Abstract
Clinical teaching generally asserts that large opacities of progressive massive fibrosis (PMF) on chest radiographs present primarily bilaterally in the upper lung zones, and with an elevated background profusion of small opacities. However, the contemporary basis for these descriptions is limited.
Radiographs taken for the Coal Workers’ Health Surveillance Program during 2000–2015 and previously determined to have large opacities (“PMF radiographs”, n = 204), and a random sample previously deemed free of large opacities (n =22), were independently reevaluated by three National Institute for Occupational Safety and Health (NIOSH) B Readers. Large opacities were noted primarily in the upper right (41%) or upper left (28%) lung zone, but 31% were in middle or lower zones. Unilateral involvement was observed in 34% of readings, with right lung predominance (82%). The median small opacity profusion category for the radiographs with PMF was 2/1. The number of large opacities was not correlated with small opacity profusion category. The “classic” descriptions of PMF as bilateral, associated with elevated background profusions of small pneumoconiotic opacities, were each absent in a third of miners.
Keywords: Epidemiology, occupational diseases, occupational lung disease
Introduction
Coal workers’ pneumoconiosis is a chronic, irreversible occupational lung disease caused by the long-term inhalation of coal mine dust, triggering inflammation of the distal airways, which eventually produces scarring and irreversible lung damage. Pneumoconiosis can range from simple to complicated with progressive massive fibrosis (PMF). PMF can be debilitating and fatal. In an effort to prevent PMF, the Federal Coal Mine Health and Safety Act of 1969 established a federal permissible exposure limit for respirable dust in underground and surface coal mines.1 The Act also established a surveillance system, the Coal Workers’ Health Surveillance Program (CWHSP), where actively working miners are eligible for periodic chest radiographs to screen for pneumoconiosis. The Act also directs the National Institute for Occupational Safety and Health (NIOSH) to use these data to study the relationships between coal mining and occupational diseases, to develop epidemiological information about pneumoconiosis and other mining-related respiratory diseases, and to periodically publish reports highlighting significant aspects of occupational diseases of miners.
Following the establishment of the permissible exposure limit in 1969, prevalence of pneumoconiosis among working underground coal miners declined markedly from 11.7% assessed during 1970–74 to 2.0% assessed during 1995–99. However, during the last 15 years pneumoconiosis prevalence has increased to 4.6% among all underground working miners and 12.7% among longer tenured miners.2–5 Additionally, PMF, the most severe form of pneumoconiosis, which was nearly eliminated before 1999, has also increased, especially in central Appalachia where prevalence has surpassed 5% among long-tenured miners.6 These recent findings have brought renewed attention to the clinical presentations and management of coal mine dust lung disease among underground and surface coal miners.
Chest radiographs are classified for changes consistent with pneumoconiosis according to the International Labour Office (ILO) Classification of Radiographs of Pneumoconiosis.7 Parenchymal abnormalities captured by an ILO classification include small and large opacities. Small opacities are fibrotic masses less than 10 mm in diameter and an ILO classification captures profusion (or concentration), affected zones of the lung, as well as shape and size of the small opacities. A large opacity is identified when the longest dimension of a fibrotic mass exceeds 10 mm. PMF is defined as the presence of one or more large opacities. Clinical teaching generally asserts that large opacities usually appear bilaterally (though some reports suggests that large opacities are more common in the right lung),8 in the upper lung zones, and often have an asymmetric shape, well-defined margins, with a background profusion of major category 2 or 3 small opacities. This conventional knowledge appears in several respiratory health resources and has been perpetuated in occupational respiratory disease articles9,10 and medical texts.11–15 However, the scientific basis underpinning these descriptions is limited and appears to rely upon individual examples and case reports, many of which are not consistent with the broad description of upper lung zone involvement.13,16 We undertook this study to provide a contemporary population-based description of PMF in coal miners, and to establish a baseline which may be useful in monitoring disease patterns over time. Our objective was to characterize the radiographic presentation of large opacities in modern coal miners, by documenting their number, location (lung zone), size, shape, and the background profusion of small opacities.
Materials and methods
All radiographs of coal miners who had participated in the CWHSP between 2000 and 2015, and that had been determined17 to show large opacities, were selected for the current study, as well as a random sample of 22 radiographs which were free of large opacities. Three B Readers, selected at random from the NIOSH CWHSP Reader pool, were asked to participate in this study. Each B Reader independently reevaluated all study radiographs for changes consistent with pneumoconiosis, according to the International Labour Office (ILO) Classification of Radiographs of Pneumoconiosis7 and recorded their readings using the standard Chest Radiograph Classification Form (CDC/NIOSH (M) 2.8) and information about large opacities, if present, using a computerized custom data collection tool. Analog film radiographs were cleaned using compressed air and/or a lint-free nonabrasive antistatic cloth and isopropyl alcohol, scanned using a Vidar Film Digitizer at default settings to DICOM format at 300 DPI, and the digitized images were inspected for digitizing artifacts. Personally identifiable information was removed, analog film radiographs were digitized, and the digital and digitized images were displayed on dual-screen high-resolution physician-quality workstations in a manner previously described.18 Data from the (M) 2.8 and the PMF characterization data collection tool were captured digitally in the NIOSH Picture Archiving and Communications System (PACS) and a database of the B Readers’ responses was automatically generated.
For each of the large opacities noted on a radiograph, Readers were asked to characterize the shape, location, and size. Readers were asked to use their best judgement in assigning opacities to shape and location categories, understanding that only one shape and one location category could be assigned to each opacity. Opacities which were more than 50% rounded were considered to be rounded, and opacities which were more than 50% asymmetric/polygonal shaped were considered to be polygonal. Each large opacity was assigned to a single lung zone, using the zones defined in the ILO Classification.7 If an opacity overlapped lung zones, the Reader assigned the zone where the majority of the opacity was located. Readers used the PACS display software measuring tool to determine the long and short axis in millimeters for each opacity. Aspect ratio of the opacity was calculated (long axis/short axis). Relationships between categorical variables were evaluated using chi-square test for independence and Pearson’s Correlation Coefficient was used for interval and ratio variables. Statistical analysis was completed using SAS 9.4 (Cary, NC, USA). This study used existing radiographs, received a waiver of informed consent, and was approved by the NIOSH Institutional Review Board (15-DRDS-03XP).
Results
A total of 226 radiographs collected between 2000 and 2015 were identified for evaluation: 204 radiographs had previously been classified as having large opacities (“PMF radiographs”; including 122 films [digitized prior to evaluation] and 82 digitally-acquired radiographs). The remaining 22 were randomly selected radiographs that had previously been classified as not having PMF (“Non-PMF radiographs”) but may have had other characteristics of interest like small opacity profusion greater than or equal to 1/0, coalescence of small opacities (AX; precursor to the development of a large opacity), or radiographs that were previously considered to be free of pneumoconiosis (small opacity profusion 0/0 or 0/1) (Table 1).
Table 1.
Summary of study radiographs.
| Characteristics | n =226 |
|---|---|
| PMF radiographsa | 204 |
| Digitized | 122 |
| Digitally acquired | 82 |
| Non-PMF radiographsb | 22 |
| ≥1/0 with AXc | 7 |
| ≥1/0 without AXc | 8 |
| No pneumoconiosis | 7 |
Radiographs with prior classification of PMF through the CWHSP.
Radiographs had a prior classification through the CWHSP that did not include PMF.
AX is the classification symbol for “coalescence of small opacities”.
B Readers did not identify large opacities among the 15 radiographs which were either previously classified as free of pneumoconiosis or radiographs previously classified as small opacity profusion ≥ 1/0 subcategory (without coalescence of small opacities). B Readers identified large opacities among 82% to 92% of the PMF radiographs and between 29% and 86% of the ≥ 1/0 small opacity profusion with AX radiographs (Table 2).
Table 2.
Number and proportion of radiographs each reader identified with large opacities by the radiograph’s previous classification.
| Radiograph previously classified as: | ||
|---|---|---|
| PMF n =204 |
Pneumoconiosis with AXa n =7 |
|
| Reader 1 noted Large Opacity | 187 (0.92) | 4 (0.57) |
| Reader 2 noted Large Opacity | 181 (0.89) | 6 (0.86) |
| Reader 3 noted Large Opacity | 167 (0.82) | 2 (0.29) |
AX is the classification symbol for “coalescence of small opacities”.
Presentation of large opacities
Because at least two Readers identified large opacities on a majority of the 7 radiographs with ≥ 1/0 small opacity profusion subcategory that had a previous indication of coalescence of small opacities (AX; Tables 1 and 2) we chose to include these radiographs in our overall characterization of large opacities. From this point forward in this manuscript, we consider a case of PMF to be ≥ 1 large opacity identified by at least one reader. Therefore, the results will include data collected from the 204 radiographs previously identified to have large opacities and 7 radiographs that had a previous indication of coalescence of small opacities (AX), for a total of 211 radiographs. Readers identified 1131 large opacities (mean of 377 large opacities per Reader) among the 211 radiographs (Table 3); 53% (600/1131) were rounded (47% [531/1131] were polygonal). Regardless of shape, large opacities occurred most frequently in the upper right lung zone (41%) followed by the upper left lung zone (28%); the most common shape and location for large opacities was rounded occurring in the upper right lung zone (22%; 251/1131). However, almost a third of large opacities were identified in the middle or lower lung zones (31%).
Table 3.
Total number and proportion of large opacities identified by three B readers by shape and lung zone.
| Lung Zone | Rounded (n =600) | Polygonal (n =531) | Total (n =1131) | |||
|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | |
| Upper | 251 (0.22) | 152 (0.13) | 217 (0.19) | 163 (0.14) | 468 (0.41) | 315 (0.28) |
| Middle | 103 (0.09) | 57 (0.05) | 63 (0.06) | 59 (0.05) | 166 (0.15) | 116 (0.10) |
| Lower | 16 (0.01) | 21 (0.02) | 14 (0.01) | 15 (0.01) | 30 (0.03) | 36 (0.03) |
Values in parentheses represent the proportion of the total number of large opacities identified (n =1131).
Of the 547 readings with a large opacity indicated in Table 2, 544 (99%) had complete information for small opacity profusion subcategory; of these, 203 (37%) were found in miners with a background of category 1 small opacity profusion (subcategory 1/0 to 1/2). The median small opacity profusion category for the radiographs with PMF was 2/1. The number of large opacities was not correlated with small opacity profusion category (Pearson Correlation Coefficient r = 0.07, p = 0.11). Shape and location of large opacities were not associated with small opacity profusion (shape p = 0.13; location p = 0.58). Median aspect ratio was 1.5 (range 0.68–13.38), with 3.89% (44/1131) large opacities having an aspect ratio greater than 3.
Individually, B Readers identified the number of large opacities and classified shape and location of large opacities in somewhat different proportions (Table 4). Reader 3 identified half as many large opacities as Reader 1 (Reader 3 n = 227 vs. Reader 1 n =483). Reader 1 and 2 identified opacities in similar proportions. Reader 3 identified proportionally more rounded large opacities in the upper right and upper left lung zones and less in the middle and lower lung zones than Readers 1 and 2.
Table 4.
Number and proportion of large opacities identified by shape and lung zone for each B reader.
| Lung zone | Reader 1 (n =483) | Reader 2 (n =421) | Reader 3 (n =227) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rounded (n =244) | Polygonal (n =239) | Rounded (n =197) | Polygonal (n =224) | Rounded (n =159) | Polygonal (n =68) | |||||||
| Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | |
| Upper | 80 (0.17) | 49 (0.10) | 92 (0.19) | 74 (0.15) | 86 (0.20) | 55 (0.13) | 90 (0.20) | 64 (0.15) | 85 (0.37) | 48 (0.21) | 35 (0.15) | 25 (0.11) |
| Middle | 61 (0.13) | 36 (0.07) | 28 (0.06) | 33 (0.07) | 30 (0.07) | 15 (0.04) | 31 (0.10) | 24 (0.06) | 12 (0.05) | 6 (0.03) | 4 (0.02) | 2 (0.01) |
| Lower | 8 (0.02) | 10 (0.02) | 5 (0.01) | 7 (0.01) | 3 (0.01) | 8 (0.02) | 8 (0.01) | 7 (0.02) | 5 (0.02) | 3 (0.01) | 1 (0.00) | 1 (0.00) |
Values in parentheses represent the proportion of the total number of large opacities identified by shape and specific reader.
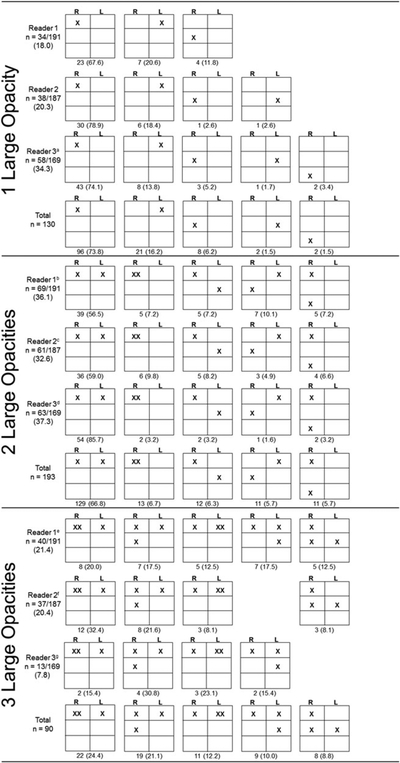
B Readers identified between one and three large opacities in a majority (86.4%), of the radiographs, the remaining radiographs had between four (9.0%) and eight (0.2%) large opacities. Repeated patterns of lung zone involvement were observed and were generally consistent among all three Readers (Figure 1). Among radiographs with one large opacity, a majority were found in the upper zones (73.8% upper right, 16.2% in the upper left lung zone); the remaining 10.0% occurred in the middle and lower zones. Over two thirds (66.8%) of radiographs with two large opacities had bilateral upper lung zone involvement. Among the most commonly observed patterns for radiographs with three large opacities, large opacities were always present in the upper lung zones.
Figure 1.
Lung zone locations of large opacities identified among 211 radiographs with PMF or AX previously indicated stratified by B Reader and number of opacities identified. “X” denotes the location of one large opacity. Boxes represent the right and left, upper, middle, and lower lung zones as defined in the ILO Classification.a 1 (1.7) additional pattern. Total patterns shown for 1 lesion is 129/130 (99.2%) of all patterns (0.8% additional pattern not shown).b 8 (11.6) additional patterns,c 7 (11.5) additional patterns,d 2 (3.2) additional patterns. Total patterns shown for 2 lesions are 176/193 (91.2%) of all patterns (8.8% additional patterns not shown).e 10 (23.3) additional patterns,f 11 (29.8) additional patterns,g 2 (15.3) additional patterns. Total patterns shown for 3 lesions are 69/93 (74.2%) of all patterns (25.8% additional patterns not shown).
B Readers identified large opacities in 79.6% to 90.5% of radiographs of the 211 radiographs previously identified to have large opacities or AX, for a total of 547 readings with at least one large opacity. Data on opacity location were complete in 513 readings; of those 173 (33.7%) had an opacity(ies) in only one lung (unilateral involvement). A total of 82.7% (143/173) of the unilateral opacities were located in the right lung. Of the 513 readings, 6.8% (35/513) had exclusively middle and/or lower lung zone involvement with no large opacities identified in the upper lung zones.
Discussion
A limited number of studies have been conducted assessing the shape, size, and location of small opacities on chest radiographs of coal mine dust exposed workers.19–21 However, to our knowledge, similar studies assessing the radiographic characteristics of PMF in these populations have not been conducted. The objective of this study was to design a straightforward empirical approach to characterize large opacities on chest radiographs of modern coal miners by describing the number, location (lung zone), size, and shape using experienced NIOSH B Readers. In general, our findings are consistent with standard clinical teaching with regard to location; among coal miners with PMF, large opacities were found predominantly, though not exclusively, in the upper lung zones with right lung predominance. However, PMF did not always present in the “classical” fashion. We found no clear association related to large opacity shape, the number of large opacities, or with small opacity profusion category. Of note we found 37% of PMF cases had a background of small opacities with profusion category 1 (subcategory 1/0 to 1/2). This observation is similar to that recently observed in a population of former miners applying for federal black lung benefits, where 40% of former miners with large opacities had a low (category 1) background small opacity profusion.22 In addition, our findings do not support the “classic” definition of PMF opacities typically presenting bilaterally; of the 513 individual readings that identified at least one large opacity and had information on location, 33.7% had large opacities in a single lung and 6.8% had solely lower or middle lung zone involvement. Often PMF is thought to present as large opacities that are elongated and less spherical than other lesions. We looked at the aspect ratio and only 4% were found to have an aspect ratio (long axis diameter/short axis diameter) greater than 3 suggesting an elongated shape.
The ILO classification system requires readers to indicate the affected zones of the lung and shape and size of small opacities. However, for large opacities, the system requires no indication of shape or location, and a single indication of opacity size, which indicates either a single large opacity or the summation of the area of multiple large opacities. Therefore, we designed a data collection tool that permitted readers to record information on the number, shape, predominant lung zone, and size of each large opacity observed.
We found that large opacities were more common in the right lung and the upper zones. Possible reasons for upper zone predominance may include slow lymphatic drainage in the upper lobes.23,24
Additionally, visceral pleural lymphatics in the upper lobes have delayed clearance which results in increased deposition of dust around bronchovascular lymphatics in this region.25 The finding that large opacities more commonly appear in the right lung is likely associated with normal airway anatomy. The right upper lobe bronchus arises shortly beyond the bifurcation of the main stem bronchi at the carina making the right upper lobe the shortest pathway for dust deposition. In some cases, the right upper lobe bronchus may arise from the distal trachea. Furthermore, particle deposition models have suggested that the upper lobes, especially the upper right lobe, may receive the highest deposition of inhaled particles.26,27
It is important to note that unilateral large opacities require a full clinical evaluation to rule out other pathologies, including lung cancer. Evaluation with CT/PET scanning and other modalities including biopsy may be required unless radiologic stability can be demonstrated. One recent study demonstrated the utility of magnetic resonance imaging (MRI) using the T2W sequence in differentiating PMF from lung cancer.28
Strengths of this study include a large sample radiographs previously identified as having PMF; three B Reader interpretations for each radiograph; and a standardized and straightforward data collection tool. No true gold standard exists for determining the presence of PMF, however plain chest radiographs are the most widely used technique to screen for pneumoconiosis. Classification of chest radiographs has subjective components; therefore, to minimize impacts reader variability three Readers characterized the radiographs in this study. The Readers did not agree on every case of PMF. However, for the metrics we were measuring (shape/size, location) the findings were overall consistent among the interpretations performed by the Readers. Classifying digitized radiographs is not an optimal practice. Therefore, a potential limitation of our study is that the performance of ILO classification of digitized radiographs originally acquired as analog film has not been rigorously studied. This change in technique may have introduced some variability relative to past classifications of film radiographs.
Since this study required collection of data beyond the standard ILO reading B Readers are asked to perform for our surveillance program, the B Readers could not be entirely blinded to the objectives of the study. The impact of this cannot be fully assessed, however given the consistency of the findings we have confidence that the overall characterization of large opacities we observed is robust.
There is some indication that current coal mine dust exposures and pneumoconiosis patterns may not be similar to historical observations. Whether the cases and characteristics represented in this report are typical of what may have been observed in the past or reflect new patterns cannot be ascertained because we limited our cases from 2000 to 2015. However, our findings are broadly consistent with the historical descriptions and textbook definitions of PMF so this is likely not a significant factor. Having a data-driven empirical understanding of the diversity of large opacity radiographic presentations is important for clinical, epidemiological, and medico-legal purposes. Our findings establish a clear pattern that can be used as a baseline to measure whether disease configurations change over time.
Acknowledgments
The authors wish to acknowledge the contribution of the NIOSH Coal Workers’ Health Surveillance Team, the B Readers who participated in this study, and Drs. Eileen Storey, and Edward (Lee) Petsonk for their helpful reviews of the manuscript.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
Agency disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. Mention of product names does not imply endorsement by NIOSH/CDC.
IRB
This study used existing radiographs, received a waiver of informed consent, and was approved by the NIOSH Institutional Review Board (15-DRDS-03XP).
References
- 1.Federal Coal Mine Health and Safety Act. Title 30 Code of Federal Regulations, United States; 1969. [Google Scholar]
- 2.Antao VC, Petsonk EL, Sokolow LZ, et al. Rapidly progressive coal workers’ pneumoconiosis in the United States: geographic clustering and other factors. Occup Environ Med. 2005;62:670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suarthana E, Laney AS, Storey E, Hale JM, Attfield MD. Coal workers’ pneumoconiosis in the United States: regional differences 40 years after implementation of the 1969 Federal Coal Mine Health and Safety Act. Occup Environ Med. 2011;68(12):908–913. doi: 10.1136/oem.2010.063594. [DOI] [PubMed] [Google Scholar]
- 4.Laney AS, Weissman DN. Respiratory diseases caused by coal mine dust. J Occup Environ Med. 2014;56: S18–S22. doi: 10.1097/JOM.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackley DJ, Halldin CN, Laney AS. Continued increase in prevalence of coal workers’ pneumoconiosis in the United States, 1970–2017. Am J Public Health. 2018;108(9):1220–1222. doi: 10.2105/AJPH.2018.304517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackley DJ, Crum JB, Halldin CN, Storey E, Laney AS. Resurgence of progressive massive fibrosis in coal miners - Eastern Kentucky, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(49):1385–1389. doi: 10.15585/mmwr.mm6549a1. [DOI] [PubMed] [Google Scholar]
- 7.International Labour Office. Guidelines for the Use of the ILO International Classification of Radiographs of Pneumoconioses. Geneva, Switzerland: International Labour Office; 2011. [Google Scholar]
- 8.Castranova V, Vallyathan V. Silicosis and coal workers’ pneumoconiosis. Environ Health Perspect. 2000; 108 (Suppl 4):675–684. doi: 10.1289/ehp.00108s4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wade WA, Petsonk EL, Young B, Mogri I. Severe occupational pneumoconiosis among West Virginian coal miners: one hundred thirty-eight cases of progressive massive fibrosis compensated between 2000 and 2009. Chest. 2011;139(6):1458–1462. doi: 10.1378/chest.10-1326. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin RA, Des Prez RM. Apical localization of pulmonary tuberculosis, chronic pulmonary histoplasmosis, and progressive massive fibrosis of the lung. Chest 1983;83(5):801–805. doi: 10.1378/chest.83.5.801. [DOI] [PubMed] [Google Scholar]
- 11.Banks DE. 2005. Silicosis In: Textbook of clinical occupational and environmental medicine. Philadelphia, PA: Elsevier Saunders. [Google Scholar]
- 12.Cole RB. Essentials of Respiratory Disease. Philadelphia, PA: Lippincott; 1972. [Google Scholar]
- 13.Cowie RL, Murray J, Becklake MR. 2010. Pnuemoconioses and other dust-related diseases In: Murray & Nadel’s Textbook of respiratory medicine. Oxford, UK: Elsevier Health Sciences. [Google Scholar]
- 14.Morgan WKC, Seaton A. Occupational lung diseases. Philadelphia, PA: Saunders; 1975. [Google Scholar]
- 15.Petsonk EA, Attfield MD. 2005. Respiratory diseases of coal miners In: Textbook of clinical occupational and environmental medicine. Philadelphia, PA: Elsevier Saunders. [Google Scholar]
- 16.Narayanasamy S, Singh J, Sathiadoss PA, Khan MS, Jamal F, Zaman N. Angel’s wing appearance on chest radiograph - progressive massive fibrosis. Clin Med (Lond). 2015;15(3):307. doi: 10.7861/clinmedicine.15-3-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Pneumoconiosis prevalence among working coal miners examined in federal chest radiograph surveillance programs-United States, 1996–2002. Morb Mortal Wkly Rep (MMWR) 2003;52:336–340. [PubMed] [Google Scholar]
- 18.Laney AS, Petsonk EL, Wolfe AL, Attfield MD. Comparison of storage phosphor computed radiography with conventional film-screen radiography in the recognition of pneumoconiosis. Eur Resp J. 2010; 36(1):122–127. doi: 10.1183/09031936.00127609. [DOI] [PubMed] [Google Scholar]
- 19.Amandus HE, Lapp NL, Morgan WK, Reger RB. Pulmonary zonal involvement in coal workers’ pneumoconiosis. J Occup Med. 1974;16(4):245–247. [PubMed] [Google Scholar]
- 20.Laney AS, Petsonk EL. Small pneumoconiotic opacities on U.S. coal worker surveillance chest radiographs are not predominantly in the upper lung zones. Am J Ind Med. 2012;55(9):793–798. doi: 10.1002/ajim.22049. [DOI] [PubMed] [Google Scholar]
- 21.Young RC Jr., Rachal RE, Carr PG, Press HC. Patterns of coal workers’ pneumoconiosis in Appalachian former coal miners. J Natl Med Assoc. 1992;84(1):41–48. [PMC free article] [PubMed] [Google Scholar]
- 22.Almberg KS, Halldin CN, Blackley DJ, et al. Progressive massive fibrosis resurgence identified in us coal miners filing for black lung benefits, 1970–2016. Ann Am Thoracic Soc. 2018;15(12): 1420–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egashira R, Tanaka T, Imaizumi T, et al. Differential distribution of lymphatic clearance between upper and lower regions of the lung. Respirology. 2013;18(2): 348–353. doi: 10.1111/resp.12006. [DOI] [PubMed] [Google Scholar]
- 24.West JB. Regional differences in the lung. Chest. 1978; 74(4):426–437. [DOI] [PubMed] [Google Scholar]
- 25.Gurney JW, Schroeder BA. Upper lobe lung disease: physiologic correlates. Review. Radiology 1988;167(2): 359–366. doi: 10.1148/radiology.167.2.3282257. [DOI] [PubMed] [Google Scholar]
- 26.Asgharian B, Hofmann W, Bergmann R. Particle deposition in a multiple-path model of the human lung. Aerosol Sci Technol. 2001;34:332–339. doi: 10.1080/02786820119122. [DOI] [Google Scholar]
- 27.Subramaniam RP, Asgharian B, Freijer JI, Miller FJ, Anjilvel S. Analysis of lobar differences in particle deposition in the human lung. Inhal Toxicol. 2003; 15(1):1–21. doi: 10.1080/08958370304451. [DOI] [PubMed] [Google Scholar]
- 28.Ogihara Y, Ashizawa K, Hayashi H, et al. Progressive massive fibrosis in patients with pneumoconiosis: utility of MRI in differentiating from lung cancer. Acta Radiol. 2018;59(1):284185117700929. [DOI] [PubMed] [Google Scholar]