Abstract
Studies on the insect pollinators diversity and their relative abundance in Eruca sativa Mill. (Arugula) and Brassica rapa L. (field mustard) was carried out during spring season from February to April consecutively during all the three years of 2016–18. Insect pollinators observed belonged to four orders i.e. Hymenoptera, Diptera, Lepidoptera, and Coleoptera. A total of 20 major species of insect pollinators were recorded. The highest abundance of pollinator species belonged to Hymenoptera. The most prominent insect pollinator species were Apis mellifera followed by other three honey bee species of A. cerana, A. florea, and A. dorsata respectively. Some species of solitary bees were also recorded. From Diptera, four species of syrphid fly and one species from Muscidae family were also recorded. Insect pollinators recorded from order Lepidoptera were Pieris brassicae, Vanessa cardui, and Papilio demoleus. Lady bird beetle Coccinella septempunctata was recorded from Coleoptera order as occasional visitor. It was noticed that E. sativa attracted more insect pollinators than B. rapa which may be attributed to different amount and chemical properties of nectar, with number of pollen grains, and flower canopy of both crops. Further studies are needed to confirm the reasons for higher pollinator visitation to E. sativa than B. rapa through chemical analysis of nectar, amount of pollens, flower physiology and phenology of both crops.
Keywords: Insect pollinators, Diversity, Hymenoptera, Diptera, Coleoptera, Lepidoptera, Brassicaceae, Cruciferous crops
1. Introduction
Brassica rapa (Field mustard) belongs to family Brassicaceae. This crop is economically important because of seed oil contents and some other plant parts like leaves which are edible and can be used as fodder crop. The oil extracted from their seeds is consumed by humans from centuries in the Asian continent. Seeds after oil extraction are changed to the shape of cake which is very nutritious and used to feed animals (Ramachandran et al., 2007). Eruca sativa (Arugula) also belongs to family Brassicaceae. Local name of E. sativa is Taramira. This crop has medicinal and economic value and can be consumed as salad and vegetable by humans. It can also be used as green fodder for feeding animals (Ghazali et al., 2014).
Insects help in pollination of these crops. Crops belonging to family Brassicaceae are predominantly dependent on insect pollination (Entomophilous). Increase in seed quality and quantity is possible through pollination (Abrol, 2007, Shakeel and Inayatullah, 2013, Shakeel and Mian Inayatullah, 2015). The role of cross-pollination between cruciferous crops is majorly played by honey bees. Absence of cross-pollination generally reduces seeds number, seeds size, and viability of seeds that can lead to decrease in yield (Delaplane et al., 2000). Pollination by insect not only increase crop yield but also improve physiochemical properties of the fruits (Bashir et al., 2018). Crops of family Brassicaceae are very attractive for insect pollinators for a good source of pollens and nectar (Masierowska, 2003).
The crops with higher number of flowers have generally larger number of insect pollinators (Westphal et al., 2003). The service of pollination provided by pollinators is endangered due to losses in pollinator abundance and diversity (Daily, 1997). Studies on insect pollinators is a hot debate globally among researchers due to losses in population of pollinators due to different stresses like climate change, unavailability of floral resources. The diversity and abundance of pollinators is negatively affected by habitat destruction and fragmentation (Ghazoul, 2005a, Ghazoul, 2005b, Kremen et al., 2002, Steffan-Dewenter et al., 2002). The impact of pollinator scarcity on the yield of crops should be evaluated precisely for assessing importance of pollinators decline due to different environmental stresses (Knight et al., 2006). Decreased population of pollinators may lead to decline of plant species diversity (Biesmeijer et al., 2006). Winter crops of family Brassicaceae provide large quantities of pollen and nectar to pollinators for their population stability (Klein et al., 2007). Different kinds of pollinators visit crops belonging to family Brassicaceae (Howlett et al., 2009a, Howlett et al., 2009b, Howlett et al., 2011, Shakeel and Mian Inayatullah, 2015).
Social bees like honey bees have been reported as major pollinators of Brassica plants (Donovan, 1980, Goodell and Thomson, 2007, Shakeel and Mian Inayatullah, 2015). Other pollinators belong to different insect orders like Diptera, Lepidoptera, and Coleoptera have been reported from Brassica crops (Brunel et al., 1992, Chaudhary, 2001, Chifflet et al., 2011, Howlett et al., 2009a, Howlett et al., 2009b, Rader et al., 2009, Walker et al., 2009). The present study was conducted to evaluate the difference of pollinators diversity and abundance between E. sativa (Arugula) and B. rapa (Field mustard) crops.
2. Materials and methods
2.1. Experiment location
The experiment was conducted at the model research farm of the university of agriculture during the months of February-April of 2016–18 consecutively.
2.2. Preparation of plots
E. sativa and B. rapa seeds were sown on two separate plots in the month of November 2016, 2017, and 2018. The dimensions of each plot were approximately 18 × 15 m2. All the standard agronomic practices were followed for sowing the crops. No pesticide was sprayed on the crops during the whole experiment.
2.3. Diversity of crop visiting insect pollinators
At flowering stage of the crops the insect pollinators visiting the flowers were observed visually and were collected through aerial net throughout the month of February to April of all 3 years. The flower morphology depth of flower, length of petals, distance between the petals were measured with help of simple inch/cm ruler. The height of plants was also measured. Insect pollinators were killed in the killing jar having drops of ethyl acetate. Collection of insect pollinators was carried out throughout the flowering season of the crops. All the collected specimens were labelled and kept in insect collection box. The unidentified specimens were identified through relevant insect identification keys of Ascher and Rasmussen, 2010, Mahmood et al., 2012. All the voucher specimens were deposited to the entomology museum of the department of entomology, faculty of crop protection sciences, the University of Agriculture Peshawar.
2.4. Abundance of pollinators visiting the crop
Abundance of the insect pollinators was recorded on Eruca sativa Mill. (Arugula) and Brassica rapa L. crops. The data were recorded on weekly basis, data were collected in morning timing from 10:00–12:00 am and afternoon time from 2:00–4:00 pm. The data were recorded on the methodology used by Shakeel and Mian Inayatullah (2015). Four-meter square area was selected randomly in the field and insect pollinators visiting the flowers of both crop species were counted by hand counter clicker. The data on the abundance of pollinators were recorded from the start of flowering season till the end. The collected data were statistically analyzed with ANOVA using SPSS® version 15.0 for Windows®. Fisher’s least significant difference (LSD) tests were used for the statistical comparisons of means for evaluating frequency of visitation and relative abundance of pollinators between the two crop species.
3. Results
3.1. Eruca sativa and Brassica rapa flowers and insect pollinators diversity
The flowers of both plants is presented in Fig. 1. Both plants have different flower morphology. The flower colors are also different. B. rapa color is yellow while E. sativa color is whitish. The petals of B. rapa is very close to each other, while in E. sativa the petals are apart from each other. In cross section the depth is shorter in B. rapa compare to E. sativa (Fig. 1). The height is also different in both plants, E. sativa height is lower than B. rapa (Fig. 1).
Fig. 1.
Flowers, cross sections, and field of Eruca sativa and Brassica rapa.
The insect pollinators collected from the both crop species included four Apis species of honey bees. A. mellifera was the dominant pollinators followed by A. cerana, A. florea, and A. cerana respectively. Other pollinators from the order Hymenoptera were the large carpenter bees of species Xylocopa fenestrata, X. pubescens, Megachile sp., Lasioglossum sp., Polistes olivaceus, and Andrena pilipes.
Insect pollinators from the order Diptera were Episyrphus balteatus, Eristalis tenax, and Eristalis aeneus. Pollinators from order Lepidoptera were Vanessa cardui, Pieris brassicae, and Papilio demoleus. Insect visiting flowers from order Coleoptera observed was Coccinella septempunctata. They mainly visited the flowers of both plants for nectar and pollen collection (Table 1). Lepidopteran were mainly visited for nectar purpose while hymenoptera and Diptera were for both. Coleoptera mainly visited for pollen collection. In Hymenoptera mostly pollinators were from family Apidae. Insects having pollen on their legs and body were marked as pollen collectors.
Table 1.
Diversity of insect pollinators on Eruca sativa and Brassica rapa.
| Order | Family | Pollinator species | Foraging purpose |
|---|---|---|---|
| Hymenoptera | Apidae | Apis mellifera | Nectar and Pollen |
| Apis cerana | |||
| Apis florea | |||
| Apis dorsata | |||
| Xylocopa fenestrata | |||
| Xylocopa pubescens | |||
| Ceratina smaragdula | |||
| Megachilidae | Megachile sp. | ||
| Halictidae | Lasioglossum sp. | ||
| Vespidae | Polistes olivaceus | ||
| Andrenidae | Andrena pilipes | ||
| Diptera | Syrphidae | Episyrphus balteatus | Nectar and Pollen |
| Eristalis tenax | |||
| Eristalinus aeneus | |||
| Syrphus ribesii | |||
| Muscidae | Musca sp. | ||
| Lepidoptera | Pieridae | Pieris brassicae | Nectar |
| Nymphalidae | Vanessa cardui | ||
| Papilionidae | Papilio demoleus | ||
| Pieridae | Colias erate | ||
| Coleoptera | Coccinellidae | Coccinella septempunctata | Pollen |
3.2. Percent relative abundance of insect pollinators
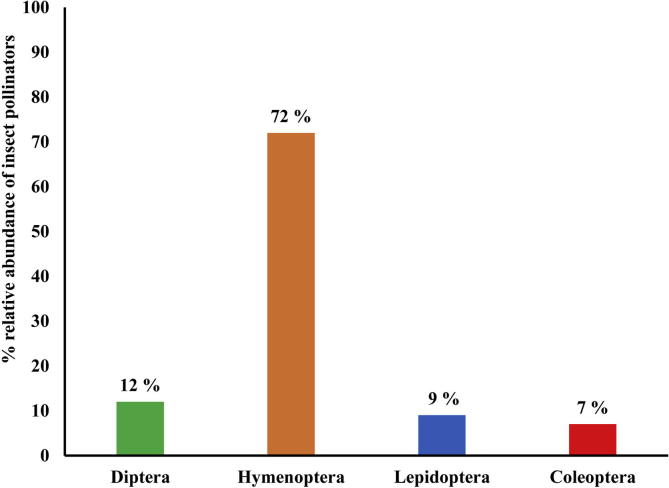
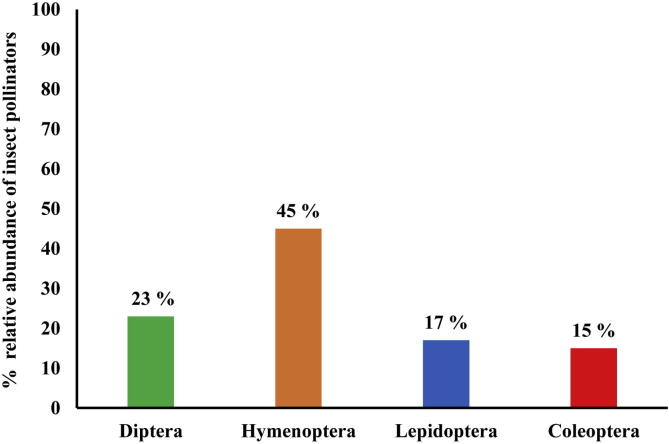
The percent relative abundance of pollinators on E. sativa is presented in Fig. 2. The recorded Hymenoptera order relative abundance was (72%) significantly higher than Diptera (12%) and Lepidoptera (9%). However, the lowest relative abundance was recorded for Coleoptera. Fig. 3 shows the relative abundance of different insect pollinator orders on B. rapa. The percent relative abundance of Hymenoptera was (45%) followed by Diptera (23%) and Lepidoptera (17%). Order Coleoptera relative abundance was lower than other three orders on B. rapa.
Fig. 2.
Percent relative abundance of pollinators on Eruca sativa at Peshawar, Pakistan.
Fig. 3.
Percent relative abundance of pollinators on Brassica rapa at Peshawar Pakistan.
On E. sativa hymenoptera percent relative abundance was (72%) significantly higher than on B. rapa (45%). On other hand Diptera, Lepidoptera, and Coleoptera relative abundance was higher on B. rapa than E. sativa. This showed that E. sativa attracted more hymenopterans than other orders (Fig. 2, Fig. 3).
3.3. Percent relative abundance on E. sativa and B. rapa at different timings
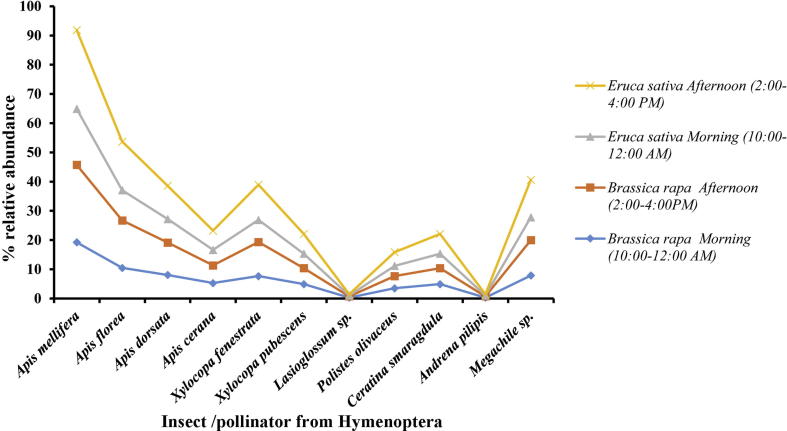
Abundance of hymenopterans during different day times on E. sativa and B. rapa is presented in Fig. 4. Their abundance was lower in the morning time on both plants. However, their abundance increased in the afternoon on both flowering plants.
Fig. 4.
Abundance of pollinators from Hymenoptera on Eruca sativa and Brassica rapa in different timings of the day during year 2016–2018. Apis mellifera (LSD = 1.04, P = 0.003), Apis florea (LSD = 1.31, P = 0.006), Apis dorsata (LSD = 0.87, P = 0.008), Apis cerana (LSD = 0.721, P = 0.004), Xylocopa fenestrata (LSD = 0.65, P = 0.04), Xylocopa pubescens (LSD = 0.76, P = 0.005), Lassioglossum sp. (LSD = 0.17, P = 0.45), Polistes olivaceus (LSD = 0.04, P = 0.007), Ceratina smaragdula (LSD = 0.72, P = 0.003), Anderna pilipis (LSD = 0.76, P = 0.005), Megachile sp. (LSD = 0.65, P = 0.04).
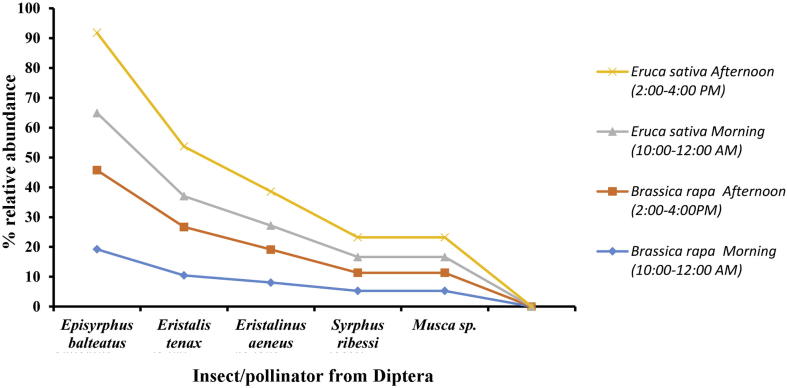
Regarding dipteran pollinators similar observation was recoded. Lower abundance was recorded in the morning time while highest abundance was recorded in afternoon timing on E. sativa and B. rapa (Fig. 5).
Fig. 5.
Abundance of pollinators from Diptera on Eruca sativa and Brassica rapa in different timings of the day during year 2016–2018. Episyrphus balteatus (LSD = 1.04, P = 0.003), Eristalis tenax (LSD = 1.31, P = 0.006), Eristalis aeneus (LSD = 0.87, P = 0.008), Syrphus ribessi (LSD = 0.721, P = 0.004).
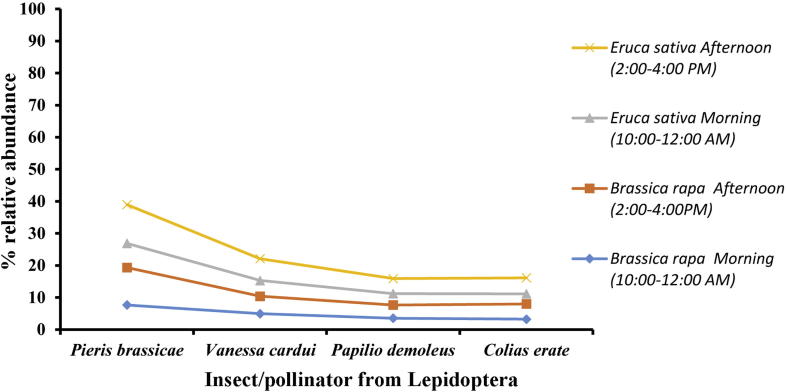
The abundance of lepidopteran pollinators is presented in Fig. 6. It shows that the abundance was low on E. sativa and B. rapa in the morning. During the afternoon timing their abundance was high comparatively.
Fig. 6.
Abundance of pollinators from Lepidoptera on Eruca sativa and Brassica rapa in different timings of the day during year 2016–2018. Pieris brassicae (LSD = 0.65, P = 0.04), Vanessa cardui (LSD = 0.76, P = 0.005), Papilio demoleus (LSD = 0.04, P = 0.007), Colias erate (LSD 0.041, P = 0.007).
4. Discussion
The color of flower has great impact on the attraction of pollinators. E. sativa mainly attracted higher no of Hymenoptera than B. rapa. Other orders of insect pollinator were greatly attracted to B. rapa than E. sativa. The reasons could be the colors of flowers. Earlier studies reported that color has great impact on the attraction of insect pollinators. Lepidopterans insects are more attracted towards bright color and this may be the reason of high number of Lepidoptera on B. rapa as its color is bright than E. sativa. Reverté et al. (2016) reported that pollinators have color preferences. Further they reported that it is not important that similar color should attract similar pollinators.
The sugar content and flower morphology also plays an important role in attracting different pollinators. Hymenopteran were mainly attracted to E. sativa than B. rapa. The reason could be high amount of nectar secretion. The other reason could the depth of flowers, which is more in E. sativa than B. rapa. More depth of E. sativa make them more feasible for hymenoptera order which having larger proboscis. Silva and Dean (2000) reported that high nectar concentrations of flower attract more honey bees compare with less nectar concentration.
In the current study, among Apis species A. mellifera abundance was higher than three species. Similar results were also reported by Shakeel and Mian Inayatullah (2015). They reported abundance and diversity of honey bee species of A. mellifera, A. cerana, A. dorsata, and A. florea on canola (B. napus) at Peshawar region. The highest abundant species was A. mellifera followed by A. cerana, A. dorsata and A. florea respectively. Studies by Kunjwal et al. (2014) on brown mustard B. juncea have also reported these four species of honey bees as pollinators in Indian region of Patnagar: other pollinators observed were Xylocopa sp., Ceratina sexmaculata, Andrena sp., and Megachile sp. Devi et al. (2017) reported different insect pollinators on Brassica juncea from Solan District of northern region of India mainly including four honey species i.e. A. mellifera, A. cerana, A. dorsata, and A. florea other wild bees included Xylocopa sp. Halictus sp. the dipterous pollinators included Eristalis sp. Episyrphus balteatus, the lepidopteran pollinators included Pieris brassicae and Colias electo. Mishra et al. (1988) reported pollinators of Brassica campestris var. Sarson from India. The major pollinators recorded were honey bee species of A. cerana indica, A. mellifera and pollinators from order Diptera i.e. Episyrphus balteatus, Eristalis spp., Musca sp. etc. Coccinella septempunctata (Coloeptera) was recorded on both plants. Although it has nothing to do with pollination of theses crops but may be it feed on pollen grains of the crops. Earlier some research reported that pollen was available in the guts of C. septempunctata (Triltsch, 1999).
High temperature in the afternoon increases the secretion of nectar which attracts more insect pollinators. Higher abundance of pollinators was also recoded in afternoon in earlier studies on sunflower (Ali et al., 2015). The observed abundance of pollinators on E. sativa and B. rapa was less in the morning while it increased in the afternoon. This may be due to the increase of temperature in afternoon timing or the amount of nectar secretion. Flowers nectar secretion has great relation with temperature.
5. Conclusion
Eruca sativa and Brassica rapa were visited by almost 20 types of insect pollinators. Among the orders hymenoptera abundance was higher than Diptera, Lepidoptera and Coleoptera. Among all pollinators Apis species was most prominent. A. mellifera abundance was higher followed by other 3 honey bee species of A. cerana, A. florea, and A. dorsata respectively. The relative abundance of all insects on both crops were higher in afternoon compare to the morning.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abrol D. Honeybees and rapeseed: a pollinator–plant interaction. Adv. Bot. Res. 2007;45:337–367. [Google Scholar]
- Ali H., Owayss A.A., Khan K.A., Alqarni A.S. Insect Visitors and Abundance of Four Species of Apis on Sunflower Helianthus annuus L. in Pakistan. Acta Zool. Bulgarica. 2015;67:235–240. [Google Scholar]
- Ascher, J., Rasmussen, C., 2010. The bee fauna and pollination in Pakistan. FAO Report, Rome, Italy.
- Bashir M.A., Alvi A.M., Khan K.A., Rehmani M.I.A., Ansari M.J., Atta S., Ghramh H.A., Batool T., Tariq M. Role of pollination in yield and physicochemical properties of tomatoes (Lycopersicon esculentum) Saudi J. Biol. Sci. 2018;25:1291–1297. doi: 10.1016/j.sjbs.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesmeijer J.C., Roberts S.P., Reemer M., Ohlemüller R., Edwards M., Peeters T., Schaffers A., Potts S.G., Kleukers R., Thomas C. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313:351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- Brunel E., Cadou D., Mesquida J. Entomofauna associated with flowering of male fertile spring rape (Brassica napus L.): Syrphidae (Insecta: Diptera) Apidologie (France) 1992 [Google Scholar]
- Chaudhary O. Abundance of wild pollinators on rapeseed and mustard. Insect Environ. 2001;7:141–142. [Google Scholar]
- Chifflet R., Klein E.K., Lavigne C., Le Feon V., Ricroch A.E., Lecomte J., Vaissiere B.E. Spatial scale of insect-mediated pollen dispersal in oilseed rape in an open agricultural landscape. J. Appl. Ecol. 2011;48:689–696. [Google Scholar]
- Daily G. Island Press; Washing D: 1997. Nature’s Service: Social Dependence on Nature Ecosystem. [Google Scholar]
- Delaplane K.S., Mayer D.R., Mayer D.F. Cabi; 2000. Crop pollination by bees. [Google Scholar]
- Devi M., Sharma H.K., Thakur R.K., Bhardwaj S.K., Rana K., Thakur M., Ram B. Diversity of insect pollinators in reference to seed set of mustard (Brassica juncea L.) Int. J. Curr. Microbiol. App. Sci. 2017;6:2131–2144. [Google Scholar]
- Donovan B. Interactions between native and introduced bees in New Zealand. N. Z. J. Ecol. 1980:104–116. [Google Scholar]
- Ghazali H.M.Z., Naz T., Fatima I., Saghir N. Morphological variations in different accessions of Eruca sativa. J. Sci. 2014;4:452–458. [Google Scholar]
- Ghazoul J. Buzziness as usual? Questioning the global pollination crisis. Trends Ecol. Evol. 2005;20:367–373. doi: 10.1016/j.tree.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Ghazoul J. Response to Steffan-Dewenter et al.: questioning the global pollination crisis. Trends Ecol. Evol. 2005;20:652–653. doi: 10.1016/j.tree.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Goodell K., Thomson J.D. Influence of bee species (Hymenoptera: Apiformes) with contrasting behaviors on pollen movement in a mustard, Brassica rapa (Brassicaceae) and the muskmelon Cucumis melo (Cucurbitaceae) Entomologia Generalis. 2007:237–252. [Google Scholar]
- Howlett B., Walker M., McCallum J., Teulon D. Small flower-visiting arthropods in New Zealand pak choi fields. New Zealand Plant Protection. 2009;62:86–91. [Google Scholar]
- Howlett B., Walker M., Newstrom-Lloyd L., Donovan B., Teulon D. Window traps and direct observations record similar arthropod flower visitor assemblages in two mass flowering crops. J. Appl. Entomol. 2009;133:553–564. [Google Scholar]
- Howlett B., Walker M., Rader R., Butler R., Newstrom-Lloyd L., Teulon D. Can insect body pollen counts be used to estimate pollen deposition on pak choi stigmas? New Zealand Plant Protection. 2011;64:25–31. [Google Scholar]
- Klein A.-M., Vaissiere B.E., Cane J.H., Steffan-Dewenter I., Cunningham S.A., Kremen C., Tscharntke T. Importance of pollinators in changing landscapes for world crops. Proc. Roy. Soc. Lond. B: Biol. Sci. 2007;274:303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T.M., Steets J.A., Ashman T.L. A quantitative synthesis of pollen supplementation experiments highlights the contribution of resource reallocation to estimates of pollen limitation. Am. J. Bot. 2006;93:271–277. doi: 10.3732/ajb.93.2.271. [DOI] [PubMed] [Google Scholar]
- Kremen C., Williams N.M., Thorp R.W. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. 2002;99:16812–16816. doi: 10.1073/pnas.262413599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunjwal N., Kumar Y., Khan M. Flower-visiting insect pollinators of Brown Mustard, Brassica juncea (L.) Czern and Coss and their foraging behaviour under caged and open pollination. Afr. J. Agric. Res. 2014;9:1278–1286. [Google Scholar]
- Mahmood K., Ullah M., Aziz A., Hasan S.A., Inayatullah M. To the knowledge of Vespidae (Hymenoptera) of Pakistan. Zootaxa. 2012;3318:26–50. [Google Scholar]
- Masierowska M. Floral nectaries and nectar production in brown mustard (Brassica juncea) and white mustard (Sinapis alba)(Brassicaceae) Plant Syst. Evol. 2003;238:97–107. [Google Scholar]
- Mishra R., Kumar J., Gupta J. The effect of mode of pollination on yield and oil potential of Brassica campestris L. var. sarson with observations on insect pollinators. J. Apic. Res. 1988;27:186–189. [Google Scholar]
- Rader R., Howlett B.G., Cunningham S.A., Westcott D.A., Newstrom-Lloyd L.E., Walker M.K., Teulon D.A., Edwards W. Alternative pollinator taxa are equally efficient but not as effective as the honeybee in a mass flowering crop. J. Appl. Ecol. 2009;46:1080–1087. [Google Scholar]
- Ramachandran S., Singh S.K., Larroche C., Soccol C.R., Pandey A. Oil cakes and their biotechnological applications – a review. Bioresour. Technol. 2007;98:2000–2009. doi: 10.1016/j.biortech.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Reverté S., Retana J., Gómez J.M., Bosch J. Pollinators show flower colour preferences but flowers with similar colours do not attract similar pollinators. Ann. Bot. 2016;118:249–257. doi: 10.1093/aob/mcw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeel M., Inayatullah M. Impact of insect pollinators on the yield of canola (Brassica napus) in Peshawar, Pakistan. J. Agric. Urban Entomol. 2013;29:1–5. [Google Scholar]
- Shakeel, M., Mian Inayatullah, H.A., 2015. Checklist of insect pollinators and their relative abundance on two canola (Brassica napus) cultivars in Peshawar, Pakistan.
- Silva E., Dean B.B. Effect of nectar composition and nectar concentration on honey bee (Hymenoptera: Apidae) visitations to hybrid onion flowers. J. Econ. Entomol. 2000;93:1216–1221. doi: 10.1603/0022-0493-93.4.1216. [DOI] [PubMed] [Google Scholar]
- Steffan-Dewenter I., Münzenberg U., Bürger C., Thies C., Tscharntke T. Scale-dependent effects of landscape context on three pollinator guilds. Ecology. 2002;83:1421–1432. [Google Scholar]
- Triltsch H. Food remains in the guts of Coccinella septempunctata (Coleoptera: Coccinellidae) adults and larvae. Eur. J. Entomol. 1999;96:355–364. [Google Scholar]
- Walker M., Howlett B., McCallum J., Wallace A., Teulon D. Small arthropods as pollinators in a New Zealand pak choi field trial. New Zealand Plant Protection. 2009;62:92–98. [Google Scholar]
- Westphal C., Steffan-Dewenter I., Tscharntke T. Mass flowering crops enhance pollinator densities at a landscape scale. Ecol. Lett. 2003;6:961–965. [Google Scholar]