Highlights
-
•
This systematic review focuses on structural and functional neuroimaging findings in PD patients with FOG.
-
•
The existing neuroimaging literature may explain several mechanisms underpinning FOG in PD.
-
•
FOG in PD reflect structural or functional damage in brain regions responsible for human locomotion.
Keywords: Parkinson's disease, Freezing of gait, MRI, PET, SPECT
Abstract
Freezing of gait (FOG) is a paroxysmal gait disorder that often occurs at advanced stages of Parkinson's disease (PD). FOG consists of abrupt walking interruption and severe difficulty in locomotion with an increased risk of falling. Pathophysiological mechanisms underpinning FOG in PD are still unclear. However, advanced MRI and nuclear medicine studies have gained relevant insights into the pathophysiology of FOG in PD. Neuroimaging studies have demonstrated structural and functional abnormalities in a number of cortical and subcortical brain regions in PD patients with FOG. In this paper, we systematically review existing neuroimaging literature on the structural and functional brain changes described in PD patients with FOG, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. We evaluate previous studies using various MRI techniques to estimate grey matter loss and white matter degeneration. Moreover, we review functional brain changes by examining functional MRI and nuclear medicine imaging studies. The current review provides up-to-date knowledge in this field and summarizes the possible mechanisms responsible for FOG in PD.
1. Introduction
Patients with Parkinson's disease (PD) often manifest freezing of gait (FOG), a paroxysmal gait disorder characterized by abrupt walking interruption and severe difficulty continuing locomotion, leading to an increased risk of falling (Giladi, 2008, 2007; Giladi et al., 2001b, 2001a; Nutt et al., 2011; Walton et al., 2015). FOG mainly occurs in advanced stages of PD, is often refractory to medical treatment, and contributes to a significant worsening of quality of life (Lopez et al., 1999; Macht et al., 2007; Nonnekes et al., 2015; Nutt et al., 2011). Although a number of clinical and experimental studies have demonstrated that cognitive (i.e. abnormal executive and visuospatial functions) as well as behavioral disorders (i.e. anxiety) contribute to FOG in patients with PD (Nutt et al., 2011), the pathophysiological mechanisms leading to FOG in PD still remain under debate (Fasano et al., 2015).
In this systematic review designed in agreement with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009), we examine and critically discuss existing literature on structural and functional neuroimaging studies in PD-FOG in order to provide an overview of up-to-date knowledge in this field. As a further novelty, the manuscript also includes a quality assessment of the previously published neuroimaging studies in PD-FOG which are systematically organized into three main sections. In the first section, we report structural magnetic resonance imaging (MRI) studies on brain alterations. We consider separately grey matter loss (i.e. voxel-based morphometry and surface-based morphometry) and white matter damage (i.e. diffusion tensor imaging). In the second section, we examine functional MRI (fMRI) studies on brain changes and consider separately results from task-based fMRI and resting-state fMRI approaches. In the third section, we evaluate nuclear medicine imaging studies with positron emission tomography (PET) and single photon emission computed tomography (SPECT) in PD-FOG. Lastly, we discuss the main pathophysiological hypotheses to explain FOG in PD.
2. Literature search and methods
According to the PRISMA guidelines (Moher et al., 2009), two electronic databases (PubMed and Scopus) were used to perform the search criteria, using the following keywords: (“Parkinson”) AND ("freezing of gait" OR “FOG”) AND (“neuroimaging” OR “MRI” OR "diffusion tensor imaging" OR "fMRI" OR "functional MRI" OR “PET” OR "positron emission tomography" OR "single photon" OR “SPECT”). The search was concluded on 31 July 2019. No restriction was applied to publication dates. First, we identified all corresponding documents in both databases. In order to identify further articles, we used the references from both original articles as well as from review articles. After identifying existing PD-FOG neuroimaging studies, we then cross-checked all the articles collected to avoid duplicates. The abstracts were examined carefully, and the following exclusion criteria were applied: reviews, case reports, articles written in languages other than English, articles on children and articles including patients with diseases other than PD including lesion-induced FOG.
In accordance with the PRISMA guidelines, in order to assess the scientific quality of the studies included in our review and any possible source of bias, we prepared a checklist of nine questions and a point was assigned to each question according to the quality criteria fulfilled. We then performed a quality assessment analysis based on the customized set of criteria used by a recent study (De Giglio et al., 2018). The overall procedure was carried out by two investigators (KB, ST). In case of disagreement, a more experienced author (PP) made the final decision. In order to evaluate the quality improvement over time of each article category (structural, functional and nuclear medicine imaging), we calculated correlation between the quality assessment score and the year of publication by using the Spearman rank test. We compared the quality assessment scores among each article category by using a two-tailed, alpha=0.05, Mann-Whitney test.
3. Results
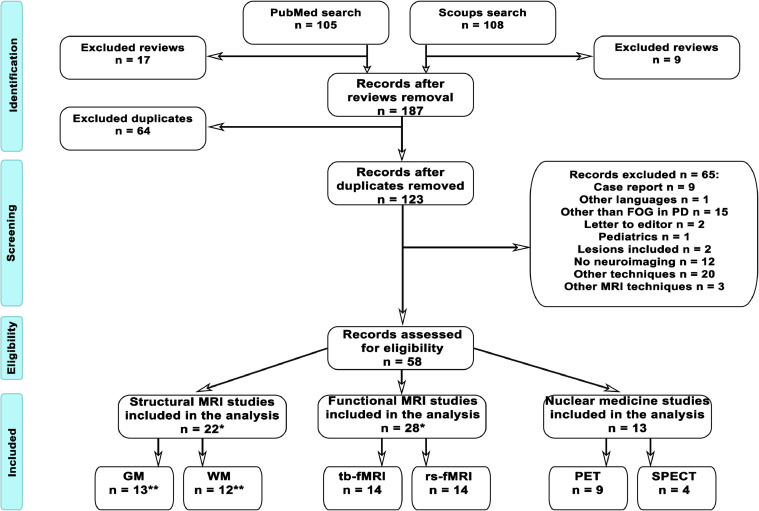
We obtained 213 articles from both databases (PubMed and Scopus). After excluding review articles, we selected 187 articles. Out of the 187 articles, 64 were present in both databases and therefore only counted once. The abstracts of the remaining 123 articles were carefully inspected to check for exclusion criteria. Finally, 58 original articles based on PD-FOG neuroimaging findings were included and further divided into 22 structural and 41 functional (28 fMRI and 13 nuclear medicine techniques) studies. Seventeen out of the 22 structural studies focused only on structural data, whereas 5 of them investigated both structural and functional PD-FOG MRI data, and their results are discussed in both Section 1 and 2 (Fig. 1).
Fig. 1.
Flow Chart. Following PRISMA guidelines, this flowchart displays the procedure to identify those studies that were suitable to be included in the systematic review.* Five studies report both structural and functional MRI analysis and are counted twice, thus total number of studies seems erroneously to be n = 63, while being n = 58.** Three studies report both grey and white matter analysis and are counted twice. Therefore, the correct number of structural MRI studies (on both grey and white matter) is n = 22, not n = 25.
Quality assessment included questions about sample clinical characteristics, statistical analysis and results (see Supplementary Materials). The analysis showed no improvement in the structural and functional MRI studies over time (all p≥0.05). Conversely, nuclear medicine studies improved significantly with time (p=0.001; rho=0.79). Group comparisons showed a higher quality assessment in structural studies as compared to functional (t=2.36, p≤0.014) and nuclear medicine studies (t=2.76, p≤0.004).
3.1. Section 1: Structural MRI studies
3.1.1. Grey matter
We identified a total of 13 structural MRI studies reporting cortical or subcortical grey matter (GM) abnormalities in PD-FOG (Table. 1.1).
Table. 1.1.
Structural MRI studies in PD-FOG - Grey Matter Studies.
| Authors (Year) | Participants (years) (mean age±SD) | Technique | Outcome measures | Imaging findings | Clinical correlation |
|---|---|---|---|---|---|
| Snijders et al. (2011) | 12 PD-FOG (59±9) 12 PD-nFOG (63±7) 21 HS (57± 9) | VBM | GM volume | GM loss in the MLR | No correlation |
| Kostic et al. (2012) | 17 PD-FOG (64±8) 20 PD-nFOG (63±5) 34 HS (64±7) | VBM | GM volume | GM loss in frontal, parietal and temporal cortices | Between GM volumes of bilateral frontal, parietal cortices and FOG-Q |
| Tessitore et al. (2012a) | 12 PD-FOG (67±5) 12 PD-nFOG (66±6) 12 HS (66±6) | VBM | GM volume | GM loss in cuneus, precuneus, lingual gyrus and PCC | Between GM loss in posterior cortical regions and FOG-Q |
| Sunwoo et al. (2013) | 16 PD-FOG (67±5) 30 PD-nFOG (69±4) | VBM | GM volume | GM loss in the thalamus | Between thalamic volume and visual recognition memory |
| Herman et al. (2014) | 30 PD-FOG (65±9) 76 PD-nFOG (64.9±9.5) | VBM | GM volume | GM loss in inferior parietal lobule | Between bilateral caudate volumes and FOG-Q |
| Rubino et al. (2014) | 13 PD-FOG (68±8) 13 PD-nFOG (68±7) | VBM | GM volume | GM loss in left posterior parietal gyrus | Not performed |
| Brugger et al. (2015) | 18 PD-FOG (61±8) 20 PD-nFOG (61±10) | VBM | GM volume | GM loss in middle frontal gyrus, superior and middle orbital gyrus, superior frontal gyrus and right middle temporal gyrus, superior and inferior parietal lobule | Between GM volume of parietal, temporal lobes and FOG-Q Between GM volume of frontal lobe and FAB |
| Canu et al. (2015) | 35 PD-FOG (68±8) 23 HS (67±8) | VBM | GM volume | No GM loss | No correlation |
| Jha et al. (2015) | 17 PD-FOG (57±7) 21 PD-nFOG (47±9) | VBM | GM volume | GM loss in right cerebellum, medial frontal, precentral cortex, and left middle temporal gyrus | Not performed |
| Myers et al. (2017) | 25 PD-FOG (67±10) 38 PD-nFOG (64±10) | VBM | Cerebellar volumes | No GM loss | No correlation |
| Vastik et al. (2017) | 11 PD-FOG (71±6) 10 PD-nFOG (69±10) | SBM | GM thickness | GM loss in left SMA, anterior cingulate cortex, temporal pole and right frontal operculum | No correlation |
| Bharti et al. (2019a) | 15 PD-FOG (71±7) 16 PD-nFOG (66±11) 16 HS (67±8) | VBM | Cerebellar volumes | No GM loss | Not performed |
| Pietracupa et al. (2018) | 21 PD-FOG (66±11) 16 PD-nFOG (70±11) 19 HS (67±8) | SBM | Cortical thickness | GM loss in superior frontal gyrus, paracentral lobule, PCC, precuneus, pericalcarine, and right dorsolateral prefrontal cortex | No correlation |
PD-FOG: Parkinson's disease patients with freezing of gait; PD-nFOG: Parkinson's disease patients without freezing of gait; HS: healthy controls; VBM: Volume-based morphometry; GM: Grey matter; MLR: Mesencephalic locomotor region; FOG-Q: Freezing of Gait Questionnaire; PCC: Posterior cingulate cortex; FAB: Frontal Assessment Battery; SBM: Surface-based morphometry; SMA: supplementary motor area.
FOG-Q represents severity of freezing of gait (FOG)
*NA: not available
Voxel-based morphometry (VBM) was the most common technique used to examine local volume changes in GM by analyzing voxel-based features (Ashburner and Friston, 2000; Good et al., 2001). The first structural study using VBM and region of interest analysis in PD-FOG (Snijders et al., 2011) focused on possible changes in subcortical brain structures, primarily the mesencephalic locomotor region (MLR). In this study, the authors found greater GM atrophy in a small portion of the MLR in PD-FOG patients as compared to PD patients without FOG (PD-nFOG) and healthy subjects (HS). Sunwoo and colleagues (2013) also analyzed subcortical brain structures and found lower thalamic volumes in PD-FOG than PD-nFOG (Sunwoo et al., 2013). Finally, Herman et al. (2014) demonstrated GM loss in the bilateral caudate nucleus of PD-FOG compared to PD-nFOG (Herman et al., 2014).
Several studies have examined GM changes in the cerebellum in PD-FOG by using the spatially unbiased infratentorial toolbox (SUIT) technique (Bharti et al., 2019a; Myers et al., 2017). While one VBM study showed GM changes in the cerebellum (Jha et al., 2015), more recently both Myers et al. (2017) and Bharti et al. (2019a) failed to find differences in cerebellar GM when comparing PD-FOG and PD-nFOG.
A different line of VBM research found GM changes in cortical rather than subcortical brain structures in PD-FOG. Kostic and colleagues (2012) first demonstrated prominent GM atrophy in left frontoparietal regions in PD-FOG compared with PD-nFOG that negatively correlated with FOG severity (Kostic et al., 2012). These findings have been confirmed by a number of studies (Brugger et al., 2015; Herman et al., 2014; Jha et al., 2015; Rubino et al., 2014; Tessitore et al., 2012) that have demonstrated prominent GM loss in anterior (frontal) and posterior (parieto-occipital) cortical areas in PD-FOG compared with PD-nFOG and HS. VBM studies also demonstrated a correlation between the amount of GM atrophy and FOG severity (Brugger et al., 2015; Herman et al., 2014; Jha et al., 2015; Rubino et al., 2014; Tessitore et al., 2012). However, Canu et al. (2015) failed to detect GM volume differences when comparing PD-FOG and PD-nFOG, possibly due to the relatively small sample size used (Canu et al., 2015).
Besides VBM, an alternative morphometric technique used to examine GM structural abnormalities is surface-based morphometry (SBM). SBM provides cortical thickness and surface area measures (Fischl, 2012). Only two recent studies (Pietracupa et al., 2018; Vastik et al., 2017) have investigated possible GM changes in PD-FOG using the SBM technique and both demonstrated prominent cortical thinning in frontoparietal regions of the medial wall in PD-FOG.
3.1.2. White matter
We selected 12 structural studies investigating white matter (WM) changes in PD-FOG that used diffusion tensor imaging (DTI) with various methodological approaches (Table. 1.2).
Table. 1.2.
Structural MRI studies in PD-FOG - White Matter Studies.
| Authors (Year) | Participants (years) (mean age±SD) | Technique | Outcome measures | Imaging findings | Clinical correlation |
|---|---|---|---|---|---|
| Schweder et al. (2010) | 2 PD-FOG (NA) 8 PD-nFOG (NA) 17 HS (NA) | Tractography (Seed on PPN) | WM connectivity | Higher PPN connectivity with anterior pontine nucleus of the fourth ventricle | Not performed |
| Fling et al. (2013) | 14 PD-FOG (67±5) 12 PD-nFOG (65±7) 15 HS (67±8) | Tractography (Seed on PPN) | WM connectivity | Lower PPN connectivity with cerebellum, thalamus, areas of frontal and prefrontal cortex | No correlation |
| Fling et al. (2014) | 8 PD-FOG (65±6) 7 PD-nFOG (64±6) 14 HS (67±8) | Tractography (ROI-ROI; right STN and SMA; left STN and SMA; right MLR and SMA; left MLR and SMA) | WM connectivity strength | Lower connectivity strength between right STN – SMA | No correlation |
| Peterson et al. (2015) | 13 PD-FOG (65±7) 12 PD-nFOG (66±5) | Tractography (Seed on PPN) | Stride length | Stride length changes within the PPN | Between asymmetry of PPN structural connectivity and dual-task interference |
| Canu et al. (2015) | 35 PD-FOG (68±8) 23 HS (67±8) | TBSS (whole brain skeleton) Tractography (Seed on PPT) | FA, MD, AD, and RD within the skeleton FA, MD, AD, and RD within the PPT | Higher FA Lower MD in motor, frontal, orbitofrontal and parietal areas Higher AD in PPT | No correlation Between AD of PPT and UPDRS-III |
| Vercruysse et al. (2015) | 11 PD-FOG (69±9) 15 PD-nFOG (68±6) 15 HS (68±6) | TBSS (whole brain skeleton) Tractography (Seed on caudate, putamen, pallidum, STN, thalamus, PPN and MCP) | FA, and MD within the skeleton FA and MD within the mask of 7 seeds | Lower FA in cerebellum and SLF; higher MD in anterior part of internal capsule Lower FA in caudate, putamen, pallidum, STN, frontal, motor and sensory areas | Between FA in the left caudate–ACC tract and UPDRS-III Between MD of left striatofrontal tracts and UPRDS-III |
| Youn et al. (2015) | 19 PD-FOG (71±6) 23 PD-nFOG (68±6) 33 HS (70±6) | TBSS (whole brain skeleton) TBSS (Seed on PPN) | FA, and MD within the skeleton FA and MD within the PPN | Higher MD in basal ganglia, thalamus and cerebellum Lower FA; higher MD in bilateral PPN | No correlation Between FA of PPN and FOG-Q |
| Trufanov et al. (2016) | 31 PD-FOG 47 PD-nFOG | Tractography (Seed on ATR, CST, ILF, and SLF) | FA, and MD within the seed | Lower FA; and higher RD along the upper longitudinal fascicles of temporal area | No correlation |
| Wang et al. (2016) | 14 PD-FOG (72±6) 16 PD-nFOG (69±6) 16 HS (69±3) | TBSS (whole brain skeleton) | FA, and MD within the skeleton | Lower FA; higher MD in left cingulum, CC, internal capsule, posterior thalamic radiations, corona radiate, external capsule, sagittal stratum and SLF Higher MD in cerebral peduncles, inferior fronto-occipital fasciculus | Not performed |
| Bharti et al. (2019a) | 15 PD-FOG (71±7) 16 PD-nFOG (66±11) 16 HS (67±8) | TBSS (Seeds on SCP, MCP, and inferior cerebellar peduncle) | FA, and MD in the cerebellar peduncles | Lower FA and higher MD in SCP, and MCP | Between FA, MD of SCP, MCP and FOG-Q |
| Hall et al. (2018) | 26 PD-FOG (64±9)24 PD-nFOG (66.9±5) | Graph theory analysis | Modularity | Higher involvement of frontal and parietal brain areas along with right caudate, thalamus, and hippocampus | No correlation result |
| Pietracupa et al. (2018) | 21 PD-FOG (66±11) 16 PD-nFOG (70±11) 19 HS (67±8) | Tractography (Seeds on CST, SLF, ATR, uncinate fasciculus, cingulum, ILF and CC) | FA, MD, AD, and RD within the seed | WM changes in SLF, uncinate fasciculus, cingulate gyrus and CC | Between several WM bundles and MMSE, FAB, H&Y and HAM-D |
PD-FOG: Parkinson's disease patients with freezing of gait; PD-nFOG: Parkinson's disease patients without freezing of gait; HS: healthy controls; FOG-Q: Freezing of Gait Questionnaire; WM: White matter; PPN: Pedunculopontine nucleus; MLR: Mesencephalic locomotor region; SMA: supplementary motor area; STN: Subthalamic nucleus; TBSS: Tract-Based Spatial Statistics; FA: Fractional anisotropy; MD: Mean diffusivity; AD: Axial diffusivity; RD: Radial diffusivity; PPT: pedunculopontine tract; UPDRS: Unified Parkinson's Disease Rating Scale; SLF: Superior longitudinal fasciculus; MCP: Medial cerebellar peduncle; CC: Corpus callosum; CST: Corticospinal tract; ATR: Anterior thalamic radiation; ILF: Inferior longitudinal fasciculus; MMSE: Mini-mental state examination; H&Y: Hoehn and Yahr scale; HAM-D: Hamilton depression rating scale; SCP: Superior cerebellar peduncle.
FOG-Q represents severity of freezing of gait (FOG)
*NA: not available
DTI examines alterations in WM (Le Bihan, 1985; Merboldt et al., 1991) by estimating the diffusivity of water molecules along the WM fiber tracts (Pierpaoli et al., 1996) by means of specific measures including fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) (Song et al., 2005, 2003). Altered patterns of FA, MD, AD, and RD changes suggest axonal loss and/or demyelination (Basser, 1995; Song et al., 2005, 2003).
One line of research to study WM changes in PD-FOG has focused on structural connections in subcortical brain regions crucially involved in locomotion, such as the peduncolopontine nucleus (PPN). First, Schweder et al., 2010 demonstrated abnormal connectivity between the PPN and a number of cortical and subcortical structures (Schweder et al., 2010). Later, several DTI studies confirmed PPN connectivity impairment when comparing PD-FOG and PD-nFOG (Canu et al., 2015; Fling et al., 2013; Peterson et al., 2015; Vercruysse et al., 2015; Youn et al., 2015). However, another study investigating the connectivity between brain structures included in the locomotor network failed to confirm WM changes when comparing PD-FOG and PD-nFOG (Fling et al., 2014). Besides the PPN, a recent study from Bharti et al. (2018) demonstrated greater WM damage in the superior and middle cerebellar peduncles in PD-FOG than in PD-nFOG (Bharti et al., 2019a). In most of the DTI studies, WM changes also correlated with FOG severity (Bharti et al., 2019a; Canu et al., 2015; Fling et al., 2013; Schweder et al., 2010; Vercruysse et al., 2015; Youn et al., 2015).
Another line of research has focused on possible damage in long associative WM bundles. Canu et al. (2015) found WM damage involving the majority of frontoparietal and temporo-occipital cortico-cortical connections, as well as the genu and splenium of the corpus callosum in PD-FOG compared with PD-nFOG (Canu et al., 2015). In keeping with these findings, a number of studies reported widespread damage to several WM tracts including the superior longitudinal fasciculus, several corticofugal and frontostriatal tracts, and thalamic radiation to frontal regions (Vercruysse et al., 2015; Wang et al., 2016). More recently, using the Tracts Constrained by UnderLying Anatomy (TRACULA) method, a three-dimensionally reconstructed tractography approach for the automatic rebuilding of major WM pathways (Trufanov et al., 2016; Pietracupa et al., 2018; Yendiki et al., 2011), changes were found in long-range associative WM bundles, such as the superior longitudinal fasciculus, uncinate fasciculus, cingulum cingulate gyrus, and inferior longitudinal fasciculus in PD-FOG. Moreover, Pietracupa et al. (2018) reported a predominant impairment in WM bundles in the right hemisphere in PD-FOG (Pietracupa et al., 2018).
Lastly, a recent article by Hall et al. (2018) investigated WM changes and structural disconnection in PD-FOG by using graph theory analysis. The authors found an altered structural organization of the WM connectome in PD-FOG mainly involving frontal and parietal cortical areas as well as the right caudate, thalamus, and hippocampus (Hall et al., 2018).
In summary, structural MRI protocols involving VBM have demonstrated that PD-FOG have GM loss in specific subcortical regions mostly in the brainstem (i.e. MLR), basal ganglia (i.e. striatum) and cerebellum (i.e. CLR). Besides subcortical structures, VBM as well as more advanced SBM techniques, have both demonstrated GM loss also in specific cortical areas and prominently in frontal and parietal regions. In addition to GM loss, a second set of structural MRI studies have focused on WM changes as examined by DTI in PD-FOG. These DTI studies have consistently demonstrated damage of widespread long associative WM bundles (i.e. SPL) and structural disconnection among cortical (i.e. SMA) and subcortical (i.e. PPN) brain regions specifically involved in locomotion. Overall, structural MRI studies have demonstrated GM loss as well as WM damage involving multiple cortical and subcortical structures in PD-FOG.
3.2. Section 2: Functional MRI studies
We identified 28 studies that reported fMRI abnormalities in PD-FOG. Fourteen studies reported functional abnormalities based on a stimulus-driven approach (task-based fMRI) (Table 2.1), whereas the remaining 14 studies adopted the resting-state fMRI technique (Table. 2.2).
Table. 2.1.
Functional MRI studies in PD-FOG - Task-Based fMRI studies.
| Authors (Year) | Participants (years) (mean age±SD) | Technique | Outcome measures | Imaging findings | Clinical correlation |
|---|---|---|---|---|---|
| Shine et al. (2011) | 1 PD-FOG | SPM (event related design) | Activations using virtual-reality walking task | During walking: Lower BOLD response in SMC and anterior cerebellum During dual-task walking: Higher BOLD response in DLPFC, ventrolateral prefrontal cortex, pre-SMA and posterior parietal regions. During the episodes of freezing: Activation of pre-SMA, motor cortices, DLPFC, ventrolateral prefrontal cortex, and posterior parietal region. | Not performed |
| Snijders et al. (2011) | 12 PD-FOG (59±9) 12 PD-nFOG (63±7) 21 HS (57±9) | SPM (event related design) Whole brain analysis and ROI on bilateral superior frontal gyrus, superior parietal lobule, right ACC, left putamen, and MLR | Activations during motor imagery of walking | Higher BOLD response in MLR and lower BOLD response in cingulate, SMA and right superior parietal lobule | Between MLR activity and FOG-Q, disease duration |
| Shine et al. (2013a) | 18 PD-FOG (67±8) | SPM (event-related block design) ROI on precentral sulcus, dorsal premotor area, dorsal caudal, putamen, DLPFC, posterior parietal cortex, caudate anterior insula, ACC, medial prefrontal cortex, ventral striatum, Globus pallidus, STN, anterior thalamus, MLR | Activations during virtual reality task of using the foot pedals to control walking | Higher BOLD response in fronto-parietal and insula Lower BOLD response in SMC, caudate, thalamus, and Globus pallidus internus Higher BOLD response in DLPFC, posterior parietal cortex, and MLR Lower BOLD response in putamen, right dorsal premotor, caudate, medial prefrontal cortex, left anterior insula, ventral striatum, Globus pallidus internus, anterior thalamus and bilateral subthalamic nucleus. | Between activity in sensorimotor regions, frontoparietal cortical regions and FOG-Q |
| Shine et al. (2013b) | 14 PD-FOG (63±7) 15 PD-nFOG (63±8) | SPM (event related design) Whole brain analysis and ROI on bilateral anterior insula, left superior frontal, bilateral ventral striatum, right pre-SMA | Activations during virtual reality task of direct and indirect cues of walking | Higher BOLD response in DLPFC, posterior parietal cortices, midline pre-SMA, anterior insula, medial temporal lobes and extra-striate visual cortex Lower BOLD response in anterior insula, ventral striatum, left STN and SMA Lower BOLD response within the anterior insula, ventral striatum, left STN | Not performed |
| Shine et al. (2013c) | 10 PD-FOG (67±6) | ||||
| 10 PD-nFOG (66±6) | SPM (Boxcar) SPM (ICA) | Activations during direct and indirect cues of walking FC during direct and indirect cues of walking | During task performances higher BOLD response in left cognitive control network and ventral attention network Functional decoupling between basal ganglia and cognitive control network | Not performed | |
| Vercruysse et al. (2014) | 16 PD-FOG (66±7) 16 PD-nFOG (67±5) 16 HS (67±6) | SPM (event related design) ROI on putamen, caudate nucleus, STN, pallidum, PPN, and MLR | Activations during bimanual finger movements | During successful movement: Lower BOLD in DLPFC; higher BOLD response in dorsal putamen, pallidum and STN During motor blocks: higher BOLD in right SMC, dorsal premotor cortex, left prefrontal cortex; lower BOLD in bilateral pallidum and putamen Higher BOLD response in bilateral STN, dorsal putamen, pallidum. | Not performed |
| Peterson et al. (2014) | 9 PD-FOG (67±7) 9 PD-nFOG (63±9) | Brain Voyager (event related design) ROI on SMA, putamen, Globus pallidus interna, MLR, and CLR | Activations during imagined walking and standing | During imagined standing: lower BOLD response in the CLR During imagined walking: lower BOLD in right Globus pallidus | Not performed |
| Hoorn et al. (2014) | 7 PD-FOG (62.1±9.5) 15 PD-nFOG (60.9±12.1) 15 HS (60.5±6.2) | SPM (block design) Psychophysiological interactions (Seed on motion sensitive area right V5) | Activations using effects of optic flow FC using effects of optic flow | Lower BOLD response in dorsal occipito-parietal and pre-SMA | No correlation |
| Gilat et al. (2015) | 17 PD-FOG (67±6) 10 PD-nFOG (65±4) | SPM (event related design) ROI on caudate nucleus, putamen, ventral striatum, MLR, Globus pallidus interna, STN, CLR seed on caudate nucleus, putamen, ventral striatum, MLR, Globus pallidus internus, STN, bilateral CLR) | Activations during virtual reality of simple walking and turning FC during virtual reality of walking and turning | During turning: Higher BOLD response in visual cortex and inferior frontal region During turning: Higher FC in MLR and Globus pallidus | Not performed |
| Agosta et al. (2017) | 13 PD-FOG (64±7) | SPM (block design) | Activations during action observation training and landscape task | During action observation training: Higher BOLD response in fronto-parietal areas | |
| During Landscape: Lower BOLD response in left postcentral gyrus, inferior parietal gyri, right Rolandic operculum and supramarginal gyrus | Not performed | ||||
| Martens et al. (2018) | 41 PD-FOG (67±6) | SPM (epoch related design) Seed on primary motor cortex, SMA, cerebellum, ACC, DLPFC, posterior parietal cortex, medial prefrontal cortex, anterior insula, amygdala | FC during virtual reality task of normal foot tapping | Higher FC between limbic network and ventral striatum Lower FC between striatum -motor and cognitive networks | No correlation |
| Myers et al. (2018) | 13 PD-FOG (65±10) 24 PD-nFOG (66±8) | Brain Voyager (event related design) ROI on regions of SMN, cerebellar regions associated with SMN, and M1 and S1 cortical regions | Activations during imaginary walking task | Lower BOLD response in cerebellum, primary motor and primary sensory cortices | Not performed |
| Nackaerts et al. (2018) | 10 PD-FOG (67±10) 27 PD-nFOG (63±7) | SPM (block design) ROI: extrastriate visual cortex, superior parietal lobule, primary motor cortex, left SMA and right cerebellar lobule VI Dynamic casual modeling (Seed on extrastriate visual cortex, superior parietal lobule, primary motor cortex, left SMA and right cerebellar lobule VI) | Activations during visually cued and uncued handwriting FC during visually cued and uncued handwriting task | Higher BOLD response in visual cortex Weak coupling strength between left extrastriate visual cortex and left superior parietal lobule; left superior parietal lobule-right superior parietal lobule; left dorsal premotor cortex-left SMA; left dorsal premotor cortex-left primary motor cortex | Between effective connectivity and handwriting quality |
| Matar et al. (2019) | 19 PD-FOG (65±6) | SPM (event related design) SPM (seed on STN and auditory cortex) | Activations during virtual reality task using foot pedals to navigate a series of doorways FC during virtual reality task using foot pedals to navigate a series of doorways | Lower BOLD response in pre-SMA Higher FC between pre-SMA and STN | Correlation between functional connectivity and delay in footstep. |
PD-FOG: Parkinson's disease patients with freezing of gait; PD-nFOG: Parkinson's disease patients without freezing of gait; HS: healthy controls; BOLD = Blood oxygenated level dependent signal; SMA: supplementary motor area; FDLPFC: dorsolateral prefrontal cortex; FOG-Q: Freezing of Gait Questionnaire; ROI: Region of interest; SMC: supplementary motor complex; ACC: anterior cingulate cortex; MLR: mesencephalic locomotor region; STN: subthalamic nucleus; ICA: Independent component analysis; FC: Functional connectivity; PPN: pedunculopontine nucleus; CLR: cerebellar locomotor region.
FOG-Q represents severity of freezing of gait (FOG)
*NA: not available
Table. 2.2.
Functional MRI studies in PD-FOG - Resting state fMRI.
| Tessitore et al. (2012b) | 16 PD-FOG (67±6) 13 PD-nFOG (66±6) 15 HS (65±6) | Brain Voyager QX (ICA) | FC | Lower FC in executive-attention and visual networks | Between executive-attention, visual networks and FOG-Q |
|---|---|---|---|---|---|
| Fling et al. (2014) | 8 PD-FOG (65±6) 7 PD-nFOG (64±6) 14 HS (67±8) | Seed: SMA, STN, MLR, and CLR. | FC | Higher FC in SMA, left CLR, and MLR | Between SMA-MLR FC and FOG-Q |
| Canu et al. (2015) | 35 PD-FOG (68±8) 23 HS (67±8) | FSL (ICA) | FC | Lower FC in SMC, DMN, and visual associative networks | No correlation |
| Lenka et al. (2016) | 15 PD-FOG (54±11) 13 PD-nFOG (50±9) 30 HS (52±10) | CONN (multiple seeds) | FC | Lower FC of left parietal opercular cortex with primary SMC and auditory areas | Between left parietal opercular cortex FC and FOG-Q |
| Vervoort et al. (2016) | 13 PD-FOG (66±10) 60 PD-nFOG (58±10) 20 HS (58±9) | SPM (seeds on motor network and FPN) | FC | Lower FC within the striatum Higher FC between the dorsal putamen and precuneus Lower FC between the caudate and superior temporal lobe | Between FC and gait kinematics |
| Wang et al. (2016) | 14 PD-FOG (72±6) 16 PD-nFOG (69±6) 16 HS (68±2) | SPM (Seeds on bilateral PPN) | FC | Lower FC of right PPN with inferior temporal gyri and cerebellum Higher FC of the left PPN with right middle temporal gyrus | Not performed |
| Mi et al. (2017) | 31 PD-FOG (61±8) 31 PD-nFOG (58±10) 32 HS (58±7) | REST version 1.8 | ALFF | Higher in right ACC and left inferior parietal lobule Lower in superior frontal gyrus, cerebellum and left thalamus | Between ALFF in superior frontal gyrus, subcortical and sensorimotor areas, ACC and FOG-Q |
| Bharti et al. (2019a) | 15 PD-FOG (71+7) 16 PD-nFOG (66+11) 16 HS (67 ± 8) | FSL (Seeds on CLR, fastigial nucleus, and dentate). | FC | Higher FC between CLR, fastigial nucleus and cerebellar, posterior cortical cortices Lower FC between dentate and brainstem, right basal ganglia, frontal, prefrontal, parieto-occipital cortices | Between CLR FC and FOG-Q |
| Gilat et al. (2018) | 19 PD-FOG (68±7) 21 PD-nFOG (67±8) | FSL (seeds on bilateral amygdala, bilateral nucleus accumbens, caudate nucleus, and putamen). | FC | Higher FC between right amygdala and right putamen; between FPN and right putamen Lower FC between FPN and left amygdala | Between limbic FC and FOG-Q and fear of falling |
| Li et al. (2018) | 21 PD-FOG (70±6) 33 PD-nFOG (66±9) 24 HS (66±4) | Voxel-mirrored homotopic approach | FC | Lower voxel-mirrored homotopic connectivity in the inferior parietal lobule | Between voxel-mirrored homotopic connectivity in inferior parietal lobe and FOG-Q |
| Zhou et al. (2018) | 14 PD-FOG (62 ±10) 20 PD-nFOG (63±8) 18 HS (64±10) | REST (regional homogeneity) | Local FC | Lower regional homogeneity in left SMA and left superior frontal region | No correlation was performed |
| Bharti et al. (2019b) | 15 PD-FOG (71±7) 16 PD-nFOG (66±11) 16 HS (67±8) | FSL (Within - and Between- network analysis) | FC | Within-network results: higher right fronto-parietal, orbitofrontal, frontal, sensorimotor and basal ganglia FC Between-network results: Lower FC between anterior default mode and sensorimotor networks, between right fronto-parietal and executive control networks | Between basal ganglia within-network FC and TUG duration during ON state Between FC right fronto-parietal versus executive-control networks and FOG-Q |
| Hu et al. (2019) | 18 PD-FOG (63±11) 18 PD-nFOG (61±8) 17 HS (61±8) | SPM12 | ALFF | In frequency band slow-5, lower ALFF in the bilateral putamen In frequency band slow-4, higher ALFF in left inferior temporal gyrus and lower ALFF in right middle frontal gyrus In classical frequency band, higher ALFF in inferior temporal gyrus | No correlation was performed |
| Maidan et al. (2019) | 26 PD-FOG (72±1) 11 PD-nFOG (74±1) 20 HS (70±1) | Graph theory analysis | FC | Lower global efficiency of dorsal attention network Higher local efficiency of dorsal attention network | Between global efficiency of dorsal attention network and FOG-Q |
PD-FOG: Parkinson's disease patients with freezing of gait; PD-nFOG: Parkinson's disease patients without freezing of gait; HS: healthy controls; FC: Functional connectivity; FOG-Q: Freezing of Gait Questionnaire; SMA: supplementary motor area; STN: subthalamic nucleus; CLR: cerebellar locomotor region; MLR: mesencephalic locomotor region; ICA: Independent component analysis; SMC: supplementary motor complex; DMN: default mode network; PPN: pedunculopontine nucleus; ACC: anterior cingulate cortex; FPN: frontoparietal network; ALFF: Amplitude of Low Frequency Fluctuation; TUG: Time Up and Go.
FOG-Q represents severity of freezing of gait (FOG)
*NA: not available
3.2.1. Task-based functional MRI
Task-based fMRI (tb-fMRI) is a technique used to measure neuronal activity through the inference of changes in the blood oxygenation level dependent (BOLD) fMRI signals during the execution of a specific task (Logothetis et al., 2009). Previous studies demonstrated that motor imagery tasks activate neural processes resembling those involved during motor performance, including gait (la Fougère et al., 2010; Stephan et al., 1995). A number of studies have therefore investigated FOG pathophysiology in PD by means of tb-fMRI with motor imagery paradigms (Agosta et al., 2017; Myers et al., 2018; Peterson et al., 2014; Snijders et al., 2011; Stephan et al., 1995). Snijders and colleagues (2011) first observed an increased subcortical activity, mainly in the MLR, during motor imagery of gait in PD-FOG that significantly correlated with FOG severity and disease duration (Snijders et al., 2011). However, other studies with similar paradigms reported decreased rather than increased activity in structures other than the MLR, such as the cerebellar locomotor region (CLR) and basal ganglia in PD-FOG as compared to PD-nFOG (Agosta et al., 2017; Myers et al., 2018; Peterson et al., 2014). In addition to subcortical changes, the same studies also found altered functional activation of a number of cortical regions during motor imagery of gait in PD-FOG compared to PD-nFOG (Agosta et al., 2017; Myers et al., 2018; Snijders et al., 2011). Snijders et al. (2011) found decreased cortical responses in mesial frontoparietal regions in PD-FOG, but Agosta et al. (2017) and Myers et al. (2018) showed that PD-FOG had altered functional recruitment involving motor, premotor, and sensory cortices.
Other tb-fMRI studies have investigated brain activity in PD-FOG using virtual reality paradigms while performing alternate stepping in place for forward progression or turning in a virtual environment (Ehgoetz Martens et al., 2018; Gilat et al., 2015; Hoorn et al., 2014; Matar et al., 2019; Shine et al., 2013b, 2013a, 2013c, 2011). Using the virtual reality paradigm of walking, Shine et al. (2011) showed lower BOLD signals in the sensorimotor cortex and anterior cerebellum and higher BOLD signals in the prefrontal cortex, motor, and posterior brain areas when patients were asked to perform dual-task walking (Shine et al., 2011). Later, Shine et al (2013a) showed higher BOLD signal in the frontoparietal and insular cortices and decreased in the SMA, caudate, and thalamus in PD-FOG (Shine et al., 2013a). In the second tb-fMRI study from the same authors BOLD signals were reduced in the anterior insula, ventral striatum, left subthalamic nucleus (STN), and presupplementary motor area (pre-SMA) in PD-FOG compared with PD-nFOG (Shine et al., 2013b). Finally, a third study of Shine et al. (2013c) reported an association between motor arrest episodes and functional decoupling between the basal ganglia network and the cognitive control network in PD-FOG (Shine et al., 2013c). During a virtual reality paradigm providing an optic wide-field flow, van der Hoorn et al. (2014) showed decreased BOLD response in the dorsal occipitoparietal and pre-SMA cortices in PD-FOG (Hoorn et al., 2014). Later, Gilat et al. (2015) found decreased BOLD responses in premotor and parietal cortices but increased responses in inferior frontal regions in PD-FOG during virtual turning. In addition, PD-FOG was also characterized by strong functional connectivity (FC) between several subcortical structures (globus pallidus internus, STN, CLR, and MLR) (Gilat et al., 2015). Recently, when comparing virtual motor arrest and normal gait, Martens et al. (2018) found decreased FC between the striatum and cognitive control network and between the striatum and motor network, whereas FC increased between the limbic network and ventral striatum (Ehgoetz Martens et al., 2018). Finally, in PD-FOG Matar et al. (2019) found lower activations in the pre-SMA and reduced FC between the pre-SMA and STN bilaterally (Matar et al., 2019).
A further tb-fMRI approach previously used in PD-FOG involves the evaluation of brain activity recorded during a finger tapping task. Vercruysse et al. (2014) found abnormal activation of cortico-subcortical areas including the basal ganglia and frontal cortical areas in PD-FOG (Vercruysse et al., 2014). More recently, by examining a specific handwriting task in PD-FOG, Nackaerts et al. (2018) also demonstrated abnormal cortical responses in frontal areas and in occipitoparietal regions (Nackaerts et al., 2018).
3.2.2. Resting-state functional MRI
Resting-state fMRI (rs-fMRI) measures the functional interactions between regional brain areas at rest, and thus represents a non-invasive method to assess FC (Biswal et al., 1995; Biswal, 2012). By analyzing low-frequency BOLD signals in the brain, this technique segregates cerebral regions that are functionally connected even if they are anatomically distant, thus identifying the so-called brain resting-state networks (RSNs) (Beckmann et al., 2005; Logothetis, 2008; Smith et al., 2009). Independent component analysis (ICA) is an unbiased approach to analyze rs-fMRI data on a whole brain basis (Beckmann and Smith, 2004). By using ICA in PD-FOG, Tessitore et al. (2012) found reduced FC within both executive-attention and visual networks in PD-FOG compared with PD-nFOG. These functional changes significantly correlated with FOG severity (Alessandro Tessitore et al., 2012). Later, Canu et al. (2015) showed reduced FC within the sensorimotor, default-mode, and visual RSNs in PD-FOG compared to HS (Canu et al., 2015). Finally, in agreement with Tessitore et al. (2012b), Bharti et al. (2019b) recently found impaired FC between the RSNs underlying attentive and executive abilities, mainly in the right hemisphere (Bharti et al., 2019b).
As opposed to the ICA method, seed-based analysis uses a priori regions of interest to evaluate resting-state FC (Fox and Raichle, 2007). With this approach, Fling et al. (2014) demonstrated greater FC between the SMA and MLR bilaterally and between the SMA and left CLR in PD-FOG than in PD-nFOG. In the same study, the authors also showed a correlation between FC changes and FOG severity (Fling et al., 2014). When examining FC between the PPN and cortical and subcortical regions, Wang et al. (2016) demonstrated a prominent impairment in the cortico-pontine-cerebellar pathway and in occipitotemporal areas (Wang et al., 2016). Conversely, Lenka et al. (2016) demonstrated abnormal FC in multiple brain regions mainly involving primary somatosensory and auditory areas (Lenka et al., 2016).
In addition to fMRI studies primarily focusing on the FC of subcortical regions, Vervoort et al. (2016) analyzed FC between regions within the motor and frontoparietal network and demonstrated decreased FC within the striatum and between the caudate and superior temporal lobe (Vervoort et al., 2016). Conversely, FC increased between the dorsal putamen and precuneus in PD-FOG compared to PD-nFOG. Recently, Gilat et al. (2018) focused on the possible involvement of limbic circuitry in the pathophysiology of FOG by assessing differences in FC between the amygdala, striatum, and frontoparietal networks (Gilat et al., 2018). They found greater FC between the right amygdala and right putamen, increased anti-coupling between the frontoparietal network and the left amygdala, and reduced anti-coupling between the frontoparietal network and the right putamen. These FC changes significantly correlated with FOG severity and fear of falling in PD-FOG. Only one study using the seed-to-voxel approach specifically investigated the cerebellum's role in the pathophysiology of FOG (Bharti et al., 2019a). In this study, decreased FC between the dentate nucleus and several cortical and subcortical areas was found in PD-FOG as compared to PD-nFOG.
Other studies analyzed rs-fMRI data in PD-FOG with alternative methods. By using a low-frequency (0.01~0.08Hz) BOLD signal amplitude (amplitude of low-frequency fluctuation, ALFF), Mi et al. (2017) showed decreased FC in the right superior frontal gyrus, bilateral cerebellum, and left thalamus, but increased FC in the right anterior cingulate cortex and left inferior parietal lobule in PD-FOG compared to PD-nFOG. In this study, Mi et al. (2017) also found a significant correlation between ALFF changes and FOG severity (Mi et al., 2017). More recently, in line with previous findings, Hu et al. (2019) found abnormal ALFF in the putamen, frontal, and temporal brain areas in PD-FOG (Hu et al., 2019). By using the voxel-mirrored homotopic connectivity (VMHC) technique (Stark et al., 2008), Li et al. (2018) also examined homotopic FC patterns in PD-FOG. In this study, Li et al. (2018) showed lower FC in the inferior parietal lobule in PD-FOG than in PD-nFOG, which negatively correlated with FOG severity scores (Li et al., 2018). More recently, Zhou et al. (2018) examined regional homogeneity, a measure of local FC (Jiang and Zuo, 2016), and demonstrated lower regional homogeneity in the frontal cortex and motor brain areas in PD-FOG (Zhou et al., 2018). Lastly, Maidan et al. (2019) examined FC changes in the attentional networks in PD-FOG by using graph theory analysis. The authors reported lower global efficiency of the dorsal attention network in PD-FOG than in PD-nFOG (Maidan et al., 2019).
In summary, most of the previous tb-fMRI and rs-fMRI studies in PD-FOG have revealed abnormal functional activation and connectivity in brain regions responsible for frontal executive and attention abilities (i.e. frontal and fronto-striatal networks). In addition, a second set of observations in PD-FOG have pointed to abnormal functional activation and connectivity in posterior rather than anterior brain areas, specifically in regions responsible for visuospatial abilities (i.e. parieto-occipital networks, prominently in the right hemisphere). A third line of research has specifically focused on functional disconnection of subcortical brain regions involved in the locomotor network (i.e. MLR and CLR). Overall, the majority of the previous tb-fMRI and rs-fMRI studies have demonstrated abnormal activation in frontal and parietal regions and their functional connections with subcortical structures involved in the locomotor network, as a relevant pathophysiological mechanism underlying FOG.
3.3. Section 3: Nuclear medicine studies
We identified a total of 13 nuclear medicine imaging studies (nine PET studies and four SPECT studies) that reported brain changes in neurotransmitter activity, brain perfusion, and glucose metabolism in PD-FOG (Table 3).
Table. 3.
Nuclear medicine studies in PD-FOG.
| Authors (Year) | Participants (years) (mean age ± SD) | Technique | Outcome measures | Imaging findings | Clinical correlation |
|---|---|---|---|---|---|
| PET | |||||
| Bartels et al. (2006) | 10 PD-FOG (NA) 7 PD-nFOG (NA) | 18 [F]-DOPA and 18 [F]-FDG | Striatal dopamine and glucose metabolism | Reduced uptake of FDOPA in putamen and caudate, bilaterally; increased uptake of FDG in putamen, bilaterally; reduced uptake of FDG in caudate, bilaterally, and right parietal regions | No correlation |
| Lyoo et al. (2007) | 10 PD-FOG (61.3±9.8) | 18 [F]-FDG | Glucose metabolism before and after STN-DBS for clinical-radiological correlations | FOG improvement associated with increased metabolic activity in parieto-temporo-occipital areas after STN-DBS | FOG improvement positively correlated with metabolic activity in parieto-temporo-occipital areas after STN-DBS |
| Bohnen et al. (2014) | 20 PD-FOG (66±6) 123 PD-nFOG (65±8) | 11[C]-DTBZ, 11[C]-PMP, 11[C]-PIB | Dopaminergic and cholinergic activity, amyloid deposition | Reduced striatal dopaminergic activity; Reduced neocortical cholinergic innervation; Increased neocortical β‐amyloid deposition | Not performed |
| Maillet et al. (2015) | 8 PD-FOG (12.3 ± 3.8) | [H2150] | Regional cerebral blood flow during motor imagery of gait in ON state of therapy compared to Off state of therapy | Levodopa increased activation in motor regions, putamen, thalamus and cerebellum, while reduced premotor‐parietal and brainstem activation compared to OFF state of therapy | Not performed |
| Tard et al. (2015) | 11 PD-FOG (61±5) 11 PD-nFOG (62±3) | 18[F]-FDG | Glucose metabolism after performing a gait trajectory involving FOG triggers | Increased metabolic activity in the paracentral lobule, Globus pallidus and left posterior parietal cortex; reduced metabolic activity in the left dorsolateral prefrontal cortex | FOG occurrence positively correlated with metabolic activity in cerebellum, paracentral lobule and frontal eye field; negatively correlated with activity in orbitofrontal area, premotor cortex, SMA and temporal lobe. |
| Ono et al. (2016) | 40 PD patients (69±8; 11 PD-nFOG) 11 HS (64±7) | 6-18[F]-FMT | AADC activity | Reduced FMT uptake in putamen, caudate and LC in PD patients than HS | Severity of FOG negatively correlated with FMT uptake in LC |
| Gallardo et al. (2018) | 9 PD-FOG (NA) 8 PD-nFOG (NA) | 18[F]-FDG | Glucose metabolism | Frontal and predominantly right-sided hypometabolism | Not performed |
| Mitchell et al. (2019) | 9 PD-FOG (68±6) 9 PD-nFOG (65±5) | 18[F]-FDG | Glucose metabolism during steering of gait compared to straight walking | Changes in cortico-thalamic circuit and hyperdirect pathway (reduced activation of parietal regions; reduced deactivation of prefrontal regions and thalamus; increased activation of supplementary motor area) during staring of gait | Activity in right dorsolateral prefrontal cortex negatively correlated with stride length during steering of gait |
| Bohnen et al. (2019) | 15 PD-FOG (73±10) 79 PD-nFOG (67±7) | 18[F]-FEOBV | VAChT expression | Reduced VAChT expression in bilateral striatum (mostly right caudate), temporal and mesiofrontal limbic regions. | No correlation |
| SPECT | |||||
| Matsui et al. (2005) | 24 PD-FOG (66±8) 31 PD-nFOG (70±7) | 123[I]‐IMP | Brain perfusion | Decreased perfusion in orbitofrontal cortex, bilaterally | FOG severity negatively correlated with perfusion rate in the orbitofrontal cortex |
| Imamura et al. (2012) | 21 PD-FOG (71±8) 34 PD-nFOG (69±10) | 123[I]‐IMP | Regional cerebral blood flow | Increased perfusion in frontal lobe (bilateral BA 10, 11 and left BA 32) | FOG severity positively correlated with perfusion increase in BA 10, 11, 32 |
| Djaldetti et al. (2018) | 15 PD-FOG (63±11) 26 PD-nFOG (61±9) | 123[I]-FP-CIT | DAT uptake | Reduced DAT uptake in putamen and striatum | Inverse association between DAT uptake in putamen and striatum and FOG |
| Kim et al. (2018) | 390 PD patients (NA) (PPMI database) | 123[I]-Ioflupane | DAT uptake | Reduced DAT uptake in caudate and putamen | Inverse association between DAT uptake in caudate and putamen and FOG |
PD-FOG: patients with Parkinson's disease and Freezing of Gait; PD-nFOG: patients with Parkinson's disease without Freezing of Gait; 18[F] DOPA: 18 [F]-6-fluoro-levodopa; 18[F] FDG: 18[F]-fluordesoxy-glucose; STN: subthalamic nucleus; DBS: Deep brain stimulation; 11[C] DTBZ: 11[C] Dihydrotetrabenazine; 11[C] PMP: 11[C]methyl4piperidyl propionate; 11[C] PIB: 11[C]-Pittsburgh compound B; On state of therapy: after Levodopa intake; Off state of therapy: after Levodopa withdrawal; HS: Healthy Subjects; 6-18[F] FMT: 6-18[F]Fluoro-l-m-tyrosine; AADC: Aromatic l-amino acid decarboxylase; LC: locus coeruleus; 18[F] FEOBV: 18[F]-fluoroethoxybenzovesamicol; VAChT: Vesicular acetylcholine transporter; 123[I]‐IMP: N-isopropyl-p-123[I]iodoamphetamine; BA: Brodmann Area; 123[I]-FP-CIT: 123[I]-2-b-carbomethoxy-3b-(4-iodophenyl)-N-(3-fluoropropyl) nortropane; DAT: Dopamine Transporter; PPMI: Parkinson's Progression Markers Initiative.
*NA: not available
PET studies consistently found lower striatal dopaminergic activity in PD-FOG than in PD-nFOG (Bartels et al., 2006; Bohnen et al., 2014), which mainly involved the right putamen and caudate (Bartels et al., 2006). SPECT studies examining dopamine transporter activities found that lower levels of dopamine transporter activities in the striatum significantly predicted FOG development in PD patients (Djaldetti et al., 2018; Kim et al., 2018).
Only a few PET studies (Bohnen et al., 2014, 2009) have examined cholinergic activity in PD-FOG. These studies demonstrated greater cholinergic denervation in the neocortex (Bohnen et al., 2014), striatum, and limbic archicortex (Bohnen et al., 2019) in PD-FOG than in PD-nFOG. Finally, Ono et al. (2016) studied the aminergic system in PD-FOG by analysing aromatic l-amino acid decarboxylase (AADC) activity. In this study, the authors found a significant reduction in AADC activity in the locus coeruleus and striatum that correlated with FOG severity (Ono et al., 2016).
SPECT studies investigating brain perfusion found lower perfusion in the prefrontal cortex (Imamura et al., 2012; Matsui et al., 2005) and in the anterior cingulate cortex (Imamura et al., 2012) in PD-FOG than in PD-nFOG. Few PET studies (Ballanger et al., 2009; Maillet et al., 2015) have examined cerebral blood flow (CBF) changes during different conditions in PD-FOG. In particular, Ballanger et al. (2009) demonstrated that deep brain stimulation of PPN increases CBF in subcortical (cerebellum, thalamus, MLR) and cortical (medial sensorimotor cortex extending into caudal SMA) areas in PD-FOG (Ballanger et al., 2009). More recently, Maillet et al. (2015) found various brain areas of either decreased or increased CBF during motor imagery of gait in PD-FOG compared to PD-nFOG. In this study, Maillet et al. (2015) also demonstrated that L-Dopa increases CBF in the primary motor cortex, basal ganglia, thalamus, and cerebellum in PD-FOG (Maillet et al., 2015).
Other PET studies compared cerebral glucose metabolism in PD-FOG and PD-nFOG (Bartels et al., 2006; Gallardo et al., 2018; Mitchell et al., 2019; Tard et al., 2015). Most of the studies demonstrated hypometabolism in frontal (Gallardo et al., 2018; Tard et al., 2015) and parietal brain regions in PD-FOG (Bartels et al., 2006; Mitchell et al., 2019) primarily involving the right hemisphere (Bartels et al., 2006; Gallardo et al., 2018). In contrast, other studies showed hyper rather than hypometabolism in specific frontal areas (e.g. paracentral lobule) (Mitchell et al., 2019; Tard et al., 2015). Studies investigating subcortical brain regions with PET also described functional abnormalities in subcortical structures such as the basal ganglia (Bartels et al., 2006; Tard et al., 2015) and MLR (Tard et al., 2015. Lyoo et al. (2007) specifically investigated cerebral glucose metabolic changes in PD-FOG associated with clinical improvement secondary to STN deep brain stimulation procedures. Lyoo et al. (2007) found a positive correlation between cerebral glucose metabolism in the parieto-temporo-occipital areas and clinical improvement of FOG (Lyoo et al., 2007). More recently, Mitchell et al. (2018) examined cerebral glucose metabolic changes during steering and straight walking and discovered cerebral glucose metabolic changes in various brain regions involving the cognitive corticothalamic circuit (Mitchell et al., 2019). Lastly, Bohnen et al. (2014) analysed amyloid deposition for in vivo detection of fibrillar plaques and found increased neocortical β‐amyloid deposition in PD-FOG compared to PD-nFOG (Bohnen et al., 2014).
In summary, nuclear medicine imaging involving PET and SPECT have demonstrated abnormal patterns of dopaminergic and cholinergic transmission as well as perfusion and metabolic changes in PD-FOG. More in detail, PET and SPECT studies have suggested decreased dopaminergic striatal activity in PD-FOG compared with PD-nFOG. Furthermore, PET studies have also demonstrated non-dopaminergic involvement in PD-FOG as reflected by cholinergic as well as noradrenergic denervation, and finally extra-nigral amyloid deposition. Overall, both perfusion and metabolic studies have shown heterogeneous results involving multiple cortical and subcortical structures, i.e. frontoparietal regions, temporo-occipital cortex, brainstem, cerebellum, basal ganglia, thalamus and limbic system. Despite a lack of consistency among studies, most of them pointed to a lower perfusion or metabolism in the frontoparietal areas.
4. Discussion
In this systematic review, we examined the available literature on structural neuroimaging findings with MRI techniques and functional brain changes as measured by fMRI and nuclear medicine techniques such as SPECT and PET in PD-FOG. MRI studies with VBM and SBM have reported structural changes in GM in a number of brainstem and cerebellar nuclei as well as in the basal ganglia in PD-FOG. In addition, MRI demonstrated cortical atrophy in various cortical regions including frontoparietal regions in PD-FOG. Structural WM changes in long associative bundles, such as the superior longitudinal fasciculus, and in subcortical brain regions specifically involved in locomotion were also prominent in PD-FOG as compared with PD-nFOG. Consistent with structural MRI findings, functional MRI studies performed during the execution of specific tasks or in resting conditions have also found abnormal functional activation and connectivity in the brainstem, cerebellum, basal ganglia, and frontoparietal cortex in PD-FOG. Lastly, nuclear medicine imaging with SPECT and PET has demonstrated functional changes in dopaminergic and cholinergic transmission and further confirmed metabolic changes in the frontoparietal regions in PD-FOG.
By systematically examining and merging all the previous neuroimaging findings on structural and functional brain changes in PD-FOG, we concisely summarize available information and discuss the current pathophysiological hypotheses regarding FOG in PD as a result of structural or functional damage in the human locomotion network.
4.1. The role of the brainstem, cerebellum, and basal ganglia in PD-FOG
Compelling evidence points to structural and functional abnormalities in the MLR, as demonstrated by GM loss (Snijders et al., 2011), abnormal structural WM connectivity (Canu et al., 2015; Fling et al., 2013; Schweder et al., 2010; Vercruysse et al., 2015; Youn et al., 2015), and functional abnormalities (Fling et al., 2014; Snijders et al., 2011; Tard et al., 2015; Wang et al., 2016) in PD-FOG. The MLR is a midbrain area composed of several nuclei (PPN, cuneiform, and subcuneiform nuclei) whose electrical stimulation determines locomotive activity in animal models (Garcia-Rill et al., 1987; Shik et al., 1966). Experimental studies have demonstrated that MLR damage is involved in several human gait disorders (Karachi et al., 2010; Snijders et al., 2016). Accordingly, in PD-FOG, the impairment of MLR cholinergic structures such as the PPN (Xiao et al., 2017) could lead to cortical and striatal cholinergic denervation and in turn to FOG (Bohnen et al., 2019, 2014). Alternatively, FOG in PD could result from a paroxysmal inhibition of MLR secondary to altered inputs driven by higher-level motor structures such as the basal ganglia (Lewis and Shine, 2016; Snijders et al., 2011).
Besides the MLR, a growing number of neuroimaging studies have demonstrated structural and functional changes in cerebellar structures in PD-FOG as evidenced by GM loss (Jha et al., 2015) and altered (increased or decreased) connectivity (Bharti et al., 2019a; Fling et al., 2014, 2013; Schweder et al., 2010; Vercruysse et al., 2015; Youn et al., 2015) and functional activation (Fling et al., 2014; Gilat et al., 2015; Mi et al., 2017; Myers et al., 2018; Peterson et al., 2014; Vervoort et al., 2016; Wang et al., 2016 ). The cerebellum is physiologically connected with multiple cortical and subcortical structures implicated in human locomotion including the PPN (Mori et al., 2016) and basal ganglia (Wu and Hallett, 2013). The cerebellum is thought to be involved in multiple locomotive functions, including internal postural models, perception of body motion, motor planning, and movement adaptation to environmental changes (Bostan et al., 2013; Middleton and Strick, 2000). In particular, the CLR is the main cerebellar structure evoking locomotion by activating the rhythm-generating system through projections to the medullary reticular formation (Takakusaki, 2017). A number of neuroimaging studies have demonstrated an abnormal cortico-pontine-cerebello-thalamo-cortical pathway (Gilman et al., 2010; Hanakawa et al., 1999; Schweder et al., 2010) as well as abnormal functional activation of the cerebellum in PD-FOG (Ballanger et al., 2008; Palmer et al., 2009). One hypothesis suggests that FOG in PD could result from impaired compensatory mechanisms exerted by the cerebellum, which assists and supports gait planning and execution (Bharti et al., 2019a; Fling et al., 2014, 2013; Gilat et al., 2015; Jha et al., 2015; Myers et al., 2018; Peterson et al., 2014; Schweder et al., 2010; Vercruysse et al., 2015; Vervoort et al., 2016; Wang et al., 2016; Youn et al., 2015). Alternatively, given the role of the cerebellum in cognitive and emotional functions (Koziol et al., 2014), structural or functional cerebellar impairment could contribute to executive and visuospatial deficits, as well as behavioural deficits in PD-FOG (Amboni et al., 2008; Cohen et al., 2014; Giladi, 2007; Giladi and Hausdorff, 2006; Lieberman, 2006; Martens et al., 2016, 2014; Vandenbossche et al., 2011).
Concerning the possible pathophysiological role of the basal ganglia in PD-FOG, a widely accepted hypothesis includes the “cross-talk model” proposed by Lewis et al. (2009) that suggests FOG occurs in PD as a result of a dysfunctional convergence and overload of normally segregated motor, cognitive, and limbic circuits in the basal ganglia (Lewis and Barker, 2009). In support of this hypothesis, a recent study reported a negative correlation between FOG severity and bilateral caudate body GM volumes (Herman et al., 2014), and several other studies demonstrated metabolic and neurotransmitter system changes in the striatum in PD-FOG (Bartels et al., 2006; Bohnen et al., 2014, 2019; Djaldetti et al., 2018; Kim et al., 2018; Pikstra et al., 2016; Tard et al., 2015).
4.2. The role of cortical brain regions in PD-FOG
Compelling evidence from neuroimaging studies has identified structural and functional changes in a number of frontal regions as key pathophysiological mechanisms in PD-FOG (Bharti et al., 2019b; Brugger et al., 2015; Fling et al., 2013; Gallardo et al., 2018; Imamura et al., 2012; Kostic et al., 2012; Maillet et al., 2015; Matsui et al., 2005; Pietracupa et al., 2018; Tard et al., 2015). These observations are consistent with clinical studies demonstrating prominent cognitive impairment primarily involving frontal functions such as attention, response inhibition, and conflict resolution abilities in PD-FOG (Matar et al., 2013; Naismith et al., 2010; Shine et al., 2012; Tard et al., 2015; Vandenbossche et al., 2012). Frontal cognitive functions are known to be relevant in human locomotion particularly when gait requires attentional resources, such as when walking in unfamiliar environments (Mirelman et al., 2018). Accordingly, cognitive impairment in frontal executive-attentive functions may contribute to FOG prominently during cognitively demanding tasks (Vandenbossche et al., 2013). This hypothesis is consistent with the clinical observation that in PD, FOG commonly occurs in the presence of challenging environmental conditions such as passage through a narrow space (Almeida and Lebold, 2010), while turning (Spildooren et al., 2013), during a dual task, or while trying to avoid obstacles (Jacobs et al., 2009; Lewis and Barker, 2009; Snijders et al., 2010; Spildooren et al., 2010). Several neuroimaging studies in PD-FOG have reported structural and functional changes in the SMA (Fling et al., 2014; Snijders et al., 2011; Vastik et al., 2017; Vercruysse et al., 2015). The SMA is thought to play a key role in gait control since it encodes specific sequences of movement and generates anticipatory postural adjustments (APAs) (Massion, 1992). In accordance with this hypothesis, several previous clinical studies have demonstrated prominent alterations in the generation of APAs, such as defective body weight shifting during step execution, in PD-FOG (Delval et al., 2014; Jacobs and Horak, 2007; Tard et al., 2014).
In addition to frontal lobe impairment, a second set of structural and functional neuroimaging studies have proposed a widespread impairment of frontoparietal networks responsible for visuospatial functions in PD-FOG (Brugger et al., 2015; Jha et al., 2015; Kostic et al., 2012; Pietracupa et al., 2018). Furthermore, some previous studies have found a prominent impairment of frontoparietal networks in the right hemisphere in PD-FOG (Bharti et al., 2019b; Fling et al., 2013; Peterson et al., 2014; Pietracupa et al., 2018; Youn et al., 2015). These findings are consistent with clinical evidence of prominent visuospatial impairment in PD-FOG (Almeida and Lebold, 2010). Hence, it is likely that in PD-FOG a visuospatial dysfunction secondary to structural or functional changes in frontoparietal networks in the non-dominant hemisphere could deteriorate online visuomotor transformations that adapt gait to abrupt environmental changes (Almeida and Lebold, 2010; Fling et al., 2013; Nantel et al., 2012; Silveira et al., 2015).
A final comment concerns a third set of studies on PD-FOG that demonstrates structural and functional changes in brain areas included in the limbic system such as the cingulate cortex and amygdala (Gilat et al., 2018; Vastik et al., 2017). Clinical studies have disclosed that anxiety and depression are common non-motor features in PD-FOG that crucially contribute to FOG occurrence (Martens et al., 2014). One possible explanation implies that emotional loading could detract attentional resources in PD-FOG, in turn leading to abrupt gait dysfunction (Gilat et al., 2018; Martens et al., 2016).
4.3. Quality Assessment
All studies presented a clear hypothesis statement. In comparison to functional and nuclear medicine imaging studies, structural investigations included larger sample sizes, a more detailed neuropsychological assessment, greater control for confoundings and clearer presentation of the results. Conversely, less than 40% of the functional studies included a detailed patients’ neurophysiological assessment, and only 57% presented a clinico-radiological correlation analysis, thus implying a lower overall quality of the analysis. Lastly, studies on nuclear medicine suffered from poor statistical analysis and result presentation, even if the latter increased over time thus contributing to improve the quality of the analysis. Overall, the results of our quality assessment would help in improving future neuroimaging studies in PD-FOG.
4.4. Conclusion
In humans, the neurophysiology of locomotion requires a distributed network including spinal and supraspinal structures (Takakusaki, 2008). More specifically, spinal central pattern generators are thought to provide a basic locomotive pattern that is influenced by sensory signals and descending pathways from supraspinal structures. Among them, specific basal ganglia, cerebellar, and brainstem locomotor regions (i.e. subthalamic locomotor region, CLR, MLR) are known to be involved in unconscious and automatic regulation of posture and gait in humans. Furthermore, when an intentional modification of gait is required, such as for volitional gait initiation or obstacle avoidance, the activation of higher order cortical networks promotes a number of cognitive aspects of motor control including visuomotor coordination and motor planning (Mirelman et al., 2018). For instance, the temporo-parietal cortex processes online multisensory signals and provides cognition of self-body and extracorporeal space to the SMA and other premotor areas responsible for motor planning (Takakusaki, 2013). Descending corticofugal projections, such as the cortico-reticulo-spinal system, regulate anticipatory posture control by transmitting motor programs to supraspinal and spinal structures to allow for fine tuning of specific kinematic and biomechanical variables such as limb trajectory and foot placement during gait (Takakusaki, 2008).
In this systematic review, we have discussed relevant advances in the current understanding of the pathophysiology of FOG in PD from both recent structural and functional neuroimaging studies. Rather than demonstrating a specific neuroanatomical substrate responsible for FOG in PD, currently available data point to a widespread structural and functional impairment in cortical and subcortical brain structures. This hypothesis is consistent with recent neuroimaging studies on lesion-induced FOG in non-PD patients demonstrating heterogeneous patterns of brain damage in patients with FOG (Fasano et al., 2017). Overall these observations strongly support the hypothesis that FOG in PD requires structural lesions or functional abnormalities of specific nodes involved in the network responsible for human locomotion (Ehgoetz Martens et al., 2018; Schaafsma et al., 2003).
Data and code availability statement
Any data and code associated with this review article will be made available by reasonable request to corresponding author (PP).
Declaration of Competing Interest
The authors report no financial conflicts of interest.
Funding
This work received no specific grant from any funding agency.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102059.
Appendix. Supplementary materials
References
- Agosta F., Gatti R., Sarasso E., Volonté M.A., Canu E., Meani A., Sarro L., Copetti M., Cattrysse E., Kerckhofs E., Comi G., Falini A., Filippi M. Brain plasticity in Parkinson's disease with freezing of gait induced by action observation training. J. Neurol. 2017;264:88–101. doi: 10.1007/s00415-016-8309-7. [DOI] [PubMed] [Google Scholar]
- Almeida Q.J., Lebold C.A. Freezing of gait in Parkinson's disease: a perceptual cause for a motor impairment? J. Neurol. Neurosurg. Psychiatry. 2010;81:513–518. doi: 10.1136/jnnp.2008.160580. [DOI] [PubMed] [Google Scholar]
- Amboni M., Cozzolino A., Longo K., Picillo M., Barone P. Freezing of gait and executive functions in patients with Parkinson's disease. Mov. Disord. 2008;23:395–400. doi: 10.1002/mds.21850. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ballanger B., Baraduc P., Broussolle E., Bars D.L., Desmurget M., Thobois S. Motor urgency is mediated by the contralateral cerebellum in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2008;79:1110–1116. doi: 10.1136/jnnp.2007.141689. [DOI] [PubMed] [Google Scholar]
- Ballanger B., Lozano A.M., Moro E., Eimeren T.van, Hamani C., Chen R., Cilia R., Houle S., Poon Y.Y., Lang A.E., Strafella A.P. Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson's disease: A [15O] H2O PET study. Hum. Brain Mapp. 2009;30:3901–3909. doi: 10.1002/hbm.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A.L., de Jong B.M., Giladi N., Schaafsma J.D., Maguire R.P., Veenma L., Pruim J., Balash Y., Youdim M.B.H., Leenders K.L. Striatal dopa and glucose metabolism in PD patients with freezing of gait. Mov. Disord. 2006;21:1326–1332. doi: 10.1002/mds.20952. [DOI] [PubMed] [Google Scholar]
- Basser P.J. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Bharti K., Suppa A., Pietracupa S., Upadhyay N., Giannì C., Leodori G., Di Biasio F., Modugno N., Petsas N., Grillea G., Zampogna A., Berardelli A., Pantano P. Abnormal cerebellar connectivity patterns in patients with Parkinson's disease and freezing of gait. Cerebellum. 2019;18:298–308. doi: 10.1007/s12311-018-0988-4. [DOI] [PubMed] [Google Scholar]
- Bharti K., Suppa A., Pietracupa S., Upadhyay N., Giannì C., Leodori G., Di Biasio F., Modugno N., Petsas N., Grillea G., Zampogna A., Berardelli A., Pantano P. Aberrant functional connectivity in patients with Parkinson's disease and freezing of gait: a within- and between-network analysis. Brain Imaging Behav. 2019 doi: 10.1007/s11682-019-00085-9. [DOI] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal B.B. Resting state fMRI: a personal history. Neuroimage. 2012;62:938–944. doi: 10.1016/j.neuroimage.2012.01.090. [DOI] [PubMed] [Google Scholar]
- Bohnen N.I., Frey K.A., Studenski S., Kotagal V., Koeppe R.A., Constantine G.M., Scott P.J.H., Albin R.L., Müller M.L.T.M. Extra-nigral pathological conditions are common in Parkinson's disease with freezing of gait: An in vivo positron emission tomography study. Mov. Disord. 2014;29:1118–1124. doi: 10.1002/mds.25929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen N.I., Kanel P., Zhou Z., Koeppe R.A., Frey K.A., Dauer W.T., Albin R.L., Müller M.L.T.M. Cholinergic system changes of falls and freezing of gait in Parkinson's disease. Ann. Neurol. 2019;85:538–549. doi: 10.1002/ana.25430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen N.I., Müller M.L.T.M., Koeppe R.A., Studenski S.A., Kilbourn M.A., Frey K.A., Albin R.L. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73:1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan A.C., Dum R.P., Strick P.L. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cognit. Sci. 2013;17:241–254. doi: 10.1016/j.tics.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugger F., Abela E., Hägele-Link S., Bohlhalter S., Galovic M., Kägi G. Do executive dysfunction and freezing of gait in Parkinson's disease share the same neuroanatomical correlates? J. Neurol. Sci. 2015;356:184–187. doi: 10.1016/j.jns.2015.06.046. [DOI] [PubMed] [Google Scholar]
- Canu E., Agosta F., Sarasso E., Volontè M.A., Basaia S., Stojkovic T., Stefanova E., Comi G., Falini A., Kostic V.S., Gatti R., Filippi M. Brain structural and functional connectivity in Parkinson's disease with freezing of gait. Hum. Brain Mapp. 2015;36:5064–5078. doi: 10.1002/hbm.22994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R.G., Klein K.A., Nomura M., Fleming M., Mancini M., Giladi N., Nutt J.G., Horak F.B. Inhibition, executive function, and freezing of gait. J. Parkinsons Dis. 2014;4:111–122. doi: 10.3233/JPD-130221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Giglio L., Tommasin S., Petsas N., Pantano P. The Role of fMRI in the Assessment of Neuroplasticity in MS: A Systematic Review [WWW Document] Neural Plast. 2018 doi: 10.1155/2019/5181649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delval A., Moreau C., Bleuse S., Tard C., Ryckewaert G., Devos D., Defebvre L. Auditory cueing of gait initiation in Parkinson's disease patients with freezing of gait. Clin. Neurophys. 2014;125:1675–1681. doi: 10.1016/j.clinph.2013.12.101. [DOI] [PubMed] [Google Scholar]
- Djaldetti R., Rigbi A., Greenbaum L., Reiner J., Lorberboym M. Can early dopamine transporter imaging serve as a predictor of Parkinson's disease progression and late motor complications? J. Neurol. Sci. 2018;390:255–260. doi: 10.1016/j.jns.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Hall J.M., Georgiades M.J., Gilat M., Walton C.C., Matar E., Lewis S.J.G., Shine J.M. The functional network signature of heterogeneity in freezing of gait. Brain. 2018;141:1145–1160. doi: 10.1093/brain/awy019. [DOI] [PubMed] [Google Scholar]
- Fasano A., Herman T., Tessitore A., Strafella A.P., Bohnen N.I. Neuroimaging of freezing of gait. J. Parkinsons Dis. 2015;5:241–254. doi: 10.3233/JPD-150536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A., Laganiere S.E., Lam S., Fox M.D. Lesions causing freezing of gait localize to a cerebellar functional network. Ann. Neurol. 2017;81:129–141. doi: 10.1002/ana.24845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling B.W., Cohen R.G., Mancini M., Carpenter S.D., Fair D.A., Nutt J.G., Horak F.B. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling B.W., Cohen R.G., Mancini M., Nutt J.G., Fair D.A., Horak F.B. Asymmetric pedunculopontine network connectivity in Parkinsonian patients with freezing of gait. Brain. 2013;136:2405–2418. doi: 10.1093/brain/awt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Gallardo M.J., Cabello J.P., Corrales M.J., Torres-Donaire J., Bravo J.J., Talavera M.P., León A., Vaamonde-Gamo J. Freezing of gait in Parkinson's disease: functional neuroimaging studies of the frontal lobe. Neurol. Res. 2018:1–6. doi: 10.1080/01616412.2018.1484985. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E., Houser C.R., Skinner R.D., Smith W., Woodward D.J. Locomotion-inducing sites in the vicinity of the pedunculopontine nucleus. Brain Res. Bull. 1987;18:731–738. doi: 10.1016/0361-9230(87)90208-5. [DOI] [PubMed] [Google Scholar]
- Giladi N. Medical treatment of freezing of gait. Mov. Disord. 2008;2:S482–S488. doi: 10.1002/mds.21914. 23 Suppl. [DOI] [PubMed] [Google Scholar]
- Giladi N. Gait and mental function: the interplay between walking, behavior and cognition. J. Neural Trans. (Vienna) 2007;114:1241–1242. doi: 10.1007/s00702-007-0802-9. [DOI] [PubMed] [Google Scholar]
- Giladi N., Hausdorff J.M. The role of mental function in the pathogenesis of freezing of gait in Parkinson's disease. J. Neurol. Sci. 2006;248:173–176. doi: 10.1016/j.jns.2006.05.015. Dementia in Parkinson's Disease: International Symposium. [DOI] [PubMed] [Google Scholar]
- Giladi N., McDermott M.P., Fahn S., Przedborski S., Jankovic J., Stern M., Tanner C., Parkinson Study Group Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology. 2001;56:1712–1721. doi: 10.1212/wnl.56.12.1712. [DOI] [PubMed] [Google Scholar]
- Giladi N., Treves T.A., Simon E.S., Shabtai H., Orlov Y., Kandinov B., Paleacu D., Korczyn A.D. Freezing of gait in patients with advanced Parkinson's disease. J. Neural Transm. (Vienna) 2001;108:53–61. doi: 10.1007/s007020170096. [DOI] [PubMed] [Google Scholar]
- Gilat M., Ehgoetz Martens K.A., Miranda-Domínguez O., Arpan I., Shine J.M., Mancini M., Lewis S.J.G., Horak F.B. Dysfunctional limbic circuitry underlying freezing of gait in parkinson's disease. Neuroscience. 2018;374:119–132. doi: 10.1016/j.neuroscience.2018.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilat M., Shine J.M., Walton C.C., O'Callaghan C., Hall J.M., Lewis S.J.G. Brain activation underlying turning in Parkinson's disease patients with and without freezing of gait: a virtual reality fMRI study. NPJ Parkinsons Dis. 2015;1:15020. doi: 10.1038/npjparkd.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S., Koeppe R.A., Nan B., Wang C.-N., Wang X., Junck L., Chervin R.D., Consens F., Bhaumik A. Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology. 2010;74:1416–1423. doi: 10.1212/WNL.0b013e3181dc1a55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J., Henson R.N., Friston K.J., Frackowiak R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Hall J.M., Shine J.M., Ehgoetz Martens K.A., Gilat M., Broadhouse K.M., Szeto J.Y.Y., Walton C.C., Moustafa A.A., Lewis S.J.G. Alterations in white matter network topology contribute to freezing of gait in Parkinson's disease. J. Neurol. 2018;265:1353–1364. doi: 10.1007/s00415-018-8846-3. [DOI] [PubMed] [Google Scholar]
- Hanakawa T., Katsumi Y., Fukuyama H., Honda M., Hayashi T., Kimura J., Shibasaki H. Mechanisms underlying gait disturbance in Parkinson's disease: a single photon emission computed tomography study. Brain. 1999;122(Pt 7):1271–1282. doi: 10.1093/brain/122.7.1271. [DOI] [PubMed] [Google Scholar]
- Herman T., Rosenberg-Katz K., Jacob Y., Giladi N., Hausdorff J.M. Gray matter atrophy and freezing of gait in Parkinson's disease: Is the evidence black-on-white? Mov. Disord. 2014;29:134–139. doi: 10.1002/mds.25697. [DOI] [PubMed] [Google Scholar]
- Hoorn A., van der Renken, Leenders R.J., Jong K.L., de B.M. Parkinson-related changes of activation in visuomotor brain regions during perceived forward self-motion. PLOS ONE. 2014;9:e95861. doi: 10.1371/journal.pone.0095861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Chen J., Huang H., Zhou C., Zhang S., Liu X., Wang L., Chen P., Nie K., Chen L., Wang S., Huang B., Huang R. Common and specific altered amplitude of low-frequency fluctuations in Parkinson's disease patients with and without freezing of gait in different frequency bands. Brain Imaging Behav. 2019 doi: 10.1007/s11682-018-0031-x. [DOI] [PubMed] [Google Scholar]
- Imamura K., Okayasu N., Nagatsu T. Cerebral blood flow and freezing of gait in Parkinson's disease. Acta Neurol. Scand. 2012;126:210–218. doi: 10.1111/j.1600-0404.2012.01652.x. [DOI] [PubMed] [Google Scholar]
- Jacobs J.V., Horak F.B. External postural perturbations induce multiple anticipatory postural adjustments when subjects cannot pre-select their stepping foot. Exp. Brain Res. 2007;179:29–42. doi: 10.1007/s00221-006-0763-5. [DOI] [PubMed] [Google Scholar]
- Jacobs J.V., Nutt J.G., Carlson-Kuhta P., Stephens M., Horak F.B. Knee Trembling During Freezing of Gait Represents Multiple Anticipatory Postural Adjustments. Exp. Neurol. 2009;215:334–341. doi: 10.1016/j.expneurol.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha M., Jhunjhunwala K., Sankara B.B., Saini J., Kumar J.K., Yadav R., Pal P.K. Neuropsychological and imaging profile of patients with Parkinson's disease and freezing of gait. Parkinsonism Relat. Disord. 2015;21:1184–1190. doi: 10.1016/j.parkreldis.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Jiang L., Zuo X.-N. Regional Homogeneity. Neuroscientist. 2016;22:486–505. doi: 10.1177/1073858415595004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachi C., Grabli D., Bernard F.A., Tandé D., Wattiez N., Belaid H., Bardinet E., Prigent A., Nothacker H.-P., Hunot S., Hartmann A., Lehéricy S., Hirsch E.C., François C. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J. Clin. Invest. 2010;120:2745–2754. doi: 10.1172/JCI42642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R., Lee J., Kim Y., Kim A., Jang M., Kim H.-J., Jeon B., Kang U.J., Fahn S. Presynaptic striatal dopaminergic depletion predicts the later development of freezing of gait in de novo Parkinson's disease: An analysis of the PPMI cohort. Parkinsonism Relat. Disord. 2018;51:49–54. doi: 10.1016/j.parkreldis.2018.02.047. [DOI] [PubMed] [Google Scholar]
- Kostic V.S., Agosta F., Pievani M., Stefanova E., Jecmenica-Lukic M., Scarale A., Spica V., Filippi M. Pattern of brain tissue loss associated with freezing of gait in Parkinson disease. Neurology. 2012;78:409–416. doi: 10.1212/WNL.0b013e318245d23c. [DOI] [PubMed] [Google Scholar]
- Koziol L.F., Budding D., Andreasen N., D'Arrigo S., Bulgheroni S., Imamizu H., Ito M., Manto M., Marvel C., Parker K., Pezzulo G., Ramnani N., Riva D., Schmahmann J., Vandervert L., Yamazaki T. Consensus paper: the cerebellum's role in movement and cognition. Cerebellum. 2014;13:151–177. doi: 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fougère C., Rominger A., Fesl G., Dieterich M., Brandt T., Strupp M., Bartenstein P., Jahn K. Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. Neuroimage. 2010;50:1589–1598. doi: 10.1016/j.neuroimage.2009.12.060. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. [Microcomputer simulation of nuclear magnetic resonance imaging contrasts] J. Radiol. 1985;66:303–308. [PubMed] [Google Scholar]
- Lenka A., Naduthota R.M., Jha M., Panda R., Prajapati A., Jhunjhunwala K., Saini J., Yadav R., Bharath R.D., Pal P.K. Freezing of gait in Parkinson's disease is associated with altered functional brain connectivity. Parkinsonism Relat. Disord. 2016;24:100–106. doi: 10.1016/j.parkreldis.2015.12.016. [DOI] [PubMed] [Google Scholar]
- Lewis S.J.G., Barker R.A. A pathophysiological model of freezing of gait in Parkinson's disease. Parkinsonism Relat. Disord. 2009;15:333–338. doi: 10.1016/j.parkreldis.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Lewis S.J.G., Shine J.M. The next step: a common neural mechanism for freezing of gait. Neuroscientist. 2016;22:72–82. doi: 10.1177/1073858414559101. [DOI] [PubMed] [Google Scholar]
- Li J., Yuan Y., Wang M., Zhang J., Zhang L., Jiang S., Wang X., Ding J., Zhang K. Decreased interhemispheric homotopic connectivity in Parkinson's disease patients with freezing of gait: a resting state fMRI study. Parkinsonism Relat. Disord. 2018;52:30–36. doi: 10.1016/j.parkreldis.2018.03.015. [DOI] [PubMed] [Google Scholar]
- Lieberman A. Are freezing of gait (FOG) and panic related? J. Neurol. Sci. 2006;248:219–222. doi: 10.1016/j.jns.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Logothetis N.K. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Logothetis N.K., Murayama Y., Augath M., Steffen T., Werner J., Oeltermann A. How not to study spontaneous activity. Neuroimage. 2009;45:1080–1089. doi: 10.1016/j.neuroimage.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Lopez O.L., Litvan I., Catt K.E., Stowe R., Klunk W., Kaufer D.I., Becker J.T., DeKosky S.T. Accuracy of four clinical diagnostic criteria for the diagnosis of neurodegenerative dementias. Neurology. 1999;53:1292–1299. doi: 10.1212/wnl.53.6.1292. [DOI] [PubMed] [Google Scholar]
- Lyoo C.H., Aalto S., Rinne J.O., Lee K.O., Oh S.H., Chang J.W., Lee M.S. Different cerebral cortical areas influence the effect of subthalamic nucleus stimulation on Parkinsonian motor deficits and freezing of gait. Mov. Disord. 2007;22:2176–2182. doi: 10.1002/mds.21609. [DOI] [PubMed] [Google Scholar]
- Macht M., Kaussner Y., Möller J.C., Stiasny-Kolster K., Eggert K.M., Krüger H.-P., Ellgring H. Predictors of freezing in Parkinson's disease: a survey of 6,620 patients. Mov. Disord. 2007;22:953–956. doi: 10.1002/mds.21458. [DOI] [PubMed] [Google Scholar]
- Maidan I., Jacob Y., Giladi N., Hausdorff J.M., Mirelman A. Altered organization of the dorsal attention network is associated with freezing of gait in Parkinson's disease. Parkinsonism Relat. Disord. 2019;63:77–82. doi: 10.1016/j.parkreldis.2019.02.036. [DOI] [PubMed] [Google Scholar]
- Maillet A., Thobois S., Fraix V., Redouté J., Bars D.L., Lavenne F., Derost P., Durif F., Bloem B.R., Krack P., Pollak P., Debû B. Neural substrates of levodopa-responsive gait disorders and freezing in advanced Parkinson's disease: A kinesthetic imagery approach. Hum. Brain Mapp. 2015;36:959–980. doi: 10.1002/hbm.22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens K.A.E., Ellard C.G., Almeida Q.J. Does anxiety cause freezing of gait in Parkinson's disease? PLOS ONE. 2014;9 doi: 10.1371/journal.pone.0106561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens K.A.E., Hall J.M., Gilat M., Georgiades M.J., Walton C.C., Lewis S.J.G. Anxiety is associated with freezing of gait and attentional set-shifting in Parkinson's disease: A new perspective for early intervention. Gait Posture. 2016;49:431–436. doi: 10.1016/j.gaitpost.2016.07.182. [DOI] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog. Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Matar E., Shine J.M., Gilat M., Martens K.A.E., Ward P.B., Frank M.J., Moustafa A.A., Naismith S.L., Lewis S.J.G. Identifying the neural correlates of doorway freezing in Parkinson's disease. Hum. Brain Mapp. 2019;40:2055–2064. doi: 10.1002/hbm.24506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matar E., Shine J.M., Naismith S.L., Lewis S.J.G. Using virtual reality to explore the role of conflict resolution and environmental salience in freezing of gait in Parkinson's disease. Parkinsonism Relat. Disord. 2013;19:937–942. doi: 10.1016/j.parkreldis.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Matsui H., Udaka F., Miyoshi T., Hara N., Tamaura A., Oda M., Kubori T., Nishinaka K., Kameyama M. Three-dimensional stereotactic surface projection study of freezing of gait and brain perfusion image in Parkinson's disease. Mov. Disord. 2005;20:1272–1277. doi: 10.1002/mds.20520. [DOI] [PubMed] [Google Scholar]
- Merboldt K.D., Hänicke W., Frahm J. Diffusion imaging using stimulated echoes. Magn. Reson. Med. 1991;19:233–239. doi: 10.1002/mrm.1910190208. [DOI] [PubMed] [Google Scholar]
- Mi T.-M., Mei S.-S., Liang P.-P., Gao L.-L., Li K.-C., Wu T., Chan P. Altered resting-state brain activity in Parkinson's disease patients with freezing of gait. Sci. Rep. 2017;7:16711. doi: 10.1038/s41598-017-16922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res. Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Mirelman A., Shema S., Maidan I., Hausdorff J.M. Gait. Handb. Clin. Neurol. 2018;159:119–134. doi: 10.1016/B978-0-444-63916-5.00007-0. [DOI] [PubMed] [Google Scholar]
- Mitchell T., Potvin-Desrochers A., Lafontaine A.-L., Monchi O., Thiel A., Paquette C. Cerebral Metabolic Changes Related to Freezing of Gait in Parkinson Disease. J. Nucl. Med. 2019;60:671–676. doi: 10.2967/jnumed.118.218248. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- Mori F., Okada K., Nomura T., Kobayashi Y. The pedunculopontine tegmental nucleus as a motor and cognitive interface between the cerebellum and basal ganglia. Front. Neuroanat. 2016;10 doi: 10.3389/fnana.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers P.S., McNeely M.E., Koller J.M., Earhart G.M., Campbell M.C. Cerebellar volume and executive function in Parkinson disease with and without freezing of gait. J. Parkinsons Dis. 2017;7:149–157. doi: 10.3233/JPD-161029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers P.S., McNeely M.E., Pickett K.A., Duncan R.P., Earhart G.M. Effects of exercise on gait and motor imagery in people with Parkinson disease and freezing of gait. Parkinsonism Relat. Disord. 2018;53:89–95. doi: 10.1016/j.parkreldis.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nackaerts E., Nieuwboer A., Broeder S., Swinnen S., Vandenberghe W., Heremans E. Altered effective connectivity contributes to micrographia in patients with Parkinson's disease and freezing of gait. J. Neurol. 2018;265:336–347. doi: 10.1007/s00415-017-8709-3. [DOI] [PubMed] [Google Scholar]
- Naismith S.L., Shine J.M., Lewis S.J.G. The specific contributions of set-shifting to freezing of gait in Parkinson's disease. Mov. Disord. 2010;25:1000–1004. doi: 10.1002/mds.23005. [DOI] [PubMed] [Google Scholar]
- Nantel J., McDonald J.C., Tan S., Bronte-Stewart H. Deficits in visuospatial processing contribute to quantitative measures of freezing of gait in Parkinson's disease. Neuroscience. 2012;221:151–156. doi: 10.1016/j.neuroscience.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Nonnekes J., Snijders A.H., Nutt J.G., Deuschl G., Giladi N., Bloem B.R. Freezing of gait: a practical approach to management. Lancet Neurol. 2015;14:768–778. doi: 10.1016/S1474-4422(15)00041-1. [DOI] [PubMed] [Google Scholar]
- Nutt J.G., Bloem B.R., Giladi N., Hallett M., Horak F.B., Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–744. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S.A., Sato T., Muramatsu S. Freezing of Gait in Parkinson's Disease Is Associated with Reduced 6-[18F]Fluoro-L-m-tyrosine Uptake in the Locus Coeruleus [WWW Document] Parkinson's Dis. 2016 doi: 10.1155/2016/5430920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S.J., Eigenraam L., Hoque T., McCaig R.G., Troiano A., McKeown M.J. Levodopa-sensitive, dynamic changes in effective connectivity during simultaneous movements in Parkinson's disease. Neuroscience. 2009;158:693–704. doi: 10.1016/j.neuroscience.2008.06.053. [DOI] [PubMed] [Google Scholar]
- Peterson D.S., Fling B.W., Mancini M., Cohen R.G., Nutt J.G., Horak F.B. Dual-task interference and brain structural connectivity in people with Parkinson's disease who freeze. J. Neurol. Neurosurg. Psychiatry. 2015;86:786–792. doi: 10.1136/jnnp-2014-308840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D.S., Pickett K.A., Duncan R., Perlmutter J., Earhart G.M. Gait-related brain activity in people with Parkinson disease with freezing of gait. PLoS ONE. 2014;9:e90634. doi: 10.1371/journal.pone.0090634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C., Jezzard P., Basser P.J., Barnett A., Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Pietracupa S., Suppa A., Upadhyay N., Giannì C., Grillea G., Leodori G., Modugno N., Biasio F.D., Zampogna A., Colonnese C., Berardelli A., Pantano P. Freezing of gait in Parkinson's disease: gray and white matter abnormalities. J. Neurol. 2018;265:52–62. doi: 10.1007/s00415-017-8654-1. [DOI] [PubMed] [Google Scholar]
- Pikstra A.R.A., van der Hoorn A., Leenders K.L., de Jong B.M. Relation of 18-F-Dopa PET with hypokinesia-rigidity, tremor and freezing in Parkinson's disease. Neuroimage Clin. 2016;11:68–72. doi: 10.1016/j.nicl.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino A., Assogna F., Piras F., Di Battista M.E., Imperiale F., Chiapponi C., Spalletta G., Meco G. Does a volume reduction of the parietal lobe contribute to freezing of gait in Parkinson's disease? Parkinsonism Relat. Disord. 2014;20:1101–1103. doi: 10.1016/j.parkreldis.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Schaafsma J.D., Balash Y., Gurevich T., Bartels A.L., Hausdorff J.M., Giladi N. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson's disease. Eur. J. Neurol. 2003;10:391–398. doi: 10.1046/j.1468-1331.2003.00611.x. [DOI] [PubMed] [Google Scholar]
- Shik M.L., Severin F.V., Orlovskiĭ G.N. [Control of walking and running by means of electric stimulation of the midbrain] Biofizika. 1966;11:659–666. [PubMed] [Google Scholar]
- Shine J.M., Matar E., Ward P.B., Bolitho S.J., Gilat M., Pearson M., Naismith S.L., Lewis S.J.G. Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson's disease. Brain. 2013;136:1204–1215. doi: 10.1093/brain/awt049. [DOI] [PubMed] [Google Scholar]
- Shine J.M., Matar E., Ward P.B., Bolitho S.J., Pearson M., Naismith S.L., Lewis S.J.G. Differential neural activation patterns in patients with Parkinson's disease and freezing of gait in response to concurrent cognitive and motor load. PLoS ONE. 2013;8:e52602. doi: 10.1371/journal.pone.0052602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J.M., Matar E., Ward P.B., Frank M.J., Moustafa A.A., Pearson M., Naismith S.L., Lewis S.J.G. Freezing of gait in Parkinson's disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain. 2013;136:3671–3681. doi: 10.1093/brain/awt272. [DOI] [PubMed] [Google Scholar]
- Shine J.M., Moore S.T., Bolitho S.J., Morris T.R., Dilda V., Naismith S.L., Lewis S.J.G. Assessing the utility of freezing of gait questionnaires in Parkinson's disease. Parkinsonism Relat. Disord. 2012;18:25–29. doi: 10.1016/j.parkreldis.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Shine J.M., Ward P.B., Naismith S.L., Pearson M., Lewis S.J.G. Utilising functional MRI (fMRI) to explore the freezing phenomenon in Parkinson's disease. J. Clin. Neurosci. 2011;18:807–810. doi: 10.1016/j.jocn.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Silveira C.R.A., Ehgoetz Martens K.A., Pieruccini-Faria F., Bell-Boucher D., Roy E.A., Almeida Q.J. Disentangling perceptual judgment and online feedback deficits in Parkinson's freezing of gait. J. Neurol. 2015;262:1629–1636. doi: 10.1007/s00415-015-7759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders A.H., Leunissen I., Bakker M., Overeem S., Helmich R.C., Bloem B.R., Toni I. Gait-related cerebral alterations in patients with Parkinson's disease with freezing of gait. Brain. 2011;134:59–72. doi: 10.1093/brain/awq324. [DOI] [PubMed] [Google Scholar]
- Snijders A.H., Takakusaki K., Debu B., Lozano A.M., Krishna V., Fasano A., Aziz T.Z., Papa S.M., Factor S.A., Hallett M. Physiology of freezing of gait. Ann. Neurol. 2016;80:644–659. doi: 10.1002/ana.24778. [DOI] [PubMed] [Google Scholar]
- Snijders A.H., Weerdesteyn V., Hagen Y.J., Duysens J., Giladi N., Bloem B.R. Obstacle avoidance to elicit freezing of gait during treadmill walking. Mov. Disord. 2010;25:57–63. doi: 10.1002/mds.22894. [DOI] [PubMed] [Google Scholar]
- Song S.-K., Sun S.-W., Ju W.-K., Lin S.-J., Cross A.H., Neufeld A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song S.-K., Yoshino J., Le T.Q., Lin S.-J., Sun S.-W., Cross A.H., Armstrong R.C. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Spildooren J., Vercruysse S., Desloovere K., Vandenberghe W., Kerckhofs E., Nieuwboer A. Freezing of gait in Parkinson's disease: the impact of dual-tasking and turning. Mov. Disord. 2010;25:2563–2570. doi: 10.1002/mds.23327. [DOI] [PubMed] [Google Scholar]
- Spildooren J., Vercruysse S., Heremans E., Galna B., Vandenbossche J., Desloovere K., Vandenberghe W., Nieuwboer A. Head-pelvis coupling is increased during turning in patients with Parkinson's disease and freezing of gait. Mov. Disord. 2013;28:619–625. doi: 10.1002/mds.25285. [DOI] [PubMed] [Google Scholar]
- Schweder P.M., Hansen P.C., Green A.L., Quaghebeur G., Stein J., Aziz T.Z. Connectivity of the pedunculopontine nucleus in Parkinsonian freezing of gait. Neuroreport. 2010;21(14):914–916. doi: 10.1097/WNR.0b013e32833ce5f1. [DOI] [PubMed] [Google Scholar]
- Stark D.E., Margulies D.S., Shehzad Z.E., Reiss P., Kelly A.M.C., Uddin L.Q., Gee D.G., Roy A.K., Banich M.T., Castellanos F.X., Milham M.P. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J. Neurosci. 2008;28:13754–13764. doi: 10.1523/JNEUROSCI.4544-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K.M., Fink G.R., Passingham R.E., Silbersweig D., Ceballos-Baumann A.O., Frith C.D., Frackowiak R.S. Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J. Neurophysiol. 1995;73:373–386. doi: 10.1152/jn.1995.73.1.373. [DOI] [PubMed] [Google Scholar]
- Sunwoo M.K., Cho K.H., Hong J.Y., Lee J.E., Sohn Y.H., Lee P.H. Thalamic volume and related visual recognition are associated with freezing of gait in non-demented patients with Parkinson's disease. Parkinsonism Relat. Disord. 2013;19:1106–1109. doi: 10.1016/j.parkreldis.2013.07.023. [DOI] [PubMed] [Google Scholar]
- Takakusaki K. Functional neuroanatomy for posture and gait control. J. Mov. Disord. 2017;10:1–17. doi: 10.14802/jmd.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K. Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov. Disord. 2013;28:1483–1491. doi: 10.1002/mds.25669. [DOI] [PubMed] [Google Scholar]
- Takakusaki K. Forebrain control of locomotor behaviors. Brain Res. Rev. 2008;57:192–198. doi: 10.1016/j.brainresrev.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Tard C., Delval A., Devos D., Lopes R., Lenfant P., Dujardin K., Hossein-Foucher C., Semah F., Duhamel A., Defebvre L., Le Jeune F., Moreau C. Brain metabolic abnormalities during gait with freezing in Parkinson's disease. Neuroscience. 2015;307:281–301. doi: 10.1016/j.neuroscience.2015.08.063. [DOI] [PubMed] [Google Scholar]
- Tard C., Dujardin K., Bourriez J.-L., Destée A., Derambure P., Defebvre L., Delval A. Attention modulates step initiation postural adjustments in Parkinson freezers. Parkinsonism Relat. Disord. 2014;20:284–289. doi: 10.1016/j.parkreldis.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Tessitore A., Amboni M., Cirillo G., Corbo D., Picillo M., Russo A., Vitale C., Santangelo G., Erro R., Cirillo M., Esposito F., Barone P., Tedeschi G. Regional gray matter atrophy in patients with Parkinson disease and freezing of gait. AJNR Am. J. Neuroradiol. 2012;33:1804–1809. doi: 10.3174/ajnr.A3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore Alessandro, Amboni M., Esposito F., Russo A., Picillo M., Marcuccio L., Pellecchia M.T., Vitale C., Cirillo M., Tedeschi G., Barone P. Resting-state brain connectivity in patients with Parkinson's disease and freezing of gait. Parkinsonism Relat. Disord. 2012;18:781–787. doi: 10.1016/j.parkreldis.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Trufanov A.G., Khalimov R.R., Yurin A.A., Litvinenko I.V. Use of magnetic resonance tractography in detecting specific features of the impairment of conduction pathways and their prognostic value in Parkinson’s disease complicated by freezing of gait. Sovremennye Tehnologii v Medicine. 2016;8:178–183. [Google Scholar]
- Vandenbossche J., Deroost N., Soetens E., Coomans D., Spildooren J., Vercruysse S., Nieuwboer A., Kerckhofs E. Freezing of gait in Parkinson's disease: disturbances in automaticity and control. Front. Hum. Neurosci. 2013;6 doi: 10.3389/fnhum.2012.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbossche J., Deroost N., Soetens E., Spildooren J., Vercruysse S., Nieuwboer A., Kerckhofs E. Freezing of gait in Parkinson disease is associated with impaired conflict resolution. Neurorehabil. Neural Repair. 2011;25:765–773. doi: 10.1177/1545968311403493. [DOI] [PubMed] [Google Scholar]
- Vandenbossche J., Deroost N., Soetens E., Zeischka P., Spildooren J., Vercruysse S., Nieuwboer A., Kerckhofs E. Conflict and freezing of gait in Parkinson's disease: support for a response control deficit. Neuroscience. 2012;206:144–154. doi: 10.1016/j.neuroscience.2011.12.048. [DOI] [PubMed] [Google Scholar]
- Vastik M., Hok P., Valosek J., Hlustik P., Mensikova K., Kanovsky P. Freezing of gait is associated with cortical thinning in mesial frontal cortex. Biomed. Pap. 2017;161:389–396. doi: 10.5507/bp.2017.035. [DOI] [PubMed] [Google Scholar]
- Vercruysse S., Leunissen I., Vervoort G., Vandenberghe W., Swinnen S., Nieuwboer A. Microstructural changes in white matter associated with freezing of gait in Parkinson's disease. Mov. Disord. 2015;30:567–576. doi: 10.1002/mds.26130. [DOI] [PubMed] [Google Scholar]
- Vercruysse S., Spildooren J., Heremans E., Wenderoth N., Swinnen S.P., Vandenberghe W., Nieuwboer A. The neural correlates of upper limb motor blocks in Parkinson's disease and their relation to freezing of gait. Cereb. Cortex. 2014;24:3154–3166. doi: 10.1093/cercor/bht170. [DOI] [PubMed] [Google Scholar]
- Vervoort G., Heremans E., Bengevoord A., Strouwen C., Nackaerts E., Vandenberghe W., Nieuwboer A. Dual-task-related neural connectivity changes in patients with Parkinson’ disease. Neuroscience. 2016;317:36–46. doi: 10.1016/j.neuroscience.2015.12.056. [DOI] [PubMed] [Google Scholar]
- Walton C.C., Shine J.M., Mowszowski L., Gilat M., Hall J.M., O'Callaghan C., Naismith S.L., Lewis S.J.G. Impaired cognitive control in Parkinson's disease patients with freezing of gait in response to cognitive load. J. Neural Trans. (Vienna) 2015;122:653–660. doi: 10.1007/s00702-014-1271-6. [DOI] [PubMed] [Google Scholar]
- Wang M., Jiang S., Yuan Y., Zhang L., Ding J., Wang Jianwei, Zhang J., Zhang K., Wang Jie. Alterations of functional and structural connectivity of freezing of gait in Parkinson's disease. J. Neurol. 2016;263:1583–1592. doi: 10.1007/s00415-016-8174-4. [DOI] [PubMed] [Google Scholar]
- Wu T., Hallett M. The cerebellum in Parkinson's disease. Brain. 2013;136:696–709. doi: 10.1093/brain/aws360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Li M., Cai J., Li N., Zhou M., Wen P., Xie Z., Wang Q., Chang J., Zhang W. Selective cholinergic depletion of pedunculopontine tegmental nucleus aggravates freezing of gait in Parkinsonian rats. Neurosci. Lett. 2017;659:92–98. doi: 10.1016/j.neulet.2017.08.016. [DOI] [PubMed] [Google Scholar]
- Yendiki A., Panneck P., Srinivasan P., Stevens A., Zöllei L., Augustinack J., Wang R., Salat D., Ehrlich S., Behrens T., Jbabdi S., Gollub R., Fischl B. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front. Neuroinform. 2011;5 doi: 10.3389/fninf.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J., Lee J.-M., Kwon H., Kim J.S., Son T.O., Cho J.W. Alterations of mean diffusivity of pedunculopontine nucleus pathway in Parkinson's disease patients with freezing of gait. Parkinsonism Relat. Disord. 2015;21:12–17. doi: 10.1016/j.parkreldis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Zhou C., Zhong X., Yang Y., Yang W., Wang L., Zhang Y., Nie K., Xu J., Huang B. Alterations of regional homogeneity in freezing of gait in Parkinson's disease. J. Neurol. Sci. 2018;387:54–59. doi: 10.1016/j.jns.2018.01.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any data and code associated with this review article will be made available by reasonable request to corresponding author (PP).