Abstract
Non-alcoholic fatty liver disease (NAFLD) is a multi-factorial disease and the most common of chronic liver diseases worldwide. The four clinical-pathological entities which are usually followed by NAFLD course include non-alcoholic steatosis, non-alcoholic steatohepatitis, advanced fibrosis/cirrhosis, and hepatocellular carcinoma. The cornerstones of NAFLD management and treatment, however, are healthy lifestyles such as dietary modifications, regular physical activity, and gradual weight loss. At present, no drugs or pharmacological agents have been approved for long-term treatment of NAFLD. Therefore, lifestyle modification is considered the main clinical recommendation and an initial step for the management of NAFLD.
Abbreviations: NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; AASLD, American Association for the Study of Liver Diseases; EASL, European Association for the Study of the Liver; EASD, European Association for the Study of Diabetes; EASO, European Association for the Study of Obesity; DDT, dichlorodiphenyltrichloroethane; ALA, alpha-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid
Keywords: Diet, Exercise, Lifestyle, Weight loss, Liver disease
1. Introduction
Unhealthy behaviors have been implicated in a significant amount of premature deaths (Saint Onge and Krueger, 2017). Ceteris paribus, a healthy lifestyle is a sine qua non of good health. Lifestyle factors (such as improper dietary habits, lack of physical activity and tobacco smoking) are largely modifiable (Masana et al., 2017). For instance, the traditional Mediterranean diet alone has been shown to be very effective in the control of obesity and the reduction of the incidence of major cardiovascular events (Marquis-Gravel et al., 2015). On the other hand, healthy lifestyles such as dietary modifications, regular physical activity, and gradual weight loss have a substantial impact on the population health and also remain the cornerstone of treatment for most diseases including the non-alcoholic fatty liver disease [NAFLD] (Dong et al., 2016, Dunkler et al., 2016), which is a universal disorder considered as the most common liver disease especially in the western world (Ahmed, 2015, Ahmed et al., 2017a, Hannah and Harrison, 2016). NAFLD is also associated with an increased risk of both diabetes and ischemic heart disease (Vilar-Gomez et al., 2015). Though the metabolic disturbances, as well as the long-term complications of NAFLD, are surmountable through lifestyle intervention, poor implementation and reduced adherence to lifestyle intervention programs would ultimately diminish its efficacy (Barrera and George, 2014, Stonerock and Blumenthal, 2017).
Admittedly, this review is not aimed at providing a comprehensive listing of all the common interventions and management methods for NAFLD which have been the subject of other reviews (Bril and Cusi, 2017, Hannah and Harrison, 2016, Kenneally et al., 2017, Paul and Davis, 2018, Zou et al., 2018), but rather to focus, using a different approach, on a representative variety of the lifestyle approaches which have been selected for their popularity and soundness. In other words, this review is mainly aimed at providing relevant but simplified opinions for both clinical and non-clinical audiences on the risk factors and the management of NAFLD vis-à-vis weight reduction, physical exercise and diet modification.
2. Non-alcoholic fatty liver disease
The non-alcoholic fatty liver disease is the leading cause of liver disease. It comprises hepatic steatosis which is defined as the liver fat deposition affecting >5% of hepatocytes in the absence of excessive alcohol intake, other liver diseases or the consumption of steatogenic drugs (Chalasani et al., 2012, Dongiovanni et al., 2016, McPherson et al., 2015). It is considered as the hepatic manifestation of the metabolic syndrome. Both the oxidative stress and insulin resistance are known to be involved in the pathogenesis of NAFLD (Dong et al., 2016, Dongiovanni et al., 2016, Nobili et al., 2008). Generally, the prevalence of NAFLD varies widely depending on the definition used as well as population studied (Chalasani et al., 2012). For instance, its prevalence is, at least, 25% of American adults, while 15–35% of the general population in China, Europe, Japan, and the Middle East are estimated to be affected by NAFLD (Orci et al., 2016). Comparatively, 2.8–46% of the world population are reportedly affected (Vilar‐Gomez et al., 2016) while about 35% of western populations are affected with this widely prevalent hepatic metabolic disorder. Individuals with type 2 diabetes and obesity are even at higher risk of getting NAFLD (Naik et al., 2013). Despite the high prevalence of NAFLD in the general population, most people only exhibit simple steatosis while only a small percentage of affected individuals eventually develops inflammation and the subsequent fibrosis as well as chronic liver disease (Buzzetti et al., 2016). The factors which are thought to be responsible for the progression from simple steatosis to non-alcoholic steatohepatitis (NASH) include the accumulation of triglycerides in the liver, the free fatty acid lipotoxicity, the activation of inflammatory, oxidative stress and fibrogenesis pathways and the failure of the hepatocytes to replace and regenerate dead cells (Naik et al., 2013). Generally, the histological spectrum of NAFLD includes non-alcoholic fatty liver steatosis (which is defined by a concentration of hepatic triglycerides exceeding 5% of liver weight without hepatocellular injury) and non-alcoholic steatohepatitis (which is characterized by fibrogenesis, hepatocellular damage, hepatocyte ballooning degeneration, lobular necroinflammation and ultimately cirrhosis. Patients who progress to cirrhosis, however, are at risk of potentially life-threatening liver-related complications such as portal hypertension, end-stage hepatic failure and hepatocellular carcinoma (Dongiovanni et al., 2016, McPherson et al., 2015). Therefore, the NAFLD course could follow one or more of its four clinical-pathological entities which are steatosis, NASH, advanced fibrosis/cirrhosis and hepatocellular carcinoma (Lonardo et al., 2017). NAFLD is also associated with atherosclerosis, increased cardiovascular risks as well as mortality (Trovato et al., 2016). In spite of the fact that currently no approved drugs or pharmacological agents for the long-term treatment of NAFLD (Dongiovanni et al., 2016, Keating and Adams, 2016, Kenneally et al., 2017, Promrat et al., 2010, Vilar-Gomez et al., 2015, Vilar-Gomez et al., 2016), the authors, therefore, opine that lifestyle modification should be considered as the main clinical recommendation and an initial step for the management of NAFLD.
3. Natural history and mortality of NAFLD
The studies available in the literature about the natural history and mortality of NAFLD/ NASH are not conclusive owing to the uncertainty and contradictory nature of the available data as well as the difficulty in measuring NAFLD/ NASH in the short-time interval (Bellentani and Marino, 2009, Bril and Cusi, 2017, Kenneally et al., 2017). Though, the presence of NAFLD tends to increase disease severity and progression in other liver diseases such as alcoholic liver disease, chronic hepatitis C infection, as well as hemochromatosis. Patients with simple steatosis also seem to have a more benign prognosis while NAFLD patients with NASH have a reduced survival when compared with a matched reference population (Bellentani and Marino, 2009). Population-based studies and those reporting sequential liver biopsies have reported liver-related mortality in 18% of NASH patients compared to only 3% of the non-NASH cohort after 18.5 years or follow-up (Matteoni et al., 1999, Rafiq et al., 2009, Vernon et al., 2011). Furthermore, the general belief that fibrosis progression in NAFLD patients with simple steatosis is uncommon as compared to the frequent progression in NASH has also been challenged by other studies which suggest that simple steatosis may not be an entirely benign condition but rather also able evolve to NASH with advanced fibrosis (McPherson et al., 2015). Nevertheless, the authors’ opinion is that NASH patients have a higher chance of developing liver-related mortality as compared to patients with only NAFLD but without NASH. Furthermore, we agree with Hannah and Harrison (2016) that the highest risk of liver-related mortality is found in patients with fibrosis.
4. Risk factors of NAFLD
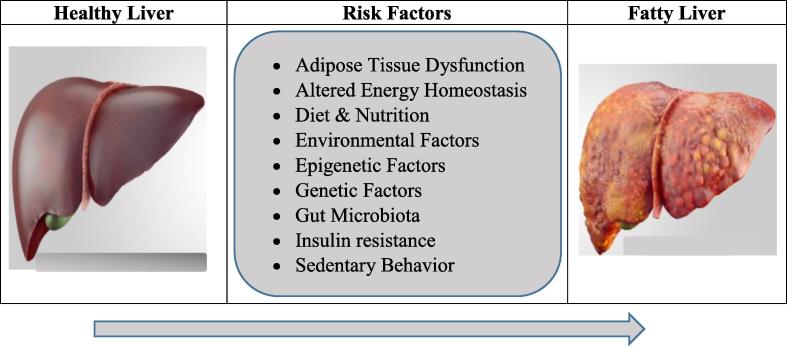
Though, NAFLD is a multi-factorial disease as a result of a complex interaction between nutrition, environmental factors and a genetic background, a sedentary behavior, a high-calorie diet, dietary excess of saturated fats, refined carbohydrates and a high fructose intake are among the major contributors to the development of NAFLD (Lonardo et al., 2017). Other factors which are thought to be involved in the pathogenesis of NAFLD (Fig. 1), in addition to genetic and nutritional factors, are gut microbiota, insulin resistance, hormones secreted from the adipose tissue and epigenetic factors (Betrapally et al., 2017, Buzzetti et al., 2016, Doulberis et al., 2017).
Fig. 1.
Common Risk Factors of non-alcoholic fatty liver disease (NAFLD).
The relationship between age, gender, NAFLD, and fibrosis also remains unsettled because the high prevalence of NAFLD and the higher stage of fibrosis and cirrhosis in NAFLD are more related to the duration of disease rather than to either age or gender (Vernon et al., 2011). Inflammation and fibrosis due to lipotoxic events of the free fatty acids and their metabolites in the liver also appear to be among the major mechanisms underlying the disease progression (Fuchs et al., 2014). The adipose tissue dysfunction is considered as the key contributor to the pathogenesis of NAFLD and not the body fat mass per se (Byrne and Targher, 2015). An altered whole-body energetic homeostasis from an unbalance between triglycerides accumulation and removal is the most common cause of NAFLD (Dongiovanni et al., 2016). In such a situation, the caloric intake exceeds its expenditure leading to a spillover of extra-energy in the form of non-esterified fatty acids from the visceral adipose tissue into ectopic fat depots such as the liver, pancreas and skeletal muscles (Lonardo et al., 2017).
5. Management of NAFLD
The ultimate goals of NAFLD management include, but not limited to, early diagnosis of the disease early, prevention of further progression of the disease, regression of the disease as much as possible as well as improvement of the underlying metabolic syndrome, in addition to prevention of recurrence of NAFLD following liver transplantation after an end-stage liver disease (Ahmed, 2015). Lifestyle intervention (Table 1) is considered as the first-line therapeutic option, particularly, in patients with NASH. Therefore, the development of effective and noninvasive methods which are able to predict NASH resolution after diet and exercise interventions is essential owing to the fact that the use of liver biopsy for monitoring patients is not recommended because it is not only expensive but also invasive and also carries an associated risk (Vilar‐Gomez et al., 2016). Furthermore, the American Association for the Study of Liver Diseases (AASLD), similar to the European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO) have offered some recommendations to suggest more reliable approaches to the diagnostic, therapeutic and preventive aspects of care of NAFLD patients (Chalasani et al., 2012, Liver and Diabetes, 2016).
Table 1.
Lifestyle intervention regiments for non-alcoholic fatty liver disease (NAFLD).
| Lifestyle | Remarks |
|---|---|
| Gradual weight reduction |
|
| Increase in physical activity |
|
| Dietary modification |
|
The main clinical recommendation for the management of NAFLD includes lifestyle counseling to achieve a gradual weight reduction, an increase in physical activity as well as dietary modification. In a standardized, protocol-based, randomized controlled trial by Promrat and colleagues, the authors reported that lifestyle modifications through behavioral changes, diet, and exercise successfully led to improvements in the liver chemistry, histological activity, degree of steatosis, as well as the overall NASH (Promrat et al., 2010). The results have been corroborated by other large prospective studies which were conducted in real-world clinical practice (Vilar-Gomez et al., 2015, Vilar-Gomez et al., 2016) in addition to other systematic review and meta-analysis studies (Katsagoni et al., 2017, Keating et al., 2012, Orci et al., 2016). Though the mechanisms by which lifestyle modification through diet modification and exercise enhance liver enzymes and histology are still poorly understood, nevertheless, lifestyle modification has been shown to improve, abdominal and body fat, hyperlipidemia, insulin resistance, and inflammatory markers. Diet modification also comprises an increased consumption of diets rich in fruits and vegetables in addition to a reduction in the intakes of fructose, total fat, saturated fatty acids and trans-fatty acids (Dongiovanni et al., 2016).
5.1. Weight reduction
Obesity is an established risk factor for NAFLD which is associated with an impairment of pro/antioxidant equilibrium leading to the inflammation in liver tissue (Kobyliak et al., 2017). On the other hand, the strongest determinant of the development of NAFLD is arguably weight gain making the weight loss as the greatest predictor of reductions in liver aminotransferases and fat (Keating and Adams, 2016). Therefore, one of the cardinal goals of weight reduction is the reduction of fatty liver and maintenance of a normal heart function (Trovato et al., 2016). The most robust and consistent predictor of NASH resolution is the degree of weight loss because a significant weight loss does not only give rise to a reduced fatty acid supply to the liver but also reduces adipose tissue inflammation and proinflammatory cytokine secretion in addition to improving insulin sensitivity and subsequent improvements in the liver histology (Vilar‐Gomez et al., 2016). It has also been reported in the literature that the consumption of probiotic yogurt, for instance, may be applicable in reducing risk factors of NAFLD through an improved body mass index and insulin levels (Nabavi et al., 2015). Generally, patients are encouraged to lose ≥10% of their body weight for significant improvement in the cardiovascular risk profile, liver histology, steatosis as well as reduction in hepatic inflammation and hepatocellular injury despite the difficulty in achieving and sustaining weight loss through energy restriction (Dongiovanni et al., 2016, Vilar-Gomez et al., 2015). While 7−10% weight loss is considered to be modest, weight losses >10% are necessary to induce significant improvements in the liver histology of obese and overweight patients with NASH because greater weight losses have been found to have with greater improvements in all histologic parameters, especially in those individuals with significant weight reduction of ≥10% (Vilar-Gomez et al., 2015, Vilar-Gomez et al., 2016). Weight loss also decreases diabetes/ cardiovascular risk, regresses liver disease, resolves near universal NASH as well as improves fibrosis by at least one stage (Romero-Gómez et al., 2017).
5.2. Exercise and physical activity
Sedentary lifestyle and physical activity and have been strongly associated with specific physiological effects such as adiposity and cardio-metabolic health outcomes (Ellis et al., 2017, Martínez-López et al., 2015). Exercise and other physical activities provide a valid low-cost therapy for NAFLD management given the paucity of current treatment options (Keating et al., 2012). Exercise has multiple benefits beyond weight loss. The efficacy of exercise for the reduction of liver fat has also been recognized owing to the difficulty of sustaining weight loss in the long-term, despite its profound effectiveness in the management of NAFLD (Keating and Adams, 2016). Physical activity and exercise also effectively decrease steatosis and intrahepatic lipids as well as improve liver function independently of weight loss (Dongiovanni et al., 2016). The focus of recent clinical investigations, however, is to demarcate the optimal ‘dose’ of exercise therapy for reducing liver fat and preventing or reversing NAFLD (Keating and Adams, 2016). Long-term weight loss is achievable through greater amounts of activity. Promrat et al. (2010), for instance, achieved 7–10% weight loss through moderate-intensity activities which included 200 min weekly exercise from 10,000 steps walking per day, in addition to aerobic dance, bicycling, and strength training. It has also been reported in the literature that physical activity exerted a moderate-to-large impact not only on the reduction of intrahepatic lipid content but also significantly reduced the markers of hepatocellular injury as well as peripheral insulin resistance. Thus, physical activity is highly recommended not only in combination with dietary changes but also as an independent effective approach to manage NAFLD (Keating et al., 2012, Orci et al., 2016). Moderate physical activity exercise (such as 30 min of aerobic exercise 4 times a week) is also considered as one of the preferred methods of weight loss (Ahmed, 2015). Aerobic exercise also does not only boost activity and expression of antioxidant enzymes but also facilitate reduction of dichlorodiphenyltrichloroethane (DDT)- induced oxidative damage primarily due to better oxygen availability (Li et al., 2017). In general, the superiority of exercise to other lifestyle interventions is noticeable in the improvement of insulin sensitivity and BMI reduction (Zou et al., 2018). Our opinion is that exercise greatly helps in containing and reducing body calorie.
5.3. Diet modification
Besides the total energy intake, the diet composition also has a significant effect on the metabolic and endocrine functions as well as the overall energy balance (Dongiovanni et al., 2016). Therefore, the consumption of diets which are rich in fruits and vegetables is highly recommended for the prevention of oxidative damage and chronic disease (Mikail et al., 2016), and NAFLD is not an exception. Plants and plant-based natural products (such as fruit, vegetables, minimally-refined cereal, medicinal and aromatic plants) are sustainable antioxidant sources containing novel biologically active compounds such as phenolic compounds and vitamins with known beneficial effects (Ahmed and Mikail, 2017, Ahmed et al., 2015, Ahmed et al., 2017a, Ahmed et al., 2017b, Ibrahim et al., 2017a, Ibrahim et al., 2017b). A significant reduction in the consumption of saturated fatty acids, total fat, trans-fatty acids, and fructose is also strongly recommended. For instance, dietary fructose, which is consumed in the form of sugar-containing soft drinks or juices, has been implicated in the pathogenesis of NAFLD (Dongiovanni et al., 2016). The high sugar consumption is considered as the major cause of obesity, while fructose-rich diets are known to impair insulin sensitivity leading to the development of NAFLD (Ahmed, 2015). On the other hand, the Mediterranean diet (such as olive oil which is high in monounsaturated fatty acid and/or omega-3 fatty acids) alone even without weight loss does not only reduce liver steatosis but also improve insulin sensitivity in an insulin-resistant population with NAFLD (Ryan et al., 2013). The deficiency of dietary omega-3 fatty acid in association with an increase in omega-6 fatty acid in the body has also been reported to cause NAFLD in rats and mice (Ahmed, 2015). The enrichment of frequently-consumed food item such as eggs with essential omega-3 fatty acids [such as alpha-linolenic acid (ALA), docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA)] has thus become a common practice in the food industry (Khan et al., 2017). The hepatoprotective potential of coffee in NAFLD patients has also been reported in the literature with underlying mechanisms such as antioxidative, anti-inflammatory, antifibrotic effects, and chemopreventive which explains the effect of coffee to drinkers with chronic liver disease (Bambha et al., 2014, Chen et al., 2014). In general, diet improves the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and thus assumes the most effective intervention in the management of patients with NAFLD (Zou et al., 2018). Our opinion is that adherence to low calorie, very low calorie, low carbohydrate and low fat diets with regular physical activity and behavioral modifications are essential for the management of NAFLD.
6. Pharmacotherapy
The authors believe that despite the advocacy for lifestyle intervention, patients with a high risk of disease progression or advanced diseases status should also be supplemented with pharmacological therapy for the additive benefit. According to Bril and Cusi (2017), complete resolution of NASH is rarely achieved with lifestyle intervention alone due to the difficulty and challenges in maintaining it over time. It is axiomatic that NAFLD is associated with metabolic syndrome, thus, the associated comorbidities like diabetes mellitus, obesity, hyperlipidemia, and hypertension are also recommended to be managed well and concurrently as part of NAFLD treatment. A comprehensive guideline based on the recommendations of the AASLD and EASL has been reported in the literature (Ahmed, 2015, Chalasani et al., 2012, Liver and Diabetes, 2016). In general, the broad categories of pharmacotherapy for the treatment and management of NAFLD are: (1) Antioxidants (such as vitamins C&E); (2) Insulin-sensitizing agents (such as Metformin); (3) Hepatoprotective and miscellaneous agents (such as thioglitazones, Ursodeoxycholic acid, statins, pentoxifylline, Orlistat, etc.); and (4) Bariatric surgery.
7. Conclusion
Healthy lifestyle modifications such as such as dietary modifications, regular physical activity, and gradual weight loss are the best approaches which should be clinically recommended and holistically implemented together as an initial step for the prevention, treatment, and management of NAFLD.
8. Future research directions
It is highly important to unravel the mechanisms by which each of the aforementioned interventions (dietary modifications, regular physical activity, and gradual weight loss) specifically resolve various stages of liver diseases. There is also a dire need for randomized controlled trials to elucidate the relative importance of different exercise doses.
9. Conflicts of interest
Authors declare no conflict of interest.
10. Authors’ contributions
All the authors jointly contributed to and approved the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed I.A., Mikail M.A. Paradigm shift: focusing on plant-based natural antimicrobials. J. Microbiol. Experiment. 2017;5(2):1–2. [Google Scholar]
- Ahmed I.A., Mikail M.A., Ibrahim M. Baccaurea angulata fruit juice ameliorates altered hematological and biochemical biomarkers in diet-induced hypercholesterolemic rabbits. Nutr. Res. 2017;42:31–42. doi: 10.1016/j.nutres.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Ahmed I.A., Mikail M.A., Ibrahim M. Baccaurea angulata fruit juice protects rabbit's liver from hypercholesterolemia-induced injury. Malaysian J. Med. Res. 2017;1(4):18–21. [Google Scholar]
- Ahmed I.A., Mikail M.A., bin Ibrahim M., bin Hazali N., Rasad M.S.B.A., Ghani R.A., Yahya M.N.A. Antioxidant activity and phenolic profile of various morphological parts of underutilised Baccaurea angulata fruit. Food Chem. 2015;172:778–787. doi: 10.1016/j.foodchem.2014.09.122. [DOI] [PubMed] [Google Scholar]
- Ahmed M. Non-alcoholic fatty liver disease in 2015. World J. Hepatol. 2015;7(11):1450. doi: 10.4254/wjh.v7.i11.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambha K., Wilson L.A., Unalp A., Loomba R., Neuschwander-Tetri B.A., Brunt E.M., Bass N.M. Coffee consumption in NAFLD patients with lower insulin resistance is associated with lower risk of severe fibrosis. Liver Int. 2014;34(8):1250–1258. doi: 10.1111/liv.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera F., George J. The role of diet and nutritional intervention for the management of patients with NAFLD. Clin. Liver Dis. 2014;18(1):91–112. doi: 10.1016/j.cld.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Bellentani S., Marino M. Epidemiology and natural history of non-alcoholic fatty liver disease (NAFLD) Ann. Hepatol. 2009;8(Suppl 1):S4–S8. [PubMed] [Google Scholar]
- Betrapally N.S., Gillevet P.M., Bajaj J.S. Gut microbiome and liver disease. Trans. Res. 2017;179:49–59. doi: 10.1016/j.trsl.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bril F., Cusi K. Management of non-alcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care. 2017;40(3):419–430. doi: 10.2337/dc16-1787. [DOI] [PubMed] [Google Scholar]
- Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65(8):1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Byrne C.D., Targher G. NAFLD: a multisystem disease. J. Hepatol. 2015;62(1 Supplement):S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Chalasani N., Younossi Z., Lavine J.E., Diehl A.M., Brunt E.M., Cusi K., Sanyal A.J. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the study of liver diseases, American college of gastroenterology, and the American gastroenterological association. Hepatology. 2012;55(6):2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- Chen S., Teoh N.C., Chitturi S., Farrell G.C. Coffee and non-alcoholic fatty liver disease: brewing evidence for hepatoprotection? J. Gastroenterol. Hepatol. 2014;29(3):435–441. doi: 10.1111/jgh.12422. [DOI] [PubMed] [Google Scholar]
- Dong F., Zhang Y., Huang Y., Wang Y., Zhang G., Hu X., Bao Z. Long-term lifestyle interventions in middle-aged and elderly men with non-alcoholic fatty liver disease: a randomized controlled trial. Scientific Reports. 2016;6 doi: 10.1038/srep36783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongiovanni P., Lanti C., Riso P., Valenti L. Nutritional therapy for non-alcoholic fatty liver disease. J. Nutr. Biochem. 2016;29:1–11. doi: 10.1016/j.jnutbio.2015.08.024. [DOI] [PubMed] [Google Scholar]
- Doulberis M., Kotronis G., Gialamprinou D., Kountouras J., Katsinelos P. Non-alcoholic fatty liver disease: an update with special focus on the role of gut microbiota. Metabolism. 2017;71:182–197. doi: 10.1016/j.metabol.2017.03.013. [DOI] [PubMed] [Google Scholar]
- Dunkler D., Kohl M., Teo K.K., Heinze G., Dehghan M., Clase C.M., Oberbauer R. Population-attributable fractions of modifiable lifestyle factors for CKD and mortality in individuals with type 2 diabetes: a cohort study. Am. J. Kidney Dis. 2016;68(1):29–40. doi: 10.1053/j.ajkd.2015.12.019. [DOI] [PubMed] [Google Scholar]
- Ellis Y.G., Cliff D.P., Janssen X., Jones R.A., Reilly J.J., Okely A.D. Sedentary time, physical activity and compliance with IOM recommendations in young children at childcare. Preventive Med. Rep. 2017;7(Supplement C):221–226. doi: 10.1016/j.pmedr.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs C.D., Claudel T., Trauner M. Role of metabolic lipases and lipolytic metabolites in the pathogenesis of NAFLD. Trends Endocrinol. Metab. 2014;25(11):576–585. doi: 10.1016/j.tem.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Hannah W.N., Jr., Harrison S.A. Lifestyle and dietary interventions in the management of non-alcoholic fatty liver disease. Dig. Dis. Sci. 2016;61(5):1365–1374. doi: 10.1007/s10620-016-4153-y. [DOI] [PubMed] [Google Scholar]
- Ibrahim M., Ahmed I.A., Mikail M.A., Ishola A.A., Draman S., Isa M.L.M., Yusof A.M. Baccaurea angulata fruit juice reduces atherosclerotic lesions in diet-induced Hypercholesterolemic rabbits. Lipids Health Dis. 2017;16(134):1–8. doi: 10.1186/s12944-017-0526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M., Mikail M.A., Ahmed I.A., Hazali N., Rasad M.S.B.A., Ghani R.A., Draman S. Comparison of the effects of three different Baccaurea angulata whole fruit juice doses on plasma, aorta and liver MDA levels, antioxidant enzymes and total antioxidant capacity. Eur. J. Nutr. 2017:1–12. doi: 10.1007/s00394-017-1466-3. [DOI] [PubMed] [Google Scholar]
- Katsagoni C.N., Georgoulis M., Papatheodoridis G.V., Panagiotakos D.B., Kontogianni M.D. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: a meta-analysis. Metabolism. 2017;68:119–132. doi: 10.1016/j.metabol.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Keating S.E., Adams L.A. Exercise in NAFLD: just do it. J. Hepatol. 2016;65(4):671–673. doi: 10.1016/j.jhep.2016.06.022. [DOI] [PubMed] [Google Scholar]
- Keating S.E., Hackett D.A., George J., Johnson N.A. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J. Hepatol. 2012;57(1):157–166. doi: 10.1016/j.jhep.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Kenneally, S., Sier, J.H., Moore, J.B., 2017. Efficacy of dietary and physical activity intervention in non-alcoholic fatty liver disease: a systematic review. 4(1), e000139. 10.1136/bmjgast-2017-000139. [DOI] [PMC free article] [PubMed]
- Khan S.A., Khan A., Khan S.A., Beg M.A., Ali A., Damanhouri G. Comparative study of fatty-acid composition of table eggs from the Jeddah food market and effect of value addition in omega-3 bio-fortified eggs. Saudi J. Biol. Sci. 2017;24(4):929–935. doi: 10.1016/j.sjbs.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobyliak N., Virchenko O., Falalyeyeva T., Kondro M., Beregova T., Bodnar P., Spivak M. Cerium dioxide nanoparticles possess anti-inflammatory properties in the conditions of the obesity-associated NAFLD in rats. Biomed. Pharmacother. 2017;90:608–614. doi: 10.1016/j.biopha.2017.03.099. [DOI] [PubMed] [Google Scholar]
- Li K., Zhu X., Wang Y., Zheng S., Dong G. Effect of aerobic exercise intervention on DDT degradation and oxidative stress in rats. Saudi J. Biol. Sci. 2017;24(3):664–671. doi: 10.1016/j.sjbs.2017.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Association for the Study of the Liver. European Association for the Study of Diabetes. European Association for the Study of Obesity EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Obesity facts. 2016;9(2):65–90. doi: 10.1159/000443344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonardo A., Nascimbeni F., Targher G., Bernardi M., Bonino F., Bugianesi E., Bellentani S. AISF position paper on non-alcoholic fatty liver disease (NAFLD): updates and future directions. Digest. Liver Dis. 2017;49(5):471–483. doi: 10.1016/j.dld.2017.01.147. [DOI] [PubMed] [Google Scholar]
- Marquis-Gravel G., Hayami D., Juneau M., Nigam A., Guilbeault V., Latour É., Gayda M. Intensive lifestyle intervention including high-intensity interval training program improves insulin resistance and fasting plasma glucose in obese patients. Prevent. Med. Rep. 2015;2:314–318. doi: 10.1016/j.pmedr.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-López E.J., Hita-Contreras F., Moral-García J.E., Grao-Cruces A., Ruiz J.R., Redecillas-Peiró M.T., Martínez-Amat A. Association of low weekly physical activity and sedentary lifestyle with self-perceived health, pain, and well-being in a Spanish teenage population. Sci. Sports. 2015;30(6):342–351. [Google Scholar]
- Masana L., Ros E., Sudano I., Angoulvant D., Ibarretxe Gerediaga D., Murga Eizagaechevarria N., Piedecausa M. Is there a role for lifestyle changes in cardiovascular prevention? What, when and how? Atherosclerosis Supplements. 2017;26:2–15. doi: 10.1016/S1567-5688(17)30020-X. [DOI] [PubMed] [Google Scholar]
- Matteoni C.A., Younossi Z.M., Gramlich T., Boparai N., Liu Y.C., McCullough A.J. Non-alcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- McPherson S., Hardy T., Henderson E., Burt A.D., Day C.P., Anstee Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J. Hepatol. 2015;62(5):1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- Mikail M.A., Ahmed I.A., Ibrahim M., Hazali N., Rasad M.S.B.A., Ghani R.A., Isa M.L.M. Baccaurea angulata fruit inhibits lipid peroxidation and induces the increase in antioxidant enzyme activities. Eur. J. Nutr. 2016;55(4):1435–1444. doi: 10.1007/s00394-015-0961-7. [DOI] [PubMed] [Google Scholar]
- Nabavi S., Rafraf M., Somi M.-h., Homayouni-Rad A., Asghari-Jafarabadi M. Probiotic yogurt improves body mass index and fasting insulin levels without affecting serum leptin and adiponectin levels in non-alcoholic fatty liver disease (NAFLD) J. Funct. Foods. 2015;18(Part A):684–691. [Google Scholar]
- Naik A., Košir R., Rozman D. Genomic aspects of NAFLD pathogenesis. Genomics. 2013;102(2):84–95. doi: 10.1016/j.ygeno.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Nobili V., Manco M., Devito R., Di Ciommo V., Comparcola D., Sartorelli M.R., Angulo P. Lifestyle intervention and antioxidant therapy in children with non-alcoholic fatty liver disease: a randomized, controlled trial. Hepatology. 2008;48(1):119–128. doi: 10.1002/hep.22336. [DOI] [PubMed] [Google Scholar]
- Orci L.A., Gariani K., Oldani G., Delaune V., Morel P., Toso C. Exercise-based interventions for non-alcoholic fatty liver disease: a meta-analysis and meta-regression. Clin. Gastroenterol. Hepatol. 2016;14(10):1398–1411. doi: 10.1016/j.cgh.2016.04.036. [DOI] [PubMed] [Google Scholar]
- Paul S., Davis A.M. Diagnosis and management of non-alcoholic fatty liver disease. JAMA. 2018;320(23):2474–2475. doi: 10.1001/jama.2018.17365. [DOI] [PubMed] [Google Scholar]
- Promrat K., Kleiner D.E., Niemeier H.M., Jackvony E., Kearns M., Wands J.R., Wing R.R. Randomized controlled trial testing the effects of weight loss on non-alcoholic steatohepatitis. Hepatology. 2010;51(1):121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiq N., Bai C., Fang Y., Srishord M., McCullough A., Gramlich T., Younossi Z.M. Long-term follow-up of patients with non-alcoholic fatty liver. Clin. Gastroenterol. Hepatol. 2009;7(2):234–238. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Romero-Gómez M., Zelber-Sagi S., Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017 doi: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Ryan M.C., Itsiopoulos C., Thodis T., Ward G., Trost N., Hofferberth S., Wilson A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013;59(1):138–143. doi: 10.1016/j.jhep.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Saint Onge J.M., Krueger P.M. Health lifestyle behaviors among U.S. adults. SSM – Population Health. 2017;3:89–98. doi: 10.1016/j.ssmph.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonerock G.L., Blumenthal J.A. Role of counseling to promote adherence in healthy lifestyle medicine: strategies to improve exercise adherence and enhance physical activity. Prog. Cardiovasc. Dis. 2017;59(5):455–462. doi: 10.1016/j.pcad.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trovato F.M., Martines G.F., Catalano D., Musumeci G., Pirri C., Trovato G.M. Echocardiography and NAFLD (non-alcoholic fatty liver disease) Int. J. Cardiol. 2016;221:275–279. doi: 10.1016/j.ijcard.2016.06.180. [DOI] [PubMed] [Google Scholar]
- Vernon G., Baranova A., Younossi Z. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011;34(3):274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L., Romero-Gomez M. Weight loss through lifestyle modification significantly reduces features of non-alcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–378. doi: 10.1053/j.gastro.2015.04.005. e365. [DOI] [PubMed] [Google Scholar]
- Vilar-Gomez E., Yasells-Garcia A., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Diago M. Development and validation of a noninvasive prediction model for non-alcoholic steatohepatitis resolution after lifestyle intervention. Hepatology. 2016;63(6):1875–1887. doi: 10.1002/hep.28484. [DOI] [PubMed] [Google Scholar]
- Zou T.T., Zhang C., Zhou Y.F., Han Y.J., Xiong J.J., Wu X.X., Zheng M.H. Lifestyle interventions for patients with non-alcoholic fatty liver disease: a network meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018;30(7):747–755. doi: 10.1097/MEG.0000000000001135. [DOI] [PubMed] [Google Scholar]