Abstract
Portal vein thrombosis (PVT) is associated with inferior pre- and post-transplantation outcomes. We aimed to create a predictive model to risk stratify transplant candidates for PVT. Data on adult transplants in the United States during the Model for End stage Liver Disease (MELD) era through September 2016 were reviewed. We constructed and validated a scoring system comprised of routine, readily available clinical information to predict the development of incident PVT at 12 months from transplantation listing. 66,568 liver transplant candidates were dichotomized into two groups to construct (n=34,751) and validate (n=31,817) a scoring system. In general, the derivation and validation cohorts were clinically similar. While nonalcoholic steatohepatitis was the strongest independent predictor of incident PVT (HR 1.29, 95% CI 1.08–1.54, p <0.001), age, MELD score, and moderate-severe ascites were associated with increased risk. African American race was associated with decreased risk. A scoring system (PVT Risk Index) of these five variables had area under the curve of 0.71 and 0.70 in both estimation and validation cohorts respectively. By applying the low cutoff score of 2.6, incident PVT could be accurately excluded (94% negative predictive value). Using the high cutoff score of 4.6 (85% positive predictive value), PVT could be diagnosed with high accuracy. The PVT-RI predicts which candidates awaiting lifesaving liver transplantation will and will not develop future PVT. Although this scoring system will require prospective validation, it provides a powerful new tool for the clinician when risk stratifying cirrhosis patients prior to liver transplantation for future PVT development.
Keywords: cirrhosis, nonalcoholic steatohepatitis, hypercoagulability, anticoagulation, prophylaxis
In patients with cirrhosis, the hemostatic environment is a precarious balance. Because of a relative deficiency of both procoagulant and anticoagulant factors, the balance can be easily tipped between bleeding and thrombosis, depending on the provocation.(1–3) This rebalance between procoagulant, anticoagulant and fibrinolytic systems in chronic liver disease is the focus of much research and remains a clinically relevant, controversial topic. Portal vein thrombosis (PVT) occurs in upwards of 30% of liver transplant candidates.(4) PVT is no longer considered to be an absolute contraindication to liver transplantation due to advances in liver transplantation technique as well as better imaging modalities and classification systems to define the extent of clot burden.(5, 6) Despite newer approaches, patient-centered outcomes remain inferior in candidates with PVT in both the pre- and post- transplant setting with greater rates of hepatic decompensation, decreased post-transplant survival, and lower health-related quality of life.(7–10) Risk factors for incident PVT include reduced portal vein blood flow, severity of liver disease, hepatocellular carcinoma, and also etiology of liver disease [e.g., nonalcoholic steatohepatitis (NASH), autoimmune hepatitis].(11–16) Acquired hypercoagulability also plays a role in PVT development through elevated levels of prohemostatic Factor VIII and decreased levels of the naturally occurring prohemostatic protein C, protein S, antithrombin as well as heparin cofactor II.(17) Rates of the JAK2 V6i7F mutation, the antiphospholid antibody syndrome, and the inherited Factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations are also more prevalent in cirrhosis patients with PVT.(18, 19)
Despite these widely accepted risk factors, a predictive scoring system has yet to be developed to determine future risk of incident PVT. An accurate predictive model would greatly aid clinical decision making and guide best clinical practices. For these reasons, we aimed to create a predictive model to stratify transplant candidates by PVT risk and to apply this prediction tool to best determine which liver transplant candidates are at both greatest and least risk of developing PVT.
Methods
Case selection and study design:
Utilizing the nationwide United Network for Organ Sharing/Organ Procurement and Transplantation Network (UNOS/OPTN) database, we performed a retrospective analysis of liver transplant candidates in the MELD era beginning February 2002 through September 2016. All recipients transplanted for acute liver failure, re-transplants and status 1a indication (urgent re-transplantation or acute liver failure) were excluded to avoid cohort heterogeneity. To ensure an adequate predictive validated study, we divided the total number individuals studied into two cohorts: a derivation cohort and a validation cohort. We sorted the first group of individuals (derivation cohort) by the time frame February 1st, 2002 through December 31st, 2009. The second group (validation cohort) included candidates transplanted between January 1st 2010 through September 30th, 2016. Baseline characteristics were reviewed, including demographics (age at listing and transplantation, body mass index, gender, ethnicity), diabetes, etiology of liver disease, hepatocellular carcinoma, hemodialysis need, laboratory values (bilirubin, international normalized ratio [INR], creatinine and albumin), MELD exception, portal hypertension manifestations (moderate to severe ascites and hepatic encephalopathy), severity of liver disease, and spontaneous bacterial peritonitis. Inherited and acquired thrombophilia data is not readily captured in the UNOS database nor is information regarding candidate anticoagulation use.
Outcome definition:
Incident PVT was defined as “no” PVT at liver transplantation listing and “yes” PVT at the time of liver transplantation, confirmed by direct examination of the explant by the liver transplant surgeon. No incident PVT was defined as no PVT identified at listing and no PVT identified at transplantation. Candidates with PVT at listing or with unknown PVT status were further excluded from the analysis as they did not meet the a priori definition of incident PVT. “Unknown” PVT status at the time of liver transplantation was also excluded. The supplementary materials contain information showing which subjects were excluded and for what reasons.
Statistical analysis:
Patient characteristics are reported as means with 95% confidence intervals of continuous data and percentages of categorical data where appropriate. Univariate analysis was completed with standard measures including Chi-square test, Fisher’s exact test, Student t-test, Wilcoxon rank test, as appropriate for categorical and continuous variables. To create a predictive model, variables were analyzed by univariate logistic regression to predict incident PVT in the derivation cohort. Variables were eliminated for (1) a p-value of >0.20 or (2) concern for a lack of clinical significance. All remaining factors were entered into a stepwise multivariable cox-proportional hazards analysis for incident PVT prediction. A p-value cutoff of 0.20 was used to stay in the final model. Ultimately, five variables were incorporated into the PVT Risk Index (PVT-RI): age, African American race, MELD score, moderate to severe ascites (ascites causing moderate symmetrical distention of the abdomen or ascites causing marked abdominal distention) and NASH. The PVT-RI was then applied to the validation cohort and concordant statistics were compared. Receiver operating characteristic curves (ROC) were constructed to assess the sensitivity and specificity of the PVT-RI in predicting incident PVT within 12-months after liver transplantation listing. The optimal cutoff for incident PVT risk was determined by the PVT-RI. Using the ROC curve for the final model, two cutoff points were selected in order for both positive predictive value and negative predictive value for incident PVT were at least 85%, on the assumption that false results of less than 15% are clinically acceptable. The diagnostic accuracy of the two cutoff points was determined by calculating sensitivity, specificity, PPV, NPV and likelihood ratios. Data management and analyses were performed using SAS (version 9.4, Cary, NC) and R (version 3.4.0.). Institutional review board approval was obtained. No organ donations from prisoners were included in this study.
Results
Comparison of derivation and validation cohorts:
66,568 candidates met inclusion criteria and were listed for liver transplantation between February 2002 through September 2016 and included in our analysis. For the overall cohort, the mean age was 54 +/− 10 years and mean MELD score at listing 18 +/− 9. The incidence rate of PVT was 6.5% (n=4,311). Candidates were divided into a derivation cohort (n=34,751) and a validation cohort (n=31,817), based on the timeframes of February 1st, 2002 through December 31st, 2009 and January 1st 2010 through September 30th, 2016, respectively. In general, the derivation and validation cohort were clinically similar with several notable exceptions (Table 1). The mean age of derivation cohort and validation cohort were 53.1 years old and 55.3 years old respectively (p<0.001). MELD score at listing in the derivation group was 17.1 versus 19.1 for the validation group (p<0.001). Etiology of liver disease was different between the two groups as well. The derivation cohort had a greater proportion of hepatitis C (39.5% versus 33.3%, p<0.001) while NASH/cryptogenic (16.2% vs. 9.4%, p<0.001) and alcoholic liver disease (17.3% vs. 14.0%, p<0.001) were more common in the validation cohort. Incident PVT while statistically different between the two groups (4.9% derivation vs. 8.2% validation, p<0.001) was not felt to be of clinical significance.
Table 1:
Comparison of baseline characteristics of both cohorts
| Derivation Cohort 2002–2009 (n=34,751) | Validation Cohort 2010–2016 (n=31,817) | p-value | |
|---|---|---|---|
| Age at listing, mean (years) | 53.1 (53.0–53.2) | 55.3 (55.2–55.4) | <0.001 |
| Male gender, n (%) | 21536 (67.7) | 23908 (68.8) | 0.01 |
| MELD score at listing, mean | 17.1 (17.0–17.2) | 19.1 (19.0–19.2) | <0.001 |
| Etiology of liver disease, n (%) | |||
| Alcohol | 4873 (14.0) | 5512 (17.3) | <0.001 |
| Autoimmune | 823 (2.4) | 815 (2.6) | |
| Cholestatic | 2646 (7.6) | 2067 (6.5) | |
| HBV | 1003 (2.9) | 661 (2.1) | |
| HCV | 13729 (39.5) | 10609 (33.3) | |
| NASH/Cryptogenic | 4176 (9.4) | 5150 (16.2) | |
| Malignancy | 3281 (9.4) | 4252 (13.4) | |
| Other | 4220 (12.1) | 2751 (8.7) | |
| Laboratories at transplantation | |||
| Albumin (g/dL) | 3.00 (2.98–3.00) | 3.08 (3.07–3.09) | <0.001 |
| Bilirubin (g/dL) | 5.1 (5.0–5.2) | 6.4 (6.3–6.5) | <0.001 |
| Creatinine (g/dL) | 1.32 (1.31–1.33) | 1.43 (1.42–1.44) | <0.001 |
| INR | 1.57 (1.56–1.58) | 1.69 (1.68–1.69) | <0.001 |
| Sodium (mEq/L) | 136.0 (136.0–136.1) | 135.8 (135.7–135.8) | <0.001 |
| BMI at listing, mean (kg/m2) | 28.5 (28.4–28.5) | 29.0 (28.9–29.1) | <0.001 |
| Waiting list time, mean (days) | 256 (251–261) | 191 (188–193) | <0.001 |
| Incident portal vein thrombosis, n (% | 1718 (4.9) | 2596 (8.2) | <0.001 |
| Ethnicity, n (%) | |||
| African American | 2999 (8.6) | 3127 (9.8) | <0.001 |
| Asian | 1920 (5.5) | 1758 (5.5) | 1.00 |
| Caucasian | 25443 (73.2) | 22636 (71.1) | <0.001 |
| Hispanic | 4389 (12.6) | 4296 (13.5) | <0.001 |
| Diabetes, n (%) | 8209 (23.6) | 8627 (27.1) | <0.001 |
| Portal hypertension, n (%) | |||
| Ascites > grade 2 | 9622 (27.7) | 9815 (30.9) | <0.001 |
| HE > grade 2 | 3445 (9.9) | 3140 (9.9) | 0.85 |
| Hepatocellular carcinoma, n (%) | 7871 (22.7) | 8021 (25.2) | <0.001 |
| MELD exception (including HCC), n (%) | 10454 (30.1) | 11411 (35.9) | <0.001 |
| SBP, n (%) | 1951 (5.6) | 2357 (7.4) | <0.001 |
BMI=Body Mass Index; HBV=Hepatitis B; HCV=Hepatitis C; HE=Hepatic Encephalopathy INR=International Normalized Ratio; MELD=Model for End Stage Liver Disease; NASH=Nonalcoholic Steatohepatitis; SBP=Spontaneous Bacterial Peritonitis
In general, the derivation and validation cohorts, while statistically different, were clinically similar. Notable exceptions included etiology of liver disease where a temporal shift was seen between HCV and NASH/Cryptogenic., severity of liver disease and a slight increase in utilization of MELD exceptions.
Comparison of recipients with incident PVT vs. recipients without incident PVT:
On unadjusted univariate analysis, recipients with incident PVT (n=4,311) were different when compared to recipients without incident PVT (n=62,254) across race, ethnicity, comorbidity, etiology and severity of liver disease as shown in Table 2. Significant predictors of incident PVT included NASH/cryptogenic (HR 1.29, 95% CI 1.08–1.54, p<0.001), moderate/severe ascites (HR 1.14, 95% CI 1.03–1.27, p=0.01), MELD score (HR 1.10, 95% CI 1.09–1.11,p<0.001) and age (HR 1.03, 95% CI 1.02–1.03, p<0.001). African American race was associated with lower risk of PVT (HR 0.77, 95% CI 0.63–0.94, p<0.001).
Table 2:
Univariate analysis of variables comparing recipients with and without incident PVT at the time of liver transplantation
| Incident PVT (n=4,311) | No Incident PVT (n=62,254) | p-value | |
|---|---|---|---|
| African American, n (%) | 260 (6.3) | 5856 (9.4) | <0.001 |
| Age, years, mean (95% CI) | 55.3 (55.0–55.6) | 54.0 (53.9–54.1) | <0.001 |
| Diabetes, n (%) | 1360 (31.5) | 15476 (24.9) | <0.001 |
| HCC, n (%) | 958 (22.2) | 14934 (24.0) | 0.01 |
| HCV, n (%) | 1066 (24.7) | 18212 (29.3) | <0.001 |
| Hispanic, n (%) | 671 (15.6) | 8014 (12.9) | <0.001 |
| MELD, mean (95% CI) | 17.3 (17.1–17.6) | 18.1 (18.0–18.2) | <0.001 |
| Moderate-severe ascites, n (%) | 1427 (33.1) | 18010 (28.9) | <0.001 |
| NASH, n (%) | 751 (17.4) | 7693 (12.4) | <0.001 |
| TIPS, n (%) | 587 (13.6) | 6253 (10.0) | <0.001 |
Hepatitis C; MELD=Model for End Stage Liver Disease; NASH=Nonalcoholic Steatohepatitis; PVT=Portal Vein Thrombosis; TIPS=Transjugular Intrahepatic Portosystemic Shunt
The proportion of NASH was greater among total candidates with incident PVT while HCV was less frequent. Race and ethnicity were important as well with greater rates of PVT in Hispanics and lower rates in Blacks.
PVT-RI Model Development:
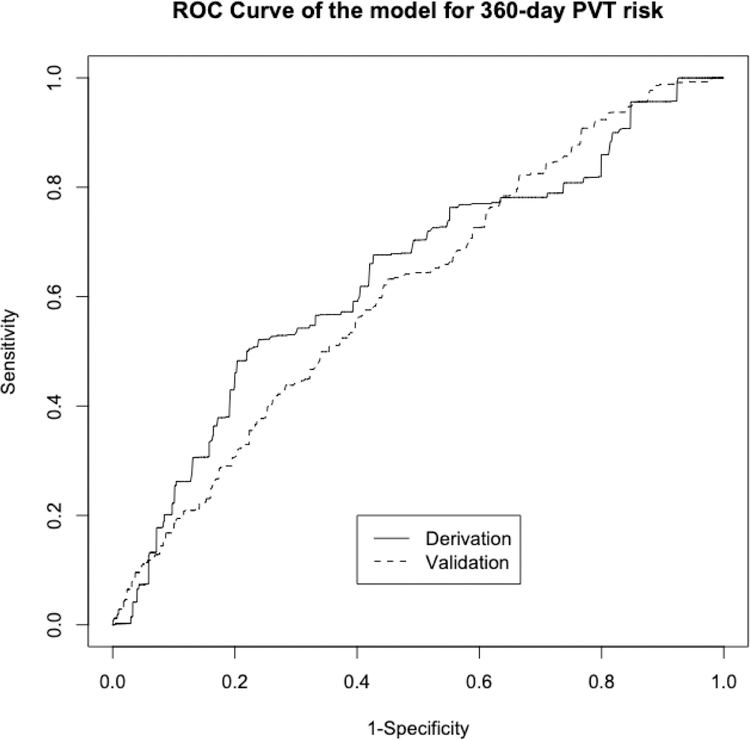
After evaluating for risk factors for incident PVT, variables with a p-value <0.20 were entered into stepwise multivariable modeling. The final model consisted of five clinical risk factors. The PVT-RI regression equation is calculated as: 0.335*NASH + 0.095*MELD score + 0.126*moderate/severe ascites + 0.028*age (years) - 0.261*African American race (Figure 1). The PVT-RI was found to have area under the ROC curve of 0.712 in the derivation cohort, which is similar to the area under the ROC curve for MELD score in predicting mortality (Figure 2).(20) Using the area under the ROC curve, two cutoff points were selected in the derivation cohort of 34,751 transplant recipients to identify high-risk (greater than 4.6) and low-risk (less than 2.6) profiles for future PVT development. The diagnostic accuracy of the PVT-RI in identifying transplant candidates who would either develop or not develop future PVT was cross-validated in the validation cohort of 31,817 recipients. The area under the ROC curve remained high in the validation cohort at 0.701. By applying the low cutoff point of <2.6, PVT could be ruled out with high accuracy (NPV 94%). Using the high cutoff point >4.6, the PVT-RI correctly predicted who would develop future PVT with high accuracy (85% PPV) (Table 3).
Figure 1. Portal Vein Thrombosis Risk Index Equation.
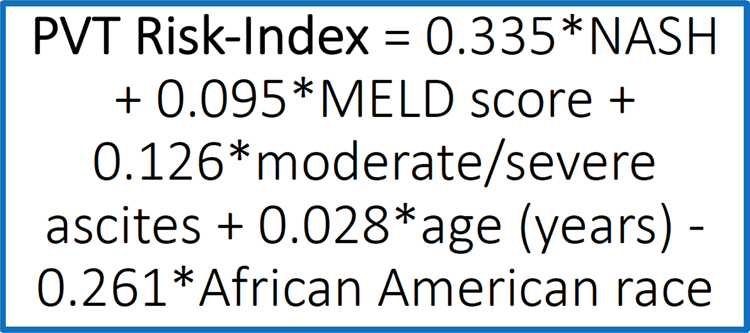
Legend: The regression equation for the PVT-RI
Figure 2. Receiver operating curve for the Portal Vein Thrombosis Risk Index.
Legend: An AUROC of 0.712 was obtained in the derivation cohort and 0.701 in the validation cohort.
Table 3-.
Predictive Value of the Scoring System Obtained from the Validation Group*
| Low cutoff point (<2.6) | Indeterminate (2.6–4.6) | High cutoff point (>4.6) | Total | |
|---|---|---|---|---|
| Total | 6434 | 21108 | 4267 | 31809** |
| No Incident PVT | 6011 | 1895 | 4267 | 29213 |
| Incident PVT | 432 | 1895 | 278 | 2596 |
| Sensitivity | 85% | 9% | ||
| Specificity | 36% | 100% | ||
| Positive predictive value | 16% | 85% | ||
| Negative predictive value | 94% | 88% | ||
| Likelihood ratio (+) | 1.3 | 38.8 | ||
| Likelihood ratio (−) | 0.4 | 0.9 | ||
| Interpretation | PVT will not develop over next 12 months (94% certainty) | PVT will develop over next 12 months (85% certainty) |
Incident PVT was 8.2% in the validation group.
PVT-RI unable to be calculated for eight recipients due to missing data
Predictive Values of PVT-RI for Difference Incident Rates of PVT:
Incidence rates of PVT widely vary across the literature and may be as high as 16%,(11) Therefore, we calculated PPV and NPV for the two cutoff points using varying incident rates from 2.5% to 20%. The NPV of the low cutoff point to exclude future PVT remained high, particularly for incidence rates above 2.5% (Table 4). The PPV of the high cutoff point to diagnose future PVT also remained high for incidence rates above 5%. Thus, these two cutoff points may be used to predict and rule out future PVT in medical centers and different geographic regions where PVT rates and management varies.
Table 4-.
Predictive Values of the Cutoff Points for Different Incidence Rates of PVT
| Incidence of PVT (%) | Low Cutoff Value (<2.6) NPV | High Cutoff Value (>4.6) PPV |
|---|---|---|
| 2.5 | 96% | 58% |
| 5 | 92% | 74% |
| 10 | 85% | 86% |
| 15 | 77% | 90% |
| 20 | 64% | 95% |
Future PVT could be excluded at incidence rates of ≥2.5% and predicted at incidence rates >5%.
Clinical Use of PVT-RI:
For ease of clinical decision making, the PVT-RI is widely available as an online calculator and can be freely accessed from anywhere at https://app-phs.hmc.psu.edu/pvtriskindex/. Screenshots of the website are included in the supplementary materials.
Discussion
PVT is a common complication in liver transplant candidates that is strongly associated with inferior outcomes in the perioperative transplant setting including increased morbidity and mortality which parallel lower health-related quality of life.(7–10) Consequently, the decision to proceed with liver transplantation listing, surveillance imaging and treatment with either anticoagulation or TIPS in transplantation candidates with PVT remains one of much debate and uncertainly. While clinical risk factors for PVT are well described, to date, data are limited regarding which liver transplant candidates are at high or low risk for the development of incident PVT and a predictive scoring system which considers multiple variables simultaneously has not yet been developed. In this study, we developed a tool that can enhance clinical decision making in liver transplant candidates, quantifying which patients are at both high and low-risk for PVT development with the goal of impacting the associated morbidity and mortality that may necessitate waiting-list removal in those candidates at risk for PVT.
Our study extends the literature on PVT in liver transplant candidates in three ways. First, we have identified ten baseline predictors of PVT risk including African American ethnicity, age, diabetes, chronic hepatitis C, hepatocellular carcinoma, Hispanic ethnicity, MELD score, moderate/severe ascites, NASH and TIPS. Second, we have used five of these predictors to construct a risk score calculator with the PVT-RI to provide a new clinical decision-making tool to more accurately predict or rule out future PVT in at risk individuals within twelve months of liver transplantation listing. Third, we have shown that NASH remains a strong independent predictor of PVT throughout the entire MELD era.
Our findings confirm that NASH remains a strong independent predictor of PVT risk in liver transplant candidates with cirrhosis.(11, 15) This is increasingly important given the worsening obesity and NASH epidemics which parallel the increasing demand for liver transplantation as evidenced by the fact that NASH is the leading indication for liver transplantation in women and simultaneous liver-kidney transplantation for all genders.(21, 22) Perhaps more importantly, recipients with NASH and PVT have inferior post-transplantation survival as demonstrated by Agbim et al.(10) who found a 37% increased risk of graft failure and 31% increased risk of overall death in NASH transplant recipient with PVT compared to NASH recipients without PVT at transplant. As more and more patients develop NASH cirrhosis, we would expect an increasing number of high-risk patients to be identified by the PVT-RI, further increasing its future clinical utility.
Race and ethnicity may also play a role in PVT risk. Confirming previous findings,(23) we documented that PVT was less common in African American recipients suggesting that there may be an unknown genetic determinant of thrombotic risk in patients with cirrhosis. While we are unaware of any studies investigating the role of genetics in PVT development, we recognize that performing genome wide association studies in this population is an intriguing avenue of investigation, especially given the shifting racial and ethnicity of the population listed for liver transplantation in the United States.(24)
Additionally, we show that moderate-severe ascites is associated with PVT risk. However, because the existing analysis is temporally limited by the UNOS data collection instrument, the question as to whether PVT leads to development of greater volumes of ascites or whether ascites is a surrogate marker for PVT risk cannot be answered. TIPS, which is often used as a treatment for PVT, was also found to be a predictor of incident PVT. However, due to limitations in the UNOS database including the temporality between TIPS placement and PVT development, again, concrete conclusions cannot be drawn from this finding. What seems most likely is that the database is capturing small intrahepatic PVTs that develop after a TIPS is placed that is of no clinical significance. In addition, the association between TIPS and incident PVT was attenuated with multivariable modeling and was no longer statistically significant. For this reason, we did not include TIPS in our final model.
Whether or not prophylactic intervention in the high-risk group identified by the PVT-RI is beneficial remains to be determined. This offers an avenue of thought provoking future prospective study with the inclusion of any transplant candidate with a PVT-RI >4.6 as a target population for intervention. Villa et al.(25) previously demonstrated that prophylactic administration of low molecular weight heparin for 12 months prevented development of de novo PVT in patients with compensated cirrhosis with an associated reduction in mortality and hepatic decompensation in the absence of any clinically relevant bleeding events. Our findings reinforce the importance of this study as preventing the development of PVT is of great interest, especially given the link between pre-transplantation PVT and early hepatic artery thrombosis with its high morbidity and mortality within 90-days following liver transplantation.(26, 27) It is plausible that by utilizing the PVT-RI prospectively to identify those patients at greatest risk for developing PVT, future therapeutic studies could be developed to offer the most clinical utility and benefit to patient outcomes. Furthermore, identifying the group of liver transplant candidates at greatest risk for PVT development may offer avenue for improved screening and surveillance practices.
Additionally, discerning which liver transplant candidates are at low risk for future PVT is also of great clinical importance. Current guidelines recommend screening all patients with cirrhosis, including those who are liver transplant candidates, for PVT with ultrasound with doppler every six months regardless of their risk profile.(1) Based on our findings, we would suggest that the lower cutoff of the PVT-RI could be utilized to avoid unnecessary testing in low-risk patients by eliminating the need for doppler testing. As nearly 20% of the cohort was low-risk, the PVT-RI in this population has the potential to lower healthcare utilization and decrease the high cost associated with cirrhosis and its complications. This may be accomplished by one, not performing screening doppler studies in low-risk populations; two, minimizing the possibility of false-positives and further downstream testing with confirmatory cross-sectional imaging; and three, avoiding additional incidental findings that require further clinical investigation.
Our findings must be interpreted in the context of the study design. The primary outcome of the UNOS database is to provide data regarding liver transplantation outcomes on a nationwide basis. With this in mind, there are notable limitations inherent in a large, secondary retrospective analysis that may be subjected to missing data as well as the possibility of confounding variables that may have not been considered (e.g., co-morbidities at time of transplant listing). Additionally, there is no information on anticoagulation status or data on thrombophilia of any individuals listed which may offer additional performance to the PVT-RI model as both of these are established to play a role in PVT development. The UNOS database also does not have data on the extent (e.g., partial vs complete), chronicity (e.g., acute, subacute or chronic) or vessels involved (e.g., splenic vein, superior mesenteric vein) by the thrombus. Furthermore, portal vein velocity has been shown to be a strong predictor of incident PVT development(12, 28) and the UNOS database also does not contain information from doppler ultrasound measurements of this parameter which again could be used to enhance the predictive ability of the PVT-RI. Lastly, although the proportion of liver transplant candidates with incident PVT is somewhat lower than that reported by others, the vast number of transplant candidates included in both the derivation and validation cohorts is enough to offset this and we performed subsequent sensitivity analysis looking at various PVT incidence thresholds (Table 3). The strict definition criteria based on direct examination of the explanted liver by the transplant surgeon is a strength of the study design in that it overcomes false positive results obtained with radiological imaging.
In conclusion, this is the first study that has developed a predictive model utilizing a large nationwide data base of transplant recipients to both predict and exclude de novo PVT formation within 12-months prior to liver transplant with high accuracy. This is important as PVT is a common occurrence that results in inferior outcomes in the pre- and post-transplant setting. The PVT-RI predicts which candidates awaiting lifesaving liver transplantation will develop future PVT and provides a new diagnostic threshold for low-risk candidates who will not develop an incident PVT. Although our model requires prospective evaluation, it offers an important new tool for the clinician to improve transplant morbidity and mortality, may decrease costly testing that is of low clinically utility, and may open doors for future investigation into the benefit of pharmacologic prophylaxis in high-risk populations.
Supplementary Material
Acknowledgments
Grants and Financial Support: This grant was funded in part by NIH grant L30 DK118601
Dr. Stine has received research funding from Target PharmaSolutions, Inc. Dr. Eyster has received research funding from Bayer, Baxalta Shire and Spark Therapeutics.
Abbreviations:
- BMI
Body Mass Index
- CI
Confidence Interval
- HBV
Hepatitis B Virus
- HCV
Hepatitis C Virus
- HE
Hepatic Encephalopathy
- INR
International Normalized Ratio
- MELD
Model for End stage Liver Disease
- PVT
Portal Vein Thrombosis
- ROC
Receiver operating characteristic curves
- RI
Risk Index
- SBP
Spontaneous Bacterial Peritonitis
- TIPS
Transvenous Intrajugular Portal Vein Shunt
- UNOS/OPTN
United Network for Organ Sharing/ Organ Procurement and Transplantation Network
Footnotes
Conflict of Interest: The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
References:
- 1.Intagliata NM, Argo CK, Stine JG, Lisman T, Caldwell SH, Violi F. Concepts and Controversies in Haemostasis and Thrombosis Associated with Liver Disease: Proceedings of the 7th International Coagulation in Liver Disease Conference. Thrombosis and haemostasis 2018;118(8):1491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tripodi A, Primignani M, Chantarangkul V, Dell’Era A, Clerici M, de Franchis R, et al. An imbalance of pro- vs anti-coagulation factors in plasma from patients with cirrhosis. Gastroenterology 2009;137(6):2105–11. [DOI] [PubMed] [Google Scholar]
- 3.Kujovich JL. Coagulopathy in liver disease: a balancing act. Hematology American Society of Hematology Education Program 2015;2015:243–9. [DOI] [PubMed] [Google Scholar]
- 4.DeLeve LD, Valla DC, Garcia-Tsao G. Vascular disorders of the liver. Hepatology (Baltimore, Md) 2009;49(5):1729–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarin SK, Philips CA, Kamath PS, Choudhury A, Maruyama H, Nery FG, et al. Toward a Comprehensive New Classification of Portal Vein Thrombosis in Patients With Cirrhosis. Gastroenterology 2016;151(4):574–7.e3. [DOI] [PubMed] [Google Scholar]
- 6.Yerdel MA, Gunson B, Mirza D, Karayalcin K, Olliff S, Buckels J, et al. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation 2000;69(9):1873–81. [DOI] [PubMed] [Google Scholar]
- 7.Englesbe MJ, Schaubel DE, Cai S, Guidinger MK, Merion RM. Portal vein thrombosis and liver transplant survival benefit. Liver Transpl 2010;16(8):999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Castro KI, Porte RJ, Nadal E, Germani G, Burra P, Senzolo M. Management of nonneoplastic portal vein thrombosis in the setting of liver transplantation: a systematic review. Transplantation 2012;94(11):1145–53. [DOI] [PubMed] [Google Scholar]
- 9.Hibi T, Nishida S, Levi DM, Selvaggi G, Tekin A, Fan J, et al. When and Why Portal Vein Thrombosis Matters in Liver Transplantation: A Critical Audit of 174 Cases. Annals of Surgery 2013. [DOI] [PubMed]
- 10.Agbim U, Jiang Y, Kedia SK, Singal AK, Ahmed A, Bhamidimarri KR, et al. Impact of Nonmalignant Portal Vein Thrombosis in Transplant Recipients With Nonalcoholic Steatohepatitis. Liver Transpl 2019;25(1):68–78. [DOI] [PubMed] [Google Scholar]
- 11.Stine JG, Argo CK, Pelletier SJ, Maluf DG, Caldwell SH, Northup PG. Advanced non-alcoholic steatohepatitis cirrhosis: A high-risk population for pre-liver transplant portal vein thrombosis. World J Hepatol 2017;9(3):139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zocco MA, Di Stasio E, De Cristofaro R, Novi M, Ainora ME, Ponziani F, et al. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. Journal of hepatology 2009;51(4):682–9. [DOI] [PubMed] [Google Scholar]
- 13.Violi F, Corazza RG, Caldwell SH, Perticone F, Gatta A, Angelico M, et al. Portal vein thrombosis relevance on liver cirrhosis: Italian Venous Thrombotic Events Registry. Intern Emerg Med 2016;30:30. [DOI] [PubMed] [Google Scholar]
- 14.Bartell N, Al-Judaibi B. The association between nonselective beta-blockers and portal venous thrombosis in cirrhotic patients: More questions on the horizon. Saudi journal of gastroenterology : official journal of the Saudi Gastroenterology Association 2018;24(1):3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stine JG, Shah NL, Argo CK, Pelletier SJ, Caldwell SH, Northup PG. Increased risk of Portal Vein Thrombosis in Patients with Cirrhosis due to Non-Alcoholic Steatohepatitis (NASH). Liver Transpl 2015. [DOI] [PMC free article] [PubMed]
- 16.Bezinover D, Iskandarani K, Chinchilli V, McQuillan P, Saner F, Kadry Z, et al. Autoimmune conditions are associated with perioperative thrombotic complications in liver transplant recipients: A UNOS database analysis. BMC anesthesiology 2016;16(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lisman T, Bongers TN, Adelmeijer J, Janssen HL, de Maat MP, de Groot PG, et al. Elevated levels of von Willebrand Factor in cirrhosis support platelet adhesion despite reduced functional capacity. Hepatology (Baltimore, Md) 2006;44(1):53–61. [DOI] [PubMed] [Google Scholar]
- 18.Saugel B, Lee M, Feichtinger S, Hapfelmeier A, Schmid RM, Siveke JT. Thrombophilic factor analysis in cirrhotic patients with portal vein thrombosis. Journal of thrombosis and thrombolysis 2015;40(1):54–60. [DOI] [PubMed] [Google Scholar]
- 19.Amitrano L, Brancaccio V, Guardascione MA, Margaglione M, Iannaccone L, D’Andrea G, et al. Inherited coagulation disorders in cirrhotic patients with portal vein thrombosis. Hepatology (Baltimore, Md) 2000;31(2):345–8. [DOI] [PubMed] [Google Scholar]
- 20.Luca A, Angermayr B, Bertolini G, Koenig F, Vizzini G, Ploner M, et al. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis. Liver Transpl 2007;13(8):1174–80. [DOI] [PubMed] [Google Scholar]
- 21.Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am J Gastroenterol 2018;113(11):1649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singal AK, Hasanin M, Kaif M, Wiesner R, Kuo YF. Nonalcoholic Steatohepatitis is the Most Rapidly Growing Indication for Simultaneous Liver Kidney Transplantation in the United States. Transplantation 2015. [DOI] [PubMed]
- 23.Bezinover D, Reeder E, Aziz F, Saner F, McQuillan P, Kadry Z, et al. African Americans have a lower prevalence of portal vein thrombosis at the time of liver transplantation. HPB : the official journal of the International Hepato Pancreato Biliary Association 2017;19(7):620–8. [DOI] [PubMed] [Google Scholar]
- 24.Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, et al. OPTN/SRTR 2017 Annual Data Report: Liver. Am J Transplant 2019;19 Suppl 2:184–283. [DOI] [PubMed] [Google Scholar]
- 25.Villa E, Camma C, Marietta M, Luongo M, Critelli R, Colopi S, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 2012;143(5):1253–60.e1–4. [DOI] [PubMed] [Google Scholar]
- 26.Stine JG, Pelletier SJ, Schmitt TM, Porte RJ, Northup PG. Pre-transplant portal vein thrombosis is an independent risk factor for graft loss due to hepatic artery thrombosis in liver transplant recipients. HPB : the official journal of the International Hepato Pancreato Biliary Association 2016;18(3):279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stine JG, Argo CK, Pelletier SJ, Maluf DG, Northup PG. Liver transplant recipients with portal vein thrombosis receiving an organ from a high-risk donor are at an increased risk for graft loss due to hepatic artery thrombosis. Transplant international : official journal of the European Society for Organ Transplantation 2016;29(12):1286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stine JG, Wang J, Shah PM, Argo CK, Intagliata N, Uflacker A, et al. Decreased Portal Vein Velocity is Predictive of the Development of Portal Vein Thrombosis: a Matched Case-Control Study. Liver international : official journal of the International Association for the Study of the Liver 2017. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.