Abstract
Different temperature zones have significant impact on the population dynamics of Plutella xylostella. Effective management of P. xylostella requires the knowledge of temperature tolerance by different life stages. In the current study, fitness parameters of diamondback moth were reported by using age-stage, two-sex life table traits at four constant temperatures (15, 20, 25 and 30 °C). The life cycle of P. xylostella was significantly longer at 15 °C. The 20 °C level of temperature was found optimal for fecundity, gross reproductive rate (51.74 offspring) and net reproductive rate (44.35 offspring per individual). The adult pre-oviposition period was statistically at par at all four level of temperatures. However, the survival was maximum at 20 °C as compared to other three temperature ranges. Based on the current study, it was concluded that temperature has a great role in population build-up of P. xylostella and effective management tactics should be applied to prevent significant damage to cabbage and other cruciferous crops when the temperature in the field is near 20 °C.
Keywords: Age-stage, Plutella xylostella, Lepidoptera, Temperature, Life table
1. Introduction
Diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae) is a one of the major pest of vegetables across the world (Garrad et al., 2016, Jaleel et al., 2017, Shakeel et al., 2017, Steinbach et al., 2017). This pest has been reported to cause up to 90% yield losses under high population levels with 4–5 billion US dollars spent for its management globally (Zalucki et al., 2012). This is due to high fecundity, short generation time, wide host range, insecticide resistance and ability to survive under wide range of temperatures (Furlong et al., 2013, Gu et al., 2010, Shelton and Nault, 2004). The relationship between temperature and insects is well studied. Being poikilothermic organisms, the growth, fecundity, survival and other biological factors of insects are highly influenced by external temperature conditions (Denlinger and Hallman, 1998). Each species has its own thermal window at which it can survive and reproduce in a particular area (Jarošík et al., 2002). Hence such relationships are very important in planning the integrated management of diamondback moth (Ahn et al., 2012, Kim and Lee, 2010, Notter-Hausmann and Dorn, 2010).
Although numerous studies have been conducted in several countries to study the biology of P. xylostella under different temperature (Golizadeh et al., 2009, Liu et al., 2002, Shirai, 2000) but such studies are lacking in Pakistan. Moreover, populations of pest in different ecological regions vary for thermal requirements (Chen et al., 2015, Gomi et al., 2003). Such variations demand to study the biology/fitness of that specific pest for its better management.
In the current study, life traits of diamondback moth were studied on four different temperatures by using the two-sex life table traits along with age-stage on Chinese cabbage (Brassica rapa var. Pekinensis). The population of P. xylostella belong to arid climatic conditions of Multan, Pakistan. We aimed to find out how arid population of P. xylostella performs at different temperatures. The purpose was to find relationship between the P. xylostella and temperature and use that information for future management of this destrctive pest in Pakistan.
2. Materials and methods
2.1. Population of P. xylostella
For growing of plants, seeds of Chinese cabbage or bok choy were sown in beds (having area = 5 m2) and plastic boxes (6 × 10 × 20 cm) at the research farm of Bahauddin Zakariya University, Multan, Pakistan. The cabbage leaves from these plants were used for laboratory studies. The larval and pupal stages of diamondback moth were collected from cabbage fields located near Central Cotton Research Institute (CCRI), Multan, Pakistan. Briefly, the collected larvae were reared on leaves of cabbage plants in glass petri dishes untill pupae. The pupae were transferred to glass jars for adult emergence. The adults moths were provided with 10% honey solution as diet until egg laying and death. They were reared in same manner untill enough numbers were obtained for experiments (Jaleel et al., 2017). In addition, they were reared on each temperature (15, 20, 25 and 30 °C) for two generations in the incubators for acclimatization (Model VELP # 90E Japan) before using for experiments.
2.2. Life table traits of P. xylostella
For this purpose, 150 leaves (5.5 cm × 1.2 cm) of cabbage were cut and placed individually in Petri dishes at 15, 20, 25, and 30 °C temperatures in incubators. A total of 150 eggs were collected from leaves at each temperature with the help of camel hair brush. They were placed in petri dishes on a piece of cabbage leaf placed at the bottom of petri dish. Each egg denoted as a replication. Fresh cabbage leaves were given after every 2 days during the experimental period. Life cycle of diamondback moth was recorded daily at each temperature.
To study the reproduction potential of diamondback moth, both male and female moths were kept together in a glass jar (14 cm × 20 cm). Thirty replications were made at each temperature. The 10% honey solution was prepared and given to adults. The cabbage leaves were placed in the glass jars for egg laying. Daily, leaves of cabbage were replaced and the numbers of eggs were counted (Jaleel et al., 2017).
Following parameters were recorded at each temperature: Adult pre-oviposition period (APOP = the time period between the female adult emergence to its first egg laying), total pre-oviposition period (TPOP = the time interval between birth to the start of egg laying), oviposition period, and daily fecundity by using the methodology of Colinet et al. (2015). Each stage of diamondback moth was weighed sensitive analytical electric balance (Model#SE-391).
2.3. Statistical analysis of experiments
Two-sex life table program, TWO-SEX-MS Chart in computer was used to analyze different biological parameters (egg, larva, pupa, adult pre-oviposition period, total pre-oviposition period, fecundity) by following the methodology of Chi, 1988, Chi, 2015. In the age-stage, two-sex life table, the lx, mx, and R0 values are calculated as
| (1) |
| (2) |
| (3) |
where k is a number of stages, sxj is the survival rate of diamondback moth where x = age in days and j = stage), fxj is the age-stage-specific fecundity, lx is age-specific survival rate, mx is age-specific fecundity, exj age-stage life expectancy, vxj age-stage reproductive value, R0 is net reproductive rate, k is finite rate of increase, and T is the mean generation).
In this study, the iterative bisection method from the Euler–Lotka formula was used to estimate r (r is intrinsic rate of increase) is using the with age indexed from 0 (Goodman, 1982) as shown in Eq. (2).
The exj is defined as the length of duration or time that an individual or insect of x and j is predictable to living, calculated by the methodology of Chi and Su (2006) as
| (4) |
where is define as the probability that individuals of x and j will survive to age i and stage y and, is found by assuming = 1 (Tuan et al., 2014).
The vxj was estimated by following the methodology of Abbas et al. (2014) and was calculated as
| (5) |
Finally, the standard errors and variances of fruit preference (number of visits and oviposition punctures) and means of different parameters were compared LSD and bootstrap technique respectively by following the methodology of Efron and Tibshirani (1994).
3. Results
3.1. Life table parameters
The egg development time of P. xylostella was longer at 15 °C in comparison to other three temperatures (P < 0.001). The 1st, 2nd, 3rd, and 4th instar of P. xylostella were longer at 15 °C than those at other three temperatures (P < 0.001). Males and females of diamondback moth lived longer at 15 °C with significant difference to other three temperatures (P < 0.001). Statistically, adult pre-oviposition period of P. xylostella was not significantly different at all four temperatures.
Differences were also observed in the total pre-reproductive period (TPRP), oviposiiton period and fecundity. TPRP was significantly longer at 15 °C, while significantly shorter at 30 °C (P < 0.001). Similarly, shortest oviposition period was observed at 15 °C while there was no significant difference regarding oviposition period among all the rest of temperatures. Mean fecundity values fecundity per female (288.09 eggs) was recorded at 20 °C temperature (P < 0.05) (Table 1).
Table 1.
Influence of four different temperature on the biological traits of the diamondback moth.
| Temperature |
||||
|---|---|---|---|---|
| Parameters | 15 °C | 20 °C | 25 °C | 30 °C |
| Egg duration (d) | 4.36 ± 0.23 a | 3.38 ± 0.14 b | 2.34 ± 0.15 c | 1.83 ± 0.14 d |
| 1st instar (d) | 5.20 ± 0.06 a | 3.17 ± 0.09 b | 2.32 ± 0.08 c | 1.14 ± 0.02 d |
| 2nd instar (d) | 4.28 ± 0.08 a | 2.76 ± 0.06 b | 2.10 ± 0.08 c | 1.44 ± 0.10 d |
| 3rd instar (d) | 3.76 ± 0.06 a | 1.87 ± 0.08 b | 1.64 ± 0.10 bc | 1.12 ± 0.02 c |
| 4th instar (d) | 3.55 ± .07 a | 2.43 ± 0.04 b | 1.76 ± 0.03 c | 1.22 ± 0.05 d |
| Pupal duration (d) | 7.26 ± 0.14 a | 6.24 ± 0.06 b | 3.86 ± 0.06 c | 3.11 ± 0.07 d |
| Male adult longevity (d) | 19.15 ± 0.28 a | 15.31 ± 0.18 b | 13.89 ± 0.18 c | 11.55 ± 0.29 d |
| Female adult longevity (d) | 17.54 ± 0.16 a | 12.76 ± 0.26 b | 11.29 ± 0.12 c | 10.15 ± 0.16 d |
| DT from egg to male adult (d) | 47.00 ± 0.63 a | 35.64 ± 0.50 b | 27.27 ± 0.18 c | 24.23 ± 0.64 d |
| DT from egg to female adult (d) | 45.91 ± 0.69 a | 33.67 ± 0.66 b | 25.83 ± 0.56 c | 23.42 ± 0.61 d |
| APOP/APRP (d) | 1.00 ± 0.33 a | 0.50 ± 0.26 a | 1.00 ± 0.33 a | 1.00 ± 0.00 a |
| TPOP/TPRP (d) | 30.00 ± 0.67 a | 21.05 ± 0.50 b | 15. 65 ± 0.98 c | 12.67 ± 0.76 c |
| Oviposition (d) | 9.91 ± 0.25 b | 10.75 ± 0.63 a | 9.67 ± 0.22 a | 8.58 ± 0.19 a |
| Fecundity (total eggs/female) | 101.93 ± 4.58 d | 288.09 ± 4.65 a | 261.93 ± 2.68 b | 194.53 ± 3.09 c |
APOP: Adult pre-oviposition period of female adult, APRP: Adult pre-reproduction period of female adult. TPOP: Total pre-oviposition period of female counted from birth, TPRP: Total pre-reproduction period of female counted from birth. Means in the same row followed by the same letter are not significantly different (P > 0.05) using bootstrap test.
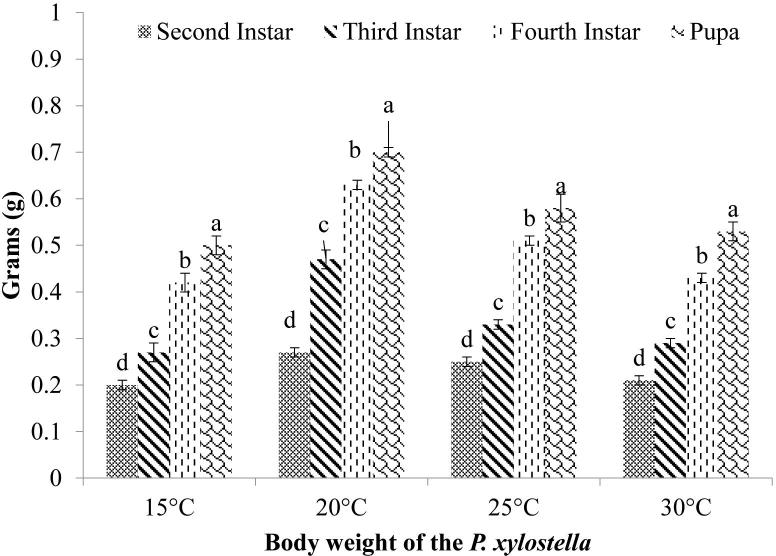
Second, third, fourth larval instars and pupae of P. xylostella were significantly different in term of weight at each temperature (Fig. 1). Overall pupal body weight was higher than fourth, third, and second instar. Each instar was significantly gained highest weight at 15 °C among other temperatures.
Fig. 1.
The body weight of the Plutella xylostella at four different constant temperatures; Different letters above each bar indicate significant differences between instar treatments using one-way ANOVA, LSD test, and at P < 0.05.
3.2. Life table parameters
The r was significantly higher at 25 and 30 °C and significantly less at 15 °C (P < 0.05) (Table 2). The GRR and R0 were significantly higher at 20 °C (P < 0.05) while decreased at 15 °C (Table 2). The T significantly decreased from 34.55 ± 0.67 to 16.67 ± 0.51 days when temperature was 15 °C to 30 °C (P < 0.05).
Table 2.
Influence of four different temperatures on the life table parameters of Plutella xylostella.
| Temperature |
||||
|---|---|---|---|---|
| Parameters | 15 °C | 20 °C | 25 °C | 30 °C |
| r | 0.11 ± 0.01 c | 0.17 ± 0.01 b | 0.22 ± 0.02 a | 0.25 ± 0.02 a |
| ʎ | 1.12 ± 0.01 c | 1.19 ± 1.17 b | 1.25 ± 0.02 a | 1.29 ± 0.02 a |
| GRR | 76.52 ± 17.43 c | 103.48 ± 22.46 a | 95.34 ± 20.41 b | 87.91 ± 18.78 c |
| R0 | 61.10 ± 14.92 c | 88.70 ± 20.03 a | 79.10 ± 17.75 b | 70.66 ± 16.05 ab |
| T | 34.55 ± 0.67 a | 26.13 ± 0.55 b | 19.21 ± 0.58 c | 16.67 ± 0.51 d |
r; The intrinsic rate of increase (per days).
ʎ; The finite rate of increase (per days).
GRR; Gross reproductive rate (offspring).
R0; The net reproductive rate (offspring/individual).
T; The mean generation time (days).
Means in the same row followed by the same letter are not significantly different (P > 0.05) using bootstrap test.
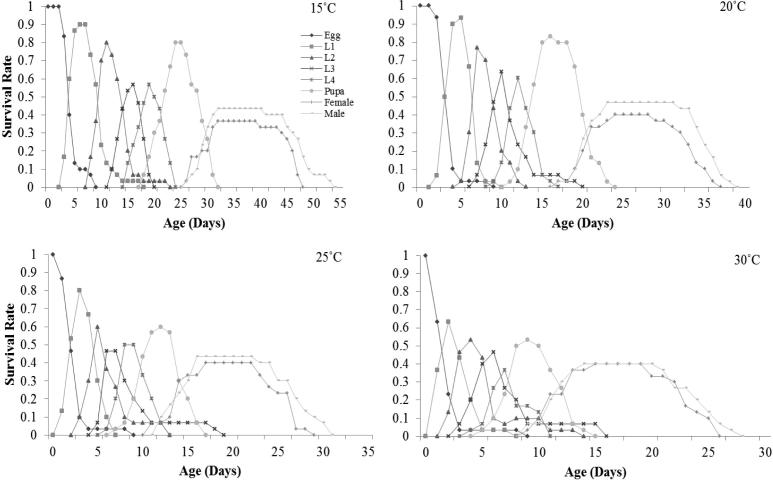
The detailed age-stage-specific survival rate (Sxj) of P. xylostella at different temperatures (15, 20, 25 and 30 °C) have shown in Fig. 2. There was significant difference in survival rates of eggs at different temperature with higher rate at 20 °C temperature and lowest at other temperature (15, 25 and 30 °C).
Fig. 2.
Influence of four different temperatures on the age-stage-specific survival rate (Sxj) of the Plutella xylostella; L1 = 1st Instar, L2 = 2nd Instar, L3 = 3rd Instar, L4 = 4th Instar.
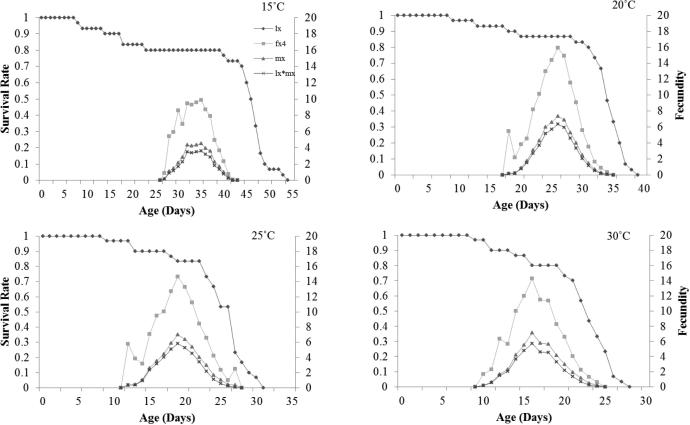
Fig. 3 showed the age-specific survival rate (lx), age-specific fecundity of whole population (mx) and their product viz. age-specific maternity (lx*mx) at different temperatures. There was inverse relationship recoded between age specific-survival rate and the temperature however age-specific fecundity was directly proportional to temperatures 15 and 20 °C only. Age-specific maternity also depicted a similar trend to age-specific fecundity.
Fig. 3.
Influence of four different temperatures on the Age-specific survival rate (lx), female age-specific fecundity (fx4), age-specific fecundity (mx), and age-specific maternity (lx*mx) of the Plutella xylostella; L1 = 1st Instar, L2 = 2nd Instar, L3 = 3rd Instar, L4 = 4th Instar.
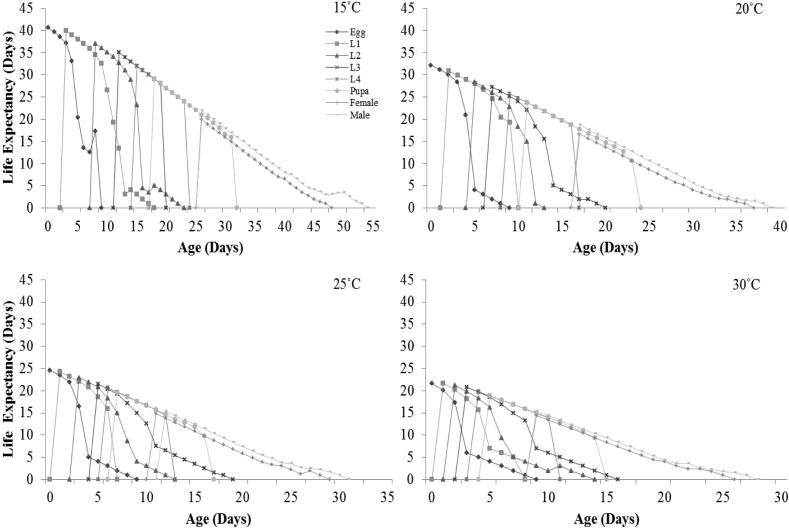
Age-stage-specific life expectancy (exj) of P. xylostella at each stage as affected by temperature is shown in Fig. 4 and was inversely proportional to temperature viz. it decreased from 40.6 to 21.67 days while moving from 15 to 30 °C (Fig. 4).
Fig. 4.
Influence of four different temperatures on the age-stage-specific life expectancy (exj) of the Plutella xylostella; L1 = 1st Instar, L2 = 2nd Instar, L3 = 3rd Instar, L4 = 4th Instar.
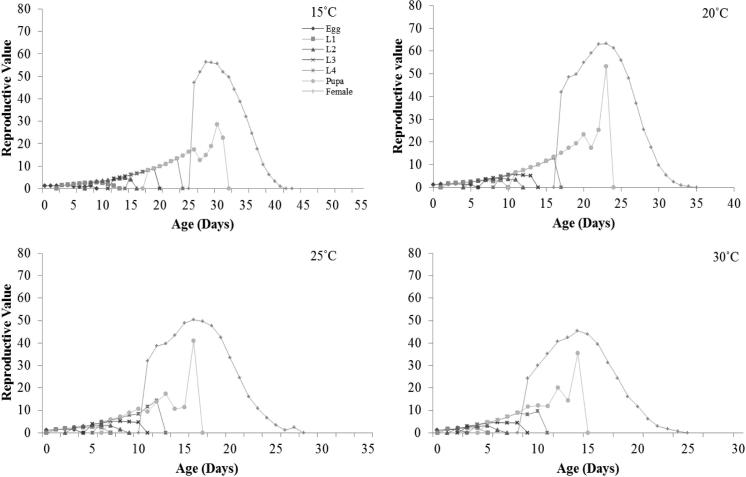
Age-stage reproductive value (vxj) which is the scale of population forecasting and is shown in Fig. 5. The curves of reproductive value at each temperature significantly increased after adult emergence from pupae up to 3–6 days depending upon the temperature. It was significantly higher at 20 °C as compared to the remaining tested temperatures.
Fig. 5.
Age-stage reproductive value (vxj) of the Plutella xylostella as affected by temperature; L1 = 1st Instar, L2 = 2nd Instar, L3 = 3rd Instar, L4 = 4th Instar.
There was inverse relationship between the T and temperature. Intrinsic rate of increase was inversely proportional to the highest finite rate of increase as compared to the T. The r was significantly higher at 25 °C followed by 30 °C while it was significantly lower at 15 °C (Table 2).
4. Discussion
Life table, an important research tool used for ecology studies, enables to study important biological components of organisms like growth rate, survival rate and reproductive potential of an organism under different conditions. Growth rate is highly dependent on first reproductive age and the age approaching the peak of reproductive value (Lewontin and Felsenstein, 1965). Due to drawbacks of Jackknife method (Huang and Chi, 2012, Huang and Chi, 2013), bootstrap technique was used with n = 100,000 to get precise estimate of population parameters. Means of all resampling (n = 100,000) were used to estimate the standard errors. A normal frequency distribution can be generated by bootstrap which in turn proves effective for analysis of variance and comparison of means (Akköprü et al., 2015).
Insect pests are ectothermic in nature, and their biology, behaviour, and fitness are greatly affected by abiotic factors (Cui et al., 2008, Jaleel et al., 2018). There are various studies showing the effect of temperature on survival and development of different insects (Garrad et al., 2016, Golizadeh et al., 2009, Powell and Bentz, 2009) but none have studied the two-sex life table traits with age stage survival and age specific fecundity of P. xylostella. Marchioro and Foerster (2011) reported that P. xylostella can tolerate wider ranges of temperatures however, temperature more than 30 °C negatively affect its survival.
It has been reported that the pre-adult and adult development rate varies at different temperatures (Folguera et al., 2010). The present study concluded that P. xylostella had short life cycle at 30 °C as compared to other temperatures (15, 20, and 25 °C), while the fecundity was highest at 20 °C (Table 1). This may be due to more feeding at 20 °C. The feeding capability of test insects was found dependent on the temperature. Shirai (2000) described that 20–25 °C temperature is more suitable for the fecundity of P. xylostella, while in our study at 20 °C fecundity and hatching percentage were found maximum indicating that 20 °C is a suitable temperature (Table 1).
Zheng et al. (2008) also reported that the feeding capability of P. xylostella was found dependent on the temperature. The present study showed that larval weight was highest at 20 °C (Table 1). This could be due to the fact that the weight of larvae is also affected by consumption, and growth rate (Gilbert et al., 2004). Lower body weight at higher temperatures attributed to rapid developmental time (Colinet et al., 2015, Keil et al., 2015) while in our study rapid development of P. xylostella was completed at 30 °C also gain less weight (Table 1).
The GRR is the indication of rapid increase of population that depends on the number of eggs laid, eggs hatched, and adult eclosion, and all these parameters are affected by temperature (Khaliq et al., 2007). In two other studies, Colinet et al., 2015, Keil et al., 2015 found positive relationship between intrinsic rate (r) increase and temperature up to certain extent due to a higher growth rate. In the present study, the GRR was highest at 20 °C and r increased from 15 to 30 °C (Table 2). Such extraordinarily high growth rates must be due to the rapid development and high fecundity of P. xylostella. The population increases only when net reproductive rate will be greater than 1 and r > 0 (Chen et al., 2017, Southwood and Henderson, 2009). Our results were also according to this above-mentioned theory.
5. Conclusion
Based on the current study, it is concluded that P. xylostella has potential to grow and reproduce on all four temperatures 15, 20, 25 and 30 °C but the most favourable temperature for P. xylostella is 20 °C. The results can be used to forecast pest population under field conditions. So, it is recommended that farmers should plan insecticide application when the field temperature is near 20 °C.
Acknowledgement
This research was done under financial project Pakistan Science Foundation (Project No. PSF/NSLP/P-BZU [130]).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbas N., Khan H.A.A., Shad S.A. Resistance of the house fly Musca domestica (Diptera: Muscidae) to lambda-cyhalothrin: mode of inheritance, realized heritability, and cross-resistance to other insecticides. Ecotoxicology. 2014;23:791–801. doi: 10.1007/s10646-014-1217-7. [DOI] [PubMed] [Google Scholar]
- Ahn J.J., Yang C.Y., Jung C. Model of Grapholita molesta spring emergence in pear orchards based on statistical information criteria. J. Asia-Pac. Entomol. 2012;15:589–593. [Google Scholar]
- Akköprü E.P., Atlıhan R., Okut H., Chi H. Demographic assessment of plant cultivar resistance to insect pests: a case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 2015;108:378–387. doi: 10.1093/jee/tov011. [DOI] [PubMed] [Google Scholar]
- Chen Q., Li N., Wang X., Ma L., Huang J.-B., Huang G.-H. Age-stage, two-sex life table of Parapoynx crisonalis (Lepidoptera: Pyralidae) at different temperatures. PLoS One. 2017;12:e0173380. doi: 10.1371/journal.pone.0173380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Fleischer S.J., Saunders M.C., Thomas M.B. The influence of diurnal temperature variation on degree-day accumulation and insect life history. PLoS One. 2015;10:e0120772. doi: 10.1371/journal.pone.0120772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988;17:26–34. [Google Scholar]
- Chi, H., 2015. TWOSEX-MSChart: a computer program for the age-stage, two-sex life table analysis. Available on: http://140.120.197.
- Chi H., Su H.-Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead)(Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer)(Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006;35:10–21. [Google Scholar]
- Colinet H., Sinclair B.J., Vernon P., Renault D. Insects in fluctuating thermal environments. Ann. Rev. Entomol. 2015;60 doi: 10.1146/annurev-ento-010814-021017. [DOI] [PubMed] [Google Scholar]
- Cui X., Wan F., Xie M., Liu T. Effects of heat shock on survival and reproduction of two whitefly species, Trialeurodes vaporariorum and Bemisia tabaci biotype B. J. Insect Sci. 2008;8:24. [Google Scholar]
- Denlinger D.L., Hallman G.J. Westview Press Boulder; CO: 1998. Temperature Sensitivity in Insects and Application in Integrated Pest Management. [Google Scholar]
- Efron B., Tibshirani R.J. CRC Press; 1994. An Introduction to the Bootstrap. [Google Scholar]
- Folguera G., Mensch J., Munoz J.L., Ceballos S.G., Hasson E., Bozinovic F. Ontogenetic stage-dependent effect of temperature on developmental and metabolic rates in a holometabolous insect. J. Insect Physiol. 2010;56:1679–1684. doi: 10.1016/j.jinsphys.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Furlong M.J., Wright D.J., Dosdall L.M. Diamondback moth ecology and management: problems, progress, and prospects. Annu. Rev. Entomol. 2013;58:517–541. doi: 10.1146/annurev-ento-120811-153605. [DOI] [PubMed] [Google Scholar]
- Garrad R., Booth D., Furlong M. The effect of rearing temperature on development, body size, energetics and fecundity of the diamondback moth. Bull. Entomol. Res. 2016;106:175–181. doi: 10.1017/S000748531500098X. [DOI] [PubMed] [Google Scholar]
- Gilbert E., Powell J.A., Logan J.A., Bentz B.J. Comparison of three models predicting developmental milestones given environmental and individual variation. Bull. Math. Biol. 2004;66:1821. doi: 10.1016/j.bulm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Golizadeh A., Kamali K., Fathipour Y., Abbasipour H. Effect of temperature on life table parameters of Plutella xylostella (Lepidoptera: Plutellidae) on two brassicaceous host plants. J. Asia-Pac. Entomol. 2009;12:207–212. [Google Scholar]
- Gomi T., Inudo M., Yamada D. Local divergence in developmental traits within a trivoltine area of Hyphantria cunea Drury (Lepidoptera: Arctiidae) Entomol. Sci. 2003;6:71–75. [Google Scholar]
- Goodman D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982;119:803–823. [Google Scholar]
- Gu, X., Tian, S., Wang, D., Fei, G., Wei, H., 2010. Interaction between short-term heat pretreatment and fipronil on 2nd instar larvae of diamondback moth, Plutella xylostella (Linn). Dose-Response 8, dose-response. 09-032. Gu. [DOI] [PMC free article] [PubMed]
- Huang Y.B., Chi H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett)(Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. 2012;19:263–273. [Google Scholar]
- Huang Y.B., Chi H. Life tables of Bactrocera cucurbitae (D iptera: Tephritidae): with an invalidation of the jackknife technique. J. Appl. Entomol. 2013;137:327–339. [Google Scholar]
- Jaleel W., Lu L., He Y. Biology, taxonomy, and IPM strategies of Bactrocera tau Walker and complex species (Diptera; Tephritidae) in Asia: a comprehensive review. Environ. Sci. Pollut. Res. 2018:1–16. doi: 10.1007/s11356-018-2306-6. [DOI] [PubMed] [Google Scholar]
- Jaleel W., Saeed S., Saeed Q., Naqqash M.N., Sial M.U., Aine Q.U., Yanyuan L., Rui Z., He Y., Lu L. Effects of three different cultivars of cruciferous plants on the age‐stage, two‐sex life table traits of Plutella xylostella (L.)(Lepidoptera: Plutellidae) Entomol. Res. 2017 [Google Scholar]
- Jarošík V., Honěk A., Dixon A.F. Developmental rate isomorphy in insects and mites. Am. Nat. 2002;160:497–510. doi: 10.1086/342077. [DOI] [PubMed] [Google Scholar]
- Keil G., Cummings E., de Magalhaes J.P. Being cool: how body temperature influences ageing and longevity. Biogerontology. 2015;16:383–397. doi: 10.1007/s10522-015-9571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq A., Attique M., Sayyed A. Evidence for resistance to pyrethroids and organophosphates in Plutella xylostella (Lepidoptera: Plutellidae) from Pakistan. Bull. Entomol. Res. 2007;97:191–200. doi: 10.1017/S0007485307004877. [DOI] [PubMed] [Google Scholar]
- Kim D.-S., Lee J.-H. A population model for the peach fruit moth, Carposina sasakii Matsumura (Lepidoptera: Carposinidae), in a Korean orchard system. Ecol. Model. 2010;221:268–280. [Google Scholar]
- Lewontin R.C., Felsenstein J. The robustness of homogeneity tests in 2 x N tables. Biometrics. 1965:19–33. [Google Scholar]
- Liu S.-S., Chen F.-Z., Zalucki M.P. Development and survival of the diamondback moth (Lepidoptera: Plutellidae) at constant and alternating temperatures. Environ. Entomol. 2002;31:221–231. [Google Scholar]
- Marchioro C., Foerster L. Development and survival of the diamondback moth, Plutella xylostella (L.)(Lepidoptera: Yponomeutidae) as a function of temperature: effect on the number of generations in tropical and subtropical regions. Neotrop. Entomol. 2011;40:533–541. [PubMed] [Google Scholar]
- Notter-Hausmann C., Dorn S. Relationship between behavior and physiology in an invasive pest species: oviposition site selection and temperature-dependent development of the oriental fruit moth (Lepidoptera: Tortricidae) Environ. Entomol. 2010;39:561–569. doi: 10.1603/EN09231. [DOI] [PubMed] [Google Scholar]
- Powell J.A., Bentz B.J. Connecting phenological predictions with population growth rates for mountain pine beetle, an outbreak insect. Landscape Ecol. 2009;24:657–672. [Google Scholar]
- Shakeel M., Farooq M., Nasim W., Akram W., Khan F.Z.A., Jaleel W., Zhu X., Yin H., Li S., Fahad S. Environment polluting conventional chemical control compared to an environmentally friendly IPM approach for control of diamondback moth, Plutella xylostella (L.), in China: a review. Environ. Sci. Pollut. Res. 2017;24:14537–14550. doi: 10.1007/s11356-017-8996-3. [DOI] [PubMed] [Google Scholar]
- Shelton A., Nault B. Dead-end trap cropping: a technique to improve management of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) Crop Prot. 2004;23:497–503. [Google Scholar]
- Shirai Y. Temperature tolerance of the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae) in tropical and temperate regions of Asia. Bull. Entomol. Res. 2000;90:357–364. doi: 10.1017/s0007485300000481. [DOI] [PubMed] [Google Scholar]
- Southwood T.R.E., Henderson P.A. John Wiley & Sons; 2009. Ecological Methods. [Google Scholar]
- Steinbach D., Moritz G., Nauen R. Fitness costs and life table parameters of highly insecticide-resistant strains of Plutella xylostella (L.)(Lepidoptera: Plutellidae) at different temperatures. Pest Manag. Sci. 2017;73:1789–1797. doi: 10.1002/ps.4597. [DOI] [PubMed] [Google Scholar]
- Tuan S.-J., Li N.-J., Yeh C.-C., Tang L.-C., Chi H. Effects of green manure cover crops on Spodoptera litura (Lepidoptera: Noctuidae) populations. J. Econ. Entomol. 2014;107:897–905. doi: 10.1603/ec13435. [DOI] [PubMed] [Google Scholar]
- Zalucki M.P., Shabbir A., Silva R., Adamson D., Shu-Sheng L., Furlong M.J. Estimating the economic cost of one of the world's major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): just how long is a piece of string? J. Econ. Entomol. 2012;105:1115–1129. doi: 10.1603/EC12107. [DOI] [PubMed] [Google Scholar]
- Zheng F.S., Du Y.Z., Wang Z.J., Xu J.J. Effect of temperature on the demography of Galerucella birmanica (Coleoptera: Chrysomelidae) Insect Science. 2008;15:375–380. [Google Scholar]