Abstract
The United States opioid use epidemic over the past decade has coincided with an increase in HCV positive donors. Using propensity score matching, and the Organ Procurement Transplant Network data files from January 2015 to June 2019, we analyzed the short-term outcomes of adult deceased donor kidney transplants of HCV uninfected recipients with two distinct groups of HCV positive donors (HCV seropositive, non-viremic n=352 and viremic n=196) compared to those performed using HCV uninfected donors (n=36,934). Compared to the reference group, the transplants performed using HCV seropositive, non-viremic and viremic donors experienced a lower proportion of delayed graft function (35.2 vs. 18.9%;P<0.001 [HCV seropositive, non-viremic donors] and 36.2 vs. 16.8%;P<0.001 [HCV viremic donors]). The recipients of HCV viremic donors had better allograft function at 6 months post-transplant (eGFR [54.1 vs. 68.3 ml/min/1.73 m2; p 0.004]. Furthermore, there was no statistical difference in the overall graft failure risk at 12 months post-transplant by propensity score matched multivariable Cox proportional analysis (HR=0.60, 95% CI 0.23 to 1.29 [HCV seropositive, non-viremic donors] and HR=0.85, 95% CI 0.25 to 2.96 [HCV viremic donors]). Further studies are required to determine the long-term outcomes of these transplants and address unanswered questions regarding the use of HCV viremic donors.
Introduction:
Kidney transplantation is the treatment of choice for end-stage renal disease (ESRD). It extends life, improves quality of life, and reduces cost compared to maintenance dialysis (1, 2). Although mortality in patients on the renal transplant waitlist has decreased, it remains unacceptably high (up to 12.7 per 100 patients-year) in certain regions of the United States (US) (3). Data in 2016 from the Scientific Registry of Transplant Recipients (SRTR) revealed that more than 25% of the 33,291 adult patients removed from the kidney transplant waitlist were withdrawn due to death or deteriorating medical condition. This withdrawal rate reflects the excessive wait times and severe organ shortage despite an increase in overall transplant numbers (3). These statistics underscore the importance of exploring opportunities to expand the donor pool while maintaining satisfactory post-transplant outcomes.
During the last decade, there has been an abrupt rise in opioid use in the US. This has coincided with a surge in intravenous drug use (IDU), hepatitis C virus (HCV) transmission and opioid-related overdose deaths (4–6). Unfortunately, the organs from HCV positive donors are underutilized (7–9). This population encompasses two distinct sub-groups based on HCV antibody (Ab) and nucleic acid testing (NAT). The HCV seropositive, non-viremic donors (HCV Ab+, NAT−) and the HCV viremic donors (HCV Ab ±, NAT+) (10). The HCV seropositive, non-viremic donors are primarily comprised of individuals with prior infection with spontaneous clearance of the infection. Concerns have been raised regarding the possibility of HCV disease transmission from this sub-group (11). An analysis of the Organ Procurement Transplant Network (OPTN) data demonstrated no cases of HCV transmission from HCV seropositive, non-viremic donors who did not have Public Health Service (PHS) increased-risk behaviors (12). This data suggests that the risk of HCV disease transmission is related to the donors’ behaviors (e.g., IDU) and not HCV seropositivity (13). Furthermore, a small single-center retrospective cohort study has demonstrated satisfactory short-term outcomes of kidney transplants using this subgroup of HCV positive donors for HCV uninfected recipients without evidence of HCV transmission (14). On the other hand, the use of HCV viremic donors for HCV uninfected recipients carries an exceedingly high risk of disease transmission, and, until recently, the use of HCV viremic donors was limited to recipients already with HCV viremia. The advent of HCV direct-acting antivirals (DAAs) has fostered the development of protocols to allow for use of these donor organs in HCV non-viremic recipients (10). However, concerns remain about the impact of HCV acquisition on graft and patient survival. Two small, open-label, non-randomized clinical trials, using HCV DAAs, demonstrate satisfactory short-term outcomes of kidney transplants in this scenario (15, 16). Notably, all recipients were able to achieve 12-week HCV sustained virologic response.
Mandatory HCV Ab and NAT donor testing were instituted in January 2014. In August 2015, DonorNet allowed centers to indicate patients’ willingness to accept an organ from an HCV seropositive and/or viremic donor. The objective of our study was to analyze the short-term outcomes of adult deceased donor kidney transplants (DDKT) of HCV uninfected recipients, comparing the two distinct populations of HCV positive donors (HCV seropositive, non-viremic donors and HCV viremic donors) against those performed using HCV uninfected donors. We analyzed the OPTN Standard Transplant Analysis Research (STAR) files using propensity score (PS) matching.
Materials and Methods
Study Population
This study used data from the OPTN STAR files administered by the United Network of Organ Sharing (UNOS) which includes data submitted by members on all donors, waitlisted candidates, and transplant recipients in the US. The Health Resources and Services Administration of the US Department of Health and Human Services oversees the activities of the OPTN and contractor. The study was approved by the University of Texas Southwestern Medical Center Institutional Review Board. The retrospective cohort study, included adult (≥18 years of age) DDKT recipients registered in the OPTN STAR files from January ,1 2015 to June 30, 2018. We excluded recipients of a kidney-pancreas and multiorgan transplant as well as donors and recipients with incomplete HCV Ab and NAT information. Figure 1 illustrates the study cohort selection process.
Figure 1.
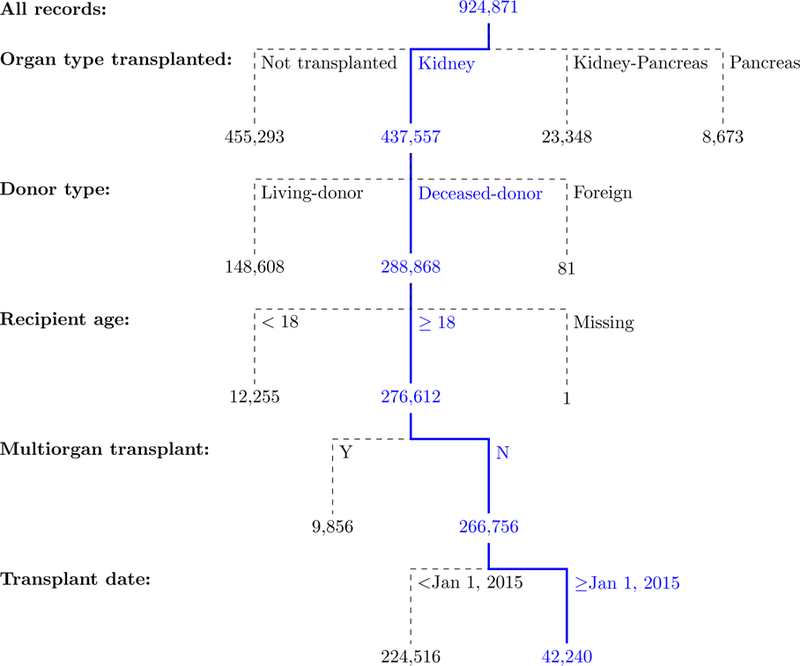
Adult DDKT reported to OPTN/UNOS from January 1, 2015 to June 30, 2018.
DDKT, Deceased donor kidney transplant; OPTN, Organ Procurement and Transplantation Network; UNOS, United Network for Organ Sharing.
Since the OPTN dataset does not include the HCV NAT status of the transplant candidates, an HCV uninfected recipient was defined as a subject with a negative HCV Ab. For practical purposes, an HCV uninfected donor was defined as a donor with a negative HCV Ab and NAT. An HCV seropositive, non-viremic donor was defined as a donor with a positive HCV Ab and a negative NAT (HCV D Ab+, NAT−). An HCV viremic donor was defined as a subject with a positive HCV NAT, regardless of the HCV Ab status (HCV D Ab±, NAT+). The term “HCV positive donor” refers to donors with either a positive HCV Ab and/or positive HCV NAT.
Outcome ascertainment
The primary outcome measures were length of stay (LOS), delayed graft function (DGF), and rejection rate, serum creatinine and estimated glomerular filtration rate (eGFR) at six months post-transplant. The LOS was defined as the time in days from admission to discharge during the transplant admission. DGF was defined as requiring dialysis during the first-week post-transplantation. The eGFR was calculated using the Modification of Diet in Renal Disease Study (MDRD) 4 variable equation. Secondary outcomes included: overall graft and patient survival at 12 months post-transplant and overall graft failure risk at 12 months by PS matched Cox proportional hazard analysis. Overall graft loss is a composite outcome of both graft loss (return to dialysis or retransplantation) and death (17). Overall graft survival was defined as 1 minus the probabilily of overall graft loss. All outcome measures were censored at the administrative end of the study (September 30th, 2018)
Statistical Methods
Donor and recipient characteristics were described using mean and standard deviation or frequencies. Comparisons between groups were made using the t-test or Wilcoxon signed–rank test (non-parametric), one-way ANOVA or the Kruskal-Wallis test by ranks (non-parametric) for continuous variables, and Chi-squared tests for categorical variables as appropriate. Holm’s correction was used to adjust for multiple pairwise testing of the baseline characteristics of the study groups. Survival curves were estimated using the Kaplan-Meier method. The log-rank test was used to compare groups.
Multivariable Cox proportional hazard regression models were used to estimate the hazard ratios associated with overall graft failure risk at 12 months post-transplant. We assessed the proportional-hazards assumption graphically using log-log plots of survival, Kaplan-Meier curves, predicted survival plots, and Schoenfeld residuals global testing. The magnitude of missing data in the PS matched cohort was minimal (<1%), so we did not use imputation.
PS is a balancing score representing the probability of receiving a, HCV D Ab+, NAT− or HCV D Ab±, NAT+ deceased donor given the patient’s covariate pattern. The PS scores were calculated using two separate models between exposure vs. control groups (model 1: HCV D Ab+, NAT−, vs. HCV D Ab−, NAT− and model 2: HCV D Ab± NAT+, vs. D Ab−, NAT−). Within each stratum, we used nearest-neighbor Mahalanobis metric matching with bias-correction term (18) to match and allocate patients to their respective groups based on their PS scores. Matching was performed using a 2:1 ratio without replacement. The PS matching was performed based on variables including recipient age (year), gender, race (African American, non-African American), cause of ESRD (diabetes, hypertension, glomerulonephritis, polycystic kidney disease, others), diabetes status, re-transplant status, share type (local, regional, national), donor after cardiac death status, induction type (no-induction, interleukin-2 receptor antagonist, rabbit antithymocyte globulin, and alemtuzumab), cold ischemia time (hours), donor Kidney Donor Profile (KDPI) score category (0–20, 21–50, 51–80, 81–100%), wait-list time (0–1, 1–3, >3 years), and dialysis vintage (preemptive, 0–1, 1–3, >3 years). We compared the distribution of PS between groups using box-and-whisker plots. In multivariable Cox proportional hazards models, the independent variables of interest were identified based on prior published literature (15, 16, 19, 20), and selected using the backward stepwise selection method to identify significant variables (p<0.10) associated with the outcomes in the final models. Statistical inference was based on the analysis within strata defined by HCV Ab and NAT status using multivariable PS-matched Cox proportional hazards models, adjusted for other covariates including BMI (BMI<35, BMI ≥35), transplant year, HLA mismatch, wait-list time, and the UNOS Region. A sensitivity analysis excluding the KDPI score was performed to evaluate the robustness of the propensity score and Cox proportional hazards models and eliminate the effect of HCV Ab positivity on the KDPI score (supplement material).
A p-value < 0.05 was considered statistically significant. Statistical analyses were performed with Stata/MP14 (StataCorp LP, College Station, TX) and R version 3.5.1.
Results
Study Population
During the study period, we identified 42,240 DDKT recipients meeting inclusion and exclusion criteria (Figure 1). The recipients’ HCV Ab status was negative in 38,976 (92.3%), positive in 2,584 (6.1%), not done in 440 (1.0%), missing in 170 (0.4%) and unknown in 70 (0.2%). Of the 40,492 donors with a negative HCV Ab, 38,862 (96.0%) had a negative HCV NAT and 55 (0.1%) a positive result. Of the 1,745 donors with a positive HCV Ab, 1,033 (59.2%) had a positive NAT while 664 (38.1%) had a negative NAT (Figure 2). Thus, we identified 36,934 DDKT from HCV uninfected donors to HCV uninfected recipients, 352 from HCV seropositive, non-viremic donors to uninfected recipients, and 196 from HCV viremic donors to uninfected recipients (Figure 2) for a total of 37,482 kidney transplants performed in HCV uninfected recipients.
Figure 2.
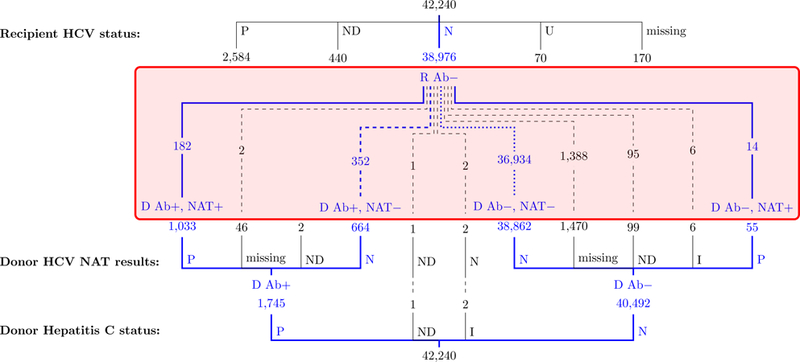
Number of adult DDKT reported to the OPTN/UNOS for HCV uninfected recipients by HCV Ab and NAT status of the donor; January 1, 2015 to June 30, 2018.
Ab, Antibody; D, Donor; DDKT, Deceased donor kidney transplant; I, Indeterminate; N, Negative; NAT: Nucleic acid testing; ND, Not done; OPTN, Organ Procurement and Transplantation Network; P, Positive; R, Recipient; U, Unknown; UNOS, United Network for Organ Sharing.
Donor and Recipients Characteristics
Table 1 summarises the donor and recipients characteristics of the study population. Compared to the reference group, the HCV seropositive non-viremic and HCV viremic donors were more likely to be White, die from anoxia, meet Public Health Services (PHS) increased risk criteria and less likely to meet extended criteria donor (ECD) definition. The HCV seropositive non-viremic donors had higher KDPI scores while the HCV viremic donors had lower proportion of hypertension and diabetes compared to the reference group. The recipients of HCV seropositive non-viremic and HCV viremic donors were older, more likely to be male and White, to have diabetes as their ESRD etiology, to receive an organ from a non-local organ procurement organization (OPO), to have a higher estimated post-transplant survival (EPTS) score, to have a shorter time on dialysis and to wait significantly shorter duration on the transplant waitlist.
Table 1.
Characteristics of deceased kidney transplant donors and recipients for HCV uninfected recipients from HCV uninfected donors (HCV D Ab−, NAT−/R Ab−), HCV seropositive, non-viremic donors (HCV D Ab+,NAT−/R Ab−) and HCV viremic (HCV Ab±, NAT+/R−) donors; US January 1, 2015 to June 30, 2018.
| All groups (n* = 37,482) | HCV D Ab−, NAT− /R Ab− (n* = 36,934) | HCV D Ab+, NAT−/R Ab− (n* = 352) | HCV D Ab±, NAT+/ R Ab− (n* = 196) | HCV D Ab−,NAT−/R Ab – vs. | ||
|---|---|---|---|---|---|---|
| p value † HCV D Ab+,NAT−/ R Ab− | p value † HCV D Ab±,NAT+/ R Ab− | |||||
| Donors | ||||||
| Age, years, n (%) | < 0.001 | <0.001 | ||||
| 18–39 | 19396 (51.7) | 19016 (51.5) | 217 (61.6) | 163 (83.2) | ||
| 40–59 | 15361 (41.0) | 15206 (41.2) | 122 (34.7) | 33 (16.8) | ||
| >=60 | 2725 (7.3) | 2712 (7.3) | 13 (3.7) | - (−) | ||
| Mean (SD) years | 38.0 (15.7) | 38.1 (15.8) | 37.8 (11.6) | 32.6 (7.7) | 0.35 | <0.001 |
| Male Gender, n (%) | 22920 (61.1) | 22620 (61.2) | 176 (50.0) | 124 (63.3) | <0.001 | 0.85 |
| Race/Ethnicity, n (%) | <0.001 | <0.001 | ||||
| White | 25387 (67.7) | 24919 (67.5) | 302 (85.8) | 166 (84.7) | ||
| Hispanic | 5235 (14.0) | 5188 (14.0) | 25 (7.1) | 22 (11.2) | ||
| Black | 5173 (13.8) | 5152 (13.9) | 16 (4.5) | 5 (2.6) | ||
| Other | 1687 (4.5) | 1675 (4.5) | 9 (2.6) | 3 (1.5) | ||
| BMI, n (%) | 0.79 | 0.15 | ||||
| <22 | 6846 (18.3) | 6764 (18.3) | 54 (15.3) | 28 (14.3) | ||
| 22–30 | 20112 (53.7) | 19757 (53.6) | 218 (61.9) | 137 (69.9) | ||
| 31–40 | 8061 (21.5) | 7978 (21.6) | 58 (16.5) | 25 (12.8) | ||
| >40 | 2413 (6.4) | 2385 (6.5) | 22 (6.2) | 6 (3.1) | ||
| Mean (SD) kg/m2 | 28.0 (7.2) | 28.0 (7.2) | 28.1 (6.7) | 26.5 (4.9) | 0.77 | 0.02 |
| Diabetes (any type), n (%) | 2682 (7.2) | 2660 (7.2) | 22 (6.3) | - (−) | 0.79 | <0.001 |
| Hypertension, n (%) | 10507 (28.2) | 10398 (28.4) | 91 (26.1) | 18 (9.2) | 0.66 | <0.001 |
| ABO, n (%) | 0.19 | 0.88 | ||||
| O | 17757 (47.4) | 17485 (47.3) | 178 (50.6) | 94 (48.0) | ||
| A | 14065 (37.5) | 13853 (37.5) | 136 (38.6) | 76 (38.8) | ||
| B | 4307 (11.6) | 4250 (11.5) | 32 (9.1) | 25 (12.8) | ||
| AB | 1353 (3.6) | 1346 (3.6) | 6 (1.7) | 1 (0.5) | ||
| Cause of death, n, (%) | <0.001 | <0.001 | ||||
| Anoxia | 15099 (40.3) | 14703 (39.8) | 248 (70.5) | 148 (75.5) | ||
| Head Trauma | 11723 (31.3) | 11639 (31.5) | 52 (14.8) | 32 (16.3) | ||
| Cerebrovascular | 9421 (25.1) | 9363 (25.4) | 45 (12.8) | 13 (6.6) | ||
| Other | 1239 (3.3) | 1229 (3.3) | 7 (2.0) | 3 (1.5) | ||
| DCD, n (%) | 8182 (21.8) | 8106 (21.9) | 59 (16.8) | 17 (8.7) | 0.06 | <0.001 |
| ECD, n (%) | 5176 (13.8) | 5146 (13.9) | 30 (8.5) | - (−) | 0.01 | <0.001 |
| PHS IRD, n (%) | 8393 (22.4) | 7954 (21.5) | 260 (73.9) | 179 (91.3) | <0.001 | <0.001 |
| KDPI %, n (%) | <0.001 | 0.53 | ||||
| 0–20% | 7726 (20.6) | 7724 (20.9) | 1 (0.3) | 1 (0.5) | ||
| 21–50% | 12583 (33.6) | 12358 (33.5) | 115 (32.7) | 110 (56.1) | ||
| 51–80% | 12253 (32.7) | 12009 (32.5) | 163 (46.3) | 81 (41.3) | ||
| 81–100% | 4919 (13.1) | 4842 (13.1) | 73 (20.7) | 4 (2.0) | ||
| Mean (SD) % | 47.1 (26.5) | 47.0 (26.6) | 61.0 (20.3) | 49.0 (14.8) | <0.001 | 0.20 |
| Recipients | ||||||
| Age, years, n (%) | <0.001 | <0.001 | ||||
| 0–39 | 7439 (19.8) | 7405 (20.0) | 28 (8.0) | 6 (3.1) | ||
| 40–59 | 17154 (45.8) | 16912 (45.8) | 154 (43.8) | 88 (44.9) | ||
| >=60 | 12889 (34.4) | 12617 (34.2) | 170 (48.3) | 102 (52.0) | ||
| Mean (SD) years | 52.1 (13.5) | 52.0 (13.6) | 57.6 (11.6) | 59.2 (10.2) | <0.001 | <0.001 |
| Male Gender, n (%) | 21943 (58.5) | 21560 (58.4) | 231 (65.6) | 152 (77.6) | 0.02 | <0.001 |
| Race/Ethnicity, n (%) | <0.001 | <0.001 | ||||
| White | 13877 (37.0) | 13625 (36.9) | 164 (46.6) | 88 (44.9) | ||
| Black | 12520 (33.4) | 12333 (33.4) | 106 (30.1) | 81 (41.3) | ||
| Hispanic | 7330 (19.6) | 7261 (19.7) | 57 (16.2) | 12 (6.1) | ||
| Other | 3755 (10.0) | 3715 (10.1) | 25 (7.1) | 15 (7.7) | ||
| BMI, kg/mg2, n, (%) | 0.52 | 0.92 | ||||
| <22 | 4606 (12.3) | 4557 (12.3) | 35 (9.9) | 14 (7.1) | ||
| 22–30 | 21224 (56.6) | 20898 (56.6) | 201 (57.1) | 125 (63.8) | ||
| 31–40 | 11042 (29.5) | 10874 (29.4) | 112 (31.8) | 56 (28.6) | ||
| >40 | 604 (1.6) | 599 (1.6) | 4 (1.1) | 1 (0.5) | ||
| Mean (SD) kg/m2 | 28.3 (5.4) | 28.3 (5.4) | 28.5 (5.2) | 28.6 (4.5) | 0.87 | 0.64 |
| ABO, count (%) | 0.06 | 0.99 | ||||
| O | 17034 (45.4) | 16770 (45.4) | 175 (49.7) | 89 (45.4) | ||
| A | 13343 (35.6) | 13145 (35.6) | 130 (36.9) | 68 (34.7) | ||
| B | 5101 (13.6) | 5036 (13.6) | 36 (10.2) | 29 (14.8) | ||
| AB | 2004 (5.3) | 1983 (5.4) | 11 (3.1) | 10 (5.1) | ||
| Region, n (%) | <0.001 | <0.001 | ||||
| 1 | 1262 (3.4) | 1251 (3.39) | 5 (1.42) | 6 (3.06) | ||
| 2 | 4507 (12.0) | 4415 (11.95) | 28 (7.95) | 64 (32.65) | ||
| 3 | 5078 (13.5) | 4977 (13.48) | 53 (15.06) | 48 (24.49) | ||
| 4 | 3774 (10.1) | 3739 (10.12) | 33 (9.38) | 2 (1.02) | ||
| 5 | 6798 (18.1) | 6740 (18.25) | 47 (13.35) | 11 (5.61) | ||
| 6 | 1464 (3.9) | 1452 (3.93) | 11 (3.12) | 1 (0.51) | ||
| 7 | 2658 (7.1) | 2626 (7.11) | 32 (9.09) | - (−) | ||
| 8 | 2402 (6.4) | 2400 (6.50) | 2 (0.57) | - (−) | ||
| 9 | 2588 (6.9) | 2561 (6.93) | 6 (1.70) | 21 (10.71) | ||
| 10 | 2889 (7.7) | 2768 (7.49) | 112 (31.82) | 9 (4.59) | ||
| 11 | 4062 (10.8) | 4005 (10.84) | 23 (6.53) | 34 (17.35) | ||
| EPTS, Mean (SD) | 48.8 (30.4) | 48.7 (30.4) | 57.6 (26.5) | 56.5 (25.6) | <0.001 | <0.001 |
| Days on waiting list, Mean (SD) | 957.6 (888.2) | 961.8 (889.6) | 818.1 (794.0) | 426.0 (536.5) | 0.01 | <0.001 |
| Dialysis duration, years, n (%) | <0.001 | <0.001 | ||||
| Preemptive | 5135 (13.7) | 5059 (13.7) | 56 (16.0) | 39 (19.9) | ||
| <= 1 year | 9145 (24.4) | 9011 (24.4) | 92 (26.1) | 56 (28.4) | ||
| 1–3 years | 11019 (29.4) | 10821 (29.3) | 119 (33.7) | 81 (41.1) | ||
| > 3 years | 12181 (32.5) | 12003 (32.5) | 85 (24.2) | 19 (9.7) | ||
| Mean‡, (SD) years | 2.7 (3.3) | 2.7 (3.3) | 2.1 (2.7) | 1.5 (1.6) | <0.001 | <0.001 |
| CIT (hours), mean (SD) | 17.9 (8.7) | 17.9 (8.7) | 18.7 (7.1) | 19.0 (7.7) | 0.01 | 0.03 |
| cPRA (%) | <0.001 | <0.001 | ||||
| 0–20 | 23937 (63.9) | 23484 (63.6) | 280 (79.5) | 173 (88.3) | ||
| 21–80 | 5974 (15.9) | 5908 (16.0) | 51 (14.5) | 15 (7.7) | ||
| 81–100 | 7571 (20.2) | 7542 (20.4) | 21 (6.0) | 8 (4.1) | ||
| Mean (SD) | 27.7 (39.0) | 27.9 (39.1) | 13.2 (26.6) | 7.7 (21.9) | <0.001 | <0.001 |
| HLA Mismatch, n (%) | 0.01 | 0.04 | ||||
| 0 | 1931 (5.2) | 1927 (5.2) | 2 (0.6) | 2 (1.0) | ||
| 1 | 645 (1.7) | 642 (1.7) | 2 (0.6) | 1 (0.5) | ||
| 2 | 2053 (5.5) | 2032 (5.5) | 11 (3.1) | 10 (5.1) | ||
| 3 | 5546 (14.8) | 5467 (14.8) | 49 (13.9) | 30 (15.3) | ||
| 4 | 10374 (27.7) | 10210 (27.6) | 111 (31.5) | 53 (27.0) | ||
| 5 | 11599 (30.9) | 11425 (30.9) | 113 (32.1) | 61 (31.1) | ||
| 6 | 5334 (14.2) | 5231 (14.2) | 64 (18.2) | 39 (19.9) | ||
| Mean (SD) | 4.1 (1.5) | 4.1 (1.5) | 4.4 (1.1) | 4.4 (1.2) | <0.001 | 0.02 |
| Diagnosis, n (%) | 0.16 | 0.90 | ||||
| Diabetes | 9853 (26.3) | 9647 (26.1) | 129 (36.6) | 77 (39.3) | ||
| Hypertension | 8532 (22.8) | 8410 (22.8) | 79 (22.4) | 43 (21.9) | ||
| Glomerulonephritis | 7209 (19.2) | 7134 (19.3) | 52 (14.8) | 23 (11.7) | ||
| Polycystic | 2757 (7.4) | 2699 (7.3) | 39 (11.1) | 19 (9.7) | ||
| Prior kidney Transplant failure | 2966 (7.9) | 2943 (8.0) | 14 (4.0) | 9 (4.6) | ||
| Other | 6165 (16.4) | 6101 (16.5) | 39 (11.1) | 25 (12.8) | ||
| Allocation type, n (%) | <0.001 | <0.001 | ||||
| Local | 26384 (70.4) | 26247 (71.1) | 98 (27.8) | 39 (19.9) | ||
| Regional | 4916 (13.1) | 4748 (12.9) | 107 (30.4) | 61 (31.1) | ||
| National | 6182 (16.5) | 5939 (16.1) | 147 (41.8) | 96 (49.0) | ||
| Induction Immunosuppression, n (%) | 0.53 | 0.24 | ||||
| No-induction | 3,536 (9.4) | 3,512 (9.5) | 17 (4.8) | 7 (3.6) | ||
| IL2-RA | 5,082 (13.6) | 4,996 (13.5) | 55 (15.6) | 31 (15.8) | ||
| r-ARTG | 21,330 (56.9) | 20,981 (56.8) | 204 (58.0) | 145 (74.0) | ||
| Alemtuzumab | 5,786 (15.4) | 5,705 (15.4) | 73 (20.7) | 8 (4.1) | ||
| Other | 1748 (4.7) | 1,740 (4.7) | 3 (0.9) | 5 (2.6) | ||
ABO, Antibodies blood group; Ab, Antibody; BMI, Body mass index; CIT, Cold ischemia time; cPRA, Calculated panel reactive antibodies; DCD, Donation after cardiac death;; D, Donor; ECD, Extended criteria donor; HCV, Hepatitis C Virus; HLA, Human leucocyte antigens; IRD, Increased risk donor; KDPI, Kidney Donor Profile Index; NAT, Nucleic acid testing; PHS, Public Health Service; SD, Standard deviation.
n denotes the total number of records in each group. Missing/unknown values in particular variables are ignored when reporting summary statistics.
Adjusted by Holm’s method for multiple pairwise testing.
If not pre-emptive.
The overall graft survival in years for adult HCV uninfected DDKT recipients, stratified by HCV Ab and NAT donor status, is presented by the Kaplan-Meier Method in Figure 3 (overall study cohort prior to propensity matching, N=37,482). At 1 year post-transplant, the overall graft survival was 94.1% (95% CI, 93.8 to 94.3) for the HCV uninfected donors to uninfected recipients, 94.1% (95% CI 90.7 to 97.6) for the HCV seropositive, non-viremic donors to uninfected recipients and 98.2 (95% CI 95.6 to 100.0) for the HCV viremic donors to uninfected recipients. There was no statistical difference for overall graft survival among three groups (p=0.77).
Figure 3.
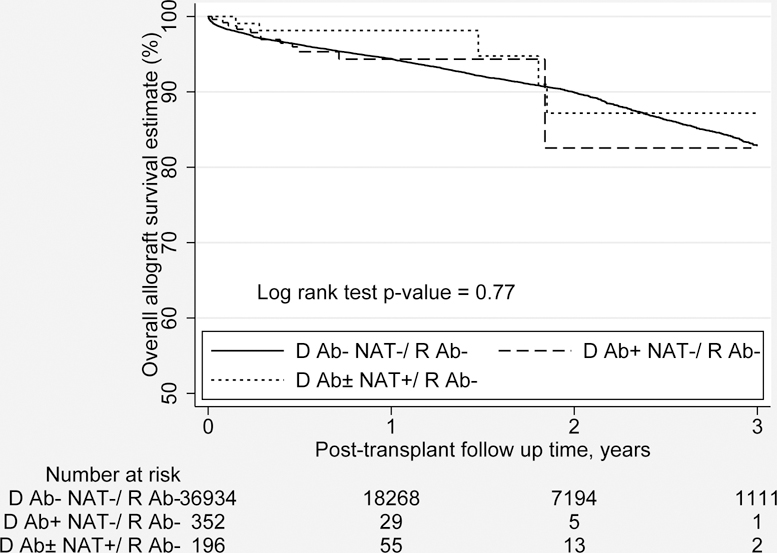
Overall graft survival (in years) by the Kaplan-Meier Method for adult HCV uninfected DDKT recipients by donor HCV Ab and HCV NAT status (the study cohort N=37,482).
Ab, Antibody; D, Donor; DDKT, Deceased donor kidney transplant; HCV, Hepatitis C virus; NAT, Nucleic acid testing; R, Recipient.
Propensity Score Matching
Table 2 summarizes the post-propensity score matched group characteristics based on the HCV uninfected recipients by HCV Ab and NAT status of the donor. There was no statistical difference between the baseline characteristics of the DDKT from HCV seropositive, non-viremic donors to uninfected recipients and HCV uninfected donors to uninfected recipients. Compared to the reference group, the DKKT performed using HCV viremic donors for uninfected recipients had shorter time on the waitlist and dialysis, and a lower KDPI Score.
Table 2.
Post-propensity matched characteristics of HCV uninfected recipients from HCV uninfected donors (HCV D Ab−, NAT−/R Ab−), vs. HCV seropositive, non-viremic donors (HCV D Ab+,NAT−/R Ab−) and HCV viremic (HCV Ab±, NAT+/R−) donors.*
| D Ab−, NAT− / R Ab− (n = 654) | D Ab+, NAT− / R Ab− (n = 349) | p value | D Ab−, NAT− / R Ab− (n = 340) | D Ab+−, NAT+/ R Ab− (n =191) | p value | |
|---|---|---|---|---|---|---|
| Age (SD) year | 57.5 (10.7) | 57.6 (11.6) | 0.83 | 57.6 (10.4) | 59.1 (10.5) | 0.12 |
| Gender male, n (%) | 430 (65.8) | 229 (65.6) | 0.97 | 259 (76.2) | 148 (77.5) | 0.73 |
| African American race, n (%) | 192 (29.4) | 104 (29.8) | 0.88 | 136 (40.0) | 77 (40.3) | 0.94 |
| Diabetes (any type), n (%) | 290 (44.3) | 157 (45.0) | 0.85 | 158 (46.7) | 93 (48.7) | 0.62 |
| Previous renal transplant, n (%) | 49 (7.5) | 25 (7.2) | 0.85 | 24 (7.1) | 12 (6.3) | 0.73 |
| Diagnosis, n (%) | 0.83 | 0.99 | ||||
| Glomerulonephritis | 108 (16.5) | 53 (15.2) | 47 (13.8) | 23 (12.0) | ||
| Hypertension | 131 (20.0) | 80 (22.9) | 77 (22.7) | 44 (23.0) | ||
| DM | 242 (37.0) | 120 (34.4) | 114 (33.5) | 66 (34.6) | ||
| PKD | 53 (8.1) | 39 (11.2) | 32 (9.4) | 18 (9.4) | ||
| Others | 120 (18.4) | 57 (16.3) | 70 (20.1) | 40 (20.9) | ||
| cPRA, n (%) | 0.66 | 0.57 | ||||
| 0–20 | 515 (78.8) | 277 (79.4) | 297 (87.4) | 169 (88.5) | ||
| 21–80 | 90 (13.8) | 51 (14.6) | 22 (6.5) | 14 (7.3) | ||
| 81–100 | 49 (7.5) | 21 (6.0) | 21 (6.2) | 8 (4.2) | ||
| mean (SD) | 14.8 (29.3) | 13.3 (26.9) | 0.42 | 10.1 (26.7) | 7.8 (22.1) | 0.29 |
| Days on waitlist, mean (SD) | 823 (847) | 816 (794) | 0.91 | 558 (711) | 429 (541) | 0.03 |
| Dialysis duration prior to listing (years), n (%) | 0.88 | 0.02 | ||||
| Preemptive | 98 (15.0) | 55 (15.8) | 70 (20.6) | 38 (19.9) | ||
| ≤1 | 185 (28.3) | 92 (26.3) | 99 (29.2) | 55 (29.0) | ||
| 1–3 | 207 (31.7) | 117 (33.5) | 103 (30.3) | 74 (38.7) | ||
| >3 | 163 (25.0) | 85 (24.4) | 60 (17.6) | 16 (8.4) | ||
| Mean† (SD) | 2.3 (3.2) | 2.1 (2.7) | 0.18 | 2.4 (2.9) | 1.5 (1.6) | <0.001 |
| Allocation type, n (%) | 0.07 | 0.08 | ||||
| Local | 229 (35.0) | 98 (28.1) | 100 (29.4) | 39 (20.4) | ||
| Regional | 186 (28.4) | 105 (30.1) | 92 (27.1) | 60 (31.4) | ||
| National | 239 (36.5) | 146 (41.8) | 148 (43.5) | 92 (48.2) | ||
| DCD, n (%) | 108 (16.5) | 59 (16.9) | 0.87 | 33 (9.7) | 17 (8.9) | 0.76 |
| KDPI %, n (%) | 0.62 | 0.01 | ||||
| 0–20 | 16 (2.5) | 1 (0.3) | 15 (0.9) | 1 (0.5) | ||
| 21–50 | 201 (30.7 | 113 (32.3) | 165 (48.5) | 106 (55.5) | ||
| 51–80 | 300 (45.8) | 163 (46.7) | 141 (41.5) | 81 (42.1) | ||
| 81–100 | 137 (21) | 72 (20.6) | 19 (5.6) | 3 (1.6) | ||
| CIT mean (SD), hours | 19.1 (7.4) | 18.7 (7.1) | 0.41 | 19.1 (7.4) | 18.8 (7.4) | 0.61 |
| Induction Immunosuppression | 0.89 | 0.78 | ||||
| No-induction | 29 (4.4) | 17 (4.9) | 15 (4.3) | 7 (3.7) | ||
| IL2-RA | 96 (14.7) | 55 (15.8) | 54 (15.4) | 31 (16.2) | ||
| r-ATG | 417 (63.8) | 204 (58.5) | 265 (75.7) | 145 (75.9) | ||
| Alemtuzumab | 112 (17.1) | 73 (20.9) | 16 (4.6) | 8 (4.2) |
Ab, Antibody; CIT, Cold Ischemia time; cPRA, Calculated panel reactive antibodies; DCD, Donation after cardiac death; D, Donor; HCV, Hepatitis C Virus; IL-2RA, Interleukin 2 receptor antagonist, KDPI %, Kidney Donor Profile Index; PKD, Polycystic Kidney Disease; r-ATG, Rabbit Antithymocyte globulin; R, Recipient; SD, Standard deviation.
The propensity matching was performed based on variables including recipient age (year), gender, race (AA, non-AA), cause of ESRD (DM, HTN, GN, PKD, others), diabetes status, re-transplant status, share type (local, regional, national), donor DCD status, induction type (no-induction, IL2-RA, r-ATG, alemtuzumab), cold ischemia time (hour), donor KDPI score category (0–20, 21–50, 51–80, 81–100%), cPRA (0–20%, 21–80%, 81–100%), wait-list time (0–1, 1–3, >3 years), and dialysis vintage (preemptive, 0–1, 1–3, >3 years).
If not pre-emptive.
Propensity Score-Matched Group Outcomes
Table 3 presents the outcomes of the propensity score matched groups. Compared to the reference group, the transplants performed using HCV seropositive, non-viremic donors for uninfected recipients had no statistically significant difference in LOS (6.3 vs. 6.1 days; p=0.70), rejection rate (4.2% vs. 2.0%; p=0.05), serum creatinine (1.49 vs. 1.29 mg/dl; p<0.19) and eGFR (55.6 vs. 60.9 ml/min/1.73 m2; p<0.25) at 6 months. However, HCV uninfected recipients of HCV seropositive, non-viremic donor kidneys, compared to those of uninfected donor kidneys, experienced a lower proportion of DGF (35.2% vs. 18.9%; p<0.001). There was no statistically significant differences in overall graft survival (92.8% vs. 94.3%; p=0.72) at 12 months post-transplant or by the Kaplan-Meier Method (Figure 4a). There was no statistical difference in the overall graft failure risk (HR 0.60; 95% CI 0.23 to 1.29; p=0.19) estimated from multivariate Cox proportional hazards analysis or patient survival at 12 months post-transplant (96.7% vs. 96.3%; p=0.85).
Table 3.
Outcomes of propensity-matched HCV uninfected recipients from HCV uninfected donors (HCV D Ab−, NAT−/R Ab−), vs. HCV seropositive, non-viremic donors (HCV D Ab+, NAT− / R Ab−) and HCV viremic (HCV Ab± ,NAT+/R Ab−) donors.*
| D Ab−,NAT−/ R Ab− (n = 659) | D Ab+, NAT− / R Ab− (n = 349) | p value | D Ab−, NAT− / R Ab− (n = 350) | D Ab±, NAT+/ R Ab− (n =191) | p value | |
|---|---|---|---|---|---|---|
| Post-transplant follow-up time, mean (SD),years | 1.2 (0.9) | 0.5 (0.5) | <0.001 | 1.2 (0.9) | 0.6 (0.8) | <0.001 |
| Primary Outcomes | ||||||
| LOS mean (SD), days | 6.3 (8.1) | 6.1 (8.3) | 0.70 | 6.0 (4.8) | 5.6 (4.1) | 0.35 |
| DGF, n (%) | 230 (35.2) | 66 (18.9) | <0.001 | 123 (36.2) | 32 (16.8) | <0.001 |
| Acute rejection at 6 months, n (%) | 25 (4.2) | 4 (2.0) | 0.05 | 13 (5.3) | 4 (4.4) | 0.72 |
| Scr at 6 months, mean (SD), mg/dl | 1.49 (0.75) | 1.29 (0.27) | 0.19 | 1.56 (0.48) | 1.26 (0.33) | 0.01 |
| eGFR† at 6 months, mean, (SD) ml/min/1.73 m2 | 55.6 (22.6) | 60.9 (14.9) | 0.25 | 54.1 (19.3) | 68.3 (17.4) | 0.004 |
| Secondary Outcomes | ||||||
| Overal graft survival at 12 months, % | 92.8 | 94.3 | 0.72 | 94.2 | 98.4 | 0.17 |
| Patient survival at 12 months, % | 96.7 | 96.3 | 0.85 | 97.2 | 100 | 0.23 |
| Overall graft failure risk at 12 months post-transplant | Reference | HR (95% CI) | Reference | HR (95% CI) | P value | |
| Univariate | - | 0.81 (0.42–1.60) | 0.55 | - | 0.61 (0.21–1.83) | 0.39 |
| Multivariable‡§ | - | 0.60 (0.23–1.29) | 0.19 | - | 0.85 (0.25–2.96) | 0.17 |
Ab, Antibody; cPRA, Calculated panel reactive antibodies; D, Donor; DGF, Delayed graft function; eGFR, Estimated Glomerular filtration rate; HCV, Hepatitis C Virus; LOS, Length of stay; NAT, Nucleic acid testing; R, Recipient; Scr, Serum Creatinine.
The propensity matching was performed based on variables including recipient age (year), gender, race (AA, non-AA), cause of ESRD (DM, HTN, GN, PKD, others), diabetes status, re-transplant status, share type (local, regional, national), donor DCD status, induction type (no-induction, IL2-RA, r-ATG, alemtuzumab), cold ischemia time (hour), and donor KDPI score category (0–20, 21–50, 51–80, 81–100%), cPRA (0–20%, 21–80%, 81–100%), wait-list time (0–1, 1–3, >3 years), and dialysis vintage (preemptive, 0–1, 1–3, >3 years).
eGFR was calculated using the Modification of Diet in Renal Disease Study (MDRD) equation.
Cox proportional hazard analysis
Multivariable proportional Cox hazard analysis adjusted for BMI (BMI<35, BMI ≥35), transplant year, HLA mismatch, time and the UNOS Region.
Figure 4a.
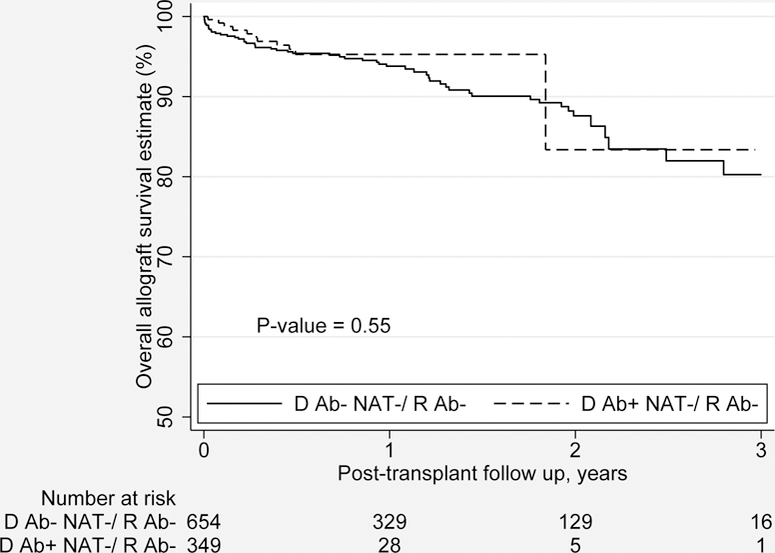
Propensity matched overall graft survival (in years) by the Kaplan-Meier Method for HCV uninfected adult DDKT recipents of HCV seropositive, non-viremic donors vs. HCV uninfected donors.
Ab, Antibody; D, Donor; HCV, Hepatitis C virus; NAT, Nucleic acid testing; R, Recipient.
Compared to the reference group, the transplants performed using HCV viremic donors for uninfected recipients also had no statistically significant difference in the LOS (6.0 vs. 5.6 days; p=0.35), and rejection rate at at 6 months (5.3% vs. 4.4%; p=0.72). However, HCV uninfected recipients of HCV viremic donor kidneys, compared to those of uninfected donor kidneys, experienced a lower proportion of DGF (36.2% vs. 16.8%; p<0.001), serum creatinine (1.56 vs. 1.26 mg/dl; p=0.01) and eGFR (54.1 vs. 68.3 ml/min/1.73 m2; p 0.004) at 6 months. There was no statistically significant difference in the overall graft survival (94.2% vs. 98.4%; p=0.17) at 12 months post-transplant or by the Kaplan-Meier Method (Figure 4b). There was no statistically significant difference in the overall graft failure risk (HR 0.85; 95% CI 0.25 to 2.96; p=0.17) estimated from multivariate Cox proportional hazards analysis or patient survival at 12 months post-transplant (97.2% vs. 100.0% p=0.23).
Figure 4b.
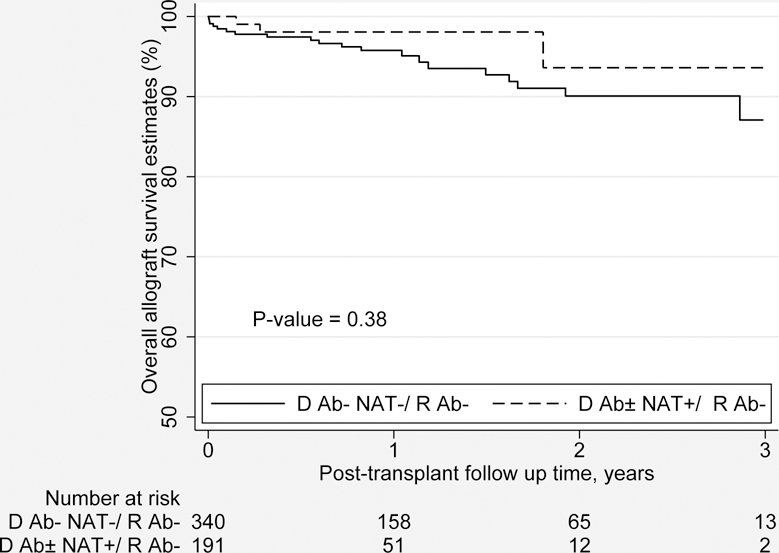
Propensity matched overall graft survival (in years) by the Kaplan-Meier Method for HCV uninfected adult DDKT recipents of HCV Viremic donors vs. HCV uninfected donors.
Ab, Antibody; D, Donor; HCV, Hepatitis C virus; NAT, Nucleic acid testing; R, Recipient.
Number of DDKT using HCV seropositive, non-viremic donors and HCV viremic donors for HCV uninfected recipients
Between January 1, 2015 and June 30, 2018, 239 transplant programs performed at least one kidney transplant. Among these programs, 183 (76.6%) did not utilize any HCV infected donors for uninfected recipients. The number of programs that utilize HCV infected donors for uninfected recipients more than doubled during our study period (12 in 2015 to 29 in the first half 2018, Table 4). Overall, during the study period, 56 (23.4%) transplant programs utilized HCV infected donors for uninfected recipients, of which 23 (41.1%) utilized HCV seropositive, non-viremic donors but no HCV viremic donors, 18 (32.1%) utilized HCV viremic donors but no HCV seropositive, non-viremic donors, and 15 (26.8%) utilized both types of donors.
Table 4.
Number of deceased donor kidney transplants performed from January 1, 2015 to June 30, 2018 by transplant program using HCV seropositive non-viremic (HCV D Ab+, NAT −) and HCV Viremic donors (HCV D Ab±, NAT+) for HCV uninfected recipients (R Ab −).
| HCV D Ab+, NAT −/ R Ab − | HCV D Ab±, NAT+ / R Ab − | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Center | 2015 | 2016 | 2017 | 2018 | ALL | Center | 2015 | 2016 | 2017 | 2018 | ALL | |
| 1 | 1 | 2 | 3 | 1 | 10 | 23 | 9 | 42 | ||||
| 2 | 3 | 3 | 2 | 37 | 37 | |||||||
| 3 | 1 | 5 | 6 | 3 | 24 | 24 | ||||||
| 5 | 1 | 1 | 4 | 14 | 14 | |||||||
| 7 | 2 | 1 | 1 | 2 | 6 | 5 | 8 | 1 | 1 | 10 | ||
| 8 | 1 | 1 | 6 | 6 | 4 | 10 | ||||||
| 9 | 1 | 1 | 7 | 4 | 1 | 1 | 6 | |||||
| 10 | 1 | 1 | 8 | 2 | 2 | 1 | 5 | |||||
| 13 | 1 | 2 | 3 | 9 | 3 | 2 | 5 | |||||
| 16 | 2 | 2 | 10 | 1 | 3 | 4 | ||||||
| 17 | 1 | 1 | 11 | 1 | 3 | 4 | ||||||
| 18 | 1 | 1 | 12 | 3 | 1 | 4 | ||||||
| 19 | 7 | 7 | 13 | 3 | 3 | |||||||
| 21 | 1 | 1 | 14 | 2 | 1 | 3 | ||||||
| 22 | 1 | 1 | 15 | 3 | 3 | |||||||
| 34 | 4 | 25 | 25 | 54 | 16 | 2 | 2 | |||||
| 35 | 28 | 13 | 41 | 17 | 1 | 1 | 2 | |||||
| 36 | 21 | 11 | 32 | 18 | 1 | 1 | 2 | |||||
| 37 | 24 | 7 | 31 | 19 | 1 | 1 | 2 | |||||
| 38 | 17 | 7 | 24 | 20 | 1 | 1 | ||||||
| 39 | 1 | 17 | 4 | 22 | 21 | 1 | 1 | |||||
| 40 | 5 | 15 | 20 | 22 | 1 | 1 | ||||||
| 41 | 18 | 18 | 23 | 1 | 1 | |||||||
| 42 | 5 | 12 | 17 | 24 | 1 | 1 | ||||||
| 43 | 1 | 15 | 16 | 25 | 1 | 1 | ||||||
| 44 | 2 | 9 | 11 | 26 | 1 | 1 | ||||||
| 45 | 1 | 4 | 5 | 27 | 1 | 1 | ||||||
| 46 | 1 | 4 | 5 | 28 | 1 | 1 | ||||||
| 47 | 4 | 4 | 29 | 1 | 1 | |||||||
| 48 | 4 | 4 | 30 | 1 | 1 | |||||||
| 49 | 2 | 2 | 31 | 1 | 1 | |||||||
| 50 | 2 | 2 | 32 | 1 | 1 | |||||||
| 51 | 1 | 1 | 33 | 1 | 1 | |||||||
| 52 | 1 | 1 | ALL | 14 | 39 | 46 | 97 | 196 | ||||
| 53 | 1 | 1 | ||||||||||
| 54 | 1 | 1 | ||||||||||
| 55 | 1 | 1 | ||||||||||
| 56 | 1 | 1 | ||||||||||
| ALL | 7 | 14 | 170 | 161 | 352 | |||||||
Ab, antibody; D, Donor; HCV, Hepatitis C Virus; NAT, Nucleic acid testing; R, Recipient.
A total of 352 adult DDKT were performed using HCV seropositive, non-viremic donors for uninfected recipients during the study period at 38 transplant hospitals (Table 4). There has been an increase in the number of such transplants over time (7 in 2015, 14 in 2016, 168 in 2017, and 161 in the first half of 2018). The year 2017 marks the beginning of increased utilization of HCV seropositive, non-viremic donor kidneys. The ten programs with the highest overall volume of such transplants performed all of these transplants starting in 2017. Overall, 11 centers have performed at least ten, with 7 of them performing at least twenty, while another 27 transplant programs have performed less than ten, with 14 of them performing only one.
A total of 196 adult DDKT were performed using HCV viremic donors for uninfected recipients during the study period at 33 transplant hospitals (Table 4). There has been an increase in the number of such transplants over time (14 in 2015, 39 in 2016, 46 in 2017, and 97 in the first half of 2018). Three of the top four programs with the highest number of such transplants started performing them in 2018. Overall, 6 transplant programs have performed at least ten, with 3 of them achieving more than twenty, while another 27 transplant programs have performed less than ten, with 14 of them performing only one.
Discussion
Using PS matching, our study demonstrates a lower proportion of DGF between the study groups (HCV seropositive, non-viremic donors to uninfected recipients, and HCV viremic donors to uninfected recipients) and the reference group (HCV uninfected donors to uninfected recipients). Furthermore, HCV uninfected recipients of HCV viremic donor kidneys had better allograft function at 6 months compared to the reference group. Conversely, there were no statistically significant differences in the secondary outcomes (overall graft survival, patient survival, and risk of overall graft failure at 12 months post-transplant). These results mirror those reported in two small, open-label, single-center clinical trials using HCV viremic donors for uninfected recipients. The first trial (THINKER) used a pre-emptive approach, using elbasvir-grazoprevir ± ribavirin for donors with genotype 1 and 4 (16). The second trial (EXPANDER) used a pre- and post-exposure approach, using grazoprevir-elbasvir ± sofosbuvir for donors with genotypes 1–4(15). These favorable outcomes may be the result of an overestimation of the KDPI scores of the HCV positive organs and/or the result of rapid control of the HCV infection with DAA in the recipients (15, 16, 21, 22). In all twenty THINKER study participants, the HCV viral load was undetectable within four weeks, and seven of the ten EXPANDER trial participants had undetectable viral loads at all measured timepoints (15, 16).
The characteristics of the HCV seropositive, non-viremic and HCV viremic donors mirror those of the victims of the opioid epidemic and are similar to those observed in prior studies using these donors (6, 14–16, 23). The recipients of these organs were older, more likely to be transplanted due to diabetes, had a higher EPTS score and had a shorter duration of time on the waitlist. The outcomes observed in our and prior studies raise concerns about the underestimation of the quality of these organs and their allocation to patients with a higher EPTS score (16, 22).
These findings are of significant and immediate clinical relevance given that patients waiting for a DDKT face a high mortality rate (24). For example, a patient over the age of 64 years has an approximately 50% chance of dying before a kidney becomes available (25). On the other hand, the survival benefit of kidney transplantation is well documented (1). However, a severe shortage of organ donors exists, driving interest in efforts to expand the donor pool. Unfortunately, the organs from HCV positive donors are underutilized (7–9). The proportion of these organs procured from non-local OPO and the shorter transplant wait times (Table 1) seen in our study and prior ones are likely to be the result of underutilization of these organs at a local level (14–16, 21). Our result, although encouraging should be interpreted with caution given the short duration of follow-up and confidence intervals of our secondary outcomes. Transplant programs and candidates, willing to accept the long-term uncertainties, could consider expanding the utilization of these organs as a potential strategy to increase the number of transplants and decrease costs (7, 19, 20). Furthermore, a survey revealed that 82% of transplant candidates would consider an HCV positive organ in some situations (26). Transplants from HCV viremic to uninfected recipients should be performed in the context of scientific institutional review board approved protocols as recommended by the American Society of Transplantation consensus conference on the use of hepatitis C viremic donors in solid organ transplantation (10).
The main strengths of our study are its large sample size and its use of PS matching to minimize confounders in the ascertainment of clinical outcomes. However, the following limitations should be noted. First, our study only provides evidence of at least comparable outcomes in the short-term (up to 12 months). Second, the OPTN/UNOS dataset lacks granularity with regards to HCV infection and treatment. Although not included, it is reasonable to assume that during the study period most recipients of HCV viremic organs received DAAs. The dataset also precludes determination of the risk of HCV transmission, the rate of HCV sustained virologic response at 12 weeks, and adverse event rates. We were also unable to compare the outcomes according to donor HCV genotype and viral load, HCV treatment strategy (pre-emptive vs. pre- and post-exposure vs. post-exposure), the timing of initiation of post-exposure treatment, and DAA used. However, other smaller published trials help address some of these knowledge gaps. In the THINKER trial (pre-emptive treatment approach), the rate of HCV transmission from viremic donors was 100% at five days. The rates of HCV sustained virologic response at 12 weeks in both the THINKER and EXPANDER trials were 100%. The only attributable adverse effect was the development of proteinuria at six months post-transplant due to focal segmental glomerulosclerosis in one recipient that improved with an angiotensin-receptor blocker.
Although our study provides evidence of at least comparable short-term clinical outcomes using HCV viremic donors for uninfected recipients, it does not provide sufficient evidence that use of these organs should become standard of care. Further studies are required to determine the long-term outcomes in this scenario and address additional unanswered questions. What is the optimal treatment strategy: pre-emptive, vs. pre- and post-exposure vs. post-exposure? Are the outcomes of post-exposure equivalent if HCV DAAs are started immediately after transplant, compared to 2 or 4 weeks after? In this regard, the transplant community needs to work in collaboration with payers to develop pathways for HCV treatment of recipients of viremic donors. Current DAAs have not been tested or approved by the FDA for the treatment of acute HCV (as would be the case of an HCV uninfected recipient of a viremic donor). Furthermore, outside of a clinical trial, the likelihood of insurance companies paying for DAA therapy of recipients of HCV viremic donors is unknown. Even in the setting of chronic HCV infection, denials are not uncommon since 65% of state Medicaid programs have hepatic fibrosis restrictions (27–30). In the future, patients consenting to receive an HCV viremic organ should receive the available preliminary data and be made aware of current areas of uncertainty to allow patient-centered decision making that balances these risks against their mortality risk while on the waitlist.
In summary, our study demonstrated a lower proportion of DGF between the study groups (HCV seropositive, non-viremic donors to uninfected recipients and HCV viremic donors to uninfected recipients) and the reference group (HCV uninfected donors to uninfected recipients). Addtionally, HCV uninfected recipients of HCV viremic donor kidneys had better allograft function at 6 months compared to the reference group. There was no statistically significant difference in the secondary outcomes. The use of propensity score matching, and multivariable Cox proportional hazards regression to reduce confounding strengthen the confidence in these results. However, further studies are required to evaluate the long-term outcomes of DDKT in these populations and to address unanswered questions regarding the use of HCV viremic donors for uninfected recipients.
Supplementary Material
Acknowledgments
We are grateful to Dr. Ank E. Nijhawan and Dr. James Cutrell for their critical review of the manuscript.
Funding
This research is partly supported by the University of Texas Southwestern George M. O’Brien Kidney Research Core Center grant (NIH P30DK079328).
Abbreviations
- Ab
antibody
- cPRA
Calculated panel reactivity antibodies
- CIT
Cold ischemia time
- D
Donor
- DDKT
Deceased donor kidney transplant
- DAAs
Direct-acting antivirals
- DGF
Delayed graft function
- eGFR
estimated glomerular filtration rate
- ESRD
End-stage renal disease
- HCV
hepatitis C virus
- IDU
Intravenous drug use
- KDPI
Kidney Donor Profile Index
- LOS
Length of stay
- MDRD
Modification of Diet in Renal Disease Study
- NAT
nucleic acid testing
- OPTN
Organ Procurement and Transplantation Network
- PHS
Public Health Services
- PS
Propensity score
- R
Recipient
- SRTR
Scientific Registry of Transplant Recipients
- UNOS
United Network for Organ Sharing
- US
United States
Footnotes
Disclosures
The authors declare no conflict of interest related to the topic.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999;341(23):1725–1730. [DOI] [PubMed] [Google Scholar]
- 2.Schnitzler MA, Lentine KL, Burroughs TE. The cost effectiveness of deceased organ donation. Transplantation 2005;80(11):1636–1637. [DOI] [PubMed] [Google Scholar]
- 3.Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Robinson A et al. OPTN/SRTR 2016 Annual Data Report: Kidney. Am J Transplant 2018;18 Suppl 1:18–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durand CM, Bowring MG, Thomas AG, Kucirka LM, Massie AB, Cameron A et al. The Drug Overdose Epidemic and Deceased-Donor Transplantation in the United States: A National Registry Study. Ann Intern Med 2018;168(10):702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez SA, Trotter JF. The rise of the opioid epidemic and hepatitis C-positive organs: A new era in liver transplantation. Hepatology 2018;67(4):1600–1608. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg DS, Blumberg E, McCauley M, Abt P, Levine M. Improving Organ Utilization to Help Overcome the Tragedies of the Opioid Epidemic. Am J Transplant 2016;16(10):2836–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kling CE, Perkins JD, Landis CS, Limaye AP, Sibulesky L. Utilization of Organs From Donors According to Hepatitis C Antibody and Nucleic Acid Testing Status: Time for Change. Am J Transplant 2017;17(11):2863–2868. [DOI] [PubMed] [Google Scholar]
- 8.Kucirka LM, Singer AL, Ros RL, Montgomery RA, Dagher NN, Segev DL. Underutilization of hepatitis C-positive kidneys for hepatitis C-positive recipients. Am J Transplant 2010;10(5):1238–1246. [DOI] [PubMed] [Google Scholar]
- 9.Reese PP, Abt PL, Blumberg EA, Goldberg DS. Transplanting Hepatitis C-Positive Kidneys. N Engl J Med 2015;373(4):303–305. [DOI] [PubMed] [Google Scholar]
- 10.Levitsky J, Formica RN, Bloom RD, Charlton M, Curry M, Friedewald J et al. The American Society of Transplantation Consensus Conference on the Use of Hepatitis C Viremic Donors in Solid Organ Transplantation. Am J Transplant 2017;17(11):2790–2802. [DOI] [PubMed] [Google Scholar]
- 11.Bari K, Luckett K, Kaiser T, Diwan T, Cuffy M, Schoech MR et al. Hepatitis C transmission from seropositive, nonviremic donors to non-hepatitis C liver transplant recipients. Hepatology 2018;67(5):1673–1682. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe C, Tlusty S, Vece G, Sawyer R, Bag R, G. B et al. HCV Ab+/NAT- Organ Donors, and the Challenges of ‘Eclipse Windows’: Analysis of the OPTN/UNOS Ad Hoc Disease Transmission Advisory Committee (DTAC) In: American Transplant Congress; 2018; 2018. [Google Scholar]
- 13.Goldberg DS, Wolfe CR. Maximizing utilization of the donor pool by appropriate classification of hepatitis C antibody-positive donors. Am J Transplant 2018;18(10):2380–2381. [DOI] [PubMed] [Google Scholar]
- 14.de Vera ME, Volk ML, Ncube Z, Blais S, Robinson M, Allen N et al. Transplantation of hepatitis C virus (HCV) antibody positive, nucleic acid test negative donor kidneys to HCV negative patients frequently results in seroconversion but not HCV viremia. Am J Transplant 2018. [DOI] [PubMed] [Google Scholar]
- 15.Durand CM, Bowring MG, Brown DM, Chattergoon MA, Massaccesi G, Bair N et al. Direct-Acting Antiviral Prophylaxis in Kidney Transplantation From Hepatitis C Virus-Infected Donors to Noninfected Recipients: An Open-Label Nonrandomized Trial. Ann Intern Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reese PP, Abt PL, Blumberg EA, Van Deerlin VM, Bloom RD, Potluri VS et al. Twelve-Month Outcomes After Transplant of Hepatitis C-Infected Kidneys Into Uninfected Recipients: A Single-Group Trial. Ann Intern Med 2018;169(5):273–281. [DOI] [PubMed] [Google Scholar]
- 17.Taber DJ, Gebregziabher M, Payne EH, Srinivas T, Baliga PK, Egede LE. Overall Graft Loss Versus Death-Censored Graft Loss: Unmasking the Magnitude of Racial Disparities in Outcomes Among US Kidney Transplant Recipients. Transplantation 2017;101(2):402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.StataCorp. Stata 15 Base Reference Manual 2017. [cited 2019 01/05/19]; Available from: https://www.stata.com/manuals13/teteffectsnnmatch.pdf
- 19.Gupta G, Zhang Y, Carroll NV, Sterling RK. Cost-effectiveness of hepatitis C-positive donor kidney transplantation for hepatitis C-negative recipients with concomitant direct-acting antiviral therapy. Am J Transplant 2018;18(10):2496–2505. [DOI] [PubMed] [Google Scholar]
- 20.Kadatz M, Klarenbach S, Gill J, Gill JS. Cost-effectiveness of using kidneys from hepatitis C nucleic acid test-positive donors for transplantation in hepatitis C-negative recipients. Am J Transplant 2018;18(10):2457–2464. [DOI] [PubMed] [Google Scholar]
- 21.Sibulesky L, Kling CE, Blosser C, Johnson CK, Limaye AP, Bakthavatsalam R et al. Are we underestimating the quality of aviremic hepatitis C-positive kidneys? Time to reconsider. Am J Transplant 2018;18(10):2465–2472. [DOI] [PubMed] [Google Scholar]
- 22.Sibulesky L, Kling CE, Limaye AP, Johnson CK. Is Kidney Donor Profile Index (KDPI) Valid for Hepatitis C Aviremic Kidneys? Ann Transplant 2017;22:663–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudd RA, Seth P, David F, Scholl L. Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010–2015. MMWR Morb Mortal Wkly Rep 2016;65(50–51):1445–1452. [DOI] [PubMed] [Google Scholar]
- 24.Saran R, Robinson B, Abbott KC, Agodoa LY, Albertus P, Ayanian J et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2017;69(3 Suppl 1):A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danovitch GM, Cohen DJ, Weir MR, Stock PG, Bennett WM, Christensen LL et al. Current status of kidney and pancreas transplantation in the United States, 1994–2003. Am J Transplant 2005;5(4 Pt 2):904–915. [DOI] [PubMed] [Google Scholar]
- 26.McCauley M, Mussell A, Goldberg D, Sawinski D, Molina RN, Tomlin R et al. Race, Risk, and Willingness of End-Stage Renal Disease Patients Without Hepatitis C Virus to Accept an HCV-Infected Kidney Transplant. Transplantation 2018;102(4):e163–e170. [DOI] [PubMed] [Google Scholar]
- 27.Do A, Mittal Y, Liapakis A, Cohen E, Chau H, Bertuccio C et al. Drug Authorization for Sofosbuvir/Ledipasvir (Harvoni) for Chronic HCV Infection in a Real-World Cohort: A New Barrier in the HCV Care Cascade. PLoS One 2015;10(8):e0135645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo Re V 3rd, Gowda C, Urick PN, Halladay JT, Binkley A, Carbonari DM et al. Disparities in Absolute Denial of Modern Hepatitis C Therapy by Type of Insurance. Clin Gastroenterol Hepatol 2016;14(7):1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vu TM, Toribio W, Riazi F, Ciprian G, Gibbs N, Giardina M et al. Increasing Access to Hepatitis C Virus Medications: A Program Model Using Patient Navigators and Specialty Pharmacy to Obtain Prior Authorization Approval. J Manag Care Spec Pharm 2018;24(4):329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Younossi ZM, Bacon BR, Dieterich DT, Flamm SL, Kowdley K, Milligan S et al. Disparate access to treatment regimens in chronic hepatitis C patients: data from the TRIO network. J Viral Hepat 2016;23(6):447–454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


