Abstract
Background:
An association between prepregnancy body mass index (BMI) and severe maternal morbidity (SMM) has been reported, but evidence has been mixed and potential explanations have not been examined.
Objective:
To evaluate the association between prepregnancy BMI and SMM in a large, diverse birth cohort and assess potential mediation by obesity-related comorbidities and cesarean birth.
Methods:
This cohort study used linked birth certificate and hospitalization discharge records from Californian births during 2007–2012. We assessed associations between prepregnancy BMI and SMM, and used inverse probability weighting for multiple mediators to estimate relative and absolute natural direct and indirect effects accounting for mediation by comorbidities (hypertensive conditions, diabetes, asthma) and cesarean birth.
Results:
Among 2,650,182 births, the prevalence of SMM was 1.42%. Adjusted risk ratios for the total association between prepregnancy BMI category and SMM were 1.12 (95% confidence interval [CI] 1.07, 1.18) for underweight, 1.02 (95% CI 0.99, 1.04) for overweight, 1.04 (95% CI 1.00, 1.07) for obesity class 1, 1.14 (95% CI 1.09, 1.20) for obesity class 2, and 1.28 (95% CI 1.22, 1.36) for obesity class 3 compared to women with normal weight. After accounting for mediation by comorbidity and cesarean birth, the risk ratios were 1.19 (95% CI 1.14, 1.26) for underweight, 0.91 (95% CI 0.89, 0.94) for overweight, 0.86 (95% CI 0.84, 0.89) for obesity class 1, 0.88 (95% CI 0.84, 0.92) for obesity class 2, and 0.89 (95% CI 0.83, 0.95) for obesity class 3.
Conclusions:
Comorbidities and cesarean birth explained an association between high prepregnancy BMI and SMM. These findings suggest that promotion of healthy prepregnancy weight, along with management of comorbidities and support of vaginal birth in pregnant women with high BMI, could reduce the risk of SMM. However, these mediators did not reduce the elevated risk of SMM observed in women with low BMI.
Keywords: cesarean section, comorbidity, maternal health, obesity, population health, pregnancy complications, severe maternal morbidity
BACKGROUND
Each year, more than 50,000 women in the United States experience severe maternal morbidity (SMM), which includes life-threatening complications such as hemorrhage, embolism, and stroke.1–3 Its prevalence has steadily increased from approximately one in 190 births in 1994 to one in 70 births in 2014.2 In addition to threatening immediate and long-term health, SMM increases the cost of medical care and reduces a mother’s ability to care for her infant and other children.1,4 A better understanding of risk factors for SMM is needed to develop effective prevention strategies.
Obesity has been proposed as a risk factor for SMM.1,4 Approximately one-quarter of women in the U.S. have obesity (body mass index (BMI) ≥ 30 kg/m2) at conception, and this number continues to grow.5 Prepregnancy obesity is known to increase the risk of adverse neonatal outcomes and gestational morbidities, such as gestational hypertension and gestational diabetes mellitus.6,7 Obesity has also been associated with an increased risk of infection, postpartum hemorrhage, and other specific complications at delivery.6–12 Although prepregnancy obesity has received more attention, women with an underweight BMI (< 18.5 kg/m2 at conception) may also be at elevated risk of hemorrhage, obstetric shock, and other serious complications.9,10
Studies of prepregnancy BMI and SMM have been limited because most datasets that include adequate numbers and well-defined cases have lacked information on BMI.1,4 As a result, few rigorous studies of prepregnancy BMI and SMM have been conducted and findings have been mixed.9–15 Further investigation is needed, ideally in large, diverse populations and using methods that help elucidate causal pathways between maternal weight status and SMM.16,17
Increased understanding of how prepregnancy BMI is related to SMM could help identify opportunities for interventions that reduce the risk of this serious outcome. In particular, high prepregnancy BMI is associated with a higher risk of comorbidities – namely hypertensive conditions, diabetes mellitus, and asthma – and cesarean birth, which in turn increase the risk of SMM.4,6,11 Women with high BMI may be biologically more prone to these events, but clinical management may also play a role.16–18
We evaluated the association between prepregnancy BMI and SMM in a large, diverse birth cohort and assessed potential mediation by obesity-related comorbidities and cesarean birth.
METHODS
Cohort Selection
This population-based cohort study was drawn from 3,390,285 California births recorded between 2007–2012 by the California Office of Statewide Health Planning and Development. These live birth and fetal death vital records were previously linked to patient discharge records from antepartum, delivery, and postpartum hospitalizations.19 California adopted the revised U.S. live birth and fetal death records in 2007, which enabled collection of maternal weight and height data. Eligibility criteria for our analytical sample included gestational age ≥20 weeks and linkage of the vital record and maternal delivery hospitalization record (eFigure 1). Because maternal data are recorded identically in multiple records if twins or multiples are delivered, we selected the first maternal record in such cases to prevent duplication of information. Implausible weight, height, and BMI values were identified and set to missing following the Centers for Disease Control and Prevention (CDC) recommendations.20
Exposure
Prepregnancy BMI (kg/m2) was calculated from weight and height self-reported on the vital record. Prepregnancy BMI was categorized as underweight (<18.5), normal weight (18.5–24.9), overweight (25–29.9), obesity class 1 (30–34.9), obesity class 2 (35–39.9), and obesity class 3 (≥40). Nonlinearity of BMI was also explored using restricted quadratic splines (described below).
Outcome
Severe maternal morbidity events occurring from delivery hospitalization to 42 days postpartum were identified using the International Classification of Disease Clinical Modification 9th Revision (ICD-9) diagnosis and procedure codes shown in eTable 1.1 These codes form the basis of an index developed by the CDC and its partners to identify SMM in administrative data,1,2 which was subsequently validated in California.21 The most common indicators of SMM reported previously in a national sample were blood transfusion, disseminated intravascular coagulation, and hysterectomy.3 Blood transfusion is the only qualifying indicator for approximately half of SMM cases because postpartum hemorrhage is by far the most common cause of SMM.1,13 In addition, less severe hemorrhage coupled with preexisting anemia could lead to a blood transfusion, which has been hypothesized as a possible explanation for increased risk of SMM in women with low BMI.9 For these reasons, we also studied an alternative definition of SMM that excluded those cases for which the only indication was a blood transfusion (“transfusion-only cases”) as a secondary outcome in this analysis. This outcome is hereafter referred to as non-transfusion SMM.
Covariates
Covariates were selected a priori from available data, based on prior knowledge,1,8–13,22–27 temporality, and directed acyclic graphs (eFigure 2). Mediators included BMI-related comorbidity (preexisting or gestational hypertension [ICD-9 diagnosis codes 401–405, 642.3], preeclampsia [ICD-9 642.4, 642.5, 642.7], preexisting or gestational diabetes mellitus [ICD-9 250, 648.0, 648.8], or asthma [ICD-9 493]), and cesarean birth [ICD-9 procedure code 74]. Prepregnancy confounders included age, height, educational attainment, race/ethnicity, expected payment type for delivery, and parity. In mediation analyses, plural birth, previous cesarean birth, trimester prenatal care began, preterm birth (<37 weeks’ gestation), and placenta previa or abruption (ICD-9 641) were additionally included as mediator-outcome confounders. Comorbidities and cesarean birth were identified using both vital record and delivery hospitalization discharge data to increase accuracy.28 The other maternal characteristics were identified in the vital record.
Statistical Analysis
Study variable distributions were compared between subjects with and without SMM. The prevalence of SMM, comorbidity, and cesarean birth was also calculated among prepregnancy BMI groups. The association between prepregnancy BMI and SMM was then modeled using logistic regression. The logit link ensured that the predicted probability of the binary outcome was within [0,1] bounds, given the rarity of SMM (<2%). Estimated odds ratios approximated risk ratios because of the rare outcome. Risk differences were calculated using marginal predicted probabilities from the regression models. The logistic regression model was adjusted for baseline confounders (age, race/ethnicity, education, health insurance, height, parity). Mediation of the association between BMI and SMM by comorbidity and cesarean birth was then assessed using an inverse probability weighting approach for assessing multiple mediators, as described in detail by VanderWeele and Vansteelandt.29 In contrast to a regression-based approach, this weighting method does not require models for the mediators. Multivariable logistic regression models for prepregnancy BMI were used to construct the inverse probability weights. Relative and absolute marginal natural direct and indirect effects (risk ratios and risk differences) were estimated for each prepregnancy BMI group in reference to normal weight, which was the lowest risk group. Interaction terms between prepregnancy BMI and each mediator were tested and retained in the regression models if the p-value was <0.1. Direct effect and risk difference calculations were bootstrapped 500 times in the full sample to construct 95% confidence intervals.
Missing Data
Women with implausible or missing values for prepregnancy weight, height, BMI, or covariates were excluded from analyses and compared to those included. The percentage of missing observations for prepregnancy BMI was 8% and ranged from 0% to 3% for each covariate.
Sensitivity Analyses
A series of additional analyses were conducted to assess robustness of the results to analytical decisions and unmeasured confounding. First, all analyses were repeated for the outcome of non-transfusion SMM. Second, anemia complicating pregnancy or delivery (ICD-9 648.20–648.23) and reported as present-on-admission for the delivery hospitalization was included as a comorbidity.30 Third, we attempted to reduce reverse causality between cesarean birth and SMM by restricting analyses to women without a maternal health indication for cesarean birth. (Details provided in eTable 4.) Fourth, we assessed nonlinearity of the association between BMI and SMM by fitting BMI with restricted quadratic splines. The spline terms were used in a logistic regression model to predict and graph the marginal probability of SMM across BMI values. Fifth, we calculated E-values to assess the robustness of the observed associations to potential unmeasured confounding.31,32 Analyses were conducted in R version 3.4.4 (Vienna, Austria).
Ethics Approval
The State of California Committee for the Protection of Human Subjects and the Stanford University Research Compliance Office approved the study protocol.
RESULTS
The final cohort included 2,650,182 births, of which 37,731 (1.42%) resulted in SMM. Prepregnancy obesity classes 2 and 3 (BMI ≥ 35 kg/m2) were more common in women with SMM than in women without SMM (Table 1). Women with SMM also had a nearly twofold higher prevalence of comorbidity and cesarean birth. A higher proportion was primiparous, ≥ 35 years old, non-Hispanic Black, had a previous cesarean birth, and delivered preterm. Women excluded from analyses because of missing or implausible variables experienced a higher prevalence of SMM than those included (1.8% vs. 1.4%) and had lower socioeconomic indicators (eTable 2).
Table 1.
Distribution of variables included in the study among women with and without severe maternal morbidity, California, 2007–2012.
| No severe maternal morbidity (n = 2,612,451) |
Severe maternal morbidity (n = 37,731) |
|||
|---|---|---|---|---|
| Study variable | Number | % | Number | % |
| Prepregnancy body mass index (kg/m2) | ||||
| Underweight (<18.5) | 105,771 | 4 | 1,609 | 4 |
| Normal weight (18.5–24.9) | 1,290,493 | 49 | 17,810 | 47 |
| Overweight (25–29.9) | 674,847 | 26 | 9,692 | 26 |
| Obese class 1 (30–34.9) | 328,575 | 13 | 4,899 | 13 |
| Obese class 2 (35–39.9) | 134,451 | 5 | 2,218 | 6 |
| Obese class 3 (≥40) | 78,315 | 3 | 1,503 | 4 |
| Comorbiditya | 429,257 | 6 | 12,047 | 11 |
| Cesarean birth | 852,375 | 33 | 22,821 | 60 |
| Anemia | 141,511 | 5 | 8,350 | 22 |
| Primiparous | 1,041,383 | 40 | 16,655 | 44 |
| Height | ||||
| <157 cm | 571,438 | 22 | 10,059 | 27 |
| ≥157 cm | 2,041,013 | 78 | 27,672 | 73 |
| Age | ||||
| <20 y | 226,173 | 9 | 3,746 | 10 |
| 20–24 y | 556,216 | 21 | 7,514 | 20 |
| 25–29 y | 702,321 | 27 | 8,753 | 23 |
| 30–34 y | 662,638 | 25 | 8,965 | 24 |
| 35–39 y | 370,464 | 14 | 6,402 | 17 |
| ≥40 y | 94,639 | 4 | 2,351 | 6 |
| Race/ethnicity | ||||
| Foreign-born Hispanic/Latina | 725,352 | 28 | 10,079 | 27 |
| U.S.-born Hispanic/Latina | 636,203 | 24 | 9,451 | 25 |
| Non-Hispanic white | 688,451 | 26 | 8,406 | 22 |
| Asian/Pacific Islander | 317,995 | 12 | 4,679 | 12 |
| Non-Hispanic black/African American | 131,220 | 5 | 3,328 | 9 |
| Other | 113,230 | 4 | 1,788 | 8 |
| Educational attainment | ||||
| Less than high school completion | 632,468 | 24 | 10,122 | 27 |
| High school degree or equivalent | 683,659 | 26 | 9,993 | 26 |
| Some college | 628,765 | 24 | 8,944 | 24 |
| College degree or higher | 667,559 | 26 | 8,672 | 23 |
| Private insurance expected as delivery payment method | 1,241,624 | 48 | 16,628 | 44 |
| Previous cesarean delivery | 521,676 | 20 | 13,515 | 36 |
| Preterm delivery (<37 weeks’ gestation) | 195,693 | 7 | 8,413 | 22 |
| Prenatal care began | ||||
| 1st trimester | 2,177,686 | 83 | 30,444 | 81 |
| 2nd trimester | 356,354 | 14 | 5,633 | 15 |
| 3rd trimester or none | 78,411 | 3 | 1,654 | 4 |
| Placenta previa or abruption | 40,990 | 2 | 3,759 | 10 |
| Twin/multiple birth | 40,835 | 2 | 2,436 | 6 |
Preexisting or gestational hypertension, preeclampsia, preexisting or gestational diabetes, or asthma.
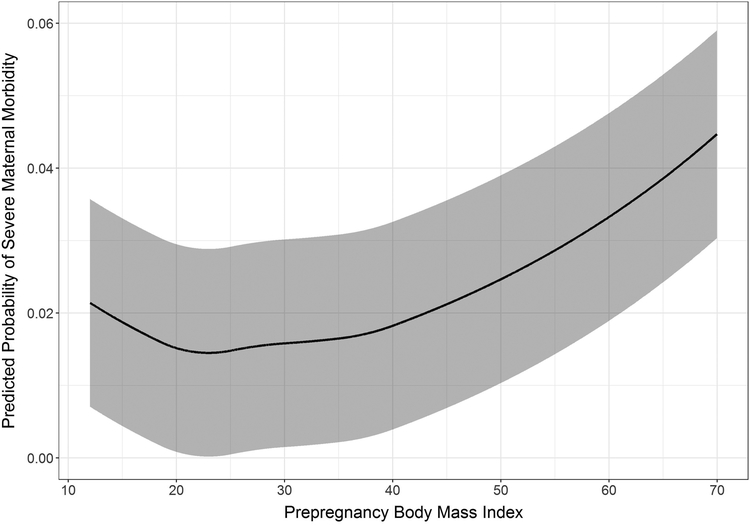
The prevalence of SMM was lowest in women with normal prepregnancy BMI, with higher prevalence in women with low or high BMI (Table 2). In contrast, the prevalence of non-transfusion SMM was similar for women with low and normal BMI, then increased linearly. Comorbidity and cesarean birth increased in prevalence across all BMI categories. The “J-shaped” relationship between prepregnancy BMI and SMM was further supported by modeling non-linear associations with quadratic splines (Figure 1). When we fit BMI with splines, the marginal predicted probability of SMM decreased from 0.021 at the lowest BMI to 0.014 at approximately 23 kg/m2 (normal weight) and then increased to 0.045 at the highest BMI.
Table 2.
Prevalence of study outcomes and mediators within prepregnancy BMI groups, California, 2007–2012.
| Prepregnancy BMI | Severe maternal morbidity | Non-transfusion severe maternal morbidity | Comorbiditya | Cesarean birth | ||||
|---|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | Number | % | |
| Underweight | 1,609 | 1.50 | 624 | 0.58 | 9,634 | 3.0 | 25,319 | 23.6 |
| Normal weight | 17,810 | 1.36 | 7,734 | 0.59 | 152,724 | 3.9 | 373,845 | 28.6 |
| Overweight | 9,692 | 1.42 | 4,540 | 0.66 | 122,322 | 6.0 | 236,282 | 34.5 |
| Obesity class 1 | 4,899 | 1.47 | 2,357 | 0.71 | 81,744 | 8.7 | 133,741 | 40.1 |
| Obesity class 2 | 2,218 | 1.62 | 1,122 | 0.82 | 43,193 | 12.7 | 62,892 | 46.0 |
| Obesity class 3 | 1,503 | 1.88 | 806 | 1.01 | 31,687 | 18.6 | 43,117 | 54.0 |
| Total | 37,731 | 1.42 | 17,183 | 0.65 | 441,304 | 5.9 | 875,196 | 33.0 |
Preexisting or gestational hypertension, preeclampsia, preexisting or gestational diabetes, or asthma.
Figure 1. Marginal predicted probability of severe maternal morbidity across prepregnancy BMI values.
Shaded bands represent 95% confidence intervals.
In unadjusted regression models, both high and low prepregnancy BMI were associated with an increased risk of SMM (Table 3). Women with obesity class 3 were at the highest risk; they were 39% (95% CI: 32%, 47%) more likely to experience SMM than women with normal weight. Among every 10,000 births to women with obesity class 3, there were 52 excess cases (95% CI: 47, 58) of SMM compared with normal weight women. The estimated risk of SMM decreased in women with overweight or obesity after adjusting for confounders (adjusted total effect), but increased in women with underweight (Table 3).
Table 3.
Estimated associations between prepregnancy BMI and severe maternal morbidity with mediation by comorbidity and cesarean birth.
| Risk Ratio (95% confidenceiInterval) | ||||
|---|---|---|---|---|
| Prepregnancy BMI | Unadjusted total effect | Adjusted total effect | Adjusted natural direct effect | Adjusted natural indirect effect |
| Underweight | 1.10 (1.05, 1.16) | 1.12 (1.07, 1.18) | 1.19 (1.14, 1.26) | 0.94 (0.93, 0.95) |
| Normal weight | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Overweight | 1.04 (1.02, 1.07) | 1.02 (0.99, 1.04) | 0.91 (0.89, 0.94) | 1.09 (1.09, 1.10) |
| Obesity Class 1 | 1.08 (1.05, 1.12) | 1.04 (1.00, 1.07) | 0.86 (0.84, 0.89) | 1.16 (1.15, 1.18) |
| Obesity Class 2 | 1.20 (1.14, 1.25) | 1.14 (1.09, 1.20) | 0.88 (0.84, 0.92) | 1.27 (1.24, 1.30) |
| Obesity Class 3 | 1.39 (1.32, 1.47) | 1.28 (1.22, 1.36) | 0.89 (0.83, 0.95) | 1.39 (1.33, 1.47) |
| Risk Differences per 10,000 births (95% confidence interval) | ||||
| Prepregnancy BMI | Unadjusted total effect | Adjusted total effect | Adjusted natural direct effect | Adjusted natural indirect effect |
| Underweight | 13.8 (6.4, 21.5) | 19.0 (10.9, 27.0) | 25.8 (17.8, 33.9) | −9.5 (−11.1, −8.1) |
| Normal weight | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) |
| Overweight | 5.3 (1.6, 8.7) | 1.5 (−2.2, 5.2) | −11.0 (−14.3, −7.7) | 11.5 (10.9, 12.2) |
| Obesity Class 1 | 10.9 (6.3, 15.1) | 4.8 (0.4, 15.1) | −17.4 (−21.9, −13.2) | 19.5 (18.0, 21.2) |
| Obesity Class 2 | 26.3 (19.4, 32.9) | 19.6 (12.5, 25.9) | −16.3 (−22.6, −9.8) | 32.4 (29.3, 36.4) |
| Obesity Class 3 | 51.8 (41.7, 61.9) | 38.9 (28.7, 48.2) | −14.5 (−23.3, −54.9) | 48.3 (41.1, 56.8) |
In mediation analyses, the estimated risk of SMM not transmitted through comorbidity and cesarean birth (natural direct effect) was lower than the total effect in women with overweight or obesity, but was higher in women with underweight (Table 3). Correspondingly, the estimated indirect effect of prepregnancy BMI—transmitted through comorbidity and cesarean birth—on SMM was higher in women with overweight or obesity, but lower in women with underweight, than the total effect.
Results differed when the outcome was restricted to non-transfusion SMM (Table 4). The risk ratios in women with overweight and obesity were higher for this outcome; risk differences were similar, but this outcome was much less common (0.65% vs. 1.42%). After accounting for mediation by comorbidity and cesarean birth, only minimal differences in risk remained among women with normal weight, overweight, or obesity.
Table 4.
Estimated associations between prepregnancy BMI and non-transfusion severe maternal morbiditya with mediation by comorbidity and cesarean birth.
| Risk Ratio (95% confidence interval) | ||||
|---|---|---|---|---|
| Prepregnancy BMI | Unadjusted total effect | Adjusted total effect | Adjusted natural direct effect | Adjusted natural indirect effect |
| Underweight | 0.98 (0.91, 1.07) | 1.01 (0.93, 1.10) | 1.10 (1.01 1.19) | 0.92 (0.91, 0.93) |
| Normal weight | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Overweight | 1.12 (1.08, 1.17) | 1.10 (1.06, 1.15) | 0.98 (0.94, 1.01) | 1.10 (1.09, 1.11) |
| Obesity Class 1 | 1.20 (1.14, 1.25) | 1.15 (1.10, 1.21) | 0.95 (0.90, 0.99) | 1.17 (1.15, 1.19) |
| Obesity Class 2 | 1.39 (1.31, 1.48) | 1.33 (1.25, 1.42) | 0.97 (0.91, 1.04) | 1.32 (1.27, 1.36) |
| Obesity Class 3 | 1.71 (1.59, 1.84) | 1.56 (1.45, 1.68) | 1.05 (0.95, 1.14) | 1.43 (1.35, 1.52) |
| Risk Difference per 10,000 births (95% confidence interval) | ||||
| Prepregnancy BMI | Unadjusted total effect | Adjusted total effect | Adjusted natural direct effect | Adjusted natural indirect effect |
| Underweight | −1.0 (−6.0, 3.9) | 2.1 (−3.0, 7.2) | 5.5 (2.8, 11.1) | −4.9 (−6.0, −3.9) |
| Normal weight | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) | 0.0 (Reference) |
| Overweight | 7.2 (0.5, 9.5) | 5.4 (3.1, 7.7) | −1.0 (−3.2, 1.2) | 6.0 (5.5, 6.5) |
| Obesity Class 1 | 11.5 (8.6, 14.2) | 8.7 (5.6, 11.6) | −2.3 (−5.3, 6.7) | 10.0 (8.8, 11.1) |
| Obesity Class 2 | 23.2 (18.0, 28.0 | 19.0 (14.2, 24.0) | −11.1 (−5.0, 2.9) | 18.6 (16.2, 21.0) |
| Obesity Class 3 | 41.9 (35.6, 48.9) | 32.4 (26.8, 38.9) | 3.2 (−2.3, 9.6) | 27.0 (23.0, 32.0) |
Excluded cases for which the only indication of severe maternal morbidity was a blood transfusion
In underweight women, the risk of non-transfusion SMM was only higher compared with normal-weight women after accounting for mediation by comorbidity and cesarean birth. However, the risk ratio and risk difference were smaller in comparison to those for the main SMM outcome. After considering preexisting anemia as a comorbidity, changes in the results were negligible except that the fully adjusted risk ratios and risk differences for SMM in women with overweight or obesity were lower (eTable 3). The prevalence of anemia was 6.1% in underweight, 5.5% in normal weight, 5.7% in overweight, 5.8% in obese class 1, 5.9% in obese class 3, and 6.5% in obese class 3 women.
In other secondary analyses, excluding women with maternal health indications for cesarean birth had a minimal impact on results (eTable 4). E-values suggested that relatively strong unmeasured confounding would be required to explain away the observed associations (eTable 5).31
COMMENT
Principal Findings
Both low and high prepregnancy BMI were associated with SMM in this population-based cohort study. However, findings from our mediation analysis suggest different causal mechanisms at each end of the BMI distribution. The prevalence of maternal comorbidity and cesarean birth increased with increasing BMI and our models suggest that these factors explained the increased risk of SMM in women with high prepregnancy BMI. In contrast, the risk of SMM increased in women with low BMI after accounting for mediation by comorbidity and cesarean birth.
Strengths of the Study
This analysis overcame several methodological challenges that have limited the study of prepregnancy BMI and SMM. The California dataset was exceptionally diverse and large, which allowed us to examine this rare outcome in relation to less common exposures (underweight and obesity class 3) and to study the rarer outcome of non-transfusion SMM. With one exception,9 studies that have previously assessed the association between BMI and SMM have either grouped together women with underweight and normal weight,13–15 excluded underweight women from analysis,11 or had few women with underweight.10,12 The linked California database included postpartum hospitalizations, enabling the inclusion of severe morbidities occurring after delivery hospitalization discharge. Cases were identified using an index created by the CDC and its partners, which has been validated and can be replicated in other datasets with ICD diagnosis and procedures codes. In addition, the inverse probability weighting approach used in this study overcomes strong assumptions required for traditional methods and was robust to interactions and unmeasured common causes of two related mediators.29
Limitations of the Data
Large, population-based datasets enable the study of rare outcomes like SMM, but have several limitations. Very rare maternal conditions, including cardiac disease, renal disease, and eclampsia, are substantially underreported in hospital discharge data.28 The SMM index used here, which combines 18 rare conditions and procedures, has been found to have a sensitivity of 0.77 and a specificity of 0.99 compared to medical records in California, with a lower sensitivity (0.53) for non-transfusion cases.21 If misclassification of such binary outcomes was non-differential, bias in measures of association would be expected toward the null. If misclassification was differential, bias could be toward or away from the null. This dataset does not contain information on how many units of blood were used in a transfusion, which limits the identification of severe postpartum hemorrhage – the most common contributor to SMM.21 Although we attempted to isolate preexisting anemia using diagnoses reported as present-on-admission for the delivery hospitalization,30 postpartum cases secondary to hemorrhage may have been miscoded as preexisting. In addition, self-reported weight in the vital records could have caused exposure misclassification. Individuals tend to underreport prepregnancy weight by 0.3 to 3 kg,33 although underweight women tend to over-report their weight.34 A recent systematic review, however, did not find these magnitudes of error to bias associations with birth outcomes.33 As discussed above, cesarean birth was treated as a mediator in this study but temporality cannot be certain. Possible selection bias was also indicated by differences in SMM and socioeconomic indicators between included and excluded subjects. Finally, other potential mediators and confounders of interest were not measured, such as stress, drug use, and nutritional status.
Interpretation
The association between high prepregnancy BMI and SMM corroborate those of other recent studies and highlight the potential value of healthy prepregnancy weight as one primary prevention strategy to improve maternal health outcomes.9,12–14 The increased risk of SMM associated with very high prepregnancy BMI reported here supports the well-known need to reverse current trends in obesity. Prepregnancy obesity classes 2 and 3 have been increasing in prevalence, affecting approximately 10% of women giving birth in the U.S.5 This increase has occurred despite policies to promote healthy weight prior to pregnancy.35 Efforts have largely focused on supporting individual women in achieving a healthy weight.36 While important, focusing on individual behaviors will continue to be an ineffective public health approach as women live and work in an increasingly obesogenic world.36
This study further suggests that improved management of common comorbidities and supporting vaginal birth when possible could be secondary prevention opportunities to lower the risk of SMM in pregnant women with high BMI. It has long been known that high maternal weight puts women at increased risk of hypertensive disorders, gestational diabetes, and cesarean birth.6,7,11 The metabolic environment associated with a high prepregnancy BMI affects placental development and maternal physiology starting at conception; these early effects set the stage for metabolic dysfunctions later in pregnancy, such as gestational hypertensive disorders and gestational diabetes.6 High BMI also increases the risk of cesarean birth, potentially due in part to reduced uterine contractility, narrowing of the birth canal by soft tissue, and higher infant weight for gestational age.11 Comorbidities and cesarean birth, in turn, increase the risk of serious complications during and after birth, including postpartum hemorrhage, venous thromboembolism, and infection.4,11
The role of cesarean birth is complex because women with high BMI are more likely to have an indication for cesarean birth (e.g., severe preeclampsia),10,11 and are at higher risk of surgical complications.6 Certain SMM conditions, such as eclampsia and amniotic fluid embolism, can also be indications for cesarean birth. Cesarean birth was treated as a mediator in this study because it is a well-known contributor to SMM,1 but temporality is not certain because the dataset does not include information on timing of events during a hospitalization. In a sensitivity analysis, we attempted to mitigate reversal causality between cesarean birth and SMM by excluding women with health indications for cesarean birth, which did not meaningfully change results. Overall, the findings suggest an important role of comorbidities and cesarean birth, which may be informative for future studies on optimizing care for obstetric patients with high BMI, of which much is still to be learned.17,18
In comparison to high prepregnancy BMI, the possible maternal health risks of low BMI have received relatively little attention in recent research and policies.5 The prevalence of SMM in this California study was 1.47% in women with obesity class 1 and 1.50% in women with underweight, which is consistent with a previous report from Washington State.9 A particularly interesting finding from our study is that because underweight women were least likely to have one of the studied comorbidities or cesarean birth, after accounting for mediation by these factors, the magnitude of the association between underweight and SMM actually increased. Lisonkova et al.9 posited that a higher prevalence of anemia in underweight women could exacerbate the effects of hemorrhage and lead to more blood transfusions, but did not examine this hypothesis with their data. We assessed this possibility by both limiting analysis to non-transfusion SMM cases and including preexisting anemia as a comorbidity. Although these changes affected the risk estimates, women with underweight remained at the highest risk of SMM after accounting for comorbidities and cesarean birth. The prevalence of anemia was also not meaningfully higher in women with underweight (approximately 6% in all BMI groups). We did not identify any other reasons why underweight women were at higher risk of SMM and our results highlight an important area of future research. One potential contributor to both low BMI and SMM is opioid-use disorder,37 which could not be adequately assessed in this dataset.
Results from this study differed when transfusion-only cases were included or excluded from the outcome. Our study used a standard measure of SMM based on ICD-9 diagnosis and procedure codes, which was previously validated in California data.21 Blood transfusion alone, however, accounts for approximately half of SMM cases using this measure. In studying non-transfusion cases, the magnitude of the association between BMI and SMM increased in women with overweight or obesity and decreased in women with underweight. In addition, the risk of SMM was actually lower in women with high BMI than in women with normal BMI after accounting for mediation by comorbidities and cesarean birth, but not after excluding transfusion-only cases from the outcome. These findings suggest that the pathway between prepregnancy BMI and SMM may differ for low and high BMI as well as by type of complication. Previous studies have reported differences in the association between prepregnancy BMI and specific morbidities, such as the highest risk of cardiac morbidities in women with obesity class 3 and the highest risk of severe postpartum hemorrhage in women with underweight.9,10 Women with high BMI are more likely to have comorbidities and deliver by cesarean, and these surgeries can be challenging, particularly in women with very high BMI.16,18 Longer operative times during cesarean birth could increase the potential for blood loss and thus increase the risk of blood transfusion. This pathway could explain why accounting for increased comorbidities and cesarean birth in women with high BMI dramatically reduced effect estimates when transfusion-only cases were included in the outcome.
CONCLUSIONS
The results of this study underscore healthy prepregnancy weight as an important component of reducing the risk of complications during and after birth. We confirmed the common view that obesity is a risk factor for SMM and extended that finding to women with underweight. Our study indicates that improved management of comorbidities and promotion of vaginal birth when appropriate may be opportunities to further reduce the risk of SMM in women with high prepregnancy BMI and deserve further exploration. However, potential strategies to prevent SMM associated with low prepregnancy BMI require further research.
Supplementary Material
SYNOPSIS.
Study Question
What are the roles of comorbidities and cesarean birth in the association between prepregnancy BMI and severe maternal morbidity?
What’s Already Known
High, and possibly low, prepregnancy BMI is associated with an increased risk of severe maternal morbidity.
What this Study Adds
Higher prevalence of comorbidities and cesarean birth may explain the association between high prepregnancy BMI and severe maternal morbidity. However, these mediators likely do not contribute to the increased risk of SMM observed in women with low BMI.
ACKNOWLEDGEMENTS
We acknowledge the California Office of Statewide Health Planning and Development for linking the data files and Louisa Smith for her input on the study analysis.
FUNDING
The National Institute of Nursing Research (R01 NR017020), the Eunice Kennedy Shriver National Institute of Child Health and Development (F32 HD091945), and Stanford Maternal and Child Health Research Institute provided funding for this study.
Footnotes
SOCIAL MEDIA QUOTE
Higher comorbidity and cesarean birth rates may be responsible for a higher risk of severe maternal morbidity in obese women
Recommendation to use Figure 1 on social media.
@steff_leonard
REFERENCES
- 1.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstetrics and Gynecology 2012;120(5):1029–1036. [DOI] [PubMed] [Google Scholar]
- 2.CDC. Severe maternal morbidity in the United States. 2017; https://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html#anchor_SMM. Accessed March 5, 2019.
- 3.Fingar KR, Hambrick MM, Heslin KC, Moore JE. Trends and disparities in delivery hospitalizations involving severe maternal morbidity, 2006–2015. Agency for Healthcare Research and Quality;2018. [PubMed] [Google Scholar]
- 4.Hirshberg A, Srinivas SK. Epidemiology of maternal morbidity and mortality. Seminars in Perinatology 2017;41(6):332–337. [DOI] [PubMed] [Google Scholar]
- 5.Deputy NP, Dub B, Sharma AJ. Prevalence and trends in prepregnancy normal weight - 48 states, New York City, and District of Columbia, 2011–2015. MMWR Morbidity and Mortality Weekly Report 2018;66:1402–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ (Clinical Research Ed) 2017;356:j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obesity Reviews 2015;16(8):621–638. [DOI] [PubMed] [Google Scholar]
- 8.Butwick AJ, Bentley J, Leonard SA, et al. Prepregnancy maternal body mass index and venous thromboembolism: A population based cohort study. BJOG 2019;126(5):581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lisonkova S, Muraca GM, Potts J, et al. Association between prepregnancy body mass index and severe maternal morbidity. JAMA 2017;318(18):1777–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schummers L, Hutcheon JA, Bodnar LM, Lieberman E, Himes KP. Risk of adverse pregnancy outcomes by prepregnancy body mass index: a population-based study to inform prepregnancy weight loss counseling. Obstetrics and Gynecology 2015;125(1):133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sebire NJ, Jolly M, Harris JP, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. International Journal of Obesity 2001;25(8):1175–1182. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui A, Azria E, Howell EA, Deneux-Tharaux C. Associations between maternal obesity and severe maternal morbidity: Findings from the French EPIMOMS population-based study. Paediatric and Perinatal Epidemiology 2019;33(1):7–16. [DOI] [PubMed] [Google Scholar]
- 13.Grobman WA, Bailit JL, Rice MM, et al. Frequency of and factors associated with severe maternal morbidity. Obstetrics and Gynecology 2014;123(4):804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pallasmaa N, Ekblad U, Gissler M, Alanen A. The impact of maternal obesity, age, pre-eclampsia and insulin dependent diabetes on severe maternal morbidity by mode of delivery-a register-based cohort study. Archives of Gynecology and Obstetrics 2015;291(2):311–318. [DOI] [PubMed] [Google Scholar]
- 15.Lindquist A, Knight M, Kurinczuk JJ. Variation in severe maternal morbidity according to socioeconomic position: a UK national case-control study. BMJ Open 2013;3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caughey AB. Prepregnancy obesity and severe maternal morbidity: What can be done? JAMA 2017;318(18):1765–1766. [DOI] [PubMed] [Google Scholar]
- 17.Creanga AA. Maternal obesity and severe maternal morbidity—It is time to ask new research questions. Paediatric and Perinatal Epidemiology 2019;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.McCall SJ, Li Z, Kurinczuk JJ, Sullivan E, Knight M. Binational cohort study comparing the management and outcomes of pregnant women with a BMI 50–59 kg/m2. BMJ Open 2018;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.California Office of Statewide Health Planning and Development. Types of OSHPD Patient-Level Data. https://www.oshpd.ca.gov/HID/Data_Request_Center/Types_of_Data.html. Accessed March 5, 2019.
- 20.Centers for Disease Control and Prevention. User Guide to the 2016 Natality Public Use File. [Google Scholar]
- 21.Main EK, Abreo A, McNulty J, et al. Measuring severe maternal morbidity: validation of potential measures. American Journal of Obstetrics and Gynecology 2016;214(5):643 e641–643 e610. [DOI] [PubMed] [Google Scholar]
- 22.Berg CJ, McKay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993–1997 and 2001–2005. Obstetrics and Gynecology 2009;113(5):1075–1081. [DOI] [PubMed] [Google Scholar]
- 23.Kuklina EV, Meikle SF, Jamieson DJ, et al. Severe obstetric morbidity in the United States: 1998–2005. Obstetrics and Gynecology 2009;113(2 Pt 1):293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer MS, Berg C, Abenhaim H, et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. American Journal of Obstetrics and Gynecology 2013;209(5):449 e441–447. [DOI] [PubMed] [Google Scholar]
- 25.Lyndon A, Lee HC, Gilbert WM, Gould JB, Lee KA. Maternal morbidity during childbirth hospitalization in California. The Journal of Maternal-Fetal & Neonatal Medicine 2012;25(12):2529–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibson C, Rohan AM, Gillespie KH. Severe maternal morbidity during delivery hospitalizations. Wisconsin Medical Journal 2017;116(5):259–264. [PMC free article] [PubMed] [Google Scholar]
- 27.Lisonkova S, Potts J, Muraca GM, et al. Maternal age and severe maternal morbidity: A population-based retrospective cohort study. PLoS Medicine 2017;14(5):e1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lydon-Rochelle MT, Holt VL, Cardenas V, et al. The reporting of pre-existing maternal medical conditions and complications of pregnancy on birth certificates and in hospital discharge data. American Journal of Obstetrics and Gynecology 2005;193(1):125–134. [DOI] [PubMed] [Google Scholar]
- 29.VanderWeele T, Vansteelandt S. Mediation analysis with multiple mediators. Epidemiology Methods 2013;2(1):95–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldman LE, Chu PW, Osmond D, Bindman A. The accuracy of present-on-admission reporting in adminstrative data. Health Services Research 2011;46(6pt1):1946–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Annals of Internal Medicine 2017;167(4):268–274. [DOI] [PubMed] [Google Scholar]
- 32.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing E-values. Epidemiology 2018;29(5):e45–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Headen I, Cohen AK, Mujahid M, Abrams B. The accuracy of self-reported pregnancy-related weight: a systematic review. Obesity Reviews 2017;18(3):350–369. [DOI] [PubMed] [Google Scholar]
- 34.Han E, Abrams B, Sridhar S, Xu F, Hedderson M. Validity of self-reported pre-pregnancy weight and body mass index classification in an integrated health care delivery system. Paediatric and Perinatal Epidemiology 2016;30(4):314–319. [DOI] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Healthy People 2020 Midcourse Review Hyattsville, MD: 2016. [Google Scholar]
- 36.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. The Lancet 2011;378:804–814. [DOI] [PubMed] [Google Scholar]
- 37.Schiff DM, Patrick SW, Terplan M. Maternal health in the United States. New England Journal of Medicine 2018;387(6):587–588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.