Abstract
Aim
The presence of Phosphorus as a macronutrient in soil is necessary for plant growth and its deficiency restricts crop yield. Therefore, the aim of current study is to isolate promising rhizospheric phosphate solubilizing bacteria presenting with plant growth promoting (PGP) traits and their utilization as biofertilizers to improve Triticum aestivum (Var. Galaxy 2013) growth and nutrition.
Method
Out of 30 isolates obtained from rhizosphere of various plants of different regions, 10 best PSRB strains (WumS-3, WumS-4, WumS-5, WumS-11, WumS-12, WumS-21, WumS-24, WumS-25, WumS-26 and WumS-28) were selected based on their high P solubilization and good PGP (auxin, psiderphore, HCN, Nitrogen fixation) activities. Triticum aestivum (Var. Galaxy 2013) was used as an experimental crop under laboratory and field conditions.
Results
In this study, P solubilization capacity of selected strains were found 4–7 solubilization index on agar plate and 30–246 µg/ml in liquid broth respectively. The optimum conditions for phosphate solubilization under in vitro condition were found 35 °C at pH 7, glucose as good carbon source and ammonium nitrate as a good nitrogen source. Furthermore, the selected strains had the ability to produces phytohormones (indole acetic acid), siderophore, ammonia and Hydrogen Cyanide. Finally, PSRB inoculum showed significant (p < 0.05) increase (50%–80%) in seed germination while 10–90% increase in root length and shoot length was found as compared to control in laboratory condition. Under natural conditions, 40–80% increase in seed germination while 5–34.8% increase in shoot length and 5–96% increase in seed weight was also observed.
Conclusion
Isolated strains are promising PSRB that enhance plant growth and this research is a base for recommending the use of these bacterial strains for biofertilizer, as an alternative of chemical fertilizer, for Triticum aestivum L. production.
Keywords: Phosphate solubilizing rhizobacteria, Indole acetic acid, Wheat plant growth, Biofertilizers, Alginate bead
Abbreviations: PSRB, phosphate solubilizing rhizobacteria; NBRIP, National Botanical Research Institute Phosphate media; TCP, tricalcium phosphate; PSI, phosphate solubilization index
1. Introduction
In developing countries, agriculture sector is considered as a main pillar of economy for their development (Zahid et al., 2015) and Phosphorus (fos’furus = light-bearing) is a second major macronutrient that required for plant growth and development because it is involved in basic biological functions such as cell division, nucleic acids synthesis, photosynthesis and respiration, energy transfer and in the formation of oils, sugars and starches (Awasthi et al., 2011). Naturally, 100–1000 g/acre phosphorus is present in soil but this P is not available for plants uptake and consequently severely affects crop productivity (Awasthi et al., 2011). Therefore, to compensate P shortage in soil and to ensure maximum crop production, an excessive use of chemical P fertilizer is a current agriculture practice in all over the world. Globally, more than 175.5 million tons of chemical fertilizer is used in agriculture to obtain optimum yield of crops (FAO, 2011). Nevertheless, only 5–25% applied P will be absorbed by plants while remaining 75–95% persist in soil in insoluble form (Stevenson and Cole, 1999). It causes adverse effects on soil matrix, on their ecological functions and activities of rhizospheric bacteria (Lemanski and Scheu, 2014, Kumar et al., 2014). In addition, the main source of phosphate is phosphate mines and top producers of phosphate fertilizers are United States, China and Morocco (FAO, 2004). Due to human interference, these natural resources of phosphate are speedily consumed and have reached at planetary limit (Rockström et al., 2009, Carpenter and Bennett, 2011). The continuous input of chemical fertilizer in particular soil not only reduces their nutrient status but also hazardous for sustainable crop production and environment (Shakeela et al., 2017). The high cost of fertilizers is another major problem for developing countries (López-Bellido et al., 2013).
The above consequences showed the requirement of effective strategies against synthetic fertilizers especially Phosphorus management in soil and it is a need of time to find out the alternate of these agrochemicals. In this regard, Soil microorganisms play remarkable role in improvement of crop productivity and soil health (Krishnaraj and Dahale, 2014, Zahid et al., 2015). Therefore, soil microorganisms are used as microbial inoculants/biofertilizer as alternative of chemical fertilizers since 1950s (Sharma et al., 2013). Because they perform various chemical and biological activities that improves crop health and productivity without causing any harm in ecosystem (Zhang et al., 2010). Phosphate solubilizing rhizobacteria play vital role in accumulation and transformation of phosphate to plant roots. Mostly phosphorus is absorbed during vegetative growth and this absorbed form of phosphorus is re-trans located in seeds and fruits (Eftekhari et al., 2010). It has been worldwide reported that by implementation of phosphate solubilizing bacteria inoculum increase growth and yield of many crops like wheat, maize and sweet potato (Calvo et al., 2010, Zahid et al., 2015, Majeed et al., 2015). In Pakistan Triticum aestivum used as a staple food and it is the third most cultivated cereal crop in all around the world. The adequate supply of phosphorus increases wheat growth while decrease in phosphate availability will reduced wheat yield (Noonari et al., 2016).
In view of above background, the aim of our study is the isolation of PSRB from different rhizosphere of plants, their identification and evaluation on the basis of plant growth promoting activities and development of sodium alginate beads biofertilizer for enhancement of wheat growth and nutrition.
2. Materials and methods
2.1. Rhizospheric soil sample collection
This research was conducted in department of Microbiology and Molecular Genetics, The Women University Multan, Pakistan. Total 70 rhizospheric soil samples were collected from various regions of south Punjab, Pakistan i.e. Multan, Khanewal, Vehari, Lodhran, Shujabad, Bahawalpur and Muzzaffergarh. These soil samples were collected in sterile polythene bags and brought into laboratory and stored at 4 °C for further analysis (Liu et al., 2016). Physiochemical characteristics of collected rhizospheric soil such as soil texture, pH, temperature, and electrical conductivity were also determined.
2.2. Isolation, selection and purification of phosphate solubilizing rhizobacteria
For isolation of bacterial colonies, one gram of rhizospheric soil was dispersed in 9 ml of distilled water for each sample separately, vortex it and made up to 10−6 folds by serial dilution method. 0.1 µl suspension was spread on NBRIP medium (Nautiyal, 1999) that amended with tri calcium phosphate (TCP 5.0 g/L) as a sole source of P and incubated at 37 °C for 7 days. The bacterial colonies with clear halo zone was picked and purified. The purified colonies were further maintained on L-agar plates and stored in refrigerator at 4 °C. Cultures were refreshed after every 15 days. Final selection of efficient PSRB was done by measuring of phosphate solubilizing index (PSI) of bacterial culture spots on NBRIP agar plate according to formula PSI = C + Z/C (Premono et al., 1996). In equation C = Colony diameter; Z = Halo zone diameter.
2.3. Quantitative estimation of phosphate solubilization
For quantitative estimation of phosphate solubilization, vanadomolybedate phosphoric method was used (Jackson, 1958). For this purpose NBRIP broth taken in 250 ml flask, inoculated with 24 h fresh bacterial cultures and incubated in shaking condition at 120 rev/min at 37 °C for 7 days. After that the broth was centrifuged for 10 min at 10,000 rpm and 0.5 ml supernatant was added in 2.5 ml Barton’s reagent then the volume was made up to 50 ml by adding distilled water, the flask was placed in dark for yellow color development. Finally, optical density was measured on UV–VIS spectrophotometer at 430 nm.
Standard solution of KH2PO4 with different concentrations (2 µg/ml) was prepared and processed in same way and standard curve was drawn for comparison to determine P solubilization in the bacterial culture supernatant.
2.4. Morphological and biochemical characterization
PSRB strains were grown on Luria Bertani agar (Gerhardt et al., 1994) for study of their morphological characteristics i.e. color, size, shape, texture and opacity under stereomicroscope while for observation of cell structure gram staining method was used (Gerhardt et al., 1994). Different biochemical tests such as catalase, macConkey’s agar, Vogus proskur, Nitrate reduction, indole production, starch hydrolysis, citrate utilization and acid production by carbohydrates were performed according to Bergey’s manual systematic (Holt et al., 1994).
2.5. Optimization of PSRB strains at different physical factors
For optimization purpose, PSRB strains were grown in NBRIP broth at temperature (25 °C, 30 °C, 35 °C, 45 °C, 50 °C), pH (5,6,7,8,9), Carbon sources (Glucose, Sucrose, lactose, mannitole, fructose), Nitrogen sources (ammonium sulphate, Urea, ammonium nitrate, potassium nitrate, calcium nitrate) and incubated in shaking condition at 120 rev/min for seven days then phosphate solubilization estimated by same method as described above.
2.6. Quantitative estimation of acid and alkaline phosphatase
For phosphatase estimation, PSRB were grown in NBRIP broth in shaking condition 120 rev/min at 37 °C for 7 days. In 1 ml supernatant 4 ml universal buffer and 1 ml of p-nitrophenyl phosphate solution were added, vortex it for few seconds then incubated for 1 h in dark at room temperature. After that to stop the reaction 4 ml NaOH and 1 ml CaCl2 were added in it. The intensity of yellow color was measured at 410 nm on UV–VIS spectrophotometer (Tabatabai and Bremner, 1969). Data were recorded as µm PNP/ml/hour.
For standard P-nitophenol solution was prepared with different concentrations and followed the same procedure as described above. Standard curve was formed between p-nitrophenol and bacterial culture p-nitrophenyl solution.
2.7. Plant growth promoting traits of PSRB
2.7.1. Auxin and siderophore production
For determination of auxin production, selected strains were grown in LB broth without L-tryptophane incubated in shaking condition 120 rev/min for 72 h at 37 °C. Bacterial cells were harvested by centrifugation at 10,000 rpm for 10 min. After centrifugation, supernatant was separated from pellet. 2 ml of supernatant was mixed with Salkowski reagent. Put it into dark for 60 min for color development. Finally, optical density measured at 535 nm by using UV spectrophotometer (Glickmann and Dessaux, 1995).
For Siderophore production, Chrome azurol S dye (CAS) and nutrient agar prepared separately then autoclaved both solutions. After that mixed both solutions and poured in petri dishes after solidification of media stab test of selected strains was performed on it. After 3 days of incubation bacteria with orange zone was considered as positive (Chauhan et al., 2015).
2.7.2. Ammonium and hydrogen cyanide production
For ammonium production, 10% peptone water was prepared, autoclaved and inoculated with selected strains. After 3 days of incubation, in 1 ml supernatant 0.5 ml Nessler’s reagent was added. Appearance of yellow to orange color was considered as positive (Kumar et al., 2012).
For Hydrogen cyanide production, selected strains were streaked on L agar plates that were amended with 0.44% glycine after that soaked the filter paper in 2% Sodium carbonate and 0.5 ml picric acid solution, placed on the agar plate and sealed with para film then incubated for 72 h at 37 °C. Change in color of filter paper from yellow to brown consider as positive (Bakker and Schippers, 1987).
2.8. Biocompatibility test
For determination of biocompatibility of selected strains, L agar plates were prepared and one strain was streaked as straight line while test strains line was drawn perpendicular of it, then the plates were incubated for 24–48 h at 37 °C. The growth of test strain that was inhibit at the intersection consider as bio-incompatible strain.
2.9. Plant microbe interaction
2.9.1. Seed germination assay
Certified seeds of Triticum aestivum (Var. Galaxy 2013) were obtained from “Punjab seed corporation Multan, Pakistan.” Healthy seeds were washed with autoclaved water, sterilized with 0.1% HgCl2 for 5 min and again washed extensively with autoclaved water. Then these Seeds were soaked in NBRIP liquid bacterial culture and incubated at 37 °C for 30 min. For control seeds were soaked in un-inoculated NBRIP broth. After that these seeds were put in petri plates on wet filter paper and placed in dark for 3 days. After seed germination, plates were transferred in room with condition (16:8 light:dark) for seven days. Experiment was performed in duplicate and following parameters were measured i.e. seed germination, root and shoot length, no of roots and root hairs.
2.9.2. Root colonization assay
Five days grown old seedlings of Triticum aestivum were taken from petri plates and dipped into NBRIP bacterial culture for seven days then the roots were washed with autoclaved water for cleaning of loosely attached bacteria. Slide was prepared with acridine orange and bacterial colonization with root was observed under light microscope (LM).
2.9.3. Preparation of sodium alginate beads with bacteria as biofertilizer
For this purpose, Liquid cultures of selected strains were prepared in NBRIP medium in both single and consortium form. 2% sodium alginate solution was prepared and autoclaved. Then bacterial culture mixed with sodium alginate solution and stirrer for 1 h. After that suspension was taken in syringe and drop wise added in CaCl2 solution, alginate beads were formed, stirrer it for 30 min. Filtered the beads, washed with water and dried. The viability of bacteria in alginate beads was determined as cfu/ml (Bashan, 1986).
2.9.4. Treatments used for pot experiment in duplicate
The experimental plan was based on following treatments: Pot1-WumS-3; Pot2-WumS-4; Pot3-WumS-5; Pot4-WumS-11; Pot5-WumS-12; Pot6-WumS-21; Pot7-WumS-24; Pot8-WumS-25; Pot9-WumS-26; Pot10-WumS-28; Pot 11-WumS-3 + WumS-4 + WumS-5, WumS-11 + WumS-12; Pot 12-WumS-21 + WumS-22 + WumS-24 + WumS-25 + WumS-28; Pot 13-all PSRB strains; Pot 14-control(un-inoculated).
2.9.5. Laboratory and field pot experiment
Before sowing the seeds soil analysis was done then soil was autoclaved and filled in pots.
For lab pot experiment, 200 g soil were filled in each plastic pot. To determine the effect of direct inoculum, sterilized seeds of wheat were dipped in NBRIP bacterial culture for 30 min at 37 °C. After that 5 seeds were sown in each pot at 2 cm depth in soil. For control un-inoculated seeds were also sown in same manner. To determine the effect of alginate bead biofertilizer treatment, the seeds were sown with 3 beads/seed in pot. After 15 days of emergence the plants were thinned out and growth parameters were measured.
For field pot experiment, 5 kg soil was filled in each pot and 15 seeds/pot were sown as described above for lab experiment. Plants were watered properly in equal manner. After 100 days when wheat was fully matured was harvested. For validation of results all experiment was conducted in duplicate. Following growth parameters such as seed germination percentage, root and shoot length, fresh and dry weight of root and shoot were measured.
2.9.6. Statistical analysis
Data were analyzed by using SPSS software, version 16.0. Comparison of means were made by Duncan’s multiple range test (P ≤ 0.05).
3. Results
3.1. Physiochemical analysis of rhizospheric soil samples
Mostly soil samples were collected from cultivated areas and some samples were collected from non-cultivated areas. The soil samples that collected from Multan and Vehari showed loamy texture but the soil collected from Bhawalpur, Khnewal and Muzaffargarh showed sandy loam texture while Lodhran and Shujabad soil have loamy clay texture. Due to different locations, variation in temperature was also observed. The minimum temperature was 25 °C and maximum temperature was 40 °C. The pH of mostly soil samples were neutral but some rhizospheric soil samples showed acidic and alkaline pH. The electric conductivity values of soil varied from 2.1 to 4.5 dSm−1.
3.2. Qualitative and quantitative screening of PSRB
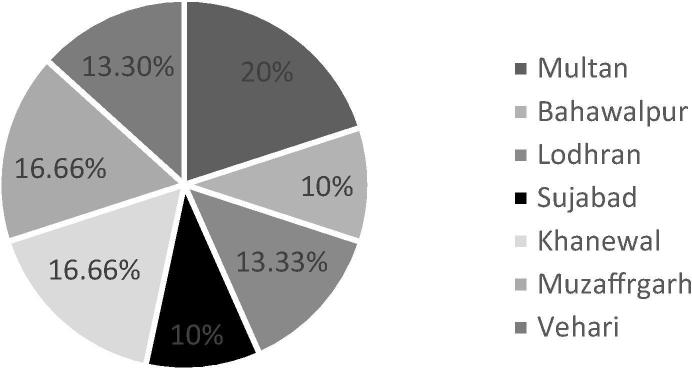
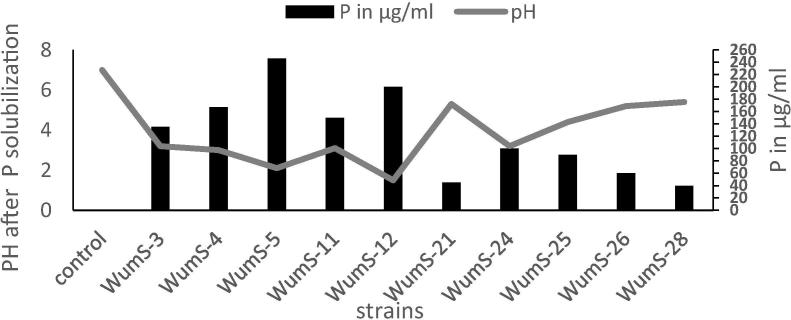
Total 30 strains were isolated, maximum 20% strains were isolated from Multan, 10% from Bhawalpur while 13.33%, 10%, 16.66%, 16.66%, 13.30% strains were obtained from Lodhran, Shujabad, Khanewal, Muzaffargarh and Vehari respectively on the basis of clear halo zone formation around colonies (Fig. 1). Out of these only 10 strains were selected on the basis of their high phosphate solubilization index (PSI) (Table 1). In NBRIP broth phosphate solubilization activity of selected strains was varied from 30 to 246 µg/ml using tricalcium phosphate as a source of insoluble P. The PS activity accompanied by decreased in medium pH (Fig. 2). No direct correlation was observed between ‘P’ solubilizing activity in solid and liquid assay.
Fig. 1.
Percentage of isolated strains exhibiting P solubilizing ability from different regions of Punjab.
Table 1.
Selected PSRB strains on the basis of phosphate solubilization index (PSI).
| Plant name | Isolated strains | Colony diameter (mm) | Halo zone diameter | Solubilization Index = C + Z/C | Location |
|---|---|---|---|---|---|
| Cicer arietinum | WumS-3 | 3.2 | 20.6 | 7.43 | Multan |
| Cicer arietinum | WumS-4 | 2.5 | 15.8 | 7.3 | Multan |
| Cicer arietinum | WumS-5 | 1.4 | 5.5 | 5.3 | Multan |
| Vigna radiata | WumS-11 | 1.4 | 9 | 7.42 | Lodhran |
| Colocasia esculenta | WumS-12 | 1.7 | 8.5 | 6.0 | Lodhran |
| Oryza sativa | WumS-21 | 1.8 | 9.2 | 6.1 | Kahanewal |
| Vigna radiate | WumS-24 | 1.5 | 6.9 | 5.60 | Muzaffargrrh |
| zea mays | WumS-25 | 1.3 | 5.8 | 5.46 | Muzaffargrrh |
| zea mays | WumS-26 | 1.2 | 7.7 | 7.4 | Muzaffargrrh |
| Allium cepa | WumS-28 | 1.2 | 7.7 | 7.41 | Vehari |
Fig. 2.
Quantitative estimation of P solubilization in NBRIP broth by selected PSRB accompanying with change in pH.
3.3. Morphological and biochemical characterization
10 selected PSRB strains were gram negative, motile and non-spore forming while their colony size varied from 0.5 mm to 0.8 mm and color of colony were mostly white (Table 2). All strains grow on macConkey’s agar and gave positive result except WumS-12. The catalase and citrase test were positive for all strains. Production of indole was positive for all strains except WumS-11 but most of the strains showed negative results for VP test while acid production by sugar utilization showed positive results for most of strain.
Table 2.
Morphological and biochemical characterization of PSRB strains.
| Morphological and biochemical characterization | Selected PSRB strains |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wums-3 | Wums-4 | Wums-5 | Wums-11 | Wums-12 | Wums-21 | Wums-24 | Wums-25 | Wums-26 | Wums-28 | ||
| Biochemical test | Size(mm) | 3.2 | 2.5 | 1.4 | 1.4 | 2.7 | 1.8 | 1.5 | 1.3 | 1.2 | 1.2 |
| Colony morphology | C,W,E,F,D,O | C,W,E,F,Mu,O | I,W,U,CO,Mo,O | C,PY,E,R,D,O | C,W,E,R,Mo,T | I,PY,L,R,Mo,O | C,OW,E,R,D,O | I,PY,L,R,Mu,O | I,W,U,CO,Mu,O | I,OW,U,CO,MO,O | |
| Gram stainig | − | − | − | − | − | − | − | − | + | − | |
| Spore staining | − | − | − | − | − | − | − | − | + | − | |
| MacConkey's agar | + | + | + | + | + | + | + | + | − | + | |
| Catalase test | + | + | + | + | + | + | + | + | + | + | |
| Motility | + | + | + | + | + | + | + | + | + | + | |
| Indole Production | + | + | + | − | + | + | + | + | + | + | |
| Citrase test | + | + | + | + | + | + | + | + | + | + | |
| Methyl red test | + | + | + | + | + | − | + | − | − | + | |
| Voges Proskauer test | + | + | + | − | − | + | − | − | − | + | |
| Starch hydrolysis | + | − | − | + | − | + | + | + | − | − | |
| Nitrate reduction | + | + | + | − | − | − | + | − | + | + | |
| Acid production from different sugars | Glucose | + | + | + | + | + | + | + | + | + | + |
| Sucrose | + | − | + | + | + | + | + | − | + | + | |
| Lactose | + | − | + | + | + | + | + | + | + | + | |
| Mannitole | + | − | + | − | + | − | + | + | + | + | |
| Maltose | + | − | + | + | + | + | + | + | + | + | |
Symbols: C = circular; I = irregular; W = white; OW = off white; PY = pale yellow; E = entire; L = lobate; U = undulate; F = flat; CO = convex; R = raised; D = dry; Mu = mucoid; Mo = moist; D = Dry; O = opaque; + = positive; − = negative.
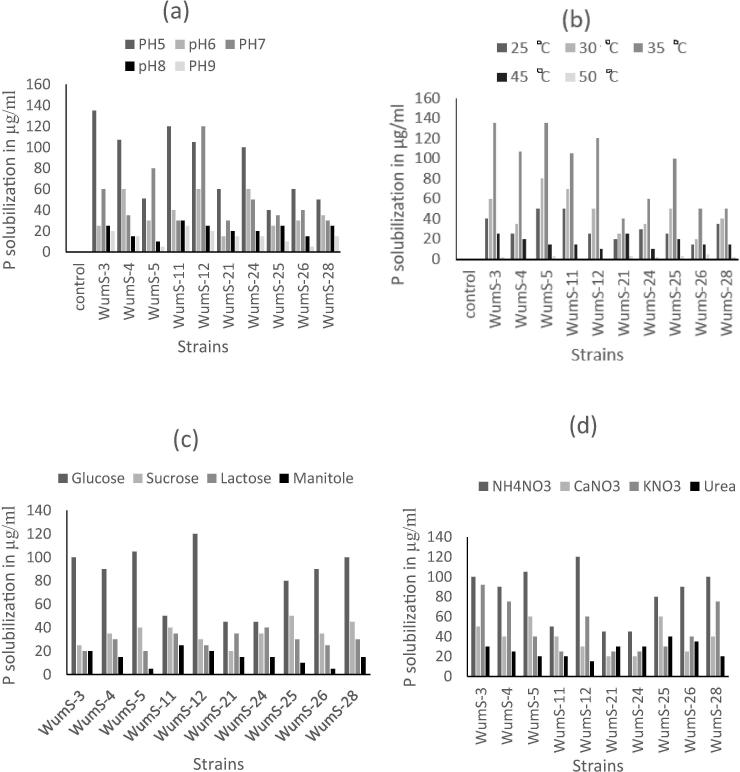
3.4. Optimization of PSRB at different pH, temperature, carbon sources and nitrogen sources
The optimum pH was 5–7 at which all the strains showed maximum phosphate solubilization activity. The highest Phosphate solubilization at pH 7 was 105 µg/ml and least 25 µg/ml (Fig. 3a). The optimum temperature was 35 °C at which all strains solubilize phosphate but the solubilization efficiency was decreased with increase in temperature 40–50 °C because only few bacteria can survive at high temperature (Fig. 3b).
Fig. 3.
Optimization of PSRB strains at different physical factors: (a) pH factor, (b) Temperature factor, (c) Carbon sources, (d) Nitrogen sources.
Glucose is the most beneficial carbon source at which all the strains showed maximum (30–120 µg/ml) phosphate solubilization while sucrose and lactose showed medium (15–25 µg/ml) and mannitole indicated minimum (5–20 µg/ml) phosphate solubilization efficiency (Fig. 3c). Different nitrogen sources also effects on the solubilization ability of selected strains. Ammonium nitrate is the best nitrogen source for hydrolysis of phosphate by all strains while urea is the least utilizable by PSRB strains (Fig. 3d).
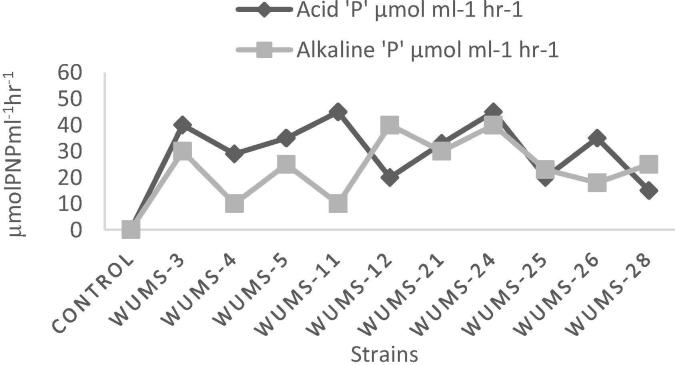
3.5. Estimation of alkaline and acid phosphatase
The highest production of acid phosphatase was indicated by WumS-11 (45 µM PNP ml−1 h−1) and least by WumS-28 (15 µM PNP ml−1 h−1) while in case of alkaline phosphatase enzyme production WumS-11 produced lowest quantity 10 µM PNP ml−1 h−1 and WumS-28 produced high quantity 25 µM PNP ml−1 h−1 of phosphatase enzyme (Fig. 4).
Fig. 4.
Acid and alkaline phosphatase enzyme production by PSRB strains in NBRIP media.
3.6. Plant growth promoting activities and biocompatibility of PSRB
Selected PSRB strains were tested for their plant growth promoting abilities (Table 3). All the strains have ability to produce IAA except WumS-11. The highest IAA 120 µg/ml produced by WumS-3 and lowest IAA 25 µg/ml produced by WumS-25. All strains give positive results for ammonium production. Except for WumS-25, mostly strains produced hydrogen cyanide and siderophore. All the strains were biocompatible with each other.
Table 3.
PGP activities and biocompatibility of selected PSRB strains.
| Strains | Auxin production (µg/ml) | Siderophore production | Ammonia production | HCN production | Biocompatibility test |
|---|---|---|---|---|---|
| WumS-3 | 120 (+++) | ++ | +++ | (++) | + |
| WumS-4 | 40 (+) | +++ | + | (+) | + |
| WumS-5 | 30(+) | − | +++ | (++) | + |
| WumS-11 | − | + | + | − | + |
| WumS-12 | 20 (+) | + | + | − | + |
| WumS-21 | 40 (+) | +++ | ++ | (+) | + |
| WumS-24 | 30 (+) | ++ | − | − | + |
| WumS-25 | 25 (+) | − | ++ | − | + |
| WumS-26 | 50 (++) | + | + | (+++) | + |
| WumS-28 | 100 (+++) | + | + | (++) | + |
Symbols: + = weak positive, ++ = medium positive, +++ = strong positive, − = negative no color.
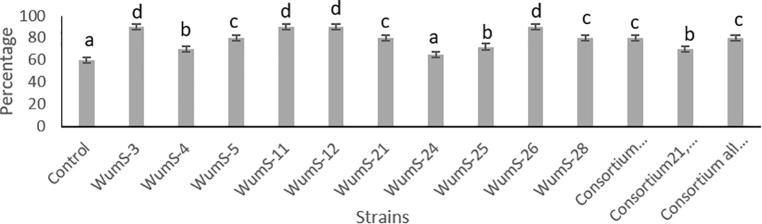
3.7. Seed germination assay on petri dish
Phosphate solubilizing rhizobacteria led significant increase (P < 0.05) in seed germination, root length, shoot length, number of roots and root hairs of wheat (Fig. 5a, Fig. 5b). Seed germination increase 65–90% as compared to control. The inoculation of consortium caused the maximum increase (76.19%) in root length followed by WumS-12 strain (73.80%), WumS-21 (69.04%) and WumS-3 (45.23%) when compared with that of non-inoculated seedlings. On comparing shoot length percentage with control, we observed that co-inoculum of all strains showed significant increase in shoot length (98.61%) followed by WumS-12 (97.22%), WumS-21 (91.66%) and WumS-3(83.33%). The number of roots were also affected by inoculated strains WumS-4 added more root number (91.66%) as compared to control (Table 4).
Fig. 5a.
Effect of PSRB strains on root and shoot length in Petri dish; in (a) root length and in (b) shoot length of control seedling compared with inoculated seedling.
Fig. 5b.
Effect of PSRB strains on seed germination percentage of wheat in petri dish; Values are the mean of n = 5; Data was expressed with standard error of means, The significant difference among different PSRB strain and control plants was expressed as different letters by using Duncan’s multiple range test, sharing the same letter do not differ significantly at the means of (P < 0.05).
Table 4.
Effect of PSRB strains inoculum on different parameters of wheat in petri dish.
| Strains | Root length (cm) | Shoot length (cm) | No of roots | Root hairs | Root colonization |
|---|---|---|---|---|---|
| Control | 8.4 ± 0.40a | 7.2 ± 0.48a | 4.8 ± 0.20a | + | + |
| WumS-3 | 12.2 ± 0.37e | 13.2 ± 0.97f | 6.0 ± 0.00c | ++ | ++ |
| WumS-4 | 9.6 ± 0.24b | 11.6 ± 0.82d | 6.8 ± 0.20c | ++ | + |
| WumS-5 | 11.2 ± 0.48d | 12.4 ± 0.24e | 9.2 ± 0.80f | +++ | +++ |
| WumS-11 | 8.6 ± 0.24 | 9.6 ± 0.24b | 5.8 ± 0.20b | +++ | +++ |
| WumS-12 | 14.6 ± 0.24f | 14.2 ± 0.20g | 6.0 ± 0.01c | +++ | +++ |
| WumS-21 | 14.2 ± 0.37f | 13.8 ± 0.20f | 5.4 ± 0.40b | ++ | ++ |
| WumS-24 | 9.7 ± 0.44b | 9.8 ± 0.20b | 5.6 ± 0.24b | +++ | +++ |
| WumS-25 | 11.4 ± 0.24d | 10.4 ± 0.24c | 9.0 ± 0.03f | +++ | +++ |
| WumS-26 | 10.4 ± 0.24c | 11.6 ± 0.24d | 6.8 ± 0.20c | ++ | ++ |
| WumS-28 | 9.8 ± 0.31b | 14.3 ± 0.24g | 6.20 ± .20c | +++ | +++ |
| Consortium 3,4,5,11,12 strains | 14.4 ± 0.24f | 13.8 ± 0.48f | 8.8 ± 0.20 e | +++ | +++ |
| Consortium 21,24,25,26, 28 strains | 12.4 ± 0.24e | 13.2 ± 0.73f | 7.6 ± 0.40 d | +++ | +++ |
| Consortium all strains | 14.8 ± 0.20f | 14.3 ± 0.80g | 8.0 ± 0.00 e | +++ | +++ |
Values are the mean of n = 5; Data was expressed with standard error of means, The significant difference among different PSRB strain and control plants was expressed as different letters by using Duncan’s multiple range test, sharing the same letter do not differ significantly at the means of (P < 0.05). Symbols: + = Positive, ++ = medium positive, +++ = strong positive.
3.8. Light microscopy and root colonization
Proliferation of root hairs of PSRB inoculated Triticum aestivum seedlings was observed under light microscope. Inoculation of PSRB strains have pronounced effect on root hairs growth as compared to control. Majority of the strains WumS-5, WumS-11, WumS-12, WumS-24, WumS-25, WumS-28, Consortium 3–12 strains, consortium 21–28 strains, consortium of all strains showed strong (+++) effect on root hairs length, and WumS-3, WumS-4, WumS-26 showed medium (++) effect on root hairs length as compared to control (Table 4).
The results of root colonization assay showed that bacteria were successfully attached with roots. In keeping view under microscope the strains divide into three categories i.e. weak, medium and strong root colonizer. So WumS-12, WumS-25, WumS-26 and WumS-28 showed strong root colonization while WumS-3, WumS-5, WumS-21, WumS-24 were medium and WumS-4 were weak root colonizers (Table 4).
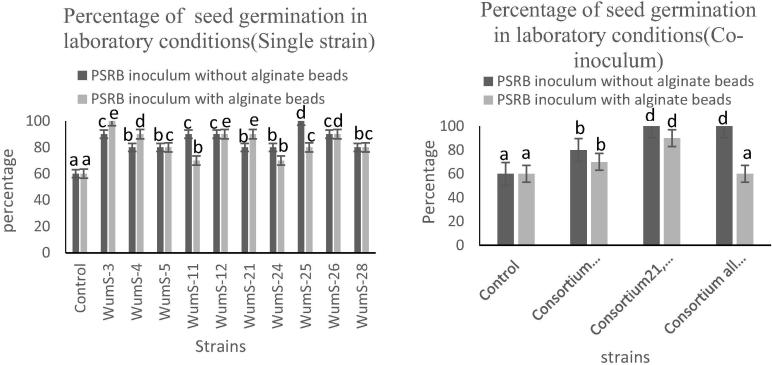
3.9. Effect of PSRB inoculum on Triticum aestivum in laboratory conditions
PSRB based biofertilizer led the significant (P < 0.05) effect on growth parameters of Triticum aestivum such as decrease seed germination date while increase shoot, root length and biomass of Triticum aestivum. With direct inoculum of PSRB, Strains WumS-12, WumS-25 and consortium of all strains gives 80% growth as compare to control while strain WumS-3 and WumS-11 90% increase seed germination rate (Fig. 6a). Maximum length of root was 16 cm and maximum length of shoot was 29 cm in single strain inoculum but in consortium based inoculum it was observed that 18 cm root and 24 cm shoot length increased that was 90% greater than control (Table 5a). With alginate beads biofertilizer, strains WumS-3 and WumS-12 given 85% increase in seed germination as compare to control but in consortium of all strains in alginate beads showed no better effect as compare to control. Single strain inoculum given maximum increase in length of root 15 cm and maximum length of shoot 28 cm, in consortium of all strains 16 cm root and 29 cm shoot increased that was 80% greater than control. If we see the biomass of root and shoot it was also remarkably increased as compared to control (Table 5b).
Fig. 6a.
Effect of PSRB with and without alginate beads on seed germination in laboratory conditions; Data was expressed with standard error of means, The significant difference among different PSRB strain and control plants was expressed as different letters by using Duncan’s multiple range test, sharing the same letter do not differ significantly at the means of (P < 0.05).
Table 5a.
Effect of PSRB direct inoculum (without alginate beads) on growth of wheat in laboratory.
| Strains | Direct a inoculum |
|||||
|---|---|---|---|---|---|---|
| R.L | S.L | F.R.W | F.S.W | D.R.W | D.S.W | |
| Control | 9.8 ± 1.2a | 15.2 ± 0.7a | 0.110 ± 0.1a | 0.160 ± 0.2a | 0.077 ± 0.1a | 0.112 ± 0.3a |
| WumS-3 | 13.8 ± 1.2d | 25.0 ± 2.9e | 0.175 ± 0.0f | 0.195 ± 0.3d | 0.105 ± 0.0b | 0.150 ± 0.00ef |
| WumS-4 | 19.0 ± 0.5h | 23.2 ± 1.2c | 0.189 ± 0.2g | 0.182 ± 0.1c | 0.140 ± 0.2d | 0.132 ± 0.01c |
| WumS-5 | 15.8 ± 0.4e | 25.0 ± 0.3e | 0.147 ± 0.2d | 0.178 ± 0.1b | 0.109 ± 0.4b | 0.120 ± 0.01b |
| WumS-11 | 16.6 ± 0.4f | 28.0 ± 0.2i | 0.182 ± 0.0g | 0.188 ± 0.4c | 0.115 ± 0.0c | 0.118 ± 0.00a |
| WumS-12 | 13.6 ± 1.1d | 25.0 ± 1.0e | 0.154 ± 0.1e | 0.342 ± 0.1f | 0.110 ± 0.1bc | 0.200 ± 0.01f |
| WumS-21 | 15.8 ± 0.2e | 21.6 ± 0.6b | 0.159 ± 0.1e | 0.190 ± 0.5d | 0.112 ± 0.4c | 0.113 ± 0.00a |
| WumS-24 | 16.2 ± 0.7f | 27.6 ± 0.6g | 0.156 ± 0.1e | 0.161 ± 0.1a | 0.100 ± 0.7b | 0.117 ± 0.01a |
| WumS-25 | 12.2 ± 0.5c | 24.6 ± 0.4d | 0.136 ± 0.0c | 0.162 ± 0.1a | 0.102 ± 0.9b | 0.115 ± 0.01a |
| WumS-26 | 11.4 ± 0.2b | 29.2 ± 0.4j | 0.187 ± 0.2g | 0.167 ± 0.2a | 0.113 ± 0.3c | 0.130 ± 0.02c |
| WumS-28 | 13.6 ± 0.4d | 27.0 ± 0.3g | 0.125 ± 0.0b | 0.224 ± 0.1e | 0.101 ± 0.3b | 0.134 ± 0.01c |
| Consortium 3,4,5,11,12 strains | 13.2 ± 0.7d | 29.0 ± 0.6j | 0.234 ± 0.3hi | 0.192 ± 0.1d | 0.178 ± 0.5f | 0.122 ± 0.01b |
| Consortium21,24,25,26,28 strains | 18.8 ± 0.4g | 24.0 ± 0.2d | 0.213 ± 0.1hi | 0.233 ± 0.1e | 0.150 ± 0.6de | 0.112 ± 0.01a |
| Consortium all strains | 16.6 ± 0.2f | 29.4 ± 0.5j | 0.206 ± 0.1h | 0.234 ± 0.1e | 0.104 ± 0.2b | 0.140 ± 0.01e |
Values are the mean of n = 5; Data was expressed with standard error of means, The significant difference among different PSRB strain and control plants was expressed as different letters by using Duncan’s multiple range test, sharing the same letter do not differ significantly at the means of (P < 0.05). Symbols: L.R: length of root; L.S = length of shoot; D.W.R = dry weight of root; D.W.S = dry weight of shoot.
Table 5b.
Effect of PSRB based alginate beads on growth of wheat in laboratory.
| Strains | Alginate beads |
|||||
|---|---|---|---|---|---|---|
| R.L | S.L | F.R.W | F.S.W | D.R.W | D.S.W | |
| Control | 8.5 ± 0.4a | 14.2 ± 0.7a | 0.120 ± 0.0a | 0.131 ± 0.2a | 0.104 ± 0.06a | 0.100 ± 0.02a |
| WumS-3 | 14.8 ± 0.4e | 22.8 ± 1.1c | 0.169 ± 0.1c | 0.175 ± 0.1d | 0.115 ± 0.00ab | 0.120 ± 0.01bc |
| WumS-4 | 14.4 ± 1.1e | 25.6 ± 0.8e | 0.170 ± 0.0d | 0.167 ± 0.0c | 0.123 ± 0.00c | 0.114 ± 0.00b |
| WumS-5 | 13.2 ± 0.7d | 27.0 ± 0.5g | 0.167 ± 0.0c | 0.183 ± 0.1e | 0.105 ± 0.00a | 0.111 ± 0.01b |
| WumS-11 | 12.8 ± 0.3c | 22.8 ± 0.7c | 0.167 ± 0.1c | 0.185 ± 0.0e | 0.112 ± 0.01ab | 0.124 ± 0.00bc |
| WumS-12 | 10.8 ± 0.8b | 19.4 ± 0.5b | 0.182 ± 0.0e | 0.178 ± 0.2d | 0.108 ± 0.00a | 0.110 ± 0.00a |
| WumS-21 | 13.0 ± 0.8d | 22.6 ± 0.6c | 0.172 ± 0.1d | 0.178 ± 0.1d | 0.103 ± 0.01a | 0.100 ± 0.01a |
| WumS-24 | 12.6. ± 0.6c | 19.6 ± 0.8b | 0.123 ± 0.1a | 0.196 ± 0.1f | 0.109 ± 0.01a | 0.115 ± 0.01b |
| WumS-25 | 15.2 ± 0.7f | 23.0 ± 0.2d | 0.156 ± 0.0b | 0.157 ± 0.1b | 0.108 ± 0.00a | 0.105 ± 0.01a |
| WumS-26 | 12.6 ± 0.2c | 26.8 ± 0.9f | 0.158 ± 0.2b | 0.168 ± 0.1c | 0.123 ± 0.02c | 0.113 ± 0.01b |
| WumS-28 | 12.0 ± 0.3c | 27.2 ± 0.8g | 0.183 ± 0.1e | 0.194 ± 0.1f | 0.116 ± 0.01ab | 0.100 ± 0.01a |
| Consortium 3,4,5,11,12 strains | 15.8 ± 0.4f | 25.4 ± 0.8e | 0.166 ± 0.1c | 0.159 ± 0.1b | 0.109 ± 0.01a | 0.112 ± 0.01b |
| Consortium 21,24,25,26,28 strains | 12.0 ± 0.4c | 26.2 ± 0.4f | 0.181 ± 0.0e | 0.175 ± 0.3d | 0.113 ± 0.00ab | 0.102 ± 0.03a |
| Consortium all strains | 13.4 ± 0.4d | 27.0 ± 0.2g | 0.197 ± 0.1ef | 0.203 ± 0.1e | 0.106 ± 0.01a | 0.120 ± 0.01bc |
Values are the mean of n = 5; Data was expressed with standard error of means, The significant difference among different PSRB strain and control plants was expressed as different letters by using Duncan’s multiple range test, sharing the same letter do not differ significantly at the means of (P < 0.05). Symbols: L.R: length of root; L.S = length of shoot; D.W.R = dry weight of root; D.W.S = dry weight of shoot.
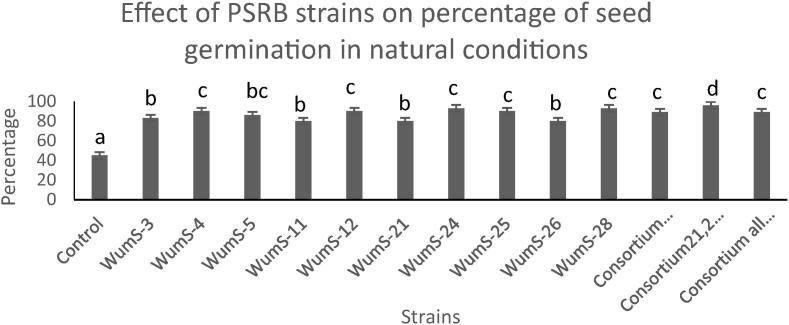
3.10. Effect of PSRB inoculum on Triticum aestivum in natural conditions
In natural condition experiment, the seed germination percentage increase 20–80% as compared to control (Fig. 6b). The Phosphate solubilizing rhizobacterial inoculum increased 5–34.8% shoot length as compared to control while 5–96% increase in seed weight was also observed. WumS-3, Wums-5 and consortium of strains were more effective than other strains in field condition but in comparison with non-inoculated all strains showed effective growth of plant (Table 5c). The rhizobacterial strains also showed good effects on spike length and seed weight.
Fig. 6b.
Effect of PSRB inoculum on seed germination in natural conditions, n = 10; Data was expressed with standard error of means, The significant difference among different PSRB strain and control seeds was expressed as different letters by using Duncan’s multiple range test, sharing the same letter do not differ significantly at the means of (P < 0.05).
Table 5c.
Effect of PSRB strains on wheat with and without alginate beads in natural conditions.
| Strains | Shoot length before alginate beads |
Shoot length after alginate beads inoculation |
|||||
|---|---|---|---|---|---|---|---|
| 7 days | 14 days | 21 days | 28 days | 42 days | 56 days | 100 days | |
| Control | 7.5 ± 1.1a | 12.6 ± 0.5a | 13.8 ± 0.6a | 15.5 ± 0.5a | 20.5 ± 0.5a | 23.5 ± 0.8a | 36.2 ± 0.3a |
| WumS-3 | 13.6 ± 1.1fg | 19.7 ± 1.4h | 21.6 ± 2.1f | 24.2 ± 0.9i | 32.2 ± 0.4g | 37.0 ± 0.8h | 45.8 ± 0.6h |
| WumS-4 | 11.05 ± 0.8b | 16.4 ± 0.5d | 18.6 ± 0.8bc | 21.4 ± 0.6e | 28.7 ± 1.0e | 34.5 ± 1.0f | 37.5 ± 1.1b |
| WumS-5 | 15.1 ± 0.1h | 16.0 ± 0.4d | 18.4 ± 0.5b | 23.3 ± 0.2g | 43.6 ± 0.4i | 40.2 ± 0.6j | 46.7 ± 0.9i |
| WumS-11 | 12.9 ± 0.8e | 15.5 ± 1.0bc | 18.5 ± 0.4b | 21.5 ± 0.5e | 29.0 ± 0.1ef | 33.1 ± 0.3f | 46.5 ± 0.8i |
| WumS-12 | 12.4 ± 0.7d | 17.6 ± 0.5f | 19.4 ± 0.4d | 20.5 ± 0.7cd | 27.5 ± 0.6d | 30.2 ± 1.4c | 38.2 ± 0.7c |
| WumS-21 | 13.1 ± 0.9ef | 17.0 ± 1.0ef | 18.0 ± 0.8b | 19.3 ± 0.8b | 25.4 ± 0.2b | 35.6 ± 07g | 41.1 ± 0.8e |
| WumS-24 | 13.2 ± 1.0ef | 14.8 ± 0.7b | 19.1 ± 0.9d | 20.6 ± 0.4d | 25.9 ± 0.9b | 30.1 ± 0.5c | 38.1 ± 0.4c |
| WumS-25 | 11.9 ± 0.8c | 17.4 ± 0.4ef | 18.6 ± 0.5bc | 20.7 ± 0.2d | 26.0 ± 0.8c | 28.9 ± 0.3b | 38.5 ± 0.28c |
| WumS-26 | 12.4 ± 0.5d | 18.6 ± 0.6g | 19.6 ± 0.4de | 22.2 ± 1.6ef | 27.2 ± 1.0d | 33.2 ± 0.8f | 40.1 ± 0.28d |
| WumS-28 | 13.4 ± 1.1f | 16.9 ± 0.9de | 19.1 ± 0.6d | 20.2 ± 0.8cd | 27.1 ± 0.9d | 39.1 ± 1.0i | 42.7 ± 0.37f |
| Consortium 3,4,5,11,12 strains | 13.6 ± 0.6f | 15.6 ± 0.5bc | 18.0 ± 0.7b | 19.0 ± 0.9b | 27.7 ± 0.3d | 31.0 ± 0.9d | 45.9 ± 0.2g |
| Consortium 21,24,25,26,28 strains | 12.3 ± 0.2d | 16.6 ± 1.3d | 18.6 ± 0.9bc | 19.9 ± 0.8bc | 29.4 ± 0.6f | 32.4 ± 0.7e | 43.2 ± 0.3fg |
| Consortium all strains | 15.3 ± 0.9h | 17.3 ± 0.9ef | 19.6 ± 0.5de | 23.8 ± 0.9gh | 37.0 ± 0.7h | 37.9 ± 0.6h | 48.1 ± 0.2j |
Values are the mean of n = 10; Data was expressed with standard error of means, The significant difference among different PSRB strain and control plants was expressed as different letters by using Duncan’s multiple range test, sharing the same letter do not differ significantly at the means of (P < 0.05).
4. Discussion
The excessive use of chemical fertilizers in agriculture field makes a country self-dependent in food production but causes worse effects on soil health, environment, economy and also harmful for living beings. Another adverse effect of increased use of fertilizers is that an important nutrients like phosphorus become unavailable for plant uptake without this fact that large amount of P is present in soil. To solve this problem, bio-agents i.e. plant growth promoting rhizobacteria or biofertilizers play an important role in providing nutrients and other necessary plant growth promoting substances without disrupting the agro-ecosystem and environment (Youssef and Eissa, 2014).
In present study total 30 strains were isolated from different rhizospheric soil samples on the basis of clear halo zone formation around bacterial colonies on NBRIP agar plates and only those strains were selected that showed high phosphate solubilization index 4–7 PSI. The result was in accordance with (Shakeela et al., 2017) and (Rfaki et al., 2015) as phosphate solubilizing bacteria were selected on the basis of halo zone formation and measure solubilizing index 3.0-4SI or 1.85–2.88SI respectively. Accompanying this Phosphate solubilization activity of selected isolates varied from 30 to 246 µg/ml in NBRIP broth. The results of this analysis were in accordance with previous studies (Rfaki et al., 2015), as soluble P range 41.20–119.95 µg/ml in NBRIP medium. After that selected PSRB strains were characterized morphologically, physiologically and biochemically. Most of the strains were gram negative cocci or rods and some of strains were gram positive rods. It is according to the study of (Khan et al., 2010) as different forms of bacteria i.e. bacilli, cocci and spiral are found in soil and important property of gram negative bacteria is mineralization of phosphate. The present investigation that 83.33% strains were gram negative is the agreement with the study of (Mujahid et al., 2015), as 90% PSRB strains were gram negative. The ability of phosphate solubilization also varied with environmental conditions. So, in this study optimization of selected strains were also done at different physical factors. The optimum temperature was 35 °C but all strains also survived at higher temperature 40–45 °C while optimum pH5-7, best nitrogen and carbon sources ammonium nitrate and glucose respectively. It showed that selected strains had ability to survive in harsh environmental conditions. The results are agreement with research of (Sharan et al., 2008, Mujahid et al., 2015) as all the strains can solubilize P at 37 °C, optimum pH 5–7, best nitrogen and carbon source are ammonium and glucose. Plant growth promotion by bacteria is a complex phenomenon and it can be achieved by activities of more than one PGP traits that performed by associated bacterium. Therefore, selected PSRB strains were further evaluated for plant growth promoting activities and results showed that maximum IAA production 120 µg/ml was exhibited by WumS-3 while WumS-4 and WumS-21 was the good producer of siderophore. Similarly, the strains WumS-3 and WumS-5 exhibited very good production of ammonia while WumS-26 had very high HCN production ability. So, if we see according to their percentage, 83.33% strains had auxin and ammonia producers while 66.66% strains had HCN and siderophore producers. All these characteristics of PSRB help in plant growth promotion and make them able to survive in harsh environmental conditions and earlier researcher already explained that single strain of phosphate solubilizing bacteria exhibited multiple PGP traits (Shakeela et al., 2017). These results are closely related to the study (Zhang et al., 2017), as 61.5% strains were auxin and 46.2% strains were siderophore producers. It also revealed from the study of (Shakeela et al., 2017) as 95% strains IAA producer, 100% strains produce siderophore and 67.5% strains were HCN producers.
We can implement the soil microorganisms i.e. PS bacteria in single or in consortium form as an alternative of costly chemical fertilizer in agriculture field (Krishnaveni, 2010, Zhu et al., 2011, Dugar et al., 2013, Sharma et al., 2013, Khan et al., 2013). So, after isolation and characterization of PSRB strains on the basis of multifarious plant growth promoting abilities, we applied these strains inoculum on Triticum aestivum and check their effect under laboratory and field conditions. We investigate the Phosphate solubilizing rhizobacteria led the significantly (P < 0.05) increase root, shoot length and number of roots of Triticum aestivum.
In lab condition, the PSRB strains showed 80–90% increase in seed germination while 10–95% increase in root length and shoot length as compared to control. Under natural conditions 80–96% increase in seed germination, in shoot length 5–34.8% increased while 5–96% increase in seed weight was observed. WumS-3, Wums-5 and consortium of strains was more effective than other strains in field condition but in comparison with non-inoculated all strains showed significant growth of plant. Many researchers also demonstrated that PSB could improve growth of wheat plant and other crops (Ahmad et al., 2014). Increases in plant root and shoot biomass were observed in different crops in response to PGPR bacteria as witnessed in this study (Yildirim et al., 2008, Yildirim et al., 2011a, Yildirim et al., 2011b). Another researcher also performed the field study and checked the consequence of PS bacteria on wheat and revealed that inoculum of PSB strains with and without P fertilizers caused significant effect on growth parameters of wheat i.e. Numbers of tillers, length of spike, grain yield and grain weight (Akhtar et al., 2013).
In this study we were able to isolate such phosphate solubilizing rhizobacteria which can promote plant growth from local rhizospheric soil samples. They improve soil fertility and productivity. Phosphate solubilizing activity varied between the PS strains. But they have potential to increase various growth parameters of Triticum aestivum. Most beneficial strains were WumS-3, WumS-4, WumS-5 and co inoculum of all strains that could be used as bio-inoculants for good growth and yield of wheat plant. However, further research is required to explore their potential as biofertilizer and genetic stability.
Acknowledgement
The author greatly acknowledges the financial support from Higher Education Commission and Department of MMG, The Women University Multan, Pakistan.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Shumaila Batool, Email: shumaila.shakeel124@gmail.com.
Atia Iqbal, Email: atia.iqbal@wum.edu.pk.
References
- Ahmad, E., Zaidi, A., Khan, M.S., 2014. Response of PSM Inoculation to Certain Legumes and Cereal Crops. vol. 1, Springer, 175–205.
- Akhtar, N., Arshad, I., Shakir, M.A., Qureshi, M.A., Sehrish, J., Ali, L., 2013. Co-inoculation with Rhizobium and Bacillus sp. to improve the phosphorus availability and yield of wheat (Triticum aestivum L.). J. Appl. P. Sci., 23, 190–197.
- Awasthi R., Tewari R., Nayyar H. Synergy between plants and p-solubilizing microbes in soils: effects on growth and physiology of crops. Int. Res. J. Microbiol. 2011;2:484–503. [Google Scholar]
- Bakker A.W., Schippers B. Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp-mediated plant growth-stimulation. Soil Biol. Biochem. 1987;19:451–457. [Google Scholar]
- Bashan Y. Alginate beads as synthetic inoculant carriers for slow release of bacteria that affect plant growth. Appl. Environ. Microbiol. 1986;51(5):1089–1098. doi: 10.1128/aem.51.5.1089-1098.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo P., Ormeño-Orrillo E., Martínez-Romero E., Zúñiga D. Characterization of Bacillus isolates of potato rhizosphere from Andean soils of Peru and their potential PGPR characteristics. Braz. J. Microbiol. 2010;41:899–906. doi: 10.1590/S1517-83822010000400008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S.R., Bennett E.M. Reconsideration of the planetary boundary for phosphorus. Environ. Res. Lett. 2011;6(1):1458–1459. [Google Scholar]
- Chauhan A., Shirkot C.K., Kaushal R., Rao D.L.N. Plant-Growth-Promoting Rhizobacteria (PGPR) and Medicinal Plants. Springer; Switzerland: 2015. Plant growth-promoting rhizobacteria of medicinal plants in NW Himalayas: current status and future prospects; pp. 381–412. [Google Scholar]
- Dugar G., Gopinath B., Arun B., Sharma S. Plant growth promoting abilities of phosphate solubilizers from the rhizosphere of Parthenium hysterophorus. Afr. J. Microbiol. Res. 2013;7:147–151. [Google Scholar]
- Eftekhari G., Fallah A.R., Akbari G.A., Mohaddesi A., Allahdadi I. Effect of phosphate solubilizing bacteria and phosphate fertilizer on rice growth parameters. Iran. J. Soil Water Res. 2010;23:229–238. [Google Scholar]
- FAO, I., IMF, O., UNCTAD, W., 2011. The World Bank, the WTO, IFPRI and the UN HLTF (2011). Price Volatility in Food and Agricultural Markets: Policy Responses. Rome, FAO.
- FAO/WHO Expert committee on food additives, & world health organization, 2004. Evaluation of certain food additives and contaminants: sixty-first report of the joint FAO/WHO expert committee on food additives. WHO, 61.
- Gerhardt, P., Murray, R.G.E., Wood, W., Krieg, N.R., 1994. Methods for general and molecular bacteriology. Amric. Soci. Microbiol., Washington, D.C. USA.
- Glickmann E., Dessaux Y. A critical examination of the specificity of the Salkowski’s reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 1995;61:793–796. doi: 10.1128/aem.61.2.793-796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J.G., Kreig N.R., Sneath P.H.A., Staley J.T., Williams S.T. Williams and Wilkins; Baltimore, USA: 1994. Bergey’s Manual of Determinative Bacteriology. [Google Scholar]
- Jackson, M.L., 1958. Soil Chemical Analysis. Prentice-Hall, Inc. Englewood Cliffs, N.J., USA, pp. 214–221.
- Khan M.S., Zaidi A., Ahemad M., Oves M., Wani P.A. Plant growth promotion by phosphate solubilizing fungi–current perspective. Arch. Agro. Soil Sci. 2010;56:73–98. [Google Scholar]
- Khan, M.S., Ahmad, E., Zaidi, A., Oves, M., 2013. Functional aspect of phosphate-solubilizing bacteria: importance in crop production. Bact. in Agrobiol.: Crop Produc., pp. 237–263.
- Krishnaraj P.U., Dahale S. Mineral phosphate solubilization: concepts and prospects in sustainable agriculture. Indian Nation. Sci. Acad. 2014;80:389–405. [Google Scholar]
- Krishnaveni M.S. Studies on phosphate solubilizing bacteria (PSB) in rhizosphere and non-rhizosphere soils in different varieties of foxtail millet (Setaria italica) Int. J. Agri. Food Sci. Technol. 2010;1:23–39. [Google Scholar]
- Kumar A., Kumar A., Devi S., Patil S., Payal C., Negi S. Isolation, screening and characterization of bacteria from rhizospheric soils for different plant growth promotion (PGP) activities: an in vitro study. Recent Res. Sci. Technol. 2012;4(1):01–05. [Google Scholar]
- Kumar A., Maurya B.R., Raghuwanshi R. Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.) Biocatal. Agri. Biotechnol. 2014;3:121–128. [Google Scholar]
- Lemanski K., Scheu S. Fertilizer addition lessens the flux of microbial carbon to higher trophic levels in soil food webs of grassland. Oecologia. 2014;176:487–496. doi: 10.1007/s00442-014-3037-0. [DOI] [PubMed] [Google Scholar]
- Liu M., Liu X., Cheng B.S., Ma X.L., Lyu X.T., Zhao X.F. Selection and evaluation of phosphate-solubilizing bacteria from grapevine rhizospheres for use as biofertilizers. Spanish J. Agri. Res. 2016;14:1106. [Google Scholar]
- López-Bellido L., Muñoz-Romero V., López-Bellido R.J. Nitrate accumulation in the soil profile: long-term effects of tillage, rotation and N rate in a mediterranean vertisol. Soil Till. Res. 2013;130:18–23. [Google Scholar]
- Majeed A., Abbasi M.K., Hameed S., Imran A., Rahim N. Isolation and characterization of plant growth promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front Microbiol. 2015;6:198. doi: 10.3389/fmicb.2015.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujahid T.Y., Subhan S.A., Wahab A., Masnoon J., Ahmed N., Abbas T. Effects of different physical and chemical parameters on phosphate solubilization activity of plant growth promoting bacteria isolated from indigenous soil. J. Pharm. Nutri. Sci. 2015;5(1):64–70. [Google Scholar]
- Nautiyal C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. Fems Microbiol Lett. 1999;170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- Noonari S., Kalhoro S.A., Ali A., Mahar A., Raza S., Ahmed M. Effect of different levels of phosphorus and method of application on the growth and yield of wheat. Natur. Sci. 2016;8:305–314. [Google Scholar]
- Premono, M.E., Moawad, A.M., Vlek, P.L.G., 1996. Effect of phosphate-solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. REP-12113.
- Rfaki, A., Nassiri, L., Ibijbijen, J., 2015. Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of faba bean (Vicia faba L.) in Meknes Region, Morocco. Brit. Microbiol Res. J., 6(5), 247–254.
- Rockström J., Steffen W., Noone K., Persson A., Chapin F.S., III, Lambin E. Planetary boundaries: exploring the safe operating space for humanity. Eco. Soci. 2009;14:1–15. [Google Scholar]
- Shakeela S., Padder S.A., Bhat Z.A. Isolation of phosphate solubilising rhizobacteria and endorhizobacteria from medicinal plant Picrorhiza kurroa and their optimization for tricalcium phosphate solubilization. The Pharma Inn. 2017;6(6):160–170. [Google Scholar]
- Sharan A., Darmwal N.S., Gaur R. Xanthomonas campestris, a novel stress tolerant, phosphate-solubilizing bacterial strain from saline–alkali soils. W. J. Microbiol. Biotechnol. 2008;24:753–759. [Google Scholar]
- Sharma S.B., Sayyed R.Z., Trivedi M.H., Gobi T.A. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springer Plus. 2013;2(1):587–602. doi: 10.1186/2193-1801-2-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson, F.J., Cole, M.A., 1999. Cycles of Soil: Carbon, nitrogen, phosphorus, sulfur, micronutrients, 2nd ed. John Wiley & Sons: New York, NY, USA, 1999. p. 427, ISBN 978-0-471-32071-5.
- Tabatabai M.A., Bremner J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969;1:301–307. [Google Scholar]
- Yildirim E.R.T.A.N., Turan M.E.T.I.N., Donmez M.F. Mitigation of salt stress in radish (Raphanus sativus L.) by plant growth promoting rhizobacteria. Rouma. Biotechnol. Lett. 2008;13:3933–3943. [Google Scholar]
- Yildirim E., Karlidag H., Turan M., Dursun A., Goktepe F. Growth, nutrient uptake and yield promotion of broccoli by plant growth promoting rhizobacteria with manure. HortScience. 2011;46(6):932–936. [Google Scholar]
- Yildirim E., Turan M., Ekinci M., Dursun A., Çakmakci R. Plant growth promoting rhizobacteria ameliorate deleterious effect of salt stress on lettuce. Sci. Res. Essays. 2011;6(20):4389–4396. [Google Scholar]
- Youssef M.M.A., Eissa M.F.M. Biofertilizers and their role in management of plant parasitic nematodes. J. Biotechnol. Pharm. Res. 2014;5:1–6. [Google Scholar]
- Zahid M., Abbasi M.K., Hameed S., Rahim N. Isolation and identification of indigenous plant growth promoting rhizobacteria from Himalayan region of Kashmir and their effect on improving growth and nutrient contents of maize (Zea mays L.) Front. Microbiol. 2015;6:1–15. doi: 10.3389/fmicb.2015.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, F., Shen, J., Jing, J., Li, L., Chen, X., 2010. Rhizosphere processes and management for improving nutrient use efficiency and crop productivity. Molec. Environ. Soil Sci. at the Interfaces in the Earth’s Critical Zone, 1, 52–54.
- Zhu F., Qu L., Hong X., Sun X. Isolation and characterization of a phosphate-solubilizing halophilic bacterium Kushneria sp. YCWA18 from Daqiao Saltern on the coast of Yellow Sea of China. Evid. Based Complement. Alternat. Med. 2011;2011:1–6. doi: 10.1155/2011/615032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang P., Fang L., Zhang Q.A., Yan C., Chen J. Isolation and characterization of phosphate-solubilizing bacteria from mushroom residues and their effect on tomato plant growth promotion. Polish J. Microbiol. 2017;66(1):57–65. doi: 10.5604/17331331.1234993. [DOI] [PubMed] [Google Scholar]