthesized by using RevertAidTM H Minus (Fermentas, life science, Pittsburgh, PA, USA).
2.7.2. Primers for experimental genes
The primers pairs were designed according to the previously published data as following AMPKα1 (ID: 65248); forward, 5′-ATCCGCAGAGAGATCCAGAA-3′ and reverse 5′-CGTCGACTCTCCTTTTCGTC-3′ (McCrimmon et al., 2006), IRA (ID: 24954); forward, 5′-TTCATTCAGGAAGACCTTCGA-3′ and reverse, 5′-AGGCCAGAGATGACA AGTGAC-3′, GLUT-2 (ID: 25351); forward, 5′-TTAGCAACTGGGTCTGCAAT-3′, and reverse 5′-TCTCTGAAGACGCCAGGA AT-3′ (Alkaladi et al., 2014), and ß-actin (ID: 81822); forward, 5′- AGCCATGTACGTAGCCAT -3′ and reverse 5- CTCTCAGCTGTGGTGGTGAA -3′ (Batalha et al., 2016).
2.7.3. Real time PCR
One μL of cDNA was mixed with 12.5 μL of 2x SYBR® Green PCR mix from BioRad, 5.5 μL of autoclaved water, and 0.5 μL (10 pmol/μL) of each forward and reverse primer for the measured genes. The house keeping gene β-actin was used as a control for normalization. Fold change was calculated using the (2−ΔΔct) method to quantitate mRNA levels, according to Litvak and Schmittgen (2001).
2.8. Statistical analysis
The obtained data was analyzed by using the statistical package for social science (SPSS, 18.0 software, 2011). Differences among groups was evaluated using one way ANOVA. Results were expressed as mean ± SE. P values less than 0.05 were considered to be significant.
3. Results
3.1. Fasting blood glucose (mg/dL) and serum insulin (µIU/mL) levels.
There were a significant decrease in the blood glucose levels in diabetic rats received ginseng as compared to diabetic non-treated rats. The insulin levels were significantly increased in diabetic rats received ginseng also if compared with diabetic non-treated groups at P < 0.05 (Table 1).
Table 1.
Fasting blood glucose and serum insulin levels in experimental rats.
| Groups | Glucose (mg/dL) | Insulin (µIU/mL) |
|---|---|---|
| Control | 80.5 ± 14.3c | 13.8 ± 0.51c |
| Normal + ginseng | 78.3 ± 24c | 13.2 ± 0.38c |
| Diabetic | 298 ± 28.17a | 7.9 ± 0.11a |
| Diabetic + ginseng | 167.9 ± 3.4b | 11.2 ± 0.23b |
Means carrying different superscripts are significant at P < 0.05.
3.2. Glucokinase (U/gm tissue) and glycogen synthase (mU/mg protein) activities in liver tissues.
The activities of glucokinase and glycogen synthase were significantly increased in diabetic rats received ginseng if compared with diabetic non-treated rats at p < 0.05 (Table 2).
Table 2.
Liver glucokinase and glycogen synthase activities in experimental rats.
| Groups | Glucokinase (U/g liver tissue) | Glycogen synthase (mU/mg protein) |
|---|---|---|
| Control | 1.21 ± 0.02a | 3.7 ± 0.08a |
| Normal + ginseng | 1.43 ± 24a | 3.5 ± 0.38a |
| Diabetic | 0.16 ± 0.008b | 1.98 ± 0.1c |
| Diabetic + ginseng | 1.03 ± 0.05a | 2.7 ± 0.22b |
Means carrying different superscripts are significant at P < 0.05.
3.3. Serum lipid profiles (mg/dL).
There were a significant decrease in the concentrations of total cholesterol, tri-acylglycerols and LDL-cholesterol (mg/dL) in diabetic rats received ginseng if compared with the diabetic non-treated rats. The level of HDL-cholesterol was significantly decreased in diabetic rats received ginseng when compared with diabetic non-treated rats at P < 0.05 (Table 3).
Table 3.
Serum lipids profile levels in experimental rats.
| Groups | Total cholesterol (mg/dL) | Triglycerides (mg/dL) | HDL-cholesterol (mg/dL) | LDL- cholesterol (mg/dL) |
|---|---|---|---|---|
| Control | 87.2 ± 1.35c | 83.4 ± 2.51b | 46.18 ± 2.93b | 23.34 ± 1.59c |
| Normal + ginseng | 81.9 ± 1.51c | 82.2 ± 3.25b | 48.87 ± 1.52b | 15.9 ± 1.44d |
| Diabetic | 171.8 ± 2.1a | 119 ± 0.11a | 38.3 ± 2.02a | 99.5 ± 2.92a |
| Diabetic + ginseng | 111.3 ± 2.83b | 85 ± 1.92b | 48.1 ± 1.56b | 45.2 ± 2.17b |
Means carrying different superscripts are significant at P < 0.05.
3.4. mRNA expression levels of AMPK, IRA, and GLUT2.
The mRNA expression levels of AMPK, IRA and GLUT2 proteins was increased in the diabetic rats received ginseng if compared with diabetic non-treated rats at P < 0.05 (Table 4).
Table 4.
mRNA expression levels of examined proteins in experimental rats.
| Groups | AMPK | IRA | GLUT2 |
|---|---|---|---|
| Control | 1.05 ± 0.20a | 1.07 ± 0.21a | 1.11 ± 0.28a |
| Normal + ginseng | 1.02 ± 0.28a | 1.03 ± 14a | 1.05 ± 0.18a |
| Diabetic | 0.45 ± 0.26b | 0.36 ± 0.08b | 0.48 ± 0.1b |
| Diabetic + ginseng | 0.96 ± 0.11a | 0.87 ± 0.05a | 0.91 ± 0.22a |
Means carrying different superscripts are significant at P < 0.05.
4. Discussion
The present work studied the molecular and biochemical effects of Panax ginseng on some enzymes and proteins related to glucose metabolism in diabetic rats. The effect on AMPK mRNA expression levels was estimated. The mRNA of AMPK showed the lowest expression level among diabetic rats (0.45 ± 0.26), while its expression level was modulated and increased in all rats received ginseng, normal (1.02 ± 0.28) and diabetic (0.96 ± 0.11) respectively. The results explained the great association between AMPK action and the incidence of diabetes mellitus the matter which making AMPK a popular target when study DM. recently it has been demonstrated that activation of AMPK could promote the glucose uptake by the cells (Na et al., 2018) and the ability of ginseng to induce an up-regulation of AMPK expression in diabetic rats lead to decline the blood glucose in experimental rats received ginseng 1.02 and 1.78 times in normal and diabetic rats respectively. The same observations have been obtained in human by Choi et al. 2018, ginseng has induced lowering in blood glucose of diabetic patients specially those of fasting blood glucose (FBG) of 110 mg/dL or more (Choi et al., 2018). We also reported the effect of ginseng on serum insulin levels. Insulin level was increased by 1.42 times in diabetic rats received ginseng when compared with non treated rats. In the same line, other studies reported the hypoglycemic effect of ginseng in diabetic/obese models (Kim et al., 2017, Lee et al., 2017b, Sun et al., 2018), it has been reported to induce an increase in serum insulin levels (Wang et al., 2017), and improved the insulin tolerance (Deng et al., 2017) in diabetic mice. There is a great prove now that the activation of AMPK can lead to increasing insulin secretion and improve its tolerance (Jung et al., 2018, Wu et al., 2018, Zhao et al., 2018). We can indicate the effect of ginseng to reduce serum blood glucose and increase serum insulin levels to its ability to induce up-regulation of AMPK expression in diabetic rats. Coming with this up-regulation there was a significant up-regulation of IRA and GLUT2 (Fig. 1). Ginseng significantly increased the expression of IRA and GLUT2 0.87 and 0.91 fold respectively in diabetic rats received ginseng when compared to diabetic non-treated rats 0.36 and 0.48 fold respectively. In a similar study the effect of ginseng on insulin receptor has been reported to induce high expression in insulin receptor B (IRB) and insulin receptor substrate-1 (IRS) (Dai et al., 2018). Another study reported the ability of ginseng to induce up-regulation of insulin receptors in muscles of metabolic syndrome rats (Kho et al., 2016). Our data regarding mRNA expression of IRs come in accordance with the study of (Cheon et al., 2015) which reported the increase IR mRNA expression in old-aged ob/ob mice received Panax ginseng for 16 weeks. In this direction, our data proved the ability of ginseng to improve insulin sensitivity in diabetic rats. There were many studies that have demonstrated the effect of ginseng on GLUTs, it have been reported that ginseng induced translocation of GLUT4 (Tabandeh et al., 2015, Ota and Ulrih, 2017), up-regulation of GLUT4 in muscles of diabetic rats (Lai et al., 2006, Kang et al., 2017), high expression levels of GLUT1 and GLUT4 in myotubes of diabetic rats (Seo et al., 2016), increase the expression of GLUT1 and GLUT4 in livers of diabetic mice (Jung and Kang, 2013, Xie et al., 2015), , and up-regulation of GLUT4 in adipose tissue of diabetic rats (Kim and Kim, 2012). Regarding GLUT2 mRNA expression levels our data come in the same line of (Kang et al., 2017) which demonstrated the high expression levels of hepatic GLUT2 hand by hand with high phosphorylation of AMPK in diabetic mice received ginseng for 4 weeks. Similar data have been come with the same line of our observations regarding high expression levels of GLUT2 in different cell lines/tissues of diabetic models received ginseng (Ohnishi et al., 1996, Gu et al., 2013, Liu et al., 2013). In general, the ability of ginseng to increase the expression levels of GLUTs and especially GLUT2 explained its ability to increase the uptake of glucose by insulin targeted-cells especially liver cells. The matter which translated to lower blood glucose in diabetic models received ginseng. Also we showed that Panax ginseng induced a significant increase in activities of hepatic GK (6.4 times) and GS (1.36 times) of diabetic rats received ginseng for 30 days if compared with non-treated rats. Their activities also non significantly increased in non-diabetic rats received ginseng when compared with control. It well-known that hepatic GK activity and expression are decreased in DM (Song et al., 2017), in the same way, we observed the lowest activity of GK in diabetic non-treated rats. It was also known that glycogen storage is impaired in livers of diabetic models due to impairment of GS (Ros et al., 2011) the matter which leads to hyperglycemia. Many targets which have been used to lower blood glucose in diabetic models targeted the activation of the two enzymes. The activation of AMPK has lead to an increase in GK and GS activities (Chen et al., 2018)). In our study, ginseng activated both GK and GS in diabetic rats. Ginseng has to increase GK expression in adipocytes (Lee et al., 2017a) and induced the expression of GK in pancreatic cells (Park et al., 2008), in the same line, ginseng have to increase hepatic or muscles glycogen deposition (Xie et al., 2015, Xu and Dou, 2016) through inhibition of Glycogen synthase kinase (GSK) in hepatocytes (Kho et al., 2016, Zhang et al., 2016, Shi et al., 2018). Others conclude that ginseng have to suppress the expression of GS (Seo et al., 2016). Although, there were a lot of data about the molecular effect of ginseng on GK and GS expressions, to the best of our knowledge, we are the first record for the assessment of direct effect of Panax ginseng on the enzymatic activities of hepatic GK and GS. On the contrary of our data, authors reported the inhibitory effect of Panax ginseng on ovarian Hexokinase (Li et al., 2015), while the study of (Chung et al., 2001) on hepatic Hexokinase agreed with us. The ability of ginseng to activate both GK and GS activities indicates how it reduces blood glucose in diabetic rats. It does so through activating both glucose oxidation (glycolysis) and increase glycogen deposition (glycogenesis) through AMPK activation pathway (Fig. 2). Also lipids profiles tended to be reduced in diabetic rats received ginseng. Usually DM is characterized by an impairment of lipids metabolism (Cantuaria et al., 2018). The progress of DM without glycemic control by the way will induce distorted blood lipids profile (dyslipidemia) (Levitt Katz et al., 2018). We tested the ability of Panax ginseng to correct this impairment. In the present study, ginseng increased the levels of HDL-c and decreased TC, TGs, and LDL-c. Our results come in accordance with the results obtained from studying the effect of different types of ginseng on dyslipidemic models. It has been reported that ginseng can reduce blood TGs (Lee et al., 2017b), plasma TGs and cholesterol, LDL-c (Cheon et al., 2015, Gui et al., 2016, Kim et al., 2016, Lee et al., 2016, Chen et al., 2017, Kang et al., 2017, Shin and Yoon, 2018). Although, others found that there was no significant change in HDL-c levels between treated and non-treated models (Gui et al., 2016), we reported an increase in serum HDL-c in diabetic rats received ginseng. It has been reported that activation of AMPK will targeted for all lipid metabolism processes leading to oxidation (Sharma et al., 2018) and so will reduce blood lipids. We relate the activity of ginseng to lower high lipid levels in diabetic rats to its ability to activate AMPK. The overall observation indicated that Panax ginseng can modulates the dyslipidemias originated from DM.
Fig. 1.
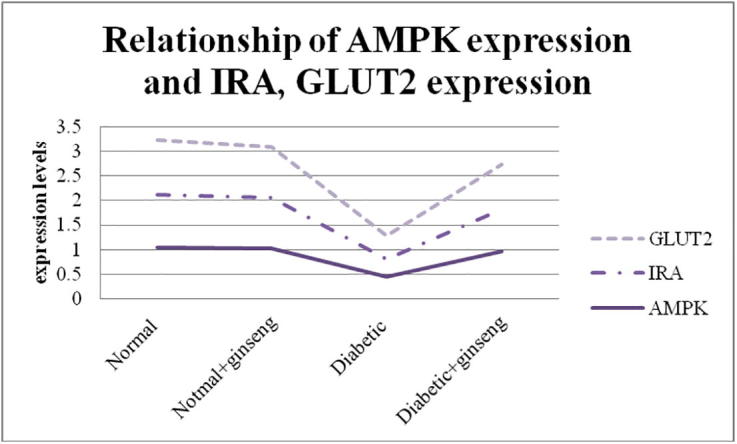
Relationship between AMPK expression and IRA and GLUT2 expression in hepatocytes.
Fig. 2.
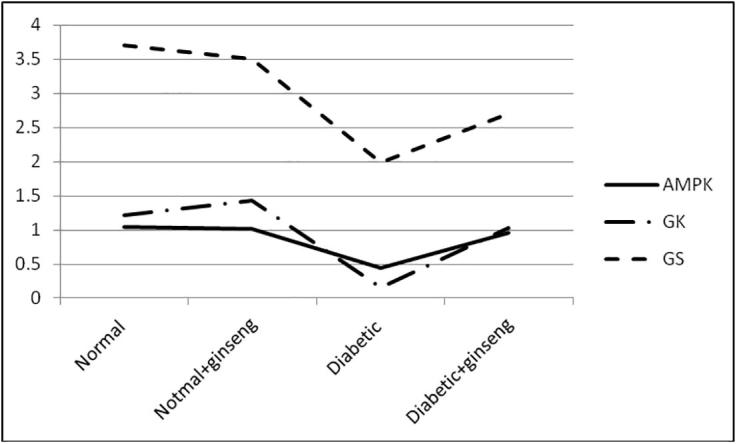
Correlation between AMPK expression and GK and GS activities in diabetic rats.
5. Conclusion
In conclusion, the study pointed to the ability of Panax ginseng to reduce blood glucose in diabetic rats through activation of AMPK pathways, increase insulin secretion and tolerance, increase glucose uptake and oxidation, and increase glycogen deposition in liver cells.
6. Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alkaladi A., Abdelazim A.M., Afifi M. Antidiabetic activity of zinc oxide and silver nanoparticles on streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2014;15:2015–2023. doi: 10.3390/ijms15022015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalha V.L., Ferreira D.G., Coelho J.E., Valadas J.S., Gomes R., Temido-Ferreira M., Shmidt T., Baqi Y., Buee L., Muller C.E., Hamdane M., Outeiro T.F., Bader M., Meijsing S.H., Sadri-Vakili G., Blum D., Lopes L.V. The caffeine-binding adenosine A2A receptor induces age-like HPA-axis dysfunction by targeting glucocorticoid receptor function. Sci. Rep. 2016;6:31493. doi: 10.1038/srep31493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund G., Dadar M., Martins N., Chirumbolo S., Goh B.H., Smetanina K., Lysiuk R. Brief challenges on medicinal plants: an eye-opening look at ageing-related disorders. Basic Clin. Pharmacol. Toxicol. 2018 doi: 10.1111/bcpt.12972. [DOI] [PubMed] [Google Scholar]
- Brady M.J. Measurement of glycogen synthesis and glycogen synthase activity in 3T3-L1 adipocytes. Methods Mol. Med. 2003;83:155–161. doi: 10.1385/1-59259-377-1:155. [DOI] [PubMed] [Google Scholar]
- Cantuaria A.P.C., Figueiredo T.M., Freire M.S., Lima S.M.F., Almeida J.A., Franco O.L., Rezende T.M.B. The effects of glucose concentrations associated with lipopolysaccharide and interferon-gamma stimulus on mediators' production of RAW 264.7 cells. Cytokine. 2018;17:S1043–S4666. doi: 10.1016/j.cyto.2017.11.008. [DOI] [PubMed] [Google Scholar]
- Chen F.C., Shen K.P., Chen J.B., Lin H.L., Hao C.L., Yen H.W., Shaw S.Y. PGBR extract ameliorates TNF-alpha induced insulin resistance in hepatocytes. Kaohsiung J. Med. Sci. 2018;34:14–21. doi: 10.1016/j.kjms.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Chen G., Li H., Zhao Y., Zhu H., Cai E., Gao Y., Liu S., Yang H., Zhang L. Saponins from stems and leaves of Panax ginseng prevent obesity via regulating thermogenesis, lipogenesis and lipolysis in high-fat diet-induced obese C57BL/6 mice. Food Chem. Toxicol. 2017;106:393–403. doi: 10.1016/j.fct.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Cheon J.M., Kim D.I., Kim K.S. Insulin sensitivity improvement of fermented Korean Red Ginseng (Panax ginseng) mediated by insulin resistance hallmarks in old-aged ob/ob mice. J. Ginseng. Res. 2015;39:331–337. doi: 10.1016/j.jgr.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevenne D., Letailleur A., Trivin F., Porquet D. Effect of hemolysis on the concentration of insulin in serum determined by RIA and IRMA. Clin. Chem. 1998;44:354–356. [PubMed] [Google Scholar]
- Choi H.S., Kim S., Kim M.J., Kim M.S., Kim J., Park C.W., Seo D., Shin S.S., Oh S.W. Efficacy and safety of Panax ginseng berry extract on glycemic control: A 12-wk randomized, double-blind, and placebo-controlled clinical trial. J. Ginseng. Res. 2018;42:90–97. doi: 10.1016/j.jgr.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S.H., Choi C.G., Park S.H. Comparisons between white ginseng radix and rootlet for antidiabetic activity and mechanism in KKAy mice. Arch. Pharm. Res. 2001;24:214–218. doi: 10.1007/BF02978260. [DOI] [PubMed] [Google Scholar]
- Dai S., Hong Y., Xu J., Lin Y., Si Q., Gu X. Ginsenoside Rb2 promotes glucose metabolism and attenuates fat accumulation via AKT-dependent mechanisms. Biomed. Pharmacother. 2018;100:93–100. doi: 10.1016/j.biopha.2018.01.111. [DOI] [PubMed] [Google Scholar]
- Deng J., Liu Y., Duan Z., Zhu C., Hui J., Mi Y., Ma P., Ma X., Fan D., Yang H. Protopanaxadiol and protopanaxatriol-type saponins ameliorate glucose and lipid metabolism in type 2 diabetes mellitus in high-fat diet/streptozocin-induced mice. Front. Pharmacol. 2017;8:506. doi: 10.3389/fphar.2017.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dial A.G., Rooprai P., Lally J.S., Bujak A.L., Steinberg G.R., Ljubicic V. The role of AMP-activated protein kinase in the expression of the dystrophin-associated protein complex in skeletal muscle. FASEB J. 2018 doi: 10.1096/fj.201700868RRR. [DOI] [PubMed] [Google Scholar]
- Fossati P., Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem. 1982;28:2077–2080. [PubMed] [Google Scholar]
- Glatter T.R. Hyperlipidemia. What is 'normal', who should be treated and how. Postgrad. Med. 1984;76(49–55):58–149. doi: 10.1080/00325481.1984.11698776. [DOI] [PubMed] [Google Scholar]
- Gu J., Li W., Xiao D., Wei S., Cui W., Chen W., Hu Y., Bi X., Kim Y., Li J., Du H., Zhang M., Chen L. Compound K, a final intestinal metabolite of ginsenosides, enhances insulin secretion in MIN6 pancreatic beta-cells by upregulation of GLUT2. Fitoterapia. 2013;87:84–88. doi: 10.1016/j.fitote.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Gui Q.F., Xu Z.R., Xu K.Y., Yang Y.M. The efficacy of ginseng-related therapies in type 2 diabetes mellitus: an updated systematic review and meta-analysis. Med. (Baltimore) 2016;95:e2584. doi: 10.1097/MD.0000000000002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhang D., Lin L., Jiang R., Dai J., Tang L., Yang Y., Ge P., Wang B., Zhang L. Potential roles of AMP-activated protein kinase in liver regeneration in mice with acute liver injury. Mol. Med. Rep. 2018 doi: 10.3892/mmr.2018.8522. [DOI] [PubMed] [Google Scholar]
- Jung D.Y., Kim J.H., Lee H., Jung M.H. Antidiabetic effect of gomisin N via activation of AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2017;494:587–593. doi: 10.1016/j.bbrc.2017.10.120. [DOI] [PubMed] [Google Scholar]
- Jung H.L., Kang H.Y. Effects of Korean red ginseng supplementation on muscle glucose uptake in high-fat fed rats. Chin. J. Nat. Med. 2013;11:494–499. doi: 10.1016/S1875-5364(13)60090-4. [DOI] [PubMed] [Google Scholar]
- Jung T.W., Kim H.C., Abd El-Aty A.M., Jeong J.H. Maresin 1 attenuates NAFLD by suppression of endoplasmic reticulum stress via AMPK-SERCA2b pathway. J. Biol. Chem. 2018 doi: 10.1074/jbc.RA117.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang O.H., Shon M.Y., Kong R., Seo Y.S., Zhou T., Kim D.Y., Kim Y.S., Kwon D.Y. Anti-diabetic effect of black ginseng extract by augmentation of AMPK protein activity and upregulation of GLUT2 and GLUT4 expression in db/db mice. BMC Complement Altern. Med. 2017;17:341. doi: 10.1186/s12906-017-1839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., Min H. Ginseng, the 'Immunity Boost': The effects of Panax ginseng on immune system. J. Ginseng. Res. 2012;36:354–368. doi: 10.5142/jgr.2012.36.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargozar R., Azizi H., Salari R. A review of effective herbal medicines in controlling menopausal symptoms. Electron. Physician. 2017;9:5826–5833. doi: 10.19082/5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho M.C., Lee Y.J., Park J.H., Kim H.Y., Yoon J.J., Ahn Y.M., Tan R., Park M.C., Cha J.D., Choi K.M., Kang D.G., Lee H.S. Fermented red ginseng potentiates improvement of metabolic dysfunction in metabolic syndrome rat models. Nutrients. 2016;8(6):369. doi: 10.3390/nu8060369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.S., Jo K., Kim J.S., Pyo M.K., Kim J. GS-E3D, a new pectin lyase-modified red ginseng extract, inhibited diabetes-related renal dysfunction in streptozotocin-induced diabetic rats. BMC Complement Altern. Med. 2017;17:430. doi: 10.1186/s12906-017-1925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.Y., Kim K. Regulation of signaling molecules associated with insulin action, insulin secretion and pancreatic beta-cell mass in the hypoglycemic effects of Korean red ginseng in Goto-Kakizaki rats. J. Ethnopharmacol. 2012;142:53–58. doi: 10.1016/j.jep.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Kim M.H., Lee E.J., Cheon J.M., Nam K.J., Oh T.H., Kim K.S. Antioxidant and hepatoprotective effects of fermented red ginseng against high fat diet-induced hyperlipidemia in rats. Lab. Anim. Res. 2016;32:217–223. doi: 10.5625/lar.2016.32.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai D.M., Tu Y.K., Liu I.M., Chen P.F., Cheng J.T. Mediation of beta-endorphin by ginsenoside Rh2 to lower plasma glucose in streptozotocin-induced diabetic rats. Planta Med. 2006;72:9–13. doi: 10.1055/s-2005-916177. [DOI] [PubMed] [Google Scholar]
- Lee H., Kim J., Park J.Y., Kang K.S., Park J.H., Hwang G.S. Processed Panax ginseng, sun ginseng, inhibits the differentiation and proliferation of 3T3-L1 preadipocytes and fat accumulation in Caenorhabditis elegans. J. Ginseng. Res. 2017;41:257–267. doi: 10.1016/j.jgr.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.B., Yoon S.J., Lee S.H., Lee M.S., Jung H., Kim T.D., Yoon S.R., Choi I., Kim I.S., Chung S.W., Lee H.G., Min J.K., Park Y.J. Ginsenoside Rg3 ameliorated HFD-induced hepatic steatosis through downregulation of STAT5-PPARgamma. J. Endocrinol. 2017;235:223–235. doi: 10.1530/JOE-17-0233. [DOI] [PubMed] [Google Scholar]
- Lee S.B., Cho H.I., Jin Y.W., Lee E.K., Ahn J.Y., Lee S.M. Wild ginseng cambial meristematic cells ameliorate hepatic steatosis and mitochondrial dysfunction in high-fat diet-fed mice. J. Pharm. Pharmacol. 2016;68:119–127. doi: 10.1111/jphp.12487. [DOI] [PubMed] [Google Scholar]
- Levitt Katz L.E., Bacha F., Gidding S.S., Weinstock R.S., El Ghormli L., Libman I., Nadeau K.J., Porter K., Marcovina S., Group T.S. Lipid profiles, inflammatory markers, and insulin therapy in youth with type 2 diabetes. J. Pediatr. 2018 doi: 10.1016/j.jpeds.2017.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Liu T., Zhao L., Chen W., Hou H., Ye Z., Li X. Ginsenoside 20(S)Rg3 inhibits the Warburg effect through STAT3 pathways in ovarian cancer cells. Int. J. Oncol. 2015;46:775–781. doi: 10.3892/ijo.2014.2767. [DOI] [PubMed] [Google Scholar]
- Liu J., Guo W.F., Ren L., Chen W.W. Effects of ginsenoside Re on glucose absorption in Caco-2 cells. Zhong Yao Cai. 2013;36:1992–1995. [PubMed] [Google Scholar]
- Liu L., Yasen M., Tang D., Ye J., Aisa H.A., Xin X. Polyphenol-enriched extract of Rosa rugosa Thunb regulates lipid metabolism in diabetic rats by activation of AMPK pathway. Biomed. Pharmacother. 2018;100:29–35. doi: 10.1016/j.biopha.2018.01.143. [DOI] [PubMed] [Google Scholar]
- Liu M.W., Wei R., Su M.X., Li H., Fang T.W., Zhang W. Effects of Panax notoginseng saponins on severe acute pancreatitis through the regulation of mTOR/Akt and caspase-3 signaling pathway by upregulating miR-181b expression in rats. BMC Complement Altern. Med. 2018;18:51. doi: 10.1186/s12906-018-2118-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopes-Virella M.F., Stone P.G., Colwell J.A. Serum high density lipoprotein in diabetic patients. Diabetologia. 1977;13:285–291. doi: 10.1007/BF01223267. [DOI] [PubMed] [Google Scholar]
- Luo H., Hu J., Wang Y., Chen Y., Zhu D., Jiang R., Qiu Z. In vivo and in vitro neuroprotective effects of Panax ginseng glycoproteins. Int. J. Biol. Macromol. pii. 2018;S0141–8130(17):33352–33354. doi: 10.1016/j.ijbiomac.2018.02.015. [DOI] [PubMed] [Google Scholar]
- Majeed F., Malik F.Z., Ahmed Z., Afreen A., Afzal M.N., Khalid N. Ginseng phytochemicals as therapeutics in oncology: Recent perspectives. Biomed. Pharmacother. 2018;100:52–63. doi: 10.1016/j.biopha.2018.01.155. [DOI] [PubMed] [Google Scholar]
- McCrimmon R.J., Fan X., Cheng H., McNay E., Chan O., Shaw M., Ding Y., Zhu W., Sherwin R.S. Activation of AMP-activated protein kinase within the ventromedial hypothalamus amplifies counterregulatory hormone responses in rats with defective counterregulation. Diabetes. 2006;55:1755–1760. doi: 10.2337/db05-1359. [DOI] [PubMed] [Google Scholar]
- Na R.S., Ma C., Liu Q.R., Wu L.M., Zheng X.L., Liu Z.W. Itraconazole attenuates hepatic gluconeogenesis and promotes glucose uptake by regulating AMPK pathway. Exp. Ther. Med. 2018;15(2):2165–2171. doi: 10.3892/etm.2017.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neamsuvan O., Madeebing N., Mah L., Lateh W. A survey of medicinal plants for diabetes treating from Chana and Nathawee district, Songkhla province Thailand. J. Ethnopharmacol. 2015;174:82–90. doi: 10.1016/j.jep.2015.07.050. [DOI] [PubMed] [Google Scholar]
- Ohnishi Y., Takagi S., Miura T., Usami M., Kako M., Ishihara E., Yano H., Tanigawa K., Seino Y. Effect of ginseng radix on GLUT2 protein content in mouse liver in normal and epinephrine-induced hyperglycemic mice. Biol. Pharm. Bull. 1996;19:1238–1240. doi: 10.1248/bpb.19.1238. [DOI] [PubMed] [Google Scholar]
- Ota A., Ulrih N.P. An overview of herbal products and secondary metabolites used for management of type two diabetes. Front. Pharmacol. 2017;8:436. doi: 10.3389/fphar.2017.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakoskey A.M., Lesher E.C., Scott D.B. Hexokinase of Escherichia coli. Assay of enzyme activity and adaptation to growth in various media. J. Gen. Microbiol. 1965;38:73–80. doi: 10.1099/00221287-38-1-73. [DOI] [PubMed] [Google Scholar]
- Park S.M., Hong S.M., Sung S.R., Lee J.E., Kwon D.Y. Extracts of Rehmanniae radix, Ginseng radix and Scutellariae radix improve glucose-stimulated insulin secretion and beta-cell proliferation through IRS2 induction. Genes Nutr. 2008;2:347–351. doi: 10.1007/s12263-007-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond W. Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin. Chem. 1973;19:1350–1356. [PubMed] [Google Scholar]
- Ros S., Garcia-Rocha M., Calbo J., Guinovart J.J. Restoration of hepatic glycogen deposition reduces hyperglycaemia, hyperphagia and gluconeogenic enzymes in a streptozotocin-induced model of diabetes in rats. Diabetologia. 2011;54:2639–2648. doi: 10.1007/s00125-011-2238-x. [DOI] [PubMed] [Google Scholar]
- Ru W., Wang D., Xu Y., He X., Sun Y.E., Qian L., Zhou X., Qin Y. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.) Drug Discov. Ther. 2015;9:23–32. doi: 10.5582/ddt.2015.01004. [DOI] [PubMed] [Google Scholar]
- Sangeetha K.N., Sujatha S., Muthusamy V.S., Anand S., Shilpa K., Kumari P.J., Sarathkumar B., Thiyagarajan G., Lakshmi B.S. Current trends in small molecule discovery targeting key cellular signaling events towards the combined management of diabetes and obesity. Bioinformation. 2017;13:394–399. doi: 10.6026/97320630013394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y.S., Shon M.Y., Kong R., Kang O.H., Zhou T., Kim D.Y., Kwon D.Y. Black ginseng extract exerts anti-hyperglycemic effect via modulation of glucose metabolism in liver and muscle. J. Ethnopharmacol. 2016;190:231–240. doi: 10.1016/j.jep.2016.05.060. [DOI] [PubMed] [Google Scholar]
- Sharma A.X., Quittner-Strom E.B., Lee Y., Johnson J.A., Martin S.A., Yu X., Li J., Lu J., Cai Z., Chen S., Wang M.Y., Zhang Y., Pearson M.J., Dorn A.C., McDonald J.G., Gordillo R., Yan H., Thai D., Wang Z.V., Unger R.H., Holland W.L. Glucagon receptor antagonism improves glucose metabolism and cardiac function by promoting AMP-mediated protein kinase in diabetic mice. Cell Rep. 2018;22:1760–1773. doi: 10.1016/j.celrep.2018.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergis J.L., Di Y.M., Zhang A.L., Vlahos R., Helliwell R., Ye J.M., Xue C.C. Therapeutic potential of Panax ginseng and ginsenosides in the treatment of chronic obstructive pulmonary disease. Complement Ther. Med. 2014;22:944–953. doi: 10.1016/j.ctim.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Shi X., Yang J., Wei G. Ginsenoside 20(S)-Rh2 exerts anti-cancer activity through the Akt/GSK3beta signaling pathway in human cervical cancer cells. Mol. Med. Rep. 2018;17:4811–4816. doi: 10.3892/mmr.2018.8454. [DOI] [PubMed] [Google Scholar]
- Shin S.S., Yoon M. Korean red ginseng (Panax ginseng) inhibits obesity and improves lipid metabolism in high fat diet-fed castrated mice. J Ethnopharmacol. 2018;210:80–87. doi: 10.1016/j.jep.2017.08.032. [DOI] [PubMed] [Google Scholar]
- Song M., Zou L., Peng L., Liu S., Wu B., Yi Z., Gao Y., Zhang C., Xu H., Xu Y., Tang M., Wang S., Xue Y., Jia T., Zhao S., Liang S., Li G. LncRNA NONRATT021972 siRNA normalized the dysfunction of hepatic glucokinase through AKT signaling in T2DM rats. Endocr Res. 2017;42:180–190. doi: 10.1080/07435800.2017.1292522. [DOI] [PubMed] [Google Scholar]
- Sun Z., Tan X., Ye H., Zou C., Ye C., Wang A. Effects of dietary Panax notoginseng extract on growth performance, fish composition, immune responses, intestinal histology and immune related genes expression of hybrid grouper (Epinephelus lanceolatus male symbol x Epinephelus fuscoguttatus female symbol) fed high lipid diets. Fish Shellfish Immunol. 2018;73:234–244. doi: 10.1016/j.fsi.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Tabandeh M.R., Jafari H., Hosseini S.A., Hashemitabar M. Ginsenoside Rb1 stimulates adiponectin signaling in C2C12 muscle cells through up-regulation of AdipoR1 and AdipoR2 proteins. Pharm Biol. 2015;53:125–132. doi: 10.3109/13880209.2014.912237. [DOI] [PubMed] [Google Scholar]
- Wang J., Hou Y., Jia Z., Xie X., Liu J., Kang Y., Wang X., Wang X., Jia W. Metabonomics approach to comparing the antistress effects of four Panax ginseng components in rats. J. Proteome Res. 2018;17:813–821. doi: 10.1021/acs.jproteome.7b00559. [DOI] [PubMed] [Google Scholar]
- Wang Y., Xie Q., Liang C.L., Zeng Q., Dai Z. Chinese medicine Ginseng and Astragalus granules ameliorate autoimmune diabetes by upregulating both CD4+FoxP3+ and CD8+CD122+PD1+ regulatory T cells. Oncotarget. 2017;8:60201–60209. doi: 10.18632/oncotarget.18732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder W.W., Hardie D.G. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am. J. Physiol. 1999;277:E1–E10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- Wu Y., Li P., Zhang D., Sun Y. Metformin and pioglitazone combination therapy ameliorate polycystic ovary syndrome through AMPK/PI3K/JNK pathway. Exp. Ther. Med. 2018;15:2120–2127. doi: 10.3892/etm.2017.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Loi Truong T., Zhang P., Xu F., Xu X., Li P. Dan-Qi prescription ameliorates insulin resistance through overall corrective regulation of glucose and fat metabolism. J. Ethnopharmacol. 2015;172:70–79. doi: 10.1016/j.jep.2015.05.041. [DOI] [PubMed] [Google Scholar]
- Xiong H., Zhang S., Zhao Z., Zhao P., Chen L., Mei Z. Antidiabetic activities of entagenic acid in type 2 diabetic db/db mice and L6 myotubes via AMPK/GLUT4 pathway. J Ethnopharmacol. 2018;211:366–374. doi: 10.1016/j.jep.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Xu M., Sun B., Li D., Mao R., Li H., Li Y., Wang J. Beneficial effects of small molecule oligopeptides isolated from Panax ginseng meyer on pancreatic beta-cell dysfunction and death in diabetic rats. Nutrients. 2017;9:1061. doi: 10.3390/nu9101061. doi:10.3390/nu9101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Dou D. The ginseng's fireness is associated with the lowering activity of liver Na(+)-K(+)-ATPase. J. Ethnopharmacol. 2016;190:241–250. doi: 10.1016/j.jep.2016.06.024. [DOI] [PubMed] [Google Scholar]
- Yu X., Zhang N., Lin W., Wang C., Gu W., Ling C., Feng Y., Su Y. Regulatory effects of four ginsenoside monomers in humoral immunity of systemic lupus erythematosus. Exp. Ther. Med. 2018;15:2097–2103. doi: 10.3892/etm.2017.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang Z., Wang H., Cai N., Zhou S., Zhao Y., Chen X., Zheng S., Si Q., Zhang W. Neuroprotective effect of ginsenoside Rg1 prevents cognitive impairment induced by isoflurane anesthesia in aged rats via antioxidant, anti-inflammatory and anti-apoptotic effects mediated by the PI3K/AKT/GSK-3beta pathway. Mol. Med. Rep. 2016;14:2778–2784. doi: 10.3892/mmr.2016.5556. [DOI] [PubMed] [Google Scholar]
- Zhao P., Wong K.I., Sun X., Reilly S.M., Uhm M., Liao Z., Skorobogatko Y., Saltiel A.R. TBK1 at the crossroads of inflammation and energy homeostasis in adipose tissue. Cell. 2018;172(731–743):e712. doi: 10.1016/j.cell.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q., Bao X.Y., Zhu P.C., Tong Q., Zheng G.Q., Wang Y. Ginsenoside Rb1 for myocardial ischemia/reperfusion injury: preclinical evidence and possible mechanisms. Oxid. Med. Cell Longev. 2017;2017:6313625. doi: 10.1155/2017/6313625. [DOI] [PMC free article] [PubMed] [Google Scholar]


