Abstract
Objective:
To evaluate trends over time in perioperative outcomes for patients undergoing hepatectomy.
Background:
As perioperative care and surgical technique for hepatectomy have improved, the indications for and complexity of liver resections have evolved. However, the resulting effect on the short-term outcomes over time has not been well described.
Methods:
Consecutive patients undergoing hepatectomy during 1998–2015 at one institution were analyzed. Perioperative outcomes, including the comprehensive complication index (CCI), were compared between patients who underwent hepatectomy in the eras 1998–2003, 2004–2009, and 2010–2015.
Results:
The study included 3707 hepatic resections. The number of hepatectomies increased in each era (794 in 1998–2003, 1402 in 2004–2009, and 1511 in 2010–2015). Technical complexity increased over time as evidenced by increases in the rates of major hepatectomy (20%, 23%, 30%, p<0.0001), two-stage hepatectomy (0%, 3%, 4%, p<0.001), need for portal vein embolization (5%, 9%, 9%, p=0.001), and preoperative chemotherapy for colorectal liver metastases (70%, 82%, 89%, p<0.001) and median operative time (180, 175, 225 min, p<0.001). Significant decreases over time were observed in median blood loss (300, 250, 200 mL, p<0.001), transfusion rate (19%, 15%, 5%, p<0.001), median length of hospitalization (7, 7, 6 days, p<0.001), and rates of CCI ≥26.2 (20%, 22%, 16%, p<0.001) and 90-day mortality (3.1%, 2.6%, 1.3%, p=0.008). On multivariable analysis, hepatectomy in the most recent era 2010–2015 was associated with lower incidence of CCI ≥26.2 (odds ratio, 0.69, 95% CI 0.57–0.84, p<0.0001).
Conclusion:
Despite increase in complexity over an 18-year period, continued improvements in surgical technique and perioperative outcomes yielded a resultant decrease in CCI in the most current era.
MINI ABSTRACT
Among 3707 consecutive hepatic resections performed during 1998–2015, significant improvements were observed over time in estimated blood loss, perioperative transfusion requirements, length of hospitalization, major complications, and 90-day mortality despite increasing case complexity.
INTRODUCTION
Hepatic resection offers the best chance of long-term survival for patients with primary liver cancer and colorectal liver metastases (CRLM) and may be indicated in patients with other pathologic conditions as a means of improving associated symptoms or survival. Over the past two decades, refinements in the surgical techniques for hepatic resection and the perioperative care of patients undergoing the procedure have expanded the proportion of patients with primary and secondary hepatobiliary malignancies whose disease is deemed “resectable”.1–3 These refinements have included advances in the preoperative estimation of future liver remnant (FLR) volume and postoperative hepatic function 4–7, refinement of preoperative portal vein embolization (PVE) techniques and indications, 8–10 advances in anesthesia to minimize intraoperative blood loss and transfusion requirements, 3,11,12 and the development of advanced surgical techniques for parenchymal transection 13,14. The degree to which surgical boundaries have been expanded varies by institution, and the impact of these changes on perioperative outcomes has not been well described.
In the present study, we reviewed our high-volume institutional experience with complex hepatic resection in order to characterize the trends in perioperative outcomes of patients undergoing hepatic resection over the past 18 years.
METHODS
Patients and data collection
The Institutional Review Board of The University of Texas MD Anderson Cancer Center approved this study protocol (PA17–0564). A prospectively maintained hepatobiliary database was reviewed to identify patients who underwent hepatic resection between 1998 and 2015. Patients who underwent only liver biopsy and/or radiofrequency ablation (RFA) were excluded. The hepatic resections were divided into three eras according to the year when resection was performed: early (1998–2003), middle (2004–2009), and late (2010–2015). The following data were extracted from the electronic patient medical record for each resection: sex, age, American Society of Anesthesiologists (ASA) physical status score, body mass index, presence of cirrhosis, histologic subtype of disease resected from the liver, number and size of any CRLM and treatment of CRLM, preoperative PVE, types of surgical procedures, use of RFA in conjunction with hepatic resection, operative time, intraoperative estimated blood loss, intraoperative red blood cell transfusion, postoperative complications, length of hospitalization, unplanned hospital readmission within 45 days, and 90-day mortality.
Preoperative management
The decision to offer hepatic resection was made upon consideration of a patients’ physical status, background liver function, tumor histology, and tumor resectability. All patients underwent a high-resolution computed tomography scan using liver protocol with rapid injection of intravenous contrast agent and acquisition of thin-cut images both before contrast agent injection and in the late arterial, portal venous, and delayed phases. 3 In patients scheduled to undergo major hepatectomy, standardized FLR volume was calculated using volumetry based on the computed tomography images and standardized liver volume as previously described. 6,15 Liver tumors were deemed resectable when hepatectomy could achieve a negative margin while preserving more than 20% to 30% of the standardized FLR, sparing two continuous hepatic segments, and maintaining vascular inflow and outflow and biliary drainage. 16 If the calculated standardized FLR volume was deemed inadequate (≤20% in patients with normal liver, ≤30% in patients with fibrosis or liver injury, or ≤40% in patients with cirrhosis), preoperative PVE, with or without extension to the segment 4 portal branch, was performed. 10,17,18 Two-stage hepatectomy was proposed to patients with advanced bilobar CRLM who responded to preoperative systemic chemotherapy. In two-stage hepatectomy, first-stage resection involved minor hepatic resections on the less affected side of the liver, followed by PVE. After confirmation of adequate hypertrophy of the FLR, second-stage, contralateral major hepatectomy was performed. 19,20 Patients presenting with obstructive jaundice underwent preoperative endoscopic and/or percutaneous biliary drainage to provide effective clearance of jaundice and/or cholangitis 21 and underwent hepatic resection when the serum total bilirubin level decreased to ≤ 2 mg/dL. 3
Surgical technique
A standardized operative technique was used, with minor technical modifications made over the study period. During the early years, an inverted “Y” subcostal incision was used; as time progressed, this was changed to an “L” right upper quadrant incision (modified Makuuchi incision).22 Intraoperative sonography was systematically performed to confirm findings on preoperative radiologic imaging, detect radiologically occult lesions, and review the intrahepatic portal and hepatic venous anatomy. For parenchymal transection, two-surgeon technique, which combines ultrasonic dissection and saline-linked cautery, was introduced at the end of 2002. 14 Hepatic inflow occlusion with the Pringle maneuver was used and consisted of periods of occlusion lasting up to 15 minutes with intervening 5-minutes periods of inflow restoration. 23 RFA was performed under ultrasonographic guidance in conjunction with resection in selected patients with tumors not amenable to resection due to their location or distribution in the remnant liver.
At the completion of hepatic resection, the cut surface was examined for hemostasis and open bile ducts by direct visualization and with application of white gauze compresses. An intraoperative air leak test was introduced in 2009.24 This technique was consisted of injection of air into the biliary tree via a transcystic cholangiogram catheter while finger compression was used to occlude the distal common bile duct. Identified leaks were closed with polypropylene suture. Surgical technique was standardized across all surgeons though the number of full-time hepatobiliary surgeons increased throughout the study period (early: 3; middle: 4; late: 5).
Evaluation of perioperative outcomes
Major hepatectomy was defined as resection of three or more hepatic segments according to the definition of the Brisbane 2000 terminology of hepatic anatomy and resection.25 Surgical complications were defined as any deviation from the normal postoperative course within 90 days after hepatic resection, graded according to Clavien-Dindo classification, and scored using the comprehensive complication index (CCI). 26,27 In previous studies, the CCI has been shown to be a more sensitive measure of postoperative complications than traditional indices. A CCI of 26.2, which corresponds to 1 postoperative complication of Clavien-Dindo grade IIIa severity, was used as the threshold between high (CCI ≥26.2) and low (CCI <26.2) complication severity. 28 Postoperative hepatic insufficiency was defined as a peak postoperative bilirubin >7 mg/dl. Postoperative bile leak was defined according to criteria established by the International Study Group of Liver Surgery. Unplanned hospital readmission within 45 days and 90-day mortality were defined and classified according to previous definitions. 29–32
Statistical analysis
All statistical test were two-sided, and p<0.05 was considered statistically significant in all analyses. Variables were presented as median (range), or number (percentage) as appropriate. Continuous variables were compared using the Kruskal-Wallis test or Mann-Whitney tests as appropriate, and categorical variables were compared using the χ2 test. To identify factors associated with postoperative major complication (CCI ≥26.2) and 90-day mortality, multivariable logistic regression models were performed using non-collinear clinical variables from the univariable analysis with p<0.05. Statistical analyses were performed with IBM SPSS software (version 24.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics
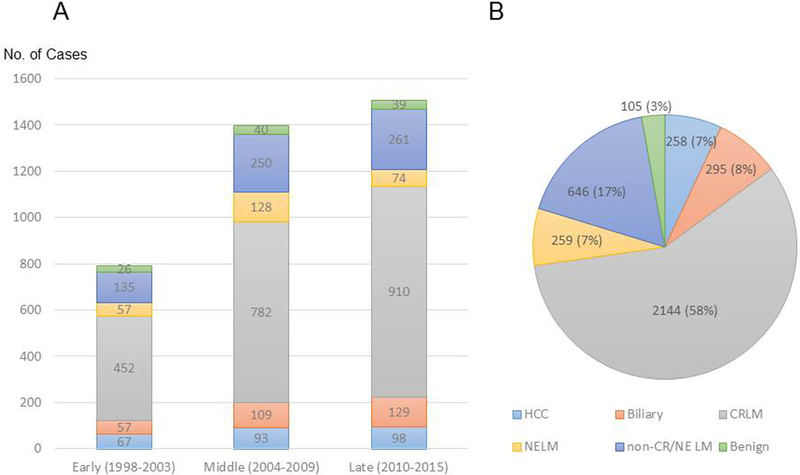
Between 1998 and 2015, 3707 hepatic resections were performed in 3348 patients. The number of hepatic resections increased over the course of the study (early period, n=794; middle period, n=1402; late period, n=1511) (Fig. 1a). The types of malignancy prompting hepatic resection, in order from most to least common, were CRLM (n=2144, 58%), non-colorectal/non-neuroendocrine liver metastasis (n=646, 17%), primary biliary malignancy (n=295, 8%), neuroendocrine liver metastasis (n=259, 7%), and hepatocellular carcinoma (n=258, 7%) (Fig. 1b). A total of 105 hepatic resections (3%) were performed for resection of benign lesions. Patient characteristics are summarized in Table 1. Compared to the patients in the early and middle study periods, the patients in the late study period were younger and were more likely to be obese and to have an ASA physical status score ≥3. Only minor differences in underlying disease histology were observed between the three time periods (Table 1). Among the 2144 hepatic resections performed for CRLM, the use of preoperative chemotherapy increased significantly over the study period.
Figure 1.
Number of hepatic resections performed for various tumor types and benign disease during 1998–2015 (A) by successive 6-year time period and (B) overall. The total number of hepatic resections performed was 3707. HCC, hepatocellular carcinoma; CRLM, colorectal liver metastasis; NELM, neuroendocrine liver metastasis; non-CR/NE LM, non-colorectal, non-neuroendocrine liver metastasis.55
Table 1.
Patient characteristics by study period*
| Early | Middle | Late | p value† | p value‡ | ||
|---|---|---|---|---|---|---|
| Characteristic | Total | (1998–2003) | (2004–2009) | (2010–2015) | (among 3 groups) | (middle vs. late) |
| n | 3707 | 794 (21) | 1402 (38) | 1511 (41) | - | - |
| Sex | ||||||
| Male | 1932 (52) | 414 (52) | 716 (51) | 802 (53) | 0.56 | 0.28 |
| Female | 1775 (48) | 380 (48) | 686 (49) | 709 (47) | ||
| Age, median (range), y | 57 (3–89) | 57 (3–88) | 58 (4–88) | 56 (4–89) | < 0.0001§ | < 0.0001# |
| ASA-PS score ≥ 3 | 2612 (71) | 314 (42) | 1036 (74) | 1262 (84) | < 0.0001 | < 0.0001 |
| BMI, median (range), kg/m2 | 27 (11–68) | 27 (11–68) | 27 (14–59) | 27 (15–55) | 0.019§ | 0.69# |
| ≥ 30 | 1080 (30) | 189 (25) | 417 (30) | 474 (31) | 0.01 | 0.36 |
| Cirrhosis | 103 (3) | 33 (4) | 40 (3) | 30 (2) | 0.04 | 0.29 |
| Disease | ||||||
| Hepatocellular carcinoma | 258 (7) | 67 (8) | 93 (7) | 98 (6) | 0.18 | 0.88 |
| Biliary carcinoma | 295 (8) | 57 (7) | 109 (8) | 129 (9) | 0.49 | 0.46 |
| CRLM | 2144 (58) | 452 (57) | 782 (56) | 910 (60) | 0.04 | 0.02 |
| NELM | 259 (7) | 57 (7) | 128 (9) | 74 (5) | < 0.0001 | <.00001 |
| Non-CR/non-NE LM | 646 (17) | 135 (17) | 250 (18) | 261 (17) | 0.87 | 0.70 |
| Benign lesion | 105 (3) | 26 (3) | 40 (3) | 39 (3) | 0.63 | 0.73 |
| CRLM (n=2144) | ||||||
| Preoperative chemotherapy | 1757 (82) | 314 (70) | 637 (82) | 806 (89) | < 0.0001 | < 0.0001 |
| Tumor number, median (range) | 2 (1–76) | 1 (1–15) | 2 (1–76) | 2 (1–41) | 0.002§ | 0.8# |
| Maximum tumor size, median (range), mm | 20 (1–210) | 20 (2–150) | 20 (1–210) | 22 (1–180) | 0.04§ | 0.5# |
Values in table are number of patients (percentage) unless indicated otherwise
χ2 test unless indicated otherwise
Fisher exact test unless indicated otherwise
Kruskal-Wallis test
Mann-Whitney U test
ASA-PS, American Society of Anesthesiologists physical status; BMI, body mass index
CRLM, colorectal liver metastasis; NELM, neuroendocrine liver metastasis
Non-CR/non-NE LM, non-colorectal/non-neuroendocrine liver metastasis
Perioperative outcomes
Perioperative outcomes are summarized in Table 2. In general, technical complexity increased over the study period as evidenced by increases in the rates of preoperative PVE, major hepatectomy, two-stage hepatectomy, and repeat hepatic resection as well as an increase in median operative time. The use of simultaneous RFA decreased over the study period, and the use of biliary reconstruction remained constant. Despite the increase in case complexity observed over time, median estimated blood loss and the rate of intraoperative red blood cell transfusion decreased significantly over time.
Table 2.
Perioperative outcomes by study period*
| Early | Middle | Late | p value† | p value† | ||
|---|---|---|---|---|---|---|
| Characteristic | Total | (1998–2003) | (2004–2009) | (2010–2015) | (among 3 groups) | (middle vs. late) |
| n | 3707 | 794 (21) | 1402 (38) | 1511 (41) | - | - |
| PVE performed | 312 (8) | 41 (5) | 132 (9) | 139 (9) | 0.001 | 0.8 |
| Hepatic resection | ||||||
| Two-stage hepatectomy completed | 112 (3) | 0 | 47 (3) | 65 (4) | < 0.0001 | 0.2 |
| Repeat resection for intrahepatic recurrence | 320 (9) | 46 (6) | 115 (8) | 159 (11) | <0.0001 | 0.03 |
| Major hepatic resection (≥ 3 segments)‡ | 931 (25) | 158 (20) | 316 (23) | 457 (30) | < 0.0001 | < 0.0001 |
| With RFA | 493 (13) | 223 (28) | 159 (11) | 111 (7) | < 0.0001 | < 0.0001 |
| With biliary reconstruction | 183 (5) | 40 (5) | 79 (6) | 64 (4) | 0.2 | 0.09 |
| Operative time, median (range), minutes | 195 (20–1336) | 180 (33–878) | 175 (25–1336) | 225 (20–929) | < 0.0001§ | < 0.0001# |
| Blood loss, median (range), mL | 200 (0–12500) | 300 (10–6800) | 250 (0–7000) | 200 (0–12500) | < 0.0001§ | < 0.0001# |
| Intraoperative red blood cell transfusion administered | 426 (12) | 153 (19) | 204 (15) | 69 (5) | < 0.0001 | < 0.0001 |
| Postoperative complication | 1471 (40) | 304 (38) | 624 (45) | 543 (36) | < 0.0001 | < 0.0001 |
| Comprehensive complication index ≥ 26.2¶ | 697 (19) | 155 (20) | 306 (22) | 236 (16) | < 0.0001 | < 0.0001 |
| ≥ grade IIIa** | 491 (13) | 125 (16) | 209 (15) | 157 (10) | < 0.0001 | < 0.0001 |
| Organ space surgical site infection requiring percutaneous drainage | 303 (8) | 63 (8) | 140 (10) | 100 (7) | 0.004 | 0.001 |
| Postoperative bile leakage ≥ grade B‡‡ | 157 (4) | 31 (4) | 78 (6) | 48 (3) | 0.005 | 0.002 |
| Postoperative hepatic insufficiency§§ | 137 (4) | 53 (7) | 63 (5) | 21 (1) | < 0.0001 | < 0.0001 |
| Length of hospital stay, median (range), days## | 6 (0–73) | 7 (2–73) | 7 (1–72) | 6 (0–63) | < 0.0001§ | < 0.0001# |
| Unplanned readmission within 45 days | 354 (10) | 75 (9) | 142 (10) | 137 (9) | 0.6 | 0.3 |
| 90-day mortality | 81 (2) | 25 (3) | 36 (3) | 20 (1) | 0.008 | 0.02 |
Values in table are number of patients (percentage) unless indicated otherwise
χ2 test unless indicated otherwise
According to the Brisbane 2000 nomenclature
Kruskal-Wallis test
Mann-Whitney U test
According to the Dindo-Clavien classification
According to the definition by Slankamenac et al.
According to the International Study Group of Liver Surgery definition
According to the definition by Mullen et al.
Calculated between the operation and the day of discharge
PVE, portal vein embolization; RFA, radiofrequency ablation
Of the 3707 hepatectomies, 1471 (40%) were associated with at least one complication, including 157 (4%) with bile leakage of at least grade B according to the International Study Group of Liver Surgery definition, and 137 (4%) with postoperative hepatic insufficiency. In addition, 697 hepatectomies (19%) were associated with CCI ≥26.2, and in 81 hepatectomies (2%), the patient died within 90 days of surgery. Of the 697 cases with CCI ≥26.2, 525 (75.3%) were associated with more than one complication. The most common complication in patients with CCI ≥26.2 was organ space surgical site infection requiring percutaneous drainage (n=303, 8%), and more than one-third of these cases (n=135, 4%) were associated with confirmed biliary leakage. The rate of any complication and the rate of a high CCI increased from the early period to the middle period and then decreased in the late period to rates below those in the early period. The 90-day mortality rate (3.1%, 2.6%, 1.3%, p=0.008) decreased significantly over time.
On multivariable regression analysis, male sex, age ≥60 years, major hepatectomy, combined biliary reconstruction, and intraoperative red blood cell transfusion were significantly associated with an increased risk of high CCI (Table 3). Similarly, male sex, age ≥60 years, major hepatectomy, combination use of RFA, and intraoperative red blood cell transfusion were significantly associated with an increased risk of 90-day mortality (Table 4). Hepatectomy performed during the late period compared to the middle period was significantly associated with a decreased risk of high CCI (Table 3). While the time period of hepatectomy was associated with 90-day mortality on univariable analysis, it was not associated with 90-day mortality on multivariable analysis.
Table 3.
Univariable and multivariable analyses of comprehensive complication index (CCI)* >26.2
| CCI ≥ 26.2 | Univariable† | Multivariable‡ | |||
|---|---|---|---|---|---|
| Characteristic | n (%) | p value | Odds ratio | 95% confidence interval | p value |
| All patients | 697 (19) | - | - | - | - |
| Sex¶ | |||||
| Male | 404 (21) | 0.001 | 1.321 | 1.109–1.574 | 0.002 |
| Female | 293 (17) | ||||
| Age, years¶ | |||||
| ≥60 | 312 (21) | 0.007 | 1.196 | 1.004–1.425 | 0.05 |
| <60 | 385 (17) | ||||
| Body mass index, kg/m2 | |||||
| <30 | 499 (19) | 0.2 | - | - | - |
| ≥30 | 188 (17) | ||||
| ASA-PS score | |||||
| <3 | 204 (19) | 0.6 | - | - | - |
| ≥3 | 487 (19) | ||||
| Disease¶ | |||||
| Primary hepatobiliary cancer | 128 (23) | 0.001 | ref | ||
| Metastatic liver cancer | 559 (18) | 1.187 | 0.917–1.536 | 0.2 | |
| Benign | 10 (10) | 0.696 | 0.342–1.413 | 0.3 | |
| Second stage hepatectomy of two stage hepatectomy | |||||
| Yes | 28 (25) | 0.09 | - | - | - |
| No | 669 (19) | ||||
| Repeated resection for intrahepatic recurrence | |||||
| No | 643(19) | 0.4 | - | - | - |
| Yes | 54 (17) | ||||
| Type of hepatectomy¶ | |||||
| Major | 259 (28) | <0.0001 | 1.813 | 1.500–2.191 | <0.0001 |
| Minor | 438 (16) | ||||
| Resection with RFA | |||||
| Yes | 98 (20) | 0.5 | - | - | - |
| No | 599 (19) | ||||
| Combined biliary reconstruction¶ | |||||
| Yes | 80 (44) | <0.0001 | 2.766 | 1.937–3.949 | <0.0001 |
| No | 617 (18) | ||||
| Operative time, minutes¶ | |||||
| ≥180 | 443 (21) | <0.0001 | 1.194 | 0.994–1.435 | 0.06 |
| <180 | 254 (16) | ||||
| Estimated blood loss, mL¶ | |||||
| ≥1000 | 76 (34) | <0.0001 | 1.049 | 0.738–1.491 | 0.8 |
| <1000 | 621 (18) | ||||
| Intraoperative red blood cell transfusion¶ | |||||
| Yes | 153 (36) | <0.0001 | 2.373 | 1.824–3.087 | <0.0001 |
| No | 544 (17) | ||||
| Period¶ | |||||
| Middle (2004–2009) | 306 (22) | <0.0001 | ref | ||
| Early (1998–2003) | 155 (20) | 0.848 | 0.676–1.063 | 0.2 | |
| Late (2010–2015) | 236 (16) | 0.690 | 0.566–0.842 | <0.0001 | |
According to the definition by Slankamenac et al.
χ2 test
Logistic regression analysis
Variables entered into the binary logistic regression analysis
ASA-PS, American Society of Anesthesiologists physical status; RFA, radiofrequency ablation
Table 4.
Univariable and multivariable analyses of 90 day mortality
| 90 days mortality | Univariable* | Multivariable† | |||
|---|---|---|---|---|---|
| Characteristic | n (%) | p value | Odds ratio | 95% confidence interval | p value |
| All patients | 81 (2.2) | ||||
| Sex‡ | |||||
| Male | 54 (2.8) | 0.008 | 1.693 | 1.042–2.750 | 0.04 |
| Female | 27 (1.5) | ||||
| Age, years‡ | |||||
| ≥60 | 54 (3.5) | <0.0001 | 2.339 | 1.455–3.761 | <0.0001 |
| <60 | 27 (1.3) | ||||
| Body mass index, kg/m2 | |||||
| <30 | 61 (2.3) | 0.4 | - | - | - |
| ≥30 | 20 (1.9) | ||||
| ASA-PS | |||||
| ≥3 | 61 (2.3) | 0.4 | - | - | - |
| <3 | 20 (1.9) | ||||
| Disease‡ | |||||
| Primary hepatobiliary cancer | 22 (4.0) | 0.003 | ref | ||
| Metastatic liver cancer | 59 (1.9) | 0.743 | 0.404–1.364 | 0.3 | |
| Benign | 0 | 0 | 0 | 1 | |
| Second stage hepatectomy of two stage hepatectomy | |||||
| Yes | 6 (5.4) | 0.02 | - | - | - |
| No | 75 (2.1) | ||||
| Repeat resection‡ | |||||
| No | 80 (2.4) | 0.02 | 5.757 | 0.792–41.83 | 0.08 |
| Yes | 1 (0.3) | ||||
| Type of hepatectomy‡ | |||||
| Major | 35 (3.8) | <0.0001 | 1.772 | 1.086–2.891 | 0.02 |
| Minor | 46 (1.7) | ||||
| Resection with RFA‡ | |||||
| Yes | 17 (3.4) | 0.04 | 2.142 | 1.180–3.889 | 0.01 |
| No | 64 (2.0) | ||||
| Combined biliary reconstruction‡ | |||||
| Yes | 13 (7.1) | <0.0001 | 1.968 | 0.919–4.214 | 0.08 |
| No | 68 (1.9) | ||||
| Operative time, minutes | |||||
| <180 | 38 (2.3) | 0.6 | |||
| ≥180 | 43 (2.1) | ||||
| Estimated blood loss, mL‡ | |||||
| ≥1000 | 18 (8.1) | <0.0001 | 1.501 | 0.763–2.955 | 0.2 |
| <1000 | 63 (1.8) | ||||
| Intraoperative red blood cell transfusion‡ | |||||
| Yes | 32 (7.5) | <0.0001 | 3.653 | 2.068–6.453 | <0.0001 |
| No | 49 (1.5) | ||||
| Period‡ | |||||
| Early (1998–2003) | 25 (3.1) | 0.008 | ref | ||
| Middle (2004–2009) | 36 (2.6) | 1.012 | 0.584–1.755 | 1 | |
| Late (2010–2015) | 20 (1.3) | 0.727 | 0.378–1.398 | 0.3 | |
χ2 test
Logistic regression analysis
Variables entered into the logistic regression analysis
ASA-PS, American Society of Anesthesiologists Physical Status; RFA, radiofrequency ablation
DISCUSSION
The results of this single-institutional review of 3707 consecutive hepatic resections over 18 years demonstrate significant trends over time both in the characteristics of patients undergoing liver surgery and in the perioperative outcomes following hepatectomy. Specifically, we found increases over the study period in the rates of major hepatectomy (20% to 30%), repeat hepatic resection (6% to 11%), two-stage hepatectomy (0 to 4%), and preoperative PVE (5% to 9%), suggesting a greater extent and/or complexity of resection in more recent years. Despite this apparent increase in case complexity, we noted significant decreases over the study period in the median estimated blood loss (300 mL to 200 mL), transfusion rate (19% to 5%), median length of hospitalization (7 days to 6 days), major postoperative complication rate (20% to 16%), and 90-day postoperative mortality rate (3.1% to 1.3%). These improvements in outcomes demonstrate that our continuous refinements in liver surgery have expanded surgical boundaries while simultaneously improving operative safety.
Our detailed evaluation of the trends in liver surgery at our institution suggests an increase in the relative case complexity over the past two decades. First, despite potential adverse effects of preoperative chemotherapy on liver function and the FLR, the percentage of hepatectomies for CRLM in which preoperative chemotherapy was delivered increased throughout the study period. Second, despite an increased emphasis at our institution on using parenchymal-sparing approaches when applicable,33 the rate of major hepatectomy increased throughout the study period. Although major versus minor hepatectomy does not necessarily represent technical complexity (i.e. some anatomic segmentectomies are technically more challenging than major hemihepatectomies), it does consistently correlate with risk of postoperative complications. Finally, the proportion of cases that were two-stage or repeat hepatectomies increased throughout the study period, whereas the use of combined ablative procedures decreased. These observations may suggest that tumors that would have been ablated or deemed unresectable in earlier time periods were either resected or percutaneously ablated as a planned multidisciplinary treatment strategy in the later time periods.34 These observations also may suggest an expansion of the indications for hepatectomy at our institution in an effort to extend the benefits of hepatic resection to as many patients as possible given the improving safety profile of major liver surgery.
Paramount in improving the outcomes of a high-volume hepatobiliary surgery service are comprehensive preoperative evaluation and meticulous patient selection. All patients seen at our institution are evaluated in three domains, physiologic, oncologic, and technical, to determine if they are candidates for surgery. The goal of the physiologic assessment is to determine if the patient can safely undergo one or more major abdominal operations. This assessment involves a thorough history and physical examination, basic laboratory analyses, frailty measurements, and consultations by appropriate internal medicine services. The assessment may include measurements that fall outside traditional tools such as ASA physical status score and body mass index, both of which increased over time in the current study. The goal of the oncologic assessment, a multidisciplinary assessment of the patient’s underlying tumor biology, is to determine if the patient is likely to benefit from major liver surgery. Factors that inform the likelihood of benefit include serum tumor marker level, disease-free interval after primary resection, presence of extrahepatic disease, tumor somatic gene mutation status 35, and response to preoperative chemotherapy. 36,37 Finally, technical resectability means that the surgeon can obtain negative microscopic margins while preserving an adequate FLR. At our institution, comprehensive liver volumetry is routinely obtained whenever major hepatectomy is anticipated. When the FLR is expected not to meet established thresholds, 16,17,38 PVE is then performed, not only to produce hypertrophy of the FLR but also to serve as a physiologic test of hepatic function. 39 PVE can also be helpful in reversing the hepatic atrophy that occurs secondary to preoperative chemotherapy 40 and is an independent predictor of postoperative hepatic insufficiency and death. 41 These systematic assessments of physiologic, oncologic, and technical resectability, in addition to improvements in surgical technique and perioperative management, has led to a reduction in the incidence of postoperative hepatic insufficiency over time, demonstrated by the low incidence (1%) of patients experiencing hepatic insufficiency in the most recent time period.
In addition to careful preoperative evaluation and selection, several intraoperative and postoperative strategies have been developed to improve postoperative outcomes at our institution. At the beginning of the middle period (2004–2009), the two-surgeon technique of parenchymal transection was developed 14,42,43. In this technique, the primary surgeon dissects using the Cavitron Ultrasonic Surgical Aspirator (Medtronic Inc, Minneapolis, MN) while the assistant provides exposure and divides vessels. A saline-cooled radiofrequency coagulation device is used for hemostasis. Other energy devices are not routinely used during parenchymal dissection. Following implementation of this technique, combined with low-central venous pressure anesthesia provided by anesthesiologists specializing in liver surgery, estimated blood loss and intraoperative transfusion of red blood cells, both of which are independent predictors of complications (high CCI) and mortality, gradually decreased over time. In addition, the systematic use of an intraoperative air leak test was introduced toward the end of the middle period, and reports show that this technique has been associated with significant reductions in postoperative biliary complication and organ space infections at our institution. 24,44 In our study, there was an increase in the rate of postoperative bile leaks between the early and middle periods, followed by a significant decrease in the rate of bile leakage between the middle and late periods, after introduction of this technique. Finally, the use of enhanced recovery protocols at our institution after both minimally invasive and open operations has led to improved perioperative outcomes, including a shortened length of hospital stay and faster return to intended oncologic therapy.45
The strength of this study is its large sample size with relatively complete patient data from a contemporary period (1998–2015). Previous studies evaluating trends in the characteristics and outcomes of patients undergoing hepatic resection over time have had mixed results. Most studies from the 2000s found evidence of increasing case complexity as the indications for hepatectomy expanded, resulting in either stable rates or slight increases in the rate of postoperative adverse events. 46–48 In contrast, the Memorial Sloan-Kettering Cancer Center group recently studied 4152 consecutive hepatic resections performed between 1993 and 2012 and reported substantial improvements in postoperative morbidity and mortality with emphasis on parenchyma-sparing approaches to hepatic resection. 49 Our group previously reported that parenchyma-sparing hepatectomy is associated with a lower incidence of postoperative hepatic insufficiency and improves salvageability and survival in the setting of intrahepatic recurrence of CRLM.33 In this regard, the results of the current study suggest that improvement in perioperative outcomes can be achieved while the complexity and aggressiveness of surgical resection are simultaneously increased.
As the current study also represents one of the largest single-institution series of its kind, an additional strength of this investigation is the opportunity to critically evaluate factors associated with postoperative major morbidity at a high-volume, experienced, hepatobiliary center. Indeed, risk factors for CCI ≥26.2 observed in the current study, namely increasing age, major hepatectomy, combined biliary reconstruction, and intraoperative red blood cell transfusion, are consistent with the results of previous studies.50 The finding that male sex was significantly associated with both major morbidity and mortality following liver resection has been found in previous studies as well and may reflect worsened underlying comorbidities.51–53
Several limitations of this retrospective single-institution study should be acknowledged. First, minimally invasive approaches to hepatic resection54 have only been recently introduced at our institution and were not detailed in this analysis. Second, the purpose of this study was to evaluate perioperative outcomes, and therefore long-term oncologic outcomes were not included. Whether changes in margin status, recurrence rates and/or overall survival have occurred as a result of improved outcome should be the subject of future investigation. Third, primary liver cancers comprised a minority of the cases in our experience which is a reflection of the referral practices at our independent cancer center as well as the meticulous patient selection used to ensure patients have adequate liver function prior to undergoing hepatectomy. A stronger focus on hepatocellular carcinoma or cholangiocarcinoma could have altered the perioperative outcomes observed. Finally, the results demonstrated in this study are the result of a multidisciplinary team focused exclusively on hepatobiliary disease at a high-volume center and it is unclear if such results are generalizable to low volume center.
In conclusion, this single-institution review of hepatic resections over the past 18 years indicates that an evolving refinement in patient selection, perioperative optimization, surgical technique, and perioperative management over time has led to improved outcomes despite escalation in case complexity. Further advances in systemic therapies and nonoperative liver-directed therapies should continue to expand the indications for liver surgery as improvements in perioperative outcomes enable safe surgery to be applied more broadly.
ACKNOWLEDGEMENTS
The authors particularly thank Stephanie Deming of the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center, for copyediting the manuscript and Ruth J. Haynes the Department of Surgical Oncology at The University of Texas MD Anderson Cancer Center, for the administrative support in the preparation of this manuscript.
Source of funding: This research was supported in part by the National Institutes of Health through MD Anderson Cancer Center’s Cancer Center Support Grant, CA016672.
REFERENCES
- 1.Imamura H, Seyama Y, Kokudo N et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg 2003;138:1198–1206. [DOI] [PubMed] [Google Scholar]
- 2.Nagino M, Ebata T, Yokoyama Y et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg 2013;258:129–140. [DOI] [PubMed] [Google Scholar]
- 3.Vauthey JN, Pawlik TM, Abdalla EK et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg 2004;239:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubota K, Makuuchi M, Kusaka K et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–1181. [DOI] [PubMed] [Google Scholar]
- 5.Nagino M, Nimura Y, Hayakawa N et al. Logistic regression and discriminant analyses of hepatic failure after liver resection for carcinoma of the biliary tract. World J Surg 1993;17:250–255. [DOI] [PubMed] [Google Scholar]
- 6.Vauthey JN, Chaoui A, Do KA et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–519. [DOI] [PubMed] [Google Scholar]
- 7.Shirabe K, Shimada M, Gion T et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg 1999;188:304–309. [DOI] [PubMed] [Google Scholar]
- 8.Nagino M, Kamiya J, Kanai M et al. Right trisegment portal vein embolization for biliary tract carcinoma: technique and clinical utility. Surgery. 2000;127:155–160. [DOI] [PubMed] [Google Scholar]
- 9.Farges O, Belghiti J, Kianmanesh R et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg 2003;237:208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madoff DC, Abdalla EK, Gupta S et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol 2005;16:215–225. [DOI] [PubMed] [Google Scholar]
- 11.Melendez JA, Arslan V, Fischer ME et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg 1998;187:620–625. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa K, Takayama T, Orii R et al. Effect of hypoventilation on bleeding during hepatic resection: a randomized controlled trial. Arch Surg 2002;137:311–315. [DOI] [PubMed] [Google Scholar]
- 13.Belghiti J, Guevara OA, Noun R et al. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg 2001;193:109–111. [DOI] [PubMed] [Google Scholar]
- 14.Aloia TA, Zorzi D, Abdalla EK et al. Two-surgeon technique for hepatic parenchymal transection of the noncirrhotic liver using saline-linked cautery and ultrasonic dissection. Ann Surg 2005;242:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vauthey JN, Abdalla EK, Doherty DA et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl 2002;8:233–240. [DOI] [PubMed] [Google Scholar]
- 16.Kishi Y, Abdalla EK, Chun YS et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg 2009;250:540–548. [DOI] [PubMed] [Google Scholar]
- 17.Zorzi D, Laurent A, Pawlik TM et al. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg 2007;94:274–286. [DOI] [PubMed] [Google Scholar]
- 18.Kishi Y, Madoff DC, Abdalla EK et al. Is embolization of segment 4 portal veins before extended right hepatectomy justified? Surgery. 2008;144:744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brouquet A, Abdalla EK, Kopetz S et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol 2011;29:1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passot G, Chun YS, Kopetz SE et al. Predictors of Safety and Efficacy of 2-Stage Hepatectomy for Bilateral Colorectal Liver Metastases. J Am Coll Surg 2016;223:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanai M, Nimura Y, Kamiya J et al. Preoperative intrahepatic segmental cholangitis in patients with advanced carcinoma involving the hepatic hilus. Surgery. 1996;119:498–504. [DOI] [PubMed] [Google Scholar]
- 22.Chang SB, Palavecino M, Wray CJ et al. Modified Makuuchi incision for foregut procedures. Arch Surg 2010;145:281–284. [DOI] [PubMed] [Google Scholar]
- 23.Pringle JH. V. Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg 1908;48:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmitti G, Vauthey JN, Shindoh J et al. Systematic use of an intraoperative air leak test at the time of major liver resection reduces the rate of postoperative biliary complications. J Am Coll Surg 2013;217:1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strasberg SM. For the International Hepato-Pancreato-Biliary Association Terminology Committee Survey. (2000) The Brisbane 2000 Terminology of Liver Anatomy and Resections. HPB. 2:333–339. [Google Scholar]
- 26.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slankamenac K, Graf R, Barkun J et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1–7. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita S, Sheth RA, Niekamp AS et al. Comprehensive Complication Index Predicts Cancer-specific Survival After Resection of Colorectal Metastases Independent of RAS Mutational Status. Ann Surg 2016; [DOI] [PubMed] [Google Scholar]
- 29.Mullen JT, Ribero D, Reddy SK et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 2007;204:854–862. [DOI] [PubMed] [Google Scholar]
- 30.Koch M, Garden OJ, Padbury R et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. [DOI] [PubMed] [Google Scholar]
- 31.Brudvik KW, Mise Y, Conrad C et al. Definition of readmission in 3,041 patients undergoing hepatectomy. J Am Coll Surg 2015;221:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mise Y, Vauthey JN, Zimmitti G et al. Ninety-day postoperative mortality is a legitimate measure of hepatopancreatobiliary surgical quality. Ann Surg 2015;262:1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mise Y, Aloia TA, Brudvik KW et al. Parenchymal-sparing hepatectomy in colorectal liver metastasis improves salvageability and survival. Ann Surg 2016;263:146–152. [DOI] [PubMed] [Google Scholar]
- 34.Odisio BC, Yamashita S, Frota L et al. Planned treatment of advanced metastatic disease with completion ablation after hepatic resection. J Gastrointest Surg 2017;21:628–635. [DOI] [PubMed] [Google Scholar]
- 35.Vauthey JN, Zimmitti G, Kopetz SE et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg 2013;258:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chun YS, Vauthey JN, Boonsirikamchai P et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shindoh J, Loyer EM, Kopetz S et al. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol 2012;30:4566–4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shindoh J, Tzeng CW, Aloia TA et al. Optimal future liver remnant in patients treated with extensive preoperative chemotherapy for colorectal liver metastases. Ann Surg Oncol 2013;20:2493–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shindoh J, Truty MJ, Aloia TA et al. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg 2013;216:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Omichi K, Yamashita S, Cloyd JM et al. Portal Vein Embolization Reduces Postoperative Hepatic Insufficiency Associated with Postchemotherapy Hepatic Atrophy. J Gastrointest Surg 2017; [DOI] [PubMed] [Google Scholar]
- 41.Yamashita S, Shindoh J, Mizuno T et al. Hepatic atrophy following preoperative chemotherapy predicts hepatic insufficiency after resection of colorectal liver metastases. J Hepatol 2017; [DOI] [PubMed] [Google Scholar]
- 42.Palavecino M, Kishi Y, Chun YS et al. Two-surgeon technique of parenchymal transection contributes to reduced transfusion rate in patients undergoing major hepatectomy: analysis of 1,557 consecutive liver resections. Surgery. 2010;147:40–48. [DOI] [PubMed] [Google Scholar]
- 43.Day RW, Brudvik KW, Vauthey JN et al. Advances in hepatectomy technique: toward zero transfusions in the modern era of liver surgery. Surgery. 2016;159:793–801. [DOI] [PubMed] [Google Scholar]
- 44.Tran Cao HS, Phuoc V, Ismael H et al. Rate of organ space infection is reduced with the use of an air leak test during major hepatectomies. J Gastrointest Surg 2017;21:85–93. [DOI] [PubMed] [Google Scholar]
- 45.Day RW, Cleeland CS, Wang XS et al. Patient-reported outcomes accurately measure the value of an enhanced recovery program in liver surgery. J Am Coll Surg 2015;221:1023–1030 e1021–1022. [DOI] [PubMed] [Google Scholar]
- 46.Cescon M, Vetrone G, Grazi GL et al. Trends in perioperative outcome after hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann Surg 2009;249:995–1002. [DOI] [PubMed] [Google Scholar]
- 47.Dokmak S, Fteriche FS, Borscheid R et al. 2012 Liver resections in the 21st century: we are far from zero mortality. HPB (Oxford). 2013;15:908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimmitti G, Roses RE, Andreou A et al. Greater complexity of liver surgery is not associated with an increased incidence of liver-related complications except for bile leak: an experience with 2,628 consecutive resections. J Gastrointest Surg 2013;17:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kingham TP, Correa-Gallego C, D’Angelica MI et al. Hepatic parenchymal preservation surgery: decreasing morbidity and mortality rates in 4,152 resections for malignancy. J Am Coll Surg 2015;220:471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aloia TA, Fahy BN, Fischer CP et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB (Oxford). 2009;11:510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Virani S, Michaelson JS, Hutter MM et al. Morbidity and mortality after liver resection: results of the patient safety in surgery study. J Am Coll Surg 2007;204:1284–1292. [DOI] [PubMed] [Google Scholar]
- 52.Abbott AM, Parsons HM, Tuttle TM et al. Short-term outcomes after combined colon and liver resection for synchronous colon cancer liver metastases: a population study. Ann Surg Oncol 2013;20:139–147. [DOI] [PubMed] [Google Scholar]
- 53.Lyu HG, Sharma G, Brovman EY et al. Unplanned reoperation after hepatectomy: an analysis of risk factors and outcomes. HPB (Oxford). 2018; [DOI] [PubMed] [Google Scholar]
- 54.Mizuno T, Sheth R, Yamamoto M et al. Laparoscopic Glissonean pedicle transection (Takasaki) for negative fluorescent counterstaining of segment 6. Ann Surg Oncol 2017;24:1046–1047. [DOI] [PubMed] [Google Scholar]
- 55.Yamashita S, Venkatesan AM, Mizuno T et al. Remnant liver ischemia as a prognostic factor for cancer-specific survival after resection of colorectal liver metastases. JAMA Surg 2017;2152(2010):e172986. doi:172910.171001/jamasurg.172017.172986. [DOI] [PMC free article] [PubMed] [Google Scholar]