Graphical abstract
Keywords: Juniperus procera, AgNPs, Splenic cells proliferation, Antibacterial, Antifungal, Plant extract
Abstract
Juniperus spp. are used as medicinal plants in many countries like Bosnia, Lebanon, and Turkey. In folk medicines, these plants have been used for treating skin and respiratory tract diseases, urinary problems, rheumatism and gall bladder stones. The objectives of this work were to synthesize silver nanoparticles (AgNPs) using a coniferous tree, Juniperus procera leaf extract and testing the synthesized AgNPs for its antimicrobial potentials, hemolytic activity, toxicity and the proliferative effects against normal and activated rat splenic cells. Leaf extract was prepared using acetone and ethanol as solvents. AgNPs were prepared using the acetone extract. AgNPs were validated using UV–Vis spectroscopy and scanning electron microscopy (SEM). Functional groups in the extract were identified using Fourier Transform Infrared (FT-IR) spectroscopy. SEM images of AgNPs showed spherical and cubic shapes with a uniform size distribution with an average size of 30–90 nm. FT-IR spectroscopy showed the presence of many functional groups in the plant extract. AgNPs showed promising antimicrobial activity against tested bacteria and fungus. AgNPs also expressed a stimulating activity towards the rat splenic cells in a dose dependent manner. Acetone as solvent was safer on cells than ethanol. Green synthesized AgNPs using J. procera might be used as a broad-spectrum therapeutic agent against microorganisms and as an immunostimulant agent.
1. Introduction
Plants are naturally bestowed with numerous bioactive compounds that have desirable medicinal and health benefits and are traditionally used for prevention of many chronic diseases (Yogalakshmi et al., 2010). Herbs are used for preventing and curing many diseases such as the fibrosis and injury of liver (Al-Attar and Shawush, 2015, Hsieh et al., 2008, Yuan et al., 2008). Some useful plant species, like the Juniperus species, are used to treat some human illness such as ulcers, hyperglycemia, bronchitis, tuberculosis, pneumonia, intestinal worms, cure liver problems and heal wounds (Burits et al., 2001, Loizzo et al., 2007). The medicinal uses of Juniperus plants are widespread in many countries such as Saudi Arabia, Bosnia, Lebanon, and Turkey and according to folk medicine was used for treating skin and respiratory tract diseases (Öztürk et al., 2011), urinary problems, rheumatism and gall bladder stones (Šarić-Kundalić et al., 2011).
The uses of silver nanoparticles (AgNPs) in the sector of pharmaceutical industries are wide (Satyavani et al., 2011) as these nanoparticles have a powerful inhibitory potential against many microorganisms (Majeed et al., 2016, Mohanta et al., 2017) and is a good drug carrier in treatment of cancer (Nayak et al., 2016). Nowadays, AgNPs are of great interest. The synthesis of AgNPs can be performed through many chemical methods and physical ways that involve chemical (Bindhu and Umadevi, 2015, Wiley et al., 2005), photochemical (Mallick et al., 2005, Pileni, 2000), and electrochemical reduction (Liu and Lin, 2004, Sandmann et al., 2000) as well as vaporization (Bae et al., 2002, Smetana et al., 2005). All of these methods utilize many toxic chemicals to act as reducing agents. So, the presence of alternatives to these toxic materials will be beneficial for the human health and environment.
To overcome the many side effects represented by the toxicity of chemicals used in the synthesis and biological applications of AgNPs, plants or their extracts have been used to replace chemicals in the AgNPs synthesis (biosynthesis process). Plants and plant extracts have many natural organic constituents; phyto-molecules that have protective and reducing properties which are very important for the reduction of silver ions depending on the natural compounds and reductive enzyme complexes. Recently, extracellular AgNPs were synthesized using different plant extracts as a potential reducing agent (Ahmed et al., 2016, Mohanta et al., 2017, Raja et al., 2017).
This work aimed to study the cell proliferative/anti-proliferative potentials as well as the anti-microbial activities found in J. procera leaf acetone extract and silver nanoparticles prepared by the extract.
2. Materials and methods
2.1. Plant material and preparation of extract
The leaves of J. procera were collected in January 2017. The plant sample was kindly identified by an expert taxonomist at the Biology Department, of the University. The active ingredients in the latex of the plant were extracted according to Ghramh et al. (2018) with some modifications. The leaves, after mild washing, were air-dried under shade at room temperature and ground into powder using an electric grinder and 300 g of the resulted powder was added to 1.5 L of either acetone or ethanol and stirred for 72 h. The resultant plant extracts were separately filtered and dried at reduced temperature using a rotary evaporator, and stored at 22 °C. Stock solution, at 1% concentration, was prepared by dissolving 1 g dried acetone extract materials in 100 mL acetone or 1 g dried ethanol extract materials in 100 mL ethanol and stored at 4 °C in the dark until used. The acetone stock is the most suitable when used in culture media (Vandhana et al., 2010).
2.2. Green biosynthesis of silver nanoparticles
Silver nanoparticles were prepared according to Ndikau et al. (2017) with little modifications. One mL of acetone plant extract stock and 0.5 mL Triton X-100 were added to 98.5 mL 1 mM AgNO3 solution in an Erlenmeyer flask and incubated for 24 h at room temperature until the color of the mixture changed.
2.3. Characterizations
UV–visible spectrophotometer (Lambda 25, PerkinElmer), at the wavelengths from 200 nm to 600 nm, was used to analyse and monitor the process of AgNPs synthesis at 1 nm resolution. The surface morphology of the prepared AgNPs was studied by using a scanning electron microscopy (SEM; Hitachi S4800) at an accelerating voltage of 90 kV. Fourier Transform infrared spectroscopy (FT-IR, JASCO 460 plus) was used at room temperature to identify the functional groups of bioreductant in the extract using the KBr pellet technique within the range 600–4000 cm−1 at a rate of 16 times and the clarity of 4 cm−1.
2.4. Antimicrobial activity
The antimicrobial potential of prepared silver nanoparticles was tested using microorganisms. The extract stock was diluted 100 times by adding 1 mL stock to 99 mL of distilled water containing 0.5 mL of Triton X-100 to match the concentration of the extract found with the nanoparticles.
2.4.1. Test microorganisms
The microorganisms utilized in this study were representative of Gram positive (Bacillus subtilis and Micrococcus luteus), Gram negative (Proteus mirabilis and Klebsiella pneumoniae) pathogenic bacteria and Candida albicans as a model of yeast. The isolates were streak plated on a nutrient agar and maintained at 4 °C.
2.4.2. Antimicrobial susceptibility testing (AST)
Well diffusion assay was adopted according to Daud et al. (2005). Into the 6 mm wells a 40 µL of each diluted plant extract and prepared nanoparticles were aseptically pipetted into the wells in separate plates. The plates were incubated at 4 °C for 3 h and then at 32 °C for an additional 18 h. Penicillin (10 µg) was used as positive control. Diameter of the zone of inhibition, in triplicate, around each of the 6-mm well was measured and the means were calculated.
2.5. Effects of plant extract and biosynthesized AgNPs on splenic cells proliferation
Adult male Sprague Dawley rats, whose weight was approximately 250–300 g, were kindly supplied by the animal rearing house at the University. This study was performed under the protocol and approval # 2017-03-38 of Research Ethics Committee, Faculty of Medicine. The animals were killed by cervical dislocation and the splenic cells were aseptically prepared in serum-free RPMI-1640 medium (SFM) [consisting of RPMI-1640 medium, augmented by 100 U/100 µg/ml penicillin/streptomycin, 2 mM L-glutamine (all from Gibco), 2 mM sodium pyruvate, 2% sodium bicarbonate, pH 7.2 (both from Seromed) and HEPES (N-2-hydroxyethylpiperazine-N-2-ehtanesulfonic acid) buffer solution (Sigma, Aldrich)] according to Gu et al. (2013). The viability of prepared cells was monitored through the exclusion of trypan blue by live cells (Böyum, 1968). Cells were enumerated to 5 × 104/mL in SFM including 10% fetal calf serum (Gibco) (culture medium). The cell culture was performed in a microwell tissue culture plate (TPP, Merk) by adding 100 μL of cell suspension (5000 cells/well) and acetone extract (10 μg/mL, 50 μg/mL, 100 μg/mL, and 200 μg/mL).
2.5.1. Study of anti-proliferative effects
To a sufficient volume of prepared cell culture, Phytohaemagglutinin (PHA, Sigma) at a final concentration of 5 μg/mL was added to stimulate cell proliferation. The anti-proliferative capability which may be found in the extract were tested by adding different concentrations of J. procera leaf extract and biosynthesized AgNPs separately at final concentrations of 10 μg/mL, 50 μg/mL, 100 μg/mL, and 200 μg/mL to wells containing cells. Cells without extract were included representing fast dividing cell control. Plates containing the cells were incubated at 37 °C for 72 h in 5% CO2 (Memmert, Gmbh) (Yashi et al., 1993). The MTT technique was used to measure the changes in cell numbers of different treated cells (Mosmann, 1983) using Vybrant® MTT Cell Proliferation Assay Kit (Thermo Fisher Scientific) according to manufacturer instructions.
2.5.2. Study of cytotoxic/proliferative effects
Cytotoxic (cell killing) or stimulating (induction of normal cell’s division) potentials which may be found in the extract were examined by incubating different concentrations of J. procera leaf extract and biosynthesized AgNPs separately with final concentrations of 10 μg/mL, 50 μg/mL, 100 μg/mL, and 200 μg/mL with normal splenic cells separately. Cells without any treatment were included as normal cell control. Culture plates were incubated and the increase or decrease in cell number was monitored as mentioned above.
The results were expressed as a percentage of increase/decrease in growth, according to Oves et al. (2013).
2.6. Red blood cell lysis test
The cytotoxicity was also determined by testing the hemolytic activity of the J. procera leaf extracts and biosynthesized AgNPs separately at final concentrations 200 μg/mL using the method defined by Oves et al. (2013) with few amendment. Fresh cow blood (50 mL) was placed in 50 mL Falcone tube containing EDTA to evade blood coagulation, slightly mixed and then divided into several sterile 15 mL Falcon tubes and placed in centrifugation for 10 min at 1500g. The blood supernatant was decanted and RBCs were washed four times with chilled (4 °C) PBS. The washed RBCs were resuspended in chilled PBS to get 10% hematocrit. A 100 µL of prepared ethanol and acetone J. procera leaf extract and biosynthesized AgNPs were added to 900 µL of prepared RBCs in 1.6 mL Eppendorf tubes separately and incubated for 60 min at 37 °C. 1.5% Triton X-100 (positive control) and phosphate buffer saline (PBS, negative control) were included in the test. All tubes were centrifuged for 10 min at 2000 RPM. After centrifugation supernatants were taken from the tubes, and the absorbance was measured at 576 nm.
2.7. Statistical analysis
All data were expressed as the mean of three triplicates. Different concentrations of the extract and extract generated AgNPs differences were analyzed with one-way analysis of variance (ANOVA) using SPSS (version 17). Differences of p ≤ 0.05 were considered to be statistically significant.
3. Results and discussion
Juniperus is considered as one of the important genera of the family Cupressaceae. It includes about 70 species of Juniperus that are distributed throughout the world (Al-Attar et al., 2016, Moein et al., 2010, Topçu et al., 1999). It is established that the Juniperus species are a source of natural products with antibacterial, antifungal and insecticidal potentials (Barrero et al., 2005, El-Sawi et al., 2007, Tumen et al., 2012). J. procera is also used as a local treatment for tuberculosis and jaundice (Samoylenko et al., 2008), intestinal worms and eye infections (Klauss and Adala, 1994). Pankaj et al. (2010) investigated several fractions isolated from Juniperus bark and leaves and found that it could inhibit aflatoxigenic Aspergillus flavus and A. niger growth. Hexane and ethanolic extracts prepared from J. lucayana aerial parts showed antifungal activities (Nuñez et al., 2007).
Sliver or silver nanoparticles were prepared by adding the plant extract to the AgNO3 solution. The mixture was incubated for 24 h at room temperature. Synthesis of AgNPs was indicated by the change of the mixture into the brown-yellow color (Fig. 1). The characteristic brown color is attributed to the excitation of Surface Plasmon Response (SPR) with the silver nanoparticles (Ranganathan et al., 2012). This color change indicates that the changes that happened in the metal oxidation state from Ag+ to the reduced form Ag0 were by biomolecules in plant extract through the nitrate reductase enzyme. The mentioned enzymes secreted into the solution may reduce the silver nitrate to silver nanoparticles by capping agents like proteins (Manivasagan et al., 2013). The power of Juniperus may be attributed in inclusion of a wide variety of phenolic acids, flavonoids, chlorogenic acid, caffeoylquinic acids, etc, which could act as reducing agents to react with silver ions and as scaffolds to direct the formation of AgNP in solution (Al-Attar, 2011, Lai et al., 2007).
Fig. 1.

Acetone extract of Juniperus procera. A: Acetone extract without AgNO3 and B: Acetone extract after adding AgNo3 for 24 h.
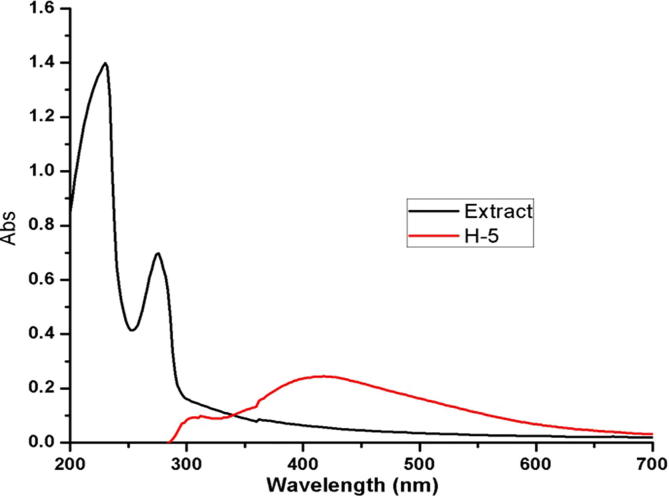
Formation of the AgNPs by the extract was monitored by the UV–visible spectrum (Fig. 2). Pure extract showed two absorption peaks at 230 and 275 nm. These two peaks may be responsible for the reduction of silver ion to silver metal due to the presence of polyphenol and other functional groups (Ranganathan et al., 2012). Testing the extract after the formation AgNPs resulted in a characteristic absorption spectrum at 417 nm. This may be due to SPR which proves the synthesis of silver nanoparticles at room temperature by using the extract (Parikh et al., 2008).
Fig. 2.
UV–Vis spectra of silver nanoparticles synthesized by the Juniperus procera leaves extract. (A) Acetone plant extract without silver nitrate; and (B) acetone plant extract-reduced silver nanoparticles.
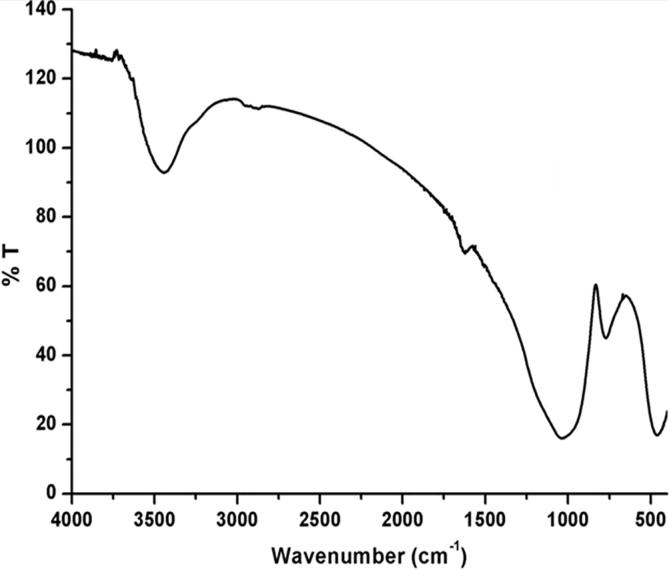
Biosynthesis of the AgNPs at room temperature was confirmed by FT-IR spectrum (Fig. 3). The broad band appeared at ∼3442 cm−1 is due to the presence of O—H stretching vibration (Li et al., 2017, Liu et al., 2006). The strong band appeared at 1026 cm−1 is ascribed to the C—O stretching vibration of OH group, which also confirms the presence of the phenolic group in the extract and interacted with the silver ion. Scissoring vibration of the NH2 group in amines and the stretching mode of the C C bonds in aromatic rings seem to be responsible for the band at 1616 cm−1. In addition to the referred modes, the vibration bending of the N—H bonds in carboxylic acids and derivatives could also contribute to the neighbouring band at 1620 cm−1. A band with stretching vibration appears at ∼452 cm−1 perhaps assigned to the interaction or adsorption of O—H on the surface of silver nanoparticles. The FT-IR results revealed that the biomolecules of the extract present on the surface of silver nanoparticles (Li et al., 2017, Prathna et al., 2011). The FT-IR results revealed that the production of AgNPs might be stabilized by the existence of polyphenols materials in the extract, which may play a role as powerful bio-reducer and good capping agent; therefore, they help in the formation of stabilized silver nanoparticles.
Fig 3.
FT-IR spectrum of biosynthesized silver nanoparticles by the Juniperus procera leaves extract.
The shape and size of the biosynthesized AgNPs were monitored by SEM analysis (Fig. 4). SEM images of AgNPs showed spherical and cubic shapes with uniform size distribution. The particles have an average size of 30–90 nm with inter-particle distances, which are magnified at ×13,000 times. The small degree of agglomeration could be seen in SEM investigations may be due to the effect of the bio extract and surfactant during synthesis. The SEM studies clearly depict that the bioorganic extract and surfactant play a momentous role for producing the spherical AgNPs materials.
Fig 4.

SEM micrograph of the Juniperus procera leaves extract prepared AgNPs.
The antimicrobial activities found in the J. procera leaves extract were tested. J. procera leaves extract showed inhibition potentials against bacterial and fungal growth (Table 1). Plant extract only showed mild activity against bacteria and fungus while silver nanoparticles generated by the plant extract showed more activities against Gram positive and negative bacteria as well as yeast. These results are in agreement with others. Some investigators (Pankaj et al., 2010) studied the different fractions isolated from Juniperus bark and leaves and found that it can inhibit Aspergillus flavus and A. niger growth (Nuñez et al., 2007). Other researchers demonstrated that J. procera; found at high altitude has antibacterial activities and antifungal potentials (Gherbawy and Elhariry, 2016).
Table 1.
Antimicrobial potentials of Juniperus procera leaves extract and extract prepared AgNPs.
| Test organisms | Inhibition zone (mm) |
||
|---|---|---|---|
| Juniperus extract | Juniperus extract + AgNPs | Penicillin (10 µg) | |
| Micrococcus luteus | 9 ± 0.7 | 28 ± 1.1 | 8 ± 0.7 |
| Bacillus subtilis | 11 ± 0.8 | 28 ± 1.2 | 10 ± 0.9 |
| Proteus mirabilis | 10 ± 0.9 | 29 ± 1.3 | 9 ± 0.9 |
| Klebsiella pneumoniae | 9 ± 0.6 | 18 ± 0.9 | 10 ± 0.7 |
| Candida albicans | NI | 24 ± 0.1.2 | 10 ± 0.8 |
NB: Diameter of zones of inhibition are expressed as means of three replicates ± SD (p < 0.05).
Muhammad et al., 1995, Muhammad et al., 1996 isolated some components, the (+)-8α-acetoxyelemol, β-peltatin A methyl ether and deoxypodophyllotoxin, that have antibacterial activities.
It was demonstrated that the active biomolecules in J. procera leaves included flavonoids and polyphenol (Ali and Elgimabi, 2015); which confer the antibiotic potential of the plant against many species of bacterial pathogens (Jung, 2009). Hence, preliminary screening revealed that the biologically synthesized AgNPs showed significant antimicrobial activity, as compared to other researches (Arokiyaraj et al., 2017, He et al., 2013). This antibacterial activity of the AgNPs that were green synthesized by Juniperus may be due the very small size leading to the large surface area of the AgNPs, resulting in binding to the bacterial cell wall and neutralizing the cellular enzymes initiating the disruption of the membrane permeability (Su et al., 2009). Coupled to this, antibacterial activity may be due to the interaction of AgNPs with groups like the thiol group of the l-cysteine protein residues leading to enzymatic dysfunction (Gordon et al., 2010). In addition, the AgNPs may induce the release of reactive oxygen species resulting in the damage to proteins and nucleic acids which cause the cell death (Abdal Dayem et al., 2017).
The immunomodulatory effects of J. procera leaf extract and extract prepared nanoparticles using the cell proliferation as a model are used for the search of new therapeutic agents with natural origin (Al-Attar et al., 2016, Arokiyaraj et al., 2007).
Both plant extract alone and with AgNPs showed no inhibitory effects on PHA-stimulated splenic cells, in contrary the extracts at all different concentrations showed stimulatory effects (Table 2). Extract containing AgNPs showed more stimulatory potential than the extract alone. Maximal stimulation was at higher concentration of extract containing nanoparticles (200 µg/mL).
Table 2.
Percent of splenic cells growth stimulation after treatment with plant extract and its silver nitrate generated nanoparticles.
| Extract concentration (µg/mL) |
% of splenic cells growth stimulation |
|||
|---|---|---|---|---|
| Normal |
PHA treated |
|||
| Extract | AgNPs | Extract | AgNPs | |
| 10 | 110 ± 0.71 | 118 ± 0.66 | 115 ± 0.97 | 130 ± 1.1 |
| 50 | 115 ± 0.82 | 122 ± 0.90 | 125 ± 1.3 | 140 ± 1.6 |
| 100 | 115 ± 0.91 | 129 ± 1.1 | 130 ± 1.4 | 145 ± 1.8 |
| 200 | 120 ± 0.87 | 140 ± 1.3 | 135 ± 1.6 | 165 ± 2.2 |
NB: Percentage of splenic cell growth stimulation was expressed as average of three replicates ± SD (p < 0.05).
Both plant extract alone and with AgNPs showed no cytotoxic effects on normal splenic cells. Both extracts at all different concentrations used showed stimulatory effects (Table 2).
The activation of the immune response in this model boosts cell proliferation/inhibition through an increase/decrease in the number of cells found in the culture for specific duration (Rocha et al., 2007) and this change can be observed with MTT assay (Mosmann, 1983). The acetone extract of J. procera leaves used in this study, induced an increase in cell proliferation both in un-induced or PHA-induced cultures in a dose-dependent response. PHA is the most frequently used lectin to activate T lymphocytes in vitro to evaluate immune response mediated by this type of cells (Rocha et al., 2007). Thus, the results obtained with the acetone extract of J. procera leaves suggest that the component(s) present in the plant could stimulate T lymphocyte proliferation, suggesting the ability of that extract to activate the cellular immune response. The phytochemical picture of these herbs demonstrates the presence of constituents J. procera flavonoids and phenol (Al-Attar et al., 2016). Although flavonoids have been described in many works as inhibitors of lymphocytes proliferation (Arokiyaraj et al., 2007, Moiteiro et al., 2001), the action of the flavonoids seems to be inhibited by other compounds with immunostimulatory effects, such as alkaloids (Sheng et al., 2000), that’s why the results of this study revealed substantial rise in cell proliferation that can be due to the stimulation of T lymphocytes.
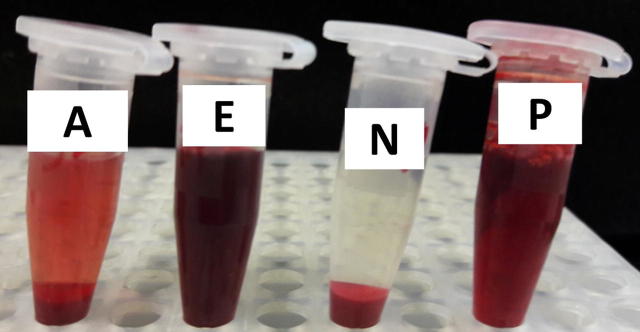
The cytotoxicity was measured by hemolytic activity against cow RBCs with Triton X-100 (positive control). The percentage of lysis was calculated by relating the absorbance of sample to the positive control. The positive control (1.5% Triton X-100) showed about 100% RBCs lysis, while the negative control (PBS) showed no lysis effects on the RBCs. The ethanolic plant extract showed 100% RBCs lysis (Fig. 5) while acetone extract alone or extract with AgNPs showed insignificant lysis effect on RBCs (about 1.75%, Fig. 6). These results supported our idea to use acetone extract rather than ethanol extract in cell culture experiments.
Fig 5.
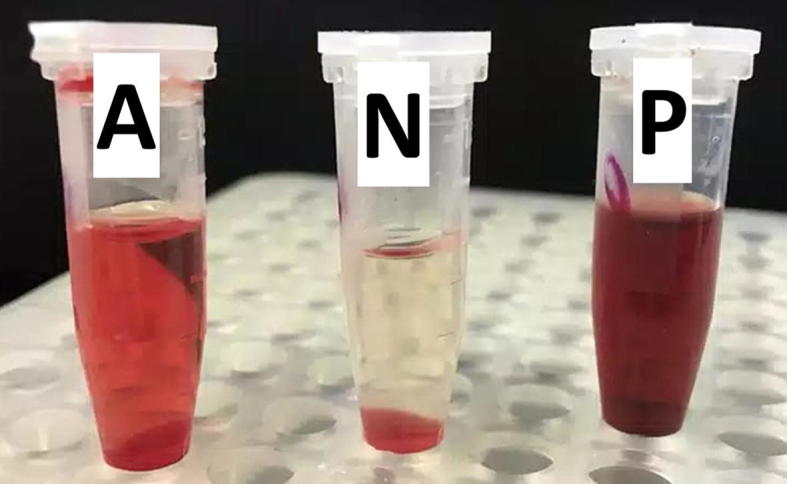
The effect of Juniperus procera leaves acetone extract containing AgNPs on cow RBCs. Where A: Acetone extract containing AgNPs; N: Negative control and P: positive control.
Fig 6.
The effect of Juniperus procera leaves acetone and ethanol extracts on cow RBCs. Where A: Acetone extract; E: ethanol extract; N: Negative control and P: positive control.
4. Conclusions
Acetone extract of J. procera leaves could be used for the green biosynthesis of AgNPs. The extract contained functional groups and metabolite that enabled the synthesis and stabilizing of AgNPs from silver ions. The extract could stimulate normal and PHA-stimulated splenocytes so it can be used as immunostimulant.
Acknowledgments
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through research group programme (R.G.P.1)-11/39.
The authors would like to express their gratitude to Research Center for Advanced Materials Science (RCAMS), King Khalid University, Abha, Saudi Arabia for support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdal Dayem A., Hossain M.K., Lee S.B., Kim K., Saha S.K., Yang G.-M., Choi H.Y., Cho S.-G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017;18:120. doi: 10.3390/ijms18010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S., Ahmad M., Swami B.L., Ikram S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016;9:1–7. [Google Scholar]
- Al-Attar A.M. Hepatoprotective influence of vitamin C on thioacetamide—induced liver cirrhosis in Wistar male rats. J. Pharmacol. Toxicol. 2011;6:218–288. [Google Scholar]
- Al-Attar A.M., Alrobai A.A., Almalki D.A. Effect of Olea oleaster and Juniperus procera leaves extracts on thioacetamide induced hepatic cirrhosis in male albino mice. Saudi J. Biol. Sci. 2016;23:363–371. doi: 10.1016/j.sjbs.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Attar A.M., Shawush N.A. Influence of olive and rosemary leaves extracts on chemically induced liver cirrhosis in male rats. Saudi J. Biol. Sci. 2015;22:157–163. doi: 10.1016/j.sjbs.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A.A., Elgimabi M.E.N. Extraction and determination of antioxidants, polyphenols, flavonoids and antioxidant activity in some plants. Int. J. Chem. Sc. 2015;13:1883–1892. [Google Scholar]
- Arokiyaraj S., Perinbam K., Agastian P., Balaraju K. Immunosuppressive effect of medicinal plants of Kolli hills on mitogen-stimulated proliferation of the human peripheral blood mononuclear cells in vitro. Indian J. Pharmacol. 2007;39:180. [Google Scholar]
- Arokiyaraj S., Vincent S., Saravanan M., Lee Y., Oh Y.K., Kim K.H. Green synthesis of silver nanoparticles using Rheum palmatum root extract and their antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa. Artif. Cells Nanomed. Biotechnol. 2017;45:372–379. doi: 10.3109/21691401.2016.1160403. [DOI] [PubMed] [Google Scholar]
- Bae C.H., Nam S.H., Park S.M. Formation of silver nanoparticles by laser ablation of a silver target in NaCl solution. Appl. Surf. Sci. 2002;197:628–634. [Google Scholar]
- Barrero A.F., del Moral J.F.Q., Lara A., Herrador M.M. Antimicrobial activity of sesquiterpenes from the essential oil of Juniperus thurifera wood. Planta Med. 2005;71:67–71. doi: 10.1055/s-2005-837753. [DOI] [PubMed] [Google Scholar]
- Bindhu M., Umadevi M. Antibacterial and catalytic activities of green synthesized silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;135:373–378. doi: 10.1016/j.saa.2014.07.045. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. A two-phase system for removal of red cells with methylcellulose as erythrocyte-aggregating agent. Scand. J. Clin. Lab. Invest. 1968;97(Supplementum):9. [PubMed] [Google Scholar]
- Burits M., Asres K., Bucar F. The antioxidant activity of the essential oils of Artemisia afra, Artemisia abyssinica and Juniperus procera. Phytother. Res. 2001;15:103–108. doi: 10.1002/ptr.691. [DOI] [PubMed] [Google Scholar]
- Daud A., Gallo A., Riera A.S. Antimicrobial properties of Phrygilanthus acutifolius. J. Ethnopharmacol. 2005;99:193–197. doi: 10.1016/j.jep.2005.01.043. [DOI] [PubMed] [Google Scholar]
- El-Sawi S.A., Motawae H.M., Ali A.M. Chemical composition, cytotoxic activity and antimicrobial activity of essential oils of leaves and berries of Juniperus phoenicea L. grown in Egypt. Afr. J. Tradit. Complement. Altern. Med. 2007;4:417–426. doi: 10.4314/ajtcam.v4i4.31236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghramh H.A., Al-Ghamdi K.M., Mahyoub J.A., Ibrahim E.H. Chrysanthemum extract and extract prepared silver nanoparticles as biocides to control Aedes aegypti (L.), the vector of dengue fever. J. Asia. Pac. Entomol. 2018;21 doi: 10.1016/j.aspen.2017.12.001. [DOI] [Google Scholar]
- Gherbawy Y., Elhariry H. Endophytic fungi associated with high-altitude Juniperus trees and their antimicrobial activities. Plant Biosyst.-Int. J. Deal. Aspects Plant Biol. 2016;150:131–140. [Google Scholar]
- Gordon O., Slenters T.V., Brunetto P.S., Villaruz A.E., Sturdevant D.E., Otto M., Landmann R., Fromm K.M. Silver coordination polymers for prevention of implant infection: thiol interaction, impact on respiratory chain enzymes, and hydroxyl radical induction. Antimicrob. Agents Chemother. 2010;54:4208–4218. doi: 10.1128/AAC.01830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L., Xiong X., Wei D., Gao X., Krams S., Zhao H. T cells contribute to stroke-induced lymphopenia in rats. PLoS ONE. 2013;8:e59602. doi: 10.1371/journal.pone.0059602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Du Z., Lv H., Jia Q., Tang Z., Zheng X., Zhang K., Zhao F. Green synthesis of silver nanoparticles by Chrysanthemum morifolium Ramat. extract and their application in clinical ultrasound gel. Int. J. Nanomed. 2013;8:1809. doi: 10.2147/IJN.S43289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C.-C., Fang H.-L., Lina W.-C. Inhibitory effect of Solanum nigrum on thioacetamide-induced liver fibrosis in mice. J. Ethnopharmacol. 2008;119:117–121. doi: 10.1016/j.jep.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Jung E.-K. Chemical composition and antimicrobial activity of the essential oil of Chrysanthemum indicum against oral bacteria. J. Bacteriol. Virol. 2009;39:61–69. [Google Scholar]
- Klauss, V., Adala, H., 1994. Traditional Herbal Eye Medicine in Kenya. [PubMed]
- Lai J.-P., Lim Y.H., Su J., Shen H.-M., Ong C.N. Identification and characterization of major flavonoids and caffeoylquinic acids in three Compositae plants by LC/DAD-APCI/MS. J. Chromatogr. B. 2007;848:215–225. doi: 10.1016/j.jchromb.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Li H., Mahyoub S.A.A., Liao W., Xia S., Zhao H., Guo M., Ma P. Effect of pyrolysis temperature on characteristics and aromatic contaminants adsorption behavior of magnetic biochar derived from pyrolysis oil distillation residue. Bioresour. Technol. 2017;223:20–26. doi: 10.1016/j.biortech.2016.10.033. [DOI] [PubMed] [Google Scholar]
- Liu Y.-C., Lin L.-H. New pathway for the synthesis of ultrafine silver nanoparticles from bulk silver substrates in aqueous solutions by sonoelectrochemical methods. Electrochem. Commun. 2004;6:1163–1168. [Google Scholar]
- Liu Z., Woo S.I., Lee W.S. In situ FT-IR studies on the mechanism of selective catalytic reduction of NOx by propene over SnO2/Al2O3 catalyst. J. Phys. Chem. B. 2006;110:26019–26023. doi: 10.1021/jp0637274. [DOI] [PubMed] [Google Scholar]
- Loizzo M.R., Tundis R., Conforti F., Saab A.M., Statti G.A., Menichini F. Comparative chemical composition, antioxidant and hypoglycaemic activities of Juniperus oxycedrus ssp. oxycedrus L. berry and wood oils from Lebanon. Food Chem. 2007;105:572–578. [Google Scholar]
- Majeed A., Ullah W., Anwar A.W., Shuaib A., Ilyas U., Khalid P., Mustafa G., Junaid M., Faheem B., Ali S. Cost-effective biosynthesis of silver nanoparticles using different organs of plants and their antimicrobial applications: a review. Mater. Technol. 2016:1–8. [Google Scholar]
- Mallick K., Witcomb M.J., Scurrell M.S. Self-assembly of silver nanoparticles in a polymer solvent: formation of a nanochain through nanoscale soldering. Mater. Chem. Phys. 2005;90:221–224. [Google Scholar]
- Manivasagan P., Venkatesan J., Senthilkumar K., Sivakumar K., Kim S.-K. Biosynthesis, antimicrobial and cytotoxic effect of silver nanoparticles using a novel Nocardiopsis sp. MBRC-1. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/287638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein M., Ghasemi Y., Moein S., Nejati M. Analysis of antimicrobial, antifungal and antioxidant activities of Juniperus excelsa M. B subsp. Polycarpos (K. Koch) Takhtajan essential oil. Pharmacogn. Res. 2010;2:128. doi: 10.4103/0974-8490.65505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanta Y.K., Panda S.K., Jayabalan R., Sharma N., Bastia A.K., Mohanta T.K. Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa (Roxb.) Front. Mol. Biosci. 2017;4:14. doi: 10.3389/fmolb.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiteiro C., Justino F., Tavares R., Marcelo-Curto M., Florêncio M.H., Nascimento M.S.J., Pedro M., Cerqueira F., Pinto M.M. Synthetic secofriedelane and friedelane derivatives as inhibitors of human lymphocyte proliferation and growth of human cancer cell lines in vitro. J. Nat. Prod. 2001;64:1273–1277. doi: 10.1021/np010217m. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Muhammad I., Mossa J.S., Al-Yahya M.A., Ramadan A.F., El-Feraly F.S. Further antibacterial diterpenes from the bark and leaves of Juniperus procera Hochst. ex Endl. Phytother. Res. 1995;9:584–588. [Google Scholar]
- Muhammad I., Mossa J.S., El-Feraly F.S. Additional antibacterial diterpenes from the bark of Juniperus procera. Phytother. Res. 1996;10:604–607. [Google Scholar]
- Nayak D., Minz A.P., Ashe S., Rauta P.R., Kumari M., Chopra P., Nayak B. Synergistic combination of antioxidants, silver nanoparticles and chitosan in a nanoparticle based formulation: characterization and cytotoxic effect on MCF-7 breast cancer cell lines. J. Colloid Interface Sci. 2016;470:142–152. doi: 10.1016/j.jcis.2016.02.043. [DOI] [PubMed] [Google Scholar]
- Ndikau M., Noah N.M., Andala D.M., Masika E. Green synthesis and characterization of silver nanoparticles using Citrullus lanatus fruit rind extract. Int. J. Anal. Chem. 2017;2017 doi: 10.1155/2017/8108504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez Y.O., Salabarria I.S., Collado I.G., Hernández-Galán R. Sesquiterpenes from the wood of Juniperus lucayana. Phytochemistry. 2007;68:2409–2414. doi: 10.1016/j.phytochem.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Oves M., Khan M.S., Zaidi A., Ahmed A.S., Ahmed F., Ahmad E., Sherwani A., Owais M., Azam A. Antibacterial and cytotoxic efficacy of extracellular silver nanoparticles biofabricated from chromium reducing novel OS4 strain of Stenotrophomonas maltophilia. PLoS ONE. 2013;8:e59140. doi: 10.1371/journal.pone.0059140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öztürk M., Tümen İ., Uǧur A., Aydoǧmuş-Öztürk F., Topçu G. Evaluation of fruit extracts of six Turkish Juniperus species for their antioxidant, anticholinesterase and antimicrobial activities. J. Sci. Food Agric. 2011;91:867–876. doi: 10.1002/jsfa.4258. [DOI] [PubMed] [Google Scholar]
- Pankaj K., Bhatt R., Sati O., Dhatwalia V., Lokendra S. In-vitro antifungal activity of different fraction of Juniperus communis leaves and bark against Aspergillus niger and Aflatoxigenic Aspergillus flavus. Int. J. Pharma Bio Sci. 2010;1:1–17. [Google Scholar]
- Parikh R.Y., Singh S., Prasad B., Patole M.S., Sastry M., Shouche Y.S. Extracellular synthesis of crystalline silver nanoparticles and molecular evidence of silver resistance from Morganella sp.: towards understanding biochemical synthesis mechanism. ChemBioChem. 2008;9:1415–1422. doi: 10.1002/cbic.200700592. [DOI] [PubMed] [Google Scholar]
- Pileni M. Fabrication and physical properties of self-organized silver nanocrystals. Pure Appl. Chem. 2000;72:53–65. [Google Scholar]
- Prathna T., Chandrasekaran N., Raichur A.M., Mukherjee A. Biomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle size. Colloids Surf., B. 2011;82:152–159. doi: 10.1016/j.colsurfb.2010.08.036. [DOI] [PubMed] [Google Scholar]
- Raja S., Ramesh V., Thivaharan V. Green biosynthesis of silver nanoparticles using Calliandra haematocephala leaf extract, their antibacterial activity and hydrogen peroxide sensing capability. Arabian J. Chem. 2017;10:253–261. [Google Scholar]
- Ranganathan R., Madanmohan S., Kesavan A., Baskar G., Krishnamoorthy Y.R., Santosham R., Ponraju D., Rayala S.K., Venkatraman G. Nanomedicine: towards development of patient-friendly drug-delivery systems for oncological applications. Int. J. Nanomed. 2012;7:1043. doi: 10.2147/IJN.S25182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha K., Goresco R., Beltrame R., Vaz A., Kioko T., Bueno E. In: Imunoensaios: fundamentos e aplicações. Vaz A.J., Kioko T., Bueno E.C., editors. Guanabara Koogan; Rio de Janeiro: 2007. Metodologia laboratorial para estudo da resposta imune celular; pp. 119–131. [Google Scholar]
- Samoylenko V., Dunbar D.C., Gafur M., Khan S.I., Ross S.A., Mossa J.S., El-Feraly F.S., Tekwani B.L., Bosselaers J., Muhammad I. Antiparasitic, nematicidal and antifouling constituents from Juniperus berries. Phytother. Res. 2008;22:1570–1576. doi: 10.1002/ptr.2460. [DOI] [PubMed] [Google Scholar]
- Sandmann G., Dietz H., Plieth W. Preparation of silver nanoparticles on ITO surfaces by a double-pulse method. J. Electroanal. Chem. 2000;491:78–86. [Google Scholar]
- Šarić-Kundalić B., Dobeš C., Klatte-Asselmeyer V., Saukel J. Ethnobotanical survey of traditionally used plants in human therapy of east, north and north-east Bosnia and Herzegovina. J. Ethnopharmacol. 2011;133:1051–1076. doi: 10.1016/j.jep.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Satyavani K., Ramanathan T., Gurudeeban S. Plant mediated synthesis of biomedical silver nanoparticles by using leaf extract of Citrullus colocynthis. Res. J. Nanosci. Nanotechnol. 2011;1:95–101. [Google Scholar]
- Sheng Y., Bryngelsson C., Pero R.W. Enhanced DNA repair, immune function and reduced toxicity of C-MED-100™, a novel aqueous extract from Uncaria tomentosa. J. Ethnopharmacol. 2000;69:115–126. doi: 10.1016/s0378-8741(99)00070-7. [DOI] [PubMed] [Google Scholar]
- Smetana A.B., Klabunde K.J., Sorensen C.M. Synthesis of spherical silver nanoparticles by digestive ripening, stabilization with various agents, and their 3-D and 2-D superlattice formation. J. Colloid Interface Sci. 2005;284:521–526. doi: 10.1016/j.jcis.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Su H.-L., Chou C.-C., Hung D.-J., Lin S.-H., Pao I.-C., Lin J.-H., Huang F.-L., Dong R.-X., Lin J.-J. The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials. 2009;30:5979–5987. doi: 10.1016/j.biomaterials.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Topçu G., Erenler R., Çakmak O., Johansson C.B., Çelik C., Chai H.-B., Pezzuto J.M. Diterpenes from the berries of Juniperus excelsa. Phytochemistry. 1999;50:1195–1199. doi: 10.1016/s0031-9422(98)00675-x. [DOI] [PubMed] [Google Scholar]
- Tumen I., Eller F.J., Clausen C.A., Teel J.A. Antifungal activity of heartwood extracts from three Juniperus species. BioResources. 2012;8:12–20. [Google Scholar]
- Vandhana, S., Deepa, P., Aparna, G., Jayanthi, U., Krishnakumar, S., 2010. Evaluation of suitable solvents for testing the anti-proliferative activity of triclosan-a hydrophobic drug in cell culture. [PubMed]
- Wiley B., Sun Y., Mayers B., Xia Y. Shape-controlled synthesis of metal nanostructures: the case of silver. Chem.-Eur. J. 2005;11:454–463. doi: 10.1002/chem.200400927. [DOI] [PubMed] [Google Scholar]
- Yashi S., Yoshiie K., Oda H., Sugano M., Imaizumi K. Dietary Curcuma xanthorrhiza Roxb. increases mitogenic responses of splenic lymphocytes in rats, and alters populations of the lymphocytes in mice. J. Nutr. Sci. Vitaminol. 1993;39:345–354. doi: 10.3177/jnsv.39.345. [DOI] [PubMed] [Google Scholar]
- Yogalakshmi B., Viswanathan P., Anuradha C.V. Investigation of antioxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology. 2010;268:204–212. doi: 10.1016/j.tox.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Yuan L.-P., Chen F.-H., Ling L., Dou P.-F., Bo H., Zhong M.-M., Xia L.-J. Protective effects of total flavonoids of Bidens pilosa L. (TFB) on animal liver injury and liver fibrosis. J. Ethnopharmacol. 2008;116:539–546. doi: 10.1016/j.jep.2008.01.010. [DOI] [PubMed] [Google Scholar]