Abstract
Vitamin D deficiency is a pandemic problem. Non-animal source of vitamin D is obtained from edible mushrooms. Oyster mushroom (Pleurotus ostreatus) was sliced into the size of 1 cm3, 4 cm3 and 9 cm3, and treated with the sun as a natural resource of UVB under subtropical settings in Ethiopia. The content of vitamin D was measured by using high-performance liquid chromatography (HPLC). After sun treatment, there was a significant increment in the content of vitamin D2 from nil to 67.4 ± 28.0 µg/g dry weight (DW). Based on the results of the overall pairwise comparisons, 1 cm3 size of slice group had the highest content of vitamin D2. Duration of sun exposure, sizes of mushroom slices and moisture content were identified as determining factors for vitamin D2 synthesis. Exposing slices of oyster mushroom to the sunlight for <30 min provides the amount that satisfies the current recommended dietary allowance (RDA) of vitamin D without any visible change in color and texture. Thus, sun treatment of oyster mushroom is an effective and economically cheap strategy in the fight against vitamin D deficiency.
Keywords: Sun-treatment, Vitamin D2, Oyster mushroom, Subtropics
Abbreviations: AI, adequate intake; DRI, dietary reference intake; IU, international unit; RDA, recommended dietary allowance; UVB, ultra violet ray B
1. Introduction
Vitamin D deficiency is a pandemic and an ever-increasing problem in human nutrition and health. It is estimated that 30–50% of individuals in Europe and the U.S. have insufficient levels of vitamin D (Battault et al., 2013, Holick, 2009, Mansoor et al., 2010). In Africa, more than 80% of the people are prone to vitamin D deficiency (Keflie et al., 2015).
Oyster and other edible mushrooms have been recognized as a source for vitamin D since 1994 (Mattila et al., 1994). Mushrooms are the only non-animal food source that contains vitamin D (Valverde et al., 2015). Ergosterol is the principal sterol and precursor for vitamin D2 in mushrooms. It is converted via absorption of ultra violet B (UVB) light energy (290–320 nm). The absorption of light causes the isomerization of the molecule and bond cleavage between carbons 9 and 10, resulting in unstable intermediate “pre-vitamin D2”. The conversion of pre-vitamin D2 to vitamin D2 then follows via a thermally catalysed process (Chen et al., 2010, Simon et al., 2011, Keegan et al., 2013).
The formation of ergocalciferol starts without delay after exposure to UVB light. As UVB light is not able to penetrate deeply into the fruiting body, the reaction rate must cease when the superficial ergosterol is used up. The yields of ergocalciferol are thus limited, but at the same time the risk of accumulating extreme doses is low (Krings and Berger, 2014). Prolonged exposure in the sun-exposed samples can significantly produce irreversible, over-exposed products by dimerization and ring cleavage of pre-vitamin D2 and tachysterol (Keegan et al., 2013, Urbain et al., 2016).
Besides of the wavelength of UV irradiation, the efficiency of vitamin D2 synthesis in mushrooms can be influenced by multiple parameters such as ambient temperature, exposure time and irradiation intensity. It is not known whether the effects of these factors are independent or interactive (Wu and Ahn, 2014).
So far, very few studies conducted until recent time focused on the natural resource of UVB irradiation for the synthesis of vitamin D2 (Simon et al., 2011, Urbain and Jakobsen, 2015, Urbain et al., 2016, Nölle et al., 2017). Of these studies, none of them addressed the potential effects of the sun on vitamin D2 production under tropical and subtropical settings. There is also a scarce information on the content of vitamin D2 in oyster mushroom. Nowadays, cultivated oyster mushroom is available in the markets of some areas in Ethiopia. Therefore, this study was designed to assess the impact of the natural resource of UVB irradiation on the content of vitamin D2 in oyster mushroom under subtropical settings.
2. Material and methods
2.1. Chemicals
The internal standard (vitamin D3) and external standard (vitamin D2) were purchased from ENZO Life Sciences (Germany), whereas potassium hydroxide was purchased from Merck and ascorbic acid from AppliChem GmbH (Germany).
2.2. Experimental design
The experiment was undertaken on oyster mushroom. The samples of oyster mushrooms were categorized into sun treated and non-treated groups. Non-treated group was used as a control group of the experiment. The third group was added for further comparisons and irradiated with artificial UVB irradiation.
2.3. Mushrooms
Samples of oyster mushrooms for sun treatment and control groups were purchased from the local super market in Addis Abeba, Ethiopia on September 24, 2016. Those samples under the group of sun treatment were immediately processed and were either left whole or sliced manually using a knife and ruler into 1 cm3 (1 cm × 1 cm × 1 cm), 4 cm3 (2 cm × 2 cm × 1 cm) and 9 cm3 (3 cm × 3 cm × 1 cm) size of slice groups. The control group was placed into black plastic bag, knotted tightly, labelled and stored at minus 80 °C. The mean ± standard deviation (SD) of single mushroom stock was 32.7 ± 4.1 g with cap diameter of 8.2 ± 0.9 cm and stem length of 10.4 ± 0.5 cm (n = 12).
2.4. Sun treatment
Sun treatment was conducted between 8:00 am and 4:00 pm on 24 and 25 September 2016 at Aleltu town. This town is situated at 9° 11′ 47 N, 39° 9′ 12 E, and 2350 m above sea level in the north-eastern part of Addis Abeba, the capital city of Ethiopia. The minimum and maximum temperatures during the process of sun treatment were 21 °C and 34 °C, respectively. On both days, the sky was cloudless. Samples were divided into the above-mentioned size groups. Each of the samples was positioned horizontally and exposed to the sun light for the durations of either 30 min, 1 h, 3 h, 8 h or 16 h. In every 15 min for the first 1 h and in every 30 min later, the samples were turned around to expose all parts equally to the sun light. Changes in color and texture were checked every time during the process of turning. Like control group, the samples were placed into black plastic bags, knotted tightly, labelled and stored at minus 80 °C immediately after their treatment period was over.
2.5. Samples transportation
The samples were packed into an insulated plastic bags together with dry ice and transported to University of Hohenheim, Stuttgart, Germany where further laboratory analyses were performed. Immediately after arrival, the samples were stored at minus 80 °C.
2.6. UVB irradiation
Samples for artificial UVB irradiation were purchased from a local supermarket in Stuttgart, Germany. Like the groups for sun treatment, the samples were divided into four groups: 1 cm3, 4 cm3, 9 cm3 size slices and whole size. All the samples including a sample of lyophilized powder were horizontally placed on the shelf of irradiation chamber equipped with UVB-lamps (Dr. Groebel, GmbH, Ettlingen, Germany). Each side of the samples was irradiated up to a UVB dose of 1.5 J/cm2 at 22 °C.
2.7. Lyophilisation, pulverization and moisture content determination
All treated and non-treated samples were lyophilized with a Telstar LyoQuest freeze drier (Azbil Telstar Technologies SLU, LyoQuest-85 year 2014, Spain) and then, pulverized and homogenised into fine powder using a grinding mill (TH. Geyer, Germany) at Institute of Biological Chemistry and Nutrition, University of Hohenheim. The powders were kept in an airtight plastic bag and stored at minus 20 °C until used for laboratory analyses. The moisture content was determined using oven drying method at the Institute of Agricultural Engineering, University of Hohenheim.
2.8. Vitamin D extraction
Triplicate samples were taken from each group for the measurement of vitamin D2. The extraction of vitamin D2 was carried out based on the method of Nölle et al. (2017) with some modifications. Samples of pulverized and homogenized powder (1 g) were mixed with 19 mL of ethanol (with a chemical purity of 99.7%), 4 mL of 50% potassium hydroxide (500 g KOH in 1 L of H2O), 1.333 mL of sodium ascorbate (1.75 g solved in 10 mL of 1 M sodium hydroxide) and 1 mL of vitamin D3 (100 mg/L) as internal standard in a 50 mL falcon tube. The mixture was vortex mixed and subsequently saponified for 1 h in a water bath at 80 °C. The mixture was then cooled to ambient temperature in ice water. To promote a better separation of the layers, 10 mL of saturated sodium chloride solution was added. Subsequently, 15 mL of n-hexane was added, vortex mixed and centrifuged for 8 min at 4500g (Heraeus, Hanau, Germany). The n-hexane layer was transferred into a new falcon tube and the extraction processes were repeated twice, one time with 15 mL of n-hexane and later with 10 mL of n-hexane. The pooled organic layers were washed three times with deionised water until neutralized. The organic layer was then transferred into a 100 mL round bottom flask and rotary evaporated to dryness. The flask was rinsed with 6 mL of n-hexane and transferred into 10 mL of round bottom flask and, rotary evaporated again. Once evaporated to dryness, the sample was immediately re-dissolved in 1 mL of tetrahydrofuran and vortex mixed. Thereafter, the samples were centrifuged for 5 min at 1600g at 20 °C to remove impurities and then used for subsequent analyses by HPLC.
2.9. HPLC analysis
A system of HPLC (Shimadzu technologies) equipped with a DGU-20A3R degassing Unit, two LC-20AT pumps, a SIL-20ACHT auto sampler and a CBM-20A communication bus module (Shimadzu GmbH, Duisburg, Germany) was used to measure vitamin D2 content at the Institute of Biological Chemistry and Nutrition. The column used was a Reprosil 80 ODS-2 analytical column, 4.6 × 250 mm, 3 µm particle size (Dr. Maisch GmbH, Ammerbuch, Germany). The mobile phase was composed of acetonitrile (77%), deionized water (14%) and tetrahydrofuran (9%) at a flow rate of 2 mL/min with a total run time of 42 min. The injection volume was 10 µl and detection was carried out by diode array detector at a wavelength of 265 nm. A set of six calibration standards of vitamin D2 and D3 were prepared with the contents of 10, 20, 100, 200, 300 and 400 µg/mL, respectively. The Lab Solution software was used for HPLC control as well as acquisition and quantification of data.
2.10. Statistical analyses
Statistical analyses were performed using IBM SPSS statistics version 23. The normality of the data distribution was checked by Shapiro-Wilk’s normality test. Graphs were generated with Microsoft Excel. The content of each analyte was described as mean ± standard deviation (SD). Pre-and post-treatment comparisons were analysed by paired two sample t-test for means. The effects of two independent variables on the continuous dependent variable were examined using two-way of analysis of variance (two-way ANOVA) and the estimated marginal means were compared with least significant difference (LSD) post hoc test. A p < 0.05 was considered as statistically significant.
3. Results and discussion
3.1. Moisture content, color and texture changes
In the present study, the overall moisture content of oyster mushroom was 92.5%. During the process of sun treatment, changes in colour, texture and moisture content were observed on the samples. Fig. 1 demonstrates the differences before and after sun treatment. The description in Table 1 shows the percentage of the loss of moisture and the extent to which the color and texture changed. In 1 cm3 size group, more than 67% of moisture content was lost within 3 h of sun exposure, followed by brown coloration and shrivelled texture. Within the same duration of sun exposure, the whole size group lost about 35% of moisture content with minor change in color and texture. In 9 cm3 size group, about 12% of the moisture content was lost within 30 min of sun exposure without any observable change in color and texture. With equal durations of sun exposure, about 19% of the moisture content was lost in 1 cm3 size group. This shows that increasing the surface area to volume ratio for sun exposure increases the loss of moisture with rapid change in color and texture.
Fig. 1.
Oyster mushroom before (A) and after (B) sun treatment.
Table 1.
Percentage of moisture loss and changes in color and texture of sun treated oyster mushroom.
| Size | Duration of sun exposure (hour) | Loss of moisture (%) | Minimum Temp. (°C) | Maximum Temp. (°C) | Color change | Texture change |
|---|---|---|---|---|---|---|
| 1 cm3 | 0.5 | 19.3 | 21 | 23 | Not observable | None |
| 1.0 | 31.4 | 21 | 27 | Very light brown | Very slight shrinking | |
| 3.0 | 67.0 | 21 | 32 | Brown | Shrank | |
| 8.0 | 91.0 | 21 | 34 | Dark brown | Totally shrank | |
| 16.0 | 90.2 | 21 | 34 | Dark brown | Totally shrank | |
| 4 cm3 | 0.5 | 11.4 | 21 | 23 | Not observable | None |
| 1.0 | 27.4 | 21 | 27 | Very light brown | Very slight shrinking | |
| 3.0 | 59.7 | 21 | 32 | Light brown | Shrank | |
| 8.0 | 89.7 | 21 | 34 | Dark brown | Totally shrank | |
| 16.0 | 90.9 | 21 | 34 | Dark brown | Totally shrank | |
| 9 cm3 | 0.5 | 12.0 | 21 | 23 | Not observable | None |
| 1.0 | 23.4 | 21 | 27 | Not observable | Not observable | |
| 3.0 | 62.2 | 21 | 32 | Brown | Shrank | |
| 8.0 | 90.2 | 21 | 34 | Dark brown | Totally shrank | |
| 16.0 | 90.7 | 21 | 34 | Dark brown | Totally shrank | |
| Whole | 0.5 | 0.5 | 21 | 23 | Not observable | None |
| 1.0 | 7.6 | 21 | 27 | Not observable | None | |
| 3.0 | 35.0 | 21 | 32 | Very light brown | Very slight shrinking | |
| 8.0 | 74.6 | 21 | 34 | Brown | Shrank | |
| 16.0 | 90.8 | 21 | 34 | Dark brown | Totally shrank | |
The loss of moisture and shrivelled texture were due to evaporation of the moisture, whereas the color change was as the results of enzymatic reaction in the tissue of the mushroom. Kalac (2013) described that the color changes are usually caused by the presence of tyrosinase enzyme. This enzyme catalyses tyrosin to be oxidized to o-dihydroxyphenylalanine (DOPA), then to brown quinonic pigments and eventually to melanins (Kalac, 2013). The reported tissue discoloration could affect the aesthetic value of mushrooms for consumption.
3.2. Vitamin D2 as the result of sun exposure
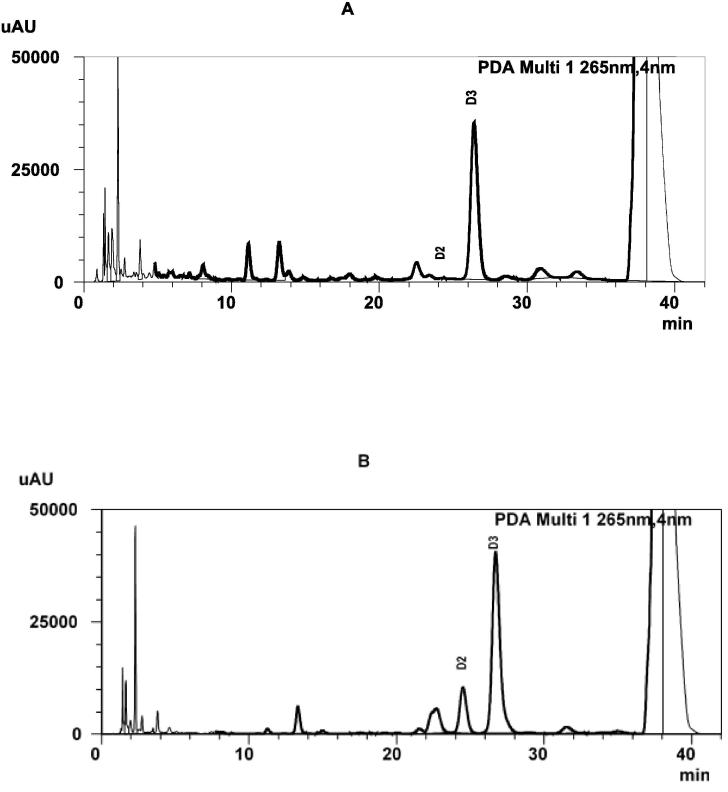
The chromatograms in Fig. 2 shows the content of vitamin D2 in non-treated and sun treated oyster mushrooms. The content of vitamin D2 increased from nil to considerable levels with different slice sizes and durations of sun exposure. The maximum content of vitamin D2 was produced in 1 cm3 size of slice group (67.4 ± 28.0 µg/g dw). Shapiro-Wilk’s test with Liliefors significance correction (p > 0.05) indicated that the contents of vitamin D2 were normally distributed in 1 cm3, 4 cm3 and 9 cm3 size of slice groups. For all vitamin D2 measurements, the coefficient of variation was <13.5%.
Fig. 2.
HPLC chromatograms of vitamin D2 analysis in non-treated oyster mushroom (A) and sun treated oyster mushroom (B). Vitamin D3 was used as internal standard.
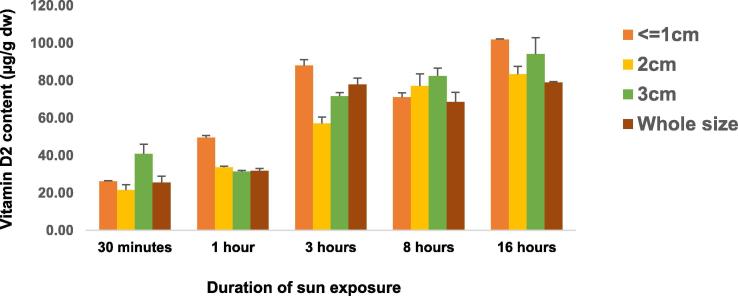
For further comparisons, the mean content of vitamin D2 in 1 cm3 size of slice group was used. The mean of vitamin D2 in 1 cm3 size in the present study was higher than the reports of Jasinghe and Perera (2005) on oyster mushrooms (45.1 ± 3.1 µg/g dw); Nölle et al. (2017) on brown button mushrooms (36 µg/g dw); and Simon et al. (2011) on white button mushrooms (3.7 ± 0.9 µg/g dw). These variations may arise from the type of mushrooms used for experiments, the differences in the duration and intensity of sun exposure, and the place of cultivation. Fig. 3 depicts the content of vitamin D2 across the durations of sun exposure and slice sizes of oyster mushrooms. The figure shows that the contents of vitamin D2 were increased with the durations of sun exposure.
Fig. 3.
Contents of vitamin D2 across different sizes groups and duration of sun exposure.
The mean ± SD of vitamin D2 contents produced in 30 min, 1 h and 3 h of sun exposure were 28.5 ± 8.5, 36.6 ± 8.7 and 73.7 ± 12.9 µg/g dw, respectively. In 1 h of sun exposure, Urbain and Jakobsen (2015) obtained 3.9 µg/g dw of vitamin D2 from white button mushroom in Germany. But, Simon et al. (2011) obtained 3.8 µg/g dw of vitamin D2 from white button mushroom exposed to the sun for 2.5 h in United States of America. These two results were by far lower than the present findings. Possible explanations for the discrepancies in vitamin D2 contents are the differences in the latitude and altitude of the locations, cultivars/types, sizes of the mushroom preparations, moisture contents and temperature as well as duration and intensity of sun exposure. Latitude and season affect both the quantity and quality of solar radiation reaching the surface of the earth which in turn have an influence on the synthesis of vitamin D (Webb et al., 1988).
During the first 3 h of sun exposure, vitamin D2 contents were rapidly increased in all size groups and the maximum content was found in 1 cm3 size of slice group with mean ± SD of 88.1 ± 3.1 µg/g dw. Some fluctuations in the distribution of vitamin D2 were observed along with the durations of sun exposure. From 3 h to 8 h of sun exposures, the content of vitamin D2 in 1 cm3 size of slice group was decreased but from 8 h to 16 h of sun exposure its content was increased again. Per the results of the overall pairwise comparisons, 1 cm3 size of slice group had significantly higher content of vitamin D2 than 4 cm3, 9 cm3 and whole size groups in 1 h, 3 h and 16 h of sun exposures (p < 0.05).
There were statistically significant differences in vitamin D2 contents among different size groups (p < 0.05). The contributions of durations of sun exposure, size of slice groups and the interaction of the two were also statistically significant on the content of vitamin D2 in oyster mushroom (p < 0.05). The overall distributions of vitamin D2 indicated that the smaller the mushroom sliced, the higher the content of vitamin D2. These results were corroborated with the findings of Urbain and Jakobsen (2015), and Urbain et al. (2016). Nölle et al. (2017) also observed the highest conversion of ergosterol to vitamin D2 in sliced fruit bodies in 2016.
The effects of durations of sun exposure on the content of vitamin D2 were also statistically analysed. More than 93% of the variations in vitamin D2 contents amongst 1 cm3, 4 cm3, 9 cm3 and whole size groups were accounted for by the duration of sun exposure. As compared to durations of sun exposure, the size of slices in the group had smaller effects on the content of vitamin D2. After 8 h of sun exposure, there was no statistically significant difference in the contents of vitamin D2 across all size groups (p > 0.05). However, in 16 h of sun exposure, the difference in the contents of vitamin D2 became significant. This showed the variabilities in the contents of vitamin D2 in extended periods of sun exposure. One of the factors that account for such variabilities is the intensity of UVA irradiation. The longer wavelength UVA present in sunlight could potentially photodegrade vitamin D (Chen et al., 2010).
Vitamin D2 may also be subjected to the action of tissue mono-oxygenases that reduce the overall conversion of ergosterol to vitamin D2 (Jasinghe and Perera, 2005). However, in the presence of ergosterol, the conversion processes might eventually resume and elevate the concentration of vitamin D2 as the case of 16 h of sun exposure in our findings. The efficacy of ergosterol to vitamin D2 conversion was positively dependent on the irradiation intensity. The content of vitamin D2 dramatically increases when the irradiation intensity increases (Wu and Ahn, 2014).
3.3. Vitamin D2 as the result of UVB irradiation
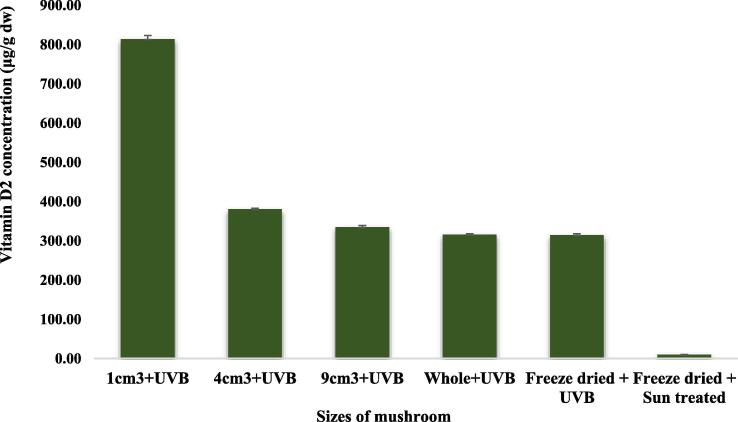
An experiment on artificial UVB irradiation was conducted for the sake of comparisons. The bar graphs clearly depicted the differences of the contents of vitamin D2 across the size of slice groups (Fig. 4). The maximum content of vitamin D2 was 814.1 ± 21.9 µg/g dw which was measured in 1 cm3 size of slice group. This amount was more than 9 folds higher than the one produced with 3 h of sun exposure in the same size group. In agreement with this, Urbain and Jakobsen (2015) noted that the content of vitamin D2 produced with artificial UVB irradiation was more than 10 folds higher than the one produced with solar radiation during the process of drying.
Fig. 4.
Vitamin D2 concentration in oyster mushroom treated with UVB irradiation up to a dose of 1.5 J/cm2.
Wu and Ahn (2014) obtained 239 ± 4.5 µg/g dw of vitamin D2 content in oyster mushroom with operational conditions of 28.2 °C, 94.28 min and 1.14 W/m2. This value was lower than the present finding in the whole size oyster mushroom (316.0 ± 5.1 µg/g dw). Urbain et al. (2016) reported the highest vitamin D2 content after the largest UVB dose of 2.01 J/cm2 and consisted of 101.5 ± 4.3 µg/g dw. However, this finding was nearly 8 times lower than our finding in 1 cm3 size of slice group (814.1 ± 21.9 µg/g dw). This difference could be emanated from the difference in moisture content, types and sizes of mushroom slices as well as the doses of UVB irradiation.
With the same operational condition, Wu and Ahn (2014) found 498.1 µg/g dw of vitamin D2 content in lyophilized powder. However, this finding was higher than our finding in lyophilized powder (314.5 ± 8.2 µg/g dw). With equal dose of UVB irradiation, the content of vitamin D2 in lyophilized sample was 2.6 times lower than the one in 1 cm3 size of slice group. One of the reasons for such a difference could be moisture content. Lyophilized powder contained very little moisture content as compared to 1 cm3 size of slice group. This in turn ascertained the essentiality of the moisture content which is important for the dilution of ergosterol in the photochemical reaction and its conversion to vitamin D2. Perera et al. (2003) reported that the conversion of ergosterol to vitamin D2 was affected by the moisture content of the mushrooms.
Further comparison was made on lyophilized powder. One group of lyophilized powder was exposed to the sun for 20 min and compared with the one irradiated with artificial UVB. The content of vitamin D2 produced in the former was about 31 times lower than in the latter. These results clearly showed how the difference in the doses of UVB irradiation influences the production of vitamin D2 in oyster mushroom.
3.4. Contribution of sun treated oyster mushroom
In general, vitamin D2 is effectively produced in oyster mushroom under sun treatment. Vitamin D2 has similarity with vitamin D3 in physiological responses such as regulation of calcium and phosphate homeostasis and, cell proliferation and differentiation (Jones et al., 1998, Jurutka et al., 2001). Although there is a slight difference in the chemical structure at the side chains, recent reports confirmed that vitamin D2 is as effective as vitamin D3 in maintaining the contents of 25-hydroxy vitamin D in the blood (Biancuzzo et al., 2010, Urbain et al., 2011, Keegan et al., 2013). Hence, its importance in the fight against vitamin D deficiency is worth noting particularly in tropical and subtropical areas.
The analyses done based on the dietary reference intakes (DRI) revealed that sun treated oyster mushroom contained a large amount of vitamin D2. For instance, a group of 9 cm3 size slices of oyster mushroom which was treated with sun for 30 min produced vitamin D2 to the levels equivalent to 40.9 ± 5.1 µg/g dw. This was comparable to the content of vitamin D3 in cod liver oil (40.3 µg per 1 tablespoon) (Bueno and Czepielewski, 2008).
A regular 100 g serving of such mushrooms could provide vitamin D2 more than 20 times of the current recommended dietary allowance (RDA) of 15 µg (600 IU) for all ages between 1 and 70 years as indicated in Table 2 (IOM, 2010, Simon et al., 2011, European Food Safety Authority (EFSA), 2016). In other words, 5 g fresh weight of such mushrooms are sufficient to satisfy the RDA of vitamin D.
Table 2.
Contribution of 100 g edible portion of oyster mushroom based on dietary reference intakes.
| Duration of sun exposure | Size of slices | Vitamin D (AIa = 15 µg) |
|
|---|---|---|---|
| Mean (µg/100 g fresh weight) | Contribution (%) | ||
| 30 min of sun exposure | 1 cm3 | 197.8 | 1318.8 |
| 4 cm3 | 162.5 | 1083.5 | |
| 9 cm3 | 308.1 | 2054 | |
| 1 h sun exposure | 1 cm3 | 373.5 | 2490.2 |
| 4 cm3 | 253.8 | 1692 | |
| 9 cm3 | 236.6 | 1577.2 | |
| 3 h sun exposure | 1 cm3 | 664.1 | 4427.1 |
| 4 cm3 | 431.2 | 2874.7 | |
| 9 cm3 | 540.9 | 3605.8 | |
AI – Adequate Intake.
Based on European Food Safety Authority (EFSA), 2016 recommendation.
3.5. Limitations
In the present study, no measurements were taken on the UVB doses of different durations of sun exposure. In addition, data on the contents of other UV sensitive substances in the oyster mushrooms like vitamin C or β-carotenes were not included in this study. However, food composition tables show that oyster mushrooms do not provide notable amount of such substances.
4. Conclusion
In conclusion, sun-treated oyster mushroom is an excellent resource of vitamin D2 which is comparable to the content of vitamin D3 in cod liver oil. Increasing the surface areas of sun exposure maximizes the synthesis of vitamin D2 in oyster mushroom. Our findings also indicated that duration of sun exposure, sizes of mushroom slices and their moisture content at the time of sun exposure are the factors that determine the synthesis of vitamin D2. Exposing slices of oyster mushroom to the sun light for a brief period (≤30 min) provide the amount that satisfies the current RDA of vitamin D2 without any visible change in color and texture. Thus, sun treatment of oyster mushroom is an effective and economically cheap strategy in the fight against vitamin D deficiency particularly in tropical and subtropical areas where there is a year-round sun shine. In the future, further researches are warranted to precisely understand the clinical importance of vitamin D2 and the changes in the chemical compositions of oyster mushrooms as imparted by sun-treatment.
Acknowledgments
Acknowledgments
The authors would like to thank Mr. Alexander Koza for his unreserved technical assistance since the commencement of the project and Sr. Seblewengel Seyoum for her contribution in samples preparation. Sarah Fleischmann, Ute Kayser and Olga Gotra were acknowledged for their support in dry oven method for moisture analysis. The principal author obtained a scholarship from Food Security Centre of University of Hohenheim which is funded by the Federal Ministry of Economic Cooperation and Development (BMZ) of Germany through the German Academic Exchange Service (DAAD).
Author contributions
T.S.K and H.K.B, study concept; T.S.K., experimental design, samples analyses; data analyses and interpretations, and drafted the manuscript; N.N., C.L., D.N., and H.K.B., critical review and approval of the final version of the manuscript.
Conflict of interest
The authors declare no competing financial interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Tibebeselassie Seyoum Keflie, Email: Tibebeselassie_Keflie@uni-hohenheim.de.
Nils Nölle, Email: noelle@uni-hohenheim.de.
Christine Lambert, Email: christine.lambert@uni-hohenheim.de.
Donatus Nohr, Email: donatus.nohr@uni-hohenheim.de.
Hans Konrad Biesalski, Email: biesal@uni-hohenheim.de.
References
- Battault S., Whiting S.J., Peltier S.L., Sadrin S., Gerber G., Maixent J.M. Vitamin D metabolism, functions and needs: from science to health claims. Eur. J. Nutr. 2013;52:429–441. doi: 10.1007/s00394-012-0430-5. [DOI] [PubMed] [Google Scholar]
- Biancuzzo R.M., Young A., Bibuld D., Cai M.H., Winter M.R., Klein E.K. Fortification of orange juice with vitamin D2 or vitamin D3 is as effective as an oral supplement in maintaining vitamin D status in adults. Am. J. Clin. Nutr. 2010;91:1621–1626. doi: 10.3945/ajcn.2009.27972. PMID: 20427729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno A.L., Czepielewski M.A. The importance for growth of dietary intake of calcium and vitamin D: review article. J. Paediatr. (Rio J.) 2008;84(5):386–394. doi: 10.2223/JPED.1816. [DOI] [PubMed] [Google Scholar]
- Chen T.C., Lu Z., Holick M.F. Photobiology of vitamin D. In: Holick M.F., editor. Vitamin D Physiology, Molecular Biology, and Clinical Applications from Nutrition and Health. Springer Science and Business Media; 2010. [Google Scholar]
- European Food Safety Authority (EFSA) European Food Safety Authority Nutrition and Allergies Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on dietary reference values for vitamin D. EFSA J. 2016;14(10):4547. [Google Scholar]
- Holick M.F. Vitamin D status: measurement, interpretation, and clinical application. Ann. Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (IOM) Dietary reference intakes for calcium and vitamin D. In: Ross A.C., Taylor C.L., Yaktine A.L., Del Valle H.B., editors. The National Academy of Science (NAS) Food and Nutrition Board; Washington, DC, USA: 2010. [Google Scholar]
- Jasinghe V.J., Perera C.O. Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV irradiation. Food. Chem. 2005;92:541–546. [Google Scholar]
- Jones G., Strugnell S.A., DeLuca H.F. Current understanding of the molecular actions of vitamin D. Physiol. Rev. 1998;78(4):1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- Jurutka P.W., Whitfield G.K., Hsieh J.C., Thompson P.D., Haussler C.A., Haussler M.R. Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev. Endocrinol. Metal. Dis. 2001;2(2):203–216. doi: 10.1023/a:1010062929140. [DOI] [PubMed] [Google Scholar]
- Kalac P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013;93:209–218. doi: 10.1002/jsfa.5960. [DOI] [PubMed] [Google Scholar]
- Keegan R.J.H., Lu Z., Bogusz J.M., Williams J.E., Holick M.F. Photobiology of vitamin D in mushrooms and its bioavailability in humans. Dermatoendocrinology. 2013;5(1):165–176. doi: 10.4161/derm.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keflie T.S., Nölle N., Lambert C., Nohr D., Biesalski H.K. Vitamin D deficiencies among tuberculosis patients in Africa: a systematic review. Nutrition. 2015;31:1204–1212. doi: 10.1016/j.nut.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Krings U., Berger R.G. Dynamics of sterols and fatty acids during UV-B treatment of oyster mushroom. Food. Chem. 2014;149(8):10–14. doi: 10.1016/j.foodchem.2013.10.064. [DOI] [PubMed] [Google Scholar]
- Mansoor S., Habib A., Ghani F., Fatmi Z., Badruddin S., Mansoor S., Siddiqui I., Jabbar A. Prevalence and significance of vitamin D deficiency and insufficiency among apparently healthy adults. Clin. Biochem. 2010;43:1431–1435. doi: 10.1016/j.clinbiochem.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Mattila P.H., Piironen V.I., Uusi-Rauva E.J., Koivistoinen P.E. Vitamin D contents in edible mushrooms. J. Agric. Food Chem. 1994;42:2449–2453. [Google Scholar]
- Nölle N., Argyropoulos D., Ambacher S., Müller J., Biesalski H.K. Vitamin D2 enrichment in mushrooms by natural or artificial UV-light during drying. LWT – Food Sci. Technol. 2017;85:400–404. [Google Scholar]
- Perera C.O., Jasinghe V.J., Ng F.L., Mujumdar A.S. The effect of moisture content on the conversion of ergosterol to vitamin D in shiitake mushrooms. Drying Technol. 2003;21:1093–1101. [Google Scholar]
- Simon R.R., Phillips K.M., Horst R.L., Munro I.C. Vitamin D mushrooms: comparison of the composition of button mushrooms (Agaricus bisporus) treated postharvest with UVB light or sunlight. J. Agric. Food Chem. 2011;59:8724–8732. doi: 10.1021/jf201255b. [DOI] [PubMed] [Google Scholar]
- Urbain P., Jakobsen J. Dose−response effect of sunlight on vitamin D2 production in Agaricus bisporus mushrooms. J. Agric. Food Chem. 2015;63:8156–8161. doi: 10.1021/acs.jafc.5b02945. [DOI] [PubMed] [Google Scholar]
- Urbain P., Singler F., Ihorst G., Biesalski H.K., Bertz H. Bioavailability of vitamin D2 from UV-B-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D: a randomized controlled trial. Eur. J. Clin. Nutr. 2011;65:965–971. doi: 10.1038/ejcn.2011.53. [DOI] [PubMed] [Google Scholar]
- Urbain P., Valverde J., Jakobsen J. Impact on vitamin D2, vitamin D4 and agaritine in Agaricus bisporus mushrooms after artificial and natural solar UV light exposure. Plant Foods Hum. Nutr. 2016;71:314–321. doi: 10.1007/s11130-016-0562-5. [DOI] [PubMed] [Google Scholar]
- Valverde M.E., Hernández-Pérez T., Paredes-López O. Edible mushrooms: improving human health and promoting quality life: review. Int. J. Microbiol. 2015:1–14. doi: 10.1155/2015/376387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb A.R., Kline L., Holick M.F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in boston and edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endocrinol. Metab. 1988;67:373. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- Wu W.J., Ahn B.Y. Statistical optimization of ultraviolet irradiate conditions for vitamin D2 synthesis in oyster mushrooms (Pleurotus ostreatus) using response surface methodology. PLoS ONE. 2014;9(4) doi: 10.1371/journal.pone.0095359. [DOI] [PMC free article] [PubMed] [Google Scholar]