Abstract
Cadmium is among the toxic and hazardous metal widely dispersed in the environment in high levels. Current studies have provided new insights into antioxidant properties of bioflavonoid which have emerged as probable therapeutic and nutraceutical agents. The present study is geared to investigate the possible role of Cymbopogon schoenanthus (L.) Spreng. (or Ethkher) on heavy metal cadmium (Cd) induced oxidative stress in mice. Mice were randomly divided into four groups and treated for 15 days as follows: group 1: normal control-treated (saline); group 2: Ethkher leaves extract-treated (100 mg/kg); group 3: cadmium chloride (CdCl2) treated; group 4: CdCl2 plus Ethkher leaves extract. The results showed a significant reduction in hemoglobin, RBC and hematocrit in cadmium-treated mice as compared to control. Exposure to Cd caused a significant increase in the number of white blood cells (P < 0.05) indicating the occurrence of systemic inflammation. The results of this study also revealed that the mice intoxicated with Cd showed a significant increase in bilirubin, aspartate aminotransferase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (GGTP) activities. Cd intoxication leads to suppression in humoral immunity. However, pretreatment with Ethkher extract reversed almost all the abnormalities in the blood parameters showing noteworthy protection against cadmium induced toxicity in mice. The outcome of the present study revealed that the Ethkher possessed significant immunomodulatory activity and had a preventive effect on the hematological alterations in Cd intoxicated mice.
Keywords: Cymbopogon schoenanthus (L.) Spreng.L, Cadmium (Cd), DPPH, Hematology, Humoral immunity
1. Introduction
Cadmium is a hazardous and toxic heavy metal that produces severe noxious health issues in humans (Lee and White, 1980, Jarup et al., 1998, ATSDR, 1999, Singh et al., 2007). It is released into the atmosphere through mining and smelting operations, fuel combustion (Zhang et al., 2012, Chen et al., 2014), burning of wastes from municipal, sludge from sewage (Edwards et al., 2013), and the application of phosphate fertilizer (Gill et al., 2013). Humans can obtain Cd from crops, leaves of vegetables and tobacco (Fan et al., 2009, García-Esquinas et al., 2014), soil (Guo and Zhou, 2006), and fruits and oily seeds (Schwarz et al., 2014). It is known for the cytotoxicity, carcinogenicity, mutagenicity and known to spur free radical production, causing oxidative degeneration of lipids, proteins, DNA, and initiating various disease conditions in humans and animals (Gunnarsson et al., 2003, Manca et al., 1991, Shaikh et al., 1999, World Health Organization, 1992).
The function of a typical cell depends on the equilibrium between the reactive oxygen species (ROS) produced and the mechanisms that propel antioxidant. This balance is obstructed by an increase in the ROS instigating an oxidative stress (Fidan and Dundar, 2008). ROS are part of life and when there are stressful situations there is an increased outlay of energy resulting in more ROS generation and when the levels of these ROS surpass the ability of the body to counteract, they start to damage cells, tissues and organs. Antioxidants possess radical-scavenging properties and prevent lipid peroxidation and other free radical mediated processes. Therefore, they can defend the human body from numerous diseases associated with the reactions of radicals (Takao et al., 1994). Recently, polyphenols have been found to possess various pharmacological actions. Numerous plant derived polyphenols have been found to possess antioxidant elements that are connected to the treatment of various ailments. Therefore, herbs are regarded as valuable resources that may inhibit or eliminate various diseases, like diabetes, atherosclerosis, hepatotoxicity and other complications.
Cymbopogon schoenanthus (L.) Spreng., (common name lemon grass) (CS) is an aromatic herb and is widespread in North Africa, Arabian Peninsula, India & Pakistan. In Saudi Arabia, there are two species of the Cymbopogon genus, Cymbopogon commutatus (Sakhbar) and the Cymbopogon schoenanthus (L.) Spreng. (or Ethkher) (Atyat, 1995, Hilo, 1996, Chaudhary, 1999). The Saudi folk medicine uses the Cymbopogon schoenanthus (L.) Spreng. for treating people with a kidney stone (Hilo, 1996). The traditional treatment has used as Antihelminthic, antidiarrhea, antirheumatic, carminative, diaphoretic stomachic, diuretic, emmenagogue, for treating fever, for treating jaundice and as tonic. Its aqueous extract is known to heal intestinal troubles, food poisoning and aids in digestion (Atyat, 1995, Khadri et al., 2010).
However, the impact of Ethkher, as a potent antioxidant on cadmium chloride induced oxidative stress on hematology and humoral immunity parameters, on mice has not been studied. Therefore, the primary goal of this evaluation is to explore the potential effect of Ethkher against cadmium chloride intoxication in mice.
2. Materials and methods
2.1. Plant and extract preparation
The dried leaves of Cymbopogon schoenanthus (L.) Spreng. (called Ethkher in Saudi folk medicine) were obtained from the local market and the process of extraction for the hydro-alcoholic extract done as highlighted by Saggu et al. (2014).
2.2. Total flavonoids content
The amounts of flavonoid contents were measured by a colorimetric assay as was emphasized by Rohman et al. (2010). For the calibration curve, rutin was used as a standard and was expressed as mg rutin equivalents (RE) per gram of sample (mg/g) (Fig. 1).
Figure 1.
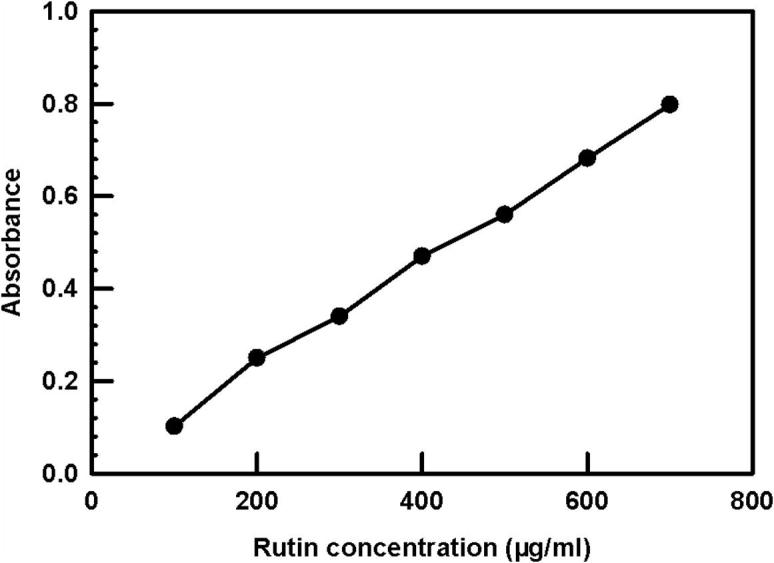
Standard rutin calibration curve for determination of total flavonoid content.
2.3. Determination of total phenolic content
The accumulated amounts of phenolic content were assessed quantitatively as reported by Jindal and Singh (1975). Gallic acid (0.1 mg/ml) was prepared and different concentrations were used for the standard curve and values were expressed in mg/g gallic acid equivalent.
2.4. Assessment of total antioxidant capacity
2,2-Diphenyl-1-Picrylhydrazyl (DPPH) method was utilized for the estimation of total antioxidant capacity as recorded by Brand-Williams et al. (1995). The presence of antioxidants lowers the concentration of DPPH at 515 nm and the engrossing nature disappears as the process continues. Analysis of samples was done in threes, and the outcomes were calculated as averages. A negative control was taken after adding DPPH solution to 0.1 ml of the methanol.
2.5. Animals
The study was conducted in Swiss albino male mice (30–32 g) in Department of Home Economics, Faculty of Specific Education, Kafr ElShaikh University, Egypt. The animals were bred and maintained under standard conditions: temperature (25 ± 2 °C) and a photoperiod of 12 h. Commercial pellet diet and water were given ad libitum.
2.6. Experimental design
Cadmium chloride salt was dissolved in double distilled water and according to the body weight of the animals (5 mg/kg) was injected into mice intraperitoneally (i.p.), daily for 15 days.
The mice were randomly assigned into four experimental groups; each group contained 6 mice, including saline group as control (1 ml/kg for 15 days), CdCl2 group (5 mg/kg body weight, i.p for 15 days), Ethkher extract (100 mg/kg for 15 days) and CdCl2 plus Ethkher extract. For co-administration of CdCl2 and Ethkher extract, Ethkher extract was gavaged at a dose of 100 mg/kg immediately after i.p. Injection of CdCl2 for 15 days. A low effective dose of 100 mg/kg of the Ethkher extract was chosen and no signs of mortality and toxicity were observed up to 3000 mg/kg (data not shown). Autopsies were done on the 15th day of post treatment.
2.7. Sample Preparation and Biochemical analysis
Overnight fasted animal blood specimens were taken from the orbital sinus (Riley, 1960) using capillary tubes under mild ether anesthesia. A blood specimen for hematology studies was collected into tubes containing ethylene-diamine-tetra-acetic acid (EDTA) as an anticoagulant. ALT, AST (Reitman and Frankel, 1957), ALP (Bessey et al., 1946), bilirubin (Pearlman and Lee, 1974), GGTP (Rosalki, 1975) were estimated.
2.8. Hematological parameters determination
The parameters such as hemoglobin (Hb), red blood cells (RBC), hematocrit (HCT), white blood cells (WBC), neutrophils, lymphocytes, monocytes, were analyzed using automated analyzer.
2.9. Humoral immunity parameters determination
Important immunological tests that include the plaque forming cell (PFC) assay and the quantitative hemolysis of sheep red blood cell, (QHS) test were carried out in the spleen as recorded by Bin-Hafeez et al. (2003) and Simpson and Gozzo (1978).
2.10. Statistical analysis
All data are presented as mean ± SE for at least six replications for each prepared sample. Statistical analysis was performed using a one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls test to assess significant differences among different groups. The results are considered to be significant when P < 0.05. All statistical analyses were performed using SigmaPlot, Systat Software program version 11.
3. Results
3.1. Analysis of phytochemical and total antioxidant potential
In this study partial characterization of the Ethkher extract was done in relation to the total phenolic contents and its total antioxidant capacity (IC50). The extract contains75 mg/g of the gallic acid equivalent of total phenols which may be responsible for its observed antioxidant activity (Table 1).
Table 1.
Total flavonoids; total phenolics contents and percentage inhibition of antioxidant activity (IC50) in Ethkher leaves extract. Value represents are mean ± S.D of four determinations.
| Parameter | Ethkher extracts |
|---|---|
| Total phenolic compound (mg/g gallic acid) | 2.8 ± 75 |
| IC50 (μg/ml) | 110.04 ± 0.35 |
| Total Flavonoids (TF) (mg/g dry weight) | 10.42 ± 0.08 |
The DPPH test was performed to evaluate the free radical scavenging effect of the Ethkher extract using the IC50 parameter. In this case, IC50 is the concentration of antioxidant required for 50% scavenging of DPPH radicals during a particular time. Ethkher extract showed the dose-dependent activity and the IC50 value of the extract were found to be 110 μg/ml. It was also found to contain high flavonoid contents (10.42 mg/g of RE) (Table 1).
3.2. Effect of Cd intoxication on the hematological parameters in swiss albino mice
Cadmium when bioaccumulated from different sources, leads to various pathological conditions. Hounkpatin et al. (2013) highlighted that the most important tissues in the human body are blood where metabolic changes are reproduced. Therefore, any variations in the parameters of blood are regarded as the most reliable indicators of toxic effects of drugs, chemicals, heavy metals, etc.
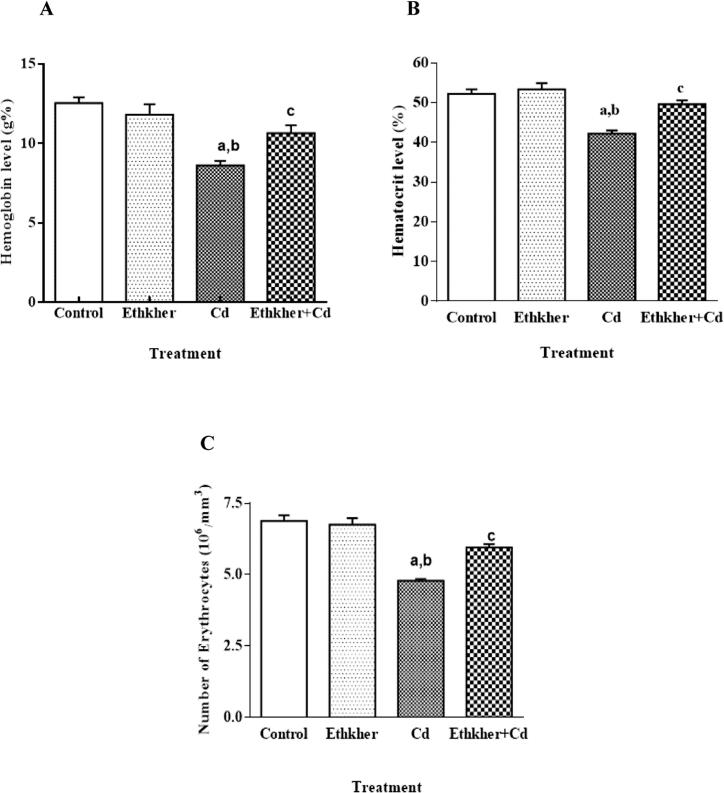
In the present study, a significant decrease was observed in hemoglobin (Hb) (F13.2,3), hematocrit and red blood cells (RBC) in the cadmium-treated group when compared to control (Fig. 2A, 2B and 2C). The levels of the hematocrit and total RBCs count indicated an adverse effect of Cd as evident in Fig. 2B and C. Cd intoxication indicated a significant decrease from the control treated group in hematocrit and total number of RBCs (F19.0,3; P < 0.001 and F33.62,3; P < 0.001) respectively. Similarly, pretreatment with Ethkher extract showed an increase in all of the above said parameters as compared to the cadmium-treated group (P < 0.001).
Figure 2.
(A) Effect of Cd intoxication on Hb, (B) hematocrit and (C) total number of erythrocytes in mice. (Values are mean ± S.E. N = 6 animals per group. Statistical significances: (aP < 0.01, compared with normal control group; bP < 0.05, compared with Ethkher extract group and cP < 0.005, compared with Cd group).
3.3. Alteration in differential counts of peripheral blood leukocytes in Cd intoxicated mice
In the case of differential WBCs count, significant decreases in the value of lymphocytes (F120.3,3; P < 0.001) were found as compared with the control group. However, the pretreatment with the Ethkher extract helps in restoring the count of lymphocytes. A similar trend was observed in monocytes when compared with the Cd-treated group but in the case of neutrophil the value increased significantly after treatment (Table 2) (F28.3,3; P < 0.001).
Table 2.
Effect of Ethkher extract 0 on differential counts of peripheral blood leucocytes in Cd intoxicated mice. Value are mean ± S.E. N = 6 animals per group. Statistical significances: (aP < 0.01, compared with normal control group; bP < 0.05, compared with Ethkher extract group and cP < 0.05, compared with Cd group, where P < 0.001 in all compared groups).
| Parameters | Control | Groups |
||
|---|---|---|---|---|
| Ethkher | Cd (5 mg/kg) | Ethkher + Cd | ||
| WBC (thousands/mm3) | 10.76 ± 0.75 | 10.68 ± 0.15 | 9.15 ± 0.07a,b | 10.07 ± 0.04 |
| Lymphocytes (%) | 71.39 ± 0.16 | 70.21 ± 0.12 | 66.7 ± 0.05a,b | 69.70 ± 0.3a,c |
| Neutrophils (%) | 31.60 ± 1.1 | 30.76 ± 0.25 | 38.80 ± 1.1a,b | 28.20 ± 0.68a,b,c |
| Monocytes (%) | 1.86 ± 0.02 | 1.76 ± 0.05 | 0.38 ± 0.02a,b | 0.83 ± 0.05a,b,c |
3.4. In vivo hepatoprotective activity of Ethkher extract
The data shown in Table 3 indicate that, AST, ALT, ALP and GGTP activities were markedly increased (P < 0.001) in mice intoxicated with Cd (group 3) and in combination with Ethkher extract (group 4) when compared with the control group (group 1). The leakage of above enzymes in the blood reflects the malfunction of liver indirectly due to Cd-induced hepatotoxicity. When compared to the control group administration of Cd to the overnight fasted animals resulted in a 3.0-fold increase in Serum ALT level and a 4.0-fold rise in AST levels (Table 2). The upsurge in the levels of ALP, total bilirubin and GGTP were also clearly evident in Cd-treated animals, indicating liver damage (P < 0.001). Pretreatment with the Ethkher extract (100 mg/kg) prevented Cd-induced elevations compared to the control group (Table 3).
Table 3.
Effect of Ethkher extract on liver function markers and total bilirubin in Cd intoxicated mice. Values are mean ± S.E. N = 6 animals per group. Statistical significances: (aP < 0.01, compared with normal control group; bP < 0.01, compared with Ethkher extract group and cP < 0.05, compared with Cd group, where P < 0.001 in all compared groups).
| Group | ALT (U/L) |
AST (U/L) |
ALP (U/L) |
GGTP (U/L) |
Total Bilirubin (mg/dl) |
|---|---|---|---|---|---|
| Control | 35.1 ± 1.4 | 42.3 ± 5.7 | 107.3 ± 4.0 | 6.5 ± 2.0 | 0.18 ± 0.02 |
| Ethkher extract | 34.0 ± 2.4 | 40.2 ± 2.2 | 102.2 ± 4.3 | 5.8 ± 1.5 | 0.19 ± 0.03 |
| CdCl2 | 105.4 ± 2.1a,b | 169.3 ± 10.2a,b | 192.7 ± 7.8a,b | 15.3 ± 1.8a,b | 1.42 ± 0.05a,b |
| CdCl2 + Ethkher extract | 82.4 ± 4.7a,b,c | 95.4 ± 2.1a,b,c | 124.4 ± 3.4a,b,c | 10.2 ± 1.42a,b,c | 0.32 ± 0.03a,b,c |
3.5. Effect of cadmium on humoral immunity parameters
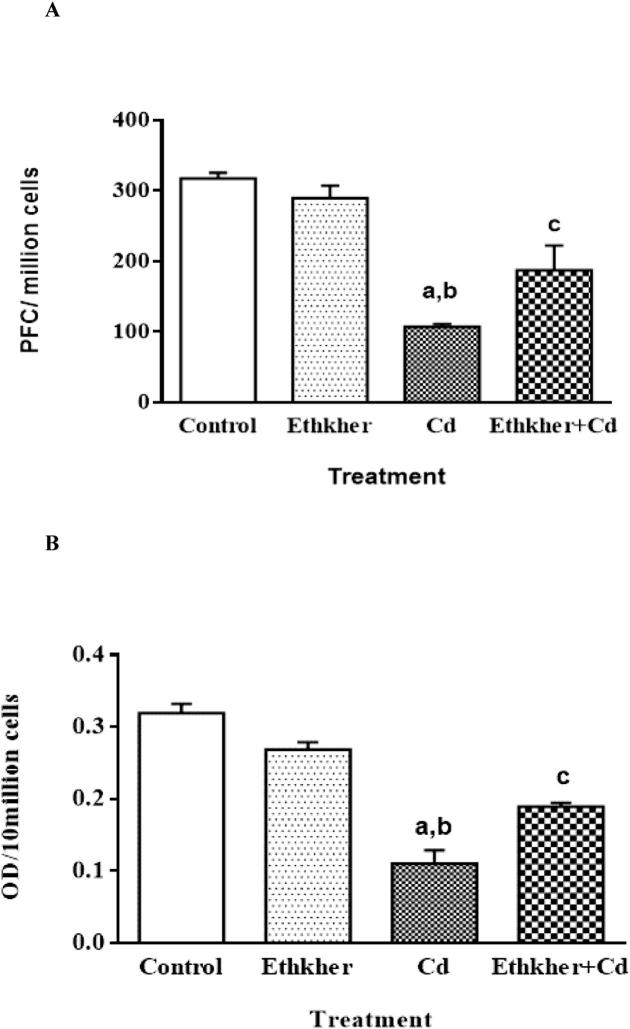
Cd intoxication leads to suppression in humoral immunity showing a reduction in all the parameters viz., PFC response (Fig. 3A), and QHS response (Fig. 3B). The decrease in PFC response and QHS was significant (P < 0.01–P < 0.001) when compared to control treated animals (group I).
Figure 3.
Effect of Cd on humoral immunity parameters (A) PFC and (B) QHS response in mice. Values are mean ± S.E. N = 6 animals per group. Statistical significances: (aP < 0.01, compared with normal control group; bP < 0.05, compared with Ethkher extract group and cP < 0.005, compared with Cd group).
4. Discussion
Tabuk is one of the important regions of Saudi Arabia, characterized by the presence of an abundant flora of medicinal value and Ethkher is one of the important therapeutic plants being used today for the extraction of different phytochemical components for the treatment of fatal diseases like, cancer, diabetes hypertension etc. The present work investigated the impact of Ethkher leaves extract against cadmium chloride-induced perturbations on hematological and humoral immunity parameters.
In the current study, the hydroalcoholic extract of Ethkher was tested for their total antioxidant activity, total phenolic and total flavonoid content (Table 1). Phenolics are non-enzymatic antioxidants and the antioxidant property owes to the redox properties, which permit their action as reducing agents, hydrogen donors and singlet oxygen quenchers (Thirugnanasampandan and Jayakumar, 2011, Buck et al., 1976).
After 48 h of Oral administration of Ethkher extract showed no signs of morbidity or mortality after 48 h in doses up to 3000 mg/kg, warranting a possibility that the extracts were safe to be used and are nontoxic. Plants with LD values past than 5000 mg/kg are regarded as nontoxic.
In our study some of the blood parameters like Hb decreased along with RBC and WBC count. This decrease might be because of the oxidative damage to the erythrocytes induced by cadmium, resulting to the damaging of the cell membrane by increasing lipid peroxidation or oxidative stress (Hounkpatin et al., 2013). The differential leukocyte count showed low lymphocyte and high neutrophil values in Cd intoxicated group. Although the peripheral cell counting is not adequate to indicate a change in quality and quantity of precursor cells, it is still the way to determine the toxicity on hematological indices.
Liver function tests revealed that the Cd intoxication in mice produced a significant (P < 0.001) rise in enzyme levels such as AST, ALT, ALP, GGTP, as well as total bilirubin, when compared to the control treated group. There was a significant (P < 0.001) retrieval of these enzyme levels on the administration of the Ethkher extract (Table 2). For the assessment of liver injury these enzymes are principally used and can be found in the cytosol and any damage liberates the enzyme into the blood circulation and hence it is determinable. Increased amounts of these enzymes are indices of cellular spillage and loss of functional integrity of cell membranes in the liver (Drotman and Lawhan, 1978). Administration of the Ethkher extract resulted in the restoration of increased serum enzymes which may be because of the membrane stabilizing action to prevent leakage of intracellular enzymes. This coincides with the generally accepted opinion that the serum levels of transaminases return to their normal state with the curing of hepatic parenchyma and the reformation of hepatocytes (Thabrew and Joice, 1987).
In this study using plaque-forming cell assay the number of antibodies secreting cells from spleen was determined. Pretreatment with the Ethkher extract secured them from Cd-induced inhibition of humoral immunity. However, there was no inhibitory effect on the identified parameters of humoral immunity by the plant extract. As a matter of fact all the parameters of humoral immunity that were inhibited as a result of Cd intoxication showed recovery in the animals treated with Ethkher extract (Fig. 3). The humoral mediated immunity involves the interaction of B cells with the antigen and their subsequent proliferation and differentiation into antibody secreting plasma cells. Antibodies perform the task as the effectors of the humoral response by binding to the antigen and neutralizing its effect by ensuring that it is eliminated through cross-linking to form clusters that can be ingested easily by phagocytic cells. The effect was significant (P < 0.01) when compared to the control. The pretreatment with Ethkher extract indicates that the immunostimulation has achieved through humoral immunity.
Cd-induced immunosuppression mechanism is not entirely known. Any alterations in the immunological parameters may reflect an intense stress effect of Cd (Sovenyi and Szakolczai, 1993). Previous reports showed that the heavy metals such as Cd prevent immune cell activity through direct toxicity to cellular molecules and structures. Nonetheless, the metals can also alter the behavior of cells through affecting specific genes by transmitting or influencing signals controlling gene expression (Koropatnick and Zalups, 1997).
5. Conclusions
Ethkher extract suffices as a possible source of natural antioxidants, phenolics, alkaloids that contain potent immunomodulatory action. The extracts may be protecting the liver through free radical scavenging activity and this may be due to the ubiety of antioxidants and flavonoids in the extract. The further precise and full inquest may shed light on this aspect to be used as in polyherbal formulations.
Acknowledgment
The authors would like to acknowledge financial support for this work, from the Deanship of Scientific Research (DSR), University of Tabuk, Tabuk, Saudi Arabia, under the no. S-1436-0010. The author would also like to thanks Department of Biology, Faculty of Sciences, Saudi Digital Library and University Library providing the facility for literature survey and collection.
Footnotes
Peer review under responsibility of King Saud University.
References
- ATSDR (Agency for Toxic Substances and Disease Registry) ATSDR/US department of Health and Human Services; Atlanta, US: 1999. Toxicological Profile for Cadmium. [Google Scholar]
- Atyat, A., 1995. Production and Processing of Medical and Aromatic Plants in the Arab world. Sakhiat Al-Jaziaar, Karloun Spire Building P.O. Box 11-5460.
- Bessey O.A., Lowry O.H., Brock M.J. A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum. J. Biol. Chem. 1946;164:321–329. [PubMed] [Google Scholar]
- Bin-Hafeez Bilal, Haque Rizwanul, Parvez Suhel, Pandey Suwarna, Sayeed Iqbal, Raisuddin S. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. Int. Immunopharmacol. 2003;3(2):257–265. doi: 10.1016/S1567-5769(02)00292-8. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. 1995;28:25–30. [Google Scholar]
- Buck W.B., Osweiter G.D., Van Gelder A.G. second ed. Kendall, Hunt Publishing Company; Iowa City: 1976. Clinical and Diagnostic Veterinary Toxicology. [Google Scholar]
- Chaudhary, S., 1999. Vegetation of the Kingdom of Saudi Arabia. National Agriculture and Water Research Center. P.O. Box 17285, Riyadh – 11484, Kingdom of Saudi Arabia, Ministry of Agriculture and Water.
- Chen Z., Wang K., Ai Y.W., Li W., Gao H., Fang C. The effects of railway transportation on the enrichment of heavy metals in the artificial soil on railway cut slopes. Environ. Monit. Assess. 2014;186(2):1039–1049. doi: 10.1007/s10661-013-3437-3. [DOI] [PubMed] [Google Scholar]
- Drotman R., Lawhan G. Serum enzymes are indications of chemical induced liver damage. Drug Chem. Toxicol. 1978;1:163–171. doi: 10.3109/01480547809034433. [DOI] [PubMed] [Google Scholar]
- Edwards C.D., Beatty J.C., Loiselle J.B., Vlassov K.A., Lefebvre D.D. Aerobic transformation of cadmium through metal sulfide biosynthesis in photosynthetic microorganisms. BMC Microbial. 2013;13(1):161. doi: 10.1186/1471-2180-13-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J.L., Ziadi N., Belanger G., Parent L.É., Cambouris A., Hu Z.Y. Cadmium accumulation in potato tubers produced in Quebec. Can. J. Soil Sci. 2009;89(4):435–443. [Google Scholar]
- Fidan A.F., Dundar Y. The effects of Yucca schidigera and Quillaja saponaria on DNA damage, protein oxidation, lipid peroxidation and some biochemical parameters in streptozotocin-induced diabetic rats. J. Diabetes Complications. 2008;22:348–356. doi: 10.1016/j.jdiacomp.2007.11.010. [DOI] [PubMed] [Google Scholar]
- García-Esquinas E., Pollan M., Tellez-Plaza M., Francesconi K.A., Goessler W., Guallar E., Umans J.G., Yeh J., Best L.G., Navas-Acien A. Cadmium exposure and cancer mortality in a prospective cohort: the strong heart study. Environ. Health Perspect. 2014;122(4):363–370. doi: 10.1289/ehp.1306587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S.S., Hasanuzzaman M., Nahar K., Macovei A., Tuteja N. Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol. Biochem. 2013;63:254–261. doi: 10.1016/j.plaphy.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Gunnarsson D., Nordberg G., Lundgren P., Selstam G. Cadmium-induced decrement of the LH receptor expression and cAMP levels in the testis of rats. Toxicology. 2003;183(1):57–63. doi: 10.1016/s0300-483x(02)00440-7. [DOI] [PubMed] [Google Scholar]
- Guo G.L., Zhou Q.X. Evaluation of heavy metal contamination in Phaeozem of northeast China. Environ. Geochem. Health. 2006;28(4):331–340. doi: 10.1007/s10653-005-9002-4. [DOI] [PubMed] [Google Scholar]
- Hilo, S., 1996. The Medicinal Properties of some Azalea Flora in Arabian Peninsula. Dar Almanar, P.O. Box 1250, Jeddah 21431, Saudi Arabia, Dar Almanar.
- Hounkpatin A.S.Y., Edorh P.A., Guédénon P., Alimba C.G., Ogunkanmi A., Dougnon T.V., Boni G., Aissi K.A., Montcho S., Loko F., Ouazzani N., Mandi L., Boko M., Creppy E.E. Haematological evaluation of Wistar rats exposed to chronic doses of cadmium, mercury and combined cadmium and mercury. Afr. J. Biotechnol. 2013;12(23):3731–3737. [Google Scholar]
- Jarup L., Alfven T., Persson B., Toss G., Elinder C.G. Cadmium may be a risk factor for osteoporosis. Occup. Environ. Med. 1998;55:435–439. doi: 10.1136/oem.55.7.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal K.K., Singh R.N. Phenolic content in male and female Carica papaya: a possible physiological marker for sex identification of vegetable seedlings. Physiol. Plant. 1975;33:104–107. [Google Scholar]
- Khadri Ayda, Neffati Mohamed, Smiti Samira, Pedro Falé A., Rosa L., Lino M, Luisa M., Serralheiro M., Eduarda M., Araujo M. Antioxidant, antiacetylcholinesterase and antimicrobial activities of Cymbopogon schoenanthus L. Spreng (lemon grass) from Tunisia. LWT – Food Sci. Technol. 2010;43:331–336. [Google Scholar]
- Koropatnick J., Zalups R.K. Effect of non-toxic mercury, zinc or cadmium pretreatment on the capacity of human monocytes to undergo lipopolysaccharide-induced activation. Br. J. Pharmacol. 1997;120(5):797–806. doi: 10.1038/sj.bjp.0700975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., White K.L. A review of health effects of cadmium. Am. J. Ind. Med. 1980;1:307–317. doi: 10.1002/ajim.4700010308. [DOI] [PubMed] [Google Scholar]
- Manca D., Ricard A.C., Trottier B., Chevalier G. Studies on lipid peroxidation in rat tissues following administration of low and moderate doses of cadmium chloride. Toxicology. 1991;67(3):303–323. doi: 10.1016/0300-483x(91)90030-5. [DOI] [PubMed] [Google Scholar]
- Pearlman F.C., Lee R.T. Detection and measurement of total bilirubin in serum, with use of surfactants as solubilizing agents. Clin. Chem. 1974;20:447–453. [PubMed] [Google Scholar]
- Reitman S., Frankel S. Colorimetric determination of GOT and GPT activity. Am. J. Clin. Path. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Riley V. Adaptation of orbital bleeding technique to rapid serial blood studies. Proc. Soc. Exp. Biol. Med. 1960;104(4):751–754. doi: 10.3181/00379727-104-25975. [DOI] [PubMed] [Google Scholar]
- Rohman A., Riyanto S., Yuniarti N., Saputra W.R., Utami R., Mulatsih W. Antioxidant activity, total phenolic, and total flavonoid of extracts and fractions of red fruit (Pandanus conoideus Lam) Int. Food Res. 2010;17:97–106. [Google Scholar]
- Rosalki S.B. Gamma-glutamyltranspeptidase. In: Bodanski O., Latner A.L., editors. vol. 17. Academic Press; New York, London: 1975. pp. 53–107. (Advances in Clinical Chemistry). [Google Scholar]
- Saggu S., Sakeran M.I., Zidan N., Tousson E., Mohan A., Rehman H. Ameliorating effect of chicory (Chichorium intybus L.) fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food Chem. Toxicol. 2014;72C:138–146. doi: 10.1016/j.fct.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Schwarz M.A., Lindtner O., Blume K., Heinemeyer G., Schneider K. Cadmium exposure from food: the German LExUKon project. Food Addit. Contam. A. 2014;31(6):1038–1051. doi: 10.1080/19440049.2014.905711. [DOI] [PubMed] [Google Scholar]
- Shaikh Z.A., Zaman K., Tang W., Vu T. Treatment of chronic cadmium nephrotoxicity by N-acetyl cysteine. Toxicol. Lett. 1999;104(1–2):137–142. doi: 10.1016/s0378-4274(98)00358-0. [DOI] [PubMed] [Google Scholar]
- Simpson M.A., Gozzo J.J. Spectrophotometric determination of lymphocyte mediated sheep red blood cell haemolysis in vitro. J. Immunol. Methods. 1978;21(1–2):159–165. doi: 10.1016/0022-1759(78)90232-6. [DOI] [PubMed] [Google Scholar]
- Singh P., Chaudhary S., Patni A., Sankhla V. Effect of cadmium chloride induced genotoxicity in bone marrow chromosomes of swiss albino mice and subsequent protective effects of Emblica officinalis and vitamin C. J. Herb. Med. Toxicol. 2007;1(2):67–71. [Google Scholar]
- Sovenyi J., Szakolczai J. Studies on the toxic and immunosuppressive effects of cadmium on the common carp. Acta Vet. Hung. 1993;41:415–426. [PubMed] [Google Scholar]
- Takao T., Kiatani F., Watanabe N., Yagi A., Sakata K. A simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish. Biosci. Biotechnol. Biochem. 1994;58:1780–1783. [Google Scholar]
- Thabrew M., Joice P.A. Comparative study of the efficacy of Pavetta indica and Osbeckia octanda in the treatment of liver dysfunction. Planta Med. 1987;53:239–241. doi: 10.1055/s-2006-962691. [DOI] [PubMed] [Google Scholar]
- Thirugnanasampandan R., Jayakumar R. Protection of cadmium chloride induced DNA damage by Lamiaceae plants. Asian Pac. J. Trop. Biomed. 2011;1(5):391–394. doi: 10.1016/S2221-1691(11)60086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . IPCS; Geneva: 1992. Environmental Health Criteria, Cadmium [EB] [Google Scholar]
- Zhang X., Yang L., Li Y., Li H., Wang W., Ye B. Impacts of lead/zinc mining and smelting on the environment and human health in China. Environ. Monit. Assess. 2012;184(4):2261–2273. doi: 10.1007/s10661-011-2115-6. [DOI] [PubMed] [Google Scholar]