Abstract
The use of natural products by communities from the Colombian Caribbean region to treat health issues, together with biodiversity and geographical features, constitute a great scenery to develop new therapies based on ethnopharmacological heritage. Here, we investigated the anti-inflammatory potential of 10 commonly used plants in Colombian folk medicine, evaluating their effect on nitric oxide (NO) production by LPS-stimulated RAW 264.7 macrophages. The most active plant was evaluated in vivo using 12-O-tetradecanoyl-phorbol-13-acetate (TPA)-induced mouse ear edema, along with its effect on the production of pro-inflammatory mediators in vitro. The extract of Physalis angulata L. calyces showed the highest activity. This extract was fractionated and its dichloromethane fraction (DF) was the most active in vitro, inhibiting the production of NO, prostaglandin E2 (PGE2), interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α and monocyte chemotactic protein (MCP)-1 (CCL2). In vivo, DF showed a significant inhibition of ear edema and myeloperoxidase (MPO) activity, with evident reduction of the leukocyte infiltration into tissue. Our results support the ethnopharmacological use of the selected plants in folk medicine. P. angulata dichloromethane fraction represents a promising source of pharmacological compounds with great potential therapeutic use to treat inflammatory illness.
Keywords: Folk medicine, Anti-inflammatory, Physalis angulata, Nitric oxide, Macrophages
1. Introduction
Inflammatory mediators are key signaling molecules implicated in the activation and regulation of inflammatory processes and are produced by various hematopoietic and non-hematopoietic cell types (Harden et al., 2013, Sofroniew, 2013). One of the most important mediators of the immune response is the nitric oxide (NO) radical produced by the inducible nitric oxide synthase (iNOS). This small molecule is not only essential for host innate immune response but also has a pivotal physiological role in the regulation of vasodilatation, neuronal transmission, cardiac contraction, stem cell differentiation and proliferation (Lei et al., 2013). However, when iNOS activity is aberrant it has detrimental consequences and promotes multiple human diseases (Pautz et al., 2010). Thus, NO is a fundamental molecule for inflammation development and resolution. Given the simplicity of the NO measure trough the Griess reaction, the employment of activated immune cells (i.e. macrophages) has been established as a fast, economic and high-throughput alternative, not only to measure the anti-inflammatory potential of a molecule, but as a great criteria for bioassay-guided fractionation (Ye et al., 2014).
Chronic anti-inflammatory therapy carries several unwanted secondary effects and poses burden on the patients, reducing therapeutic adherence and compromising the effectiveness of the treatment (Tabas and Glass, 2013). To address this, drug discovery research has focused on synthetic and computational methods, leaving aside bioprospecting as an alternative to identify new chemical entities (Macilwain, 1998, Rishton, 2008). Despite this, medicinal plants have been a major source of bioactive molecules for years and continue to be an essential alternative when it comes to health care in many places around the world, especially the rural areas of developing countries, where socio-economic factors difficult the access to modern drugs and health care systems (Cragg and Newman, 2013). In countries like Colombia, with more than 10% of the global biodiversity, folk medicine is used as a primary health care, by communities with knowledge and regular access to medicinal plants (Gómez-Estrada et al., 2011). In this context, ethnopharmacological research and bio-guided isolation of active secondary metabolites remain a crucial strategy in the discovery of safer and more effective drugs (Mishra and Tiwari, 2011).
The Colombian Caribbean region has unique geographical and topographical features that had molded not only an extremely diverse flora and fauna, but also a great cultural diversity based on a wide ethnopharmacological knowledge centered on natural resources (Gómez-Estrada et al., 2011). Despite Colombian government efforts to protect natural biodiversity and traditions related to medicinal plants, factors like discrimination to minorities, forced migrations and lack of policies, have affected the preservation of unique traditions to Colombian culture (Gómez-Estrada et al., 2011, Regalado, 2013, Velásquez-Tibatá et al., 2013).
In an effort to document and increase the attention to biodiversity conservation and ethnopharmacology, our research group started a bioprospecting program based on traditional knowledge of anti-inflammatory plants from the Colombia Caribbean region. The aim of this work was to assess the effect of 10 plants frequently used in the folk medicine to treat inflammation related diseases and that have been previously reported for its traditional uses (Table 1.). Anti-inflammatory potential was assessed using the NO production in lipopolysaccharide (LPS)-stimulated macrophages, the most active plant was fractionated and evaluated using 12-O-tetradecanoyl-phorbol-13-acetate (TPA)-induced mouse ear edema, measuring histopathological parameters and myeloperoxidase activity (MPO). Complementarily, the most active plant was further studied regarding its effects on the production of prostaglandin (PG) E2, cytokines and chemokines by LPS-stimulated macrophages. Additionally, the free radical scavenging potential was evaluated using NO, ABTS and DPPH scavenging assays.
Table 1.
List of selected medicinal plant species employed in Colombian Caribbean Region to treat inflammatory related diseases including their ethno-pharmacological characteristics.
| Scientific name | Local name | ethnopharmacological use | Part used | Mode of preparation/administration route | Reference |
|---|---|---|---|---|---|
|
Calotropis procera (Aiton) Dryand. (Apocynaceae) |
Algodón de seda | Headache Rheumatism |
Roots Leaves |
Exudate/Oral Cataplasm/Topic |
Lal and Yadav (1983) Kumar Vijay et al. (2011) |
|
Ceratopteris pteridoides (Hook.) Hieron. (Pteridaceae) |
Cola de caballo | Respiratory Conditions Diuretic and Cholelithiasic |
Leaves | Infusion/Oral |
Alviz et al. (2013) Beltrán Villanueva et al. (2013) |
|
Cordia alba (Jacq.) Roem, & Schult. (Boraginaceae) |
Sauce sabanero | Bronchitis | Bark, Buds | Oil Decoction, Infusion/Oral | Waizel-Bucay (2005) |
|
Croton malambo H.Karst. (Euphorbiaceae) |
Croton | Rheumatism Pain Inflammation |
Bark | Infusion/Oral |
Salatino et al. (2007) Suárez et al. (2003) |
|
Cryptostegia grandiflora Roxb. ex R.Br. (Apocynaceae) |
Flor de muerto | Inflammation Infection |
Leaves | Fresh plant in maceration/Cataplasm |
Castro et al. (2014) Rivera et al. (2015) |
|
Dracontium dubium Kunth (Araceae) |
Chupadera | Snakebite Inflammation |
Root | Poultice/Topic | Lévi-Strauss (1952) |
|
Maclura tinctoria (L.) D. Don. ex Steud. (Moraceae) |
Palomora | Inflammation Toothache Rheumatism |
Bark | Latex Drops/Topic Decoction, /Oral |
Beltrán Villanueva et al. (2013) |
|
Merremia umbellata (L.) Hallier f. (Convolvulaceae) |
Juan de Dios | Inflammation and Infections Antiulcer Rheumatism |
Leaves Whole Plant |
Decoction/Oral |
Castro-Guerrero et al. (2013) Rivera et al. (2015) |
|
Physalis angulata L. (Solanaceae) |
Topo toropo | Inflammation and Infections Abdominal Cramps Asthma, Dermatitis, Rheumatism |
Calyces Aerial Parts Whole Plant |
Poultice/Topic Infusion/Oral Infusion/Oral |
Rivera et al. (2015) Soares et al. (2003) |
|
Tabebuia rosea (Bertol.) Bertero ex A.DC. (Bignoniaceae) |
Palo de rosa | Inflammation Fever |
Bark Stem, Bark |
Decoction/Oral |
Gómez-Estrada et al. (2011) |
2. Materials and methods
2.1. Chemicals
12-O-tetradecanoyl-phorbol-13-acetate (TPA), indomethacin, ethylenediaminetetraacetic acid (EDTA), O-dianisidine, dulbecco’s modified eagle medium (DMEM), penicillin–streptomycin, trypan blue, lipopolysaccharide (LPS), N-([3-(aminomethyl)phenyl]methyl) ethanimidamide dihydrochloride (1400W), sodium nitrite, N-(1,1-naphthyl) ethylenediamine dihydrochloride, sulfanilamide, hematoxylin-eosin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), potassium persulfate (K2S2O8) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Dichloromethane, dimethylsulfoxide, ethanol, and petroleum ether were obtained from Merck (Kenilworth, NJ, USA) or Carlo Erba (Val-de-Reuil, France). Hexadecyltrimethylammonium (HTAB), hydrogen peroxide (H2O2), bromide of 3(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) from Calbiochem® (San Diego, CA, USA). Fetal bovine serum (FBS) was obtained from GIBCO (Gaithersburg, MD, USA). All other chemicals and reagents used were of analytical grade.
2.2. Plant material and preparation of the extracts
The plant material was collected in the Caribbean coast of Colombia and plants were identified by a botanist at the Herbarium of the University of Antioquia (HUA, Medellin, Antioquia, Colombia) and the Botanical Garden Guillermo Piñeres (JBC, Túrbaco, Bolivar, Colombia) where voucher specimens were deposited (Table 2).
Table 2.
Plant information: list of evaluated plants, collection sites, vouchers, organs used, extraction solvent and yields.
| Scientific name | Collection site | Voucher number | Used part | Extract yields (%) |
|---|---|---|---|---|
| Calotropis procera | Galerazamba, Bolívar (10°47′52″N 75°15′41″W) | HUA175327 | Leaves | 9,73 |
| Ceratopteris pteridoides | San Bernardo del Viento, Cordoba (9°21′18″N 75°57′16″W) | HUA166134 | Whole Plant | 6,79 |
| Cordia alba | Pueblo Nuevo, Bolívar (10°44′26″N 75°15′43″W) | HUA175329 | Flowers | 11,59 |
| Croton malambo | Galerazamba, Bolívar (10°47′22″N 75°15′36″W) | JBC 12,008 | Bark | 8,82 |
| Cryptostegia grandiflora | Pueblo Nuevo, Bolívar (10°44′33″N 75°15′28″W) | HUA175331 | Leaves | 6,92 |
| Dracontium dubium | La Unión, Sucre (8°51′37″N 75°16′48″W) | HUA 189,121 | Root | N.D. |
| Maclura tinctoria | Pueblo Nuevo, Bolívar (10°44′42″N 75°15′48″W) | JBC 12,013 | Bark | 5,33 |
| Merremia umbellata | Pueblo Nuevo, Bolívar (10°44′22″N 75°15′35″W) | HUA175331 | Leaves | 14,44 |
| Physalis angulata | Pueblo Nuevo, Bolívar (10°44′N 75°15′W) | HUA175328 | Calyces | 16,05 |
| Tabebuia rosea | Túrbaco, Bolívar (10°19′55″N 75°24′51″W) | HUA196871 | Bark | 9,20 |
All extracts were obtained by maceration with ethanol 96% and concentrated in a rotaryevaporator. The yields were calculated as: [Dry Concentrated Extract (g)/Dry Material (g)] × 100. N.D.: Not determined.
Except for Dracontium dubium, all plant material were dried at room temperature, powdered and extracted by maceration with ethanol at room temperature until exhaustion. The crude ethanolic extract was filtered and the solvent evaporated under reduced pressure at 40 ± 5 °C using a rotary evaporator (Heidolph VV 2000, Heidolph, Kelheim, Germany). Tubers of D. dubium were washed, peeled and crushed in a minimum amount of water, then lyophilized (FreeZone® Dry 2.5, Labconco, Kansas, MO, USA). The powder obtained was finally extracted by maceration as described before.
Physalis angulata extract was fractionated through liquid-liquid partition with petroleum ether, dichloromethane and methanol-water (9:1) to obtain a petroleum ether (EF), dichloromethane (DF) and methanol fraction (MF).
Test extracts/fractions were dissolved in dimethyl sulfoxide (DMSO) or ethanol to obtain a stock solution, and stored at -20 °C. The final percentage of vehicle was adjusted to 0.1% (v/v) in cell culture experiments.
2.3. Phytochemical screening, flavonoids and polyphenols quantification
The phytochemical screening for presence of terpenes, steroids, quinones, glycosides, alkaloids and tannins was performed as reported by Herrera et al. (2014). Polyphenols were quantified using the Folín-Ciocalteu method with some modifications (Del-Toro-Sánchez et al., 2014). Briefly, the sample was mixed with Folín-Ciocalteu reagent (0.1 M), and 10 min later a solution of sodium carbonate (7.5%) was added. After incubating the mixture for 2 h at room temperature (RT), the Optic Density at 760 nm (OD760) was determined using a microplate reader (Multiskan EX Thermo, Waltham, MA, USA). Results are expressed as mg of gallic acid equivalents (GAE) per gram of extract.
Flavonoids were determined by the aluminum trichloride method with some modifications (Adebayo et al., 2015). Samples were mixed with sodium nitrite (1.5%) and stirred for 10 min at RT, aluminum trichloride (3%) was then added and the mixture was now stirred for 5 min, before finally adding sodium hydroxide (1N). After 10 min, the OD450 was measured. Results are expressed as mg of catechin equivalents (QE) per gram of extract.
2.4. In vitro anti-inflammatory activity
2.4.1. Cell culture
RAW 264.7 (TIB-71™), murine macrophages were obtained from American Type Culture Collection (Manassas, VA, USA). The cells were cultured in DMEM supplemented with 2 mM l-glutamine, 10% FBS and penicillin (10 UI/mL)/streptomycin (10 μg/mL) at 37 °C and maintained in a humidified atmosphere containing 5% CO2. For all experiments, the cells were grown to 80–90% confluence.
2.4.2. Cell viability
The reduction of MTT to formazan was used to assess the effects of test extracts/fractions on cell viability following the protocol described by Mosmann, 1983. Cells (2 × 105 cells/mL) were seeded in 96-well microplates and treated with vehicle (DMSO/ethanol) or extracts/fractions (100–6.25 µg/mL) for 30 min, then stimulated with LPS (10 µg/mL). After 24 h, cells were incubated with MTT solution (0.25 mg/mL), 4 h later the formazan was dissolved in DMSO and the OD550 was measured. Triton X-100 (2%) was used as positive control. Percentages of cell survival relative to control group were calculated, as well as the lethal concentration 50% (LC50).
2.4.3. Pro-inflammatory mediators assessment
Cells (2 × 105 cells/mL) were seeded in 24-well microplates and treated with vehicle, test extracts/fractions (100–6.25 µg/mL), 1400 W (2.50 µg/mL), dexamethasone (7.85 µg/mL), or rofecoxib (6.29 µg/mL) for 30 min, then stimulated with LPS (10 µg/mL). After 24 h, culture supernatants were collected and kept at -20 °C until further analysis.
NO release was determined using the Griess reaction (Green et al., 1982). Equal volumes of supernatants and Griess reagent (1:1 mixture of 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride and 1% sulfanilamide in 5% H3PO4) were mixed and incubated at RT for 5 min. The OD550 was measured and compared with a sodium nitrite standard curve (1–200 µM). Percentage of inhibition was calculated using control cells and the IC50 was determined. To corroborate that NO depletion was caused by biological inhibition, instead of a scavenging effect of the extracts, the scavenging activity was measured using the method by Franco et al., 2014.
Production of PGE2, IL-1β, IL-6, TNF-α and CCL2 was measured using ELISA following the instructions of the manufacturer (eBiosciences, San Diego, CA, USA).
2.5. In vivo anti-edema activity
2.5.1. Experimental animals
Female ICR mice (20–25 g) provided by the Instituto Nacional de Salud (Bogotá, Colombia) were allowed to acclimatize for at least two weeks before use and fed standard rodent food and water ad libitum. They were housed in filtered-capped polycarbonate cages and kept in a controlled environment (22 ± 3 °C and relative humidity: 65 to 75%), under a 12 h light/darkness cycle. All experiments were designed and conducted in accordance with the European Union regulations (CEC council 86/809) and approved by the Ethics Committee of the University of Cartagena (Minutes No. 32 August 4, 2011).
2.5.2. TPA-induced acute ear edema
The edema was induced by topical application of TPA (2.5 μg/ear) in acetone, according to the method described by De Young et al., 1989. Groups (n = 6) were treated on the inner and outer surfaces of the right ear with the acetone dissolved treatment (1 or 05 mg in 20 μL of vehicle) or indomethacin (0.5 mg in 20 μL of vehicle), as reference drug. Immediately after, TPA was applied on both faces of the right ear. The left ear received only acetone, as a control. After 4 h, the animals were sacrificed, and a disk (6 mm diameter) removed from both ears (treated and untreated) was individually weighed on an analytical scale (Ohaus PioneerTM, Parsippany, NJ, USA). Edema was indicated by the weight increase of the right ear disk over the left, and the anti-edema activity was expressed as inhibition percentage of edema.
2.5.3. Histopathology
Tissue samples were preserved in buffered formalin, embedded in paraffin, 5 μm sections were cut and stained with hematoxylin and eosin (H&E). The presence of edema, epidermal hyperplasia/hypertrophy, infiltration of mononuclear and polymorphonuclear cells, connective tissue disruption and dermal fibrosis was assessed by a blind pathologist using light microscopy (Axio Lab A1, Zeiss, Oberkochen, Germany). Parameters were scored from 0 to 3 (0, none; 1, mild; 2, moderate; 3, severe).
2.5.4. Myeloperoxidase (MPO) assay
MPO activity was measured according to the method described by Bradley et al. (1982). Ear tissue was weighed and homogenized in phosphate-buffered saline (PBS), pH 7.4. The homogenate was centrifuged at 2576g for 30 min at 4 °C. The pellet was again homogenized PBS, pH 6.0, containing 0.5% hexadecyl-trimethylammonium bromide (HTAB). This suspension was subjected to cycles of freezing/thawing and brief periods of sonication (15 s) and centrifuged at 2576g for 10 min at 4 °C. Supernatants were mixed with O-dianisidine dihydrochloride (0.067%) and hydrogen peroxide (0.003%), and incubated for 5 min before measuring the OD450. Enzyme activity was expressed as enzyme activity units, where 1 activity unit is defined as the amount of enzyme capable of metabolizing 1 µmol of hydrogen peroxide in 1 min at 25 °C.
2.6. Radical scavenging activity
The ABTS-scavenging effect was determined using the method described by Re et al. (1999), with modifications. ABTS (3.5 mM; in ethanol) and K2S2O8 (1.25 mM) were mixed with ascorbic acid (positive control) or test extracts/fractions and incubated at RT for 5 min. The scavenging of the ABTS radical was determined by measuring the OD405. The DPPH-scavenging effect was determined using the method described by Brand-Williams et al. (1995), with some modifications. In brief, DPPH (100 µg/mL), was mixed with ascorbic acid or test extracts/fractions, and then incubated at RT for 30 min. The scavenging of the DPPH radical was determined by measuring the OD550. Vehicles were used as negative controls.
2.7. Statistical analysis
Results are expressed as the mean ± standard error of the mean (S.E.M) of at least two independent experiments. IC50 and LC50 were calculated employing non-linear regression and expressed as mean and its 95% confidence interval. Data were analyzed using one-way analysis of variance (ANOVA), followed by Dunnett’s and Tukey’s post hoc tests. Values of P < 0.05 were considered significant.
3. Results and discussion
Ethnopharmacological studies allow not only to document traditional knowledge of plants, but to engage policy-makers in designing appropriate strategies for the conservation of biodiversity and cultural knowledge related to medicinal plants. We contribute to this endeavor through our bioprospecting program, focused on isolation of bioactive compounds or therapeutically useful extracts/fractions from plants of the Colombian Caribbean region. In the first part of our work the anti-inflammatory potential of 10 plants traditionally used to alleviate inflammation (Table 1, Table 2) were evaluated based on their capacity to inhibit NO production by LPS-stimulated RAW 264.7 macrophages. In the second part, we focused on the fractionation and evaluation of the most active extract, as well as some insights into the mechanism of action.
3.1. Effect of the plant extracts on the NO production by LPS-stimulated RAW 264.7 macrophages
Stimulation of RAW 264.7 macrophages with LPS (10 μg/mL) elicited a significant accumulation of nitrite in the medium (41.36 ± 0.63 μM). As can be seen in Table 3, NO production was reduced by test extracts at different degrees. We employed the NO inhibition rates (IR) to classify plants among four categories: inactive (−), when the IR was below 20%; weakly active (+), when between 20 and 40%; moderately active (++), with IRs between 40 and 60%; and highly active (+++) when the IR was higher than 60%. Extracts were evaluated at non-toxic concentrations (cell viability above 85%) as determined by the MTT assay (Table 3), and did not show any significant NO-scavenging effect (data not shown). Therefore, the NO inhibition presented by the extracts is due to an effect on the cellular processes and not an in vitro scavenging effect or cytotoxicity.
Table 3.
Evaluation of the cell viability and NO inhibition potential of extracts obtained from Colombian Caribbean Coast plants on LPS-stimulated RAW 264.7 macrophages.
| Plant | Concentration (µg/mL) | % Cell viability | % Nitrite inhibition | Rank | |
|---|---|---|---|---|---|
| 1 | Calotropis procera | 100 | 100 ± 6.70** | 45.11 ± 4.50 | ++ |
| 2 | Ceratopteris pteridoides | 100 | 99.10 ± 1.23*** | 33.48 ± 2.29 | + |
| 3 | Cordia alba | 100 | 100 ± 8.91*** | 33.15 ± 2.69 | + |
| 4 | Croton malambo | 50 | 86.56 ± 1.66*** | 97.05 ± 2.15 | +++ |
| 5 | Cryptostegia grandiflora | 100 | 98.48 ± 0.63*** | 70.47 ± 0.81 | +++ |
| 6 | Dracontium dubium | 100 | 96.45 ± 0.65*** | 7.23 ± 1.52 | – |
| 7 | Maclura tinctoria | 100 | 102.11 ± 1.61*** | 41.07 ± 2.12 | ++ |
| 8 | Merremia umbellata | 100 | 100 ± 0.81*** | 56.06 ± 1.74 | ++ |
| 9 | Physalis angulata | 25 | 93.84 ± 1.41*** | 91.94 ± 1.97 | +++ |
| 10 | Tabebuia rosea | 100 | 96.65 ± 0.60*** | 9.23 ± 1.89 | – |
| 11 | 1400W | 2.50 | 100 ± 1.59*** | 83.88 ± 1.07 | +++ |
The cell viability was measured using the MTT assay and the NO• inhibition was assessed with the Griess assay as described in Methods. Extracts were classified by their activity as highly active (IR ≥ 60%, +++), moderately active (60%>IR ≥ 40%, ++), weakly active (40%>IR ≥ 20%, +), and inactive (IR ≤ 20%, −). 1400 W: N-(3-(aminomethyl) benzyl)acetamidine. Each value represents mean ± SEM (n = 8) from at least two independent experiments.*P < 0.05, **P < 0.01, ***P < 0.001 compared with control group (One way ANOVA).
At the evaluated concentrations, D. dubium and T. rosea showed the lowest activity with IRs below 10%. Although previous studies demonstrated the anti-inflammatory activity of T. rosea (Franco Ospina et al., 2013) and the anti-edema properties of Dracontium species (Gomes et al., 2010), our results are in agreement with several studies that demonstrated that NO production is not inhibited by extracts from plants belonging to the Tabebuia genus at concentrations below 200 µg/mL (Byeon et al., 2008). This suggests that the inhibition of NO production might not be a decisive target involved in the effect of these plants. On the other hand, the plants classified as weakly active, C. pteridoides and C. alba; have no reports of anti-inflammatory activity. However, there are reports of compounds isolated from other plants of the Cordia genus with this biological activity (Al-Musayeib et al., 2011, Geller et al., 2010). In this sense, their potential as treatment for inflammation could not be denied, as we employed a stringent top test concentration (100 µg/mL) and focused only on NO production.
To our knowledge, the anti-inflammatory effect of M. tinctoria (moderately active) has not been previously reported, positioning our study as the first precedent of the potential of this plant to treat inflammation. On the other hand, the results for M. umbellata, also classified as moderately active, are consistent with our previous report that determined its effectiveness reducing the edema and MPO activity in the TPA-induced mouse ear edema model; and supporting the traditional use given to this species (Castro-Guerrero et al., 2013). Likewise, C. procera leaves have also been reported to reduce edema and itching on different in vivo models (Saba et al., 2013), our results corroborate these findings and place the inhibition of the NO production as a possible target, partially responsible for M. umbellata and C. procera leaves anti-inflammatory activity.
The extracts of C. grandiflora, C malambo and P. angulata were catalogued as highly active. C. grandiflora extract inhibited NO production by 70%, in accordance with our previous findings that proved its capacity to reduce edema, MPO activity and leukocyte infiltration (Castro et al., 2014). Due to the cytotoxic effect of the C. malambo and P. angulata extracts, they were evaluated at the lower concentrations. Despite this, both extracts were highly active, with IRs above 90%, making them the most promising extracts in the group. The efficacy of NO inhibition was estimated with the Selectivity Index (SI), defined as LC50/IC50, and used as criteria to choose the best fractionation candidate. As seen in Table 4, C. malambo and P. angulata had the lowest IC50 values. Nevertheless, the SI for P. angulata (32.15) doubled the SI for C. malambo (16.05), suggesting the potential of P. angulata as a safer anti-inflammatory agent and a better candidate for fractionation.
Table 4.
Assessment of the LC50, IC50 (NO), and SI of the extracts with the highest NO inhibition potential among the tested plants group.
| Plant | LC50 ± confidence intervals (µg/mL) | IC50 ± confidence intervals (µg/mL) | SI | |
|---|---|---|---|---|
| 1 | Croton malambo | 113.907 (97.537–131.361) | 7.097 (6.229–8.199) | 16.05 |
| 2 | Cryptostegia grandiflora | >100 | 43.442 (41.748–45.136) | >2.30 |
| 4 | Physalis angulata | 130.628 (114.505–144.937) | 4.063 (3.541–4.665) | 32.15 |
The cell viability was measured using the MTT assay and the NO inhibition was assessed with the Griess assay as described in Methods. IC50 and LC50 were calculated with non-linear regression and expressed as mean and its 95% confidence interval (n = 8) from at least two independent experiments.
The anti-inflammatory activity of P. angulata has been widely studied, proving its capacity to reduce inflammation and modulate NO production by extracts from roots (Bastos et al., 2008) and aerial parts (Choi and Hwang, 2003, Sun et al., 2011) as well as by different isolated compounds (Pinto et al., 2010, Soares et al., 2006, Sun et al., 2010). However, our results constitute the first report of anti-inflammatory potential of its calyces. The distinctive calyx of the Physalis genus is an important structure formed by sepals as a source of carbohydrates for development and protection of the fruit against environmental threats (Puente et al., 2011). Hence, it represents a rich source of novel bioactive metabolites with a vast pharmacological potential, this is supported by other recent reports (Franco et al., 2014, Takimoto et al., 2014).
3.2. Assessment of the anti-inflammatory effect of the primary fractions obtained from P. angulata calyces
The total ethanolic extract of the calyces of P. angulata was fractionated through liquid-liquid partition, obtaining three primary fractions, a petroleum ether (EF), dichloromethane (DF) and methanol fraction (MF). These fractions were evaluated for its potential to inhibit NO production on RAW 264.7 macrophages.
EF and MF were evaluated both at 75 µg/mL whereas DF was evaluated at 25 µg/mL, in accordance with the MTT cell viability test (data not shown). Despite the difference in concentration, DF had a significantly higher NO IR (97%), compared to EF and MF, with IR below 50% (Table 5), indicating that the bioactive metabolites were concentrated in the medium polarity fraction. Moreover, DF displayed a significant concentration-dependent inhibition with IC50 of 2.48 (2.23–2.77) µg/mL, showing a comparable activity than the iNOS selective inhibitor 1400 W (IC50 of 3.72 (2.98–4.57) µg/mL). Moreover, none of the fractions displayed NO scavenging effects (data not shown).
Table 5.
Evaluation of the NO inhibition potential of fractions obtained from Physalis angulata calyces on LPS-stimulated RAW 264.7.
| P. angulata fractions | Concentration (µg/mL) | % Nitrite inhibition | Rank | |
|---|---|---|---|---|
| 1 | Ether (EF) | 75 | 42.897 ± 1.791 | ++ |
| 2 | Dichloromethane (DF) | 25 | 97.079 ± 0.494 | +++ |
| 3 | Methanol (MF) | 75 | 49.232 ± 1.138 | + |
The cell viability was measured using the MTT assay and the NO inhibition was assessed with the Griess assay. Fractions were classified by their activity as highly active (IR ≥ 60%, +++), moderately active (60%>IR ≥ 40%, ++), weakly active (40%>IR ≥ 20%, +), and inactive (IR ≤ 20%, −). Each value represents mean ± SEM (n = 8) from at least two independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control group (One way ANOVA)
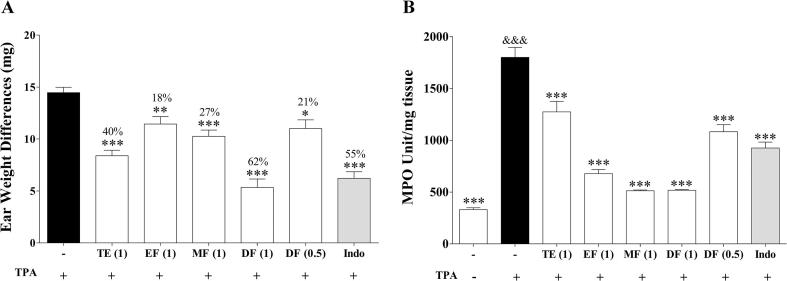
The extract and fractions were also evaluated on the TPA-induced ear edema model. TPA is an irritating agent that activates protein kinase C (PKC), increasing the levels of pro-inflammatory mediators such as PGE2, TNF-α, IL-1β, and IL-6; which are accompanied by edema, epidermal hyperplasia and immune cell infiltration (Gábor, 2003, Murakawa et al., 2006). TPA application increased the ear thickness after 4 h (Fig. 1A), ear edema was significantly ameliorated by treatment with extract and fractions from P. angulata calyces. DF showed a dose-dependent reduction of the edema, with the dose of 1 mg/ear inhibiting up to 60% (P < 0.001), an effect comparable to the produced by indomethacin (0.5 mg/ear). None of the tested extract/fractions produced evident local or systemic adverse effects, suggesting that their topical administration is well tolerated.
Fig. 1.
In vivo evaluation of the anti-inflammatory effect of the extract and fractions obtained from the calyces of P. angulata in the TPA-induced mice ear edema. TE(1): Total extract (1 mg/ear), EF(1): Ether fraction (1 mg/ear), MF(1): Methanol fraction (1 mg/ear), DF(1): Dichloromethane fraction (1 mg/ear), DF(0,5): Dichloromethane fraction (0,5 mg/ear), Indo: Indomethacin. (A): Ear weight difference (mg) and inflammation inhibition percentage, circular 6 mm diameter sections of both treated and non-treated ear were weighted. (B): Enzymatic activity measured in ear tissue homogenized expressed as MPO units/mg tissue. Each value represents mean ± S.E.M. (n = 6) from at least two independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 compared with TPA group (One way ANOVA). &&&P < 0,001 compared with Control group (One way ANOVA).
The neutrophil accumulation is a characteristic feature of TPA-induced edema (Sánchez and Moreno, 1999) and is measured indirectly by the increase of MPO activity (P < 0.001). As seen in Fig. 1B, treatment with extract and fractions from P. angulata significantly decreased the MPO activity relative to the TPA group (P < 0.001). The highest activity was exerted by both MF and DF, which inhibited the enzymatic activity by over 71% at 1 mg/ear (P < 0.001). Additionally, DF also showed MPO inhibition at 0.5 mg/ear (39% inhibition P < 0.001) indicating a dose-dependent effect.
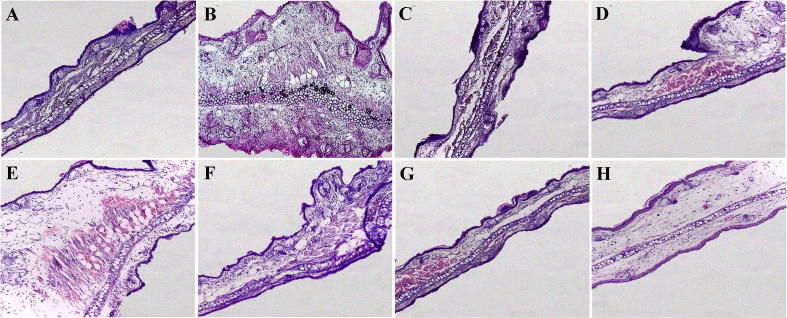
The anti-edema effect of the test extract and fractions was confirmed by histological examination of the ear tissue (Fig. 2). The edema on the TPA group (score: 16), was accompanied by increased ear thickness, disruption of tissue structure, immune cell infiltration, hyperplasia and hypertrophy of dermis, and epidermis (Fig. 2B). Administration of extract and fractions reduced the tissue damage induced by TPA (Fig. 2D–H), DF showed the highest anti-edema activity, restoring tissue architecture and diminishing the edema, cell infiltration which is reflected in the reduced lower histological score (Score: 4) (Fig. 2G and H) similar to the effect produced by indomethacin with a histological score of 2 (Fig. 2C).
Fig. 2.
Histology samples from the TPA-induced ear edema test, ear tissue was dehydrated, embedded in paraffin and stained with Hematoxylin-Eosin. All pictures were taken in an Axio Lab A1 microscope (Zeiss, Oberkochen, Germany) and correspond to the 10X zoom. (A) Control group, (B) TPA group, (C) Indomethacin (0,5 mg/ear), (D) TE (1 mg/ear), (E) EF (1 mg/ear), (F) MF (1 mg/ear), (G) DF (1 mg/ear) and (H) DF (0,5 mg/ear).
Taken together, observations at macroscopic and histopathological level reveal that DF has the highest anti-inflammatory potential and represents a prominent source of bioactive metabolites. These in vivo results support the use of the NO determination by the Griess assay as a suitable method for a bio-guided fractionation of potentially anti-inflammatory plant extracts, in accordance with Dirsch et al. (1998).
3.3. Effect of DF on the production of pro-inflammatory mediators by LPS-stimulated RAW 264.7 macrophages
Macrophages are critical effectors and regulators of inflammation and the immune response. When stimulated with LPS, macrophages undergo classical M1 activation through activation of Toll-like receptor 4 (TLR4) (Kayagaki et al., 2013). TLR4 modulation by LPS promotes activation effector functions, NO production and reactive oxygen intermediates (ROI), PGE2, cytokines (IFN-β, IL-1β, IL-6, IL-12, and TNF-α) and chemokines like the monocyte chemotactic protein-1 (MCP-1, CCL2), as well as other important mediators of the inflammatory response (Bode et al., 2012). M1 macrophages and high NO levels positively correlate with many inflammatory diseases (Gupta et al., 2014). CCL2 provokes mast cell, eosinophil, and macrophage recruitment and activation in rat skin (Sun et al., 2013) and has a critical role in the onset of inflammation and fibrosis (Yamamoto, 2008).
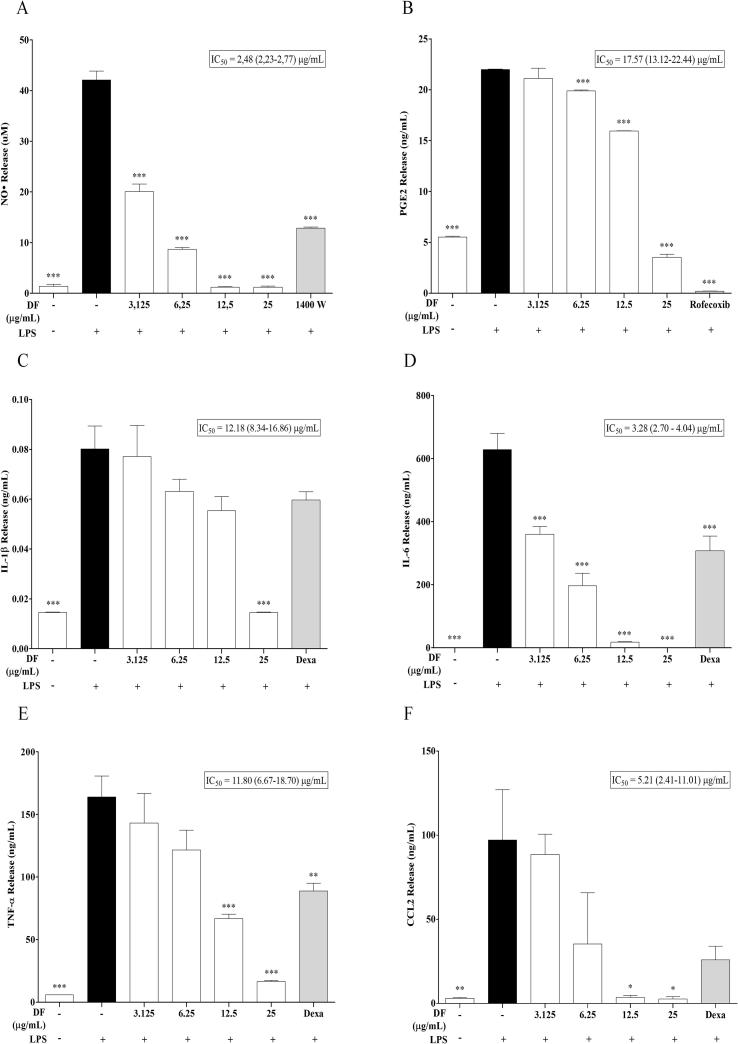
Considering the results showed by DF in vivo, we evaluated its effect on pro-inflammatory mediators using LPS-stimulated RAW 264.7 macrophages. As shown in Fig. 3A–F, high concentration of NO, PGE2, IL-1β, IL-6, TNF-α, and CCL2 was observed after LPS stimulation, with at least a 4-fold increase (P < 0.001). In the absence of LPS, incubation with DF did not modify the release of pro-inflammatory mediators (data not shown). DF significantly inhibited the LPS-induced release of all the evaluated pro-inflammatory mediators in a concentration-dependent manner (Fig. 3A–F), with IC50 values below 20 µg/mL, suggesting a potential immunomodulatory effect.
Fig. 3.
Effect of the dichloromethane fraction (DF) on the production of pro-inflammatory mediators by LPS-stimulated RAW 264.7 macrophages. (A): NO•, (B): PGE2, (C): IL-1 β, (D): IL-6, (E): TNF-α, (F): MCP-1. Cells were incubated for 24 hours after stimulation and treatment with DF. Then supernatants were collected and mediators were measured through ELISA kits, except the NO• which was measured with the Griess reaction, as described in Methods. Each value represents mean ± SEM (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 compared with LPS group (One way ANOVA).
Collectively, we have demonstrated that calyces of P. angulata promote an anti-inflammatory state of macrophages, decreasing the release of pro-inflammatory mediators. Such effects are, consistent with previous in vitro and in vivo reports of extracts and compounds obtained from other parts of the P. angulata plant, which inhibited NO, PGE2, IL-6, IL-1β, IL-12, and TNF-α production (Bastos et al., 2008, Sun et al., 2010); or increased IL-4 and IL-10 levels (Sun et al., 2011, Yu et al., 2010), distinctive cytokines of M2 macrophages. We hypothesized that the effects of DF could be related with the blockage of the TLR4 signaling pathway or a profile transition from M1 to M2 macrophages (Martinez and Gordon, 2014), whichever the case might be, further research is needed to determine the actual mechanisms.
Compounds isolated from the calyces of species of Physalis genus as physalins (Physalis alkekengi L.), withanolides (Physalis pruinosa L.) and sucrose esters (Physalis peruviana L.), have also show anti-inflammatory potential by the modulation of stimulated macrophages and expression of NO, PGE2, IL-6, CCL2 or TNF-α, through the inhibition of NF-κB activation (Franco et al., 2014, Ji et al., 2012, Takimoto et al., 2014). Although, we did not study in detail the chemical composition of DF, the phytochemical screening of the fraction showed presence of glycosides, steroids and terpenes (Table 6) and colorimetric quantification revealed a low quantity of polyphenols (8.78 ± 0.165 mg Gallic acid/g extract) whereas flavonoids were not detectable. Due to the low concentration of these pharmacologically important secondary metabolites, we think that the biological effects of the fraction may be more influenced by glycosides as they appear to be the most abundant.
Table 6.
Phytochemical characterization of the dichloromethane fraction obtained from the total ethanolic extract of the calyces of Physalis angulata.
| Metabolite | P. angulata DF |
|---|---|
| Flavonoids | − |
| Terpenes and/or steroids | ++ |
| Quinones | − |
| Glycosides | +++ |
| Steroidal core | ++ |
| Alkaloids | − |
| Tannins | − |
(−) Not detected, (+) Weak presence, (++) Moderate presence and (+++) Strong presence.
As reactive oxygen species are commonly known as triggers of inflammatory processes and its production is considered a potential target of anti-inflammatory drugs (Debnath et al., 2013), we evaluated the potential scavenging effect of P. angulata calyces, using DPPH and ABTS assays. The results showed that the total extract and fractions have no radical scavenging effect (100 µg/mL), suggesting that the effects observed, are not related to antioxidant activity (data not shown).
4. Conclusions
In short, our results confirm and support the ethnopharmacological employment of the selected plants in the Colombian folk medicine, constituting the first scientific report of anti-inflammatory potential for Ceratopteris pteridoides, Cordia alba, and Maclura tinctoria. The extract obtained from calyces of Physalis angulata had the highest anti-inflammatory potential according to the in vitro results, and the dichloromethane fraction inhibited the production of inflammatory mediators (NO, PGE2, IL-6, IL-1β, TNF-α, and CCL2) by LPS-induced macrophages, as well as reducing the edema, cell infiltration and MPO activity in a TPA-induced mice ear edema assay. To our knowledge, this is the first scientific report of the anti-inflammatory potential of the calyces of P. angulata, placing them as promising source of bioactive metabolites. Nevertheless, a bio-guided isolation of bioactive compounds is still necessary.
Author contributions
D. Rivera and Y. Ocampo, performed the experiments, analyzed data, and wrote the first draft of the manuscript. J. Castro collaborated with plant collection, extract preparation, experiments and data analysis. L. Barrios oversaw tissue processing and histological analysis. F. Diaz, performed plant collection and extract preparation. L. Franco, principal investigator, coordinated the project, conceived and designed the experiments, assisted in data analysis, revised the manuscript and approved the final draft.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the University of Cartagena, Colombia (grants 054-2013, 003-2015, 097-2015). We are grateful to Lilia Anillo who collaborated with her technical assistance with cell culture experiments. We thank people from Colombian Caribbean region who shared their knowledge about medicinal plants and some of their own crops to our study. Yanet Ocampo is sponsored by Colciencias (Grant 597-2012) and the University of Cartagena (Resolution 1446-2011).
Footnotes
Peer review under responsibility of King Saud University.
References
- Adebayo S.A., Dzoyem J.P., Shai L.J., Eloff J.N. The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern African. BMC Complement. Altern. Med. 2015;15(1):1–10. doi: 10.1186/s12906-015-0669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Musayeib N., Perveen S., Fatima I., Nasir M., Hussain A. Antioxidant, anti-glycation and anti-inflammatory activities of phenolic constituents from Cordia sinensis. Molecules. 2011;16(12):10214–10226. doi: 10.3390/molecules161210214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alviz A.A., Salas R.D., Franco L.A. Acute diuretic effect of ethanolic and aqueous extracts of Ceratopteris pteridoides (Hook) in normal rats. Biomédica. 2013;33(1):115–121. doi: 10.1590/S0120-41572013000100014. [DOI] [PubMed] [Google Scholar]
- Bastos G.N.T., Silveira A.J.A., Salgado C.G., Picanço-Diniz D.L.W., do Nascimento J.L.M. Physalis angulata extract exerts anti-inflammatory effects in rats by inhibiting different pathways. J. Ethnopharmacol. 2008;118(2):246–251. doi: 10.1016/j.jep.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Beltrán Villanueva C.E., Díaz Castillo F., Gómez Estrada H. Tamizaje fitoquímico preliminar de especies de plantas promisorias de la Costa Atlántica Colombiana. Rev. Cubana Plant. Med. 2013;18(4):619–631. [Google Scholar]
- Bode J.G., Ehlting C., Häussinger D. The macrophage response towards LPS and its control through the p38 MAPK–STAT3 axis. Cell. Signal. 2012;24(6):1185–1194. doi: 10.1016/j.cellsig.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Bradley P.P., Priebat D.A., Christensen R.D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. 1995;28(1):25–30. [Google Scholar]
- Byeon S.E., Chung J.Y., Lee Y.G., Kim B.H., Kim K.H., Cho J.Y. In vitro and in vivo anti-inflammatory effects of taheebo, a water extract from the inner bark of Tabebuia avellanedae. J. Ethnopharmacol. 2008;119(1):145–152. doi: 10.1016/j.jep.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Castro-Guerrero J.P., Ocampo-Buendia Y.C., Franco-Ospina L.A. Actividad antiinflamatoria y antioxidante de Merremia umbellata (L.) Hallier f. Revista Ciencias Biomedicas. 2013;4(1):13–19. [Google Scholar]
- Castro J.P., Ocampo Y.C., Franco L.A. In vivo and in vitro anti-inflammatory activity of Cryptostegia grandiflora Roxb. ex R. Br. leaves. Biol. Res. 2014;47(1):1–8. doi: 10.1186/0717-6287-47-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg G.M., Newman D.J. Natural products: a continuing source of novel drug leads. Biochim. Biophys. Acta. 2013;1830(6):3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E.-M., Hwang J.-K. Investigations of anti-inflammatory and antinociceptive activities of Piper cubeba, Physalis angulata and Rosa hybrida. J. Ethnopharmacol. 2003;89(1):171–175. doi: 10.1016/s0378-8741(03)00280-0. [DOI] [PubMed] [Google Scholar]
- De Young L.M., Kheifets J.B., Ballaron S.J., Young J.M. Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions. 1989;26(3):335–341. doi: 10.1007/BF01967298. [DOI] [PubMed] [Google Scholar]
- Debnath T., Kim D., Lim B. Natural products as a source of anti-inflammatory agents associated with inflammatory bowel disease. Molecules. 2013;18(6):7253–7270. doi: 10.3390/molecules18067253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del-Toro-Sánchez C.L., Bautista-Bautista N., Blasco-Cabal J.L., Gonzalez-Ávila M., Gutiérrez-Lomelí M., Arriaga-Alba M. Antimutagenicity of methanolic extracts from Anemopsis californica in relation to their antioxidant activity. Evid. Based Complement Alternat. Med. 2014;2014:8. doi: 10.1155/2014/273878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirsch V.M., Stuppner H., Vollmar A.M. The Griess assay: suitable for a bio-guided fractionation of anti-inflammatory plant extracts? Planta Med. 1998;64(5):423–426. doi: 10.1055/s-2006-957473. [DOI] [PubMed] [Google Scholar]
- Franco L.A., Ocampo Y.C., Gómez H.A., De la Puerta R., Espartero J.L., Ospina L.F. Sucrose esters from Physalis peruviana calyces with anti-inflammatory activity. Planta Med. 2014;80(17):1605–1614. doi: 10.1055/s-0034-1383192. [DOI] [PubMed] [Google Scholar]
- Franco Ospina L.A., Castro Guerrero J.P., Ocampo Buendía Y.C., Pájaro Bolívar I.B., Díaz Castillo F. Actividad antiinflamatoria, antioxidante y antibacteriana de dos especies del género Tabebuia. Rev. Cubana Plant. Med. 2013;18(1):34–46. [Google Scholar]
- Gábor M. Models of acute inflammation in the ear. In: Winyard P.G., Willoughby D.A., editors. Inflammation Protocols. Humana Press; 2003. pp. 129–137. [Google Scholar]
- Geller F., Schmidt C., Göttert M., Fronza M., Schattel V., Heinzmann B., Werz O., Flores E.M.M., Merfort I., Laufer S. Identification of rosmarinic acid as the major active constituent in Cordia americana. J. Ethnopharmacol. 2010;128(3):561–566. doi: 10.1016/j.jep.2010.01.062. [DOI] [PubMed] [Google Scholar]
- Gomes A., Das R., Sarkhel S., Mishra R., Mukherjee S., Bhattacharya S., Gomes A. Herbs and herbal constituents active against snake bite. Indian J. Exp. Biol. 2010;48:865–878. [PubMed] [Google Scholar]
- Gómez-Estrada H., Díaz-Castillo F., Franco-Ospina L., Mercado-Camargo J., Guzmán-Ledezma J., Domingo Medina J., Gaitán-Ibarra R. Folk medicine in the northern coast of Colombia: an overview. J Ethnobiol. Ethnomed. 2011;7(1):1–11. doi: 10.1186/1746-4269-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Gupta N., Goel N., Kumar R. Correlation of exhaled nitric oxide, nasal nitric oxide and atopic status: a cross-sectional study in bronchial asthma and allergic rhinitis. Lung India. 2014;31(4):342–347. doi: 10.4103/0970-2113.142107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden L.M., Rummel C., Luheshi G.N., Poole S., Gerstberger R., Roth J. Interleukin-10 modulates the synthesis of inflammatory mediators in the sensory circumventricular organs: implications for the regulation of fever and sickness behaviors. J. Neuroinflammation. 2013;10(1):1–4. doi: 10.1186/1742-2094-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera Herrera A., Franco Ospina L., Fang L., Díaz Caballero A. Susceptibility of Porphyromonas gingivalis and Streptococcus mutans to antibacterial effect from Mammea americana. Adv. Pharmacol. Sci. 2014;2014:6. doi: 10.1155/2014/384815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L., Yuan Y., Luo L., Chen Z., Ma X., Ma Z., Cheng L. Physalins with anti-inflammatory activity are present in Physalis alkekengi var. franchetii and can function as Michael reaction acceptors. Steroids. 2012;77(5):441–447. doi: 10.1016/j.steroids.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Kayagaki N., Wong M.T., Stowe I.B., Ramani S.R., Gonzalez L.C., Akashi-Takamura S., Miyake K., Zhang J., Lee W.P., Muszyński A., Forsberg L.S., Carlson R.W., Dixit V.M. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341(6151):1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- Kumar Vijay L., Chaudhary P., Ramos Marcio V., Mohan M., Matos Mayara P.V. Protective effect of proteins derived from the latex of Calotropis procera against inflammatory hyperalgesia in monoarthritic rats. Phytother. Res. 2011;25(9):1336–1341. doi: 10.1002/ptr.3428. [DOI] [PubMed] [Google Scholar]
- Lal S.D., Yadav B.K. Folk medicines of kurukshetra district (Haryana), India. Econ. Bot. 1983;37(3):299–305. [Google Scholar]
- Lei J., Vodovotz Y., Tzeng E., Billiar T.R. Nitric oxide, a protective molecule in the cardiovascular system. Nitric Oxide. 2013;35:175–185. doi: 10.1016/j.niox.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Lévi-Strauss C. The use of wild plants in tropical South America. Econ. Bot. 1952;6(3):252–270. [Google Scholar]
- Macilwain C. When rhetoric hits reality in debate on bioprospecting. Nature. 1998;392(6676):535–540. doi: 10.1038/33237. [DOI] [PubMed] [Google Scholar]
- Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:1–13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra B.B., Tiwari V.K. Natural products: an evolving role in future drug discovery. Eur. J. Med. Chem. 2011;46(10):4769–4807. doi: 10.1016/j.ejmech.2011.07.057. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murakawa M., Yamaoka K., Tanaka Y., Fukuda Y. Involvement of tumor necrosis factor (TNF)-α in phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin edema in mice. Biochem. Pharmacol. 2006;71(9):1331–1336. doi: 10.1016/j.bcp.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Pautz A., Art J., Hahn S., Nowag S., Voss C., Kleinert H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide. 2010;23(2):75–93. doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Pinto N.B., Morais T.C., Carvalho K.M.B., Silva C.R., Andrade G.M., Brito G.A.C., Veras M.L., Pessoa O.D.L., Rao V.S., Santos F.A. Topical anti-inflammatory potential of Physalin E from Physalis angulata on experimental dermatitis in mice. Phytomedicine. 2010;17(10):740–743. doi: 10.1016/j.phymed.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Puente L.A., Pinto-Muñoz C.A., Castro E.S., Cortés M. Physalis peruviana Linnaeus, the multiple properties of a highly functional fruit: a review. Food Res. Int. 2011;44(7):1733–1740. [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Regalado A. Venturing back into Colombia. Science. 2013;341(6145):450–452. doi: 10.1126/science.341.6145.450. [DOI] [PubMed] [Google Scholar]
- Rishton G.M. Natural products as a robust source of new drugs and drug leads: past successes and present day issues. Am. J. Cardiol. 2008;101(10, Suppl.):S43–S49. doi: 10.1016/j.amjcard.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Rivera D.E., Ocampo Y.C., Castro J.P., Caro D., Franco L.A. Antibacterial activity of Physalis angulata L., Merremia umbellata L., and Cryptostegia grandiflora Roxb. Ex R.Br. - medicinal plants of the. Colombian Northern Coast. Orient. Pharm. Exp. Med. 2015;15(1):95–102. [Google Scholar]
- Saba A.B., Oguntoke P.C., Oridupa O.A. Anti-inflammatory and analgesic activities of ethanolic leaf extract of Calotropis procera. Afr. J. Biomed. Res. 2013;14(3):203–208. [Google Scholar]
- Salatino A., Salatino M.L.F., Negri G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae) J. Br. Chem. Soc. 2007;18:11–33. [Google Scholar]
- Sánchez T., Moreno J.J. Role of leukocyte influx in tissue prostaglandin H synthase-2 overexpression induced by phorbol ester and arachidonic acid in skin. Biochem. Pharmacol. 1999;58(5):877–879. doi: 10.1016/s0006-2952(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Soares M.B.P., Bellintani M.C., Ribeiro I.M., Tomassini T.C.B., Ribeiro dos Santos R. Inhibition of macrophage activation and lipopolysaccaride-induced death by seco-steroids purified from Physalis angulata L. Eur. J. Pharmacol. 2003;459(1):107–112. doi: 10.1016/s0014-2999(02)02829-7. [DOI] [PubMed] [Google Scholar]
- Soares M.B.P., Brustolim D., Santos L.A., Bellintani M.C., Paiva F.P., Ribeiro Y.M., Tomassini T.C.B., Ribeiro dos Santos R. Physalins B, F and G, seco-steroids purified from Physalis angulata L., inhibit lymphocyte function and allogeneic transplant rejection. Int. Immunopharmacol. 2006;6(3):408–414. doi: 10.1016/j.intimp.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Sofroniew M.V. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist. 2013;20(2):160–172. doi: 10.1177/1073858413504466. [DOI] [PubMed] [Google Scholar]
- Suárez A.R.I., Compagnone R.S., Salazar-Bookaman M.M., Tillett S., Delle Monache F., Di Giulio C., Bruges G. Antinociceptive and anti-inflammatory effects of Croton malambo bark aqueous extract. J. Ethnopharmacol. 2003;88(1):11–14. doi: 10.1016/s0378-8741(03)00179-x. [DOI] [PubMed] [Google Scholar]
- Sun G., Wang Y., Yin B., Zhu L., Liu Y. Tumor necrosis factor-α, monocyte chemoattractant protein-1 and intercellular adhesion molecule-1 increase during the development of a 2,4-dinitrofluorobenzene-induced immediate-type dermatitis in rats. Inflamm. Res. 2013;62(6):589–597. doi: 10.1007/s00011-013-0611-6. [DOI] [PubMed] [Google Scholar]
- Sun L., Liu J., Cui D., Li J., Yu Y., Ma L., Hu L. Anti-inflammatory function of Withangulatin A by targeted inhibiting COX-2 expression via MAPK and NF-κB pathways. J. Cell. Biochem. 2010;109(3):532–541. doi: 10.1002/jcb.22430. [DOI] [PubMed] [Google Scholar]
- Sun L., Liu J., Liu P., Yu Y., Ma L., Hu L. Immunosuppression effect of Withangulatin A from Physalis angulata via heme oxygenase 1-dependent pathways. Process Biochem. 2011;46(2):482–488. [Google Scholar]
- Tabas I., Glass C.K. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339(6116):166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto T., Kanbayashi Y., Toyoda T., Adachi Y., Furuta C., Suzuki K., Miwa T., Bannai M. 4β-Hydroxywithanolide E isolated from Physalis pruinosa calyx decreases inflammatory responses by inhibiting the NF-κB signaling in diabetic mouse adipose tissue. Int. J. Obesity. 2014;38:1432–1439. doi: 10.1038/ijo.2014.33. [DOI] [PubMed] [Google Scholar]
- Velásquez-Tibatá J., Salaman P., Graham C. Effects of climate change on species distribution, community structure, and conservation of birds in protected areas in Colombia. Reg. Environ. Change. 2013;13(2):235–248. [Google Scholar]
- Waizel-Bucay J. Some plants from arid zones used by homeopathy. Am. J. Hom. Med. 2005;98(3):179. [Google Scholar]
- Yamamoto T. Pathogenic role of CCL2/MCP-1 in scleroderma. Front. Biosci. 2008;13:2686–2695. doi: 10.2741/2875. [DOI] [PubMed] [Google Scholar]
- Ye H., Wu W., Liu Z., Xie C., Tang M., Li S., Yang J., Tang H., Chen K., Long C., Peng A., Wei Y., Chen L. Bioactivity-guided isolation of anti-inflammation flavonoids from the stems of Millettia dielsiana Harms. Fitoterapia. 2014;95:154–159. doi: 10.1016/j.fitote.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Yu Y., Sun L., Ma L., Li J., Hu L., Liu J. Investigation of the immunosuppressive activity of Physalin H on T lymphocytes. Int. Immunopharmacol. 2010;10(3):290–297. doi: 10.1016/j.intimp.2009.11.013. [DOI] [PubMed] [Google Scholar]